- Section of Endocrinology and Investigative Medicine, Imperial College London, Hammersmith Hospital, London, United Kingdom
Kisspeptin and its receptor are central to reproductive health acting as key regulators of the reproductive endocrine axis in humans. Kisspeptin is most widely recognised as a regulator of gonadotrophin releasing hormone (GnRH) neuronal function. However, recent evidence has demonstrated that kisspeptin and its receptor also play a fundamental role during pregnancy in the regulation of placentation. Kisspeptin is abundantly expressed in syncytiotrophoblasts, and its receptor in both cyto- and syncytio-trophoblasts. Circulating levels of kisspeptin rise dramatically during healthy pregnancy, which have been proposed as having potential as a biomarker of placental function. Indeed, alterations in kisspeptin levels are associated with an increased risk of adverse maternal and foetal complications. This review summarises data evaluating kisspeptin’s role as a putative biomarker of pregnancy complications including miscarriage, ectopic pregnancy (EP), preterm birth (PTB), foetal growth restriction (FGR), hypertensive disorders of pregnancy (HDP), pre-eclampsia (PE), gestational diabetes mellitus (GDM), and gestational trophoblastic disease (GTD).
Introduction
Kisspeptin is best known for its role as a hypothalamic neuropeptide that regulates gonadotrophin releasing hormone (GnRH) secretion (1). Indeed, early studies showed that inactivating variants of the kisspeptin receptor result in pubertal failure due to hypogonadotrophic hypogonadism, confirming the importance of kisspeptin signalling to reproductive health (2, 3).
During pregnancy, kisspeptin is produced in large amounts by the placenta and thus there is significant interest in evaluating its potential as a novel marker of pregnancy complications (4). Kisspeptin is a peptide encoded by the KISS-1 gene that binds to a G-protein coupled kisspeptin receptor (KISS-1R, previously known as the orphan receptor GPR54) (5). Kisspeptin levels in the circulation are several hundred fold higher during healthy pregnancy compared to the non-pregnant state (6, 7). This review will summarise data evaluating kisspeptin’s role as a putative biomarker of pregnancy complications including miscarriage, ectopic pregnancy (EP), preterm birth (PTB), foetal growth restriction (FGR), hypertensive disorders of pregnancy (HDP), pre-eclampsia (PE), gestational diabetes mellitus (GDM), and gestational trophoblastic disease (GTD).
Kisspeptin
The gene encoding kisspeptin (KISS-1) was first identified in 1996 as a metastasis tumour-suppressor gene in malignant melanoma cell lines and its peptide product was initially termed ‘metastin’ (8). Subsequently, it became known as kisspeptin in homage to its discovery in Hershey, Pennsylvania, USA, the hometown of the famous chocolate Hershey’s kisses (8). The KISS-1 gene, located on chromosome 1q32, encodes a 145 amino acid prepropeptide that is post-translationally cleaved into biologically active kisspeptin peptides of different amino acid lengths indicated by their suffix: e.g. kisspeptin -54, -14, -13, and -10 (5, 9, 10). All of these peptides bind and activate the kisspeptin receptor through their shared C-terminal region decapeptide motif (Arg-Phe-NH2) (5, 10). Kisspeptin is expressed in multiple tissues including the hypothalamus, limbic system, gonads, pancreas, and liver, but is particularly abundant in the placenta, and thus is believed to play an important role in pregnancy (10, 11).
Kisspeptin in Healthy Pregnancy
Kisspeptin plays a key role in implantation and decidualisation. Kisspeptin promotes embryo attachment to the endometrium through interaction with cell adhesion molecules, and stimulates stromal decidualisation by up-regulating leukaemia inhibitory factor (LIF) (12) (Figure 1). Kisspeptin also attenuates the excessive migration and invasion of trophoblasts through inhibition of the matrix metalloproteinases (MMP) 2 and 9 (13–15). Kisspeptin may also impact angiogenesis and uterine spiral artery modelling (16–18). A further relevant mechanism of kisspeptin in pregnancy relates to the maternal immune tolerance needed to avoid foetal rejection. Indeed, in vitro incubation with kisspeptin at levels corresponding to those found in pregnancy, results in increased differentiation of human naive T cells into T-regulatory cells (19).
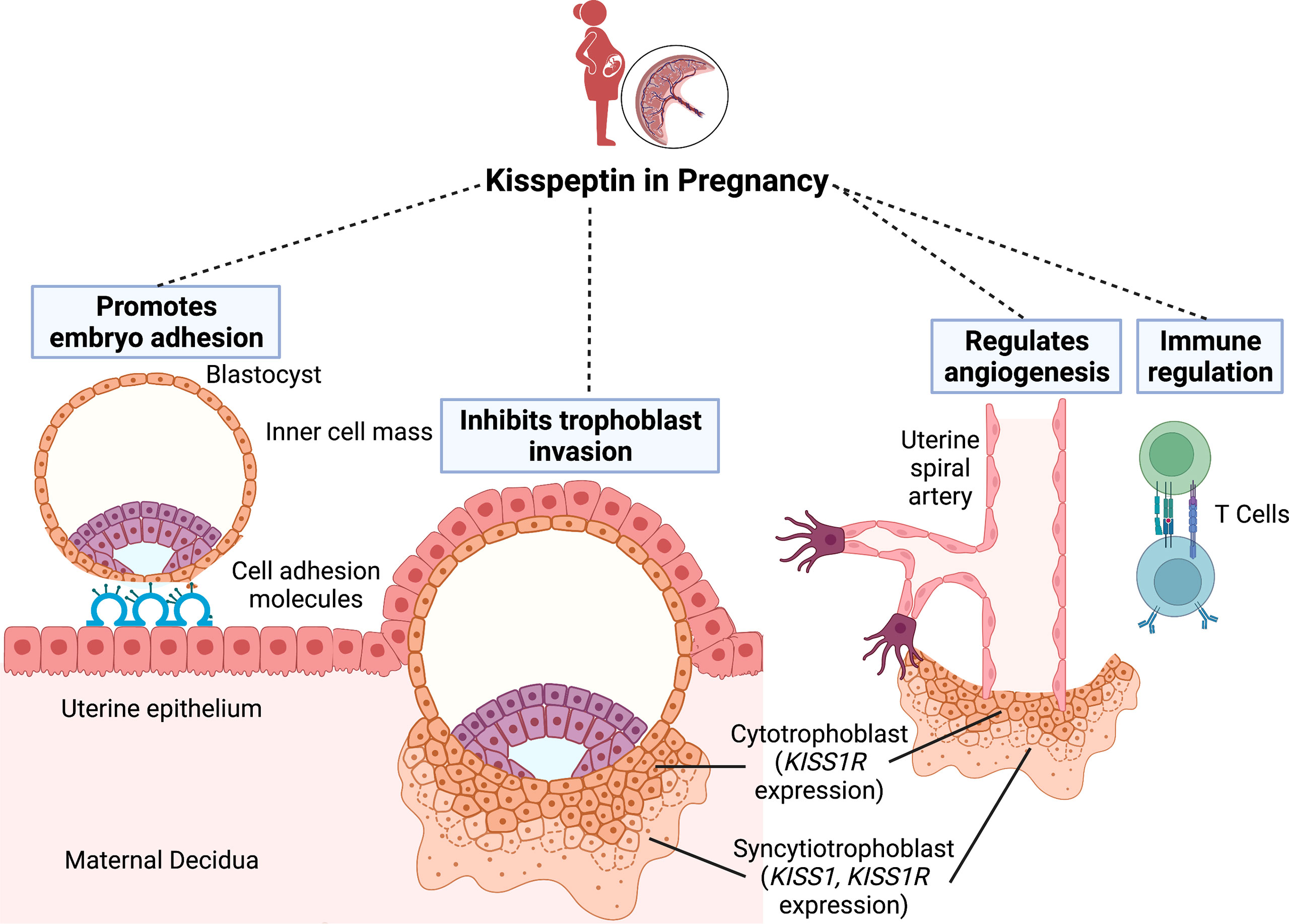
Figure 1 The role of kisspeptin in embryo implantation. Successful implantation requires communication between the blastocyst and a receptive uterine epithelium. Kisspeptin initially promotes embryo attachment to the endometrial epithelium through interaction with cell adhesion molecules. Once the blastocyst penetrates the epithelium, the trophoblast cells differentiate into the inner cytotrophoblast and outer syncytiotrophoblast cells. Whilst the cytotrophoblast cells express the kisspeptin receptor (KISS-1R), the syncytiotrophoblast cells express both KISS-1R and the kisspeptin gene (KISS-1). Kisspeptin subsequently regulates implantation by inhibiting excessive trophoblast invasion into the endometrium. Finally, kisspeptin also has roles in uterine spiral artery remodelling and immune regulation to avoid maternal foetal rejection. Figure created with BioRender.com.
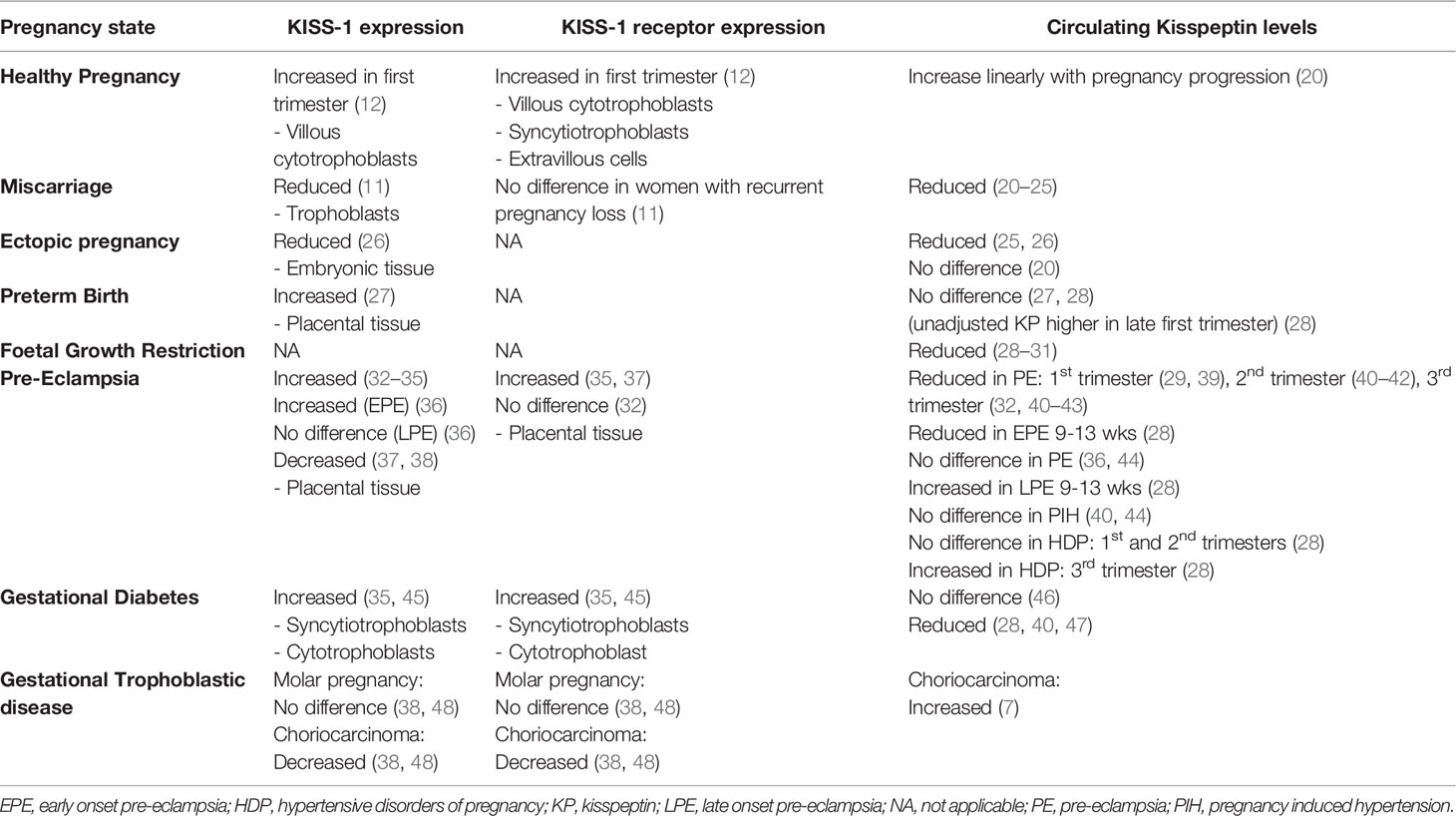
The placenta is considered the main source of kisspeptin during pregnancy and the KISS-1/Kiss-1 gene is expressed in syncytiotrophoblasts, whereas its receptor is expressed in both cytotrophoblasts and syncytiotrophoblasts (12) (Table 1). Expression of kisspeptin and its receptor is high during early pregnancy and declines as the placenta matures, thus highlighting kisspeptin’s role in placentation (14). Interestingly, circulating kisspeptin levels increase linearly with advancing gestation and kisspeptin-54 immunoreactivity dramatically rises from 1230 pmol/L during the first trimester to 9590 pmol/L during the third trimester and returns to non-pregnant levels (<100 pmol/L) soon after birth (8 pmol/L) (6, 7, 20).
Circulating kisspeptin levels are affected by several variables in healthy pregnancy (20). Whilst gestational and maternal age are associated with raised kisspeptin levels, Afro-Caribbean ethnicity, smoking during pregnancy, and high body mass index (BMI) are associated with reduced kisspeptin levels (20). Additionally, kisspeptin levels have been shown to be lower in serum compared to plasma samples, and are influenced by pre-analytical factors such as collection tube type, processing time and time to sample storage (49).
Kisspeptin in Pregnancy Complications
1. Kisspeptin in Miscarriage
Miscarriage is the spontaneous loss of an intrauterine pregnancy before 24 weeks of gestation and affects 1 in 5 clinical pregnancies (50). Miscarriage predominantly occurs during the first trimester of pregnancy and the majority of early miscarriages are due to a genetic abnormality of the developing embryo, however other causes include endocrine, anatomical, and immunological factors (51).
Miscarriage diagnosis can be challenging as often a pregnancy is failing for a time before pregnancy loss has conclusively been confirmed. This uncertainty can exacerbate the psychological burden related to investigating possible miscarriage, with up to 6% of women suffering from moderate-severe depression, 17% from moderate-severe anxiety and 18% from post-traumatic stress disorder (52). To date, there is no clinical predictor of miscarriage, however recent data demonstrates a potential for kisspeptin as a biomarker of miscarriage.
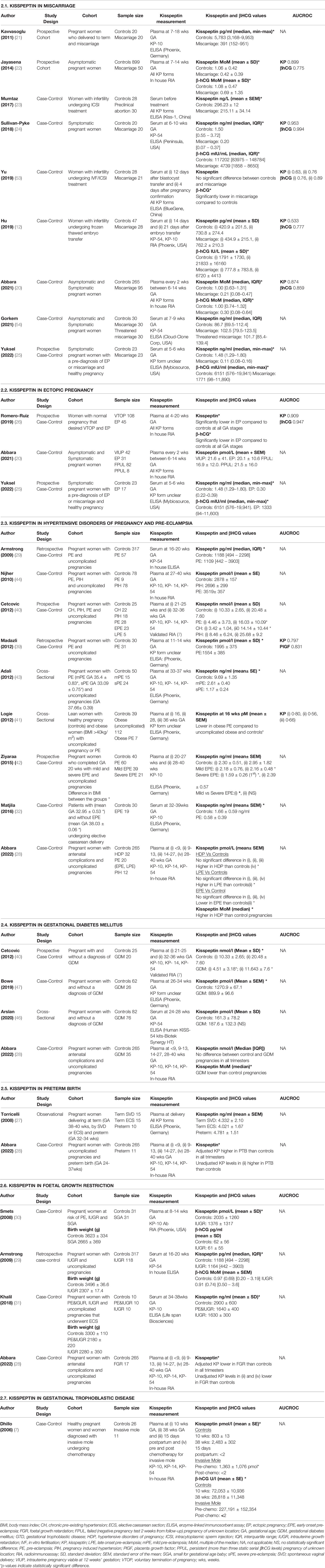
Kisspeptin levels (adjusted for gestation) are markedly reduced by 60-79% in women with miscarriage compared to healthy pregnancy (20–25) (Table 2.1). Above average levels, when corrected for gestational age, are reassuring with a <1% chance of miscarriage (20), whereas kisspeptin levels 95% lower than the median for that gestation are associated with up to an 85% chance of miscarriage. Concordantly, KISS-1 expression is decreased in the placentae of women with recurrent spontaneous abortion compared to those who undergo voluntary termination of pregnancy (11). Furthermore, whilst kisspeptin’s high diagnostic performance for identifying miscarriage is maintained in late-first trimester pregnancies (>8 weeks of gestation), that of β-human chorionic gonadotrophin (β-hCG) worsens (20). Thus, the combination of both kisspeptin and β-hCG can be used to ensure high diagnostic accuracy at all gestations (AUCROC 0.92, 95% CI 0.89-0.95) (20, 22, 24). Kisspeptin has also been shown to reflect different types of miscarriage, with lower levels reported in complete (no retained products of conception) versus incomplete (retained products of conception) or missed (empty gestational sac or a foetal pole with no heartbeat) miscarriage (20). Additionally, both kisspeptin and β-hCG levels decline with closer proximity to miscarriage confirmation, and therefore repeat measurements every 1-2 weeks could enable further risk-stratification of miscarriage risk in clinical practice (20).
Studies involving women with infertility who undergo assisted reproductive techniques (in vitro fertilisation, intracytoplasmic sperm insemination (ICSI) or frozen thawed embryo transfer) have found reduced β-hCG levels in miscarriage compared to controls, but no difference in kisspeptin levels (53, 55). These findings may be due to the very early gestations at which kisspeptin levels were assessed (2-3 weeks following, or even before, pregnancy confirmation) (53, 55). Indeed, kisspeptin may not be expressed in the placenta at high levels prior to 6 weeks of gestation, suggesting that β-hCG levels may be more useful at these very early gestations (26).
2. Kisspeptin in Ectopic Pregnancy
Ectopic pregnancy (EP) affects 2% of pregnancies and occurs when a fertilised ovum implants and develops outside the uterine cavity, most commonly within the fallopian tube (56). EP can result in tubal rupture and accounts for 9-13% of all pregnancy-related deaths in developed countries and can compromise a woman’s future fertility (57). EP is currently diagnosed by serial β-hCG measurements in combination with ultrasound, although laparoscopy is often required to provide a definitive diagnosis (58). The sensitivity and specificity of these tests significantly decrease in the case of pregnancies of unknown location (PUL) as false positive or negative diagnoses may occur. This is important as an incorrect diagnosis may lead to termination of a healthy pregnancy (59). Accordingly, different biomarkers have been investigated in an attempt to improve the diagnostic accuracy of EP, including kisspeptin.
Some studies have found that kisspeptin levels in EP are lower than in healthy pregnancy but higher than in miscarriage (25, 26). However, another study demonstrated that kisspeptin levels are not significantly altered between women with viable intrauterine pregnancies (VIUPs) and those with either EP or failing or persistent PUL, after adjusting for confounding variables (20) (Table 2.2). Current evidence remains limited, and larger studies are required to determine kisspeptin’s performance as a diagnostic marker in EP at early gestations (<6 weeks).
3. Kisspeptin in Hypertensive Disorders of Pregnancy and Pre-Eclampsia
Hypertensive disorders affect 5% of all pregnancies (60) and include pre-existing chronic hypertension (CH), pregnancy induced hypertension (PIH) and pre-eclampsia (PE). PIH is defined as new onset hypertension (BP ≥140/90mmHg) occurring after 20 weeks of gestation, PE is PIH with proteinuria (urine >3g/24 hours) or significant end-organ dysfunction, and severe PE is the presence of at least one of: hypertension (BP≥160/110 mmHg), visual disturbance, chest pain, dyspnoea, pulmonary oedema, seizures, or neonatal distress (61). PE is further classified, according to the onset of clinical features, into early-onset PE (EPE <34 weeks of gestation) and late-onset PE (LPE ≥34 weeks of gestation). EPE is associated with impaired trophoblast invasion, defective spiral artery remodelling and adverse perinatal complications including IUGR (62). LPE occurs due to hypoxic stress and impaired perfusion but is less likely to compromise foetal growth (63, 64). Currently, PE diagnosis is based on early pregnancy risk factor screening, uterine artery Doppler velocimetry and biomarkers such as PPAP-A or placental growth factor (PlGF) (61). Kisspeptin has been implicated in the pathogenesis of PE through reduced angiogenesis, decreased cytotrophoblast invasion and increased trophoblast apoptosis, and thus could have potential in predicting PE (16–18).
Levels of circulating kisspeptin in HDP vary in the literature, and largely differ according to HDP subtype, severity, and onset (Table 2.3). Most of the studies report reduced circulating kisspeptin levels in PE compared to normotensive pregnant controls (29, 32, 40–43, 65) and therefore kisspeptin is considered to reflect placental dysfunction. However, expression of KISS-1, which inhibits trophoblast invasion and results in defective transformation of the spiral arteries, is increased in the placentae of PE pregnancies, thus supporting its role in the pathophysiology of PE (32–34, 36, 66) (Table 1). Nonetheless, there are also some reports of decreased KISS-1 expression in PE placentae (37, 38) (Table 1). Furthermore, evidence suggests that circulating kisspeptin levels decline as the severity of PE increases, which could also reflect reduced placental mass in more severe disease. Indeed, both circulating kisspeptin levels and placental mass is reduced in EPE compared to LPE (28, 42, 67). Additionally, pregnant women with pre-existing hypertension and PE, states associated with a higher burden of disease, have reduced kisspeptin levels compared to PIH (40).
Whilst most studies demonstrate reduced kisspeptin levels in PE, a recent study found that kisspeptin levels are increased in HDP during the third trimester of pregnancy (Table 2.3). However, there was no association between circulating kisspeptin levels and severity of PET (28). It is likely that complexity in the categorisation, severity, and onset of PET, and the need for correction for possible confounders such as BMI and gestational age, could explain differences between kisspeptin levels observed in the current studies. Larger observational studies that are carefully designed to address these and look at each PET-subset throughout pregnancy would therefore be valuable in resolving these inconsistencies.
4. Kisspeptin in Gestational Diabetes Mellitus
During pregnancy a physiological rise in maternal insulin resistance provides glucose to the developing foetus (68, 69). This insulin resistance leads to maternal pancreatic β-cell adaptation and increased insulin secretion. Failure of these changes results in gestational diabetes mellitus (GDM), which affects up to 20% of pregnancies worldwide (70).
Kisspeptin receptors are expressed in pancreatic β-cells (71) and have been implicated in β-cell adaptation during pregnancy. Exogenous kisspeptin administration has variable physiological effects on the glucose-dependent regulation of pancreatic beta-cells. For instance, KISS-1 peptide (KP-145) (71), KP-13 (72), KP-10 (72–74) potentiates glucose-stimulated insulin secretion (GSIS) in animal and human islets in-vitro. KP-54 increases GSIS in healthy men following an intravenous glucose tolerance test (IVGTT), which induces high glucose levels (75). On the other hand, Vikam and colleagues have found that KP-13 and KP-54 drives dose-dependent inhibitory effects on insulin secretion in mouse islets in the presence of lower glucose concentrations (2.8-11.1 mmol/l), compared to controls, which is not observed at higher glucose concentrations (76). Furthermore, chronic administration of KP-10 in non-pregnant mice enhances GSIS and improves glucose tolerance (47). Interestingly, hyperlipidaemia, impaired glucose tolerance (IGT) and weight gain develops in Kiss-1r-null female mice exclusively, thus suggesting sexual dimorphism in kisspeptin’s effects on metabolism and glucose homeostasis (77).
In late gestation murine pregnancy, β-cell specific Kiss-1r-knockout models and pharmacological inhibition of Kiss-1r leads to reduced GSIS and development of IGT, which is not observed in non-pregnant states or wild-type controls (47). This supports a role for β-cell kisspeptin signalling in the regulation of glucose homeostasis during pregnancy. Loss of kisspeptin signalling in the ß-cell-specific Kiss-1r-knockout models also attenuates the increased ß-cell proliferation normally seen during murine pregnancy when assessed with bromodeoxyuridine (BrdU) labelling. Nonetheless, the levels are not reduced to non-pregnant levels, suggesting contribution of other signals in pancreatic β-cell proliferation during pregnancy (47, 78).
In human pregnancies with GDM, placental KISS-1 and KISS-1R expression is elevated in the third trimester (35, 45) (Table 1), whereas circulating kisspeptin levels have been either lower (40, 47) or not significantly altered (28, 46) (Table 2.4). Finally, Bowe and colleagues have demonstrated a positive correlation between third trimester kisspeptin levels and oral glucose–stimulated insulin levels at 60 minutes (r2 = 0.18; P < 0.0001) and AUC serum insulin over the OGTT (r2 = 0.13; P=0.0013) in women with GDM (47).
5. Kisspeptin in Pre-Term Birth
Pre-term birth (PTB) is defined as delivery prior to 37 weeks of gestation and affects 11% of pregnancies (79, 80). Kisspeptin has been proposed to initiate labour through increased oxytocin neuronal firing rate in pregnant rats and thus may play a potential role in PTB (81). Gestation adjusted kisspeptin levels are higher in PTB-affected pregnancies than in control pregnancies during the late-first trimester, with the adjusted odds of PTB being increased by 20% (95% CI, 1-42%) for every 1 nmol/L increase in plasma kisspeptin (28) (Table 2.5). Furthermore, KISS-1 mRNA expression is higher in preterm placentae than in term placentae delivered vaginally or by Caesarean section thus indicating that increased kisspeptin expression could be involved in the induction of labour (27) (Table 1)W. However, no alteration in circulating kisspeptin levels have been reported to date during the third trimester between healthy pregnancy and PTB and thus more data is needed to elucidate whether there are changes in kisspeptin levels preceding and around the time of spontaneous labour (27, 28).
6. Kisspeptin in Foetal Growth Restriction
Foetal growth restriction (FGR) encompasses both intrauterine growth restriction (IUGR, foetal weight <10th centile for gestational age with abnormal umbilical artery doppler results) and small for gestation age (SGA, delivery weight <10th percentile for gestational age) (82, 83). FGR is thought to arise from abnormal trophoblast invasion and spiral artery remodelling that limits oxygen supply to the placenta (84, 85). The resulting ischemic injury generates reactive oxygen species which lead to apoptosis and restriction of placental and foetal growth (84, 85). To date, four studies have demonstrated significantly reduced kisspeptin levels in FGR versus healthy pregnancy in all three trimesters (28–31) (Table 2.6). Thus, low circulating kisspeptin levels could reflect low placental mass in pregnancies affected by FGR.
7. Kisspeptin in Gestational Trophoblastic Disease
Gestational trophoblastic disease (GTD) is characterised by an abnormal proliferation of placental tissue and comprises of choriocarcinoma, invasive mole, placental site trophoblastic tumour and epithelioid trophoblastic tumour (86). Molar pregnancy is a benign form of GTD, whereas choriocarcinomas are more aggressive, however both exhibit high β-hCG levels and respond well to chemotherapy (87). Serum β-hCG measurement aids with GTD diagnosis, staging and prognostication before and after chemotherapy (88).
KISS-1 and KISS-1R expression is significantly lower in malignant choriocarcinoma cells compared to molar and healthy pregnancies (38, 48) (Table 1). Conversely, circulating kisspeptin levels are elevated in malignant GTD compared to healthy pregnancies but significantly decline following chemotherapy (7) (Table 2.7). The increased circulating kisspeptin levels could reflect an increased malignant trophoblast mass rather than an elevation in cellular KISS-1 expression (89). Thus, kisspeptin levels can be altered in choriocarcinomas and other GTDs, which is interesting when considering the original identification of KISS-1 as an anti-metastatic gene.
Conclusion
Kisspeptin levels are markedly reduced in miscarriage; and whilst the performance of kisspeptin levels to identify women at high risk of miscarriage is maintained throughout the first trimester, that of β-hCG falls during the latter part of the first trimester. Nevertheless, kisspeptin levels are only mildly elevated at early gestations (< 6 weeks) and therefore can be difficult to detect using current collection and assay methods. Thus, measuring kisspeptin in combination with β-hCG levels could potentially overcome this deficiency at early gestations. Due to the current difficulty in miscarriage diagnosis and the lack of available biomarkers, the high performance of plasma kisspeptin suggests that it has significant potential for further development in this context. Given that kisspeptin has been proposed as a biomarker of healthy placentation, it could potentially be used to recognise late pregnancy complications characterised by abnormal placentation during the first trimester. Regarding HDP, most studies have suggested lower circulating kisspeptin levels but increased placental kisspeptin expression. Kisspeptin levels in pregnancy complications such as PE are confounded by factors such as BMI, disease severity, time of onset, and concomitant FGR, and thus could limit the use of kisspeptin diagnostically.
Overall, current evidence suggests that circulating kisspeptin levels are consistently reduced in miscarriage, EP, FGR, GDM, and increased in PTB and GTD. Larger datasets with adequately sized control cohorts that accurately adjust for gestation, BMI, ethnicity, detailed disease severity phenotype and onset are needed to enable more precise characterisation of the utility of kisspeptin levels in these settings. In summary, circulating kisspeptin is a promising biomarker for early pregnancy loss and further research is needed to assess its potential in other pregnancy complications.
Author Contributions
BP, JT wrote the manuscript, designed the figures and tables. AA, WSD, ANC reviewed and edited the manuscript and are the corresponding authors. All authors have made a substantial, direct and intellectual contribution to the work and approved the manuscript prior to its submission.
Funding
This work was supported by grants from the National Institute of Health Research (NIHR), the NIHR/Wellcome Trust Imperial Clinical Research Facility, and the NIHR Imperial Biomedical Research Centre. The Section of Endocrinology and Investigative Medicine was funded by grants from the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council (BBSRC), NIHR and was supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the authors and not necessarily those of the MRC, BBSRC, the NHS, the NIHR, or the Department of Health. BP is supported by an MRC Clinical Training Research Fellowship (Grant Ref: MR/W024144/1). AC is supported by the National Health Service. WD is supported by an NIHR Senior Investigator Award (NIHR RP-2014-05-001). AA is supported by an NIHR Clinician Scientist Award (No. CS-2018-18-ST2-002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abbara A, Clarke SA, Dhillo WS. Clinical Potential of Kisspeptin in Reproductive Health. Trends Mol Med (2021) 27(8):807–23. doi: 10.1016/j.molmed.2021.05.008
2. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic Hypogonadism Due to Loss of Function of the KiSS1-Derived Peptide Receptor GPR54. Proc Natl Acad Sci U S A (2003) 100(19):10972–976. doi: 10.1073/pnas.1834399100
3. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JSJ, Shagoury JK, et al. The GPR54 Gene as a Regulator of Puberty. N Engl J Med (2003) 349(17):1614–27. doi: 10.1056/NEJMoa035322
4. Savaris RF. Kisspeptin as a Biomarker for Miscarriage: Let’s Wait! Fertil Steril (2018) 109:67. doi: 10.1016/j.fertnstert.2017.10.014
5. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-Coupled Receptor Gpr54. J Biol Chem (2001) 276(37):34631–636. doi: 10.1074/jbc.M104847200
6. Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, et al. Dramatic Elevation of Plasma Metastin Concentrations in Human Pregnancy: Metastin as a Novel Placenta-Derived Hormone in Humans. J Clin Endocrinol Metab (2003) 88(2):914–19. doi: 10.1210/jc.2002-021235
7. Dhillo WS, Savage P, Murphy KG, Chaudhri OB, Patterson M, Nijher GM, et al. Plasma Kisspeptin is Raised in Patients With Gestational Trophoblastic Neoplasia and Falls During Treatment. Am J Physiol - Endocrinol Metab (2006) 291(5):878–84. doi: 10.1152/ajpendo.00555.2005
8. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. J Natl Cancer Inst (1996) 88(23):1731–37. doi: 10.1093/jnci/88.23.1731
9. West A, Vojta PJ, Welch DR, Weissman BE. Chromosome Localization and Genomic Structure of the KiSS-1 Metastasis Suppressor Gene (KISS1). Genomics (1998) 54(1):145–8. doi: 10.1006/geno.1998.5566
10. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis Suppressor Gene KiSS-1 Encodes Peptide Ligand of a G-Protein-Coupled Receptor. Nat (2001) 411(6837):613–7. doi: 10.1038/35079135
11. Park DW, Lee SK, Hong SR, Han AR, Kwak-Kim J, Yang KM. Expression of Kisspeptin and its Receptor GPR54 in the First Trimester Trophoblast of Women With Recurrent Pregnancy Loss. Am J Reprod Immunol (2012) 67(2):132–9. doi: 10.1111/j.1600-0897.2011.01073.x
12. Hu KL, Chang HM, Zhao HC, Yu Y, Li R, Qiao J. Potential Roles for the Kisspeptin/Kisspeptin Receptor System in Implantation and Placentation. Hum Reprod Update (2019) 25(3):326–43. doi: 10.1093/humupd/dmy046
13. Francis VA, Abera AB, Matjila M, Millar RP, Katz AA. Kisspeptin Regulation of Genes Involved in Cell Invasion and Angiogenesis in First Trimester Human Trophoblast Cells. PLoS One (2014) 9(6):1–10. doi: 10.1371/journal.pone.0099680
14. Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/Metastin-Derived Decapeptide, is a Physiological Invasion Inhibitor of Primary Human Trophoblasts. J Cell Sci (2004) 117(8):1319–28. doi: 10.1242/jcs.00971
15. Roseweir AK, Katz AA, Millar RP. Kisspeptin-10 Inhibits Cell Migration In Vitro via a Receptor-GSK3 Beta-FAK Feedback Loop in HTR8SVneo Cells. Placenta (2012) 33(5):408–15. doi: 10.1016/j.placenta.2012.02.001
16. Maynard SE, Ananth Karumanchi S. Angiogenic Factors and Preeclampsia. Semin. Nephrol (2010) 31(1):33–46. doi: 10.1016/S2210-7789(10)60068-2
17. Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First Trimester Placental Growth Factor and Soluble Fms-Like Tyrosine Kinase 1 and Risk for Preeclampsia. J Clin Endocr Metab (2004) 89(2):770–75. doi: 10.1210/jc.2003-031244
18. Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N Engl J Med (2004) 12:672–83. doi: 10.1056/NEJMoa031884
19. Williams Z. Inducing Tolerance to Pregnancy. N Engl J Med (2012) 367(12):1159–61. doi: 10.1056/NEJMcibr1207279
20. Abbara A, Al-Memar M, Phylactou M, Kyriacou C, Eng PC, Nadir R, et al. Performance of Plasma Kisspeptin as a Biomarker for Miscarriage Improves With Gestational Age During the First Trimester. Fertil Steril (2021) 116(3):809–19. doi: 10.1016/j.fertnstert.2021.04.031
21. Kavvasoglu S, Ozkan ZS, Kumbak B, Simsek M, Ilhan N. Association of Kisspeptin-10 Levels With Abortus Imminens: A Preliminary Study. Arch Gynecol Obstet (2012) 285(3):649–53. doi: 10.1007/s00404-011-2061-0
22. Jayasena CN, Abbara A, Izzi-Engbeaya C, Comninos AN, Harvey RA, Gonzalez Maffe J, et al. Reduced Levels of Plasma Kisspeptin During the Antenatal Booking Visit are Associated With Increased Risk of Miscarriage. J Clin Endocrinol Metab (2014) 99(12):E2652–60. doi: 10.1210/jc.2014-1953
23. Mumtaz A, Khalid A, Jamil Z, Fatima SS, Arif S, Rehman R. Kisspeptin: A Potential Factor for Unexplained Infertility and Impaired Embryo Implantation. Int J Fertil Steril (2017) 11(2):99–104. doi: 10.22074/ijfs.2017.4957
24. Sullivan-Pyke C, Haisenleder DJ, Senapati S, Nicolais O, Eisenberg E, Sammel MD, et al. Kisspeptin as a New Serum Biomarker to Discriminate Miscarriage From Viable Intrauterine Pregnancy. Fertil Steril (2018) 109(1):137–41. doi: 10.1016/j.fertnstert.2017.09.029
25. Yuksel S, Ketenci Gencer F. Serum Kisspeptin, to Discriminate Between Ectopic Pregnancy, Miscarriage and First Trimester Pregnancy. J Obstet Gynaecol (Lahore) (2022) 0(0):1–5. doi: 10.1080/01443615.2022.2028747
26. Romero-Ruiz A, Avendaño MS, Dominguez F, Lozoya T, Molina-Abril H, Sangiao-Alvarellos S, et al. Deregulation of miR-324/KISS1/kisspeptin in Early Ectopic Pregnancy: Mechanistic Findings With Clinical and Diagnostic Implications. Am J Obstet Gynecol (2019) 220(5):e1–e17. doi: 10.1016/j.ajog.2019.01.228
27. Torricelli M, Galleri L, Voltolini C, Biliotti G, Florio P, De Bonis M, et al. Changes of Placental Kiss-1 mRNA Expression and Maternal/Cord Kisspeptin Levels at Preterm Delivery. Reprod Sci (2008) 15(8):779–84. doi: 10.1177/1933719108322442
28. Abbara A, Al-Memar M, Phylactou M, Daniels E, Patel B, Eng PC, et al. Changes in Circulating Kisspeptin Levels During Each Trimester in Women With Antenatal Complications. J Clin Endocrinol Metab (2022) 107(1):E71–83. doi: 10.1210/clinem/dgab617
29. Armstrong RA, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased Serum Levels of Kisspeptin in Early Pregnancy are Associated With Intra-Uterine Growth Restriction and Pre-Eclampsia. Prenat Diagn (2009) 29(10):982–5. doi: 10.1002/pd.2328
30. Smets EML, Deurloo KL, Go ATJI, Van Vugt JMG, Blankenstein MA, Oudejans CBM. Decreased Plasma Levels of Metastin in Early Pregnancy are Associated With Small for Gestational Age Neonates. Prenat Diagn (2008) 28(4):299–303. doi: 10.1002/pd.1969
31. . Abulfadle KA, Khalil SS, Elnagar WM. Serum Kisspeptin-10 Levels in Pregnant Women Complicated With Intrauterine Growth Restriction With or Without Preeclampsia. Med J Cairo Univ (2018) 86(6):1975–82. doi: 10.21608/mjcu.2018.56929
32. Matjila M, Millar R, van der Spuy Z, Katz A. Elevated Placental Expression at the Maternal-Fetal Interface But Diminished Maternal Circulatory Kisspeptin in Preeclamptic Pregnancies. Pregnancy Hypertens (2016) 6(1):79–87. doi: 10.1016/j.preghy.2015.11.001
33. Zhang H, Long Q, Ling L, Gao A, Li H, Lin Q. Elevated Expression of KiSS-1 in Placenta of Preeclampsia and its Effect on Trophoblast. Reprod Biol (2011) 11(2):99–115. doi: 10.1016/S1642-431X(12)60048-5
34. Vazquez-Alaniz F, Galaviz-Hernandez C, Marchat LA, Salas-Pacheco JM, Chairez-Hernandez I, Guijarro-Bustillos JJ, et al. Comparative Expression Profiles for KiSS-1 and REN Genes in Preeclamptic and Healthy Placental Tissues. Eur J Obstet Gynecol Reprod Biol (2011) 159(1):67–71. doi: 10.1016/j.ejogrb.2011.07.019
35. Kapustin RV, Drobintseva AO, Alekseenkova EN, Onopriychuk AR, Arzhanova ON, Polyakova VO, et al. Placental Protein Expression of Kisspeptin-1 (KISS1) and the Kisspeptin-1 Receptor (KISS1R) in Pregnancy Complicated by Diabetes Mellitus or Preeclampsia. Arch Gynecol Obstet (2020) 301(2):437–45. doi: 10.1007/s00404-019-05408-1
36. Qiao C, Wang C, Zhao J, Liu C, Shang T. Elevated Expression of KiSS-1 in Placenta of Chinese Women With Early-Onset Preeclampsia. PLoS One (2012) 7(11):1–9. doi: 10.1371/journal.pone.0048937
37. Cartwright JE, Williams PJ. Altered Placental Expression of Kisspeptin and its Receptor in Pre-Eclampsia. J Endocrinol (2012) 214(1):79–85. doi: 10.1530/JOE-12-0091
38. Qiao C, Wang C, Shang T, Lin Q. Clinical Significance of KiSS-1 and Matrix Metalloproteinase-9 Expression in Trophoblasts of Women With Preeclampsia and Their Relation to Perinatal Outcome of Neonates. Zhonghua Fu Chan Ke Za Zhi (2005) 40(9):585–90.
39. Madazli R, Bulut B, Tuten A, Aydin B, Demirayak G, Kucur M. First-Trimester Maternal Serum Metastin, Placental Growth Factor and Chitotriosidase Levels in Pre-Eclampsia. Eur J Obstet Gynecol Reprod Biol (2012) 164(2):146–9. doi: 10.1016/j.ejogrb.2012.06.016
40. Ćetković A, Miljic D, Ljubić A, Patterson M, Ghatei M, Stamenkoví J, et al. Plasma Kisspeptin Levels in Pregnancies With Diabetes and Hypertensive Disease as a Potential Marker of Placental Dysfunction and Adverse Perinatal Outcome. Endocr Res (2012) 37(2):78–88. doi: 10.3109/07435800.2011.639319
41. Logie JJ, Denison FC, Riley SC, Ramaesh T, Forbes S, Norman JE, et al. Evaluation of Kisspeptin Levels in Obese Pregnancy as a Biomarker for Pre-Eclampsia. Clin Endocrinol (Oxf) (2012) 76(6):887–93. doi: 10.1111/j.1365-2265.2011.04317.x
42. Ziyaraa MA, Hamdan FB, Mousa LR. Correlation of Kisspeptin-10 Level and Fetal Well-Being in Preeclamptic Patients. Taiwan J Obstet Gynecol (2016) 55(6):840–6. doi: 10.1016/j.tjog.2015.10.028
43. Adali E, Kurdoglu Z, Kurdoglu M, Kamaci M, Kolusari A, Yildizhan R. Metastin Levels in Pregnancies Complicated by Pre-Eclampsia and Their Relation With Disease Severity. J Matern Neonatal Med (2012) 25(12):2671–5. doi: 10.3109/14767058.2012.708369
44. Nijher GMK, Chaudhri OB, Ramachandran R, Murphy KG, Zac-Varghese SEK, Fowler A, et al. The Effects of Kisspeptin-54 on Blood Pressure in Humans and Plasma Kisspeptin Concentrations in Hypertensive Diseases of Pregnancy. Br J Clin Pharmacol (2010) 70(5):674–81. doi: 10.1111/j.1365-2125.2010.03746.x
45. Loegl J, Nussbaumer E, Cvitic S, Huppertz B, Desoye G, Hiden U. GDM Alters Paracrine Regulation of Feto-Placental Angiogenesis via the Trophoblast. Lab Investig (2017) 97:409–18. doi: 10.1038/labinvest.2016.149
46. Arslan E, Gorkem U, Togrul C. Is There An Association Between Kisspeptin Levels And Gestational Diabetes Mellitus? Gynecol Obstet Reprod Med (2020) 26(3):179–83. doi: 10.21613/GORM.2019.946
47. Bowe JE, Hill TG, Hunt KF, Smith LIF, Simpson SJS, Amiel SA, et al. A Role for Placental Kisspeptin in β Cell Adaptation to Pregnancy. JCI Insight (2019) 4(20):1–14. doi: 10.1172/jci.insight.124540
48. Janneau J-L, Maldonado-Estrada J, Tachdjian G, Miran I, Motté N, Saulnier P, et al. Transcriptional Expression of Genes Involved in Cell Invasion and Migration by Normal and Tumoral Trophoblast Cells. J Clin Endocrinol Metab (2002) 87(11):5336–9. doi: 10.1210/jc.2002-021093
49. Ramachandran R, Patterson M, Murphy KG, Dhillo WS, Patel S, Kazarian A, et al. Preanalytical Factors Affecting RIA Measurement of Plasma Kisspeptin. Clin Chem (2008) 54:615–17. doi: 10.1373/clinchem.2007.093005
50. Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage Matters: The Epidemiological, Physical, Psychological, and Economic Costs of Early Pregnancy Loss. Lancet (2021) 397:1658–67. doi: 10.1016/S0140-6736(21)00682-6
51. Colak E, Ozcimen EE, Erinanç OH, Tohma YA, Ceran MU. Is Placental KISS-1 Expression Associated With First Trimester Abortion Spontaneous? Obstet Gynecol Sci (2020) 63(4):490–96. doi: 10.5468/ogs.19242
52. Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic Stress, Anxiety and Depression Following Miscarriage and Ectopic Pregnancy: A Multicenter, Prospective, Cohort Study. Am J Obstet Gynecol (2020) 222(4):367.e1–367.e22. doi: 10.1016/j.ajog.2019.10.102
53. Yu H, Liu J, Guo H, Chen C, Han Y, Cui Y. Prognostic Value of Repeated Serum Kisspeptin Measurements in Early First Trimester Pregnancy: A Preliminary Study. Reprod BioMed Online (2019) 38(3):465–71. doi: 10.1016/j.rbmo.2018.11.014
54. Gorkem U, Kan O, Bostanci MO, Taskiran D, Inal HA. Kisspeptin and Hematologic Parameters as Predictive Biomarkers for First-Trimester Abortions. Medeni Med J (2021) 36(2):98–105. doi: 10.5222/MMJ.2021.32549
55. Hu KL, Zhang Y, Yang Z, Zhao H, Xu H, Yu Y, et al. Predictive Value of Serum Kisspeptin Concentration at 14 and 21 Days After Frozen–Thawed Embryo Transfer. Reprod BioMed Online (2019) 39(1):161–7. doi: 10.1016/j.rbmo.2019.03.202
56. Petrini A, Spandorfer S. Recurrent Ectopic Pregnancy: Current Perspectives. Int J Women’s Health (2020) 12:597–600 . doi: 10.2147/IJWH.S223909
57. Goyaux N, Leke R, Keita N, Thonneau P. Ectopic Pregnancy in African Developing Countries. Acta Obstetricia Gynecol Scand (2003) 82:305–12. doi: 10.1034/j.1600-0412.2003.00175.x
58. Taran FA, Kagan KO, Hübner M, Hoopmann M, Wallwiener D, Brucker S. The Diagnosis and Treatment of Ectopic Pregnancy. Dtsch Arztebl Int (2015) 112(41):693–704. doi: 10.3238/arztebl.2015.0693
59. Doubilet PM, Benson CB, Bourne T, Blaivas M. Diagnostic Criteria for Nonviable Pregnancy Early in the First Trimester. N Engl J Med (2013) 369(15):1443–51. doi: 10.1056/NEJMra1302417
60. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and Regional Estimates of Preeclampsia and Eclampsia: A Systematic Review. Eur J Obstet Gynecol Reprod Biol (2013) 170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005
61. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 International Society for the Study of Hypertension in Pregnancy Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens (2022) 27:148–69. doi: 10.1016/j.preghy.2021.09.008
62. Bischof P, Meisser A, Campana A. Paracrine and Autocrine Regulators of Trophoblast Invasion— A Review. Placenta (2000) 21(SUPPL.1):S55–60. doi: 10.1053/plac.2000.0521
63. Staff AC. The Two-Stage Placental Model of Preeclampsia: An Update. J Reprod Immunol (2019) 134–135(March):1–10. doi: 10.1016/j.jri.2019.07.004
64. Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental Pathology Suggesting That Preeclampsia is More Than One Disease. Am J Obstet Gynecol (2014) 210(1):66.e1–7. doi: 10.1016/j.ajog.2013.09.010
65. Kucur M, Madazli R, Bulut B, Tuten A, Aydin B. First-Trimester Maternal Serum Metastin , Placental Growth Factor and Chitotriosidase Levels in Pre-Eclampsia. Eur J Obstetrics Gynecol Reprod Biol (2012) 164(2012):146–9. doi: 10.1016/J.EJOGRB.2012.06.016
66. Gomes VCL, Sones JL. From Inhibition of Trophoblast Cell Invasion to Proapoptosis: What are the Potential Roles of Kisspeptins in Preeclampsia? Am J Physiol - Regul Integr Comp Physiol (2021) 321(1):R41–8. doi: 10.1152/ajpregu.00258.2020
67. Krielessi V, Papantoniou N, Papageorgiou I, Chatzipapas I, Manios E, Zakopoulos N, et al. Clinical Study Placental Pathology and Blood Pressure’s Level in Women With Hypertensive Disorders in Pregnancy. Obstet Gynecol Int (2012) 2012:6. doi: 10.1155/2012/684083
68. Ryan EA, Enns L. Role of Gestational Hormones in the Induction of Insulin Resistance. J Clin Endocrinol Metab (1988) 67(2):341–7. doi: 10.1210/jcem-67-2-341
69. Buchanan TA. Pancreatic B-Cell Defects in Gestational Diabetes: Implications for the Pathogenesis and Prevention of Type 2 Diabetes. J Clin Endocr Metab (2001) 86(3):989–93. doi: 10.1210/jcem.86.3.7339
70. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global Estimates of the Prevalence of Hyperglycaemia in Pregnancy. Diabetes Res Clin Pract (2014) 103(2):176–85. doi: 10.1016/j.diabres.2013.11.003
71. Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A Role for Kisspeptin in Islet Function. Diabetologia (2006) 49:2131–35. doi: 10.1007/S00125-006-0343-Z
72. Bowe JE, Foot VL, Amiel SA, Huang GC, Lamb M, Lakey J, et al. GPR54 Peptide Agonists Stimulate Insulin Secretion From Murine, Porcine and Human Islets. Islets (2012) 4(1):20–23. doi: 10.4161/isl.18261
73. Schwetz TA, Reissaus CA, Piston DW. Differential Stimulation of Insulin Secretion by GLP-1 and Kisspeptin-10. PloS One (2014) 9(11):113020. doi: 10.1371/journal.pone.0113020
74. Bowe JE, King AJ, Kinsey-Jones JS, Foot VL, Li XF, O’byrne KT, et al. Kisspeptin Stimulation of Insulin Secretion: Mechanisms of Action in Mouse Islets and Rats. Diabetologia (2009) 52. doi: 10.1007/S00125-009-1283-1
75. Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, et al. The Effects of Kisspeptin on β-Cell Function, Serum Metabolites and Appetite in Humans. Diabetes, Obesity and Metabolism (2018) 20(12):2800–10. doi: 10.1111/dom.13460
76. Vikman J, Ahrén B. Inhibitory Effect of Kisspeptins on Insulin Secretion From Isolated Mouse Islets. Diabetes, Obesity and Metabolism (2009) 11(4):197–201. doi: 10.1111/j.1463-1326.2009.01116.x
77. Tolson KP, Marooki N, Wolfe A, Smith JT, Kauffman AS. Cre/lox Generation of a Novel Whole-Body Kiss1r KO Mouse Line Recapitulates a Hypogonadal, Obese, and Metabolically-Impaired Phenotype HHS Public Access. Mol Cell Endocrinol (2019) 498:110559. doi: 10.1016/j.mce.2019.110559
78. Smith LIF, Bowe JE. The Pancreas and the Placenta: Understanding Gestational Diabetes and Why Some Islets Fail to Cope With Pregnancy. Biochem (Lond) (2021) 43(2):42–6. doi: 10.1042/bio_2021_115
79. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The Global Epidemiology of Preterm Birth. Best Pract Research: Clin Obstetrics Gynaecol (2018) 52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003
80. Tucker J, McGuire W. Epidemiology of Preterm Birth. Br Med J (2004) 329(7467):387–91. doi: 10.1136/bmj.329.7467.675
81. Seymour AJ, Scott V, Augustine RA, Bouwer GT, Campbell RE, Brown CH. Development of an Excitatory Kisspeptin Projection to the Oxytocin System in Late Pregnancy. J Physiol (2017) 595(3):825–38. doi: 10.1113/JP273051
82. Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, et al. Optimizing the Definition of Intrauterine Growth Restriction: The Multicenter Prospective PORTO Study. Am J Obstet Gynecol (2013) 208(4):e1–6. doi: 10.1016/j.ajog.2013.02.007
83. McCowan LME, Roberts CT, Dekker GA, Taylor RS, Chan EHY, Kenny LC, et al. Risk Factors for Small-for-Gestational-Age Infants by Customised Birthweight Centiles: Data From an International Prospective Cohort Study. BJOG Int J Obstet Gynaecol (2010) 117(13):1599–1607. doi: 10.1111/j.1471-0528.2010.02737.x
84. Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal Growth is Directly Related to Maternal Anthropometry and Placental Volume. Eur J Clin Nutr (2004) 58(6):894–900. doi: 10.1038/sj.ejcn.1601909
85. Hafner E, Metzenbauer M, Höfinger D, Munkel M, Gassner R, Schuchter K, et al. Placental Growth From the First to the Second Trimester of Pregnancy in SGA-Foetuses and Pre-Eclamptic Pregnancies Compared to Normal Foetuses. Placenta (2003) 24(4):336–42. doi: 10.1053/plac.2002.0918
86. Biscaro A, Braga A, Berkowitz RS. Diagnóstico, Classificação E Tratamento Da Neoplasia Trofoblástica Gestacional. Rev Bras Ginecol e Obstet (2014) 37(1):42–51. doi: 10.1590/SO100-720320140005198
87. Goldstein DP, Berkowitz RS. Current Management of Gestational Trophoblastic Neoplasia. Hematol Oncol Clin North Am (2012) 26(1):111–31. doi: 10.1016/j.hoc.2011.10.007
88. Yang J, Xiang Y, Wan X, Yang X. The Prognosis of Gestational Trophoblastic Neoplasia Patient With Residual Lung Tumor After Completing Treatment. Gynecol Oncol (2006) 103(2):479–82. doi: 10.1016/j.ygyno.2006.03.015
Keywords: gestational trophoblastic disease (GTD), gestational diabetes mellitus (GDM), pre-ecalmpsia (PET), foetal growth restriction (FGR), hypertensive disorders of pregnancy (HDP), preterm (birth), miscarriage, kisspeptin
Citation: Tsoutsouki J, Patel B, Comninos AN, Dhillo WS and Abbara A (2022) Kisspeptin in the Prediction of Pregnancy Complications. Front. Endocrinol. 13:942664. doi: 10.3389/fendo.2022.942664
Received: 12 May 2022; Accepted: 16 June 2022;
Published: 19 July 2022.
Edited by:
Junping Wen, Fujian Provincial Hospital, ChinaReviewed by:
Juan Scheun, University of South Africa, South AfricaCopyright © 2022 Tsoutsouki, Patel, Comninos, Dhillo and Abbara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waljit S. Dhillo, dy5kaGlsbG9AaW1wZXJpYWwuYWMudWs=; Ali Abbara, YWxpLmFiYmFyYUBpbXBlcmlhbC5hYy51aw==
†These authors share first authorship
‡These authors share senior authorship
 Jovanna Tsoutsouki
Jovanna Tsoutsouki Bijal Patel
Bijal Patel Alexander N. Comninos
Alexander N. Comninos Waljit S. Dhillo
Waljit S. Dhillo Ali Abbara
Ali Abbara