- 1Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan
- 2Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
- 3Departments of Medical Imaging, National Taiwan University Hospital Yun-lin Branch, Douliu, Taiwan
- 4Departments of Internal Medicine, National Taiwan University Hospital Yun-lin Branch, Douliu, Taiwan
- 5Department of Business Administration and Graduate School of Service Management, Chihlee University of Technology, New Taipei City, Taiwan
- 6Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 7Department of Cardiovascular Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 8Department of Obstetrics and Gynecology, National Taiwan University Hospital, Taipei, Taiwan
- 9Department of Internal Medicine, National Taiwan University Hospital Hsin-Chu Branch, HsinChu, Taiwan
- 10Department of Computer Science and Engineering, Yuan Ze University, Taoyuan City, Taiwan
- 11Cardiovascular Center, National Taiwan University Hospital, Taipei, Taiwan
Objective: Primary aldosteronism (PA) is the most common type of secondary hypertension, and it is associated with a higher rate of cardiovascular complications. KCNJ5 somatic mutations have recently been identified in aldosterone-producing adenoma (APA), however their influence on vascular remodeling and injury is still unclear. The aim of this study was to investigate the association between KCNJ5 somatic mutation status and vascular status.
Methods: We enrolled 179 APA patients who had undergone adrenalectomy from a prospectively maintained database, of whom 99 had KCNJ5 somatic mutations. Preoperative clinical, biochemical and imaging data of abdominal CT, including abdominal aortic calcification (AAC) score, aortic diameter and wall thickness at levels of superior (SMA) and inferior (IMA) mesenteric arteries were analyzed.
Results: After propensity score matching for age, sex, body mass index, triglycerides and low-density lipoprotein, there were 48 patients in each KCNJ5 (+) and KCNJ5 (-) group. Mutation carriers had a lower AAC score (217.3 ± 562.2 vs. 605.6 ± 1359.1, P=0.018), higher aortic wall thickness (SMA level: 2.2 ± 0.6 mm vs. 1.8 ± 0.6 mm, P=0.006; IMA level: 2.4 ± 0.6 mm vs. 1.8 ± 0.7 mm, P<0.001) than non-carriers. In multivariate analysis, KCNJ5 mutations were independently associated with AAC score (P=0.014) and aortic wall thickness (SMA level: P<0.001; IMA level: P=0.004). After adrenalectomy, mutation carriers had less aortic wall thickness progression than non-carriers (Δthickness SMA: -0.1 ± 0.8 mm vs. 0.9 ± 0.6 mm, P=0.024; IMA: -0.1 ± 0.6 mm vs. 0.8 ± 0.7 mm, P=0.04).
Conclusion: KCNJ5 mutation carriers had less calcification burden of the aorta, thickened aortic wall, and less wall thickness progression than non-carriers.
Introduction
Primary aldosteronism (PA) is characterized by abnormal aldosterone hypersecretion, and it is the most common cause of secondary hypertension. Approximately 5-10% of general hypertensive patients may have PA (1), and this rate can be as high as 20% in patients with resistant hypertension (2). Clinically, PA patients are associated with higher cardiovascular events than those with essential hypertension (3, 4).
Excessive aldosterone is related to various cardiovascular injuries. Animal studies have shown that the infusion of aldosterone can cause increased arterial stiffness and vascular fibronectin accumulation, and that the damage can be reversed by an aldosterone antagonist (5). In human studies, higher pulse wave velocity has been reported in PA patients compared to those with essential hypertension, indicating increased arterial stiffness in PA patients (6, 7). These aldosterone-induced cardiovascular injuries may be reversible, as studies have shown that adrenalectomy can ameliorate increased carotid intima-media thickness and arterial stiffness in patients with aldosterone-producing adenoma (APA) (7, 8).
APA is one of the most common subtypes of PA and is surgically correctable (9). KCNJ5 (coding for potassium channel GIRK4) mutations are the most frequently identified somatic mutations in both Western and Asian countries, with a prevalence rate of 34-45% (10–12) and 55-75%, respectively (13–16). APA patients with KCNJ5 mutations tend to be younger, have a higher aldosterone level, lower potassium level, and higher cure rate after adrenalectomy (17). Regarding the effect of KCNJ5 mutations on the cardiovascular system, mutation carriers have been shown to exhibit greater post-operative regression of observed left ventricular remodeling and improvement in arterial stiffness compared to non-carriers (18–20).
Aortic calcification is considered to be an irreversible endpoint of vascular atherosclerosis and an ideal indicator of vascular injury (21), and it can be quantitatively evaluated on CT. Aortic wall thickness of the common carotid artery, thoracic aorta and abdominal aorta is also regarded to be an important marker of vascular atherosclerosis with the ability to predict cardiovascular events (22, 23), and it has been associated with cardiovascular risk factors such as old age, male sex, smoking, elevated systolic blood pressure and low-density lipoprotein.
The aim of this study was to assess the influence of KCNJ5 mutations on atherosclerotic parameters on CT and use propensity score matching (PSM) analysis to balance possible confounding factors.
Materials and Methods
Patient Enrollment
In this study, we retrospectively analyzed 179 APA patients who underwent adrenalectomy between September 2006 and March 2019 at National Taiwan University Hospital (NTUH) from a prospectively maintained database. This study was approved by the Institutional Review Board of NTUH, and the need for written informed consent was waived. The clinical information of the patients, including demographic data, atherosclerotic parameters of abdominal CT, and histopathological results of APAs were recorded.
Laboratory Measurements
Plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were measured using specific radioimmunoassay kits (Aldosterone Maia Kit; Adaltis Italia, Bologna, Italy and DiaSorin, Stillwater, Minnesota, USA, respectively). The aldosterone-to-renin ratio (ARR) was calculated as PAC/PRA.
Diagnostic Criteria for APA
The diagnosis of APA was established based on the modified four corner criteria as reported previously (19, 24, 25), including: (1) excess aldosterone production confirmed according to an ARR > 35, TAIPAI score > 60%; and seated post-saline loading PAC > 16 ng/dl or urine aldosterone > 12 μg/24 h; (2) identification of adrenal nodules on CT; (3) lateralization of aldosterone hypersecretion by adrenal venous sampling (AVS) or dexamethasone suppression NP-59 single-photon emission CT; (4) pathological evidence of adenoma after adrenalectomy, and subsequent clinical improvement defined by either complete resolution of hypertension or partial resolution of hypertension, potassium, PAC, and PRA.
In this study, successful AVS was defined as a sampled adrenal plasma cortisol concentration (PCC) similar to or two-fold greater than sampled peripheral PCC. The lateralization of PA was determined on the basis of a lateralization index of ≥2.0, which was estimated as the ratio of the sampled adrenal PAC/PCC on the dominant side to the PAC/PCC on the contralateral side.
Imaging Analysis
Abdominal CT data were available for all APA patients and were obtained using routine techniques (helical CT, 5-mm slices). One radiologist (V.J.K) with 3 years of experience independently evaluated the images on a standard imaging workstation and was unaware of the KCNJ5 mutation status. Aortic wall thickness and diameter were measured at superior mesenteric artery (SMA) and inferior mesenteric artery (IMA) levels, respectively. The thickest portion of the aortic wall and maximum aortic transverse diameter were estimated using reconstructed contrast-enhanced CT images perpendicular to the vascular centerline, to avoid overestimation due to tortuous segments of the aorta. Abdominal aortic calcification (AAC) score was calculated using the CT-based Agatston method from unenhanced abdominal CT axial view images using commercial software (Philips IntelliSpace Portal, Best, Netherlands). Vascular calcifications with attenuation greater than the predefined 130-HU threshold were estimated. A region of interest was manually selected so that only calcifications in the abdominal aorta were included. In this study, we used the Agatston score as described by Agatston for abdominal aorta (26).
Histopathologic Study and Sequencing of the KCNJ5 Gene
Laparoscopic adrenalectomy was used for all APA patients in this study. The resected adrenal specimens were blindly inspected by a pathologist. Nodules consisting of adrenal cells and a clearly demarcated pseudo-capsule were defined as adenomas. Before DNA extraction, fresh APA specimens were frozen at −80°C until use. Genomic DNA was prepared using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) for each tissue sample. Exome sequencing was used to assess the coding region of the genomic DNA. Four sets of gene-specific primers were used to amplify and sequence the whole coding sequence (exons 2–3) and the flanking regions of KCNJ5 (27). The PCR reactions was set at 58°C for primer annealing using GoTaq® Master Mix (Promega Corporation, Madison, USA), and DNA fragments were extracted using a GenepHlow™ Gel/PCR Kit (Geneaid, Taipei, ROC). The PCR products were sent for Sanger sequencing using a 3730 DNA Analyzer (Applied Biosystems, Foster City, USA).
Statistical Analysis
All statistical analyses were performed using MedCalc statistical software (MedCalc version 15.4.0.0, Frank Schoonjans, Mariakerke, Belgium). Differences between categorical variables were compared using Fisher’s exact test. For independent continuous variables, the differences were compared using an independent two-sample t-test. Continuous variables with skewed distribution such as PAC, ARR, and calcium score were compared using the Mann-Whitney U test. We used propensity score analysis to eliminate possible confounders between the KCNJ5 mutation carrier and non-carrier groups. Clinical variables including age, sex, body mass index (BMI) and low-density lipoprotein (LDL) were included to generate propensity scores. The maximum allowable difference was 8 for age, 6 for BMI, 14 for LDL, and an exact match for sex, with 1:1 matching to select patients from both groups for subsequent analysis. Independent associations between KCNJ5 mutation status and AAC score, aortic wall thickness, aortic diameter progression, aortic wall thickness progression and AAC score progression were investigated using multivariable regression analyses. For all statistical analyses, the significance was 2-tailed with a threshold for significance of P<0.05.
Results
Clinical Data in All APA Patients Before and After Matching
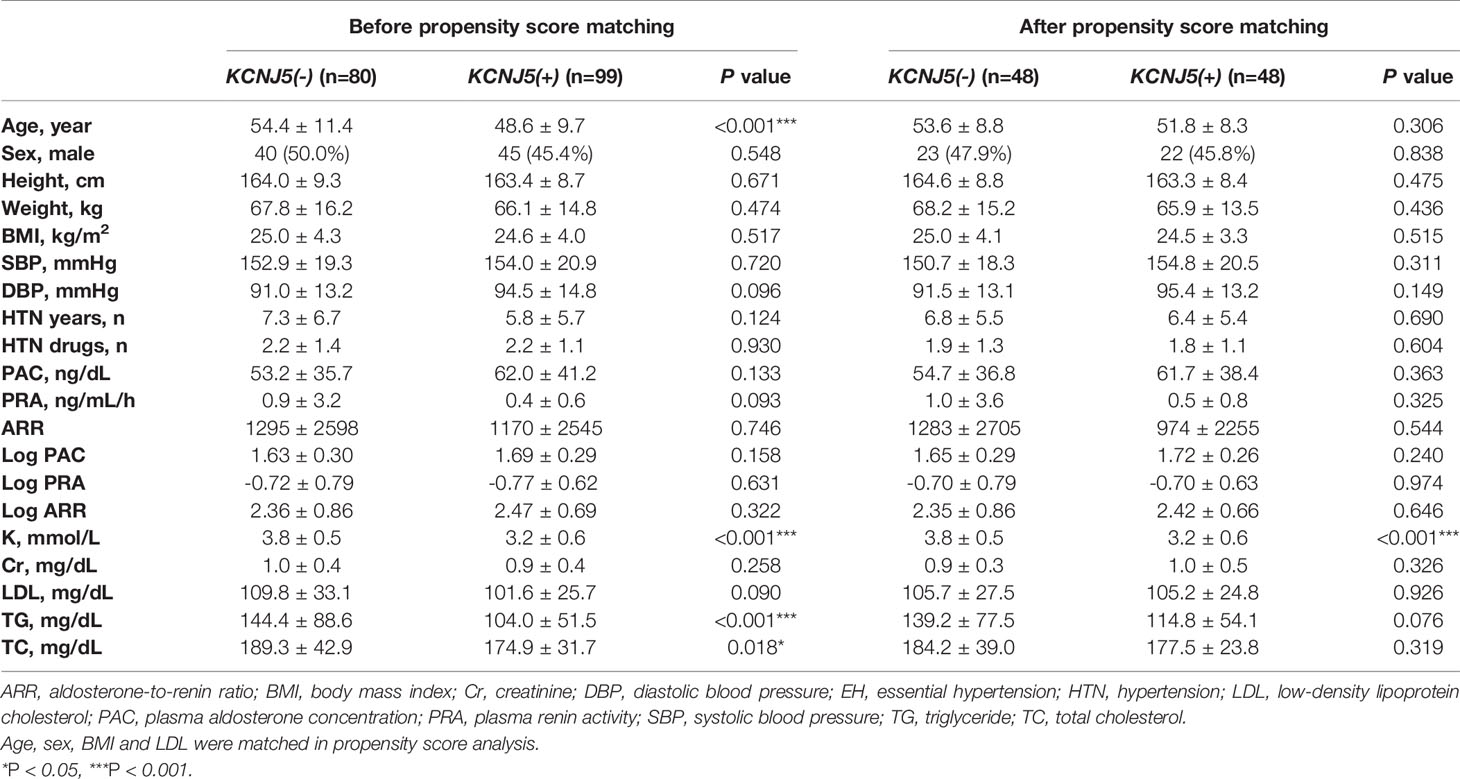
Of the 179 APA patients, 99 had KCNJ5 mutations and the other 80 did not. A comparison of the pre-operative clinical variables of the two groups are summarized in Table 1. The mutation carriers were younger (P<0.001) and had lower serum levels of potassium (P<0.001) and triglycerides (P<0.001) than the non-carriers. The other clinical variables including sex, BMI, blood pressure, duration of hypertension, and number of hypertensive medications were similar between both groups. After 1:1 PSM for age, sex, BMI and serum triglycerides, each group had 48 patients remained in each group. The matched mutation carriers had a lower serum potassium level (P<0.001) than the non-carriers, while other clinical variables were balanced without significant difference (Table 1).
Atherosclerotic Parameters Before and After Matching
The atherosclerotic parameters on abdominal CT, including aortic diameter, aortic wall thickness and AAC score, were evaluated for both groups in this study. Before PSM, the mutation carriers had an increased aortic wall thickness at the SMA (P=0.001) and IMA levels (P=0.001), but a lower rate of mitral valve calcification (P=0.026) and lower AAC score (P<0.001) compared to the non-carriers (Table 2). After PSM, the mutation carriers still had a higher aortic wall thickness at the SMA (P=0.006) and IMA levels (P<0.001), but a lower AAC score (P=0.018) than the non-carriers (Table 2).
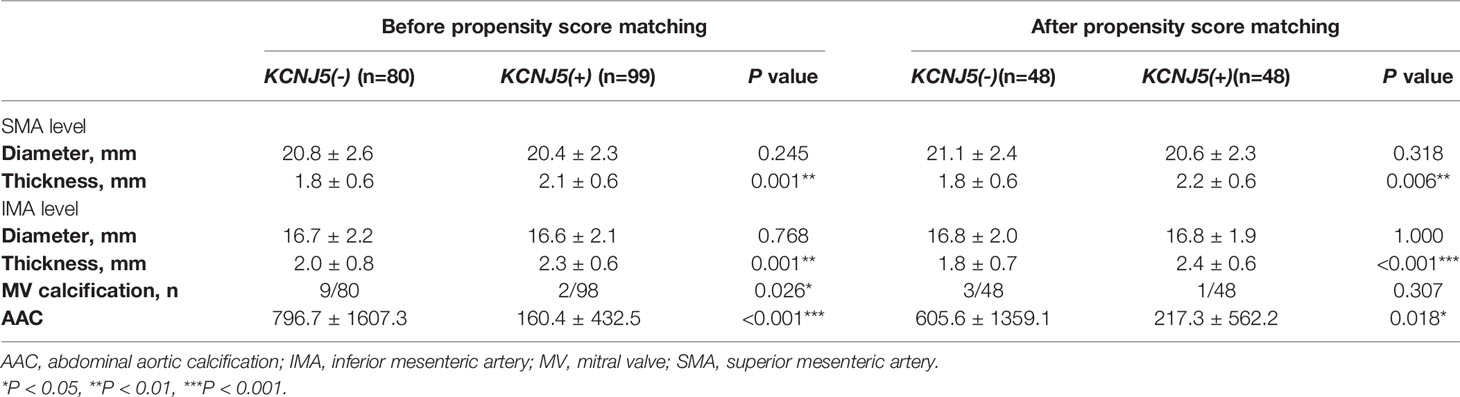
Table 2 Abdominal aortic calcification, diameter and thickness in patient with APA before and after propensity score matching.
Factors Associated With Baseline Aortic Wall Thickness
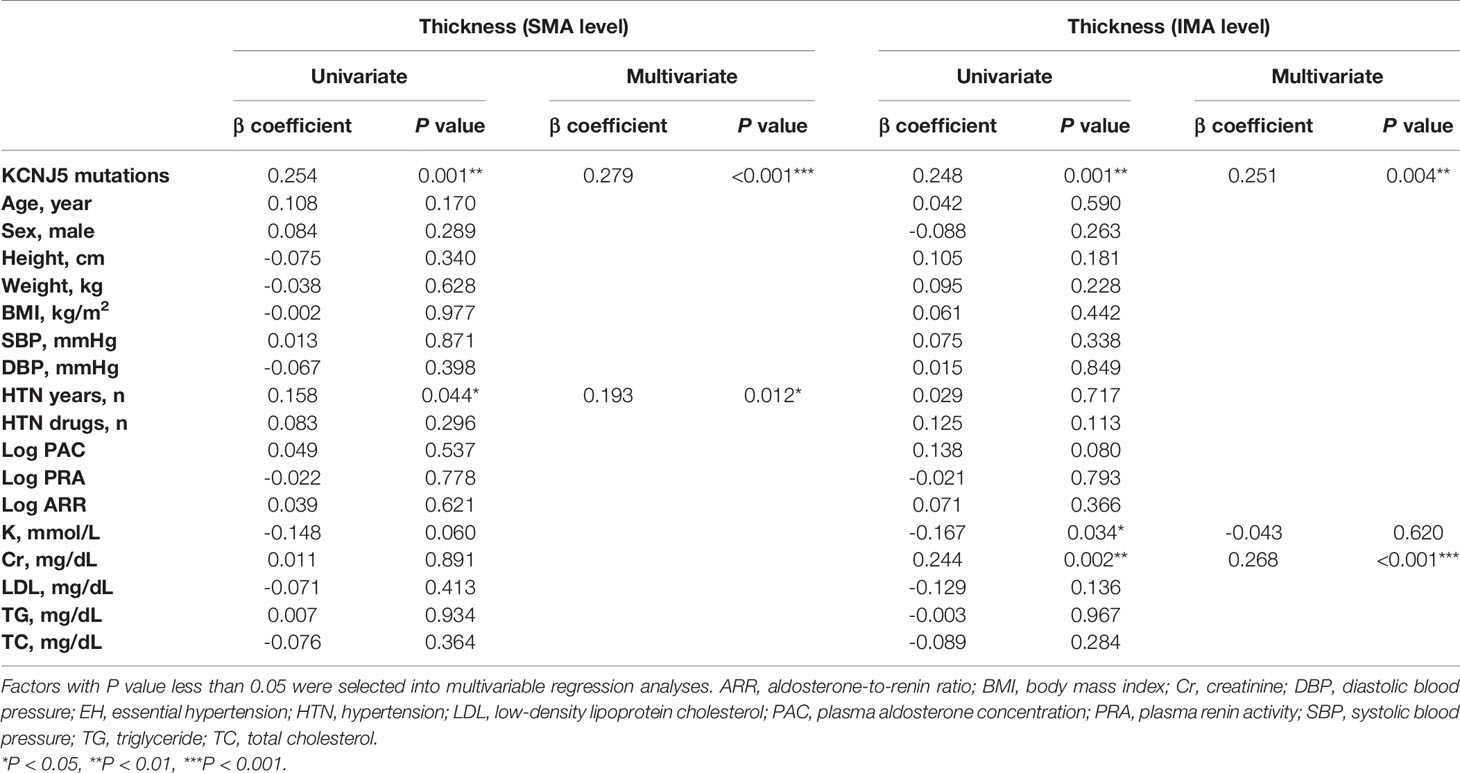
In univariate analysis (Table 3), aortic wall thickness at the SMA level was associated with KCNJ5 mutations (P=0.001) and the duration of hypertension (P=0.044), and aortic wall thickness at the IMA level was associated with KCNJ5 mutations (P=0.001), potassium (P=0.034) and creatinine (P=0.002) levels. In multivariable analysis, KCNJ5 mutations were independently associated with aortic wall thickness at the SMA (β=0.279, P<0.001) and IMA (β=0.251, P=0.004) levels.
Factors Associated With Baseline Aortic Calcification
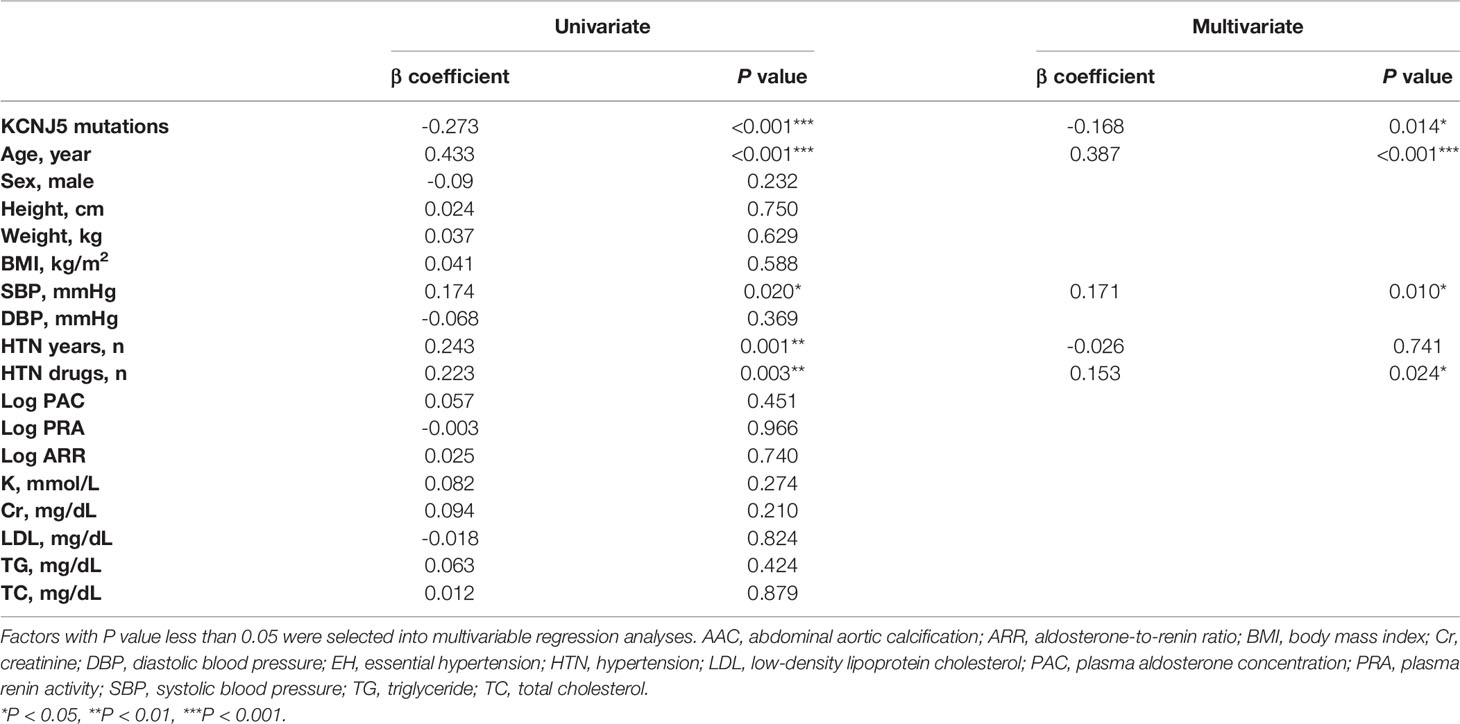
As shown in Table 4, AAC score was related to several factors including KCNJ5 mutations (P<0.001), age (P<0.001), systolic blood pressure (P=0.020), duration of hypertension (P=0.001) and number of hypertension drugs (P=0.003) in univariate analysis. In multivariable analysis, AAC score was independently associated with KCNJ5 mutations (β=-0.168, P=0.014), age (β=0.387, P<0.001), systolic blood pressure (β=0.171, P=0.010), and number of hypertension drugs (β=0.153, P=0.024).
Changes in Atherosclerotic Parameters on Abdominal CT After Adrenalectomy
Twenty-six patients had post-operative CT images. After adjusting for age, sex, and follow-up examination interval in multivariable analysis, the non-carriers had higher aortic wall thickness progression after adrenalectomy at the SMA (Δthickness: -0.1 ± 0.8 mm vs. 0.9 ± 0.6 mm, P=0.024) and IMA (Δthickness: -0.1 ± 0.6 mm vs. 0.8 ± 0.7 mm, P=0.040) levels than the carriers (Table 5). The progression of AAC score was similar between the two groups (P=0.732).
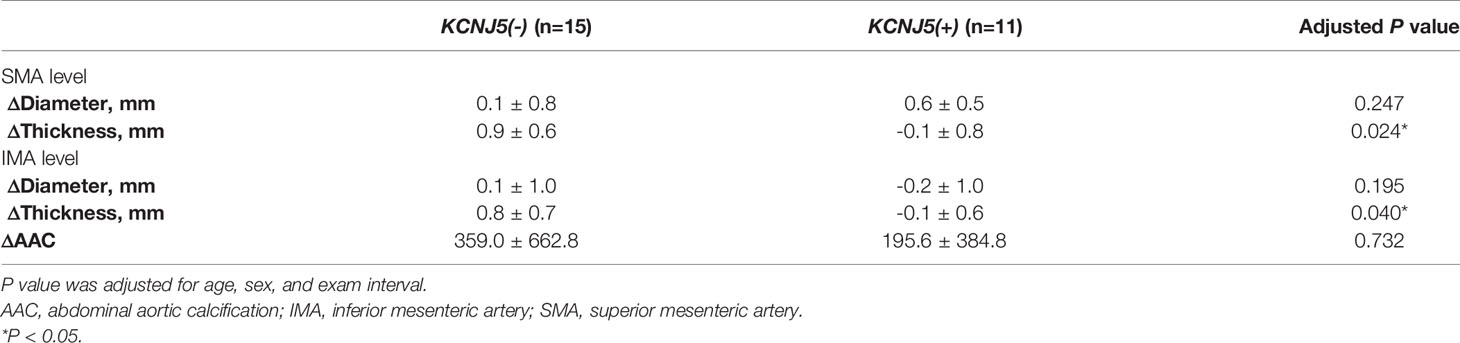
Table 5 Change of aortic calcification, diameter and thickness in patient with APA before and after adrenalectomy.
Discussion
There are several major findings in this study. First, APA patients with KCNJ5 somatic mutations had a thicker aortic wall and less aortic calcification compared to those without KCNJ5 mutations, even after matching for age, sex and blood pressure by PSM. Second, in multivariate analysis, the presence of KCNJ5 mutations was an independent factor associated with aortic wall thickness and aortic calcification. Third, after adrenalectomy, the patients with KCNJ5 mutations had less progression of aortic wall thickness compared to those without KCNJ5 somatic mutations.
The wall of the aorta is composed of tunica intima, tunica media, and tunica adventitia (28). The intima and media of the aortic wall can thicken due to adaptive collagen redistribution from aging, hypertension and pathological atherosclerosis with the formation of fat streaks and plaques (29, 30), and this has been shown to be a good marker of atherosclerosis and coronary artery disease (31, 32). Excessive aldosterone induces chronic inflammation of the vessel walls by increasing reactive oxygen species and proinflammatory transcription factors production (33). Enhanced monocytes and macrophages infiltration and adhesion on the endothelium are noted under high concentration of aldosterone (34), and the infiltrated inflammatory cells again worsen vascular inflammation (35). Aldosterone also promotes vascular remodeling of small arteries by increased collagen, fibronectin and ICAM-1 deposition in the vessel wall (36). PA is clinically associated with increased intima–media thickness of the carotid artery (37, 38), which may regress after adrenalectomy or spironolactone treatment. In this study, the APA patients with KCNJ5 somatic mutations had a thicker abdominal aorta compared to those without KCNJ5 mutations, which is probably due to higher blood pressure and more severe aldosteronism in the mutation carriers (19). In our previous study, APA patients with KCNJ5 somatic mutations had a higher degree of left ventricular hypertrophy and worse diastolic function (19). These findings may also have been due to similar reasons.
During the atherosclerotic process, atheroma or fibrous fatty plaques are formed, followed by calcium deposition in the latter stages (39). In the present study, we found more calcified plaques in those without KCNJ5 somatic mutations. The mechanism of aortic calcification formation is complex and involves multifactorial vascular inflammation (40). In our prior study we investigated serum CRP levels in PA patients, and showed that KCNJ5 mutations were associated with lower levels of pro-inflammation factors (41), metabolic syndrome and abdominal obesity (42). This may explain why the patients without KCNJ5 somatic mutations had a higher burden of aortic calcified plaques despite lower blood pressure and less severe aldosteronism compared to those with KCNJ5 somatic mutations.
Several somatic mutations including ATP1A1, ATP2B3, CACNA1D and KCNJ5 may contribute to the pathogenesis of APA (43–47). These mutations activate calcium signaling and lead to aldosterone production by increasing the expression of aldosterone synthase. In APA patient with KCNJ5 mutations, opened calcium channels and the overexpression of CYP11B2 lead to excessive aldosterone secretion from the adrenal tumor (47, 48). These mutation carriers have been found to have more severe aldosteronism compared to non-carriers (11, 12). Recently, CYP11B2 immunohistochemistry (IHC)-guided biopsy has been shown to increase the diagnostic performance of somatic mutations to around 87-94% in APA patients (49–51). One study demonstrated that an IHC-guided biopsy could increase the detection of somatic mutations in frozen adenoma tissue from 71% to 94% compared to a random biopsy by Sanger sequencing (51). In the present study, a random biopsy was used to sequence KCNJ5 mutations without the aid of CYP11B2 IHC guidance. However, an IHC-guided biopsy may have limited value in detecting KCNJ5 mutations, as they could be detected regardless of CYP11B2 expression in a random biopsy. Thus, we believe that the rate of KCNJ5 mutations identified by random biopsy is reliable in our study. Notably, the prevalence of other somatic mutations besides KCNJ5 were low (<6%) in our prior studies (13), indicating that the detection performance for other somatic mutations may be inferior by random biopsy compared to IHC-guided biopsy. As a result, the non-carriers of KCNJ5 mutations in the present study may have had various types of somatic mutations besides KCNJ5. However, we focused on the influence of KCNJ5 mutations with regards to aortic atherosclerosis in this study; therefore, our conclusions may not be affected by the lack of IHC-guided biopsy.
There are several limitations to this study. First, somatic mutations besides KCNJ5 such as ATP1A1, ATP2B3, CACNA1D and other new genes were not evaluated in this study, and the patients were grouped as being non-carriers of KCNJ5 mutations (44, 46, 52, 53). In addition, we did not use CYP11B2 IHC-guided biopsy to detect somatic mutations, which may have performed better than random biopsy. Second, despite the use of PSM to ameliorate imbalances in age, sex, BMI and LDL between the two groups of APA patients, other unknown discrepancies may have contributed to the increased aortic calcification in the non-carriers, and we do not have pathological data regarding the atherosclerotic burden of resected adrenal samples. Third, the number of patients with available follow-up abdominal CT was relatively small (n=26). However, the progression of aortic wall thickness remained significant after adjusting for age, sex and examination interval, indicating that small number of patients only had a limited impact. Finally, the CT images used to generate AAC and aortic wall thickness used 5.0 mm slice reconstruction. Although the correlation between the measured AAC from 2.5mm and 5.0mm slice thickness was almost perfect (r = 0.999, P < 0.0001), a 10% underestimation of AAC maybe expected due to partial volume effect (54, 55).
In conclusion, APA patients with KCNJ5 somatic mutations had a thicker abdominal aortic wall but less atherosclerotic calcification compared to those without KCNJ5 mutations. After surgery, the APA patients with KCNJ5 somatic mutations had less wall thickness progression than the non-carriers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of NTUH. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
B-CL: project concept and design, data collection, imaging analysis, data analysis, and write up. VK: data collection, imaging analysis, and write up. C-CC project concept and design. J-ZH: data collection, imaging analysis. Y-YC: project concept and design. C-HT: project concept and design. Z-WC: project concept and design. Y-LL: project concept and design. C-HC: critical revisions. C-WL: critical revisions. C-TP: critical revisions. C-SH: critical revisions. V-CW: project concept and design, data collection, and critical revisions. Y-HL: project concept and design, data collection, imaging analysis, and critical revisions. All authors contributed to the article and approved the submitted version.
Appendix
Membership of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group: Che-Hsiung Wu, MD (Chi-Taz Hospital, PI of Committee); V-CW, MD (NTUH, PI of Committee); Y-HL, MD (NTUH, PI of Committee); Hung-Wei Chang, MD, PhD (Far Eastern Clinics, PI of Committee); Lian-Yu Lin MD, PhD (NTUH, PI of Committee); Fu-Chang Hu, MS, ScD, (Harvard Statistics, Site Investigator); Kao-Lang Liu, MD (NTUH, PI of Committee); Shuo-Meng Wang, MD (NTUH, PI of Committee); Kuo-How Huang, MD (NTUH, PI of Committee); Yung-Ming Chen, MD (NTUH, PI of Committee); C-CC, MD (NTUH, PI of Committee); Shih-Cheng Liao, MD (NTUH, PI of Committee); Ruoh-Fang Yen, MD, PhD (NTUH, PI of Committee); and Kwan-Dun Wu, MD, PhD (NTUH, Director of Coordinating Center).
Funding
This study was supported by National Taiwan University Hospital (NTUH 108-A141), Ministry of Science and Technology (MOST 106-2314-B-002-169-MY3), and Department of Health, Executive Yuan, R.O.C. (PTH10744). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staff of the Second Core Lab of the Department of Medical Research at National Taiwan University Hospital for technical assistance.
References
1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J Am Coll Cardiol (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
2. Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, et al. Prevalence of Primary Hyperaldosteronism in Resistant Hypertension: A Retrospective Observational Study. Lancet (2008) 371:1921–6. doi: 10.1016/S0140-6736(08)60834-X
3. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic Outcomes and Mortality in Medically Treated Primary Aldosteronism: A Retrospective Cohort Study. Lancet Diabetes Endocrinol (2018) 6:51–9. doi: 10.1016/S2213-8587(17)30367-4
4. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an Increased Rate of Cardiovascular Events in Patients With Primary Aldosteronism. J Am Coll Cardiol (2005) 45:1243–8. doi: 10.1016/j.jacc.2005.01.015
5. Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased Carotid Wall Elastic Modulus and Fibronectin in Aldosterone-Salt-Treated Rats: Effects of Eplerenone. Circulation (2002) 106:2848–53. doi: 10.1161/01.CIR.0000039328.33137.6C
6. Strauch B, Petrak O, Zelinka T, Wichterle D, Holaj R, Kasalicky M, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens (2008) 21:1086–92. doi: 10.1038/ajh.2008.243
7. Lin YH, Lin LY, Chen A, Wu XM, Lee JK, Su TC, et al. Adrenalectomy Improves Increased Carotid Intima-Media Thickness and Arterial Stiffness in Patients With Aldosterone Producing Adenoma. Atherosclerosis (2012) 221:154–9. doi: 10.1016/j.atherosclerosis.2011.12.003
8. Liao CW, Lin LY, Hung CS, Lin YT, Chang YY, Wang SM, et al. Time Course and Factors Predicting Arterial Stiffness Reversal in Patients With Aldosterone-Producing Adenoma After Adrenalectomy: Prospective Study of 102 Patients. Sci Rep (2016) 6:20862. doi: 10.1038/srep20862
9. Amar L, Plouin PF, Steichen O. Aldosterone-Producing Adenoma and Other Surgically Correctable Forms of Primary Aldosteronism. Orphanet J Rare Dis (2010) 5:9. doi: 10.1186/1750-1172-5-9
10. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic Spectrum and Clinical Correlates of Somatic Mutations in Aldosterone-Producing Adenoma. Hypertension (2014) 64:354–61. doi: 10.1161/HYPERTENSIONAHA.114.03419
11. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, et al. Prevalence, Clinical, and Molecular Correlates of KCNJ5 Mutations in Primary Aldosteronism. Hypertension (2012) 59:592–8. doi: 10.1161/HYPERTENSIONAHA.111.186478
12. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab (2015) 100:E1089–95. doi: 10.1210/jc.2015-2149
13. Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, et al. The Prevalence of CTNNB1 Mutations in Primary Aldosteronism and Consequences for Clinical Outcomes. Sci Rep (2017) 7:39121. doi: 10.1038/srep39121
14. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and Mutations of KCNJ5 mRNA in Japanese Patients With Aldosterone-Producing Adenomas. J Clin Endocrinol Metab (2012) 97:1311–9. doi: 10.1210/jc.2011-2885
15. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical Characteristics of Somatic Mutations in Chinese Patients With Aldosterone-Producing Adenoma. Hypertension (2015) 65:622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
16. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, et al. Genetics of Aldosterone-Producing Adenoma in Korean Patients. PloS One (2016) 11:e0147590. doi: 10.1371/journal.pone.0147590
17. Vilela LAP, Rassi-Cruz M, Guimaraes AG, Moises CCS, Freitas TC, Alencar NP, et al. KCNJ5 Somatic Mutation Is a Predictor of Hypertension Remission After Adrenalectomy for Unilateral Primary Aldosteronism. J Clin Endocrinol Metab (2019) 104:4695–702. doi: 10.1210/jc.2019-00531
18. Rossi GP, Cesari M, Letizia C, Seccia TM, Cicala MV, Zinnamosca L, et al. KCNJ5 Gene Somatic Mutations Affect Cardiac Remodelling But do Not Preclude Cure of High Blood Pressure and Regression of Left Ventricular Hypertrophy in Primary Aldosteronism. J Hypertens (2014) 32:1514–21; discussion 1522. doi: 10.1097/HJH.0000000000000186
19. Chang YY, Tsai CH, Peng SY, Chen ZW, Chang CC, Lee BC, et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated With a Worse Baseline Status and Better Recovery of Left Ventricular Remodeling and Diastolic Function. Hypertension (2021) 77:114–25. doi: 10.1161/HYPERTENSIONAHA.120.15679
20. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of Cardiovascular Complications in Patients With and Without KCNJ5 Gene Mutations Harboring Aldosterone-Producing Adenomas. J Atheroscler Thromb (2015) 22:191–200. doi: 10.5551/jat.24455
21. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-Term Prognosis Associated With Coronary Calcification: Observations From a Registry of 25,253 Patients. J Am Coll Cardiol (2007) 49:1860–70. doi: 10.1016/j.jacc.2006.10.079
22. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation (2007) 115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875
23. Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH. Sex, Race, and Age Distributions of Mean Aortic Wall Thickness in a Multiethnic Population-Based Sample. J Vasc Surg (2011) 53:950–7. doi: 10.1016/j.jvs.2010.10.073
24. Rossi GP, Belfiore A, Bernini G, Desideri G, Fabris B, Ferri C, et al. Comparison of the Captopril and the Saline Infusion Test for Excluding Aldosterone-Producing Adenoma. Hypertension (2007) 50:424–31. doi: 10.1161/HYPERTENSIONAHA.107.091827
25. Wu VC, Chang HW, Liu KL, Lin YH, Chueh SC, Lin WC, et al. Primary Aldosteronism: Diagnostic Accuracy of the Losartan and Captopril Tests. Am J Hypertens (2009) 22:821–7. doi: 10.1038/ajh.2009.89
26. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J Am Coll Cardiol (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-T
27. Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, et al. Somatic Mutations Affecting the Selectivity Filter of KCNJ5 are Frequent in 2 Large Unselected Collections of Adrenal Aldosteronomas. Hypertension (2012) 59:587–91. doi: 10.1161/HYPERTENSIONAHA.111.186239
28. Jana S, Hu M, Shen M, Kassiri Z. Extracellular Matrix, Regional Heterogeneity of the Aorta, and Aortic Aneurysm. Exp Mol Med (2019) 51:1–15. doi: 10.1038/s12276-019-0286-3
29. Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic Modelling of Arterial Layers With Distributed Collagen Fibre Orientations. J R Soc Interface (2006) 3:15–35. doi: 10.1098/rsif.2005.0073
30. Finn AV, Kolodgie FD, Virmani R. Correlation Between Carotid Intimal/Medial Thickness and Atherosclerosis: A Point of View From Pathology. Arterioscler Thromb Vasc Biol (2010) 30:177–81. doi: 10.1161/ATVBAHA.108.173609
31. Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal Echocardiographically Detected Atherosclerotic Aortic Plaque Is a Marker for Coronary Artery Disease. J Am Coll Cardiol (1993) 21:144–50. doi: 10.1016/0735-1097(93)90729-K
32. Nishino M, Masugata H, Yamada Y, Abe H, Hori M, Kamada T. Evaluation of Thoracic Aortic Atherosclerosis by Transesophageal Echocardiography. Am Heart J (1994) 127:336–44. doi: 10.1016/0002-8703(94)90122-8
33. Fiebeler A, Schmidt F, Muller DN, Park JK, Dechend R, Bieringer M, et al. Mineralocorticoid Receptor Affects AP-1 and Nuclear Factor-Kappab Activation in Angiotensin II-Induced Cardiac Injury. Hypertension (2001) 37:787–93. doi: 10.1161/01.HYP.37.2.787
34. Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, et al. Functional Mineralocorticoid Receptors in Human Vascular Endothelial Cells Regulate Intercellular Adhesion Molecule-1 Expression and Promote Leukocyte Adhesion. Circ Res (2008) 102:1359–67. doi: 10.1161/CIRCRESAHA.108.174235
35. Cathcart MK. Regulation of Superoxide Anion Production by NADPH Oxidase in Monocytes/Macrophages: Contributions to Atherosclerosis. Arterioscler Thromb Vasc Biol (2004) 24:23–8. doi: 10.1161/01.ATV.0000097769.47306.12
36. Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL Endothelin Antagonism on Aldosterone-Induced Oxidative Stress and Vascular Remodeling. Hypertension (2003) 42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC
37. Holaj R, Zelinka T, Wichterle D, Petrak O, Strauch B, Widimsky J Jr. Increased Intima-Media Thickness of the Common Carotid Artery in Primary Aldosteronism in Comparison With Essential Hypertension. J Hypertens (2007) 25:1451–7. doi: 10.1097/HJH.0b013e3281268532
38. Holaj R, Rosa J, Zelinka T, Strauch B, Petrak O, Indra T, et al. Long-Term Effect of Specific Treatment of Primary Aldosteronism on Carotid Intima-Media Thickness. J Hypertens (2015) 33:874–82; discussion 882. doi: 10.1097/HJH.0000000000000464
39. Rudelli S, Viriato SP, Meireles TL, Frederico TN. Treatment of Displaced Neck Fractures of the Femur With Total Hip Arthroplasty. J Arthroplasty (2012) 27:246–52. doi: 10.1016/j.arth.2011.04.041
40. Chen NC, Hsu CY, Chen CL. The Strategy to Prevent and Regress the Vascular Calcification in Dialysis Patients. BioMed Res Int (2017) 2017:9035193. doi: 10.1155/2017/9035193
41. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and Clinical Correlates of Somatic Mutation in Aldosterone Producing Adenoma-Taiwanese Population. Sci Rep (2015) 5:11396. doi: 10.1038/srep11396
42. Chen KM, Chang YL, Wu TH, Lee BC, Chen PT, Liu KL, et al. Aldosterone-Producing Adenoma-Harbouring KCNJ5 Mutations Is Associated With Lower Prevalence of Metabolic Disorders and Abdominal Obesity. J Hypertens (2021) 39(12):2353–60. doi: 10.1097/HJH.0000000000002948
43. Stowasser M, Gordon RD. Primary Aldosteronism: Changing Definitions and New Concepts of Physiology and Pathophysiology Both Inside and Outside the Kidney. Physiol Rev (2016) 96:1327–84. doi: 10.1152/physrev.00026.2015
44. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic Mutations in ATP1A1 and ATP2B3 Lead to Aldosterone-Producing Adenomas and Secondary Hypertension. Nat Genet (2013) 45:440–4, 444e1-2. doi: 10.1038/ng.2550
45. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, et al. Somatic Mutations in ATP1A1 and CACNA1D Underlie a Common Subtype of Adrenal Hypertension. Nat Genet (2013) 45:1055–60. doi: 10.1038/ng.2716
46. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and Germline CACNA1D Calcium Channel Mutations in Aldosterone-Producing Adenomas and Primary Aldosteronism. Nat Genet (2013) 45:1050–4. doi: 10.1038/ng.2695
47. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science (2011) 331:768–72. doi: 10.1126/science.1198785
48. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium Channel Mutant KCNJ5 T158A Expression in HAC-15 Cells Increases Aldosterone Synthesis. Endocrinology (2012) 153:1774–82. doi: 10.1210/en.2011-1733
49. Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab (2018) 103:3869–76. doi: 10.1210/jc.2018-01004
50. Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension (2019) 73:885–92. doi: 10.1161/HYPERTENSIONAHA.118.12070
51. De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, Cellular, and Molecular Heterogeneity in Adrenals With Aldosterone-Producing Adenoma. Hypertension (2020) 75:1034–44. doi: 10.1161/HYPERTENSIONAHA.119.14177
52. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel Somatic Mutations in Primary Hyperaldosteronism are Related to the Clinical, Radiological and Pathological Phenotype. Clin Endocrinol (Oxf) (2015) 83:779–89. doi: 10.1111/cen.12873
53. Prada ETA, Burrello J, Reincke M, Williams TA. Old and New Concepts in the Molecular Pathogenesis of Primary Aldosteronism. Hypertension (2017) 70:875–81. doi: 10.1161/HYPERTENSIONAHA.117.10111
54. van der Bijl N, de Bruin PW, Geleijns J, Bax JJ, Schuijf JD, de Roos A, et al. Assessment of Coronary Artery Calcium by Using Volumetric 320-Row Multi-Detector Computed Tomography: Comparison of 0.5 Mm With 3.0 Mm Slice Reconstructions. Int J Cardiovasc Imaging (2010) 26:473–82. doi: 10.1007/s10554-010-9581-8
Keywords: hypertension, humans, KCNJ5 somatic mutation, primary aldosteronism, aortic calcification
Citation: Lee B-C, Kang VJ-W, Pan C-T, Huang J-Z, Lin Y-L, Chang Y-Y, Tsai C-H, Chou C-H, Chen Z-W, Liao C-W, Chiu Y-W, Wu V-C, Hung C-S, Chang C-C and Lin Y-H (2022) KCNJ5 Somatic Mutation Is Associated With Higher Aortic Wall Thickness and Less Calcification in Patients With Aldosterone-Producing Adenoma. Front. Endocrinol. 13:830130. doi: 10.3389/fendo.2022.830130
Received: 06 December 2021; Accepted: 07 February 2022;
Published: 02 March 2022.
Edited by:
Norlela Sukor, Universiti Kebangsaan Malaysia Medical Center (UKMMC), MalaysiaReviewed by:
Yao-chou Tsai, Taipei Medical University Hospital, TaiwanXin Gao, Tohoku University, Japan
Copyright © 2022 Lee, Kang, Pan, Huang, Lin, Chang, Tsai, Chou, Chen, Liao, Chiu, Wu, Hung, Chang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Chen Chang, bWFjb3RvY2NAZ21haWwuY29t
 Bo-Ching Lee
Bo-Ching Lee Victor Jing-Wei Kang
Victor Jing-Wei Kang Chien-Ting Pan
Chien-Ting Pan Jia-Zheng Huang1
Jia-Zheng Huang1 Cheng-Hsuan Tsai
Cheng-Hsuan Tsai Chia-Hung Chou
Chia-Hung Chou Che-Wei Liao
Che-Wei Liao Vin-Cent Wu
Vin-Cent Wu Chi-Sheng Hung
Chi-Sheng Hung Chin-Chen Chang
Chin-Chen Chang Yen-Hung Lin
Yen-Hung Lin