- 1School of Veterinary Medicine and Biomedical Sciences, University of Nebraska-Lincoln, Lincoln, NE, United States
- 2Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE, United States
Gonadotropin-releasing hormone (GnRH1) and its receptor (GnRHR1) drive reproduction by regulating gonadotropins. Another form, GnRH2, and its receptor (GnRHR2), also exist in mammals. In humans, GnRH2 and GnRHR2 genes are present, but coding errors in the GnRHR2 gene are predicted to hinder full-length protein production. Nonetheless, mounting evidence supports the presence of a functional GnRHR2 in humans. GnRH2 and its receptor have been identified throughout the body, including peripheral reproductive tissues like the ovary, uterus, breast, and prostate. In addition, GnRH2 and its receptor have been detected in a wide number of reproductive cancer cells in humans. Notably, GnRH2 analogues have potent anti-proliferative, pro-apoptotic, and/or anti-metastatic effects on various reproductive cancers, including endometrial, breast, placental, ovarian, and prostate. Thus, GnRH2 is an emerging target to treat human reproductive cancers.
Introduction
GnRH2
Hypothalamic GnRH1 binds GnRHR1 on gonadotropes, promoting gonadotropin [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] synthesis/secretion. Another form, GnRH2, is also present in mammals (1). GnRH2 is ubiquitously expressed (1) and originates from the GnRH2 gene (chromosome 20) in humans (2). Both decapeptides, GnRH2 and GnRH1 have a 70% sequence identity (3). Amino acid substitutions in GnRH2 enhance its stability (4) and half-life (5, 6) compared with GnRH1.
GnRHR2
A 7-transmembrane (TM) G-protein coupled receptor (GPCR) specific to GnRHR2 is present in mammals and ubiquitously expressed (1). GnRH2 binds its cognate receptor with greater affinity than GnRHR1 (24-fold increase (7)), leading to greater activity (up to 440-fold increase (8–10)). In contrast, GnRH1 exhibits 12-fold greater activity at GnRHR1 compared to GnRH2 (9, 11). Thus, GnRHR2 displays greater selectivity for GnRH2, whereas GnRHR1 binds/activates both decapeptides reasonably well (8). Both receptors utilize Gαq/11 to trigger IP3 synthesis and activate protein kinase C (PKC (8, 12, 13)), but downstream signaling pathways diverge (7). Additionally, GnRH2 activation of GnRHR1 initiates different signaling pathways than GnRH1 (14), suggesting that GnRH2 elicits different physiological effects than GnRH1 at GnRHR1.
Humans maintain a full-length GnRHR2 gene (chromosome 1 (10)), although a frameshift mutation and premature stop codon are predicted to prevent full-length receptor production (15, 16). Nevertheless, there is mounting evidence for a functional GnRHR2 in humans (12, 17–23), potentially via production of a 5-TM GnRHR2 (17). Mammals, including humans, produce functional 5-TM GPCRs (11, 24–26). Notably, pigs produce 5-TM GnRHR2 transcripts with translatable protein characteristics, resulting from alternative splicing and an alternative start codon (27, 28). In addition to the full-length GnRHR2 gene (chromosome 1), a truncated GnRHR2 gene (chromosome 14) is also present in humans (10), which is more transcriptionally active and widely expressed (29).
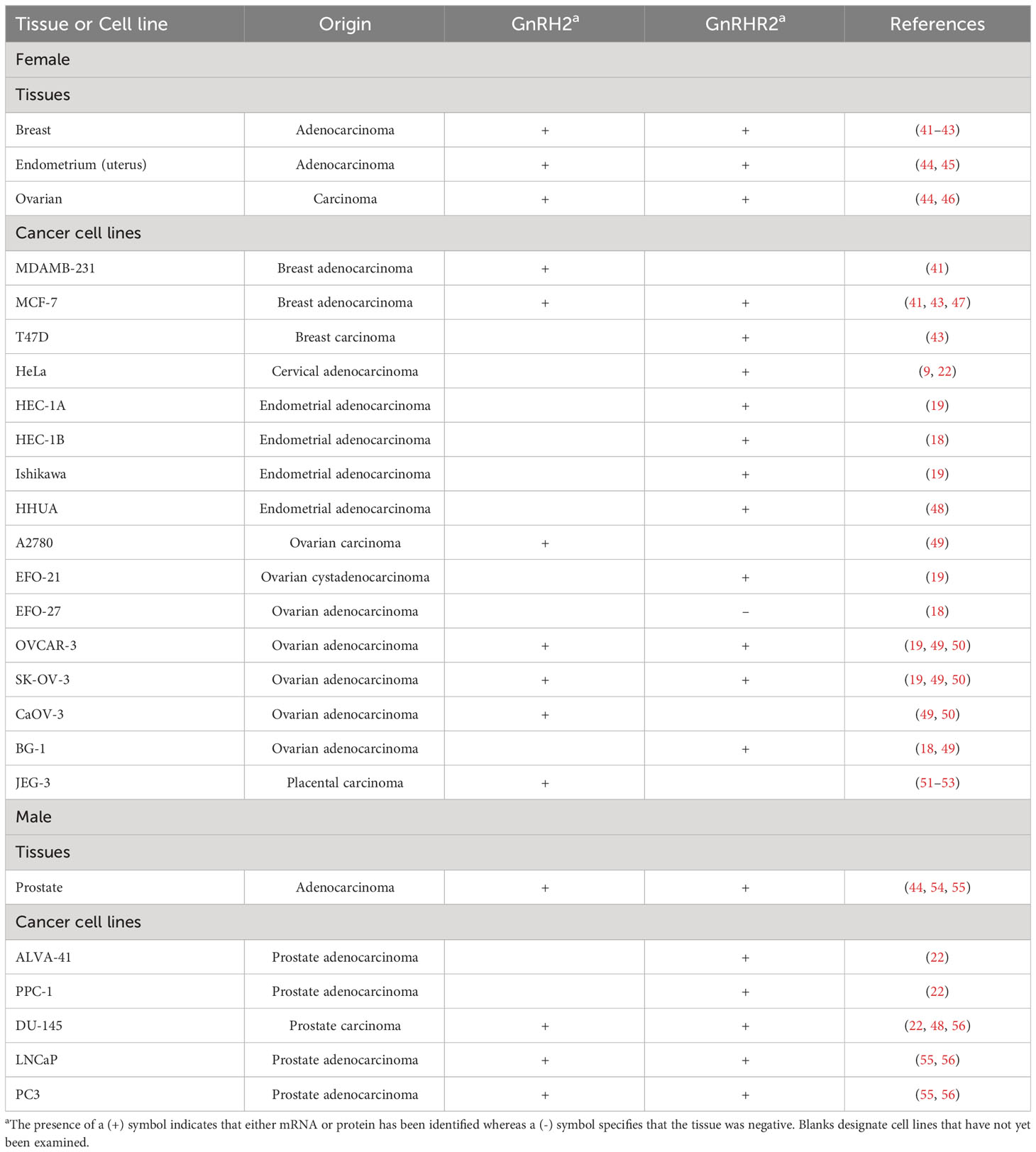
GnRH2 and GnRHR2 in human reproductive cancers
The tumor microenvironment is dependent on unchecked cell division, cytokines, anti-apoptotic mediators, and immune cell recruitment, which are controlled by a variety of important biomolecules like hormones, growth factors, cytokines, and immune mediators (e.g., Toll-like receptors) (30–32). For decades, we have known that GnRH1 and GnRHR1 are expressed in reproductive tumors and GnRH1 analogues inhibit cancer cell proliferation (19, 33–38). In fact, the newest approaches utilize GnRH1-tagged nanoparticles to directly target chemotherapeutics into cancer cells (39). In addition, elevated expression of GnRH1 and GnRHR1 in bladder cancer is linked with better survival in men but worse survival in women, suggesting possible regulation of the GnRH1/GnRHR1 system by gonadal steroids in non-reproductive tissues (40). Recently, GnRH2 and GnRHR2 have been detected in reproductive cancer cells (Table 1). Like GnRH1, GnRH2 analogues inhibit cancer cell proliferation; however, GnRH2 is often more potent (18, 19, 21, 44, 57). It remains unclear if GnRHR2 or GnRHR1 are mediating these effects. A role for GnRHR2 seems plausible due to difficulty detecting high affinity receptors for GnRH1 in peripheral reproductive tissues (34). Also, high concentrations of GnRH1 analogues are required to suppress cancer cell proliferation (35). Indeed, many independent groups have reported evidence for a functional GnRHR2 in reproductive cancer cells (12, 18, 19, 21, 44, 58). Moreover, new data suggests that differential methylation of GnRH2 may affect cancer progression in non-reproductive organs (59, 60). Likewise, gene polymorphisms are increasingly being linked with the onset of cancer (31). GnRH2 gene polymorphisms have been linked with bone cancer (61, 62). Thus, GnRH2 and GnRHR2 may be novel, unexploited cancer targets.
Breast
In 2002, Chen et al. (41) demonstrated that GnRH2 mRNA was overexpressed in cancerous versus normal breast tissue. In another study, GnRH2 expression was 2-fold greater in malignant compared to normal tissue (42). Moreover, GnRH2 expression in breast cancer samples correlated with indices of a poorer prognosis (42). GnRHR2 immunostaining is detectable in breast cancer cells (Table 1 (43)), suggesting autocrine/paracrine interactions. Others identified direct anti-proliferative effects of GnRH2 on breast cancer cells (47, 63). In MCF-7 and T47D cells, GnRH2 agonist pre-treatment interrupted epidermal growth factor (EGF) signaling, ablating EGF-mediated autophosphorylation of EGF receptor and the induction of the mitogen-activated protein kinase (MAPK), extracellular-signal-regulated kinase 1/2 (ERK1/2 (43)). Furthermore, a GnRH2 agonist reversed 4OH-tamoxifen insensitivity of breast cancer cells (43). In MCF-7 cells, GnRH2 downregulated proteins required for translation and cell proliferation (41).
In addition to reducing cell proliferation, GnRH2 analogues induce apoptosis. GnRH2 antagonists stimulated loss of mitochondrial membrane potential and apoptosis in breast cancer cells via p38 MAPK and c-Jun N-terminal kinase (JNK) pathways, culminating in activation of the pro-apoptotic protein, BAX (47). The same antagonists failed to activate protein kinase B (also known as AKT) or ERK1/2 (47). In a different study, GnRH2 antagonists induced apoptosis in triple negative MDA-MB-231 breast cancer cells [lack estrogen receptors, progesterone receptors, and human EGF receptor 2 (HER2)], which was mediated by p38 MAPK signaling, loss of mitochondrial membrane potential, and capsase-3 activation (23). GnRHR1 knockdown failed to fully ablate GnRH2 antagonist-mediated apoptosis, implicating GnRHR2 (23). Moreover, GnRH2 antagonists completely inhibited breast cancer tumor growth in nude mice (23).
Notably, MCF-7 cells take up fluorescently labeled GnRH2-conjugates effectively, which is under investigation as a method of targeted drug delivery (64). Indeed, GnRH2 analogues conjugated to cytotoxic drugs (e.g., daunorubicin) have shown promising anti-tumor effects in vitro (64, 65). In addition to anti-proliferative and pro-apoptotic effects, GnRH2 analogues also have anti-metastatic properties. For example, breast cancer cell migration to bone and invasion of an artificial basement membrane were attenuated by GnRH2, although to a lesser extent than GnRH1 (66).
Uterine
Endometrium
GnRH2 has been detected in endometrial carcinomas (45), whereas GnRHR2 mRNA is present in both endometrial carcinomas (45) and endometrial cancer cells (HEC-1A, HEC-1B, HHUA, and Ishikawa (18, 19, 48; Table 1)). Further, GnRHR2 protein was identified in endometrial cells (44). Co-expression of GnRH2 and GnRHR2 suggest an autocrine/paracrine role in endometrial cancers (67). Several independent groups determined that GnRH2 analogues reduced endometrial cancer cell growth (18, 19, 21, 47, 57, 58, 68). GnRH2 more effectively inhibited the growth of HEC-1A and Ishikawa cells compared to the same dose of a potent GnRH1 agonist (triptorelin (18)). Triptorelin, cetrorelix (pan GnRHR antagonist), and GnRH2 exerted anti-proliferative effects on endometrial cancer cells (Ishikawa, HEC-1A, and HEC-1B) that produce GnRHR1 and GnRHR2 transcripts (18). GnRHR1 knockdown ablated anti-proliferative effects of triptorelin but failed to ablate the efficacy of cetrorelix and GnRH2 (18).
The same group detected protein corresponding to the 5-TM GnRHR2 (43-kDA) in Ishikawa and HEC-1A cells (44). Their antibody was validated in part via detection of a 7-TM GnRHR2 band (54-kDa) in ovarian protein from marmoset monkeys (44), a species that produces a full-length GnRHR2 (9). Interestingly, radiolabeled GnRH2 binds a 43-kDA protein in human endometrial cancer cells (44). Both native GnRH2 and cetrorelix (pan GnRHR antagonist) were able to displace 125I-labeled GnRH2, but not triptorelin (GnRH1 agonist (44)). Of note, cetrorelix binds both GnRHR1 and GnRHR2 reasonably well (22, 69, 70), whereas triptorelin is highly specific for GnRHR1 (10). The authors hypothesized that these results occurred due to a functional 5-TM GnRHR2 (44). Indeed, both low and high affinity binding sites for GnRH1 were detectable in human endometrial cancer cells (71), implicating the presence of GnRHR1 (high) and GnRHR2 (low).
GnRH2 also has anti-proliferative effects in endometrial cancer cells. For example, a GnRH2 agonist attenuated the proliferative effects of growth factors on Ishikawa and HEC-1A cells (21). Specifically, the GnRH2 agonist activated phosphotyrosine phosphatase, which reduces the autophosphorylation of activated EGF receptors, and downregulated genes associated with EGF-mediated transcription, leading to reduced cell proliferation (21). Importantly, these effects persisted following GnRHR1 deletion, implicating GnRHR2 (21). Furthermore, Park et al. (58) demonstrated reduced proliferation of HEC-1A cells after GnRH2 treatment, which was more effective than GnRH1.
The efficacy of GnRH analogues to induce apoptosis has been tested in endometrial cancer cell lines. Analogues of GnRH1 (agonist and antagonist) failed to induce apoptosis (57). In contrast, antagonists of GnRH2 induced apoptosis via caspase-3 activation, which appeared to be mediated by GnRHR1 (47). Likewise, GnRH2 induced apoptosis in Ishikawa cells via GnRHR1 (72). GnRH2 induced apoptosis and suppressed cell proliferation in endometrial carcinoma cell lines, with a greater effect observed in cells with PTEN knockdown (68). Additionally, GnRH2 reduced protein kinase B (AKT) and ERK1/2 activity in HEC-1A-ND cells (68). In animal models, growth of xenotransplants from HEC-1B cells in nude mice was suppressed by GnRH2 (57). Researchers have also found that a GnRH2 agonist enhanced cell migration through GnRHR1-mediated phosphorylation of ERK1/2 and JNK, leading to MAPK-dependent activation of matrix metalloproteinase-2 (MMP-2) in Ishikawa and ECC-1 cells (73). Notably, GnRH2 analogues have a more potent inhibitory effect than GnRH1 on proliferation of endometrial cancer cells (18, 19, 21, 44, 57). Interestingly, a metabolite of GnRH1 [GnRH-(1–5)] also regulates the progression of endometrial cancer (74); however, effects of GnRH2 metabolites on endometrial cancer cells have not yet been explored.
Myometrium
GnRH analogues are clinically utilized to treat leiomyomas, benign fibroids of the myometrium (75). Induction of a hypoestrogenic state is thought to drive fibroid involution (75). However, transcripts for both GnRH2 and GnRHR2, as well as protein for GnRH2, were detected in normal myometrial tissue and leiomyomas of women, suggesting a direct effect of GnRH analogues on fibroid growth (76).
Ovarian
Both GnRH2 and/or GnRHR2 have been detected in cancerous ovarian cells (Table 1). GnRH2 was overexpressed in malignant compared to benign ovarian tumors or normal ovarian tissue (46). GnRH2 expression in ovarian cancer cells appears to be mediated in part by gonadotropins. Choi et al. (49) reported that gonadotropin treatment (FSH or LH) reduced GnRH2 expression in the majority of ovarian cancer cell lines tested (including OVCAR-3 cells); however, GnRH1 mRNA was unaffected by treatment (49). GnRHR1 mRNA was downregulated by FSH or LH in most ovarian cancer cell lines but GnRHR2 expression was not examined (49). Converse to this data, Ling Soon et al. (51) found that a GnRH2 promoter-luciferase reporter gene construct was activated by8-bromoadenosine-cAMP in OVCAR-3 cells (cAMP is a second messenger of LH and FSH (51)). The cause of the discrepancy has not been resolved in the literature but may relate to differences in treatment (LH/FSH versus 8-bromoadenosine-cAMP), dose, culture conditions, and/or testing of the endogenous cellular machinery versus luciferase assay. Notably, in post-menopausal women with ovarian tumors, there was a positive correlation between serum LH and FSH concentrations and GnRH2 expression in ovarian tumor samples (46), suggesting a stimulatory role of the gonadotropins on GnRH2 expression in vivo. GnRH2 expression is also regulated by EGF in ovarian cancer cells. For example, EGF upregulates GnRH2 promoter activity in OVCAR-3 cells; an effect that is abolished in the presence of an EGF receptor inhibitor (77).
Due to its potent anti-proliferative effects, GnRH2 has garnered attention as a possible therapeutic for ovarian cancer treatment. Early studies demonstrated that treatment of both non-tumorigenic (IOSE-29) and tumorigenic (IOSE-29EC) cells with GnRH2 reduced cell proliferation (19, 50). Choi et al. (49) reported that GnRH2 agonists inhibited growth of ovarian cancer cells, an effect that was reversed by LH or FSH pre-treatment. Grundker et al. (19) showed that GnRH2 reduced ovarian cancer cell proliferation, outperforming equimolar triptorelin (a GnRH1 agonist) treatment. In SK-OV-3 ovarian cancer cells (expressing GnRHR2 but not GnRHR1), GnRH2 exhibited powerful anti-proliferative effects, unlike triptorelin (19), suggesting that GnRHR2 is mediating these effects. However, others contested the assertion that GnRHR1 is not expressed in SK-OV-3 cells (78), although they acknowledged that it may be present, but expressed at low levels (77). In another study, anti-proliferative effects of triptorelin were abolished after GnRHR1 knockdown in ovarian cancer cells (EFO-21 and OVCAR-3), but effects of GnRH2 and the pan GnRHR antagonist, cetrorelix (binds both GnRHR1 and GnRHR2 (70)), persisted (18). Together, these findings suggest that GnRHR2 is functional in certain ovarian cancer cells. However, other groups provided evidence that GnRHR1 is involved in mediating anti-proliferative effects of GnRH2 (78). Thus, the exact receptor (GnRHR1 and/or GnRHR2) eliciting anti-proliferative effects of GnRH2 remains controversial.
Data from Eicke et al. (44) supports the presence of GnRHR2 protein in humans; immunostaining identified GnRHR2 in ovarian cancer samples. Both immunoblotting of protein and photo labeling studies of cell membrane fractions from ovarian cancer cells (EFO-21, SK-OV-3) resulted in a band corresponding to the 5-TM GnRHR2 isoform (43-kDa (44)). Competition experiments showed that triptorelin weakly competed for the binding site (43-kDa) compared to the stronger effect of cetrorelix (44) (pan GnRHR antagonist (70)). However, GnRH2 was the most potent competitor, indicating the presence of a functional 5-TM GnRHR2 in human ovarian cancer cells (44).
Subsequent studies have been conducted to determine the mechanism underlying anti-proliferative effects of GnRH2 on ovarian cancer cells. For example, GnRH2 treatment led to p38 MAPK activation, an effect which was reversed by SB203580 (p38 MAPK inhibitor (79)). Likewise, activator protein-1 was stimulated by GnRH2 but reduced in the presence of SB203580 (79). In OVCAR-3 cells, GnRH2 treatment inhibited cell growth, but this effect was abolished when cells were pre-treated with SB203580 (79). GnRH2 treatment also enhanced apoptosis, which was reversed with SB203580 pre-treatment (79). The same group showed that ERK1/2 (but not JNK) is involved in mediating anti-proliferative effects of GnRH2 (63). Others reported that GnRH2 mediated cell proliferation is dependent on PKC (78). In this study, however, data suggested that GnRHR1 (not GnRHR2) mediated these effects (78). Additional research demonstrated that GnRH2 treatment inhibited mitogenic effects of EGF in ovarian cancer cells (21). GnRHR1 knockdown failed to prevent these effects, suggesting the contribution of GnRHR2.
Many researchers have investigated pro-apoptotic activities of GnRH2 analogues on ovarian cancer cells (47, 57, 79, 80). GnRH2 antagonists induced apoptosis by activating caspase-3 and effectively inhibited growth of human ovarian cancer xenotransplants in nude mice (57). Furthermore, GnRH2 antagonists activated p38 MAPK and JNK, resulting in activation of BAX mitochondrial dysfunction (loss of membrane potential, release of cytochrome C), and caspase-3 activation (47). Recent data also demonstrated that co-treatment of ovarian cancer cells with a glycolysis inhibitor and a GnRH2 antagonist reduced cell viability and increased apoptosis to a greater extent than each treatment individually (80).
The role of GnRH2 in ovarian cancer metastases has also been explored. Chen et al. (81) found that low doses of GnRH1 and GnRH2 promoted invasion of OVCAR-3 cells but had the opposite effect in SKOV-3 cells (both GnRH1 and GnRH2 inhibited invasion but only at high doses). GnRHR1 knockdown abolished the effect of treatment in both cell types; however, GnRHR2 expression was not examined (81). Treating SKOV-3 cells with either GnRH1 or GnRH2 led to reduced MMP-2 expression and increased secretion of tissue inhibitor of MMP-2 (TIMP2), both important mediators of ovarian carcinoma metastasis (81). Furthermore, GnRH1 and GnRH2 disrupted activation of the phosphatidylinositol-3-kinase (PI3K)/AKT pathway, which promotes proteolysis and invasion in ovarian cancer cells (81). Thus, in SKOV-3 cells, GnRH2 inhibits ovarian cancer invasion by regulating the balance of MMP2/TIMP2, and disrupting AKT-mediated proteolysis and invasion (81). In contrast, others reported that GnRH2 enhanced membrane type I metalloproteinase production via the PI3K/AKT pathway and phosphorylation of GSK3β in OVCAR-3 and CaOV-3 cells (82).
Chen et al. (81) hypothesized several different mechanisms that might enable two lines of ovarian cancer cells to exhibit different invasive responses to GnRH2 (presumably both via GnRHR1). Possible explanations include differences in inherent cell invasiveness and/or receptor expression levels, which is a known driver of differential cellular responses (83). For example, Chen et al. (81), found that low doses of GnRH2 promoted invasion in OVCAR-3 cells (with elevated GnRHR1 expression) unlike SKOV-3 cells (with low GnRHR1 expression). Interestingly, both SKOV-3 and OVCAR-3 cells express GnRHR2 (Table 1), but the level of expression has not been compared to our knowledge.
Interestingly, GnRH2 and EGF worked synergistically to promote invasion of OVCAR-3 and CaOV-3 cells, but not SKOV-3 cells (reduced endogenous GnRHR1 expression (77)). GnRHR1 knockdown in OVCAR-3 and CaOV-3 cells only partially inhibited invasiveness mediated by EGF (77), suggesting that GnRHR2 may be involved. Later studies demonstrated that EGF increased GnRH2 expression in OVCAR-3 and CaOV-3 cells, potentially enhancing autocrine signaling (mediated by GnRHR1 (84)). Enhanced GnRHR1 signaling leads to increased production of the 37-kDa laminin receptor precursor, more tumor cell interactions with laminin in the extracellular matrix, and enhanced MMP-2 production (84). These data suggest that GnRH2 modulates pro- and anti-metastatic effects depending on the ovarian cancer cell type. This discrepancy has not yet been resolved but may be related to expression differences in GnRHR1 and/or GnRHR2 among cell types.
Placental
GnRH2 mRNA is present in the choriocarcinoma cell line, JEG-3 ((51–53); Table 1), and cAMP treatment activated the GnRH2 promoter (51). Both GnRH1 and GnRH2 enhanced JEG-3 cell invasion (12) but GnRHR1 knockdown only inhibited GnRH1-mediated effects, not GnRH2 (12), implicating GnRHR2. Furthermore, GnRH2 treatment of JEG-3 cells reduced cell proliferation, results which were ascribed to GnRHR1 (85). GnRHR2 has not been investigated in JEG-3 cells, although Eicke et al. (44) demonstrated evidence for a functional 5-TM GnRHR2 in human placentae.
Prostate
GnRH1 agonists are commonly used to treat prostate cancer but increase the risk of adverse cardiovascular events (86), which highlights the need for more therapeutic options. In addition to normal tissue, GnRH2 is also present in hyperplastic and neoplastic prostate tissues ((54, 55); Table 1). Eicke et al. (44) reported immunoreactive GnRHR2 in prostate adenocarcinomas, specifically within epithelial (not stromal) cells. A recent study found an association between prostate cancer progression and a GnRH2 gene polymorphism in Japanese men (87), although a separate study did not observe this link in Caucasian men (54). Therefore, GnRH2 and GnRHR2 may be involved in autocrine/paracrine regulation of prostate cancer progression.
Notably, GnRH2 and GnRHR2 are expressed in normal and cancerous prostate cell lines (22, 48, 55, 56). GnRH2 treatment reduced proliferation of all tested prostate cancer cell lines; these results were ascribed to GnRHR1 and activation of cAMP (56). Others showed that GnRH2 increased intracellular calcium levels via activation of the ryanodine receptor in androgen independent DU-145 cells (22). Likewise, a GnRH2-specific antagonist (trptorelix-1) induced cell death and prevented GnRH2-mediated calcium influx (22). Photoaffinity labeling suggested that GnRH2 binds with high affinity to a protein in prostate cancer cells (22), implicating GnRHR2.
Androgens enhanced GnRH2 expression in prostate tumors by binding a putative androgen response element on the 5’ flanking region of the human GnRH2 gene (55). Thus, anti-androgen therapy reduces GnRH2 expression in tumor biopsies (55). Studies using a prostate xenograft model demonstrated that androgens enhanced GnRH2 expression, whereas androgen deprivation reduced GnRH2 expression (55). Consistent with this, GnRH2 expression is elevated in prostate cancer cells (e.g., LNCaP cells) that produce androgen receptors (ARs) compared to those lacking ARs (e.g., PC3 cells (55)) and AR inhibition blocked androgen-mediated increases in GnRH2 expression in LNCaP cells. Interestingly, GnRH2 treatment of LNCaP (AR positive) and PC3 (AR negative) cells led to reduced cell proliferation and migration, suggesting that these actions are not dependent on AR signaling (55).
The anti-proliferative activity of GnRH2 has garnered increasing attention as a therapeutic target. For example, Kim et al. (88) developed a GnRH2 specific antagonist, trptorelix-1, that effectively inhibited growth of PC3 cells in vitro and ex vivo (88). Moreover, trptorelix-1 decreased mitochondrial membrane potential and enhanced reactive oxygen species (ROS) within the cytoplasm and mitochondria (88). Antioxidant co-treatment partially protected against trptorelix-1-mediated growth inhibition. Furthermore, autophagosome formation was observed in the absence of apoptosis markers in prostate cancer cells treated with trptorelix-1, which induced cell signaling cascades consistent with autophagy (88).
The same group developed another GnRHR2 antagonist, SN09-2 (89). When compared to trptorelix-1, SN09-2 suppressed growth of prostate cancer cells, even at low concentrations, and was an effective inhibitor of PC3 xenograft growth. These effects were associated with mitochondrial accumulation of SN09-2, leading to mitochondrial dysfunction and ROS generation (89). Furthermore, SN09-2 induced markers of apoptosis in PC3 cells (89).
Researchers have also investigated the potential for targeted tumor treatment by incubating LNCaP cells with selectively labeled, fluorescent derivatives of GnRH analogues, including GnRH2 (64). Effective cellular uptake of GnRH2 conjugates were observed in LNCaP cells, which was ascribed to GnRHR1 (64). However, GnRHR2 is also expressed in these cells (56), so it remains unclear which receptor mediated these effects since GnRHR2 was not examined (64). Of note, uptake of GnRH2 conjugates by LNCaP cells was greater than GnRH1 conjugates or any other cell type tested (human breast, colon, pancreas (64)).
Other reproductive cancers
Cervical
There is a severe lack of information about the potential role of GnRH2 and GnRHR2 in cervical cancer despite the detection of GnRHR2 mRNA in HeLa cells ( (9, 22); Table 1), an important cell line for biomedical and oncology research (90). There is a critical need to better understand the potential function of GnRH2 and GnRHR2 in these cells since cervical cancer is the second most common cancer in women (91).
Testis
Although GnRH2 and GnRHR2 have been investigated in the regulation of many different reproductive cancers, there is a gap in our knowledge regarding the potential influence of GnRH2 and GnRHR2 as a therapeutic to treat testicular cancer. This gap is surprising given that GnRH2 and GnRHR2 are both present within the human testis (29, 92) and highly abundant in swine testes (93), an important biomedical model (94). Likewise, GnRHR2 expression was greatest in marmoset monkey testes compared to 30 other tissues (7). To our knowledge, neither GnRH2 nor GnRHR2 have been evaluated as possible regulators of testicular cancer. Further study is especially important given the recent discovery that a single nucleotide polymorphism in the GnRH2 gene is associated with both GnRH2 expression in the testis as well as bone cancer risk (62). Furthermore, GnRH2 gene polymorphisms were associated with elevated testosterone levels and an increased prostate cancer risk (87).
Conclusions
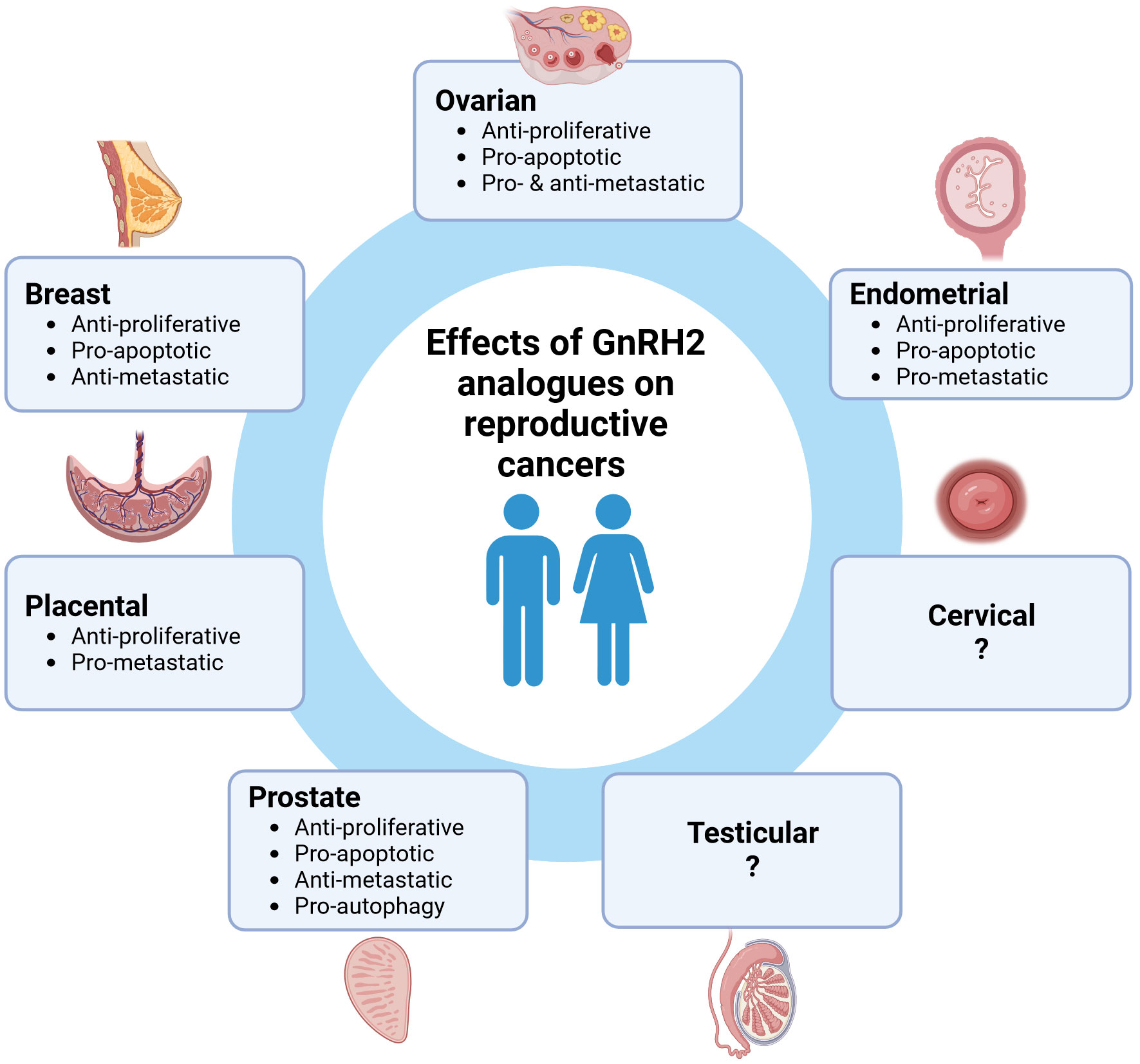
GnRH2 and GnRHR2 are expressed in a wide range of human reproductive cancers suggesting an autocrine/paracrine role. Notably, GnRH2 and its analogues mediate potent anti-proliferative and pro-apoptotic activities in many different reproductive cancer cells suggesting an overall inhibitory role (Figure 1). However, the metastatic effects of GnRH2 are variable based upon cell type, which remains unresolved. To date, the most widely studied cells have been derived from cancers of the ovary, endometrium, prostate, and breast. However, GnRH2 and/or GnRHR2 are also expressed in other reproductive cancer cells (cervical, placenta), warranting further study. Importantly, the anti-tumor effects of GnRH2 are often more robust than GnRH1, enhancing therapeutic potential. Of concern, the ubiquitous expression of both GnRH2 and GnRHR2 could result in more off-target effects unless GnRH2 analogues could be delivered directly to tumorigenic reproductive tissues (e.g., nanoparticle drug delivery). In addition, further exploration of the connection between methylations/mutations in the GnRH2 gene with the onset of cancer is essential. Although controversial, the effects of GnRH2 may indeed be mediated via a unique GnRHR2 (e.g., 5-TM). Thus, GnRH2 and GnRHR2 are negative paracrine/autocrine regulators of human reproductive cancers and represent emerging oncological targets.
Author contributions
AD: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. BW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by institutional start-up funds from the University of Nebraska-Lincoln to AD.
Acknowledgments
The authors thank Jackie Shelton for copy editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Desaulniers AT, Cederberg RA, Lents CA, White BR. Expression and role of gonadotropin-releasing hormone 2 and its receptor in mammals. FrontEndocrinol(Lausanne) (2017) 8:269. doi: 10.3389/fendo.2017.00269
2. Stewart AJ, Katz AA, Millar RP, Morgan K. Retention and silencing of prepro-GnRH-II and type II GnRH receptor genes in mammals. Neuroendocrinology (2009) 90:416–32. doi: 10.1159/000233303
3. Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. ProcNatlAcadSciUSA (1984) 81:3874–8. doi: 10.1073/pnas.81.12.3874
4. Siler-Khodr TM, Grayson M. Action of chicken II GnRH on the human placenta. JClinEndocrinolMetab (2001) 86:804–10. doi: 10.1210/jc.86.2.804
5. Tsai PS, Licht P. In vivo GnRH responsiveness of LH secretion in the female turtle, Trachemys scripta, in relation to the reproductive stage. GenCompEndocrinol (1993) 90:328–37. doi: 10.1006/gcen.1993.1088
6. Licht P, Tsai PS, Sotowska-Brochocka J. The nature and distribution of gonadotropin-releasing hormones in brains and plasma of ranid frogs. GenCompEndocrinol (1994) 94:186–98. doi: 10.1006/gcen.1994.1075
7. Millar R, Lowe S, Conklin D, Pawson A, Maudsley S, Troskie B, et al. A novel mammalian receptor for the evolutionarily conserved type II GnRH. ProcNatlAcadSciUSA (2001) 98:9636–41. doi: 10.1073/pnas.141048498
8. Millar RP. GnRH II. and type II GnRH receptors. Trends EndocrinolMetab (2003) 14:35–43. doi: 10.1016/S1043-2760(02)00016-4
9. Neill JD, Duck LW, Sellers JC, Musgrove LC. A gonadotropin-releasing hormone (GnRH) receptor specific for GnRH II in primates. BiochemBiophysResCommun (2001) 282:1012–8. doi: 10.1006/bbrc.2001.4678
10. Neill JD. GnRH and GnRH receptor genes in the human genome. Endocrinology (2002) 143:737–43. doi: 10.1210/endo.143.3.8705
11. Perron A, Sarret P, Gendron L, Stroh T, Beaudet A. Identification and functional characterization of a 5-transmembrane domain variant isoform of the NTS2 neurotensin receptor in rat central nervous system. JBiolChem (2005) 280:10219–27. doi: 10.1074/jbc.M410557200
12. Liu J, Maccalman CD, Wang YL, Leung PC. Promotion of human trophoblasts invasion by gonadotropin-releasing hormone (GnRH) I and GnRH II via distinct signaling pathways. MolEndocrinol (2009) 23:1014–21. doi: 10.1210/me.2008-0451
13. Kang SK, Tai CJ, Cheng KW, Leung PC. Gonadotropin-releasing hormone activates mitogen-activated protein kinase in human ovarian and placental cells. MolCellEndocrinol (2000) 170:143–51. doi: 10.1016/S0303-7207(00)00320-8
14. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. EndocrRev (2004) 25:235–75. doi: 10.1210/er.2003-0002
15. Gault PM, Morgan K, Pawson AJ, Millar RP, Lincoln GA. Sheep exhibit novel variations in the organization of the mammalian type II gonadotropin-releasing hormone receptor gene. Endocrinology (2004) 145:2362–74. doi: 10.1210/en.2003-1625
16. Morgan K, Sellar R, Pawson AJ, Lu ZL, Millar RP. Bovine and ovine gonadotropin-releasing hormone (GnRH)-II ligand precursors and type II GnRH receptor genes are functionally inactivated. Endocrinology (2006) 147:5041–51. doi: 10.1210/en.2006-0222
17. Neill JD, Musgrove LC, Duck LW. Newly recognized GnRH receptors: function and relative role. Trends EndocrinolMetab (2004) 15:383–92. doi: 10.1016/j.tem.2004.08.005
18. Grundker C, Schlotawa L, Viereck V, Eicke N, Horst A, Kairies B, et al. Antiproliferative effects of the GnRH antagonist cetrorelix and of GnRH-II on human endometrial and ovarian cancer cells are not mediated through the GnRH type I receptor. EurJEndocrinol (2004) 151:141–9. doi: 10.1530/eje.0.1510141
19. Grundker C, Gunthert AR, Millar RP, Emons G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. JClinEndocrinolMetab (2002) 87:1427–30. doi: 10.1210/jcem.87.3.8437
20. Chou CS, MacCalman CD, Leung PC. Differential effects of gonadotropin-releasing hormone I and II on the urokinase-type plasminogen activator/plasminogen activator inhibitor system in human decidual stromal cells in vitro. JClinEndocrinolMetab (2003) 88:3806–15. doi: 10.1210/jc.2002-021955
21. Eicke N, Gunthert AR, Emons G, Grundker C. GnRH-II agonist [D-Lys6]GnRH-II inhibits the EGF-induced mitogenic signal transduction in human endometrial and ovarian cancer cells. IntJOncol (2006) 29:1223–9. doi: 10.3892/ijo.29.5.1223
22. Maiti K, Oh DY, Moon JS, Acharjee S, Li JH, Bai DG, et al. Differential effects of gonadotropin-releasing hormone (GnRH)-I and gnRH-II on prostate cancer cell signaling and death. J Clin Endocrinol Metab (2005) 90:4287–98. doi: 10.1210/jc.2004-1894
23. Grundker C, Fost C, Fister S, Nolte N, Gunthert AR, Emons G. Gonadotropin-releasing hormone type II antagonist induces apoptosis in MCF-7 and triple-negative MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast Cancer Res (2010) 12:R49. doi: 10.1186/bcr2606
24. Sanchez C, Escrieut C, Clerc P, Gigoux V, Waser B, Reubi JC, et al. Characterization of a novel five-transmembrane domain cholecystokinin-2 receptor splice variant identified in human tumors. MolCellEndocrinol (2012) 349:170–9. doi: 10.1016/j.mce.2011.10.010
25. Ling K, Wang P, Zhao J, Wu YL, Cheng ZJ, Wu GX, et al. Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors. ProcNatlAcadSciUSA (1999) 96:7922–7. doi: 10.1073/pnas.96.14.7922
26. Bokaei PB, Ma XZ, Byczynski B, Keller J, Sakac D, Fahim S, et al. Identification and characterization of five-transmembrane isoforms of human vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors. Genomics (2006) 88:791–800. doi: 10.1016/j.ygeno.2006.07.008
27. Neill JD, Duck LW, Musgrove LC. GnRH II receptor is encoded in genomes of human, monkey, and pig but not mouse. Abstracts 84th Annu Meeting of the Endocrine Soc. San Francisco, CA (2002). p. 177 (Abstract P-1-97).
28. Neill JD, Duck LW, Musgrove LC. Potential regulatory role for GnRH II in gonadotropin secretion: molecular characterization of a GnRH II receptor in the pig pituitary. Abstracts 32nd Annu Meeting of the Society for Neuroscience. Orlando, FL (2002). Abstract 1–97. Abstract 74.9.
29. van Biljon W, Wykes S, Scherer S, Krawetz SA, Hapgood J. Type II gonadotropin-releasing hormone receptor transcripts in human sperm. BiolReprod (2002) 67:1741–9. doi: 10.1095/biolreprod.101.002808
30. Mukherjee S, Patra R, Behzadi P, Masotti A, Paolini A, Sarshar M. Toll-like receptor-guided therapeutic intervention of human cancers: molecular and immunological perspectives. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1244345
31. Behzadi P, Sameer AS, Nissar S, Banday MZ, Gajdács M, García-Perdomo HA, et al. The interleukin-1 (IL-1) superfamily cytokines and their single nucleotide polymorphisms (SNPs). J Immunol Res (2022) 2022:e2054431. doi: 10.1155/2022/2054431
32. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res (2019) 79:4557–66. doi: 10.1158/0008-5472.CAN-18-3962
33. Grundker C, Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. ReprodBiolEndocrinol (2003) 1:65. doi: 10.1186/1477-7827-1-65
34. Limonta P, Moretti RM, Montagnani Marelli M, Motta M. The biology of gonadotropin hormone-releasing hormone: role in the control of tumor growth and progression in humans. FrontNeuroendocrinol (2003) 24:279–95. doi: 10.1016/j.yfrne.2003.10.003
35. Emons G, Müller V, Ortmann O, Schulz KD. Effects of LHRH-analogues on mitogenic signal transduction in cancer cells. J Steroid Biochem Mol Biol (1998) 65:199–206. doi: 10.1016/S0960-0760(97)00189-1
36. Emons G, Weiss S, Ortmann O, Gründker C, Schulz KD. LHRH might act as a negative autocrine regulator of proliferation of human ovarian cancer. Eur J Endocrinol (2000) 142:665–70. doi: 10.1530/eje.0.1420665
37. Völker P, Gründker C, Schmidt O, Schulz K-D, Emons G. Expression of receptors for luteinizing hormone-releasing hormone in human ovarian and endometrial cancers: frequency, autoregulation, and correlation with direct antiproliferative activity of luteinizing hormone-releasing hormone analogues. Am J Obstet Gynecol (2002) 186:171–9. doi: 10.1067/mob.2002.119633
38. Leung PC, Cheng CK, Zhu XM. Multi-factorial role of GnRH-I and GnRH-II in the human ovary. MolCellEndocrinol (2003) 202:145–53. doi: 10.1016/S0303-7207(03)00076-5
39. Garrido MP, Hernandez A, Vega M, Araya E, Romero C. Conventional and new proposals of GnRH therapy for ovarian, breast, and prostatic cancers. Front Endocrinol (2023) 14. doi: 10.3389/fendo.2023.1143261
40. Song Y, Qin C, Zhang C, Peng Y, Yang W, Du Y, et al. GNRH family genes contributed to gender-specific disparity of bladder cancer prognosis through exerting opposite regulatory roles between males and females. J Cancer Res Clin Oncol (2023) 149:6827–40. doi: 10.1007/s00432-023-04640-2
41. Chen A, Kaganovsky E, Rahimipour S, Ben-Aroya N, Okon E, Koch Y. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: a putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res (2002) 62:1036–44.
42. Pazaitou-Panayiotou K, Chemonidou C, Poupi A, Koureta M, Kaprara A, Lambropoulou M, et al. Gonadotropin-releasing hormone neuropeptides and receptor in human breast cancer: correlation to poor prognosis parameters. Peptides (2013) 42:15–24. doi: 10.1016/j.peptides.2012.12.016
43. Günthert AR, Gründker C, Olota A, Läsche J, Eicke N, Emons G. Analogs of GnRH-I and GnRH-II inhibit epidermal growth factor-induced signal transduction and resensitize resistant human breast cancer cells to 4OH-tamoxifen. Eur J Endocrinol (2005) 153:613–25. doi: 10.1530/eje.1.01996
44. Eicke N, Gunthert AR, Viereck V, Siebold D, Behe M, Becker T, et al. GnRH-II receptor-like antigenicity in human placenta and in cancers of the human reproductive organs. EurJEndocrinol (2005) 153:605–12. doi: 10.1530/eje.1.02005
45. Jankowska AG, Andrusiewicz M, Fischer N, Warchol PJB. Expression of hCG and GnRHs and their receptors in endometrial carcinoma and hyperplasia. Int J Gynecol Cancer (2010) 20:92–101. doi: 10.1111/IGC.0b013e3181bbe933
46. Serin IS, Tanriverdi F, Ata CD, Akalin H, Ozcelik B, Ozkul Y, et al. GnRH-II mRNA expression in tumor tissue and peripheral blood mononuclear cells (PBMCs) in patients with Malignant and benign ovarian tumors. Eur J Obstet Gynecol Reprod Biol (2010) 149:92–6. doi: 10.1016/j.ejogrb.2009.11.009
47. Fister S, Gunthert AR, Aicher B, Paulini KW, Emons G, Grundker C. GnRH-II antagonists induce apoptosis in human endometrial, ovarian, and breast cancer cells via activation of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax. Cancer Res (2009) 69:6473–81. doi: 10.1158/0008-5472.CAN-08-4657
48. Enomoto M, Endo D, Kawashima S, Park MK. Human type II GnRH receptor mediates effects of GnRH on cell proliferation. Zoolog Sci (2004) 21:763–70. doi: 10.2108/zsj.21.763
49. Choi JH, Choi KC, Auersperg N, Leung PC. Differential regulation of two forms of gonadotropin-releasing hormone messenger ribonucleic acid by gonadotropins in human immortalized ovarian surface epithelium and ovarian cancer cells. EndocrRelatCancer (2006) 13:641–51. doi: 10.1677/erc.1.01057
50. Choi KC, Auersperg N, Leung PC. Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. JClinEndocrinolMetab (2001) 86:5075–8. doi: 10.1210/jcem.86.10.8100
51. Poon SL, An B-S, So W-K, Hammond GL, Leung PCK. Temporal recruitment of transcription factors at the 3’,5’-cyclic adenosine 5’-monophosphate-response element of the human GnRH-II promoter. Endocrinology (2008) 149:5162–71. doi: 10.1210/en.2008-0481
52. Hoo RLC, Chan KYY, Leung FKY, Lee LTO, Leung PCK, Chow BKC. Involvement of NF-κB subunit p65 and retinoic acid receptors, RARα and RXRα, in transcriptional regulation of the human GnRH II gene. FEBS J (2007) 274:2695–706. doi: 10.1111/j.1742-4658.2007.05804.x
53. Lee HJ, Snegovskikh VV, Park JS, Foyouzi N, Han KT, Hodgson EJ, et al. Role of GnRH-GnRH receptor signaling at the maternal-fetal interface. FertilSteril (2010) 94:2680–7. doi: 10.1016/j.fertnstert.2010.03.016
54. Sissung TM, Lochrin S, Liu T, Schmidt K, Strope J, Risdon E, et al. GNRH2 polymorphism in men with prostate cancer treated with androgen deprivation therapy. Anticancer Res (2023) 43:4023–30. doi: 10.21873/anticanres.16590
55. Darby S, Stockley J, Khan MM, Robson CN, Leung HY, Gnanapragasam VJ. Expression of GnRH type II is regulated by the androgen receptor in prostate cancer. EndocrRelatCancer (2007) 14:613–24. doi: 10.1677/ERC-07-0041
56. Montagnani Marelli M, Moretti RM, Mai S, Januszkiewicz-Caulier J, Motta M, Limonta P. Type I gonadotropin-releasing hormone receptor mediates the antiproliferative effects of GnRH-II on prostate cancer cells. JClinEndocrinolMetab (2009) 94:1761–7. doi: 10.1210/mend.23.4.9997
57. Fister S, Gunthert AR, Emons G, Grundker C. Gonadotropin-releasing hormone type II antagonists induce apoptotic cell death in human endometrial and ovarian cancer cells in vitro and in vivo. Cancer Res (2007) 67:1750–6. doi: 10.1158/0008-5472.CAN-06-3222
58. Park DW, Choi K-C, MacCalman CD, Leung PC. Gonadotropin-releasing hormone (GnRH)-I and GnRH-II induce cell growth inhibition in human endometrial cancer cells: Involvement of integrin beta3 and focal adhesion kinase. Reprod Biol Endocrinol (2009) 7:81. doi: 10.1186/1477-7827-7-81
59. Ammerpohl O, Pratschke J, Schafmayer C, Haake A, Faber W, von Kampen O, et al. Distinct DNA methylation patterns in cirrhotic liver and hepatocellular carcinoma. Int J Cancer (2012) 130:1319–28. doi: 10.1002/ijc.26136
60. Zhang C, Wang F, Guo F, Ye C, Yang Y, Huang Y, et al. A 13-gene risk score system and a nomogram survival model for predicting the prognosis of clear cell renal cell carcinoma. Urol Oncol (2020) 38:74.e1–74.e11. doi: 10.1016/j.urolonc.2019.12.022
61. Kushlinskii NE, Tivofeev YS, Generozov EV, Naumov VA, Soloviev YN, Boulytcheva IV, et al. Associations of single nucleotide polymorphisms with Malignant and borderline bone tumors. Klin Lab Diagn (2013), 58–60, 22–4.
62. Feng W, Wang Z, Feng D, Zhu Y, Zhang K, Huang W. The effects of common variants in MDM2 and GNRH2 genes on the risk and survival of osteosarcoma in Han populations from Northwest China. Sci Rep (2020) 10:15939. doi: 10.1038/s41598-020-72995-4
63. Kim KY, Choi KC, Park SH, Auersperg N, Leung PC. Extracellular signal-regulated protein kinase, but not c-Jun N-terminal kinase, is activated by type II gonadotropin-releasing hormone involved in the inhibition of ovarian cancer cell proliferation. JClinEndocrinolMetab (2005) 90:1670–7. doi: 10.1210/jc.2004-1636
64. Murányi J, Gyulavári P, Varga A, Bökönyi G, Tanai H, Vántus T, et al. Synthesis, characterization and systematic comparison of FITC-labelled GnRH-I, -II and -III analogues on various tumour cells. J Pept Sci (2016) 22:552–60. doi: 10.1002/psc.2904
65. Szabo I, Bosze S, Orban E, Sipos E, Halmos G, Kovacs M, et al. Comparative in vitro biological evaluation of daunorubicin containing GnRH-I and GnRH-II conjugates developed for tumor targeting. JPeptSci (2015) 21:426–35. doi: 10.1002/psc.2775
66. von Alten J, Fister S, Schulz H, Viereck V, Frosch K-H, Emons G, et al. GnRH analogs reduce invasiveness of human breast cancer cells. Breast Cancer Res Treat (2006) 100:13–21. doi: 10.1007/s10549-006-9222-z
67. Emons G, Gründker C. The role of gonadotropin-releasing hormone (GnRH) in endometrial cancer. Cells (2021) 10:292. doi: 10.3390/cells10020292
68. Zhao L, Liu N, Li X, Wang J, Wei L. Phosphatase and tensin homolog gene inhibits the effect induced by gonadotropin-releasing hormone subtypes in human endometrial carcinoma cells. Chin Med J (Engl) (2010) 123:1170–5. doi: 10.3760/cma.j.issn.0366-6999.2010.09.013
69. Wang AF, Li JH, Maiti K, Kim WP, Kang HM, Seong JY, et al. Preferential ligand selectivity of the monkey type-II gonadotropin-releasing hormone (GnRH) receptor for GnRH-2 and its analogs. MolCellEndocrinol (2003) 209:33–42. doi: 10.1016/j.mce.2003.08.004
70. Maiti K, Li JH, Wang AF, Acharjee S, Kim WP, Im WB, et al. GnRH-II analogs for selective activation and inhibition of non-mammalian and type-II mammalian GnRH receptors. MolCells (2003) 16:173–9.
71. Emons G, Schroder B, Ortmann O, Westphalen S, Schulz KD, Schally AV. High affinity binding and direct antiproliferative effects of luteinizing hormone-releasing hormone analogs in human endometrial cancer cell lines. JClinEndocrinolMetab (1993) 77:1458–64. doi: 10.1210/jcem.77.6.8263128
72. Wu HM, Cheng JC, Wang HS, Huang HY, MacCalman CD, Leung PC. Gonadotropin-releasing hormone type II induces apoptosis of human endometrial cancer cells by activating GADD45alpha. Cancer Res (2009) 69:4202–8. doi: 10.1158/0008-5472.CAN-08-4591
73. Wu H-M, Wang H-S, Huang H-Y, Lai C-H, Lee C-L, Soong Y-K, et al. Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the invasiveness of endometrial cancer cells through the GnRH-I receptor and mitogen-activated protein kinase (MAPK)-dependent activation of matrix metalloproteinase (MMP)-2. BMC Cancer (2013) 13:300. doi: 10.1186/1471-2407-13-300
74. Cho-Clark MJ, Watkins A, Wu TJ. The role of GnRH metabolite, GnRH-(1-5), in endometrial cancer. Front Endocrinol (2023) 14. doi: 10.3389/fendo.2023.1183278
75. Robboy SJ, Bentley RC, Butnor K, Anderson MC. Pathology and pathophysiology of uterine smooth-muscle tumors. Environ Health Perspect (2000) 108:779–84. doi: 10.1289/ehp.00108s5779
76. Parker JD, Malik M, Catherino WH. Human myometrium and leiomyomas express gonadotropin-releasing hormone 2 and gonadotropin-releasing hormone 2 receptor. FertilSteril (2007) 88:39–46. doi: 10.1016/j.fertnstert.2006.11.098
77. Poon SL, Hammond GT, Leung PC. Epidermal growth factor-induced GnRH-II synthesis contributes to ovarian cancer cell invasion. MolEndocrinol (2009) 23:1646–56. doi: 10.1210/me.2009-0147
78. Kim KY, Choi KC, Auersperg N, Leung PC. Mechanism of gonadotropin-releasing hormone (GnRH)-I and -II-induced cell growth inhibition in ovarian cancer cells: role of the GnRH-I receptor and protein kinase C pathway. EndocrRelatCancer (2006) 13:211–20. doi: 10.1677/erc.1.01033
79. Kim KY, Choi KC, Park SH, Chou CS, Auersperg N, Leung PC. Type II gonadotropin-releasing hormone stimulates p38 mitogen-activated protein kinase and apoptosis in ovarian cancer cells. JClinEndocrinolMetab (2004) 89:3020–6. doi: 10.1210/jc.2003-031871
80. Reutter M, Emons G, Gründker C. Starving tumors: inhibition of glycolysis reduces viability of human endometrial and ovarian cancer cells and enhances antitumor efficacy of GnRH receptor-targeted therapies. Int J Gynecol Cancer (2013) 23:34–40. doi: 10.1097/IGC.0b013e318275b028
81. Chen CL, Cheung LW, Lau MT, Choi JH, Auersperg N, Wang HS, et al. Differential role of gonadotropin-releasing hormone on human ovarian epithelial cancer cell invasion. Endocrine (2007) 31:311–20. doi: 10.1007/s12020-007-0041-8
82. Ling Poon S, Lau MT, Hammond GL, Leung PC. Gonadotropin-releasing hormone-II increases membrane type I metalloproteinase production via beta-catenin signaling in ovarian cancer cells. Endocrinology (2011) 152:764–72. doi: 10.1210/en.2010-0942
83. Kang SK, Cheng KW, Nathwani PS, Choi K-C, Leung PCK. Autocrine role of gonadotropin-releasing hormone and its receptor in ovarian cancer cell growth. Endocrine (2000) 13:297–304. doi: 10.1385/ENDO:13:3:297
84. Poon SL, Klausen C, Hammond GL, Leung PC. 37-kDa laminin receptor precursor mediates GnRH-II-induced MMP-2 expression and invasiveness in ovarian cancer cells. MolEndocrinol (2011) 25:327–38. doi: 10.1210/me.2010-0334
85. Maudsley S, Davidson L, Pawson AJ, Chan R, Lopez de Maturana R, Millar RP. Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res (2004) 64:7533–44. doi: 10.1158/0008-5472.CAN-04-1360
86. Gu L, Li X, Liu W. Adverse cardiovascular effect following gonadotropin-releasing hormone antagonist versus GnRH agonist for prostate cancer treatment: A systematic review and meta-analysis. Front Endocrinol (2023) 14. doi: 10.37766/inplasy2023.2.0009
87. Shiota M, Fujimoto N, Takeuchi A, Kashiwagi E, Dejima T, Inokuchi J, et al. The association of polymorphisms in the gene encoding gonadotropin-releasing hormone with serum testosterone level during androgen deprivation therapy and prognosis of metastatic prostate cancer. J Urol (2018) 199:734–40. doi: 10.1016/j.juro.2017.09.076
88. Kim DK, Yang JS, Maiti K, Hwang JI, Kim K, Seen D, et al. A gonadotropin-releasing hormone-II antagonist induces autophagy of prostate cancer cells. Cancer Res (2009) 69:923–31. doi: 10.1158/0008-5472.CAN-08-2115
89. Park S, Han JM, Cheon J, Hwang JI, Seong JY. Apoptotic death of prostate cancer cells by a gonadotropin-releasing hormone-II antagonist. PloS One (2014) 9:e99723. doi: 10.1371/journal.pone.0099723
90. Masters JR. HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer (2002) 2:315–9. doi: 10.1038/nrc775
92. Lin YM, Poon SL, Choi JH, Lin JS, Leung PC, Huang BM. Transcripts of testicular gonadotropin-releasing hormone, steroidogenic enzymes, and intratesticular testosterone levels in infertile men. FertilSteril (2008) 90:1761–8. doi: 10.1016/j.fertnstert.2007.08.078
93. Desaulniers AT, Cederberg RA, Mills GA, Ford JJ, Lents CA, White BR. LH-independent testosterone secretion is mediated by the interaction between GnRH2 and its receptor within porcine testes. BiolReprod (2015) 93:45. doi: 10.1095/biolreprod.115.128082
Keywords: GnRH2, GnRHR2, reproductive cancer, breast, prostate, endometrial, ovarian, placental
Citation: Desaulniers AT and White BR (2024) Role of gonadotropin-releasing hormone 2 and its receptor in human reproductive cancers. Front. Endocrinol. 14:1341162. doi: 10.3389/fendo.2023.1341162
Received: 19 November 2023; Accepted: 13 December 2023;
Published: 08 January 2024.
Edited by:
Tatiana Fiordelisio, National Autonomous University of Mexico, MexicoReviewed by:
Rahul Pal, National Institute of Immunology (NII), IndiaPayam Behzadi, Islamic Azad University, ShahreQods, Iran
Copyright © 2024 Desaulniers and White. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy T. Desaulniers, ZGVzYXVsbmllcnNAdW5sLmVkdQ==
 Amy T. Desaulniers
Amy T. Desaulniers Brett R. White
Brett R. White