- 1The Committee of the Evaluation and Dissemination for Certified Kidney Disease Educator, Japan Kidney Association, Tokyo, Japan
- 2Division of Nephrology, Hypertension and Endocrinology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan
- 3Department of Medicine, Hatta Medical Clinic, Kyoto, Japan
- 4Department of Nephrology, Nissan Tamagawa Hospital, Tokyo, Japan
- 5Department of Nephrology and Hypertension, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan
- 6Department of Nephrology and Rheumatology, Kyorin University School of Medicine, Tokyo, Japan
Background: Multidisciplinary care is necessary to prevent worsening renal function and all-cause mortality in patients with chronic kidney disease (CKD) but has mostly been investigated in the outpatient setting. In this study, we evaluated the outcome of multidisciplinary care for CKD according to whether it was provided in an outpatient or inpatient setting.
Methods: This nationwide, multicenter, retrospective, observational study included 2954 Japanese patients with CKD stage 3–5 who received multidisciplinary care in 2015–2019. Patients were divided into two groups: an inpatient group and an outpatient group, according to the delivery of multidisciplinary care. The primary composite endpoint was the initiation of renal replacement therapy (RRT) and all-cause mortality, and the secondary endpoints were the annual decline in the estimated glomerular filtration rate (ΔeGFR) and the changes in proteinuria between the two groups.
Results: Multidisciplinary care was provided on an inpatient basis in 59.7% and on an outpatient basis in 40.3%. The mean number of health care professionals involved in multidisciplinary care was 4.5 in the inpatient group and 2.6 in the outpatient group (P < 0.0001). After adjustment for confounders, the hazard ratio of the primary composite endpoint was significantly lower in the inpatient group than in the outpatient group (0.71, 95% confidence interval 0.60-0.85, P = 0.0001). In both groups, the mean annual ΔeGFR was significantly improved, and proteinuria significantly decreased 24 months after the initiation of multidisciplinary care.
Conclusion: Multidisciplinary care may significantly slow deterioration of eGFR and reduce proteinuria in patients with CKD and be more effective in terms of reducing initiation of RRT and all-cause mortality when provided on an inpatient basis.
1 Introduction
Increasing numbers of patients have chronic kidney disease (CKD) worldwide (1). In Japan, nearly 15 million adults were estimated to have CKD in 2015 (2), and increasing numbers of patients with end-stage kidney disease are starting renal replacement therapy (RRT) each year, with more than 340,000 patients now receiving dialysis (3). The prevalence of dialysis in Japan is 2682 per million population, second only to Taiwan (4). A comprehensive approach to management is needed because CKD increases the risk of not only ESKD but also cardiovascular mortality. Thus it is necessary to control blood pressure, glycemic status, anemia, bone mineral status, and low-density lipoprotein cholesterol alongside lifestyle modification, dietary guidance, and measures to ensure adherence with medication (5, 6). It has been reported that comprehensive multidisciplinary care can reduce all-cause mortality, the likelihood of temporary catheterization for patients on dialysis, and the hospitalization rate as well as slow decline in the estimated glomerular filtration rate (eGFR) (7–10). In these studies, comprehensive multidisciplinary care was provided by teams that included nephrologists, specialist nurses, dieticians, pharmacists, and social workers.
The Certified Kidney Disease Educator (CKDE) system was established in Japan by the Japan Kidney Association in 2017 with the aims of preventing progression of CKD and improving and maintaining quality of life for patients with CKD. Nurses, registered dieticians, and pharmacists who meet certain requirements are eligible to qualify as a CKDE. All CKDEs have acquired the basic skills for managing patients with CKD, including providing guidance on lifestyle modification, dietary counseling, and medical therapy according to disease stage. Generally, multidisciplinary care for patients with CKD and diabetes is performed on an outpatient basis, as reflected in the Steno-2 and MASTERPLAN studies (11–14). However, in Japan, widespread multidisciplinary care for patients with CKD is provided not only on an outpatient basis but also on an inpatient basis because of lack of time during outpatient appointments to cover lifestyle modification, dietary restriction, and medication adherence in sufficient depth. Currently, however, there is limited information on whether these multidisciplinary interventions in the inpatient setting improve the prognosis of CKD.
We conducted this nationwide study to assess the outcome of multidisciplinary intervention in patients with CKD according to whether it was provided in an outpatient or inpatient setting.
2 Methods
2.1 Study design and participants
This nationwide multicenter retrospective cohort study was performed by members of the Japan Kidney Association Committee for Evaluation and Dissemination of CKDE. To reflect practice patterns across most of Japan, around 3000 Japanese patients were participated at any of 24 selected health care institutions in Japan that play a central role in the treatment of patients with CKD. All-cause mortality and the start of RRT were tracked until the end of 2020 for patients with CKD who had data on kidney function available for the 12 months before to and 24 months after receiving multidisciplinary therapy between January 2015 and December 2019. The following exclusion requirement were used: age < 20 years; CKD stage 1 and 2 (i.e., eGFR ≥ 60 mL/min/1.73 m2); patients who were hospitalized for another reason other than CKD; short-term follow-up of 6 months or less; received multidisciplinary care in the past; active malignant disease; transplant recipient; history of long-term dialysis; and data missing for age, sex, kidney function, or results. In Japan, multidisciplinary care for patients with CKD was conducted in outpatient or inpatient settings based on the hospital functions, nephrologists’ judgment, and the patient’s wishes. As a result, the enrolled patients were classified into an outpatient and an inpatient group based on the approach and place of intervention by the multidisciplinary care team at the start of the intervention (baseline). They were further divided into subgroups based on whether they had diabetes. A group of inpatient patients were admitted to the hospital and received multidisciplinary care in accordance with each facility’s inpatient educational program.
The main efficacy composite endpoint was the initiation of RRT and all-cause mortality at the end of 2020. The secondary efficacy endpoint was the annual decline in eGFR (ΔeGFR) and the annual change in urinary protein level between 12 months before and 6, 12, and 24 months after the initiation of multidisciplinary intervention.
The study was approved by the ethics committee of Nihon University Itabashi Hospital and conducted in accordance with the Declaration of Helsinki, Japanese privacy protection laws, and the Ethical Guidelines for Medical and Health Research Involving Human Subjects published by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare in 2015. The need for informed consent was waived in view of the use of de-identified data. The study is registered in the University Hospital Medical Information Network (UMIN000049995).
2.2 Multidisciplinary care
The definition of multidisciplinary care adopted was (1) a multidisciplinary care team composed of nephrologists and other professionals (i.e., specialist nurses, registered dieticians, pharmacists, physical therapists, social workers, clinical engineers, and clinical laboratory technicians) and (2) an operational model of multidisciplinary care comprising patient education, medical management, and lifestyle modification according to CKD stage. The quality of the educational content provided was maintained based on the text created by the Japanese Society of Nephrology, the Japanese Society for Dialysis Therapy, the Japan Society for Transplant, and the Japanese Society for Clinical Renal Transplantation or the CKD Teaching Guidebook for Certified Kidney Disease Educators published by the Japan Kidney Association (15, 16).
2.3 Data collection
Patient demographics and data on clinical characteristics were collected, including age, sex, history of cardiovascular disease (CVD), primary etiology of CKD, body mass index (BMI), hemoglobin, serum albumin, urea nitrogen, creatinine (Cr), eGFR, and urinary protein. Information on glycated hemoglobin (HbA1c) was also collected for patients with diabetes at baseline. CVD was defined as coronary artery disease, ischemic stroke, hemorrhagic stroke, or limb amputation. eGFR was calculated according to the following formula for Japanese patients: eGFR (mL/min/1.73 m2) = 194 × serum Cr−1.094 × age−0.287 (× 0.739 for women) (17). Urinary protein was calculated as the urinary protein to Cr ratio (UPCR). The data on method and place of intervention (outpatient or inpatient), the number or duration of interventions (number of visits for outpatient intervention or the number of hospitalization days for inpatients), and the type and number of health care professionals involved in the multidisciplinary care team were also collected. Data were collected for the primary composite endpoint, which included the date attained or the end of 2020, whichever came first (initiation of RRT and all-cause mortality). Also noted was the RRT’s kind (kidney transplantation, peritoneal dialysis, or hemodialysis).
2.4 Statistical analysis
Data are reported as the number and proportion, mean ± standard deviation, or median [interquartile range] as appropriate. Intragroup comparisons were made using two-tailed paired t-tests. Categorical variables were examined using the chi-squared test and continuous variables using the t-test. The composite outcome was estimated using the Kaplan–Meier method and compared between groups using the log-rank test. A univariate analysis was performed according to the method and place of intervention (i.e., outpatient-based or inpatient-based). Multivariate survival analyses were performed using Cox proportional hazards models with adjustment for confounding factors to examine the method and place of intervention and the composite outcome during the 6 years of follow-up. Model 1 was used to calculate hazard ratios (HRs) adjusted for basic factors, including age, sex, history of CVD, eGFR, and UPCR at baseline, and model 2 was adjusted for BMI, hemoglobin, and serum albumin level in addition to the factors included in model 1. A subgroup analysis was performed according to the diabetes status and the CKD stage (G3a, G3b, G4, or G5) at baseline. A further subdivision analysis in the inpatient group based on the presence or absence of physical therapists was performed. In patients with diabetes, model 1 was used to calculate the HRs with adjustment for basic factors (e.g., age, sex, history of CVD, HbA1c, eGFR, and UPCR at baseline), and model 2 was adjusted for BMI, hemoglobin, and serum albumin level in addition to the factors included in model 1. In patients without diabetes, model 1 was used to calculate the HRs adjusted for basic factors, including age, sex, history of CVD, eGFR, and UPCR at baseline and model 2 was adjusted for BMI, hemoglobin, and serum albumin level in addition to the factors included in model 1. The results from the models are reported as HRs with 95% confidence intervals (CIs) and P-values. For the regression analyses, imputation of missing data was performed by conventional methods as appropriate. All analyses were performed using JMP® version 13.0 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P-values less than 0.05.
3 Results
3.1 Patient characteristics at time of initiation of multidisciplinary care
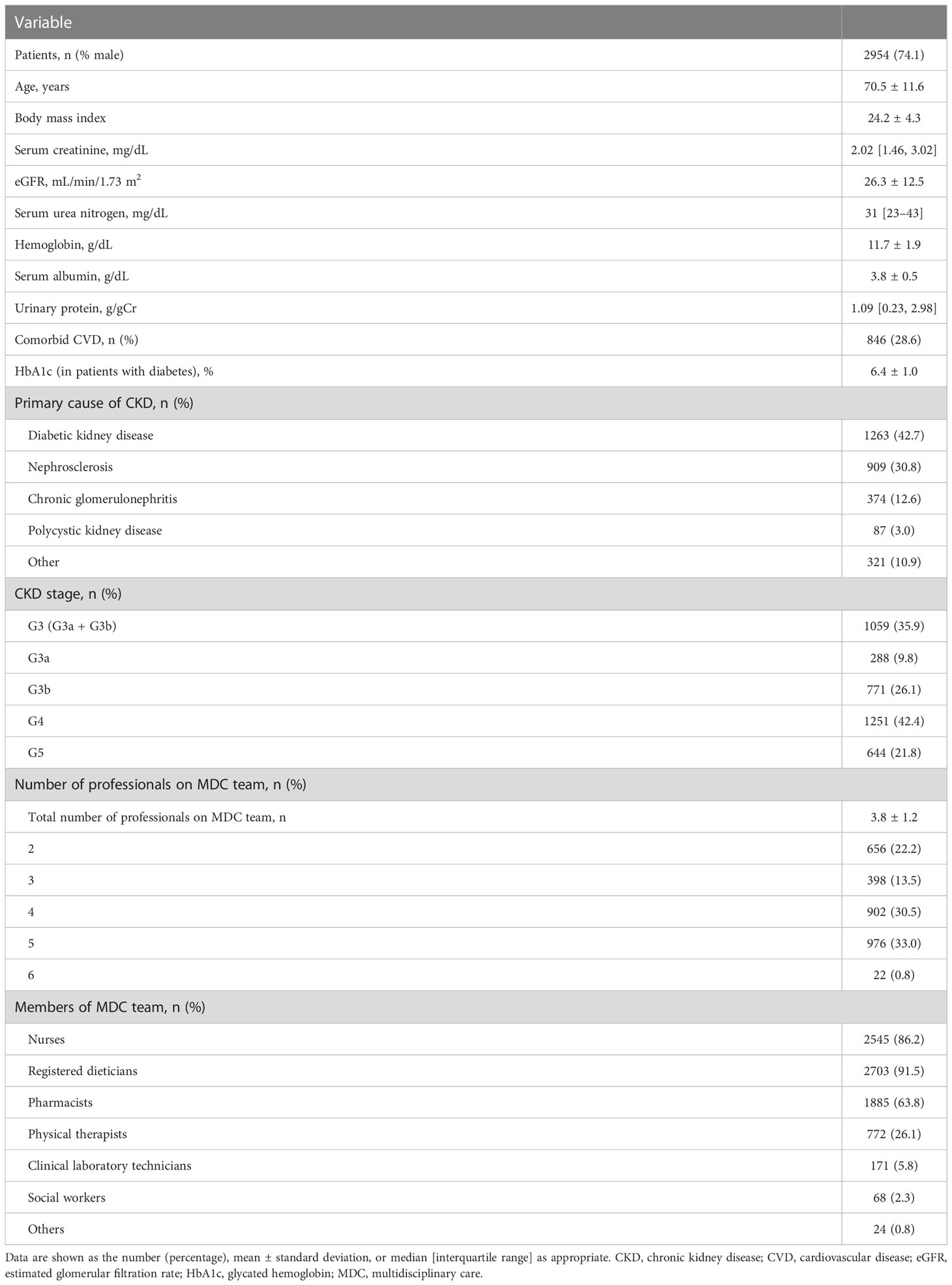
Overall, of the 3296 patients enrolled, 342 were removed (CKD stage 1 or 2, n = 118; age younger than 20 years, n = 3; follow-up for 6 months or less, n = 124; lack of data for baseline kidney function, n = 13), which left 2954 patients for inclusion in the analysis. Patient characteristics at the time of initiation of multidisciplinary care are shown in Table 1. The mean age was 70.5 ± 11.6 years, and 74.1% of the patients were male. The mean eGFR was 26.3 ± 12.5 mL/min/1.73 m2 and the median UPCR was 1.09 g/gCr [0.23, 2.98]. The most common etiology of CKD was diabetic kidney disease (42.7%), followed by nephrosclerosis (30.8%) and chronic glomerulonephritis (12.6%). The most common CKD stage was G4 (42.4%), followed by G3b (26.1%) and G5 (21.8%).
3.2 Type and number of professionals in the multidisciplinary care team
Details of the interventions implemented by the multidisciplinary care team are shown in Table 1. The mean number of multidisciplinary care team members, including nephrologists, was 3.8 ± 1.2. It was most common for the multidisciplinary care team to include five professionals (33.0%), followed by four (30.5%) and the two (22.2%). Registered dieticians were the most common members of the multidisciplinary care team (91.5%), followed by specialist nurses (86.2%), pharmacists (63.8%), and physical therapists (26.1%).
3.3 Outcomes
The median observation period was 36 months [22, 52], during which 128 patients (4.3%) died, 648 (21.9%) initiated RRT, and 66 (2.2%) were lost to follow-up; 2112 (71.6%) of all patients were alive without RRT at the end of the study period. RRT consisted of hemodialysis in 559 patients (86.2%), peritoneal dialysis in 66 (10.2%), and kidney transplantation in 23 (3.6%).
3.3.1 Comparison between outpatient and inpatient groups
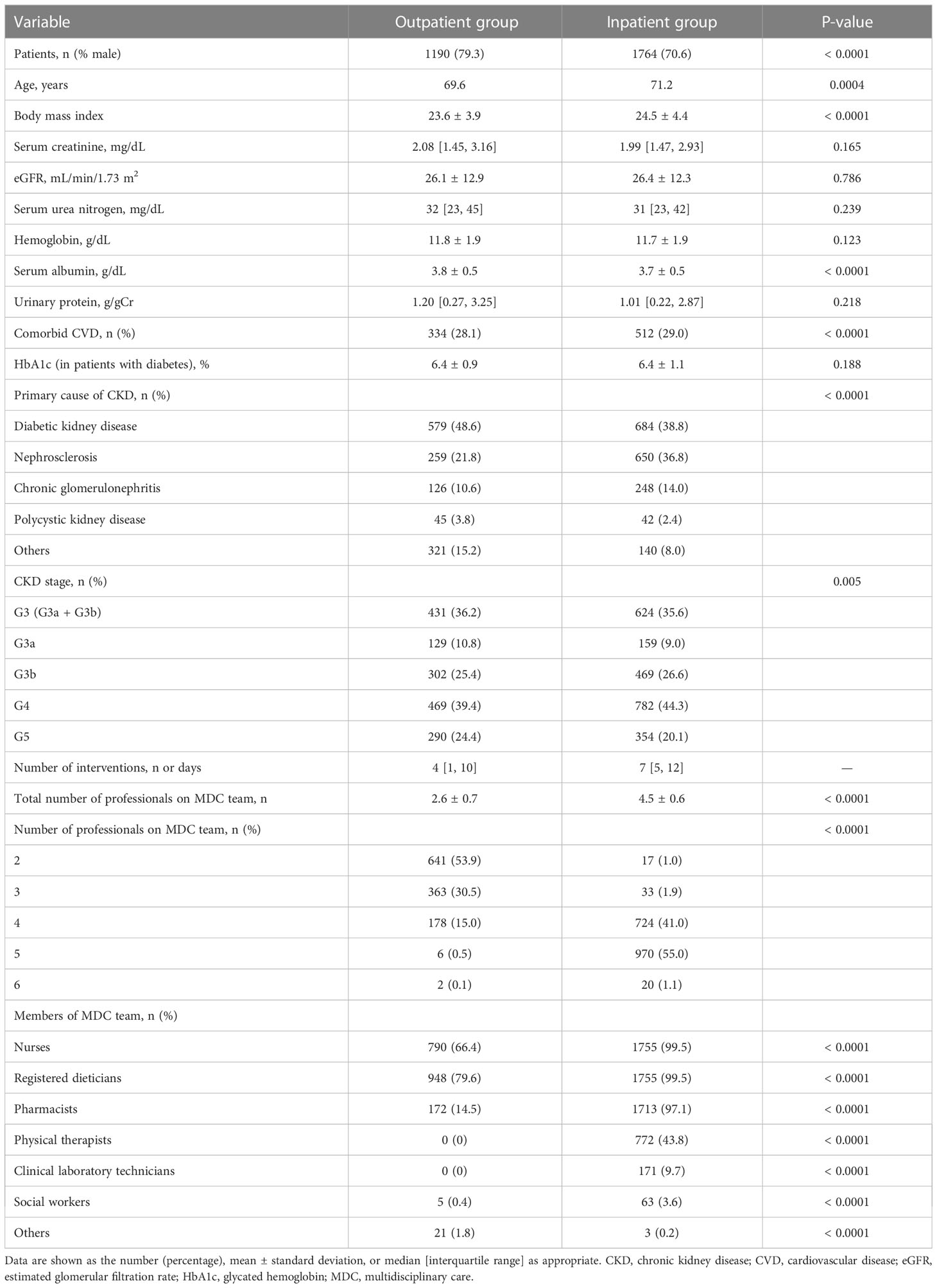
The baseline characteristics of the patients in the inpatient and outpatient groups are shown in Table 2. Intervention was provided in an inpatient setting for more than half of the patients (59.7%) and on an outpatient basis for the remainder (40.3%). The baseline kidney function, including eGFR, serum Cr and UPCR, was comparable between the two groups, but patients in the inpatient group were more likely to be female and older and to have a higher BMI and comorbid CVD. However, rates of diabetic kidney disease and CKD stage G5 were lower in the inpatient group than in the outpatient group. The mean number of multidisciplinary care team members was significantly higher in the inpatient group (4.5 ± 0.6 vs. 2.6 ± 0.7, P < 0.0001).
Kaplan–Meier analysis for the composite endpoint (initiation of RRT and all-cause mortality) revealed a significant difference between the outpatient and inpatient groups (P = 0.0003, log-rank test; Figure 1). Compared with the outpatient (reference) group, the inpatient group had a significantly lower unadjusted HR for the composite endpoint (0.78, 95% CI 0.68–0.91, P = 0.0004). After adjustment for basic factors, including age, sex, history of CVD, eGFR, and UPCR at baseline, the HR in the inpatient group was 0.73 (95% CI 0.63–0.88, P = 0.0001). After further adjustment for basic factors and BMI, hemoglobin, and serum albumin at baseline, the HR was significantly lower in the inpatient group (0.71, 95% CI 0.60–0.85, P = 0.0001) (Table 3).
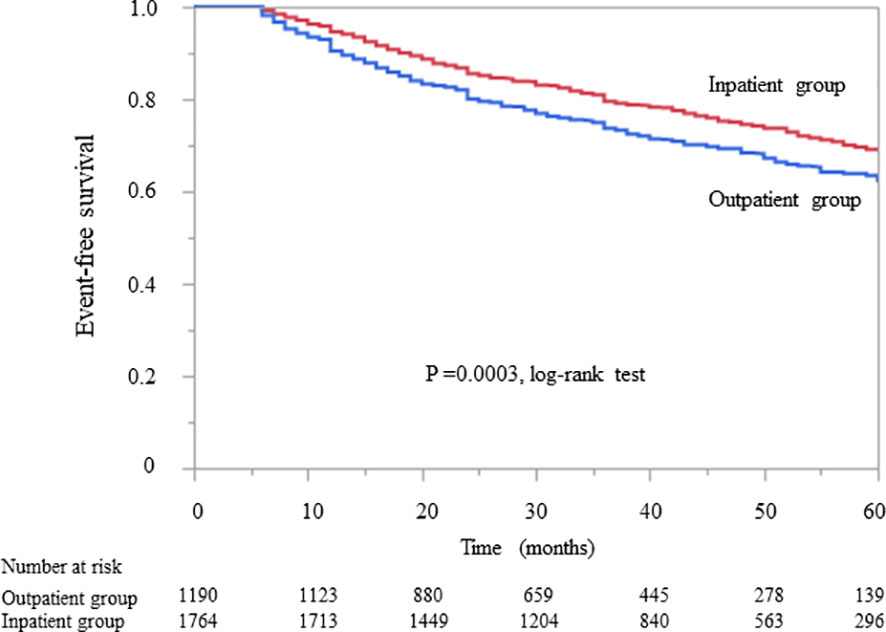
Figure 1 Kaplan–Meier curves showing the incidence of initiation of renal replacement therapy and all-cause mortality in Japanese patients with chronic kidney disease according to whether they received outpatient or inpatient multidisciplinary care.
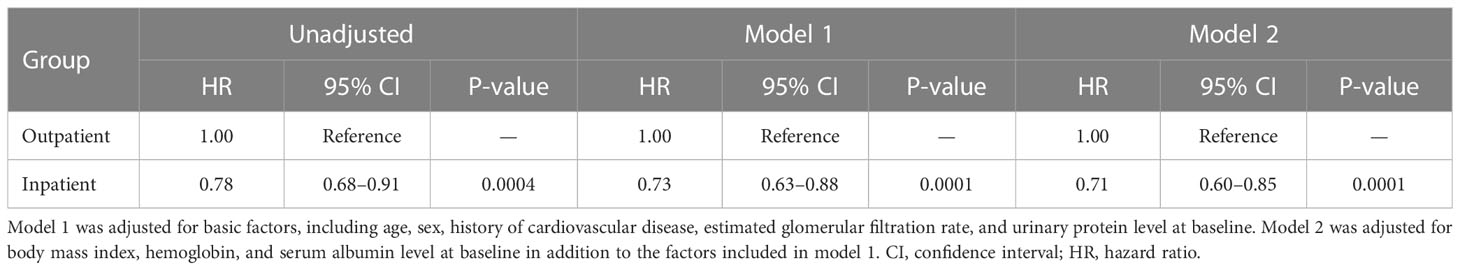
Table 3 Comparison of initiation of renal replacement therapy and all-cause mortality between the outpatient and inpatient groups in Cox proportional hazards models adjusted for confounding factors in Japanese patients with chronic kidney disease.
3.4 Subgroup analysis according to diabetes status
Kaplan–Meier analysis revealed that there was no significant difference in the composite endpoint between patients with diabetes in the outpatient group and those in the inpatient group (P = 0.133, log-rank test; Figure 2). Cox proportional analysis revealed no significant difference in the unadjusted HR for the composite endpoint between the inpatient and outpatient groups (Table 4). However, after adjustment for basic factors, including age, sex, history of CVD, HbA1c, eGFR, and UPCR at baseline, the HR in the inpatient group was 0.75 (95% CI 0.61–0.93, P = 0.010). After further adjustment for basic factors and BMI, hemoglobin, and serum albumin level at baseline, the inpatient group had a significantly lower HR (0.74, 95% CI 0.59–0.95, P = 0.018) (Table 4).
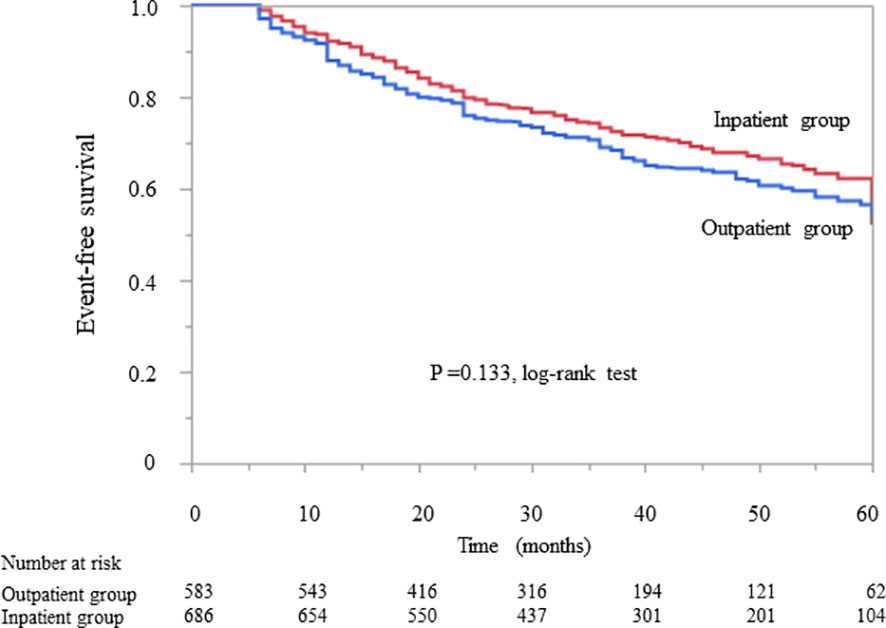
Figure 2 Kaplan–Meier curves showing the incidence of initiation of renal replacement therapy and all-cause mortality in Japanese diabetes patients with chronic kidney disease according to whether they received outpatient or inpatient multidisciplinary care.
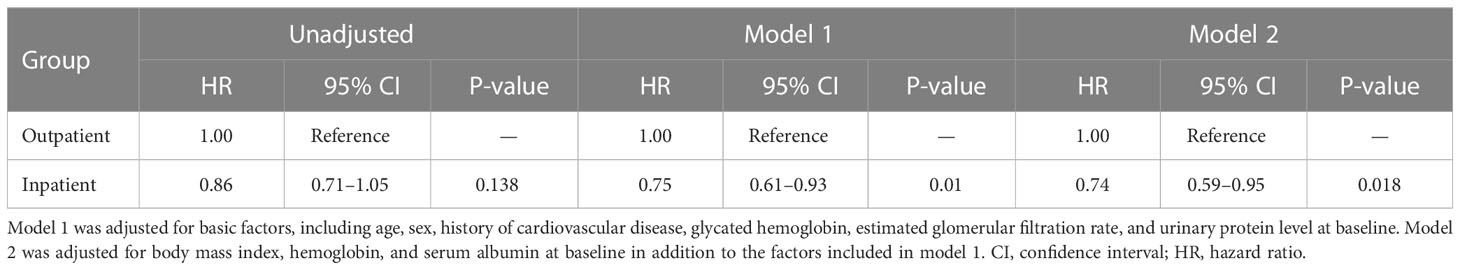
Table 4 Comparison of initiation of renal replacement therapy and all-cause mortality between the outpatient and inpatient groups in Cox proportional hazards models adjusted for confounding factors in Japanese patients with chronic kidney disease and diabetes.
In patients without diabetes, Kaplan–Meier analysis revealed a significant difference in the composite endpoint between the outpatient and inpatient groups (P = 0.009, log-rank test; Figure 3). Compared with the outpatient group, the inpatient group had a significantly lower unadjusted HR for the composite endpoint (0.75, 95% CI 0.61–0.93, P = 0.009). After adjustment for basic factors, including age, sex, history of CVD, eGFR, and UPCR at baseline, the HR in the inpatient group was 0.75 (95% CI 0.59–0.94, P = 0.015). After further adjustment for basic factors and BMI, hemoglobin, and serum albumin level at baseline, the inpatient group had a significantly lower HR (0.76, 95% CI 0.59–0.98, P = 0.034) (Table 5).
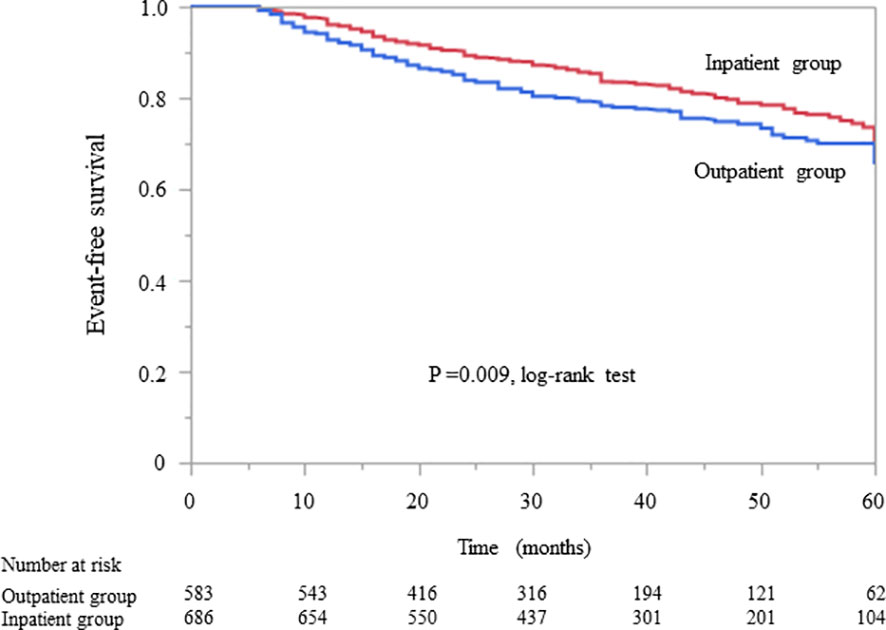
Figure 3 Kaplan–Meier curves for the incidence of initiation of renal replacement therapy and all-cause mortality in Japanese non-diabetes patients with chronic kidney disease in the outpatient and inpatient groups.
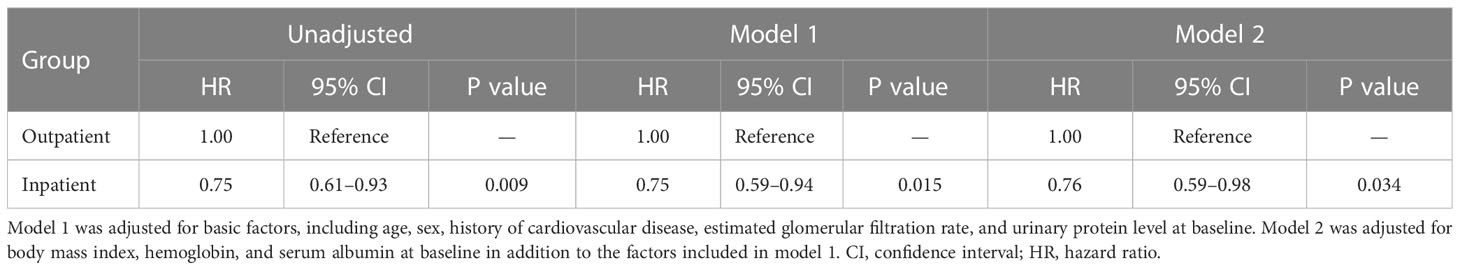
Table 5 Comparison of all-cause mortality between the outpatient and inpatient groups according to Cox proportional hazards models adjusted for confounding factors in Japanese patients with chronic kidney disease but no diabetes.
3.5 Subgroup analysis according to the CKD stage at baseline
All-cause mortality and RRT initiation were dependent on the disease stage. The Kaplan–Meier analysis revealed that the composite endpoint varied significantly depending on the CKD stage at baseline in both groups (P < 0.0001, log-rank test; Figure 4). After the adjustment of basic factors, including age, sex, comorbid CVD, and the presence or absence of diabetes, the HRs in the G3b, G4, and G5 groups were compared with the G3a (reference) group and were significantly higher in both. However, after the adjustment of basic factors and laboratory data, including BMI, hemoglobin, serum albumin, and UPCR level, the G4 and G5 groups had significantly higher HRs (Tables 6, 7).
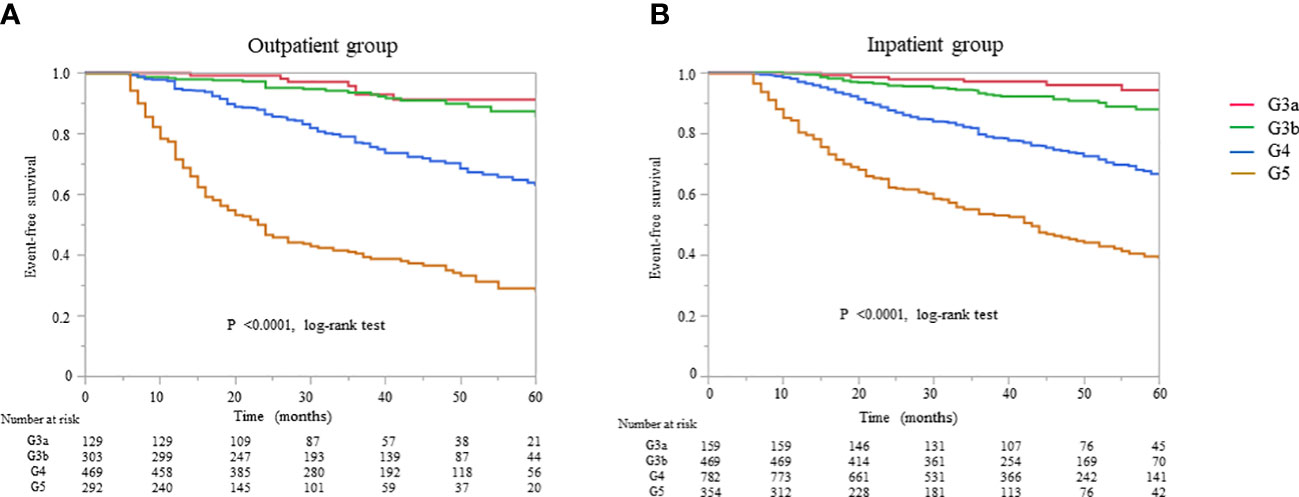
Figure 4 Kaplan–Meier curves for the incidence of initiation of renal replacement therapy and all-cause mortality in Japanese patients with chronic kidney disease according to the baseline stages in the (A) outpatient and (B) inpatient groups.
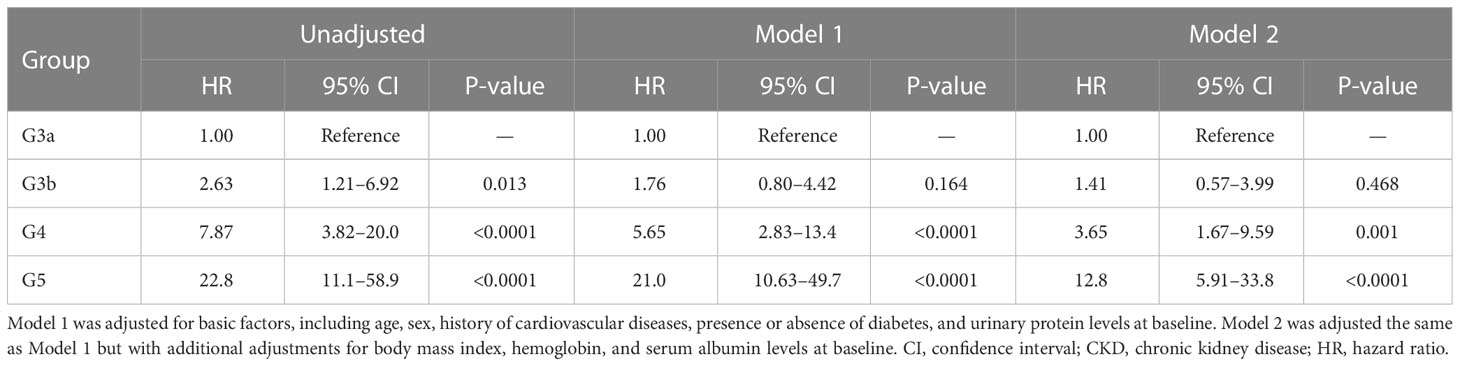
Table 6 All-cause mortality and initiation of renal replacement therapy according to the CKD stage at baseline in Cox proportional hazards models adjusted for confounding factors in the outpatient group.
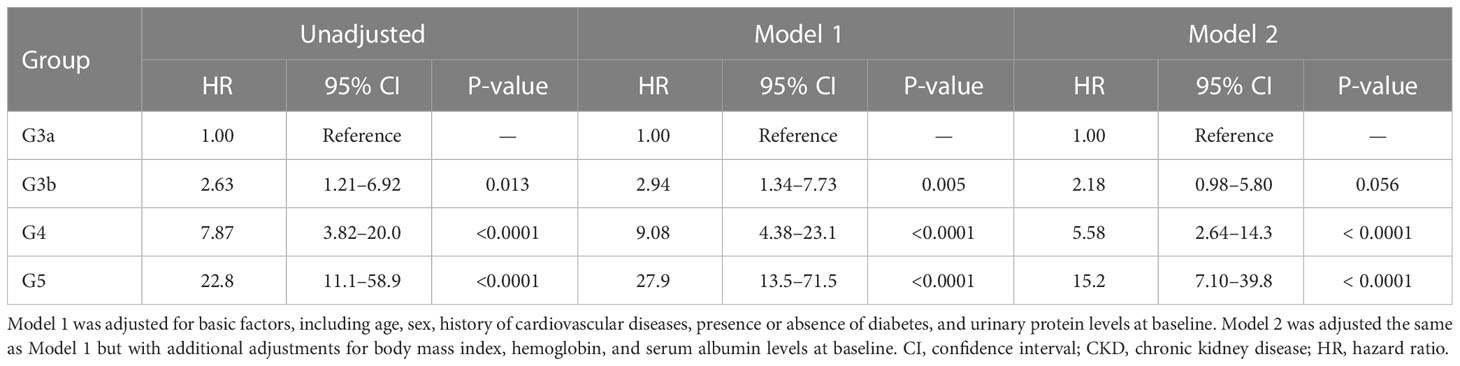
Table 7 All-cause mortality and initiation of renal replacement therapy according to the CKD stage at baseline in Cox proportional hazards models adjusted for confounding factors in the inpatient group.
3.6 Subgroup analysis based on the presence or absence of physical therapists in the inpatient group
The patients in the inpatient group were subdivided into two groups with and without a physical therapist in the multidisciplinary care team. The baseline characteristics of the two groups are shown in Supplementary Table 1. The group with physical therapists had higher eGFR and lower proteinuria at baseline, with a higher rate of comorbid CVD and diabetic kidney disease. The Kaplan–Meier analysis revealed a significant difference in the composite endpoint between the two groups (P < 0.0001, log-rank test; Figure 5). Compared with the group without physical therapists, the group with physical therapists had a significantly lower unadjusted HR for the composite endpoint (0.52, 95% CI 0.42–0.63, P < 0.0001). After the adjustment of basic factors, including age, sex, history of CVD, eGFR, and UPCR at baseline, the HR in the group with physical therapists was 0.51 (95% CI 0.41–0.64, P < 0.0001). After further adjustment of basic factors and BMI, hemoglobin, and serum albumin level at baseline, the group with physical therapists had a significantly lower HR (0.55, 95% CI 0.42–0.71, P < 0.0001) (Supplementary Table 2).

Figure 5 Kaplan–Meier curves for the initiation of renal replacement therapy and all-cause mortality in Japanese patients with chronic kidney disease based on the presence or absence of physical therapists in the inpatient subgroups.
3.7 ΔeGFR and change in UPCR before and after multidisciplinary care in all patients
The mean ΔeGFR was significantly improved from –5.89 ± 7.17 before multidisciplinary intervention to –0.44 ± 5.21 at 6 months, –1.52 ± 6.09 at 12 months, and –1.48 ± 3.78 at 24 months after intervention (all P < 0.0001; Figure 6A). The median UPCR was significantly decreased from 1.09 g/gCr [0.23, 2.98] at baseline to 1.00 g/gCr [0.24, 2.71] at 6 months, 0.89 g/gCr [0.21, 2.38] at 12 months, and 0.82 g/gCr [0.20, 2.22] at 24 months (all P < 0.0001; Figure 6B).
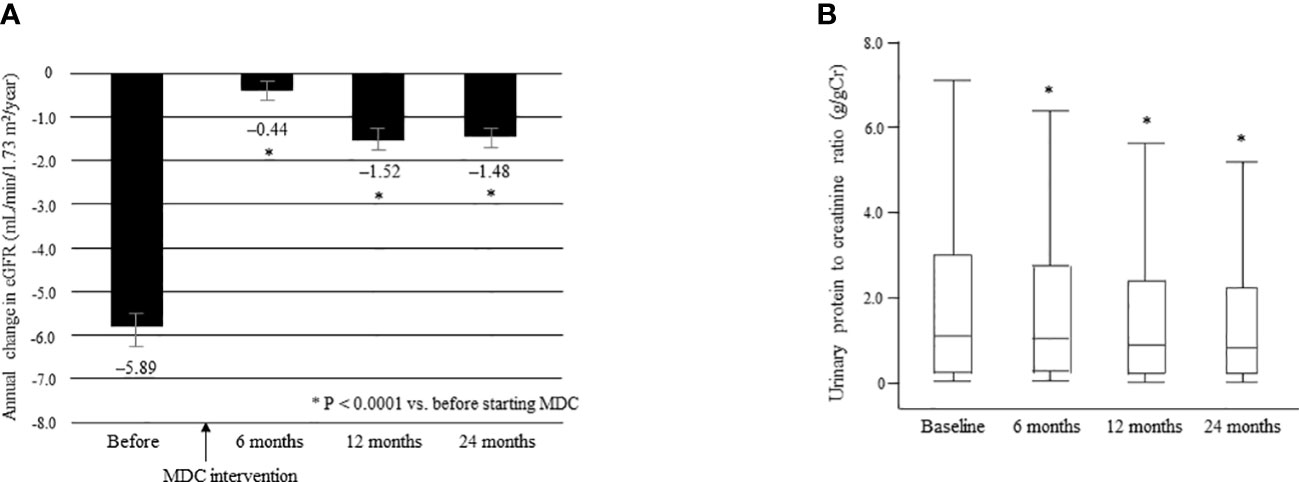
Figure 6 Annual change in eGFR in the 12 months before and 24 months after starting multidisciplinary care in all patients (A). Data are shown as the mean. Bars indicate the 95% confidence interval. *P < 0.0001 vs. before start of MDC. Changes in the urinary protein level between time of initiation of MDC and 24 months later (B). Data are shown as the median and interquartile range. *P < 0.0001 vs. baseline. ΔeGFR, change in eGFR; eGFR, estimated glomerular filtration rate; MDC, multidisciplinary care.
3.7.1 ΔeGFR and change in UPCR before and after multidisciplinary care in the two groups
The mean ΔeGFR before and after multidisciplinary intervention in each group is shown in Figure 7. There was no significant between-group difference in mean ΔeGFR before intervention (Supplementary Table 3). The mean ΔeGFR was -6.09 ± 7.65 before intervention and -0.52 ± 5.23 at 6 months, -1.32 ± 6.01 at 12 months, and -1.32 ± 3.64 at 24 months after intervention in the outpatient group (all P < 0.0001; Figure 7A); the respective values in the inpatient group were -5.81 ± 7.43, -0.40 ± 5.20, -1.63 ± 6.15, and -1.56 ± 3.84 (all P < 0.0001; Figure 7B). There was no significant between-group difference in mean ΔeGFR at any time point after intervention (Supplementary Table 3).
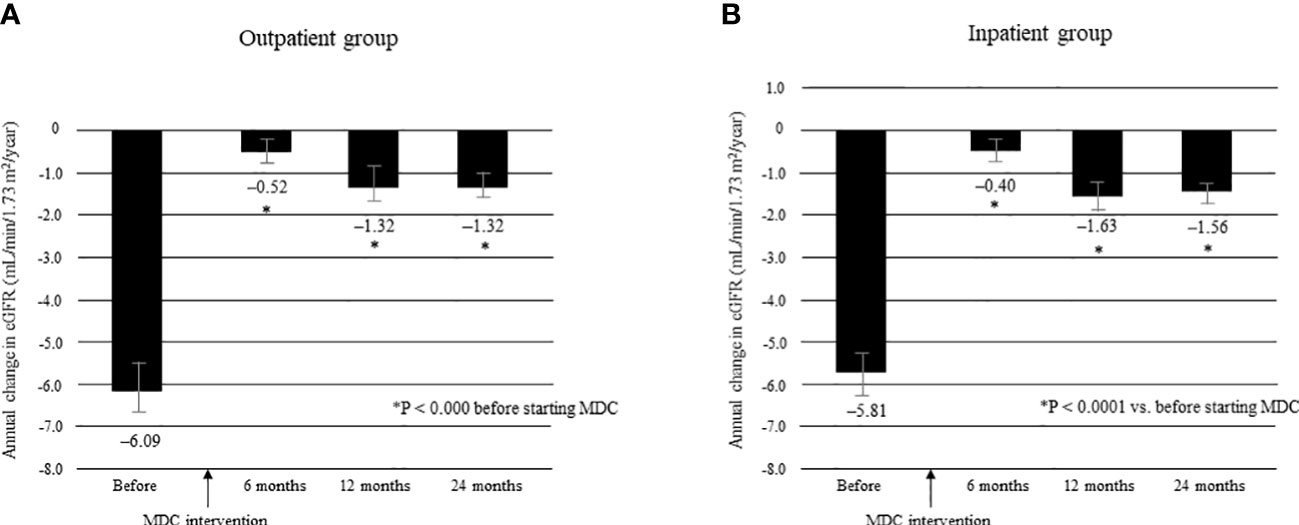
Figure 7 Annual change in eGFR in the 12 months before and 24 months after starting MDC in the outpatient group (A) and in the inpatient group (B). *P < 0.0001 vs. before start of MDC. Data are shown as the mean. Bars indicate the 95% confidence interval. ΔeGFR, change in eGFR; eGFR, estimated glomerular filtration rate; MDC, multidisciplinary care.
Changes in the median UPCR after intervention by the multidisciplinary care team are shown for each group in Figure 8. There was no significant between-group difference in UPCR at baseline. However, in the outpatient group, the median UPCR decreased significantly from 1.20 g/gCr [0.27, 3.25] at baseline to 1.10 g/gCr [0.29, 2.98] at 6 months, 0.94 g/gCr [0.22, 2.42] at 12 months, and 0.88 g/gCr [0.24, 2.36] at 24 months (all P <0.0001; Figure 8A); the respective values in the inpatient group were 1.01 g/gCr [0.22, 2.87], 0.92 g/gCr [0.21, 2.61], 0.82 g/gCr [0.21, 2.37], and 0.79 g/gCr [0.17, 2.28] (all P < 0.0001; Figure 8B). Furthermore, there was no significant between-group difference in the median UPCR at any time point after intervention (Supplementary Table 4).
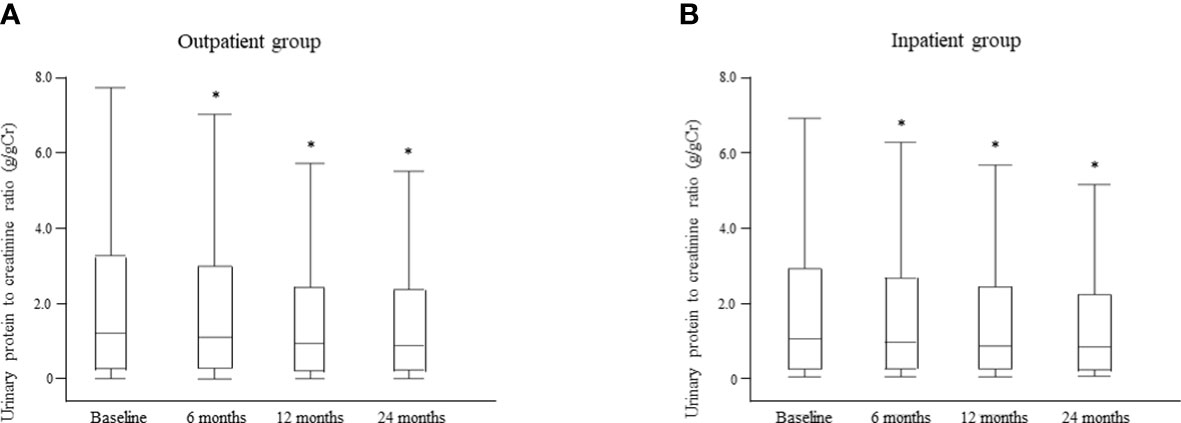
Figure 8 Changes in the urinary protein level between the time of starting multidisciplinary care and 24 months later in the outpatient (A) and inpatient (B) groups. Data are shown as the median and interquartile range. *P < 0.0001 vs. baseline.
4 Discussion
This nationwide cohort study included 2954 individuals from 24 facilities in Japan. We found that patients with CKD currently receive multidisciplinary care more often in hospitals (59.7%) than in an outpatient setting (40.3%) in Japan. The major strengths of this study are its large sample population recruited from multiple centers, the relatively long observation period, and inclusion of a comparatively high number of elderly patients. Although the mean age of patients in the previous studies was younger than 70 years, our mean age was 70.5 years, reflecting our aging CKD population in Japan (5, 7–10). This study is the first to suggest that multidisciplinary care may be able to prevent worsening kidney function in Japanese patients with CKD regardless of whether it is provided on an outpatient or inpatient basis. The rate of RRT initiation and all-cause mortality over the longer observation period of 6 years were the key composite endpoints, and although there was no significant difference between the two groups’ baseline eGFR levels, there was a significant between-group difference in both variables. Therefore, our results suggest that multidisciplinary care for patients with CKD might be more beneficial in terms of outcomes in the inpatient setting than in the outpatient setting. Furthermore, multidisciplinary care was effective for patients with CKD regardless of whether or not they had diabetes and should be provided at CKD stage G4 at the latest. A multidisciplinary care team should include a nephrologist, a specialist nurse, a physical therapist, and professionals from other fields and is recommended for the management of patients with CKD.
Inpatient education programs have been reported to improve glycemic control, prevent diabetic complications, and reduce hospitalization rates in patients with diabetes (18–20). However, there is little information on the efficacy of multidisciplinary intervention for patients with CKD according to whether the intervention is inpatient-based or outpatient-based. This is the first study to indicate that inpatient multidisciplinary care improves the all-cause mortality risk and initiation of RRT in patients with CKD. Inpatient education programs for patients with CKD have not been implemented extensively in Western countries, probably reflecting differences in the medical insurance system between Japan and Western countries. Although education provided in an outpatient setting is reimbursed for patients with diabetic kidney disease in Japan, it is not reimbursed for patients with other etiologies of CKD. However, full reimbursement is available for these patients if they are admitted to hospital. A few single-center studies in Japan have evaluated the effectiveness of education programs for CKD to date. One study found that the annual rate of decline in eGFR was improved by an inpatient education program, which was continued for 2 years (21). Furthermore, the interval between the start of stage G5 and the start of RRT was longer in patients who received an inpatient education program than in those who did not (22). The patients who received an inpatient education program also had better survival after initiation of dialysis (23). Therefore, multidisciplinary care would be associated with a decreased hospitalization rate, a longer time until initiation of dialysis, and a shorter hospital stay at the start of dialysis, which could lead to a reduction of medical costs. However, the content of the education program and the delivered systems varied according to each facility. Nevertheless, the number of days of hospitalization and the time spent on education should be analyzed. Also, the reasons why it could not be achieved on an outpatient basis should be confirmed. Therefore, further research is required to confirm that the cost-effectiveness of the inpatient setting is superior to that of the outpatient setting.
A meta-analysis revealed that the reduction in all-cause mortality depended on the disciplines represented in the multidisciplinary care team and the stage (24). With only nephrologists and specialist nurses on the team, there was no significant difference in all-cause mortality between patients receiving multidisciplinary care and those who were not. By contrast, when the multidisciplinary care team comprised nephrologists, specialist nurses, and professionals from other disciplines (e.g., dieticians, pharmacists, or social workers), multidisciplinary care was associated with a lower risk of all-cause mortality (25). The FROM-J (Frontier of Renal Outcome Modifications in Japan) study reported that lifestyle and dietary advice provided by a registered dietician in an outpatient setting slowed the rate of deterioration of kidney function in patients with CKD when compared with controls (26). However, the findings were not significant for all stages of CKD and were limited to stage 3; moreover, the multidisciplinary care team comprised only doctors and registered dieticians. In our study, multidisciplinary care was provided by a mean of 4.5 ± 0.6 professionals in the inpatient group and by 2.6 ± 0.7 in the outpatient group. A possible explanation for this result is that when the multidisciplinary care team consists of nephrologists and nurses, the multidisciplinary care model is similar to a conventional model, in which non-multidisciplinary care may be provided by nephrologists and nurses. When the multidisciplinary care group does not include other professionals (e.g., registered dieticians and pharmacists), the education provided for patients with CKD may be insufficient, such that guidelines for dietary protein restriction and other targets are not met, thereby contributing to worsening of kidney function. Patients with CKD require holistic care and support, including dietary modification, maintenance and improvement of medication adherence, education on self-monitoring and early detection of complications, and adequate financial resources to continue treatment. These supports cannot be provided by nephrologists alone and must be implemented by a medical team consisting of multiple professionals. To achieve good outcomes, multidisciplinary care teams that include nephrologists, nurses, registered dieticians, pharmacists, physical therapists, and medical social workers should be involved and have shared goals in terms of individual patients. However, we have no definitive conclusions on how many different cooperating disciplines are needed to achieve optimal outcomes, and further investigations are required to confirm this.
This study has several limitations. First, it did not include a non-multidisciplinary control group. Although multidisciplinary care was not associated with a lower risk of all-cause mortality in previous randomized controlled trials, the risk was found to be reduced in one cohort study (14, 26–28). In addition, the patients could not be randomly allocated to outpatient and inpatient groups because the environment in which multidisciplinary care could be provided varied depending on each facility. Therefore, further prospective randomized controlled trials and large epidemiological studies that include control groups are needed to confirm the efficacy of multidisciplinary care in patients with CKD. Second, we did not investigate changes in blood pressure or laboratory findings other than for kidney function. Salt restriction by multidisciplinary intervention may have lowered blood pressure, reduced proteinuria, and maintained kidney function. We were unable to investigate whether there was any difference in the reduction of salt intake or blood pressure between the study groups. Third, adding or changing medications during the observation period might have affected laboratory findings and kidney function. Renin–angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors are recommended for patients with albuminuria, and statins are recommended for all patients with diabetes and CKD (29). Treatment of renal anemia with erythropoiesis-stimulating agents plays an important role in kidney survival (30, 31). Further investigations are needed to determine the contribution of improved adherence with prescribed medication and dietary modification to prevention of worsening kidney function. Finally, there may have been some degree of patient selection and facility bias. Inpatient programs are longer and more expensive than outpatient programs. It is possible that the inpatient group included patients with high self-management ability and a strong desire to prevent progression of their CKD. Therefore, multidisciplinary care in an inpatient setting may be associated with improved patient health literacy. In this study, the participants were divided into two groups by the first intervention method. Therefore, some patients may have been treated in both the inpatient and outpatient settings. Patients might have received multidisciplinary care as an inpatient first, followed by an outpatient setting, or vice versa. However, most facilities in this study provided outpatient or inpatient educational programs based on the hospital functions and human resources. In addition, the content of the education program and the makeup of the patient population varied between the outpatient and inpatient groups from facility to facility. Therefore, the effects of simultaneous participation in outpatient and inpatient sessions should be verified, and educational programs should be standardized to improve the level of care for patients with CKD.
In conclusion, our findings indicate that multidisciplinary care may significantly slow the decline of eGFR, reduce proteinuria in patients with CKD and be effective regardless of diabetes status. Furthermore, this study suggests that multidisciplinary care might be more effective when inpatient-based than when outpatient-based in terms of reducing the all-cause mortality risk and initiation of RRT. Further research is needed to devise a standardized program of multidisciplinary care for both outpatients and inpatients with CKD and to determine which professionals should be involved to achieve the best outcomes for these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The ethics committee of Nihon University Itabashi Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
MA wrote the manuscript and analyzed the data. TH, YI, TS, and SK designed the study and contributed to data collection. MA, TH, YI, TS, and SK discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript. All authors contributed to the article.
Funding
This work was supported by Health Labour Sciences Research Grant 20FD1003.
Acknowledgments
We thank the members of the Committee of the Evaluation and Dissemination for Certified Kidney Disease Educator, Japan Kidney Association for all their efforts, as well as the staff members at the participating facilities. In particular, we thank the following medical professionals and hospitals: Takashi Maruyama, Division of Nephrology, Hypertension and Endocrinology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan; Toshihiro Shindo, Department of Nephrology, Hiroshima University Hospital, Hiroshima, Japan; Kunihiro Yamagata and Chie Saito, Department of Nephrology, Faculty of Medicine, University of Tsukuba, Ibaraki, Japan; Kimihiko Nakatani, Department of Nephrology, Kyoto Yamashiro General Medical Center, Kyoto, Japan; Isao Ohsawa, Saiyu Soka Hospital, Saitama, Japan; Hiroaki Io, Department of Nephrology, Juntendo University Nerima Hospital, Tokyo, Japan; Hiroshi Kado, Oumihachiman Community Medical Center, Shiga, Japan; Katsuhito Mori, Department of Nephrology, Osaka Metropolitan University Graduate School of Medicine, Osaka, Japan; Aya Sakurai, St. Marianna University School of Medicine Yokohama City Seibu Hospital, Yokohama, Japan; Akira Ishii and Motoko Yanagita, Department of Nephrology, Graduate School of Medicine, Kyoto University, Kyoto, Japan; Tatsuo Yamamoto, Fujieda Municipal General Hospital, Shizuoka, Japan; Seigo Hiraga, JCHO Mishima General Hospital, Shizuoka, Japan; Mamiko Shimamoto, Division of Nephrology, Sapporo City General Hospital, Sapporo, Japan; Masaru Matsui, Department of Nephrology, Nara Prefecture General Medical Center, Nara, Japan; Yuriko Yonekura, Divison of Nephrology, Department of Medicine, AIJINKAI Healthcare Corporation Akashi Medical Center, Hyogo, Japan; Katsuyuki Tanabe, Okayama University Hospital, Okayama, Japan; Tatsuo Tsukamoto, Department of Nephrology and Dialysis, Medical Research Institute Kitano Hospital, PIIF Tazuke-Kofukai, Osaka, Japan; Ryouhei Horii and Satoshi Suzuki, Seirei Sakura Citizen Hospital, Chiba, Japan; Katsuhiko Morimoto, Nara Prefectural Seiwa Medical Center, Nara, Japan; Kenta Torigoe and Tomoya Nishino, Nagasaki University Hospital, Nagasaki, Japan; Saori Nishio, Department of Rheumatology, Endocrinology, and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan; Kazue Ueki and Takayuki Matsumoto, Dialysis and Nephrology Center, Sanshikai TOHO Hospital, Gunma, Japan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1180477/full#supplementary-material
References
1. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
2. Nagai K, Asahi K, Iseki K, Yamagata K. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin Exp Nephrol (2021) 25:885–92. doi: 10.1007/s10157-021-02049-0
3. Nitta K, Goto S, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, et al. Annual dialysis data report for 2018, JSDT renal data registry: survey methods, facility data, incidence, prevalence, and mortality. Ren Replace Ther (2020) 6:41. doi: 10.1186/s41100-020-00286-9
4. United States Renal Data System. 2020 USRDS annual data report: epidemiology of kidney disease in the united states. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2020).
5. Chen PM, Lai TS, Chen PY, Lai CF, Yang SY, Wu V, et al. Multidisciplinary care program for advanced chronic kidney disease: reduces renal replacement and medical costs. Am J Med (2015) 128:68–76. doi: 10.1016/j.amjmed.2014.07.042
6. Wang SM, Hsiao LC, Ting IW, Yu TM, Liang CC, Kuo HL, et al. Multidisciplinary care in patients with chronic kidney disease: a systematic review and meta-analysis. Eur J Intern Med (2015) 26:640–5. doi: 10.1016/j.ejim.2015.07.002
7. Chen YR, Yang Y, Wang SC, Chiu PF, Chou WY, Lin CY, et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: a 3-year prospective cohort study. Nephrol Dial Transplant (2013) 28:671–82. doi: 10.1093/ndt/gfs469
8. Chen YR, Yang Y, Wang SC, Chou WY, Chiu PF, Lin C, et al. Multidisciplinary care improves clinical outcome and reduces medical costs for pre-end-stage renal disease in Taiwan. Nephrol (Carlton) (2014) 19:699–707. doi: 10.1111/nep.12316
9. Wu IW, Wang SY, Hsu KH, Lee CC, Sun CY, Tsai CJ, et al. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality–a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant (2009) 24:3426–33. doi: 10.1093/ndt/gfp259
10. Imamura Y, Takahashi Y, Hayashi T, Iwamoto M, Nakamura R, Goto M, et al. Usefulness of multidisciplinary care to prevent worsening renal function in chronic kidney disease. Clin Exp Nephrol (2019) 23:484–92. doi: 10.1007/s10157-018-1658-z
11. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med (2003) 348:383–93. doi: 10.1056/NEJMoa021778
12. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med (2008) 358:580–91. doi: 10.1056/NEJMoa0706245
13. Fogelfeld L, Hart P, Miernik J, Ko J, Calvin D, Tahsin B, et al. Combined diabetes-renal multifactorial intervention in patients with advanced diabetic nephropathy: proof-of-concept. J Diabetes Complications (2017) 31:624–30. doi: 10.1016/j.jdiacomp.2016.11.019
14. Peeters MJ, van Zuilen AD, van den Brand JA, Bots ML, van Buren M, Ten Dam MA, et al. Nurse practitioner care improves renal outcome in patients with CKD. J Am Soc Nephrol (2014) 25:390–8. doi: 10.1681/ASN.2012121222
15. Japan Kidney Association. Chronic kidney disease (2018). Available at: https://j-ka.or.jp/ckd/download/ (Accessed March 05, 2023).
16. Japanese Society of Nephrology. Guide for kidney disease (2020). Available at: https://jsn.or.jp/jsn_new/iryou/kaiin/free/primers/pdf/2021allpage.pdf (Accessed March 05, 2023).
17. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis (2009) 53:982–92. doi: 10.1053/j.ajkd.2008.12.034
18. Wexler DJ, Beauharnais CC, Regan S, Nathan DM, Cagliero E, Larkin ME. Impact of inpatient diabetes management, education, and improved discharge transition on glycemic control 12 months after discharge. Diabetes Res Clin Pract (2012) 98:249–56. doi: 10.1016/j.diabres.2012.09.016
19. Fritsche A, Stumvoll M, Goebbel S, Reinauer KM, Schmülling RM, Häring HU. Long term effect of a structured inpatient diabetes teaching and treatment programme in type 2 diabetic patients: influence of mode of follow-up. Diabetes Res Clin Pract (1999) 46:135–41. doi: 10.1016/s0168-8227(99)00081-9
20. Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care (2013) 36:2960–87. doi: 10.2337/dc13-0108
21. Machida S, Shibagaki Y, Sakurada T. An inpatient educational program for chronic kidney disease. Clin Exp Nephrol (2019) 23:493–500. doi: 10.1007/s10157-018-1660-5
22. Takagi WH, Osako K, Machida S, Koitabashi K, Shibagaki Y, Sakurada T. Inpatient educational program delays the need for dialysis in patients with chronic kidney disease stage G5. Clin Exp Nephrol (2021) 25:166–72. doi: 10.1007/s10157-020-01979-5
23. Yoshida K, Shimizu S, Kita Y, Takagi WH, Shibagaki Y, Sakurada T. Impact of inpatient educational programs on mortality after the start of dialysis therapy. Clin Exp Nephrol (2022) 26:819–26. doi: 10.1007/s10157-022-02211-2
24. Shi Y, Xiong J, Chen Y, Deng J, Peng H, Zhao J, et al. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol (2018) 50:301–12. doi: 10.1007/s11255-017-1679-7
25. Yamagata K, Makino H, Iseki K, Ito S, Kimura K, Kusano E, et al. Effect of behavior modification on outcome in early- to moderate-stage chronic kidney disease: a cluster-randomized trial. PloS One (2016) 11:e0151422. doi: 10.1371/journal.pone.0151422
26. Barrett BJ, Garg AX, Goeree R, Levin A, Molzahn A, Rigatto C, et al. A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trial. Clin J Am Soc Nephrol (2011) 6:1241–7. doi: 10.2215/CJN.07160810
27. Yu YJ, Wu IW, Huang CY, Hsu KH, Lee CC, Sun CY, et al. Multidisciplinary predialysis education reduced the inpatient and total medical costs of the first 6 months of dialysis in incident hemodialysis patients. PloS One (2014) 9:e112820. doi: 10.1371/journal.pone.0112820
28. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care (2009) 32:977–82. doi: 10.2337/dc08-1908
29. de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: a consensus report by the American diabetes association (ADA) and kidney disease: improving global outcomes (KDIGO). Kidney Int (2022) 102:974–89. doi: 10.1016/j.kint.2022.08.012.
30. Akizawa T, Tsubakihara Y, Hirakata H, Watanabe Y, Hase H, Nishi S, et al. A prospective observational study of early intervention with erythropoietin therapy and renal survival in non-dialysis chronic kidney disease patients with anemia: JET-STREAM study. Clin Exp Nephrol (2016) 20:885–95. doi: 10.1007/s10157-015-1225-9
Keywords: certified kidney disease educator, chronic kidney disease, estimated glomerular filtration rate, inpatient educational program, multidisciplinary care, outpatient guidance, renal replacement therapy
Citation: Abe M, Hatta T, Imamura Y, Sakurada T and Kaname S (2023) Inpatient multidisciplinary care can prevent deterioration of renal function in patients with chronic kidney disease: a nationwide cohort study. Front. Endocrinol. 14:1180477. doi: 10.3389/fendo.2023.1180477
Received: 06 March 2023; Accepted: 24 May 2023;
Published: 20 June 2023.
Edited by:
Jun Wada, Okayama University, JapanReviewed by:
Masao Toyoda, Tokai University School of Medicine, JapanYoshinari Yasuda, Nagoya University, Japan
Copyright © 2023 Abe, Hatta, Imamura, Sakurada and Kaname. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masanori Abe, YWJlLm1hc2Fub3JpQG5paG9uLXUuYWMuanA=
 Masanori Abe
Masanori Abe Tsuguru Hatta1,3
Tsuguru Hatta1,3 Shinya Kaname
Shinya Kaname