- 1Unit of Pediatric Endocrinology and Diabetology, Pediatric Service, Woman-Mother-Child Department, Lausanne University Hospital, Lausanne, Switzerland
- 2Obstetric Service, Woman-Mother-Child Department, Lausanne University Hospital, Lausanne, Switzerland
- 3Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland
- 4Institute of Higher Education and Research in Healthcare (IUFRS), University of Lausanne, Lausanne, Switzerland
- 5Neonatology Service, Woman-Mother-Child Department, Lausanne University Hospital, Lausanne, Switzerland
Introduction: Gestational Diabetes Mellitus (GDM) carries an increased risk for adverse perinatal and longer-term cardiometabolic consequences in offspring. This study evaluated the utility of maternal anthropometric, metabolic and fetal (cord blood) parameters to predict offspring anthropometry up to 1 year in pregnancies with GDM.
Materials and methods: In this prospective analysis of the MySweetheart study, we included 193/211 women with GDM that were followed up to 1 year postpartum. Maternal predictors included anthropometric (pre-pregnancy BMI, gestational weight gain (GWG), weight and fat mass at the 1st GDM visit), and metabolic parameters (fasting insulin and glucose, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Quantitative insulin-sensitivity check index (QUICKI), HbA1c, triglycerides, and high-density lipoprotein (HDL) at the 1st visit and HbA1c at the end of pregnancy). Fetal predictors (N=46) comprised cord blood glucose and insulin, C-Peptide, HOMA-IR, triglycerides and HDL. Offspring outcomes were anthropometry at birth (weight/weight z-score, BMI, small and large for gestational age (SGA,LGA)), 6-8 weeks and 1 year (weight z-score, BMI/BMI z-score, and the sum of 4 skinfolds).
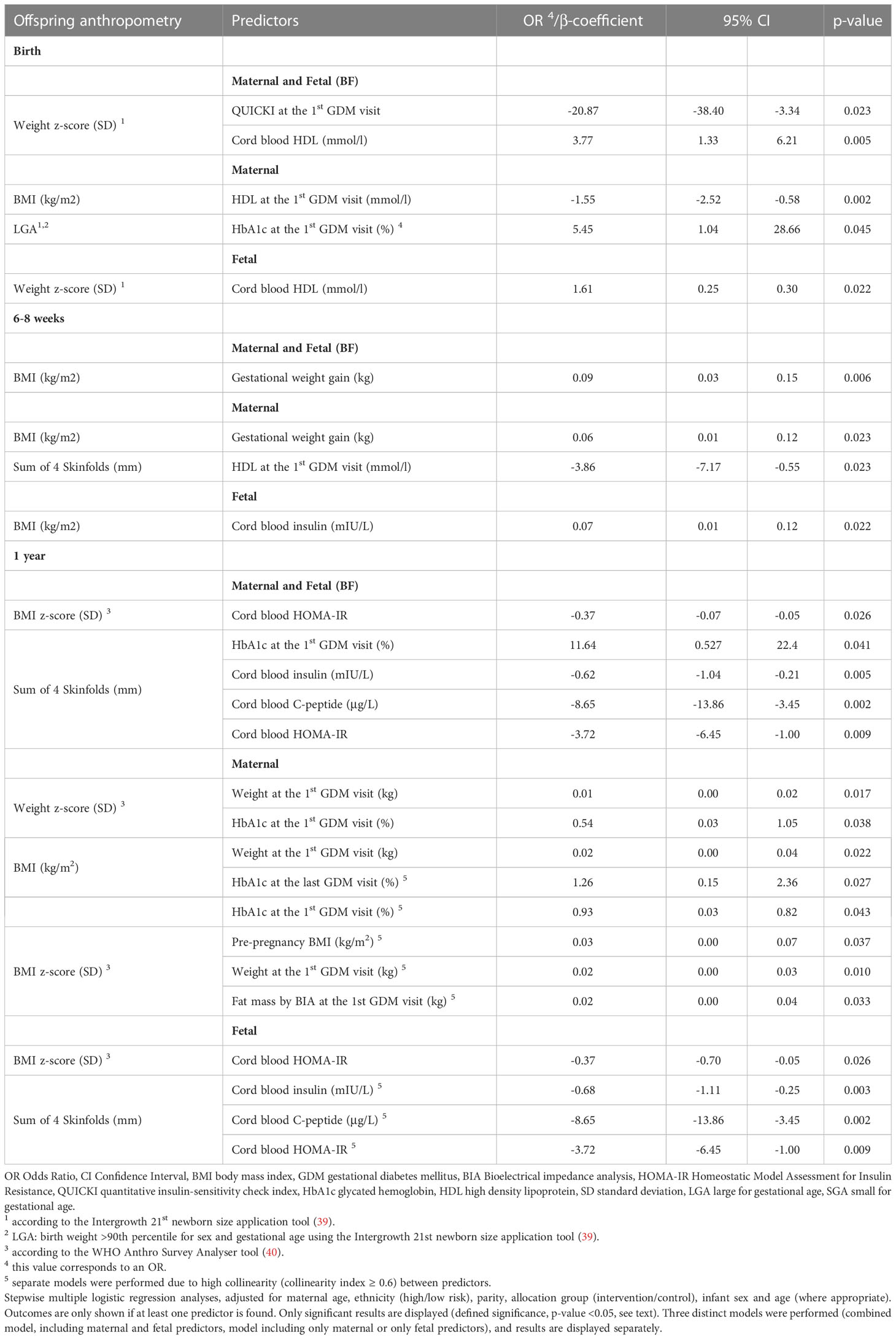
Results: In multivariate analyses, birth anthropometry (weight, weight z-score, BMI and/or LGA), was positively associated with cord blood HDL and HbA1c at the 1st GDM visit, and negatively with maternal QUICKI and HDL at the 1st GDM visit (all p ≤ 0.045). At 6-8 weeks, offspring BMI was positively associated with GWG and cord blood insulin, whereas the sum of skinfolds was negatively associated with HDL at the 1st GDM visit (all p ≤0.023). At 1 year, weight z-score, BMI, BMI z-score, and/or the sum of skinfolds were positively associated with pre-pregnancy BMI, maternal weight, and fat mass at the 1st GDM visit and 3rd trimester HbA1c (all p ≤ 0.043). BMI z-score and/or the sum of skinfolds were negatively associated with cord blood C-peptide, insulin and HOMA-IR (all p ≤0.041).
Discussion: Maternal anthropometric, metabolic, and fetal metabolic parameters independently affected offspring anthropometry during the 1st year of life in an age-dependent manner. These results show the complexity of pathophysiological mechanism for the developing offspring and could represent a base for future personalized follow-up of women with GDM and their offspring.
1 Introduction
Gestational Diabetes Mellitus (GDM) is defined as diabetes first diagnosed during the second or third trimester of pregnancy, not fulfilling the criteria of pre-existing diabetes (1). The prevalence of GDM varies significantly worldwide, ranging from 1% to > 30% and is ~ 11% in Switzerland (2). It has been suggested that the intrauterine environment of GDM may affect fetal programming and future health in offspring of mothers with GDM (3–5). GDM carries an increased risk for adverse perinatal outcomes, such as large for gestational age (LGA) and increased adiposity, birth trauma, respiratory distress syndrome, jaundice, hypoglycemia, and admission to the neonatal intensive care unit (6–8). The impact of GDM on offspring anthropometry is present at birth and in later childhood (4, 8, 9), but data during the 1st year of life in this population are lacking. A higher body mass index (BMI) as well as an increased risk for overweight and obesity during childhood has been found in GDM-exposed offspring in most studies (4, 10, 11). In the HAPO study, GDM was positively associated with childhood overweight or obesity at a mean age of 11.4 years and this was mediated by the maternal BMI during pregnancy (9). These findings are in agreement with the KiGGS study (12). Data on cardiometabolic consequences of GDM in the offspring have been in part inconsistent. They might include elevated blood pressure (13) and a higher risk for dyslipidemia (14). They have a higher risk of impaired glucose metabolism (15, 16) during childhood and adolescence and an increased risk for obesity, insulin resistance, metabolic syndrome, prediabetes, and type 2 diabetes during early adulthood (17, 18).
In women with GDM, maternal anthropometric (pre-pregnancy BMI, GWG) and metabolic parameters (glucose values during oGTT, HbA1c, C-peptide and lipids) during the 2nd and 3rd trimester of pregnancy, have been associated with neonatal anthropometry including birth weight, BMI, macrosomia, large and small for gestational age (LGA, SGA) (19–23). Although data on the impact of cord blood metabolic parameters on neonatal anthropometry and adiposity are available in the general population (24, 25), they are limited in the GDM population. In pregnancies with GDM, cord blood insulin, C-peptide, and glucose values have been associated with weight, BMI, the sum of skinfolds and fat mass at birth, whereas the impact of fetal lipid metabolism remains controversial (26–29). Previous studies have assessed these associations only in older offspring, i.e., during childhood and adolescence (9, 11, 12, 30). The impact of maternal and fetal metabolism on infant anthropometry at different time points during the 1st year of life has not been studied in the GDM population and data in the general population are scarce. This might shed light on different pathophysiological processes regarding developmental aspects of metabolic health.
The aim of this study was to evaluate the utility of maternal anthropometric and metabolic, as well as fetal (cord blood) parameters as predictors of infant anthropometric and adiposity outcomes at different time points during the 1st year of life in pregnancies with GDM.
2 Materials and methods
2.1 Study design and follow-up
This study is a secondary analysis of the MySweetheart trial, a randomized-controlled intervention trial of 211 women with GDM and their offspring (Clinicaltrials.gov NCT02890693) (31). They were followed during pregnancy up to one year postpartum between 2016 and 2021, in the Diabetes and Pregnancy Unit in the Lausanne University Hospital, Switzerland. The intervention consisted of a multidimensional interdisciplinary lifestyle and psychosocial intervention in women with GDM and their offspring compared with an active lifestyle and guidelines-based usual care. The allocation ratio was 1:1 using a block randomization method (blocks of 4) after stratification. Details of the study protocol have been described elsewhere (31).
2.1.1 Participant recruitment and consent
Women ≥18 years diagnosed with GDM between 24 and 32 weeks of gestational age (GA), who understood French or English, and consented to participate were included in MySweetheart trial. Women on strict bed rest, with severe mental disorder and pre-existing diabetes were excluded from the study. Signed informed consent was obtained from all participating women. The study was conducted in accordance with the guidelines of the declaration of Helsinki, and good clinical practice. The Human Research Ethics Committee of the Canton de Vaud approved the study protocol (study number 2016-00745).
2.1.2 Diagnosis of gestational diabetes
GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Group (IADPSG Criteria). GDM was confirmed if fasting blood glucose was ≥5.1 mmol/l and/or 1-h blood glucose was ≥10.0 mmol/l and/or 2-h blood glucose was ≥8.5 mmol/l, following a 75 g oGTT (32). Included women were randomized to the control or intervention group after the baseline visit and signing of an informed consent.
2.1.3 Follow-up
2.1.3.1 Control group
Women randomized to usual care (N=106 women with GDM) received a very active guideline-based treatment-as-usual clinical follow-up based on the American Diabetes Association and on the Endocrine Society guidelines for the management of GDM (33, 34). They had regular appointments every 1-3 weeks with a medical doctor, a diabetes-specialist nurse and/or a dietician after the GDM diagnosis. During the 1st visit, women were counselled on GDM and taught how to perform self-monitoring of blood glucose control 4 times during the day (fasting and 2 hours post-prandial). They were also advised on gestational weight gain (GWG) based on the Institute of Medicine (IOM) 2009 recommendations (35, 36). Patients had one appointment with a dietician for a personalized dietary counselling,and were encouraged to increase physical activity according to the Endocrine Society Guidelines (33). If glucose values remained above targets two or more times during a 1 to 2-week period (fasting glucose >5.3 mmol/l, 1-h postprandial glucose >8 mmol/l and/or 2-h postprandial glucose >7 mmol/l) despite lifestyle changes, insulin treatment (or very rarely metformin) was introduced depending on patient’s glucose values and preference. After delivery, capillary glucose measures and glucose-lowering treatments were stopped and women saw a physician and a dietician at the 6-8 weeks postpartum after an oGTT test to discuss further management and receive lifestyle counselling.
2.1.3.2 Intervention group
Women randomized to the intervention group (N=105 women) received a multidimensional, interdisciplinary lifestyle and psychosocial intervention on top of the usual care. The focus was on eating behavior and a balanced food intake as well as physical activity and breastfeeding. The intervention also included a psychosocial component including the assessment of depression during and after pregnancy. Throughout the period of pregnancy and up to 1 year postpartum, patients were supported by a lifestyle coach (see (31) for more details).
2.1.3.3 Visits
Women were evaluated at different moments during the study, i.e., at the 1st GDM visit at 24-32 weeks (baseline; visit 1), at birth (visit 2), at 6-8 weeks postpartum (visit 3), and at 1 year postpartum (visit 4). Offspring were evaluated at birth, 6-8 weeks and at 1 year. At each visit, several measures were assessed. In the following section, we only mention measures that were analyzed in this present study.
Visit 1-1st GDM visit: At 24-32 weeks of GA information on maternal socio-demographic characteristics were collected and maternal anthropometric parameters and fasting metabolic biomarkers were measured.
Visit 2- Birth: Immediately after childbirth, blood was drawn from the umbilical cord to measure laboratory biomarkers. Offspring anthropometric parameters were obtained from the hospital birth record.
Visit 3- 6-8 Weeks: At 6-8 weeks of life, offspring’s anthropometric measures including weight, length, BMI and skinfold measures were obtained.
Visit 4- 1 year: At this visit, offspring’s anthropometric measures including weight, BMI, skinfold measures were collected.
2.2 Maternal and offspring parameters
2.2.1 Maternal sociodemographic and anthropometric parameters
Maternal socio-demographic parameters, including age, ethnicity, and parity were collected during a structured face-to-face interview at the 1st GDM clinical visit. Ethnicity was classified in Low (Europe, North America) and High Risk (Asia, Central and South America, Africa, Oceania) ethnic groups (37). Pre-pregnancy BMI was calculated based on self-reported pre-pregnancy weight or retrieved from medical charts and measured height on the 1st visit at the GDM clinic1. Weight was measured at the 1st GDM visit to the nearest 0.1 kg in women wearing light clothes and no shoes with an electronic Seca ® scale. Height was measured at the 1st GDM visit to the nearest 0.1 cm with a regularly calibrated Seca ® height scale. GWG was determined as the difference between the weight at the end of pregnancy and pre-pregnancy weight. At the 1st GDM visit, Bioelectrical Impedance Analysis (BIA) was performed (Akern BIA 101) to estimate fat free mass (FFM) using the Kyle equation (38), and fat mass was calculated using the formula: Fat Mass = Weight – FFM. Maternal medical treatment for GDM was classified in 2 categories (no treatment, treatment with insulin and/or very rarely metformin).
2.2.2 Offspring anthropometric parameters
Birth growth parameters such as weight (g) and length (cm) were documented at birth; percentiles and z-scores for each of the above-mentioned parameters were calculated using the Intergrowth 21st newborn size application tool (39) and BMI was calculated. LGA was defined as birth weight >90th percentile and SGA as birth weight <10th percentile for sex and gestational age. Gestational age was calculated according to the date of the last menstruation, or as assessed by the fetal ultrasound in the cases where gestational age was corrected during the early in-utero ultrasound evaluation. Neonatal data were obtained from patient medical chart for all newborns born in the Lausanne University Hospital. In the cases where delivery took place in another hospital or clinic, anthropometric parameters at birth were provided by the respective hospital.
At the 6-8 weeks and 1 year visits, offspring weight (kg) and length (cm) were measured. Weight was measured to the closest 0.1 kg, with a calibrated scale (Seca ® model 336). Babies were weighed without any clothes or in nappy. If the weight was measured with the nappy, the respective weight of the nappy was subtracted. Length was measured to the closest 0.1 cm with the same scale (Seca ® model 336) and BMI was calculated. Z-scores for weight, length and BMI were calculated using the using the WHO Anthro Survey Analyser tool -Offline version (40).
Skinfold thickness was measured to the nearest 0.1mm at 4 anatomical sites (biceps, triceps, subscapular, and iliac) using a Harpenden Skinfold Caliper. Skinfolds were measured three times at each anatomical site and mean value of each skinfold measure was used to calculate the sum of the 4 skinfolds.
2.2.3 Maternal and fetal (cord blood) metabolic parameters
At the 1st GDM visit, maternal metabolic health parameters, including fasting glucose, insulin, HbA1c, high-density lipoprotein (HDL), and triglycerides were measured. Maternal HbA1c also were measured at the end of pregnancy (last visit before delivery). At birth, glucose, insulin, C-peptide, high-density lipoprotein (HDL), and triglycerides were measured in the cord blood. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the formula (Fasting insulin in mIU/L x Fasting glucose in mmol/l)/22.5 (41). The Quantitative insulin-sensitivity check index (QUICKI) was calculated using the formula QUICKI = 1/[log(Fasting insulin in mIU/L) + log(Fasting glucose in mg/dl)] (42).
2.2.4 Laboratory methods
Plasma glucose was measured using a Hexokinase/Glucose-6-Phosphat-Dehydrogenase (HK/G6P-DH) assay. Insulin and C-peptide were measured with an electrochemiluminescence Immunoassay. HbA1c was measured using a chemical photometric method (conjugation with boronate; Afinion®). HDL cholesterol and triglycerides were measured with an enzymatic colorimetric method (CHOD-PAP and GPO-PAP respectively).
2.2.5 Predictors and outcomes
Maternal anthropometric parameters comprised of pre-pregnancy BMI, 1st GDM visit weight and fat mass (BIA) and GWG. Maternal metabolic parameters included fasting glucose, insulin, HOMA-IR, QUICKI, HDL, triglycerides as well as HbA1c at the 1st and last GDM visit. Cord blood metabolic parameters included glucose, insulin, C-peptide, HOMA-IR, HDL, and triglycerides. Outcomes comprised offspring anthropometric parameters at birth, 6-8 weeks and 1 year. More precisely, birth outcomes included weight z-score, BMI, LGA, and SGA, and outcomes at 6-8 weeks and 1 year, weight z-score, BMI, BMI z-score, and the sum of 4 skinfolds.
2.3 Statistical analysis
All data were analysed using Stata/SE 16.0 (StataCorp LLC, TX, USA). The normality of continuous variables was assessed using histograms and Q-Q plots. Outcomes variables were normally distributed. For consistency all continuous variables were described as mean and standard deviation. Binary outcomes were described as N (percentages) (Table 1). Comparisons between the intervention and control group and between the two ethnicity group categories (low/high-risk) were done using the unpaired t-test for normally distributed continuous variables, the Mann-Whitney test for continuous variables with non-normal distribution and the Fisher’s exact test for binary variables. In all analyses, predictors and outcomes did not differ in the groups (intervention vs control group, low vs high ethnicity group category) and the effect sizes were similar. Therefore, women from intervention and control groups and of low- and high-risk ethnicity were pooled together and adjusted for group allocation in all analyses. All analyses were also adjusted for infant age and sex, where appropriate.
We initially performed univariate linear and logistic regression analyses with infant anthropometric parameters as the dependent variables (Supplementary Tables 1–3).
Maternal and fetal (cord blood) predictors with a p-value < 0.05 in univariate analysis were included in stepwise multiple regression analyses models. We performed three different multivariate models, a first one including both maternal and fetal predictors, and a second and third one including only maternal or only fetal predictors, respectively. Fetal predictors were available for N = 46 participants. These analyses were adjusted for group allocation, infant age and sex where appropriate, as well as maternal ethnicity group category, parity and maternal age. These analyses were performed in order to identify the most significant maternal and fetal predictors of infant anthropometric parameters at birth, 6-8 weeks and 1 year (Table 2). We tested for collinearity for all predictors and separate models were performed for predictors with a collinearity index ≥ 0.6. More specifically, as the collinearity index was ≥ 0.6 between HbA1c at the 1st and last GDM visit, between pre-pregnancy BMI, weight and fat mass by BIA at the 1st GDM visit, between insulin, HOMA-IR and QUICKI, as well as between cord blood insulin, C-peptide and HOMA-IR, separate multivariate models were performed for these predictors if more than one of them were significantly related to the respective outcome variable in univariate analyses (see Supplementary Tables 1–3). For all analyses, β-coefficients (for continuous outcomes) and adjusted odds ratios (aORs-for binary outcomes) are reported along with their 95% confidence intervals (CIs), and statistical significance was set at 0.05.
3 Results
The initial population included 211 women with GDM participating in the randomized-controlled intervention, and their offspring. One woman was excluded because the diagnosis of GDM was done before 13 weeks of gestation, and 17 were excluded due to multiple gestation (N=4), and/or because their offspring were born premature (< 37 weeks of gestational age, N=16). Thus, 193 women (93 intervention group/100 control group) were included in the final analysis.
3.1 Maternal, fetal and infant characteristics
Detailed information on the maternal characteristics, cord blood metabolic parameters, and offspring anthropometric outcomes at birth, 6-8 weeks, and 1 year are shown in Table 1. Briefly, mean maternal age was 33.6 ± 4.8 years, pre-pregnancy BMI was 25.9 ± 5.1 kg/m2 and GWG 12.6 ± 6.5 kg. GA at birth was 39.7 ± 1.1 weeks, mean weight z-score at birth was +0.18 ± 1.1 standard deviations (SD) and 11.8% of newborns were LGA. At 6-8 weeks and 1 year, mean offspring BMI z-score was -0.2 ± 1.3 and +0.23 ± 1.1 SD respectively.
3.2 Associations between maternal and fetal predictors and offspring anthropometry at birth, 6-8 weeks, and 1 year in univariate analyses
Different maternal metabolic and fetal predictors were associated with offspring outcomes at birth, while maternal anthropometric and metabolic as well as fetal predictors were associated with offspring outcomes at 6-8 weeks and 1 year in univariate analyses (Supplementary Tables 1–3).
3.3 Associations between maternal and fetal predictors and offspring anthropometry at birth, 6-8 weeks and 1 year in multivariate analyses
The significant results of all multivariate univariate analyses are shown in Table 2.
3.3.1 Birth
In the models including only maternal predictors, HDL at the 1st GDM visit was negatively associated with offspring BMI, and HbA1c at the 1st GDM visit was positively associated with LGA (p ≤0.045). In the models including only fetal predictors, cord blood HDL was positively associated with the weight z-score (p ≤0.022). In the combined maternal and fetal model, maternal QUICKI at the 1st GDM visit was negatively associated and cord blood HDL was positively associated with the weight z-score (p ≤0.023).
3.3.2 6-8 weeks
In the models including only maternal predictors, HDL at the 1st GDM visit was negatively associated with the sum of skinfolds, whereas GWG showed a positive association with BMI (both p =0.023). In the model including only fetal predictors, cord blood insulin presented a positive association with offspring BMI (p =0.022). Lastly, in the combined model, GWG was positively associated with BMI (p =0.006).
3.3.3 1 year
In the models including only maternal predictors, pre-pregnancy BMI, as well as weight and fat mass at the 1st GDM visit showed a positive association with the offspring BMI z-score (all p ≤0.037). Weight at the 1st GDM visit and HbA1c at the 1st and last GDM visit presented a positive association with BMI (both p ≤0.043). Moreover, weight and HbA1c at the 1st GDM visit were positively correlated the weight z-score (both p ≤0.038). In models including only fetal predictors, cord blood HOMA-IR, C-peptide and insulin showed a negative association with the sum of 4 skinfolds (all p ≤0.009). Cord blood HOMA-IR presented a negative association with the BMI z-score (p =0.026). In the combined maternal and fetal models, HbA1c at the 1st GDM visit showed a positive, whereas cord blood insulin, C-peptide and HOMA-IR a negative association with the sum of 4 skinfolds (all p ≤0.041). Finally, cord blood HOMA-IR was negatively associated with the BMI z-score (p =0.026).
4 Discussion
This prospective, observational study of women with GDM and their offspring found that maternal anthropometric, metabolic and fetal metabolic parameters distinctively predicted offspring anthropometry during the 1st year of life. Maternal metabolic parameters during the 3rd trimester including QUICKI and HDL were negatively associated with offspring anthropometry at birth;HDL was negatively associated with offspring anthropometry at 6-8 weeks,whereas HbA1c was positively associated with offspring anthropometry at 1 year. Maternal anthropometric parameters, such as pre-pregnancy BMI, weight and fat mass during the 3rd trimester, and GWG predicted higher offspring anthropometry, but only after birth. Cord blood HDL correlated positively with weight z-score at birth, while cord blood insulin, C-peptide and HOMA-IR were associated with anthropometry during the 1st year of life in an age dependent pattern, showing positive associations at birth and 6-8 weeks and negative associations at 1 year.
4.1 Impact of maternal and fetal metabolism on birth anthropometry
Maternal HDL and QUICKI at the 1st GDM visit, both observed in situations of increased insulin sensitivity, were negatively associated with weight z-score and BMI respectively. In contrast, no associations were found between maternal HDL levels and birth anthropometry in another population with GDM (19). However, they found that maternal triglyceride levels were positively associated with adjusted birth weight centiles and LGA in their insulin-treated subpopulation; similar results have been documented in studies in the general pregnant population (19, 22, 23). In insulin-resistant states, when triglycerides are elevated, HDL is often decreased and may be a more stable marker than triglycerides (43). An adverse maternal lipid profile programs offspring regarding obesity in human and animal studies at and beyond birth, by multiple mechanisms including the offspring’s eating behavior and energy expenditure, adipocyte development, genetics, epigenetics and shared post-natal environment and the inverse could be true for markers of favorable lipid profiles such as higher HDL (44, 45). HbA1c reflects overall maternal and thus subsequent fetal glucose exposure and was positively associated with LGA. This is in agreement with previous data in women with GDM (19), but has not been found in all studies (20, 21). As increased adiposity at birth represents a risk factor for obesity and the metabolic syndrome later in life (46), higher HbA1c levels, but also lower HDL levels in pregnancy may be used for risk stratification in these pregnancies with the aim to reduce the metabolic risk of the offspring (21). In contrast to previous studies in GDM populations, maternal anthropometric parameters had no impact on birth anthropometry (19–21). This may be explained by the smaller variability of maternal anthropometric parameters in our cohort and the fact that the impact of maternal anthropometry may be reduced by the strict monitoring, which is represented by the low % of LGA in our sample.
In terms of fetal metabolism, cord blood HDL showed a positive association with birth weight z-score, in agreement with a study in the general population (25), but on contrast to other studies (19, 23). Interestingly, cord blood HDL influenced birth anthropometry independently from maternal parameters in our study, highlighting the importance of fetal lipid metabolism for fetal growth, that might be distinct from, albeit also dependent of, the impact of maternal parameters. The findings of two previous studies in GDM showing inverse correlations between cord blood triglyceride and birth anthropometry further underline our results (19, 28). Large molecules such as HDL cannot cross the placenta directly, but they could affect the metabolism of other lipids, depending on the concentration and presence (47, 48). As proposed by Ye et al. (48), this potential interaction between lipid particles in the fetus could be critical for the fetal and neonatal metabolism, and may have a lasting impact on the metabolic health of the offspring. Cord blood markers including insulin, C-peptide and HOMA-IR were related to birth anthropometry in univariate, but not in multivariate analyses. Studies in healthy pregnancies, with mild untreated hyperglycemia (HAPO) or GDM have found a positive association between cord blood insulin and/or C-peptide and birth anthropometry, but they did not adjust for cord blood HDL (19, 24, 25, 27, 28). In agreement with previous studies, cord blood glucose was not related to birth anthropometry (26, 28).
4.2 Impact of maternal and fetal metabolism on infant anthropometry at 6-8 weeks of life
GWG was positively associated with infant BMI and maternal HDL negatively associated with the sum of skinfolds at 6-8 weeks in multivariate analyses (maternal and combined model). Maternal metabolic parameters related to insulin resistance were only correlated to higher anthropometric parameters in univariate analyses. Similarly, another study also found no relationship between maternal 3rd trimester C-peptide levels and the offspring’s adipose tissue at 6 weeks and 4 months of age in adjusted models (49). Lastly, our study found a positive association between cord blood insulin and infant BMI.
4.3 Impact of maternal and fetal metabolism on infant anthropometry at 1 year
Regarding maternal metabolic parameters, HbA1c at the 1st GDM visit was positively associated with weight z-score, BMI, and the sum of skinfolds and HbA1c at the last GDM visit was positively associated with BMI at 1 year. Similarly, the HAPO-FUS study found a positive association between 3rd trimester HbA1c and offspring anthropometry, including BMI, body fat and the presence of overweight/obesity later in childhood, at 10-14 years (16).
Maternal anthropometric parameters, including pre-pregnancy BMI, 1st GDM visit weight and fat mass were also positively associated with weight z-score, BMI and BMI z-score at 1 year. This is in accordance with data in the general population where higher pre-pregnancy BMI was associated with higher offspring BMI and adiposity at 4-7 years (16, 44, 50). These findings may be explained by fetal programming and/or lifestyle and genetic characteristics (50, 51).
In terms of fetal metabolism, cord blood insulin, C-Peptide, and HOMA-IR were inversely associated with infant BMI z-score and the sum of skinfolds at 1 year, in agreement with a study in the general population which found a negative association between cord blood insulin and the sum of 4 skinfolds and % body fat at 3 years of age, but not in older children (52). Similarly, another study showed an inverse association between cord blood C-peptide and weight at 1 year in girls (53). In contrast, other studies in the general population have found a positive or no correlation between cord blood insulin and infant anthropometry or adiposity at 1-2 years (54, 55). Lastly, cord blood C-peptide was positively associated with offspring adiposity parameters at a mean age of 11.4 years in the HAPO Study and FUS (56).
The switch in the effect of cord blood insulin, C-peptide and HOMA-IR on offspring anthropometry during the 1st years of life (positive association at birth and 1st months of life, negative association at 1 year and the positive association in later childhood and adolescence) is intriguing and needs more research. A hypothesis for our findings is that at birth and the 1st months of life cord blood insulin, C-peptide and HOMA-IR may be markers of metabolic (glucose) overload in these babies and thus the impact of the maternal metabolism and fuel overload is significant. This may lead to fat accretion and body fat accumulation (mostly maternally-driven and not a clear marker of initial fetal or infant insulin resistance). However, as infants of mothers with GDM are insulin resistant and get even more insulin-resistant with increasing fat accretion, the insulin resistance at the adipose tissue level, could then prevent further fat accumulation in the subcutaneous adipose tissue, and might foster fat deposition in ectopic tissues (57). Thereby, fat cell lipolysis may also play a role (57). Further investigation is necessary to unravel these pathophysiological mechanisms. In later childhood, mechanisms related to excess energy intake and insulin resistance could again become more dominant for fat accretion.
4.4 Strengths and limitations
This is one of the rare studies assessing the impact of maternal and fetal parameters on infant anthropometric and adiposity parameters at different time points during the 1st year of life in pregnancies with GDM. Its prospective nature allowed us to include detailed information on various maternal and fetal parameters and to assess complex associations between maternal and fetal metabolism and growth during the 1st year of life. However, some limitations can also be noted. Cord blood parameters were available for a small proportion of our population (46 patients), which may have under or overestimated some of our correlations. Moreover, due to the small sample size, particularly regarding cord blood parameters, we were not able to perform separate analyses for the intervention and control group or according to low and high-risk ethnicity. However, maternal and fetal predictors and infant anthropometric parameters were not different between groups. Moreover, we did not assess the correlation between fetal anthropometry and birth and infancy anthropometry as the majority of fetal ultrasounds were not performed at our center. Lastly, collinearity was present between multiple maternal and fetal predictors. Therefore, in multivariate analyses, multiple testing models were necessary, which may have under- or overestimated some of our results.
5 Conclusions
Maternal anthropometric, metabolic and fetal metabolic parameters distinctively influenced offspring anthropometry during the 1st year of life and this in an age-dependent manner. Distinct age-dependent associations were particularly present for cord blood metabolic parameters. These observations show the complexity of the pathophysiological mechanism for the developing offspring and need further investigation. In the future, these predictors could be used for a more personalized follow-up of women with GDM and their offspring, to reduce the risks associated with an unfavorable in-utero environment and to foster a favorable fetal programming.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of the Canton de Vaud (study number 2016-00745). The study was conducted in accordance with the guidelines of the declaration of Helsinki, and good clinical practice. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
JP and AH conceived the study, designed the trial, obtained grant funding, and oversaw management of the trial. LG and DQ helped in designing parts of the study and participated in the implementation of the study. M-CA performed the data analysis, and interpretation and wrote the draft manuscript, under the supervision of JP. AL and BS participated in data analysis. DQ, LG, AH and JP revised the manuscript for important intellectual content and gave final approval for the version to be published. All authors contributed to the article and approved the submitted version.
Funding
This study is funded by a project grant from the Swiss National Science Foundation (SNF 32003B_176119) and by an unrestricted educational grant from Novo Nordisk, the Gottfried und Julia Bangerter-Rhyner-Stiftung Foundation, and the Dreyfus Foundation. The funding bodies did not take part in the design of the study, the collection, analysis, interpretation of data or in the writing of the manuscript.
Acknowledgments
We are very grateful to our study participants and their children and partners for their time and participation. We thank Deborah Degen, Dominique Stulz and Isabelle Cohen-Salmon who helped with data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1144195/full#supplementary-material
Abbreviations
AGA, Appropriate for Gestational Age; BIA, Bioelectrical impedance analysis; BMI, body mass index; CI, confidence interval; DS, standard deviation, GA, Gestational age; GDM, gestational diabetes mellitus; GWG, gestational weight gain; HAPO FUS, Hyperglycemia and Adverse Pregnancy Outcome Follow-Up Study; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; IADPSG, International Association of Diabetes and Pregnancy Study Group; IDL, intermediate density lipoprotein; IOM, institute of medicine; LDL, low density lipoprotein; LGA, Large for gestational age; N, Number; oGTT, oral glucose tolerance test; QUICKI, quantitative insulin-sensitivity check index; OR, odds ratio; SGA, Small for gestational age.
References
1. Association AD. Gestational diabetes mellitus. Diabetes Care (2003) 26(suppl_1):s103–s5. doi: 10.2337/diacare.26.2007.S103
2. Ryser Rüetschi J, Jornayvaz FR, Rivest R, Huhn EA, Irion O, Boulvain M. Fasting glycaemia to simplify screening for gestational diabetes. BJOG (2016) 123(13):2219–22. doi: 10.1111/1471-0528.13857
3. Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care (2007) 30(Supplement_2):S169–S74. doi: 10.2337/dc07-s211
4. Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen ACB, et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: A clinical study within the Danish national birth cohort. Diabetes Care (2017) 40(12):1746–55. doi: 10.2337/dc17-0514
5. Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta (2016) 48:S54–60. doi: 10.1016/j.placenta.2015.11.015
6. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers (2019) 5(1):47. doi: 10.1038/s41572-019-0098-8
7. Wiechers C, Balles LS, Kirchhof S, Weber R, Avellina V, Pauluschke-Fröhlich J, et al. Body composition in term offspring after maternal gestational diabetes does not predict postnatal hypoglycemia. BMC Pediatr (2021) 21(1):111. doi: 10.1186/s12887-021-02578-3
8. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
9. Lowe JR. WL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA (2018) 320(10):1005–16. doi: 10.1001/jama.2018.11628
10. Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of pima Indian women with diabetes during pregnancy. N Engl J Med (1983) 308(5):242–5. doi: 10.1056/NEJM198302033080502
11. Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabetes Med (2013) 30(12):1449–56. doi: 10.1111/dme.12286
12. Beyerlein A, Nehring I, Rosario AS, von Kries R. Gestational diabetes and cardiovascular risk factors in the offspring: results from a cross-sectional study. Diabetes Med (2012) 29(3):378–84. doi: 10.1111/j.1464-5491.2011.03454.x
13. Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, et al. The diabetic pregnancy and offspring blood pressure in childhood: A systematic review and meta-analysis. Diabetologia (2012) 55(11):3114–27. doi: 10.1007/s00125-012-2689-8
14. Tam WH, Ma RC, Yang X, Ko GT, Tong PC, Cockram CS, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics (2008) 122(6):1229–34. doi: 10.1542/peds.2008-0158
15. Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care (2017) 40(5):679–86. doi: 10.2337/dc16-2397
16. Lowe WL Jr., Lowe LP, Kuang A, Catalano PM, Nodzenski M, Talbot O, et al. Maternal glucose levels during pregnancy and childhood adiposity in the hyperglycemia and adverse pregnancy outcome follow-up study. Diabetologia (2019) 62(4):598–610. doi: 10.1007/s00125-018-4809-6
17. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab (2009) 94(7):2464–70. doi: 10.1210/jc.2009-0305
18. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes (2000) 49(12):2208–11. doi: 10.2337/diabetes.49.12.2208
19. Barrett HL, Gatford KL, Houda CM, De Blasio MJ, McIntyre HD, Callaway LK, et al. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: Responses to maternal metformin versus insulin treatment. Diabetes Care (2013) 36(3):529–36. doi: 10.2337/dc12-1097
20. Barnes RA, Edghill N, Mackenzie J, Holters G, Ross GP, Jalaludin BB, et al. Predictors of large and small for gestational age birthweight in offspring of women with gestational diabetes mellitus. Diabetes Med (2013) 30(9):1040–6. doi: 10.1111/dme.12207
21. Antoniou MC, Gilbert L, Gross J, Rossel JB, Fischer Fumeaux CJ, Vial Y, et al. Potentially modifiable predictors of adverse neonatal and maternal outcomes in pregnancies with gestational diabetes mellitus: Can they help for future risk stratification and risk-adapted patient care? BMC Preg Childbirth (2019) 19(1):469. doi: 10.1186/s12884-019-2610-2
22. Whyte K, Kelly H, O’Dwyer V, Gibbs M, O’Higgins A, Turner MJ. Offspring birth weight and maternal fasting lipids in women screened for gestational diabetes mellitus (GDM). Eur J Obstet Gynecol Reprod Biol (2013) 170(1):67–70. doi: 10.1016/j.ejogrb.2013.04.015
23. Geraghty AA, Alberdi G, O’Sullivan EJ, O’Brien EC, Crosbie B, Twomey PJ, et al. Maternal blood lipid profile during pregnancy and associations with child adiposity: Findings from the ROLO study. PloS One (2016) 11(8):e0161206. doi: 10.1371/journal.pone.0161206
24. Carlsen EM, Renault KM, Jensen RB, Nørgaard K, Jensen JE, Nilas L, et al. The association between newborn regional body composition and cord blood concentrations of c-peptide and insulin-like growth factor I. PloS One (2015) 10(7):e0121350. doi: 10.1371/journal.pone.0121350
25. Wang J, Shen S, Price MJ, Lu J, Sumilo D, Kuang Y, et al. Glucose, insulin, and lipids in cord blood of neonates and their association with birthweight: Differential metabolic risk of Large for gestational age and small for gestational age babies. J Pediatr (2020) 220:64–72.e2. doi: 10.1016/j.jpeds.2020.01.013
26. Hou R-L, Jin W-Y, Chen X-Y, Jin Y, Wang X-M, Shao J, et al. Cord blood c-peptide, insulin, HbA1c, and lipids levels in small- and large-for-gestational-age newborns. Med Sci monitor Int Med J Exp Clin Res (2014) 20:2097–105. doi: 10.12659/MSM.890929
27. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes (2009) 58(2):453–9. doi: 10.2337/db08-1112
28. Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care (2008) 31(9):1858–63. doi: 10.2337/dc08-0039
29. Couch SC, Philipson EH, Bendel RB, Wijendran V, Lammi-Keefe CJ. Maternal and cord plasma lipid and lipoprotein concentrations in women with and without gestational diabetes mellitus. Predict birth weight? J Reprod Med (1998) 43(9):816–22.
30. Tam WH, Ma RC, Yang X, Li AM, Ko GT, Kong AP, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: A 15-year follow-up study. Diabetes Care (2010) 33(6):1382–4. doi: 10.2337/dc09-2343
31. Horsch A, Gilbert L, Lanzi S, Gross J, Kayser B, Vial Y, et al. Improving cardiometabolic and mental health in women with gestational diabetes mellitus and their offspring: Study protocol for MySweetHeart trial, a randomised controlled trial. BMJ Open (2018) 8(2):e020462. doi: 10.1136/bmjopen-2017-020462
32. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33(3):676–82. doi: 10.2337/dc09-1848
33. Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2013) 98(11):4227–49. doi: 10.1210/jc.2013-2465
34. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 15. management of diabetes in pregnancy: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S232–s43. doi: 10.2337/dc22-S015
35. Sox HC, Greenfield S. Comparative effectiveness research: a report from the institute of medicine. Ann Intern Med (2009) 151(3):203–5. doi: 10.7326/0003-4819-151-3-200908040-00125
36. Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. (2009) 21(6):521–6. doi: 10.1097/GCO.0b013e328332d24e
37. 13. management of diabetes in pregnancy: Standards of medical care in diabetes–2018. Diabetes Care (2018) 41(Supplement 1):S137–S43. doi: 10.2337/dc18-S013
38. Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition (2001) 17(7-8):534–41. doi: 10.1016/S0899-9007(01)00555-X
39. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the INTERGROWTH-21st project. Lancet (2014) 384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6
40. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. (2006) 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x
41. Haffner SM, Gonzalez C, Miettinen H, Kennedy E, Stern MP. A prospective analysis of the HOMA model: The Mexico city diabetes study. Diabetes Care (1996) 19(10):1138–41. doi: 10.2337/diacare.19.10.1138
42. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab (2000) 85(7):2402–10. doi: 10.1210/jcem.85.7.6661
43. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
44. Gademan MGJ, Vermeulen M, Oostvogels AJJM, Roseboom TJ, Visscher TLS, van Eijsden M, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring's body composition at age 5–6 years: The ABCD study. PloS One (2014) 9(4):e94594. doi: 10.1371/journal.pone.0094594
45. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol (2007) 92(2):287–98. doi: 10.1113/expphysiol.2005.032854
46. Hong YH, Lee JE. Large For gestational age and obesity-related comorbidities. J Obes Metab Syndr (2021) 30(2):124–31. doi: 10.7570/jomes20130
47. McConihay JA, Honkomp AM, Granholm NA, Woollett LA. Maternal high density lipoproteins affect fetal mass and extra-embryonic fetal tissue sterol metabolism in the mouse. J Lipid Res (2000) 41(3):424–32. doi: 10.1016/S0022-2275(20)34481-3
48. Ye Q-Q, Kong S-M, Yin X, Gao C, Lu M-S, Ramakrishnan R, et al. Associations of cord blood lipids with childhood adiposity at the age of three years: A prospective birth cohort study. Metabolites (2022) 12(6):522. doi: 10.3390/metabo12060522
49. Uebel K, Pusch K, Gedrich K, Schneider K-TM, Hauner H, Bader BL. Effect of maternal obesity with and without gestational diabetes on offspring subcutaneous and preperitoneal adipose tissue development from birth up to year-1. BMC Preg Childbirth (2014) 14(1):138. doi: 10.1186/1471-2393-14-138
50. Daraki V, Georgiou V, Papavasiliou S, Chalkiadaki G, Karahaliou M, Koinaki S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: The rhea pregnancy cohort Crete, Greece. PloS One (2015) 10(5):e0126327. doi: 10.1371/journal.pone.0126327
51. Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond) (2015) 39(4):633–41. doi: 10.1038/ijo.2015.13
52. Meyer DM, Brei C, Stecher L, Brunner S, Hauner H. Maternal insulin resistance, triglycerides and cord blood insulin are not determinants of offspring growth and adiposity up to 5 years: A follow-up study. Diabetes Med (2018) 35(10):1399–403. doi: 10.1111/dme.13765
53. Regnault N, Botton J, Heude B, Forhan A, Hankard R, Foliguet B, et al. Higher cord c-peptide concentrations are associated with slower growth rate in the 1st year of life in girls but not in boys. Diabetes (2011) 60(8):2152–9. doi: 10.2337/db10-1189
54. Zhang DL, Du Q, Djemli A, Julien P, Fraser WD, Luo ZC. Cord blood insulin, IGF-I, IGF-II, leptin, adiponectin and ghrelin, and their associations with insulin sensitivity, β-cell function and adiposity in infancy. Diabetes Med (2018) 35(10):1412–9. doi: 10.1111/dme.13671
55. Brunner S, Schmid D, Hüttinger K, Much D, Heimberg E, Sedlmeier E-M, et al. Maternal insulin resistance, triglycerides and cord blood insulin in relation to post-natal weight trajectories and body composition in the offspring up to 2 years. Diabetes Med (2013) 30(12):1500–7. doi: 10.1111/dme.12298
56. Josefson JL, Scholtens DM, Kuang A, Catalano PM, Lowe LP, Dyer AR, et al. Newborn adiposity and cord blood c-peptide as mediators of the maternal metabolic environment and childhood adiposity. Diabetes Care (2021) 44(5):1194–202. doi: 10.2337/dc20-2398
Keywords: gestational diabetes, cord blood, offspring anthropometry, fetal metabolism, maternal metabolism
Citation: Antoniou M-C, Quansah DY, Mühlberg S, Gilbert L, Arhab A, Schenk S, Lacroix A, Stuijfzand B, Horsch A and Puder JJ (2023) Maternal and fetal predictors of anthropometry in the first year of life in offspring of women with GDM. Front. Endocrinol. 14:1144195. doi: 10.3389/fendo.2023.1144195
Received: 13 January 2023; Accepted: 13 March 2023;
Published: 28 March 2023.
Edited by:
Giulio Frontino, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Andrew Whatmore, The University of Manchester, United KingdomJayonta Bhattacharjee, Bangladesh Agricultural University, Bangladesh
Copyright © 2023 Antoniou, Quansah, Mühlberg, Gilbert, Arhab, Schenk, Lacroix, Stuijfzand, Horsch and Puder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jardena Jacqueline Puder, amFyZGVuYS5wdWRlckBjaHV2LmNo
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Maria-Christina Antoniou
Maria-Christina Antoniou Dan Yedu Quansah
Dan Yedu Quansah Suzanne Mühlberg
Suzanne Mühlberg Leah Gilbert
Leah Gilbert Amar Arhab
Amar Arhab Sybille Schenk2
Sybille Schenk2 Antje Horsch
Antje Horsch Jardena Jacqueline Puder
Jardena Jacqueline Puder