- 1Department of Pharmacy, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pain, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Nephrology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Traditional Chinese Medicine and Ethnic Medicine, Guangxi Institute for Food and Drug Control, Nanning, China
- 5Department of Endocrinology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Hubei Key Laboratory of Diabetes and Angiopathy, Hubei University of Science and Technology, Xianning, China
Maturity-onset diabetes of the young (MODY) is rare monogenic diabetes. However, MODY is often undiagnosed or misdiagnosed. In this study, we aimed to investigate the pathogenic gene for diabetes and provide precise treatment for diabetes patients in three families. Three families with suspected MODY were enrolled and screened for germline mutations using Whole exome sequencing (WES). Candidate pathogenic variants were validated in other family members and non-related healthy controls. Three heterozygous missense mutations in the ABCC8 gene (NM_001287174), c.1555 C>T (p.R519C), c.3706 A>G (p.I1236V), and c.2885 C>T (p.S962L) were found in families A, B, and C, respectively. All mutation sites cosegregated with diabetes, were predicted to be harmful by bioinformatics and were not found in non-related healthy controls. Two probands (onset ages, 8 and 12 years) were sensitive to glimepiride. However, an insufficient dose (2 mg/day) led to ketoacidosis. When the dosage of glimepiride was increased to 4 mg/day, blood sugar remained under control. A dose of 4 mg glimepiride daily also effectively controlled blood sugar in an adult patient 25-year-old. In addition, all patients were sensitive to liraglutide, which could control blood sugar better. These data suggest that ABCC8 was the pathogenic gene in three families with diabetes. Glimepiride (2 mg/day) was not effective in controlling blood sugar in children with ABCC8 mutations, however, 4 mg/daily glimepiride was effective in both adults and children. Moreover, liraglutide was effective in controlling blood sugar in both adults and children with ABCC8 mutations.
Introduction
Maturity-onset diabetes of the young (MODY) is an autosomal dominant mode of inheritance and a monogenic form of diabetes, characterized by an early age at onset (usually at ≤25 years of age) and impaired insulin secretion with minimal or no defects in insulin action (in the absence of coexisting obesity) (1, 2). To date, 14 genes have been identified to be responsible for MODY: HNF4A (MODY1), GCK(MODY2), HNF1A (MODY3), PDX1 (MODY4), HNF1B(MODY5), NEUROD1 (MODY6), KLF11 (MODY7), CEL(MODY8), PAX4(MODY9), INS (MODY10), BLK(MODY11), ABCC8 (MODY12), KCNJ11 (MODY13), and APPL1(MODY14) (2–4). MODY3 and MODY2 accounted for 80 – 90% of MODY in Caucasians (5, 6). MODY3 accounted for 30– 50% of the MODY cases, MODY1 and MODY5, respectively, accounted for 5% of the cases, and only a small proportion (< 1%) of the MODY cases were caused by other MODY subtypes (7–9). However, MODY was detected in only 9% of the patients in China with diabetes, therefore, most of the MODY genes in the Chinese population are still unknown (5).
The pathogenesis of MODY is highly genetically heterogeneous; thus, available treatment options are very different. For instance, GCK-MODY patients do not require treatment, whereas HNF1A- and HNF4A-MODY patients can be treated with low dose sulfonylurea and HNF1B-MODY patients require insulin treatment (10–13). Therefore, personalized medicine in MODY subtypes is essential. However, diagnosing MODY poses a challenge for physicians, with most cases remaining unidentified. This makes rapid diagnosis of MODY subtype of vital importance to patients and their families, because it provides a basis for individualized treatment and prognosis.
In this study, we report three heterozygous missense mutations, c.3706 A>G (p.I1236V) in family A, c.1555 C>T (p.R519C) in family B, and c.2885 C>T (p.S962L) in family C in the ABCC8 gene (NM_001287174). Blood sugar levels were better controlled in these families after adjusting the drug treatment plan according to the patient’s genetic test results.
Materials and methods
Participants
This study was approved by the Ethics Committee of the Central Hospital of Wuhan (2016–2). Informed consent was obtained from the patients and their families. Researchers had access to clinical information and medical records of the participants. Patients with a clinical diagnosis of MODY were included in the study and the criteria were age at diagnosis of ≤25 years, negative islet autoantibodies, and a family history of diabetes.
Whole-exome sequencing (WES)
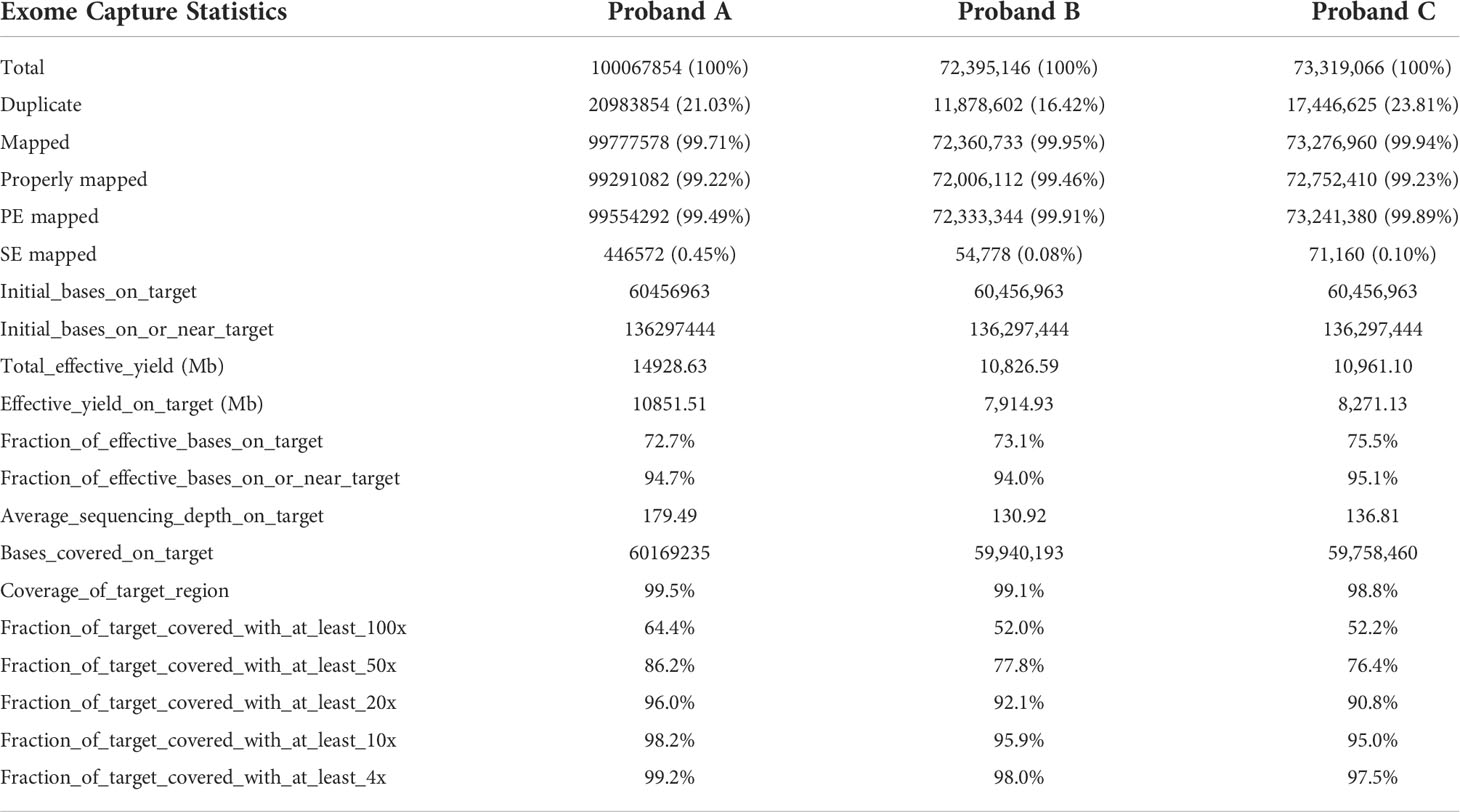
Genomic DNA was extracted from the participants’ peripheral blood samples (TIANGEN DNA extraction kit, Beijing, China). We used the Agilent SureSelect Human All Exon V6 kit for capture, followed by sequencing on the Illumina hiSeq2500 system, reaching an average depth of approximately 50×. The reference genome for aligning WES was human GRCh37/hg19. SNPs and indels were called by SAMtools, correcting for overestimated mapping quality from Burrows-Wheeler Alignment tool (14).
Genetic analysis
Variants were filtered to retain protein-altering variants (including non-synonymous, frame shift and splicing, and excluding non-exonic and synonymous variants), and to exclude those with minor allele frequency (<0.001) according to certain public databases (1000genomes, ExAc, dbSNP, ESP and gnomAD). The effects of the identified variants were evaluated using silico prediction tools, namely SIFT, PolyPhen2_HDIV, PolyPhen2_HVAR, LRT, Mutation Taster, Mutation Assessor and FATHMM (15). We selected genes previously implicated in monogenic diabetes, genes with important roles in the beta cell, and genes associated with type 2 diabetes or fasting glucose (16). Variants of uncertain significance (VUS), defined as functional significance, are not recorded in the public database and there are no relevant reports in the literature, such as novel missense variants, variants with conflicting evidence, or variants with insufficient evidence (17).
Sanger sequencing
Candidate variants in the proband and his family were confirmed by direct Sanger sequencing of the PCR products. Afterwards, the candidate variants that cosegregated with diabetes were selected for further analysis. The ABCC8 gene was amplified by PCR using forward primer (proband A) 5′-TCAAGGCCTCCTGCTTCTGT-3′ and reverse primer 5′-TGTCCAGGCCTCAGCTTCTT-3′, forward primer (proband B) 5′- AGCAGGAAGGCTTGGTGT-3′andreverse primer 5′- TTGGAGGAGGTGGGGATT-3′, forward primer (proband C) 5′- ACACCCAGAACCCCAAACCT-3′and reverse primer 5′- GTGTCTGTCTGCCCCTCCCT-3′. The PCR conditions were as follows: 95 C for 5 min; 35 cycles of 95 °C for 1 min, 60 °C for 30 sec, and 72 °Cfor 1 min; and then 72 °C for 10 min. The PCR products were directly sequenced (Beijing Genomics Institute, Wuhan, China) (18).
Medication plan adjustments and follow-up
The patients’ treatment plans were adjusted based on genetic test results and previous literature reports. For example, patients with ABCC8 gene mutations are more sensitive to sulfonylurea drugs. Therefore, according to the results of the gene test, patients with ABCC8 mutations can use sulfonylureas for hypoglycemic treatment. Blood glucose levels were regularly monitored (every day), and the HbA1C levels were controlled for two months.
Results
Medical history
Proband A was an eight-year-old female, who was admitted to the hospital for a stuffed nose and sleep snoring and was diagnosed with type 1 diabetes at the age of eight. Her body mass index (BMI) was14.0 kg/m2. The proband’s parents (III-1 and III-2) were 38 years old and non-diabetics, their BMIs were 26.1 kg/m2and 22.0kg/m2. The proband’s grandmother (II-1) was diagnosed with diabetes at the age of 51, and her BMI was 20.0kg/m2.The father of the proband’s grandmother (I-1) died from diabetes-related causes.
The proband B was a 12-year-old male, who was admitted to the hospital for the following symptoms: dry mouth, polydipsia, polyuria, and fatigue. The physical examination revealed fasting blood glucose of 14.28mmol/Land a BMI of 26.9 kg/m2. The proband’s father (III-1), mother (III-2), grandfather (II-1), maternal grandfather (II-4), and maternal grandfather’s sister (II-5) and brother (II-6) were diagnosed with diabetes at the ages of 43, 41, 59, 69, 59, and 61, respectively, and their BMIs were less than 28.0 kg/m2.
Proband C was a 25-year-old male, who was admitted to the hospital for dry mouth, polydipsia, polyuria, and fatigue, and was diagnosed with diabetes and ketoacidosis. His BMI was 27.8 kg/m2. He was prescribed insulin, which resulted in well-controlled blood sugar levels. The proband’s mother (II-4) and maternal grandfather (I-4) were diagnosed with diabetes at the ages of 41 and 60, respectively, and the proband’s mother (II-4) had a BMI of 22.8kg/m2.
The BMI of all family members of the diabetes patients was in the normal range or had not reached the obese range (> 28.0kg/m2). The pedigree of the three families is presented in Figure 1.
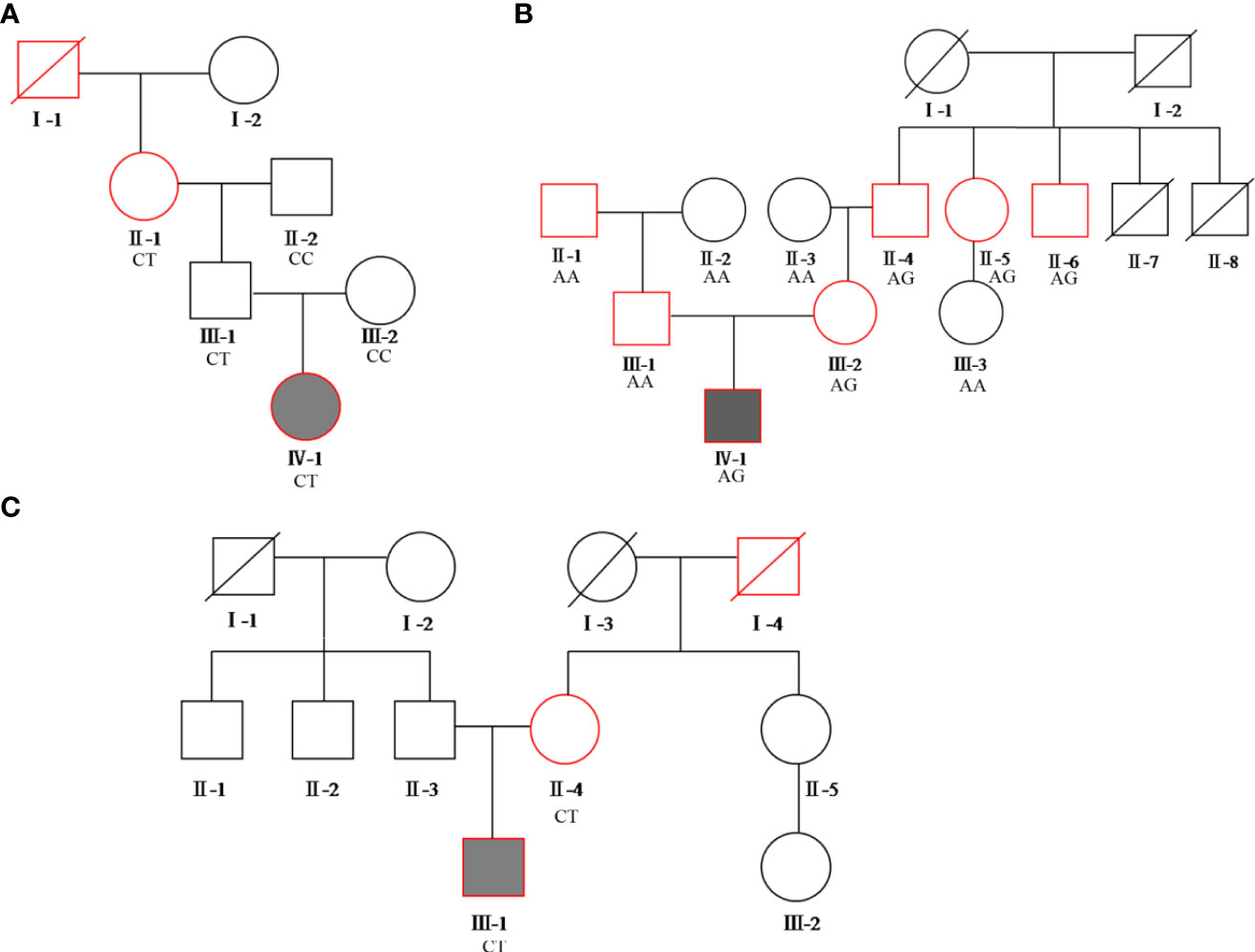
Figure 1 Pedigree of the three families. Squares represent males, while circles represent females. The red border represents diabetes, the blackened box represents the proband. (A) proband A’s family pedigree; (B) proband B’s family pedigree; (C) proband C’s family pedigree. The genotypes of each variation of c.1555 C>T, c.3706 A>G, and c.2885 C>T in the ABCC8 gene are shown in families (A–C), respectively.
Clinical characteristics
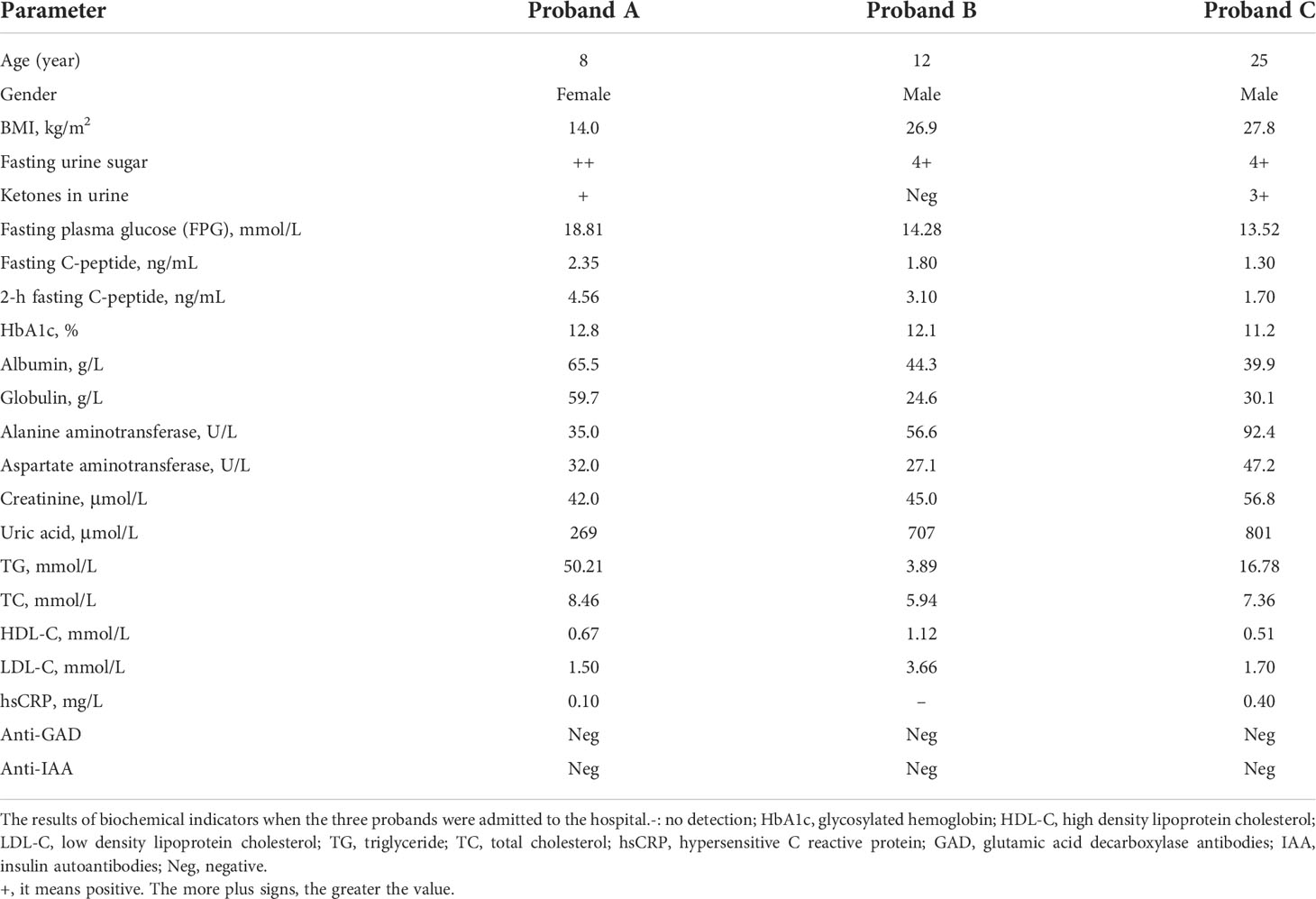
The clinical characteristics of the three probands are shown in Table 1. Proband A’s laboratory analysis revealed that her HbA1C level was 12.8%, her fasting plasma glucose (FPG) level was 18.81 mmol/L, and her fasting c-peptide was 2.35 ng/ml. She tested positive for urine glucose (2+) and urine ketones (1+), and her triglyceride levels were high (50.21 mmol/L), whereas she tested negative for all diabetes autoantibodies. Ultrasound examination of the liver, kidney, pancreas, bladder, ureter, spleen, and gall showed that organ morphology was normal. Although she was prescribed high-dose insulin and oral hypoglycemic agents (insulin glulisine, 20 units, three times a day; degludec, 30 units/night; metformin tablets, 850 mg twice a day; pioglitazone hydrochloride and metformin hydrochloride tablets, 15 mg/500mg, twice a day, and notified her guardian) for two days, her FPG levels did not drop. However, her triglyceride levels decreased to 12.6 mmol/L. The test results of proband A’s grandmother (II-1) were as follows: HbA1C, 7.1%; FPG, 11.7 mmol/L; fasting c-peptide, 1.60 ng/ml, and positive (1+) urine glucose, whereas ketones in the urine and diabetes autoantibodies were all negative. She (II-1) had diabetic retinopathy and diabetic neuropathy and was previously prescribed acarbose and insulin. However, her (II-1) blood sugar was not well controlled.
Physical examination revealed that proband B presented with hyperlipidemia, hyperuricemia (707 μmol/L), and mild fatty liver. Additionally, HbA1C was 12.1%, urine glucose was positive (4+), and fasting c-peptide was 1.8 ng/ml, while ketones in the urine and diabetic autoantibodies were all negative. There were no obvious abnormalities in other assessments. Although proband B’s islet function was normal, his fasting and two-hour postprandial blood glucose were high for a child. Hence, the doctor had been administering insulin, which led to the patient’s blood glucose being under control. Proband B’s father (III-1) was prescribed metformin and gliclazide sustained-release tablets, his HbA1C levels were 7.4%, FPG was 9.87 mmol/L, and he additionally presented with diabetic nephropathy and neuropathy. Proband B’s mother (III-2) and maternal grandfather (II-4) were prescribed insulin, metformin, and linagliptin. However, they (III-2 and III-4) did not achieve optimal outcomes since their HbA1C and FPG levels were approximately 10% and11 mmol/L, respectively. The maternal grandfather’s sister (II-5) and brother (II-6) of proband B were prescribed insulin and acarbose, respectively, their FPG levels reached 23 mmol/L at times, and both had diabetic retinopathy and neuropathy.
Proband C’s laboratory analysis revealed that his HbA1C level was 11.2%, FPG level was 13.52 mmol/L, fasting c-peptide was 1.30 ng/ml, urine glucose tested positive (4+), as well as ketones in urine (3+), and triglyceride levels were high (16.78 mmol/L). However, diabetic autoantibodies were all negative. Ultrasound examination indicated that all organs were normal and he exhibited no diabetes-related complications. Proband C was prescribed liraglutide for three days (0.6 mg/daily). Nonetheless, FPG and triglyceride levels decreased to 9.64 mmol/L and 6.11 mmol/L, respectively. The results of proband C’s mother (II-4) were as follows: HbA1C was 8.6%, the FPG level was 9.65 mmol/L, and she presented with complicated diabetic neuropathy. Although she (II-4) had been using metformin and acarbose, her blood sugar level was not under control.
Genetic and bioinformatic analyses
WES details are shown in Table 2. WES showed that the variants of each subject ranged from 110,000 to 130,000, including SNPs and InDels in probands A, B, and C. Variants were filtered as previously described and the defined panel of MODY genes (HNF4A, GCK, HNF1A, PDX1, HNF1B, NEUROD1, KLF11, CEL, PAX4, INS, BLK, ABCC8, KCNJ11, and APPL1) were firstly evaluated (15). ABCC8 was found to be a candidate pathogenic gene in all three families, and no other known MODY pathogenic variants were identified. The details are as follows. A known mutation was found in proband A: chromosome 11, position 17464342, c.C1555T, NM_001287174, p. R519C, namely, rs1057467571. This mutation has a very low frequency (25/251142, GnomAD_exome; 11/121224, ExAC; 1/31398, GnomAD) in the public database, but has not been reported in Chinese populations, and its clinical significance is not reported in ClinVar. Despite being a known mutation, its function remains unclear. In proband B, we found a novel mutation, chromosome 11, position 17419936, c.A3706G, NM_001287174, p. I1236V. In proband C, we discovered a known mutation, chromosome 11, position 17428939, c.C2885T, NM_001287174, p. S962L, namely, rs748931549.There is no reported frequency for this mutation in the public database. Its clinical significance is not reported in ClinVar. Despite being a known mutation, its function remains unclear. The three mutation sites were then sequenced and validated in other members of their respective families (Figure 2). Although we did not find them in non-related healthy controls (n=200), among the members of family A, the proband A’s father (currently 38 years old) also carried this mutation site (c.C1555T) but did not have diabetes. In family B, the mutation site (c.A3706G) was inherited from the mother (III-2), and the other patients (II-4, II-5, and II-6) all carried this mutation. Although proband B’s father may also have a genetic history of diabetes, no suspicious pathogenic site has been found so far; therefore, the pathogenic site of diabetes should come from probands B’s mother. In family C, the mutation site (c.C2885T) co-segregated with diabetes (Supplementary Table 1). However, all three mutation sites were predicted to be harmful mutations by multiple bioinformatics software, and the mutation sites were highly conserved in several species (Supplementary Tables 2, 3). Consequently, we speculated that these three mutation sites are pathogenic mutations in these families, and that they all belong to the ABCC8-MODY12 type.
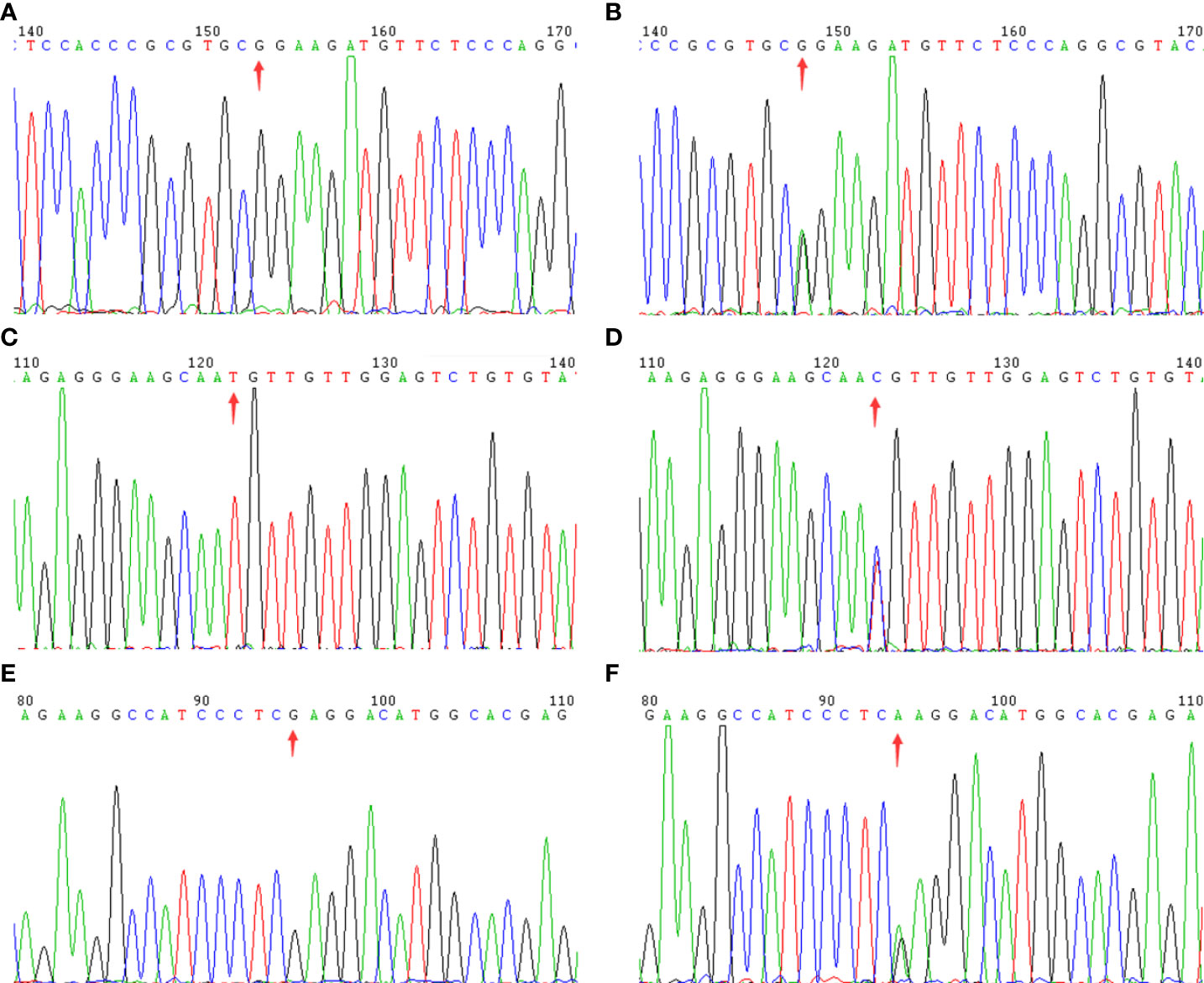
Figure 2 The sequencing chromatogram. Arrows indicate the changed position of the mutation in ABCC8 gene. Family A: (A) (wild type) and (B) (mutant type); Family B: (C) (wild type) and (D) (mutant type); Family C: (E) (wild type) and (F) (mutant type).
Precise treatment based on the patient’s genotype
Genetic testing confirmed that the above mentioned families belong to the MODY12 subtype. This information, combined with results reported in the literature, led to recommending that these patients replace current medical treatment with sulfonylurea drugs.
The proband A was administered insulin (insulin aspart injection, 12 units, subcutaneous injection before meals, and insulin glargine injection, 12 units, subcutaneous injection before 9 pm) due to high fasting blood sugar and positive urine ketones, as he was a child. However, the proband A’s fasting blood sugar was still high after two days of treatment. Then, the insulin dose was increased to 50 units, but the fasting blood glucose still did not decrease (the fasting blood sugar levels remained between 12 and 18 mmol/L), even when acarbose and metformin enteric-coated tablets were used. The results of the genetic test suggested that proband A was classified as MODY12, and there is no evidence of sulfonylureas being used previously to treat pediatric diabetes in China. Thus, we tried to utilize the relatively safe glimepiride combined with insulin on the child. When glimepiride was added, the child’s fasting blood glucose rapidly decreased. After a week, the child’s fasting blood glucose level was in the normal range (< 6.1 mmol/L).We then slowly reduced the dose of insulin (by 5 units a week), and shortly the insulin administration ceased, and only glimepiride (2 mg/day) was used. Nonetheless, after two weeks, proband A developed diabetic ketosis, necessitating the use of glimepiride (2 mg/day) in combination with a low-dose insulin aspart injection (5 units/day, twice a day) for hypoglycemia. Then, the dosage of glimepiride (4 mg/day) was increased and insulin administration was stopped. The results from follow-up tests in the following two months showed that the child’s fasting blood sugar (< 6.1 mmol/L) and glycation levels (< 6.5%) were within the normal ranges.
Proband B, who is currently 13 years old, presented with type 2 diabetes. Therefore, we replaced the previously prescribed insulin aspart with a new type of hypoglycemic drug, liraglutide injections at 1.2 mg/day, and notified his guardian. This drug’s hypoglycemic properties produced very effective results. After two months of follow-up, the patient’s blood sugar levels were under control and within the normal range (HbA1c < 6.5%). Taking into consideration that this proband B is MODY12 and a pediatric patient, liraglutide injections yielded very positive effects in terms of blood sugar reduction. Liraglutide was then gradually reduced. When glimepiride was added (2 mg/day), until liraglutide administration ceased, the blood glucose levels remained within a controlled range. However, ketosis appeared shortly, and thus, liraglutide use was continued. Then, the dosage of glimepiride was increased (4 mg/day) and liraglutide was stopped. The patient’s blood glucose returned to the normal range (HbA1c < 6.5%).
Proband C was also very sensitive to liraglutide injections (1.2 mg/day). Thus, this hypoglycemic treatment was very effective. Since injections are more problematic, whereas oral medication is relatively simple to administer, we recommended glimepiride 4mg/day as a medication plan based on the patient’s MODY12 type. After two months of follow-up, the patient’s blood glucose returned to a reasonable level (HbA1c < 6.5%).
Currently, other adult MODY12 patients in these three families were mainly using insulin or insulin combined with metformin or acarbose, with deficient results on blood sugar control. However, according to each patient’s genotype, they were slowly switched to glimepiride (4 mg/day), and their blood glucose levels were controlled after 2-3 months and the HbA1Clevels approached normal ranges(< 6.5%). All diabetes patients continued to be treated with glimepiride (4 mg daily).
Discussion
We have identified three heterozygous ABCC8 mutations in three different families. Two of these mutations are known mutations (p. R519C and p. S962L). However, their functional significance has not been reported yet. The remaining mutation was novel (p. I1236V). The three mutations (p. R519C, p. I1236V, and p. S962L) are highly likely to be pathogenic for the following reasons: their mutation sites were predicted to be harmful by bioinformatics software, the residues are highly conserved, and carriers of these mutation sites are very sensitive to glimepiride. The mutation p. R519Cwas located in the domain of ABC transmembrane type-1 1, whereas the mutation p. I1236Vwas located in the domain of ABC transmembrane type-1 2.Yet, functional studies are required to demonstrate that these mutations increase KATP channel activity or may result in loss-of-function and cause diabetes.
The ABCC8gene encodes the sulfonylurea receptor 1 (SUR1) subunit of the ATP-sensitive potassium (KATP) channel in pancreatic beta cells, which regulate insulin secretion. Mutations in the ABCC8 gene cause the overactivity or underactivity of the KATP channel, and thereby, a variety of phenotypes (19).Previous studies suggested an association between adult-onset diabetes and mutations in the ABCC8 gene (20). ABCC8 mutations can cause MODY in patients whose clinical features are similar to those with HNF1A/4AMODY (19), and some patients with ABCC8 mutations were able to change from insulin to oral glibenclamide therapy (21, 22), which means that patients with ABCC8presented with T2DM-like phenotypes. Exome sequencing identified many variants, most of which lacked the corresponding functional annotations and had no functional significance. However, a few variants were functionally significant. ABCC8 variants are associated with T2DM. Therefore, exome sequencing studies identified rare variants with functional significance in ABCC8, which were enriched in patients with T2DM.
At present, very limited types of drugs exist for the treatment of childhood diabetes. Metformin is the only oral therapy for youth with type 2 diabetes, even though up to 50% require additional agents within two years of diagnosis (23). Therefore, the current status of available treatments for children with type 2 diabetes is not optimistic. Insulin is generally indicated, despite being inconvenient to use and prone to causing hypoglycemia. Sulfonylurea sensitivity is a main feature of ABCC8 MODY (24). However, no studies have reported whether sulfonylureas are safe as a treatment for pediatric diabetes patients in China, especially in children with the MODY12 subtype. In this study, we found that the patients belonged to the MODY12 subtype, and consequently we tried to recommend oral sulfonylureas as medication, although there is no relevant experience or evidence of the use of sulfonylureas in children. Proband A, an eight-year-old female, exhibited high levels of both fasting blood glucose and glycosylated hemoglobin, and was, therefore, prescribed high-dose insulin and oral hypoglycemic agents (insulin glulisine, 20 units, three times daily; degludec, 30 units/night; metformin tablets, 850 mg, twice daily; and pioglitazone hydrochloride and metformin hydrochloride tablets, 15 mg/500mg twice daily) for two days. Nonetheless, her FPG levels did not decrease and we added a small dose of glimepiride, which caused her blood sugar to drop promptly. Thus, the insulin dose was gradually reduced, while the dose of glimepiride was increased to the point where insulin administration was completely ceased and only glimepiride (2 mg/day) was administered. However, proband A developed ketosis, a phenomenon that also occurred in proband B, moreover, the dosage of glimepiride was increased to 4 mg/day in both patients, and the patients’ blood glucose levels returned to the normal range. In summary, individuals with ABCC8 mutations are sensitive to sulfonylureas. The dosage of sulfonylureas is very important for children with ABCC8 mutations, and 4mg/day glimepiride was effective in both adults and children. In the cases where fasting blood sugar levels are abnormally high and the use of insulin cannot control blood sugar, it is recommended to add a low dose of sulfonylurea such as glimepiride, which has mild hypoglycemic effect. In addition, sulfonylureas are not currently recommended for juvenile diabetes patients in China. Therefore, China’s guidelines should recommend using sulfonylureas to prevent and treat childhood diabetes in patients with MODY12.
Glucagon-like peptide 1 (GLP-1) is a proglucagon cleavage product produced in intestinal L cells, which is secreted primarily after ingestion of glucose or a mixed meal. It increases glucose-stimulated insulin secretion at physiological plasma concentrations (25). GLP-1 receptor agonists provide the possibility to improve glycemic control and reduce body weight in type 2 diabetes without the risk of hypoglycemia, and its blood sugar reduction effect is significantly better than that of currently used drugs (25, 26).Although GLP-1 receptor agonists are currently used only for the treatment of type 2 diabetes (27), GLP-1 receptor agonists are also used to treat MODY patients, especially MODY4 (28) since this subtype is mainly characterized by type 2 diabetes. Although there is no relevant experience or evidence of usingGLP-1 receptor agonists in children in China, expert guidance on the clinical application of GLP-1 receptor agonists does not recommend using GLP-1 receptor agonists to treat adolescents with diabetes. In the present study, the patients presented with type 2 diabetes as well and were very sensitive to GLP-1 receptor agonists. Therefore, we postulate that GLP-1 receptor agonists are effective hypoglycemic drugs for treating children with type 2 diabetes, consistent with reports abroad (29). Current GLP-1 receptor agonists are mainly administered by subcutaneous injections. However, semaglutide is the first orally administered GLP-1 receptor agonist, which was approved by the U.S. Food and Drug Administration in September 2019 for adults with type 2 diabetes, and has been approved by the European Medicines Agency (30). Oral GLP-1 receptor agonists may exhibit greater tolerability than their subcutaneous counterparts.
In family A, although proband A’s father (currently 38 years old) carried this mutation site (c.C1555T), he did not have diabetes (FPG, 5.12 mmol/L; HbA1C, 5.4%; urine glucose, negative). According to reports in the literature (31, 32), we speculate that, first, individuals with ABCC8 mutations are diagnosed with diabetes across a wide age range (33); second, the onset of diabetes may be modified by certain genes (32, 34), delaying the age of onset in individuals with ABCC8 mutations or even suppressing diabetes symptoms. In addition, proband A did not respond to 50 units of insulin/day, and her triglyceride levels were 50.21 mmol/L, so she might be in a state of insulin resistance. In family B, proband B’s father may also have a genetic history of diabetes, or the child could possibly be a compound heterozygote with an unidentified mutation in the other chromosome from his mother, which would aggravate the diabetic phenotype. This could also be a coincidence, and the ABCC8 mutation might play a leading role in the occurrence of diabetes. In addition, patients with MODY12 subtype presented with diabetic retinopathy or neuropathy, which reinforces the need for precise treatment for these patients. This would contribute to controlling blood sugar in a targeted manner, to prevent complications and to improve the quality of life of patients. However, the functional significance of the above three variants in the ABCC8 gene was unclear (VUS). Although we found that these variants may be pathogenic mutations, their function needs to be further verified and should not be used to guide clinical management. In this study, three mutations (p. R519C, p. I1236V, and p. S962L) were rare according to the public database. However, patients with these mutations are sensitive to sulfonylureas or liraglutide. Therefore, patients with an age of onset of younger than 25 years old, a significant family history of diabetes, and negative test results for diabetes autoantibodies, should undergo diabetes-related genetic screening, and appropriate drugs and dosages should be selected according to the patient’s genotype.
To date, 14 different genes have been reported to cause diabetes with MODY-like phenotypes, and each MODY subtype has different patterns, such as the age at onset, clinical features, and therapeutic strategies (35). MODY accounts for 1-5% of all cases of diabetes (8).With improved knowledge and awareness of monogenic diabetes and the development of more accurate and inexpensive molecular diagnostic techniques, the diagnosis rate of MODY will increase, and the results of genetic diagnosis will provide the most precise treatment plans for the different types of MODY patients.
In conclusion, ABCC8 gene mutations c.1555 C>T (p.R519C), c.3705 A>G (p.I1236V), and c.2885 C>T (p.S962L) were the respective pathogenic mutations in three ABCC8-MODY pedigrees. Glimepiride (2 mg/day) was not effective in controlling blood sugar in children with ABCC8 mutations. However, 4 mg/day glimepiride was effective in both adults and children. Moreover, liraglutide was effective in controlling blood sugar in patients with ABCC8 mutations in both adults and children. Precise treatment based on individual genotype not only improves treatment effects and reduces adverse reactions, but also saves limited medical resources.
Authors contributions
Conceived and designed the experiments, JL, HZ, and YL. Performed the experiments, JL, YL, and AD. Analyzed the data, JL, LW, CL, HM, and XW. Wrote the paper, JL. JL, XW, and HM contributed equally. All authors contributed to the article and approved the submitted version.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of the Central Hospital of Wuhan. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81900719, No. 81800704 and No. 81870173) and the Health and Family Planning Commission of Wuhan City (No. WX18M02).
Acknowledgments
We thank the probands and their families for their support and participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.858096/full#supplementary-material
Supplementary Table 1 | Sanger sequencing results of candidate gene loci (ABCC8) in the three family members. HGVSc: human genome variation societycDNA; HGVSp: human genome variation society protein; ALT: alternative; REF: reference
Supplementary Table 2 | Pathogenicity results of candidate gene mutation sites (ABCC8) predicted by bioinformatics. HGVSc:human genome variation societycDNA; SIFT: Deleterious(<0.05); PolyPhen2_HVAR: Probably damaging (>=0.909), possibly damaging (0.447<=pp2_hdiv<=0.909); benign (<=0.446); PolyPhen2_HDIV: Probably damaging (>=0.957), possibly damaging (0.453<=pp2_hdiv<=0.956); benign (<=0.452); MutationTaster: Deleterious (>0.5); LRT: lower scores are more deleterious; MutationAssessor: Deleterious (>1.938); FATHMM: Deleterious (<-1.5); SiPhy_29way, PhyloP46way and PhyloP100way: higher scores are more deleterious; CADD: Deleterious (>15); Gerp++: Deleterious (>2); MCAP: Deleterious (>0.025); REVEL: reference range 0-1.
Supplementary Table 3 | Evolutionary conservation analysis for the three mutations in ABCC8.NP_001274103.1 match to NM_001287174.3; Proban (A) ABCC8:NM_001287174:exon10:c.C1555T:p.R519C; Proban (B) ABCC8:NM_001287174:exon30:c.A3706G:p.I1236V; Proban (C) ABCC8:NM_001287174:exon24:c.C2885T:p.S962L.
References
1. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PloS Med (2007) 4:e118. doi: 10.1371/journal.pmed.0040118
2. Fu J, Wang T, Zhai X, Xiao X. Primary hepatocellular adenoma due to biallelic HNF1A mutations and its co-occurrence with MODY 3: case-report and review of the literature. Endocrine (2019) 67:544–51. doi: 10.1007/s12020-019-02138-x
3. Gao R, Liu Y, Gjesing AP, Hollensted M, Wan X, He S, et al. Evaluation of a target region capture sequencing platform using monogenic diabetes as a study-model. BMC Genet (2014) 15:13. doi: 10.1186/1471-2156-15-13
4. Delvecchio M, Pastore C, Giordano P. Treatment options for MODY patients: A systematic review of literature. Diabetes Ther (2020) 11:1667–85. doi: 10.1007/s13300-020-00864-4
5. Xu JY, Dan QH, Chan V, Wat NM, Tam S, Tiu SC, et al. Genetic and clinical characteristics of maturity-onset diabetes of the young in Chinese patients. Eur J Hum Genet EJHG (2005) 13:422–7. doi: 10.1038/sj.ejhg.5201347
6. Anik A, Catli G, Abaci A, Bober E. Maturity-onset diabetes of the young (MODY): an update. J Pediatr Endocrinol Metab JPEM (2015) 28:251–63. doi: 10.1515/jpem-2014-0384
7. Kim SH. Maturity-onset diabetes of the young: What do clinicians need to know? Diabetes Metab J (2015) 39:468–77.doi: 10.4093/dmj.2015.39.6.468
8. Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, et al. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian childhood diabetes registry. Diabetologia (2017) 60:625–35. doi: 10.1007/s00125-016-4167-1
9. Mozzillo E, Salzano G, Barbetti F, Maffeis C, Lombardo F, Franzese A, et al. Survey on etiological diagnosis of diabetes in 1244 Italian diabetic children and adolescents: impact of access to genetic testing. Diabetes Res Clin Pract (2015) 107:e15–18. doi: 10.1016/j.diabres.2015.01.003
10. Stride A, Shields B, Gill-Carey O, Chakera AJ, Colclough K, Ellard S, et al. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia (2014) 57:54–6. doi: 10.1007/s00125-013-3075-x
11. Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. Jama (2014) 311:279–86. doi: 10.1001/jama.2013.283980
12. Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, et al. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care (2015) 38:1383–92. doi: 10.2337/dc14-2769
13. Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia (2017) 60:769–77. doi: 10.1007/s00125-017-4226-2
14. Bomba L, Walter K, Guo Q, Surendran P, Kundu K, Nongmaithem S, et al. Soranzo n: Whole-exome sequencing identifies rare genetic variants associated with human plasma metabolites. Am J Hum Genet (2022) 109:1038–54. doi: 10.1016/j.ajhg.2022.04.009
15. Li J, Sun S, Wang X, Li Y, Zhu H, Zhang H, et al. A missense mutation in IRS1 is associated with the development of early-onset type 2 diabetes. Int J Endocrinol (2020) 2020:1–8. doi: 10.1155/2020/9569126
16. Johansson S, Irgens H, Chudasama KK, Molnes J, Aerts J, Roque FS, et al. Exome sequencing and genetic testing for MODY. PloS One (2012) 7:e38050. doi: 10.1371/journal.pone.0038050
17. Brnich SE, Rivera-Munoz EA, Berg JS. Quantifying the potential of functional evidence to reclassify variants of uncertain significance in the categorical and Bayesian interpretation frameworks. Hum Mutat (2018) 39:1531–41. doi: 10.1002/humu.23609
18. Fu Y, Zeng Y, Chen T, Chen H, Lin N, Lin J, et al. Characterization and clinical significance of natural variability in hepatitis b virus reverse transcriptase in treatment-naive Chinese patients by Sanger sequencing and next-generation sequencing. J Clin Microbiol (2019) 57:e00119–19. doi: 10.1128/JCM.00119-19
19. Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia (2012) 55:123–7. doi: 10.1007/s00125-011-2319-x
20. Tarasov AI, Nicolson TJ, Riveline JP, Taneja TK, Baldwin SA, Baldwin JM, et al. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive k+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes (2008) 57:1595–604. doi: 10.2337/db07-1547
21. Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. New Engl J Med (2006) 355:456–66. doi: 10.1056/NEJMoa055068
22. Hartemann-Heurtier A, Simon A, Bellanne-Chantelot C, Reynaud R, Cave H, Polak M, et al. Mutations in the ABCC8 gene can cause autoantibody-negative insulin-dependent diabetes. Diabetes Metab (2009) 35:233–5. doi: 10.1016/j.diabet.2009.01.003
23. Meyers AG, Hudson J, Cravalho CKL, Matta ST, Villalobos-Perez A, Cogen F, et al. Metformin treatment and gastrointestinal symptoms in youth: Findings from a large tertiary care referral center. Pediatr Diabetes (2021) 22:182–91. doi: 10.1111/pedi.13148
24. Bowman P, Mathews F, Barbetti F, Shepherd MH, Sanchez J, Piccini B, et al. Neonatal diabetes international collaborative G: Long-term follow-up of glycemic and neurological outcomes in an international series of patients with sulfonylurea-treated ABCC8 permanent neonatal diabetes. Diabetes Care (2021) 44:35–42. doi: 10.2337/dc20-1520
25. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol (2012) 8:728–42. doi: 10.1038/nrendo.2012.140
26. Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: A systematic review and meta-analysis. Jama (2018) 319:1580–91. doi: 10.1001/jama.2018.3024
27. Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, Mikami T, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol (2021) 20:14. doi: 10.1186/s12933-020-01197-z
28. Ostoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care (2014) 37:1797–805. doi: 10.2337/dc13-3007
29. Tamborlane WV, Barrientos-Perez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in children and adolescents with type 2 diabetes. New Engl J Med (2019) 381:637–46. doi: 10.1056/NEJMoa1903822
30. Isaacs DM, Kruger DF, Spollett GR. Optimizing therapeutic outcomes with oral semaglutide: A patient-centered approach. Diabetes Spectr Publ Am Diabetes Assoc (2021) 34:7–19. doi: 10.2337/ds20-0016
31. Bellanne-Chantelot C, Carette C, Riveline JP, Valero R, Gautier JF, Larger E, et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes (2007) 57:503–8. doi: 10.2337/db07-0859
32. Klupa T, Warram JH, Antonellis A, Pezzolesi M, Nam M, Malecki MT, et al. Determinants of the development of diabetes (maturity-onset diabetes of the young-3) in carriers of HNF-1alpha mutations: evidence for parent-of-origin effect. Diabetes Care (2002) 25:2292–301. doi: 10.2337/diacare.25.12.2292
33. Aarthy R, Aston-Mourney K, Mikocka-Walus A, Radha V, Amutha A, Anjana RM, et al. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY) - a review. J Diabetes its complications (2021) 35:107640. doi: 10.1016/j.jdiacomp.2020.107640
34. Bellanne-Chantelot C, Carette C, Riveline JP, Valero R, Gautier JF, Larger E, et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes (2008) 57:503–8. doi: 10.2337/db07-0859
Keywords: ABCC8, MODY12, precision therapy, mutation, whole exome sequencing
Citation: Li J, Wang X, Mao H, Wen L, Deng A, Li Y, Zhang H and Liu C (2022) Precision therapy for three Chinese families with maturity-onset diabetes of the young (MODY12). Front. Endocrinol. 13:858096. doi: 10.3389/fendo.2022.858096
Received: 22 January 2022; Accepted: 13 July 2022;
Published: 03 August 2022.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Maurizio Delvecchio, Giovanni XXIII Children’s Hospital, ItalyFeng Chen, Children’s Hospital of Nanjing Medical University, China
Oksana Rymar, Institute of Internal Medicine (RAS), Russia
Guidong Dai, Anshun University, China
Copyright © 2022 Li, Wang, Mao, Wen, Deng, Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juyi Li, bGp5d3hmMTEwQDE2My5jb20=; Yarong Li, eWF5YTc2OTkxMDQwOUAxNjMuY29t; Hongmei Zhang, emhtNzAwMUAxNjMuY29t; Chao Liu, bGl1Y2hhb0BoYnVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Juyi Li
Juyi Li Xiufang Wang2†
Xiufang Wang2† Aiping Deng
Aiping Deng Hongmei Zhang
Hongmei Zhang Chao Liu
Chao Liu