- Department of Orthopaedics, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Editorial on the Research Topic
Bone-organ axis: an expanding universe
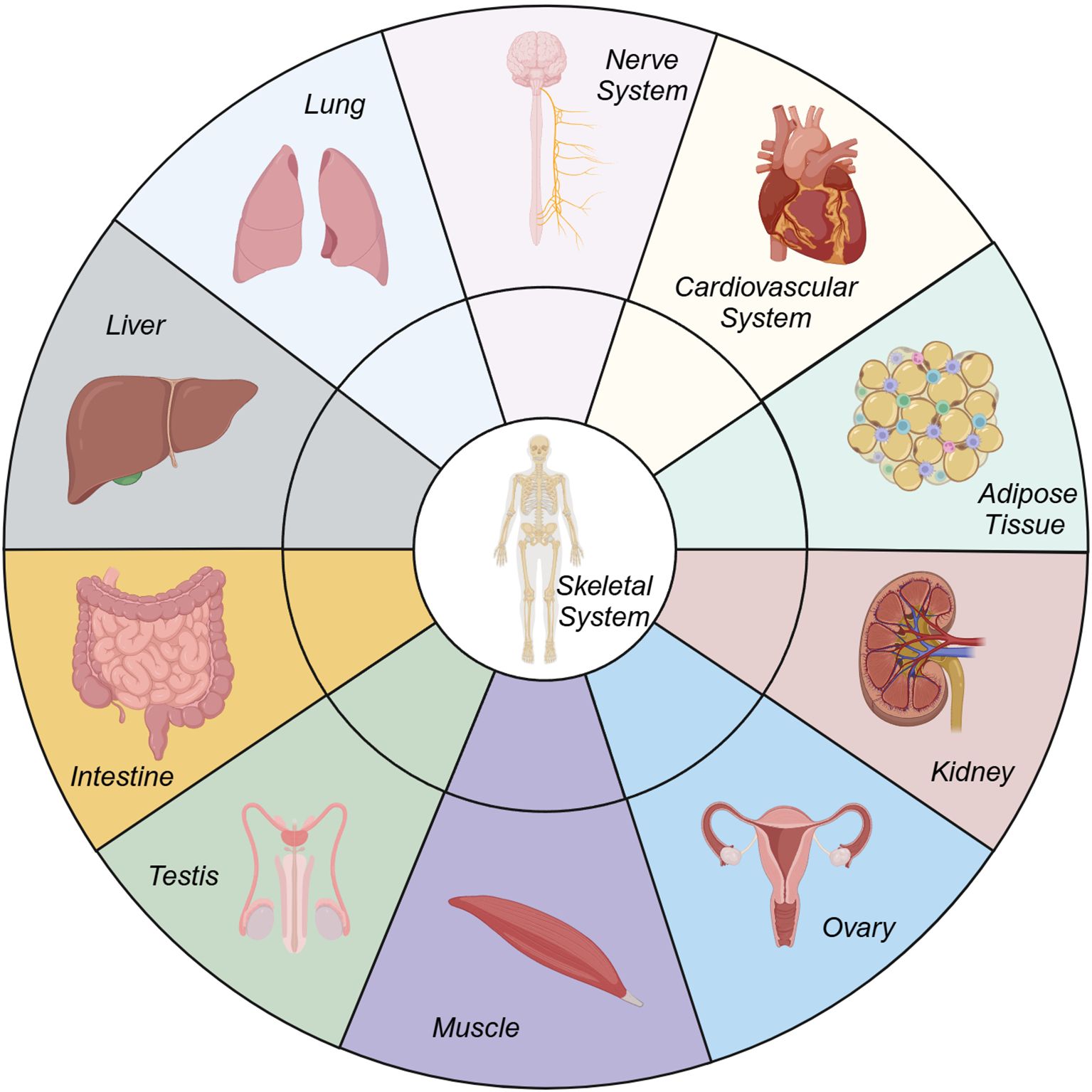
The musculoskeletal system, long regarded as a biomechanical scaffold, is increasingly recognized as a dynamic player in systemic physiology, profoundly interconnected with other organ systems (Figure 1). From a physiological perspective, bone functions as an endocrine organ that coordinates bodily functions, thereby enhancing the perception of the surrounding environment and improving athletic performance (1). Pathologically, degenerative musculoskeletal diseases such as osteoporosis, osteoarthritis (OA) and intervertebral disc degeneration (IVDD) are not merely localized bone/cartilage disorders but manifestations of broader systemic imbalances such as metabolic syndrome (2), sarcopenia (3, 4), senescence (5), and systemic inflammation. The crosstalk between bone and other organs—mediated by a complex interplay of biochemical signals, metabolic pathways, and endocrine functions—offers new perspectives on the pathogenesis of these diseases and potential avenues for therapeutic innovation. This Research Topic of Frontiers in Endocrinology explores these intricate relationships, with a particular emphasis on the bone-organ axis.
The bone-endocrine interface: a central regulator
Osteokines such as osteocalcin, fibroblast growth factor 23 (FGF23), and sclerostin serve as critical mediators in the crosstalk between bone and other organs. For instance, osteocalcin, particularly its undercarboxylated form, has been shown in animal studies to regulate glucose metabolism and insulin sensitivity in peripheral tissues, thereby establishing a link between bone health and metabolic disorders like diabetes mellitus (DM) (6, 7). Of note, these effects have yet to be conclusively demonstrated in human. Conversely, metabolic derangements seen in DM, such as chronic hyperglycemia and advanced glycation end products (AGEs), impair bone remodeling, increase fracture risk, and exacerbate degenerative joint diseases (8, 9). In this Research Topic, the link between metabolic dysfunction and bone disorders was revealed in several publications. Azami et al. conducted a systematic review that underscored the reciprocal relationship by identifying musculoskeletal complications as underdiagnosed yet significant comorbidities in DM. Additionally, Zhang et al. demonstrated an association between primary frozen shoulder and dyslipidemia, especially in individuals with underlying conditions such as diabetes and thyroid dysfunction, suggesting a potential metabolic contribution to its pathogenesis. Furthermore, Fu et al. reviewed the mechanisms by which gut microbiota metabolites influence bone health. Collectively, these findings advocate for a comprehensive approach to musculoskeletal health that incorporates metabolic regulation as a fundamental component.
Bone-muscle crosstalk: a synergistic partnership
The relationship between bone and muscle extends beyond mere mechanical interaction to encompass biochemical and endocrine communication. Myokines, such as irisin, which are released during muscle contraction, play a significant role in influencing bone density and remodeling by promoting osteoblast activity (10). Conversely, signals derived from bone, including transforming growth factor-beta (TGF-β) and sclerostin, modulate muscle mass and function (11). This bidirectional relationship highlights the interdependence of muscle and bone in the maintenance of musculoskeletal integrity. Disruption of this balance contributes to conditions such as sarcopenia and osteoporosis, which frequently coexist and exacerbate the progression of degenerative diseases. In their review of the current literature, Hurly-Novatny et al. summarized the implications of Duchenne muscular dystrophy (DMD) on bone complications. Li et al. investigated the relationship between degeneration of cervical intervertebral disc and paravertebral muscles. Additionally, Jiang et al. explored the potential link between fat infiltration in paraspinal muscles and spinal degeneration.
The bone-adipose tissue axis: inflammatory mediators in degeneration
Adipose tissue plays a dual role in musculoskeletal health through the secretion of adipokines like leptin, adiponectin, and resistin. While leptin enhances osteoblast activity and bone formation under physiological conditions, its overexpression in obesity contributes to low-grade systemic inflammation, accelerating bone resorption and cartilage degeneration (12–14). In the context of IVDD, He et al. reviewed the role of adipokines in mediating the inflammatory and catabolic cascades that disrupt intervertebral disc homeostasis. Adiponectin, traditionally regarded as protective, displays context-dependent effects, highlighting the nuanced interplay between adipose tissue and skeletal structures. Targeting this axis through modulation of adipokine signaling holds promise for mitigating the impact of obesity on degenerative musculoskeletal diseases.
Bone and the central nervous system: the neural-endocrine loop
Emerging research also highlights a neural-endocrine connection between bone and the central nervous system (CNS). Sensory neurons in bone regulate bone remodeling via neuromodulators such as calcitonin gene-related peptide (CGRP) and substance P (15–17). Simultaneously, bone-derived osteocalcin crosses the blood-brain barrier, influencing memory and mood through CNS pathways. In degenerative conditions like OA and IVDD, chronic pain originating from bone and joint pathology can alter neural signaling, leading to a feedback loop of neurogenic inflammation that exacerbates tissue damage (18, 19). Understanding this connection opens the door to integrated treatments addressing both nociceptive and systemic contributors to disease progression.
Bone and other organs: the expanding horizon
The influence of bone extends beyond muscle, adipose tissue, and the central nervous system to encompass additional physiological systems. Bone marrow functions as a reservoir for immune cells, while inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), exert a direct impact on bone resorption (20). Chronic systemic inflammation, commonly observed in autoimmune diseases, accelerates degenerative changes in both bone and cartilage. Notably, a Mendelian randomization study conducted by Li et al. established a link between hepatitis B virus (HBV) infection and the development of osteoporosis. Furthermore, bone-derived fibroblast growth factor 23 (FGF23) plays a critical role in regulating phosphate homeostasis and vitamin D metabolism, thereby creating a feedback loop with renal function (21, 22). Impaired renal function disrupts mineral metabolism, which contributes to bone loss and vascular calcification. Vascular calcification, a characteristic feature of atherosclerosis, is associated with dysregulated pathways of bone mineralization, mediated by common factors such as matrix Gla-protein and osteoprotegerin (23). These interconnected pathways highlight the systemic nature of both cardiovascular and skeletal diseases. Additionally, the role of bone in reproductive health has gained increasing attention. Osteocalcin, beyond its metabolic functions, has been implicated in the regulation of male gonadal function. Notably, Oury et al. demonstrated that osteocalcin acts through a pancreas-bone-testis axis to influence testosterone synthesis and fertility in both mice and humans (24). This emerging axis underscores the endocrine capacity of bone in orchestrating systemic physiological processes beyond traditional musculoskeletal functions.
Implications for precision medicine
The insights presented in this Research Topic emphasize the need for a systemic perspective in understanding and treating degenerative musculoskeletal diseases. Integrating bone health into the broader context of metabolic, endocrine, and inflammatory regulation offers a paradigm shift in degenerative disease management. Thus, the future of research lies in unraveling the molecular mechanisms of bone-organ interactions using advanced technologies like single-cell sequencing and organ-on-a-chip models. Longitudinal studies are needed to establish causal links and identify early biomarkers of systemic dysregulation. Finally, translating these findings into clinical practice requires multidisciplinary collaborations that integrate endocrinology, orthopedics, rheumatology, and metabolic medicine. By recognizing the bone-organ axis as a central player in systemic health, this Research Topic of Frontiers in Endocrinology sets the stage for holistic approaches to understanding and managing degenerative musculoskeletal diseases.
Author contributions
PZ: Writing – original draft, Visualization. YW: Writing – original draft, Visualization. YG: Writing – review & editing. XL: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Distinguished Young Scholars Fund of the National Natural Science Foundation of China and the Young Scientists Fund of the National Natural Science Foundation of China (82302740).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berger JM, Singh P, Khrimian L, Morgan DA, Chowdhury S, Arteaga-Solis E, et al. Mediation of the acute stress response by the skeleton. Cell Metab. (2019) 30:890–902. doi: 10.1016/j.cmet.2019.08.012
2. Farrag Y, Farrag M, Varela-Garcia M, Torrijos-Pulpon C, Capuozzo M, Ottaiano A, et al. Adipokines as potential pharmacological targets for immune inflammatory rheumatic diseases: Focus on rheumatoid arthritis, osteoarthritis, and intervertebral disc degeneration. Pharmacol Res. (2024) 205:107219. doi: 10.1016/j.phrs.2024.107219
3. Jin Z, Wang R, Jin L, Wan L, and Li Y. Causal relationship between sarcopenia with osteoarthritis and the mediating role of obesity: a univariate, multivariate, two-step Mendelian randomization study. BMC Geriatr. (2024) 24:469. doi: 10.1186/s12877-024-05098-8
4. Kitsuda Y, Wada T, Tanishima S, Osaki M, Nagashima H, and Hagino H. Impact of sarcopenia on spinal spondylosis: A literature review. J Clin Med. (2023) 12(16):5401. doi: 10.3390/jcm12165401
5. Liu X and Wan M. A tale of the good and bad: Cell senescence in bone homeostasis and disease. Int Rev Cell Mol Biol. (2019) 346:97–128. doi: 10.1016/bs.ircmb.2019.03.005
6. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. (2010) 142:296–308. doi: 10.1016/j.cell.2010.06.003
7. Kanazawa I. Osteocalcin as a hormone regulating glucose metabolism. World J Diabetes. (2015) 6:1345–54. doi: 10.4239/wjd.v6.i18.1345
8. Giri B, Dey S, Das T, Sarkar M, Banerjee J, and Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. BioMed Pharmacother. (2018) 107:306–28. doi: 10.1016/j.biopha.2018.07.157
9. Wang B and Vashishth D. Advanced glycation and glycoxidation end products in bone. Bone. (2023) 176:116880. doi: 10.1016/j.bone.2023.116880
10. Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell. (2018) 175:1756–68. doi: 10.1016/j.cell.2018.10.025
11. Girardi F, Taleb A, Ebrahimi M, Datye A, Gamage DG, Peccate C, et al. TGFbeta signaling curbs cell fusion and muscle regeneration. Nat Commun. (2021) 12:750. doi: 10.1038/s41467-020-20289-8
12. Motyl KJ and Rosen CJ. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie. (2012) 94:2089–96. doi: 10.1016/j.biochi.2012.04.015
13. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. (2002) 111:305–17. doi: 10.1016/s0092-8674(02)01049-8
14. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. (2005) 434:514–20. doi: 10.1038/nature03398
15. Xiao Y, Han C, Wang Y, Zhang X, Bao R, Li Y, et al. Interoceptive regulation of skeletal tissue homeostasis and repair. Bone Res. (2023) 11:48. doi: 10.1038/s41413-023-00285-6
16. Appelt J, Baranowsky A, Jahn D, Yorgan T, Kohli P, Otto E, et al. The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine. (2020) 59:102970. doi: 10.1016/j.ebiom.2020.102970
17. Niedermair T, Schirner S, Seebroker R, Straub RH, and Grassel S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci Rep. (2018) 8:9199. doi: 10.1038/s41598-018-27432-y
18. Sun K, Jiang J, Wang Y, Sun X, Zhu J, Xu X, et al. The role of nerve fibers and their neurotransmitters in regulating intervertebral disc degeneration. Ageing Res Rev. (2022) 81:101733. doi: 10.1016/j.arr.2022.101733
19. Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. (2023) 8:56. doi: 10.1038/s41392-023-01330-w
20. Azuma Y, Kaji K, Katogi R, Takeshita S, and Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. (2000) 275:4858–64. doi: 10.1074/jbc.275.7.4858
21. Guo Y and Yuan Q. Fibroblast growth factor 23 and bone mineralisation. Int J Sci. (2015) 7:8–13. doi: 10.1038/ijos.2015.1
22. Agoro R and White KE. Regulation of FGF23 production and phosphate metabolism by bone-kidney interactions. Nat Rev Nephrol. (2023) 19:185–93. doi: 10.1038/s41581-022-00665-x
23. Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, and Liakopoulos V. Biomarkers of vascular calcification in serum. Adv Clin Chem. (2020) 98:91–147. doi: 10.1016/bs.acc.2020.02.004
Keywords: bone metabolism, degenerative diseases, musculoskeletal disorder, organ crosstalk, endocrine dysfunction
Citation: Zhang P, Wang Y, Guo Y and Liu X (2025) Editorial: Bone-organ axis: an expanding universe. Front. Endocrinol. 16:1574370. doi: 10.3389/fendo.2025.1574370
Received: 10 February 2025; Accepted: 22 May 2025;
Published: 05 June 2025.
Edited and Reviewed by:
Alberto Falchetti, Grande Ospedale Metropolitano Niguarda, ItalyCopyright © 2025 Zhang, Wang, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaonan Liu, bGl1X2xpdXhuQHFxLmNvbQ==
 Peilin Zhang
Peilin Zhang Yicheng Wang
Yicheng Wang Xiaonan Liu
Xiaonan Liu