- Department of Spine Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, Shanxi, China
Objective: Recent evidence indicates that cervical paraspinal muscle degeneration (PMD) is a prevalent and age-related condition in patients with cervical disc degenerative disease (CDDD). However, the relationship between surgery selection and post-operative outcomes in this population remains unclear. Consequently, this study aims to investigate the disparities in clinical outcomes, radiological findings, and complications between two frequently utilized anterior cervical surgical procedures. The objective is to offer guidance for the management of PMD in conjunction with CDDD.
Methods: A total of 140 patients who underwent single-level anterior cervical discectomy and fusion (ACDF) at our department were included in this study. The patients were divided into three groups based on the severity of PMD: mild (n=40), moderate (n=54), and severe (n=46), as determined by Goutalier fat infiltration grade. The subjects of interest were those with moderate-severe PMD, and their clinical outcomes, radiological parameters, and complications were compared between those who received a stand-alone zero-profile anchored cage (PREVAIL) and those who received a plate-cage construct (PCC).
Results: The JOA, NDI, and VAS scores exhibited significant improvement at all postoperative intervals when compared to baseline, and there were no discernible differences in clinical outcomes between the two groups. While the PCC group demonstrated more pronounced enhancements and maintenance of several sagittal alignment parameters, such as the C2-7 angle, FSU angle, C2-7 SVA, and T1 slope, there were no statistically significant differences between the two groups. The incidence of dysphagia in the zero-profile group was 22.41% at one week, which subsequently decreased to 13.79% at three months and 3.45% at the final follow-up. In contrast, the plate cage group exhibited a higher incidence of dysphagia, with rates of 47.62% at one week, 33.33% at three months, and 11.90% at the final follow-up. Notably, there were significant differences in the incidence of dysphagia between the two groups within the first three months. However, the fusion rate, occurrence of implant subsidence, and adjacent segment degeneration (ASD) were comparable at the final follow-up.
Conclusion: For patients with one-level cervical disc degenerative disease combined with paraspinal muscle degeneration, both the zero-profile technique and PCC have demonstrated efficacy in ameliorating clinical symptoms and maintaining the postoperative sagittal balance. Although no significant disparities were observed between these two technologies in terms of complications such as adjacent segment degeneration and implant subsidence, the zero-profile technique exhibited superior performance over PCC in relation to dysphagia during the early stages of postoperative recovery. To validate these findings, studies with longer follow-up periods and evaluations of multilevel cervical muscles are warranted.
Introduction
Cervical degenerative disc disease (CDDD) is a pathological condition characterized by the degeneration of intervertebral discs and subsequent degeneration of the adjacent intervertebral joints, resulting in detrimental effects on the surrounding essential tissues, including the spinal cord, nerve roots, sympathetic nerves, and vertebral arteries (1, 2). It typically presents as discomfort in the neck and shoulders, accompanied by stiffness, radiating sensations towards the head, pillow, or upper limbs. In more severe instances, it may lead to spasms in both lower limbs, hindered mobility, quadriplegia, and other related symptoms (3–5). According to a cohort study involving 47,560 patients, the incidence of CDDD is 13.1% (6), with a peak incidence in the fourth and fifth decades of life (7). Reportedly, total annual treatment costs for neck pain were estimated at $686 million in Netherlands and $800 million in China (8, 9).
In cases where conservative treatments prove ineffective, surgical intervention is advised for patients with CDDD. Since anterior cervical discectomy and fusion (ACDF) was first reported by Smith and Cloward in 1958, the procedure has gradually become one of the dominant surgical strategies in the treatment of single and double level CDDD (10), and previous literature revealed that ACDF indeed could provide favorable clinical outcomes and maintain the reconstruction of the cervical spine (11–13). It was reported that more than 100 000 patients receive this treatment in the US annually (3) and is projected to increase by more than 10% in the next 20 years (14). The ACDF with traditional plate-cage construct (PCC) with screws was the primary spinal surgical approach for addressing symptomatic cervical disc disease. This method offers several benefits, including the preservation or enhancement of cervical sagittal alignment and stability, improved fusion rates, decreased likelihood of graft extrusion, as well as reduced micromotion and subsidence of implanted cages (15–18). The placement of an anterior cervical plate in close proximity to the esophagus may lead to mechanical irritation and subsequent soft tissue swelling, ultimately resulting in secondary dysphagia (19–21). Consequently, the utilization of a novel stand-alone device featuring a zero-profile device has become prevalent in ACDF procedures as a means of mitigating plate-related complications. The zero-profile devices represent a viable substitute for traditional ACDF implants, as they have demonstrated efficacy in diminishing the incidence of adjacent segment degeneration, circumventing contact with the cervical spine’s anterior soft tissue, and potentially averting postoperative dysphagia (22). Nonetheless, scholars have discovered that patients who undergo ACDF with zero-profile device may encounter postoperative axial pain, loss of cervical curvature, and sagittal imbalance as a result of the absence of supplementary plate fixation (23).
The cervical paraspinal muscle (CPM) is a vital component of the dynamic spinal stabilization system, serving a critical function in preserving the stability and mobility of the neck (24). Through the recruitment of muscles and reflex responses of the nervous system, the neck muscles and tendons provide sufficient stability and regulate cervical motion. Recent research has revealed that degeneration of the CPM, in addition to bony structural alterations, is a significant contributor to persistent neck pain, sagittal imbalance, and the development of CDDD (25–27). Numerous patients with CDDD exhibit varying degrees of neck muscle degeneration, as evidenced by two abnormal indicators on MR images: a reduction in myofiber size (muscle atrophy) and an increase in fat deposition (fatty infiltration) (28). The co-occurrence of muscle atrophy and fatty infiltration is frequently observed due to the inclination of myosatellite cells to differentiate into adipocytes under pathological conditions (29). However, there is a paucity of research investigating the correlation between surgical selection and postoperative outcomes among patients afflicted with concurrent CDDD and CPM degeneration.
Given the limited availability of clinical data in this domain, a retrospective analysis was conducted to determine the more advantageous surgical approach for these patients. Specifically, the clinical and radiological outcomes of ACDF with zero-profile device versus ACDF with PCC system were compared. The findings of this study are anticipated to furnish valuable insights and practical recommendations for the management of CDDD patients with CPM degeneration in the foreseeable future.
Materials and methods
Study design
In our department, a retrospective review was conducted on patients who underwent single-level ACDF from C3 to C7 between January 2016 and May 2020. The decision to proceed with surgery was determined by a clinical presentation that was consistent with recent magnetic resonance imaging (MRI) findings of root or spinal cord compression. This study received approval from the Medical Ethics Committee of our hospital and all patients provided informed consent for the analysis of their clinical data.
Inclusion and exclusion criteria for patients
The study’s inclusion criteria encompassed patients who exhibited radiculopathy or myelopathy stemming from single-level cervical disc disease, with corresponding magnetic resonance imaging evidence and a lack of response to conservative treatment for a minimum of six weeks. Additionally, eligible patients were over 18 years of age and had undergone ACDF utilizing either the zero-profile device or PCC system from C3 to C7. Furthermore, patients were required to have comprehensive postoperative anteroposterior and lateral X-rays, as well as clinical data, and had agreed to participate in at least one year of follow-up. The present study employed specific exclusion criteria, which included the following: cervical disc replacement (CDR) or hybrid surgery (CDR with ACDF); ACDF utilizing alternative types of devices; multilevel surgery; local or systemic infection; severe osteoporosis (T score < -2.5); pathological vertebral fracture or spinal deformity; allergy to the device material; ankylosing spondylitis; rheumatoid arthritis; or prior cervical spine surgery.
All surgical procedures were performed by one senior spinal surgeon in our department using a standard, right Smith- Robinson approach after the induction of general anesthesia (30, 31). The selection of the zero-profile device or PCC device was based on the patient’s condition and willingness. The zero-profile device group received a stand-alone cervical fusion implant (PREVAIL Medtronic Sofamor Danek, Memphis, TN) filled with a composite synthetic bone graft for ACDF, while the PCC group underwent ACDF using the VENTURE™ anterior cervical plate system (Medtronic Sofamor Danek, Memphis, Tennessee, USA) with an allograft.
Clinical evaluation
The patients’ arm and neck pain were evaluated using the visual analogue scale (VAS), which measures pain on a scale of 0 to 10 points, with 0 representing the absence of pain and 10 representing the highest level of pain. The neck disability index (NDI) scores were used to evaluate the function of the neck. The NDI is a validated 10-item questionnaire, with each item rated on a 6-point scale (32). This study used the Chinese version of the NDI proposed by Wu et al. (33), which is specifically targeted at Chinese-speaking individuals with neck pain. It also uses a 6-point Likert scale that ranges from 0 (no disability) to 5 (complete disability) for each item. Disability ratings are assigned as follows: 0 to 4, no disability; 5 to 14, mild disability; 15 to 24, moderate disability; 25 to 34, severe disability; and above 34, complete disability. The Japanese orthopedic association (JOA) scores were used to assess the neurological status of patients with myelopathy, the myelopathy severity is considered mild if the JOA score is higher than 13, moderate if the JOA score ranges from 9 to 13, and severe if the JOA score is lower than 9 (34).
Radiological evaluation
Evaluation of CPM degeneration
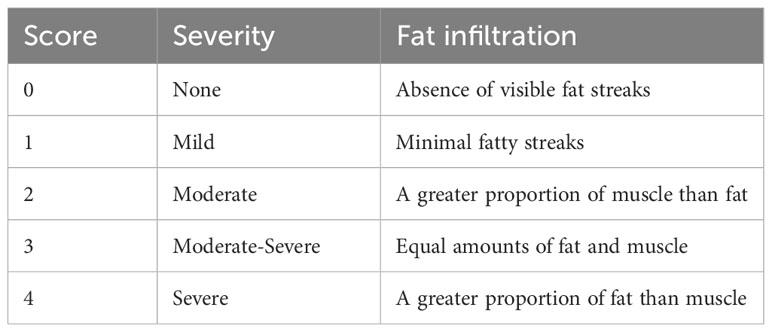
The study employed lateral X-ray images, computed tomography (CT) and MRI images to conduct radiological analysis. Prior to the operation, qualitative and quantitative evaluations of CPM were performed on an axial T2 weight section obtained from MRI. The degree of muscle fat infiltration at the C5/6 level was chosen as a representative measure of the cervical muscle, consistent with established practice in prior research (24, 35). The Goutallier classification was employed to assess the degree of fatty infiltration in the CPM prior to ACDF surgery, as documented in previous studies (24, 35). The Goutallier grading system utilizes scores ranging from 0 to 4, with 0 indicating the absence of visible fat streaks in the multifidus, 1 indicating minimal fatty streaks, 2 indicating a greater proportion of muscle than fat, 3 indicating equal amounts of fat and muscle, and 4 indicating a greater proportion of fat than muscle (Table 1, Figure 1). The multifidus muscles on both the right and left sides were evaluated separately, and the average of the scores was used for the final classification. Furthermore, the medial fascia boundaries of multifidus, semispinalis cervicis, semispinalis capitis, and splenius capitis at the C5-6 level on both sides were manually delineated using ImageJ software (v2.1.4.7; National Institutes of Health, USA) and quantified as the cross-sectional area (CSA) of each muscle Figure 2.
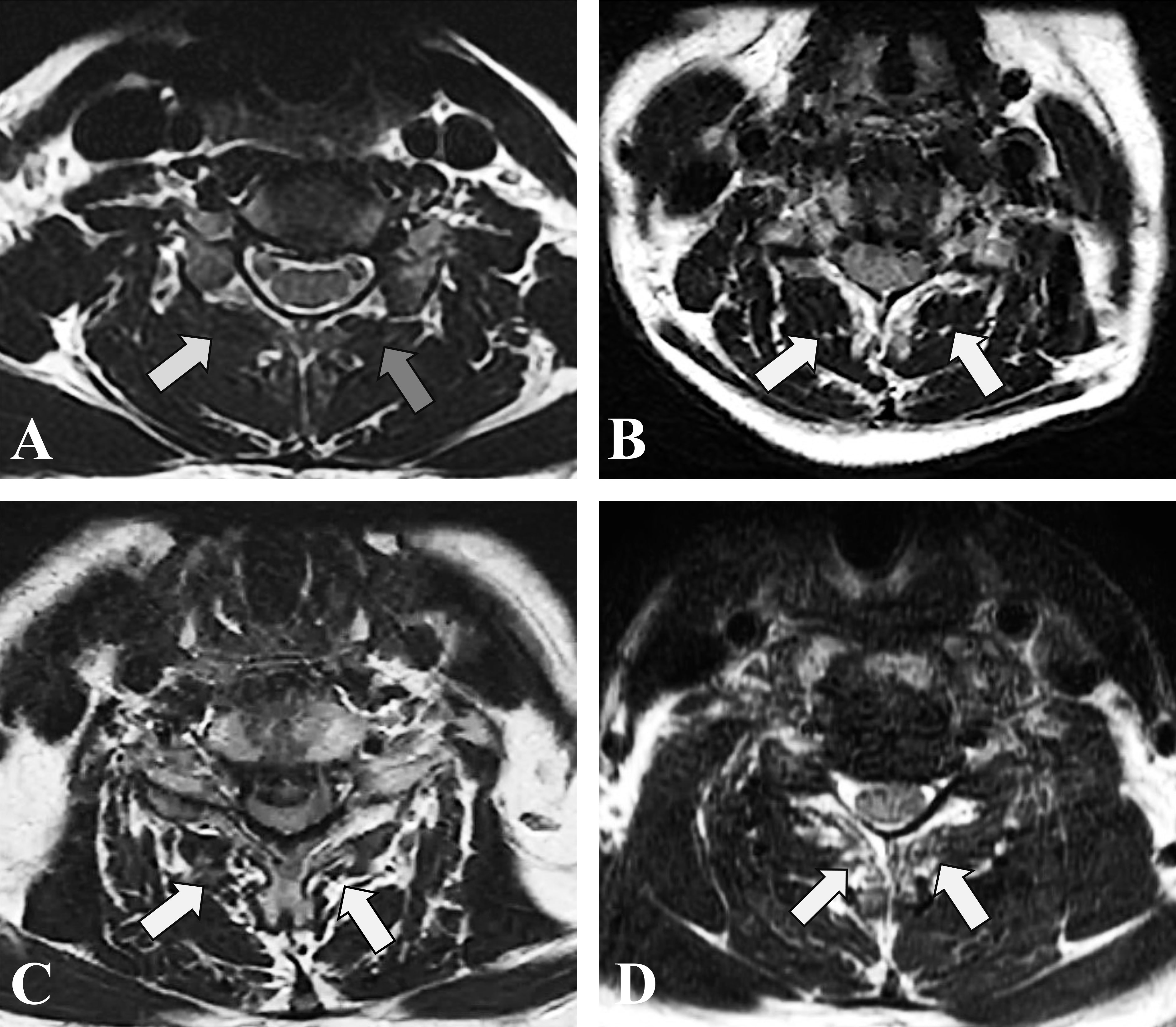
Figure 1 T2-weighted axial MRI section demonstrating fatty infiltration of muscle multifidus belly at C5/6. (A) Goutalier Grade 0 (white arrow), Goutalier Grade 1 (grey arrow); (B) Goutalier Grade 2. (C) Goutalier Grade 3. (D) Goutalier Grade 4.
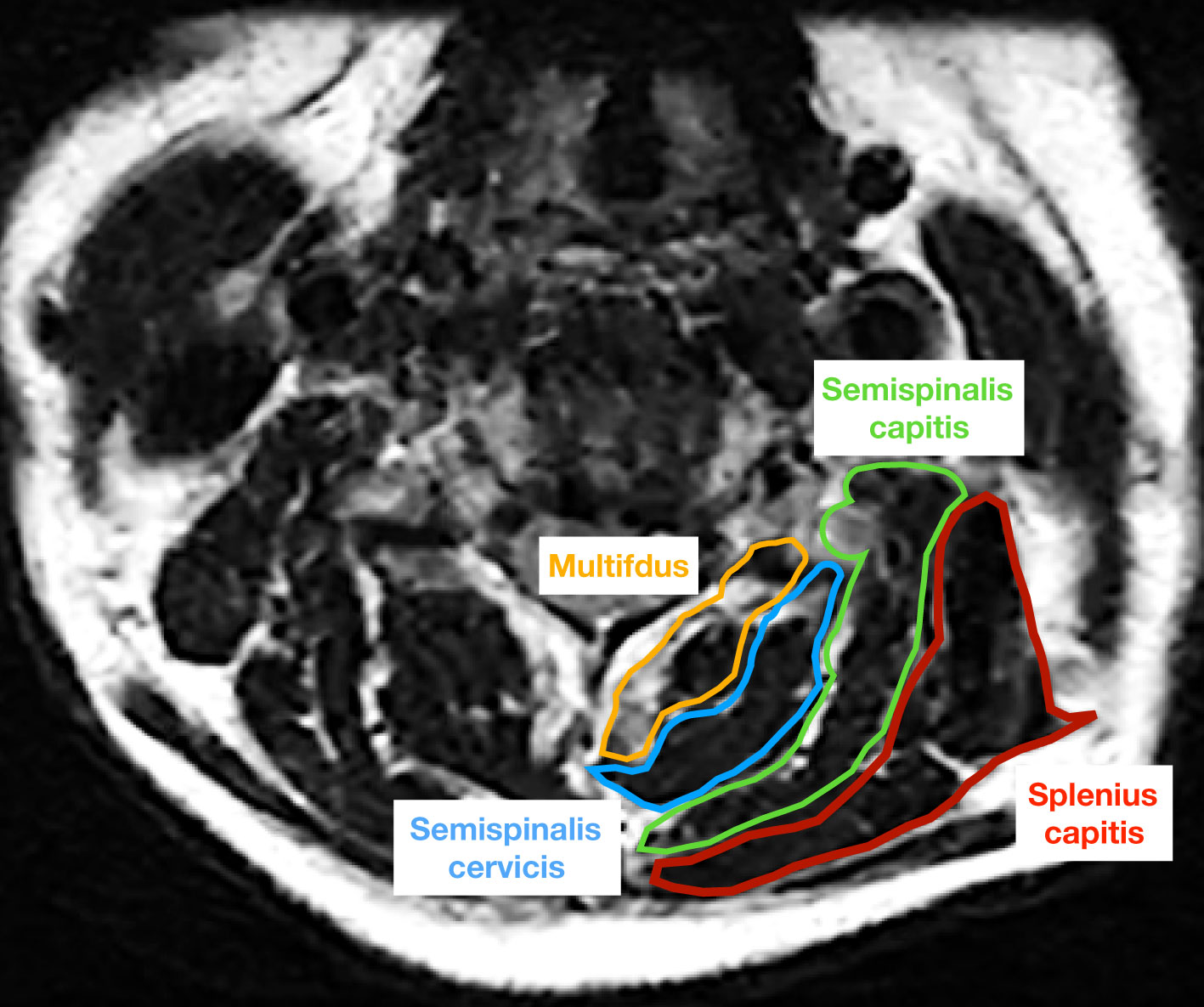
Figure 2 The cross-sectional area of multifdus (yellow), semispinalis cervicis (blue), semispinalis capitis (green), and splenius capitis (red) was measured on an axial T2 weighted image at the C5/6 level.
Measurement of cervical sagittal alignment
The present study recorded various parameters related to cervical spine, including cervical lordosis (CL), range of motion (ROM) of C2-C7, functional spinal unit angle (FSUA), sagittal vertical axis (C2-7 SVA), center of the sella turcica–C7 sagittal vertical axis (St-SVA), and T1 slope. The measurement techniques employed in this study were consistent with those described in previous literature (36, 37). Specifically, CL was determined by measuring the angle between the inferior margin of the C2 vertebrae and the inferior margin of the C7 vertebrae. The calculation of the FSU angle involved the utilization of the Cobb angle of the vertebrae adjacent to the intervertebral disc in question. The determination of the C2-C7 SVA was based on the measurement of the distance between the posterosuperior corner of C7 and the vertical line originating from the center of the C2 body. The center of the St-SVA was established as the distance between a plumb line originating from the center of the sellar turcica and the center of the C7 body. The T1 slope was defined as the angle formed between the T1 superior endplate and a horizontal line (Figure 3).
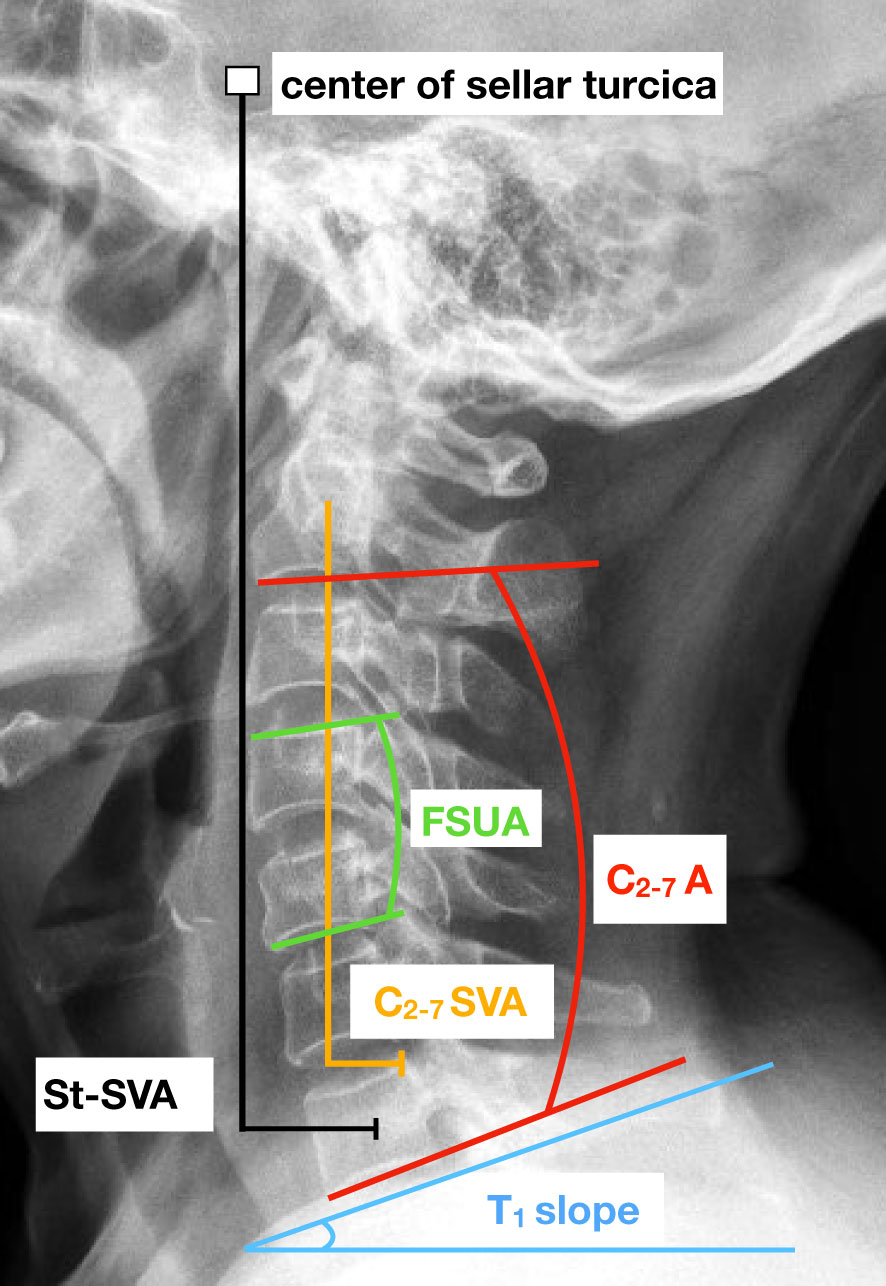
Figure 3 Lateral cervical spine radiograph with an illustration of key cervical sagittal alignment measurements. FSUA indicates the functional spinal unit angle. C2-7 A represents the C2-C7 angle. C2-7 SVA indicates the sagittal vertical axis and St-SVA indicates the center of the sella turcica – C7 sagittal vertical axis.
Complications
The study documented postoperative complications, namely dysphagia, adjacent segment degeneration (ASD) and implant subsidence. Dysphagia was evaluated by using the Bazaz grading system and the scores of the Bazaz grading system were ranked as follows: 0-none, 1-mild, 2-moderate and 3-severe, representing no episodes of swallowing problems, rare episodes of dysphagia, occasional swallowing difficulties with specific foods and frequent swallowing difficulties with most foods, respectively. ASD was characterized by the emergence of new or enlarged ossification of the anterior longitudinal ligament, new or increased narrowing of the disc space by more than 30%, new or obvious enlarging osteophyte formation, and endplate sclerosis (38). Implant subsidence pertains to a reduction in the height of the functional spinal unit (FSU) by more than 2 mm (39).
Statistical analysis
The retrospective nature of the study predetermines the fixed sample size based on the available data. The statistical software SPSS version 22.0 (IBM Corp., Armonk, NY) was utilized for all analyses. Continuous variables were presented as the mean ± standard deviation, while categorical variables were presented as the rate and ratio index. The normality of the parameters was assessed through a Shapiro-Wilk test. To examine significant differences among the groups, one-way analysis of variance (ANOVA) and Kruskal–Wallis tests were conducted based on the distribution of variables. The Chi-squared test was employed for categorical variables. The preoperative and post-operative parameters were compared using either the paired t-test or the Wilcoxon signed-rank test. Statistical significance was determined by a p-value of less than 0.05.
Results
Patient demographic data
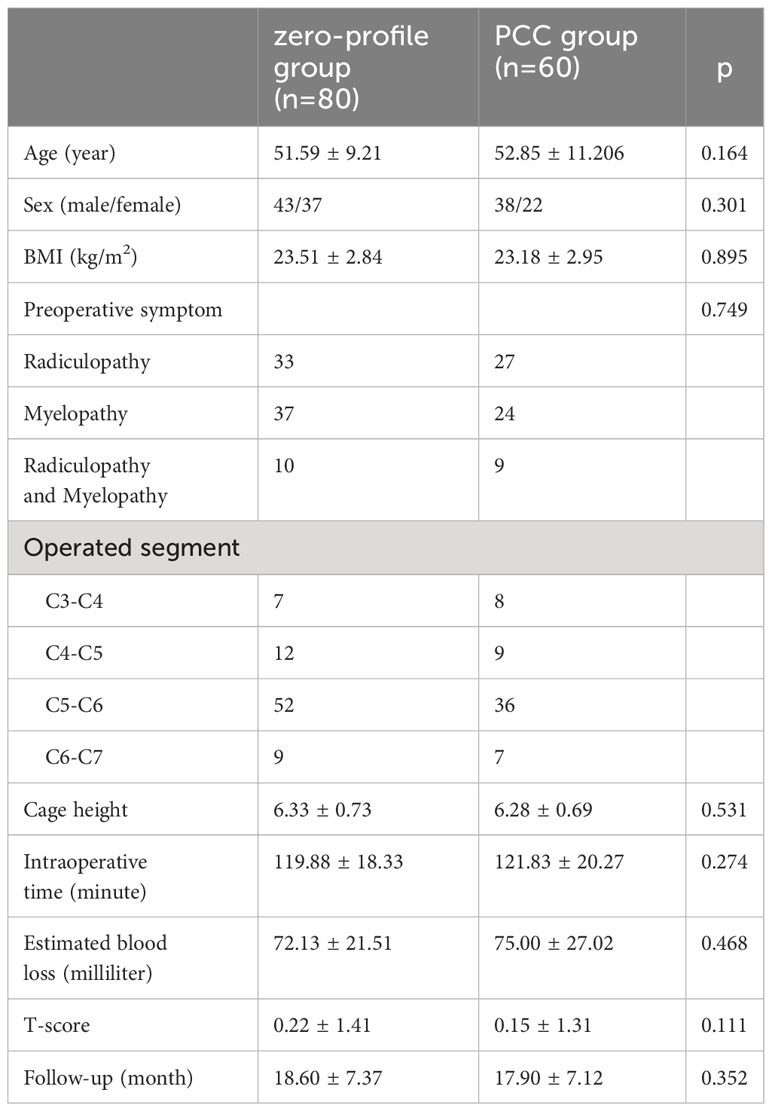
The study consisted of a total of 140 patients, with 80 patients (43 men and 37 women) in the zero-profile group and 60 patients (38 men and 22 women) in the PCC group. The average age of the zero-profile group was 51.54 ± 9.19 years, while the average age of the PCC group was 52.30 ± 10.96 years. Statistical analysis revealed no significant differences between the two groups in terms of age, sex, body mass index (BMI), bone mineral density (BMD), operated level, intraoperative time, intraoperative blood loss, median time of hospital stay, or follow-up period (Table 2).
Degrees of fatty infiltration and grouping method
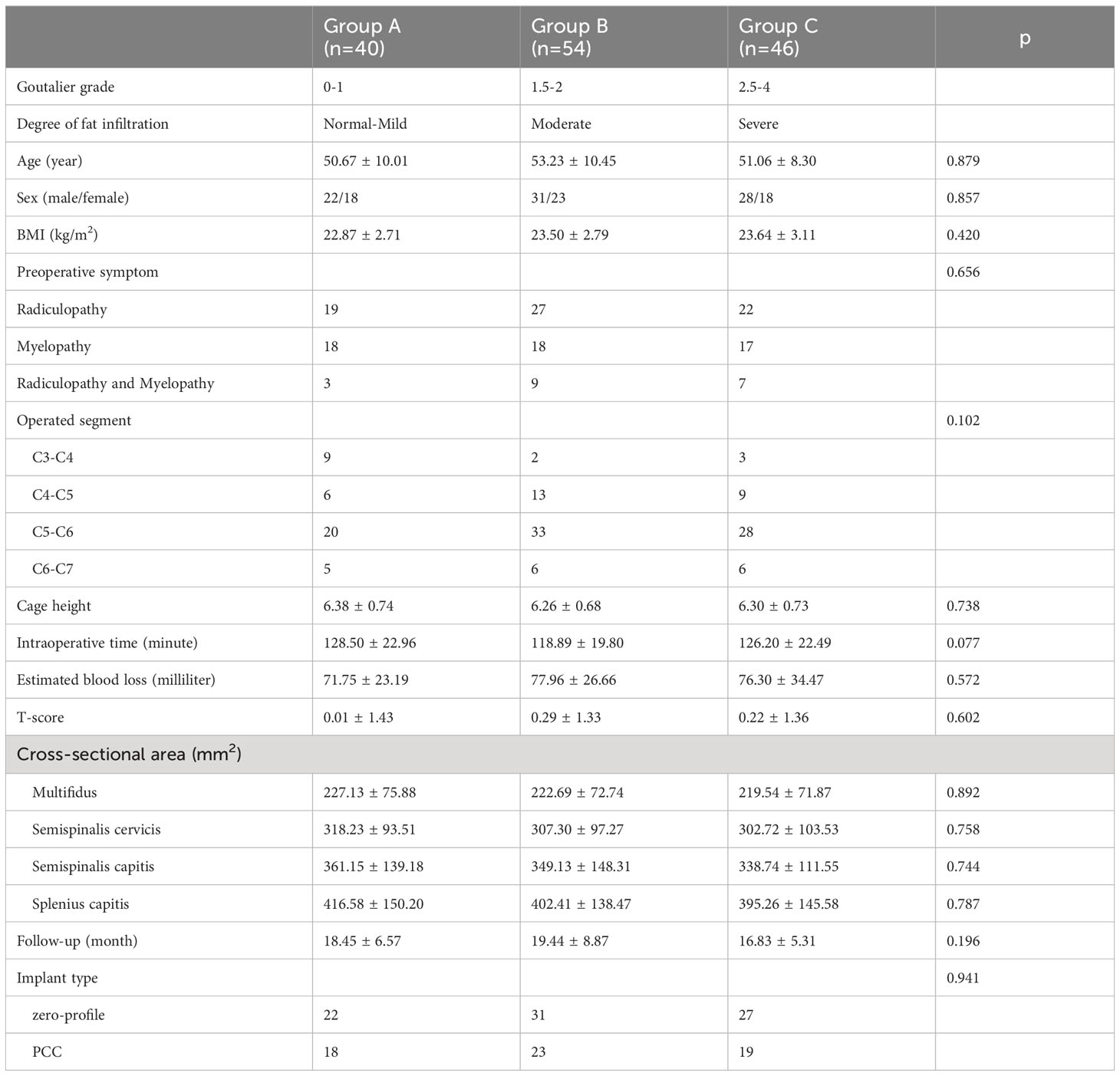
Table 3 displays the categorization of all patients based on the Goutallier classification, with three distinct groups established. The fatty infiltration of the multifidus was graded as 0-1 Goutallier grade for group A, 1.5-2 Goutalier grade for group B, and 2.5-4 Goutallier grade for group C. The patient population for group A consisted of 40 individuals (23 male and 17 females; average age=45.06 ± 6.31years), while group B comprised 54 patients (31 male and 23 female; average age = 43.21 ± 7.53 years), and group C included 46 patients (24 male and 22 female; average age = 46.51 ± 7.6 years). The study revealed that in Group A, 22 patients (55.0%) underwent ACDF with a zero-profile implant, while 18 patients (45.0%) received ACDF with a PCC fixation. Similarly, in Group B, 31 patients (57.41%) underwent ACDF with a zero-profile implant, and 23 patients (42.59%) received ACDF with a PCC fixation. In Group C, 27 patients (57.41%) underwent ACDF with a zero-profile implant, while 19 patients (42.59%) received ACDF with a PCC fixation.
Mean CSA of the paraspinal muscles
The mean CSA of the multifidus muscle was found to be 227.13 ± 75.88 mm2 in group A, 222.69 ± 72.74 mm2 in group B, and 219.54 ± 71.87 mm2 in group C. Similarly, the CSA of the semispinalis cervicis muscle was 318.23 ± 93.51 mm2 in group A, 307.30 ± 97.27 mm2 in group B, and 302.72 ± 103.53 mm2 in group C. The CSA of the semispinalis capitis muscle was 361.15 ± 139.18 mm2 in group A, 349.13 ± 148.31 mm2 in group B, and 338.74 ± 111.55 mm2 in group C. Lastly, the CSA of the splenius capitis muscle was 416.58 ± 150.20 mm2 in group A, 402.41 ± 138.47 mm2 in group B, and 395.26 ± 145.58 mm2 in group C. No statistically significant differences were observed in the mean CSA of the paraspinal muscles across the three groups (Table 3).
Zero-profile versus PCC
To explore the most effective treatment strategy for patients with CDDD and severe CPM degeneration (Goutallier grade 1.5-2 and Goutallier grade 2.5-4), we conducted a comparative analysis of therapeutic efficacy, sagittal parameters, and complications between the zero-profile group and the PCC group.
Clinical outcomes
The preoperative clinical outcomes did not exhibit any significant differences between the two groups. However, all patients experienced a marked improvement in clinical symptoms following the operation. The mean JOA score increased in all groups, while the mean VAS score and NDI significantly decreased. Postoperative clinical outcomes did not demonstrate any significant differences between the zero-profile and PCC groups, as evidenced by Table 4.
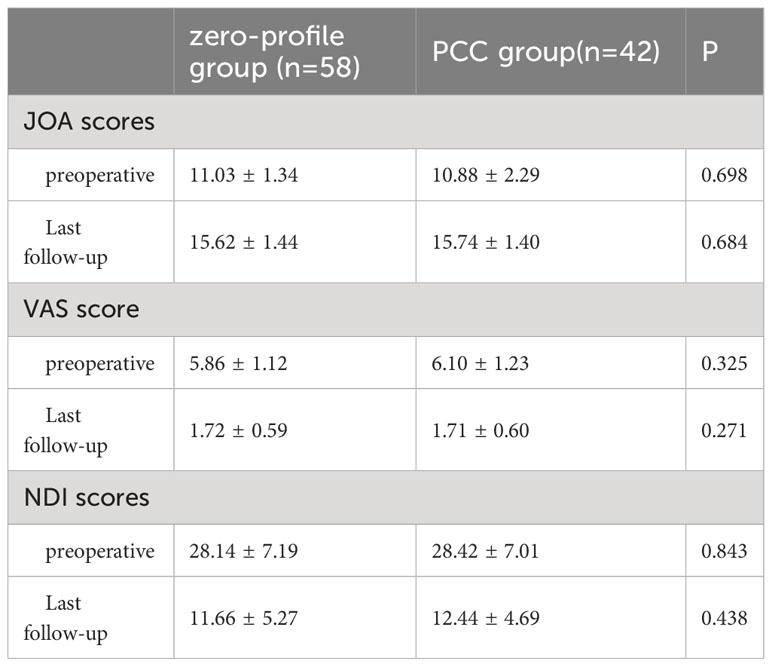
Table 4 Comparison of clinical outcomes after ACDF with a zero-profile implant and PCC in patients with severe muscle degeneration.
Radiological findings
Table 5 presents the imaging results, indicating that, with the exception of St-SVA at the final follow-up, the other sagittal alignment parameters were comparable across various time points Figure 4. Specifically, the St-SVA in the zero-profile group remained stable from 28.11 ± 7.17 mm pre-surgery to 26.45 ± 9.42 mm at the last follow-up, with a mean change value of -0.28 ± 6.65 mm. In contrast, the PCC group experienced a decrease in St-SVA from 27.86 ± 7.55 mm pre-surgery to 21.91 ± 8.61 mm at the last follow-up, with a mean change value of 2.09 ± 13.31 mm. Notably, there were significant differences between the groups in the St-SVA at the last follow-up (p=0.023).
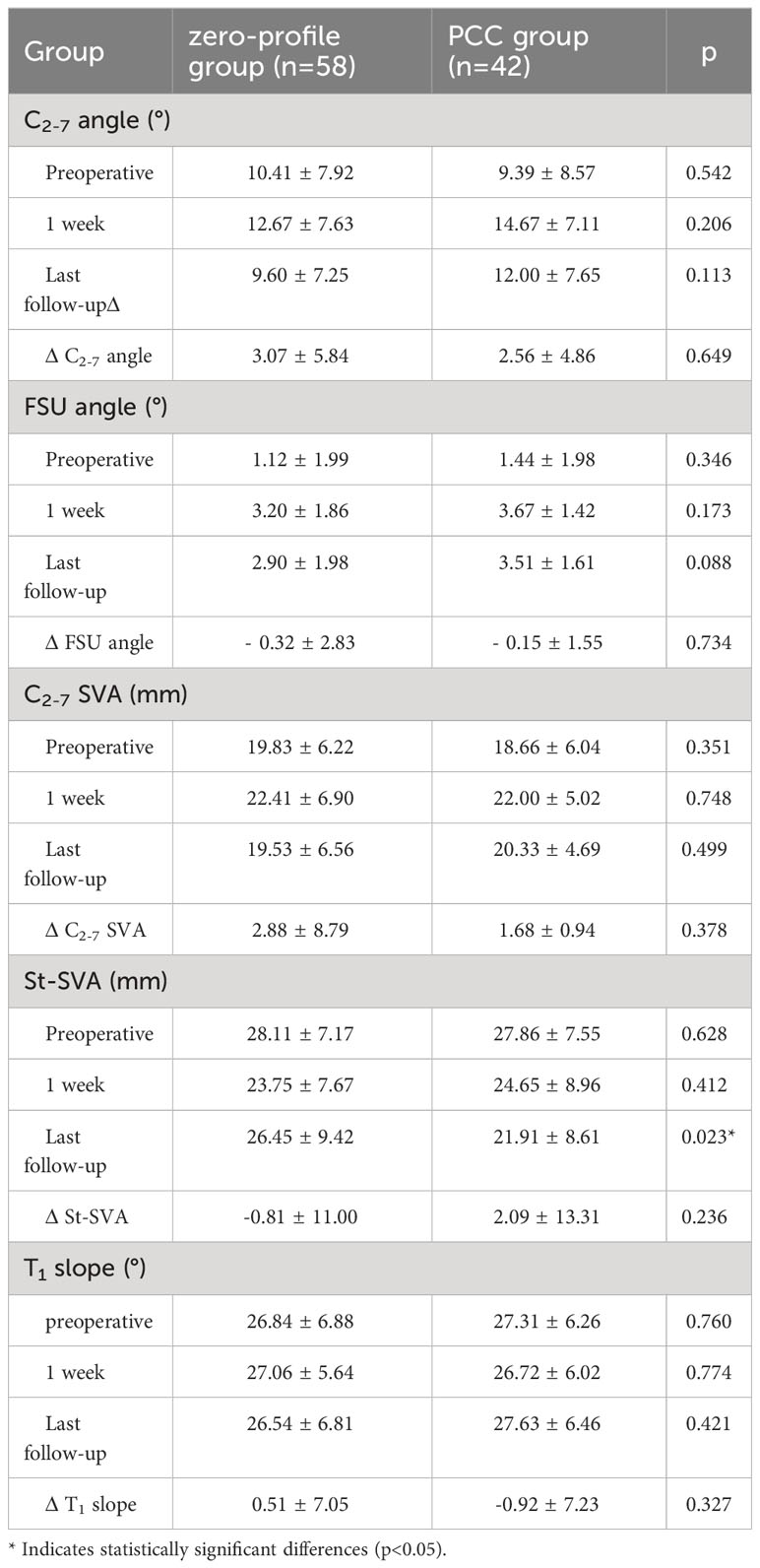
Table 5 Comparison of radiographic assessments after ACDF with a zero-profile implant and PCC in patients with severe muscle degeneration.
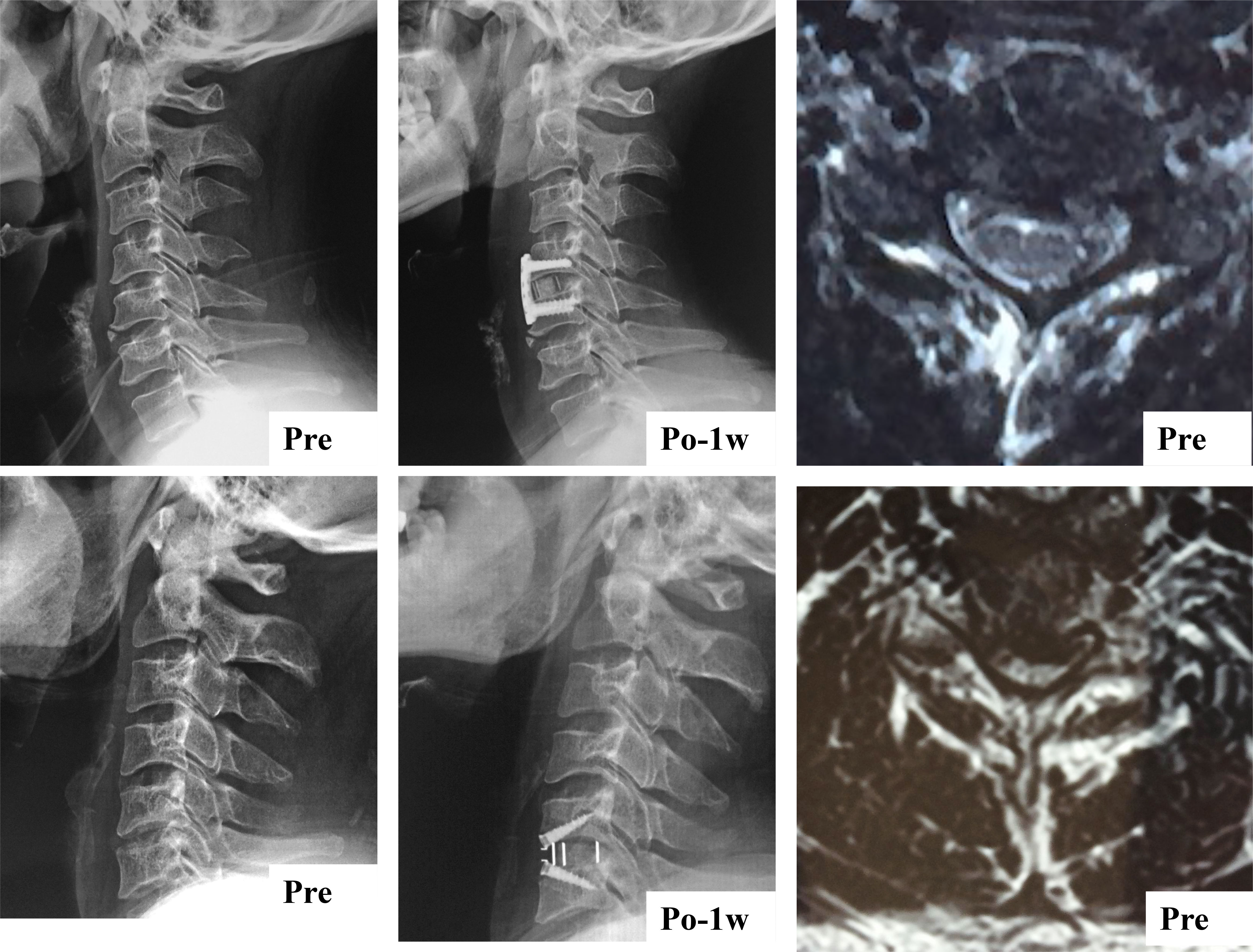
Figure 4 Sagittal parameters were improved whether cervical paraspinal muscle degeneration patients underwent ACDF using a Zero-profile device or conventional plate cage construct.
Complications
Over the course of several months following surgery, the overall occurrence of dysphagia exhibited a gradual decline in both groups. Specifically, the zero-profile group demonstrated a dysphagia incidence of 22.41% at one week, which decreased to 13.79% at three months and 3.45% at the final follow-up. In contrast, the plate cage group exhibited a dysphagia incidence of 47.62% at one week, which decreased to 33.33% at three months and 11.90% at the final follow-up. Notably, there were statistically significant differences between the groups in terms of dysphagia incidence within the initial three months (Table 6).
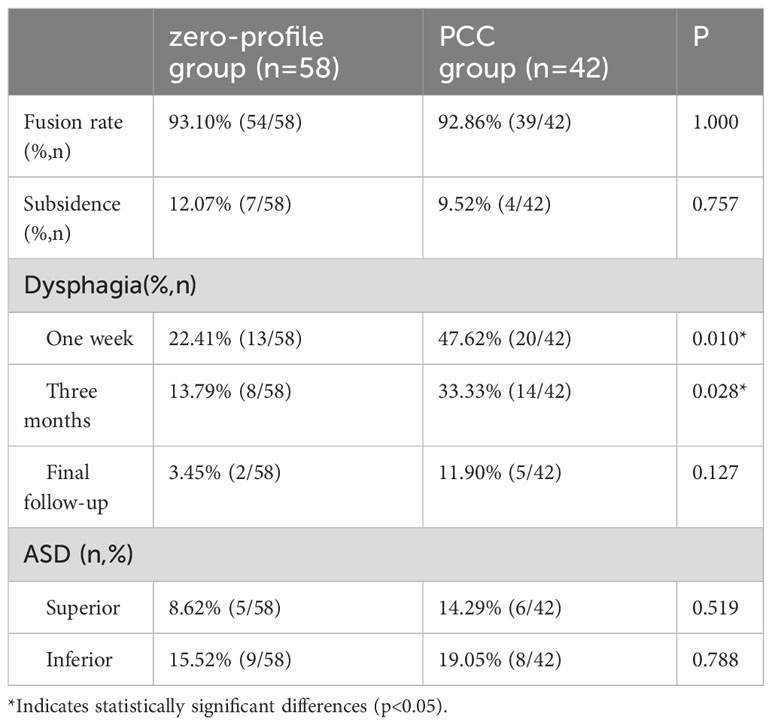
Table 6 Comparison of fusion rates and complications after ACDF with a zero-profile implant and PCC in patients with severe muscle degeneration.
Discussion
Despite the widespread prevalence of cervical muscle degeneration, it has not garnered commensurate attention relative to the lumbar spine (40–44). He et al. observed a degeneration rate of 69.1% in the paraspinal muscles (Goutallier Grade ≥1.5) among patients with two-level cervical disc degenerative disease (24). They also identified a significant positive correlation between severe paraspinal muscle degeneration and postoperative sagittal balance disorder. Similarly, Wang et al. found that 67.33% (68/101) of patients with single-level cervical disc degenerative disease and severe fatty infiltration of paravertebral muscles experienced improved cervical sagittal alignment, which was comparable to those with strong cervical extensor muscles (39). The findings of our investigation align with prior research indicating that paraspinal muscle fatty degeneration can reach a prevalence of 71.43%. As a result, it is crucial to consider which ACDF procedure would be most advantageous for this demographic. Nevertheless, there is a dearth of literature on surgical decision-making for this cohort in previous studies.
The present study reports on the incidence of dysphagia in two groups, namely the zero-profile group and Plate group. The incidence of dysphagia in the zero-profile group was found to be 22.41% and 47.62% at one week, 13.79% and 33.33% at three months, and 3.45% and 11.90% at the final follow-up, respectively. It is noteworthy that all cases of dysphagia were mild or moderate and showed a decreasing trend over time. However, the increasing use of zero-profile implant was found to be associated with a higher risk of kyphotic deformity and poor dynamic stability due to the lack of anterior support, as reported in previous studies (15, 45). Lee et al. conducted a comparative analysis of postoperative retention and motion stabilization following ACDF utilizing three distinct implants (46). Their findings suggested that patients requiring robust postoperative motion stabilization should receive a plate-cage construct rather than a Zero-profile implant. Our investigation found that a single-level ACDF procedure utilizing a zero-profile or plate cage construct, with varying degrees of multifidus fatty infiltration, did not impact sagittal balance. One possible explanation for why no differences were seen between groups is that anterior surgery results in less obstruction to the paraspinal muscle (47). ACDF has the advantage of preserving the posterior muscles and avoiding injuring the posterior structures, such as the posterior ligaments, compared with posterior surgery. In the previous literature, most of the results that paravertebral muscle fat infiltration has an effect on cervical curvature and sagittal position parameters mainly focus on posterior cervical surgery. Preserving of the posterior structures in turn has an enormous impact on the mechanical stability of the cervical spine (48, 49). Our other hypothesis is that although cross-sectional area and degree of fat infiltration are now commonly used to evaluate paravertebral muscle degeneration, whether these indicators fully reflect paravertebral muscle function remains to be verified. Therefore, the indicators that can reflect the paravertebral muscle function should be explored in the future studies. However, the parameters of the final follow-up indicated that the sagittal vertical axis (St-SVA) was worse. Our hypothesis posits that a novel sagittal balance is established following single-level anterior cervical discectomy and fusion, thereby preserving the optimal horizontal plane of the preoptic system and maintaining the head axis. The degeneration of a solitary muscle within a singular segment may not be adequate to disrupt and exacerbate the state of equilibrium. Therefore, a comprehensive and extensive study of multiple-level ACDF procedures involving multiple cervical muscles, with long-term follow-up, is imperative. Furthermore, notable advancements have been achieved in the advancement of finite element (FE) models pertaining to cervical spine in recent decades. Consequently, employing the FE model of ACDF surgery to investigate the impact of cervical paravertebral muscle degeneration on postoperative biomechanical characteristics and sagittal balance emerges as one of the crucial and efficacious avenues for future scholarly inquiry (50).
According to estimations, cervical paraspinal muscles maintain approximately 80% of the mechanical stability of the cervical spine (51), which is essential for holding posture and stabilizing the head. The cervical paraspinal muscle is categorized into superficial, intermediate, and deep layers, forming a crucial dynamic equilibrium system of the cervical spine (52). The superficial layer of cervical paraspinal muscles comprises the trapezius, rhomboid, and levator scapulae muscles, while the intermediate layer primarily consists of the head clamp muscle, neck clamp muscle, and longest neck muscle, the deep layer primarily comprises the semi-spinous muscle and neck multifidus muscle. The flexion of the neck is primarily regulated by muscles such as the scalene muscle, longissimus capitis, and longissimus cervicalis, while extension is mainly controlled by the multifidus muscle, longissimus capitis, and suboccipital muscle. Lateral flexion is primarily governed by the head clamp muscle, neck clamp muscle, sternocleidomastoid muscle, and scalene muscle. Additionally, the lateral rotation of the neck is predominantly controlled by the sternocleidomastoid muscle, multifidus muscle, erector spine muscle, and head and neck clamp muscle (53–56).
The comprehensive examination and investigation of the molecular mechanism governing adipogenesis in muscle cells will enhance our comprehension of the interconversion between muscle and adipose tissues, the metabolic roles of muscle tissues, and the etiology of muscular disorders. Despite an incomplete understanding of the molecular mechanism underlying the muscle-adipose conversion, recent advancements have provided us with fresh perspectives on adipogenesis in muscle cells. To the best of our understanding, the development of muscle, bone, and adipose tissues encompasses a complex series of steps, beginning with the specification of a shared progenitor mesodermal cell towards a particular differentiation pathway, and subsequently leading to the manifestation of diverse terminal differentiation phenotypes (57). In vitro investigations have confirmed the pluripotent capacity of muscle-derived stem cells or precursor cells to differentiate in multiple directions (58, 59). To the best of our understanding, the development of muscle, bone, and adipose tissues encompasses a complex series of steps, beginning with the specification of a shared progenitor mesodermal cell towards a particular differentiation pathway, and subsequently leading to the manifestation of diverse terminal differentiation phenotypes (60). In vitro investigations have confirmed the pluripotent capacity of muscle-derived stem cells or precursor cells to differentiate in multiple directions. The multi-directional differentiation potential of muscle-derived stem cells or precursor cells has been demonstrated in in vitro studies (61). Additionally, lineage-tracing experiments have revealed that brown adipocytes, skeletal muscle cells, and dorsal dermal cells all originate from the same multi-potential progenitor cells derived from the central dermomyotome (62). Myoblasts have the potential to transdifferentiate into adipocytes or adipocyte-like cells under specific induction conditions (i.e., drug stimulation, cytokine treatment). Our findings also suggest that adipogenesis in muscle cells is prevalent among patients with cervical disc degenerative disease. Previous studies have reported the involvement of coding genes and non-coding genes, particularly miRNAs, in regulating the adipogenic transdifferentiation of myocytes (63). For example, miR-199a has been shown to regulate the transdifferentiation of C2C12 myoblasts by targeting the FATP1 gene (64). Moreover, the elimination of the interaction between slincRAD and the DNMT1 gene is anticipated to lead to impaired epigenetic regulation, thereby compromising the process of adipogenesis (65). Additionally, Qi et al. have documented that the lncRNA-GM43652 gene exhibits potential as a regulator of adipogenesis in muscle cells (66). However, this current level of understanding is insufficient to fully elucidate the regulatory functions of coding and non-coding genes in the process of transformation. Therefore, additional research is necessary to investigate the complex mechanisms involved in adipogenesis in muscle cells and to evaluate its association with prognostic outcomes in individuals suffering from cervical disc degenerative disease.
The current investigation is subject to certain limitations. Firstly, given that the study is retrospective, selection bias was unavoidable. Another limitation is the relatively small number of patients. Although we selected patients from January 2016 and May 2020, we limited the sample to only operations performed by the same doctor. Therefore, multicenter prospective design studies with a larger sample size are needed to verify our results. Secondly, the degree of muscle fat infiltration at the C5/6 level was exclusively chosen as a surrogate for the entirety of cervical muscle. While this approach has been employed in prior research (24, 35), it may not accurately reflect the actual mass of cervical muscles. Besides, although we measured muscle fat infiltration based on previously published reports, we acknowledge that potentially inherent radiographic imaging error might be a significant limitation. Another limitation of our study was the exact mechanism of the paraspinal muscle degeneration was did not explored. Additionally, the duration of the follow-up period was brief. Nonetheless, given that clinical outcomes, radiological parameters, and fusion rates stabilized and complications such as dysphagia and subsidence manifested within 12 months, the timeframe was deemed adequate for assessing short-term outcomes. However, a more extensive duration of follow-up would be required to examine the degeneration of adjacent segments and assess the long-term results.
Conclusion
For patients with one-level cervical disc degenerative disease combined with paraspinal muscle degeneration, both the zero-profile technique and PCC have demonstrated efficacy in ameliorating clinical symptoms and maintaining the postoperative sagittal balance. Although no significant disparities were observed between these two technologies in terms of complications such as adjacent segment degeneration and implant subsidence, the zero-profile technique exhibited superior performance over PCC in relation to dysphagia during the early stages of postoperative recovery. To validate these findings, studies with longer follow-up periods and evaluations of multilevel cervical muscles are warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HA: Writing – original draft. HX:. JY: Resources, Supervision, Writing – review & editing. MY: Methodology, Validation, Writing – review & editing. KL: Methodology, Software, Writing – original draft. SW: Formal analysis, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
HA and HX contributed equally to this work and they are the co‐first authors of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Friedmann A, Baertel A, Schmitt C, Ludtka C, Milosevic J, Meisel HJ, et al. Intervertebral disc regeneration injection of a cell-loaded collagen hydrogel in a sheep model. Int J Mol Sci (2021) 22(8):4248. doi: 10.3390/ijms22084248
2. Sang D, Xiao B, Rong T, Wu B, Cui W, Zhang J, et al. Depression and anxiety in cervical degenerative disc disease: Who are susceptible? Front Public Health (2023) 10:1002837. doi: 10.3389/fpubh.2022.1002837
3. Johansen TO, Sundseth J, Fredriksli OA, Andresen H, Zwart JA, Kolstad F, et al. Effect of arthroplasty vs fusion for patients with cervical radiculopathy: A randomized clinical trial. JAMA Netw Open (2021) 4(8):e2119606. doi: 10.1001/jamanetworkopen.2021.19606
4. Tian X, Zhang L, Zhang X, Meng L, Li X. Correlations between preoperative diffusion tensor imaging and surgical outcome in patients with cervical spondylotic myelopathy. Am J Transl Res (2021) 13(10):11461–71.
5. Badhiwala JH, Ahuja CS, Akbar MA, Witiw CD, Nassiri F, Furlan JC, et al. Degenerative cervical myelopathy-update and future directions. Nat Rev Neurol (2020) 16:108–24. doi: 10.1038/s41582-019-0303-0
6. Schairer WW, Carrer A, Lu M, Hu SS. The increased prevalence of cervical spondylosis in patients with adult thoracolumbar spinal deformity. J Spinal Disord Tech (2014) 27:E305–8. doi: 10.1097/BSD.0000000000000119
7. Safaee MM, Nichols NM, Yerneni K, Zhang Y, Riew KD, Tan LA. Safety and efficacy of direct nerve root decompression via anterior cervical discectomy and fusion with uncinectomy for cervical radiculopathy. J Spine Surg (2020) 6(1):205–9. doi: 10.21037/jss.2019.12.04
8. Liu F, Fang T, Zhou F, Zhao M, Chen M, You J, et al. Association of depression/anxiety symptoms with neck pain: A systematic review and meta-analysis of literature in China. Pain Res Manage (2018) 2018:3259431. doi: 10.1155/2018/3259431
9. Miyamoto GC, Lin CC, Cabral CMN, van Dongen JM, van Tulder MW. Cost-effectiveness of exercise therapy in the treatment of non-specific neck pain and low back pain: a systematic review with meta-analysis. Br J Sports Med (2019) 53(3):172–81. doi: 10.1136/bjsports-2017-098765
10. Gendreau JL, Kim LH, Prins PN, D'Souza M, Rezaii P, Pendharkar AV, et al. Outcomes after cervical disc arthroplasty versus stand-alone anterior cervical discectomy and fusion: A meta-analysis. Global Spine J (2020) 10(8):1046–56. doi: 10.1177/2192568219888448
11. Sasso WR, Ye J, Foley DP, Vinayek S, Sasso RC. 20-year clinical outcomes of cervical disc arthroplasty: A prospective, randomized, controlled trial. Spine (Phila Pa 1976). (2023). doi: 10.1097/BRS.0000000000004811
12. Sheng XQ, Yang Y, Ding C, Wang BY, Hong Y, Meng Y, et al. Uncovertebral joint fusion versus end plate space fusion in anterior cervical spine surgery: A prospective randomized controlled trial. J Bone Joint Surg Am (2023) 105(15):1168–74. doi: 10.2106/JBJS.22.01375
13. Wang Y, Liu Y, Zhang A, Han Q, Jiao J, Chen H, et al. Biomechanical evaluation of a novel individualized zero-profile cage for anterior cervical discectomy and fusion: a finite element analysis. Front Bioeng Biotechnol (2023) 11:1229210. doi: 10.3389/fbioe.2023.1229210
14. Xu S, Liang Y, Zhu Z, Qian Y, Liu H. Adjacent segment degeneration or disease after cervical total disc replacement: a meta-analysis of randomized controlled trials. J Orthop Surg Res (2018) 13(1):244. doi: 10.1186/s13018-018-0940-9
15. Huang CY, Meng Y, Wang BY, Yu J, Ding C, Yang Y, et al. The effect of the difference in C2-7 angle on the occurrence of dysphagia after anterior cervical discectomy and fusion with the zero-profile implant system. BMC Musculoskelet Disord (2020) 21(1):649. doi: 10.1186/s12891-020-03691-7
16. Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) (2009) 34(26):2886–92. doi: 10.1097/BRS.0b013e3181b64f2c
17. Abudouaini H, Huang C, Liu H, Hong Y, Wang B, Ding C, et al. Change in the postoperative intervertebral space height and its impact on clinical and radiological outcomes after ACDF surgery using a zero-profilerofile device: a single-Centre retrospective study of 138 cases. BMC Musculoskelet Disord (2021) 22(1):543. doi: 10.1186/s12891-021-04432-0
18. Lin M, Shapiro SZ, Doulgeris J, Engeberg ED, Tsai CT, Vrionis FD. Cage-screw and anterior plating combination reduces the risk of micromotion and subsidence in multilevel anterior cervical discectomy and fusion-a finite element study. Spine J (2021) 21(5):874–82. doi: 10.1016/j.spinee.2021.01.015
19. Li ZH, Zhao YT, Tang JG, Ren D, Guo J, Wang H. A comparison of a new zero- profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: a minimum 2-year follow- up study. Eur Spine J (2017) 26(4):1129–39. doi: 10.1007/s00586-016-4739-2
20. Min Y, Kim WS, Kang SS, Choi JM, Yeom JS, Paik NJ. Incidence of dysphagia and serial videofluoroscopic swallow study findings after anterior cervical discectomy and fusion: a prospective study. Clin Spine Surg (2016) 29(4):E177. doi: 10.1097/BSD.0000000000000060
21. Riley LH, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: prevalence and risk fac- tors from a longitudinal cohort study. Spine (Phila Pa 1976). (2005) 30:2564–9. doi: 10.1097/01.brs.0000186317.86379.02
22. Abudouaini H, Wu T, Liu H, Wang B, Chen H, Li L. Comparison of the postoperative motion stabilization between anterior cervical decompression and fusion with a zero-profile implant system and a plate-cage construct. World Neurosurg (2022) 166:e484–e494.172-181. doi: 10.1016/j.wneu.2022.07.033
23. Li T, Yang JS, Wang XF, Meng CY, Wei JM, Wang YX, et al. Can zero-profile cage maintain the cervical curvature similar to plate-cage construct for single-level anterior cervical diskectomy and fusion? World Neurosurg (2020) 135:e300–6. doi: 10.1016/j.wneu.2019.11.153
24. He J, Wu T, Ding C, Wang B, Hong Y, Liu H. The fatty infiltration into cervical paraspinal muscle as a predictor of postoperative outcomes: A controlled study based on hybrid surgery. Front Endocrinol (Lausanne). (2023) 14:1128810. doi: 10.3389/fendo.2023.1128810
25. Peterson G, Leary SO, Nilsson D, Moodie K, Tucker K, Trygg J, et al. Ultrasound imaging of dorsal neck muscles with speckle tracking analyses - the relationship between muscle deformation and force. Sci Rep (2019) 9(1):13688. doi: 10.1038/s41598-019-49916-1
26. Fortin M, Wilk N, Dobrescu O, Martel P, Santaguida C, Weber MH, et al. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract (2018) 38:1–7. doi: 10.1016/j.msksp.2018.07.003
27. Tamai K, Grisdela P Jr, Romanu J, Paholpak P, Nakamura H, Wang JC, et al. The impact of cervical spinal muscle degeneration on cervical sagittal balance and spinal degenerative disorders. Clin Spine Surg (2019) 32(4):E206–13. doi: 10.1097/BSD.0000000000000789
28. Dallaway A, Kite C, Griffen C, Duncan M, Tallis J, Renshaw D, et al. Age-related degeneration of the lumbar paravertebral muscles: Systematic review and three-level meta-regression. Exp Gerontol (2020) 133:110856. doi: 10.1016/j.exger.2020.110856
29. Reza M, Subramaniyam N, Sim C, Ge X, Sathiakumar D, McFarlane C, et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun (2017) 8(1):1104. doi: 10.1038/s41467-017-01131-0
30. Deng Y, Wang B, Hong Y, Yang Y, Xing R, Wang X, et al. Anterior bone loss: A common phenomenon which should be considered as bone remodeling process existed not only in patients underwent cervical disk replacement but also those with anterior cervical diskectomy and fusion. Eur Spine J (2023) 32(3):977–85. doi: 10.1007/s00586-022-07504-4
31. Peng Z, Liu L, Sheng X, Liu H, Ding C, Wang B, et al. Risk factors of nonfusion after anterior cervical decompression and fusion in the early postoperative period: A retrospective study. Orthop Surg (2023) 15(10):2574–81. doi: 10.1111/os.13835
32. Vernon H. The neck disability index: state-of-the-art, 1991–2008. J Manipulative Physiol Ther (2008) 31(7):491–502. doi: 10.1016/j.jmpt.2008.08.006
33. Wu S, Ma C, Mai M, Li G. Translation and validation study of Chinese versions of the neck disability index and the neck pain and disability scale. Spine J (2010) 35(16):1575–9. doi: 10.1097/BRS.0b013e3181c6ea1b
34. Li C, Mei Y, Li L, Li Z, Huang S. Posterior decompression and fusion with vertical pressure procedure in the treatment of multilevel cervical OPLL with kyphotic deformity. Orthop Surg (2022) 14(9):2361–8. doi: 10.1111/os.13433
35. Pinter ZW, Wagner SC, Fredericks DR Jr, Xiong A, Freedman BA, Elder BD, et al. Higher paraspinal muscle density effect on outcomes after anterior cervical discectomy and fusion. Global Spine J (2021) 11(6):931–5. doi: 10.1177/2192568220935108
36. Noh SH, Park JY. Association of complete uncinate process removal on 2-year assessment of radiologic outcomes: subsidence and sagittal balance in patients receiving one-level anterior cervical discectomy and fusion. BMC Musculoskelet Disord (2020) 21(1):439. doi: 10.1186/s12891-020-03443-7
37. Abudouaini H, Wu T, Liu H, Wang B, Chen H, Huang C, et al. Partial uncinatectomy combined with anterior cervical discectomy and fusion for the treatment of one-level cervical radiculopathy: analysis of clinical efficacy and sagittal alignment. BMC Musculoskelet Disord (2021) 22(1):777. doi: 10.1186/s12891-021-04680-0
38. Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. (2005) 3(6):417–23. doi: 10.3171/spi.2005.3.6.0417
39. Wang XJ, Huang KK, He JB, Wu TK, Rong X, Liu H. Fatty infiltration in cervical extensor muscle: is there a relationship with cervical sagittal alignment after anterior cervical discectomy and fusion? BMC Musculoskelet Disord (2022) 23(1):641. doi: 10.1186/s12891-022-05606-0
40. Park MW, Park SJ, Chung SG. Relationships between skeletal muscle mass, lumbar lordosis, and chronic low back pain in the elderly. Neurospine (2023) 20(3):959–68. doi: 10.14245/ns.2346494.247
41. Schönnagel L, Guven AE, Camino-Willhuber G, Caffard T, Tani S, Zhu J, et al. Examining the role of paraspinal musculature in post-operative disability after lumbar fusion surgery for degenerative spondylolisthesis. Spine (Phila Pa 1976) (2023). doi: 10.1097/BRS.0000000000004840
42. Shahidi B, Padwal JA, Su JJ, Regev G, Zlomislic V, Allen RT, et al. The effect of fatty infiltration, revision surgery, and sex on lumbar multifidus passive mechanical properties. JOR Spine (2023) 6(3):e1266. doi: 10.1002/jsp2.1266
43. Song J, Shahsavarani S, Vatsia S, Katz AD, Ngan A, Fallon J, et al. Association between history of lumbar spine surgery and paralumbar muscle health: a propensity score-matched analysis. Spine J (2023) 11:S1529–9430(23)03260-6. doi: 10.1016/j.spinee.2023.07.004
44. Singh S, Shahi P, Asada T, Kaidi A, Subramanian T, Zhao E, et al. Poor muscle health and low preoperative ODI are independent predictors for slower achievement of MCID after minimally invasive decompression. Spine J (2023) 23(8):1152–60. doi: 10.1016/j.spinee.2023.04.004
45. Kaiser MG, Haid RW Jr, Subach BR, Barnes B, Rodts GE Jr. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery (2002) 50(2):229–36. doi: 10.1097/00006123-200202000-00001
46. Lee YS, Kim YB, Park SW. Does a zero-profilerofile anchored cage offer additional stabilization as anterior cervical plate? Spine (Phila Pa 1976) (2015) 40(10):E563–70. doi: 10.1097/BRS.0000000000000864
47. Siasios I, Samara E, Fotiadou A, Tsoleka K, Vadikolias K, Mantatzis M, et al. The role of cervical muscles morphology in the surgical treatment of degenerative disc disease: clinical correlations based on magnetic resonance imaging studies. J Clin Med Res (2021) 13(7):367–76. doi: 10.14740/jocmr4551
48. Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, et al. Changes in the cross-sectional area of deep posterior extensor muscles of the cervical spine after anterior decompression and fusion: 10-year follow-up study using MRI. Eur Spine J (2012) 21(2):304–8. doi: 10.1007/s00586-011-1978-0
49. Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine (Phila Pa 1976). (2005) 30(4):E86–91. doi: 10.1097/01.brs.0000153700.97830.02
50. Lin M, Paul R, Dhar UK, Doulgeris J, O'Connor TE, Tsai CT, et al. A review of finite element modeling for anterior cervical discectomy and fusion. Asian Spine J (2023) 17(5):949–63. doi: 10.31616/asj.2022.0295
51. Gorla C, Martins TS, Florencio LL, Pinheiro-Araújo CF, Fernández-de-Las-Peñas C, Martins J, et al. Reference values for cervical muscle strength in healthy women using a hand-held dynamometer and the association with age and anthropometric variables. Healthcare (Basel) (2023) 11(16):2278. doi: 10.3390/healthcare11162278
52. Panjabi MM, Lydon C, Vasavada A, Grob D, Crisco JJ 3rd, Dvorak J. On the understanding of clinical instability. Spine (Phila Pa 1976). (1994) 19(23):2642–50. doi: 10.1097/00007632-199412000-00008
53. Suvarnnato T, Puntumetakul R, Uthaikhup S, Boucaut R. Effect of specific deep cervical muscle exercises on functional disability, pain intensity, craniovertebral angle, and neck-muscle strength in chronic mechanical neck pain: a randomized controlled trial. J Pain Res (2019) 12:915–25. doi: 10.2147/JPR.S190125
54. Jull GA, Falla D, Vicenzino B, Hodges PW. The effect of therapeutic exercise on activation of the deep cervical flexor muscles in people with chronic neck pain. Man Ther (2009) 14(6):696–701. doi: 10.1016/j.math.2009.05.004
55. Moon H, Lee SK, Kim WM, Seo YG. Effects of exercise on cervical muscle strength and cross-sectional area in patients with thoracic hyperkyphosis and chronic cervical pain. Sci Rep (2021) 11(1):3827. doi: 10.1038/s41598-021-83344-4
56. Lin IH, Chang KH, Liou TH, Tsou CM, Huang YC. Progressive shoulder-neck exercise on cervical muscle functions in middle-aged and senior patients with chronic neck pain. Eur J Phys Rehabil Med (2018) 54(1):13–21. doi: 10.23736/S1973-9087.17.04658-5
57. Frasch M. Dedifferentiation, redifferentiation, and transdifferentiation of striated muscles during regeneration and development. Curr Top Dev Biol (2016) 106:331–55. doi: 10.1016/bs.ctdb.2015.12.005
58. Jiang J, Zhou P, Ling H, Xu Z, Yi B, Zhu S. MiR-499/PRDM16 axis modulates the adipogenic differentiation of mouse skeletal muscle satellite cells. Hum Cell (2018) 31:282–91. doi: 10.1007/s13577-018-0210-5
59. Buckingham M. Gene regulatory networks and cell lineages that underlie the formation of skeletal muscle. Proc Natl Acad Sci USA (2017) 114:5831–7. doi: 10.1073/pnas.1610605114
60. Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci USA (2008) 105:1226–31. doi: 10.1073/pnas.0711402105
61. Kook SH, Choi KC, Son YO, Lee KY, Hwang IH, Lee HJ, et al. Satellite cells isolated from adult hanwoo muscle can proliferate and differentiate into myoblasts and adipose-like cells. Mol Cells (2006) 22:239–45.
62. Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol (2006) 296:164–76. doi: 10.1016/j.ydbio.2006.04.449
63. Wang H, Li X, Gao S, Sun X, Fang H. Transdifferentiation via transcription factors or microRNAs: Current status and perspective. Differentiation (2015) 90:69–76. doi: 10.1016/j.diff.2015.10.002
64. Qi R, Long D, Wang J, Wang Q, Huang X, Cao C, et al. MicroRNA-199a targets the fatty acid transport protein 1 gene and inhibits the adipogenic trans-differentiation of C2C12 myoblasts. Cell Physiol Biochem (2016) 39:1087–97. doi: 10.1159/000447817
65. Yi F, Zhang P, Wang Y, Xu Y, Zhang Z, Ma W, et al. Long non-coding RNA slincRAD functions in methylation regulation during the early stage of mouse adipogenesis. RNA Biol (2019) 19:1–13. doi: 10.1080/15476286.2019.1631643
Keywords: cervical paraspinal muscle, fatty infiltration, cross-sectional area, cervical disc degenerative disease, anterior cervical discectomy and fusion, stand-alone anchored cage
Citation: Abudouaini H, Xu H, Yang J, Yi M, Lin K and Wang S (2023) Comparison of the effectiveness of zero-profile device and plate cage construct in the treatment of one-level cervical disc degenerative disease combined with moderate to severe paraspinal muscle degeneration. Front. Endocrinol. 14:1283795. doi: 10.3389/fendo.2023.1283795
Received: 27 August 2023; Accepted: 15 November 2023;
Published: 06 December 2023.
Edited by:
Kenju Shimomura, Fukushima Medical University, JapanReviewed by:
Nikolaos C. H. Syrmos, Aristotle University of Thessaloniki, GreeceMaohua Lin, Florida Atlantic University, United States
Copyright © 2023 Abudouaini, Xu, Yang, Yi, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sibo Wang, d2FuZ3Nic3BpbmUxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Haimiti Abudouaini†
Haimiti Abudouaini† Sibo Wang
Sibo Wang