- Endocrinology Department, St George Public Hospital, Sydney, NSW, Australia
Ectopic thyroid-stimulating hormone (TSH)oma located outside the sella turcica is exceedingly rare and can be associated with significant diagnostic delay. The clinical presentation depends on the anatomical location and size of the ectopic tumor and the degree of thyrotoxicosis. A 71-year-old woman presented with goiter and thyrotoxicosis. Initial investigations revealed elevated free thyroxine (fT4) and tri-iodothyronine (fT3) with inappropriately high-normal TSH. Assay interference was unlikely, pituitary magnetic resonance imaging (MRI) scan was reported as “normal,” and germline sequencing was negative for thyroid hormone receptor ß pathogenic variants. One year later, total thyroidectomy for enlarging symptomatic goiter and suspicious nodule revealed multifocal microscopic papillary thyroid carcinoma. Six years later, she presented to an ear, nose, and throat surgeon with nasal congestion, and a sphenoid bone mass was discovered on nasoendoscopy and imaging. Ectopic TSHoma was confirmed on surgical resection, and a review of the initial pituitary MRI scan revealed the mass which had initially been missed. This is the first reported case of an ectopic TSHoma located in the sphenoid bone. Ectopic TSHoma should be considered in patients with inappropriate TSH secretion when more common differentials are excluded including thyroid hormone resistance or pituitary TSHoma.
Introduction
Pituitary adenomas originate from the adenohypophysis, and ectopic pituitary adenomas (EPAs) are extrasellar and are separate from the hypophysis and infundibulum (1, 2). EPA pathogenesis largely relates to the embryological development and migration of the adenohypophysis (1, 2). EPAs are rare with approximately 180 cases described according to a recent extensive literature review (1), and clinical manifestations vary widely, depending on their secretory profile, size, and anatomical location. Symptoms often mimic those of the skull base or nasopharyngeal tumors and occur due to mass effect on adjacent structures, resulting in visual disturbance, facial paraesthesia, headache, and nasal congestion (2, 3). Specifically, nasopharyngeal and sphenoid sinus EPAs commonly present with epistaxis and nasal congestion, while sphenoid bone EPAs can also present with headache and cranial neuropathy (4). According to a review of published cases, most EPAs (85%) are hormonally active, of which ACTH (36%), prolactin (28%), and GH (22%) secretions are the most common (3, 4). TSH-secreting EPAs (ectopic TSHomas) are the rarest, and to our knowledge, only 13 such cases have been reported in the literature to date (5–17). TSHomas manifest as secondary hyperthyroidism, with elevated serum free thyroxine (fT4) and tri-iodothyronine (fT3) and non-suppressed serum TSH, and can present clinically with symptoms and signs of thyrotoxicosis and diffuse goiter which can often lead to misdiagnosis or delayed diagnosis. We present the first report of an ectopic TSHoma located within the sphenoid bone in a 71-year-old woman. A review of all reported cases of ectopic TSHoma provides key insights into the presentation, diagnosis, and treatment of this rare and often missed disorder.
Case description
A 71-year-old woman was referred to our endocrinology service in 2014 with a longstanding history of goiter for the past 10 years and recent intermittent dysphagia with solids without symptoms of thyrotoxicosis. She had a background of Hashimoto’s thyroiditis, was born in Australia, and had no prior neck irradiation or family history of thyroid-related conditions. She had no history of atrial fibrillation or osteoporosis. Examination confirmed a large, palpable non-obstructive goiter. She was normocardic and in normal sinus rhythm.
Diagnosis, treatment, and outcomes
Thyroid function tests (TFTs) revealed elevated serum fT4 of 21.6 pmol/L (reference range: 9–19 pmol/L), high-normal fT3 of 5.8 pmol/L (reference range: 2.6–6.0 pmol/L), and inappropriately high-normal TSH of 3.79 mIU/L (reference range: 0.40–4.00 mIU/L). A review of previous biochemistry using different assays (including Roche and Abbott Architect) 6 years prior demonstrated a similar pattern of thyroid function derangement, and hence, assay interference was deemed unlikely. Other investigations in 2014 showed elevated anti-thyroid peroxidase antibodies of >1,000 IU/ml (reference range: 0–120 IU/ml) and elevated anti-thyroglobulin antibodies of 1,331 IU/ml (reference range: 0–80 IU/ml), consistent with Hashimoto’s thyroiditis. Serum TSH α-subunit level was considered normal at 1.04 IU/L (postmenopausal reference range: 0–1.3 IU/L). The remainder of the pituitary panel was unremarkable: postmenopausal range follicle-stimulating hormone (FSH) of 61.3 IU/L (reference range: 25–130 IU/L) and luteinizing hormone (LH) of 24.7 IU/L (reference range: 5–62 IU/L), insulin-like growth factor 1 (IGF-1) of 11 nmol/L (reference range: 10–28 nmol/L), prolactin of 302 mIU/L (reference range: 85–500 mIU/L), cortisol of 353 nmol/L (reference range: 138–650 nmol/L), and adrenocorticotrophin hormone (ACTH) of 7.1 pmol/L (reference range: 0–12 pmol/L). Further investigations were undertaken to elucidate the underlying cause for inappropriate TSH secretion. No pathogenic variants were detected on germline DNA polymerase chain reaction (PCR) and Sanger sequencing of exons 7–10 and flanking intronic sequences of the thyroid hormone receptor ß (THRß) gene. Magnetic resonance imaging (MRI) of the pituitary suggested a normal pituitary gland and unperturbed infundibulum, optic chiasm, and cavernous sinuses.
One year later, she underwent total thyroidectomy for progressively enlarging symptomatic goiter and suspicious 2.2 cm right inferior thyroid nodule of Bethesda category III on fine needle aspiration biopsy. Histopathology revealed four foci of microscopic papillary thyroid cancer (micro-PTC) ranging between 0.5 and 3 mm in diameter with clear resection margins and no evidence of lymphovascular invasion or extrathyroidal extension. Post-thyroidectomy radioactive iodine ablation was not required. Thyroxine replacement was commenced but subsequent TFTs showed persistently elevated or high-normal serum TSH despite increasing doses of thyroxine to as much as 1,200 mcg/week (2.0 mcg/kg/day).
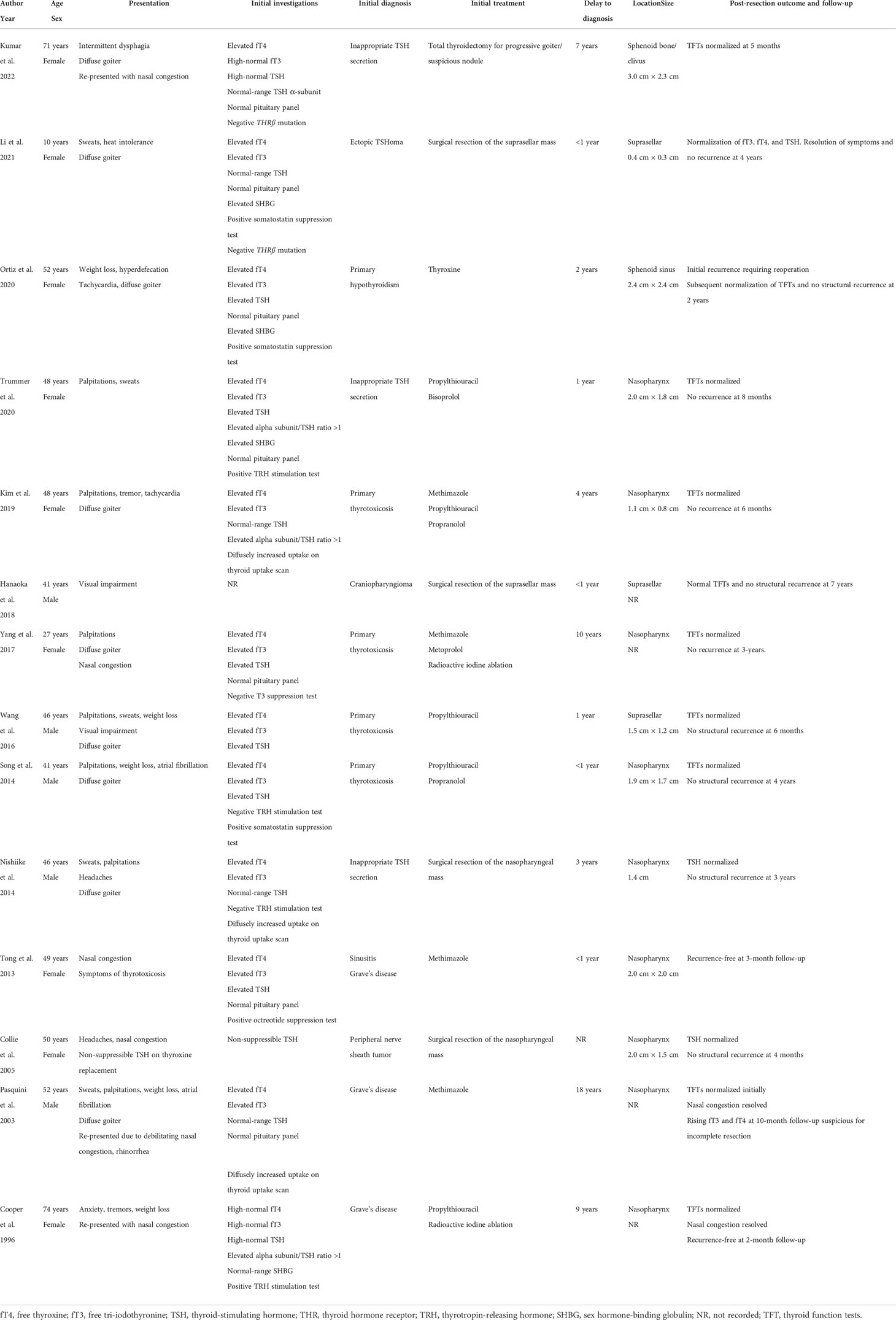
She then presented 6 years later with nasal congestion to an ear, nose, and throat surgeon who suspected a nasopharyngeal mass on nasoendoscopy. Computed tomography (CT) of the paranasal sinuses showed a 2.6-cm × 1.4-cm × 2.5-cm lytic lesion expanding the sphenoid bone and separate from the sella turcica. A subsequent MRI scan of the head demonstrated a 3.0-cm × 2.3-cm × 2.3-cm well-marginated T2-hyperintense and T1-hypointense lesion with heterogeneous contrast enhancement located within the sphenoid bone without extension into the sphenoid sinus or nasopharynx (Figure 1). Endonasal endoscopic resection and tumor histopathology confirmed an ectopic pituitary TSH-producing neuroendocrine tumor with invasion of the clivus and nasopharyngeal mucosa (Figure 2). Tumor cells had small amounts of lightly eosinophilic, granular cytoplasm and small–intermediate-sized round nuclei without any mitoses identified and with a Ki67 index of 0.4%. Immunohistochemistry showed strong and diffuse staining for chromogranin A, synaptophysin, Pit1 transcription factor, TSH (Figure 2), and TSH α-subunit. Growth hormone and prolactin showed focal, weak staining, and transcription factors T-pit and SF1 were negative. Following tumor resection, her serum TSH decreased to 0.01 mIU/L. A retrospective review of her initial pituitary MRI scan images in 2014 revealed that a 2.2-cm × 2.1-cm × 2.3-cm mass within the sphenoid bone had initially been missed (Figure 1). At 5 months post-resection, thyroxine requirements were reduced to 800 mcg/week (1.3 mcg/kg/day) with normalized TFTs.
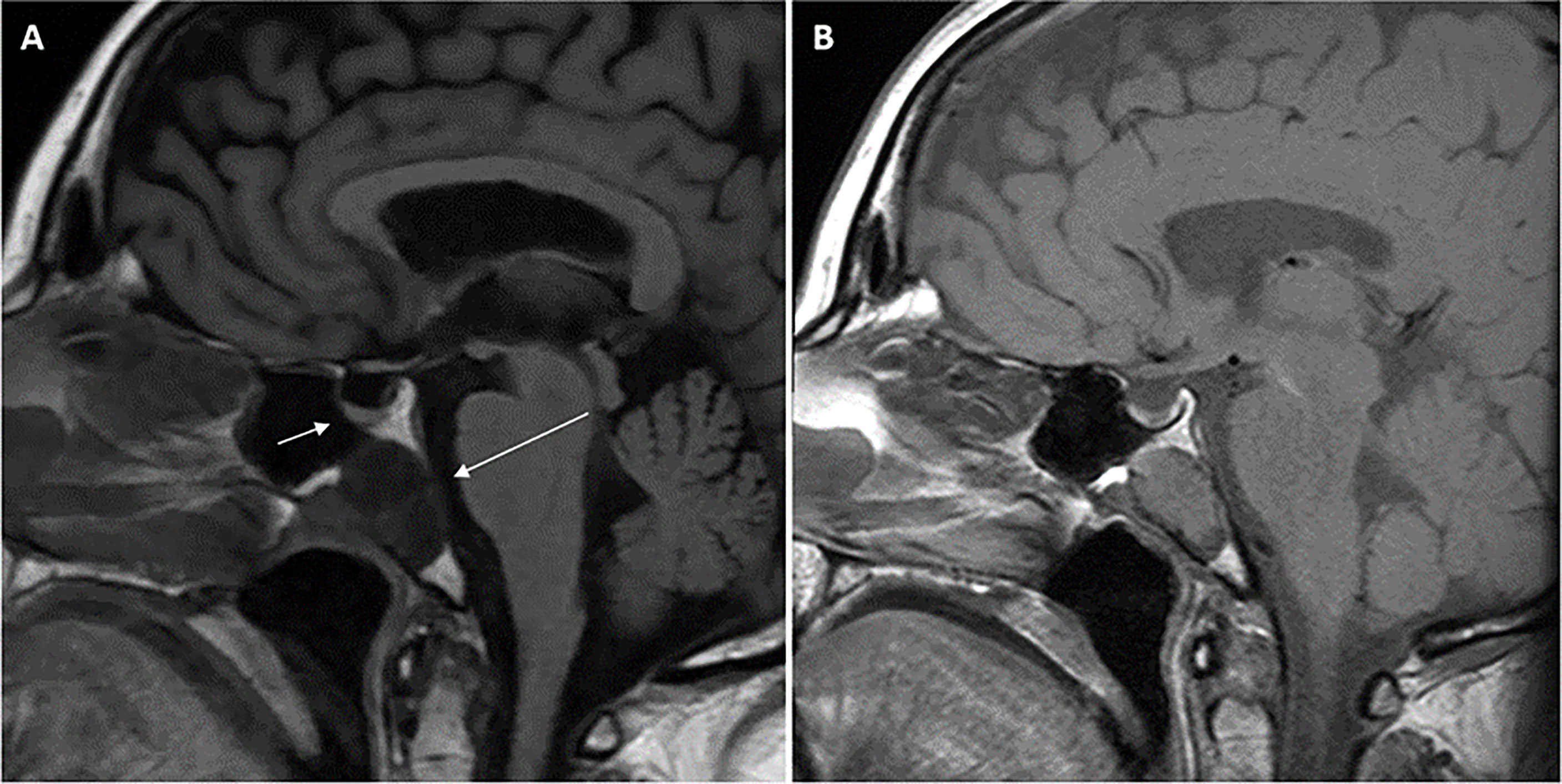
Figure 1 Magnetic resonance imaging (MRI) scans of the head demonstrating midline sphenoid bone mass. T1-weighted (FLAIR) sagittal view pituitary magnetic resonance imaging (MRI) scan performed in 2021 (A) demonstrating normal pituitary gland (short arrow) and adjacent midline T1-hypointense 3.0 cm × 2.3 cm × 2.3 cm mass (long arrow) located within the sphenoid bone extending to the clival, sphenoid sinus, and nasopharyngeal surfaces of the sphenoid bone with no extension into the sphenoid sinus or nasopharynx. Retrospective review of the T1-weighted sagittal view initial pituitary MRI scan performed in 2014 (B) demonstrated the same lesion within the sphenoid bone measuring 2.2 cm × 2.1 cm × 2.3 cm. Arrows are not included in (B) so the image is unperturbed and seen in the same way the radiologist viewed the scan.
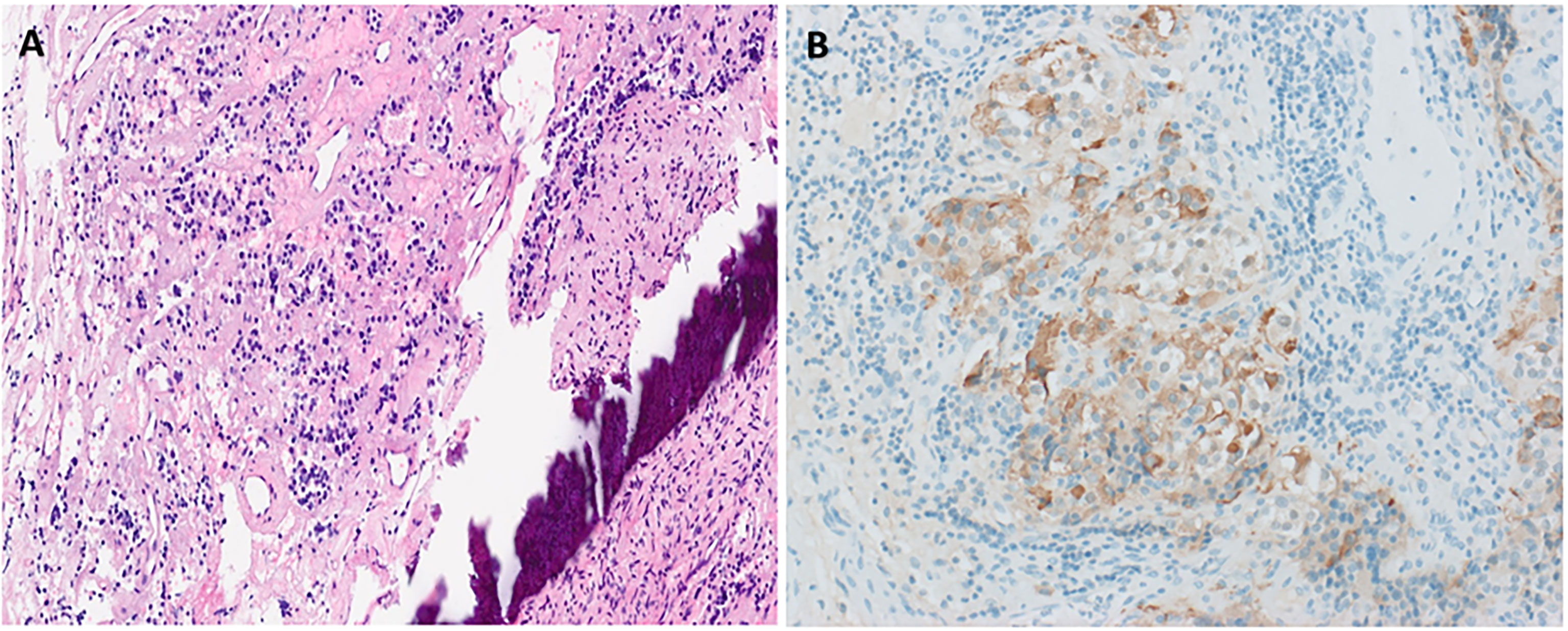
Figure 2 Histopathological confirmation of ectopic TSHoma. Histopathology (obtained from tumor resection) with hematoxylin and eosin staining (A) showing small irregularly shaped islands of cells separated by fibrovascular stroma beside the bone (dark purple streak). Cells have small amounts of lightly eosinophilic, granular cytoplasm and small–intermediate-sized round nuclei with no mitoses identified. Strong diffuse positive tumor cell staining on immunohistochemistry for TSH is also seen (B).
Timeline

Discussion
The pathogenesis of EPAs relates to the embryological development of the hypophysis (13). Between the fourth and eighth week of gestation, Rathke’s pouch develops from the ascending invagination of extracranial ectoderm from the primitive oral cavity, and it fuses with the descending neuroectoderm to form the adenohypophysis and neurohypophysis, respectively (3). During this process, embryological cells of the adenohypophysis migrate through the craniopharyngeal canal to the nasopharynx or sphenoid sinus before uniting with the neurohypophysis in the sella turcica of the sphenoid bone. Infrasellar EPAs can thus be a result of persisting embryological cells along the migration pathway, manifesting in the sphenoid sinus or nasopharynx (1, 2). Alternatively, suprasellar EPAs may be derived from cells of the supradiaphragmatic portion of the pars tuberalis in the suprasellar region (1, 9, 13). Another potential explanation is aberrant embryological cells migrating through the craniopharyngeal canal to both extracranial (infrasellar—nasopharynx, sphenoid sinus, clivus) and intracranial (supra- and parasellar) areas (2, 13). The most common location for EPAs is the suprasellar and sphenoid sinus followed by the nasopharynx.
A literature review yielded 14 published case reports of ectopic TSHoma including our report (Table 1), using PubMed/Medline search terms “ectopic” AND “TSHoma/thyrotropinoma,” “ectopic” AND “TSH/thyrotropin” AND “pituitary,” “ectopic” AND “TSH/thyrotropin” AND “adenoma,” “ectopic” AND “TSH/thyrotropin” AND “pituitary” AND “adenoma.” The first case was reported in 1996; however, 50% of the cases were published in the past 5 years suggesting greater recent awareness of ectopic TSHoma. The majority of the cases were women (9/14) and the median age at diagnosis was 48 years (range 10–78 years). Unlike other EPAs, the nasopharynx was the most common location (9/14), followed by the suprasellar (3/14), sphenoid sinus (1/14), and sphenoid bone (1/14) which may represent a unique nasopharyngeal predisposition of ectopic TSHoma. Tumor size was recorded in 10 cases, with a median maximal diameter of 2.0 cm (range 0.4–3.0 cm, one microadenoma). Symptomatic thyrotoxicosis (11/14) was the most common complaint followed by diffuse goiter (9/14) reflecting TSH hypersecretion. Mass effects differed based on tumor location with nasopharyngeal/sphenoid sinus/sphenoid bone lesions presenting with nasal congestion (6/11) and suprasellar lesions with visual impairment (2/3). Biochemically, the vast majority of patients had elevated fT4 and fT3, while 50% had elevated TSH (100% of the cases had non-suppressed TSH). Primary thyrotoxicosis/Graves’ disease was the most common initial diagnosis (7/14) which led to inappropriate treatment with anti-thyroid drugs (8/14) and radioactive iodine ablation (2/14). Our patient is the first to undergo total thyroidectomy; however, this was performed for enlarging symptomatic goiter and suspicious nodule rather than inappropriate treatment of presumed Graves’ disease. The median diagnostic delay from the initial presentation was 2.5 years (range <1 to 18 years). The single most important investigation which led to the correct diagnosis was an MRI brain scan (11/14) with the majority ordered as part of the investigation algorithm for inappropriate TSH secretion to exclude TSHoma. Three cases, including ours, required the identification of the mass by otolaryngological examination for nasal congestion to facilitate the correct diagnosis. The ectopic TSHoma was initially missed on an MRI brain scan in one case; however, this was rectified within <1 year. Our patient, however, experienced a 7-year diagnostic delay despite the midline sphenoid bone mass being visible in the same sagittal and coronal slice as the pituitary gland under investigation, suggesting a lack of awareness for ectopic TSHoma. The interval growth of the ectopic TSHoma may reflect natural history or could have been exacerbated by thyroidectomy and the resulting impaired feedback inhibition by the thyroid hormone. All patients underwent tumor resection with positive biochemical and structural outcomes at a median follow-up of 9 months (range 2 months to 7 years), likely reflecting the lack of concern for postoperative hypopituitarism allowing for more extensive complete surgical resection. Histopathological confirmation of ectopic TSH-secreting neuroendocrine tumor was achieved in all cases supported by features of the neuroendocrine tumor and positive tumor cell immunohistochemistry for TSH (14/14).
The differential diagnosis for inappropriate TSH secretion includes assay interference, thyroid hormone resistance, familial dysalbuminemic hyperthyroxinemia (FDH), and TSHoma (18). Laboratory assay interferences should first be excluded, such as the presence of heterophile antibodies, thyroid hormone antibodies, and high biotin levels (19). A useful approach to exclude assay interference is to repeat TFTs using a different assay in a different laboratory and, if other common differentials are excluded, to consider consultation with the biochemical department for exclusion of interfering antibodies. FDH occurs via autosomal dominant inheritance of pathogenic variants in the gene encoding albumin. The resulting mutant albumin has a higher binding affinity to T4 resulting in two- to three-fold elevated total T4 and mildly elevated total T3 concentrations. The mutant albumin also has greater binding to labeled T4 analogs, the assay tracer, and causes more antibodies to bind fT4 instead, giving abnormally high fT4 measurements (20). Thyroid hormone resistance is caused by autosomal dominant inheritance of mutations in the thyroid hormone receptor (THR) genes THRα and THRß which encode intranuclear T3 receptors, resulting in T3 receptor dysfunction. THRß mutation is by far the most prevalent subtype with biochemical findings of elevated fT4 and inappropriately normal or elevated TSH levels, biochemically indistinct from TSHoma (21). To help differentiate between the two conditions, family history should be sought and germline DNA sequencing for THRß mutations performed. Investigations that support the diagnosis of TSHoma over thyroid hormone resistance include elevated α-subunit/TSH ratio (>1.0) and serum sex hormone-binding globulin level (peripheral tissue marker of thyroid hormone action). Dynamic investigation results include the positive somatostatin suppression test (due to predominantly somatostatin receptor 2 expression), negative TRH stimulation test, and negative T3 suppression test (21, 22). It is unclear due to the rarity of cases whether the same conclusions can be drawn regarding ectopic TSHoma. However, we have observed certain clinical, biochemical, and radiological characteristics from the published case reports in comparison to pituitary TSHoma (Table 2).
Previous cases of ectopic TSHomas have mostly been reported as a nasopharyngeal mass, and this is the first case where it was discovered in the sphenoid bone/clivus. Primary clival EPAs, as seen in our patient, are defined as EPAs being contained within the clivus and have been reported to be exceedingly rare (2). A clival tumor, however, has a broad differential diagnosis, including chordoma, chondrosarcoma, intraosseous meningioma, craniopharyngioma, lymphoma, myeloma, and solid organ metastases, with chordoma being the most prevalent (1). Clival EPAs have also been observed to be associated with malignant transformation, bone invasion, and a concurrent empty sella (1, 4), of which only bone invasion was observed in our patient. In addition, our patient also has a concurrent history of PTC, and this has only been described in another case report of ectopic TSHoma. Given the presence of goiter in other patients with ectopic TSHoma, the ectopic TSH is promoting the growth and proliferation of thyroid follicular cells, which may explain the association with PTC in our case and another case reported by Yang et al. (12). However, our patient’s underlying Hashimoto’s thyroiditis may also have been a contributing factor to PTC pathogenesis. Furthermore, incidental micro-PTC in thyroidectomy specimens is not uncommon, and the clinical relevance of this finding is unclear (23–25). To our knowledge, there is insufficient evidence at this stage to directly link ectopic TSHoma with PTC.
In summary, we present the first reported case of sphenoid bone ectopic TSHoma. Initial investigations revealed elevated fT3 and fT4, with an inappropriate high-normal TSH indicative of inappropriate TSH secretion. The initial diagnosis was not obtained in the setting of negative germline DNA testing for TRHß gene mutation, normal post-menopausal range TSH α-subunit level, and a pituitary MRI scan that was reported as “normal.” The patient underwent total thyroidectomy 1 year later for progressive symptomatic goiter and suspicious nodule which revealed multifocal micro-PTC. Six years later, she presented with nasal congestion which directed the investigation to her nasopharynx, facilitating the discovery of a sphenoid bone mass. After surgical resection and histopathological tumor examination, the final correct diagnosis of ectopic TSHoma was obtained. Retrospective analysis of the initial pituitary MRI scan 7 years prior revealed that the sphenoid bone mass had been present since her initial presentation. Although ectopic TSHoma is exceedingly rare, we advocate that this diagnosis should be considered in patients with inappropriate TSH secretion and exclusion of other more common differential diagnoses such as assay interference, thyroid hormone resistance, and pituitary TSHoma, to minimize the diagnostic and therapeutic delay. Ectopic TSHoma may be clinically and biochemically indistinguishable from pituitary TSHoma, and one should have high clinical suspicion if no pituitary adenoma is identified on imaging and to look outside the sella turcica for an ectopic tumor in the nasopharynx, suprasellar, sphenoid sinus, and sphenoid bone.
Patient perspective
The patient declined to provide her perspective on the case report; however, she provided signed written informed consent for this report to be published.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SK conceptualized, drafted, and critically reviewed the manuscript. CP and HN assisted in drafting the manuscript. TD managed the patient and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge Dr. Peter Earls, Anatomical Pathology Department, SydPath (St Vincents Pathology), NSW, Australia, for his assistance in the preparation and interpretation of tumor histopathology slides.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu J, Wang Z, Zhang Y, Li X, Liu J, Deng K. Ectopic pituitary adenomas: clinical features, diagnostic challenges and management. Pituitary (2020) 23(6):648–64. doi: 10.1007/s11102-020-01071-x
2. Riccio L, Donofrio CA, Tomacelli G, De Blasi R, Melatini A. Ectopic GH-secreting pituitary adenoma of the clivus: systematic literature review of a challenging tumour. Pituitary (2020) 23(4):457–66. doi: 10.1007/s11102-020-01057-9
3. Agely A, Okromelidze L, Vilanilam GK, Chaichana KL, Middlebrooks EH, Gupta V. Ectopic pituitary adenomas: common presentations of a rare entity. Pituitary (2019) 22(4):339–43. doi: 10.1007/s11102-019-00954-y
4. Shuman W, Loewenstern J, Pai A, Bederson J, Shrivastava R. Variability in clinical presentation and pathologic implications of ectopic pituitary tumors: Critical review of literature. World Neurosurg (2019) 122:397–403. doi: 10.1016/j.wneu.2018.10.200
5. Cooper DS, Wenig BM. Hyperthyroidism caused by an ectopic TSH-secreting pituitary tumor. Thyroid (1996) 6(4):337–43. doi: 10.1089/thy.1996.6.337
6. Pasquini E, Faustini-Fustini M, Sciarretta V, Saggese D, Roncaroli F, Serra D. Ectopic TSH-secreting pituitary adenoma of the vomerosphenoidal junction. Eur J Endocrinol (2003) 148(2):253–7. doi: 10.1530/eje.0.1480253
7. Collie RB, Collie MJ. Extracranial thyroid-stimulating hormone-secreting ectopic pituitary adenoma of the nasopharynx. Otolaryngol Head Neck Surg (2005) 133(3):453–4. doi: 10.1016/j.otohns.2004.10.015
8. Tong A, Xia W, Qi F, Jin Z, Yang D, Zhang Z. Hyperthyroidism caused by an ectopic thyrotropin-secreting tumor of the nasopharynx: A case report and review of the literature. Thyroid (2013) 23(9):1172–7. doi: 10.1089/thy.2012.0574
9. Nishiike S, Tatsumi K-i, Shikina T, Masumura C, Inohara H. Thyroid-stimulating hormone-secreting ectopic pituitary adenoma of the nasopharynx. Auris Nasus Larynx (2014) 41(6):586–8. doi: 10.1016/j.anl.2014.07.004
10. Song M, Wang H, Song L, Tian H, Ge Q, Li J. Ectopic TSH-secreting pituitary tumor: a case report and review of prior cases. BMC Cancer (2014) 14:544. doi: 10.1186/1471-2407-14-544
11. Wang Q, Lu X-j, Sun J, Wang J, Huang CY, Wu ZF. Ectopic suprasellar thyrotropin-secreting pituitary adenoma: Case report and literature review. World Neurosurg (2016) 95:617.e613–617.e618. doi: 10.1016/j.wneu.2016.08.062
12. Yang J, Liu S, Yang Z, Shi YB. Ectopic thyrotropin secreting pituitary adenoma concomitant with papillary thyroid carcinoma: Case report. Med (Baltimore) (2017) 96(50):e8912. doi: 10.1097/MD.0000000000008912
13. Hanaoka Y, Ogiwara T, Kakizawa Y, Nagm A, Seguchi T, Aoyama T. Calcified ectopic TSH-secreting pituitary adenoma mimicking craniopharyngioma: a rare case report and literature review. Acta Neurochir (Wien) (2018) 160(10):2001–5. doi: 10.1007/s00701-018-3638-1
14. Kim S, Dillon WP, Hope TA, El-Sayed IH, van Zante A, Wu K. Ectopic thyroid-stimulating hormone–secreting pituitary adenoma of the nasopharynx diagnosed by gallium 68 DOTATATE positron emission Tomography/Computed tomography. World Neurosurg (2019) 125:400–4. doi: 10.1016/j.wneu.2019.02.022
15. Ortiz E, Peldoza M, Monnier E, Gejman R, Henriquez M, Barra MI. Ectopic pituitary adenoma of the TSH-secreting sphenoidal sinus with excellent response to somatostatin analogs. theory of the embryogenesis and literature review from a clinical case. Steroids (2020) 154:108535. doi: 10.1016/j.steroids.2019.108535
16. Trummer C, Reiher H, Theiler-Schwetz V, Pandis M, Gstettner C, Potzinger P. Secondary hyperthyroidism due to an ectopic thyrotropin-secreting neuroendocrine pituitary tumor: A case report. Eur Thyroid J (2020) 9(2):106–12. doi: 10.1159/000505020
17. Li X, Zhao B, Hou B, Wang J, Zhu J, Yao Y, et al. Case report and literature review: Ectopic thyrotropin-secreting pituitary adenoma in the suprasellar region. Front Endocrinol (Lausanne) (2021) 12:619161. doi: 10.3389/fendo.2021.619161
18. Campi I, Covelli D, Moran C, Fugazzola L, Cacciatore C, Orlandi F. The differential diagnosis of discrepant thyroid function tests: Insistent pitfalls and updated flow-chart based on a long-standing experience. Front Endocrinol (Lausanne) (2020) 11:432. doi: 10.3389/fendo.2020.00432
19. Soh SB, Aw TC. Laboratory testing in thyroid conditions - pitfalls and clinical utility. Ann Lab Med (2019) 39(1):3–14. doi: 10.3343/alm.2019.39.1.3
20. Khoo S, Lyons G, McGowan A, Gurnell M, Oddy S, Visser WE. Familial dysalbuminaemic hyperthyroxinaemia interferes with current free thyroid hormone immunoassay methods. Eur J Endocrinol (2020) 182(6):533–8. doi: 10.1530/EJE-19-1021
21. Pappa T, Refetoff S. Resistance to thyroid hormone beta: A focused review. Front Endocrinol (Lausanne) (2021) 12:656551. doi: 10.3389/fendo.2021.656551
22. Carvalho Cunha N, Gomes L, Bastos M. Challenging diagnosis of resistance to thyroid hormone in a patient with pituitary adenoma. BMJ Case Rep (2019) 12(7):e229430. doi: 10.1136/bcr-2019-229430
23. Slijepcevic N, Zivaljevic V, Marinkovic J, Sipetic S, Diklic A, Paunovic I. Retrospective evaluation of the incidental finding of 403 papillary thyroid microcarcinomas in 2466 patients undergoing thyroid surgery for presumed benign thyroid disease. BMC Cancer (2015) 15:330. doi: 10.1186/s12885-015-1352-4
24. Vasileiadis I, Karatzas T, Vasileiadis D, Kapetanakis S, Charitoudis G, Karakostas E. Clinical and pathological characteristics of incidental and nonincidental papillary thyroid microcarcinoma in 339 patients. Head Neck (2014) 36(4):564–70. doi: 10.1002/hed.23333
Keywords: thyrotoxicosis, thyroid-stimulating hormone, TSHoma, ectopic TSHoma, thyrotropinoma, ectopic thyrotropin (TSH) secreting pituitary adenoma
Citation: Kumar S, Phang CA, Ni H and Diamond T (2022) A patient with an ectopic sphenoid bone TSH secretory adenoma: Case report and review of the literature. Front. Endocrinol. 13:961256. doi: 10.3389/fendo.2022.961256
Received: 04 June 2022; Accepted: 14 July 2022;
Published: 08 August 2022.
Edited by:
Barbara Altieri, University Hospital of Wuerzburg, GermanyReviewed by:
Elizabeth McAninch, Rush University Medical Center, United StatesWaiel Bashari, University of Cambridge, United Kingdom
Copyright © 2022 Kumar, Phang, Ni and Diamond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shejil Kumar, c2hlamlsX2t1bWFyQGhvdG1haWwuY29t
 Shejil Kumar
Shejil Kumar Cun An Phang
Cun An Phang Terrence Diamond
Terrence Diamond