- 1Embryo Implantation Laboratory, Department of Obstetrics and Gynecology, The University of Melbourne, Parkville, VIC, Australia
- 2Gynecology Research Centre, The Royal Women’s Hospital, Parkville, VIC, Australia
- 3Centre for Reproductive Health, Hudson Institute of Medical Research, Clayton, VIC, Australia
Introduction: A healthy pregnancy requires successful blastocyst implantation into an adequately prepared or ‘receptive’ endometrium. Decidualization of uterine endometrial stromal fibroblast cells (hESF) is critical for the establishment of a healthy pregnancy. microRNAs (miRs) are critical regulators of cellular function that can be released by a donor cell to influence the physiological state of recipient cells. We aimed to determine how decidualization affects hESF miR release and investigated the function of one decidualization regulated miR, miR-19b-3p, previously shown to be associated with recurrent pregnancy loss.
Method: miR release by hESF was determined by miR microarray on culture media from hESF decidualized in vitro for 3 and 14 days by treatment with oestradiol and medroxyprogesterone acetate. Cellular and whole endometrial/decidual tissue miR expression was quantified by qPCR and localized by in situ hybridization. The function of miR-19b-3p in HTR8/Svneo trophoblast cells was investigated using real time cell analysis (xCELLigence) and gene expression qPCR.
Results: From our miR screen we found that essentially all hESF miR release was reduced following in vitro decidualization, significantly so for miR-17-5p, miR-21-3p, miR-34c-3p, miR-106b-5p, miR-138-5p, miR-296-5p, miR-323a-3p, miR-342-3p, miR-491-5p, miR-503-5p and miR-542-5p. qPCR demonstrated that miR-19b-3p, 181a-2-3p and miR-409-5p likewise showed a significant reduction in culture media following decidualization but no change was found in cellular miR expression following decidualization. In situ hybridization localized miR-19b-3p to epithelial and stromal cells in the endometrium and qPCR identified that miR-19b-3p was significantly elevated in the cycling endometrium of patients with a history of early pregnancy loss compared to normally fertile controls. Functionally, overexpression of miR-19b-3p significantly reduced HTR8/Svneo trophoblast proliferation and increased HOXA9 expression.
Discussion: Our data demonstrates that decidualization represses miR release by hESFs and overexpression of miR-19b-3p was found in endometrial tissue from patients with a history of early pregnancy loss. miR-19b-3p impaired HTR8/Svneo proliferation implying a role in trophoblast function. Overall we speculate that miR release by hESF may regulate other cell types within the decidua and that appropriate release of miRs by decidualized hESF is essential for healthy implantation and placentation.
1 Introduction
A healthy pregnancy requires successful blastocyst implantation into an adequately prepared or ‘receptive’ endometrium. Decidualization of human uterine human uterine endometrial stromal fibroblast (hESF) is critical for the establishment of a healthy pregnancy (1, 2); impaired decidualization is associated with poor pregnancy outcomes including recurrent early pregnancy loss and preeclampsia (3–5). Decidualization is initiated post-ovulation by corpus luteum-secreted progesterone and involves the reprogramming of hESF, including significant phenotypic and functional changes: hESF become rounded, highly secretory and with altered extellular matrix expression (1). In women, decidualization begins each menstrual cycle regardless of the presence of a functional blastocyst (1). Decidual cells interact with the implanting blastocyst to facilitate implantation and placentation: they regulate extravillous trophoblast (EVT) proliferation, migration and invasion (6–8), shield the conceptus from environmental stress signals (1), regulate the recruitment and differentiation of the uterine-resident immune cell population (9–11) and are thought to ‘sense’ the quality of the conceptus, facilitating rejection of incompetent embryos (12, 13).
microRNAs (miRs) are critical regulators of cellular function and have been most intensively investigated in cancer, where they regulate metastasis, angiogenesis and inflammation (14, 15). miRs can also act as ‘hormones’ – donor cells (which release the miR) can influence the physiological state of recipient cells (cells which take up the miR) over short (cell to neighboring cell) and long (effects on a different organ) distances (15, 16). In pregnancy, miRs are produced by cells within the decidua (decidual cells, leucocytes and endothelial cells) (17) and placental villous trophoblast (18). miR expression is altered in the decidua of early pregnancy loss compared to healthy pregnancies (18).
Less is known about miRs during endometrial remodelling. In vitro, decidual cellular miRs regulate decidualization (19), however little known about how secreted endometrial miRs may regulate other cells within the decidua including trophoblast. We aimed to determine how decidualization affected hESF miR release and determine the expression and function of one hESF released miR, miR-19b-3p, previously associated with recurrent early pregnancy loss (20).
2 Methods
2.1 Primary tissue collection
This study followed the NHMRC guidelines for ethical conduct in human research. Ethics approvals for this study were provided by The Royal Women’s Hospital and Monash Health Human Research and Ethics Committees (#90317B, #06014C and #03066B). Written and informed consent was obtained from each participant.
Endometrial biopsies were collected by dilatation and curettage (n=26 women; Table 1). Five biopsies were used for decidualization experiments (1 with history of early pregnancy loss), 3 for in situ (2 with history of early pregnancy loss) and 18 for RNA extraction (12 fertile, 6 with a history of early pregnancy loss). The women had no hormonal treatment for ≥ 3 months before tissue collection.
First trimester products of conception were collected following elective termination of pregnancy by evacuation for psychosocial reasons (n=4; amenorrhea 6-11 weeks). Term placental villous and decidual tissue was donated by healthy women following spontaneous labor at term (>37 weeks; n=4).
Serum was collected from women aged >18 years (n=5/group) attending an IVF clinic, who had successful pregnancies following IVF and those who had repeated pregnancy loss following IVF. Serum was collected from women undergoing oocyte collection, two days after induction of ovulation by human chorionic gonadotrophin. Subsequent details of outcomes of embryo transfer in the same cycle were recorded.
2.2 Cell culture
All cells were cultured at 37°C in a 5% CO2 humidified culture incubator. hESF were maintained in DMEM/F12 (Gibco, Thermo Fisher Scientific, Inc.) plus 10% charcoal stripped Fetal Bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) and 1% antibiotics (penicillin, streptomycin, amphoceterin B; Gibco, Thermo Fisher Scientific, Inc.). HTR8/SVneo cells (CRL-3271) were from the ATCC and cultured with RPMI (Gibco, Thermo Fisher Scientific, Inc.) plus 10% heat inactivated FBS (Gibco, Thermo Fisher Scientific, Inc.).
2.3 Decidualization
hESF were isolated using collagenase digestion and filtration as previously described (21), resulting in a 97% stromal fibroblast population (22). hESF were decidualized as previously described (21) by treatment for 14 days with oestradiol (E, 10-8M; Sigma) and medroxyprogesterone acetate (MPA, 10-7M; Sigma) in DMEM/F12 containing 2% charcoal stripped FBS and 1% antibiotics. The media was refreshed every 2-3 days, on a Monday, Wednesday and Friday. Cells and culture media were collected on Day 3 and Day 14, both after 72h of culture. Cells were pelleted by centrifugation at 500xg then snap-frozen. Culture media was centrifuged at 500xg for 5 minutes to pellet cell debris then the supernatant snap-frozen.
2.4 Prolactin ELISA
PRL secretion by decidualized hESF (culture media collected on days 3 and 14) was quantified by ELISA as per the manufacturer’s instructions (DuoSet kit #DY682, R&D systems) (23).
2.5 RNA isolation
Decidual culture media: RNA was isolated from 200uL culture media collected on Day 3 and Day 14 of culture and media only control using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions.
hESF & HTR8/Svneo cells, endometrial and decidual tissue: RNA extraction was performed as previously described using Tri Reagent according to the manufacturer's instructions (Sigma-Aldrich, Merck).
Serum: RNA extraction (from 250uL serum) was performed using the TRIzol LS reagent (Ambion, Life Technologies) as per the manufacturer’s instructions.
Genomic DNA was removed from isolated RNA using the DNAfree kit (Ambion; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. A spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific, Inc.), was used at an absorbance ratio of 260/280 nm to analyze RNA sample concentration, yield and purity.
2.6 microRNA array
cDNA synthesis was performed using the miRCURY LNA™ Universal RT microRNA PCR system (Qiagen) and microRNA PCR Human Panel (I) as previously described (24). cDNA products diluted 60-fold were plated on the microRNA PCR Human Panel (I) plate and qPCR was performed using a 7900HT thermocycler (Applied Biosystems) using the recommended parameters (Qiagen). Raw CT values were normalized (ΔCT) to the average of the control wells (UniSP3) on the plate, then ΔΔCT calculated by normalizing the ΔCT to the average of the day 3 samples for each gene (Supplementary Table 1). A media only control was run to enable exclusion of miRs present in the treatment media.
2.7 miR RT-qPCR
cDNA was synthesized from 10ng total RNA using the TaqMan reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc), and specific TaqMan miR primer sets (cat no. #4427975; miR-19b-3p #000396; miR-181a-2-3p #002317; miR-409-5p #002331; rnU6, #001973; Applied Biosystems; Thermo Fisher Scientific, Inc.) on the Veriti 7 fast block real-time qPCR system (Applied Biosystems). miR qPCR was performed in triplicate (final reaction volume, 10 μl) in 384-well micro- optical plates (Applied Biosystems; Thermo Fisher Scientific, Inc.) on the ABI 7900HT fast block or Viia 7 qPCR systems (Applied Biosystems; Thermo Fisher Scientific, Inc.). A template-free negative control and RNase-free water only was added for each run. The qPCR conditions were: 95°C for 10 min and 40 cycles of 95°C for 15s followed by 60°C for 1 min. Relative expression levels were calculated as per the manufacturer’s instructions using the comparative cycle threshold method (ΔΔCT).
2.8 mRNA RT-qPCR
Total RNA (250ng) was reverse transcribed using Superscript III (Invitrogen) (0.5 µL per reaction) as previously described (25). qPCR was performed as previously described (25) using Power SYBR Green master mix (Applied Biosystems) on the Veriti 7 fast block real-time qPCR system (Applied Biosystems). Primer sequences are as follows: 18s Fwd: 5`GATCCATTGGAGGGCAAGTCT3`, Rev: 5`CCAAGATCCACCTACGAGCTT3`; Fwd: HOXA9 5`TACGTGGACTCGTTCCTGCT3`, Rev: 5`CGTCGCCTTGGACTGGAAG3`; PTEN Fwd: 5`TCCATCCTGCAGAAGAAGCC3`, Rev: 5`AGGATATTGTGCAACTCTGCAA3`; (Sigma-Aldrich). A template-free negative control in the presence of primers and RNase-free water only negative controls were added for each run. The qPCR conditions were: 95°C for 10 min and 40 cycles of 95°C for 15s followed by 60°C for 1 min. Relative expression levels (normalized to 18s ribosomal RNA) were calculated as per the manufacturer’s instructions using the comparative cycle threshold method (ΔΔCT).
2.9 In situ hybridization
In situ hybridization was performed as previously described (26). Briefly, 4 μm thickness endometrial sections were deparaffinized and rehydrated in xylene, neat ethanol, 96% ethanol, and 70% ethanol and then placed in PBS Proteinase K (15 μg/mL) digestion was performed at 37°C for 15 min. Following PBS wash, 100 nM miR-19b-3p detection probe (#339111, YD00619863-BCG; Qiagen) or scramble control probe (cat no. #339111 YD00699004-BCG) was applied to sections and placed in a 60°C incubator for 1 h. Slides were then washed in 5x sodium-saline citrate (SSC), 1x SSC and 0.2x SSC buffers at 60°C for 5 min, and 0.2x SSC at room temperature (RT) for 5 min, then placed in PBS. Blocking solution of 10% CAS block (008120, Thermo), 2% sheep serum, 1% bovine serum albumin (BSA) in PBS-Tween (T) was applied to sections and incubated at RT for 15 min. After incubation, sections were treated with anti-DIG-fluorescein 1:50 in 0.5% BSA/PBS at RT for 1 h. Following additional washes in PBS-T, sections were counterstained with DAPI to indicate the cell nuclei (blue). Sections were visualized using Olympus BX63 fluorescence microscope and cellSense software. All images were taken under the same exposure and settings.
2.10 Real time cell analysis
The real-time cell analyser (RTCA) MP xCELLigence instrument (ACEA Biosciences; Agilent Technologies GmbH) was used to interrogate the effect of miR-19b-3p on HTR8/Svneo adhesion and proliferation. HTR8/Svneo were transfected with 100nM miR-19b-3p mimic (cat no. 339173 YM00470545-ADB) or negative control (cat no. 339173 YM00479902-ADB) using Lipofectamine RNAiMAX (13778100, Thermo Fisher) and Opti-MEM medium (11524456, Fisher) following manufacturer’s instructions for 72 h. After transfection cells were seeded into E-plate 96 (ACEA Biosciences; Agilent Technologies GmbH) at ~10,000 cells/well in RPMI supplemented with 5% FCS. Data was collected ever 15 minutes for a total of 96h.
2.11 Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.5.0. Paired t-tests, one-way ANOVA and repeated measures ANOVA were performed. All data is presented as mean ± SEM. P<0.05 was considered statistically significant.
3 Results
3.1 hESF miR release is repressed by decidualization
We identified 98 miRs released by hESF into the culture media (Figure 1A; Supplementary Table 1). The most highly expressed miRs were miR-125b-5p, -23a-3p and let-7b-5p. miR release into the culture media was highly repressed following in vitro hESF decidualization (Figure 1A): 11 miRs showed a significant reduction at Day 14 of in vitro decidualization (Figure 1B). Decidualization was confirmed by PRL secretion (Figure 1C).
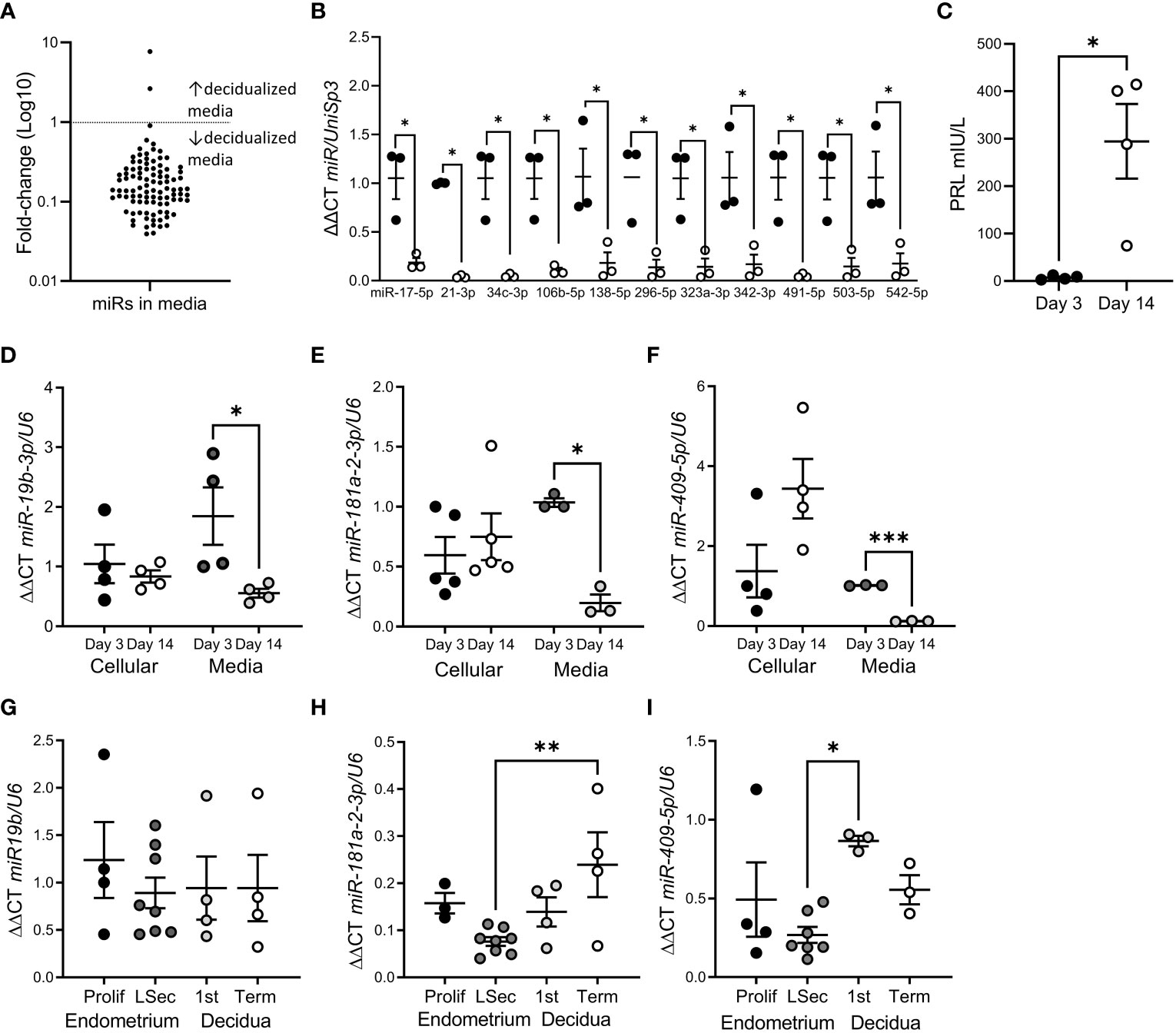
Figure 1 miR release was reduced following hESF decidualization. (A) Fold-change of all miRs identified in hESF culture media by microarray from Day 3 to Day 14. (B) miRs with significantly reduced levels in hESF culture media between Day 3 and Day 14. (C). Prolactin (PRL) secretion by hESF on Day 3 and Day 14 of decidualization. (D–F). qPCR of miR-19b-3p (D), miR-181a-2-3p (E) and miR-409-5p (F) in hESF cells and culture media on Day 3 and Day 14 of decidualization. (G–I). qPCR of miR-19b-3p (G), miR-181a-2-3p (H) and miR-409-5p (I) in whole tissue biopsies collected during the proliferative (prolif) and late secretory (LSec) stages of the menstrual cycle and 1st trimester and term decidua. Data shows mean ± SEM; *P<0.05; **P<0.01; ***P<0.001; (C–F), paired t-test; (G–I), one-way ANOVA.
To confirm the array data, we investigated the expression of 3 different miRs in hESF matched cellular and culture media RNA (Figures 1D–F). Interestingly, although the cellular levels of miRs-19b-3p, -181a-2-3p and -409-5p were not altered by decidualization, miR concentration in culture media was significantly reduced (Figures 1D–F). In contrast when we investigated whether decidualization altered miR-19b-3p, -181a-2-3p or -409-5p expression in whole endometrial tissue biopsies (non-decidualized:proliferative endometrium; decidualized: late secretory endometrium, 1st trimester or term decidua) (Figures 1G–I) we found no difference in expression between non-decidualized and decidualized tissue, although miR-181a-2-3p was significantly elevated in term decidua compared to late secretory endometrium (Figure 1H) and miR-409-5p was significantly elevated in 1st trimester decidua compared to late secretory endometrium (Figure 1I).
3.2 Endometrial miR-19b-3p is increased in women with a history of early pregnancy loss
In situ hybridization of cycling endometrial tissue biopsies localized miR-19b-3p to most cell types in the endometrium, although endometrial glandular epithelial cell expression was variable even within adjacent glands (Figure 2A). Using qPCR, we found that expression miR-19b-3p in endometrial tissue biopsies was significantly increased in patients with a history of early pregnancy loss compared to fertile controls (Figure 2B). This increase was not found in serum from women undergoing IVF with a history of repeated early pregnancy loss (Figure 2C).
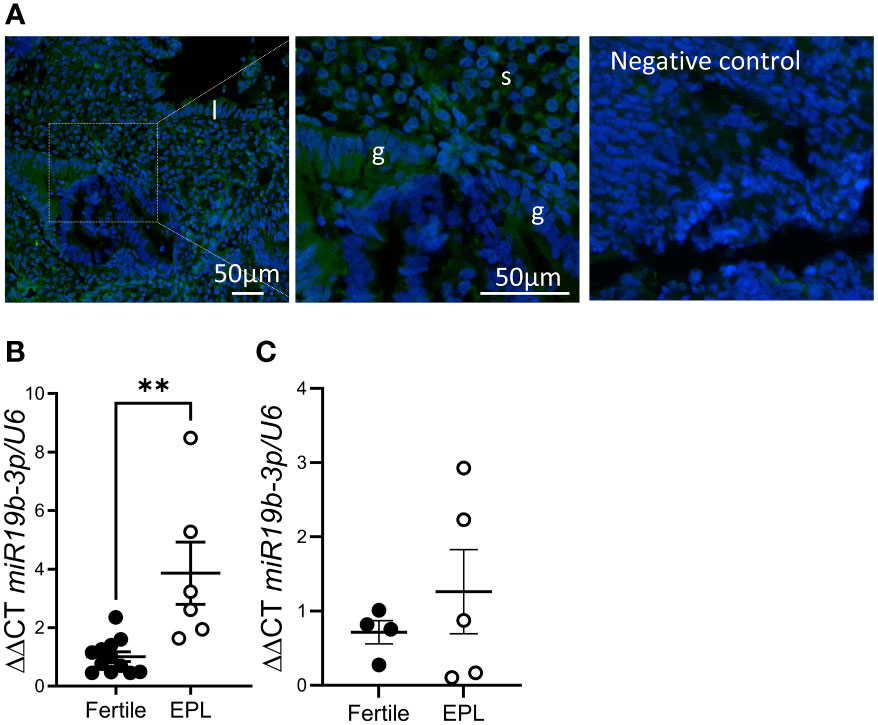
Figure 2 miR-19b-3p expression is elevated in endometrium from women with a history of early pregnancy loss. (A) In situ hybridization of miR-19b-3p in endometrium. Localization of miR-19b-3p indicated by green fluorescent staining. DAPI (blue) counterstaining identifies nuclei. (B) qPCR of miR-19b-3p in endometrium from fertile patients and patients with a history of early pregnancy loss (EPL). (C) qPCR of miR-19b-3p in serum from fertile patients and patients with a history of early pregnancy loss. g, glandular epithelium; l, luminal epithelium; s, stroma; Data shows mean ± SEM; **P<0.01; (B, C), paired t-test.
3.3 miR-19b-3p reduces HTR8/Svneo trophoblast proliferation
As impaired decidualization is associated with recurrent pregnancy loss and miR-19b-3p release was suppressed by decidualization, we investigated the effect of miR-19b-3p on trophoblast function using the HTR8/Svneo cell line. HTR8/Svneo transfected with miR-19b-3p mimic showed elevated miR-19b-3p expression in the cell pellet (Figure 3A), suggesting that miR-19b-3p is taken up from the media. Using a Real-Time Cell Analysis system (xCELLigence) we found there was no effect of miR-19b-3p on HTR8/Svneo adhesion (Figure 3B) but after 60h miR-19b-3p significantly inhibited HTR8/Svneo proliferation compared to control (Figure 3C). We investigated whether transfection with the miR-19b-3p affected HTR8/Svneo production of predicted miR-19b-3p targets (27, 28): miR-19b-3p increased HOXA9 mRNA but had no effect on PTEN mRNA (Figure 3D).
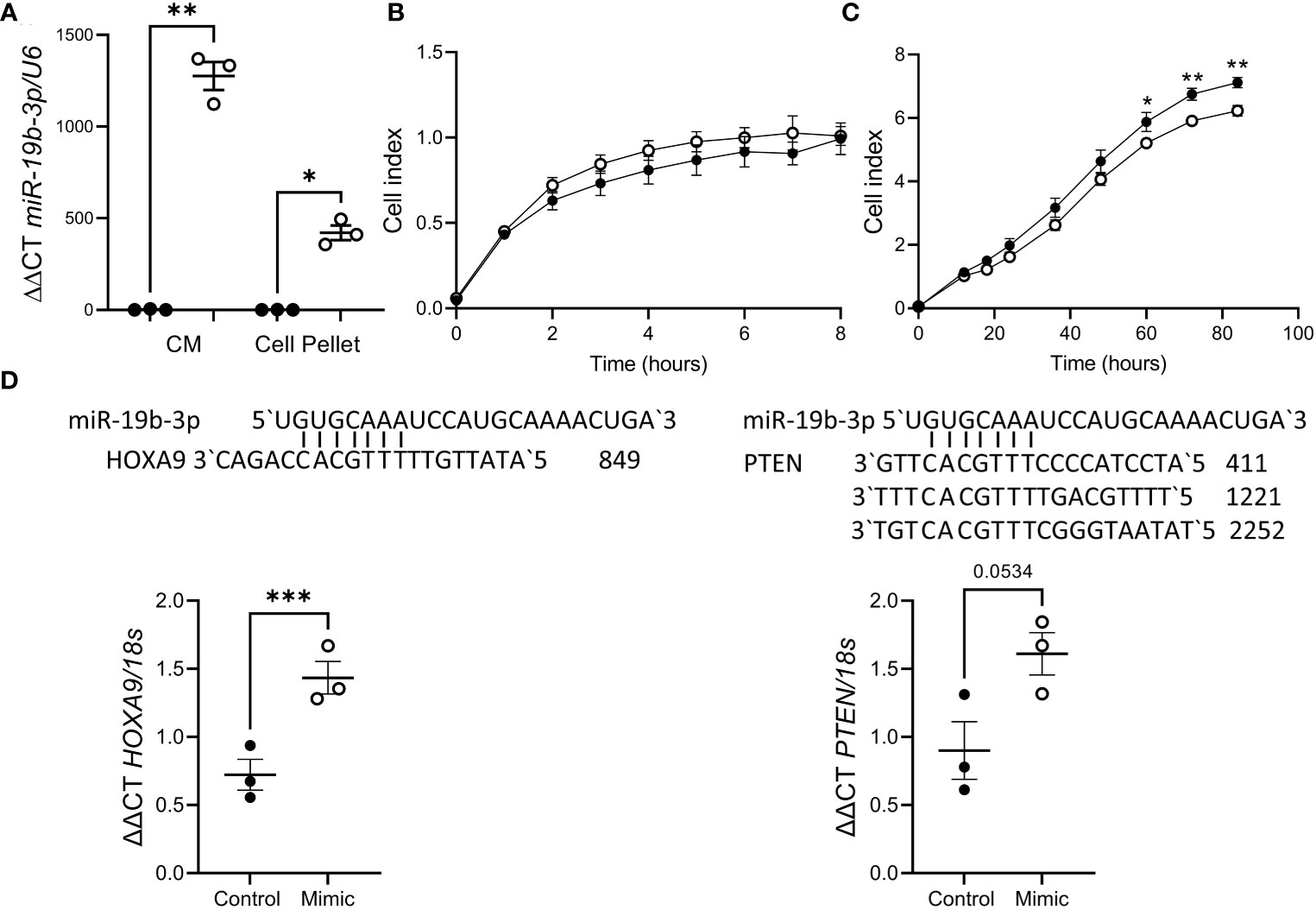
Figure 3 miR-19b-3p overexpression in HTR8/Svneo cells impaired proliferation. (A) Treatment with miR-19b-3p mimic (○) significantly increased miR-19b-3p levels in HTR8/Svneo culture media (CM) and cell pellet compared to scramble control (●). (B) miR-19b-3p mimic had no effect on HTR8/Svneo adhesion (n=3/group). (C) miR-19b-3p mimic significantly reduced HTR8/Svneo proliferation after 60h (n=3/group). (D) miR-19b-3p mimic significantly increased HOXA9 expression but had no effect on PTEN. Alignment of miR-19b-3p and the 3`UTR of HOXA9 and PTEN is also shown. Data shows mean ± SEM; *P<0.05; **P<0.01; ***P<0.001; (A–C), repeated measures ANOVA; (D), paired t-test.
4 Discussion
Here we showed for the first time that decidualization was associated with a global repression of miR release by hESFs. We found that endometrial tissue collected from women with a history of early pregnancy loss had significantly higher mir-19b-3p production conmpared to fertile controls and transfection of miR-19b-3p mimic to HTR8/Svneo trophoblast cells significantly impaired cell proliferation and increased HOXA9 mRNA production.
Our observation that global miR release was reduced in decidualized hESF is striking. Released miRs can be transferred to another cell, triggering actions in target cells (15). Certainly, decidualized cell secretions promote decidualization of surrounding stromal cells (1), regulate uterine-resident lympocyte recruitment and differentiation (29) and promote trophoblast invasion (8, 30, 31). We hypothesize hESF-released miRs would be taken up by surrounding cells (eg. other decidual cells, trophoblast, immune and endothelial cells) and our data suggests that decidualization may release these other cells from hESF mediated control. We did not investigate the mechanism by which this repression in miR release occurs, however extracellular vesicle production is increased following decidualization with cAMP (32), suggesting that there may be a change in other methods of release (eg argonaute proteins). Whether argonaute proteins in hESF are regulated by decidualization has not been investigated.
It was somewhat surprising that we didn’t see a change in cellular miRs using this in vitro model as have been seen in other models that investigated only cellular miRs following in vitro decidualization, including miR-181a (downregulated 3-fold) and miR-409-5p (upregulated 2.3-fold) (17). We saw a non-significant trend to increased miR-409-5p cellular expression and a significant increase in production in the 1st trimester decidua compared to late secretory phase endometrium. To exclude the direct effect of oestradiol or MPA on miR release in this study we collected cells and culture media 3 days after initiating the decidualization treatments. Although there is negligible PRL secretion on day 3, it is possible that alterations to miR production are initiated early in decidualization and we may have seen an effect on miR production if we compared hESF before and after decidualization hormone treatment as is done in other studies.
Collectively, previous studies and our results suggest that dysregulated miR-19b-3p production may be involved in the etiology of recurrent pregnancy loss. We found that miR-19b-3p was significantly elevated in the cycling endometrium of patients with a history of early pregnancy loss and Tian et al. showed that miR-19b-3p is decreased in the placental villous of patients with a history of recurrent early pregnancy loss (20). Furthermore, miR-19b-3p is dysregulated in monocytes from patients with antiphospholipid syndrome (33), an acquired thrombophilia diagnosed in 15-20% of patients with recurrent early pregnancy loss (5).
The function of miR-19b-3p appears highly cell type specific. In trophoblast miR-19b-3p overexpression prevents syncytialization of primary human cytotrophoblasts (34), decreased PTEN production in JEG-3 (20) and here we found miR-19b-3p impaired HTR8/Svneo trophoblast proliferation. In other tissues miR-19b-3p mostly promotes proliferation (35–39). The inhibition of proliferation by miR-19b-3p mimic seen here may be due to the increase in HOXA9 production also stimulated by the miR-19b-3p mimic: HOXA9 inhibits HTR8/Svneo proliferation (40), migration and invasion (41).
A role for miR-19b-3p in inflammation is also proposed, however again the function of miR-19b-3p in regulating inflammatory responses is not clear. miR-19b-3p increases apoptosis and intracellular reactive oxygen species in endothelial cells (42), enhances Th1/M1 inflammatory responses (43–45) and inhibits Treg differentiation (43), but may also promote M2 polarization (46, 47). That miR-19b-3p may be pro-inflammatory in pregnancy is suggested by elevated levels in maternal plasma of pregnancies with gestational diabetes mellitus (48, 49), and preterm birth (50), both inflammatory conditions. Loss of miR-19b-3p in the endometrium during decidualization therefore may be crucial to promote trophoblast differentiation and maternal tolerance.
In conclusion, we found that in vitro decidualization was associated with reduced miR release and that overexpression of miR-19b-3p was found in endometrial tissue from patients with history of early pregnancy loss. Finally, we found that miR-19b-3p impaired HTR8/Svneo proliferation implying a role for this decidual-released miR in trophoblast function. Overall we speculate that decidualization may act to reduce endometrial stromal cell regulation of other cell types within the decidua, particularly trophoblast, enabling healthy implantation and placentation during early pregnancy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Royal Women’s Hospital Research Ethics Committee Monash Health Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EM conception, design, experiments, wrote manuscript. TS experiments. KR experiments. SB experiments. WZ experiments, edited manuscript. TE design, samples, edited manuscript. ED design, edited manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Rebecca L Cooper Medical Research Foundation (project grant PG2018130 to EM), the Trevor B. Kilvington Bequest, the NHMRC (Australia) Fellowship (#550905 to ED) and the Victorian Government’s Operational Infrastructure support. The funders had no involvement in the conduct of research, preparation of the manuscript or the decision to publish.
Acknowledgments
We are grateful to the women who donated tissue and thank Emily-Jane Bromley RN, Judi Hocking RN and Leilani Santos for their contribution to this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1149786/full#supplementary-material
References
1. Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol (2016) 12:654–67. doi: 10.1038/nrendo.2016.116
2. Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua - the maternal bed embracing the embryo-maintains the pregnancy. Sem Immunopath (2016) 38:635–49. doi: 10.1007/s00281-016-0574-0
3. Founds S, Conley YP, Lyons-Weiler JF, Jeyabalan A, Allen Hogge W, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta (2009) 30:15–24. doi: 10.1016/j.placenta.2008.09.015
4. Lucas ES, Dyer NP, Murakami K, Lee YH, Chan YW, Grimaldi G, et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cells (2016) 34:346–56. doi: 10.1002/stem.2222
5. Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers (2020) 6:98. doi: 10.1038/s41572-020-00228-z
6. Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-β at the human fetal-maternal interface: Role in trophoblast growth and Differentiation1. Biol Reproduction. (1992) 46:561–72. doi: 10.1095/biolreprod46.4.561
7. Xu G, Guimond M-J, Chakraborty C, Lala PK. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual Product1. Biol Reprod (2002) 67:681–9. doi: 10.1095/biolreprod67.2.681
8. Menkhorst EM, Van Sinderen M, Correia J, Dimitriadis E. Trophoblast function is altered by decidual factors in gestational-dependant manner. Placenta (2019) 80:8–11. doi: 10.1016/j.placenta.2019.03.013
9. Keskin D, Allan DSJ, Rybalov B, Andzelm MM, Stern JNH, Kopcow HD, et al. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. PNAS (2007) 104:3378–83. doi: 10.1073/pnas.0611098104
10. Xu X, Wang Q, Deng B, Wang H, Dong Z, Qu X, et al. Monocyte chemoattractant protein-1 secreted by decidual stromal cells inhibits NK cells cytotoxicity by up-regulating expresssion of SOCS3. PloS One (2012) 7:e41869. doi: 10.1371/journal.pone.0041869
11. Wheeler K, Jena MK, Pradhan BS, Nayak N, Das S, Hsu C-D, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PloS One (2018) 13:e0191040. doi: 10.1371/journal.pone.0191040
12. Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PloS One (2010) 5:e10287. doi: 10.1371/journal.pone.0010287
13. Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, et al. Uterine selection of human embryos at implantation. Sci Rep (2014) 4:3894–4. doi: 10.1038/srep03894
14. Chew C, Conos S, Unal B, Tergaonkar V. Noncoding RNAs: master regulators of inflammatory signaling. Trends Mol Med (2018) 24:66–84. doi: 10.1016/j.molmed.2017.11.003
15. Fabbri M. MicroRNAs and miRceptors: a new mechanism of action for intercellular communication. Philos Trans R Soc Lond B Biol Sci (2018) 373:20160486.2016040486. doi: 10.1098/rstb.2016.0486
16. Park Y. MicroRNA exocytosis by vesicle fusion in neuroendocrine cells. Front Endocrinol (2017) 8:355. doi: 10.3389/fendo.2017.00355
17. Estella C, Herrer I, Moreno-Moya JM, Quiñonero A, Martínez S, Pellicer A, et al. miRNA signature and dicer requirement during human endometrial stromal decidualization in vitro. PloS One (2012) 7:e41080–0. doi: 10.1371/journal.pone.0041080
18. Wang JM, Gu Y, Zhang Y, Yang Q, Zhang X, Yin L, et al. Deep-sequencing identification of differentially expressed miRNAs in decidua and villus of recurrent miscarriage patients. Arch Gynecol Obstet (2016) 293:1125–35. doi: 10.1007/s00404-016-4038-5
19. Hong L, Yu T, Xu H, Hou N, Cheng Q, Lai L, et al. Down-regulation of miR-378a-3p induces decidual cell apoptosis: a possible mechanism for early pregnancy loss. Hum Reprod (2018) 33:11–22. doi: 10.1093/humrep/dex347
20. Tian S, Yu J, Zhang Y, Bian Y, Ma J, Yan J. Overexpression of PTEN regulated by miR-19b and miR-494 in the villous of recurrent spontaneous abortion patients. J Reprod Immunol (2020) 140:103133. doi: 10.1016/j.jri.2020.103133
21. Menkhorst EM, Van Sinderen ML, Rainczuk K, Cuman C, Winship A, Dimitriadis E. Invasive trophoblast promote stromal fibroblast decidualization via profilin 1 and ALOX5. Sci Rep (2017) 7:8690. doi: 10.1038/s41598-017-05947-0
22. Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod (2002) 8:636–43. doi: 10.1093/molehr/8.7.636
23. Grbac E, So T, Varshney S, Williamson N, Dimitriadis E, Menkhorst E. Prednisolone alters endometrial decidual cells and affects decidual-trophoblast interactions. Front Cell Dev Biol (2021) 9:647496. doi: 10.3389/fcell.2021.647496
24. Cuman C, Van Sinderen M, Gantier MP, Rainczuk K, Sorby K, Rombauts L, et al. Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine (2015) 2:1528–35. doi: 10.1016/j.ebiom.2015.09.003
25. Menkhorst E, Zhou W, Santos L, Delforce S, So T, Rainczuk K, et al. Galectin-7 impairs placentation and causes preeclampsia features in mice. Hypertension (2020) 76:1185–94. doi: 10.1161/HYPERTENSIONAHA.120.15313
26. Barton S, Zhou W, Santos L, Menkhorst E, Yang G, Tinn Teh W, et al. miR-23b-3p regulates human endometrial epithlial cell adhesion implying a role in implantation. Reproduction (2023) 165:407–16. doi: 10.1530/REP-22-0338
27. Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol (2019) 20:18. doi: 10.1186/s13059-019-1629-z
28. Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res (2020) 48:D127–d131. doi: 10.1093/nar/gkz757
29. Vinketova K, Mourdjeva M, Oreshkova T. Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J Pregnancy (2016) 2016:8689436. doi: 10.1155/2016/8689436
30. Menkhorst EM, Lane N, Winship AL, Li P, Yap J, Meehan K, et al. Decidual-secreted factors alter invasive trophoblast membrane and secreted proteins implying a role for decidual cell regulation of placentation. PloS One (2012) 7:e31418. doi: 10.1371/journal.pone.0031418
31. Menkhorst E, Winship A, Van Sinderen M, Dimitriadis E. Human extravillous trophoblast invasion: intrinsic and extrinsic regulation. Reprod Fertil Dev (2016) 28:406–15. doi: 10.1071/RD14208
32. Ma Q, Beal JR, Bhurke A, Kannan A, Yu J, Taylor RN, et al. Extracellular vesicles secreted by human uterine stromal cells regulate decidualization, angiogenesis, and trophoblast differentiation. PNAS (2022) 119:. doi: 10.1073/pnas.2200252119
33. Juárez-Vicuña Y, Guzmán-Martín CA, Martínez-Martínez LA, Hernández-Díazcouder A, Huesca-Gómez C, Gamboa R, et al. miR-19b-3p and miR-20a-5p are associated with the levels of antiphospholipid antibodies in patients with antiphospholipid syndrome. Rheumatol Int (2021) 41:1329–35. doi: 10.1007/s00296-021-04864-w
34. Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol (2013) 33:1782–96. doi: 10.1128/MCB.01228-12
35. Jiang T, Ye L, Han Z, Liu Y, Yang Y, Peng Z, et al. miR-19b-3p promotes colon cancer proliferation and oxaliplatin-based chemoresistance by targeting SMAD4: validation by bioinformatics and experimental analyses. J Exp Clin Cancer Res (2017) 36:131–1. doi: 10.1186/s13046-017-0602-5
36. Daguia Zambe JC, Zhai Y, Zhou Z, Du X, Wei Y, Ma F, et al. miR-19b-3p induces cell proliferation and reduces heterochromatin-mediated senescence through PLZF in goat male germline stem cells. J Cell Physiol (2018) 233:4652–65. doi: 10.1002/jcp.26231
37. Xiaoling G, Shuaibin L, Kailu L. MicroRNA-19b-3p promotes cell proliferation and osteogenic differentiation of BMSCs by interacting with lncRNA H19. BMC Med Genet (2020) 21:11–1. doi: 10.1186/s12881-020-0948-y
38. Wang Q, Dong Y, Wang H. microRNA-19b-3p-containing extracellular vesicles derived from macrophages promote the development of atherosclerosis by targeting JAZF1. J Cell Mol Med (2022) 26:48–59. doi: 10.1111/jcmm.16938
39. Li ZL, Li D, Yin GQ. MiR-19b-3p promotes tumor progression of non-small cell lung cancer via downregulating HOXA9 and predicts poor prognosis in patients. Histol Histopathol (2022) 37:779–89. doi: 10.14670/HH-18-448
40. Shi Z, Liu B, Li Y, Liu F, Yuan X, Wang Y. MicroRNA-652-3p promotes the proliferation and invasion of the trophoblast HTR-8/SVneo cell line by targeting homeobox A9 to modulate the expression of ephrin receptor B4. Clin Exp Pharmacol Physiol (2019) 46:587–96. doi: 10.1111/1440-1681.13080
41. Liu X, Liu X, Liu W, Luo M, Tao H, Wu D, et al. HOXA9 transcriptionally regulates the EPHB4 receptor to modulate trophoblast migration and invasion. Placenta (2017) 51:38–48. doi: 10.1016/j.placenta.2017.01.127
42. Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, Zheng S, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis (2015) 241:671–81. doi: 10.1016/j.atherosclerosis.2015.06.031
43. Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, et al. Molecular dissection of the miR-17-92 cluster's critical dual roles in promoting Th1 responses and preventing inducible treg differentiation. Blood (2011) 118:5487–97. doi: 10.1182/blood-2011-05-355644
44. Yin L, Song C, Zheng J, Fu Y, Qian S, Jiang Y, et al. Elevated expression of miR-19b enhances CD8+ T cell function by targeting PTEN in HIV invected long term non-progressors with sustained viral suppression. Front Immunol (2019) 9:3140. doi: 10.3389/fimmu.2018.03140
45. Lv L-L, Feng Y, Wu M, Wang B, Li Z-L, Zhong X, et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ (2020) 27:210–26. doi: 10.1038/s41418-019-0349-y
46. Chen J, Zhang K, Zhi Y, Wu Y, Chen B, Bai J, et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via hippo pathway. Clin Transl Med (2021) 11:e478–8. doi: 10.1002/ctm2.478
47. Jiahui C, Jiadai Z, Nan Z, Rui Z, Lipin H, Jian H, et al. miR-19b-3p/PKNOX1 regulates viral myocarditis by regulating macrophage polarization. Front Genet (2022) 13:902453. doi: 10.3389/fgene.2022.902453
48. Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int J Gynecology Obstetrics (2015) 130:49–53. doi: 10.1016/j.ijgo.2015.01.010
49. Li J, Gan B, Lu L, Chen L, Yan J. Expression of microRNAs in patients with gestational diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol (2022). doi: 10.1007/s00592-022-02005-8
Keywords: decidua, decidualization, microRNA release, miR-19b-3p, trophoblast, early pregnancy loss
Citation: Menkhorst E, So T, Rainczuk K, Barton S, Zhou W, Edgell T and Dimitriadis E (2023) Endometrial stromal cell miR-19b-3p release is reduced during decidualization implying a role in decidual-trophoblast cross-talk. Front. Endocrinol. 14:1149786. doi: 10.3389/fendo.2023.1149786
Received: 23 January 2023; Accepted: 03 March 2023;
Published: 16 March 2023.
Edited by:
Reinaldo Marín, Instituto Venezolano de Investigaciones Científicas (IVIC), VenezuelaReviewed by:
Mikihiro Yoshie, Tokyo University of Pharmacy and Life Sciences, JapanMartin Mueller, University Hospital Bern, Switzerland
Copyright © 2023 Menkhorst, So, Rainczuk, Barton, Zhou, Edgell and Dimitriadis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen Menkhorst, ZWxsZW4ubWVua2hvcnN0QHVuaW1lbGIuZWR1LmF1
 Ellen Menkhorst
Ellen Menkhorst Teresa So1,2
Teresa So1,2 Siena Barton
Siena Barton Wei Zhou
Wei Zhou Evdokia Dimitriadis
Evdokia Dimitriadis