Editorial on the Research Topic
A year in review: discussions in developmental endocrinology
Molecular endocrinology increases our understanding of endocrine diseases and informs the clinical management of complex medical disorders. Frontiers in Developmental Endocrinology bridges fundamental molecular insights with broader global public health concerns. Here, leading researchers report the latest scientific insights in endocrine physiology and metabolism. The aim is a deeper understanding of the developmental aspects of clinical disease pathogenesis, diagnosis, and treatment. We are here to integrate concepts from medical investigators, basic scientists, and clinicians to improve global health care and advance health equity.
This Research Topic reports on new insights, challenges, and future perspectives across critical biological issues. For example, we learn about endocrine-disrupting chemicals, regulation of ovarian follicle steroidogenesis, testicular Leydig cell development, and human embryonic development related to pregnancy and neonatal outcomes.
Yan et al. review the effects of endocrine-disrupting chemicals on placental development in humans. Endocrine-disrupting chemicals (EDCs) are environmental compounds interacting with the endocrine system to alter a range of critical biological processes, including immunity, metabolism, organogenesis, reproduction, and behavior. (Figure 1) Scientific evidence demonstrates the human health impacts of exposure to EDCs. There are considerable global economic costs to adverse health outcomes induced by EDC exposure (1). There is a need for a multifaceted international program to address the effects of EDCs on human health and to identify, proactively, hazards for effective regulation. The International Agency for Research in Cancer is an excellent model for such a program (2).
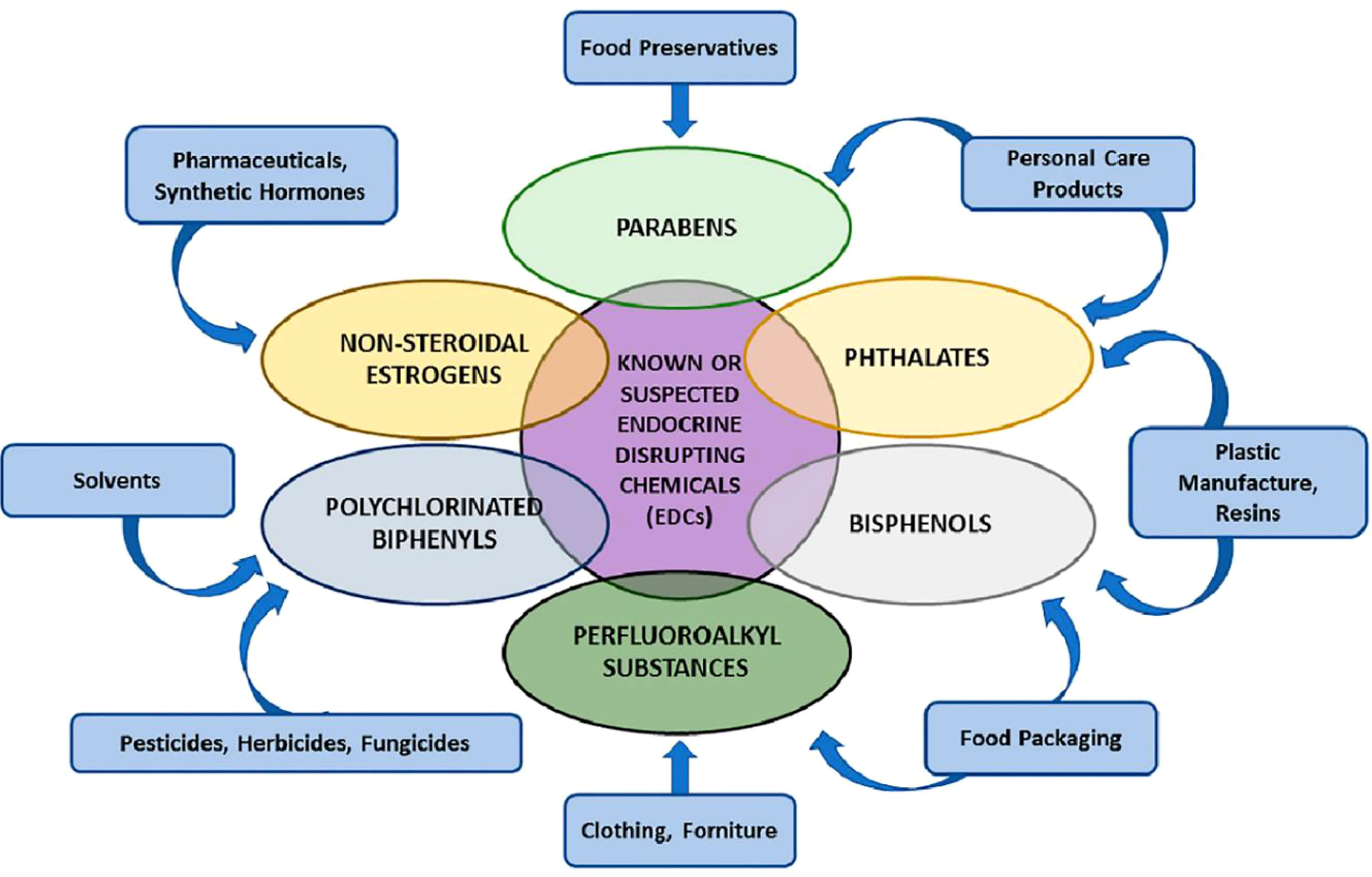
Figure 1 Various groups of endocrine-disrupting chemicals (EDCs) Yan et al.
Devillers et al. investigate molecular aspects of ovarian physiology and examine ovarian follicle estradiol synthesis pathways in mice. Their work provides insight into the expression of several critical intra-ovarian regulators, including the FSH receptor (Fshr), the aromatase enzyme (Cyp19a1), and the cell cycle inhibitor p27KIP1 (Cdkn1b).
Estradiol plays an essential role in women’s health across the lifespan. Early onset of estradiol deficiency in women is associated with morbidity and early mortality. These important roles of estradiol include establishing and maintaining bone mineral density and health of the cardiovascular and neurological systems (3–5). The menopause transition to postmenopausal serum estradiol levels is a critical period for cardiovascular health. In women, cardiovascular disease is the most frequent cause of death. Importantly, there is a notable increase in the risk for cardiovascular disease after menopause (6). Intriguingly, arterial stiffness increases within one year of the final menopausal menstrual period (7).
Most estrogen receptors, known as nuclear signaling receptors, regulate gene expression in the cell nucleus. However, a subpopulation of cell membrane estrogen receptors initiates rapid estradiol intracellular signaling, which does not involve transcription, known as “non-nuclear signaling.” The recent development of genetically modified animal models provides new insights into the non-nuclear signaling of estradiol and its role in the cardiovascular system (8).
Women are five years older than men on average when experiencing a first stroke, yet women suffer from more severe strokes (9). A recent systematic review and meta-analysis investigated differences in the presentation of stroke symptoms between the sexes. Women were more likely than men to present with nontraditional symptoms of stroke, a potential factor in delay in diagnosis and treatment. Of note, there was a paucity of studies conducted outside Europe and North America (Supplementary Figure 1). The authors called for enhanced equity in global stroke research (9).
There is a critical health need for education and advocacy to put the health benefits of estradiol replacement in proper perspective (10). A recent report in the New York Times, “Women have been misled about menopause,” (11) demonstrates that shared decision-making remains a difficult conversation in this area of care (12). Women younger than 45 with estradiol deficiency have a metabolic derangement associated with increased morbidity and mortality (13). Generally, prospective, randomized, double-blind, controlled studies provide the best evidence for making clinical decisions (14). In the past 20 years, the US National Institutes of Health (NIH) Intramural Research Program (NIH-IRP) conducted the only such study on hormone replacement in women with primary ovarian insufficiency (POI) (15). The study provided women with POI with the average daily production rate of estradiol (100 micrograms per day) by transdermal patch and cyclic, monthly oral progestogen. The NIH-IRP hormone replacement regimen restored bone mineral density to normal over three years, and women tolerated the treatment well. Oral estrogen treatment has a higher risk of thromboembolism than transdermal estradiol replacement, yet this more physiologic approach is underutilized in clinical practice (16). Rather than starting from a position of equipoise, future studies of estradiol replacement therapy would best start by considering the benefits of physiologic estradiol replacement by skin patch or vaginal ring compared to oral estrogens.
Bhattacharya et al. publish a review on testicular Leydig cell development in fetal and adult testes. They highlight the cellular progenitor/stemcell origins with associated functional significance in rodents and primates. Recent studies suggest that a small fraction (5-20%) of fetal Leydig cells persist in adult testis. Progress in ex vivo cell/organ culture, genome-wide analysis, genetically manipulated mouse models, lineage tracing, and single-cell RNA-seq experiments reveal different steroidogenic outputs of these two populations of Leydig cells.
Chen et al. report on human embryo vacuoles, cytoplasmic inclusions containing liquids from the perivitelline space. Vacuolization in human embryos on Days 3 and 4 are associated with impaired blastocyst development. However, in cases where the rejection of the vacuole-containing cells occurs during the compaction process, blastocysts had a low mosaicism rate. This noteworthy finding supports the hypothesis that excluding abnormal blastomeres during compaction is a self-correction mechanism. Furthermore, the pregnancy rates and neonatal outcomes of vacuole-positive embryos were similar to those of vacuole-negative embryos. Thus, clinicians may consider vacuole-positive embryos an option for embryo transfer.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1213095/full#supplementary-material
References
1. Kassotis CD, Trasande L. Endocrine disruptor global policy. Adv Pharmacol (2021) 92:1–34. doi: 10.1016/bs.apha.2021.03.005
2. Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol (2020) 8(8):719–30. doi: 10.1016/S2213-8587(20)30128-5
3. Svejme O, Ahlborg HG, Nilsson JÅChecktae, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG (2012) 119(7):810–6. doi: 10.1111/j.1471-0528.2012.03324.x
4. Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi-ethnic study of atherosclerosis. Menopause (2012) 19(10):1081–7. doi: 10.1097/gme.0b013e3182517bd0
5. Russell JK, Jones CK, Newhouse PA. The role of estrogen in brain and cognitive aging. Neurotherapeutics (2019) 16(3):649–65. doi: 10.1007/s13311-019-00766-9
6. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation (2020) 142(25):e506–32. doi: 10.1161/CIR.0000000000000912
7. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, et al. Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN heart study. Arterioscler Thromb Vasc Biol (2020) 40(4):1001–8. doi: 10.1161/ATVBAHA.119.313622
8. Hiroyuki T, Kazutaka U, Eiki T. The emerging role of estrogen’s non-nuclear signaling in the cardiovascular disease. Front Cardiovasc Med (2023) 10:1127340. doi: 10.3389/fcvm.2023.1127340
9. Hosman FL, Engels S, den Ruijter HM, Exalto LG. Call to action for enhanced equity: Racial/Ethnic diversity and sex differences in stroke symptoms. Front Cardiovasc Med (2022) 9:874239. doi: 10.3389/fcvm.2022.874239
10. Spencer H, Simon JA, Nelson LM. Difficult conversations: management of estradiol deficiency. Maturitas (2023) 171:24. doi: 10.1016/j.maturitas.2023.03.001
11. Dominus S. Women have been misled about menopause. New York, New York: The New York Times (2023). Available at: https://www.nytimes.com/2023/02/01/magazine/menopause-hot-flashes-hormone-therapy.html.
12. Prober CG, Grousbeck HI, Meehan WF 3rd. Managing difficult conversations: an essential communication skill for all professionals and leaders. Acad Med (2022) 97(7):973–6. doi: 10.1097/ACM.0000000000004692
13. Asllanaj E, Bano A, Glisic M, Jaspers L, Ikram MA, Laven JSE, et al. Age at natural menopause and life expectancy with and without type 2 diabetes. Menopause (2019) 26(4):387–94. doi: 10.1097/GME.0000000000001246
14. Centre for Evidence-Based Medicine (CEBM). University of Oxford. Available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (Accessed February 12, 2023).
15. Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab (2014) 99(9):3418–26. doi: 10.1210/jc.2013-4145
Keywords: estradiol (17ß-estradiol), endocrine disruptors, policy & institutional actions, longevity, women’s health activism, preventative medicine/care/services, cardiovascular disease, hormone replacement estradiol 17 beta
Citation: Nelson LM (2023) Editorial: A year in review: discussions in developmental endocrinology. Front. Endocrinol. 14:1213095. doi: 10.3389/fendo.2023.1213095
Received: 27 April 2023; Accepted: 17 May 2023;
Published: 25 May 2023.
Edited and Reviewed by:
Jeff M. P. Holly, University of Bristol, United KingdomCopyright © 2023 Nelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lawrence M. Nelson, RG9jQENvbm92ZXJGb3VuZGF0aW9uLm9yZw==
 Lawrence M. Nelson
Lawrence M. Nelson