- 1Department of Epidemiology, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Department of Thyroid and Breast Surgery, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an, China
- 3Department of Pathology, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an, China
- 4Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 5Department of Pathology, The Affiliated Hospital of Nantong University, Nantong, China
Introduction: Differentiation between benign and malignant thyroid nodules has been a challenge in clinical practice. We aim to explore a novel biomarker to determine the malignancy of thyroid nodules.
Methods: In the discovery study, 32 tissue samples from benign thyroid nodule (BTN) and thyroid cancer (TC) patients were analyzed by Methylation 850K array and RNA-Sequencing. TC associated FABP3 methylation was further verified by mass spectrometry in two independent studies (221 BTN vs. 222 TC in Validation I and 191 BTN vs. 256 TC in Validation II). Logistic regression analysis and non-parametric tests were used for the analysis between groups.
Results: Altered and inversely correlated methylation and expression in the FABP3 gene in TC was found in the discovery study (P = 2.90E-05 for the methylation and P = 0.040 for the expression), and verified in the two validation studies (P values range from 0.012 to 6.30E - 10-12). FABP3 methylation could sufficiently differentiate TC from BTN (AUC = 0.77), and could be further improved when combined with the BRAFV600E mutations (AUC = 0.87). The association between FABP3 hypomethylation and TC was enhanced in women, in patients with younger age, with larger tumor size and with lower FT3. FABP3 methylation was varied in BTN and TC subtypes, with the highest level in adenoma and the lowest in anaplastic thyroid cancer.
Conclusion: Our study suggested that altered FABP3 methylation in tissue samples as a potential biomarker to distinguish malignant and benign thyroid nodules, and might be helpful for the pathological classification of TC.
1 Introduction
Thyroid cancer (TC) is the most common endocrine malignant tumors in adults, with an estimated total of more than 9.2 million new cases in 2020 worldwide (1). In China, the incidence of TC increased from 2.40/100,000 in 2003 to 13.75/100,000 in 2012, with an average annual increase of 20% (2, 3). In some provinces, such as Zhejiang Province, the incidence rate of TC ranks first among all cancers in women (4, 5). Differentiated thyroid cancer (DTC) has two subtypes, papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), which are the most common subtype of TC. The rarer subtypes are medullary thyroid cancer (MTC) and anaplastic thyroid cancer (ATC) (6–8). Thyroid nodules have a high incidence and a low malignant rate in the population. Although 60% of ultrasonography showed the presence of thyroid nodules, only 5% were eventually confirmed as malignant (9).
Accurate diagnosis of TC, including the judgment of benign and malignant thyroid nodules and classification of TC, is one of the current clinical challenges. Ultrasound-guided fine-needle aspiration biopsy (FNAB) remains crucial in the preoperative diagnosis of thyroid nodules (10). However, up to 15 - 30% of thyroid nodules evaluated by FNAB were cytologically indeterminate, which were usually diagnosed by postoperative pathological diagnosis (11). Ultrasound-guided FNAB, while clinically essential, faces critical limitations, including operator-dependent sampling errors that may miss malignant foci, frequent insufficient cellular material (occurring in ~20% of procedures), high infrastructure costs, requiring specialized cytopathology expertise, and prolonged turnaround times delaying clinical decisions (12, 13). These constraints reduce diagnostic accuracy for indeterminate nodules. Patients with uncertain nodules will be at risk of overdiagnosis or misdiagnosis. Therefore, reliable and practical biomarkers are urgently needed.
Determining the malignancy of thyroid nodules is becoming increasingly dependent on molecular pathological techniques (14), including genetic detection and epigenetic detection (15, 16). The role of BRAF, RAS, and RET gene mutations in TC has been widely recognized. BRAF mutation is known to be specific for PTC, and the reported positive rate ranges from 29 to 83% (17, 18). Because of the low sensitivity, compensating molecular diagnostic methods are needed (19). Afirma gene expression classifiers and ThyroSeq v3 are suitable for fresh aspirates and are expensive (20, 21). In addition, they are associated with a high rate of overdiagnosis due to low positive predictive value (22). These facts point to the need for a highly accurate diagnostic test for thyroid nodules using time-insensitive materials.
DNA methylation catalyzed by DNA methyltransferases (DNMTs) is one of the important epigenetic modifications controlling gene expression and maintaining genome structure. Alterations of DNA methylation are early molecular changes in human cancers and play an important role in tumorigenesis. As has been reported, the DNA methylation biomarkers offer early detection capability as epigenetic changes precede histopathological alterations, high specificity for tumor subtyping, and increasingly cost-effective analysis with standardized protocols (23). As a potential biomarker, changes in the methylation profile have been found in all types of cancer including TC (24). For example, Stephen et al. used quantitative methylation-specific polymerase chain reaction (PCR) to detect the promoter methylation status of 21 candidate genes on 329 formalin-fixed paraffin-embedded (FFPE), demonstrating that combined abnormal gene methylation helps clinically differentiate FTC and PTC from benign thyroid nodules (BTN) (25). Yim et al. evaluated DNA methylation in 109 thyroid specimens from BTN, PTCs and adjacent normal thyroid tissues using the Reduced Representation Bisulfite Sequencing (RRBS) and then performed validation in a retrospective cohort containing 65 thyroid nodules, suggesting epigenetic testing as a new molecular approach for thyroid diagnostics (26). Nevertheless, these studies were based on candidate approaches and were also limited by small sample size. So far, there are few screenings for the variant methylation signatures aiming to differentiate malignant and benign thyroid tumors, especially in large sample sizes.
To establish reliable methylation signatures overcoming FNAB’s technical barriers, this study utilized surgically resected FFPE tissues, which provide both definitive histopathological classification and sufficient DNA for the development of robust biomarkers. Here, we intended to discover and validate the abnormal DNA methylation alterations in TC compared to BTN in the Chinese population. Combining the genome-wide screening assay of Methylation 850K BeadChip array and RNA-Sequencing, we discovered TC associated differential methylation in the FABP3 gene and performed further validations via mass spectrometry in case-control studies with a total of 890 patients from two clinical centers.
2 Materials and methods
2.1 Study design and population
The studies were approved by the Ethics Committee of the Nanjing Medical University. Informed consent was obtained from each recruited participant. In the discovery study, we collected 32 fresh-frozen tissue samples from 17 BTN subjects and 15 TC cases at the Affiliated Huai’an Hospital of Xuzhou Medical University from 2019 to 2020. Two independent studies were conducted on 890 FFPE tissue samples for validation. Validation Ⅰ: 222 TC patients and 221 age- and gender-matched BTN subjects were collected from the Affiliated Huai’an Hospital of Xuzhou Medical University and the Jiangsu Provincial Hospital of Chinese Medicine from 2013 to 2023. In the BTN group, 78.70% (174/221) were female, with a median (interquartile range, IQR) age of 53.00 (45.50 - 60.00) years; while in the TC group, 72.10% (160/222) were female, with a median (IQR) age of 50.00 (41.75 - 58.00) years. Validation Ⅱ: a total of 256 TC cases and 191 age- and gender-matched BTN subjects were collected from the Affiliated Hospital of Nantong University from 2017 to 2022. The median (IQR) age of BTN subjects and TC cases was 49.00 (35.00 - 55.00) and 50.00 (38.00 - 57.00) years old, and the proportions of females were 77.50% (148/191) and 77.30% (198/256), respectively. The detailed clinical characteristics of the participants are shown in Table 1, including tumor length, tumor size, lymph node involvement, tumor stage, thyroid-stimulating hormone (TSH) levels, free triiodothyronine (FT3) levels, free tetraiodothyronine acid (FT4) levels, and classification of BTN and TC.
In the discovery study and two independent validations, the inclusion criteria for malignant nodules were as follows: (1) no distant metastases or other co-occurring cancers, (2) before any related treatment, and (3) complete clinical records. BTN patients were matched to TC cases by age, gender, and the year of diagnosis. Histopathological diagnosis was performed by two qualified pathologists in all cases, and the clinical TNM stage of each malignant case was determined according to the 8th edition American Joint Committee on Cancer Staging System (27).
2.2 EquationsIllumina methylation EPIC 850K BeadChip array and RNA-sequencing
The study design and flow chart were shown in Figure 1. In the discovery round, FastPure Blood/Cell/Tissue/Bacteria DNA Isolation Kit (DC112, Vazyme, Nanjing, China) and FastPure Cell/Tissue Total RNA Isolation Kit (RC101, Vazyme, Nanjing, China) were used to isolate genomic DNA and total RNA from 32 fresh-frozen tissue samples, respectively.
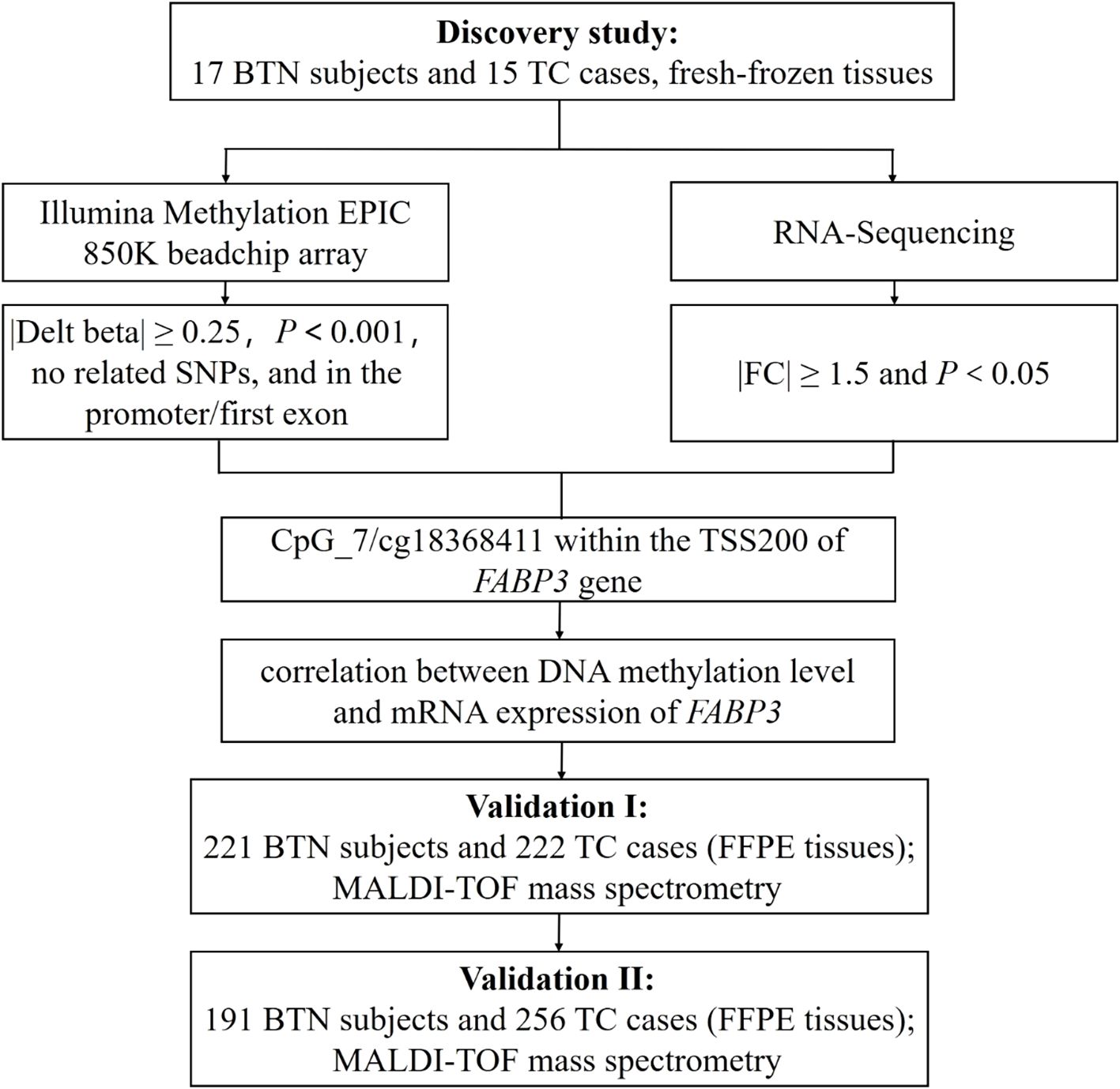
Figure 1. Study design and flow chart. The 32 fresh-frozen tissue samples in the discovery study were subjected to Illumina Methylation EPIC 850K BeadChip array and RNA-Sequencing. In the discovery study, there was a good correlation between DNA methylation and mRNA expression of FABP3 in fresh-frozen tissue samples. Further validations with FFPE tissue samples were conducted in two independent studies (Validation Ⅰ and Validation Ⅱ) by MALDI-TOF mass spectrometry. BTN, benign thyroid nodule; TC, thyroid cancer; SNP, single nucleotide polymorphism; FC, fold change; TSS200, transcription start site 200 bp; FFPE, formalin-fixed and paraffin-embedded; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight.
Genome-wide DNA methylation profiles were analyzed at single nucleotide resolution by the Illumina Methylation EPIC 850K BeadChip array. Probes that meet the following criteria were considered differentially methylated: (1) methylation difference between BTN and TC groups (|delta-beta|) ≥ 0.25, (2) P value < 0.001, (3) no adjacent single nucleotide polymorphisms (SNPs), and (4) on the promoter region or the 1st exon of gene body. At the same time, mRNA expression was measured by RNA-Sequencing. Genes with a fold change of expression ≥ 1.5 and P value < 0.05 were thought to be differentially expressed.
2.3 Gene set enrichment analysis
GSEA was performed using the clusterProfiler R package (v4.4.4) on transcriptomic DEGs ranked by log2FoldChange. Gene IDs were converted to Entrez IDs via org.Hs.eg.db, with BH-adjusted P < 0.05. Key enriched pathways were visualized by enrichplot: gseaplot2 displaying enrichment score curves, gene positions, and ranking metrics. The tidyverse ecosystem was used for data processing.
2.4 Matrix-assisted laser desorption ionization time-of-flight mass spectrometry
DNA was extracted from FFPE tissue samples using FastPure FFPE DNA Isolation Kit (DC105, Vazyme, Nanjing, China). The isolated DNA was further bisulfite converted by EZ - 96 DNA Methylation Gold Kit (D5007, Zymo Research, Orange, USA) according to the manufacturer’s protocol. After bisulfite treatment, all non-methylated cytosine (C) bases in CpG sites were converted to uracil (U), whereas all methylated (C) bases remained unchanged. The CpG methylation levels of FFPE tissue samples were then determined by MALDI-TOF mass spectrometry, as described by Yang et al. and Yin et al. (28–30). Briefly, a 183 bp amplicon including 5 of the 7 significant FABP3 CpG sites with adj.P < 0.05 from the 850K array (cg08877374, cg14407437, cg07345934, cg18368411 and cg15833534, Supplementary Table S1) was designed for analyses by MALDI-TOF mass spectrometry. The amplicons containing cg19316148 and cg20318096 failed in primer design. The target 183 bp amplicon was amplified by PCR using bisulfite-specific primers, forward primer: aggaagagagTTATAGTGATGTTGGGTTAGGTTGA, reverse primer: cagtaatacgactcactatagggagaaggctCAACCCCTCCTAAATAAACCCT. Upper case letters present the sequence-specific primer regions, and non-specific tags are shown in lower case letters. The sequence of the amplicon was presented in Supplementary Table S2, cg08877374 was referred to CpG_1, cg14407437 was referred to CpG_4, cg07345934 was referred to CpG_6, cg18368411 was referred to as CpG_7, and cg15833534 was referred to CpG_9. There are no SNPs overlapped with any of the CpG sites in the amplicon. Among the 11 CpG sites in this amplicon, the methylation levels of 7 sites were measurable by the Epityper assay including CpG_1/cg08877374, CpG_7/cg18368411 and CpG9/cg15833534, whereas the CpG_4/cg14407437 and CpG_6/cg07345934 were unmeasurable due to high mass. The 7 CpG sites yielded 5 distinguishable mass peaks by the mass spectrometry. CpG_9, CpG_10, and CpG_11 sites were located at the same fragment, and thus the mass peak shows the average methylation level of the three loci, which was presented as CpG_9.10.11. The other mass peaks have only one CpG locus. Next, the amplification products were treated with Shrimp Alkaline Phosphatase and followed by T-cleavage using RNase A. After cleaning residual ions with resin, the methylation level of each sample was quantified and collected by the MassARRAY system.
2.5 BRAFV600E mutation detection
A single hotspot mutation in nucleotide 1799 of the BRAF gene (corresponding to p.V600E) has been recognized as the most frequent genetic event in PTC, with an incidence of 29 - 83%, and accounts for more than 90% of BRAF-mutated TC (31). A total of 711 patients from two validations were analyzed for BRAF gene mutations. Forward primer: 5’-TCATAATGCTTGCTGATAGGA-3’ and reverse primer: 5’-GGCCAAAAATTTAATCAGTGGA-3’ were used for amplification by PCR. The sequences of the amplified fragments were then analyzed by Sanger-Sequencing.
2.6 Statistical analyses
All data analyses were performed using SPSS (version 25.0) and GraphPad Prism (version 8.0). Mann-Whitney U test and Chi-square test were used to compare the differences between TC and BTN groups. Spearman correlation was used to determine the relationship between variables. Binary logistic regression analysis was performed to calculate odds ratios (ORs) and their 95% confidence intervals (95% CIs), adjusted for age, gender, TSH, FT3, and FT4 levels. In addition, subgroup analyses were stratified by gender adjusted for age, TSH, FT3, and FT4. Kruskal-Wallis test and Mann-Whitney U test were used to analyze the correlation between FABP3 methylation levels and different clinical characteristics. Receiver operating characteristic (ROC) curve was used to evaluate goodness of fit. A two‐tailed P value < 0.05 was considered statistically significant.
3 Results
3.1 Discovery of TC associated FABP3 hypomethylation in tissue
Genome-wide Illumina Methylation 850K BeadChip array and RNA-Sequencing were used to screen methylation sites and genes with significant differences between TC and BTN in a total of 32 fresh-frozen samples (15 TC and 17 BTN). Gene Set Enrichment Analysis (GSEA) was applied to explore potential associations with pathways. As shown in Supplementary Figure S1, enrichment in fatty acid transport was observed. Fatty acid-binding protein 3 (FABP3), one of the central regulators of lipid metabolism and energy homeostasis, containing multiple TC-related aberrant methylation sites and also over-expressed in TC was considered for further investigation in our study. Cg18368411 within the transcription start site 200 bp (TSS200) of the FABP3 gene (Figure 1) showed the most significant difference between TC cases and BTN subjects (median methylation: TC cases = 0.28, BTN subjects = 0.56, P = 2.90E-05; Figure 2A). Alterations in DNA methylation may affect gene expression. Consistently, we found that compared with BTN subjects, TC cases showed increased expression of FABP3 mRNA (fold change =1.83, P = 0.040; Figure 2B). In addition, the methylation level of cg18368411 was significantly negatively correlated with the expression level of FABP3 with the Spearman correlation coefficient value of -0.62 (P = 1.34E-04; Figure 2C). The Cancer Genome Atlas (TCGA)-THCA dataset (32–34) also supported our results that FABP3 promoter methylation was marginally lower and the inversely correlated expression was higher in TC tissues compared to adjacent paired normal thyroid tissues (Supplementary Figure S2).
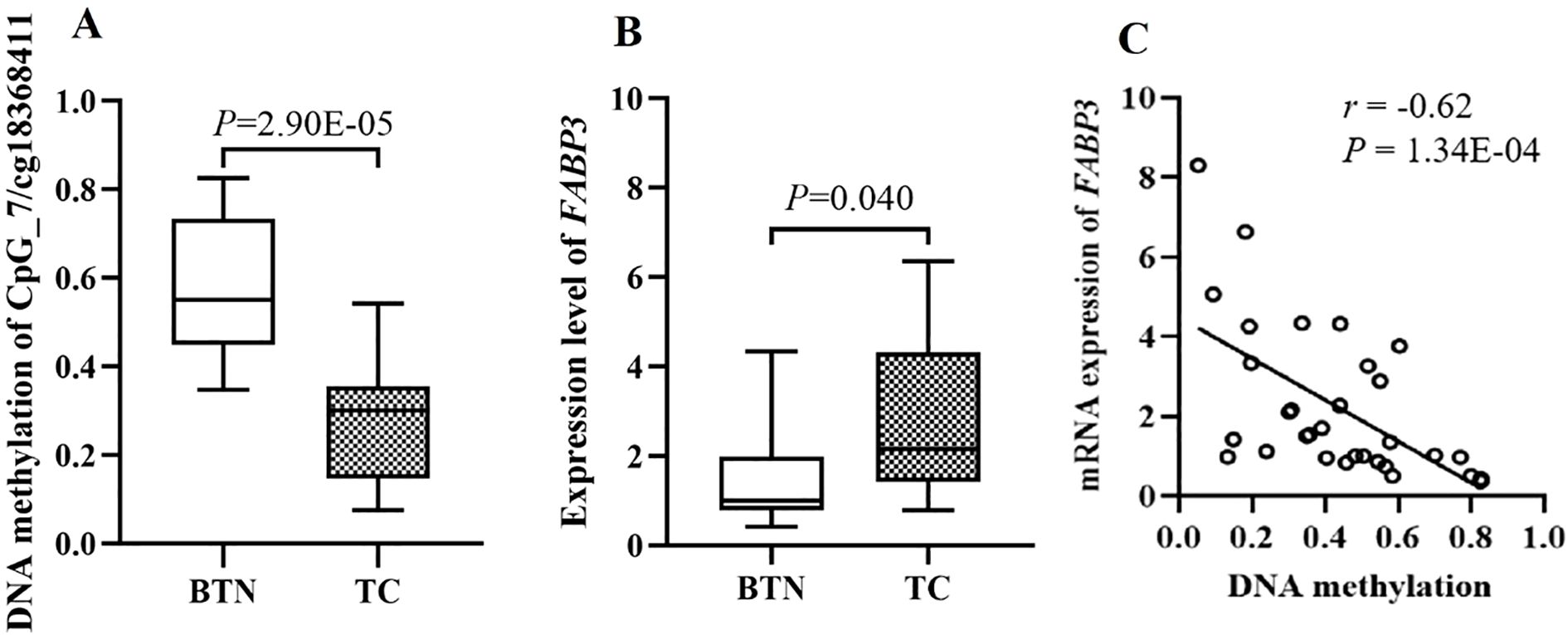
Figure 2. The discovery study showed significantly correlation between cg18368411 methylation and mRNA expression of the FABP3 gene in fresh-frozen tissue samples. (A) Box plots for methylation levels of cg18368411 detected by Methylation 850K BeadChip array. (B) Box plots for mRNA expression levels of FABP3 gene measured by RNA-Sequencing. (C) Correlations of FABP3 expression with methylation levels of cg18368411.
3.2 Validation of the differential FABP3 methylation in BTN and TC by two independent case-control studies
To validate FABP3 hypomethylation in TC cases compared to BTN subjects, two independent case-control studies were conducted using FFPE tissue samples. A 183 bp amplicon containing cg18368411 site and flanking CpG sites was designed for analyses by MALDI-TOF mass spectrometry (Figure 3A).
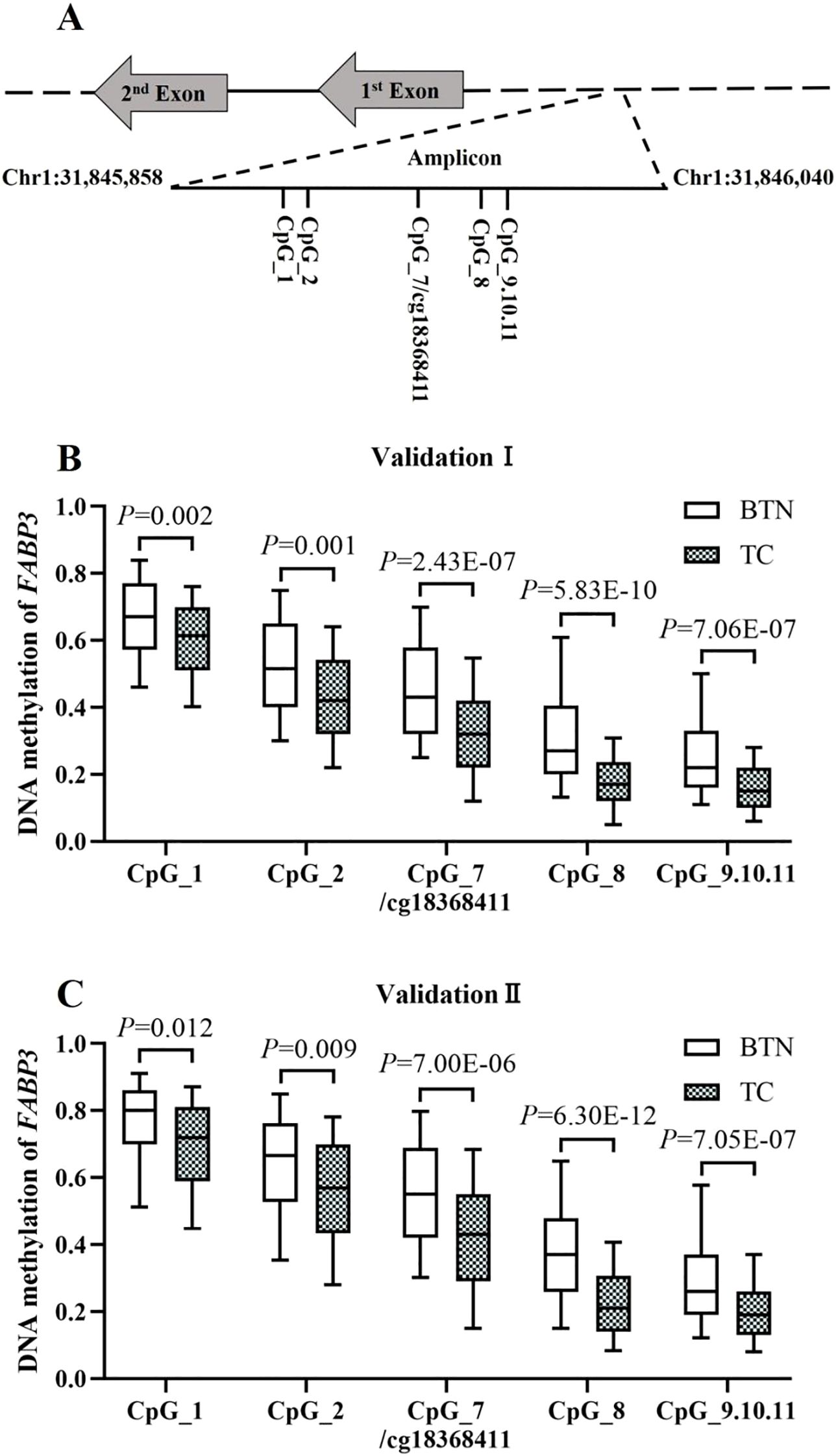
Figure 3. Validation of FABP3 hypomethylation in TC cases compared to BTN subjects in two independent studies. (A) Schematic diagram of the target amplicon within the FABP3 gene. A 183bp amplicon covers seven measurable CpG sites of the FABP3 gene (Chr1: 31845858-31846040, build GRCh37/hg19, defined by the UCSC Genome Browser). Cg18368411 was referred to CpG_7. (B, C) Box plots for the methylation levels of the seven CpG sites in FABP3 amplicon in Validation Ⅰ (B) and Validation Ⅱ (C). All the P values were calculated by logistic regression adjusted for covariates. BTN, benign thyroid nodule; TC, thyroid cancer.
In Validation I (222 TC cases and 221 age- and gender-matched BTN subjects), all the seven CpG sites in the FABP3 amplicon showed significantly lower methylation levels in TC than in BTN, among which CpG_8 was the most significant loci (methylation values of BTN and TC: 0.27 vs. 0.17, P = 5.83E-10 adjusted for age, gender, TSH, FT3, and FT4). Moreover, there was a significant association between FABP3 hypomethylation and TC cases. After adjusting for covariates, the odds ratios (ORs) per 10% reduced methylation of all FABP3 CpG sites ranged from 1.24 to 1.81 (P ≤ 0.002 for all; Figure 3B, Supplementary Table S3). Consistent results were observed in Validation II (256 TC and 191 BTN). All seven FABP3 CpG sites were hypomethylated in TC cases than those in BTN patients. Similarly, CpG_8 showed the most significant reduction (methylation values of BTN and TC: 0.37 vs. 0.21, P = 6.30E-12). Hypomethylation of all FABP3 CpG sites was significantly associated with TC (the ORs per -10% methylation ranged from 1.17 to 1.79, P ≤ 0.012 for all by binary logistic regression adjusted for age, gender, TSH, FT3, and FT4; Figure 3C, Supplementary Table S3).
3.3 The association between FABP3 hypomethylation in tissues and TC stratified by gender and age
To eliminate the confounding effects of gender and age, we further evaluated the association between FABP3 methylation and TC cases by stratified regression analyses. To avoid possible bias due to the small sample size, the subjects in the two validations were combined (478 TC vs. 412 BTN).
When stratified by gender, in males, four out of seven FABP3 CpG sites presented a significant association with TC (CpG_8 and CpG_9.10.11, the ORs per -10% methylation ranged from 1.39 to 1.56, all the P values ≤ 0.011; Figure 4A, Supplementary Table S4). In females, all CpG sites showed significantly lower methylation levels in TC cases than in BTN subjects (the ORs per -10% methylation ranged from 1.20 to 1.85, all the P values ≤ 0.001; Figure 4B, Supplementary Table S4) by logistic regression adjusted for age, TSH, FT3, and FT4. Moreover, the methylation differences between the cases and controls were larger in females than in males. When stratified by the age of 55 years old, which is the cutoff age for staging DTC, there was a significant association between all the seven CpG sites methylation and TC in the group less than 55 years old (the ORs per 10% reduced methylation ranged from 1.26 to 1.82, all the P values ≤ 3.70E - 05) by logistic regression adjusted for age, gender, TSH, FT3, and FT4 (Figure 4C, Supplementary Table S5). In contrast, in subjects older than or equal to 55 years old, only CpG_7/cg18368411, CpG_8, and CpG_9.10.11 sites showed association with TC (the ORs per -10% methylation ranged from 1.22 to 1.72, all the P values ≤ 0.009; Figure 4D, Supplementary Table S5).

Figure 4. Combination analysis of the association between FABP3 hypomethylation in FFPE tissues and TC stratified by age and gender. (A, B) Box plots for the FABP3 methylation levels in the male group (A) and female group (B). (C, D) Box plots for the FABP3 methylation levels in the less than 55 years old group (C) and older than or equal to 55 years old group (D). All the P values were calculated by logistic regression with the adjustment of covariates. BTN, benign thyroid nodule; TC, thyroid cancer.
3.4 The diagnostic efficiency of FABP3 hypomethylation alone and combined with BRAFV600E in differentiating TC from BTN
To estimate the potential clinical utility of FABP3 methylation as a biomarker for TC, ROC curve analyses were performed and adjusted for age, gender, TSH, FT3, and FT4 by logistic regression. As shown in Figures 5A, FABP3 hypomethylation based on FFPE tissue samples combining two validations has high credibility and accuracy in distinguishing TC cases from BTN patients (the area under the ROC curve (AUC) = 0.77, 95% CI: 0.73 - 0.80). The BRAFV600E mutation has been reported to be a potential biomarker for PTC. Sanger-Sequencing was performed to evaluate the BRAFV600E mutation status of the patients in two validations (Supplementary Figure S3). A total of 711 patients (381 BTN patients and 330 TC cases) were analyzed for mutations in the BRAF gene. No mutations were found in any of the BTN patients, while BRAF mutations were detected in 180 (54.5%) of the TC cases. We observed good predictability of BRAFV600E to distinguish TC cases from BTN subjects (AUC = 0.77, 95% CI: 0.74 - 0.81; Figure 5B). Next, whether FABP3 methylation in combination with BRAFV600E could improve the diagnostic value of differentiating TC from BTN was investigated. The combined application of FABP3 hypomethylation and BRAFV600E achieved higher diagnostic accuracy (AUC = 0.87, 95% CI: 0.85 - 0.90; Figure 5C).

Figure 5. The diagnostic efficiency of FABP3 hypomethylation alone and combined with BRAFV600E in differentiating TC from BTN. (A) ROC curve analyses for the discriminatory power of the seven FABP3 CpG sites to distinguish TC cases from BTN subjects. (B) ROC curve analysis for the discriminatory power of BRAFV600E to distinguish TC cases from BTN subjects. (C) ROC curve analyses for the discriminatory power of FABP3 methylation in combined with BRAFV600E mutation to differentiate TC cases from BTN subjects. All the above 95% CI of AUC were calculated by logistic regression with covariates-adjusted. ROC, receiver operating characteristic; AUC, the area under the ROC curve; CI, confidence interval.
3.5 Histological classification of BTN and TC subtypes by FABP3 methylation in tissue
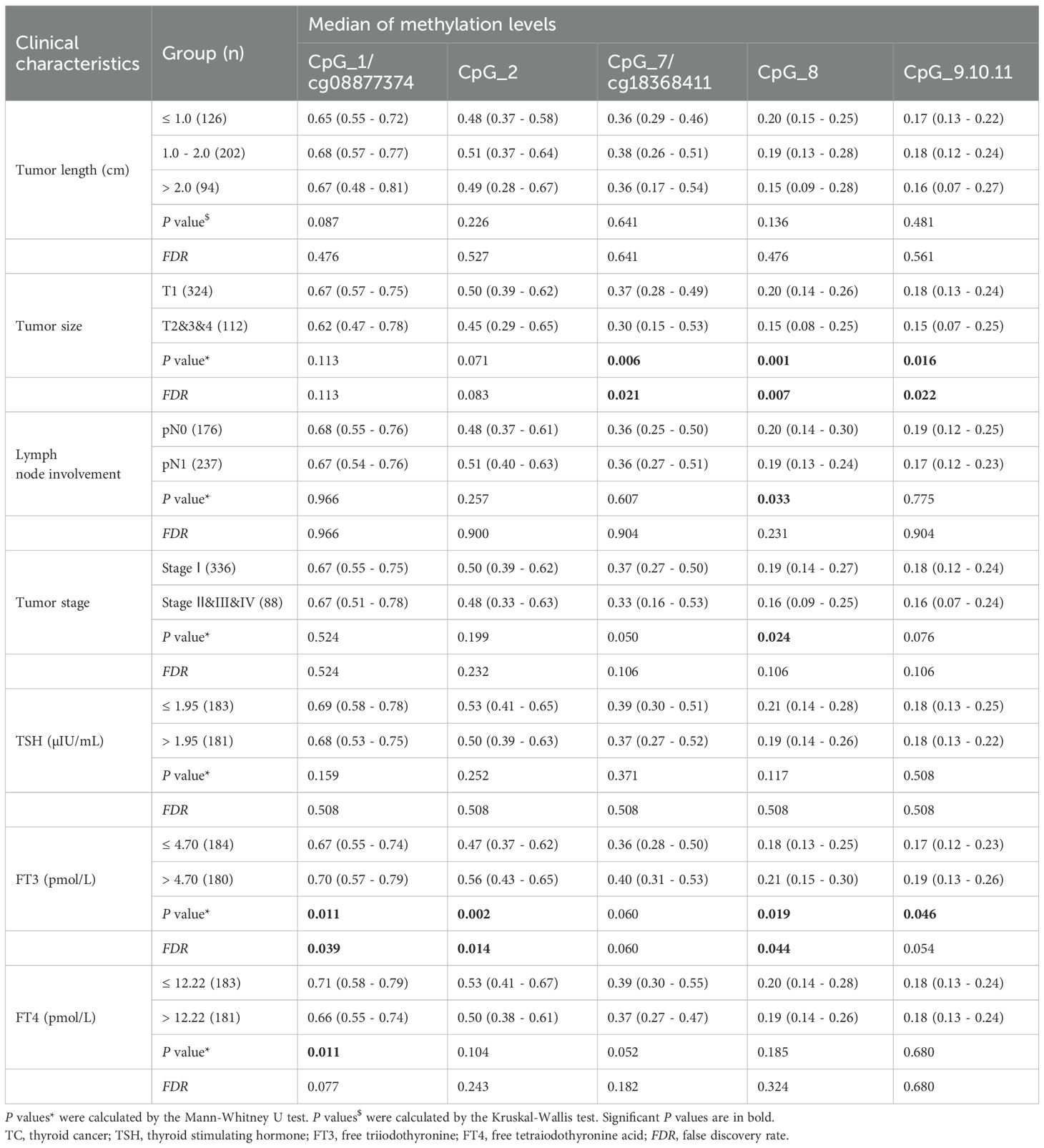
Furthermore, we analyzed the methylation levels of FABP3 in 478 TC cases and 412 BTN patients stratified by subtype. All seven FABP3 CpG sites were hypomethylated in PTC, MTC, and ATC cases than those in adenoma patients (all the P values ≤ 5.90E - 05; Figure 6, Supplementary Table S6). However, we did not observe a difference in FABP3 methylation between adenoma subjects and FTC cases (all the P values > 0.05; Figure 6, Supplementary Table S6). As for BTN, the methylation level of FABP3 in patients with lymphatic thyroiditis was lower than that in patients with adenoma (all the P values ≤ 0.002; Figure 6, Supplementary Table S6). The most significant reduction was in CpG_7/cg18368411 (methylation values in adenoma and lymphatic thyroiditis: 0.53 vs. 0.32, P = 1.41E-04; Figure 6, Supplementary Table S6). In addition, we observed a correlation between five FABP3 CpG sites methylation and tumor size (CpG_7/cg18368411, CpG_8, and CpG_9.10.11, all the P values ≤ 0.016; Table 2). Next, we evaluated the correlation between FABP3 methylation and thyroid-related hormones with their median values as cutoff points (1.95 µIU/mL for TSH, 4.70 pmol/L for FT3, and 12.22 pmol/L for FT4). A positive correlation was observed between FT3 levels and the methylation levels of CpG_1/cg08877374, CpG_2, CpG_8, and CpG_9.10.11 sites (all the P values ≤ 0.046; Table 2).
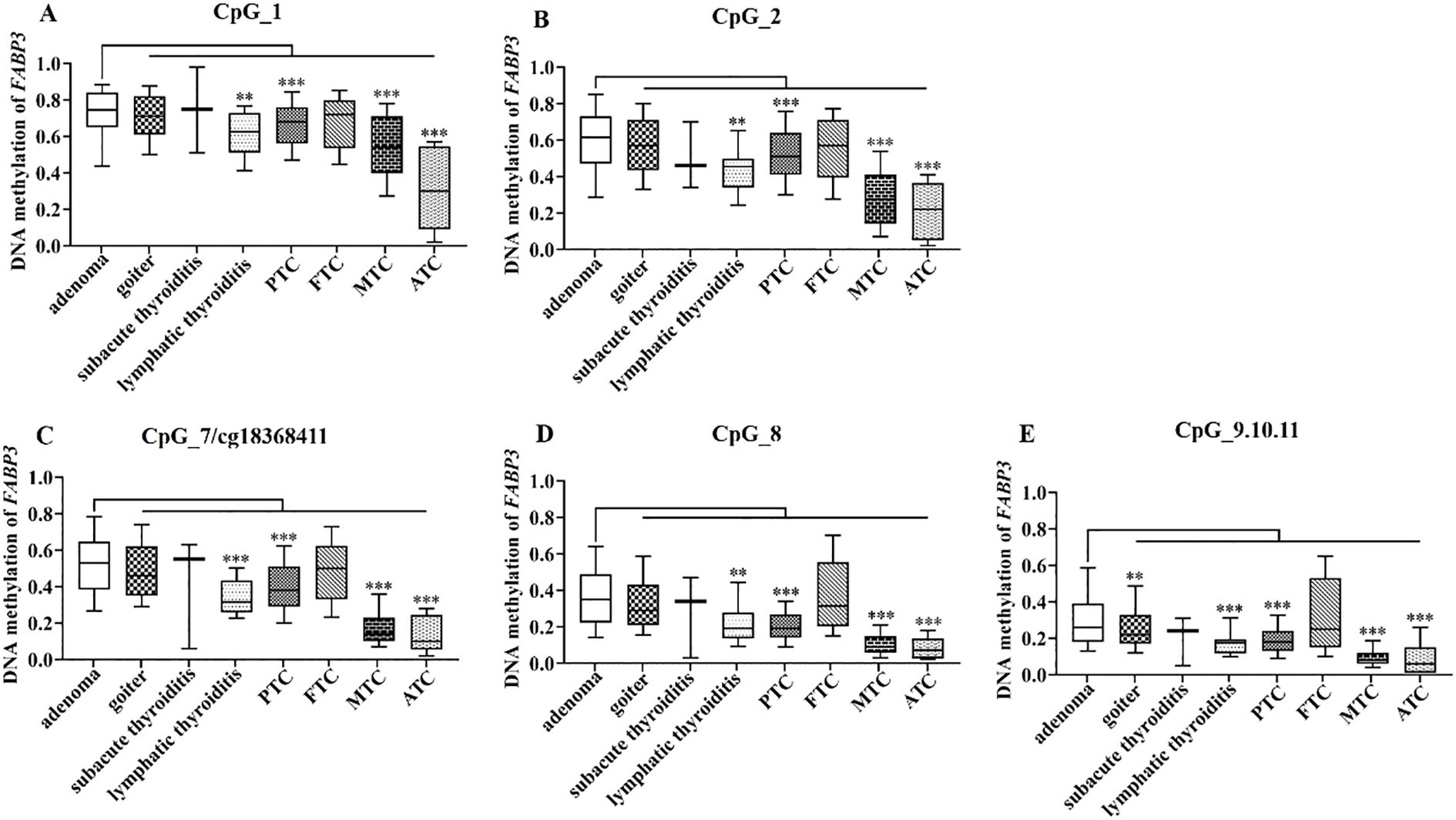
Figure 6. FABP3 methylation in BTN and TC subtypes. (A–E) Box plots of methylation levels of seven CpG sites of FABP3 in BTN and TC subtypes. All the P values were calculated by the Mann-Whitney U test referred to adenoma. *: P < 0.05, **: P < 0.01, ***: P < 0.001. BTN, benign thyroid nodule; TC, thyroid cancer; PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; MTC, medullary thyroid cancer; ATC, anaplastic thyroid cancer.
4 Discussion
Fatty acid-binding proteins (FABPs) are expressed in most major tissues (35), and have been proposed to be central regulators of lipid metabolism and energy homeostasis through their control of fatty acid transport (36). FABP3, which is mainly expressed in skeletal muscle, the heart, and the placenta, plays a role in the intracellular transport of long-chain fatty acids and their acyl-CoA esters (37). Recent reports have shown that FABP3 may be involved in the pathogenesis of various diseases (38, 39). For example, knockdown of FABP3 caused mitochondrial dysfunction and increased the apoptosis of cardiac cell lines (40). Bensaad et al. found that FABP3 knockdown impaired the growth of glioblastoma xenograft by reducing fatty acid uptake and oxidation (41). The methylation of FABP3 is associated with insulin, lipids, and cardiovascular phenotypes of the metabolic syndrome (42). As a fatty acid transporter, FABP3 hypomethylation and consequently overexpression could enhance intracellular lipid flux and activate PPARγ/RXRα signaling (43), which is implicated in tumor proliferation of thyroid cancer (44). Till now, there have been no reports on FABP3 methylation alterations in TC. Here, we discovered hypomethylation of the FABP3 gene in TC cases compared to BTN subjects together with a markedly elevated FABP3 mRNA expression via joint analyses of 850K BeadChip array and RNA-Sequencing, and performed further validations using mass spectrometry in two independent studies with large sample size.
Age and gender have been identified as risk factors for the incidence of TC (45). TC is the only malignancy with age as a prognostic indicator in the majority of staging systems (46), which is the most frequently diagnosed malignancy among adolescents and young adults (47, 48). Deng et al. reported that the most common onset age in persons who developed TC decreased, and the age at death of those with TC increased worldwide (49). A retrospective cohort evaluation of TC cases and deaths during 2005 – 2015 showed the rate of increasing incidence trend was higher in the younger age group and lower in the older age group (50). Our results revealed the association between altered FABP3 methylation and TC risk, especially in younger people. Since global methylation levels decline with age (51), larger methylation differences could be expected among the younger individuals. As for gender, TC is the only non-reproductive cancer that occurs more often in women than in men, with a 3 - 4-fold higher incidence among women than men (52). We observed the methylation differences of the FABP3 gene between the TC cases and BTN subjects were larger in females than in males. Estrogen may participate in the initiation of tumorigenesis in TC. It has been reported that estrogen has a positive effect on the proliferation of thyroid cells, which is essential for the development of TC, and may contribute to the mechanisms that cause DNA damage (53). In addition, estrogen has also been shown to enhance the secretion of VEGF (vascular endothelial growth factor) by thyroid cells (54, 55), thereby regulating the vascular environment and allowing further tumor growth (56, 57). Moreover, estrogen receptor plays a role in cell migration and invasion in TC. The observed stronger association between FABP3 hypomethylation and malignancy in females may reflect hormonal related epigenetic influences. Biologically, FABP3 operates within the PPAR signaling pathway (PPARγ/RXRα), which is modulated by sex hormones (58–60). Estrogen enhances PPARγ activity, potentially amplifying FABP3’s metabolic role in thyroid tissue and strengthening its methylation changes in females (61). While our study did not directly probe these mechanisms, future work should investigate hormonal regulation (e.g., ESR1/PPARγ crosstalk) and age-related FABP3 epigenetic drift in thyroid carcinogenesis.
Not only for the differentiation of BTN and TC, we also found interesting variation of FABP3 methylation in variant subtypes of BTN (adenoma, goiter, subacute thyroiditis, lymphatic thyroiditis) and TC (PTC, FTC, MTC, ATC). Adenoma showed the highest FABP3 methylation among all the BTN and TC subgroups, followed by goiter and subacute thyroiditis. To our surprise, the methylation level of FABP3 in most lymphocytic thyroiditis is similar to that of malignancy but not as other BTN subgroups. Chronic inflammation has been considered as a pro-tumor effect in TC (62, 63). Lymphocytic thyroiditis is the most common autoimmune disorder. Several studies have shown an epidemiological correlation between lymphocytic thyroiditis and TC, especially PTC (64, 65). Our study hereby supported that chronic inflammation may be a precancerous lesion or at least a highly correlated risk factor. However, we have to be aware that our finding is based on limited sample size of lymphocytic thyroiditis. Since most benign nodules especially the individuals with thyroiditis do not undergo surgery, we would call for enlarged studies based on multi-centers for further validation. In addition, we found that the methylated level of FABP3 was correlated with the grade of malignancy. The methylation of FABP3 gradually declined from low malignant PTC to the highly aggressive ATC. The methylation level of FABP3 of MTC, the intermediate subtypes of TC, is around 50% lower than PTC, but higher than ATC. Follicular adenoma is the early stage of FTC with a slightly different DNA methylation pattern than that observed in the normal thyroid, implying a role in the initiation of malignancy (66). Sharing a common genetic background, follicular adenoma and FTC represents a significant diagnostic challenge at pre- and postsurgical differentiation (67). Our study showed similar FABP3 methylation level in FTC with adenoma, indicating that follicular adenoma may share common epigenetic background with FTC as well (68). Taken together, the epigenetic alterations in TC subtypes could be helpful for the diagnosis and classification of tumors, and may even indicate the precancerous lesion and the malignant grade of TC.
Our current study intentionally focused on the diagnostic performance of FABP3 methylation especially for early-stage cancer (stage I and II), and thus, in lack of the prognostic information while early-stage TC is often curable upon timely diagnosis and treatment. Nevertheless, we have observed lower levels of FABP3 methylation in TC patients with larger tumors, suggesting that the decreasing methylation level of FABP3 may also be associated with the proliferation of tumors. Previous study has shown that high expression of FABPs in gastric cancer is associated with disease progression, tumor aggressiveness, and poor patient survival (69). Further investigation in larger studies is necessary to validate the correlations between FABP3 hypomethylation and TC progression as well as its prognostic value. Pan-cancer evidence suggests that while certain methylation markers can serve as standalone prognostic factors, robust prognostic power frequently emerges from integrated models combining multiple molecular features (70, 71). Future studies should evaluate FABP3 methylation and other methylation markers in conjunction with established prognostic co-factors, such as specific microRNAs or immune-context markers (72, 73), to develop enhanced prognostic tools. Interestingly, we discovered that the methylation level of FABP3 steadily rose as FT3 levels rose and fell as FT4 levels rose. The relationship between changed thyroid hormone levels and the risk of TC has been widely studied (74–76). Low FT3 and high FT4 concentrations are associated with shorter survival in patients with TC (77). Moreover, serum FT3 levels are negatively correlated with inflammation, which is also associated with TC (78). FT4 levels play different roles in different cancer types. In liver cancer, reduced FT4 levels indicate an increased risk of death; whereas in patients with primary breast cancer, elevated FT4 levels are associated with poor prognosis (76). To date, few studies have been conducted on the relationship between thyroid-related hormones and alterations in gene methylation (79). Thus, future prospective studies are warranted.
The present study is among the largest studies on differential diagnosis of TC and BTN with a total of 890 samples, which suggests hypomethylation of the FABP3 gene might be biomarkers to differentiate benign and malignant thyroid tumors. Compared to the other established tissue-based markers for the diagnosis of TC (80–83), such as GNB5 expression (AUC = 0.67), PLIN3 expression (AUC = 0.81) and EPHB2 (no difference between TC and normal thyroid tissue), FABP3 methylation showed a sufficient diagnostic performance (AUC = 0.77). Moreover, the combination of FABP3 methylation with existing diagnostic techniques, such as BRAFV600E mutation, the diagnostic efficacy can be further improved (AUC = 0.87). The combination with additional TC-related mutations may further improve the diagnostic value of FABP3 methylation. However, the TCGA dataset retrieved from cBioPortal shows that the occurrence of RAS mutations in only 6.9% of PTCs, while RET/PTC fusions are even rarer (1.88%) (32, 84, 85). Considering our current sample size, there is no sufficient statistical power to support combinatorial modeling with rare mutations. In addition, although our study based on Chinese population was in agreement with the TCGA database with samples from heterogenous genetic background, the possible bias on population heterogeneity could not be excluded. We look forward to the future international studies with multi-ethnic cohorts considering rare TC-related mutations for further exploration.
5 Conclusion
To sum up, this study revealed and proved hypomethylation of FABP3 gene, together with a markedly elevated FABP3 mRNA expression in TC cases compared to BTN subjects. FABP3 showed significant methylation differences between TC and BTN, and among TC subtypes, suggesting the potential of DNA methylation as a novel pathological biomarker not only in discriminating malignant and BTN, but also in distinguishing between TC subtypes. Moreover, by combining FABP3 methylation with BRAFV600E mutation, the diagnostic efficacy can be significantly improved. Further investigation in a prospective multi-center study is needed before clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HH: Investigation, Writing – review & editing, Writing – original draft, Formal Analysis. YY: Resources, Validation, Methodology, Supervision, Writing – review & editing. YM: Methodology, Supervision, Validation, Writing – review & editing. HL: Writing – review & editing, Supervision, Validation. JL: Visualization, Supervision, Writing – review & editing. ML: Visualization, Supervision, Writing – review & editing. YZ: Supervision, Writing – review & editing. XH: Writing – review & editing, Supervision. YFZ: Writing – review & editing, Supervision. CJ: Supervision, Conceptualization, Writing – review & editing, Project administration, Resources. RY: Funding acquisition, Supervision, Resources, Writing – review & editing, Writing – original draft, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Research Grant of Nanjing TANTICA Co. Ltd (Grant No. 2021TC01.1). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1630001/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Du L, Li R, Ge M, Wang Y, Li H, Chen W, et al. Incidence and mortality of thyroid cancer in China, 2008-2012. Chin J Cancer Res. (2019) 31:144–51. doi: 10.21147/j.issn.1000-9604.2019.01.09
3. Seib CD and Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. (2019) 48:23–35. doi: 10.1016/j.ecl.2018.10.002
4. Zhou Y, Du J, Pan L, Li H, and Du L. Analysis of cervical cancer incidence and mortality in cancer registries of Zhejiang province, 2000 to 2009. Zhonghua Yu Fang Yi Xue Za Zhi. (2014) 48:674–7.
5. Wang YQ, Li HZ, Gong WW, Chen YY, Zhu C, Wang L, et al. Cancer incidence and mortality in Zhejiang Province, Southeast China, 2016: a population-based study. Chin Med J (Engl). (2021) 134:1959–66. doi: 10.1097/CM9.0000000000001666
6. Vuong HG, Le MK, Hassell L, Kondo T, and Kakudo K. The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck. (2022) 44:926–32. doi: 10.1002/hed.26987
7. Raue F and Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res. (2016) 22:5012–21. doi: 10.1158/1078-0432.CCR-16-0484
8. Hu J, Yuan IJ, Mirshahidi S, Simental A, Lee SC, and Yuan X. Thyroid carcinoma: phenotypic features, underlying biology and potential relevance for targeting therapy. Int J Mol Sci. (2021) 22(4):1950. doi: 10.3390/ijms22041950
9. Grani G, Sponziello M, Pecce V, Ramundo V, and Durante C. Contemporary thyroid nodule evaluation and management. J Clin Endocrinol Metab. (2020) 105:2869–83. doi: 10.1210/clinem/dgaa322
10. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, and Cooper DS. The diagnosis and management of thyroid nodules: A review. JAMA. (2018) 319:914–24. doi: 10.1001/jama.2018.0898
11. Stewart R, Leang YJ, Bhatt CR, Grodski S, Serpell J, and Lee JC. Quantifying the differences in surgical management of patients with definitive and indeterminate thyroid nodule cytology. Eur J Surg Oncol. (2020) 46:252–7. doi: 10.1016/j.ejso.2019.10.004
12. Chen Y, Yin M, Zhang Y, Zhou N, Zhao S, Yin H, et al. Imprinted gene detection effectively improves the diagnostic accuracy for papillary thyroid carcinoma. BMC Cancer. (2024) 24:359. doi: 10.1186/s12885-024-12032-z
13. Ferraz C. Molecular testing for thyroid nodules: Where are we now? Rev Endocr Metab Disord. (2024) 25:149–59. doi: 10.1007/s11154-023-09842-0
14. Moses W, Weng J, Sansano I, Peng M, Khanafshar E, Ljung BM, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. (2010) 34:2589–94. doi: 10.1007/s00268-010-0720-0
15. Pupilli C, Pinzani P, Salvianti F, Fibbi B, Rossi M, Petrone L, et al. Circulating BRAFV600E in the diagnosis and follow-up of differentiated papillary thyroid carcinoma. J Clin Endocrinol Metab. (2013) 98:3359–65. doi: 10.1210/jc.2013-1072
16. Eze OP, Starker LF, and Carling T. The role of epigenetic alterations in papillary thyroid carcinogenesis. J Thyroid Res. (2011) 2011:895470. doi: 10.4061/2011/895470
17. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. (2005) 12:245–62. doi: 10.1677/erc.1.0978
18. Santos JC, Bastos AU, Cerutti JM, and Ribeiro ML. Correlation of MLH1 and MGMT expression and promoter methylation with genomic instability in patients with thyroid carcinoma. BMC Cancer. (2013) 13:79. doi: 10.1186/1471-2407-13-79
19. Chang H, Shin BK, Kim A, Kim HK, and Kim BH. DNA methylation analysis for the diagnosis of thyroid nodules - a pilot study with reference to BRAF(V) (600E) mutation and cytopathology results. Cytopathology. (2016) 27:122–30. doi: 10.1111/cyt.12248
20. Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A prospective blinded multicenter study. JAMA Oncol. (2019) 5:204–12. doi: 10.1001/jamaoncol.2018.4616
21. Nikiforova MN, Mercurio S, Wald AI, Barbi de Moura M, Callenberg K, Santana-Santos L, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. (2018) 124:1682–90. doi: 10.1002/cncr.31245
22. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. (2012) 367:705–15. doi: 10.1056/NEJMoa1203208
23. Barros-Filho MC, Dos Reis MB, Beltrami CM, de Mello JBH, Marchi FA, Kuasne H, et al. DNA methylation-based method to differentiate Malignant from benign thyroid lesions. Thyroid. (2019) 29:1244–54. doi: 10.1089/thy.2018.0458
24. Arantes LM, de Carvalho AC, Melendez ME, Carvalho AL, and Goloni-Bertollo EM. Methylation as a biomarker for head and neck cancer. Oral Oncol. (2014) 50:587–92. doi: 10.1016/j.oraloncology.2014.02.015
25. Stephen JK, Chen KM, Merritt J, Chitale D, Divine G, and Worsham MJ. Methylation markers differentiate thyroid cancer from benign nodules. J Endocrinol Invest. (2018) 41:163–70. doi: 10.1007/s40618-017-0702-2
26. Yim JH, Choi AH, Li AX, Qin H, Chang S, Tong ST, et al. Identification of tissue-specific DNA methylation signatures for thyroid nodule diagnostics. Clin Cancer Res. (2019) 25:544–51. doi: 10.1158/1078-0432.CCR-18-0841
27. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO2). Endocr Relat Cancer. (2018) 25:L7–L11. doi: 10.1530/ERC-17-0453
28. Yang R, Stöcker S, Schott S, Heil J, Marme F, Cuk K, et al. The association between breast cancer and S100P methylation in peripheral blood by multicenter case-control studies. Carcinogenesis. (2017) 38:312–20. doi: 10.1093/carcin/bgx004
29. Yin Q, Yang X, Li L, Xu T, Zhou W, Gu W, et al. The association between breast cancer and blood-based methylation of S100P and HYAL2 in the chinese population. Front Genet. (2020) 11:977. doi: 10.3389/fgene.2020.00977
30. Yang R, Pfutze K, Zucknick M, Sutter C, Wappenschmidt B, Marme F, et al. DNA methylation array analyses identified breast cancer-associated HYAL2 methylation in peripheral blood. Int J Cancer. (2015) 136:1845–55. doi: 10.1002/ijc.29205
31. Rowe LR, Bentz BG, and Bentz JS. Detection of BRAF V600E activating mutation in papillary thyroid carcinoma using PCR with allele-specific fluorescent probe melting curve analysis. J Clin Pathol. (2007) 60:1211–5. doi: 10.1136/jcp.2006.040105
32. Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
33. Ding W, Chen G, and Shi T. Integrative analysis identifies potential DNA methylation biomarkers for pan-cancer diagnosis and prognosis. Epigenetics. (2019) 14:67–80. doi: 10.1080/15592294.2019.1568178
34. Ding W, Chen J, Feng G, Chen G, Wu J, Guo Y, et al. DNMIVD: DNA methylation interactive visualization database. Nucleic Acids Res. (2020) 48:D856–D62. doi: 10.1093/nar/gkz830
35. Wu S, Kong X, Sun Y, Dai X, Yu W, Chen R, et al. FABP3 overexpression promotes vascular fibrosis in Takayasu’s arteritis by enhancing fatty acid oxidation in aorta adventitial fibroblasts. Rheumatol (Oxford). (2022) 61:3071–81. doi: 10.1093/rheumatology/keab788
36. Kawaguchi M, Tamura Y, Kakehi S, Takeno K, Sakurai Y, Watanabe T, et al. Association between expression of FABPpm in skeletal muscle and insulin sensitivity in intramyocellular lipid-accumulated nonobese men. J Clin Endocrinol Metab. (2014) 99:3343–52. doi: 10.1210/jc.2014-1896
37. Islam A, Kagawa Y, Sharifi K, Ebrahimi M, Miyazaki H, Yasumoto Y, et al. Fatty Acid Binding Protein 3 Is Involved in n-3 and n-6 PUFA transport in mouse trophoblasts. J Nutr. (2014) 144:1509–16. doi: 10.3945/jn.114.197202
38. Viswanathan K, Kilcullen N, Morrell C, Thistlethwaite SJ, Sivananthan MU, Hassan TB, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. (2010) 55:2590–8. doi: 10.1016/j.jacc.2009.12.062
39. Thumser AE, Moore JB, and Plant NJ. Fatty acid binding proteins: tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care. (2014) 17:124–9. doi: 10.1097/MCO.0000000000000031
40. Shen Y, Song G, Liu Y, Zhou L, Liu H, Kong X, et al. Silencing of FABP3 inhibits proliferation and promotes apoptosis in embryonic carcinoma cells. Cell Biochem Biophys. (2013) 66:139–46. doi: 10.1007/s12013-012-9462-y
41. Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF - 1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. (2014) 9:349–65. doi: 10.1016/j.celrep.2014.08.056
42. Zhang Y, Kent JW 2nd, Lee A, Cerjak D, Ali O, Diasio R, et al. Fatty acid binding protein 3 (fabp3) is associated with insulin, lipids and cardiovascular phenotypes of the metabolic syndrome through epigenetic modifications in a Northern European family population. BMC Med Genomics. (2013) 6:9. doi: 10.1186/1755-8794-6-9
43. Liu ZZ, Hong CG, Hu WB, Chen ML, Duan R, Li HM, et al. (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy. (2021) 17:2766–82. doi: 10.1080/15548627.2020.1839286
44. Ping P, Ma Y, Xu X, and Li J. Reprogramming of fatty acid metabolism in thyroid cancer: Potential targets and mechanisms. Chin J Cancer Res. (2025) 37:227–49. doi: 10.21147/j.issn.1000-9604.2025.02.09
45. Carling T and Udelsman R. Thyroid cancer. Annu Rev Med. (2014) 65:125–37. doi: 10.1146/annurev-med-061512-105739
46. Dean DS and Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. (2000) 7:229–39. doi: 10.1177/107327480000700302
47. Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, and Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. (2020) 70:443–59. doi: 10.3322/caac.21637
48. Di Giuseppe G, Zuk AM, Wasserman JD, and Pole JD. Pulmonary and overall healthcare utilization after childhood and young adult thyroid cancer. Endocr Relat Cancer. (2020) 27:391–402. doi: 10.1530/ERC-19-0463
49. Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open. (2020) 3:e208759. doi: 10.1001/jamanetworkopen.2020.8759
50. Wang J, Yu F, Shang Y, Ping Z, and Liu L. Thyroid cancer: incidence and mortality trends in China, 2005 - 2015. Endocrine. (2020) 68:163–73. doi: 10.1007/s12020-020-02207-6
51. Zheng Z, Li J, Liu T, Fan Y, Zhai QC, Xiong M, et al. DNA methylation clocks for estimating biological age in Chinese cohorts. Protein Cell. (2024) 15:575–93. doi: 10.1093/procel/pwae011
52. Shobab L, Burman KD, and Wartofsky L. Sex differences in differentiated thyroid cancer. Thyroid. (2022) 32:224–35. doi: 10.1089/thy.2021.0361
53. Suteau V, Munier M, Briet C, and Rodien P. Sex bias in differentiated thyroid cancer. Int J Mol Sci. (2021) 22(23):12992. doi: 10.3390/ijms222312992
54. de Araujo LF, Grozovsky R, dos Santos Pereira MJ, de Carvalho JJ, Vaisman M, and Carvalho DP. Expressions of vascular endothelial growth factor and nitric oxide synthase III in the thyroid gland of ovariectomized rats are upregulated by estrogen and selective estrogen receptor modulators. Thyroid. (2010) 20:85–92. doi: 10.1089/thy.2009.0246
55. Kamat A, Rajoria S, George A, Suriano R, Shanmugam A, Megwalu U, et al. Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg. (2011) 137:1146–53. doi: 10.1001/archoto.2011.194
56. Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, et al. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J Clin Endocrinol Metab. (1997) 82:3741–7. doi: 10.1210/jc.82.11.3741
57. Soh EY, Eigelberger MS, Kim KJ, Wong MG, Young DM, Clark OH, et al. Neutralizing vascular endothelial growth factor activity inhibits thyroid cancer growth in vivo. Surgery. (2000) 128:1059–65. doi: 10.1067/msy.2000.110430
58. Ku T, Hu J, Zhou M, Xie Y, Liu Y, Tan X, et al. Cardiac energy metabolism disorder mediated by energy substrate imbalance and mitochondrial damage upon tebuconazole exposure. J Environ Sci (China). (2024) 136:270–8. doi: 10.1016/j.jes.2022.10.012
59. Park HJ and Choi JM. Sex-specific regulation of immune responses by PPARs. Exp Mol Med. (2017) 49:e364. doi: 10.1038/emm.2017.102
60. Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. (2020) 31:101435. doi: 10.1016/j.redox.2020.101435
61. Sato H, Ishikawa M, Sugai H, Funaki A, Kimura Y, Sumitomo M, et al. Sex hormones influence expression and function of peroxisome proliferator-activated receptor gamma in adipocytes: pathophysiological aspects. Horm Mol Biol Clin Investig. (2014) 20:51–61. doi: 10.1515/hmbci-2014-0026
62. Ferrari SM, Fallahi P, Galdiero MR, Ruffilli I, Elia G, Ragusa F, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. (2019) 20(18):4413. doi: 10.3390/ijms20184413
63. Cunha LL, Marcello MA, and Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. (2014) 21:R85–R103. doi: 10.1530/ERC-13-0431
64. Di Pasquale M, Rothstein JL, and Palazzo JP. Pathologic features of Hashimoto’s-associated papillary thyroid carcinomas. Hum Pathol. (2001) 32:24–30. doi: 10.1053/hupa.2001.21138
65. Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, et al. The incidence of thyroid carcinoma in Hashimoto’s thyroiditis. Am Surg. (1987) 53:442–5.
66. Chmielik E, Rusinek D, Oczko-Wojciechowska M, Jarzab M, Krajewska J, Czarniecka A, et al. Heterogeneity of thyroid cancer. Pathobiology. (2018) 85:117–29. doi: 10.1159/000486422
67. Borowczyk M, Dobosz P, Szczepanek-Parulska E, Budny B, Debicki S, Filipowicz D, et al. Follicular thyroid adenoma and follicular thyroid carcinoma-A common or distinct background? Loss of heterozygosity in comprehensive microarray study. Cancers (Basel). (2023) 15(3):638. doi: 10.3390/cancers15030638
68. Marczyk VR, Recamonde-Mendoza M, Maia AL, and Goemann IM. Classification of thyroid tumors based on DNA methylation patterns. Thyroid. (2023) 33:1090–9. doi: 10.1089/thy.2023.0074
69. Hashimoto T, Kusakabe T, Sugino T, Fukuda T, Watanabe K, Sato Y, et al. Expression of heart-type fatty acid-binding protein in human gastric carcinoma and its association with tumor aggressiveness, metastasis and poor prognosis. Pathobiology. (2004) 71:267–73. doi: 10.1159/000080061
70. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. (2005) 352:997–1003. doi: 10.1056/NEJMoa043331
71. Xi Y, Lin Y, Guo W, Wang X, Zhao H, Miao C, et al. Multi-omic characterization of genome-wide abnormal DNA methylation reveals diagnostic and prognostic markers for esophageal squamous-cell carcinoma. Signal Transduct Target Ther. (2022) 7:53. doi: 10.1038/s41392-022-00873-8
72. Liu Z, Gao Z, Li B, Li J, Ou Y, Yu X, et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology. (2022) 11:2085432. doi: 10.1080/2162402X.2022.2085432
73. Ferraz C, Cunha GB, de Oliveira MMB, Tenorio LR, Cury AN, Padovani RDP, et al. The diagnostic and prognostic role of miR-146b-5p in differentiated thyroid carcinomas. Front Endocrinol (Lausanne). (2024) 15:1390743. doi: 10.3389/fendo.2024.1390743
74. Falstie-Jensen AM, Kjaersgaard A, Lorenzen EL, Jensen JD, Reinertsen KV, Dekkers OM, et al. Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality - a Danish population-based study. Breast Cancer Res. (2019) 21:44. doi: 10.1186/s13058-019-1122-3
75. Berghoff AS, Wippel C, Starzer AM, Ballarini N, Wolpert F, Bergen E, et al. Hypothyroidism correlates with favourable survival prognosis in patients with brain metastatic cancer. Eur J Cancer. (2020) 135:150–8. doi: 10.1016/j.ejca.2020.05.011
76. Sohn W, Chang Y, Cho YK, Kim Y, Shin H, and Ryu S. Abnormal and euthyroid ranges of thyroid hormones in serum and liver cancer mortality: A cohort study. Cancer Epidemiol Biomarkers Prev. (2020) 29:2002–9. doi: 10.1158/1055-9965.EPI-20-0283
77. Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. (2005) 103:1122–8. doi: 10.1002/cncr.20881
78. Gao R, Chen RZ, Xia Y, Liang JH, Wang L, Zhu HY, et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int J Cancer. (2018) 143:466–77. doi: 10.1002/ijc.31327
79. Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, et al. Thyroid-stimulating hormone, thyroid hormones, and risk of papillary thyroid cancer: A nested case-control study. Cancer Epidemiol Biomarkers Prev. (2017) 26:1209–18. doi: 10.1158/1055-9965.EPI-16-0845
80. Guo Q, Zhong X, Dang Z, Zhang B, and Yang Z. Identification of GBN5 as a molecular biomarker of pan-cancer species by integrated multi-omics analysis. Discov Oncol. (2025) 16:85. doi: 10.1007/s12672-025-01840-9
81. Yang S, Liu H, Zheng Y, Chu H, Lu Z, Yuan J, et al. The role of PLIN3 in prognosis and tumor-associated macrophage infiltration: A pan-cancer analysis. J Inflammation Res. (2025) 18:3757–77. doi: 10.2147/JIR.S509245
82. Zhou L, Zhou Q, Guo Q, Lai P, Rui C, Li W, et al. Dual role of Cathepsin S in cutaneous melanoma: insights from mendelian randomization and bioinformatics analysis. BMC Cancer. (2025) 25:104. doi: 10.1186/s12885-025-13481-w
83. Xu S, Zheng Y, Ye M, Shen T, Zhang D, Li Z, et al. Comprehensive pan-cancer analysis reveals EPHB2 is a novel predictive biomarker for prognosis and immunotherapy response. BMC Cancer. (2024) 24:1064. doi: 10.1186/s12885-024-12843-0
84. Cheng PF, Dummer R, and Levesque MP. Data mining The Cancer Genome Atlas in the era of precision cancer medicine. Swiss Med Wkly. (2015) 145:w14183. doi: 10.4414/smw.2015.14183
Keywords: benign thyroid nodule, thyroid cancer, DNA methylation, FABP3, biomarker, classification
Citation: Huang H, Yin Y, Mao Y, Li H, Li J, Li M, Zhang Y, Huang X, Zhang Y, Jiang C and Yang R (2025) FABP3 methylation as a novel biomarker for the differentiation and classification of benign and malignant thyroid nodules. Front. Endocrinol. 16:1630001. doi: 10.3389/fendo.2025.1630001
Received: 16 May 2025; Accepted: 20 August 2025;
Published: 11 September 2025.
Edited by:
Marina Muzza, Italian Auxological Institute (IRCCS), ItalyReviewed by:
Shengshan Xu, Jiangmen Central Hospital, ChinaVanessa Suñé Mattevi, Federal University of Health Sciences of Porto Alegre, Brazil
Copyright © 2025 Huang, Yin, Mao, Li, Li, Li, Zhang, Huang, Zhang, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongxi Yang, cm9uZ3hpeWFuZ0Buam11LmVkdS5jbg==; Chenxia Jiang, amlhbmdjeDMwNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Haixia Huang1†
Haixia Huang1† Mengxia Li
Mengxia Li Yifen Zhang
Yifen Zhang Chenxia Jiang
Chenxia Jiang Rongxi Yang
Rongxi Yang