- 1Laboratory on Thymus Research, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 2Brazilian National Institute of Science and Technology on Neuroimmunomodulation, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 3Rio de Janeiro Research Network on Neuroinflammation, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 4INOVA-IOC Network on Neuroimmunomodulation, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 5Institute for Glycomics, Griffith University, Gold Coast, QLD, Australia
The thymus gland is a central lymphoid organ in which developing T cell precursors, known as thymocytes, undergo differentiation into distinct type of mature T cells, ultimately migrating to the periphery where they exert specialized effector functions and orchestrate the immune responses against tumor cells, pathogens and self-antigens. The mechanisms supporting intrathymic T cell differentiation are pleiotropically regulated by thymic peptide hormones and cytokines produced by stromal cells in the thymic microenvironment and developing thymocytes. Interestingly, in the same way as T cells, thymic hormones (herein exemplified by thymosin, thymulin and thymopoietin), can circulate to impact immune cells and other cellular components in the periphery. Evidence on how thymic function influences tumor cell biology and response of patients with cancer to therapies remains unsatisfactory, although there has been some improvement in the knowledge provided by recent studies. Herein, we summarize research progression in the field of thymus-mediated immunoendocrine control of cancer, providing insights into how manipulation of the thymic microenvironment can influence treatment outcomes, including clinical responses and adverse effects of therapies. We review data obtained from clinical and preclinical cancer research to evidence the complexity of immunoendocrine interactions underpinning anti-tumor immunity.
1 Introduction
T lymphocytes (T cells) are critical orchestrators of the adaptive immune response that optimally eliminates tumor cells. The thymus is uniquely committed to T cell production by providing an inductive microenvironment in which bone marrow-derived progenitors undergo proliferation, T cell receptor (TCR) gene rearrangements and differentiation into mature T cells. Normal thymic architecture is essential for the proper development of T cells, which is mediated by interactions between resident thymic T cells (thymocytes) and thymic epithelial cells (TEC). TEC expressing the autoimmune regulator (AIRE) maintain immune central expressing the autoimmune regulator (AIRE) to maintain immune central tolerance by guiding the clonal deletion of autoreactive thymocytes and development of regulatory T cells (Treg) (1). Mature T cells egress from the thymus and enter the bloodstream, participating as a central component of the adaptive immune system as mediators of anti-tumor immunity (2, 3). Accordingly, therapies harnessing cytotoxic T cells have markedly improved the care of patients with multiple types of cancer (3, 4). Different from immunotherapies based on adoptive T cell therapy and immune-checkpoint inhibitors (ICI, designed to target the immune-inhibitory receptors CTLA-4, PD1 and TIGIT on T cells), conventional which are designed to target the immune-inhibitory receptors CTLA-4, PD1 and TIGIT on T cells, conventional cyto-ablative cancer therapies (i.e. chemotherapy and radiotherapy) directly target the tumor cell. These conventional cancer therapies lead to damage of the thymic structure, with significant impairment of mature TEC generation and the naïve T cell repertoire, a hallmark of thymus atrophy (Figure 1A) (5, 6).
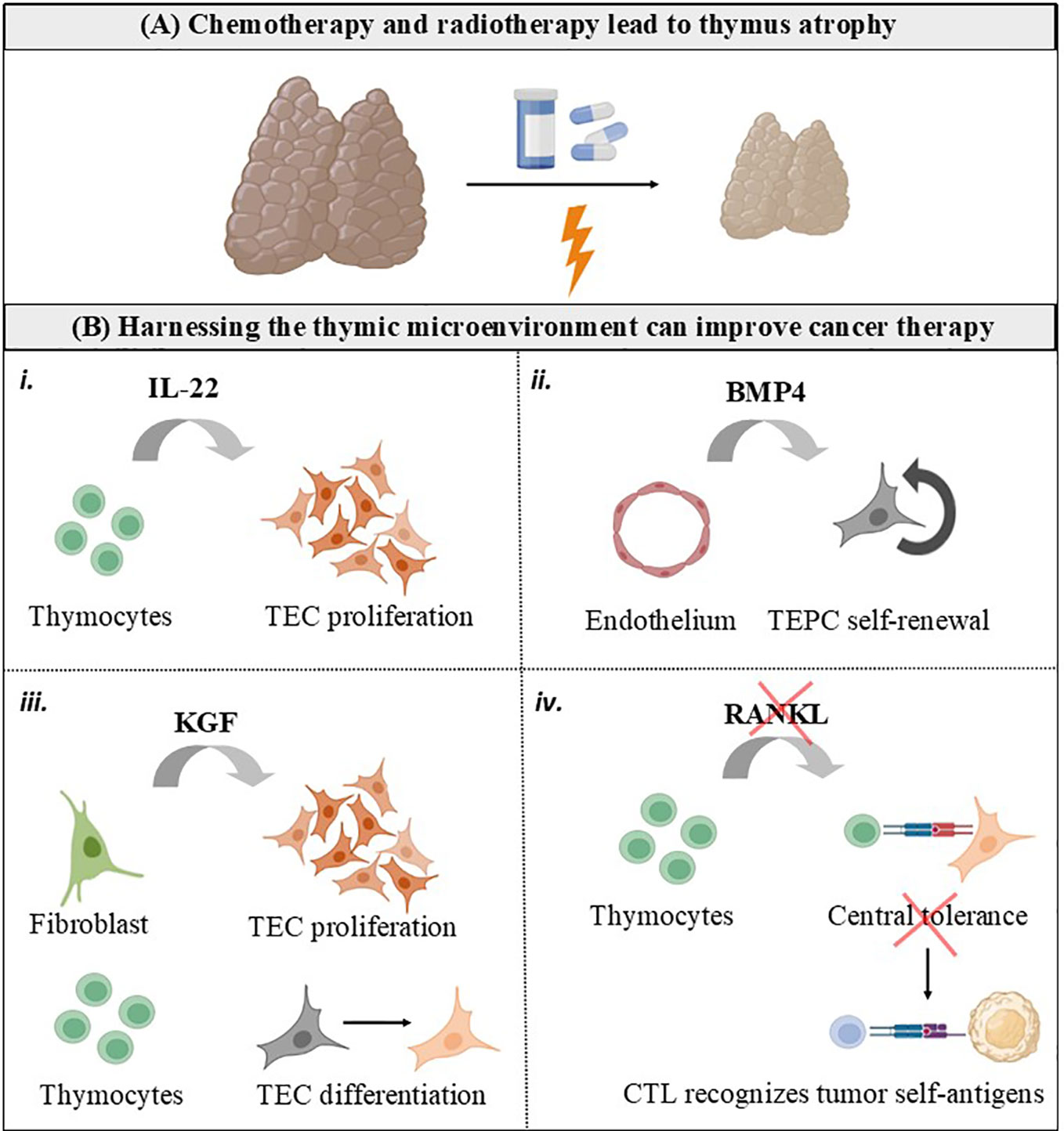
Figure 1 Harnessing the thymic microenvironment to improve cancer therapy. (A) Conventional cancer therapies, including chemotherapy and radiotherapy, causes the thymus to transiently involute due to loss of TEC and thymocytes. (B) Cytokines and growth factors produced by the thymic microenvironment can be used as adjuvants for cancer therapy. i. IL-22 is produced intrathymically by T cells and drive thymic regeneration following radiation damage, by inducing TEC proliferation. ii. BMP4 is produced by thymic endothelial cells in response to radiation and stimulate self-renewal of TEC progenitors (TEPC). iii. KGF is produced by thymic fibroblasts in the fetus and by mature thymocytes in the adult thymus. KGF has an important role in repairing epithelial tissues, inducing TEC proliferation and differentiation. iv. RANKL is mainly provided by positively selected CD4+ thymocytes and innate lymphoid cells, and controls central tolerance by inducing mTEC proliferation, differentiation, and regeneration. It is possible that RANKL blockade interrupts central tolerance and unleashes cytotoxic T cells (CTL) possessing TCR with self-reactive features to recognize tumor cells expressing self-antigens.
In comparison to T cells, the role of thymic hormones in controlling tumor progression is understudied. Thymic peptide hormones, including thymosins, thymulin and thymopoietin, are secreted by TEC and can either act locally on thymocytes or circulate in the periphery to impact the differentiation and function of T cells and other immune subsets (1, 7, 8). Purified peptides from the thymus as well as thymic peptide analogues and cytokines have been trialled in several studies investigating their impact on the response and tolerability of standard chemotherapy, radiotherapy, or both (9, 10). As such, revealing the relationship between thymic hormones/cytokines, T cells and tumor cells may lead to novel strategies to improve the outcome of patients with cancer.
2 Intrathymic T cell differentiation
The thymus, a primary lymphoid organ where differentiation of T cells take place, is a source of a variety of soluble immune-related moieties, including cytokines, chemokines, classic hormones, and neurotransmitters. Additionally, key interactions occur through cell-cell and cell-matrix interactions. Intrathymic T cell development has been extensively reviewed in the last few years by several research groups (11–15). During intrathymic T cell differentiation, bone marrow-derided T cell precursors enter the thymus through blood vessels at the corticomedullary junction where they encounter the thymic microenvironment. The thymic tridimensional network is constituted of cellular components, such as TEC, thymic dendritic cells, macrophages and fibroblasts, as well as secreted non-soluble and soluble molecules such as the extracellular matrix proteins (including among other fibronectins and laminins), cytokines (as interleukin (IL)-2, IL-6, IL-7, IL-22 and RANKL), chemokines (CXCL12, CCL4, CCL7, CCL19 and CCL25), thymic hormones (as thymosin, thymopoietin, and thymulin), growth factors (as BMP4, KGF and FLT3) and different typical soluble components of nervous tissues, including as neuropeptides and neurotransmitters.
The thymus is histologically divided in lobules, each one comprising two main regions: the cortex and the medulla. Immature CD4-CD8- double-negative (DN) and CD4+CD8+ double-positive (DP) thymocytes are in the cortex, whereas mature CD4+ or CD8+ single-positive (SP) thymocytes are in the medulla. In physiological conditions, these SP cells leave the thymus to colonize the T-dependent regions of secondary lymphoid organs. This process is under the control of the thymic microenvironment. During intrathymic T cell development, thymocytes are exposed to interactions involving the TCR and major histocompatibility complex (MHC) proteins expressed by TEC and dendritic cells. TCR-expressing DP developing thymocytes are rescued from programmed cell death during positive selection by interaction with self-antigens presented to the TCR by MHC molecules. Those thymocytes that were positively selected move towards the medulla to interact with self-antigens presented by AIRE-expressing medullary TEC (mTEC), being thus tested for negative selection. If this is the case, differentiating thymocytes will undergo apoptosis, due to high avidity interaction of the TCR with self-antigens presented by the MHC class I or class II molecules expressed by microenvironmental cells.
Positioning of developing thymocytes to specialized thymic microdomains depend on multiple interactions including cell-cell and cytokine/chemokine-mediated interactions. For example, CXCL12 is secreted by TEC, and preferentially attracts immature DN and DP cells, by ligation with the receptor CXCR4. The chemokine CCL25 also attracts immature thymocytes and its receptor, CCR9, is expressed at all stages of murine thymocyte differentiation. Notably, alike the thymic microenvironment that guide the development of thymocytes, reciprocally thymocytes control the differentiation and organization of TEC (Figure 1B), a process commonly known as thymic cross-talk (16, 17). One of the classical examples of this bidirectional interaction between thymocytes and the thymic stroma is provided by the interactions between thymocytes, mTEC and dentric cells, which result in deletion of autoreactive T cells and the generation of natural regulatory T cells at the same time that the developing thymocytes control the composition and complex three-dimensional organization of the thymic medulla (16).
Overall, in physiological conditions, mature CD4+ and CD8+ SP thymocytes exit the thymus to populate the peripheral lymphoid organs and participate in adaptive immune responses, including those underpinning anti-tumor immunity. Of note, thymic-derived hormones, cytokines, and growth factors can be manipulated to improve responses to cancer therapy, as summarized in Figures 1B, 2.
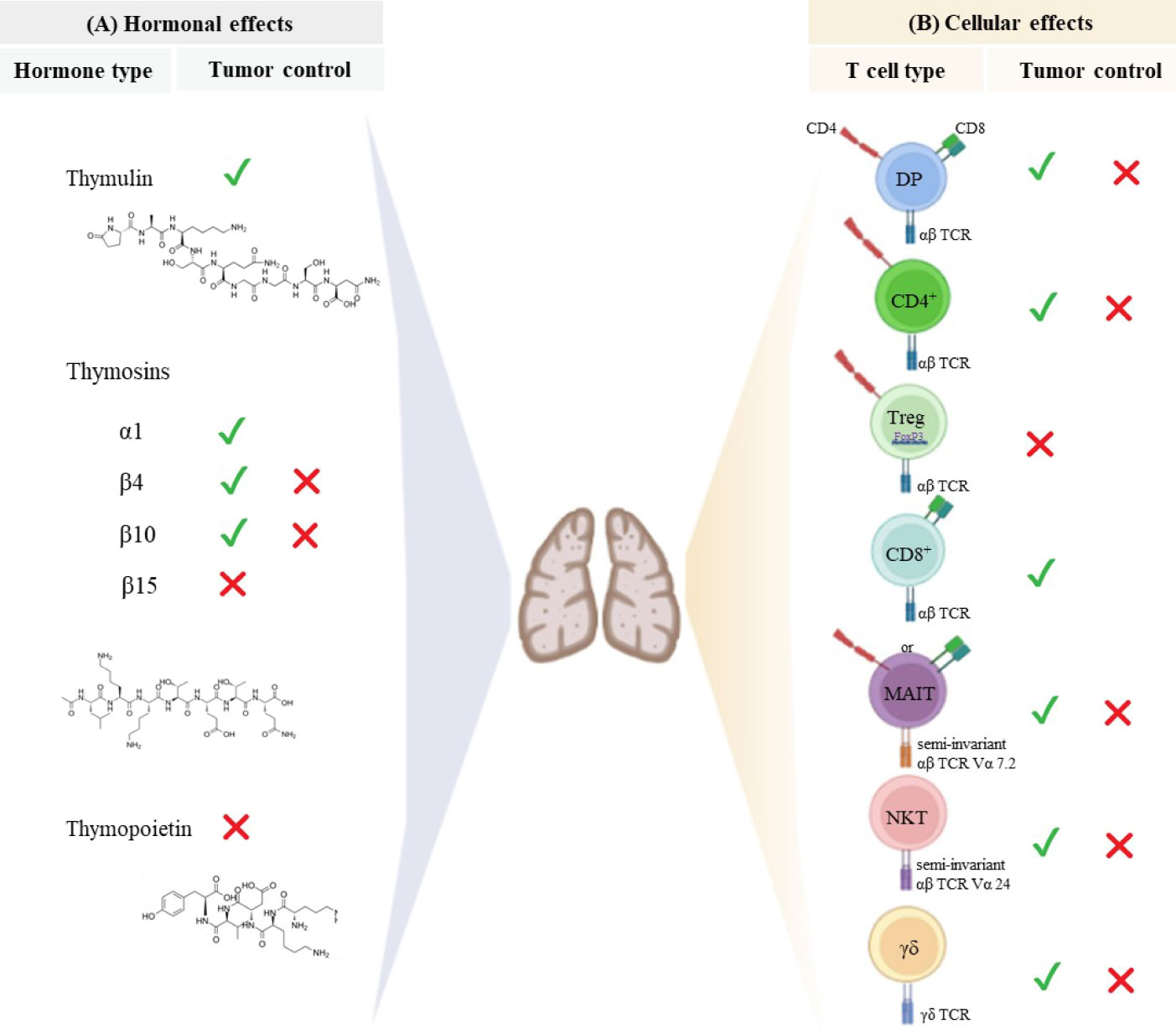
Figure 2 Thymus-derived peptides and immune cells regulate tumor growth. (A) Thymic peptide hormones are synthesized within the thymus and exert important regulatory effects on tumor cells. Thymosin α1 can induce anti-tumor immune responses and directly act on tumor cells to retrain tumor growth. Although β-thymosins (herein exemplified by β4, β10 and β15) can facilitate tumor progression, the β4 and β10 isoforms have also been reported to present suppressive tumor effect is some disease settings. Despite the paucity of available data, thymulin appears to promote anti-tumor effects, while high levels of thymopoietin have been associated with tumor cell proliferation and survival. The chemical structure of synthetic thymosin β4, thymulin and thymopoietin is shown. (B) The thymus provides the physiological microenvironment critical for the development of different types of T cells. It is possible that immature T cells expressing both CD4 and CD8 co-receptors can migrate to the immune periphery exerting either cytotoxic or immunosuppressive effects depending on the disease context. Similarly, conventional CD4+ T cells and unconventional T cells (MAIT, NKT and γδ T cells) can exert dual effect on tumor cells. Cytotoxic CD8 + T cells are the main effector cells to promote anti-tumor immunity, while regulatory T cells expressing both CD4 and the transcription factor Foxp3 have been largely investigated for its immunosuppressive properties and ability to hamper effective anti-tumor immune responses. The signals represent the role of hormones and T cells in controlling (✓) or inducing (✗) tumor progression.
3 Thymic hormones affect tumor cell biology via direct and immune-related mechanisms
In addition to the above cited molecules secreted intrathymically, we can find the thymic peptides, being considered as part of a heterogenous family of polypeptide hormones synthesized within the thymus. Studies with purified native thymic peptides and analogue peptides have shown a variety of regulatory effects on oncogenic diseases, mediated both by interactions with the host’s immune compartment (18, 19) and direct interaction with tumor cells (20–22). The effect of the main thymic peptides able to modulate tumor progression is summarized in Figure 2A and described below.
3.1 Thymosins
Includes two major families termed as α and β, which are classically regarded as main regulators of intrathymic T cell differentiation (1, 23). Besides, thymosins can also impact other immune cell types and tumor cells.
3.1.1 Thymosin α1 (Tα1)
Tα1 represents a varied range of targets for its immune-enhancing activity and has shown promising results in improving immune responses in different types of malignancies (10). Tα1 increases the number of tumor-infiltrating CD4+ and CD8+ T cells in melanoma and breast cancer xenograft models (24) due to its role in induction of T cell differentiation, enhancement of IFN-γ and IL-2 production, and downregulation of T cell apoptosis (25, 26). Besides its effect on T cells, Tα1 has been reported to accelerate the replenishment and maturation of macrophages in the bone marrow of mice severely damaged by the chemotherapy (27) and block the intratumoral accumulation of myeloid suppressor cells in a mouse subcutaneous xenograft tumor model (28). Besides modulating the host immune system, Tα1 can act directly on tumor cells, exhibiting the ability to restrain tumor growth by its proapoptotic and anti-proliferative properties demonstrated in human leukemia cells lines (20) and preclinical cancer models (29), including prevention of tumor progression in immunosuppressed mice (30, 31).
3.1.2 Thymosin β4 (Tβ4)
It is the most abundant thymic hormone among the thymosin family, with regenerative and anti-inflammatory properties, being expressed not only in the thymus but also in many other tissues (32). Tβ4-derived synthetic peptides have been shown to induce the angiogenesis, invasion and metastasis of melanoma tumor cells when administrated in vivo (21). Corroborating with its pro-tumorigenic role, Tβ4 gene silencing suppressed proliferation and invasion of NSCLC cells (22). Inversely, decreased expression of this hormone has been associated with poor prognosis in patients with multiple myeloma (20), while overexpression of Tβ4 led to decreased proliferative and migratory capacities of tumor cells in a mouse model of multiple myeloma (33). These data evidence a dual role of Tβ4 in cancer, either promoting or inhibiting tumor progression.
3.1.3 Thymosin β10
Thymosin β10 has multiple pro-tumorigenic roles. Its overexpression correlates with disease progression in bladder cancer (34), hepatocellular carcinoma (35), NSCLC (36), and pancreatic cancer (37). Thymosin β10 has been shown to promote tumor-associated macrophage conversion into immunosuppressive M2 types and favor progression of lung adenocarcinoma (38). On the other hand, administration of thymosin β10 to a xenograft model of human ovarian cancer inhibited tumor cell invasion and metastasis (39) and induced a high rate of apoptosis in ovarian cancer cell lines (40).
3.1.4 Thymosin β15
It has been pointed as a novel regulator of tumor cell motility and is upregulated in metastatic prostate cancer (41). In addition, high expression of thymosin β15 has been correlated with the metastatic potential of mouse lung carcinoma and human breast carcinoma cells (42).
Therefore, while Tα1 presents vast anti-tumors effects and have been shown synergic effects with multiple anti-cancer therapies (reviewed in section 7), the members of the beta thymosin family can either stimulate or inhibit tumor progression, differentially interfering with prognosis according to the type of cancer.
3.2 Thymopoietin
It is a thymus-derived polypeptide involved in the modulation of tumor cell biology. Studies conducted in gastric cancer patients showed that high levels of thymopoietin were associated with a significantly poorer overall survival, and that knockdown of this hormone suppressed gastric cancer and glioblastoma cell proliferation and survival (43, 44). In pancreatic cancer cells, knockdown of thymopoietin not only inhibited cell proliferation but also suppressed migration, invasion, and metastasis (45). Accordingly, long non-coding thymopoietin RNA 1 (TMPO-AS1) is highly expressed in hepatocellular carcinoma cells and promotes tumor development by enhancing cell viability, proliferation, and stemness and inhibiting apoptosis (46). Besides its contribution to tumor cell expansion and metastasis thymopoietin induced non‐significant reduction in the number of patients experiencing neutropenia during chemotherapy (47). Overall, the scarce data on the role of thymopoietin in cancer outcome remains to be better defined.
3.3 Thymulin
The thymic hormone thymulin is a zinc-containing nonapeptide specifically produced by TEC, which can be simultaneously detected with Tα1 and thymopoietin in the epithelial network of the human thymus (48, 49). A non-exhaustive list of thymulin biological functions can be seen in Box 1.
Box 1 Biological Functions of the Thymic Hormone thymulin.
● Increase of CD3, CD4 and CD8 expression in immunodeficient children
● Upregulation of NK activity in humans and mice
● Stimulation and activation of mouse intraepithelial T lymphocytes
● Increase in IL-2 production by normal mouse thymocytes and nude mouse splenocytes
● Increase in IL-1 and decrease in IL-6 and TNF-α production by peripheral blood mononuclear cells from normal volunteers
● Increase in IgA and IgE synthesis in patients with ataxia telangectasia
● Increase of LPS-induced polyclonal B cell responses of CBA/N mice
● Enhancement of T cell-dependent macrophage-mediated killing of microorganisms
● Delayed skin graft rejection time in normal mice
● Reduction in anti-DNA antibody production and glomerulonephritis in mice undergoing lúpus erithematosus
● In vitro modulation of T cell markers in human rheumatoid arthritis and systemic lupus erythematosus patients,
● In vivo prevention of encephalomyocarditis virus-induced diabetes and myocarditis in mice
● Decrease in hind paw swelling and anti-type II collagen antibody production in experimental arthritis in rats
● Protection against chronic septic inflammation in mice
● Anti-inflammatory and analgesic action in neuropathic pain
● Therapeutic reversion of asthma-induced pathology in the respiratory tract
The role of thymulin in cancer is poorly understood and the data available so far suggest that thymulin can induce anti-tumor immunity and inhibits proliferation of tumor cells. An early study investigating young patients affected by acute lymphoblastic leukemia demonstrated a positive correlation between the levels of zinc-bound active thymulin and lymphocyte proliferative responses to mitogens, at the same time the proliferation rate of human lymphoblastoid cells reduced when active thymulin was added to the cultures (52). Interestingly, we have previously identified thymus atrophy in a mouse model of lung cell carcinoma, which was abolished after treatment with zinc chloride (a stimulator of thymulin secretion) (53), indicating that thymulin and tumor cells may play a two-way regulatory role in the thymic microenvironment.
4 Thymus-derived immune cells play a complex role in cancer, either promoting or inhibiting tumor progression
T cells are regarded as the main players in tumor immunity. The thymus generates naïve T cells able to differentiate into populations of effector and memory T cells, providing long-lasting immune responses to diverse tumor antigens. Among the immune cells that contribute to anti-tumoral responses, conventional T cells (T cells that express an αβ T cell receptor, as well as a co-receptor CD4 or CD8), have attracted the attention of tumor immunologists and clinical scientists. These cells present highly diverse TCR, each composed of a heterodimeric αβ chain, to recognize processed antigenic peptide presented by MHC molecules on other cells (Figure 1B). Besides conventional T cells, other types of T lymphocytes also developed in the thymus, such as Treg and innate-like unconventional T cells (including MAIT, NKT and γδ T cells), orchestrate the immune responses against tumor cells (54, 55), as summarized in Figure 2B and reviewed below.
4.1 CD8+ T cells
Cytotoxic T cells expressing cell-surface CD8 are the most powerful effectors in the anti-tumor immunity and constitute a critical determinant of response to cancer immunotherapies (2, 56). Once conjugated to a tumor cell, secretory granules in the CD8+ T cell cytoplasm traffic to the immunological synapse and release a cargo of deadly cytotoxic proteins (mostly represented by perforin and granzymes) leading to membrane damage, induction of reactive oxygen species, nuclear envelope rupture and DNA damage, and resulting in tumor cell death (57). Besides cytotoxic events unleashed by direct interaction with tumor cells, the protective role of CD8+ T cells in the tumor microenvironment is associated with release of effector cytokines (including IFN-γ, TNF-α and IL-2) that sculpts the local immune response to cancer (58). Primary CD8+ T cells isolated from the human blood and modified with chimeric antigen receptors (CAR) to express high affinity to tumor antigens have been large used in the clinical practice to treat patients with hematological malignancies (59, 60). However, CD8+ T cell exhaustion is a major limitation to the efficacy of cytotoxic anti-tumor responses, particularly limiting the application of CAR T cells to solid tumors, due to persistent TCR activation in the tumor microenvironment (61). To overcome exhaustion in primary CD8+ T cells and CAR T cells, combination therapy with ICI has emerged as an attractive strategy for increasing efficacy (62, 63). Whereas CAR T cell therapy targeting CD8+ T cells to tumor antigen has shown remarkable efficacy for treating patients with certain B cell driven hematological malignancies (64), ICI has significantly enhanced life-expectation in patients with a broad range of solid tumors (65–68) and became the first-line therapy for advanced melanoma patients, given its improved clinical efficacy and improved safety profile in comparison with conventional cancer therapies (65).
4.2 Conventional CD4+ T cells
CD4+ T cells have largely been neglected because most tumors lack MHC II expression and cannot directly be recognized by these T cells. However, CD4+ T cells play helper functions through secretion of cytokines that orchestrate the immune response and have a dual role in the tumor microenvironment. They can destroy the tumor vasculature, induce cellular senescence of cancer cells, and help CD8+ T cells in the effector phase, a role mostly associated with T-helper (Th)1 responses mediated by IL-2 and IFN-γ producing CD4+ T cells (69). Besides Th1 cells, IL-17-producing Th17 cells may also have potent anti-tumor immune effects by recruiting immune cells into tumors, activating effector CD8+ T cells, or even directly by converting toward Th1 phenotype and producing IFN-γ (70, 71). Th2 cells have also been shown to destroy tumor cells by inducing necrosis (72) and the therapeutic effectiveness of Th2 CD4+ CAR T cells has been demonstrated in a preclinical model of myeloma (73). Contrarily to these effects, Th2 and Th17 CD4+ T cells are also thought to have pro-tumorigenic activities mainly involving induction of cytokines that can promote growth, proliferation, and invasion of tumor cells, including IL-4 and IL-17, respectively (74–76).
4.3 Treg cells
These are an immunosuppressive subset of CD4+ T cells characterized by the constitutive expression of the transcription factor forkhead box protein 3 (FoxP3) and present essential roles in maintaining central tolerance. FoxP3+ natural Treg cells are generated in the thymus as a functionally mature T cell subpopulation specialized in immune suppression, hindering immunosurveillance against cancer development and hampering effective anti-tumor immune responses (77, 78).
Treg cells exert their immunosuppressive effects through various cellular and humoral mechanisms: (i) consumption of IL-2, thus inhibiting the proliferation and differentiation of conventional T cells (79), (ii) production of the inhibitory immune cytokines, IL-10 and TGF-β (80) and (iii) high expression of CTLA-4, which induces suppression of antigen-presenting cells (81). Treg expansion is associated with induction of c-Fos and elevated transcription of FoxP3 (82). FoxP3+ Treg cells with an activated phenotype can be enriched in tumors in comparison with peripheral blood and are associated with a poor prognosis in patients with various types of cancer, including cervical, renal, melanomas, and breast cancers (83, 84). Inversely, Tregs have also been associated with improved survival in colorectal, head and neck, and esophageal cancers (85, 86). This apparent paradoxical role of Treg may be associated with the fact that most of the studies rely on the solely detection of FoxP3 expression, which is transiently increased in activated conventional CD4+ T cells and cloud the specific identification of Treg (87, 88).
4.4 CD4+CD8+ double positive (DP) T cells
Classically, DP T cells are considered as a developmental stage in the thymus, before maturation as either CD4+ or CD8+ SP cells, but have been described in the peripheral blood and tissues in various settings, including in human cancers and some infectious diseases (89–91). The role of DP T cells in the periphery remains largely understudied, and it is unclear whether these cells escape the thymus in their immature stage expressing both co-receptors or if they originate from mature SP thymocytes that re-express the opposite co-receptor. DP T cells have been described in blood of patients with melanoma, bladder, prostate, kidney cancers (89, 92). The conflicting literature regarding the role of DP T cells — cytotoxic vs. immunosuppressive (93) — may indicate that these cells are heterogeneous and/or show pleiotropic functions that need to be investigated in each disease context. DP T cells favor the polarization of naïve CD4+ T cells into a Th2 functional profile (89). This previously unrecognized capacity of DP T cells was observed in healthy donors and exacerbated in patients with urologic cancer, who also showed elevated levels of circulating DP T cells (89).
4.5 Mucosal-associated invariant T (MAIT) cells
These are a class of innate-like T cells that exists in a pre-primed memory state and express a semi-invariant TCR that recognizes non-peptide antigens presented by the non-polymorphic MHC class I-like molecule, MRI (94). MAIT cells express CD8 in humans and either CD4 or CD8 in mice (95). Due to their multiple functions often associated with successful anti-tumor immune responses, MAIT cells represent an attractive population to explore for their potential roles in anti-tumor immunity (96). However, these cells represent a controversial topic in the field of tumor immunology with studies showing conflicting results regarding as to whether they contribute to tumor growth, tumor regression, or play a neutral role in human cancers (97, 98). Once activated, tumor-infiltrating MAIT cells display decreased IFN-γ and TNF-α and increase IL-17 production. Early investigations of MAIT cells in cancer suggested they may represent a potential positive prognostic marker. Indeed, a study screening of ~18,000 human tumors across 39 malignancies found a significant association of the KLRB1 gene (encoding CD161, a marker of MAIT cells), indicating a favorable prognosis (99).
4.6 Natural killer T (NKT) cells
These are a small population of true thymus-dependent T cells which are distinct from conventional T cells. Their TCR recognizes lipids rather than peptides and is restricted by a non-classical class I-like (class Ib) molecule CD1d (100, 101). Both type I and type II NKT cells play critical roles in tumor immunity; most often type I NKT cells promote anti-tumor immunity and type II NKT cells suppress it (102, 103). Moreover, both type I and II NKT cells have a myriad of interactions with other immune effector and regulatory cells, forming a complex web of immune regulation.
Type I and II NKT cells can cross-regulate each other, forming an immunoregulatory circuit that comes into play in the early steps of immune responses (104). When type I NKT cells are absent, both Tregs and type II NKT cells can exert suppressive activity upon the same tumor, and this situation mimics that often found in cancer patients, in which type I NKT cells are deficient in numbers or function. The role of type I NKT cells in protection against cancer has been found to be largely dependent on production of Th1 cytokines, especially IFNγ, even though NKT cells have lytic activity and could potentially directly lyse tumors expressing CD1d (102, 105).
4.7 TCRγδ T cells
These are the non-classical thymus-derived cell subgroup characterized by expression of γδ heterodimeric T cell receptor (TCRγδ) on cell surface, playing important roles in tumor immunity. Depending on the microenvironment, different γδ T cell subsets can have anti-tumor or pro-tumor activities (106). TCRγδ T cells can enhance the anti-tumor ability of other immune cells by secreting cytokines or expressing costimulatory molecules (107). Accordingly, this cell population has been safely used in clinics for the treatment of NSCLC and breast cancer (108). γδ gd T cell-based immunotherapy appeared to be safe and well-tolerated in patients (109, 110). However, TCRγδ T cells represent one of the main source of IL-17 in the tumor microenvironment, promoting ovarian cancer and pancreatic cancer progression (111, 112). In these studies infiltrating TCRγδ T cells could also directly induce the apoptosis of anti-tumor immune cells (112) at the same time they have shown to promote tumor development and metastasis by enhancing angiogenesis and recruiting inhibitory cells to the tumor site (113). Thus, it urges that the mechanisms controlling the anti-tumor versus pro-tumor activity by this cell type must be clarified, so that to better design therapeutic strategies targeting TCRγδ T cells.
In summary, unconventional T cells differ from their conventional counterparts in the rapidity of their initial response, the way they recognize and respond to nonpeptidic molecules, as well as their tissue distribution within the body (114). As the complexities of the immune system continue to be elucidated, it has become increasingly apparent that conventional and unconventional T cells operate in fundamentally different ways to mediate and coordinate host’s immune response to tumor cells.
5 Thymic cancer and its impact on T cell development
Thymomas are rare neoplasms of the thymic epithelial cells and present several abnormalities that may affect normal T cell development (91). Theories attempting to explain the association between autoimmune disorders and thymomas are based on the failure of positive and negative selection of thymocytes, the absence of regulatory mechanisms provided by AIRE, and on a Treg-poor environment in the neoplastic thymus (115). Myasthenia gravis is the most common disorder associated with thymoma, often linked to T cell-mediated autoimmunity in 30% of patients with thymoma (116, 117). Pioneer work screening tumors excised from patients with thymomas revealed their endocrine contents and MHC molecules, evidencing that thymoma epithelial cells contained large amounts of thymulin, thymosin α 1 and thymopoietin (48) and did not express the MHC class II-encoded molecules, HLA II-DR and -DC (117). These were the first studies showing that thymoma epithelial cells are endocrinologically active but present a defective antigen-presenting cell function, a potential mechanism for thymoma-associated autoimmunity. The finding that immunosurveillance towards cancer cells may be impaired before the diagnosis of thymoma (118) may challenge current theories attempting to explain immune disorders in patients with thymoma, suggesting that some immune events may precede the thymoma itself. It is likely that a combination of mechanisms, yet to be elucidated, is responsible for immune disorders in patients with thymoma. Again, further studies are crucial for design therapeutic alternatives to this cancer.
6 Manipulation of the thymic microenvironment to support anti-tumor immunity
Strategies aiming to protect the thymus are required to decrease side-effects of cyto-ablative cancer therapies (Figure 1A). Cytokines and soluble mediators secreted by the thymic microenvironment can impact prognosis of patients suffering from multiple types of cancer and have been manipulated to promote thymus regeneration, as reviewed below:
6.1 IL-22
Although IL-22 is not required for the formation or maintenance of the thymus under steady-state physiological conditions, it is produced intrathymically by T cells and innate lymphoid cells, presenting a paracrine role in driving thymic regeneration following radiation damage through induction of TEC survival and proliferation (119). Although the regenerative function of IL-22 is beneficial, its expression is confined to a period of tissue repair and persistence in the microenvironment helps tumors to escape cell cycle control and eradication by cytotoxic drugs. IL-22 is increased in the tumor of patients with non-small cell lung cancer (NSCLC), pancreatic cancer, gastric cancer and hepatocellular carcinoma and predicts a poor prognosis, higher disease stage, and faster tumor progression (120–123). Tumor cells from both murine and human lungs promote IL-22 production by memory T cells via induction of IL-1 (124). Expression of its cognate receptor, IL-22R1, is restricted to the non-hematopoietic cells, which makes the IL-22-IL-22R1 pathway an attractive target for cancer therapy.
6.2 Bone morphogenetic protein 4 (BMP4)
It is produced by thymic endothelial cells in response to radiation, and acts as a regulator of thymic regeneration after acute injury due to its role in stimulating bipotent TEC progenitors (TEPC) present in the adult thymus (125, 126). Accordingly, certain chemotherapeutic drugs reduce production of BMP4 and further damage the thymus (125, 126). BMP signalling has both tumor-promoting as well as -suppressing effects: at the same time that BMP4 is an important regulator of cell migration and invasion and induces epithelial–mesenchymal transition (EMT), an event that is crucial for the ability of cancer cells to acquire mobility and eventually metastasize, BMP4 can promote anti-tumor effects (127). TGFβ-mediated inhibition of BMP4 has been reported to promote breast cancer stem cell self-renewal activity, response to chemotherapy and is a good prognostic marker for patients with triple negative breast cancer (128). In mice, BMP4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy (129). High expression of BMP4 in serous ovarian cancer is an independent prognostic factor for longer progression-free survival and overall survival (130). Contrarily to breast, colorectal and ovarian cancer, high expression of BMP4 in hepatocellular carcinoma promotes tumor progression (131).
6.3 Keratinocyte growth factor (KGF)
Keratinocyte growth factor (KGF) is a member of the fibroblast growth factor family mostly produced by cells of mesenchymal origin and plays an important role in protecting and repairing epithelial tissues. In the thymus, mesenchymal cells (fibroblasts) enhance the proliferation of TEC via the production of KGF during fetal development (132). In the adult thymus, KGF is produced by mature thymocytes, which mediates thymic epithelial cell proliferation and differentiation (133). KGF knockout mice are more vulnerable to sublethal irradiation, and endogenous administration of KGF attenuates the negative effects of acute thymic injury caused by chemotherapy and irradiation in middle-aged mice (134). In a phase III trial involving patients with hematologic malignancies who were treated with chemoradiotherapy before autologous peripheral blood progenitor cell adoptive transfer, recombinant human KGF (palifermin) treatment significantly reduced both the incidence and duration of severe oral mucositis (135). These data suggest that KGF can be used as a strategy to decrease adverse effects associated with cancer therapies.
6.4 RANK ligand (RANKL)
It is a TNF family member produced intrathymically by positively selected thymocytes and lymphoid tissue inducer cells (136). RANKL binds to its cognate receptor activator of nuclear factor kappa-B (RANK) expressed on the surface of mTEC to induce cellular expansion and differentiation into AIRE+ cells, establishing central tolerance (137). Clinically, increase in serum RANKL levels is associated with incidence of breast cancer in postmenopausal women (138). RANKL inhibition with Denosumab, a fully humanized antibody, improves bone-metastasis free survival in patients with breast cancer, prostate cancer and other solid tumors, an effect known to be associated with prevention of RANKL-RANK signaling on osteoclasts (139–141). A recent study using a poorly immunogenic murine melanoma model show that transient RANKL blockade interrupt central tolerance and unleashes T cells possessing immature TCR to recognize tumor self-antigens and improve response to immunotherapy (142). These data evidence that the therapeutic value of blocking the RANKL/RANK axis for cancer therapy is both due to its direct action in preventing skeletal-related adverse effect and in shaping intrathymic T cell development.
6.5 Interruption of thymic activity
Therapeutical thymectomy for thymic epithelial tumors has been an established procedure for more than 40 years and is associated with several paraneoplastic autoimmune syndromes due to a loss of central tolerance (143, 144). Although it is assumed that thymectomy renders immunosuppression due to disturbance in the pool of conventional T cells in the periphery, a recent study conducted in a preclinical model of primary melanoma documented that cessation of thymic activity in adult mice causes preferential reduction of Treg exports to the periphery, thus increasing the efficacy of anti-tumor immunotherapies targeting the immune checkpoint inhibitor CTLA-4 (145). Corroborating these findings, therapeutical thymectomy for thymoma prevents the increase of Treg cells in the circulation following immunosuppressive therapy (146).
Apart from its role in Treg export, interruption of thymus activity by genotoxic chemotherapy induces secretion of molecules by the thymic microenvironment and creates a chemoprotective niche harboring surviving lymphoma cells following chemotherapy (147). In support to these findings, there is evidence that tumor cells can hide in the thymus and acquire chemo-resistance. The presence of cancer cells within the thymus has been demonstrated in mice injected with 3LL lung tumors (148), while athymic mice presented significantly fewer chemo-resistant lymphoma cells and lived considerably longer than immunocompetent mice (147).
These data are from pre-clinical experimental models, and future studies in humans will provide more information on the impact of thymectomy in anti-tumor immunity and response to chemotherapy.
7 Efficacy of thymic peptides in clinical and preclinical cancer research
Purified thymus extracts are thought to enhance the immune system of patients with cancer, promoting elimination of tumor cells and resistance to opportunistic infections, which are often associated with the use of conventional therapies for cancer (9). Derivatives of thymic peptides, mostly of thymosins, have been detected as products of neoplastically transformed TEC and employed in the early diagnosis and treatment of neoplasms. Besides, studies in animal models and human patients have shown promising results in different types of malignancies, especially when Tα1 was used in combination with other cancer therapies, as reviewed below:
7.1 Clinical use of Tα1 in solid tumors
The therapeutic use of the Tα1 synthetic analogue, thymalfasin, for treating several diseases is currently approved in over 35 countries worldwide for its immunomodulatory activities and safety (10). Approved indications for medical use include adjuvant to chemotherapy. Combination of Tα1 with chemotherapy has been of value to treat oncologic patients, with reference to melanoma, non-small cell lung cancer (NSCLC) and hepatocellular carcinoma.
7.1.1 Melanoma
Dacarbazine (DTIC) is considered the benchmark treatment for advanced melanoma, despite response rates of less than 10% in contemporary trials. A phase II study analyzing the effect of combination of DTIC+Tα1+IL-2 in treating patients with metastatic melanoma showed objective responses in 36% of the patients analyzed and no safety concerns (149). A following up study aiming to analyze the effect of DTIC+Tα1+low dose IFN-α in treating melanoma patients demonstrated response rate of 50%, associated with increase in CD4+ T cells and NK cell numbers in the peripheral blood (150). Due to the low cohort size and absence of Tα1 monotherapy arm for direct comparison, both studies failed to demonstrate a survival benefit for patients receiving Tα1. In larger following up randomized trial involving 488 patients with metastatic melanoma randomly assigned to 5 groups including DTIC+Tα1+IFN-α; DTIC+Tα1; DTIC+IFN-α (control group), 10 and 12 tumor responses were observed in the DTIC+IFN-α+Tα1 and DTIC+Tα1 groups, respectively, versus 4 in the control. Response rates ranged from 1.9 to 23.2 months in patients given Tα1 and from 4.4 to 8.4 months in the control group (151). The high rates of stable disease (26% to 37%) observed in patients treated with Tα1 are characteristic of immunotherapy, where the decline in tumor volume tends to occur slowly and progressively with continued treatment (152). Although the mechanism underlying the activity of DTIC+Tα1 is not fully understood, it is possible that Tα1 potentiates T cell–mediated immune responses directed against tumor antigens.
7.1.2 NSCLC
Tα1 has been used with chemotherapy to treat NSCLC. A study performing a systematic review and meta-analysis of 27 randomized controlled trials in China containing 1925 patients with NSCLC demonstrated clinical efficacy and safety of combination therapy with synthetic thymic peptides and chemotherapy. Optimal conditions for Tα1 treatment included combination with gemcitabine or navelbine and cisplatin, twice a week, with one 3-week cycle (153). In another retrospective study, 5746 patients with margin-free-resected NSCLC patients were divided into the Tα1 group and the control group according to whether Tα1 was used or not after surgery (154). The 5-year disease-free survival and overall survival rates were significantly higher in the Tα1 group compared with the control group (77.3% versus 57.9% and 83.3% versus 65.6%, respectively). This was observed in all subgroups of age, sex, smoking status, and pathological tumor-node-metastasis stage, especially for patients with non-squamous cell NSCLC and without targeted therapy (154). More recently, phase 2 trial where 69 patients received Tα1 during and after chemoradiotherapy based on docetaxel and nedaplatin demonstrated significant reductions in radiation-induced pneumonitis and lymphopenia compared with control group (36.2% versus 53.6% and 19.1% versus 62.1%, respectively) (153).
7.1.3 Hepatocellular carcinoma
In a small phase II randomized trial for unresectable hepatocellular carcinoma where 25 patients were enrolled, Tα1 administration to patients that have gone through transarterial chemoembolization (a combination of regional chemotherapy and some form of hepatic artery occlusion) 5 times weekly for 24 weeks resulted in numerically higher rates (although not statistically significant) of tumor response (155). Through 72 weeks, 57.1% (8/14) of patients in the group receiving TACE + thymalfasin became responders to cytoablative therapy versus 45.5% (5/11) in the group receiving TACE only. Among the 8 responders in the group receiving TACE + thymalfasin, 4 patients became eligible for liver transplant whereas none of the 5 responders in the TACE-only group became eligible for transplant. A larger study enrolling a total of 206 patients with small hepatocellular carcinoma who received liver resections to evaluate the effect of Tα1 as an adjuvant therapy demonstrated a statistically significant increase in 5-years overall survival and recurrence-free survival (82.9% versus 62.9% and 53.3% versus 32.1%, respectively) for patients that received Tα1 in comparison to those that went through resection only (156). Therefore, Tα1 as an adjuvant therapy may improve the prognosis of hepatocellular carcinoma patients.
7.2 Preclinical studies on thymic hormone using murine models
The first observations that Tα1 could play a protective role in melanoma came from the work published in 1983 showing that Tα1 was able to protect mice immunosuppressed with 5-flurouracil chemotherapy from infection by opportunistic pathogens (157). In two other publications from the same year the group showed that Tα1 could similarly protect mice immunosuppressed with cytostatics or X-ray irradiation from metastatic growth and increase survival (30, 31). A more recent study showed that combination of Tα1 with cyclophosphamide significantly increased the median survival time of treated mice, and cured an average of 23% of animals, while none was cured in mice treated with cyclophosphamide only. This was associated with increase of T cell numbers, expression of IL-2 receptor and cytotoxic responses (158). The relevance of IL-2 in potentiating anti-tumor activity of Tα1 in combination with cyclophosphamide was further evidenced in mouse model of Lewis lung carcinoma, where depletion of T cells abolished the positive response to combination therapy (159). Further, chemo-immunotherapy with 5-fuorouracil (5-FU)+Tα1+IL-2 had superior activity over all treatments tested as monotherapies in preventing liver metastases in a rat colorectal cancer model (160). To improve Tα1 targeting of tumor cells, Tα1 was combined with RGD (Arg-Gly-Asp), which has been utilized in delivering anticancer drugs to tumor sites. Results showed that Tα1-RGD had remarkable anti-tumor effects, and its tumor targeting was better than that of Tα1 (161).
Further, several lines of evidence converge to the notion that Tα1 represents a plausible candidate to improve the safety of ICI. This is evidenced in a murine model of ICI-induced colitis where Tα1 administration prevented intestinal toxicity by promoting the indoleamine 2,3-dioxygenase (IDO) 1-dependent tolerogenic immune pathway (162). Despite improving safety of ICI, Tα1 monotherapy showed clear anti-metastatic benefit in a mouse melanoma lung metastasis model but no increase in effectiveness was observed upon addition of anti-PD1 (163).
Altogether, these results provide direct evidence that Tα1 can significantly affect tumor development in humans and murine models. As mentioned above, contrasting with Tα1, thymopoietin expression positively correlates with tumor development (30, 31). In this respect, it is conceivable that neutralizing thymopoietin expression might bring therapeutic advances. As regards thymulin, despite its anti-inflammatory activity well defined (51, 164), data are still lacking in terms of its potential modulatory role on cancer, and to our knowledge no clinical trials have been engaged on this aspect.
6 Conclusion and future direction
We have briefly reviewed some of the potential impacts of thymic-mediated immune and endocrine effects on modulating cancer evolution, emphasizing the relevance of future studies aiming to improve cancer prognosis and reducing side effects of treatments through manipulation of the thymus.
Since thymic-derived immune cells, hormones and cytokines can circulate in the blood impacting diverse aspects of the host’s immune system and tumor cell biology, it is important to understand the immunoendocrine interactions during oncogenic diseases in humans and in preclinical models. Further, the possibility of purifying and manipulating thymic peptides and immature T cells able to recognize tumor self-antigens for clinical use, makes the thymus an attractive target for cancer therapy.
Author contributions
AL, conceptualization and writing the original draft. WS, visualization and proofreading. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocr Rev (2000) 21(4):412–43. doi: 10.1210/edrv.21.4.0402
2. Braun M, Aguilera AR, Sundarrajan A, Corvino D, Stannard K, Krumeich S, et al. Cd155 on tumor cells drives resistance to immunotherapy by inducing the degradation of the activating receptor Cd226 in Cd8(+) T cells. Immunity (2020) 53(4):805–23.e15. doi: 10.1016/j.immuni.2020.09.010
3. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20(11):651–68. doi: 10.1038/s41577-020-0306-5
4. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. Pd-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
5. Choyke PL, Zeman RK, Gootenberg JE, Greenberg JN, Hoffer F, Frank JA. Thymic atrophy and regrowth in response to chemotherapy: ct evaluation. AJR Am J Roentgenol (1987) 149(2):269–72. doi: 10.2214/ajr.149.2.269
6. Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, et al. Constraints on Cd4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral Cd4 cells. Blood (1997) 90(9):3789–98. doi: 10.1182/blood.V90.9.3789
7. Shoham J, Eshel I. Thymic hormonal effect on human peripheral blood lymphocytes in vitro. iii. conditions for mixed lymphocyte-tumor culture assay. J Immunol Methods (1980) 37(3-4):261–72. doi: 10.1016/0022-1759(80)90312-9
8. Consolini R, Cei B, Cini P, Bottone E, Casarosa L. Circulating thymic hormone activity in young cancer patients. Clin Exp Immunol (1986) 66(1):173–80.
9. Wolf E, Milazzo S, Boehm K, Zwahlen M, Horneber M. Thymic peptides for treatment of cancer patients. Cochrane Database Syst Rev (2011) 2011(2):CD003993. doi: 10.1002/14651858.CD003993.pub3
10. Costantini C, Bellet MM, Pariano M, Renga G, Stincardini C, Goldstein AL, et al. A reappraisal of thymosin Alpha1 in cancer therapy. Front Oncol (2019) 9:873. doi: 10.3389/fonc.2019.00873
11. Savino W, Mendes-da-Cruz DA, Lepletier A, Dardenne M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol (2016) 12(2):77–89. doi: 10.1038/nrendo.2015.168
12. Savino W, Duraes J, Maldonado-Galdeano C, Perdigon G, Mendes-da-Cruz DA, Cuervo P. Thymus, undernutrition, and infection: approaching cellular and molecular interactions. Front Nutr (2022) 9:948488. doi: 10.3389/fnut.2022.948488
13. Francelin C, Veneziani LP, Farias ADS, Mendes-da-Cruz DA, Savino W. Neurotransmitters modulate intrathymic T-cell development. Front Cell Dev Biol (2021) 9:668067. doi: 10.3389/fcell.2021.668067
14. Cosway EJ, James KD, Lucas B, Anderson G, White AJ. The thymus medulla and its control of alphabetat cell development. Semin Immunopathol (2021) 43(1):15–27. doi: 10.1007/s00281-020-00830-z
15. Shichkin VP, Antica M. Key factors for thymic function and development. Front Immunol (2022) 13:926516. doi: 10.3389/fimmu.2022.926516
16. Lopes N, Serge A, Ferrier P, Irla M. Thymic crosstalk coordinates medulla organization and T-cell tolerance induction. Front Immunol (2015) 6:365. doi: 10.3389/fimmu.2015.00365
17. van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today (1994) 15(5):214–7. doi: 10.1016/0167-5699(94)90246-1
18. Cordero OJ, Maurer HR, Nogueira M. Novel approaches to immunotherapy using thymic peptides. Immunol Today (1997) 18(1):10–3. doi: 10.1016/s0167-5699(97)80007-2
19. Morozov VG, Khavinson VK. Natural and synthetic thymic peptides as therapeutics for immune dysfunction. Int J Immunopharmacol (1997) 19(9-10):501–5. doi: 10.1016/s0192-0561(97)00058-1
20. Fan YZ, Chang H, Yu Y, Liu J, Wang R. Thymosin Alpha1 suppresses proliferation and induces apoptosis in human leukemia cell lines. Peptides (2006) 27(9):2165–73. doi: 10.1016/j.peptides.2006.03.012
21. Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin Beta4 in tumor metastasis and angiogenesis. J Natl Cancer Inst (2003) 95(22):1674–80. doi: 10.1093/jnci/djg100
22. Huang D, Wang S, Wang A, Chen X, Zhang H. Thymosin beta 4 silencing suppresses proliferation and invasion of non-small cell lung cancer cells by repressing Notch1 activation. Acta Biochim Biophys Sin (Shanghai) (2016) 48(9):788–94. doi: 10.1093/abbs/gmw070
23. Dominari A, Hathaway Iii D, Pandav K, Matos W, Biswas S, Reddy G, et al. Thymosin alpha 1: a comprehensive review of the literature. World J Virol (2020) 9(5):67–78. doi: 10.5501/wjv.v9.i5.67
24. Wang F, Yu T, Zheng H, Lao X. Thymosin Alpha1-fc modulates the immune system and down-regulates the progression of melanoma and breast cancer with a prolonged half-life. Sci Rep (2018) 8(1):12351. doi: 10.1038/s41598-018-30956-y
25. Baumann CA, Badamchian M, Goldstein AL. Thymosin alpha 1 antagonizes dexamethasone and Cd3-induced apoptosis of Cd4+ Cd8+ thymocytes through the activation of camp and protein kinase c dependent second messenger pathways. Mech Ageing Dev (1997) 94(1-3):85–101. doi: 10.1016/s0047-6374(96)01860-x
26. Hsia J, Sarin N, Oliver JH, Goldstein AL. Aspirin and thymosin increase interleukin-2 and interferon-gamma production by human peripheral blood lymphocytes. Immunopharmacology (1989) 17(3):167–73. doi: 10.1016/0162-3109(89)90045-3
27. Ohta Y, Sueki K, Yoneyama Y, Tezuka E, Yagi Y. Immunomodulating activity of thymosin fraction 5 and thymosin alpha 1 in immunosuppressed mice. Cancer Immunol Immunother (1983) 15(2):108–13. doi: 10.1007/BF00199700
28. Yang Z, Guo J, Cui K, Du Y, Zhao H, Zhu L, et al. Thymosin alpha-1 blocks the accumulation of myeloid suppressor cells in nsclc by inhibiting vegf production. BioMed Pharmacother (2020) 131:110740. doi: 10.1016/j.biopha.2020.110740
29. Moody TW. Thymosin Alpha1 as a chemopreventive agent in lung and breast cancer. Ann N Y Acad Sci (2007) 1112:297–304. doi: 10.1196/annals.1415.040
30. Umeda Y, Sakamoto A, Nakamura J, Ishitsuka H, Yagi Y. Thymosin alpha 1 restores nk-cell activity and prevents tumor progression in mice immunosuppressed by cytostatics or X-rays. Cancer Immunol Immunother (1983) 15(2):78–83. doi: 10.1007/BF00199694
31. Ishitsuka H, Umeda Y, Sakamoto A, Yagi Y. Protective activity of thymosin alpha 1 against tumor progression in immunosuppressed mice. Adv Exp Med Biol (1983) 166:89–100. doi: 10.1007/978-1-4757-1410-4_9
32. Xing Y, Ye Y, Zuo H, Li Y. Progress on the function and application of thymosin Beta4. Front Endocrinol (Lausanne) (2021) 12:767785. doi: 10.3389/fendo.2021.767785
33. Caers J, Hose D, Kuipers I, Bos TJ, Van Valckenborgh E, Menu E, et al. Thymosin Beta4 has tumor suppressive effects and its decreased expression results in poor prognosis and decreased survival in multiple myeloma. Haematologica (2010) 95(1):163–7. doi: 10.3324/haematol.2009.006411
34. Wang B, Wang Z, Zhang T, Yang G. Overexpression of thymosin Beta10 correlates with disease progression and poor prognosis in bladder cancer. Exp Ther Med (2019) 18(5):3759–66. doi: 10.3892/etm.2019.8006
35. Song C, Su Z, Guo J. Thymosin beta 10 is overexpressed and associated with unfavorable prognosis in hepatocellular carcinoma. Biosci Rep (2019) 39(3):BSR20182355. doi: 10.1042/BSR20182355
36. Gu Y, Wang C, Wang Y, Qiu X, Wang E. Expression of thymosin Beta10 and its role in non-small cell lung cancer. Hum Pathol (2009) 40(1):117–24. doi: 10.1016/j.humpath.2008.06.023
37. Zhang Y, Feurino LW, Zhai Q, Wang H, Fisher WE, Chen C, et al. Thymosin beta 4 is overexpressed in human pancreatic cancer cells and stimulates proinflammatory cytokine secretion and jnk activation. Cancer Biol Ther (2008) 7(3):419–23. doi: 10.4161/cbt.7.3.5415
38. Zeng J, Yang X, Yang L, Li W, Zheng Y. Thymosin Beta10 promotes tumor-associated macrophages M2 conversion and proliferation Via the Pi3k/Akt pathway in lung adenocarcinoma. Respir Res (2020) 21(1):328. doi: 10.1186/s12931-020-01587-7
39. Lee SH, Son MJ, Oh SH, Rho SB, Park K, Kim YJ, et al. Thymosin Beta(10) inhibits angiogenesis and tumor growth by interfering with ras function. Cancer Res (2005) 65(1):137–48. doi: 10.1158/0008-5472.137.65.1
40. Lee SH, Zhang W, Choi JJ, Cho YS, Oh SH, Kim JW, et al. Overexpression of the thymosin beta-10 gene in human ovarian cancer cells disrupts f-actin stress fiber and leads to apoptosis. Oncogene (2001) 20(46):6700–6. doi: 10.1038/sj.onc.1204683
41. Bao L, Loda M, Janmey PA, Stewart R, Anand-Apte B, Zetter BR. Thymosin beta 15: a novel regulator of tumor cell motility upregulated in metastatic prostate cancer. Nat Med (1996) 2(12):1322–8. doi: 10.1038/nm1296-1322
42. Bao L, Loda M, Zetter BR. Thymosin Beta15 expression in tumor cell lines with varying metastatic potential. Clin Exp Metastasis (1998) 16(3):227–33. doi: 10.1023/a:1006540824969
43. Sun DP, Liew PL, Lin CC, Hung ST, Chen TC, Fang CL, et al. Clinicopathologic and prognostic significance of thymopoietin-alpha overexpression in gastric cancer. J Cancer (2019) 10(21):5099–107. doi: 10.7150/jca.30738
44. Zhang L, Wang G, Chen S, Ding J, Ju S, Cao H, et al. Depletion of thymopoietin inhibits proliferation and induces cell cycle Arrest/Apoptosis in glioblastoma cells. World J Surg Oncol (2016) 14(1):267. doi: 10.1186/s12957-016-1018-y
45. Kim HJ, Hwang SH, Han ME, Baek S, Sim HE, Yoon S, et al. Lap2 is widely overexpressed in diverse digestive tract cancers and regulates motility of cancer cells. PloS One (2012) 7(6):e39482. doi: 10.1371/journal.pone.0039482
46. Liu X, Shen Z. Lncrna tmpo-As1 aggravates the development of hepatocellular carcinoma Via mir-429/Got1 axis. Am J Med Sci (2020) 360(6):711–20. doi: 10.1016/j.amjms.2020.08.010
47. Gebbia V, Valenza R, Testa A, Cannata G, Borsellino N, Gebbia N. A prospective randomized trial of thymopentin versus granulocyte–colony stimulating factor with or without thymopentin in the prevention of febrile episodes in cancer patients undergoing highly cytotoxic chemotherapy. Anticancer Res (1994) 14(2B):731–4.
48. Savino W, Dardenne M. Thymic hormone-containing cells vi. immunohistologic evidence for the simultaneous presence of thymulin, thymopoietin and thymosin alpha 1 in normal and pathological human thymuses. Eur J Immunol (1984) 14(11):987–91. doi: 10.1002/eji.1830141105
49. Savino W, Dardenne M, Papiernik M, Bach JF. Thymic hormone-containing cells. characterization and localization of serum thymic factor in young mouse thymus studied by monoclonal antibodies. J Exp Med (1982) 156(2):628–33. doi: 10.1084/jem.156.2.628
50. Novoselova EG, Lunin SM, Glushkova OV, Khrenov MO, Parfenyuk SB, Zakharova NM, et al. Thymulin, free or bound to pbca nanoparticles, protects mice against chronic septic inflammation. PloS One (2018) 13(5):e0197601. doi: 10.1371/journal.pone.0197601
51. da Silva AL, de Oliveira GP, Kim N, Cruz FF, Kitoko JZ, Blanco NG, et al. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci Adv (2020) 6(24):eaay7973. doi: 10.1126/sciadv.aay7973
52. Mocchegiani E, Paolucci P, Granchi D, Cavallazzi L, Santarelli L, Fabris N. Plasma zinc level and thymic hormone activity in young cancer patients. Blood (1994) 83(3):749–57. doi: 10.1182/blood.V83.3.749.749
53. Kaiserlian D, Savino W, Hassid J, Dardenne M. Studies of the thymus in mice bearing the Lewis lung carcinoma. iii. possible mechanisms of tumor-induced thymic atrophy. Clin Immunol Immunopathol (1984) 32(3):316–25. doi: 10.1016/0090-1229(84)90275-7
54. Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity (2018) 48(3):453–73. doi: 10.1016/j.immuni.2018.03.009
55. Barsac E, de Amat Herbozo C, Gonzalez L, Baranek T, Mallevaey T, Paget C. Regulation and functions of protumoral unconventional T cells in solid tumors. Cancers (Basel) (2021) 13(14):3578. doi: 10.3390/cancers13143578
56. van der Leun AM, Thommen DS, Schumacher TN. Cd8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer (2020) 20(4):218–32. doi: 10.1038/s41568-019-0235-4
57. Weigelin B, Friedl P. T Cell-mediated additive cytotoxicity - death by multiple bullets. Trends Cancer (2022) 8(12):980–7. doi: 10.1016/j.trecan.2022.07.007
58. Hoekstra ME, Vijver SV, Schumacher TN. Modulation of the tumor micro-environment by Cd8(+) T cell-derived cytokines. Curr Opin Immunol (2021) 69:65–71. doi: 10.1016/j.coi.2021.03.016
59. Jacobson CA, Ritz J. Time to put the car-T before the horse. Blood (2011) 118(18):4761–2. doi: 10.1182/blood-2011-09-376137
60. Xin Yu J, Hubbard-Lucey VM, Tang J. The global pipeline of cell therapies for cancer. Nat Rev Drug Discovery (2019) 18(11):821–2. doi: 10.1038/d41573-019-00090-z
61. Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. Cd8(+) T cell exhaustion in cancer. Front Immunol (2021) 12:715234. doi: 10.3389/fimmu.2021.715234
62. Xiao X, Wang Y, Zou Z, Yang Y, Wang X, Xin X, et al. Combination strategies to optimize the efficacy of chimeric antigen receptor T cell therapy in haematological malignancies. Front Immunol (2022) 13:954235. doi: 10.3389/fimmu.2022.954235
63. Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, et al. Pembrolizumab for b-cell lymphomas relapsing after or refractory to Cd19-directed car T-cell therapy. Blood (2022) 139(7):1026–38. doi: 10.1182/blood.2021012634
64. Abbasi S, Totmaj MA, Abbasi M, Hajazimian S, Goleij P, Behroozi J, et al. Chimeric antigen receptor T (Car-T) cells: novel cell therapy for hematological malignancies. Cancer Med (2023) 12(7):7844–58. doi: 10.1002/cam4.5551
65. Lepletier A, Madore J, O'Donnell JS, Johnston RL, Li XY, McDonald E, et al. Tumor Cd155 expression is associated with resistance to anti-Pd1 immunotherapy in metastatic melanoma. Clin Cancer Res (2020) 26(14):3671–81. doi: 10.1158/1078-0432.CCR-19-3925
66. Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. Tigit and pd-1 impair tumor antigen-specific Cd8(+) T cells in melanoma patients. J Clin Invest (2015) 125(5):2046–58. doi: 10.1172/JCI80445
67. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11(1):3801. doi: 10.1038/s41467-020-17670-y
68. Rousseau A, Parisi C, Barlesi F. Anti-tigit therapies for solid tumors: a systematic review. ESMO Open (2023) 8(2):101184. doi: 10.1016/j.esmoop.2023.101184
69. Poncette L, Bluhm J, Blankenstein T. The role of Cd4 T cells in rejection of solid tumors. Curr Opin Immunol (2022) 74:18–24. doi: 10.1016/j.coi.2021.09.005
70. Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T Helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity (2009) 31(5):787–98. doi: 10.1016/j.immuni.2009.09.014
71. Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated Cd8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother (2011) 60(10):1473–84. doi: 10.1007/s00262-011-1054-y
72. Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med (1999) 190(5):617–27. doi: 10.1084/jem.190.5.617
73. Lorvik KB, Hammarstrom C, Fauskanger M, Haabeth OA, Zangani M, Haraldsen G, et al. Adoptive transfer of tumor-specific Th2 cells eradicates tumors by triggering an in situ inflammatory immune response. Cancer Res (2016) 76(23):6864–76. doi: 10.1158/0008-5472.CAN-16-1219
74. Vitiello GA, Miller G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J Exp Med (2020) 217(1):e20190456. doi: 10.1084/jem.20190456
75. Bankaitis KV, Fingleton B. Targeting Il4/Il4r for the treatment of epithelial cancer metastasis. Clin Exp Metastasis (2015) 32(8):847–56. doi: 10.1007/s10585-015-9747-9
76. Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, et al. Interleukin 17, a T-Cell-Derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res (1999) 59(15):3698–704.
77. Owen DL, Sjaastad LE, Farrar MA. Regulatory T cell development in the thymus. J Immunol (2019) 203(8):2031–41. doi: 10.4049/jimmunol.1900662
78. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol (2019) 16(6):356–71. doi: 10.1038/s41571-019-0175-7
79. Onda M, Kobayashi K, Pastan I. Depletion of regulatory T cells in tumors with an anti-Cd25 immunotoxin induces Cd8 T cell-mediated systemic antitumor immunity. Proc Natl Acad Sci U.S.A. (2019) 116(10):4575–82. doi: 10.1073/pnas.1820388116
80. Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by il-10 and tgf-Beta-Producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of Cd4+ and Cd8+ regulatory T cells. J Immunol (2006) 177(2):896–904. doi: 10.4049/jimmunol.177.2.896
81. Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed ctla-4 depletes Cd80/Cd86 by trogocytosis, releasing free pd-L1 on antigen-presenting cells. Proc Natl Acad Sci USA (2021) 118(30):e2023739118. doi: 10.1073/pnas.2023739118
82. Li Q, Jiang N, Zhang Y, Liu Y, Su Z, Yuan Q, et al. Dihydroartemisinin imposes positive and negative regulation on treg and plasma cells Via direct interaction and activation of c-fos. Commun Biol (2023) 6(1):52. doi: 10.1038/s42003-023-04454-5
83. Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol (2013) 4:190. doi: 10.3389/fimmu.2013.00190
84. Saleh R, Elkord E. Foxp3(+) T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett (2020) 490:174–85. doi: 10.1016/j.canlet.2020.07.022
85. Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating Foxp3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol (2009) 27(2):186–92. doi: 10.1200/JCO.2008.18.7229
86. Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating Cd4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res (2006) 12(2):465–72. doi: 10.1158/1078-0432.CCR-05-1886
87. Weerakoon H, Straube J, Lineburg K, Cooper L, Lane S, Smith C, et al. Expression of Cd49f defines subsets of human regulatory T cells with divergent transcriptional landscape and function that correlate with ulcerative colitis disease activity. Clin Transl Immunol (2021) 10(9):e1334. doi: 10.1002/cti2.1334
88. Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of Foxp3 in human activated nonregulatory Cd4+ T cells. Eur J Immunol (2007) 37(1):129–38. doi: 10.1002/eji.200636435
89. Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias SC, Dartiguenave F, Burruni R, et al. Double positive Cd4(+)Cd8(+) T cells are enriched in urological cancers and favor T helper-2 polarization. Front Immunol (2019) 10:622. doi: 10.3389/fimmu.2019.00622
90. Schad SE, Chow A, Mangarin L, Pan H, Zhang J, Ceglia N, et al. Tumor-induced double positive T cells display distinct lineage commitment mechanisms and functions. J Exp Med (2022) 219(6):e20212169. doi: 10.1084/jem.20212169
91. Morrot A, Terra-Granado E, Perez AR, Silva-Barbosa SD, Milicevic NM, Farias-de-Oliveira DA, et al. Chagasic thymic atrophy does not affect negative selection but results in the export of activated Cd4+Cd8+ T cells in severe forms of human disease. PloS Negl Trop Dis (2011) 5(8):e1268. doi: 10.1371/journal.pntd.0001268
92. Desfrancois J, Moreau-Aubry A, Vignard V, Godet Y, Khammari A, Dreno B, et al. Double positive Cd4cd8 alphabeta T cells: a new tumor-reactive population in human melanomas. PloS One (2010) 5(1):e8437. doi: 10.1371/journal.pone.0008437
93. Overgaard NH, Jung JW, Steptoe RJ, Wells JW. Cd4+/Cd8+ double-positive T cells: more than just a developmental stage? J Leukoc Biol (2015) 97(1):31–8. doi: 10.1189/jlb.1RU0814-382
94. Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of mait cells. Nat Immunol (2019) 20(9):1110–28. doi: 10.1038/s41590-019-0444-8
95. Nel I, Bertrand L, Toubal A, Lehuen A. Mait cells, guardians of skin and mucosa? Mucosal Immunol (2021) 14(4):803–14. doi: 10.1038/s41385-021-00391-w
96. Ruf B, Catania VV, Wabitsch S, Ma C, Diggs LP, Zhang Q, et al. Activating mucosal-associated invariant T cells induces a broad antitumor response. Cancer Immunol Res (2021) 9(9):1024–34. doi: 10.1158/2326-6066.CIR-20-0925
97. Cogswell DT, Gapin L, Tobin HM, McCarter MD, Tobin RP. Mait cells: partners or enemies in cancer immunotherapy? Cancers (Basel) (2021) 13(7):1502. doi: 10.3390/cancers13071502
98. Vorwald VM, Davis DM, Van Gulick RJ, Torphy RJ, Borgers JS, Klarquist J, et al. Circulating Cd8(+) mucosal-associated invariant T cells correlate with improved treatment responses and overall survival in anti-Pd-1-Treated melanoma patients. Clin Transl Immunol (2022) 11(1):e1367. doi: 10.1002/cti2.1367
99. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med (2015) 21(8):938–45. doi: 10.1038/nm.3909
100. Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DIA, et al. (Nkt) cell developmental pathway iinvolving a thymus-dependent Nk1.1(-)Cd4(+) Cd1d-dependent precursor stage. J Exp Med (2002) 195(7):835–44. doi: 10.1084/jem.20011544
101. Macho-Fernandez E, Brigl M. The extended family of Cd1d-restricted nkt cells: sifting through a mixed bag of tcrs, antigens, and functions. Front Immunol (2015) 6:362. doi: 10.3389/fimmu.2015.00362
102. Terabe M, Berzofsky JA. The role of nkt cells in tumor immunity. Adv Cancer Res (2008) 101:277–348. doi: 10.1016/S0065-230X(08)00408-9
103. Terabe M, Berzofsky JA. Tissue-specific roles of nkt cells in tumor immunity. Front Immunol (2018) 9:1838. doi: 10.3389/fimmu.2018.01838
104. Robertson FC, Berzofsky JA, Terabe M. Nkt cell networks in the regulation of tumor immunity. Front Immunol (2014) 5:543. doi: 10.3389/fimmu.2014.00543
105. Hix LM, Shi YH, Brutkiewicz RR, Stein PL, Wang CR, Zhang M. Cd1d-expressing breast cancer cells modulate nkt cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PloS One (2011) 6(6):e20702. doi: 10.1371/journal.pone.0020702
106. Li Y, Li G, Zhang J, Wu X, Chen X. The dual roles of human gammadelta T cells: anti-tumor or tumor-promoting. Front Immunol (2020) 11:619954. doi: 10.3389/fimmu.2020.619954
107. Chan KF, Duarte JDG, Ostrouska S, Behren A. Gammadelta T cells in the tumor microenvironment-interactions with other immune cells. Front Immunol (2022) 13:894315. doi: 10.3389/fimmu.2022.894315
108. Kakimi K, Matsushita H, Murakawa T, Nakajima J. Gammadelta T cell therapy for the treatment of non-small cell lung cancer. Transl Lung Cancer Res (2014) 3(1):23–33. doi: 10.3978/j.issn.2218-6751.2013.11.01
109. Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with gammadelta T cells: many paths ahead of us. Cell Mol Immunol (2020) 17(9):925–39. doi: 10.1038/s41423-020-0504-x
110. Saura-Esteller J, de Jong M, King LA, Ensing E, Winograd B, de Gruijl TD, et al. Gamma delta T-cell based cancer immunotherapy: past-Present-Future. Front Immunol (2022) 13:915837. doi: 10.3389/fimmu.2022.915837
111. Chen X, Shang W, Xu R, Wu M, Zhang X, Huang P, et al. Distribution and functions of gammadelta T cells infiltrated in the ovarian cancer microenvironment. J Transl Med (2019) 17(1):144. doi: 10.1186/s12967-019-1897-0
112. Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, et al. Gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell (2016) 166(6):1485–99.e15. doi: 10.1016/j.cell.2016.07.046
113. Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, et al. Tumor-infiltrating il-17-Producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol (2010) 40(7):1927–37. doi: 10.1002/eji.200940157
114. Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol (2015) 16(11):1114–23. doi: 10.1038/ni.3298
115. Scarpino S, Di Napoli A, Stoppacciaro A, Antonelli M, Pilozzi E, Chiarle R, et al. Expression of autoimmune regulator gene (Aire) and T regulatory cells in human thymomas. Clin Exp Immunol (2007) 149(3):504–12. doi: 10.1111/j.1365-2249.2007.03442.x
116. Yamada Y, Weis CA, Thelen J, Sticht C, Schalke B, Strobel P, et al. Thymoma associated myasthenia gravis (Tamg): differential expression of functional pathways in relation to mg status in different thymoma histotypes. Front Immunol (2020) 11:664. doi: 10.3389/fimmu.2020.00664
117. Savino W, Manganella G, Verley JM, Wolff A, Berrih S, Levasseur P, et al. Thymoma epithelial cells secrete thymic hormone but do not express class ii antigens of the major histocompatibility complex. J Clin Invest (1985) 76(3):1140–6. doi: 10.1172/JCI112069
118. Weksler B, Nason KS, Mackey D, Gallagher A, Pennathur A. Thymomas and extrathymic cancers. Ann Thorac Surg (2012) 93(3):884–8. doi: 10.1016/j.athoracsur.2011.05.089
119. Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science (2012) 336(6077):91–5. doi: 10.1126/science.1218004
120. Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of Stat3. Hepatology (2011) 54(3):900–9. doi: 10.1002/hep.24486
121. Guillon A, Gueugnon F, Mavridis K, Dalloneau E, Jouan Y, Diot P, et al. Interleukin-22 receptor is overexpressed in nonsmall cell lung cancer and portends a poor prognosis. Eur Respir J (2016) 47(4):1277–80. doi: 10.1183/13993003.01580-2015
122. Chen X, Wang Y, Wang J, Wen J, Jia X, Wang X, et al. Accumulation of T-helper 22 cells, interleukin-22 and myeloid-derived suppressor cells promotes gastric cancer progression in elderly patients. Oncol Lett (2018) 16(1):253–61. doi: 10.3892/ol.2018.8612
123. Xu X, Tang Y, Guo S, Zhang Y, Tian Y, Ni B, et al. Increased intratumoral interleukin 22 levels and frequencies of interleukin 22-producing Cd4+ T cells correlate with pancreatic cancer progression. Pancreas (2014) 43(3):470–7. doi: 10.1097/MPA.0000000000000055
124. Markota A, Endres S, Kobold S. Targeting interleukin-22 for cancer therapy. Hum Vaccin Immunother (2018) 14(8):2012–5. doi: 10.1080/21645515.2018.1461300
125. Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, et al. Production of Bmp4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol (2018) 3(19):eaal2736. doi: 10.1126/sciimmunol.aal2736
126. Lepletier A, Hun ML, Hammett MV, Wong K, Naeem H, Hedger M, et al. Interplay between follistatin, activin a, and Bmp4 signaling regulates postnatal thymic epithelial progenitor cell differentiation during aging. Cell Rep (2019) 27(13):3887–901.e4. doi: 10.1016/j.celrep.2019.05.045
127. Kallioniemi A. Bone morphogenetic protein 4-a fascinating regulator of cancer cell behavior. Cancer Genet (2012) 205(6):267–77. doi: 10.1016/j.cancergen.2012.05.009
128. Yan G, Dai M, Zhang C, Poulet S, Moamer A, Wang N, et al. Tgfbeta/Cyclin D1/Smad-mediated inhibition of Bmp4 promotes breast cancer stem cell self-renewal activity. Oncogenesis (2021) 10(3):21. doi: 10.1038/s41389-021-00310-5
129. Lombardo Y, Scopelliti A, Cammareri P, Todaro M, Iovino F, Ricci-Vitiani L, et al. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology (2011) 140(1):297–309. doi: 10.1053/j.gastro.2010.10.005
130. Laatio L, Myllynen P, Serpi R, Rysa J, Ilves M, Lappi-Blanco E, et al. Bmp-4 expression has prognostic significance in advanced serous ovarian carcinoma and is affected by cisplatin in ovcar-3 cells. Tumour Biol (2011) 32(5):985–95. doi: 10.1007/s13277-011-0200-7
131. Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol (2009) 218(4):520–9. doi: 10.1002/path.2563
132. Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, et al. Keratinocyte growth factor (Kgf) enhances postnatal T-cell development Via enhancements in proliferation and function of thymic epithelial cells. Blood (2007) 109(9):3803–11. doi: 10.1182/blood-2006-10-049767
133. Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, et al. Regulation of thymic epithelium by keratinocyte growth factor. Blood (2002) 100(9):3269–78. doi: 10.1182/blood-2002-04-1036
134. Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, et al. Keratinocyte growth factor (Kgf) is required for postnatal thymic regeneration. Blood (2006) 107(6):2453–60. doi: 10.1182/blood-2005-07-2831
135. Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med (2004) 351(25):2590–8. doi: 10.1056/NEJMoa040125
136. Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, et al. The cytokine rankl produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity (2008) 29(3):438–50. doi: 10.1016/j.immuni.2008.06.018
137. Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, et al. The tumor necrosis factor family receptors rank and Cd40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity (2008) 29(3):423–37. doi: 10.1016/j.immuni.2008.06.015
138. Kiechl S, Schramek D, Widschwendter M, Fourkala EO, Zaikin A, Jones A, et al. Aberrant regulation of Rankl/Opg in women at high risk of developing breast cancer. Oncotarget (2017) 8(3):3811–25. doi: 10.18632/oncotarget.14013
139. Castellano D, Sepulveda JM, Garcia-Escobar I, Rodriguez-Antolin A, Sundlov A, Cortes-Funes H. The role of rank-ligand inhibition in cancer: the story of denosumab. Oncologist (2011) 16(2):136–45. doi: 10.1634/theoncologist.2010-0154
140. Henry D, Vadhan-Raj S, Hirsh V, von Moos R, Hungria V, Costa L, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer (2014) 22(3):679–87. doi: 10.1007/s00520-013-2022-1
141. Pang L, Gan C, Xu J, Jia Y, Chai J, Huang R, et al. Bone metastasis of breast cancer: molecular mechanisms and therapeutic strategies. Cancers (Basel) (2022) 14(23):5727. doi: 10.3390/cancers14235727
142. Dhuey E, Oldridge O, Roshan R, Hannah D, Yongjun Y, Anderson MS, et al. Therapeutic interruption of T cell development generates high-affinity T cells that escape exhaustion and improve cancer immunotherapy. bioRxiv (2022). doi: 10.1101/2022.01.19.476935
143. Keynes G. The results of thymectomy in myasthenia gravis. Br Med J (1949) 2(4628):611–6. doi: 10.1136/bmj.2.4628.611
144. Wilkins EW Jr., Castleman B. Thymoma: a continuing survey at the Massachusetts general hospital. Ann Thorac Surg (1979) 28(3):252–6. doi: 10.1016/s0003-4975(10)63114-1
145. Almeida-Santos J, Bergman ML, Cabral IA, Demengeot J. Interruption of thymic activity in adult mice improves responses to tumor immunotherapy. J Immunol (2021) 206(5):978–86. doi: 10.4049/jimmunol.2000626
146. Fattorossi A, Battaglia A, Buzzonetti A, Ciaraffa F, Scambia G, Evoli A. Circulating and thymic Cd4 Cd25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology (2005) 116(1):134–41. doi: 10.1111/j.1365-2567.2005.02220.x
147. Gilbert LA, Hemann MT. DNA Damage-mediated induction of a chemoresistant niche. Cell (2010) 143(3):355–66. doi: 10.1016/j.cell.2010.09.043
148. Kaiserlian D, Savino W, Bach JF. Studies of the thymus in mice bearing the Lewis lung carcinoma. i. thymic natural killer cell activity in 3ll tumor-bearing mice. Cell Immunol (1983) 80(1):187–97. doi: 10.1016/0008-8749(83)90105-3
149. Lopez M, Carpano S, Cavaliere R, Di Lauro L, Ameglio F, Vitelli G, et al. Biochemotherapy with thymosin alpha 1, interleukin-2 and dacarbazine in patients with metastatic melanoma: clinical and immunological effects. Ann Oncol (1994) 5(8):741–6. doi: 10.1093/oxfordjournals.annonc.a058979
150. Rasi G, Terzoli E, Izzo F, Pierimarchi P, Ranuzzi M, Sinibaldi-Vallebona P, et al. Combined treatment with thymosin-Alpha1 and low dose interferon-alpha after dacarbazine in advanced melanoma. Melanoma Res (2000) 10(2):189–92. doi: 10.1097/00008390-200010020-00012
151. Maio M, Mackiewicz A, Testori A, Trefzer U, Ferraresi V, Jassem J, et al. Large Randomized study of thymosin alpha 1, interferon Alfa, or both in combination with dacarbazine in patients with metastatic melanoma. J Clin Oncol (2010) 28(10):1780–7. doi: 10.1200/JCO.2009.25.5208
152. Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. Ctla-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res (2012) 18(7):2039–47. doi: 10.1158/1078-0432.CCR-11-1823
153. Zeng FL, Xiao Z, Wang CQ, Jiang Y, Shan JL, Hu SS, et al. Clinical efficacy and safety of synthetic thymic peptides with chemotherapy for non-small cell lung cancer in China: a systematic review and meta-analysis of 27 randomized controlled trials following the prisma guidelines. Int Immunopharmacol (2019) 75:105747. doi: 10.1016/j.intimp.2019.105747
154. Guo CL, Mei JD, Jia YL, Gan FY, Tang YD, Liu CW, et al. Impact of thymosin Alpha1 as an immunomodulatory therapy on long-term survival of non-small cell lung cancer patients after R0 resection: a propensity score-matched analysis. Chin Med J (Engl) (2021) 134(22):756. doi: 10.1097/CM9.0000000000001819
155. Gish RG, Gordon SC, Nelson D, Rustgi V, Rios I. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int (2009) 3(3):480–9. doi: 10.1007/s12072-009-9132-3
156. He C, Peng W, Li C, Wen TF. Thymalfasin, a promising adjuvant therapy in small hepatocellular carcinoma after liver resection. Med (Baltimore) (2017) 96(16):e6606. doi: 10.1097/MD.0000000000006606
157. Ishitsuka H, Umeda Y, Nakamura J, Yagi Y. Protective activity of thymosin against opportunistic infections in animal models. Cancer Immunol Immunother (1983) 14(3):145–50. doi: 10.1007/BF00205352
158. Pica F, Fraschetti M, Matteucci C, Tuthill C, Rasi G. High doses of thymosin alpha 1 enhance the anti-tumor efficacy of combination chemo-immunotherapy for murine B16 melanoma. Anticancer Res (1998) 18(5A):3571–8.
159. Mastino A, Favalli C, Grelli S, Rasi G, Pica F, Goldstein AL, et al. Combination therapy with thymosin alpha 1 potentiates the anti-tumor activity of interleukin-2 with cyclophosphamide in the treatment of the Lewis lung carcinoma in mice. Int J Cancer (1992) 50(3):493–9. doi: 10.1002/ijc.2910500327
160. Silecchia G, Guarino E, Sinibaldi-Vallebona P, Pierimarchi P, Restuccia A, Spaziani E, et al. Efficacy of repeated cycles of chemo-immunotherapy with thymosin Alpha1 and interleukin-2 after intraperitoneal 5-fluorouracil delivery. Cancer Immunol Immunother (1999) 48(4):172–8. doi: 10.1007/s002620050562
161. Peng R, Xu C, Zheng H, Lao X. Modified thymosin alpha 1 distributes and inhibits the growth of lung cancer in vivo. ACS Omega (2020) 5(18):10374–81. doi: 10.1021/acsomega.0c00220
162. Renga G, Bellet MM, Pariano M, Gargaro M, Stincardini C, D'Onofrio F, et al. Thymosin Alpha1 protects from ctla-4 intestinal immunopathology. Life Sci Alliance (2020) 3(10):202000662. doi: 10.26508/lsa.202000662
163. King RS, Tuthill C. Evaluation of thymosin alpha 1 in nonclinical models of the immune-suppressing indications melanoma and sepsis. Expert Opin Biol Ther (2015) 15(Suppl 1):S41–9. doi: 10.1517/14712598.2015.1008446
Keywords: thymus, thymus-derived peptide hormones, T cell subsets, immunoendocrine interactions, cancer therapy
Citation: Savino W and Lepletier A (2023) Thymus-derived hormonal and cellular control of cancer. Front. Endocrinol. 14:1168186. doi: 10.3389/fendo.2023.1168186
Received: 17 February 2023; Accepted: 26 June 2023;
Published: 17 July 2023.
Edited by:
Benoit Pourcet, Université de Lille, FranceReviewed by:
Philipp Stroebel, University Medical Center Göttingen, GermanyQilong Li, Shenyang Agricultural University, China
Copyright © 2023 Savino and Lepletier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wilson Savino, d2lsc29uLnNhdmlub0BmaW9jcnV6LmJy; Ailin Lepletier, YS5sZXBsZXRpZXJkZW9saXZlaXJhQGdyaWZmaXRoLmVkdS5hdQ==
 Wilson Savino
Wilson Savino Ailin Lepletier
Ailin Lepletier