- 1Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University, College of Medicine, Seoul, Republic of Korea
- 2Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Republic of Korea
- 4Department of Internal Medicine, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Gangwon-do, Republic of Korea
Background: In patients undergoing incident hemodialysis, increased fibroblast growth factor-23 (FGF-23) levels are associated with the development of cardiovascular disease (CVD), but the influence of residual kidney function (RFK) on this association is unclear. This study aimed to investigate the association between FGF-23 levels, RKF, and CVD in patients undergoing prevalent hemodialysis.
Methods: This cross-sectional and longitudinal observational study included 296 patients undergoing maintenance hemodialysis for at least three months who were followed up for a median of 44 months. RKF was defined as 24-h urine output >200 mL, left ventricular (LV) diastolic dysfunction as E/E’ >15 on echocardiographic parameters. CVD was defined as hospitalization or emergency room visits due to cardiovascular causes, such as angina, myocardial infarction, or congestive heart failure.
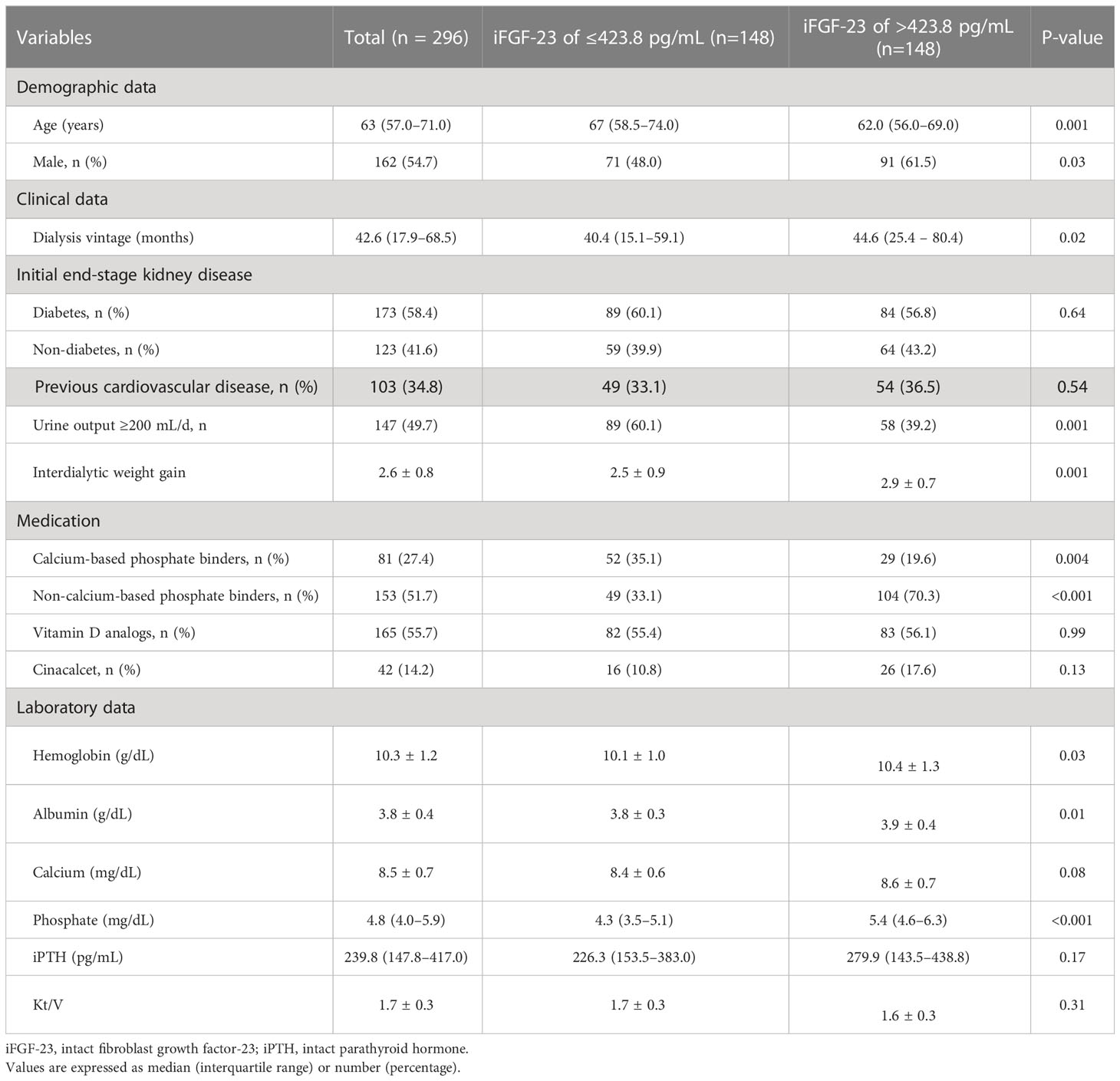
Results: The median intact FGF-23 (iFGF-23) level was 423.8 pg/mL (interquartile range, 171–1,443). Patients with an FGF-23 level > 423.8 pg/mL significantly had a lower proportion of RKF (39.2% vs. 60.1%, P < 0.001) and a higher proportion of LV diastolic dysfunction (54. 1% vs. 29.1%, P < 0.001) than those with an iFGF-23 level ≤ 423.8 pg/mL. The odds ratio (OR) for LV diastolic dysfunction was significantly higher in patients with RFK (OR per one-unit increase in the natural log-transformed iFGF-23 levels, 1.80; 95% confidence interval [CI]: 1.11–2.93) than in patients without RKF (OR per one-unit increase in the natural log-transformed iFGF-23 levels: 1.42; 95% CI: 1.01–1.99) in multivariate analysis (p < 0.001). During the follow-up period, 55 patients experienced CVD. The hazard ratio (HR) for CVD development was also significantly higher in patients with RKF (HR per one-unit increase in the natural log-transformed iFGF-23 levels, 2.64; 95% CI: 1.29–5.40) than those without RKF (HR per one-unit increase in the natural log-transformed iFGF-23 levels: 1.44; 95% CI: 1.04–1.99) in multivariate analysis (p = 0.05).
Conclusions: Increased iFGF-23 levels were associated with LV diastolic dysfunction and CVD development in patients undergoing prevalent hemodialysis; however, the loss of RKF attenuated the magnitude of these associations. Therefore, in these patients, RKF strongly influenced the detrimental role of iFGF-23 in the development of CVD.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with end-stage kidney disease (ESKD) undergoing hemodialysis (1, 2). Traditional risk factors for CVD, such as hypertension, diabetes, and dyslipidemia, do not fully account for the high prevalence of CVD in this population (3). Non-traditional risk factors for CVD, including vascular calcification, elevated asymmetrical dimethylarginine, anemia, volume overload, and others, contribute to the increased cardiovascular risk observed in patients with ESKD (4, 5). Fibroblast growth factor-23 (FGF-23), a hormone secreted by osteoblasts, plays an essential role in the development of CKD and MBD (6). In patients with CKD, serum FGF-23 levels gradually increase with decreasing kidney clearance. Increased FGF-23 levels help maintain a normal serum phosphate concentration range by enhancing urinary phosphate excretion and reducing phosphate absorption through decreased 1,25-dihydroxy vitamin D production (6). End-organ resistance to the phosphaturic action of FGF-23 due to a deficiency of Klotho, the required cofactor, may also increase FGF-23 levels (7). Usually, when these patients reach end-stage kidney disease (ESKD), FGE-23 levels can be up to 1,000-fold above the normal range (8).
Recent studies have found an association between elevated FGF-23 levels and CVD, including hypertension, LV hypertrophy, acute coronary syndrome, stroke, transient ischemic attack, and heart failure (9–13). In line with these findings, some observational studies on patients undergoing incident hemodialysis have shown that high FGF-23 levels are associated with a greater risk of CVD (14, 15). Interestingly, a few large-scale studies on patients undergoing prevalent hemodialysis also showed that high FGF-23 levels were associated with a greater risk of CVD (16, 17). However, the magnitude of this association was not as strong as that observed in patients undergoing incident hemodialysis. When hemodialysis is initiated, most patients with ESKD have substantial residual kidney function (RKF). Although these studies did not investigate RKF, the difference in the magnitude of this association might be related to RKF. Therefore, this study aimed to evaluate the association between increased FGF-23 levels and CVD development in patients undergoing prevalent hemodialysis, focusing on the differences in RKF.
2 Materials and methods
2.1 Ethics statement
The Institutional Review Boards of Kangam Sacred, Kangdong Sacred, and Chuncheon Sacred Heart Hospitals approved this study (Refs. 2018-03-19, 2018-07-01, 2018-96). This study was performed in accordance with the Declaration of Helsinki. All the patients provided written informed consent before enrolment in the study.
2.2 Patients
This study was conducted between January 2018 and January 2022 at three dialysis clinics: Kangam Sacred, Kangdong Sacred, and Chuncheon Sacred Heart Hospitals. Inclusion criterion was patients who had undergone hemodialysis for at least 3 months, three times a week for 4 h. Exclusion criteria were patients who were planning to transfer to other centers and those who did not undergo echocardiography.
2.3 Measurement of intact FGE-23 (iFGF-23)
Blood samples for iFGF-23, the full-length biological molecule, were collected in serum separator tubes, clotted at room temperature, centrifuged, frozen at -70 °C, and shipped on dry ice to Seoul Clinical Laboratories for measurement. The levels of iFGF-23 were measured using a commercial enzyme-linked immunosorbent assay kit (Kainos Laboratories Inc., Tokyo, Japan).
2.4 Data collection
Baseline characteristics, including demographic and clinical information, and biochemical parameters were collected from the medical records at the time of blood collection for iFGF-23 measurement. We conducted echocardiography within 1 week at baseline and calculated the mean interdialytic weight gain (IDWG) as an average of 10 values before baseline.
2.5 Echocardiographic measurements
This study used an ultrasound machine (Vivid 7; GE Vingmed Ultrasound AS, Horten, Norway) with a 2.5 MHz probe to perform comprehensive echocardiographic measurements based on the imaging protocols of the American Society of Echocardiography guidelines. This study calculated the LV ejection fraction and LV mass using modified biplane Simpson’s and Devereus and Reichek’s methods, respectively (18, 19). The LV mass index (LVMI) was calculated as follows: LVMI = LV mass/body surface area. This study assessed mitral inflow from an apical four-chamber view using Doppler echocardiography. Peak mitral in-flow velocities at early (E) and late (A) diastole and deceleration time were measured using mitral inflow profiles. The peak mitral annular velocities at early (E’) and late (A’) diastole were measured using Doppler tissue imaging.
2.6 Outcomes
Based on echocardiographic parameters, LV diastolic dysfunction was defined as E/E’ >15. Additionally, we designated CVD as an event requiring hospitalization or emergency room visits due to cardiovascular causes, such as angina, myocardial infarction, or congestive heart failure.
2.7 Statical analyses
We used descriptive statistics to compare baseline characteristics according to iFGF-23. Normally distributed variables were described as mean ± standard deviation and compared using the t-test for two groups. Nonnormally distributed variables were presented as a median and interquartile range, and compared using Mann–Whitney U test for the two groups. Categorical variables were described as frequencies and percentages, and compared using a chi-square test or Fisher’s exact test. Kaplan–Meier product estimation method was used to calculate the cumulative incidence of CVD according to the median iFGF-23 level. We used logistic regression to examine the association between iFGF-23 and LV diastolic dysfunction, and Cox proportional hazards analysis to investigate the association between iFGF-23 and CVD development. We used multivariate models to adjust for three models (1): demographic and clinical factors: age, sex, dialysis vintage, Kt/V, diabetes, and prior CVD (e.g., coronary artery disease and congestive heart failure); (2) markers of mineral metabolism: serum levels of calcium, phosphorus, and intact parathyroid hormone (iPTH); and (3) active vitamin D treatment: prescription of active or analog vitamin D. We analyzed the results of laboratory data on a continuous scale. Therefore, nonnormally distributed laboratory data (iPTH and iFGF-23 levels) were log-transformed. Hazard ratios (HR) or odds ratios (OR) and 95% confidence intervals (CI), corresponding to a one-unit increase in the natural log-transformed iFGF-23 levels, were provided. We also investigated the potential interactions of iFGF23 with RKF (defined as 24-h urine output >200 mL) for clinical outcomes. The correlation between mean IDWG and iFGF-23 was calculated using Pearson analysis. Statistical analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Study population
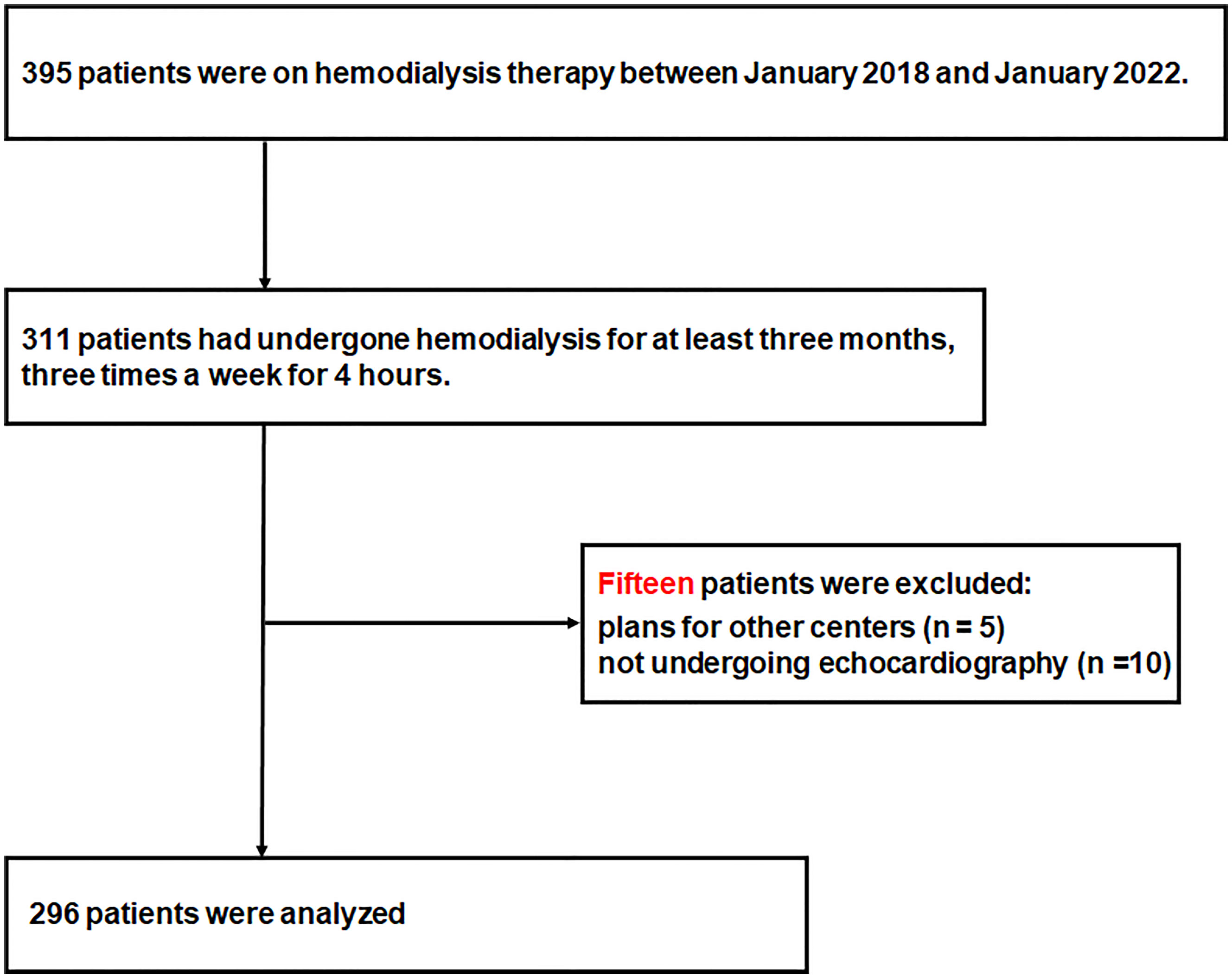
In total, 123, 140, and 132 patients were receiving hemodialysis therapy in Kangnam Sacred, Kangdong Sacred, and Chuncheon Sacred Heart Hospitals, respectively, between January 2018 and January 2019. Of these, 311 patients were eligible for inclusion in the study, and 15 patients were excluded because of plans of transfer to other centers (n=5) or because they did not undergo echocardiography (n=10). Thus, 296 patients were included in the study (Figure 1).
3.2 Baseline characteristics
Diabetes was the cause of ESKD in 173 (58.4%) patients. The median age was 63 years (interquartile range, 57–71 years), and 162 (54.7%) patients were men. The median iFGF-23 level was 423.8 pg/mL (interquartile range, 171.09–1,143.85 pg/mL) (Figure 2). Patients with iFGF-23 levels > 423.8 pg/mL had a longer hemodialysis vintage, lower proportion of RKF, greater mean IDWG, and were prescribed more noncalcium-based phosphate binders than those with iFGF-23 levels ≤423.8 pg/mL. Additionally, phosphate levels were higher in patients with iFGF-23 levels > 423.8 pg/mL (Table 1).
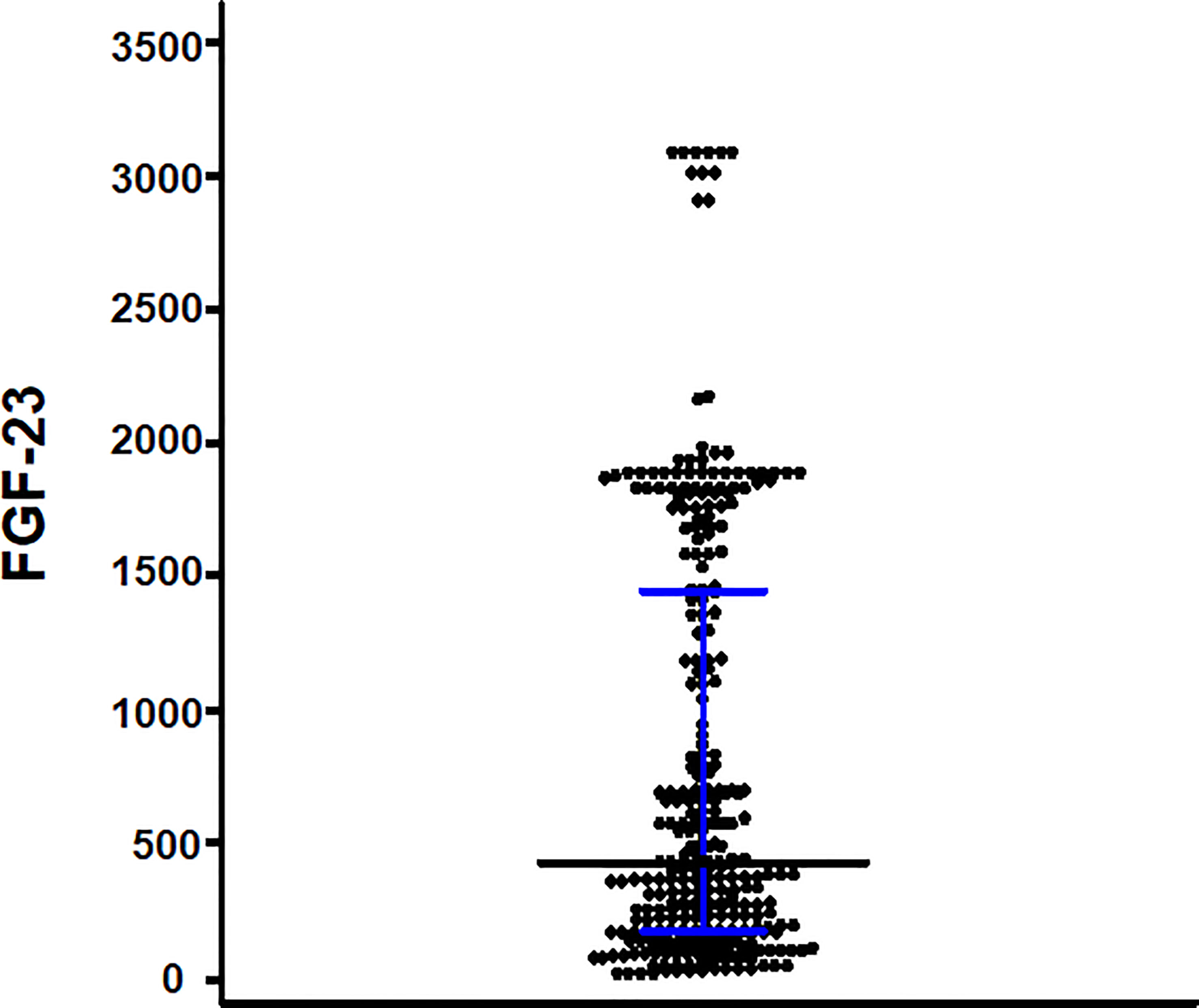
Figure 2 Scattered plots of the intact fibroblast growth factor-23 (iFGF-23) levels. The bar and error bars show the median and interquartile range.
3.3 Intact FGF-23 and LV diastolic dysfunction
Table 2 presents the echocardiographic parameters of the two groups: patients with iFGF-23 levels ≤423.8 pg/mL and those with iFGF-23 levels > 423.8 pg/mL. The proportion of LV diastolic dysfunction was significantly higher in patients with iFGF-23 levels > 423.8 pg/mL than in those with iFGF-23 levels ≤ 423.8 pg/mL (Table 2). In univariate logistic regression analysis, increased iFGF-23 levels were significantly associated with LV diastolic dysfunction (OR per unit increase in the natural log-transformed iFGF-23 levels, 1.60; 95% CI, 1.30–1.95). After adjusting for model 1 covariates, increased iFGF-23 levels were significantly associated with LV diastolic dysfunction. Even after adjusting for models 2 and 3, increased iFGF-23 levels were significantly associated with LV diastolic dysfunction (Table 3).
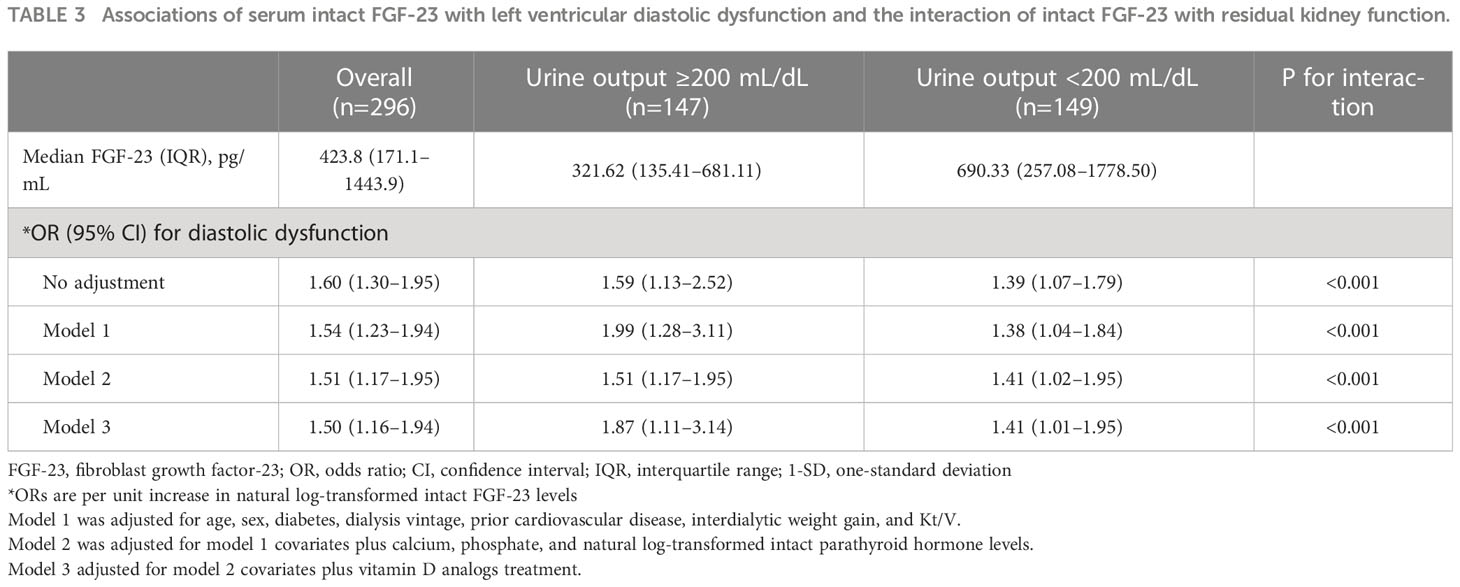
Table 3 Associations of serum intact FGF-23 with left ventricular diastolic dysfunction and the interaction of intact FGF-23 with residual kidney function.
3.4 Intact FGF-23 and CVD
During a median follow-up of 44 months (interquartile range, 41.0–44.6 months), 55 patients experienced CVD, with 17 cases of angina, 15 cases of myocardial infarction, and 23 cases of congestive heart failure. Cardiovascular disease was significantly higher in patients with iFGF-23 levels > 423.8 pg/mL than in those with iFGF-23 levels ≤ 423.8 pg/mL (26.4% vs. 10.8%, P = 0.001) (Figure 2). The cumulative probability of incident CVD was also significantly higher in patients with iFGF-23 levels > 423.8 pg/mL than in those with iFGF-23 levels ≤ 423.8 pg/mL (Figure 3). In the univariate Cox regression analysis, increased iFGF-23 levels were significantly associated with the development of CVD. Additionally, increased iFGF-23 levels were significantly associated with the development of CVD, even when the analysis was adjusted for models 1, 2, and 3 (Table 4).
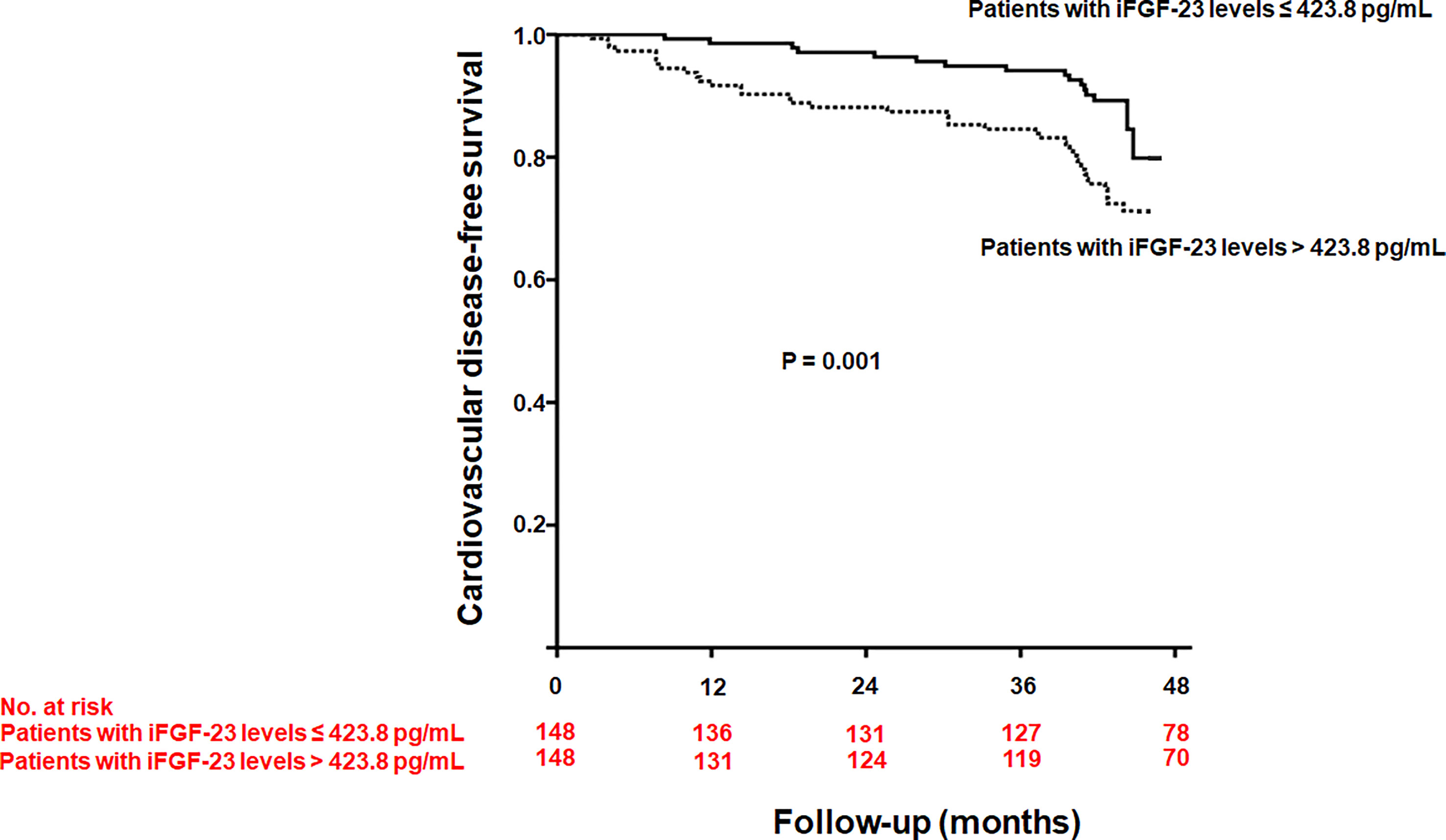
Figure 3 Kaplan-Meier curve of developing cardiovascular disease according to the median intact fibroblast growth factor-23 (iFGF-23) levels (P = 0.001).
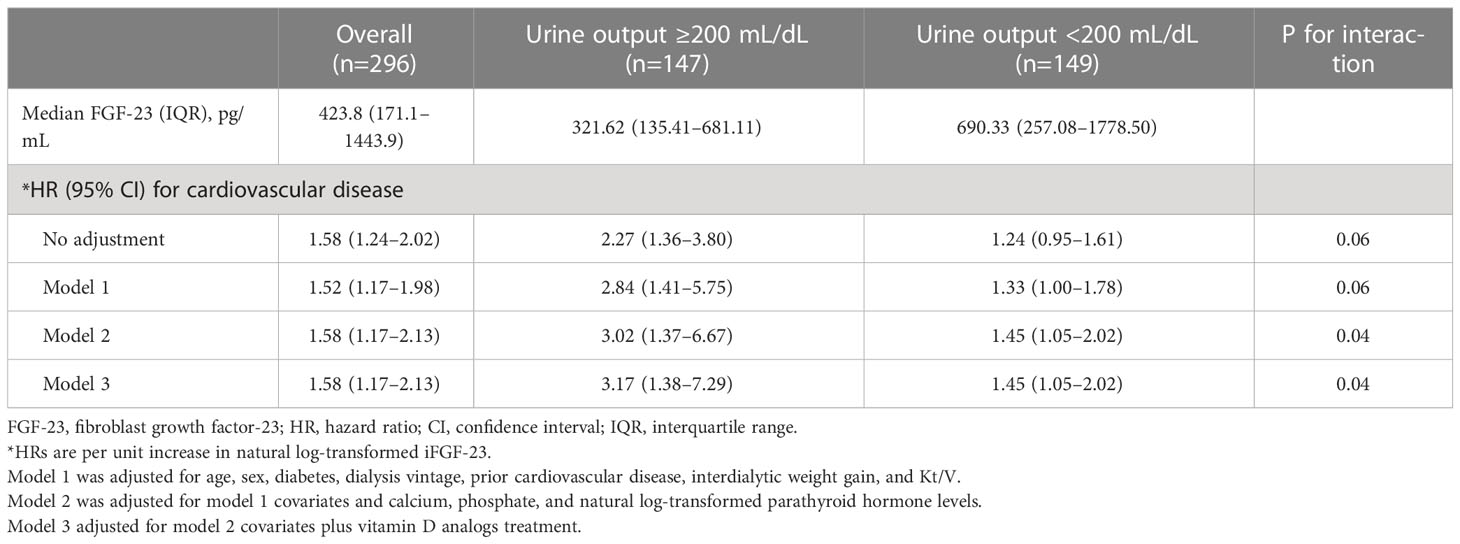
Table 4 Associations of serum intact FGF-23 with cardiovascular diseases and the interaction of intact FGF-23 with residual kidney function.
3.5 Interaction with RKF
Patients with RKF had lower iFGF-23 levels than those without RKF. However, the OR for LV diastolic dysfunction (OR per one-unit increase in the natural log-transformed iFGF-23 levels, 1.59; 95% CI: 1.13–2.52) in patients with RKF was more robust than that in patients without RKF (OR per one-unit increase in the natural log-transformed iFGF-23, 1.39; 95% CI: 1.07–1.79; p < 0.001). The higher magnitude of OR for LV diastolic dysfunction in patients with RKF than in patients without RKF was also maintained significantly by further adjustments for models 1, 2, and 3 (Table 3). Interestingly, the HR for developing CVD was significantly higher in patients with RKF than in patients without RKF when the analysis was adjusted for models 2 and 3.
3.6 Intact FGF-23, IDWG, and RKF
Although the mean IDWG was positively correlated with iFGF-23 levels in patients with RKF (r = 0.24, p = 0.004), there was no correlation between the mean IDWG and iFGF-23 levels in patients without RKF (r = -0.27, p = 0.74). However, in patients without RKF, the high mean IDWG was significantly associated with an increased risk of LV diastolic dysfunction and CVD development in the univariate and multivariate analyses (Supplemental Tables 1, 2).
4 Discussion
This cross-sectional and longitudinal observational study confirmed that increased iFGF-23 levels were significantly associated with LV diastolic dysfunction in patients undergoing prevalent hemodialysis. Additionally, increased iFGF-23 levels are significantly associated with the development of CVD. However, the loss of RKF attenuated the magnitude of these associations, even though the median iFGF-23 levels were higher in patients without RKF than in those with RKF.
Preservation of RKF has long been recognized as a significant beneficial factor in cardiovascular and overall mortality in hemodialysis patients. Even a minimal level of RKF has been associated with better fluid and electrolyte balance, enhanced clearance of middle molecules, lower inflammation levels, and improved quality of life (20, 21). Thus, understanding the role of RKF in mitigating the detrimental effects of FGF-23 on CVD is essential to developing potential therapeutic strategies for this patient population.
Observational cohort studies have shown a strong association between increased FGF-23 levels and the development of CVD in patients undergoing incident hemodialysis. These studies divided patients into three or four groups according to FGF-23 levels and demonstrated HRs of developing CVD for the highest FGF-23 level group versus the lowest FGF-23 level group, which ranged between 3–5 (14, 15). Meanwhile, in cohort studies on patients undergoing prevalent hemodialysis, HRs of developing CVD for the highest FGF-23 level group versus the lowest FGF-23 level group ranged between 1–1.2 (16, 17). Interestingly, a Japanese cohort study showed that long-term dialysis attenuated the association between increased FGF-23 levels and CVD development (22). Therefore, differences in the magnitude of this association might be related to differences in baseline characteristics between patients enrolled in the study, and RKF may be one of the differences. To understand these findings, we first evaluated the association between increased iFGF-23 levels and LV diastolic dysfunction in patients undergoing prevalent hemodialysis, focusing on differences in RFK.
Previous studies have indicated that LV diastolic dysfunction is associated with CVD, and is an independent predictor of CVD development in patients (23–25). Therefore, investigating LV diastolic dysfunction in these patients will help understand the association between increased FGF-23 levels and CVD development. Although several observational clinical studies showing that increased FGF-23 levels were associated with LV diastolic dysfunction cannot prove causality (26–29), in vitro and experimental animal studies have demonstrated that FGF-23 induced LV hypertrophy is related to LV diastolic dysfunction (11). FGF-23 causes pathological hypertrophy of isolated rat cardiomyocytes via FGF receptor-dependent activation of the calcineurin-nuclear factor of activated T cells signaling pathway, but this effect is independent of Klotho (11). These findings suggest that the pathogenesis of FGF-23-induced LV hypertrophy may be ongoing in patients undergoing hemodialysis, thereby promoting LV diastolic dysfunction. However, in this study, the magnitude of the association between increased iFGF-23 levels and LV diastolic dysfunction in patients without RKF was not as strong as that in those with RKF. Although iFGF-23 is associated with LV diastolic dysfunction, age, sex, diabetes, dialysis vintage, prior CVD, calcium, phosphate, and iPTH play significant roles in inducing LV diastolic dysfunction (30–35). Especially mechanical stress, such as volume overload, causes LV hypertrophy as the primary maladaptive response (36). Persistent fluid overload is commonly observed in patients with ESKD without RKF. In our study, the high mean IDWG was significantly associated with the risk of LV diastolic dysfunction in patients without RKF. Therefore, in patients without RKF, IDWG may diminish the effect of increased iFGF-23 levels on LV diastolic dysfunction.
In recent years, it has become clear that FGF-23 directly influences calcium and sodium handling in the distal nephron of the kidney. In the distal tubular epithelium, FGF-23 regulates the apical membrane abundance of epithelial transient receptor potential vanilloid-5 and sodium-chloride cotransporter (9). In our study, iFGF 23 and mean IDWG were positively correlated in patients with RKF but not significantly in patients without RKF. This is probably because the direct effect of increased FGF-23 on the distal nephron of the kidney resulted in sodium retention and blood volume expansion in patients with RKF. Therefore, the absence of RKF would reduce the effect of increased FGF-23 levels on LV diastolic dysfunction. Another possible explanation for how the loss of RKF reduces the effect of elevated FGF-23 levels on left ventricular diastolic dysfunction is that it leads to decreased levels of certain hormones that contribute to the detrimental effects of FGF-23 on the cardiovascular system. For example, a study has suggested that the renin-angiotensin-aldosterone system (RAAS) may play a role in the adverse effects of FGF-23 on the heart and that the attenuation of these effects with the loss of RKF may be due to a decrease in RAAS activity (37). However, it is essential to remember that these are just hypotheses and that other mechanisms may also be involved. The exact mechanism of this attenuating effect has yet to be fully explored and requires further investigation.
Therefore, RKF plays a pivotal role in the detrimental effects of increased FGF-23 levels on the development of CVD. FGF-23 is an approximately 32-kDa glycoprotein with N- and C-terminal regions (38). There are two main types of assays for measuring FGF-23 levels in humans. The iFGF-23 assay binds two epitopes that flank the proteolytic cleavage site, presumably detecting only biologically active, full-length FEG-23 (~32 kDa) (39). In contrast, the C-terminal FGF-23 (cFGF-23) assay binds to epitopes within the C-terminal region of the FGF-23 protein, and detects both full-length and processed C-terminal fragments (~14kDa) (40). Based on the idea that the iFGF-23 assay might be superior because it detects the full-length FGF23 molecule and not a mixture of full-length FGF23 and degradation products (41), we used the iFGF-23 assay in this study. However, most prior studies have reported associations between FGF-23 and clinical outcomes using the cFGF-23 assay (14, 15, 42, 43). Additionally, a few studies have shown that the iFGF-23 assay showed much weaker associations with similar endpoints than the cFGF-23 assay (44, 45). Therefore, in our study, the association between increased FGF-23 levels and clinical outcomes may have been weakened because of the usage of an iFGF-23 assay. Nevertheless, we showed the importance of RKF by showing that the loss of RKF attenuated the magnitude of the associations between increased iFGF-23 and LV diastolic dysfunction and CVD development.
Our study had several limitations. First, this was a small-scale observational study. Second, we did not measure cFGF-23 levels, which might have provided additional information on the regulation of FGF-23. Third, we could not determine whether the association between increased iFGF-23 levels and LV diastolic dysfunction is causal. Lastly, there were numerous potential confounders that could affect the relationship between iFGF-23 levels, RKF, and CVD. Although we attempted to account for several of these factors in our multivariable model, there may be other unmeasured confounding factors that we could not adjust for.
In conclusion, we showed that increased iFGF-23 levels are associated with LV diastolic dysfunction and CVD development in patients undergoing prevalent hemodialysis. The loss of RKF attenuated the magnitude of these associations, highlighting the strong influence of RKF on the detrimental role of iFGF-23 in the development of CVD. Nonetheless, the observed associations between iFGF-23 levels, RKF, and CVD should be interpreted with caution, as there may be other unmeasured confounding factors. Further large-scale studies are needed to understand the complex relationship between these factors fully. If confirmed, FGF–receptor blocker treatment could be considered in patients undergoing hemodialysis with RKF to prevent CVD development.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Kangam Sacred, Kangdong Sacred, and Chuncheon Sacred Heart Hospitals. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DHS conceived and designed the study. YKK, HJJ, YKL, AC., JWY, and HK acquired data. DHS, AC, and YKK analyzed and interpreted the data. DHS and YKK wrote the paper. DHS, YKL, and JWY reviewed the manuscript for important intellectual content and approved the final version. During the revision, THY provided invaluable advice and assistance in addressing the reviewers' comments and concerns.
Acknowledgments
The authors thank the dedicated volunteers who participated in this study, and the research nurse for collecting clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1099975/full#supplementary-material
References
1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med (2004) 351(13):1296–305. doi: 10.1056/NEJMoa041031
2. Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al. Us renal data system 2016 annual data report: epidemiology of kidney disease in the united states. Am J Kidney Dis (2017) 69(3 Suppl 1):A7–a8. doi: 10.1053/j.ajkd.2016.12.004
3. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation (2003) 108(17):2154–69. doi: 10.1161/01.Cir.0000095676.90936.80
4. Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Salvatore Malatino L, et al. Asymmetric dimethylarginine, c-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol JASN (2002) 13(2):490–6. doi: 10.1681/asn.V132490
5. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol dialysis Transplant (2003) 18(9):1731–40. doi: 10.1093/ndt/gfg414
6. Wolf M. Forging forward with 10 burning questions on Fgf23 in kidney disease. J Am Soc Nephrol JASN (2010) 21(9):1427–35. doi: 10.1681/asn.2009121293
7. Seiler S, Heine GH, Fliser D. Clinical relevance of fgf-23 in chronic kidney disease. Kidney Int Supplement (2009) 114):S34–42. doi: 10.1038/ki.2009.405
8. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol JASN (2005) 16(7):2205–15. doi: 10.1681/asn.2005010052
9. Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, et al. Fgf23 regulates renal sodium handling and blood pressure. EMBO Mol Med (2014) 6(6):744–59. doi: 10.1002/emmm.201303716
10. Fyfe-Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, et al. Serum fibroblast growth factor-23 and incident hypertension: the atherosclerosis risk in communities (Aric) study. J hyper (2016) 34(7):1266–72. doi: 10.1097/hjh.0000000000000936
11. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. Fgf23 induces left ventricular hypertrophy. J Clin Invest (2011) 121(11):4393–408. doi: 10.1172/jci46122
12. Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab (2015) 22(6):1020–32. doi: 10.1016/j.cmet.2015.09.002
13. Tuñón J, Fernández-Fernández B, Carda R, Pello AM, Cristóbal C, Tarín N, et al. Circulating fibroblast growth factor-23 plasma levels predict adverse cardiovascular outcomes in patients with diabetes mellitus with coronary artery disease. Diabetes/metab Res Rev (2016) 32(7):685–93. doi: 10.1002/dmrr.2787
14. Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol (2015) 42(1):25–34. doi: 10.1159/000438999
15. Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. New Engl J Med (2008) 359(6):584–92. doi: 10.1056/NEJMoa0706130
16. Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet hcl therapy to lower cardiovascular events (Evolve) trial. Circulation (2015) 132(1):27–39. doi: 10.1161/circulationaha.114.013876
17. Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin d and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the hemo study. J Am Soc Nephrol JASN (2016) 27(1):227–37. doi: 10.1681/asn.2014101009
18. Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. a comparison of cineangiographic and radionuclide techniques. Circulation (1979) 60(4):760–6. doi: 10.1161/01.cir.60.4.760
19. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. anatomic validation of the method. Circulation (1977) 55(4):613–8. doi: 10.1161/01.cir.55.4.613
20. Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol JASN (2016) 27(12):3758–68. doi: 10.1681/asn.2015101142
21. Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int (2016) 90(2):262–71. doi: 10.1016/j.kint.2016.02.037
22. Komaba H, Fuller DS, Taniguchi M, Yamamoto S, Nomura T, Zhao J, et al. Fibroblast growth factor 23 and mortality among prevalent hemodialysis patients in the Japan dialysis outcomes and practice patterns study. Kidney Int Rep (2020) 5(11):1956–64. doi: 10.1016/j.ekir.2020.08.013
23. Han JH, Han JS, Kim EJ, Doh FM, Koo HM, Kim CH, et al. Diastolic dysfunction is an independent predictor of cardiovascular events in incident dialysis patients with preserved systolic function. PloS One (2015) 10(3):e0118694. doi: 10.1371/journal.pone.0118694
24. Yoon HE, Chung S, Whang HC, Shin YR, Hwang HS, Chung HW, et al. Abdominal aortic calcification is associated with diastolic dysfunction, mortality, and nonfatal cardiovascular events in maintenance hemodialysis patients. J Korean Med Sci (2012) 27(8):870–5. doi: 10.3346/jkms.2012.27.8.870
25. Iwabuchi Y, Ogawa T, Inoue T, Otsuka K, Nitta K. Elevated E/E' predicts cardiovascular events in hemodialysis patients with preserved systolic function. Internal Med (Tokyo Japan) (2012) 51(2):155–60. doi: 10.2169/internalmedicine.51.6250
26. Sharma S, Hanudel MR, Ix JH, Salusky IB, Ganz T, Nguyen KL. Elevated fibroblast growth factor 23 levels are associated with greater diastolic dysfunction in esrd. Kidney Int Rep (2019) 4(12):1748–51. doi: 10.1016/j.ekir.2019.07.022
27. Milovanova LY, Shvetsov MY, Milovanova SY, Lysenko Kozlovskaya LV, Kamyshova ES, Taranova MV, et al. Elevated fibroblast growth factor 23 and decreased klotho levels are associated with diastolic dysfunction in ckd G4-5d patients. Kidney Int Rep (2020) 5(7):1118. doi: 10.1016/j.ekir.2020.04.022
28. Negishi K, Kobayashi M, Ochiai I, Yamazaki Y, Hasegawa H, Yamashita T, et al. Association between fibroblast growth factor 23 and left ventricular hypertrophy in maintenance hemodialysis patients. comparison with b-type natriuretic peptide and cardiac troponin T. Circ J (2010) 74(12):2734–40. doi: 10.1253/circj.cj-10-0355
29. Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis (2013) 231(1):114–9. doi: 10.1016/j.atherosclerosis.2013.09.002
30. Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, et al. Left ventricular diastolic dysfunction in normotensive diabetic patients in various age strata. Diabetes Res Clin Pract (2008) 79(1):91–6. doi: 10.1016/j.diabres.2007.08.006
31. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation (2005) 112(15):2254–62. doi: 10.1161/circulationaha.105.541078
32. Chatterjee K. Primary diastolic heart failure. Am J geriatric Cardiol (2002) 11(3):178–87. doi: 10.1111/j.1076-7460.2002.00051.x
33. Utescu MS, Couture V, Mac-Way F, De Serres SA, Marquis K, Larivière R, et al. Determinants of progression of aortic stiffness in hemodialysis patients: a prospective longitudinal study. Hyper (Dallas Tex 1979) (2013) 62(1):154–60. doi: 10.1161/hypertensionaha.113.01200
34. Poudel K, Shah AM, Michos ED, Folsom AR, Konety S, Lutsey PL. Association of serum calcium and phosphorus with measures of left ventricular structure and function: the aric study. Nutri metab Cardiovasc Dis NMCD (2020) 30(5):758–67. doi: 10.1016/j.numecd.2020.01.003
35. Ohara N, Hiramatsu K, Shigematsu S, Hayashi Y, Ishihara F, Aizawa T, et al. Effect of parathyroid hormone on left ventricular diastolic function in patients with primary hyperparathyroidism. Mineral electrol Metab (1995) 21(1-3):63–6.
36. Carabello BA, Zile MR, Tanaka R, Cooper Gt. Left ventricular hypertrophy due to volume overload versus pressure overload. Am J Physiol (1992) 263(4 Pt 2):H1137–44. doi: 10.1152/ajpheart.1992.263.4.H1137
37. Leifheit-Nestler M, Kirchhoff F, Nespor J, Richter B, Soetje B, Klintschar M, et al. Fibroblast growth factor 23 is induced by an activated renin-Angiotensin-Aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dialysis (2018) 33(10):1722–34. doi: 10.1093/ndt/gfy006
38. Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol JASN (2007) 18(6):1637–47. doi: 10.1681/asn.2007010068
39. Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol hyper (2014) 23(4):411–9. doi: 10.1097/01.mnh.0000447020.74593.6f
40. Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. New Engl J Med (2003) 348(17):1656–63. doi: 10.1056/NEJMoa020881
41. Bożentowicz-Wikarek M, Owczarek A, Kocełak P, Olszanecka-Glinianowicz M, Więcek A, Chudek J. C-terminal to intact fibroblast growth factor 23 ratio in relation to estimated glomerular filtration rate in elderly population. Kidney Blood Pressure Res (2016) 41(5):519–26. doi: 10.1159/000443452
42. Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJ, de Borst MH. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol CJASN (2013) 8(11):1968–78. doi: 10.2215/cjn.01880213
43. Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: chs (Cardiovascular health study). J Am Coll Cardiol (2012) 60(3):200–7. doi: 10.1016/j.jacc.2012.03.040
44. Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the multi-ethnic study of atherosclerosis. Circ Heart failure (2014) 7(3):409–17. doi: 10.1161/circheartfailure.113.000952
Keywords: fibroblast growth factor-23, cardiovascular disease, left ventricular diastolic dysfunction, hemodialysis patients, residual kidney function
Citation: Kee YK, Jeon HJ, Oh J, Cho A, Lee Y-K, Yoon J-W, Kim H, Yoo TH and Shin DH (2023) Fibroblast growth factor-23 and cardiovascular disease among prevalent hemodialysis patients focusing on residual kidney function. Front. Endocrinol. 14:1099975. doi: 10.3389/fendo.2023.1099975
Received: 16 November 2022; Accepted: 28 April 2023;
Published: 11 July 2023.
Edited by:
Anil Kumar Pasupulati, University of Hyderabad, IndiaReviewed by:
Seung Hyeok Han, Yonsei University Health System, Republic of KoreaAnupama Janardhana, Nanjappa Hospital, India
Gopalakrishnan Natarajan, Madras Medical College, India
Copyright © 2023 Kee, Jeon, Oh, Cho, Lee, Yoon, Kim, Yoo and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Ho Shin, aXNhYWM5NzEzQGdtYWlsLmNvbQ==
 Youn Kyung Kee
Youn Kyung Kee Hee Jung Jeon
Hee Jung Jeon Jieun Oh
Jieun Oh Ajin Cho
Ajin Cho Young-Ki Lee3
Young-Ki Lee3 Dong Ho Shin
Dong Ho Shin