- Department of Endocrinology, The Seventh Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China
Background: Thyroid cancer (TC) has shown a rising prevalence worldwide. While numerous studies have explored the relationship between vitamin D levels and TC risk, their conclusions remain inconsistent.
Objective: This meta-analysis aims to evaluate the association between serum vitamin D levels, vitamin D deficiency, and TC based on existing evidence.
Methods: We systematically searched the Embase, Web of Science, and PubMed databases for human studies investigating the relationship between vitamin D and TC including a control group. A random-effects model with forest plots was employed to calculate the mean difference (MD) in serum vitamin D levels, the odds ratio (OR) for vitamin D deficiency, and the risk difference (RD) between TC cases and controls. Meta-regressions and subgroup analyses were conducted based on the season of serum 25(OH)D sampling, source of controls, timing of measurement, study type, and testing methods of 25(OH)D. A p-value <0.05 was considered statistically significant.
Results: A total of 23 studies were included. The meta-analysis revealed that TC patients had significantly lower serum vitamin D compared to the controls [SMD = −0.38 (95% CI: −0.62 to −0.14)].Additionally, vitamin D deficiency was significantly more prevalent among TC patients (OR = 1.33, 95% CI: 1.02 to 1.73, P < 0.05). The subgroup analyses demonstrated significant differences across most subgroups, except for post-operative measurements. Seasonal variation in 25(OH)D sampling was identified as a key source of heterogeneity.
Conclusions: The meta-analysis suggests that lower serum vitamin D levels and vitamin D deficiency are significantly associated with an increased risk of TC. However, further studies with standardized protocols for seasonal sampling of vitamin D, source of control, measurement timing, study type, and testing methods of 25(OH)D are needed to clarify this relationship and its underlying mechanisms.
1 Introduction
Thyroid cancer is becoming increasingly prevalent worldwide, ranking as the seventh most common cancer among women in the United States and the ninth globally as of 2023 (1). While differentiated thyroid cancer (DTC) generally has a favorable prognosis and low mortality, medullary thyroid cancer (MTC) and especially anaplastic thyroid cancer (ATC) are associated with poor outcomes despite advancements in therapy (1).Hence, substantial challenges persist in the prevention, optimal management, and early detection of suboptimal outcomes. Vitamin D, a fat-soluble vitamin synthesized in the epidermis of the skin via the energy of ultraviolet radiation (UV-B) or obtained via diet or supplements, regulates more than 1,000 genes in a wide assembly of different cells and tissues involved in malignant cells’ biochemical pathways (2, 3). Vitamin D also regulates immune responses, cell proliferation, differentiation, and apoptosis (3). Therefore, the role of vitamin D in influencing malignant and tumor cells is undeniable. It is found to be a crucial factor in cancer pathology through its regulatory and metabolic roles in the body (4).
The pathogenesis and progression of thyroid cancer are still under discussion. Some scholars believed that potential risk factors for TC include chemical toxins, insulin resistance, metabolic syndrome, and vitamin D deficiency (5–7). There are a lot of studies about the risk of TC and vitamin D. Some studies found that vitamin D levels are significantly associated with the risk of TC and may be a protective factor in the development of TC (8). However, the conclusions are mixed (9–11). Moreover, seasonal variations in sunlight exposure, which affects vitamin D3 synthesis, may affect the results. At the same time, age, gender, ethnicity, types of thyroid cancer, timing of measurement, study type, and testing methods of vitamin D further complicate these associations. There was no unified attention on season and other factors mentioned above in previous meta-analysis. This meta-analysis aims to consolidate existing evidence and explore the impact of these variables on the vitamin D and TC relationship.
2 Methods
2.1 Literature search and extraction strategy
PubMed, Web of Science, and Embase were searched from their respective inception to September 28, 2024. The search terms used included (“thyroid cancer[Title/Abstract]” OR “thyroid tumor[Title/Abstract]” OR “thyroid carcinoma[Title/Abstract]” OR “TC[Title/Abstract]” OR “PTC[Title/Abstract]” OR “FTC[Title/Abstract]” OR “MTC[Title/Abstract]” OR “ATC[Title/Abstract]”) AND (“25-hydroxyvitamin D[Title/Abstract]” OR “vitamin D[Title/Abstract]” OR “25(OH)D[Title/Abstract]” OR “(25 OHD[Title/Abstract]” OR “cholecalciferol[Title/Abstract]”).
2.2 Eligibility criteria and study selection
The criteria for inclusion were as follows (1): randomized clinical trials (RCT), non-randomized clinical trial, observational studies (including cohort and case–control studies), or cross-sectional studies, (2) studies published in English, (3) studies conducted in human subjects, (4) patients with TC in the case group and healthy individuals (or those with benign thyroid diseases) in the control group, and (5) availability of complete data. We excluded the following types of studies: animal models, in vitro studies, narrative and systematic reviews, opinion papers, case reports, or abstract and conference papers.
2.3 Literature screening and data extraction
The following information was extracted independently using a predesigned form: study design, first author, year of publication, location, sample size, study population characteristics (number, age), vitamin D status, main statistical analysis methods, and, if applicable, adjustment variables included in statistical models, levels of vitamin D in both TC cases and controls, and adjusted odds ratios (OR) with 95% confidence intervals (CIs) for every category of vitamin D levels. Continuous variables are depicted as mean and standard deviation (SD); categorical variables are depicted as number and percentages.
2.4 Risk of Bias Analysis and Certainty of Evidence
The study quality was evaluated using the Newcastle–Ottawa Scale (NOS) for case–control studies, and the scale of the Agency for Healthcare Research and Quality (AHQR) was applied to cross-sectional studies.
2.5 Statistical analysis
Meta-analysis was performed using STATA software (version 15.0). According to whether the heterogeneity was low (I2 < 50%) or high (I2≥ 50%), we used fixed- or random-effects models, respectively. The OR was used as a summary statistic for dichotomous variables. The 95% CI was calculated for all mean values. Statistical significance was set at p ≤0.05, with p-values >0.05 considered non-significant.
3 Result
3.1 Literature search, study characteristics, and quality assessment
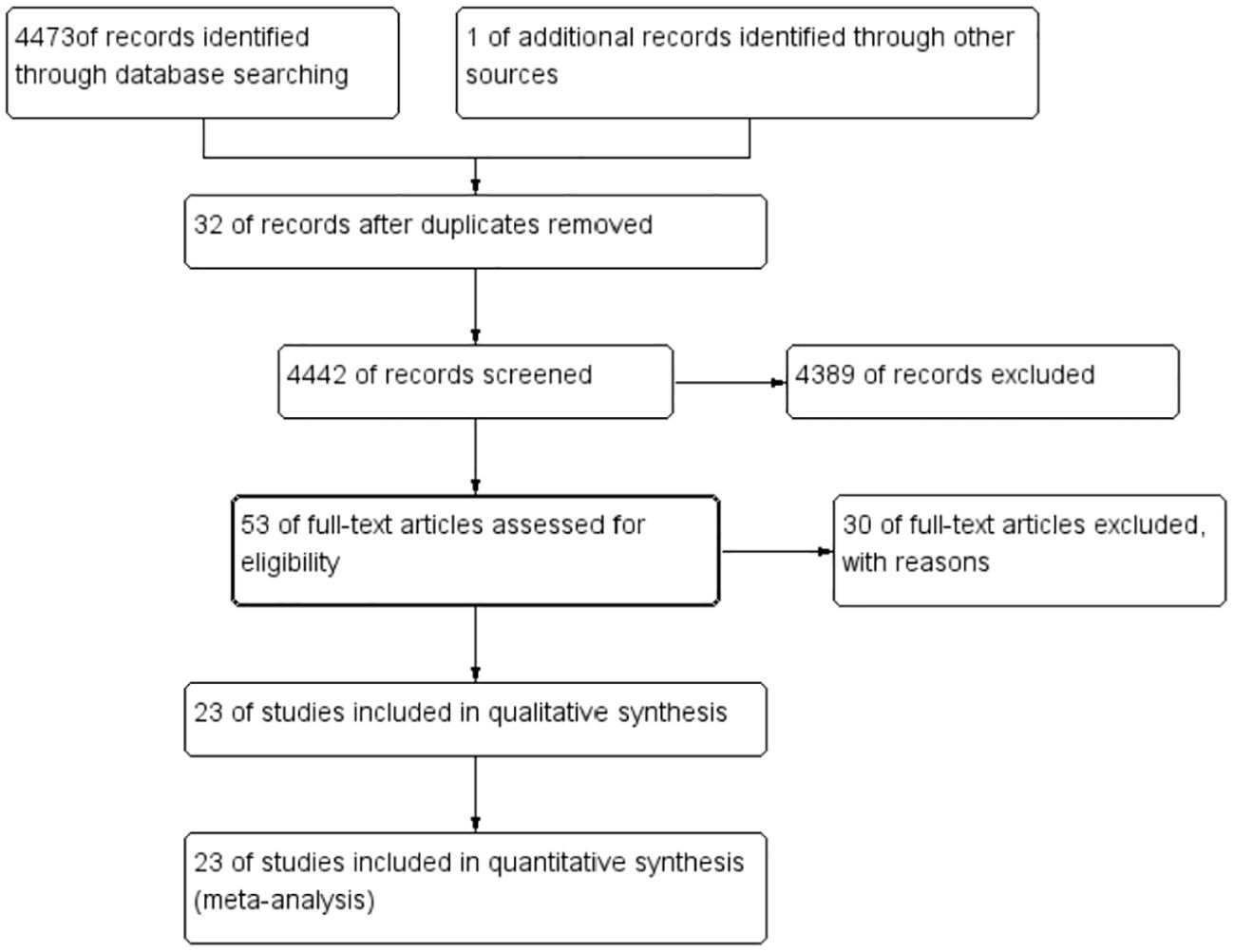
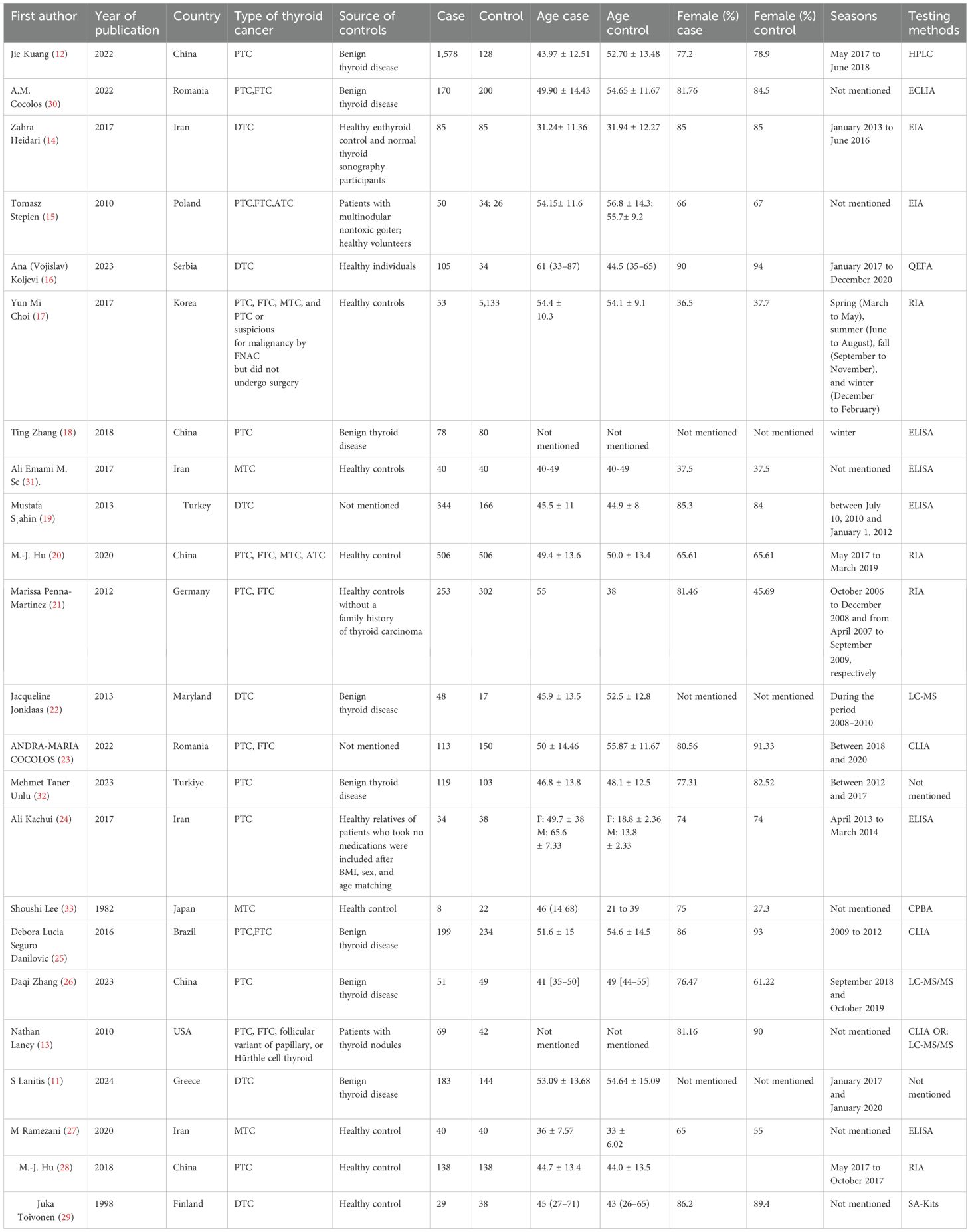
A total of 4,474 were identified in the initial examination. After removing duplicates and conducting a systematic screening of titles, abstracts, and full texts, 23 studies meeting the inclusion criteria were selected for final analysis, comprising 18 case–control studies (12–29) and five cross-sectional studies (11, 30–33). The literature search process and results are shown in Figure 1. The 23 included articles comprised 12,153 participants (ranging from 30 to 5,186), 4,362 cases, and 7,791 controls. The included studies were published between 1982 (33) and 2024 (11) with geographical distribution as follows: China (n = 5) (12, 18, 20, 26, 28), Iran (n = 4) (14, 24, 27, 31), Romania (n = 2) (23, 30), and Turkey (n = 2) (19, 32) and one study each from the USA (13), Poland (15), Serbia (16), Korea (17), Germany (21), MS (22), Japan (33), Brazil (25), Greece (15), and Finland (30). Detailed characteristics of the 23 included studies are presented in Table 1. The quality of all included studies was relatively high, with quality scores ranging from 6 to 9 (Tables 2, 3).
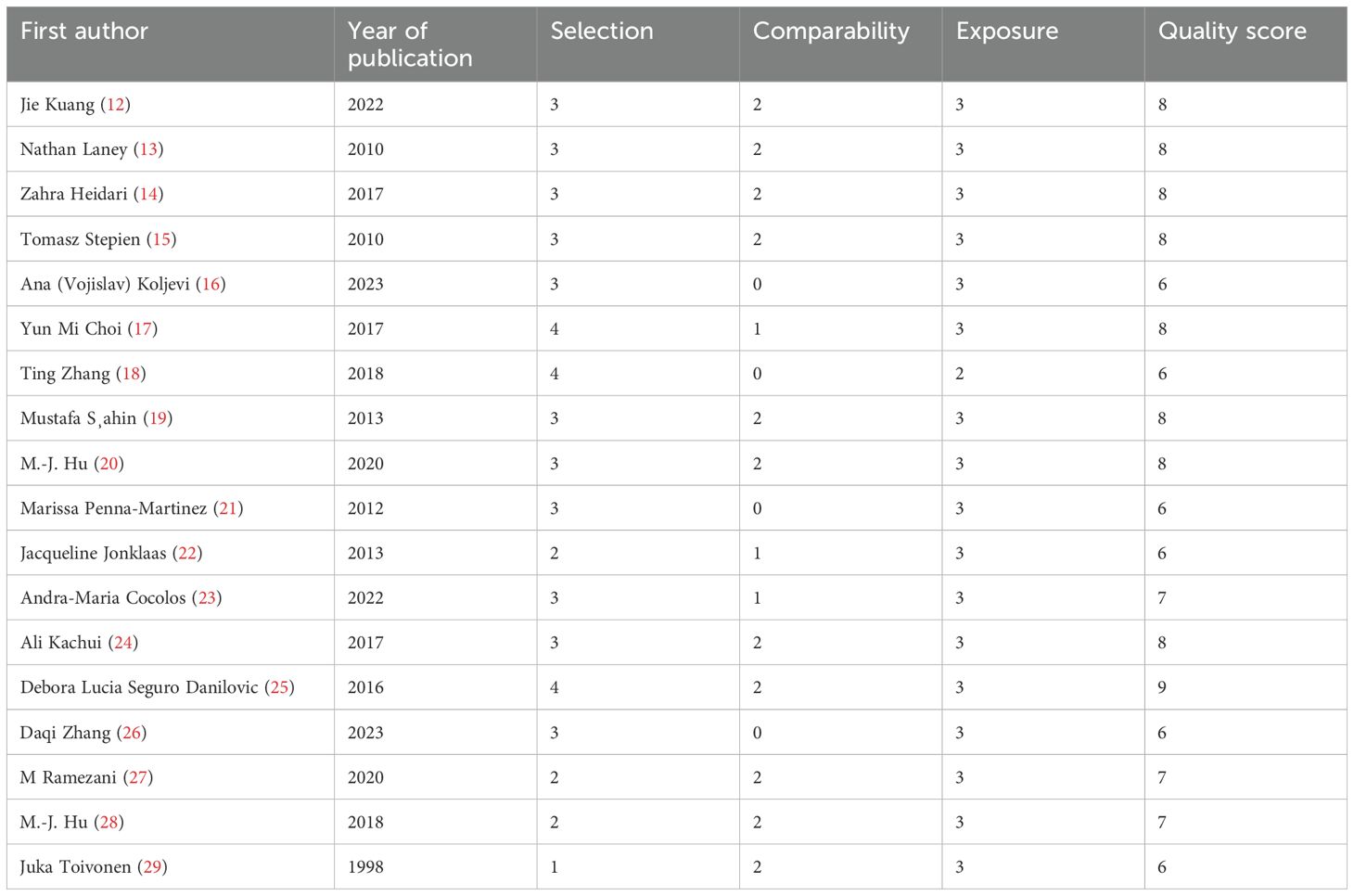
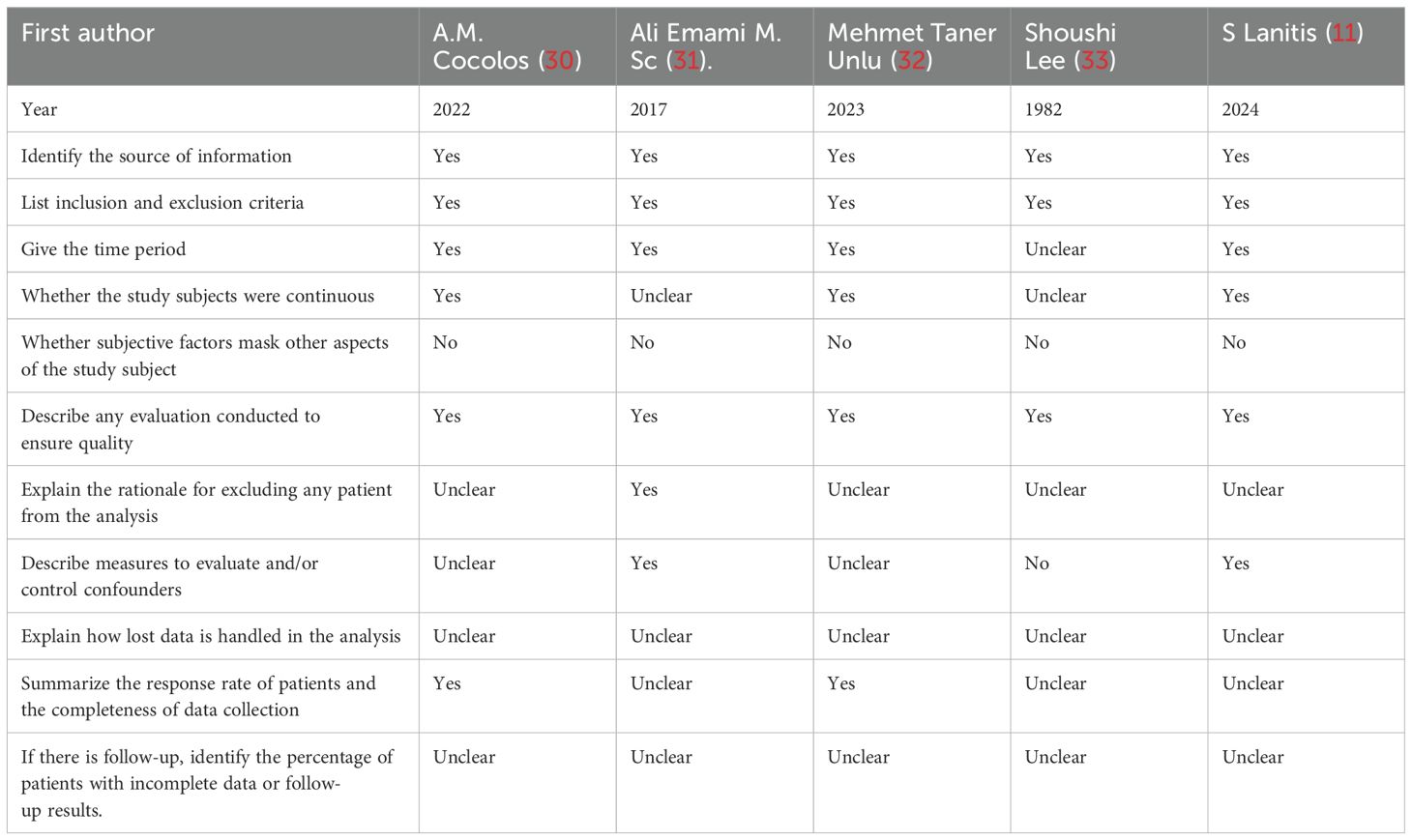
Table 2. Quality assessment for the included case–control studies according to the Newcastle–Ottawa Scale.
At the same time, we rigorously screened for duplicate datasets by comparing the authors, institutions, and participants’ demographics. Two studies from China (20, 28) shared overlapping authorship, but upon performing a meta-analysis, there are no repeated data. No other population overlaps were identified. Geographical clustering (e.g., five studies from China) reflects regional research activity rather than duplicate sampling.
3.2 Meta-analysis results
3.2.1 Comparison of 25(OH)D3 levels in TC patients and controls
Among the 23 included studies, 19 reported serum 25-hydroxyvitamin D(25-OHD) levels in both TC patients and controls (11, 12, 14–20, 22–25, 27, 29–33). Due to variations in measurement units across studies, standardized mean difference (SMD) was used. In general, of the 19 studies, the serum vitamin D levels were significantly lower in TC patients.
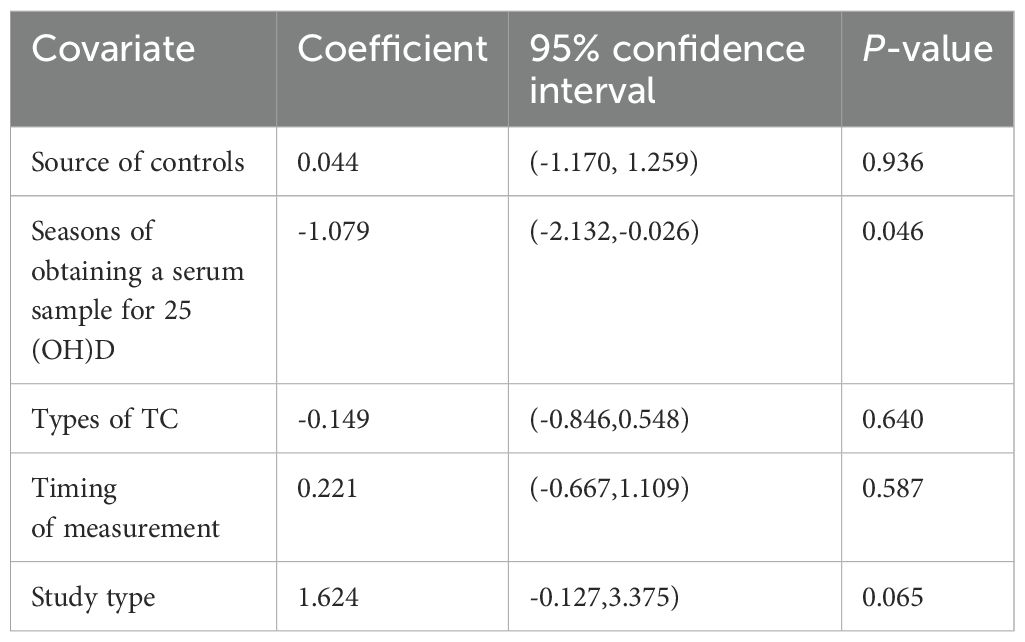
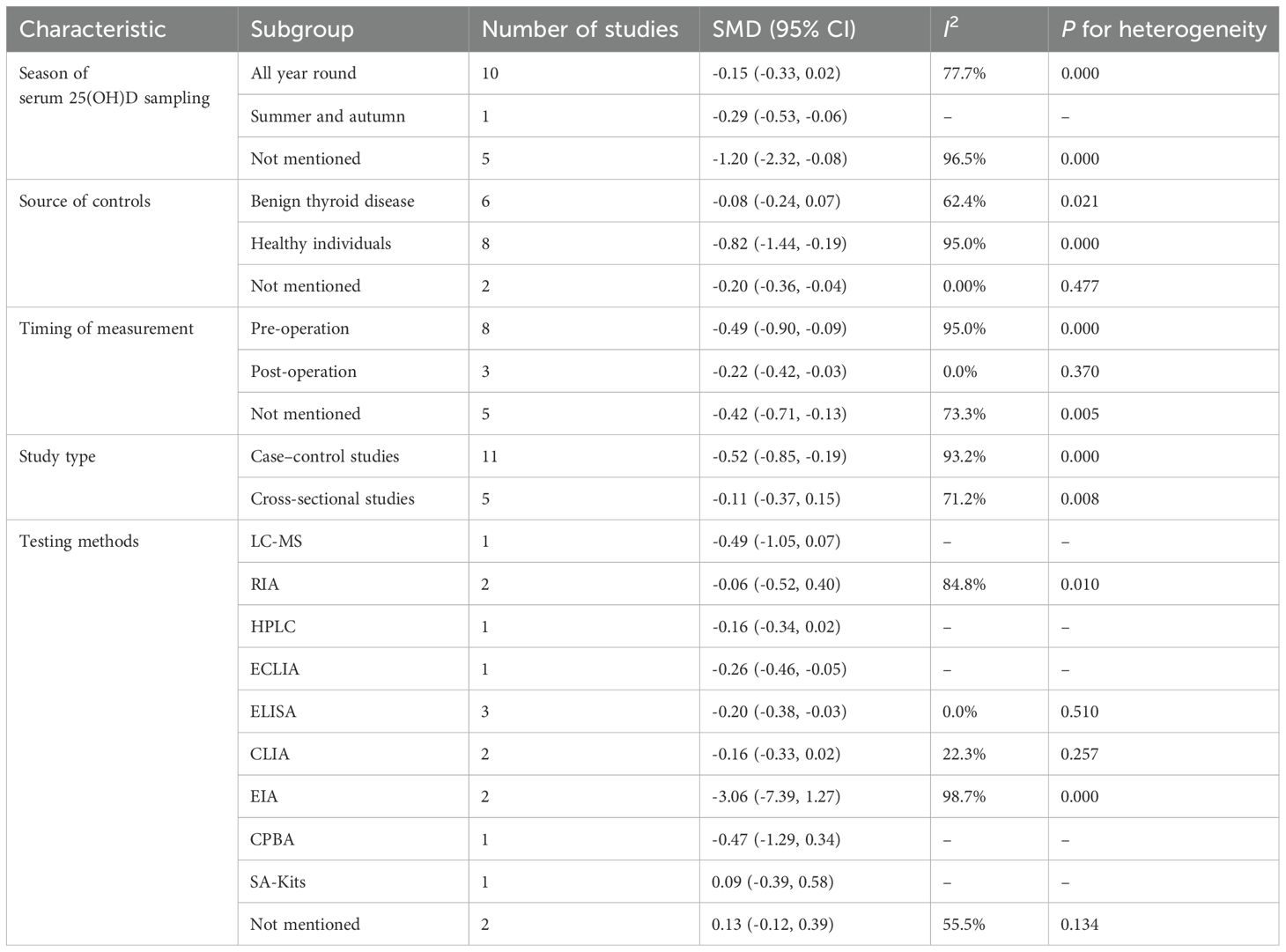
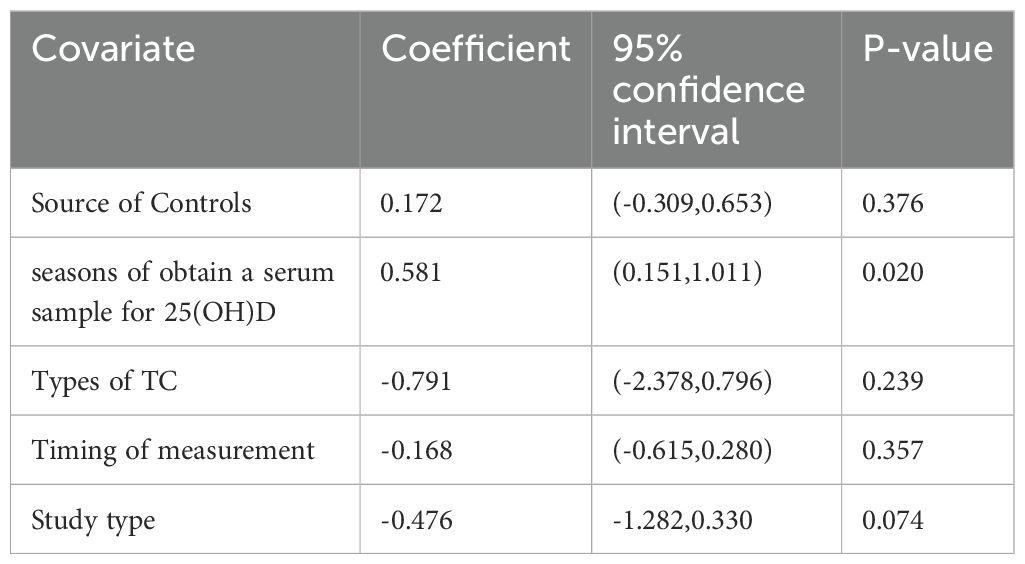
Owing to high heterogeneity (I2 = 90.9%, P = 0.000), a random-effects model was adopted. A meta-analysis demonstrated a weighted mean difference of −0.38 (95% CI(−0.62 to−0.14), indicating significantly reduced 25(OH)D levels in TC patients (Figure 2). To investigate heterogeneity sources, a meta-regression analysis was performed, evaluating covariates including control group source, season of serum 25(OH)D sampling, thyroid cancer subtypes, timing of 25(OH)D measurement relative to diagnosis/treatment, and study design. Seasonal variation in blood sampling emerged as a significant contributor to heterogeneity (P = 0.046), while other factors showed no statistically significant associations (Table 4).
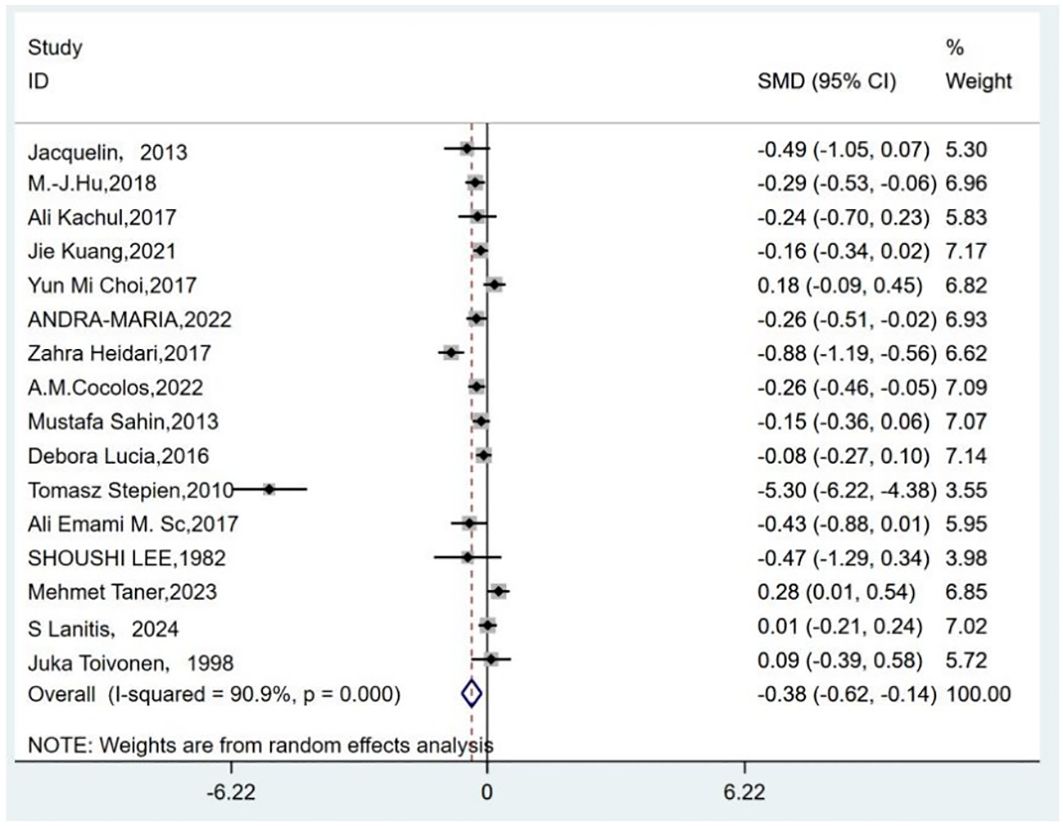
Figure 2. Forest plots of the standardized mean difference between the vitamin D levels in patients with thyroid cancer and control.
The results of subgroup analyses stratified by control source, sampling season, cancer subtype, measurement timing, study type, and testing methods were not statistically significant in certain subgroups (Table 5).
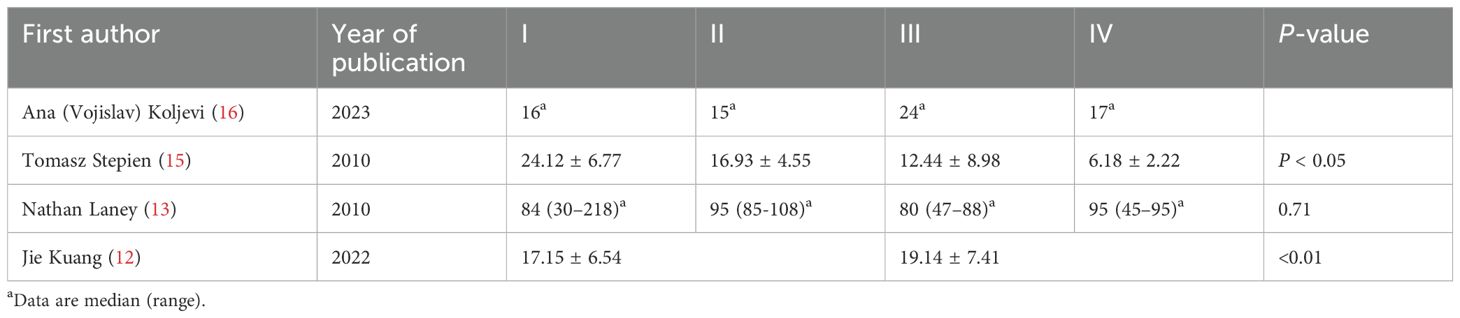
Subgroup analysis based on the pathological characteristics of thyroid cancer: A subgroup analysis was further conducted to evaluate the relationship between serum 25-hydroxyvitamin D (25[OH]D) levels and pathological characteristics (TNM stage and lymph node metastasis) in thyroid cancer (TC) patients. Limited studies addressed this association. According to the data that we summarized (Table 6), Tomasz Stepien et al. (15) reported significantly lower serum 25[OH]D levels in patients with advanced TNM stages, whereas Jie Kuang et al. (12) observed an inverse correlation with higher serum 25-OHD levels associated with advanced TNM staging. The remaining two studies (13, 16) showed no consistent trends in 25[OH]D levels relative to TNM stage or lymph node metastasis.
Assessment of publication bias and sensitivity analysis: Publication bias was assessed using funnel plots (Figure 3) and statistical tests. Begg’s tests did not reveal any significant publication bias (P = 0.192). However, Egger’s tests suggested a potential publication bias (P = 0.045). To address this discrepancy, a sensitivity analysis was conducted. The results of the sensitivity analysis showed that the removal of one trial (15) marginally influenced the effect size but did not alter the statistical significance or overall conclusions (Figure 4), confirming the robustness of our findings.
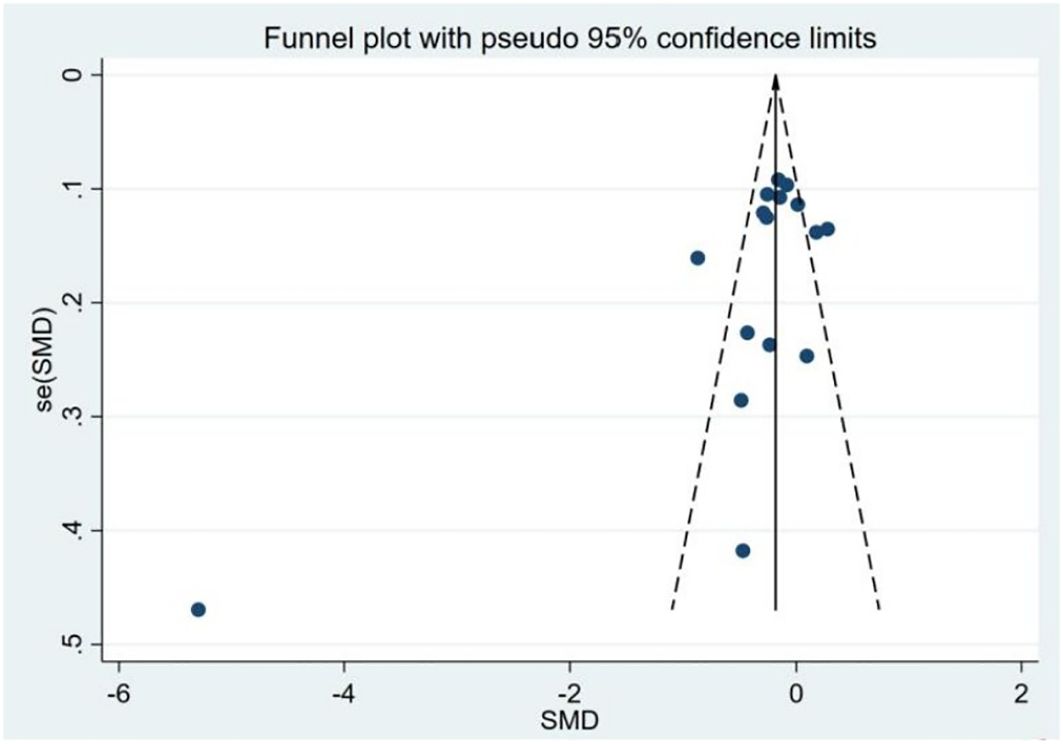
Figure 3. Funnel plot of the studies included in the meta-analysis of the standardized mean difference between the 25(OH)D levels in patients with thyroid cancer and controls.
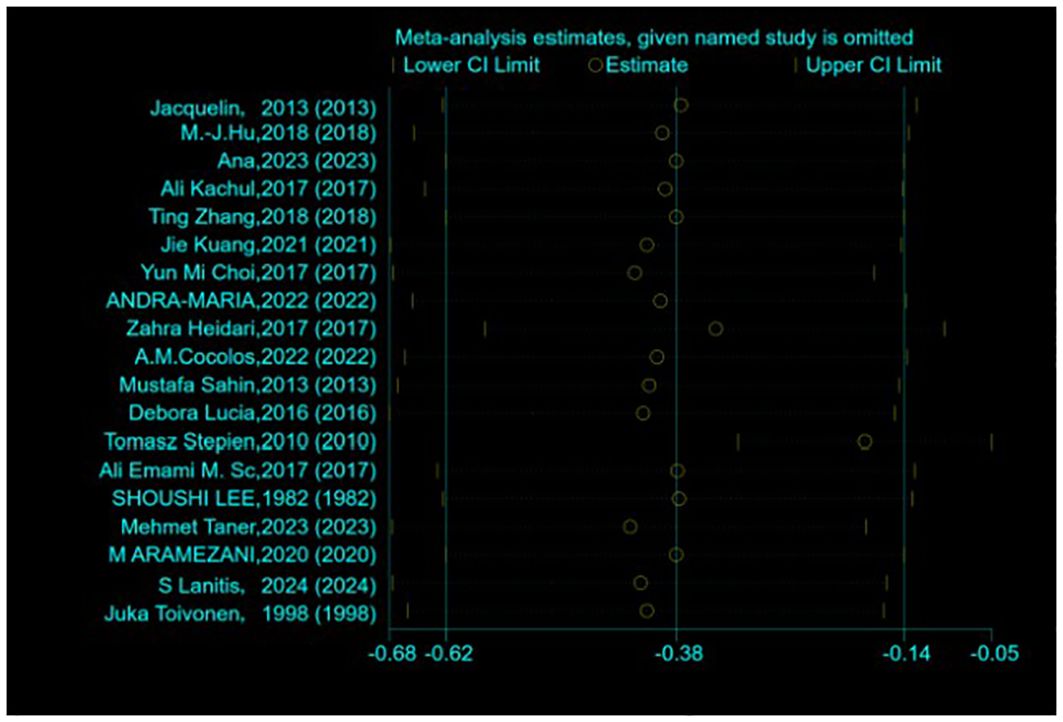
Figure 4. Sensitivity analysis of the standardized mean difference between the vitamin D levels in patients with thyroid cancer and control.
3.2.2 Association between vitamin D deficiency and risk of TC
A total of 10 studies reported the incidence of vitamin D deficiency in individuals with TC compared to the controls (11–13, 16, 19, 21, 24, 28, 30, 32). Owing to high heterogeneity (I2 = 53.9%, P = 0.021), a random-effects model was adopted. The meta-analysis revealed that vitamin D deficiency was significantly associated with an increased risk of TC (pooled OR: 1.33, 95% CI(1.02–1.73), P < 0.05) (Figure 5). Vitamin D deficiency could increase the risk of thyroid cancer by 33% compared with individuals who are not deficient in vitamin D.
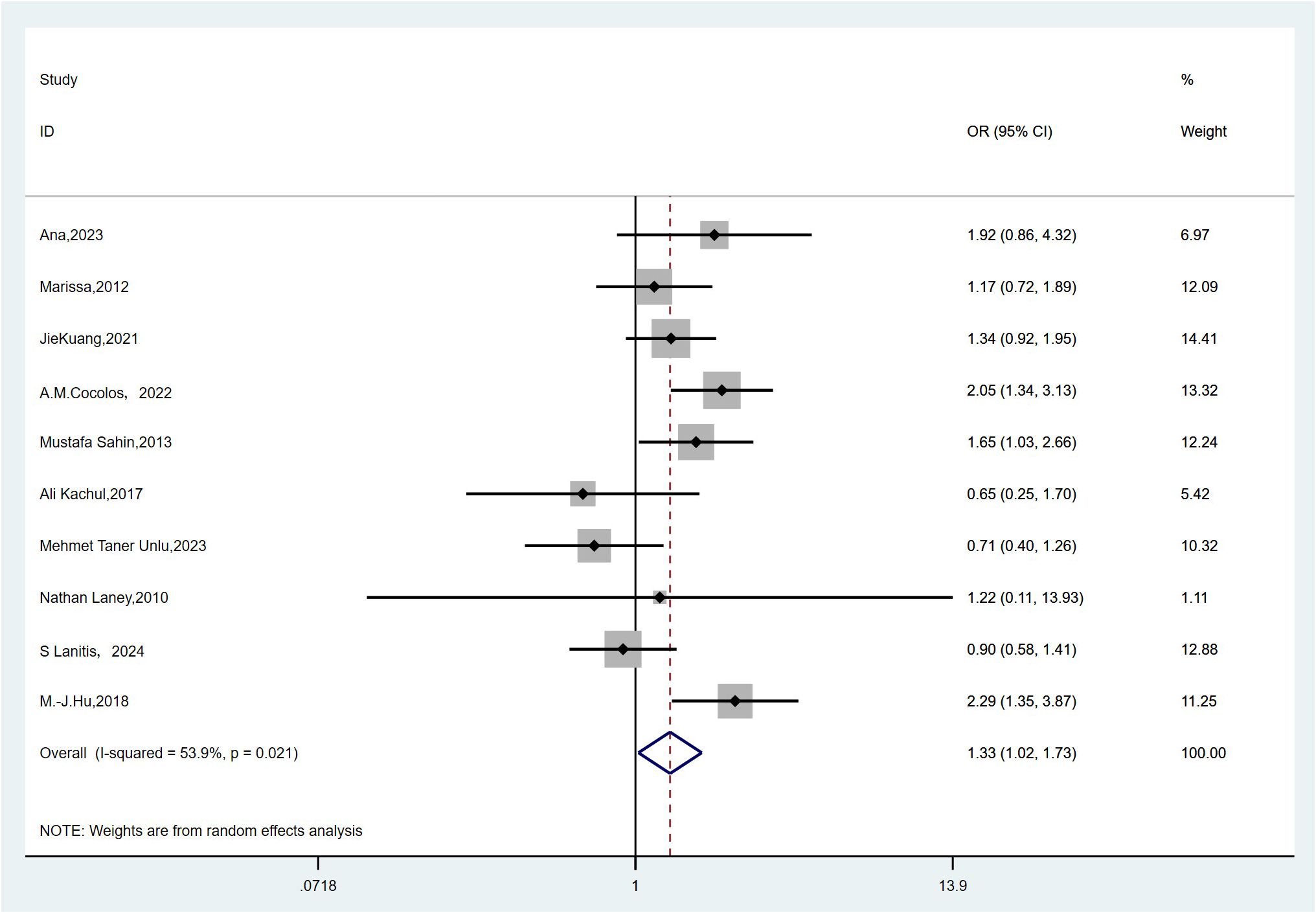
Figure 5. Forest plots and pooled estimates of the effect for the meta-analysis of the association between vitamin D deficiency and the risk of thyroid cancer.
Because significant heterogeneity was detected, meta-regression was performed, evaluating potential influences from control source, season of serum 25(OH)D sampling, timing of measurement, and study type. As shown in Table 7, season of serum 25(OH)D sampling emerged as the only statistically significant moderator of heterogeneity (P = 0.020). Subsequent subgroup analyses further demonstrated that the association between vitamin D deficiency and TC risk was not statistically significant in certain subgroups (Table 8). Publication bias assessment through funnel plot visualization (Figure 6) combined with formal statistical testing (Egger’s test: p = 0.775; Begg’s tests: p = 0.858) indicated no substantial evidence of publication bias in the analyzed literature.
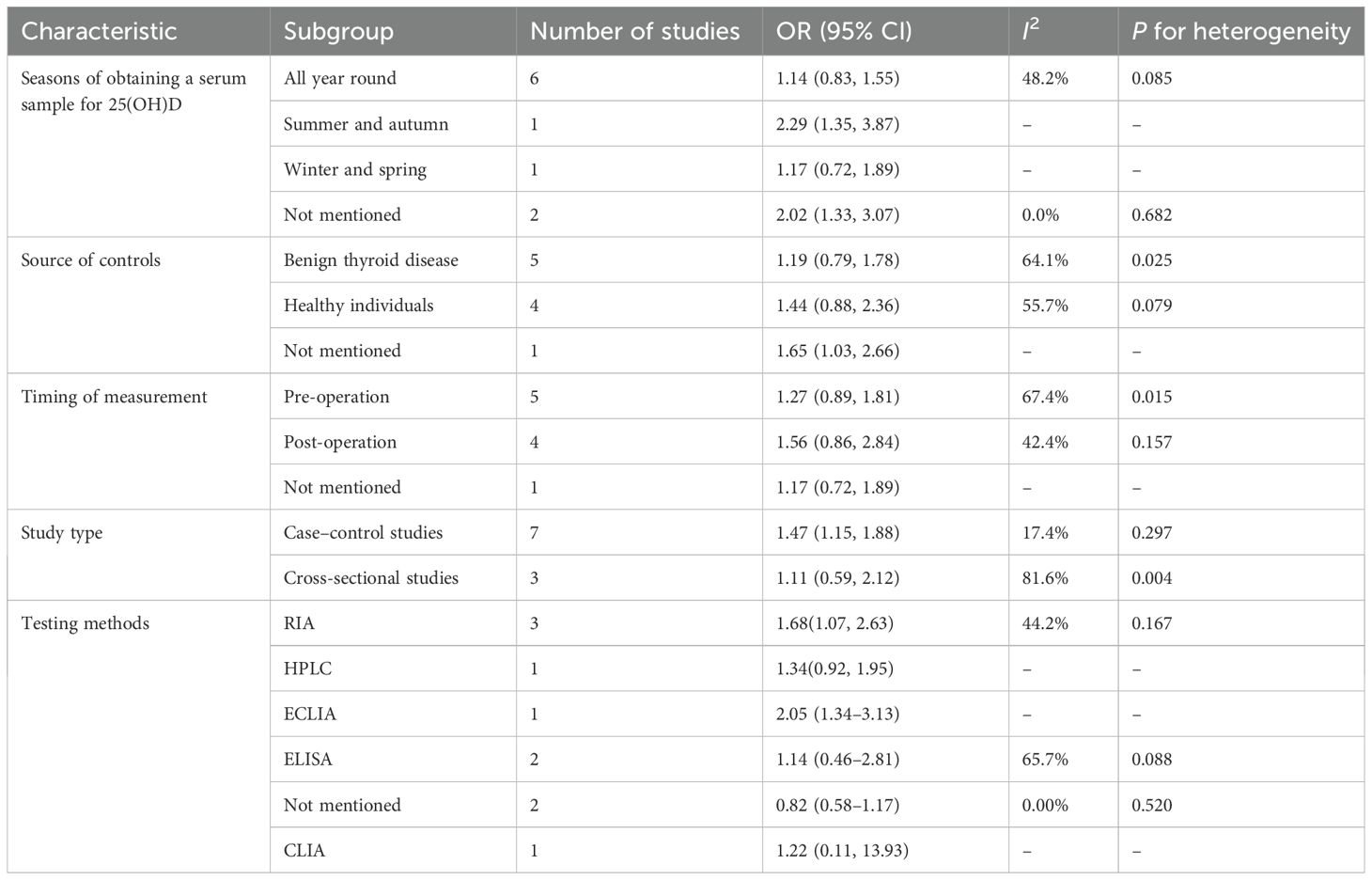
Table 8. Results of the subgroup analysis for the association between vitamin D deficiency and the risk of thyroid cancer.
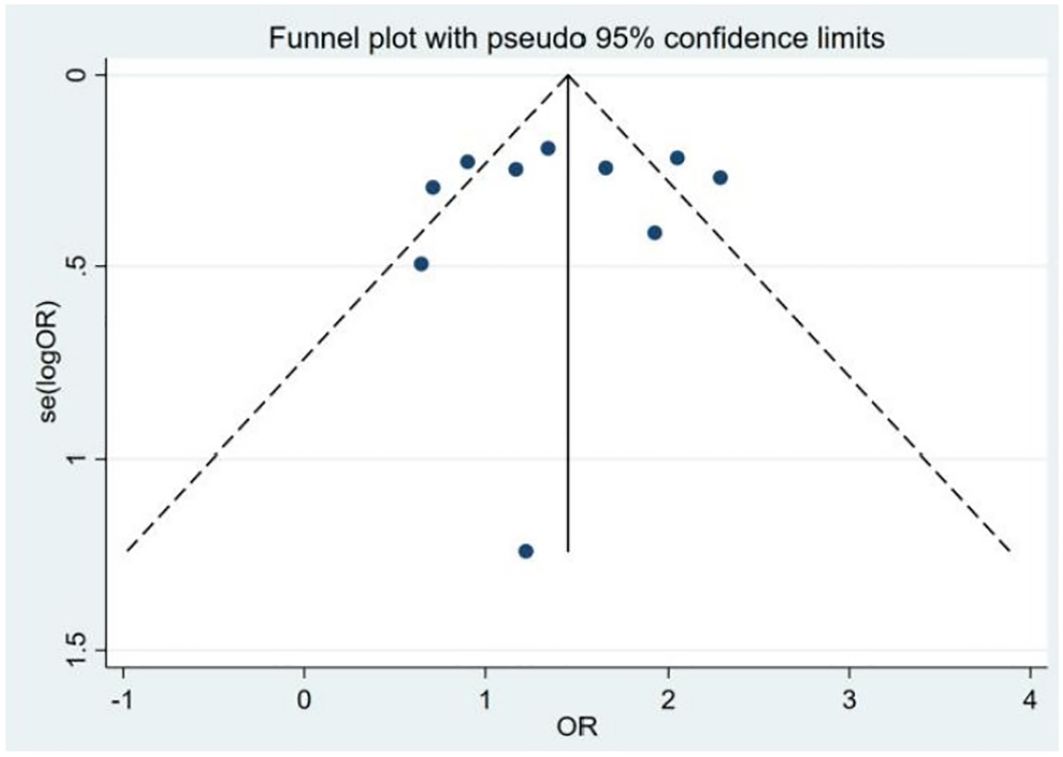
Figure 6. Funnel plot of the studies included in the meta-analysis of the deficiency of the 25(OH)D between patients with thyroid cancer and controls.
4 Discussion
Vitamin D is well known for its association with calcium absorption and bone health. Pathologies due to vitamin D deficiency are characterized by hypocalcemia, hypophosphatemia, and dental and skeletal alterations. For several decades, it has been shown that vitamin D, in addition to maintaining bone and tooth health, also plays a key role in inflammatory and immune regulation (34). Vitamin D achieves this by interacting with immune cells and signaling pathways, ultimately contributing to the body’s ability to defend against infections and maintain immune homeostasis (35). Vitamin D and its metabolites are inexpensive natural compounds that may have a significant impact on the direct and indirect prevention of cancer (36). Increasingly, scholars are focusing on the relationship between vitamin D and tumors. Researchers believe that a low level of vitamin D is related to an increased risk of several cancers (37). Its anti-tumor effect is underpinned by a multitude of molecular mechanisms that impact cell growth, differentiation, and apoptosis (38). Given the increasing incidence of thyroid cancer, its pathogenesis and influencing factors have attracted increasing attention. The expression levels of the vitamin D receptor (VDR) and other genes involved in vitamin D signaling are increased in malignant thyroid cells (39, 40), suggesting a potential anti-tumor response of vitamin D in cancer. In vivo studies have shown that treatment with calcitriol reduces tumor size in mouse models of follicular thyroid cancer and metastatic follicular thyroid cancer (41). In human studies, some have shown that vitamin D deficiency may be a risk factor for thyroid cancer (14, 15, 30), while some studies have the opposite results or found no relevance (11, 32).
Our meta-analysis identified that TC was significantly associated with lower vitamin D, and the prevalence and odds of vitamin D deficiency are significantly higher among TC patients than among participants without TC. Eventually, the results of this study may be applied to clinical scenarios: attaining sufficient levels of vitamin D3 could be associated with a decreased risk of developing thyroid cancer.
However, our meta-analysis identified significant heterogeneity in the pooled estimates of serum 25(OH)D levels and vitamin D deficiency (I² = 90.9% and 53.9%, respectively). The results showed that seasonal variation in blood sampling was the source of the heterogeneity, indicating that it was necessary to obtain a serum sample for vitamin D in the same season to assess its impact on thyroid cancer. When it comes to subgroup analysis, there were limited studies that obtained a serum sample for 25(OH)D in the same season. M.−J. Hu et al. (28) reported that TC has a lower level of serum 25(OH)D when obtaining a serum sample in summer and autumn, which indicated that attention to and screening for vitamin D levels are also necessary during the summer and autumn seasons.
Although seasonal variation in blood sampling emerged as a key source of heterogeneity through subgroup analyses and meta-regression, further interpretation of its implications is warranted. The substantial heterogeneity may stem from methodological variations across studies (e.g., vitamin D measurement techniques, seasonal timing of sampling), population characteristics (e.g., geographic regions, BMI, sunlight exposure habit, dietary intake, history of vitamin D supplementation, and other factors), and diversity in study designs (case–control vs. cross-sectional studies). Despite the high heterogeneity, the subgroup analyses demonstrated consistent findings across all subgroups except for postoperative measurements, suggesting robustness in the primary conclusions.
Nevertheless, the observed heterogeneity may limit the generalizability of the results. Future research should prioritize standardized protocols for seasonal sampling, rigorous control of confounding variables, and harmonized methodologies to minimize heterogeneity and enhance comparability.
Our Egger’s test suggested a potential publication bias (P = 0.045), suggesting that smaller studies showing null or negative associations may remain unpublished. This bias could inflate the observed effect size. A sensitivity analysis excluding one outlier study marginally reduced the heterogeneity but did not alter the significance, supporting result robustness. Nonetheless, unpublished data gaps may limit the generalizability of our findings. We advocate for the prospective registration of observational studies to mitigate publication bias.
5 Limitations
There were some limitations in this meta-analysis. Firstly, we encountered substantial heterogeneity across the included studies. While we addressed this through detailed subgroup analyses based on various factors—with heterogeneity becoming statistically non-significant in some subgroups—the underlying variations remain a concern. Secondly, the cutoff values for 25-OHD varied considerably across eligible studies, complicating cross-study comparisons. Thirdly, Reza Tabrizi et al. (42) thought that, in addition to hypovitaminosis, VDR gene polymorphism can also negatively affect the action of vitamin D, and numerous studies have shown that the VDR level was increased in the case of DTC (mainly PTC was assessed) in comparison to the normal thyroid (18, 43). However, due to limited data, 25(OH)D and VDR polymorphisms were not involved in our meta-analysis. Meanwhile, given that all included studies in our meta-analysis were observational, causal relationships cannot be inferred. Consequently, more studies with standardized protocols for these factors are needed to better assess and clarify the mechanisms underlying our findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LY: Data curation, Writing – original draft. PY: Methodology, Writing – original draft, Writing – review & editing. FL: Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jarząb B, Dedecjus M, Lewiński A, Adamczewski Z, Bakuła-Zalewska E, Bałdys-Waligórska A, et al. Diagnosis and treatment of thyroid cancer in adult patients - Recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update [Diagnostyka i leczenie raka tarczycy u chorych dorosłych - Rekomendacje Polskich Towarzystw Naukowych oraz Narodowej Strategii Onkologicznej. Aktualizacja na rok 2022. Endokrynol Pol. (2022) 73:173–300. doi: 10.5603/EP.a2022.0028
2. Schömann-Finck M and Reichrath J. Umbrella review on the relationship between vitamin D levels and cancer. Nutrients. (2024) 16:2070. doi: 10.3390/nu16162720
3. Carlberg C. Vitamin D: A micronutrient regulating genes. Curr Pharm Des. (2019) 25:1740–46. doi: 10.2174/1381612825666190705193227
4. Sobhi P, Bahrami M, Mahdizadeh F, Fazaeli A, Babaei G, and Rezagholizadeh L. Vitamin D and potential effects on cancers: a review. Mol Biol Rep. (2024) 51:190. doi: 10.1007/s11033-023-09111-y
5. Biondi B, Arpaia D, Montuori P, Ciancia G, Ippolito S, Pettinato G, et al. Under the shadow of vesuvius: a risk for thyroid cancer? Thyroid. (2012) 22:1296–97. doi: 10.1089/thy.2012.0002
6. Roskies M, Dolev Y, Caglar D, Hier MP, Mlynarek A, Majdan A, et al. Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg. (2012) 41:160–63.
7. Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control. (2011) 22:291–99. doi: 10.1007/s10552-010-9697-0
8. Shen J, Zhang H, Jiang H, Lin H, He J, Fan S, et al. The effect of micronutrient on thyroid cancer risk: a Mendelian randomization study. Front Nutr. (2024) 11:1331172. doi: 10.3389/fnut.2024.1331172
9. Hu Y, Xue C, Ren S, Dong L, Gao J, and Li X. Association between vitamin D status and thyroid cancer: a meta-analysis. Front Nutr. (2024) 11:1423305. doi: 10.3389/fnut.2024.1423305
10. Zhao J, Wang H, Zhang Z, Zhou X, Yao J, Zhang R, et al. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition. (2019) 57:5–11. doi: 10.1016/j.nut.2018.04.015
11. Lanitis S, Gkanis V, Peristeraki S, Chortis P, Kalogeris N, and Vryonidou A. Vitamin D deficiency and thyroid cancer: is there a true association? A prospective observational study. Ann R Coll Surg Engl. (2025) 107:423–8. doi: 10.1308/rcsann.2024.0041
12. Kuang J. Serum 25-hydroxyvitamin D level is unreliable as risk factor and prognostic marker in papillary thyroid cancer. Ann Transl Med. (2022) 10:193. doi: 10.21037/atm-22-10
13. Laney N. The prevalence of vitamin D deficiency is similar between thyroid nodule and thyroid cancer patients. Int J Endocrinol. (2010) 805716. doi: 10.1155/2010/805716
14. Heidari Z. Vitamin D deficiency associated with differentiated thyroid carcinoma:A case-control study. Asian Pac J Cancer Prev. (2017) 18:3419–22. doi: 10.22034/APJCP.2017.18.12.3419
15. Stepine T. Decreased 1–25 dihydroxyvitamin D3 concentration in peripheral blood serum of pationts with thyroid cancer. Asian Pac J Cancer Prev. (2017) 18:3419–22. doi: 10.22034/APJCP.2017.18.12.3419
16. Markovi AVK. Evaluation of Vitamin D3 levels and dietary management in pationts with papillary thyroid cancer. Hell J Nucl Med. (2023) 26:194–200. doi: 10.1967/s002449912604.
17. Choi YM. Serum vitamin D3 level are not associated with thyroid cancer prevalence in euthyroid subjects without autoimmune thyroid disease. Korean J Intern Med. (2017) 32:102–8. doi: 10.3904/kjim.2015.090
18. Zhang T. Potential use of 1-25-dihydroxyvitamin D in the diagnosis and treatment of papillary thyroid cancer. Cancer Med Sci Monit. (2018) 24:1614–23. doi: 10.12659/msm.909544
19. Sahin M. Vitamin D3 levels and insulin resistance in papillary thyroid cancer patients. Med Oncol. (2013) 30:589. doi: 10.1007/s12032-013-0589-5
20. Hu MJ. Association of thyroid cancer risk with plasma 25-hydroxyvitamin D and vitamin D binding protein:a case-control study in China. J Endocrinol Invest. (2020) 43:799–808. doi: 10.1007/s40618-019-01167-7
21. Penna-Martinez M. Imparied vitamin D activation and association with CYP24A1 haplotypes in differentiated thyroid carcinoma. Thyroid. (2012) 22:709–16. doi: 10.1089/thy.2011.0330
22. Jonklaas J. A pilot study of serum selenium,Vitamin D,and thyrotropin concentrations in patients with thyroid cancer. Thyroid. (2013) 23:1079–86. doi: 10.1089/thy.2012.0548
23. Cocolos A-M. Vitamin D status and VDR polymorphisms as Prognostic Factors in Differentiated Thyroid Carcinomas. In Vivo. (2022) 36:2434–41. doi: 10.21873/invivo.12977
24. Kachul A. Evaluation of Bone Density,Serum Total and lonized Calcium,Alkaline Phosphatase and 25-hydroxy Vitamin D in Pipallary Thyroid Carcinoma and their Relationship with TSH Suppression by Levothyroxine. Adv Biomed Res. (2017) 6:94. doi: 10.4103/2277-9175.211799
25. Danilovic DLS. 25-hydroxyvitamin D and TSH as risk factors or prognostic markers in thyroid carcinoma. PLoS One. (2016) 11:e0164550. doi: 10.1371/journal.pone.0164550
26. Zhang D. Relationship between serum levels of vitamins and papillary thyroid cancer:a single center case-control study. Gland Surg. (2023) 12:805–15. doi: 10.21037/gs-22-520
27. Ramezani M. Medullary thyroid cancer is associated with high serum vitamin D level and polymorphism of vitamin D receptors. Physiol Int. (2020) 107:120–33. doi: 10.1556/2060.2020.00011
28. Hu MJ. Association between vitamin D deficiency and risk of thyroid cancer:a case-control study and a meta-analysis. J Endocrinologiacl Invest. (2018). 41:1199–210. doi: 10.1007/s40618-018-0853-9
29. Toivonen J. Markers of bone turnover in patients with differentiated thyroid cancer with and following withdrawal of thyroxine suppressive therapy. Eur J Endocrinol. (1998), 667–73. doi: 10.1530/eje.0.1380667
30. Cocolos AM. Vitamin D level and its relationship with cancer stage in patients with differentiated thyroid carcinoma.Acta Endocrinol (Buchar). (2022) 18:168–73. doi: 10.4183/aeb.2022.168
31. Emami A. Micronutrient status(calcium,zinc,vitamin D and E)in patients with medullary thyroid carcinoma:A cross-sectional study. Nutrition. (2017) 41:86–9. doi: 10.1016/j.nut.2017.04.004
32. Unlu MT. The relationship of pre-operative vitamin D and TSH levels with pipallary thyroid cancer. North Clin Istanb. (2023) 10:697–703. doi: 10.14744/nci.2022.09699
33. Lee S. Normal serum 1,25-Dihydroxyvitamin D in patients with medullary carcinoma of thyroid. J Clin Endocrionlogy Metab. (1982) 55:361–3. doi: 10.1210/jcem-55-2-361
34. Wang J-G. Vitamin D levels in children and adolescents are associated with coronavirus disease-2019 outcomes:A systematic review and meta-analysis. Med (Baltimore). (2024) 44:e40245. doi: 10.1097/MD.0000000000040245
35. Ismailova A. Vitamin D, infections and immunity. Rev Endocr Metab. (2022) 2:265–77. doi: 10.1007/s11154-021-09679-5
36. Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. (2012), 1476–78. doi: 10.1126/science.337.6101.1476
37. Zhang R. Association between vitamin D supplementation and cancer mortality: A systematic review and meta-analysis.Cancers (Basel). (2022) 14:3717. doi: 10.3390/cancers14153717
38. Guo J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. (2022) 7:391. doi: 10.1038/s41392-022-01251-0
39. Clinckspoor I. Altered expression of key players in vitamin D metabolism and signaling in Malignant and benign thyroid tumors. J Histochem Cytochem. (2012) 60:502–11. doi: 10.1369/0022155412447296
40. Izkhakov E. Vitamin D receptor expression is linked to potential markers of human thyroid papillary carcinoma. J Steroid Biochem Mol Biol. (2016) 159:26–30. doi: 10.1016/j.jsbmb.2016.02.016
41. Liu W. 1alpha,25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol Endocrinol. (2005) 9:2349–57. doi: 10.1210/me.2005-0117
42. Tabrizi R. Vitamin D serum levels and temporomandibular disorders: A systematic review and meta-analysis. Arch Oral Biol. (2025) 169:106108. doi: 10.1016/j.archoralbio.2024.106108
Keywords: vitamin D level, vitamin d deficiency, thyroid cancer, meta-analysis, subgroup analyses
Citation: Yang L, Yun P and Li F (2025) Association between vitamin D serum levels and thyroid cancer: a meta-analysis. Front. Endocrinol. 16:1602844. doi: 10.3389/fendo.2025.1602844
Received: 30 March 2025; Accepted: 30 June 2025;
Published: 24 July 2025.
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyReviewed by:
Moumita Chakraborty, National Institutes of Health (NIH), United StatesGabriela Mintegui, Hospital of Clinics Dr. Manuel Quintela, Uruguay
Copyright © 2025 Yang, Yun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Yun, eXVucGVuZ0BzeXN1c2guY29t; Fangping Li, bGlmYW5ncGluZ0BzeXN1c2guY29t
 Lili Yang
Lili Yang Peng Yun*
Peng Yun*