- Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Progressive loss of physiological integrity and accumulation of degenerative changes leading to functional impairment and increased susceptibility to diseases are the main features of aging. The ovary, the key organ that maintains female reproductive and endocrine function, enters aging earlier and faster than other organs and has attracted extensive attention from society. Ovarian aging is mainly characterized by the progressive decline in the number and quality of oocytes, the regulatory mechanisms of which have yet to be systematically elucidated. This review discusses the hallmarks of aging to further highlight the main characteristics of ovarian aging and attempt to explore its clinical symptoms and underlying mechanisms. Finally, the intervention strategies related to aging are elaborated, especially the potential role of stem cells and cryopreservation of embryos, oocytes, or ovarian tissue in the delay of ovarian aging.
Introduction
Ovarian aging is characterized by the gradual decline in the quantity and quality of oocytes, mainly due to the low number of primordial follicles (PMFs) at birth and high monthly depletion during the reproductive period (1, 2). Ovarian aging manifests as reproductive decline until the loss of fertility, accompanied by endocrine dysfunction, menstrual cycle abnormalities, and other clinical symptoms (3, 4). Ovarian aging includes age-related physiological aging and pathological failure caused by different factors (5, 6). Age-related ovarian aging is a natural and inevitable physiological aging process. The age-dependent decline in oocyte quality accelerates between 35 and 40 years, and the natural menopause transition usually occurs between 40 and 45 years, with an average age of menopause between 50 and 52 years (7). In terms of pathological failure, ovarian aging mainly refers to premature ovarian insufficiency (POI), which is divided into primary or secondary POI (6, 8). POI affects approximately 1 percent of women under 40 years of age and 0.1 percent of women under 30 years of age (9). However, the etiology of primary POI remains unclear and may involve chromosomal abnormalities, gene mutations, enzyme deficiencies, and autoimmune disorders (10, 11). Secondary POI is associated with factors such as unhealthy lifestyles, chemotherapy, radiotherapy, reproductive system surgery, surgical menopause, endocrine disrupting chemicals, viral infection, or certain infectious diseases (12). This review focuses on the histomorphology and function of the ovary, clinical symptoms, pathogenesis and intervention strategies of ovarian aging, aiming to reveal the key regulatory factors of ovarian aging in the subfertility period.
Hallmarks of aging
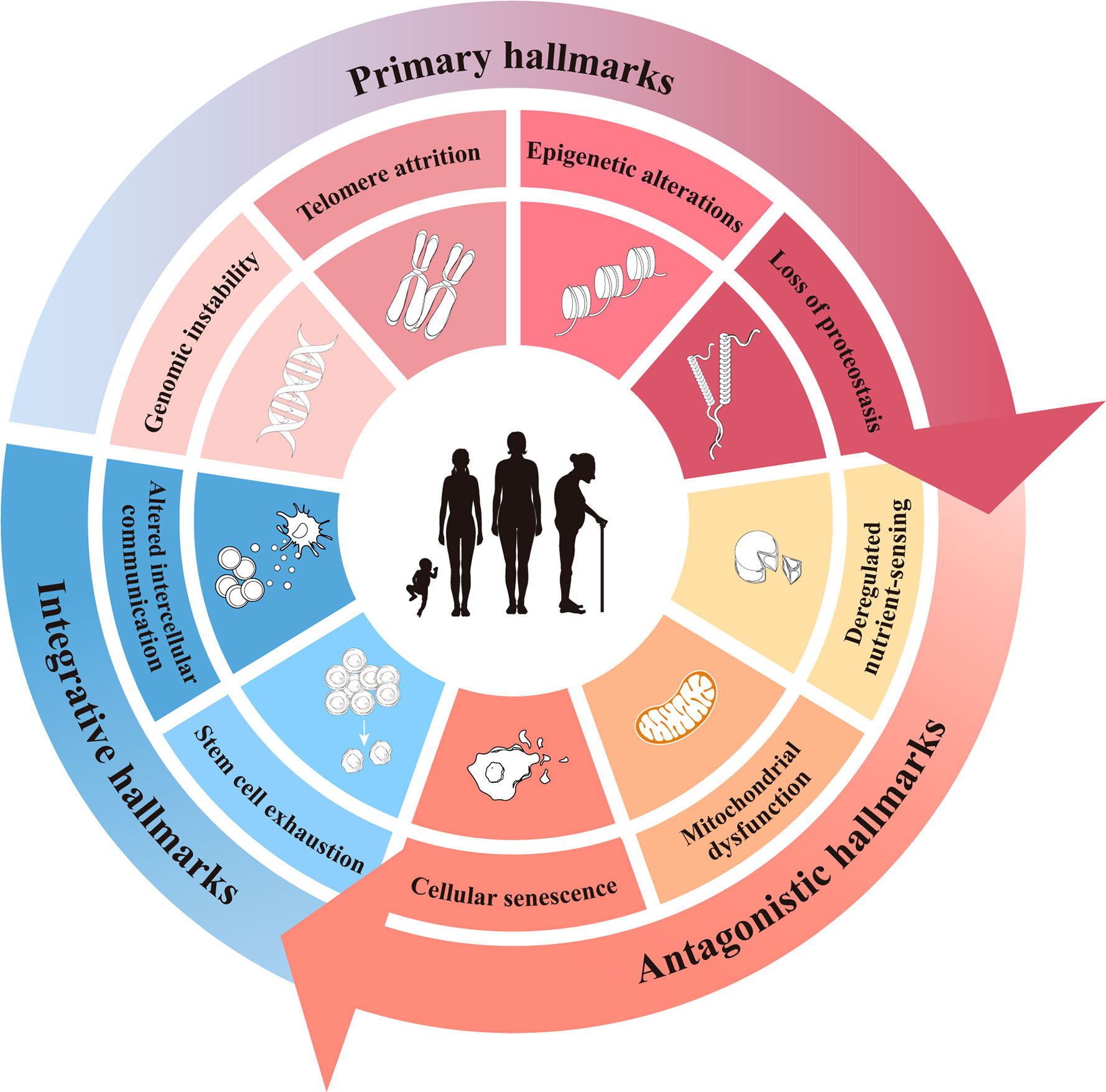
Aging can be defined as the progressive accumulation of degenerative changes that ultimately leads to an increased probability of functional impairment and mortality. Lopez-Otin C and Blasco MA et al. proposed that the current molecular and cellular hallmarks of aging be grouped into three categories: primary, antagonistic, and integrative hallmarks (13). While these hallmarks of aging have been presented as nine separate hallmarks in various research disciplines and there is a certain degree of cross-linking between them, we still hoped to summarize them by hierarchical relationship. Here, the hallmarks of aging are reviewed to provide some insight into the initiation of ovarian aging (Figure 1).
Primary hallmarks
The primary hallmarks are considered to be the actual cause of aging and have a clear negative impact on deoxyribonucleic acid (DNA) (14). They first initiate cell damage, resulting in cumulative damage and gradual loss of function over time, which manifest as genomic instability, telomere attrition, epigenetic alterations, and loss of proteostasis (15–18).
Sustaining genome integrity requires the integration of multiple mechanisms and signaling pathways, and its stability is crucial for individual growth and human health (19). Genome instability is an increasing trend of genomic alteration during the cell life cycle, driven by a variety of endogenous and exogenous damage (20). Furthermore, genomic instability is reflected in gene mutation, replication, and transcription blockage, as well as DNA repair defects, which lead to the decline of organ function and disease progression, such as xeroderma pigmentosum, Cockayne syndrome, and other aging-related diseases (21–23).
The main function of telomeres is to protect the ends of chromosomes to maintain genomic integrity (24–26). Age and environmental factors such as inflammation, reactive oxygen species (ROS), and exposure to radiation or toxins can accelerate telomere attrition (27). The indispensable components in telomere maintenance, such as telomerase, telomerase ribonucleic acid (RNA) components, and shelterin complex, are closely correlated with aging and age-related diseases, such as premature aging syndromes and cancer (28).
Epigenetic alterations in DNA methylation, histone modification, and chromatin remodeling affect aging and longevity (29). Among various epigenetic alterations, DNA methylation is directly related to ROS metabolism through key redox intermediates such as 2-oxoglutarate, S-adenosylmethionine (SAM), and nicotine adenine dinucleotide (NAD) (30). These intermediate fluctuations directly affect epigenetic characteristics, resulting in detectable changes in gene expression and protein modification. Studies have shown that mouse quiescent satellite cells and teleost fish brain tissues display epigenetic repression of trimethylated histone H3 lysine 27 (H3K27me3) during aging (31, 32). Another study showed that the trimethylated histone H3 lysine 4 (H3K4me3) complex regulates the lifespan of Caenorhabditis elegans (33, 34). In addition, epigenetic alterations during aging also cause obvious chromatin structural changes, including heterochromatin region loss, global histone loss, and chromatin spatial interaction changes (35).
In addition, the maintenance of proteostasis is the key to ensuring normal organism development, resisting environmental stress, and promoting healthy aging and longevity (36, 37). As part of the proteostasis network, the ubiquitin-proteasome system and the autophagy-lysosome pathway are two major mechanisms of intracellular protein degradation (38–40). Studies have shown that the failure of autophagy in physiological aging satellite cells causes the loss of proteostasis, increased mitochondrial dysfunction, and ROS and ultimately leads to a decline in satellite cell number and function (41). In addition, loss of protein solubility and accumulation of aggregates are the histopathological hallmarks of several neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease (42–44).
Antagonistic hallmarks
Antagonistic hallmarks, including cell senescence, mitochondrial dysfunction, and deregulated nutrient sensing, respond to the damage caused by primary hallmarks and are considered to be part of the compensatory or antagonistic response to damage. These responses initially mitigate the damage, and if enlarged or aggravated, they ultimately become deleterious and cause further damage.
Cell senescence is a generally irreversible and permanent state in the cell cycle caused by different stresses (45), including continuous DNA damage, irradiation, ROS, and viral infection (46). Cell senescence is accompanied by a low response to mitogenic stimuli, an inability to re-enter the cell cycle, and an enhanced secretory phenotype (47). The main structural and functional changes in aging cells include decreased membrane selective permeability, decreased responsiveness to mitogenic stimuli, disordered immune function, reduced enzyme activity, enhanced lysosomal activity, and decreased antioxidant capacity (48, 49). Cell senescence can damage tissue repair and regeneration, leading to age-dependent diseases, such as osteoporosis, pulmonary fibrosis, renal diseases, hepatic steatosis, and cardiovascular and neurodegenerative diseases (50).
As the primary site of oxidation of carbohydrates, fats, and amino acids to produce adenosine triphosphate (ATP), the quality and activity of mitochondria are essential for homeostasis maintenance, cell cycle control, and programmed cell death (51–53). Mitochondrial signaling pathways involving aging have been studied, such as mitochondrial dynamics, mitochondrial protein synthesis, mitochondrial autophagy, oxidative phosphorylation, ROS, and mitochondrial DNA damage (54, 55). Severe mitochondrial dysfunction can lead to biosynthesis disorders, insufficient energy supply, and increased ROS, thereby aggravating tissue and organ damage and even causing a variety of aging-related pathological changes (56–58).
The ability of cells to respond to nutrient-sensitive signaling pathways is tightly linked with nutrient availability and metabolic homeostasis, affecting the acquisition and maintenance of cell growth, cellular senescence, metabolism, and other physiological processes (59–62). Multiple signaling pathways are known to be involved in the regulation of nutrients, especially insulin/insulin-like growth factor 1 (IGF-1), the mammalian target of rapamycin (mTOR), and adenosine monophosphate-activated protein kinase (AMPK) signaling systems (63, 64). However, the dysregulation of nutrient sensing and energy metabolic pathways is closely related to metabolic diseases, such as obesity, type 2 diabetes mellitus, metabolic syndrome, and other age-associated diseases (65, 66).
Integrative hallmarks
Stem cell exhaustion and altered intercellular communication are thought to be integrative hallmarks because they directly affect tissue homeostasis and function. When the cumulative damage caused by the primary and antagonistic hallmarks cannot be compensated for through tissue homeostatic mechanisms, integrative hallmarks arise and inevitably lead to the functional decline associated with aging.
Stem cell exhaustion, including the loss of stem cell number or function, is a progressive process and a comprehensive result of multiple aging-related injuries, leading to sustained and irreversible changes in the inherent features of stem cells (67–69). Throughout the life cycle, tissue-derived adult stem cells are essential for tissue homeostasis maintenance and regeneration by balancing self-renewal with lineage selection (70, 71). Therefore, adult stem cell exhaustion is considered to be an important driving factor behind the decline in tissue and organ function observed during aging. Beyond intracellular autonomous changes, aging also changes intercellular and intertissue communication (13, 72). These communications involve multiple independent or simultaneous processes that depend on physiological or pathological conditions to affect and maintain tissue homeostasis (35). Altered intercellular communication in the immune system is a progressive exacerbation of the proinflammatory state and reduced immune surveillance and immune response (73–75). As an acute and transient response to harmful conditions, the inflammatory response is conducive to the defense, repair, turnover, and adaptation of many tissues (76). However, with age, the innate and adaptive immune systems change (77). A chronic and low-grade inflammatory state termed inflammaging is likely to have a detrimental effect on the effectiveness of the immune system (78, 79). Studies have shown that changes in redox balance, increased senescence-associated secretory phenotype (SASP), and reduced effective autophagy trigger inflammasomes, suggesting that aging-related diseases and aging itself may be delayed by inhibiting proinflammatory molecular mechanisms (80).
Histomorphological and functional changes in the ovary at different stages
Histomorphological aspects of the ovary
In a study of human ovarian histomorphology, the ovaries of fetuses at 9-40 weeks of gestation are mostly almond-shaped and arranged obliquely in the fetal period (81). In the abdominal cavity, the ovaries usually descend slowly from the anterior ureter and above the common iliac artery with increasing volume until after birth. The ovaries have a smooth surface without ovulation until puberty, and menarche is an important sign of the onset of ovulation. In adolescence, the ovaries have a grayish-white, flattened oval appearance, begin to ovulate, and gradually have periodicity (82). Due to the extrusion of oocytes, the ovarian surface becomes uneven, and empty ovulation can be observed (83). In the absence of pregnancy, the ovaries of healthy adult women undergo extensive dynamic tissue remodeling during each menstrual cycle throughout the reproductive period (approximately 40 years) (84). The ovary consists of four layers from the outer to the central section: the germinal epithelium layer, the nonvascularized and thick fibrous-rich layer called tunica albuginea, the cortex containing ovarian follicles, and the medulla containing loose connective tissue and blood vessels (85). Studies have shown that ovarian fibrosis and stiffness increase with age in the mammalian ovary and depend on the age-related increase in collagen and the decrease in hyaluronan matrices (86, 87). As women enter the perimenopausal period from the reproductive period, obvious morphological and structural degeneration of the ovary occurs. Owing to the decrease in the number and diameter of follicles, aging ovaries shrink and show a wrinkled, nonglossy appearance (88).
Ovarian follicles are structural and functional units of the ovary, in which somatic cells and germ cells are well interrelated and interdependent (89). Human primordial germ cells differentiate into oogonia and proliferate, and this differentiation occurs continuously through mitosis and meiosis, stopping at the diplotene stage of meiotic prophase I (MI), and may last for decades until the oocyte is ovulated (90). Follicular development includes oocyte development, extensive proliferation and differentiation of granulosa cells (GCs), and theca cells with highly vascularized and specialized tissue layers generated by stromal cells (84). At the 20th week of fetal development, approximately 6-7 million oocytes in the ovary are surrounded by a layer of flat granulosa cells to form PMFs (91). After that, most PMFs are rapidly lost via apoptosis in the second half of fetal life, leaving only 1-2 million PMFs at birth (92). After birth, this high rate of follicle loss slows somewhat, and some PMFs can be recruited into the growing follicular pool and develop into antral follicles, most of which will inevitably enter the atresia stage. Most PMFs undergo degeneration or atresia at any stage of ovarian folliculogenesis. With the help of HPO axis regulation, ovarian function gradually matures, and approximately 300,000-400,000 PMFs are retained at menarche (93). Menstruation gradually became regular from the beginning of irregularity, with the decline of PMFs stabilized, but then gradually accelerated (94). Only 400-500 follicles reach the ovulatory phase during the reproductive span of healthy women (84). The ovulation process involves the expansion of the oocyte-cumulus complex, digestion of the follicle wall, resumption of oocyte meiosis, extrusion of meiotic prophase II oocytes, remodeling of the extracellular matrix, etc. (95–97). Among them, cumulus expansion and oocyte maturation are the key processes of ovulation (98).
Functional aspects of the ovary
The ovary has both reproductive and endocrine functions. Ovarian reproductive function is mainly controlled by the hypothalamic-pituitary-ovarian (HPO) axis during the regular menstrual cycle (99). Ovarian endocrine function involves the secretion of steroid hormones, including estrogen, progesterone, a small amount of androgen, and various cytokines (100). These substances affect the development and function of the female vagina, uterus, oviduct, breast, and other organs.
Ovary reproductive function is the result of numerous interactions, among which follicular reserve plays a fundamental role. It is known that the PMF pool constitutes the ovarian reserve, and both reproductive and endocrine functions are limited by the PMF pool (4). Multiple cellular components of follicles coordinate and interact to regulate ovarian hormone secretion and affect oocyte development and maturation. As the number of follicles decreases, the quality of oocytes diminishes as well, especially after the age of 35 years (93). Research has shown that the decline in follicle numbers is a biexponential function of age, and this change occurs at the critical value of 25 000 follicles at the age of 37.5 years (94). The menopause transition marks a period of physiological change, during which the ovaries undergo incremental natural aging, and the PMF pool experiences a continuous irreversible decline (101). Subsequently, the rate of ovarian aging was unexpectedly accelerated. At the age of 51 years, the number of follicles decreased to 1000, which can be used as a threshold for menopause, as it corresponds to the average age of menopause in women (93, 102).
Ovarian endocrine function is jointly affected by ovarian sympathetic innervation, feedback regulation of the HPO axis, and complex interactions of the hormone axis (99). Follicles are not only the source of supply for female germ cells but also secrete essential hormones necessary to maintain normal endocrine function (103). The accelerated depletion of follicles in the PMF pool during the subfertility period may be related to increased sympathetic nerve excitement. Currently, studies have confirmed the presence of sympathetic innervation of the ovary, including the ovarian plexus nerve (projecting to the ovarian vasculature) and the superior ovarian nerve (projecting to the follicle), which can regulate ovarian blood flow and directly regulate steroid hormone production (104).
Ovarian aging is linked to changes in the HPO axis and a progressive decline in ovarian endocrine function, especially disorders of sex hormone levels. Among many sex hormones, anti-Müllerian hormone (AMH) is still the preferred ovarian reserve indicator in various clinical situations (105). AMH is produced by granulosa cells of small antral follicles in the ovary, not controlled by the hypothalamus or by gonadotropins, and independent of the menstrual cycle (106). A clinical trial showed that serum AMH levels in women aged 21-41 years declined by 5.6% per year (107). In addition, the complex interaction of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the hypothalamus, anterior pituitary gland, and reproductive organs has complementary effects on ovarian folliculogenesis and ovulation (108). With age, women will experience early menopause transition, characterized by fluctuations in estrogen levels but generally sufficient, and ovulation generally occurs during the menstrual cycle (109). Finally, menopause is the final manifestation and hallmark event of ovarian aging, which manifests as a permanent cessation of the menstrual cycle and a serious decline in hormone secretion after the loss of follicular activity, especially the decline in estrogen levels (110).
Clinical symptoms and mechanisms of ovarian aging
Clinical symptoms of ovarian aging
The female reproductive system, especially the ovaries, is aging before any other organ system. This phenomenon has obvious clinical significance and may lead to infertility, abortion, birth defects, menstrual cycle disorders, or even amenorrhea and systemic deterioration caused by estrogen deficiency (111). A cohort study of 751 women who were artificially inseminated showed that the probability of pregnancy decreased rapidly after 31 years of age, and the probability of adverse pregnancy outcomes began to increase (112). In addition, ovarian aging may manifest as a shortened or prolonged menstrual cycle, irregular cycle, excessive or insufficient menstrual volume, and perimenopausal abnormal uterine bleeding (113). Ovarian aging leads to estrogen deficiency, which not only directly affects the tissues and organs with estrogen receptors, such as the ovary, endometrium, vaginal epithelium, skin, hypothalamus, and urinary tract but also influences other aspects of the organism, including the cardiovascular, musculoskeletal, and immune systems, emotional and sleep patterns, cognitive ability, and energy metabolism (114). For example, degenerative skin changes occur with estrogen deficiency, characterized by skin atrophy and accelerated skin aging, including collagen atrophy, elasticity and epidermal thickness decrease, elongation increase, wrinkles, and dryness (115). In the cardiovascular system, estrogen deficiency can downregulate the production of nitric oxide, reduce endothelial-dependent vascular function and adversely affect cytokine-mediated cell adhesion and antiatherosclerosis activity (116). Estrogen deficiency can also lead to bone loss, articular cartilage degeneration, and increased risk of fracture, possibly causing pain, loss of mobility, and the development of osteoporosis (117). In muscle, estrogen has a significant effect on the stability of muscle membranes and can reduce or delay leukocyte infiltration after muscle injury (118). In the central nervous system, decreased estrogen levels influence cognition, sleep and mood and affect many neuropsychiatric disorders, including Alzheimer’s disease, schizophrenia, and depression (119, 120).
Potential mechanism of ovarian aging
The lifespan of the ovary depends on the delicate balance between the survival and death of oocytes. PMF activation is the basis for ovarian folliculogenesis and maintenance of fertility (121). However, damage during ovarian folliculogenesis, including the activation, recruitment, and development of early follicles, has a complex relationship with ovarian aging. In addition, depletion of the PMF pool caused by massive follicular atresia and periodic ovulation is the fundamental cause of ovarian aging.
Since the first use of [3H]-thymidine ([3H]-TdR) incubation to distinguish between slow-growing follicles and dormant PMFs, studies related to ovarian folliculogenesis have been conducted for more than 30 years. The regulatory mechanism of folliculogenesis, especially PMF activation, remains difficult to uncover due to the sophisticated process. Otsuka and Shimasaki demonstrated that the mitotic activities of oocyte-derived bone morphogenetic protein-15 and GC-derived kit ligand depend on the oocyte-GC communication system, which may play a pivotal role in ovarian folliculogenesis (122). Additionally, a series of protein and polypeptide hormones secreted by the pituitary gland, such as prolactin (PRL), growth hormone (GH), FSH, and LH, are essential for the activation and initial recruitment of PMFs and the development of growing follicles (123, 124). Studies also suggest that cytokines such as epidermal growth factor (EGF), transforming growth factor-alpha (TGF alpha), basic fibroblast growth factor (bFGF), and IGF-1 are involved in folliculogenesis and ovulation (125–128).
Forkhead box L2 (FOXL2), a winged helix/forkhead domain transcription factor, is preferentially expressed in the ovary, eyelids, and pituitary gland (129). Studies have shown that FOXL2 participates in multiple stages of ovarian development and function and the differentiation of pregranulosa cells (91). A sufficient number of pregranulosa cells expressing FOXL2 and primary oocytes arrested at the diploid stage of MI are two indispensable prerequisites for pregranulosa cells to break through germline cysts and move around primary oocytes (130). Additionally, research has demonstrated that the short-term treatment of mouse ovaries with a phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor or a phosphatidylinositol-3-kinase (PI3K) activator could increase the phosphorylation of protein kinase B (AKT) and the nuclear export of downstream forkhead box O3 (FOXO3) protein, thereby effectively activating dormant PMFs (131, 132).
Obviously, abnormal follicular activation and atresia are the intrinsic mechanisms of ovarian aging, which may involve endocrine, paracrine, or autocrine signaling pathways. Follicular atresia is closely related to autophagy or apoptosis (133). Autophagy, a highly conserved intracellular process that maintains homeostasis by removing useless, senescent organelles and macromolecules, is a unique pathway to cell death as well as an adaptive response that promotes cell survival (134). Numerous autophagosomes and autophagic responses were reported to be mainly observed and detected in dead oocytes, especially in primordial and primary follicle oocytes (135). Cell apoptosis appeared only in GCs around the secondary or antral follicles, suggesting that both apoptosis and autophagy can mediate the onset of follicular atresia, while cell apoptosis may be the main form of postnatal follicular atresia (136).
Cell apoptosis, especially the intrinsic mitochondrial pathway, is regulated by B-cell lymphoma-2 (BCL-2) family proteins (137). Myeloid cell leukemia-1 (MCL-1), an antiapoptotic protein of BCL-2 family members, has a prosurvival effect in various cell types (138). MCL-1 deficiency activated apoptosis in early PMFs, increased markers of mitochondrial dysfunction and autophagy in growing oocytes, activated the apoptosis cascade reaction, and increased sensitivity to cell fragmentation in ovulated oocytes (139). Therefore, MCL-1 is considered to be a basic survival factor for maintaining the postnatal ovarian reserve, survival of growing follicles, and effective mitochondrial function of oocytes (139). Endogenous advanced glycation end products (AGEs) and the receptor for AGEs are expressed in luteinized and theca cells as well as GCs derived from ovaries, and AGEs can induce ROS chain reactions and increase inflammation, resulting in protein, lipid, and nucleotide damage during aging in the ovarian microenvironment (140–142). The accumulation of age-related AGEs in ovarian follicles triggers ovarian aging, which may be related to the regulation of AMH and AMHRII expression to affect ovarian reserve, reduce ovarian vascular supply and decrease glucose uptake in GCs (143, 144).
Potential retrieval strategies for ovarian aging
Delaying childbearing among women has become a universal phenomenon due to sociodemographic, economic, medical, lifestyle and behavioral factors (145, 146). Human female fertility and reproductive lifespan decline with physiological aging and pathological failure, accompanied by a decline in birth rates and an increase in the number of childless adults (147–149). Attention to aging, especially ovarian aging, and finding potential retrieval strategies to improve fertility and healthy life expectancy has become an urgent task in the reproductive field (4, 150).
Dietary interventions
Calorie restriction, the reduction of dietary intake to below energy requirements while maintaining optimal nutrition, is considered one of the most promising nutritional interventions to attenuate aging (151, 152). Despite its simplicity, a constant reduction in calorie or food intake is not easy to maintain in the long run. Recently, fasting-related interventions, such as prolonged fasting, time-restricted eating, and intermittent fasting, have emerged as alternatives to calorie restriction (153). Many studies based on animal models confirm that calorie restriction can delay the progression of diabetes, stroke, neurodegeneration, sarcopenia, and cardiovascular disease; reduce the cytotoxicity of chemotherapy; and alleviate immune system disorders (154, 155). In addition, calorie restriction can reduce the activation of PMFs and increase the number of quiescent PMFs in mice, which is beneficial to the protection of ovarian reserve and may be a potential way to delay menopause (156).
GH/IGF-1 axis interventions
The mechanistic links between GH and aging mainly involve the evolutionarily conserved insulin/IGF-1 and mTOR signaling pathways that affect growth, immunity, metabolism, homeostasis and aging (157, 158). The secretion of both GH and IGF-1 peaks at puberty and gradually decreases in adulthood until only low levels are detectable in individuals aged ≥60 years (159, 160). IGF-1 levels in vivo are regulated by GH, and IGF-1 also has a negative feedback regulation on GH secretion (161, 162). Studies have shown that IGF-1 has important effects on the healthy growth and function of cells and tissue in model organisms (163–165). GH can not only directly affect human oocytes and cumulus cells but also indirectly influence oocyte quality and maintain oocyte DNA integrity by activating IGF-1 synthesis or promoting ovarian steroidogenesis (166–168). Studies confirm that the GH/IGF-1 axis not only inhibits ROS accumulation and apoptosis in GCs but also regulates steroidogenesis and follicular proliferation in polycystic ovary syndrome (169, 170).
mTOR/S6 kinase pathway interventions
mTOR is a highly conserved serine/threonine-protein kinase (171). mTOR acts as a signal transduction center that integrates environmental and intracellular nutrients and growth factor signals and regulates various processes, including cell proliferation, metabolism, immunity, cellular senescence, and protein synthesis (172, 173). In addition, mTOR can phosphorylate and activate ribosomal protein S6 kinase (S6K), which is the key regulatory element of cellular transcript translation and protein synthesis (174). The AKT/mTOR signaling pathway controls ovarian folliculogenesis by maintaining the PMF pool, including PMF activation, GC proliferation, and oocyte-GC intercellular communication (175). Studies have shown significant activation of the AKT/mTOR/S6K signaling pathway in humans with POI (176). Therefore, regulating the AKT/mTOR/S6K signaling pathways, such as AKT activators and mTOR activators, can induce further follicular maturation and development in women with POI (176).
AMPK or specific sirtuin interventions
AMPK, a serine/threonine-protein kinase, is one of the key energy sensors in eukaryotic cells and organisms and plays critical roles in regulating growth and reprogramming metabolic processes, such as autophagy, mitochondrial biogenesis, and lipid, cholesterol, and glucose metabolism (177). For example, AMPK activation in multiple tissues can autonomously and involuntarily induce autophagy, allowing cellular components to be recycled for energy production under nutrient-limited conditions (178, 179). With increasing age, the responsiveness of AMPK signaling decreases, and the aging process increases, leading to impaired maintenance of cellular homeostasis (180). Studies have confirmed that modulation of AMPK signaling can activate autophagy in GCs, affect human ovarian function, and lead to abnormal folliculogenesis (181). Furthermore, AMPK is necessary for the normal response to steroid hormones, and its intervention has potential significance for delaying ovarian aging (182).
Sirtuins are a family of NAD-dependent histone deacetylases that modulate cellular functions, such as genomic stability, mitochondrial biogenesis, cellular metabolism, autophagy or apoptosis, and the inflammatory response (183). Seven sirtuin isoforms catalyze specific lysine substrate deacetylation in mammals (184, 185). As a promising target for the prevention of aging-related diseases, sirtuin 1 is the most commonly studied isoform (186, 187). Several plant-derived polyphenol compounds, including resveratrol, butein, fisetin, and quercetin, can activate sirtuin 1 and exert beneficial effects on longevity (188). Studies have shown that resveratrol can enhance luteinization-related gene expression and ovarian progesterone secretion and improve the quality of cryopreserved ovarian tissue and embryo outcome in mice after transplantation through anti-inflammatory and antioxidant mechanisms (189, 190).
Stem cell interventions
The exploration of stem cell and stem cell-derived extracellular vesicle therapy in reproductive medicine has shown great promise and availability in preclinical and clinical trials to delay, prevent or even reverse ovarian aging (191, 192). In preclinical trials, rhesus monkeys provide a suitable model for studying ovarian aging (193). Research has observed that using juvenile bone-marrow-derived mesenchymal stem cells (BM-MSCs) to treat macaques with ovarian aging can increase ovarian volume, strengthen hormonal regulation, and promote follicular regeneration in senescent macaques (194). Recently, several clinical trials using autologous BM-MSCs and allogeneic human umbilical cord-derived mesenchymal stem cells (UC-MSCs) in the treatment of patients with premature ovarian failure have demonstrated encouraging preliminary data in the rescue of overall ovarian function, as evidenced by increased ovarian volume, resumed menstruation, improved levels of estradiol and AMH, increased number of stimulating antral follicles, and alleviated menopausal symptoms (195–198).
Embryos, oocytes, or ovarian tissue cryopreservation
The possibility of freezing oocytes and embryos has been available for a long time, and the first birth with thawed oocytes was achieved in 1983 (199). However, ovarian translocation and cryopreservation of embryos and oocytes are not suitable for prepubertal girls and women requiring urgent initiation of cancer treatment (85, 200). At present, ovarian tissue cryopreservation is a potential therapeutic option for ovarian function recovery in POI patients without ovarian stimulation or subsequent delay in the start of cancer treatment (201). The cryopreserved ovarian cortex can be thawed and autotransplanted, which has been proven to restore fertility, preserve ovarian endocrine function, and avoid the incidence of premature menopause, thereby delaying ovarian aging (202). However, the main challenges for ovarian transplantation are the massive loss of PMFs during ischemia and hypoxia and the risk of reintroduction of malignant cells with transplanted tissues (203, 204).
Conclusion and perspectives
The ovary is the core reproductive organ of women and is crucial for maintaining normal reproductive and endocrine function stability. With the increase in lifespan expectancy, ovarian aging has gradually become a key health problem for women and is associated with a progressive age-related decline in the number and quality of oocytes. When these processes occur earlier or accelerate, their clinical correlation is the diminished ovarian reserve and/or triggers POI. Therefore, clarifying the hallmarks of aging, further studying the molecular mechanisms of ovarian aging and optimizing ovarian aging interventions are of profound significance for inhibiting aging-related diseases, reversing or preventing ovarian aging, and promoting female health and longevity.
Author contributions
JW: Conceptualization, Writing-Original Draft; YL, YS: Resources, Data Curation; LW: Supervision, Project administration, Funding acquisition; JA, KL: Resources, Methodology. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project of the National Natural Science Foundation of China (82101700).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update (2013) 19:67–83. doi: 10.1093/humupd/dms043
2. Smits M, Janssens GE, Goddijn M, Hamer G, Houtkooper RH, Mastenbroek S. Longevity pathways are associated with human ovarian ageing. Hum Reprod Open (2021) 2:hoab020. doi: 10.1093/hropen/hoab020
3. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev (2009) 30:465–93. doi: 10.1210/er.2009-0006
4. Llarena N, Hine C. Reproductive longevity and aging: Geroscience approaches to maintain long-term ovarian fitness. J Gerontol A Biol Sci Med Sci (2021) 76:1551–60. doi: 10.1093/gerona/glaa204
5. Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. detection and clinical relevance. Hum Reprod (2003) 18:1137–9. doi: 10.1093/humrep/deg245
6. Li CJ, Lin LT, Tsai HW, Chern CU, Wen ZH, Wang PH, et al. The molecular regulation in the pathophysiology in ovarian aging. Aging Dis (2021) 12:934–49. doi: 10.14336/AD.2020.1113
7. Mishra GD, Chung HF, Cano A, Chedraui P, Goulis DG, Lopes P, et al. EMAS position statement: Predictors of premature and early natural menopause. Maturitas (2019) 123:82–8. doi: 10.1016/j.maturitas.2019.03.008
8. European Society for Human, R., Embryology Guideline Group On, P.O.I, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod (2016) 31:926–37. doi: 10.1093/humrep/dew027
9. Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: Past, present, and future. Front Cell Dev Biol (2021) 9:672890. doi: 10.3389/fcell.2021.672890
10. Vujovic S, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, Moen MH, et al. EMAS position statement: Managing women with premature ovarian failure. Maturitas (2010) 67:91–3. doi: 10.1016/j.maturitas.2010.04.011
11. Jiao SY, Yang YH, Chen SR. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update (2021) 27:154–89. doi: 10.1093/humupd/dmaa034
12. Ding T, Yan W, Zhou T, Shen W, Wang T, Li M, et al. Endocrine disrupting chemicals impact on ovarian aging: Evidence from epidemiological and experimental evidence. Environ pollut (2022) 305:119269. doi: 10.1016/j.envpol.2022.119269
13. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
14. Lagunas-Rangel FA. SIRT7 in the aging process. Cell Mol Life Sci (2022) 79:297. doi: 10.1007/s00018-022-04342-x
15. González-Quiroz M, Blondel A, Sagredo A, Hetz C, Chevet E, Pedeux R. When endoplasmic reticulum proteostasis meets the DNA damage response. Trends Cell Biol (2020) 30:881–91. doi: 10.1016/j.tcb.2020.09.002
16. Chakravarti D, Labella KA, Depinho RA. Telomeres: history, health, and hallmarks of aging. Cell (2021) 184:306–22. doi: 10.1016/j.cell.2020.12.028
17. Siametis A, Niotis G, Garinis GA. DNA Damage and the aging epigenome. J Invest Dermatol (2021) 141:961–7. doi: 10.1016/j.jid.2020.10.006
18. Vougioukalaki M, Demmers J, Vermeij WP, Baar M, Bruens S, Magaraki A, et al. Different responses to DNA damage determine ageing differences between organs. Aging Cell (2022) 21:e13562. doi: 10.1111/acel.13562
19. Chen Y, Geng A, Zhang W, Qian Z, Wan X, Jiang Y, et al. Fight to the bitter end: DNA repair and aging. Ageing Res Rev (2020) 64:101154. doi: 10.1016/j.arr.2020.101154
20. Laffon B, Bonassi S, Costa S, Valdiglesias V. Genomic instability as a main driving factor of unsuccessful ageing: Potential for translating the use of micronuclei into clinical practice. Mutat Res Rev Mutat Res (2021) 787:108359. doi: 10.1016/j.mrrev.2020.108359
21. Pardo B, Moriel-Carretero M, Vicat T, Aguilera A, Pasero P. Homologous recombination and Mus81 promote replication completion in response to replication fork blockage. EMBO Rep (2020) 21:e49367. doi: 10.15252/embr.201949367
22. De Majo F, Martens L, Hegenbarth JC, Rühle F, Hamczyk MR, Nevado RM, et al. Genomic instability in the naturally and prematurely aged myocardium. Proc Natl Acad Sci USA (2021) 118(36):e2022974118. doi: 10.1073/pnas.2022974118.
23. Kajitani GS, Nascimento LLS, Neves MRC, Leandro GDS, Garcia CCM, Menck CFM. Transcription blockage by DNA damage in nucleotide excision repair-related neurological dysfunctions. Semin Cell Dev Biol (2021) 114:20–35. doi: 10.1016/j.semcdb.2020.10.009
24. Aguado J, D'adda Di Fagagna F, Wolvetang E. Telomere transcription in ageing. Ageing Res Rev (2020) 62:101115. doi: 10.1016/j.arr.2020.101115
25. Brenner KA, Nandakumar J. Small molecules restore telomeres in patient stem cells. Trends Pharmacol Sci (2020) 41:506–8. doi: 10.1016/j.tips.2020.05.003
26. Myler LR, Kinzig CG, Sasi NK, Zakusilo G, Cai SW, De Lange T. The evolution of metazoan shelterin. Genes Dev (2021) 35:1625–41. doi: 10.1101/gad.348835.121
27. Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry (2013) 73:15–23. doi: 10.1016/j.biopsych.2012.06.025
28. Lin J, Epel E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res Rev (2022) 73:101507. doi: 10.1016/j.arr.2021.101507
30. Sundar V, Ramasamy T, Doke M, Samikkannu T. Psychostimulants influence oxidative stress and redox signatures: the role of DNA methylation. Redox Rep (2022) 27:53–9. doi: 10.1080/13510002.2022.2043224
31. Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep (2013) 4:189–204. doi: 10.1016/j.celrep.2013.05.043
32. Baumgart M, Groth M, Priebe S, Savino A, Testa G, Dix A, et al. RNA-Seq of the aging brain in the short-lived fish n. furzeri - conserved pathways and novel genes associated with neurogenesis. Aging Cell (2014) 13:965–74. doi: 10.1111/acel.12257
33. Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in c. elegans. Nature (2010) 466:383–7. doi: 10.1038/nature09195
34. Saul D, Kosinsky RL. Epigenetics of aging and aging-associated diseases. Int J Mol Sci (2021) 22(1):401. doi: 10.3390/ijms22010401
35. Peters A, Nawrot TS, Baccarelli AA. Hallmarks of environmental insults. Cell (2021) 184:1455–68. doi: 10.1016/j.cell.2021.01.043
36. Kruta M, Sunshine MJ, Chua BA, Fu Y, Chawla A, Dillingham CH, et al. Hsf1 promotes hematopoietic stem cell fitness and proteostasis in response to ex vivo culture stress and aging. Cell Stem Cell (2021) 28:1950–65.e1956. doi: 10.1016/j.stem.2021.07.009
37. Meller A, Shalgi R. The aging proteostasis decline: From nematode to human. Exp Cell Res (2021) 399:112474. doi: 10.1016/j.yexcr.2021.112474
38. Kwon YT, Ciechanover A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem Sci (2017) 42:873–86. doi: 10.1016/j.tibs.2017.09.002
39. Bourdenx M, Martín-Segura A, Scrivo A, Rodriguez-Navarro JA, Kaushik S, Tasset I, et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell (2021) 184:2696–714.e2625. doi: 10.1016/j.cell.2021.03.048
40. Yang C, Wang X. Lysosome biogenesis: Regulation and functions. J Cell Biol (2021) 220(6):e202102001. doi: 10.1083/jcb.202102001
41. Kaushik S, Tasset I, Arias E, Pampliega O, Wong E, Martinez-Vicente M, et al. Autophagy and the hallmarks of aging. Ageing Res Rev (2021) 72:101468. doi: 10.1016/j.arr.2021.101468
42. Hartl FU. Protein misfolding diseases. Annu Rev Biochem (2017) 86:21–6. doi: 10.1146/annurev-biochem-061516-044518
43. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer's disease. Lancet (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
44. Stojkovska I, Wani WY, Zunke F, Belur NR, Pavlenko EA, Mwenda N, et al. Rescue of α-synuclein aggregation in parkinson's patient neurons by synergistic enhancement of ER proteostasis and protein trafficking. Neuron (2022) 110:436–51.e411. doi: 10.1016/j.neuron.2021.10.032
45. Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A senescence-centric view of aging: Implications for longevity and disease. Trends Cell Biol (2020) 30:777–91. doi: 10.1016/j.tcb.2020.07.002
46. Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: Aging, cancer, and injury. Physiol Rev (2019) 99:1047–78. doi: 10.1152/physrev.00020.2018
47. Di Micco R, Krizhanovsky V, Baker D, D'adda Di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol (2021) 22:75–95. doi: 10.1038/s41580-020-00314-w
48. Childs BG, Durik M, Baker DJ, Van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med (2015) 21:1424–35. doi: 10.1038/nm.4000
49. Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update (2022) 28:172–89. doi: 10.1093/humupd/dmab038
50. Kaur J, Farr JN. Cellular senescence in age-related disorders. Transl Res (2020) 226:96–104. doi: 10.1016/j.trsl.2020.06.007
51. Kasapoglu I, Seli E. Mitochondrial dysfunction and ovarian aging. Endocrinology (2020) 161(2). doi: 10.1210/endocr/bqaa001
52. Yan W, Diao S, Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther (2021) 12:140. doi: 10.1186/s13287-021-02194-z
53. Miwa S, Kashyap S, Chini E, Von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest (2022) 132(13):e158447. doi: 10.1172/JCI158447
54. Akbari M, Kirkwood TBL, Bohr VA. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev (2019) 54:100940. doi: 10.1016/j.arr.2019.100940
55. Van Der Rijt S, Molenaars M, Mcintyre RL, Janssens GE, Houtkooper RH. Integrating the hallmarks of aging throughout the tree of life: A focus on mitochondrial dysfunction. Front Cell Dev Biol (2020) 8:594416. doi: 10.3389/fcell.2020.594416
56. Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell (2016) 61:654–66. doi: 10.1016/j.molcel.2016.01.028
57. Chiang JL, Shukla P, Pagidas K, Ahmed NS, Karri S, Gunn DD, et al. Mitochondria in ovarian aging and reproductive longevity. Ageing Res Rev (2020) 63:101168. doi: 10.1016/j.arr.2020.101168
58. Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol (2022) 18:243–58. doi: 10.1038/s41574-021-00626-7
59. Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res (2014) 24:42–57. doi: 10.1038/cr.2013.166
60. Aiello A, Accardi G, Candore G, Gambino CM, Mirisola M, Taormina G, et al. Nutrient sensing pathways as therapeutic targets for healthy ageing. Expert Opin Ther Targets (2017) 21:371–80. doi: 10.1080/14728222.2017.1294684
61. De Lucia C, Murphy T, Steves CJ, Dobson RJB, Proitsi P, Thuret S. Lifestyle mediates the role of nutrient-sensing pathways in cognitive aging: cellular and epidemiological evidence. Commun Biol (2020) 3:157. doi: 10.1038/s42003-020-0844-1
62. King KE, Losier TT, Russell RC. Regulation of autophagy enzymes by nutrient signaling. Trends Biochem Sci (2021) 46:687–700. doi: 10.1016/j.tibs.2021.01.006
63. Templeman NM, Murphy CT. Regulation of reproduction and longevity by nutrient-sensing pathways. J Cell Biol (2018) 217:93–106. doi: 10.1083/jcb.201707168
64. Chun Y, Kim J. AMPK-mTOR signaling and cellular adaptations in hypoxia. Int J Mol Sci (2021) 22(18):9765. doi: 10.3390/ijms22189765
65. Carroll B, Korolchuk VI. Nutrient sensing, growth and senescence. FEBS J (2018) 285:1948–58. doi: 10.1111/febs.14400
66. Hu X, Guo F. Amino acid sensing in metabolic homeostasis and health. Endocr Rev (2021) 42:56–76. doi: 10.1210/endrev/bnaa026
67. Sarkar TJ, Quarta M, Mukherjee S, Colville A, Paine P, Doan L, et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun (2020) 11:1545. doi: 10.1038/s41467-020-15174-3
68. Deng P, Yuan Q, Cheng Y, Li J, Liu Z, Liu Y, et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell (2021) 28:1057–1073.e1057. doi: 10.1016/j.stem.2021.01.010
69. Shaker A. Is avoiding stem cell exhaustion the new therapeutic approach in colitis? Cell Mol Gastroenterol Hepatol (2021) 11:1204–6. doi: 10.1016/j.jcmgh.2020.12.001
70. Goodell MA, Rando TA. Stem cells and healthy aging. Science (2015) 350:1199–204. doi: 10.1126/science.aab3388
71. Jayarajan J, Milsom MD. The role of the stem cell epigenome in normal aging and rejuvenative therapy. Hum Mol Genet (2020) 29:R236–r247. doi: 10.1093/hmg/ddaa167
72. Da Silva PFL, Schumacher B. Principles of the molecular and cellular mechanisms of aging. J Invest Dermatol (2021) 141:951–60. doi: 10.1016/j.jid.2020.11.018
73. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
74. Frasca D, Diaz A, Romero M, Garcia D, Blomberg BB. B cell immunosenescence. Annu Rev Cell Dev Biol (2020) 36:551–74. doi: 10.1146/annurev-cellbio-011620-034148
75. Longo VD, Cortellino S. Fasting, dietary restriction, and immunosenescence. J Allergy Clin Immunol (2020) 146:1002–4. doi: 10.1016/j.jaci.2020.07.035
76. Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev (2017) 40:95–119. doi: 10.1016/j.arr.2017.09.001
77. Budde J, Skloot G. Aging and susceptibility to pulmonary disease. Compr Physiol (2022) 12:3509–22. doi: 10.1002/cphy.c210026
78. Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Semin Immunopathol (2020) 42:545–57. doi: 10.1007/s00281-020-00806-z
79. Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol (2021), 18 1–14. doi: 10.1007/s12016-021-08899-6
80. Rea IM, Gibson DS, Mcgilligan V, Mcnerlan SE, Alexander HD, Ross OA. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol (2018) 9:586. doi: 10.3389/fimmu.2018.00586
81. Sulak O, Malas MA, Esen K, Cetin E, Tagil SM. Size and location of the fetal human ovary. Fetal Diagn Ther (2006) 21:26–33. doi: 10.1159/000089044
82. Karapanou O, Papadimitriou A. Determinants of menarche. Reprod Biol Endocrinol (2010) 8:115. doi: 10.1186/1477-7827-8-115
83. Soygur B, Laird DJ. ). ovary development: Insights from a three-dimensional imaging revolution. Front Cell Dev Biol (2021) 9:698315. doi: 10.3389/fcell.2021.698315
84. Fan X, Bialecka M, Moustakas I, Lam E, Torrens-Juaneda V, Borggreven NV, et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat Commun (2019) 10:3164. doi: 10.1038/s41467-019-11036-9
85. Dadashzadeh A, Moghassemi S, Shavandi A, Amorim CA. A review on biomaterials for ovarian tissue engineering. Acta Biomater (2021) 135:48–63. doi: 10.1016/j.actbio.2021.08.026
86. Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell (2020) 19:e13259. doi: 10.1111/acel.13259
87. Mccloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, et al. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res (2020) 26:632–42. doi: 10.1158/1078-0432.CCR-19-0603
88. Sokalska A, Valentin L. Changes in ultrasound morphology of the uterus and ovaries during the menopausal transition and early postmenopause: a 4-year longitudinal study. Ultrasound Obstet Gynecol (2008) 31:210–7. doi: 10.1002/uog.5241
89. Canipari R. Oocyte–granulosa cell interactions. Hum Reprod Update (2000) 6:279–89. doi: 10.1093/humupd/6.3.279
90. Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol (2019) 17:8. doi: 10.1186/s12958-018-0445-8
91. Niu W, Spradling AC. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci USA (2020) 117:20015–26. doi: 10.1073/pnas.2005570117
92. Sun YC, Sun XF, Dyce PW, Shen W, Chen H. The role of germ cell loss during primordial follicle assembly: a review of current advances. Int J Biol Sci (2017) 13:449–57. doi: 10.7150/ijbs.18836
93. Te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update (2002) 8:141–54. doi: 10.1093/humupd/8.2.141
94. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod (1992) 7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570
95. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels with inflammatory processes. Endocr Rev (2019) 40:369–416. doi: 10.1210/er.2018-00075
96. Laisk T, Tsuiko O, Jatsenko T, Horak P, Otala M, Lahdenpera M, et al. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update (2019) 25:34–50. doi: 10.1093/humupd/dmy031
97. Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update (2021) 27:27–47. doi: 10.1093/humupd/dmaa043
98. Yerushalmi GM, Salmon-Divon M, Yung Y, Maman E, Kedem A, Ophir L, et al. Characterization of the human cumulus cell transcriptome during final follicular maturation and ovulation. Mol Hum Reprod (2014) 20:719–35. doi: 10.1093/molehr/gau031
99. Mikhael S, Punjala-Patel A, Gavrilova-Jordan L. Hypothalamic-Pituitary-Ovarian axis disorders impacting female fertility. Biomedicines (2019) 7(1):5. doi: 10.3390/biomedicines7010005
100. Zhang K, Zeitlian G, Adel G, Santoro NF, Pal L. Enhanced hypothalamic-pituitary sensitivity to estrogen in premenopausal women with diminished ovarian reserve compared with older perimenopausal controls. Menopause (2011) 18:880–5. doi: 10.1097/gme.0b013e31820cc564
101. Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: Signs, symptoms, and management options. J Clin Endocrinol Metab (2021) 106:1–15. doi: 10.1210/clinem/dgaa764
102. Gottschalk MS, Eskild A, Hofvind S, Bjelland EK. The relation of number of childbirths with age at natural menopause: a population study of 310 147 women in Norway. Hum Reprod (2022) 37:333–40. doi: 10.1093/humrep/deab246
103. Jones ASK, Shikanov A. Follicle development as an orchestrated signaling network in a 3D organoid. J Biol Eng (2019) 13:2. doi: 10.1186/s13036-018-0134-3
104. Uchida S. Sympathetic regulation of estradiol secretion from the ovary. Auton Neurosci (2015) 187:27–35. doi: 10.1016/j.autneu.2014.10.023
105. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update (2014) 20:688–701. doi: 10.1093/humupd/dmu020
106. Moolhuijsen LME, Visser JA. Anti-mullerian hormone and ovarian reserve: Update on assessing ovarian function. J Clin Endocrinol Metab (2020) 105(11):3361–3373. doi: 10.1210/clinem/dgaa513
107. Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab (2013) 98:1602–11. doi: 10.1210/jc.2012-1829
108. Bosch E, Alviggi C, Lispi M, Conforti A, Hanyaloglu AC, Chuderland D, et al. Reduced FSH and LH action: implications for medically assisted reproduction. Hum Reprod (2021) 36:1469–80. doi: 10.1093/humrep/deab065
109. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause (2012) 19:387–95. doi: 10.1097/gme.0b013e31824d8f40
110. Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, et al. Menopause. Nat Rev Dis Primers (2015) 1:15004. doi: 10.1038/nrdp.2015.4
111. Machlin JH, Barishansky SJ, Kelsh J, Larmore MJ, Johnson BW, Pritchard MT, et al. Fibroinflammatory signatures increase with age in the human ovary and follicular fluid. Int J Mol Sci (2021) 22(9):4902. doi: 10.3390/ijms22094902
112. Van Noord-Zaadstra Bm LC, Alsbach H, Habbema JD, Te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ (1991) 302:1361–5. doi: 10.1136/bmj.302.6789.1361
113. Jewson M, Purohit P, Lumsden MA. Progesterone and abnormal uterine bleeding/menstrual disorders. Best Pract Res Clin Obstet Gynaecol (2020) 69:62–73. doi: 10.1016/j.bpobgyn.2020.05.004
114. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev (2017) 1:CD004143. doi: 10.1002/14651858.CD004143.pub5
115. Liu T, Li N, Yan YQ, Liu Y, Xiong K, Liu Y, et al. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother Res (2020) 34:435–47. doi: 10.1002/ptr.6538
116. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ (2017) 8:33. doi: 10.1186/s13293-017-0152-8
117. Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol (2022) 123:14–21. doi: 10.1016/j.semcdb.2021.05.014
118. Tiidus PM. Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev (2003) 31:40–4. doi: 10.1097/00003677-200301000-00008
119. Russell JK, Jones CK, Newhouse PA. The role of estrogen in brain and cognitive aging. Neurotherapeutics (2019) 16:649–65. doi: 10.1007/s13311-019-00766-9
120. Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep (2021) 23:73. doi: 10.1007/s11920-021-01285-1
121. Mi X, Jiao W, Yang Y, Qin Y, Chen ZJ, Zhao S. HGF secreted by mesenchymal stromal cells promotes primordial follicle activation by increasing the activity of the PI3K-AKT signaling pathway. Stem Cell Rev Rep (2022) 18:1834–50. doi: 10.1007/s12015-022-10335-x
122. Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: Its role in regulating granulosa cell mitosis. Proc Natl Acad Sci United States America (2002) 99:8060–5. doi: 10.1073/pnas.122066899
123. Wang XN, Greenwald GS. Hypophysectomy of the cyclic mouse. II. effects of follicle-stimulating hormone (FSH) and luteinizing hormone on folliculogenesis, FSH and human chorionic gonadotropin receptors, and steroidogenesis. Biol Reprod (1993) 48:595–605. doi: 10.1095/biolreprod48.3.595
124. Buratini J, Dellaqua TT, Dal Canto M, La Marca A, Carone D, Mignini Renzini M, et al. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: from fertility prognosis to mechanisms underlying age-related subfertility. Hum Reprod Update (2022) 28:232–54. doi: 10.1093/humupd/dmab044
125. Ben-Haroush A, Abir R, Ao A, Jin S, Kessler-Icekson G, Feldberg D, et al. Expression of basic fibroblast growth factor and its receptors in human ovarian follicles from adults and fetuses. Fertil Steril (2005) 84 Suppl 2:1257–68. doi: 10.1016/j.fertnstert.2005.05.018
126. Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update (2018) 24:1–14. doi: 10.1093/humupd/dmx029
127. Poulsen LC, Bøtkjær JA, Østrup O, Petersen KB, Andersen CY, Grøndahl ML, et al. Two waves of transcriptomic changes in periovulatory human granulosa cells. Hum Reprod (2020) 35:1230–45. doi: 10.1093/humrep/deaa043
128. Abbassi L, El-Hayek S, Carvalho KF, Wang W, Yang Q, Granados-Aparici S, et al. Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation. Nat Commun (2021) 12:1438. doi: 10.1038/s41467-021-21644-z
129. Georges A, Auguste A, Bessiere L, Vanet A, Todeschini AL, Veitia RA. FOXL2: a central transcription factor of the ovary. J Mol Endocrinol (2014) 52:R17–33. doi: 10.1530/JME-13-0159
130. Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development (2004) 131:933–42. doi: 10.1242/dev.00969
131. Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA (2010) 107:10280–4. doi: 10.1073/pnas.1001198107
132. Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: Implications for primordial follicle activation, oocyte quality and ageing. Cells (2020) 9(1):200. doi: 10.3390/cells9010200
133. Pelosi E, Forabosco A, Schlessinger D. Genetics of the ovarian reserve. Front Genet (2015) 6:308. doi: 10.3389/fgene.2015.00308
134. Kanninen TT, De Andrade Ramos BR, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. Eur J Obstet Gynecol Reprod Biol (2013) 171:3–8. doi: 10.1016/j.ejogrb.2013.07.020
135. Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy (2021) 17:2706–33. doi: 10.1080/15548627.2021.1938914
136. Hulas-Stasiak M, Gawron A. Follicular atresia in the prepubertal spiny mouse (Acomys cahirinus) ovary. Apoptosis (2011) 16:967–75. doi: 10.1007/s10495-011-0626-9
137. Deng J. How to unleash mitochondrial apoptotic blockades to kill cancers? Acta Pharm Sin B (2017) 7:18–26. doi: 10.1016/j.apsb.2016.08.005
138. Goodwin CM, Rossanese OW, Olejniczak ET, Fesik SW. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ (2015) 22:2098–106. doi: 10.1038/cdd.2015.73
139. Omari S, Waters M, Naranian T, Kim K, Perumalsamy AL, Chi M, et al. Mcl-1 is a key regulator of the ovarian reserve. Cell Death Dis (2015) 6:e1755. doi: 10.1038/cddis.2015.95
140. Stensen MH, Tanbo T, Storeng R, Fedorcsak P. Advanced glycation end products and their receptor contribute to ovarian ageing. Hum Reprod (2014) 29:125–34. doi: 10.1093/humrep/det419
141. Pertynska-Marczewska M, Diamanti-Kandarakis E, Zhang J, Merhi Z. Advanced glycation end products: A link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metabolism (2015) 64:1564–73. doi: 10.1016/j.metabol.2015.08.010
142. Mouanness M, Merhi Z. Impact of dietary advanced glycation end products on female reproduction: Review of potential mechanistic pathways. Nutrients (2022) 14(5):966. doi: 10.3390/nu14050966
143. Pertynska-Marczewska M, Diamanti-Kandarakis E. Aging ovary and the role for advanced glycation end products. Menopause (2017) 24:345–51. doi: 10.1097/GME.0000000000000755
144. Zhu JL, Cai YQ, Long SL, Chen Z, Mo ZC. The role of advanced glycation end products in human infertility. Life Sci (2020) 255:117830. doi: 10.1016/j.lfs.2020.117830
145. Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A, Reproduction E, Society Task F. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update (2012) 18:29–43. doi: 10.1093/humupd/dmr040
146. Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nat Rev Dis Primers (2019) 5:7. doi: 10.1038/s41572-018-0058-8
147. Birch Petersen K, Hvidman HW, Sylvest R, Pinborg A, Larsen EC, Macklon KT, et al. Family intentions and personal considerations on postponing childbearing in childless cohabiting and single women aged 35-43 seeking fertility assessment and counselling. Hum Reprod (2015) 30:2563–74. doi: 10.1093/humrep/dev237
148. Urrutia RP, Polis CB. Fertility awareness based methods for pregnancy prevention. Bmj (2019) 366:l4245. doi: 10.1136/bmj.l4245
149. Wasielak-Politowska M, Kordowitzki P. Chromosome segregation in the oocyte: What goes wrong during aging. Int J Mol Sci (2022) 23(5):2880. doi: 10.3390/ijms23052880
150. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. Interventions to slow aging in humans: Are we ready? Aging Cell (2015) 14:497–510. doi: 10.1111/acel.12338
151. Flanagan EW, Most J, Mey JT, Redman LM. Calorie restriction and aging in humans. Annu Rev Nutr (2020) 40:105–33. doi: 10.1146/annurev-nutr-122319-034601
152. Longo VD, Anderson RM. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell (2022) 185:1455–70. doi: 10.1016/j.cell.2022.04.002
153. Hwangbo DS, Lee HY, Abozaid LS, Min KJ. Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients (2020) 12(4):1194. doi: 10.3390/nu12041194
154. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev (2017) 39:46–58. doi: 10.1016/j.arr.2016.10.005
155. Giacomello E, Toniolo L. The potential of calorie restriction and calorie restriction mimetics in delaying aging: Focus on experimental models. Nutrients (2021) 13(7):2346. doi: 10.3390/nu13072346
156. Garcia DN, Saccon TD, Pradiee J, Rincon JAA, Andrade KRS, Rovani MT, et al. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience (2019) 41:395–408. doi: 10.1007/s11357-019-00087-x
157. Bartke A. Growth hormone and aging. Rev Endocr Metab Disord (2021) 22:71–80. doi: 10.1007/s11154-020-09593-2
158. Li WJ, Wang CW, Tao L, Yan YH, Zhang MJ, Liu ZX, et al. Insulin signaling regulates longevity through protein phosphorylation in caenorhabditis elegans. Nat Commun (2021) 12:4568. doi: 10.1038/s41467-021-24816-z
159. Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol (2013) 9:366–76. doi: 10.1038/nrendo.2013.67
160. Papadimitriou A, Marakaki C, Papadimitriou DT. Growth variations with opposite clinical outcomes and the emerging role of IGF-1. Trends Endocrinol Metab (2022) 33:359–70. doi: 10.1016/j.tem.2022.02.004
161. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci (2012) 67:626–39. doi: 10.1093/gerona/gls102
162. Bartke A. Growth hormone and aging: Updated review. World J Mens Health (2019) 37:19–30. doi: 10.5534/wjmh.180018
163. Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol (2013) 87:201–23. doi: 10.1016/j.critrevonc.2013.01.005
164. Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, et al. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience (2017) 39:129–45. doi: 10.1007/s11357-017-9971-0
165. Zhang WB, Ye K, Barzilai N, Milman S. The antagonistic pleiotropy of insulin-like growth factor 1. Aging Cell (2021) 20:e13443. doi: 10.1111/acel.13443
166. Xu YM, Hao GM, Gao BL. Application of growth hormone in in vitro fertilization. Front Endocrinol (Lausanne) (2019) 10:502. doi: 10.3389/fendo.2019.00502
167. Scheffler F, Vandecandelaere A, Soyez M, Bosquet D, Lefranc E, Copin H, et al. Follicular GH and IGF1 levels are associated with oocyte cohort quality: A pilot study. Front Endocrinol (Lausanne) (2021) 12:793621. doi: 10.3389/fendo.2021.793621
168. Saccon TD, Rovani MT, Garcia DN, Pradiee J, Mondadori RG, Cruz LAX, et al. Growth hormone increases DNA damage in ovarian follicles and macrophage infiltration in the ovaries. Geroscience (2022) 44:1071–81. doi: 10.1007/s11357-021-00380-8
169. Gong Y, Luo S, Fan P, Zhu H, Li Y, Huang W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod Biol Endocrinol (2020) 18:121. doi: 10.1186/s12958-020-00677-x
170. Mancini A, Bruno C, Vergani E, D'abate C, Giacchi E, Silvestrini A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int J Mol Sci (2021) 22(4):1667. doi: 10.3390/ijms22041667
171. Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol (2019) 59:92–111. doi: 10.1016/j.semcancer.2019.07.003
172. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: A mini-review. Gerontology (2018) 64:127–34. doi: 10.1159/000484629
173. Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev (2021) 101:1371–426. doi: 10.1152/physrev.00026.2020
174. Rehnitz J, Capp E, Messmer B, Nguyen XP, Germeyer A, Freis A, et al. FMR1 and AKT/mTOR signaling in human granulosa cells: Functional interaction and impact on ovarian response. J Clin Med (2021) 10(17):3892. doi: 10.3390/jcm10173892
175. Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update (2011) 17:525–40. doi: 10.1093/humupd/dmr009
176. Rehnitz J, Messmer B, Bender U, Nguyen XP, Germeyer A, Hinderhofer K, et al. Activation of AKT/mammalian target of rapamycin signaling in the peripheral blood of women with premature ovarian insufficiency and its correlation with FMR1 expression. Reprod Biol Endocrinol (2022) 20:44. doi: 10.1186/s12958-022-00919-0
177. Liu H, Ding J, Köhnlein K, Urban N, Ori A, Villavicencio-Lorini P, et al. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy (2020) 16:1618–34. doi: 10.1080/15548627.2019.1695399
178. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol (2011) 13:132–41. doi: 10.1038/ncb2152
179. Tang C, Livingston MJ, Liu Z, Dong Z. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol (2020) 16:489–508. doi: 10.1038/s41581-020-0309-2
180. Smith HJ, Sharma A, Mair WB. Metabolic communication and healthy aging: Where should we focus our energy? Dev Cell (2020) 54:196–211. doi: 10.1016/j.devcel.2020.06.011
181. Lin M, Hua R, Ma J, Zhou Y, Li P, Xu X, et al. Bisphenol a promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ Int (2021) 147:106298. doi: 10.1016/j.envint.2020.106298
182. Lopez M, Tena-Sempere M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: A gateway for obesity treatment? Pharmacol Ther (2017) 178:109–22. doi: 10.1016/j.pharmthera.2017.03.014
183. Watroba M, Dudek I, Skoda M, Stangret A, Rzodkiewicz P, Szukiewicz D. Sirtuins, epigenetics and longevity. Ageing Res Rev (2017) 40:11–9. doi: 10.1016/j.arr.2017.08.001
184. Garg G, Singh AK, Singh S, Rizvi SI. Promising drug discovery strategies for sirtuin modulators: what lessons have we learnt? Expert Opin Drug Discov (2021) 16:915–27. doi: 10.1080/17460441.2021.1915980
185. Roichman A, Elhanati S, Aon MA, Abramovich I, Di Francesco A, Shahar Y, et al. Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nat Commun (2021) 12:3208. doi: 10.1038/s41467-021-23545-7
186. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab (2014) 25:138–45. doi: 10.1016/j.tem.2013.12.001
187. Zhang W, Feng Y, Guo Q, Guo W, Xu H, Li X, et al. SIRT1 modulates cell cycle progression by regulating CHK2 acetylation-phosphorylation. Cell Death Differ (2020) 27:482–96. doi: 10.1038/s41418-019-0369-7
188. Martel J, Ojcius DM, Ko YF, Chang CJ, Young JD. Antiaging effects of bioactive molecules isolated from plants and fungi. Med Res Rev (2019) 39:1515–52. doi: 10.1002/med.21559
189. Morita Y, Wada-Hiraike O, Yano T, Shirane A, Hirano M, Hiraike H, et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: an implicative role of SIRT1 in the ovary. Reprod Biol Endocrinol (2012) 10:14. doi: 10.1186/1477-7827-10-14
190. Wang D, Geng M, Gan D, Han G, Gao G, Xing A, et al. Effect of resveratrol on mouse ovarian vitrification and transplantation. Reprod Biol Endocrinol (2021) 19:54. doi: 10.1186/s12958-021-00735-y
191. Zhang S, Huang B, Su P, Chang Q, Li P, Song A, et al. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther (2021) 12:178. doi: 10.1186/s13287-021-02255-3
192. Zhang S, Zhu D, Li Z, Huang K, Hu S, Lutz H, et al. A stem cell-derived ovarian regenerative patch restores ovarian function and rescues fertility in rats with primary ovarian insufficiency. Theranostics (2021) 11:8894–908. doi: 10.7150/thno.61690
193. Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell (2020) 180:585–600.e19. doi: 10.1016/j.cell.2020.01.009
194. Tian C, He J, An Y, Yang Z, Yan D, Pan H, et al. Bone marrow mesenchymal stem cells derived from juvenile macaques reversed ovarian ageing in elderly macaques. Stem Cell Res Ther (2021) 12:460. doi: 10.1186/s13287-021-02486-4
195. Ding L, Yan G, Wang B, Xu L, Gu Y, Ru T, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci (2018) 61:1554–65. doi: 10.1007/s11427-017-9272-2
196. Herraiz S, Romeu M, Buigues A, Martinez S, Diaz-Garcia C, Gomez-Segui I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril (2018) 110:496–505:e491. doi: 10.1016/j.fertnstert.2018.04.025
197. Igboeli P, El Andaloussi A, Sheikh U, Takala H, Elsharoud A, Mchugh A, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep (2020) 14:108. doi: 10.1186/s13256-020-02426-5
198. Xie Y, Liu W, Liu S, Wang L, Mu D, Cui Y, et al. The quality evaluation system establishment of mesenchymal stromal cells for cell-based therapy products. Stem Cell Res Ther (2020) 11:176. doi: 10.1186/s13287-020-01696-6
199. Estudillo E, Jiménez A, Bustamante-Nieves PE, Palacios-Reyes C, Velasco I, López-Ornelas A. Cryopreservation of gametes and embryos and their molecular changes. Int J Mol Sci (2021) 22(19):10864. doi: 10.3390/ijms221910864
200. Gale J, Clancy AA, Claman P. Elective egg freezing for age-related fertility decline. Cmaj (2020) 192:E142. doi: 10.1503/cmaj.191191
201. Dolmans MM, Von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril (2021) 115:1102–15. doi: 10.1016/j.fertnstert.2021.03.008
202. Oktay KH, Marin L, Petrikovsky B, Terrani M, Babayev SN. Delaying reproductive aging by ovarian tissue cryopreservation and transplantation: Is it prime time? Trends Mol Med (2021) 27:753–61. doi: 10.1016/j.molmed.2021.01.005
203. Dolmans MM, Donnez J, Cacciottola L. Fertility preservation: The challenge of freezing and transplanting ovarian tissue. Trends Mol Med (2021) 27:777–91. doi: 10.1016/j.molmed.2020.11.003
Keywords: oocyte, ovary, aging, ovarian aging, interventions
Citation: Wu J, Liu Y, Song Y, Wang L, Ai J and Li K (2022) Aging conundrum: A perspective for ovarian aging. Front. Endocrinol. 13:952471. doi: 10.3389/fendo.2022.952471
Received: 25 May 2022; Accepted: 28 July 2022;
Published: 19 August 2022.
Edited by:
Marcello Pinti, University of Modena and Reggio Emilia, ItalyReviewed by:
Xiaokui Yang, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, ChinaYing Zheng, Sichuan University, China
Copyright © 2022 Wu, Liu, Song, Wang, Ai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kezhen Li, dGprZWtlQDEyNi5jb20=; Jihui Ai, amlodWlhaUB0amgudGptdS5lZHUuY24=; Lingjuan Wang, bGp3YW5nZ0BnbWFpbC5jb20=
 Jiachen Wu
Jiachen Wu Yang Liu
Yang Liu Kezhen Li
Kezhen Li