- 1Department of Endocrinology, Beijing Institute of Geriatrics, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital, Capital Medical University, Beijing, China
- 3Department of Neurobiology, Neurology and Geriatrics, Beijing Institute of Geriatrics, Xuanwu Hospital of Capital Medical University, Beijing, China
- 4Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
- 5Key Laboratory for Neurodegenerative Disease of the Ministry of Education, Clinical Center for Parkinson’s Disease, Capital Medical University, Beijing, China
- 6Beijing Key Laboratory for Parkinson’s Disease, Xuanwu Hospital, Capital Medical University, Beijing, China
- 7Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China
Objectives: To investigate the association between body fat (BF%) and sarcopenia in older adults with type 2 diabetes mellitus (T2DM) and potential link with increased levels of inflammatory indicators and insulin resistance.
Methods: A total of 543 older adults with T2DM were included in this cross-sectional study. Appendicular skeletal muscle (ASM), handgrip strength and gait speed were measured to diagnose sarcopenia according to the updated Asian Working Group for Sarcopenia (AWGS) 2019 criteria. Body composition data were tested using dual-energy X-ray absorptiometry (DEXA). Levels of serum high-sensitive C-reactive protein (hs-CRP), interleukin-6, fasting blood insulin (FINS), hemoglobin A1c (HbA1c), 25-hydroxyvitamin D3 [25(OH) D3] were also determined.
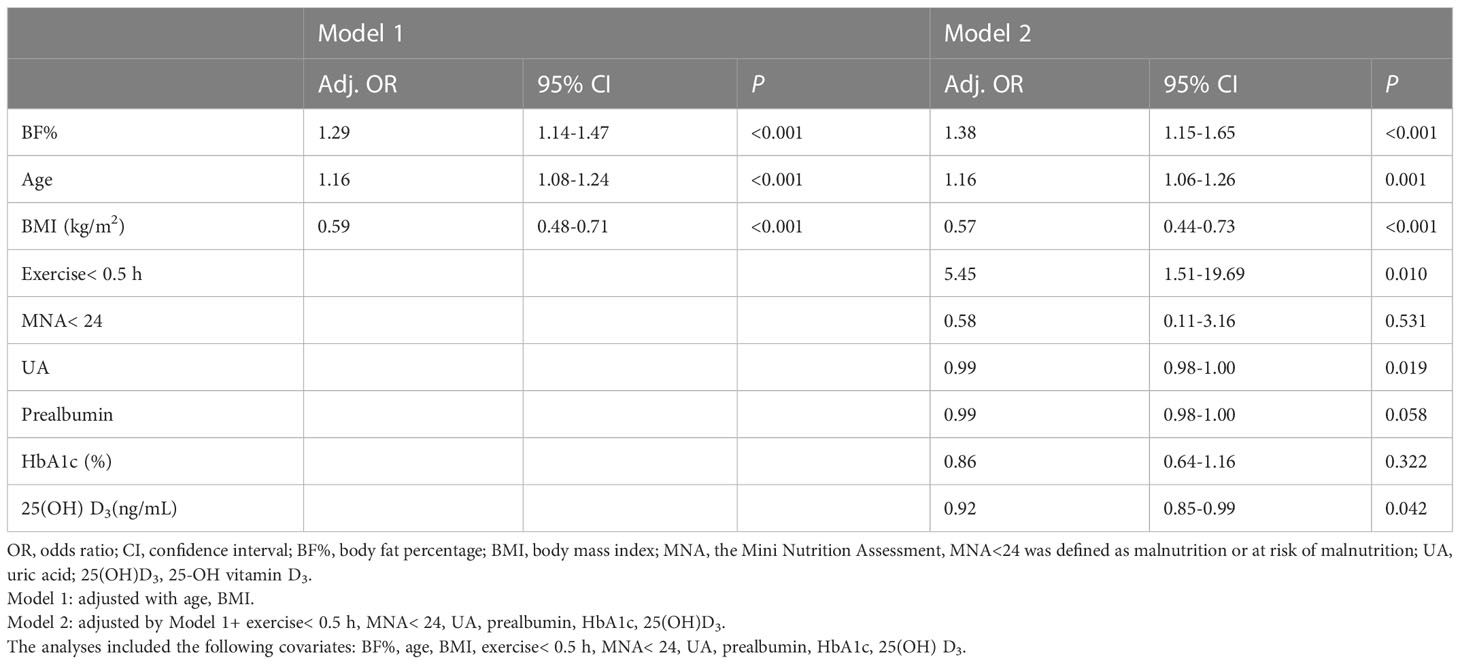
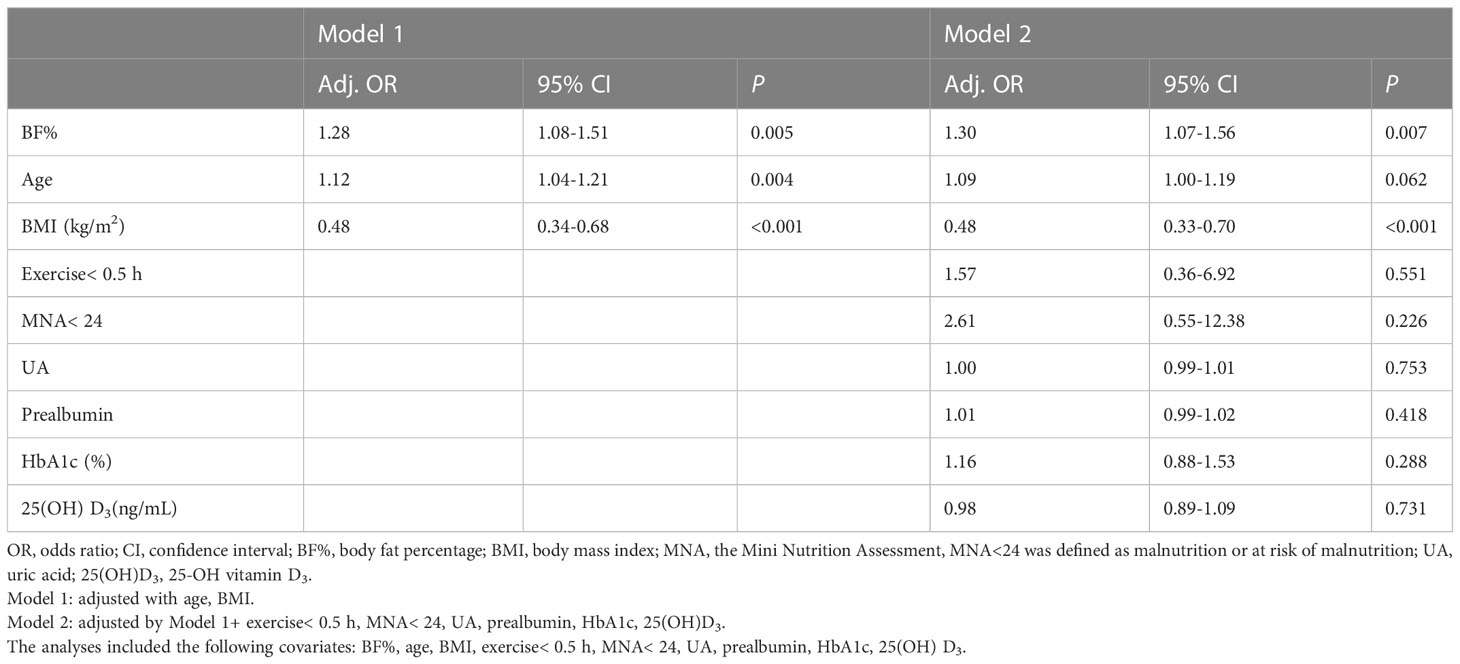
Results: The prevalence of sarcopenia in all participants was 8.84%, of which 11.90% were male and 5.84% females. The Pearson’s correlation analysis revealed that BF% was negatively correlated with gait speed in men and women (R =-0.195, P=0.001; R = -0.136, P =0.025, respectively). After adjusting for all potential confounders, sarcopenia was positive associated with BF% (male, OR: 1.38, 95% CI: 1.15–1.65, P< 0.001; female, OR: 1.30, 95% CI: 1.07–1.56, P=0.007), and negatively associated with body mass index (BMI) (male, OR: 0.57, 95% CI: 0.44–0.73, P<0.001; female, OR: 0.48, 95% CI: 0.33–0.70, P<0.001). No significant differences were found in hs-CRP, interleukin-6, and insulin resistance between older T2DM adults with and without sarcopenia.
Conclusion: Higher BF% was linked to an increased risk of sarcopenia in older adults with T2DM, suggesting the importance of assessing BF% rather than BMI alone to manage sarcopenia.
Introduction
Sarcopenia and obesity are two highly prevalent conditions in older adults related to body composition and are known to be associated with poor physical function (1, 2). Aging leads to increased fat mass along with loss of muscle mass and strength, even in older adults with stable body weight (3). Sarcopenia is defined as a loss of muscle mass, decline in muscle strength and/or physical function that is associated with increased risks of falls, fractures (4), physical disability, morbidity (5), and even mortality (6). Sarcopenic obesity (SO), a disorder characterized by the coexistence of obesity and sarcopenia, is associated with a higher risk of adverse outcomes than individuals with sarcopenia or obesity alone (7). To prevent the adverse outcomes of sarcopenia and/or SO, it is important to understand the impact of obesity on sarcopenia and the underlying mechanisms. An excessive amount of adipose tissue may lead to decline of muscle mass and function through oxidative stress, inflammation, and insulin resistance, suggesting that fat mass might play a role in the onset and development of sarcopenia (8).
Type 2 diabetes mellitus (T2DM) is another prevalent disease in the elderly population, affecting approximately 25% of people aged over 65 years (9). Previous studies have reported that individuals with T2DM presented a greater decline of muscle mass and strength compared with euglycemic subjects (10). A recent meta-analysis also demonstrated T2DM patients had a 55% higher risk of sarcopenia compared with those without diabetes (11). In addition, individuals with T2DM are often overweight or obese. Several studies have shown that high body mass index (BMI) has a protective effect on sarcopenia, possibly because high BMI reflects good nutritional status (12, 13). Although BMI can reflect overall nutritional status, BMI cannot differentiate between muscle and fat mass, nor can it reflect fat distribution (14). Some studies have shown that increased body fat was associated with lower muscle mass and muscle quality in general population (15, 16). However, data investigating the effect of fat mass on sarcopenia in patients with T2DM are very limited. To our knowledge, only two studies have investigated the impact of body fat percentage (BF%) on sarcopenia in subjects with T2DM. One study of 87 participants with T2DM has shown BF% derived from dual-energy X-ray absorptiometry(DEXA) is inversely correlated with muscle strength (17). The other study has also suggested that T2DM patients with a high BF% in addition to low BMI might develop sarcopenia (18). However, the above two studies have limitations, such as small sample size and lack of adequate confounders such as nutritional status, insulin resistance, and markers of inflammation. Therefore, understanding the effect and underlying mechanisms of fat mass on sarcopenia in patients with T2DM is crucial to implement appropriate management strategies for sarcopenia.
Therefore, the aim of our study was to assess the effect of BF% on sarcopenia in older adults with T2DM. Additionally, we aimed to explore whether the link between BF% and sarcopenia could be explained by the increased levels of inflammatory indicators and insulin resistance.
Materials and methods
Study population
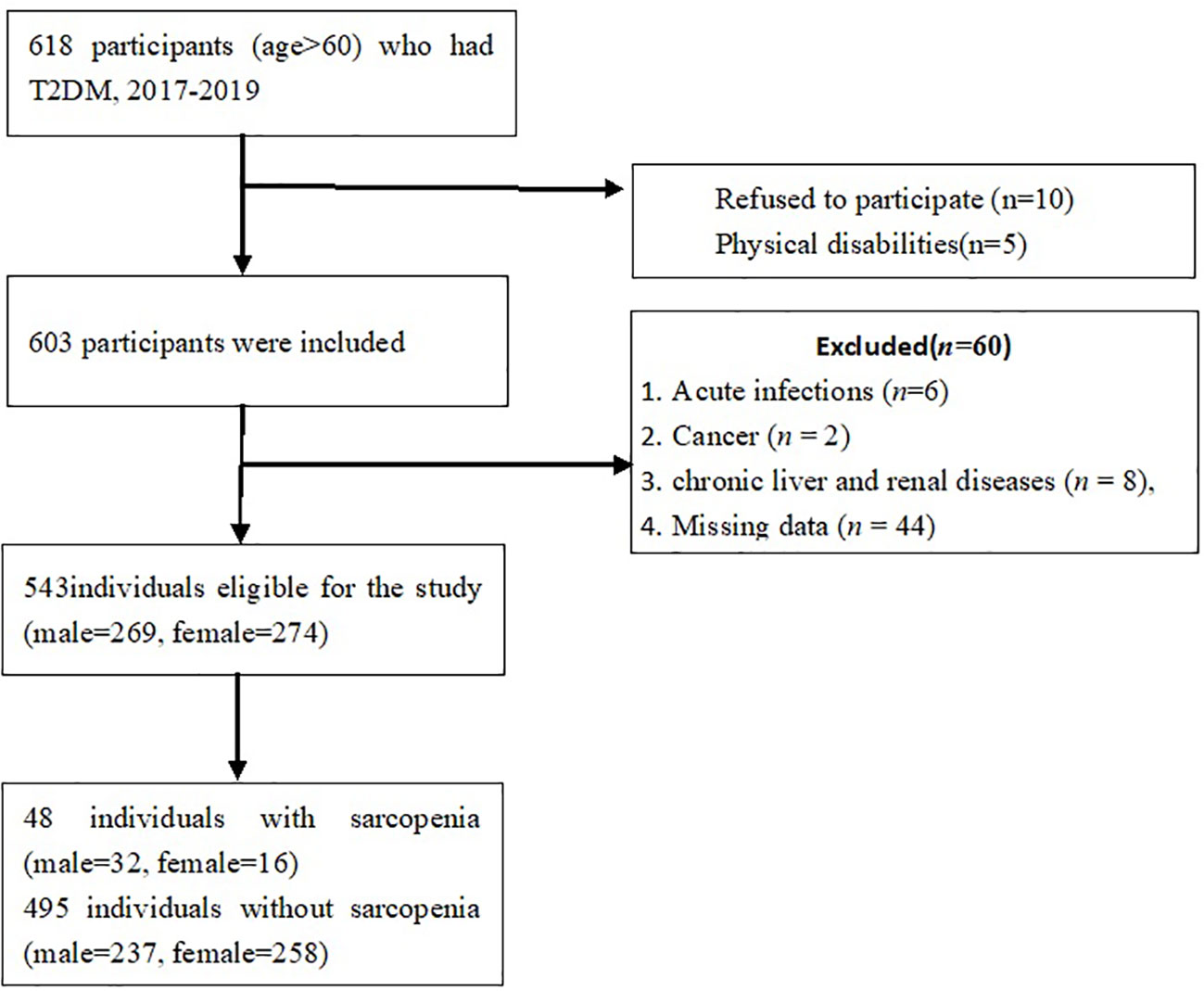
This was a cross-sectional study of older adult with T2DM, recruited from the wards of Department of Endocrinology, Xuanwu Hospital, Capital Medical University. Participants who were aged > 60 years with a confirmed diagnosis of T2DM were consecutively recruited from Oct 2017 to July 2019. The inclusion criteria were:(1) adults aged over 60 years;(2) met the criteria of the American Diabetes Association for T2DM (19). The exclusion criteria were: (1) the presence of cancer, acute inflammatory diseases, renal and chronic liver diseases;(2) diabetic ketoacidosis and hyperosmolar hyperglycemia; acute myocardial infarction; acute cerebrovascular disease; (3) gastrointestinal diseases such as gastrointestinal bleeding and ulcers, acute and chronic pancreatitis;(4) disability, poor cognitive function. A total of 618 older adults with T2DM were fulfilled the inclusion criteria, among whom, 75 were excluded from this analysis, including 5 subjects with physical disability, 6 with acute infections, 2 with cancers, 8 with renal or liver diseases, 10 refused to participate and 44 with missing data. Thus, a total of 543 subjects(male, n=269; female, n=274)were recruited (Figure 1). The post-hoc power of the study was estimated using G*power software program (20). The sample sizes revealed > 99% power to detect a significant association (α < 0.05), given an effect size index of 0.1.
The Research Ethics Boards at Xuanwu Hospital of Capital Medical University approved the study protocol (approval number: CTR-IPR-2019002). All participants signed written informed consent.
Clinical characteristics and biochemical indicators
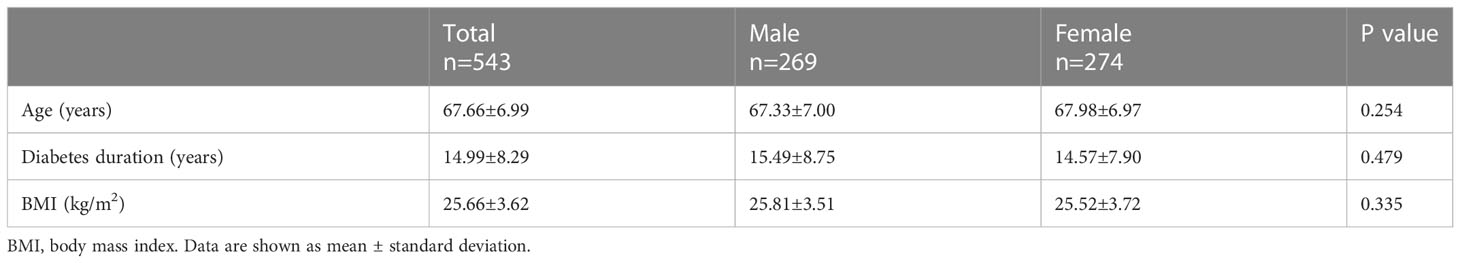
The participants were interviewed by trained staff. Each participant completed a general questionnaire including demographic variables including age, medical history, and medication records. Height and body weight were measured, and body mass index (BMI) was calculated as weight (kg)/height (m)2. The nutritional status of patients was assessed by The Mini Nutrition Assessment (MNA). The sum of the MNA score≥24 were defined as adequate nutritional status, while MNA<24 were defined as malnutrition or at risk of malnutrition (21). Physical activity was assessed by daily exercise: exercise< 0.5 h and exercise≥0.5 h.
Blood samples of the participants were obtained after overnight fasting and were measured in the biochemistry laboratory of Xuanwu Hospital of Capital Medical University. Biochemical parameters, including fasting blood glucose (FBG), hemoglobin A1c (HbA1c), total cholesterol (TCH), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), uric acid (UA), albumin, prealbumin, 25-hydroxyvitamin D3 [25(OH)D3] and hemoglobin (Hb) were measured. As inflammation indicators, high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 were tested. Fasting blood insulin (FINS), fasting blood C peptide (FCP) were also measured. Insulin resistance (IR) index (HOMA-IR) was calculated by using Homeostasis Model Assessment (HOMA) formula (HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5).
According to the Asian Working Group for Sarcopenia (AWGS) criteria, individuals with low muscle mass and low muscle strength or low physical performance were defined as having sarcopenia (22).
Body composition data were measured using DEXA (LUNAR iDXA, USA), which measured muscle mass and fat mass for total body, both arms, both legs and the torso. All the scans were performed by a single experienced technologist. Appendicular skeletal muscle (ASM, kg) was determined as the sum of skeletal muscle mass in both arms and legs. Appendicular Skeletal muscle index (ASMI) was defined as ASM divided by height squared (m2) (22, 23). Low muscle mass was defined as SMI<7.0 kg/m2 for men and < 5.4 kg/m2 for women (22). Body fat percentage(BF%) was calculated as total body fat divided by total body mass multiplied by 100. High BF% was defined as BF%≥27% for men or≥40% for women (24).
Muscle strength was defined by handgrip strength using the Jamar® Hydraulic Hand Dynamometer (Patterson Medical, Warrenville, IL, USA). Participants were directed to exert maximum effort three times with each hand. The maximal value was used for further analyses. The cut-off value for low grip strength was set at 28 kg for men and 18kg for women (22).
The physical performance of the participants was measured using usual gait speed (m/s) on a 6- meter course (25). Each participant was instructed to test two times and the faster one was used for defining sarcopenia. The cutoff value for low gait speed was set at <1.0m/s (22).
Statistical analyses
Statistical analysis was done by using SPSS 21.0. The Kolmogorov-Smirnov test was performed to assess the distribution of the variables. For continuous variables, the data was represented as mean ± SD or median (interquartile range). Categorical variables were reported as frequencies. The Student t-test, Mann-Whitney U test or Chi-square test was used for comparisons between variables where appropriate. The Pearson correlation was utilized to assess the correlations between BF% and ASMI, handgrip strength and gait speed. The independent effects of BF% on sarcopenia were assessed using multiple logistic regression analyses. Mixed effects models adjusting for (1) BF%, age and BMI; (2) BF%, age, BMI, UA, prealbumin, HbA1c, 25(OH)D3 were used to evaluate the relationship between BF% and sarcopenia. The results of the regression modeling were presented as odds ratios (ORs) and 95% confidence interval (CI). Two-sided P<0.05 was considered statistically significant.
Results
Characteristics of participants
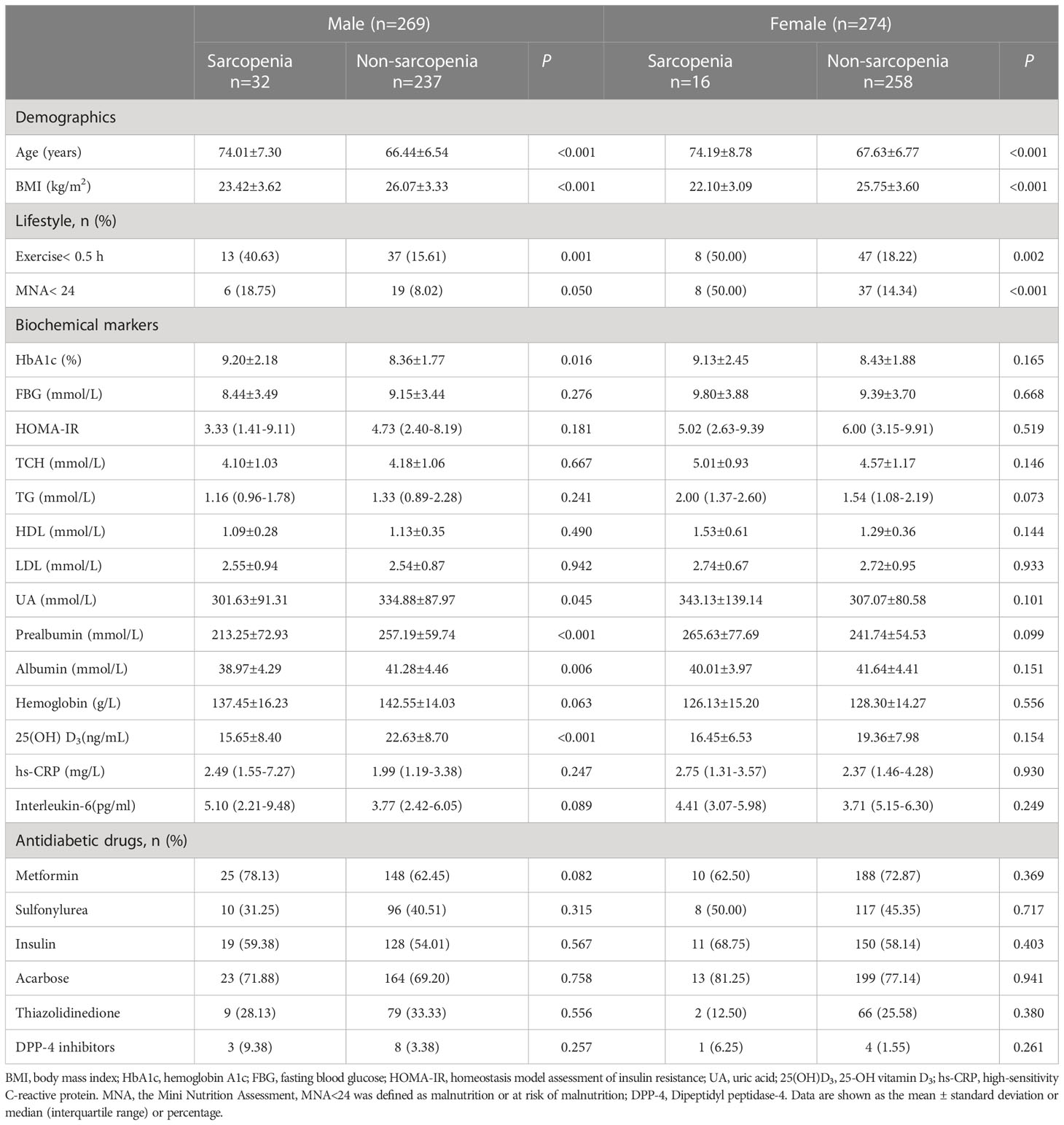
A total of 543 participants with T2DM (67.68 ± 7.06 years; male, n=269; female, n=274) were included in this study. There was no significant difference in age, BMI and duration of diabetes according to sexes (Table 1). The biochemical variables of the participants by sex and sarcopenia status were presented in Table 2. The overall prevalence of sarcopenia was 8.84%, of which 11.90% for men and 5.84% for women. Compared to the subjects without sarcopenia, those with sarcopenia were significantly older, had lower BMI, higher percentage of exercise <0.5 h and MNA< 24 in both sexes. For men, participants with sarcopenia had higher HbA1c levels, while lower prealbumin, albumin, UA and 25(OH) D3 levels. However, no significant difference was found between individuals with and without sarcopenia concerning the biochemical parameters in female participants. There were no significant differences in levels of hs-CRP, interleukin-6, and HOMA-IR and the percentage of usage of antidiabetic drugs between the two groups of either gender (Table 2).
Comparison of body composition and muscle function
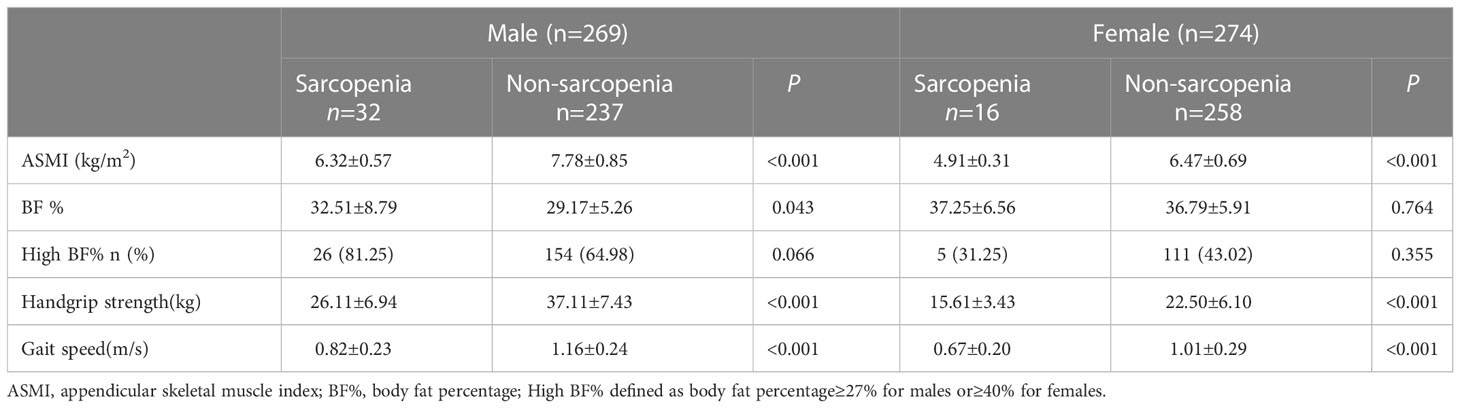
A comparison of body composition and muscle function was presented in Table 3. As expected, subjects with sarcopenia had lower ASMI, handgrip strength and gait speed for both sexes (P <0.001). For men, BF% was higher in people with sarcopenia compared to those without. However, there was no significant difference in BF% for women.
Correlations between BF% and the components of sarcopenia
The Pearson correlation analysis revealed that BF% were negatively correlated with gait speed in men and women (R = -0.195, P =0.001; R = -0.136, P =0.025, respectively). For men, BF% was also negatively correlated with handgrip strength (R = -0.230, P < 0.001). However, no significant association was found between BF% and ASMI of either gender (Figure 2).
Logistic regression models for sarcopenia in both sexes
The logistic regression models for the association between BF% and sarcopenia for men and women were presented in Table 4A, 4B. Age, BMI, exercise< 0.5 h, MNA< 24, UA, prealbumin, HbA1c, 25(OH)D3 and BF% were included in the multivariate logistic regression model. In the adjusted model 2, higher BF% was still associated with an increased risk of sarcopenia in both sexes (men, OR: 1.38, 95% CI: 1.15–1.65; women, OR: 1.30, 95% CI: 1.07–1.56), while higher BMI was associated with a decreased risk of sarcopenia (men, OR: 0.57, 95% CI: 0.44–0.73; women, OR: 0.48, 95% CI: 0.33–0.70). In addition, higher UA and 25(OH) D3 levels appeared to be protective against sarcopenia in male participants (OR = 0.99, 95% CI: 0.98-1.00; OR = 0.92, 95% CI: 0.85-0.99, respectively), while exercise< 0.5 h was associated with an increased risk of sarcopenia (men, OR: 5.45, 95% CI: 1.51–19.69). However, the other confounders (such as prealbumin, HbA1c) were not related to sarcopenia in both sexes (Figures 3A, B).
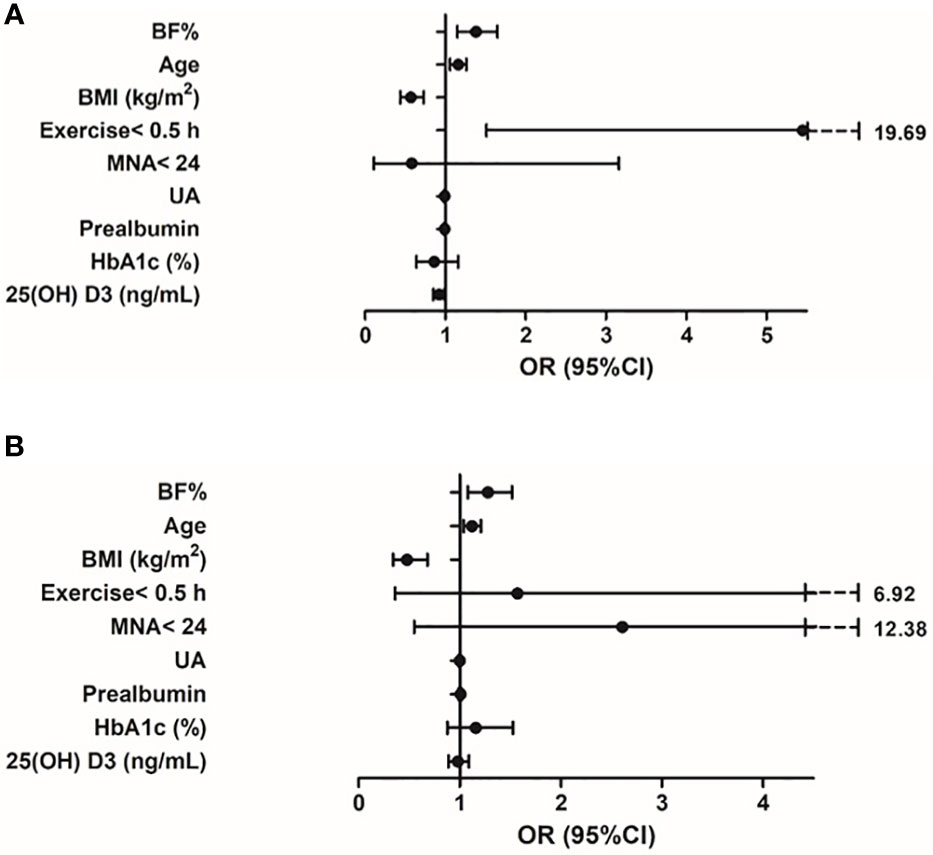
Figure 3 (A) Variables associated with sarcopenia in older adults with diabetes (male). Logistic regression model adjusted for BF%, age, BMI, exercise< 0.5 h, MNA<24, UA, prealbumin, HbA1c, 25(OH) D3. (B) Variables associated with sarcopenia in older adults with diabetes (female). Logistic regression model adjusted for BF%, age, BMI, exercise< 0.5 h, MNA<24, UA, prealbumin, HbA1c, 25(OH) D3.
Discussion
In the present study, we assessed the impact of BF% on risk of sarcopenia in a larger group of older adults with T2DM. Our main findings showed that high level of BF% was associated with an increased risk of sarcopenia in both men and women, while higher BMI was protective against sarcopenia, suggesting the importance of assessing BF% instead of assessing BMI alone to manage sarcopenia in T2DM adults.
Sarcopenia has been known as an age-related syndrome and has attracted worldwide attention in recent years. Due to the lack of a single diagnostic criterion, the prevalence of sarcopenia varied considerably with different diagnostic criteria and using different methods applied to measure muscle mass and in different study populations. A recent systematic review and meta-analysis reported the prevalence of sarcopenia ranged from 10%-27% in older adults≥60 years using different classifications and cut-off points (26). Among older Chinese adults, the prevalence was 14% in men and 15% in women based on the AWGS criterion (27). Chung et al. (28) reported that diabetics were associated with a 51% increase in risk for sarcopenia than non-diabetics in a systematic review. Another study in community-dwelling elderly with T2DM, in which sarcopenia was defined using the old AWGS algorithm, reported the prevalence of sarcopenia was 14.8% (29). In our study, the overall prevalence of sarcopenia was 8.84%, which was slightly higher than the prevalence reported by Mori H et al. in a Japanese T2DM population (7.2%) (30), but was lower than that in other populations (31, 32). These differences may be explained by the different diagnostic criteria used and heterogeneous study populations. In our study, DEXA was used to measure muscle mass and the new AWGS algorithm was applied to define sarcopenia.
Additionally, our study suggested that the prevalence of sarcopenia in man (11.90%) was higher than that in women (5.84%). These finding was consistent with the data from Hai et al., which showed the prevalence of sarcopenia was 11% and 9% in community-dwelling men and women aged≥60 years, respectively (33). The decreased levels of insulin-like growth factor 1 (IGF-1) in males and the hormone differences between sexes might be the reason (34). Sex steroid hormones such as testosterone, estrogen and progesterone play important roles in maintaining skeletal muscle quality and function including hypertrophy and the regeneration of damaged muscles (35). Testosterone promotes protein synthesis in muscle, counteracts muscle proteolysis, promotes muscle regeneration, and increases intramuscular insulin-like growth factor-1 (IGF-1) levels (36). Estrogen and progesterone directly exert their effects on muscle tissue through their receptors expressed in skeletal muscle tissue (37). The possible reasons for the higher prevalence of sarcopenia in men than in women are as follows: (1) testosterone levels in healthy men fall by 1% annually from the age of 30 and testosterone deficiency leads to a more robust catabolic response in men than women during aging (38). (2) With aging, the absolute and relative decrease in muscle mass and increases in total fat were more prominent in men than in women (39). In addition, the loss of muscle strength with aging is greater and faster in men (40).
Nutritional status might play a great role on the onset and development of sarcopenia. Several studies have indicated that lower BMI is associated with loss of muscle mass, decline of muscle strength and physical function (41). Older adults are at higher risk of sarcopenia because they are more likely to suffer from malnutrition. Our study also suggested that participants with sarcopenia had higher percentage of MNA< 24 and higher BMI was protective against sarcopenia of either gender. The reason for this finding could be that older adults with higher BMI might have higher protein intake to offset the loss of muscle mass and muscle performance (42). In addition, albumin and prealbumin have been considered as indicators for malnutrition. Higher albumin levels were reported to significantly predict a lower risk of pre-sarcopenia over 4 years in community-dwelling women over the age of 75 (43). One of our previous studies demonstrated that low prealbumin levels are associated with an increased risk of sarcopenia in older men with T2DM (13). In the present study, the levels of albumin and prealbumin were significantly lower in men with sarcopenia compared with those without sarcopenia. However, after adjusting for other confounders, prealbumin levels were no longer associated with the presence of sarcopenia. We also found no association between prealbumin and sarcopenia in older women with T2DM. The inconsistent findings may be due to different confounders applied and the low prevalence of sarcopenia in women (only 16 individuals with sarcopenia). There is growing evidence that sufficient total calorie intake and adequate protein intake, specifically leucine, are important for muscle maintenance (44, 45). Older adults with T2DM may be at additional risk of malnutrition due to excessive dietary restriction to control blood glucose. Thus, ensuring adequate intake of calorie and protein is essential to prevent the development of sarcopenia.
Several previous studies showed that obesity was inversely associated with muscle strength and muscle function in older adults. The data from the Health, Aging, and Body Composition study showed that high fatness was related to lower muscle quality, and it predicted accelerated loss of lean mass (16). Another study also reported that obesity, assessed by BMI, was a risk factor for functional decline in older persons in both sexes (1). However, our study indicated that higher BMI was protective against sarcopenia, which was in line with the findings of several previous studies (46, 47). Although BMI is the most commonly used method to estimate obesity due to the relative ease of application, BMI does not distinguish between fat mass and fat-free mass and may misclassify some older adults (48). Therefore, the reported association of better physical function with higher BMI may be due to the limitation of BMI to define obesity. Thus, BMI was a rough method to evaluate excess body fat, while the body composition measured by DEXA allows us to determine the real fat percentage (49). In the present study, we found BF% were not associated with ASMI, but negatively correlated with gait speed in both sexes, suggesting BF% may be more associated with muscle or physical function rather than muscle mass. Barrett et al. (17) also reported adiposity, assessed as BF% or WC, was inversely associated with muscle strength in community-dwelling older adults with T2DM. The possible explanation for such findings could be that adiposity leads to inter and intramuscular adipose tissue infiltration, which mainly affects muscle function rather than muscle volume. Intramuscular adipose tissue infiltration can induce mitochondrial dysfunction, increase reactive oxygen species formation, and secrete some pro-inflammatory myokines (50). All these mechanisms are involved in the development and progression of sarcopenia. Several studies demonstrated that intramuscular adipose tissue infiltration was associated with decrease in muscle density, loss of muscle quality and physical performance (51, 52). The association between higher BF% and sarcopenia could be explained by increased cytokines levels and insulin resistance. Cytokines secreted by adipose tissue such as interleukin-6, tumor necrosis factor-α may have catabolic effect on muscle (53). In addition, excess adiposity may reduce insulin action, which has pro-catabolic effects on muscle (8). However, we found no significant differences in cytokines and HOMA-IR between the two groups, suggesting that the link between BF% and sarcopenia could not be explained by inflammatory indicators and insulin resistance. This finding coincided with that from Koster et al. (16) who also failed to find the association between adipocytokines, insulin resistance and sarcopenia. The possible explanation for such results could be that the pathophysiological mechanisms linking adiposity to sarcopenia are complex, including hormones, such as testosterone and growth hormone, physical inactivity, etc. Thus, further well-designed studies are needed to investigate the possible mechanisms underlying the link between fat mass and sarcopenia.
Exercise and vitamin D are also important for muscle health. Physical activity and exercise are shown to attenuate age-related decreases in muscle mass, strength, and slow or prevent the development of sarcopenia (54). Our study also showed exercise < 0.5 h was associated with an increased risk of sarcopenia in male participants. Therefore, exercise should be emphasized as part of the lifestyle necessary to maintain muscle health. Previous studies reported that vitamin D levels were positively associated with muscle mass, muscle strength and physical function (55). Our study also indicated that higher levels of vitamin D were protective against sarcopenia in male participants with T2DM. Moreover, in vivo studies suggested vitamin D receptors (VDR) overexpression with skeletal muscle hypertrophy. However, mixed findings have been reported for relationships between vitamin D supplementation and sarcopenia indices. A recent systematic review and meta-analysis showed that Vitamin D monotherapy had no effect on any sarcopenia indices in community-dwelling older adults (56). Another meta-analysis suggests that vitamin D plus protein supplementation improves muscle strength in individuals with sarcopenia but has no impact on muscle mass or performance (57). The discrepant findings between studies may be due to heterogeneity between older populations and co-supplementation.
Our study also indicated that uric acid had a protective effect against sarcopenia in older men. Previous studies exploring the associations between uric acid and sarcopenia indices have reported inconsistent results. Beavers et al. (58) demonstrated that low relative skeletal muscle mass was associated with elevated uric acid levels. Allopurinol, a well-known inhibitor of xanthine oxidase (XO), has been reported to play a possible role in the treatment of sarcopenia (59). However, Xu et al. (60), found that higher levels of uric acid were associated with higher muscle mass and muscle strength. Another study showed that higher uric acid levels were linked to better muscle function in the oldest old (61). However, no association between uric acid and sarcopenia was found in women in our study, possibly due to the fewer cases of sarcopenia in females. Since uric acid has both protective antioxidant effect and harmful inflammatory effect, further research is needed to explore its optimal serum levels to maintain good muscle health in older adults.
Although there are several strengths to this study, such as reliable assessments of muscle mass, relatively sufficient confounding factors, certain limitations should be considered. First, causal relationships could not be determined due to the cross-sectional nature of this study. Second, we excluded those individuals with disability and/or poor cognitive function, which may lead to an underestimation of sarcopenia prevalence. Thirdly, our sample was relatively small and was only selected from Xuanwu Hospital, so the results cannot be generalizable to the wider T2DM population. Further well-designed studies with large sample size are needed to assess the impact of BF% on sarcopenia in older adults with T2DM. Fourthly, although the multiple regression analysis was adjusted, some residual confounders such as socio-economic status, comorbidities, and complications were not included in the model. Nonetheless, this study enriches our knowledge of the association between fat mass and sarcopenia.
In conclusion, our results demonstrated that higher BF% was associated with an increased risk of sarcopenia in older adults with T2DM, suggesting the importance of assessing BF% rather than BMI alone to manage sarcopenia. This finding has clinical implications due to the close relationship between sarcopenia and T2DM, suggesting that achieving proper body composition in older adults with T2DM is important for preserving muscle function. Thus, early exercise and dietary interventions aimed at maintaining muscle health might be helpful for preventing sarcopenia in older adults with high BF%.
Data availability statement
Requests for access to datasets should be directed to eGl1c2h1YW5nbGluZ0AxMjYuY29t.
Ethics statement
The studies involving human participants were reviewed and approved by The Research Ethics Boards at Xuanwu Hospital of Capital Medical University approved the study protocol (approval number: CTR-IPR-2019002). All participants signed written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SX and PC were responsible for study conception and design. LS organized the database and drafted the manuscript. JF performed the statistical analysis. LS, XD and ZM summarized the clinical data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Beijing Municipal Administration of Hospitals Incubating Program (PX2020034), the Pilot Project for Public Welfare Development and Reform of Beijing-affiliated Medical Research Institutes-Research on Intelligent Management and Innovative Model of Chronic Diseases in Elderly (Beijing Medical Research 2021-8), the National Key R&D Program of China (2021YFC2501200, 2018YFC1312001, 2017YFC0840105, 2017YFC1310200), and Key Realm R&D Program of Guangdong Province (2018B030337001).
Acknowledgments
The authors thank all the doctors, and participants who were involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jensen GL, Friedmann JM. Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc (2002) 50:918–23. doi: 10.1046/j.1532-5415.2002.50220.x
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48:16–31. doi: 10.1093/ageing/afy169
3. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol (2018) 14:513–37. doi: 10.1038/s41574-018-0062-9
4. Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle (2019) 10:485–500. doi: 10.1002/jcsm.12411
5. Di Iorio A, Abate M, Di Renzo D, Russolillo A, Battaglini C, Ripari P, et al. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int J Immunopathol Pharmacol (2006) 19:703–19. doi: 10.1177/039463200601900401
6. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PloS One (2017) 12:e0169548. doi: 10.1371/journal.pone.0169548
7. Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts (2022) 15:321–35. doi: 10.1159/000521241
8. Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci (2020) 21:494. doi: 10.3390/ijms21020494
9. American Diabetes Association. 12. older adults: Standards of medical care in diabetes-2019. Diabetes Care (2019) 42:S139–s147. doi: 10.2337/dc19-S012
10. Scott D, de Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in australia's ageing population? Med J Aust (2016) 205:329–33. doi: 10.5694/mja16.00446
11. Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: A systematic review and meta-analysis. Calcif Tissue Int (2020) 107:453–63. doi: 10.1007/s00223-020-00742-y
12. Nasimi N, Dabbaghmanesh MH, Sohrabi Z. Nutritional status and body fat mass: Determinants of sarcopenia in community-dwelling older adults. Exp Gerontol (2019) 122:67–73. doi: 10.1016/j.exger.2019.04.009
13. Xiu S, Sun L, Mu Z, Fu J. Low prealbumin levels are associated with sarcopenia in older men with type 2 diabetes mellitus: A cross-sectional study. Nutrition (2021) 91-92:111415. doi: 10.1016/j.nut.2021.111415
14. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: Clinical challenges and implications for management. Circulation (2018) 137:1391–406. doi: 10.1161/CIRCULATIONAHA.117.029617
15. Kao TW, Peng TC, Chen WL, Han DS, Chen CL, Yang WS. Impact of adiposity on muscle function and clinical events among elders with dynapenia, presarcopenia and sarcopenia: a community-based cross-sectional study. Aging (Albany NY) (2021) 13:7247–58. doi: 10.18632/aging.202581
16. Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci (2011) 66:888–95. doi: 10.1093/gerona/glr070
17. Barrett M, McClure R, Villani A. Adiposity is inversely associated with strength in older adults with type 2 diabetes mellitus. Eur Geriatr Med (2020) 11:451–8. doi: 10.1007/s41999-020-00309-y
18. Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig (2019) 10:322–30. doi: 10.1111/jdi.12908
19. American Diabetes Association. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care (2019) 42:S13–s28. doi: 10.2337/dc19-S002
20. Faul F, Erdfelder E, Lang AG, Buchner AG. Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods (2007) 39:175–91. doi: 10.3758/BF03193146
21. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition (1999) 15:116–22. doi: 10.1016/S0899-9007(98)00171-3
22. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc (2020) 21:300–307.e302. doi: 10.1016/j.jamda.2019.12.012
23. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in new Mexico. Am J Epidemiol (1998) 147:755–63. doi: 10.1093/oxfordjournals.aje.a009520
24. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, SJ B. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the national health and nutrition examination survey III. Eur J Clin Nutr (2014) 68:1001–7. doi: 10.1038/ejcn.2014.117
25. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an international academy on nutrition and aging (IANA) task force. J Nutr Health Aging (2009) 13:881–9. doi: 10.1007/s12603-009-0246-z
26. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle (2022) 13:86–99. doi: 10.1002/jcsm.12783
27. Xin C, Sun X, Lu L, Shan L. Prevalence of sarcopenia in older Chinese adults: a systematic review and meta-analysis. BMJ Open (2021) 11:e041879. doi: 10.1136/bmjopen-2020-041879
28. Chung SM, Moon JS. Chang MC.Prevalence of sarcopenia and its association with diabetes: A meta-analysis of community-dwelling Asian population. Front Med (Lausanne) (2021) 8:681232. doi: 10.3389/fmed.2021.681232
29. Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep (2016) 6:38937. doi: 10.1038/srep38937
30. Mori H, Kuroda A, Ishizu M, Ohishi M, Takashi Y, Otsuka Y, et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J Diabetes Investig (2019) 10:1332–40. doi: 10.1111/jdi.13014
31. Fung FY, Koh YLE, Malhotra R, Ostbye T, Lee PY, Shariff Ghazali S, et al. Prevalence of and factors associated with sarcopenia among multi-ethnic ambulatory older asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr (2019) 19:122. doi: 10.1186/s12877-019-1137-8
32. Sazlina SG, Lee PY, Chan YM, MS AH, Tan NC. The prevalence and factors associated with sarcopenia among community living elderly with type 2 diabetes mellitus in primary care clinics in Malaysia. PloS One (2020) 15:e0233299. doi: 10.1371/journal.pone.0233299
33. Hai S, Cao L, Wang H, Zhou J, Liu P, Yang Y, et al. Association between sarcopenia and nutritional status and physical activity among community-dwelling Chinese adults aged 60 years and older. Geriatr Gerontol Int (2017) 17:1959–66. doi: 10.1111/ggi.13001
34. Albani D, Batelli S, Polito L, Vittori A, Pesaresi M, Gajo GB, et al. A polymorphic variant of the insulin-like growth factor 1 (IGF-1) receptor correlates with male longevity in the Italian population: a genetic study and evaluation of circulating IGF-1 from the "Treviso longeva (TRELONG)" study. BMC Geriatr (2009) 9:19. doi: 10.1186/1471-2318-9-19
35. Huang LT, Wang JH. The therapeutic intervention of sex steroid hormones for sarcopenia. Front Med (Lausanne) (2021) 8:739251. doi: 10.3389/fmed.2021.739251
36. Martín AI, Priego T, López-Calderón A. Hormones and muscle atrophy. Adv Exp Med Biol (2018) 1088:207–33. doi: 10.1007/978-981-13-1435-3_9
37. Hansen M. Female hormones: do they influence muscle and tendon protein metabolism? Proc Nutr Soc (2018) 77:32–41. doi: 10.1017/S0029665117001951
38. Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism (1997) 46:410–3. doi: 10.1016/S0026-0495(97)90057-3
39. Anderson LJ, Liu H, Garcia JM. Sex differences in muscle wasting. Adv Exp Med Biol (2017) 1043:153–97. doi: 10.1007/978-3-319-70178-3_9
40. Bian A, Ma Y, Zhou X, Guo Y, Wang W, Zhang Y, et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet Disord (2020) 21:214. doi: 10.1186/s12891-020-03236-y
41. Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F, et al. Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients (2021) 13:4291. doi: 10.3390/nu13124291
42. Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, et al. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab (2014) 32:78–88. doi: 10.1007/s00774-013-0468-3
43. Kim H, Suzuki T, Kim M, Kojima N, Yoshida Y, Hirano H, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc (2015) 16(1), 16:85.e81–88. doi: 10.1016/j.jamda.2014.10.006
44. Martin-Cantero A, Reijnierse EM, Gill BMT, Maier AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta-analysis. Nutr Rev (2021) 79:315–30. doi: 10.1093/nutrit/nuaa064
45. Chen LK, Arai H, Assantachai P, Akishita M, Chew STH, Dumlao LC, et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian working group for sarcopenia. J Cachexia Sarcopenia Muscle (2022) 13:1653–72. doi: 10.1002/jcsm.12981
46. Imai K, Gregg EW, Chen YJ, Zhang P, de Rekeneire N, Williamson DF. The association of BMI with functional status and self-rated health in US adults. Obes (Silver Spring) (2008) 16:402–8. doi: 10.1038/oby.2007.70
47. Bahat G, Tufan F, Saka B, Akin S, Ozkaya H, Yucel N, et al. Which body mass index (BMI) is better in the elderly for functional status? Arch Gerontol Geriatr (2012) 54:78–81. doi: 10.1016/j.archger.2011.04.019
48. Holmes CJ, Racette SB. The utility of body composition assessment in nutrition and clinical practice: An overview of current methodology. Nutrients (2021) 13:2493. doi: 10.3390/nu13082493
49. Bahat G, Kilic C, Topcu Y, Aydin K, Karan MA. Fat percentage cutoff values to define obesity and prevalence of sarcopenic obesity in community-dwelling older adults in Turkey. Aging Male (2020) 23:477–82. doi: 10.1080/13685538.2018.1530208
50. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
51. Aversa Z, Zhang X, Fielding RA, Lanza I, LeBrasseur NK. The clinical impact and biological mechanisms of skeletal muscle aging. Bone (2019) 127:26–36. doi: 10.1016/j.bone.2019.05.021
52. Scott D, Johansson J, McMillan LB, Ebeling PR, Nordstrom A, Nordstrom P. Mid-calf skeletal muscle density and its associations with physical activity, bone health and incident 12-month falls in older adults: The healthy ageing initiative. Bone (2019) 120:446–51. doi: 10.1016/j.bone.2018.12.004
53. Mu ZJ, Fu JL, Sun LN, Chan P, Xiu SL. Associations between homocysteine, inflammatory cytokines and sarcopenia in Chinese older adults with type 2 diabetes. BMC Geriatr (2021) 21:692. doi: 10.1186/s12877-021-02622-y
54. Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med (2018) 8:a029785. doi: 10.1101/cshperspect.a029785
55. Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH Jr. Vitamin d, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition (2019) 60:66–9. doi: 10.1016/j.nut.2018.09.031
56. Prokopidis K, Giannos P, Katsikas Triantafyllidis K, Kechagias KS, Mesinovic J, Witard OC, et al. Effect of vitamin d monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle (2022) 13:1642–52. doi: 10.1002/jcsm.12976
57. Gkekas NK, Anagnostis P, Paraschou V, Stamiris D, Dellis S, Kenanidis E, et al. The effect of vitamin d plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas (2021) 145:56–63. doi: 10.1016/j.maturitas.2021.01.002
58. Beavers KM, Beavers DP, Serra MC, Bowden RG, Wilson RL. Low relative skeletal muscle mass indicative of sarcopenia is associated with elevations in serum uric acid levels: findings from NHANES III. J Nutr Health Aging (2009) 13:177–82. doi: 10.1007/s12603-009-0054-5
59. Ferrando B, Olaso-Gonzalez G, Sebastia V, Viosca E, Gomez-Cabrera MC, Viña J. Allopurinol and its role in the treatment of sarcopenia. Rev Esp Geriatr Gerontol (2014) 49:292–8. doi: 10.1016/j.regg.2014.05.001
60. Xu ZR, Zhang Q, Chen LF, Xu KY, Xia JY, Li SM, et al. Characteristics of hyperuricemia in older adults in China and possible associations with sarcopenia. Aging Med (Milton) (2018) 1:23–34. doi: 10.1002/agm2.12004
Keywords: body fat, sarcopenia, body composition, obesity, diabetes, China
Citation: Sun L, Fu J, Mu Z, Duan X, Chan P and Xiu S (2023) Association between body fat and sarcopenia in older adults with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 14:1094075. doi: 10.3389/fendo.2023.1094075
Received: 09 November 2022; Accepted: 17 January 2023;
Published: 27 January 2023.
Edited by:
Fabio Castellana, National Institute of Gastroenterology S. de Bellis Research Hospital (IRCCS), ItalyReviewed by:
Duarte Miguel Henriques-Neto, University of Maia, PortugalSaba Tariq, University of Faisalabad, Pakistan
Hiroyuki Hirai, Fukushima Medical University Hospital, Japan
Copyright © 2023 Sun, Fu, Mu, Duan, Chan and Xiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piu Chan, cGJjaGFuQGhvdG1haWwuY29t; Shuangling Xiu, eGl1c2h1YW5nbGluZ0AxMjYuY29t
 Lina Sun1
Lina Sun1 Junling Fu
Junling Fu Zhijing Mu
Zhijing Mu Xiaoye Duan
Xiaoye Duan Piu Chan
Piu Chan