- 1Sport and Health, Guangzhou Sport University, Guangzhou, China
- 2Research Center for Innovative Development of Sports and Healthcare Integration, Guangzhou Sport University, Guangzhou, China
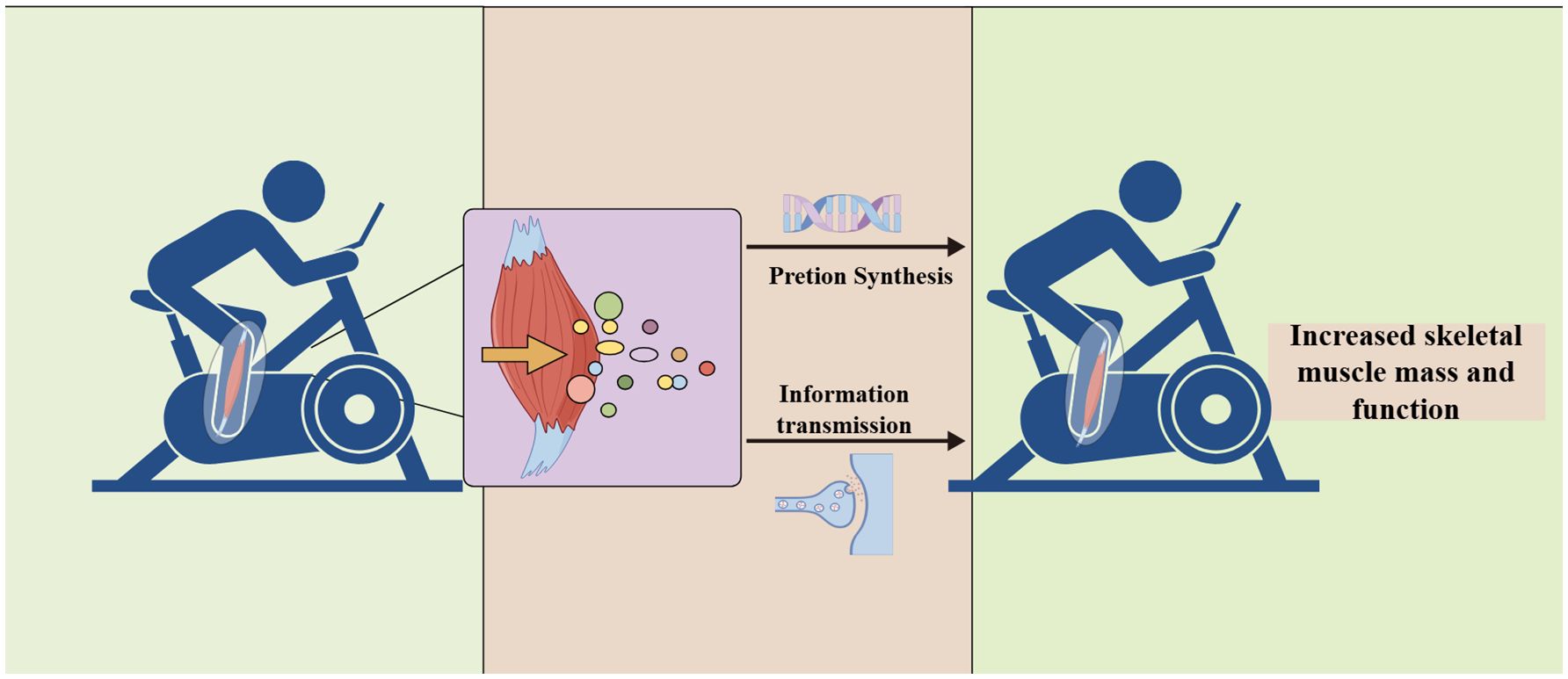
Aging sarcopenia is an unavoidable condition that affects the majority of older adults in their later years. Exercise has been extensively researched as an effective intervention for sarcopenia. In particular, the release of exerkines and myokines during physical activity has beneficial effects on the body, which, as mediators, offer a novel therapeutic strategy for elucidating how exercise enhances skeletal muscle mass and function. In this review article, we summarize how exerkines exert protective effects on aging skeletal muscle mainly through the following mechanisms: (1) mediating energy diversion to skeletal muscle, ensuring more energy supply to the muscle; (2) enhancing the activity of skeletal muscle satellite cells to promote muscle repair and regeneration; (3) upregulating the expression of genes associated with muscle regeneration and, at the same time, inhibiting the expression of those genes that contribute to the atrophy of skeletal muscle; and (4) improving the function of the neuromuscular junction to improve the neural control of skeletal muscle. These combined effects constitute the protective mechanism of myokines on aging skeletal muscle.
1 Introduction
The growing number and proportion of older individuals within the total population is one of the most significant demographic challenges worldwide. The United Nations projects that, by 2030, 16.9% of China’s population will be over the age of 65, while the global elderly population is anticipated to exceed 1.5 billion by 2050 (1, 2). The U.S. Census Bureau and the National Center for Health Statistics predict that by 2040, 80.8 million Americans will be 65 years old, representing approximately 21.6% of citizens. Among them, 14.4 million will be 85 years old, which will be a 123% increase from 2017 (3). With the improvement of people’s life expectancy, society has entered the stage of aging gradually, which results in various diseases in the elderly and leads to the aggravation of the social economy and medical burdens (4). Additionally, aging is a multifaceted biological process characterized by a progressive decline in physiological function and an increased susceptibility to disease, and this process is accompanied by both functional and structural changes within the organism (1), such as increased genetic instability, loss of protein homeostasis, and cellular senescence-induced neurodegenerative changes during aging (5). However, decreases in skeletal muscle mass and strength are common in the older population. This progressive decline, known as sarcopenia, leads to impaired physical mobility and disability in older adults (6). The pathogenesis of aging sarcopenia is accompanied by a reduction in skeletal muscle mass and impaired contractile function and it also involves systemic metabolic, inflammatory, and endocrine abnormalities (7). When the rate of protein decomposition in skeletal muscle exceeds its rate of synthesis, this imbalance leads to muscle atrophy; thus, maintaining protein homeostasis during aging is essential to prevent muscle loss (8, 9). However, with aging, a combination of disturbances in muscle homeostasis and neuronal degeneration results in the preferential loss of type II (fast) muscle fibers. This selective loss is accompanied by a reduction in motor units, which further exacerbates the weakening of muscle strength, ultimately resulting in muscle weakness and bradykinesia (10, 11).
Exercise, as a non-pharmacological intervention, has great potential to improve age-related diseases (12). In particular, resistance exercise is effective in activating the nervous system and accelerating muscle protein synthesis to increase skeletal muscle mass and strength (13). During exercise, skeletal muscles secrete various molecules that participate in the crosstalk between organs and play an active role in neurological, metabolic, cardiovascular, and immune processes (12, 14). These small molecules synthesized and secreted by skeletal muscle are known as myokines (15). Furthermore, during and/or after exercise, peptides, metabolites, and nucleic acids released into circulation are exerkines (16), which regulate numerous physiological and pathological processes within the body, ultimately influencing metabolism to promote health (17). Some skeletal muscles secrete factors that are both exerkines and myokines, such as interleukin-6 (IL-6), irisin, fibroblast growth factor 21 (FGF21), and brain-derived neurotrophic factor (BDNF) (17). Mobility exerkines are key molecules mediating the link between exercise, metabolism, and inflammation, and minor changes induced by exercise may affect the whole body (17).
Exerkines have ameliorative effects on age-related diseases and may be a potential avenue for improving them through exercise. Of these, myokines are most closely related to skeletal muscle (18, 19). Therefore, the aim of this paper is to review the research progress on exerkines and aging sarcopenia in recent years, to explore the causal relationship between sarcopenia and myokines’ plasma levels, and to provide a reference for in-depth research on the homeostasis of skeletal muscle and rejuvenation therapy of skeletal muscle in the elderly.
2 Overview of aging sarcopenia
Sarcopenia was first recognized as an age-related loss of lean body mass, and in 2010, it was recognized as a separate condition (20). The European Working Group on Sarcopenia in Older People (EWGSOP) defines sarcopenia as a progressive, generalized skeletal muscle disease involving reduced muscle mass and dysfunction, the prevalence of which increases with age (21, 22). According to its pathogenesis, it can be categorized as primary sarcopenia, which is a loss of muscle mass and dysfunction associated with aging, or secondary sarcopenia, which has significant predisposing factors, such as chronic diseases and malnutrition (23, 24). Sarcopenia evolves from muscle atrophy to muscle dysfunction and then to muscle strength decline (21). In addition, we should also emphasize the difference between sarcopenia and skeletal muscle atrophy. Muscle atrophy occurs when muscle mass and fiber size decrease (25). At this point, it is important to emphasize that sarcopenia is a progressive decline in muscle mass, strength, and function with age due to environmental or genetic factors (26). However, both conditions involve reductions in skeletal muscle mass and muscle fiber size, and both are associated with an imbalance between protein synthesis and degradation within the muscle fibers (25). The mechanisms of skeletal muscle atrophy involve the ubiquitin–proteasome and autophagy-lysosome machinery, IGF1-protein kinase B-forkhead box O (IGF1–AKT–FoxO) signaling, inflammatory cytokines, nuclear factor-kappa B (NF-kB) signaling, and other signaling pathways. The underlying mechanisms of sarcopenia have been extensively studied, and we can confirm that reduced physical activity, hormonal imbalances, decreased absorption, and chronic inflammation affect the release of myokines and influence the development of sarcopenia (27).
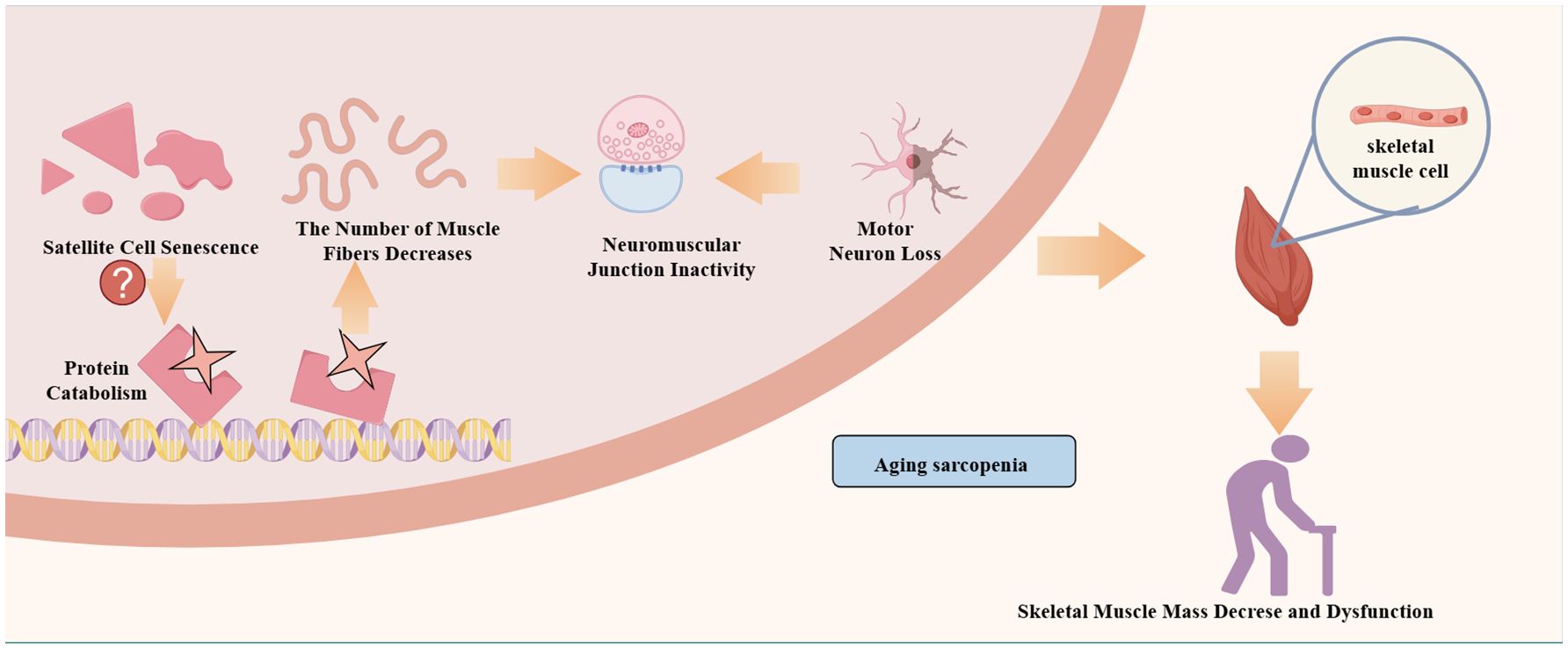
Additionally, the pathogenesis of aging sarcopenia may involve satellite cell senescence, motor neuron loss, neuromuscular junction inactivity, mitochondrial function, hormonal status, and abnormal muscle factor production (28) (Figure 1). First, with aging, protein synthesis and catabolism are abnormal. When skeletal muscle protein catabolism exceeds synthesis, a negative protein balance occurs with muscle atrophy (8). Second, the breakdown of muscle homeostasis and neuronal degeneration lead to satellite cell senescence and a reduction in the number and size of skeletal muscles (11, 29, 30). Finally, mitochondria play a crucial role in muscle mass and function, and their dysfunction is a driver of sarcopenia (29, 31–33). Changes in the above factors also affect patient mobility. However, physical activity and exercise are effective countermeasures against skeletal muscle aging and delay or prevent metabolic muscle damage (34). Because exercise stimulates and promotes skeletal muscle contraction, it releases a variety of myokines that maintain skeletal muscle mass and enhance skeletal muscle regeneration (18, 35). Furthermore, changing myokine signaling in patients with sarcopenia leads to muscle atrophy and decreased fitness, while muscle atrophy also decreases myokine expression (36, 37).
3 Overview of exerkines
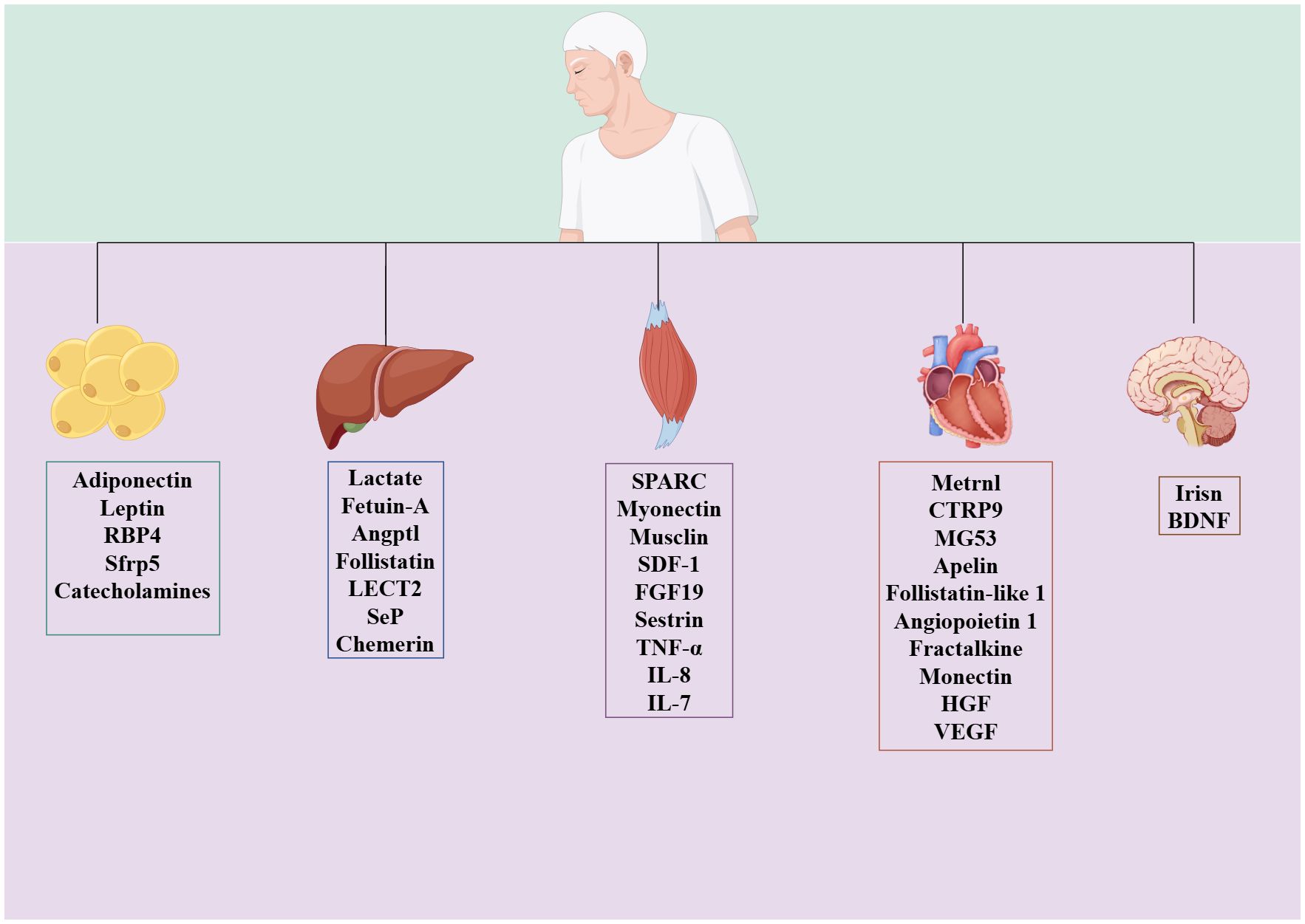
During or after exercise, tissues secrete different kinds of peptides, lipids, and nucleic acid substances, which are exerkines (16). Based on the site of exerkine release, researchers have designated exerkines released from skeletal muscle as myokines, those from the heart as cardiokines, those from the liver as hepatokines, those from white adipose tissue as adipokines, those from brown adipose tissue as batokines, and those from neurons as neurokines (16) (Figure 2). Exerkines can be secreted directly into the circulation or indirectly with the help of extracellular vehicles. Meanwhile, their molecular targets and receptors are found throughout the whole body, including the heart, brain, pancreas, bones, fat tissue, immune system, and skeletal muscle (38).
Human and animal experiments have found that exerkines circulate in the blood to achieve the crosstalk between organs and tissues. In the cardiovascular system, exerkines enhance the metabolic health in the heart (39). The majority of cardiokines are also considered to be important mediators in maintaining cardiac homeostasis and responding to myocardial injury (40). Exercise promotes the secretion of cathepsin B from skeletal muscle, which, together with BDNF, mediates neuronal maturation and improves cognitive function in the brain (41). The beneficial effects of exerkines on the brain may manifest as improvements in mitochondrial function, reductions in oxidative damage, maintenance of protein homeostasis, and promotion of synaptic plasticity (42). Exerkines are involved in the function of osteoblasts and osteoclasts and improve metabolic disorders. For example, irisin and BAIBA promote bone anabolism, while myostatin (MSTN) affects the activity of osteoblasts and osteoclasts, and promotes bone catabolism (43, 44).
Skeletal myogenesis is a multistep process involving the proliferation, migration, and differentiation of myoblasts (45). Exerkines are involved in the process of skeletal myogenesis, and even a single exerkine acting on multiple organs promotes skeletal myogenesis synergistically (46). For example, irisin affects adipose tissue and pancreatic function and coordinates the energy supply to skeletal muscle indirectly (17). Various exerkines act synergistically on a single organ to maintain homeostasis in the internal environment of skeletal muscle. Presently, we are unable to elucidate the specific mechanisms and effects of these actions, which limits our ability to maximize the efficacy of exerkine interventions.
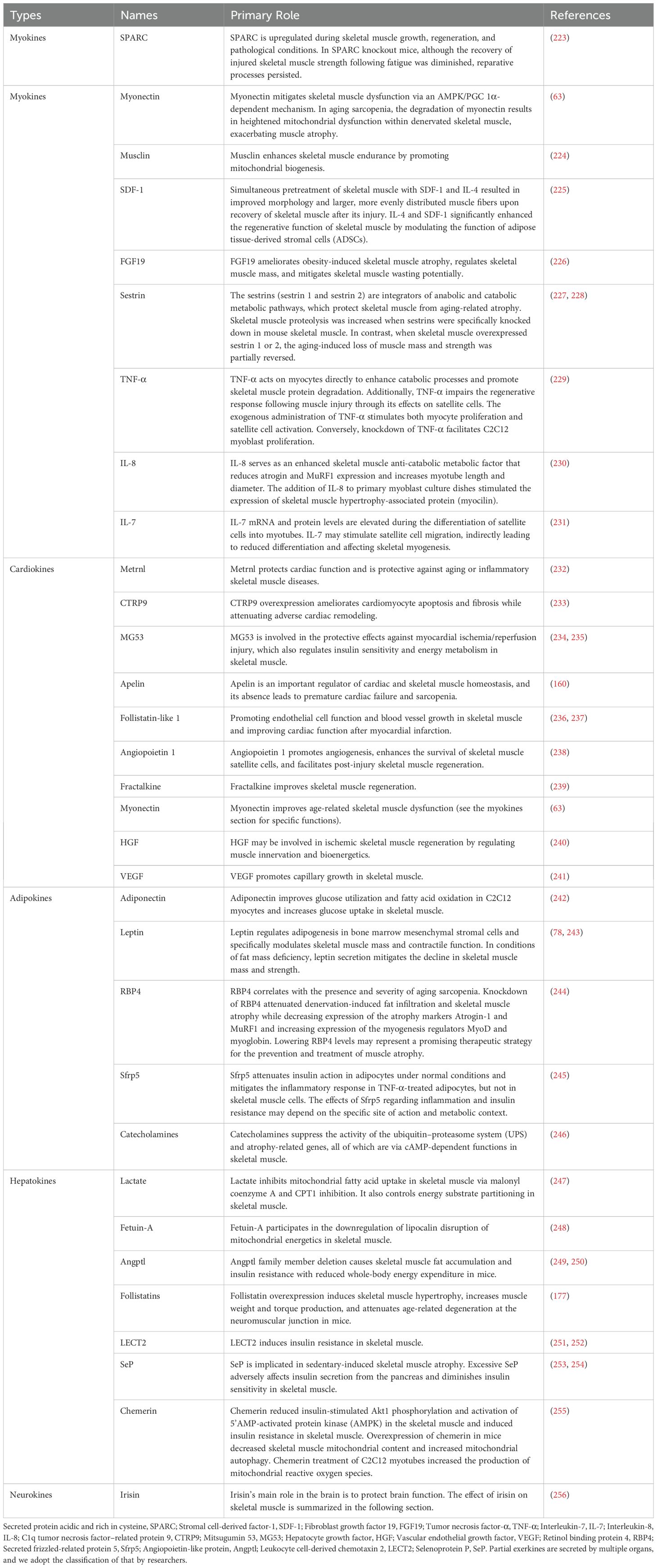
Exerkines are intricately associated with skeletal muscle function, and the identification or isolation of exerkines may offer a novel strategy for specific diseases. Because of the diversity of exerkines, we classified them according to their release sites (see Table 1). Additionally, we conducted a comprehensive search of databases such as PubMed, Web of Science, and Sci-Hub to provide an extensive overview of the molecular relationship between exercise and skeletal muscle. For the myokines that have been studied extensively and have great potential for treating aging sarcopenia, we will provide a detailed explanation of their role in sarcopenia in the following section.
4 Overview of myokines
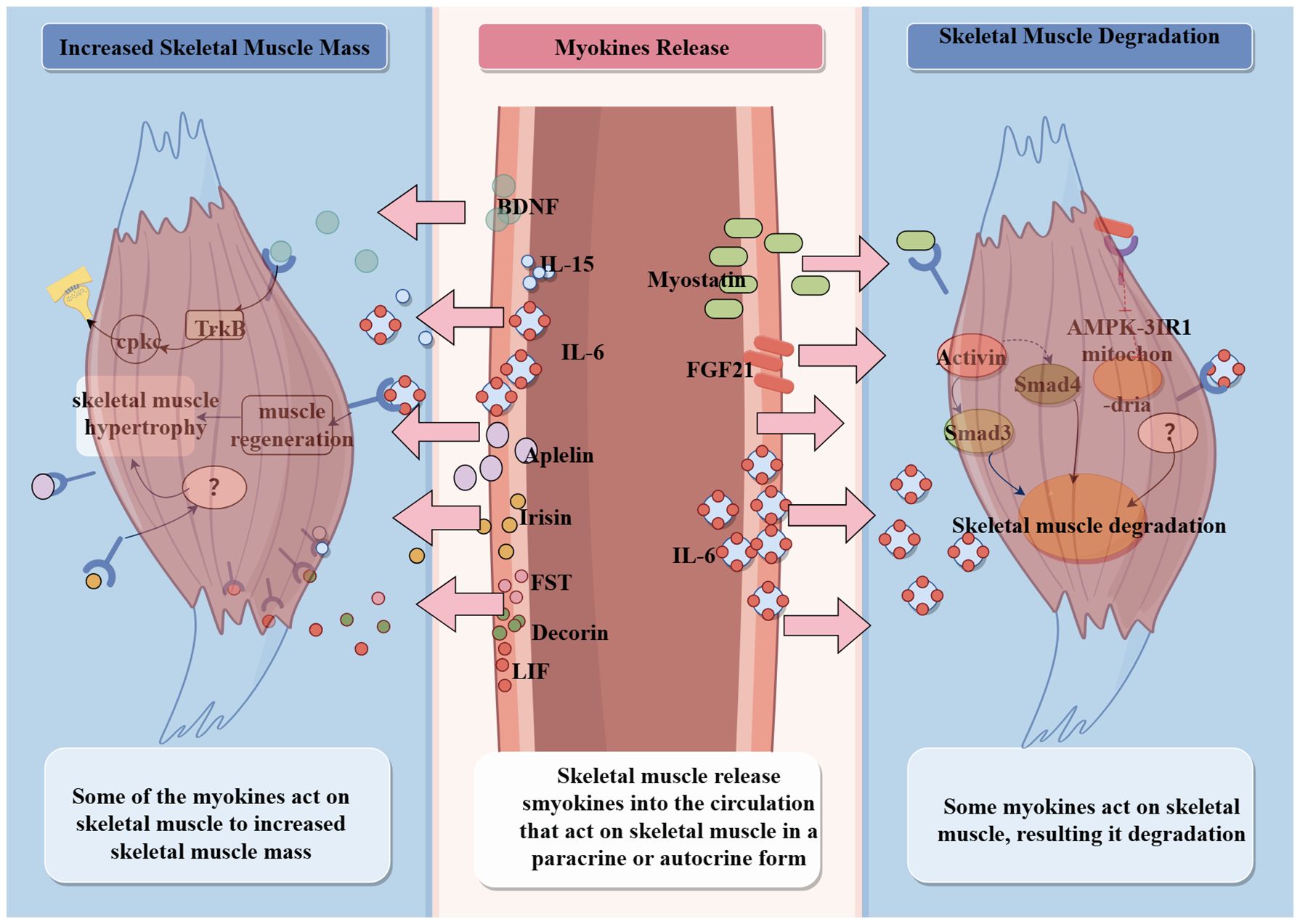
Skeletal muscle is one of the endocrine organs, and myocytes synthesize and secrete a variety of cytokines during contraction, named myokines, which exert autocrine and paracrine effects (15, 47). Multiple myokines have been identified by targeted analysis of skeletal muscle biopsy protein levels in a single acute exercise or long-term post-exercise population (18). Changes in the abundance of different myokines in the intermuscular fluid can be observed after exercise stimulation, and different exercise types stimulate different kinds of myokines (18). For example, muscles performing centripetal contractions induce the release of IL-6, interleukin-8 (IL-8), etc.; strength training induces the production of interleukin-15 (IL-15). A single exercise induces the release of IL-6, IL-1ra, and IL-8, and multiple strenuous exercises induce the release of tumor necrosis factor-α (TNF-α) (47, 48). In addition, different muscle fibers release different types of myokines; for example, glycolytic fibers mainly produce actin, angiopoietin, and Muscarinic Acetylcholine Receptors (mAChRs), whereas oxidative fibers mainly produce myosin and irisin (49–51). More than 600 myokines have been identified by non-quantitative tagging proteomic approaches. Some of them act in multiple organs throughout blood circulation to participate in metabolic processes, such as promoting glucose uptake, enhancing insulin sensitivity, improving cognitive functions in the brain, stimulating osteoblast differentiation, controlling blood pressure, and regulating myocardial contractility (52–54). Some are involved in combating acute inflammation caused by infection or low-grade inflammation due to aging (47). However, the most important physiological function of myokines is to protect skeletal muscle function and enhance skeletal muscle motility (18) (Figure 3).
During skeletal muscle cell proliferation, myoblasts secrete myokines that are more inclined to inhibit neurogenesis and adipogenesis, whereas during differentiation, myoblasts release some myokines that have the ability to promote myotube formation, vascular differentiation, and neurogenesis (35). Myokines, as signaling molecules, are important carriers in skeletal muscle and other organs, and have a complex regulatory network throughout the organism, including with the muscle (53), fat tissue (55, 56), the pancreas (57, 58), the brain (59), the vasculature (60), and bone (61), among others.
5 The role and mechanism of exerkines in aging sarcopenia
In this section, we aimed to elucidate the relationship between physical activity and aging sarcopenia from exerkines. When we searched Medline, PubMed, and Cochrane Library with the keywords “exerkines”, “aging sarcopenia”, and “skeletal muscle”, we found that a large number of exerkines were related to skeletal muscle (outlined in Table 1). We found that many researches focus on exerkiens related to skeletal muscle regeneration or functionn, with fewer studies report that exerkines improve aging sarcopenia. We selected myonectin, Metrnl, adiponectin (ApN), and leptin, which are more related to aging sarcopenia. Consequently, we will conduct an exploration of the relationship between these exerkines and aging sarcopenia, with the objective of identifying a novel therapeutic direction for aging sarcopenia from a systemic perspective.
5.1 Myonectin and aging sarcopenia
Myonectin, also known as C1q tumor necrosis factor-α-related protein isoform 15 (CTRP15), has the function of maintaining body homeostasis (62). Myonectin knockout reduces muscle strength in aging mice, which exacerbates muscle atrophy by downregulating the AMP-activated protein kinase/peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α (AMPK/PGC-1α) pathway (63). When exogenous myonectin supplementation is added, skeletal muscle atrophy is prevented by the AMPK α2/PGC-1α4/IGF-1-dependent pathway (63); however, in human trials, there were no limiting differences in serum myonectin levels among 142 older adults with or without sarcopenia (62). Myonectin plays an important role in maintaining homeostasis and preventing muscle atrophy in vivo, but its serum levels in the elderly are not significantly associated with sarcopenia.
5.2 Metrnl and aging sarcopenia
Metrnl, also known as meteorin-like hormone, cometin, subfatin, and IL-39, is expressed in a variety of tissues, including the liver, heart, stromal cells, macrophages, spleen, and the central nervous system (64). Although Metrnl is released by a variety of tissues, Lee et al. (65) found that myofiber-specific expression of Metrnl is not necessary for muscle regeneration, but when produced from macrophages, it can promote muscle regeneration. Macrophages change during aging, in which impairment of innate immune signaling leads to age-related muscle degeneration when skeletal muscle regenerates (66). Skeletal muscle injury decreased the expression of Metrnl and improved muscle regeneration in aged mice (66). However, in young mice, Metrnl is expressed in macrophages and induces the expression of regeneration genes (IL-10, IL-6, and IGF-1) to activate the proliferation of satellite cells when skeletal muscle is damaged (67). Thus, the decline in muscle regenerative function may be associated with changes in Metrnl concentration induced by macrophage changes during aging. Serum Metrnl also positively correlates with weight loss and the severity of cardiac insufficiency in elderly patients with chronic heart failure (CHF) (68). Intraperitoneal injection of recombinant Metrnl in mice can activate the activating cyclic AMP/protein kinase A/Sirtuin1 pathway and reduce ischemia/reperfusion injury-induced cardiomyocyte apoptosis via activation of AMP-activated protein kinase/p21 activated kinase 2 signaling for reduction of ischemia–reperfusion-induced apoptosis in cardiomyocytes (68). Recent experiments have identified that Metrnl promotes myosatellite cell proliferation to achieve the initial stage of skeletal muscle regeneration and also has a muscle regeneration-promoting function in aged, skeletal muscle-atrophic mice.
5.3 Adiponectin and aging sarcopenia
The majority of ApNs are secreted by white adipose tissue, skeletal muscle cells, cardiac muscle cells, liver parenchyma cells, and osteoblasts. ApN can be classified as full-length ApN (fApN) and globular ApN (gApN) according to its structure and function (69). During skeletal muscle injury, immune cells are recruited to the site of injury and release elastase, which cleaves fApN to gAPN. ApN is a protective factor against aging sarcopenia, and it is negatively correlated with skeletal muscle density, physical function, and bone density (70, 71). We found that ApN improves aging skeletal muscle in two aspects. On the one hand, gApN induces the expression of myogenic differentiation antigen (MyoD) to promote myoblast proliferation and differentiation and activates myoblastogenin and myoregulatory factor 4 to promote muscle differentiation and fusion into multinucleated myotubes (69). On the other hand, the ApN/adiponectin receptor 1-AMPK axis mediates exercise-induced satellite cell proliferation and improves locomotor activity in aging mice (69). At the same time, the improvements exhibit muscle-type specificity. Tail vein injection of the ApN receptor agonist (AdipoRon) three times a week for 6 weeks in 25-month-old mice significantly enhances the function and metabolism of fast-twitch fibers but does not affect slow-twitch fibers (72). All in all, ApN affects mitochondrial metabolism, muscle fiber regeneration, and skeletal muscle autophagy against skeletal muscle dysfunction in aged individuals.
5.4 Leptin and aging sarcopenia
Leptin is mainly derived from adipose tissue and correlates with total fat mass and plays a key role in energy balance regulation, appetite control, insulin sensitivity, and glucose metabolism (73, 74). Higher leptin levels are associated with a higher risk of sarcopenia in the elderly (75). In a cross-sectional study of 4,062 subjects (≥69 years old), serum leptin levels were found to be associated with the risk of obesity-related sarcopenia (76). In contrast, Kao et al. (77) found that higher serum leptin levels were correlated with a lower risk of sarcopenia. Such contradictory findings may be related to the body fat content of the subjects, and leptin receptor sensitivity is higher in obese individuals. Elevated levels of leptin significantly influence fatty acid oxidation and lipid metabolism in skeletal muscle, consequently leading to skeletal muscle dysfunction (76). In order to explore the relationship between leptin, Collins created fat-free (FF) mice by crossing ApN-Cre mice. The resulting FF mice constitutively have a complete absence of adipose tissue from birth. FF mice have low leptin levels and exhibit muscle mass defects driven by fast fiber atrophy (78). The effects and mechanisms of leptin on skeletal muscle homeostasis are limited.
6 The role and mechanism of myokines in aging sarcopenia
Exercise is an effective strategy to ameliorate sarcopenia. A number of studies have found that myokines improve skeletal muscle mass and strength. Based on the bidirectional effects of that on skeletal muscle mass, myokines can be categorized into two types that increase skeletal muscle mass and cause skeletal muscle atrophy. Myokines, as key mediators, may be a breakthrough in the study of the mechanisms by which exercise improves aging sarcopenia. The ameliorative effect of myokines on aging sarcopenia is characterized by the promotion of skeletal muscle protein synthesis, modulation of energy uptake in skeletal muscle, and enhancement of signaling at neuromuscular junctions (Figure 4).
6.1 Myostatin and aging sarcopenia
MSTN belongs to the transforming growth factor beta(TGF-β) superfamily (79). Skeletal muscle, cardiac muscle, adipose tissue, the brain, the kidneys, and even leukocytes can express MSTN (80–82). MSTN is first synthesized as an inactive precursor protein and hydrolyzed into active MSTN in two steps. First, the furin family removes potential MSTN complex signal peptides, and then bone morphogenetic protein 1 (BMP1)/Tolloid matrix metalloproteinases separate the muscle growth inhibitory ligand from the inhibitory N-terminal prepeptide structural domain, exposing the active site of MSTN binding to the receptor (83).
MSTN regulates skeletal muscle mass and function (84). Compared to younger men (mean age, 20 years), older men (mean age, 70 years) have approximately 100% higher expression of the MSTN gene, accompanied by an approximately 40% reduction in the cross-sectional area of type II muscle fibers. If the elders have elevated levels of serum MSTN, their grip strength will reduce by approximately 7.5% (85). It is hypothesized that MSTN level increases with age and loss of skeletal muscle, and a serum MSTN increase of 1 ng/mL is associated with an 11% increase in the odds of developing sarcopenia in older men (85–87). The mechanisms of MSTN that affect skeletal muscle mass are shown as MSTN binds to activins 2A and 2B and activates intracellular Smad2 and Smad3 signaling to negatively regulate skeletal muscle growth (88, 89). Specifically, the hydrolyzed active MSTN binds to the transmembrane hormone type IIB receptor (ActRIIB) through dimerization of disulfide bonds. This leads to the phosphorylation of Smad3 and Smad4, which then bind to activin receptor-like kinase 4 or 5 (ALK4 and ALK5) for signaling, affecting transcription factors such as the myocyte-specific enhancer factor (MEF2) and the myoblast determination protein 1 (MyoD1), which inhibit myoblast proliferation and differentiation (83, 90). Additionally, MSTN inhibits muscle hypertrophy through mammalian target of rapamycin (mTOR) signaling and increases muscle degradation through the forkhead box protein 01 (Fox01) pathway (83). As a negative regulator of muscle mass, MSTN knockdown or inhibition results in increased muscle mass. Therefore, blocking muscle growth inhibitor signaling or regulating its MSTN gene expression may be a therapeutic strategy for MSTN-related diseases, such as age-related sarcopenia.
6.2 Interleukin-6 and aging sarcopenia
IL-6 is a multifunctional cytokine released by multicellular cells during inflammation. IL-6 participates in innate and adaptive immune responses, and in the activation of anabolic and catabolic pathways to regulate cell growth, differentiation, and survival (91–93). IL-6 has three signaling pathways: the classical signaling pathway, the trans-signaling pathway, and the cluster signaling pathway (91, 94, 95). IL-6 regulates myosatellite cell proliferation and migration through these three signaling pathways and thus regulates physiological muscle hypertrophy (92). At the same time, IL-6 signaling is tightly regulated by feedback suppressors, such as suppressor of cytokine signaling, SH2-domain containing protein tyrosine phosphatase 2 (SHP2), and T-cell protein tyrosine phosphatase (TC-PTP), among others (92). Exercise induces IL-6 transcriptional upregulation through skeletal muscle contraction, contributing to increased serum IL-6 concentrations (91, 96).
Patients with aging sarcopenia have higher blood concentrations of IL-6 than healthy individuals, and elevated plasma IL-6 may increase fat deposition within skeletal muscle (97). In animal experiments, IL-6 injection into the tibialis anterior muscle of mice caused the upregulation of some genes related to immunity, and the downregulation of some genes related to energy metabolism (98). Pelosi and coworkers (99) constructed NSE/IL-6 transgenic mice and found that IL-6 overexpression in NSE/IL-6 mice resulted in a significant reduction in the rate of muscle growth in early postnatal life. In adulthood, these mice have severe muscle atrophy accompanied by low muscle mass and a significant reduction in muscle cross-sectional areas, individual muscle fiber cross-sectional areas, and total number of muscle fibers. Pelosi’s experiment demonstrated that elevated levels of IL-6 during the first and middle stages of life are an important factor in shifting muscle fiber type and skeletal muscle atrophy. However, elevated IL-6 has two opposite effects; slowly increasing the concentration of IL-6 in plasma result skeletal muscle atropht, but exercise-induced increase in plasma IL-6 promotes skeletal muscle regeneration (100, 101). On the one hand, the increased IL-6 concentration may activate satellite cells, enhancing muscle regeneration and growth (91, 102, 103). Additionally, IL-6 mediates short-term energy allocation, diverting energy to skeletal muscle (104). On the other hand, a slow and sustained increase in IL-6 inhibits skeletal myogenesis and protein synthesis (105), and accelerates skeletal muscle degeneration and atrophy (106–108). Whether the slow and sustained increase of IL-6 in the blood of elderly patients with sarcopenia causes damage to muscle fibers by influencing myofiber type transition and energy supply processes, thereby leading to atrophy of the whole skeletal muscle, still needs to be further explored.
6.3 Irisin and aging sarcopenia
In 2012, Bostrom et al. (109) found that exercise activation of PPAR-γ co-activator-1α (PGC-1α) upregulated its downstream target—fibronectin type III domain containing 5 (FNDC5). FNDC5 is a membrane protein present in the brain and skeletal muscle that is cleaved by an unknown protein hydrolase and releases irisin after exercise (110, 111). Animal studies have shown that irisin can enhance the activity of skeletal muscle satellite cells, reduce protein degradation, alleviate skeletal muscle fibrosis, and improve the stability of muscle membrane, thereby alleviating sarcopenia in different animal models, including those with denervated muscle loss, hindlimb suspension, and hereditary muscular dystrophy (46, 112, 113). During skeletal muscle differentiation, elevated levels of irisin upregulate p-Erk expression to promote the skeletal muscle protein synthesis pathway (46). During growth, intraperitoneal administration of irisin to 5-week-old mice resulted in an increase in skeletal muscle weight via the Akt and mTOR signaling pathways, subsequently translating this augmented muscle mass into enhanced grip strength. It may also downregulate the expression of Atrogin-1 and MuRF-1, thereby inhibiting the catabolic processes associated with skeletal muscle (46). During aging, Guo (12) found that irisin and its precursor FNCD5 in muscle tissue decreased with aging. Irisin knockout mice showed more severe muscle atrophy and smaller muscle mass and grip strength. However, injecting recombinant irisin protein into the abdominal cavity of aging mice can improve grip strength and muscle mass, thus improving symptoms of sarcopenia (12). Targeting the AMPK-PGC-1α-FNDC5 and the IGF-1/Akt/mTOR pathway with irisin prevents the onset of muscle disease in the elderly (114). In contrast, Baek et al. (115) found that there was no difference in plasma irisin levels between patients with aging sarcopenia and healthy older individuals, and even an increase in blood irisin levels could not change the state of reduced muscle mass, muscle dysfunction, and physical performance in patients with aging sarcopenia. These contradictory results may be related to the source of the subjects. The latter subjects came from outpatient geriatrics and endocrinology clinics. The baseline status of the subjects in the two studies was not consistent. In animal experiments, irisin produced positive effects on skeletal muscle mass and function in growth and developmental stages, and in aging. Replication of these results in animal studies in humans requires further investigation.
6.4 Fibroblast growth factor 21 and aging sarcopenia
FGF21 is a family of 22 related proteins that can be classified as six subfamilies based on genetic and functional similarities (116), which has a complex molecular mechanism of signaling, involving multiple FGF receptors (FGFRs) and a dedicated co-receptor, β-klotho (117–119). FGF21 expression is low in healthy skeletal muscle; however, fasting, endoplasmic reticulum stress, mitochondrial myopathy, and metabolic disorders induce increased expression of FGF21 in skeletal muscle, especially in dystrophic mice, where mRNA and protein expression of skeletal muscle FGF21 are significantly upregulated (120–122). In the elderly, elevated serum FGF21 levels were significantly associated with the risk of sarcopenia, low muscle mass, and low grip strength (123). Elevated levels of FGF21 are accompanied by decreased mitochondrial autophagy and skeletal muscle protein synthesis, resulting in loss of muscle mass and strength (124). Interestingly, inhibition of FGF21 levels in blood improves the aging phenotype (125). In animal experiments, skeletal muscle-specific FGF21 deficiency protects muscle from atrophy and weakness induced by starvation (121). These effects may be closely related to the state of mitochondria. FGF21 regulates mitochondrial function by activating the AMPK-SIRT1 pathway, which, in turn, activates peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), thereby affecting skeletal muscle energy supply (126, 127).
FGF21 secretion is increased in myasthenia gravis and mitochondrial stress states, and FGF21 regulates skeletal muscle glucose uptake (128) and protein synthesis (129). It has been hypothesized that increased FGF21 expression in sarcopenia may promote mitochondrial stress, reduce skeletal muscle glucose uptake, and disrupt skeletal muscle proteostasis. These states further stimulate FGF21 overexpression, thus creating a vicious cycle.
6.5 β-Aminoisobutyric acid and aging sarcopenia
Aminoisobutyric acid originates from different organs and can be classified as three isomers, α-aminobutyric acid (AABA), β-aminobutyric acid (BABA), and γ-aminobutyric acid (GABA). A pair of these isomers, which have the same molecular weight and related structures, mirror isomers, and are also known as enantiomers (130). β-Aminoisobutyric acid (BAIBA) is a PGC-1α-dependent myokine produced by skeletal muscle during exercise. It is mainly found as two enantiomers, L-BAIBA and D-BAIBA, which originate from different pathways (131–134). Skeletal muscle contraction stimulates young and old mice to release BAIBA to improve muscle mass and strength (132). In another study, plasma BAIBA levels were higher in adult subjects than in the elderly, and that BAIBA expression was regulated by PGC-1α (135, 136). It has also been suggested that BAIBA secretion is similar in young and old muscles, and the reduced BAIBA function may result from a significant decrease in its receptor with age (132). BAIBA has now been shown to promote osteoblast survival under oxidative stress and maintain bone quality (44, 132). The development of sarcopenia in older age groups is often accompanied by osteoporosis and dysfunction in muscle, leading to a reduction in skeletal muscle loading and bone mass (10). As sarcopenia is closely linked to endocrine and mechanical risk factors for osteoporosis, and muscles and bones act synergistically mechanically and biologically (10, 135), it is hypothesized that the effect of BAIBA on delaying skeletal muscle atrophy in sarcopenia in older adults may be through maintenance of bone mass and an increase in the muscle attachment points, attenuating the decline in muscle strength.
6.6 Brain-derived neurotrophic factor and aging sarcopenia
The mammalian neurotrophic factor family includes nerve growth factor (NGF), neurotrophin-3, neurotrophin-4/5, and BDNF (137). BDNF is secreted out of the cell as Pro-BDNF after being translated and cut into mature BDNF. BDNF and Pro-BDNF act on specific receptors in the nucleus and cell membrane (138, 139). Different skeletal muscles secrete BDNF in response to different exercise durations and intensities. Post-exercise plasma BDNF levels depend on exercise duration, its intensity, type of exercise, the level of previous training, and the functional status of the body (140–145). In skeletal muscle, BDNF regulates glycolytic fiber-type recognition, fatty acid oxidation, and satellite cell differentiation, and strengthens the neuromuscular junction (143, 146). BDNF takes part in the generation of regenerating muscle fibers after injury and is necessary for the formation of regenerating muscle fibers after injury or damage (147, 148). In contrast, plasma BDNF levels are significantly lower in patients with aging sarcopenia and in debilitated patients with diminished muscle strength and physical activity (149). In animal models, intramuscular injection of BDNF promotes functional repair after nerve injury. BDNF plays an important role in protecting the neuromuscular junction (150). The ameliorative effect of BDNF on aging sarcopenia involves muscle repair signal cascades and neuromuscular signaling connections. Activation of TrkB receptors by BDNF enhances presynaptic protein kinase C family (cPKCα, cPKCβI, and cPKCϵ), and activation of PKCs enhances synaptic vesicle fusion and neurotransmitter release (143), which enhances the functional innervation of the muscles (150).
Muscle wasting during aging is partly due to the retraction and death of motor neurons, resulting in the detachment of muscle fibers from neuronal innervation. Muscle fiber degeneration and muscle atrophy can only be avoided if these muscle fibers are re-innervated by neighboring neurons (151). Perhaps we may speculate that BDNF delays skeletal muscle atrophy during aging by enhancing neuromuscular connections in patients with aging sarcopenia.
6.7 Apelin and aging sarcopenia
Apelin belong to cardiac factor, myokine and adipokine (152), and is extensively distributed across various organs and tissues, including skeletal muscle, adipose tissue, the central nervous system, the gastrointestinal tract, lungs, liver, and heart (153). Apelin-13 and apelin-17 are the predominant isomers of apelin found in human plasma, with apelin-13 exhibiting greater biological activity and receiving more extensive research attention (154). Exercise and aging are important factors influencing apelin secretion. Exercise induces apelin, while overall levels decrease with aging (155, 156). However, the effect of exercise on apelin levels is controversial. One study demonstrated that prolonged aerobic exercise significantly elevated plasma apelin levels, whereas another study indicated that it did not influence apelin expression (152, 157).
Apelin enhances myocyte metabolism and stem cell function to stimulate skeletal muscle formation during aging (155, 158, 159). The Apelin/Apelin receptor system stimulates skeletal muscle stem cells through the Forkhead box 03–MuRF-1–Atrogin axis and simultaneously activates the AMP-activated protein kinase (AMPK) and P7050K pathways to promote protein production in myofibers, which together promote skeletal muscle regeneration (159). Knockdown of the apelin gene in the skeletal muscle of aged mice led to muscle mass reduction, muscle weakness, and motor dysfunction (160), whereas aged mice that are administered apelin or that are subjected to adenovirus-mediated enhancement of the apelin gene expression in skeletal muscle exhibit improved muscle function and hypertrophy of muscle fibers (155). Apelin-administered mice had increased expression of the markers Pax7, Myf5, and Myogenin in satellite cells and target muscle stem cells to promote muscle regeneration (156). Furthermore, apelin deficiency resulted in changes in skeletal muscle fiber types. Compared to wild-type mice, apelin knockout induces a shift from fast type II to slow-oxidizing type I fiber in mice and increases the proportion of MHC-1 type fibers (161). Additionally, apelin knockout mice exhibited a decreased number of mitochondria in myogenic fibers, a significant reduction in mitochondria-related enzyme activities, and diminished muscle tonic contractility and grip strength compared to wild-type mice (155).
Exercise-induced apelin enhances skeletal muscle function and alleviates sarcopenia, which makes apelin a potential target for the treatment of myofibrillar atrophy, muscle weakness, and oxidative stress in aging mice (159, 162). However, with age, both systemic and local apelin levels show a decreasing trend. Exogenous administration of apelin can lead to significant improvements in age-related pathologies. Apelin may serve not only as a novel tool for the early diagnosis of sarcopenia but also as a prognostic marker for evaluating the benefits of exercise in older adults.
6.8 Insulin-like growth factor-1 and aging sarcopenia
Insulin-like growth factor-1 (IGF-1) is an anabolic growth factor that facilitates tissue development, maturation, cellular adaptation, and regeneration during growth and development (163). In skeletal muscle, IGF-1 is secreted by muscle fibers into the extracellular matrix, subsequently binding to insulin-like growth factor binding proteins (IGFBPs) (164, 165). Age and exercise are key factors influencing circulating IGF-1 concentrations. Serum IGF-1 levels decrease with age, but exercise promotes ICF-1 secretion (166–169). Aerobic exercise, resistance exercise, whole-body vibration, and electrical stimulation all activate the IGF-1 pathway, increase protein synthesis and skeletal muscle mass, inhibit protein degradation and apoptosis, and enhance the exercise capacity of skeletal muscle in early aging mice (170). In animal experiments, IGF-1 knockout in mouse monocytes/macrophages resulted in impaired muscle regeneration after injury, with reduced size of regenerating muscle fibers, enlarged interstitial gaps, and deposition of lipid tissue (171). In contrast, IGF-1-overexpressing mice maintain high IGF-1 levels even in old age, thereby maintaining skeletal muscle function (6). IGF-1 achieves its protective effects on skeletal muscle by activating muscle signaling responses and skeletal myogenesis (6, 172). Elevated plasma levels of IGF-1 lead to IGF-1 Akt/Protein Kinase B-mTOR pathway stress, which promotes ribosomal biosynthesis and facilitates the formation of new myofibrillar proteins to provide a condition for skeletal muscle remodeling. Additionally, a high level of IGF-1 inhibits skeletal muscle via the ubiquitin ligases MuRF1 and MAFbx (173, 174). Skeletal muscle secretion of IGF-1 decreases during aging, which results in a decline in skeletal muscle mass and function. However, high plasma IGF-1 levels can increase muscle mass to decrease the incidence of aging sarcopenia.
6.9 Other myokines and aging sarcopenia
Follistatin (FST) is a multifunctional protein whose main function is to antagonize the TGB-β superfamily, such as the muscle growth inhibitors, activin, and BMPs (175). FST, as a cytokine expressed systemically, is particularly abundant in skeletal muscle, the heart, adipose tissue, the kidneys, and the lungs (176). FST overexpression through gene transfer or a transgene induces skeletal muscle hypertrophy, myofiber regeneration, and satellite cell proliferation (177, 178). Circulating MSTN and FST are negatively correlated with muscle function in older women (179). In patients with severe muscle atrophy, the MSTN pathway was found to be significantly downregulated with a progressive increase in FST, which may partially delay muscle atrophy (180). Although FST overexpression increases muscle mass and excitability, it does not prevent the age-related decline in motor unit function (177).
Decorin is an exercise-induced muscle factor expressed in various tissues, including intestinal tissue, heart, adipose tissue, and skeletal muscle. It plays a role in regulating autophagy, inflammation, and glucose homeostasis, and has been shown to effectively prevent muscle atrophy by inhibiting MSTN (181). Decorin is an anti-fibrotic and pro-myogenic generating agent. When it is injected into damaged skeletal muscle directly, it can promote the process of complete skeletal muscle regeneration and reduce the formation of fibrotic scar tissue (182). Decorin reduces MSTN-induced phosphorylation of Smad2 and inhibits the activation of the Smad signaling pathway in a dose-dependent manner (183). Intramuscular injection of recombinant Decorin may significantly enhance muscle mass in dystrophic mice by activating skeletal muscle cell differentiation (184, 185). Leukemia inhibitory factor (LIF), which belongs to the IL-16 family, regulates skeletal muscle growth and regeneration and is associated with skeletal muscle after prolonged exercise (49, 186). Aerobic exercise upregulates LIF expression in human skeletal muscle to inhibit myasthenia gravis and improve muscle performance. Exogenous LIF intervention may enable human myoblast proliferation by inducing the cell proliferation factors c-Myc and JunB (49). IL-15 is a contraction-induced myokine that improves energy metabolism in skeletal muscle locally (187, 188). High IL-15 levels protect against high-fat diet-induced obesity, glucose intolerance, and insulin resistance (188). A comparison of wild-type, IL-15 knockout, and IL-15 transgenic mice reveals that IL-15 promotes muscle protein synthesis and myofiber regeneration by activating critical regulators of skeletal muscle autophagy (189, 190). IL-15 and its cognate receptor α (IL-15 receptor α) are involved in the regulation of anabolic and catabolic homeostasis in skeletal muscle. IL-15Rα may play a role in the increased synthesis of myofibrillar proteins in skeletal muscle after a single bout of resistance exercise (191). However, few studies have reported an association between the above muscle factors and sarcopenia in aging sarcopenia, but they all have the function of maintaining muscle mass and enhancing muscle strength.
7 Strategies to improve aging sarcopenia
7.1 Exercise improves aging sarcopenia
Diminished function and impaired remodeling of motor units are commonly observed in elderly patients with sarcopenia (192). Liu (193) synthesized different exercise intensities in elderly patients with sarcopenia and found that all exercises improved muscle strength and mass, and high intensity was more effective in increasing strength than low or moderate intensity. The high-intensity interval training (HIIT) model, which alternates high-intensity intervals with low-intensity recovery periods, provides physiological benefits quickly (194). HIIT induces transcriptional co-activation of PGC-1α via mTOR and rps6 phosphorylation, which promotes muscle hypertrophy to mitigate skeletal muscle atrophy and enhance overall exercise performance (193). HIIT also enhances locomotor performance in aged mice, which includes muscle mass and strength, grip strength, and mitochondrial biomass (195).
Resistance exercise, also known as weight training and strength training, requires the muscles to resist applied external force or weight. Resistance exercise can improve muscle strength, mass, and physical performance in middle-aged and older adults (196). Twice-weekly resistance exercise is an appropriate prescription for patients with aging sarcopenia (196). A 10-week resistance training in 70-year-old male and female patients with sarcopenia found that the resistance training intervention resulted in an increase in both the mass of lean and limb skeletal muscle (197). The mechanism by which resistance exercise effectively promotes skeletal muscle protein synthesis may involve regulating the secretion of myokines and participating in microRNA regulatory processes (198). Active resistance training also played a favorable role on the lumbar spine, lean body mass, and muscle strength (199). Additionally, resistance training affects visceral fat loss, blood pressure, glucose, and fat metabolism beneficially (200). While numerous studies have documented the beneficial effects of resistance exercise in older individuals with sarcopenia, high-intensity resistance training may not be appropriate for elderly populations and those with lower fitness levels (201). It is important to find ways to improve skeletal muscle mass and strength more safely and effectively while significantly reducing mechanical stress. Low-load resistance training with blood flow restriction (L-BFR) induces similar increases in muscle mass but has less effect on skeletal muscle strength compared to H-RT (high-load resistance training), which is considered an effective countermeasure for sarcopenia (201). Static stretching has the potential to increase skeletal muscle strength and endurance, which leads to significant increases in skeletal muscle suppleness (202).
Now, it is widely acknowledged that physical activity plays a crucial role in the prevention and treatment of sarcopenia (200). Various exercise modalities—including resistance training, aerobic training, and balance training—are all effective in promoting muscle health among older adults. Among them, high-volume, high-intensity resistance training is the most significant in improving skeletal muscle mass and physical function in older adults (200). For elderly patients with sarcopenia, the choice of specific exercise modes should be based on individual characteristics, limitations, and needs, where safety is always a key factor (196, 199).
7.2 Nutrients improve aging sarcopenia
A healthy diet with adequate protein, vitamins, antioxidant nutrients, and long-chain unsaturated fatty acids may alleviate sarcopenia (203). Vitamin D/vitamin D receptor signal affects all stages of myogenesis by stimulating skeletal muscle fiber proliferation, differentiation, and maintenance and improving skeletal muscle mass and strength (204, 205). On the one hand, vitamin D directly inhibits skeletal muscle atrophy. On the other hand, it inhibits MSTN expression via IGF-independent signal indirectly, which prevents skeletal muscle degeneration and improves myofilament and muscle strength (204, 205). Chronic hypovitaminosis D may lead to upregulation of muscle atrophy markers (Murf1 and MaFbx), VD receptor loss, and a dramatic reduction in cellular remodeling capacity (205). VD receptor deficiency in mouse myocytes is associated with lean body mass, sarcopenia, reduced grip strength, and exercise capacity (206). Human studies also reported that vitamin D deficiency reduces skeletal muscle grip strength and stride speed, particularly pronounced in the elderly (207, 208), whereas oral nutritional supplementation with protein and vitamin D in elderly patients with sarcopenia effectively enhances skeletal muscle mass, although it does not improve physical mobility (209). However, in older adults with vitamin D deficiency, a longer duration of supplementation or a higher dosage of vitamin D may be necessary compared to younger adults to achieve the beneficial effects on skeletal muscle (204). Vitamin D supplementation during resistance training will improve skeletal muscle mass and positively impact skeletal muscle remodeling in both older and younger adults (210).
Essential amino acids (EAAs) stimulate skeletal muscle protein synthesis and turnover, play a key role in replacing degraded or damaged skeletal muscle proteins, and lay the metabolic foundation for enhanced skeletal muscle function (211). Several studies of EAA supplementation in elderly patients with sarcopenia have found that EAA interventions have a positive effect on both skeletal muscle mass and strength (212). Supplementation of an animal protein diet containing EAA during resistance training synergistically promotes increased skeletal muscle mass (213). Cuyul-Vásquez and coworkers (214) demonstrated that whey protein supplementation during resistance training significantly increased skeletal muscle mass and grip strength in elderly patients with sarcopenia. However, if protein supplementation is stopped at the end of 12 weeks of exercise, it may result in a skeletal muscle protein synthesis decline (215). Appropriate intake of energy, protein, long-chain saturated fatty acids, amino acids, vitamin D, and antioxidants may reduce the decline in skeletal muscle mass and strength in older adults (216). Furthermore, there are also some natural products that have been suggested for the treatment and prevention of sarcopenia, such as ursolic acid and pentacyclic triterpene acid fruits (apple peel and tomatidine) (217). In animal models, these natural products increase muscle mass and grip strength in mice by decreasing age-related muscle atrophy mediator activity (217). β-Hydroxy β-methylbutyrate (HMB) has been shown to improve muscle mass without affecting muscle strength and function in sarcopenic or debilitated older adults (218). β-Sitosterol is widely found in various parts of plants and has various effects, such as anti-inflammatory, anti-alcoholic fatty liver, and antioxidant. It can protect mice from the muscle atrophy induced by dexamethasone (9).
Daily dietary structure may influence protein intake and interfere with skeletal muscle protein metabolism and transcription, thereby accelerating skeletal muscle mass loss in older adults (219). Plant-based dietary patterns are becoming more popular in improving skeletal muscle atrophy, and related studies have shown that plant-based dietary patterns are more beneficial and effective than animal-based dietary patterns in maintaining muscle mass in functionally independent Chinese older adults (220). There is less research on the effects of vegan diets on physical performance and body composition. However, one study of a vegan diet in young women showed that dietary changes would lead to alterations in overall macronutrient compartmentalization that would impair skeletal muscle mass (221). In the elderly population, vegan diets increase the risk of inadequate protein intake, negatively affecting skeletal muscle mass (222).
Aging sarcopenia has attracted widespread attention as a skeletal muscle disease in the elderly, but there are no suitable clinical interventions. Nutrition and exercise are the main methods for its prevention and treatment. Appropriate intake of animal and vegetable proteins and a balanced dietary structure combined with appropriate physical activity are important strategies to improve sarcopenia in the elderly.
8 Conclusion
In this paper, we summarize the current state of research on aging sarcopenia and the effects of various exerkines on skeletal muscle. Exercise-induced exerkines improve aging sarcopenia mainly by increasing skeletal muscle energy supply, activating satellite cells involved in skeletal muscle repair and regeneration, improving the function of the neuromuscular junction, and enhancing the neural control of skeletal muscle. Daily dietary changes affect skeletal muscle regeneration and repair by altering protein intake. Therefore, a rational diet combined with exercise training is one of the most effective measures to improve aging sarcopenia. However, there is a lack of research on the mechanisms of combining myokines with nutrients to improve aging sarcopenia. Meanwhile, further research is needed to study the effects of multiple exerkines to improve aging sarcopenia.
Author contributions
HW: Writing – original draft, Writing – review & editing, Formal Analysis, Project administration. WH: Writing – review & editing. PC: Writing – review & editing. HZW: Software, Writing – review & editing. HGW: Software, Writing – original draft. LZ: Funding acquisition, Resources, Writing – original draft. XL: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 32300964), the GuangDong Basic and Applied Basic Research Foundation (No. 2022A1515111105), the GuangZhou Basic and Applied Basic Research Foundation (No. 2023A04J0552), and the National Office of Philosophy and Social Science of China (No. 23ATY007).
Acknowledgments
The authors would like to thank Prof. Liu, Guangzhou Sport University, for his linguistic advice, as well as Figdraw (www.figdraw.com) for the assistance in creating the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rex N, Melk A, and Schmitt R. Cellular senescence and kidney aging. Clin Sci (London England: 1979). (2023) 137:1805–21. doi: 10.1042/cs20230140
2. Man W, Wang S, and Yang H. Exploring the spatial-temporal distribution and evolution of population aging and social-economic indicators in China. BMC Public Health. (2021) 21:966. doi: 10.1186/s12889-021-11032-z
3. Fang Y, Gong AY, Haller ST, Dworkin LD, Liu Z, and Gong R. The ageing kidney: molecular mechanisms and clinical implications. Ageing Res Rev. (2020) 63:101151. doi: 10.1016/j.arr.2020.101151
4. Partridge L, Deelen J, and Slagboom PE. Facing up to the global challenges of ageing. Nature. (2018) 561:45–56. doi: 10.1038/s41586-018-0457-8
5. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, and Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. (2019) 571:183–92. doi: 10.1038/s41586-019-1365-2
6. Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, et al. Effects of igf-1 isoforms on muscle growth and sarcopenia. Aging Cell. (2019) 18:e12954. doi: 10.1111/acel.12954
7. Kalinkovich A and Livshits G. Sarcopenia–the search for emerging biomarkers. Ageing Res Rev. (2015) 22:58–71. doi: 10.1016/j.arr.2015.05.001
8. Dao T, Green AE, Kim YA, Bae SJ, Ha KT, Gariani K, et al. Sarcopenia and muscle aging: A brief overview. Endocrinol Metab (Seoul Korea). (2020) 35:716–32. doi: 10.3803/EnM.2020.405
9. Hah YS, Lee WK, Lee S, Kim EJ, Lee JH, Lee SJ, et al. β-sitosterol attenuates dexamethasone-induced muscle atrophy via regulating foxo1-dependent signaling in C2c12 cell and mice model. Nutrients. (2022) 14. doi: 10.3390/nu14142894
10. Bonewald L. Use it or lose it to age: A review of bone and muscle communication. Bone. (2019) 120:212–8. doi: 10.1016/j.bone.2018.11.002
11. Wilkinson DJ, Piasecki M, and Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. (2018) 47:123–32. doi: 10.1016/j.arr.2018.07.005
12. Guo M, Yao J, Li J, Zhang J, Wang D, Zuo H, et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J Cachexia Sarcopenia Muscle. (2023) 14:391–405. doi: 10.1002/jcsm.13141
13. Geng Q, Zhai H, Wang L, Wei H, and Hou S. The efficacy of different interventions in the treatment of sarcopenia in middle-aged and elderly people: A network meta-analysis. Medicine. (2023) 102:e34254. doi: 10.1097/md.0000000000034254
14. Barbalho SM, Prado Neto EV, De Alvares Goulart R, Bechara MD, Baisi Chagas EF, Audi M, et al. Myokines: A descriptive review. J Sports Med Phys Fitness. (2020) 60:1583–90. doi: 10.23736/s0022-4707.20.10884-3
15. Ramírez-Vélez R, González A, García-Hermoso A, Amézqueta IL, Izquierdo M, and Díez J. Revisiting skeletal myopathy and exercise training in heart failure: emerging role of myokines. Metabol: Clin Exp. (2023) 138:155348. doi: 10.1016/j.metabol.2022.155348
16. Ahmadi Hekmatikar A, Nelson A, and Petersen A. Highlighting the idea of exerkines in the management of cancer patients with cachexia: novel insights and a critical review. BMC Cancer. (2023) 23:889. doi: 10.1186/s12885-023-11391-3
17. Zhou N, Gong L, Zhang E, and Wang X. Exploring exercise-driven exerkines: unraveling the regulation of metabolism and inflammation. PeerJ. (2024) 12:e17267. doi: 10.7717/peerj.17267
18. Hoffmann C and Weigert C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harbor Perspect Med. (2017) 7. doi: 10.1101/cshperspect.a029793
19. Kwon JH, Moon KM, and Min KW. Exercise-induced myokines can explain the importance of physical activity in the elderly: an overview. Healthc (Basel Switzerland). (2020) 8. doi: 10.3390/healthcare8040378
20. Cruz-Jentoft AJ and Sayer AA. Sarcopenia. Lancet (London England). (2019) 393:2636–46. doi: 10.1016/s0140-6736(19)31138-9
21. Sayer AA and Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. (2022) 51. doi: 10.1093/ageing/afac220
22. Bastiaanse LP, Hilgenkamp TI, Echteld MA, and Evenhuis HM. Prevalence and associated factors of sarcopenia in older adults with intellectual disabilities. Res Dev Disabil. (2012) 33:2004–12. doi: 10.1016/j.ridd.2012.06.002
23. Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: A time for action. An scwd position paper. J Cachexia Sarcopenia Muscle. (2019) 10:956–61. doi: 10.1002/jcsm.12483
24. Yang Q and Chan P. Skeletal muscle metabolic alternation develops sarcopenia. Aging Dis. (2022) 13:801–14. doi: 10.14336/ad.2021.1107
25. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, and Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. (2013) 280:4294–314. doi: 10.1111/febs.12253
26. Zhong Q, Zheng K, Li W, An K, Liu Y, Xiao X, et al. Post-translational regulation of muscle growth, muscle aging and sarcopenia. J Cachexia Sarcopenia Muscle. (2023) 14:1212–27. doi: 10.1002/jcsm.13241
27. Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F, et al. Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients. (2021) 13. doi: 10.3390/nu13124291
28. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Directors Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
29. Csete ME. Basic science of frailty-biological mechanisms of age-related sarcopenia. Anesth Analgesia. (2021) 132:293–304. doi: 10.1213/ane.0000000000005096
30. Shang GK, Han L, Wang ZH, Liu YP, Yan SB, Sai WW, et al. Sarcopenia is attenuated by trb3 knockout in aging mice via the alleviation of atrophy and fibrosis of skeletal muscles. J Cachexia Sarcopenia Muscle. (2020) 11:1104–20. doi: 10.1002/jcsm.12560
31. Jimenez-Gutierrez GE, Martínez-Gómez LE, Martínez-Armenta C, Pineda C, Martínez-Nava GA, and Lopez-Reyes A. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. (2022) 11. doi: 10.3390/cells11152359
32. Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, and Arosio B. Role of age-related mitochondrial dysfunction in sarcopenia. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21155236
33. Alway SE, Mohamed JS, and Myers MJ. Mitochondria initiate and regulate sarcopenia. Exercise Sport Sci Rev. (2017) 45:58–69. doi: 10.1249/jes.0000000000000101
34. Distefano G and Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harbor Perspect Med. (2018) 8. doi: 10.1101/cshperspect.a029785
35. Li F, Li Y, Duan Y, Hu CA, Tang Y, and Yin Y. Myokines and adipokines: involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. (2017) 33:73–82. doi: 10.1016/j.cytogfr.2016.10.003
36. Coelho-Junior HJ, Picca A, Calvani R, Uchida MC, and Marzetti E. If my muscle could talk: myokines as a biomarker of frailty. Exp Gerontol. (2019) 127:110715. doi: 10.1016/j.exger.2019.110715
37. Barbalho SM, Flato UAP, Tofano RJ, Goulart RA, Guiguer EL, Detregiachi CRP, et al. Physical exercise and myokines: relationships with sarcopenia and cardiovascular complications. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21103607
38. Magliulo L, Bondi D, Pini N, Marramiero L, and Di Filippo ES. The wonder exerkines-novel insights: A critical state-of-the-art review. Mol Cell Biochem. (2022) 477:105–13. doi: 10.1007/s11010-021-04264-5
39. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. (2022) 18:273–89. doi: 10.1038/s41574-022-00641-2
40. Senesi P, Luzi L, and Terruzzi I. Adipokines, myokines, and cardiokines: the role of nutritional interventions. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21218372
41. Mazo CE, Miranda ER, Shadiow J, Vesia M, and Haus JM. High intensity acute aerobic exercise elicits alterations in circulating and skeletal muscle tissue expression of neuroprotective exerkines. Brain Plasticity (Amsterdam Netherlands). (2022) 8:5–18. doi: 10.3233/bpl-220137
42. Lee B, Shin M, Park Y, Won SY, and Cho KS. Physical exercise-induced myokines in neurodegenerative diseases. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22115795
43. Cariati I, Bonanni R, Onorato F, Mastrogregori A, Rossi D, Iundusi R, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. (2021) 6. doi: 10.3390/jfmk6020055
44. Hamrick MW and McGee-Lawrence ME. Blocking bone loss with L-baiba. Trends Endocrinol Metabol: TEM. (2018) 29:284–6. doi: 10.1016/j.tem.2018.03.005
45. Wang Y, Song J, Liu X, Liu J, Zhang Q, Yan X, et al. Multiple effects of mechanical stretch on myogenic progenitor cells. Stem Cells Dev. (2020) 29:336–52. doi: 10.1089/scd.2019.0286
46. Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. (2017) 8:1104. doi: 10.1038/s41467-017-01131-0
47. Gomarasca M, Banfi G, and Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. (2020) 94:155–218. doi: 10.1016/bs.acc.2019.07.010
48. Lombardi G, Ziemann E, and Banfi G. Physical activity and bone health: what is the role of immune system? A narrative review of the third way. Front Endocrinol. (2019) 10:60. doi: 10.3389/fendo.2019.00060
49. Jia D, Cai M, Xi Y, Du S, and Zhenjun T. Interval exercise training increases lif expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. (2018) 193:77–86. doi: 10.1016/j.lfs.2017.12.009
50. Ishiuchi Y, Sato H, Komatsu N, Kawaguchi H, Matsuwaki T, Yamanouchi K, et al. Identification of ccl5/rantes as a novel contraction-reducible myokine in mouse skeletal muscle. Cytokine. (2018) 108:17–23. doi: 10.1016/j.cyto.2018.03.012
51. Rutti S, Dusaulcy R, Hansen JS, Howald C, Dermitzakis ET, Pedersen BK, et al. Angiogenin and osteoprotegerin are type ii muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci Rep. (2018) 8:10072. doi: 10.1038/s41598-018-28117-2
52. Carson BP. The potential role of contraction-induced myokines in the regulation of metabolic function for the prevention and treatment of type 2 diabetes. Front Endocrinol. (2017) 8:97. doi: 10.3389/fendo.2017.00097
53. Chen W, Wang L, You W, and Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. J Cell Physiol. (2021) 236:2393–412. doi: 10.1002/jcp.30033
54. Henningsen J, Pedersen BK, and Kratchmarova I. Quantitative analysis of the secretion of the mcp family of chemokines by muscle cells. Mol Biosyst. (2011) 7:311–21. doi: 10.1039/c0mb00209g
55. Rodríguez A, Becerril S, Méndez-Giménez L, Ramírez B, Sáinz N, Catalán V, et al. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (2005). (2015) 39:397–407. doi: 10.1038/ijo.2014.166
56. Stanford KI and Goodyear LJ. Muscle-adipose tissue cross talk. Cold Spring Harbor Perspect Med. (2018) 8. doi: 10.1101/cshperspect.a029801
57. Barlow JP and Solomon TP. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am J Physiol Endocrinol Metab. (2018) 314:E297–e307. doi: 10.1152/ajpendo.00353.2017
58. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. (2011) 17:1481–9. doi: 10.1038/nm.2513
59. Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. (2016) 24:332–40. doi: 10.1016/j.cmet.2016.05.025
60. Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. (2014) 103:111–20. doi: 10.1093/cvr/cvu105
61. Lee JY, Park SJ, Han SA, Lee SH, Koh JM, Hamrick MW, et al. The effects of myokines on osteoclasts and osteoblasts. Biochem Biophys Res Commun. (2019) 517:749–54. doi: 10.1016/j.bbrc.2019.07.127
62. Ji S, Park SJ, Lee JY, Baek JY, Jung HW, Kim K, et al. Lack of association between serum myonectin levels and sarcopenia in older asian adults. Exp Gerontol. (2023) 178:112229. doi: 10.1016/j.exger.2023.112229
63. Ozaki Y, Ohashi K, Otaka N, Kawanishi H, Takikawa T, Fang L, et al. Myonectin Protects against Skeletal Muscle Dysfunction in Male Mice through Activation of Ampk/Pgc1α Pathway. Nat Commun. (2023) 14:4675. doi: 10.1038/s41467-023-40435-2
64. Lee JO, Byun WS, Kang MJ, Han JA, Moon J, Shin MJ, et al. The myokine meteorin-like (Metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting ampkα2. FEBS J. (2020) 287:2087–104. doi: 10.1111/febs.15301
65. Lee DE, McKay LK, Bareja A, Li Y, Khodabukus A, Bursac N, et al. Meteorin-like is an injectable peptide that can enhance regeneration in aged muscle through immune-driven fibro/adipogenic progenitor signaling. Nat Commun. (2022) 13:7613. doi: 10.1038/s41467-022-35390-3
66. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. (2016) 44:355–67. doi: 10.1016/j.immuni.2016.01.009
67. Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, et al. Meteorin-like facilitates skeletal muscle repair through a stat3/igf-1 mechanism. Nat Metab. (2020) 2:278–89. doi: 10.1038/s42255-020-0184-y
68. Cai J, Wang QM, Li JW, Xu F, Bu YL, Wang M, et al. Serum meteorin-like is associated with weight loss in the elderly patients with chronic heart failure. J Cachexia Sarcopenia Muscle. (2022) 13:409–17. doi: 10.1002/jcsm.12865
69. Lu W, Feng W, Lai J, Yuan D, Xiao W, and Li Y. Role of adipokines in sarcopenia. Chin Med J. (2023) 136:1794–804. doi: 10.1097/cm9.0000000000002255
70. Walowski CO, Herpich C, Enderle J, Braun W, Both M, Hasler M, et al. Analysis of the adiponectin paradox in healthy older people. J Cachexia Sarcopenia Muscle. (2023) 14:270–8. doi: 10.1002/jcsm.13127
71. Komici K, Dello Iacono A, De Luca A, Perrotta F, Bencivenga L, Rengo G, et al. Adiponectin and sarcopenia: A systematic review with meta-analysis. Front Endocrinol. (2021) 12:576619. doi: 10.3389/fendo.2021.576619
72. Balasubramanian P, Schaar AE, Gustafson GE, Smith AB, Howell PR, Greenman A, et al. Adiponectin receptor agonist adiporon improves skeletal muscle function in aged mice. eLife. (2022) 11. doi: 10.7554/eLife.71282
73. Park HK and Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabol: Clin Exp. (2015) 64:24–34. doi: 10.1016/j.metabol.2014.08.004
74. Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. (2011) 301:E567–84. doi: 10.1152/ajpendo.00315.2011
75. Lana A, Valdés-Bécares A, Buño A, Rodríguez-Artalejo F, and Lopez-Garcia E. Serum leptin concentration is associated with incident frailty in older adults. Aging Dis. (2017) 8:240–9. doi: 10.14336/ad.2016.0819
76. Yang ZY and Chen WL. Examining the association between serum leptin and sarcopenic obesity. J Inflammation Res. (2021) 14:3481–7. doi: 10.2147/jir.S320445
77. Kao TW, Peng TC, Chen WL, Chi YC, Chen CL, and Yang WS. Higher Serum Leptin Levels Are Associated with a Reduced Risk of Sarcopenia but a Higher Risk of Dynapenia among Older Adults. J Inflammation Res. (2021) 14:5817–25. doi: 10.2147/jir.S335694
78. Collins KH, Gui C, Ely EV, Lenz KL, Harris CA, Guilak F, et al. Leptin mediates the regulation of muscle mass and strength by adipose tissue. J Physiol. (2022) 600:3795–817. doi: 10.1113/jp283034
79. McPherron AC, Lawler AM, and Lee SJ. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature. (1997) 387:83–90. doi: 10.1038/387083a0
80. Yang M, Liu C, Jiang N, Liu Y, Luo S, Li C, et al. Myostatin: A potential therapeutic target for metabolic syndrome. Front Endocrinol. (2023) 14:1181913. doi: 10.3389/fendo.2023.1181913
81. Lee SJ. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. (2021) 131. doi: 10.1172/jci148372
82. Zhou H, Meng J, Malerba A, Catapano F, Sintusek P, Jarmin S, et al. Myostatin inhibition in combination with antisense oligonucleotide therapy improves outcomes in spinal muscular atrophy. J Cachexia Sarcopenia Muscle. (2020) 11:768–82. doi: 10.1002/jcsm.12542
83. Baczek J, Silkiewicz M, and Wojszel ZB. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients. (2020) 12. doi: 10.3390/nu12082401
84. Abati E, Manini A, Comi GP, and Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci: CMLS. (2022) 79:374. doi: 10.1007/s00018-022-04408-w
85. Consitt LA and Clark BC. The vicious cycle of myostatin signaling in sarcopenic obesity: myostatin role in skeletal muscle growth, insulin signaling and implications for clinical trials. J Frailty Aging. (2018) 7:21–7. doi: 10.14283/jfa.2017.33
86. Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, and Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutrition Health Aging. (2002) 6:343–8. doi: 10.1016/S0531-5565(02)00196-3
87. Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordrecht Netherlands). (2015) 37:121. doi: 10.1007/s11357-015-9860-3
88. McFarlane C, Langley B, Thomas M, Hennebry A, Plummer E, Nicholas G, et al. Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev Biol. (2005) 283:58–69. doi: 10.1016/j.ydbio.2005.03.039
89. Eilers W, Chambers D, Cleasby M, and Foster K. Local myostatin inhibition improves skeletal muscle glucose uptake in insulin-resistant high-fat diet-fed mice. Am J Physiol Endocrinol Metab. (2020) 319:E163–e74. doi: 10.1152/ajpendo.00185.2019
90. Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci: CMLS. (2014) 71:4361–71. doi: 10.1007/s00018-014-1689-x
91. Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, and Vannier E. Skeletal muscle wasting and renewal: A pivotal role of myokine il-6. SpringerPlus. (2016) 5:619. doi: 10.1186/s40064-016-2197-2
92. Forcina L, Miano C, and Musarò A. The physiopathologic interplay between stem cells and tissue niche in muscle regeneration and the role of il-6 on muscle homeostasis and diseases. Cytokine Growth Factor Rev. (2018) 41:1–9. doi: 10.1016/j.cytogfr.2018.05.001
93. Pinto AP, Muñoz VR, da Rocha AL, Rovina RL, Ferrari GD, Alberici LC, et al. Il-6 deletion decreased rev-erbα Protein and influenced autophagy and mitochondrial markers in the skeletal muscle after acute exercise. Front Immunol. (2022) 13:953272. doi: 10.3389/fimmu.2022.953272
94. Del Giudice M and Gangestad SW. Rethinking il-6 and crp: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. (2018) 70:61–75. doi: 10.1016/j.bbi.2018.02.013
95. Mihara M, Hashizume M, Yoshida H, Suzuki M, and Shiina M. Il-6/il-6 receptor system and its role in physiological and pathological conditions. Clin Sci (London England: 1979). (2012) 122:143–59. doi: 10.1042/cs20110340
96. Hennigar SR, McClung JP, and Pasiakos SM. Nutritional interventions and the il-6 response to exercise. FASEB J: Off Publ Fed Am Societies Exp Biol. (2017) 31:3719–28. doi: 10.1096/fj.201700080R
97. Grosicki GJ, Barrett BB, Englund DA, Liu C, Travison TG, Cederholm T, et al. Circulating interleukin-6 is associated with skeletal muscle strength, quality, and functional adaptation with exercise training in mobility-limited older adults. J Frailty Aging. (2020) 9:57–63. doi: 10.14283/jfa.2019.30
98. Lightfoot AP, Sakellariou GK, Nye GA, McArdle F, Jackson MJ, Griffiths RD, et al. Ss-31 attenuates tnf-α Induced cytokine release from C2c12 myotubes. Redox Biol. (2015) 6:253–9. doi: 10.1016/j.redox.2015.08.007
99. Pelosi L, Berardinelli MG, Forcina L, Ascenzi F, Rizzuto E, Sandri M, et al. Sustained systemic levels of il-6 impinge early muscle growth and induce muscle atrophy and wasting in adulthood. Cells. (2021) 10. doi: 10.3390/cells10071816
100. Wu C, Tang L, Ni X, Xu T, Fang Q, Xu L, et al. Salidroside attenuates denervation-induced skeletal muscle atrophy through negative regulation of pro-inflammatory cytokine. Front Physiol. (2019) 10:665. doi: 10.3389/fphys.2019.00665
101. Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exercise Immunol Rev. (2006) 12:6–33. doi: 10.1055/s-2006-948307
102. Tanaka T, Narazaki M, and Kishimoto T. Il-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
103. Sun H, Sun J, Li M, Qian L, Zhang L, Huang Z, et al. Transcriptome analysis of immune receptor activation and energy metabolism reduction as the underlying mechanisms in interleukin-6-induced skeletal muscle atrophy. Front Immunol. (2021) 12:730070. doi: 10.3389/fimmu.2021.730070
104. Kistner TM, Pedersen BK, and Lieberman DE. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. (2022) 4:170–9. doi: 10.1038/s42255-022-00538-4
105. Crescioli C. Targeting age-dependent functional and metabolic decline of human skeletal muscle: the geroprotective role of exercise, myokine il-6, and vitamin D. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21031010
106. Tanaka T, Narazaki M, Masuda K, and Kishimoto T. Regulation of il-6 in immunity and diseases. Adv Exp Med Biol. (2016) 941:79–88. doi: 10.1007/978-94-024-0921-5_4
107. da Rocha AL, Teixeira GR, Pinto AP, de Morais GP, Oliveira LDC, de Vicente LG, et al. Excessive training induces molecular signs of pathologic cardiac hypertrophy. J Cell Physiol. (2018) 233:8850–61. doi: 10.1002/jcp.26799
108. Muñoz-Cánoves P, Scheele C, Pedersen BK, and Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. (2013) 280:4131–48. doi: 10.1111/febs.12338
109. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A pgc1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. (2012) 481:463–8. doi: 10.1038/nature10777
110. Chen JQ, Huang YY, Gusdon AM, and Qu S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. (2015) 14:2. doi: 10.1186/1476-511x-14-2
111. Li H, Wang F, Yang M, Sun J, Zhao Y, and Tang D. The effect of irisin as a metabolic regulator and its therapeutic potential for obesity. Int J Endocrinol. (2021) 2021:6572342. doi: 10.1155/2021/6572342
112. Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. (2017) 7:2811. doi: 10.1038/s41598-017-02557-8
113. Reza MM, Sim CM, Subramaniyam N, Ge X, Sharma M, Kambadur R, et al. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget. (2017) 8:98553–66. doi: 10.18632/oncotarget.21636
114. Alizadeh Pahlavani H. Exercise therapy for people with sarcopenic obesity: myokines and adipokines as effective actors. Front Endocrinol. (2022) 13:811751. doi: 10.3389/fendo.2022.811751
115. Baek JY, Jang IY, Jung HW, Park SJ, Lee JY, Choi E, et al. Serum irisin level is independent of sarcopenia and related muscle parameters in older adults. Exp Gerontol. (2022) 162:111744. doi: 10.1016/j.exger.2022.111744
116. Sun H, Sherrier M, and Li H. Skeletal muscle and bone - emerging targets of fibroblast growth factor-21. Front Physiol. (2021) 12:625287. doi: 10.3389/fphys.2021.625287
117. Kaur N, Gare SR, Shen J, Raja R, Fonseka O, and Liu W. Multi-organ fgf21-fgfr1 signaling in metabolic health and disease. Front Cardiovasc Med. (2022) 9:962561. doi: 10.3389/fcvm.2022.962561
118. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, and Lee MS. Acute exercise induces fgf21 expression in mice and in healthy humans. PloS One. (2013) 8:e63517. doi: 10.1371/journal.pone.0063517
119. Arias-Calderón M, Casas M, Balanta-Melo J, Morales-Jiménez C, Hernández N, Llanos P, et al. Fibroblast growth factor 21 is expressed and secreted from skeletal muscle following electrical stimulation via extracellular atp activation of the pi3k/akt/mtor signaling pathway. Front Endocrinol. (2023) 14:1059020. doi: 10.3389/fendo.2023.1059020
120. Salminen A, Kauppinen A, and Kaarniranta K. Fgf21 activates ampk signaling: impact on metabolic regulation and the aging process. J Mol Med (Berlin Germany). (2017) 95:123–31. doi: 10.1007/s00109-016-1477-1
121. Oost LJ, Kustermann M, Armani A, Blaauw B, and Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. (2019) 10:630–42. doi: 10.1002/jcsm.12409
122. Zhou S, Qian B, Wang L, Zhang C, Hogan MV, and Li H. Altered bone-regulating myokine expression in skeletal muscle of duchenne muscular dystrophy mouse models. Muscle Nerve. (2018) 58:573–82. doi: 10.1002/mus.26195
123. Jung HW, Park JH, Kim DA, Jang IY, Park SJ, Lee JY, et al. Association between serum fgf21 level and sarcopenia in older adults. Bone. (2021) 145:115877. doi: 10.1016/j.bone.2021.115877
124. Roh E, Hwang SY, Yoo HJ, Baik SH, Cho B, Park YS, et al. Association of plasma fgf21 levels with muscle mass and muscle strength in a national multicentre cohort study: korean frailty and aging cohort study. Age Ageing. (2021) 50:1971–8. doi: 10.1093/ageing/afab178
125. Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, et al. Age-associated loss of opa1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. (2017) 25:1374–89.e6. doi: 10.1016/j.cmet.2017.04.021
126. Chau MD, Gao J, Yang Q, Wu Z, and Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the ampk-sirt1-pgc-1alpha pathway. Proc Natl Acad Sci United States America. (2010) 107:12553–8. doi: 10.1073/pnas.1006962107
127. Ost M, Coleman V, Voigt A, van Schothorst EM, Keipert S, van der Stelt I, et al. Muscle mitochondrial stress adaptation operates independently of endogenous fgf21 action. Mol Metab. (2016) 5:79–90. doi: 10.1016/j.molmet.2015.11.002
128. Rosales-Soto G, Diaz-Vegas A, Casas M, Contreras-Ferrat A, and Jaimovich E. Fibroblast growth factor-21 potentiates glucose transport in skeletal muscle fibers. J Mol Endocrinol. (2020) 65(3):85–9. doi: 10.1530/jme-19-0210
129. Homer-Bouthiette C, Xiao L, and Hurley MM. Gait disturbances and muscle dysfunction in fibroblast growth factor 2 knockout mice. Sci Rep. (2021) 11:11005. doi: 10.1038/s41598-021-90565-0
130. Wang Z, Bian L, Mo C, Shen H, Zhao LJ, Su KJ, et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun Biol. (2020) 3:39. doi: 10.1038/s42003-020-0766-y
131. Lyssikatos C, Wang Z, Liu Z, Warden S, Brotto M, and Bonewald L. The L-enantiomer of β- aminobutyric acid (L-baiba) as a potential marker of bone mineral density, body mass index, while D-baiba of physical performance and age. Res Square. (2023). doi: 10.21203/rs.3.rs-2492688/v1
132. Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, Yi J, et al. β-aminoisobutyric acid, L-baiba, is a muscle-derived osteocyte survival factor. Cell Rep. (2018) 22:1531–44. doi: 10.1016/j.celrep.2018.01.041
133. Feng J, Wang X, Lu Y, Yu C, Wang X, and Feng L. Baiba involves in hypoxic training induced browning of white adipose tissue in obese rats. Front Physiol. (2022) 13:882151. doi: 10.3389/fphys.2022.882151
134. Shimba Y, Katayama K, Miyoshi N, Ikeda M, Morita A, and Miura S. β-aminoisobutyric acid suppresses atherosclerosis in apolipoprotein E-knockout mice. Biol Pharm Bull. (2020) 43:1016–9. doi: 10.1248/bpb.b20-00078
135. Yang YJ and Kim DJ. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: osteoporosis, sarcopenia, and osteoporotic sarcopenia. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22052604
136. Hangelbroek RW, Fazelzadeh P, Tieland M, Boekschoten MV, Hooiveld GJ, van Duynhoven JP, et al. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J Cachexia Sarcopenia Muscle. (2016) 7:604–14. doi: 10.1002/jcsm.12099
137. Huang EJ and Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. (2001) 24:677–736. doi: 10.1146/annurev.neuro.24.1.677
138. Gibon J, Barker PA, and Séguéla P. Opposing presynaptic roles of bdnf and probdnf in the regulation of persistent activity in the entorhinal cortex. Mol Brain. (2016) 9:23. doi: 10.1186/s13041-016-0203-9
139. Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, and Lu B. Role of pro-brain-derived neurotrophic factor (Probdnf) to mature bdnf conversion in activity-dependent competition at developing neuromuscular synapses. Proc Natl Acad Sci United States America. (2012) 109:15924–9. doi: 10.1073/pnas.1207767109
140. Murawska-Ciałowicz E, Wiatr M, Ciałowicz M, Gomes de Assis G, Borowicz W, Rocha-Rodrigues S, et al. Bdnf impact on biological markers of depression-role of physical exercise and training. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph18147553
141. Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of amp-activated protein kinase. Diabetologia. (2009) 52:1409–18. doi: 10.1007/s00125-009-1364-1
142. Just-Borràs L, Cilleros-Mañé V, Hurtado E, Biondi O, Charbonnier F, Tomàs M, et al. Running and swimming differently adapt the bdnf/trkb pathway to a slow molecular pattern at the nmj. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22094577
143. Rentería I, García-Suárez PC, Fry AC, Moncada-Jiménez J, MaChado-Parra JP, Antunes BM, et al. The molecular effects of bdnf synthesis on skeletal muscle: A mini-review. Front Physiol. (2022) 13:934714. doi: 10.3389/fphys.2022.934714
144. Walsh JJ, Edgett BA, Tschakovsky ME, and Gurd BJ. Fasting and exercise differentially regulate bdnf mrna expression in human skeletal muscle. Appl Physiol Nutrition Metab. (2015) 40:96–8. doi: 10.1139/apnm-2014-0290
145. Ahuja P, Ng CF, Pang BPS, Chan WS, Tse MCL, Bi X, et al. Muscle-generated bdnf (Brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy. (2022) 18:1367–84. doi: 10.1080/15548627.2021.1985257
146. Nagahisa H and Miyata H. Influence of hypoxic stimulation on angiogenesis and satellite cells in mouse skeletal muscle. PloS One. (2018) 13:e0207040. doi: 10.1371/journal.pone.0207040
147. Guilherme J, Semenova EA, Borisov OV, Kostryukova ES, Vepkhvadze TF, Lysenko EA, et al. The bdnf-increasing allele is associated with increased proportion of fast-twitch muscle fibers, handgrip strength, and power athlete status. J Strength Conditioning Res. (2022) 36:1884–9. doi: 10.1519/jsc.0000000000003756
148. Lee JH and Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. (2019) 10:42. doi: 10.3389/fphys.2019.00042
149. Miyazaki S, Iino N, Koda R, Narita I, and Kaneko Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatrics Gerontol Int. (2021) 21:27–33. doi: 10.1111/ggi.14089
150. Halievski K, Xu Y, Haddad YW, Tang YP, Yamada S, Katsuno M, et al. Muscle bdnf improves synaptic and contractile muscle strength in kennedy’s disease mice in a muscle-type specific manner. J Physiol. (2020) 598:2719–39. doi: 10.1113/jp279208
151. Karim A, Muhammad T, and Qaisar R. Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J Personalized Med. (2021) 11. doi: 10.3390/jpm11090919
152. Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (2005). (2014) 38:707–13. doi: 10.1038/ijo.2013.158
153. Mannelli M, Gamberi T, Magherini F, and Fiaschi T. The adipokines in cancer cachexia. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21144860
154. Le Moal E, Liu Y, Collerette-Tremblay J, Dumontier S, Fabre P, Molina T, et al. Apelin stimulation of the vascular skeletal muscle stem cell niche enhances endogenous repair in dystrophic mice. Sci Trans Med. (2024) 16:eabn8529. doi: 10.1126/scitranslmed.abn8529
155. Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, Le Gonidec S, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. (2018) 24:1360–71. doi: 10.1038/s41591-018-0131-6
156. Son JS, Kim HJ, Son Y, Lee H, Chae SA, Seong JK, et al. Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve. (2017) 56:1155–63. doi: 10.1002/mus.25596
157. Kadoglou NP, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monitor: Int Med J Exp Clin Res. (2012) 18:Cr290–5. doi: 10.12659/msm.882734
158. Frontera WR and Ochala J. Skeletal muscle: A brief review of structure and function. Calcified Tissue Int. (2015) 96:183–95. doi: 10.1007/s00223-014-9915-y
159. Luo J, Liu W, Feng F, and Chen L. Apelin/apj system: A novel therapeutic target for locomotor system diseases. Eur J Pharmacol. (2021) 906:174286. doi: 10.1016/j.ejphar.2021.174286
160. Ligetvári R, Szokodi I, Far G, Csöndör É, Móra Á, Komka Z, et al. Apelin as a potential regulator of peak athletic performance. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24098195
161. Kon M, Tanimura Y, and Yoshizato H. Effects of acute endurance exercise on follistatin-like 1 and apelin in the circulation and metabolic organs in rats. Arch Physiol Biochem. (2022) 128:1254–8. doi: 10.1080/13813455.2020.1764050
162. McKenna CF, Salvador AF, Keeble AR, Khan NA, De Lisio M, Konopka AR, et al. Muscle strength after resistance training correlates to mediators of muscle mass and mitochondrial respiration in middle-aged adults. J Appl Physiol (Bethesda Md: 1985). (2022) 133:572–84. doi: 10.1152/japplphysiol.00186.2022
163. Goldspink G. Mechanical signals, igf-I gene splicing, and muscle adaptation. Physiol (Bethesda Md). (2005) 20:232–8. doi: 10.1152/physiol.00004.2005
164. Hameed M, Orrell RW, Cobbold M, Goldspink G, and Harridge SD. Expression of igf-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. (2003) 547:247–54. doi: 10.1113/jphysiol.2002.032136
165. Barclay RD, Burd NA, Tyler C, Tillin NA, and Mackenzie RW. The role of the igf-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. (2019) 6:146. doi: 10.3389/fnut.2019.00146
166. Hofmann M, Halper B, Oesen S, Franzke B, Stuparits P, Tschan H, et al. Serum concentrations of insulin-like growth factor-1, members of the tgf-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol. (2015) 64:35–45. doi: 10.1016/j.exger.2015.02.008
167. Hata R, Miyamoto K, Abe Y, Sasaki T, Oguma Y, Tajima T, et al. Osteoporosis and sarcopenia are associated with each other and reduced igf1 levels are a risk for both diseases in the very old elderly. Bone. (2023) 166:116570. doi: 10.1016/j.bone.2022.116570
168. Jiang JJ, Chen SM, Chen J, Wu L, Ye JT, and Zhang Q. Serum igf-1 levels are associated with sarcopenia in elderly men but not in elderly women. Aging Clin Exp Res. (2022) 34:2465–71. doi: 10.1007/s40520-022-02180-2
169. Bian A, Ma Y, Zhou X, Guo Y, Wang W, Zhang Y, et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskeletal Disord. (2020) 21:214. doi: 10.1186/s12891-020-03236-y
170. Li B, Feng L, Wu X, Cai M, Yu JJ, and Tian Z. Effects of different modes of exercise on skeletal muscle mass and function and igf-1 signaling during early aging in mice. J Exp Biol. (2022) 225. doi: 10.1242/jeb.244650
171. Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, et al. Monocyte/macrophage-derived igf-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther: J Am Soc Gene Ther. (2015) 23:1189–200. doi: 10.1038/mt.2015.66
172. Yoshida T and Delafontaine P. Mechanisms of igf-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. (2020) 9. doi: 10.3390/cells9091970
173. O’Neill BT, Lee KY, Klaus K, Softic S, Krumpoch MT, Fentz J, et al. Insulin and igf-1 receptors regulate foxo-mediated signaling in muscle proteostasis. J Clin Invest. (2016) 126:3433–46. doi: 10.1172/jci86522
174. Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, et al. Insulin-like growth factor-1 (Igf-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin (Pi3k/akt/mtor) pathway. J Biol Chem. (2005) 280:2737–44. doi: 10.1074/jbc.M407517200
175. Choi K, Jang HY, Ahn JM, Hwang SH, Chung JW, Choi YS, et al. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. (2020) 26:492–505. doi: 10.3350/cmh.2020.0005
176. Xi Y, Hao M, Liang Q, Li Y, Gong DW, and Tian Z. Dynamic resistance exercise increases skeletal muscle-derived fstl1 inducing cardiac angiogenesis via dip2a-smad2/3 in rats following myocardial infarction. J Sport Health Sci. (2021) 10:594–603. doi: 10.1016/j.jshs.2020.11.010
177. Iyer CC, Chugh D, Bobbili PJ, Iii AJB, Crum AE, Yi AF, et al. Follistatin-induced muscle hypertrophy in aged mice improves neuromuscular junction innervation and function. Neurobiol Aging. (2021) 104:32–41. doi: 10.1016/j.neurobiolaging.2021.03.005
178. Chan ASM, McGregor NE, Poulton IJ, Hardee JP, Cho EH, Martin TJ, et al. Bone geometry is altered by follistatin-induced muscle growth in young adult male mice. JBMR Plus. (2021) 5:e10477. doi: 10.1002/jbm4.10477
179. Fife E, Kostka J, Kroc Ł, Guligowska A, Pigłowska M, Sołtysik B, et al. Relationship of muscle function to circulating myostatin, follistatin and gdf11 in older women and men. BMC Geriatr. (2018) 18:200. doi: 10.1186/s12877-018-0888-y
180. Mariot V, Joubert R, Hourdé C, Féasson L, Hanna M, Muntoni F, et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat Commun. (2017) 8:1859. doi: 10.1038/s41467-017-01486-4
181. Kawaguchi T, Yoshio S, Sakamoto Y, Hashida R, Koya S, Hirota K, et al. Impact of decorin on the physical function and prognosis of patients with hepatocellular carcinoma. J Clin Med. (2020) 9. doi: 10.3390/jcm9040936
182. Goetsch KP and Niesler CU. The extracellular matrix regulates the effect of decorin and transforming growth factor beta-2 (Tgf-β2) on myoblast migration. Biochem Biophys Res Commun. (2016) 479:351–7. doi: 10.1016/j.bbrc.2016.09.079
183. El Shafey N, Guesnon M, Simon F, Deprez E, Cosette J, Stockholm D, et al. Inhibition of the myostatin/smad signaling pathway by short decorin-derived peptides. Exp Cell Res. (2016) 341:187–95. doi: 10.1016/j.yexcr.2016.01.019
184. Kishioka Y, Thomas M, Wakamatsu J, Hattori A, Sharma M, Kambadur R, et al. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J Cell Physiol. (2008) 215:856–67. doi: 10.1002/jcp.21371
185. Guiraud S, van Wittenberghe L, Georger C, Scherman D, and Kichler A. Identification of decorin derived peptides with a zinc dependent anti-myostatin activity. Neuromuscular Disorders: NMD. (2012) 22:1057–68. doi: 10.1016/j.nmd.2012.07.002
186. Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, et al. Lif is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol (Bethesda Md: 1985). (2011) 111:251–9. doi: 10.1152/japplphysiol.01399.2010
187. Nadeau L and Aguer C. Interleukin-15 as a myokine: mechanistic insight into its effect on skeletal muscle metabolism. Appl Physiol Nutrition Metab Physiol Appliquee Nutr Metabol. (2019) 44:229–38. doi: 10.1139/apnm-2018-0022
188. Nadeau L, Patten DA, Caron A, Garneau L, Pinault-Masson E, Foretz M, et al. Il-15 improves skeletal muscle oxidative metabolism and glucose uptake in association with increased respiratory chain supercomplex formation and ampk pathway activation. Biochim Biophys Acta Gen Subj. (2019) 1863:395–407. doi: 10.1016/j.bbagen.2018.10.021
189. Quinn LS. Interleukin-15: A muscle-derived cytokine regulating fat-to-lean body composition. J Anim Sci. (2008) 86:E75–83. doi: 10.2527/jas.2007-0458
190. Tanaka M, Sugimoto K, Akasaka H, Yoshida S, Takahashi T, Fujimoto T, et al. Effects of interleukin-15 on autophagy regulation in the skeletal muscle of mice. Am J Physiol Endocrinol Metab. (2024) 326:E326–e40. doi: 10.1152/ajpendo.00311.2023
191. Pérez-López A, McKendry J, Martin-Rincon M, Morales-Alamo D, Pérez-Köhler B, Valadés D, et al. Skeletal muscle il-15/il-15rα and myofibrillar protein synthesis after resistance exercise. Scandinavian J Med Sci Sports. (2018) 28:116–25. doi: 10.1111/sms.12901
192. Lazarus NR, Lord JM, and Harridge SDR. The relationships and interactions between age, exercise and physiological function. J Physiol. (2019) 597:1299–309. doi: 10.1113/jp277071
193. Liu QQ, Xie WQ, Luo YX, Li YD, Huang WH, Wu YX, et al. High intensity interval training: A potential method for treating sarcopenia. Clin Interventions Aging. (2022) 17:857–72. doi: 10.2147/cia.S366245
194. Gillen JB and Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutrition Metab Physiol Appliquee Nutr Metabol. (2014) 39:409–12. doi: 10.1139/apnm-2013-0187
195. Seldeen KL, Lasky G, Leiker MM, Pang M, Personius KE, and Troen BR. High intensity interval training improves physical performance and frailty in aged mice. J Gerontol Ser A Biol Sci Med Sci. (2018) 73:429–37. doi: 10.1093/gerona/glx120
196. Hurst C, Robinson SM, Witham MD, Dodds RM, Granic A, Buckland C, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. (2022) 51. doi: 10.1093/ageing/afac003
197. Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, et al. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J Am Med Directors Assoc. (2019) 20:28–34. doi: 10.1016/j.jamda.2018.09.011
198. Liang J, Zhang H, Zeng Z, Lv J, Huang J, Wu X, et al. Microrna profiling of different exercise interventions for alleviating skeletal muscle atrophy in naturally aging rats. J Cachexia Sarcopenia Muscle. (2023) 14:356–68. doi: 10.1002/jcsm.13137
199. Kemmler W, Kohl M, Fröhlich M, Jakob F, Engelke K, von Stengel S, et al. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia-one-year results of the randomized controlled franconian osteopenia and sarcopenia trial (Frost). J Bone Mineral Res: Off J Am Soc Bone Mineral Res. (2020) 35:1634–44. doi: 10.1002/jbmr.4027
200. Burtscher J, Strasser B, D’Antona G, Millet GP, and Burtscher M. How much resistance exercise is beneficial for healthy aging and longevity? J Sport Health Sci. (2023) 12:284–6. doi: 10.1016/j.jshs.2022.11.004
201. Yasuda T. Selected methods of resistance training for prevention and treatment of sarcopenia. Cells. (2022) 11. doi: 10.3390/cells11091389
202. Arntz F, Markov A, Behm DG, Behrens M, Negra Y, Nakamura M, et al. Chronic effects of static stretching exercises on muscle strength and power in healthy individuals across the lifespan: A systematic review with multi-level meta-analysis. Sports Med (Auckland NZ). (2023) 53:723–45. doi: 10.1007/s40279-022-01806-9
203. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr (Edinburgh Scotland). (2018) 37:1121–32. doi: 10.1016/j.clnu.2017.08.016
204. Remelli F, Vitali A, Zurlo A, and Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. (2019) 11. doi: 10.3390/nu11122861
205. Crescioli C. Vitamin D restores skeletal muscle cell remodeling and myogenic program: potential impact on human health. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22041760
206. Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle. (2019) 10:1228–40. doi: 10.1002/jcsm.12460
207. Agoncillo M, Yu J, and Gunton JE. The role of vitamin D in skeletal muscle repair and regeneration in animal models and humans: A systematic review. Nutrients. (2023) 15. doi: 10.3390/nu15204377
208. Hernández-Lepe MA, Miranda-Gil MI, Valbuena-Gregorio E, and Olivas-Aguirre FJ. Exercise programs combined with diet supplementation improve body composition and physical function in older adults with sarcopenia: A systematic review. Nutrients. (2023) 15. doi: 10.3390/nu15081998
209. Cummings SR, Kiel DP, and Black DM. Vitamin D supplementation and increased risk of falling: A cautionary tale of vitamin supplements retold. JAMA Internal Med. (2016) 176:171–2. doi: 10.1001/jamainternmed.2015.7568
210. Agergaard J, Trøstrup J, Uth J, Iversen JV, Boesen A, Andersen JL, et al. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? - a randomized controlled trial. Nutr Metab. (2015) 12:32. doi: 10.1186/s12986-015-0029-y
211. Jeong D, Park K, Lee J, Choi J, Du H, Jeong H, et al. Effects of resistance exercise and essential amino acid intake on muscle quality, myokine, and inflammation factors in young adult males. Nutrients. (2024) 16. doi: 10.3390/nu16111688
212. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporosis Int: J Established Result Coop Between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA. (2017) 28:1817–33. doi: 10.1007/s00198-017-3980-9
213. Nilsson MI, Mikhail A, Lan L, Di Carlo A, Hamilton B, Barnard K, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. (2020) 12. doi: 10.3390/nu12082391
214. Cuyul-Vásquez I, Pezo-Navarrete J, Vargas-Arriagada C, Ortega-Díaz C, Sepúlveda-Loyola W, Hirabara SM, et al. Effectiveness of whey protein supplementation during resistance exercise training on skeletal muscle mass and strength in older people with sarcopenia: A systematic review and meta-analysis. Nutrients. (2023) 15. doi: 10.3390/nu15153424
215. Zhu LY, Chan R, Kwok T, Cheng KC, Ha A, and Woo J. Effects of exercise and nutrition supplementation in community-dwelling older chinese people with sarcopenia: A randomized controlled trial. Age Ageing. (2019) 48:220–8. doi: 10.1093/ageing/afy179
216. Wang Z, Xu X, Gao S, Wu C, Song Q, Shi Z, et al. Effects of internet-based nutrition and exercise interventions on the prevention and treatment of sarcopenia in the elderly. Nutrients. (2022) 14. doi: 10.3390/nu14122458
217. Ebert SM, Dyle MC, Bullard SA, Dierdorff JM, Murry DJ, Fox DK, et al. Identification and small molecule inhibition of an activating transcription factor 4 (Atf4)-dependent pathway to age-related skeletal muscle weakness and atrophy. J Biol Chem. (2015) 290:25497–511. doi: 10.1074/jbc.M115.681445
218. Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SDR, Hart N, et al. β-hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am J Clin Nutr. (2019) 109:1119–32. doi: 10.1093/ajcn/nqy373
219. Li CY, Fang AP, Ma WJ, Wu SL, Li CL, Chen YM, et al. Amount rather than animal vs plant protein intake is associated with skeletal muscle mass in community-dwelling middle-aged and older chinese adults: results from the guangzhou nutrition and health study. J Acad Nutr Dietetics. (2019) 119:1501–10. doi: 10.1016/j.jand.2019.03.010
220. Ren L, Tang Y, Yang R, Hu Y, Wang J, Li S, et al. Plant-based dietary pattern and low muscle mass: A nation-wide cohort analysis of chinese older adults. BMC Geriatr. (2023) 23:569. doi: 10.1186/s12877-023-04265-7
221. Isenmann E, Trojak I, Lesch A, Schalla J, Havers T, Diel P, et al. The influence of a vegan diet on body composition, performance and the menstrual cycle in young, recreationally trained women- a 12-week controlled trial. J Int Soc Sports Nutr. (2024) 21:2413961. doi: 10.1080/15502783.2024.2413961
222. Domić J, Grootswagers P, van Loon LJC, and de Groot L. Perspective: vegan diets for older adults? A perspective on the potential impact on muscle mass and strength. Adv Nutr (Bethesda Md). (2022) 13:712–25. doi: 10.1093/advances/nmac009
223. Jørgensen LH, Jepsen PL, Boysen A, Dalgaard LB, Hvid LG, Ørtenblad N, et al. Sparc Interacts with Actin in Skeletal Muscle In vitro and In vivo. Am J Pathol. (2017) 187:457–74. doi: 10.1016/j.ajpath.2016.10.013
224. Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci United States America. (2015) 112:16042–7. doi: 10.1073/pnas.1514250112
225. Zimowska M, Archacka K, Brzoska E, Bem J, Czerwinska AM, Grabowska I, et al. Il-4 and sdf-1 increase adipose tissue-derived stromal cell ability to improve rat skeletal muscle regeneration. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21093302
226. Benoit B, Meugnier E, Castelli M, Chanon S, Vieille-Marchiset A, Durand C, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. (2017) 23:990–6. doi: 10.1038/nm.4363
227. Martyn JAJ and Kaneki M. Muscle atrophy and the sestrins. New Engl J Med. (2020) 383:1279–82. doi: 10.1056/NEJMcibr2003528
228. Segalés J, Perdiguero E, Serrano AL, Sousa-Victor P, Ortet L, Jardí M, et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nat Commun. (2020) 11:189. doi: 10.1038/s41467-019-13832-9
229. Fu Y, Nie JR, Shang P, Zhang B, Yan DW, Hao X, et al. Tumor necrosis factor α Deficiency promotes myogenesis and muscle regeneration. Zool Res. (2024) 45:951–60. doi: 10.24272/j.issn.2095-8137.2024.039
230. Milewska M, Domoradzki T, Majewska A, Błaszczyk M, Gajewska M, Hulanicka M, et al. Interleukin-8 enhances myocilin expression, akt-foxo3 signaling and myogenic differentiation in rat skeletal muscle cells. J Cell Physiol. (2019) 234:19675–90. doi: 10.1002/jcp.28568
231. Haugen F, Norheim F, Lian H, Wensaas AJ, Dueland S, Berg O, et al. Il-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol Cell Physiol. (2010) 298:C807–16. doi: 10.1152/ajpcell.00094.2009
232. Alizadeh H. Meteorin-like protein (Metrnl): A metabolic syndrome biomarker and an exercise mediator. Cytokine. (2022) 157:155952. doi: 10.1016/j.cyto.2022.155952
233. Du Y, Zhang S, Yu H, Wu Y, Cao N, Wang W, et al. Autoantibodies against β(1)-adrenoceptor exaggerated ventricular remodeling by inhibiting ctrp9 expression. J Am Heart Assoc. (2019) 8:e010475. doi: 10.1161/jaha.118.010475
234. Shan D, Guo S, Wu HK, Lv F, Jin L, Zhang M, et al. Cardiac ischemic preconditioning promotes mg53 secretion through H(2)O(2)-activated protein kinase C-Δ Signaling. Circulation. (2020) 142:1077–91. doi: 10.1161/circulationaha.119.044998
235. Wu HK, Zhang Y, Cao CM, Hu X, Fang M, Yao Y, et al. Glucose-sensitive myokine/cardiokine mg53 regulates systemic insulin response and metabolic homeostasis. Circulation. (2019) 139:901–14. doi: 10.1161/circulationaha.118.037216
236. Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. (2008) 283:32802–11. doi: 10.1074/jbc.M803440200
237. Xi Y, Gong DW, and Tian Z. Fstl1 as a potential mediator of exercise-induced cardioprotection in post-myocardial infarction rats. Sci Rep. (2016) 6:32424. doi: 10.1038/srep32424
238. Mofarrahi M, McClung JM, Kontos CD, Davis EC, Tappuni B, Moroz N, et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regulatory Integr Comp Physiol. (2015) 308:R576–89. doi: 10.1152/ajpregu.00267.2014
239. Arnold L, Perrin H, de Chanville CB, Saclier M, Hermand P, Poupel L, et al. Cx3cr1 deficiency promotes muscle repair and regeneration by enhancing macrophage apoe production. Nat Commun. (2015) 6:8972. doi: 10.1038/ncomms9972
240. Stafeev II, Boldyreva MA, Michurina SS, Agareva MY, Radnaeva AV, Menshikov MY, et al. The efficacy of hgf/vegf gene therapy for limb ischemia in mice with impaired glucose tolerance: shift from angiogenesis to axonal growth and oxidative potential in skeletal muscle. Cells. (2022) 11. doi: 10.3390/cells11233824
241. Milkiewicz M, Hudlicka O, Brown MD, and Silgram H. Nitric oxide, vegf, and vegfr-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circulatory Physiol. (2005) 289:H336–43. doi: 10.1152/ajpheart.01105.2004
242. Achari AE and Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18061321
243. Yue R, Zhou BO, Shimada IS, Zhao Z, and Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. (2016) 18:782–96. doi: 10.1016/j.stem.2016.02.015
244. Zhang KZ, Li JW, Xu JS, Shen ZK, Lin YS, Zhao C, et al. Rbp4 promotes denervation-induced muscle atrophy through stra6-dependent pathway. J Cachexia Sarcopenia Muscle. (2024) 15:1601–15. doi: 10.1002/jcsm.13518
245. Carstensen M, Wiza C, Röhrig K, Fahlbusch P, Roden M, Herder C, et al. Effect of sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PloS One. (2014) 9:e85906. doi: 10.1371/journal.pone.0085906
246. Silveira WA, Gonçalves DA, MaChado J, Lautherbach N, Lustrino D, Paula-Gomes S, et al. Camp-dependent protein kinase inhibits foxo activity and regulates skeletal muscle plasticity in mice. FASEB J: Off Publ Fed Am Societies Exp Biol. (2020) 34:12946–62. doi: 10.1096/fj.201902102RR
247. Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. (2020) 35:101454. doi: 10.1016/j.redox.2020.101454
248. Das S, Chattopadhyay D, Chatterjee SK, Mondal SA, Majumdar SS, Mukhopadhyay S, et al. Increase in pparγ Inhibitory phosphorylation by fetuin-a through the activation of ras-mek-erk pathway causes insulin resistance. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166050. doi: 10.1016/j.bbadis.2020.166050
249. Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. (2005) 11:400–8. doi: 10.1038/nm1214
250. Zhang R. The angptl3-4–8 model, a molecular mechanism for triglyceride trafficking. Open Biol. (2016) 6:150272. doi: 10.1098/rsob.150272
251. Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, et al. Lect2, a ligand for tie1, plays a crucial role in liver fibrogenesis. Cell. (2019) 178:1478–92.e20. doi: 10.1016/j.cell.2019.07.021
252. Kim J, Lee SK, Kim D, Lee E, Park CY, Choe H, et al. Adipose tissue lect2 expression is associated with obesity and insulin resistance in korean women. Obes (Silver Spring Md). (2022) 30:1430–41. doi: 10.1002/oby.23445
253. Ye X, Toyama T, Taguchi K, Arisawa K, Kaneko T, Tsutsumi R, et al. Sulforaphane decreases serum selenoprotein P levels through enhancement of lysosomal degradation independent of nrf2. Commun Biol. (2023) 6:1060. doi: 10.1038/s42003-023-05449-y
254. Abuduwaili H, Kamoshita K, Ishii KA, Takahashi K, Abuduyimiti T, Qifang L, et al. Selenoprotein P deficiency protects against immobilization-induced muscle atrophy by suppressing atrophy-related E3 ubiquitin ligases. Am J Physiol Endocrinol Metab. (2023) 324:E542–e52. doi: 10.1152/ajpendo.00270.2022
255. Becker M, Rabe K, Lebherz C, Zugwurst J, Göke B, Parhofer KG, et al. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in ldl receptor knockout mice on high-fat diet. Diabetes. (2010) 59:2898–903. doi: 10.2337/db10-0362
Keywords: sarcopenia, exerkines, myokines, exercise, skeletal muscle
Citation: Wang H, He W, Chen P, Wang H, Wang H, Zhu L and Liu X (2025) Exerkines and myokines in aging sarcopenia. Front. Endocrinol. 16:1592491. doi: 10.3389/fendo.2025.1592491
Received: 21 March 2025; Accepted: 02 July 2025;
Published: 29 July 2025.
Edited by:
Rita Cassia Menegati Dornelles, São Paulo State University, BrazilReviewed by:
Peggy Biga, University of Alabama at Birmingham, United StatesZiyue Wang, University of Massachusetts Medical School, United States
Copyright © 2025 Wang, He, Chen, Wang, Wang, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiguo Wang, d2FuZ2h1aWd1bzE2M0AxNjMuY29t; Lin Zhu, MTEyNTFAZ3pzcG9ydC5lZHUuY24=; Xiaoguang Liu, bGl1eGdAZ3pzcG9ydC5lZHUuY24=
 Huan Wang
Huan Wang Wenbi He1
Wenbi He1