- Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
Objective: Adult-onset hypogonadism (AOH) is a common disease for males >40 years old and is closely associated with age-related comorbidities. Phthalates are compounds widely used in a number of products with endocrine-disrupting effects. However, little is known about the association between exposure to phthalates and the risk of AOH. Thus, we conducted this study to explore the potential association using the 2013-2016 National Health and Nutrition Examination Survey (NHANES) data.
Method: Data on AOH and urinary phthalate metabolites were collected, and univariable and multivariable logistic regression analyses were adapted to evaluate the association. The concentrations of each metabolite were calculated and grouped according to their quartiles for the final analysis.
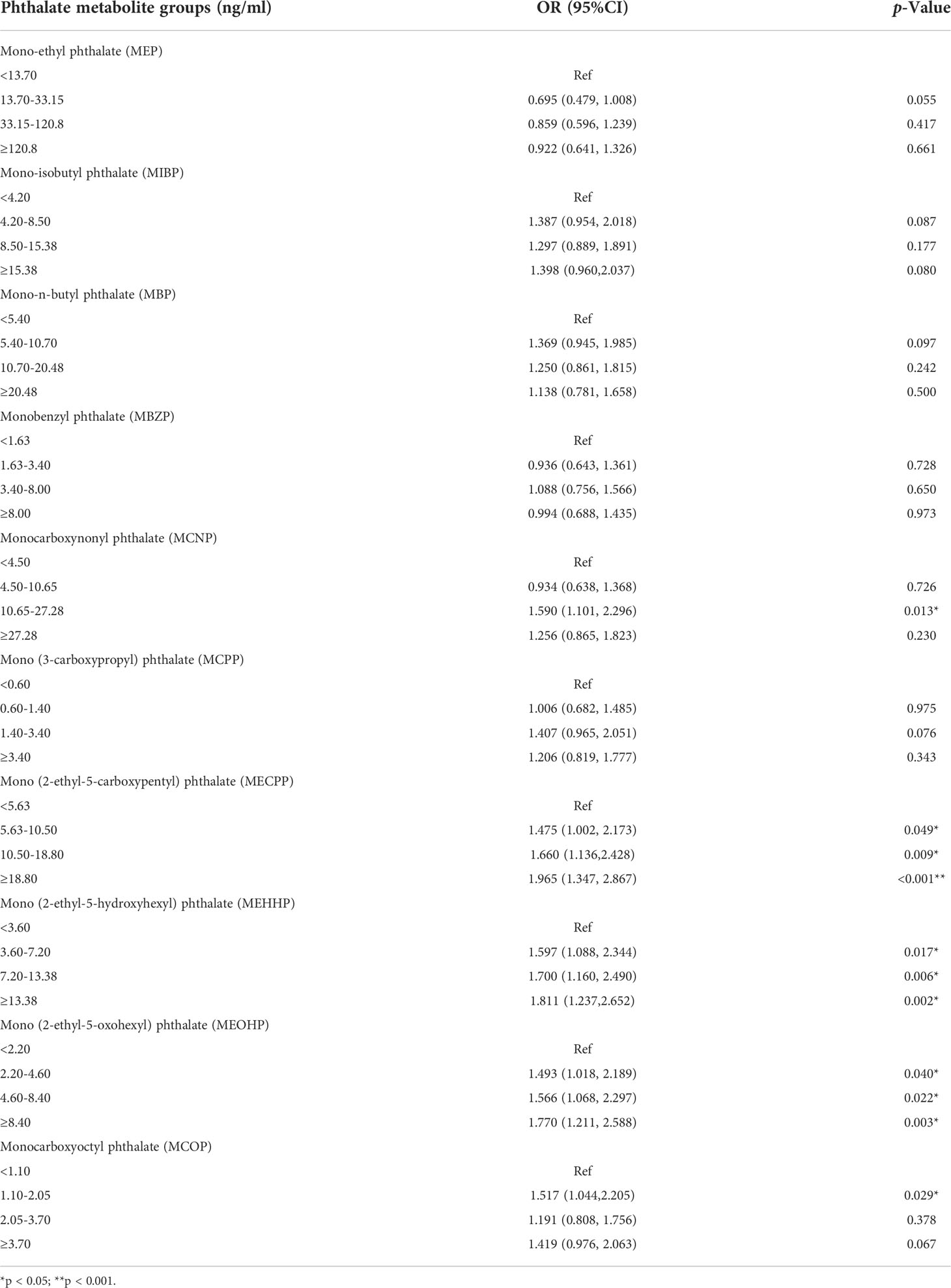
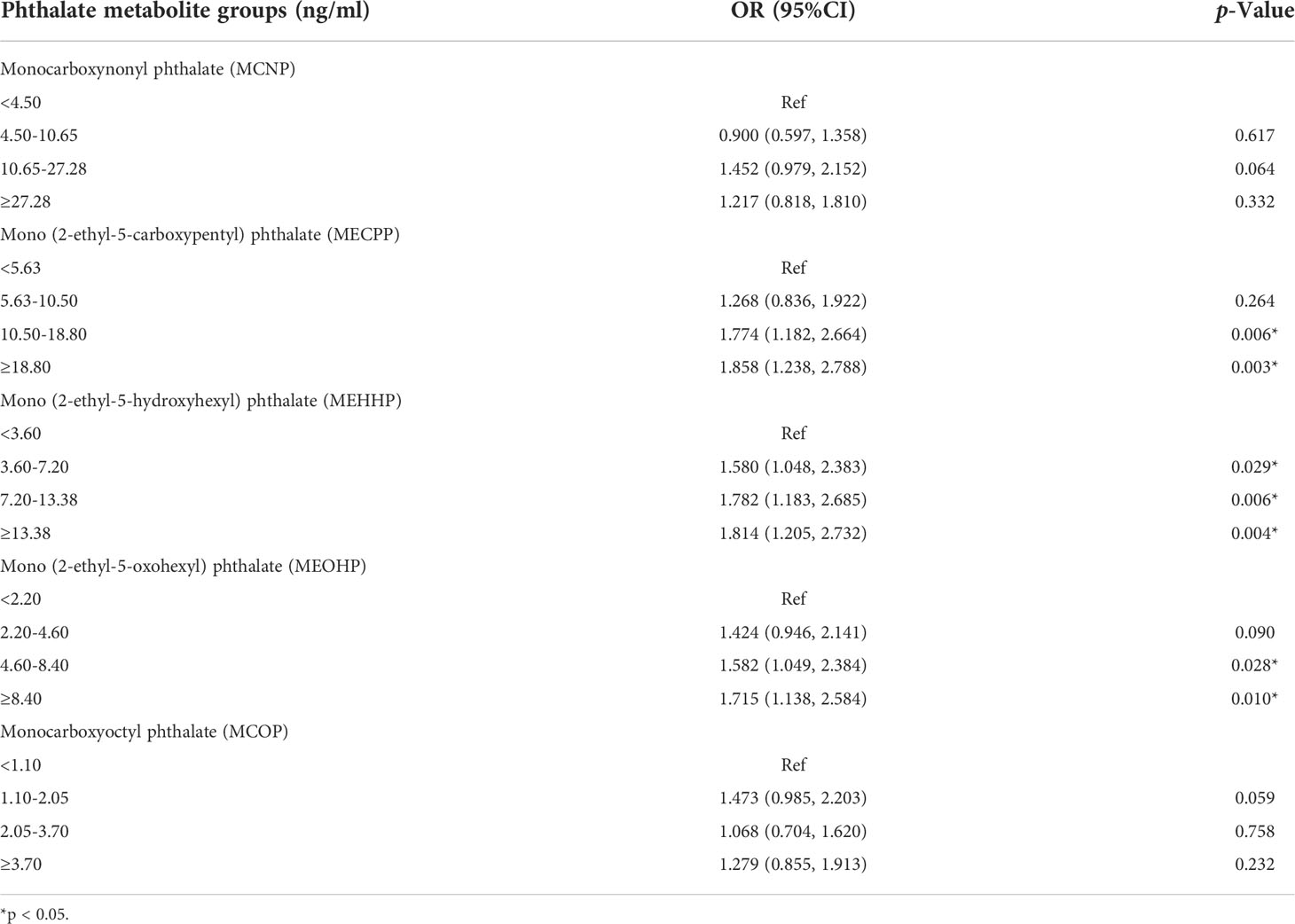
Result: Finally, we found that the odds ratio (OR) increased with increased concentrations of di-(2-ethylhexyl) phthalate (DEHP) metabolites, including mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP). Simultaneously, a significant dose-dependent effect was also observed. The OR for the fourth quartile was highest among all three groups. Specifically, the ORs for the third quartile and fourth quartile were 1.774 and 1.858, respectively, in the MECPP group. For the MEHHP group, the OR increased from 1.580 for the second quartile to 1.814 for the fourth quartile. Similarly, the OR for the higher three quartiles varied from 1.424 to 1.715 in the MEOHP group.
Conclusion: This study first revealed that there was a positive association between exposure to DEHP metabolites and the risk of AOH. These findings add limited evidence to study this topic, while further studies are needed to explain the potential molecular mechanisms.
Introduction
The hypothalamic-pituitary-gonadal (HPG) axis plays an important role in many processes related to the development, maturation and aging of males (1). Any congenital or acquired disturbances of the HPG axis could lead to the clinical syndrome of hypogonadism. Male hypogonadism is defined as a disorder associated with decreased functional activity of the testes, with decreased production of sexual hormones (2). When hypogonadism is specifically associated with aging among adult males, adult-onset hypogonadism (AOH) is usually named late-onset hypogonadism (LOH). In heathy, young eugonadal males (defined as men younger than 30 years old with normal testosterone levels), serum testosterone levels vary between 10.4 and 36.4 nmol/L (300-1050 ng/dl), with a slightly gradual decline when older than 40 years old (3). Biochemical hypogonadism is defined as a serum total testosterone level < 11 nmol/L (317 ng/dl), and approximately 12, 20, 30 and 50% of men in their 50 s, 60 s, 70 s and 80 s, respectively, have biochemical hypogonadism (3). According to the biochemical hypogonadism criteria, AOH is a relatively common disease among males > 40 years of age, with a 2-15% prevalence within the general population (4). And AOH is usually closely associated with age-related comorbidities (5, 6). However, the mechanism of AOH still remains unclear.
Phthalates are a kind of synthetic chemicals applied to cosmetics, food packaging and medical products (7). Widespread application of phthalates could lead to pervasive human exposure (8, 9). Moreover, evidence based on experimental or observational data has shown the connection between exposure to phthalates and endocrine function, especially for sexual hormone dysregulation (10). Thus, we conducted this study to explore the relationship between exposure to phthalate metabolism and AOH using data from the National Health and Nutrition Examination Survey (NHANES) database in 2013-2016.
Methods
Study population
NHANES is a nationally representative survey among the noninstitutionalized American population, released in two-year cycles. NHANES was conducted by the CDC’s National Center for Health Statistics and was approved by the NCHS Research Ethics Review Board (ERB). We used the data from two cycles (2013-2014 and 2015-2016) of the NHANES database. The study sample was restricted to males older than 40 years old, without taking sexual hormone medications in the NHANES questionnaire, and had measured levels of urinary phthalate metabolite concentrations as well as serum total testosterone at the same time. We identified males with AOH using the definition of biochemical hypogonadism, which was a serum total testosterone level < 11 nmol/L (317 ng/dl). The data used in this study are publicly accessible on the NHANES website.
Phthalate exposure
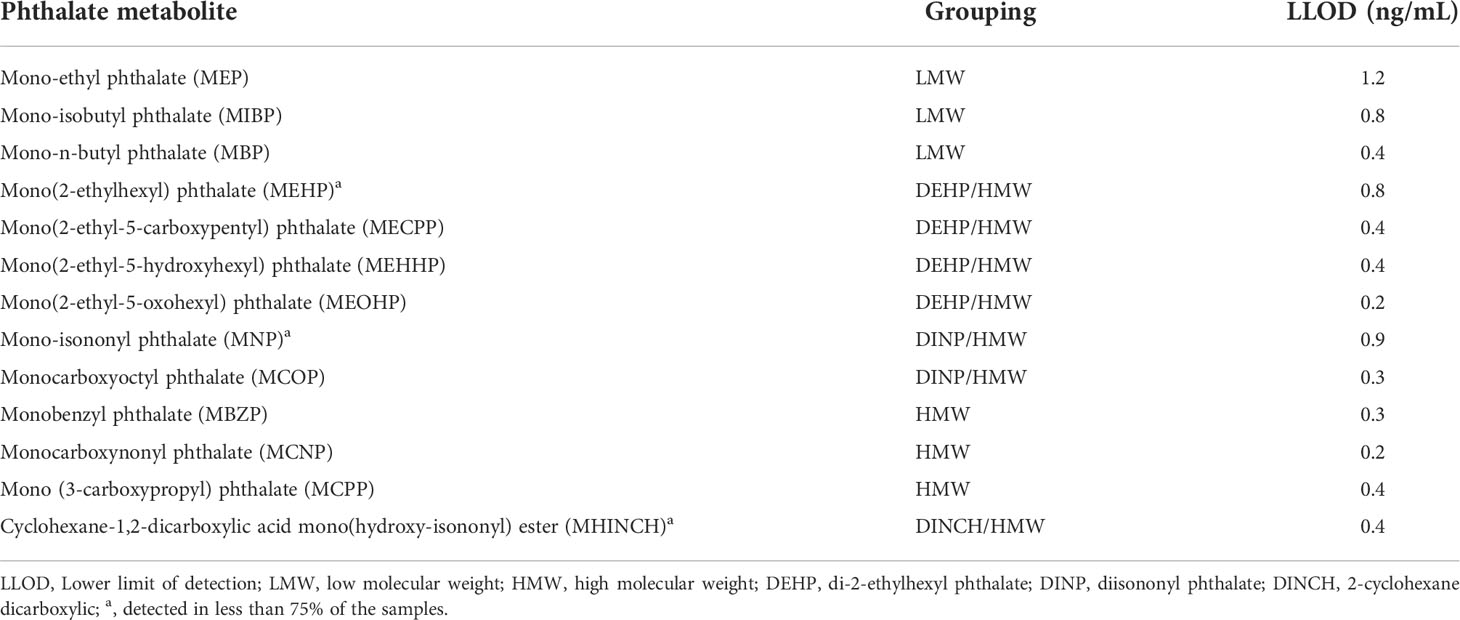
NHANES laboratories evaluated phthalate metabolite concentrations by using spot urine samples. The methods for exposure assessment have been described in detail in another study (11). Phthalate metabolites were grouped by parent phthalate molecule and the weight of the parent molecule according to NHANES guidelines. We divided phthalate metabolites into low molecular weight (LMW), high molecular weight (HMW), di-(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DINP) and 2-cyclohexane dicarboxylic (DINCH) groups. In our study, only the phthalate metabolites detected in at least 75% of the samples were considered in the final analysis to avoid bias due to results below the limit of detection (LOD). The phthalate metabolites were grouped according to their quartiles for the final analysis.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 24 software (IBM Corp., Armonk, NY, USA), and a p value <0.05 was considered statistically significant. Data from the two cycles were combined for analysis via appropriate weighting methods (12). The environmental subsample B sample weights were used among all results. Univariate and multivariate logistic regression analyses were conducted to evaluate the association between AOH and exposure to phthalate metabolite concentrations. The potential confounding factors were identified based on previous studies, including age, race/ethnicity body mass index (BMI) and poverty-to-income ratio (PIR). PIR was grouped according to its quartiles. BMI was grouped into four categories based on the published standards of the National Institutes of Health (13).
Result
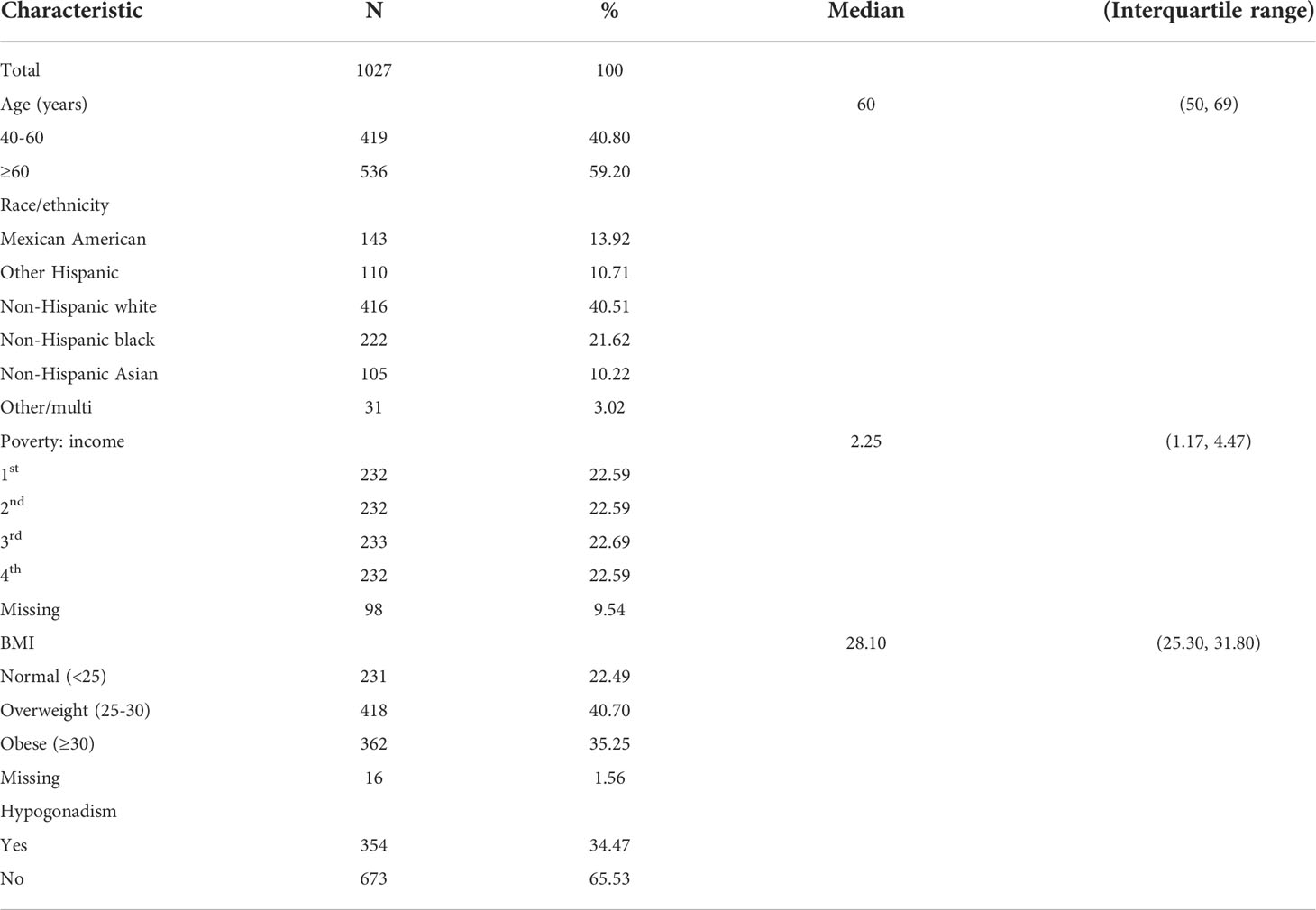
Finally, we enrolled 1027 eligible cases in this study. Table 1 demonstrates the characteristics of the study population in detail. The median age was 60 years old. Most of the population was non-Hispanic white (n=416). In our study, the median BMI was 28.10, indicating that most of the population was overweight. There were 354 cases with AOH, while 673 cases were without AOH. As shown in Table 2, there were 13 kinds of phthalate metabolites measured in this study. Three metabolites, including mono-isonoynyl phthalate (MNP), mono(2-ethylhexyl) phthalate and cyclohexane-1,2-dicarboxylic acid mono(hydroxy-isononyl) ester (MHINCH), were detected in less than 75% of the samples. Thus, we excluded these phthalate metabolites from the following analysis. Table 3 summarizes the phthalate metabolite concentrations between different age groups. Metabolite concentrations did not vary significantly by age across most phthalate groups except for the monoethyl phthalate (MEP) and mono-n-butyl phthalate (MBP) groups. Men aged 40-60 had lower MEP concentrations than those aged >60 (31.55 vs 36.55, p=0.044). Similarly, men aged 40-60 had lower MBP (10.20 vs 11.20, p=0.009). The median exposure with corresponding interquartile ranges stratified by AOH is shown in Table 4. All DEHP-related metabolites were higher among men with AOH than those without, including mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) (11.90 vs 9.80, p=0.001), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) (8.00 vs 6.70, p=0.001) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) (4.90 vs 4.20, p=0.003). In addition, men with AOH also had greater monocarboxynonyl phthalate (MCNP) levels than those without (12.50 vs 9.60, p=0.04). The other phthalate metabolites were not different between these two groups.
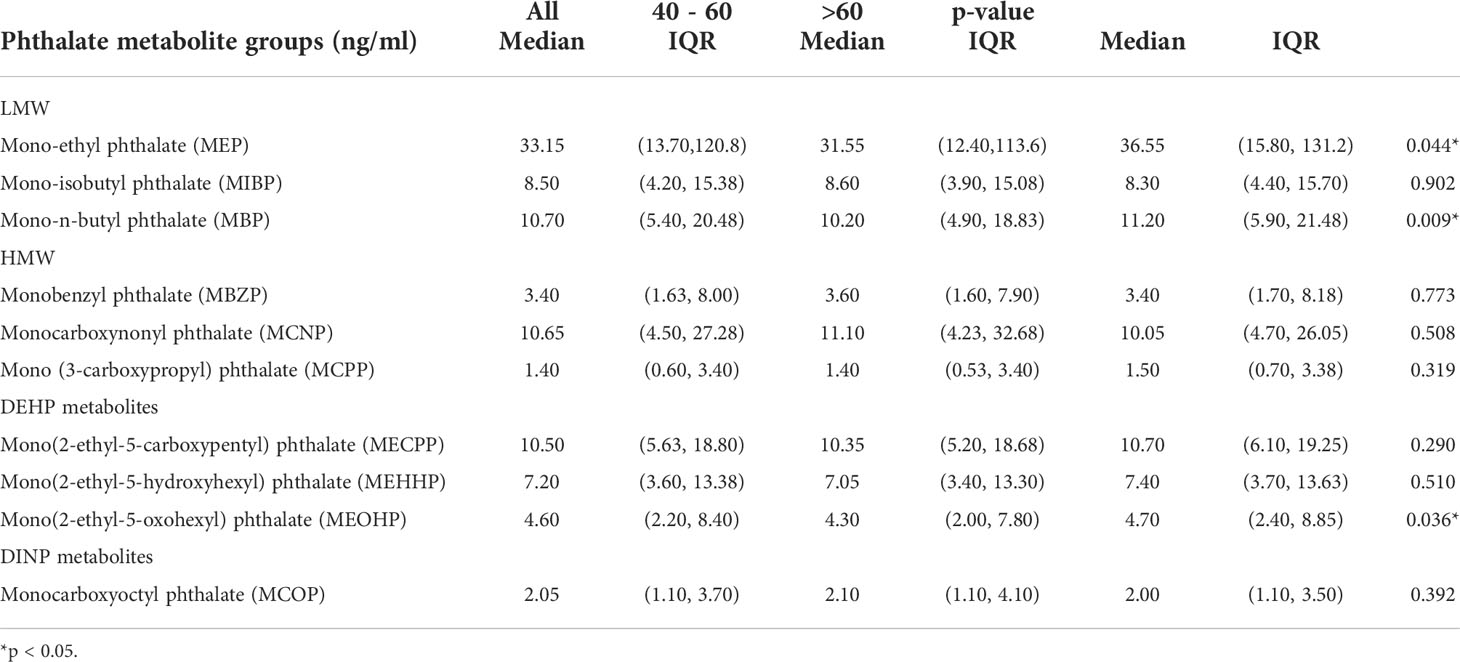
Table 3 Phthalate metabolite group medians and interquartile ranges (IQR) stratified by age, NHANES 2013-2016. *<0.05.

Table 4 Phthalate metabolite group medians and interquartile ranges (IQR) stratified by adult-onset hypogonadism, NHANES 2013-2016.
The results of the univariable and multivariable logistic regression are shown in Tables 5, 6, respectively. During the process, we divided the phthalate metabolites into four groups according to quartiles. The univariable logistic regression revealed significant associations between exposure to MCNP as well as DEHP metabolites (including MECPP, MEHHP, MEOHP) and AOH, consistent with the results shown in Table 4. In addition, there was also a significant relationship between monocarboxyoctyl phthalate (MCOP) exposure and AOH. However, after adjusting for age, PIR, and BMI, the associations between exposure to MCNP as well as MCOP and AOH disappeared. However, the relationships between exposure to DEHP metabolites were still significant. Men with higher MECPP, MEHHP or MHEOP concentrations had a higher risk of AOH. The odds ratio (OR) for the fourth quartile was highest among all three groups. Specifically, the ORs for the third quartile and fourth quartile were 1.774 and 1.858, respectively, in the MECPP group. For the MEHHP group, the OR increased from 1.580 for the second quartile to 1.814 for the fourth quartile. Similarly, the OR for the higher three quartiles varied from 1.424 to 1.715 in the MEOHP group.
Discussion
In this study, a nationally representative cross-sectional study was adapted to explore whether urinary phthalate metabolites were associated with AOH. The results indicated a clear relationship between urinary phthalate metabolites, especially DEHP metabolite exposure and AOH. Simultaneously, a significant dose response trend was observed; that is, the OR increased with increasing DEHP metabolite concentrations compared with the lowest quartile.
AOH is defined as a clinical and biochemical syndrome featuring testosterone deficiency with signs or symptoms caused by HPG dysfunction (14). Biochemical hypogonadism is defined as a serum total testosterone level<317 ng/dl, which we applied in our study to identify AOH cases (3). However, the prevalence of AOH is still unclear. Many epidemiological studies vary in whether symptoms are considered and how androgen deficiency is defined. According to the data based on EMAS, the prevalence of hypogonadism was 13.8%. Moreover, the specific mechanism of AOH is also unclear. Phthalate is positively associated with endocrine dysfunction (10). A previous study showed that phthalate and its metabolite are associated with lower testosterone levels (15). Because AOH is characterized by lower total serum testosterone levels, we conducted this study to explore the association between AOH and phthalate metabolite exposure.
Phthalates are typically synthetic compounds widely applied as plasticizers, solvents and additives in many consumer products (16). Additionally, DEHP is one of the most widely used plasticizers and is regarded as an important endocrine-disrupting compound (17). Due to the widespread use of DEHP, humans tend to be exposed to DEHP and its metabolites (9). The metabolites of DEHP can be absorbed by the body in different ways, including dermal exposure, ingestion and inhalation (18). Importantly, various studies have revealed a positive association between exposure to DEHP and injury to the male reproductive system, including structure and function, especially in the testis (19). According to the European Food Safety Authority (EFSA) guidelines, male reproductive system injury could be caused by a daily DEHP exposure dose of 50 µg/kg, while there is more than a 300 µg/kg concentration of DEHP found in daily food (20). Furthermore, a previous study demonstrated that infertility patients have higher concentrations of DEHP and its metabolites in blood, urine and semen than normal males (19). Importantly, animal studies have revealed that DEHP and its metabolite could damage the structure of the testis, such as blood testicular barrier destruction and atrophy of seminiferous tubules, and furthermore lead to dysfunction of Leydig cells, including testosterone synthesis and secretion (21–24). As the results showed, the metabolites of DEHP were positively associated with a significantly higher risk of AOH, presenting a relevant dose-dependent effect. There are several potential mechanisms explaining the phenomenon. The previous study has shown the phthalates could inhibit stem Leydig cells differentiating into Leydig lineage cells while promote adipocyte differentiation (25). Besides, our study also indicates DEHP could induce the methylation of Sod2, Gpx1 and igf1, leading to testicular injury and dysfunction of Leydig cell (26). furthermore, DEHP could also induce apoptosis and autophagy of Leydig cell both in vivo and in vitro, through activating oxidative stress and p53 signaling pathway (27, 28). And testosterone is the major hormone in men, produced by Leydig cells, playing a vital role in maintaining normal reproductive and sexual function (29). For example, the spermatogenesis is totally dependent on testosterone level (30). When lacking of testosterone stimulation, the spermatogenesis would stay at meiosis stage (31). Thus, exposure to DEHP metabolites might increase the risk of AOH by damaging the structure and function of the male reproductive system, especially the testis. Further studies are needed to explore the specific mechanisms.
The strengths of this study include a relatively large, nationally representative sample with AOH and individually quantified urinary phthalate metabolites. Moreover, this analysis is the first study exploring the association between exposure to phthalates, especially DEHP metabolites, and the risk of AOH. And this finding could suggest that we might improve the management of AOH via decreasing the exposure of DEHP metabolites. However, there are still some limitations in our study. First, there is a lack of data on the symptoms caused by HPG dysfunction, such as erectile dysfunction, loss of morning erections and reduced sexual desire. Thus, we identified males with AOH using the definition of biochemical hypogonadism. Second, there is an inherent weakness due to its cross-sectional nature. Thus, further studies are needed to confirm the findings in our study and explain the related molecular mechanisms.
In summary, our study revealed a clear association between DEHP metabolite exposure and the risk of AOH in a dose-dependent manner. This brings us some insights, reducing the use of DEHP by replacement with structural analogues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Z-HL and L-CY conceived and designed the study, collected and analyzed the data and wrote the manuscript. PS and J-HC analyzed the data. Z-FP and QD reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
The work is supported by the Project of Science and Technology Department of Chengdu (grant no. 2021-YF05-00717-SN) and the Project of Science and Technology Department of Sichuan Province (2021YFS0117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corradi PF, Corradi RB, Greene LW. Physiology of the hypothalamic pituitary gonadal axis in the Male. Urol Clin North Am (2016) 43(2):151–62. doi: 10.1016/j.ucl.2016.01.001
2. Rey RA, Grinspon RP, Gottlieb S, Pasqualini T, Knoblovits P, Aszpis S, et al. Male Hypogonadism: An extended classification based on a developmental, endocrine physiology-based approach. Andrology (2013) 1(1):3–16. doi: 10.1111/j.2047-2927.2012.00008.x
3. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab (2001) 86(2):724–31. doi: 10.1210/jcem.86.2.7219
4. Salonia A, Rastrelli G, Hackett G, Seminara SB, Huhtaniemi IT, Rey RA, et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers (2019) 5(1):38. doi: 10.1038/s41572-019-0087-y
5. Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: Focus on holistic management. J Clin Endocrinol Metab (2017) 102(3):1067–75. doi: 10.1210/jc.2016-3580
6. Maseroli E, et al. Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: A comparative study. J Sex Med (2015) 12(4):956–65. doi: 10.1111/jsm.12832
7. Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics (2019) 7(2). doi: 10.3390/toxics7020021
8. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev (2009) 30(4):293–342. doi: 10.1210/er.2009-0002
9. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol (2011) 127(3-5):204–15. doi: 10.1016/j.jsbmb.2011.08.007
10. Radke EG, Braun JM, Meeker JD, Cooper GS. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ Int (2018) 121(Pt 1):764–93. doi: 10.1016/j.envint.2018.07.029
11. Silva MJ, Samandar E, Jr Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol BioMed Life Sci (2007) 860(1):106–12. doi: 10.1016/j.jchromb.2007.10.023
12. Chen TC, Parker JD, Clark J, Shin HC, Rammon JR, Burt VL. National health and nutrition examination survey: Estimation procedures, 2011-2014. Vital Health Stat (2018) 177):1–26.
13. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National institutes of health. Obes Res (1998) 6 Suppl 2:51s–209s.
14. Khera M, Broderick GA, Carson CC 3rd, Dobs AS, Faraday MM, Goldstein I, et al. Adult-onset hypogonadism. Mayo Clin Proc (2016) 91(7):908–26. doi: 10.1016/j.mayocp.2016.04.022
15. Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res (2008) 108(2):177–84. doi: 10.1016/j.envres.2008.08.007
16. Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res (2007) 51(7):899–911. doi: 10.1002/mnfr.200600243
17. Posnack NG. The adverse cardiac effects of Di(2-ethylhexyl)phthalate and bisphenol a. Cardiovasc Toxicol (2014) 14(4):339–57. doi: 10.1007/s12012-014-9258-y
18. Koch HM, Rossbach B, Drexler H, Angerer J. Internal exposure of the general population to DEHP and other phthalates–determination of secondary and primary phthalate monoester metabolites in urine. Environ Res (2003) 93(2):177–85. doi: 10.1016/S0013-9351(03)00083-5
19. Mariana M, Feiteiro J, Verde I, Cairrao E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ Int (2016) 94:758–76. doi: 10.1016/j.envint.2016.07.004
20. Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ Health (2014) 13(1):43. doi: 101186/1476-069x-13-43
21. Wong JS, Gill SS. Gene expression changes induced in mouse liver by di(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol (2002) 185(3):180–96. doi: 10.1006/taap.2002.9540
22. Shen L, Tang X, Wei Y, Long C, Tan B, Wu S, et al. Vitamin e and vitamin c attenuate di-(2-ethylhexyl) phthalate-induced blood-testis barrier disruption by p38 MAPK in immature SD rats. Reprod Toxicol (2018) 81:17–27. doi: 10.1016/j.reprotox.2018.06.015
23. Dobrzyńska MM, Tyrkiel EJ, Derezińska E, Pachocki KA, Ludwicki JK. Two generation reproductive and developmental toxicity following subchronic exposure of pubescent male mice to di(2-ethylhexyl)phthalate. Ann Agric Environ Med (2012) 19(1):31–7.
24. Yi WEI, Xiang-Liang T, Yu Z, Bin L, Lian-Ju S, Chun-Lan L, et al. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis (2018) 5(3):263–74. doi: 10.1016/j.gendis.2018.06.004
25. Hao X, Guan X, Zhao X, Ji M, Wen X, Chen P, et al. Phthalate inhibits leydig cell differentiation and promotes adipocyte differentiation. Chemosphere (2021) 262:127855. doi: 10.1016/j.chemosphere.2020.127855
26. Yang L, Lu D, Yang B, Peng Z, Fang K, Liu Z, et al. DEHP-induced testicular injury through gene methylation pathway and the protective effect of soybean isoflavones in sprague-dawley rats. Chem Biol Interact (2021) 348:109569. doi: 10.1016/j.cbi.2021.109569
27. Sun Y, Shen J, Zeng L, Yang D, Shao S, Wang J, et al. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse leydig cells. Environ Pollut (2018) 243(Pt A):563–72. doi: 10.1016/j.envpol.2018.08.089
28. Wang J, Zhao T, Chen J, Kang L, Wei Y, Wu Y, et al. Multiple transcriptomic profiling: p53 signaling pathway is involved in DEHP-induced prepubertal testicular injury via promoting cell apoptosis and inhibiting cell proliferation of leydig cells. J Hazard Mater (2021) 406:124316. doi: 10.1016/j.jhazmat.2020.124316
29. Kaufman JM, Lapauw B, Mahmoud A, T'Sjoen G, Huhtaniemi IT. Aging and the Male reproductive system. Endocr Rev (2019) 40(4):906–72. doi: 10.1210/er.2018-00178
30. Goto T, Hirabayashi M, Watanabe Y, Sanbo M, Tomita K, Inoue N, et al. Testosterone supplementation rescues spermatogenesis and in vitro fertilizing ability of sperm in Kiss1 knockout mice. Endocrinology (2020) 161(9). doi: 10.1210/endocr/bqaa092
Keywords: adult-onset hypogonadism (AOH), phthalate, di-(2-ethylhexyl) phthalate (DEHP), NHANES, exposure
Citation: Liu Z-H, Yang L-C, Song P, Chen J-H, Peng Z-F and Dong Q (2022) The relationship between exposure to phthalate metabolites and adult-onset hypogonadism. Front. Endocrinol. 13:991497. doi: 10.3389/fendo.2022.991497
Received: 11 July 2022; Accepted: 01 August 2022;
Published: 18 August 2022.
Edited by:
Chunhua Deng, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Ren-Shan Ge, Wenzhou Medical University, ChinaShengde Wu, College of Pediatrics, Chongqing Medical University, China
Copyright © 2022 Liu, Yang, Song, Chen, Peng and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Dong, ZHFpYW5nNjY2QDE2My5jb20=
†These authors have contributed equally to this work
 Zheng-Huan Liu
Zheng-Huan Liu Lu-Chen Yang†
Lu-Chen Yang† Pan Song
Pan Song Zhu-Feng Peng
Zhu-Feng Peng Qiang Dong
Qiang Dong