- 1Department of Pharmacy, Taizhou Hospital of Zhejiang Province affiliated to Wenzhou Medical University, Taizhou, Zhejiang, China
- 2Key Laboratory of Pathobiology, Ministry of Education, Nanomedicine and Translational Research Center, The Third Bethune Hospital of Jilin University, Changchun, Jilin, China
- 3Department of Orthopedic, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China
- 4Department of Orthopedic, Taizhou Hospital of Zhejiang Province affiliated to Wenzhou Medical University, Taizhou, Zhejiang, China
Objective: Diabetes mellitus is a worldwide health problem, and it remains unclarified whether fruit is beneficial in glycemic control. This study aimed to analyze evidence from randomized controlled trials evaluating the effect of fruit consumption on glucose control.
Methods: We searched the PubMed, EMBASE, Ovid, Web of Science, and Cochrane Central Register of Controlled Trials databases from the respective database inception dates to December 30, 2022, to identify randomized controlled trials that evaluated the effects of fruit consumption on glucose control. Two researchers independently screened the studies in accordance with the inclusion and exclusion criteria, and performed the literature quality evaluation and data extraction. RevMan 5.4 software was used to perform the data analysis.
Results: Nineteen randomized controlled trials with 888 participants were included. Fruit consumption significantly decreased the fasting blood glucose concentration (MD -8.38, 95% CI -12.34 to -4.43), but it showed no significant difference in the glycosylated hemoglobin (MD -0.17, 95% CI -0.51 to 0.17). Subgroup analyses further suggested that the consumption of both fresh and dried fruit decreased the fasting blood glucose concentration.
Conclusions: Increasing the fruit intake reduced fasting blood glucose concentration. Therefore, we recommend that patients with diabetes eat more fruits while ensuring that their total energy intake remains unchanged.
Introduction
According to the International Diabetes Federation Diabetes Atlas of 2021, there are 536.6 million adults living with diabetes worldwide (1). Complications arising from poor glycemic control are a major risk factor against healthy survival in patients with diabetes, and it is unclear whether glycemic control is influenced by fruit consumption. The Australian Diabetes, Obesity and Lifestyle Study of 7675 participants with an average age of 54 years showed that those who ate about two servings of fruit (including apples, bananas, and oranges) per day had a 36% lower risk of developing type II diabetes over the subsequent 5 years than those who ate less than half a serving of fruit per day (2). There is also a clear negative correlation between fruit intake and markers of insulin sensitivity, meaning that people who eat more fruit need to produce less insulin to lower their blood glucose concentration (2). This is important because abnormal metabolism in diabetes, hypertension, vascular sclerosis, obesity, and heart disease is strongly associated with hyperinsulinemia (3, 4). Furthermore, an unhealthy diet lacking fruits and vegetables increases the risks of cardiovascular disease and tumorigenesis (5).
Fresh fruits are rich in dietary fiber, organic acids, minerals, and antioxidants (e.g., vitamins, polyphenols), and contain small amounts of fat and calories, which helps regulate the composition and metabolic activity of intestinal microbes and reduce the incidence of complications in patients with diabetes (6). Polyphenols have a variety of important biological activities, including antiviral, antibacterial, anti-inflammatory, anticancer, and antioxidant. The most important of these activities is antioxidation, which converts free radicals into more stable free radicals to promote their scavenging ability, or reduces reactive oxygen species production by inhibiting mitochondrial oxidative stress to maintain cellular redox homeostasis (7). A 7-year prospective study of 500,000 Chinese adults showed a 17% reduction in mortality among patients with diabetes who consumed fruit on 3 or more days per week, as well as a 28% and 13% reduction in the risks of diabetic microvascular complications and macrovascular complications, respectively (8). Recent studies of patients with diabetes have shown that flavonoid-rich fruit intake is associated with lower glycosylated hemoglobin (HbA1c) and fasting blood glucose concentrations, and that increased flavonoid fruit intake reduces the risk of retinopathy by 30% (9).
In recent years, increasing attention has been paid to the impact of fresh fruit intake on type 2 diabetes. Fruit has a high sugar content and fruit consumption may affect the blood glucose management. Traditional beliefs have led patients with diabetes to eat only a limited variety of fruits with a low sugar content, such as cucumbers. However, recent studies have shown that fruit consumption has no significant effect on the fasting blood glucose concentration and the blood glucose concentration at 2 hours after meals compared with a control group (10). Furthermore, fruit intake reportedly helps control the HbA1c concentration (11). In addition, another study reported that fruits not only improve the postprandial concentrations of glucose and triglycerides, but also reduce inflammation and cardiovascular risk. Although many clinical studies have assessed the impact of fruits on the fasting blood glucose concentration, most were not large-scale, randomized, controlled trials (RCTs). Therefore, the present meta-analysis combined the previous research findings to explore the relationship between fruit intake and blood glucose control in patients with diabetes, to provide a reference for the consumption of fruits by patients with type 2 diabetes.
Materials and methods
According to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement, this meta-analysis was performed in agreement (12). The protocol for this meta-analysis was registered on PROSPERO (Registration No: CRD42021237276).
Inclusion criteria
(1) RCTs with human participants; (2) studies evaluating fruit intake and control of blood glucose concentration; (3) studies including patients diagnosed with diabetes or prediabetes; (4) studies with a follow-up of at least 1 month; (5) studies that assessed the BPG or HbA1c concentration; (6) studies that assessed the intake of fresh fruit, fruit juice, dry fruit, or fruit powder.
Exclusion criteria
(1) Letters, case reports, reviews, observational studies, animal trials, or republished studies; (2) cohort studies; (3) studies that assessed the intake of fruit extract; (4) studies that included patients diagnosed with type 1 diabetes.
Outcomes
The primary outcome was the difference in the blood glucose response to fruit consumption compared with control. The second outcome was the difference in the HbA1c concentration between a group that consumed fruit versus a control group. The serum lipid and lipoprotein concentrations were also analyzed.
Search strategy
Two researcher searched the PubMed, Ovid, Web of Science, and Cochrane Central Register of Controlled Trials databases from their respective inception dates to December 30, 2022, using the keywords “(blood glucose or serum glucose or plasma glucose or glycemic or glycaemic or HbA1c or Hemoglobin A1c) and (fruit or juice) and (diabetes or diabetic or prediabetes or insulin resistance or impaired glucose tolerance) and (randomized controlled trial OR controlled clinical trial OR randomized OR randomly)”. We also searched the International Clinical Trials Registry Platform maintained by the World Health Organization to identify ongoing or unpublished eligible trials, and searched the reference lists of articles retrieved in the database search to identify related articles. No language restrictions were applied during the literature search.
Study selection
After the removal of duplicates, two independent researchers (SS and YR) screened all titles and abstracts and determined the study eligibility in accordance with the inclusion and exclusion criteria. For studies considered eligible, the researchers obtained the full text and performed further screening. When the two researchers had differing opinions regarding the eligibility of a study for inclusion and could not reach a consensus, the senior researcher made the final decision after a group discussion.
Data collection
Two researchers (HL and YR) used a standard data extraction form to independently extract all related data from the selected RCTs. When the RCTs had more than two groups and permitted multiple comparisons, we pooled only the data and information of interest reported in the original article. If a study included more than one intervention, we combined groups to create a single pair-wise comparison. The extracted data included the first author’s name, year of publication, type of study, sample size, interventions in the control and experimental groups, and follow-up duration. Disagreements were resolved by consensus.
Assessment of risk of bias
Two researchers (YWS and YR) independently assessed the quality of all included RCTs based on the Cochrane risk-of-bias criteria (13).
Data synthesis
The meta-analysis was performed using RevMan software (version 5.4; The Cochrane Collaboration). The heterogeneity was assessed by the Q test and I2 value. If heterogeneity was not present (P>0.1 and I2<50%), the data were combined with a fixed effect model. If heterogeneity was present (P<0.1 or I2 >50%), the random effects model was used. The mean difference (MD) and associated 95% confidence interval (CI) were used to assess outcomes, and P<0.05 was taken to indicate a significant difference. The possibility of small study effects was assessed qualitatively by visual estimations of funnel plots.
Subgroup analyses
We performed several subgroup analyses to test interactions in accordance with the intake of fresh fruit or dry fruit.
Sensitivity analyses
We performed sensitivity analyses by excluding the largest trial, excluding cluster-randomized or quasi-randomized trials, excluding trials with a high risk of bias, and using random effect models.
Results
Eligible studies and study characteristics
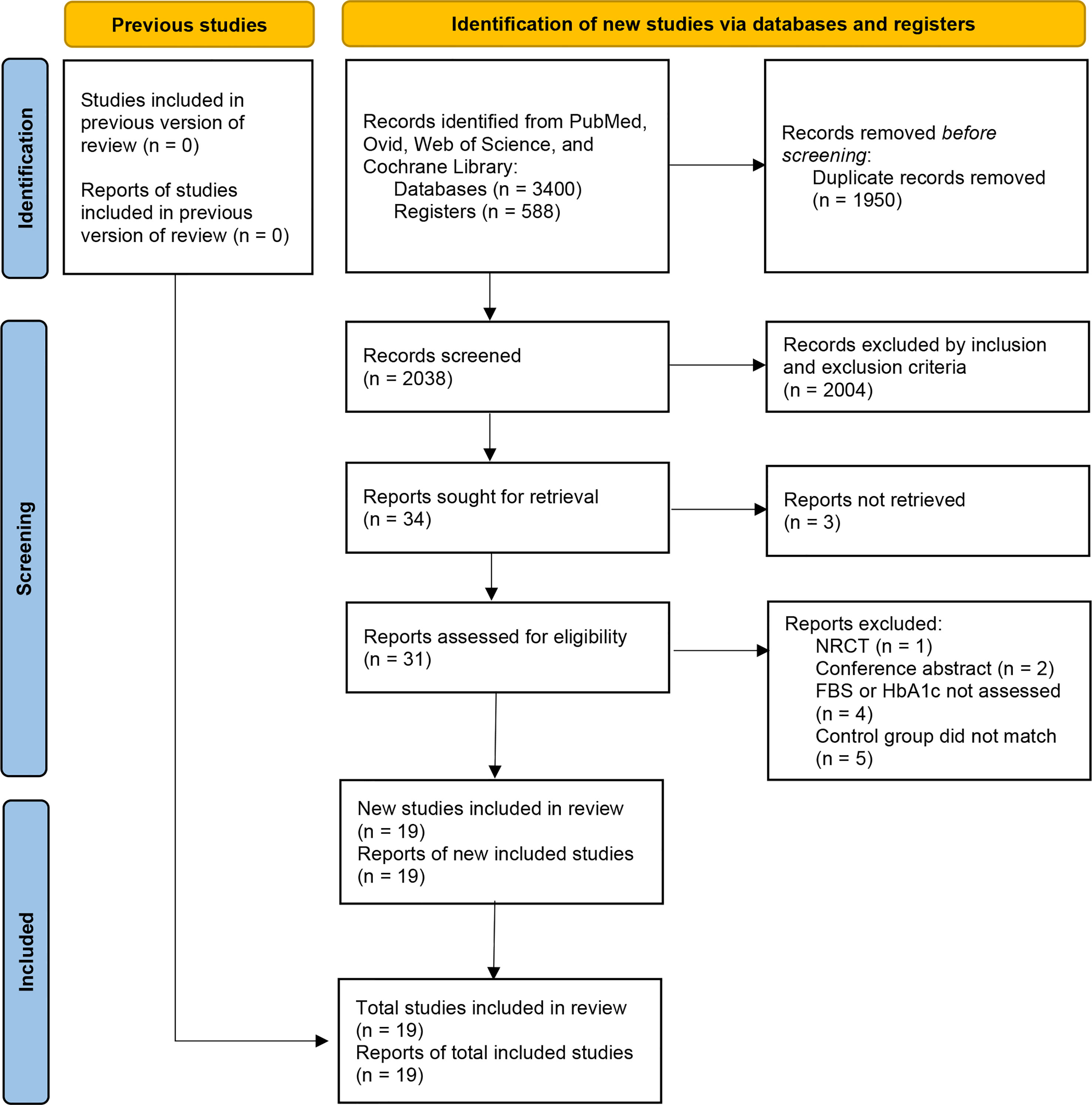
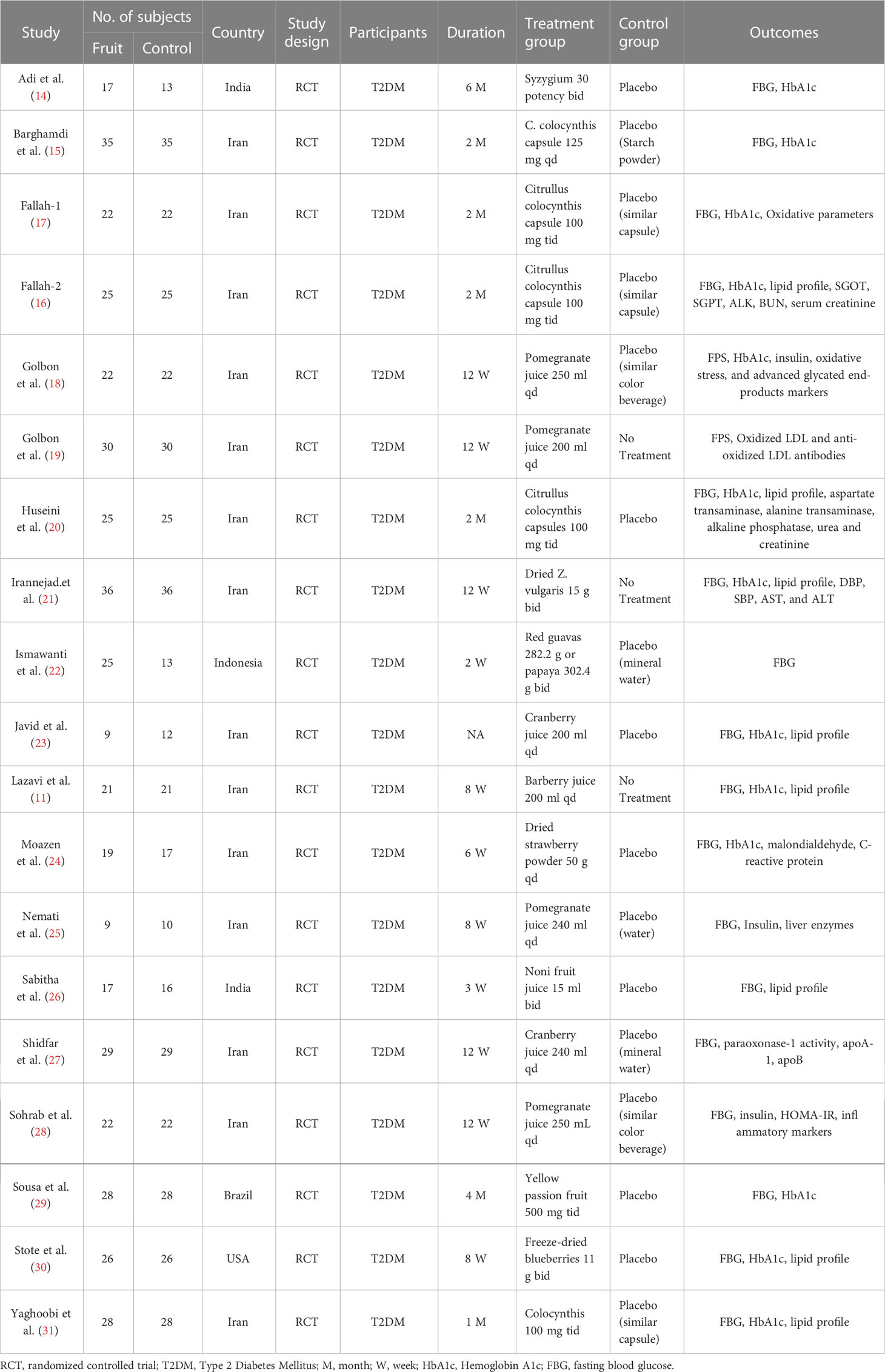
We initially identified a total of 3988 articles, and finally included nineteen eligible RCTs with 888 participants in the meta-analysis (11, 14–31). The study flow chart is shown in Figure 1. A summary of the included RCTs is shown in Table 1. The risk of bias of each of the included RCTs is shown in Figure 2.
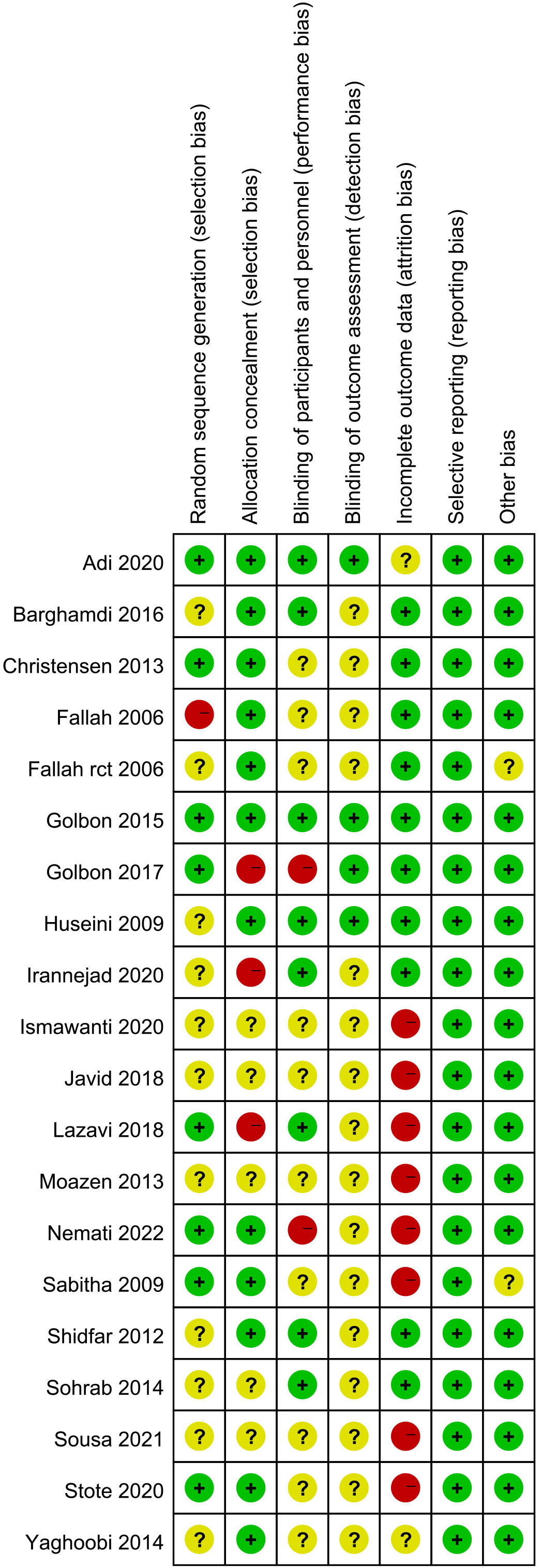
Figure 2 Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Primary outcome: effect of fruit on glycemic control
All nineteen RCTs reported the fasting blood glucose concentration. Compared with the control group, fruit consumption reduced the fasting blood glucose concentration (MD -8.38, 95% CI -12.34 to -4.43, I2 = 32%; Figure 3A). Stratified subgroup analyses were conducted to determine whether the effect on the fasting blood glucose concentration differed for the intake of fresh fruit/fruit juice compared with dry fruit. Compared with control, the pooled result showed a significant reduction in fasting blood glucose concentration after the consumption of both fresh fruit (MD -12.82, 95% CI -22.24 to -3.4, I2 = 48%; Figure 3B1) and dry fruit (MD -5.03, 95% CI -7.15 to -2.90, I2 = 0%; Figure 3B2).
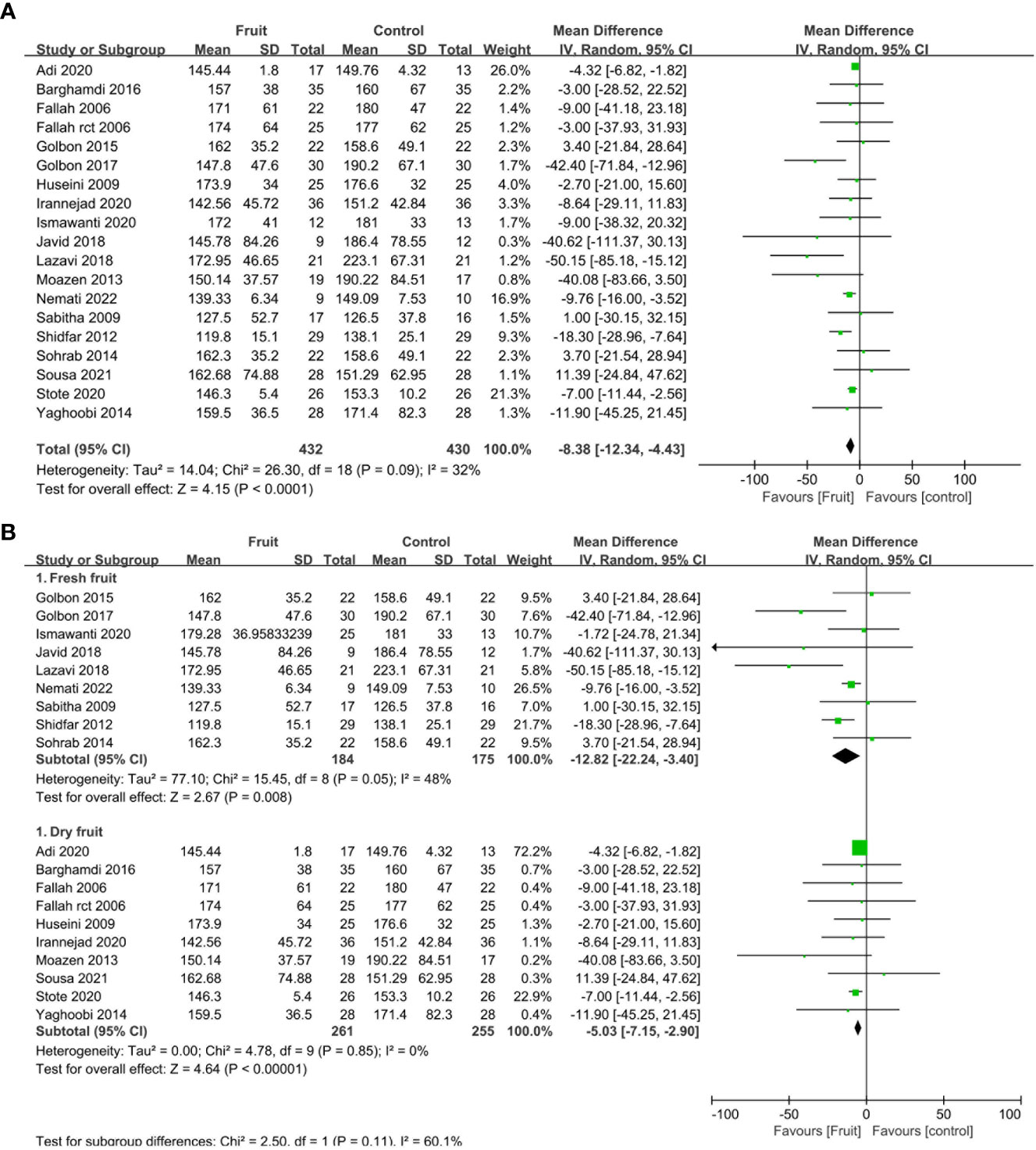
Figure 3 (A) Forest plot of comparison: fruit versus placebo; outcome: fasting blood glucose; (B) Forest plot of subgroup comparison: fruit versus placebo; outcome: fasting blood glucose.
Secondary outcomes
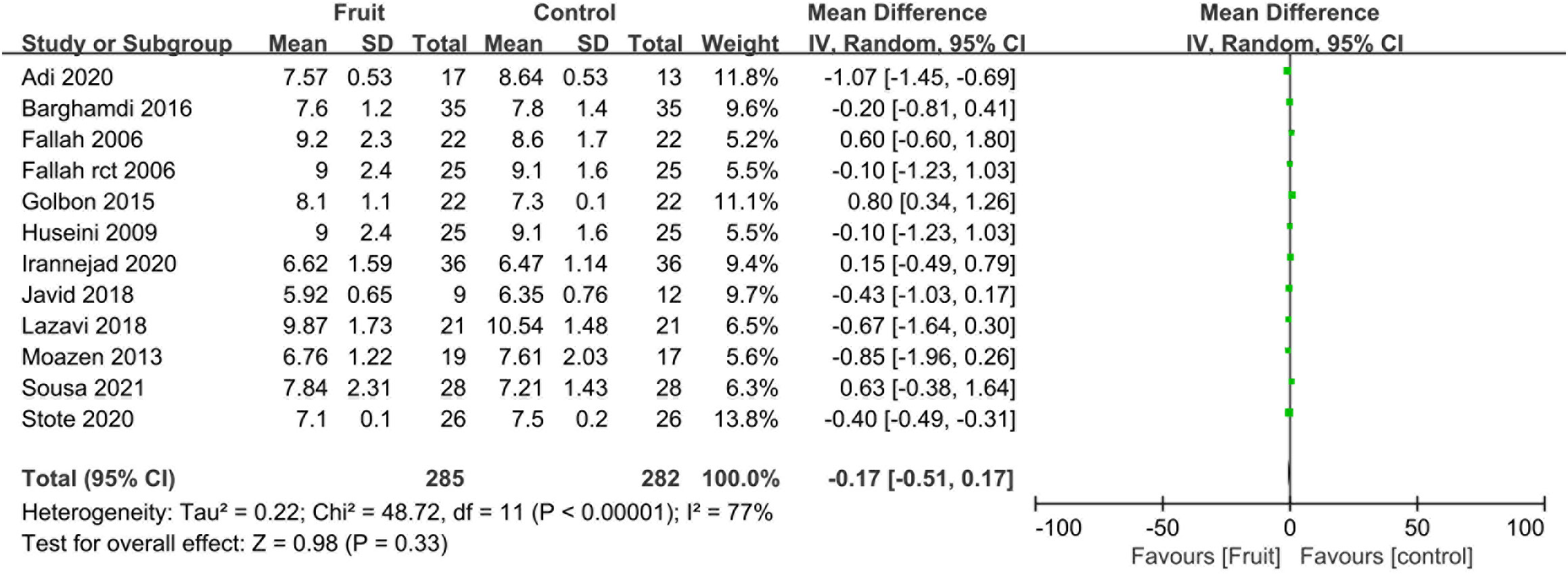
Pooled analysis showed no difference in the HbA1c concentration between the fruit consumption group and the control group (MD -0.17, 95% CI -0.51 to 0.17, I2 = 77%; Figure 4).
Discussion
Our meta-analysis showed that fruit consumption significantly reduced fasting blood glucose concentrations but had no significant effect on Hb1Ac concentrations in people with diabetes. We speculate that because the monosaccharides in fruits are mainly fructose-based, have a low blood glucose response, and are slow to absorb, the metabolism of fruit does not require the participation of insulin, and can be quickly cleared and transferred after reaching the liver (32). Another possible reason may be that some of the included fruits have anti-diabetic effects to some extent (14–17, 20, 21, 29, 31). Although the dry fruit had an increased sugar content compared with fresh fruit due to water loss, a subgroup analysis suggested that both dried and fresh fruit reduced blood glucose concentrations, and dried fruit has the characteristics of a long shelf-life and good transportability compared with fresh fruit.
Du et al. found that fruit consumption had a weak inverse, instead of positive, association with levels of blood glucose (8). Our results also suggest that eating fruit reduced the fasting blood glucose concentration. The available evidence suggests that the increase in the fruit intake should be accompanied by a corresponding decrease in the carbohydrate intake, ensuring that the total energy intake remained unchanged (6). The energy produced per 100 grams of fresh fruit is about 21–282 kcal (33). Our experience is that patients with stable blood glucose control who eat 200–250 g of fresh fruit per day need to reduce their daily intake of staple food by 25 g to avoid exceeding their total daily energy intake limit. Increasing the fruit intake is limited to patients with diabetes with stable glycemic control, while patients with diabetes with unstable blood glucose concentrations need to be cautious when increasing their fruit intake. A good method is to measure the urine glucose concentration at 2 hours after eating fruit. If the urine sugar increases, the patient needs to reduce the amount of fruit eaten; if the urine glucose is still high after reducing the fruit consumption, then the amount of staple food eaten needs to be appropriately reduced. In short, fruits should be considered as a part of a diet that has been comprehensively considered to achieve the purpose of not only supplementing nutrition but also controlling diabetes.
Diabetes is currently one of the main conditions that leads to a variety of macrovascular and microvascular complications (34). In severe cases, diabetes lead to disability and is even life-threatening. Although fruit is an important part of the daily diet, the high sugar content of most fruits has led to the conventional belief that fruit consumption may exacerbate diabetes, and thus the fruit intake of patients with diabetes is often severely restricted (35). However, fruits provide a lot of nutrients such as vitamins, fruit acids, minerals, and antioxidants, which help prevent the development and progression of many diseases; therefore, diabetes guidelines in the United States recommend eating more fruits (36). Antioxidants in fruits prevent cell damage by inhibiting lipid peroxidation and having lipotoxic effects (22). Furthermore, fruits may contain resistant starches and oligosaccharides that cannot be digested in the stomach and need to enter the colon for bacterial fermentation (37), which can stimulate the growth and/or activity of one or more beneficial bacteria, thereby improving human health (38). An 8-year follow-up study showed that diabetic retinopathy was significantly reduced in the group that consumed 253 g of fruit daily compared with the group that consumed 23 g of fruit daily (39). Increasing the fruit intake also reduces the incidences of stroke, coronary heart disease, and cancer (40–42). Other studies have shown beneficial effects of various fruits. For example, eating fresh grapefruit before meals significantly reduces weight while improving insulin resistance (43), polyphenols in strawberry fruits improve insulin sensitivity (44), and carambola juice may lower the blood glucose concentration and improve liver function in mice with diabetes (45).
Although studies have shown that the sugar load is higher after fruit juice is changed, the blood glucose concentration is easily increased by fruit juice consumption, which is not conducive to blood glucose control and causes vitamin and fiber loss, decreased satiety, and increased total energy; therefore, fruit juice is not recommended for patients with diabetes (46). However, Weisel et al. reported that fruit juice resulted in a decrease in oxidative DNA damage, an increase in reduced glutathione and glutathione status, a return to break-in levels during the subsequent elution period, and a significant reduction in oxidative damage in healthy proband cells in the juice (47). Other studies have shown that watermelon juice exerts antidiabetic effects in experimental animal models of diabetes by modulating glucose transporters, anti-inflammatory activity, antioxidant defense systems, and inhibiting multiple pathways such as α-glucosidase and α-amylase (48). Furthermore, the homeostasis of the gut flora is regulated by the ingestion of bioactive compounds found in citrus fruits and orange juice, such as hesperidin and naringenin (49).
To our knowledge, this is the first study to systematically review the potential effects of fruit consumption on glycemic control in patients with diabetes. The relatively large number of pooled participants achieved a greater statistical power than a single RCT. However, this study has some limitations. First, although this meta-analysis focused on studies evaluating the benefits of fruit consumption on human mechanisms, most of the included studies were assessed in Iran, which may increase the bias in the result. Different fruits have different sugar contents, and the types of fruits eaten varies between countries and regions. The sugar content can even vary between the same fruits with different levels of ripeness. Second, most of the fruits included in this meta-analysis have a certain medicinal value, which warrants further research. Third, most guidelines recommend a diet with a high intake of fiber-rich food, including fruit. This is based on the many positive effects of fruit on human health. However, some health professionals are concerned that fruit intake has a negative impact on glycemic control and therefore recommend restricting the fruit intake. We found no studies addressing this important clinical question (6). Fourth, the I2 value is the percentage of total variation across studies due to heterogeneity (between-study variability) rather than chance, with higher values indicating higher levels of heterogeneity between studies and higher inconsistency of results. As there was a high heterogeneity in the studies that assessed the Hb1Ac concentration, this conclusion needs to be further demonstrated by higher quality studies.
Conclusions
Increasing the fruit intake reduced the fasting blood glucose concentration of patients with diabetes. Therefore, we still recommend that patients with diabetes eat more fruits while ensuring that their total energy intake remains unchanged. We concluded that moderate amounts of practical fruit products reduce blood glucose concentration, but the specific amounts of daily practical fruits need to be investigated in multicenter RCTs.
Author contributions
YR and SS performed literature review. YR and HL collected data, performed data analysis, interpreted results, and wrote the first draft of the manuscript. YS and YR assessed risk of bias, reviewed the protocol, screened articles, and reviewed the results and manuscript. CY contributed to the systematic review protocol and critically reviewed the results and manuscript. HL contributed to the protocol development and reviewed the manuscript. YR, SS, YS, CY, and HL critically revised successive drafts of the manuscript and approved the final version. HL has affirmed that this manuscript is an honest, accurate, and transparent account of the study being reported. that no important aspects of the study have been omitted. and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. HL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Bondonno NP, Davey RJ, Murray K, Radavelli-Bagatini S, Bondonno CP, Blekkenhorst LC, et al. Associations between fruit intake and risk of diabetes in the AusDiab cohort. J Clin Endocrinol Metab (2021) 106(10):e4097–108. doi: 10.1210/clinem/dgab335
3. Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism onblood vessels. Cell Biochem Biophys (2014) 69(2):213–8. doi: 10.1007/s12013-013-9810-6
4. Jia G, Sowers JR. Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension (2021) 78(5):1197–205. doi: 10.1161/hypertensionaha.121.17981
5. Gao M, Jebb SA, Aveyard P, Ambrosini GL, Perez-Cornago A, Carter J, et al. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK biobank: a prospective cohort study. BMC Med (2021) 19(1):83. doi: 10.1186/s12916-021-01958-x
6. Christensen AS, Viggers L, Hasselstrom K, Gregersen S. Effect of fruit restriction on glycemic control in patients with type 2 diabetes - a randomized trial. Nutr J (2013) 12. doi: 10.1186/1475-2891-12-29
7. Ola MS, Al-Dosari D, Alhomida AS. Role of oxidative stress in diabetic retinopathy and the beneficial effects of flavonoids. Curr Pharm Des (2018) 24(19):2180–7. doi: 10.2174/1381612824666180515151043
8. Du H, Li L, Bennett D, Guo Y, Turnbull I, Yang L, et al. Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: a 7-y prospective study of 0.5 million Chinese adults. PloS Med (2017) 14(4):e1002279. doi: 10.1371/journal.pmed.1002279
9. Matos AL, Bruno DF, Ambrósio AF, Santos PF. The benefits of flavonoids in diabetic retinopathy. Nutrients (2020) 12(10). doi: 10.3390/nu12103169
10. Poolsup N, Suksomboon N, Paw NJ. Effect of dragon fruit on glycemic control in prediabetes and type 2 diabetes: a systematic review and meta-analysis. PloS One (2017) 12(9):e0184577. doi: 10.1371/journal.pone.0184577
11. Lazavi F, Mirmiran P, Sohrab G, Nikpayam O, Angoorani P, Hedayati M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: a randomized clinical trial. Complement Ther Clin Pract (2018) 31:170–4. doi: 10.1016/j.ctcp.2018.01.009
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
13. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
14. Adi BS, Vangani A, Siva Rami Reddy E. Effect of syzygium cumini in type 2 diabetes mellitus by assessing glycosylated haemoglobin and blood glucose levels: a double blind randomized controlled trial. J Global Trends Pharm Sci (2020) 11(4):8546–51.
15. Barghamdi B, Ghorat F, Asadollahi K, Sayehmiri K, Peyghambari R, Abangah G. Therapeutic effects of citrullus colocynthis fruit in patients with type II diabetes: a clinical trial study. J Pharm Bioallied Sci (2016) 8(2):130–4. doi: 10.4103/0975-7406.171702
16. Fallah Huseini H, Heshmat R, Larijani B, Fakhrzadeh, Darvishzadeh F, Jafariazar Z, et al. The clinical investigation of citrullus colocynthis (L.) schrad. fruit in treatment of type II diabetic patients; a randomized, double-blind, placebo-controlled study. J Med Plants (2006) 5(SUPPL. 2):31–5.
17. Fallah Huseini H, Zaree A, Heshmat R, Larijani B, Fakhrzadeh H, Rezaii Sharifabadi R, et al. The effect of citrullus colocynthis (L.) schrad. fruit on oxidative stress parameters in type II diabetic patients. [Persian]. J Med Plants (2006) 5(SUPPL. 2):55–60.
18. Golbon S, Pooneh A, Maryam T, Hadi T, Masoud K, Javad N. Pomegranate (Punica granatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: a randomized double-blind clinical trial. Food Nutr Res (2015) 59:28551. doi: 10.3402/fnr.v59.28551
19. Golbon S, Samira E, Giti S, Reza Neyestani T, Pooneh A, Mehdi H, et al. Effects of pomegranate juice consumption on oxidative stress in patients with type 2 diabetes: a single-blind, randomized clinical trial. Int J Food Sci Nutr (2017) 68(2):249–55. doi: 10.1080/09637486.2016.1229760
20. Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Phytother Res (2009) 23(8):1186–9. doi: 10.1002/ptr.2754
21. Irannejad Niri Z, Shidfar F, Jabbari M, Zarrati M, Hosseini A, Malek M, et al. The effect of dried ziziphus vulgaris on glycemic control, lipid profile, apo-proteins and hs-CRP in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. J Food Biochem (2021) 45(3):e13193. doi: 10.1111/jfbc.13193
22. Ismawanti Z, Suparyatmo JB, Wiboworini B. The comparative effect of red guava (Psidium guajava. l.) with papaya (Carica papaya) on blood glucose level of type 2-diabetic patients. Romanian J Diabetes Nutr Metab Dis (2020) 27(3):209–13.
23. Javid AZ, Maghsoumi-Norouzabad L, Ashrafzadeh E, Yousefimanesh HA, Zakerkish M, Angali KA, et al. Impact of cranberry juice enriched with omega-3 fatty acids adjunct with nonsurgical periodontal treatment on metabolic control and periodontal status in type 2 patients with diabetes with periodontal disease. J Am Coll Nutr (2018) 37(1):71–9. doi: 10.1080/07315724.2017.1357509
24. Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial [Randomized controlled trial research support, non-U.S. gov't]. Ann Nutr Metab (2013) 63(3):256–64. doi: 10.1159/000356053
25. Nemati S, Tadibi V, Hoseini R. Pomegranate juice intake enhances the effects of aerobic training on insulin resistance and liver enzymes in type 2 diabetic men: a single-blind controlled trial. BMC Nutr (2022) 8(1):48. doi: 10.1186/s40795-022-00538-3
26. Sabitha P, Adhikari Prabha MR, Shetty Rukmini MS, Anupama H, Asha K. The beneficial effects of noni fruit juice in diabetic patients. J Clin Diagn Res (2009) 3(6):1822–6.
27. Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, lp(a), and paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci (2012) 17(4):355–60.
28. Sohrab G, Nasrollahzadeh J, Zand H, Amiri Z, Tohidi M, Kimiagar M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: a randomized, placebo-controlled trial. J Res Med Sci (2014) 19(3):215–20.
29. Sousa D, Araujo M, Mello V, Damasceno MMC, Freitas R. Cost-effectiveness of passion fruit albedo versus turmeric in the glycemic and lipaemic control of people with type 2 diabetes: randomized clinical trial. J Am Coll Nutr (2021) 40(8):679–88. doi: 10.1080/07315724.2020.1823909
30. Stote KS, Wilson MM, Hallenbeck D, Thomas K, Rourke JM, Sweeney MI, et al. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: an 8-week, double-blind, randomized, placebo-controlled trial. Curr Dev Nutr (2020) 4(4). doi: 10.1093/cdn/nzaa030
31. Yaghoobi M, Miri-Moghaddam E, Navidian A, Nikbakht R, Mehrafarin A, Fallah Huseini H. Safety and efficacy of processed citrullus colocynthis l. fruit in treatment of hyperlipidemic type II diabetic patients: a randomized, placebo-controlled clinical trial. [Persian]. J Med Plants (2014) 13(52):81–8.
32. Braunstein CR, Noronha JC, Khan TA, Mejia SB, Wolever TM, Josse RG, et al. Effect of fructose and its epimers on postprandial carbohydrate metabolism: a systematic review and meta-analysis. Clin Nutr (2020) 39(11):3308–18. doi: 10.1016/j.clnu.2020.03.002
33. Rouman Ahmad. (2020). Available at: https://www.healthynex.com/fruit-calories-chart.html.
34. Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care (2019) 42(4):674–81. doi: 10.2337/dc18-2047
35. Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis (2006) 187(2):363–71. doi: 10.1016/j.atherosclerosis.2005.09.006
36. American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes (2022) 40(1):10–38. doi: 10.2337/cd22-as01
37. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr (2015) 54(3):325–41. doi: 10.1007/s00394-015-0852-y
38. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr (2012) 3(4):506–16. doi: 10.3945/an.112.002154
39. Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, et al. Japan Diabetes complications study g. fruit intake and incident diabetic retinopathy with type 2 diabetes [Randomized controlled trial research support, non-U.S. gov't]. Epidemiology (2013) 24(2):204–11. doi: 10.1097/EDE.0b013e318281725e
40. He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet (2006) 367(9507):320–6. doi: 10.1016/s0140-6736(06)68069-0
41. Yoshizaki T, Ishihara J, Kotemori A, Yamamoto J, Kokubo Y, Saito I, et al. Association of vegetable, fruit, and okinawan vegetable consumption with incident stroke and coronary heart disease. J Epidemiol (2020) 30(1):37–45. doi: 10.2188/jea.JE20180130
42. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European prospective investigation into cancer and nutrition (EPIC). Am J Clin Nutr (2014) 100 Suppl 1:394s–8s. doi: 10.3945/ajcn.113.071357
43. Fujioka K, Greenway F, Sheard J, Ying Y. The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food (2006) 9(1):49–54. doi: 10.1089/jmf.2006.9.49
44. Paquette M, Medina Larque AS, Weisnagel SJ, Desjardins Y, Marois J, Pilon G, et al. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial [Randomized controlled trial]. Br J Nutr (2017) 117(4):519–31. doi: 10.1017/S0007114517000393
45. Ying-xia Y, Tian-min H, Ren-bin H, Xiao-hui X. Study on the liver tissue anti-oxidative stress and hypoglycemic effects of carambola fruit juice. Food Res Dev (2018) 39(9):148–51. doi: 10.3969/j.issn.1005-6521.2018.09.028
46. Wojcicki JM, Heyman MB. Reducing childhood obesity by eliminating 100% fruit juice. Am J Public Health (2012) 102(9):1630–3. doi: 10.2105/ajph.2012.300719
47. Weisel T, Baum M, Eisenbrand G, Dietrich H, Will F, Stockis JP, et al. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol J (2006) 1(4):388–97. doi: 10.1002/biot.200600004
48. Ajiboye BO, Shonibare MT, Oyinloye BE. Antidiabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats. J Diabetes Metab Disord (2020) 19(1):343–52. doi: 10.1007/s40200-020-00515-2
49. Lima ACD, Cecatti C, Fidelix MP, Adorno MAT, Sakamoto IK, Cesar TB, et al. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: controlled clinical trials [Controlled clinical trial]. J Med Food (2019) 22(2):202–10. doi: 10.1089/jmf.2018.0080
Keywords: fruit, glucose, diabetes, glycosylated hemoglobin, meta-analaysis
Citation: Ren Y, Sun S, Su Y, Ying C and Luo H (2023) Effect of fruit on glucose control in diabetes mellitus: a meta-analysis of nineteen randomized controlled trials. Front. Endocrinol. 14:1174545. doi: 10.3389/fendo.2023.1174545
Received: 26 February 2023; Accepted: 10 April 2023;
Published: 05 May 2023.
Edited by:
João Soares Felício, Federal University of Pará, BrazilReviewed by:
Daniela Lopes Gomes, Federal University of Pará, BrazilPatrizio Tatti, Istituto Neurotraumatologico Italiano (INI), Italy
Copyright © 2023 Ren, Sun, Su, Ying and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Luo, MTg3MzIxOTY2NjBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yu Ren
Yu Ren Shuang Sun2†
Shuang Sun2† Hua Luo
Hua Luo