- 1Department of Environmental Biology, Sapienza University of Rome, Rome, Italy
- 2Unit of Cognitive Primatology, Institute of Cognitive Sciences and Technologies, National Research Council of Italy, Rome, Italy
- 3Department of Biology, University of Tor Vergata, Rome, Italy
- 4Department of Life Sciences and Systems Biology, University of Turin, Turin, Italy
- 5Department of Ancient World Studies, Sapienza University of Rome, Rome, Italy
How animals respond to novelty may have important outcomes in terms of fitness. On the one hand, aversion to novel stimuli may reduce the risks of consuming potentially toxic food or encountering predators. On the other hand, the propensity to approach novel stimuli may allow individuals to explore novel food sources and more flexibly adapt to novel challenges. Different species and individuals may find different ways to balance the costs and benefits that novelty posits. To date, however, little is known on how response to novel food varies across individuals of the same species depending on their previous experience with novelty, risk attitude and presence of higher-ranking conspecifics. In this study, we assessed individual variation in response to novel food by testing captive capuchin monkeys (Sapajus spp.) in an unconstrained social context, where all individuals in a group were able to access the testing area on a voluntary basis. We provided familiar and novel food to 23 study subjects belonging to four social groups differing in (i) previous experience with novel food, (ii) risk attitude (as assessed by a previous risky decision-making task), and (iii) dominance rank. We predicted that, as individuals may generalize their previous experience to novel contexts, those with more previous experience with novel food would be less neophobic than those with less experience. Moreover, if neophobia is a facet of the individual’s risk attitude, we predicted that more risk-prone individuals would be less neophobic than less risk-prone ones. Finally, individuals might flexibly modify their food choices according to the presence of conspecifics; in this respect, we predicted that, in response to monopolization of preferred resources by higher-ranking individuals, lower-ranking individuals would prefer familiar over novel food in the absence of higher-ranking individuals, but would modify their preference in favor of novel food in the presence of higher-ranking individuals. None of these predictions were supported by our results. We observed, however, that neophobia, measured as the latency to retrieve a food item, was more pronounced in lower-ranking than higher-ranking individuals, and that males showed a generally stronger bias than females toward a quicker retrieval of familiar food.
Introduction
Animals face a variety of stimuli during their everyday life, some of which entail a degree of novelty because they have never been previously experienced. On the one hand, aversion to novel stimuli may be adaptive to, for instance, reduce the risks of consuming novel and potentially toxic food (Greenberg and Mettke-Hofmann, 2001; Ferrari et al., 2015). On the other hand, individuals attracted by novel stimuli may be more explorative and innovative, and thus more likely to encounter novel resources, solve novel problems and flexibly adapt to novel challenges (birds: Greenberg, 2003; Overington et al., 2011; Sol et al., 2011; Griffin and Diquelou, 2015; primates: Webster and Lefebvre, 2001; Day et al., 2003; Amici et al., 2020a). Therefore, how animals react to novelty may have important outcomes in terms of fitness (Crane et al., 2020), and different species and individuals may find different ways to balance the costs and benefits of novelty, depending on the socio-ecological challenges they face (Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann et al., 2002; Forss et al., 2017; for a review see Mettke-Hofmann, 2014; Miller et al., 2022).
Animals may avoid novel stimuli (i.e., showing neophobia), they may be attracted by novelty (i.e., showing neophilia) or they may neutrally respond to novel stimuli. Although neophobia and neophilia may be considered two extremes of the same continuum, they are best conceptualized as independent responses (Russell, 1973), resulting from the dynamic balance of fear and curiosity (Hughes, 2007). Here, we will define neophobia as the avoidance behavior that arises when the novel stimulus generates a fear response (Greggor et al., 2015). Neophobia can be context-specific (e.g., gustatory, predator, social, object or spatial neophobia; Crane et al., 2020), and can strongly vary depending on the specific stimuli experienced (Greggor et al., 2015). Different methods can be used to assess neophobia, including the time individuals spend in proximity to the novel stimulus, the latency to approach or retrieve the novel stimulus or the number of novel food items consumed. Therefore, response to novelty may strongly vary within individuals, depending on the context, the stimuli and methods employed to assess this behavior.
To date, the vast majority of studies have compared neophobia across different species, to investigate the socio-ecological conditions that may be linked to the emergence of higher neophobia in certain species (e.g., birds: Mettke-Hofmann et al., 2002; ungulates: Schaffer et al., 2021; primates: Addessi et al., 2004; Addessi and Visalberghi, 2006; Bergman and Kitchen, 2009; Forss et al., 2019; Amici et al., 2020b). Other studies, however, have focused on individual variation in neophobia (e.g., birds: Mettke-Hofmann et al., 2005; Fox and Millam, 2007; Ensminger and Westneat, 2012; ungulates: Schaffer et al., 2021; primates: Amici et al., 2020b). Neophobia, for instance, may vary across conspecifics depending on their age, sex or rank. In birds, Biondi et al. (2010) have shown that adults are more neophobic than juveniles toward novel objects. A similar effect has been found in primates tested with novel food (wild capuchin monkeys (Cebus apella): Visalberghi et al., 2003a; captive chimpanzees (Pan troglodytes): Addessi and Visalberghi, 2006), although other studies have found no significant relation between age and neophobia (wild capuchin monkeys (Cebus libidinosus): Sabbatini et al., 2007; captive great apes (Pan troglodytes, Gorilla gorilla, Pongo pygmaeus, Pongo abelii): Gustafsson et al., 2014) or, in contrast, the opposite pattern was observed (captive marmosets (Callithrix jacchus): Yamamoto and de Araújo Lopes, 2004; Voelkl et al., 2006). Moreover, sex may predict differences in neophobia: some authors argued that males should be less neophobic than females, because competition through sexual selection is more intense for males, who would thus need higher behavioral flexibility (Schuett et al., 2010; Crane et al., 2020). However, since in mammals males are usually higher-ranking than females, sex-rank effects might be confounded (Crane et al., 2020). Indeed, studies on primates have found contrasting results: in captive capuchin monkeys, for instance, sex and rank were not found to affect preference toward novel food (Visalberghi et al., 2003b) but, in a study on wild capuchin monkeys, females showed a less neophobic response toward novel objects than males, while rank had no significant effect (Visalberghi et al., 2003a).
In terms of sociality, different factors may explain individual variation in response to novelty. In social species, for example, higher-ranking individuals usually have priority of access to resources (Ellis, 1995; Altmann, 1998; Hohmann et al., 2006). Moreover, primates who are more integrated in their social group may also have better access to resources (Amici et al., 2020b; Dell’Anna et al., 2020). Therefore, higher-ranking or more socially-integrated individuals may receive a lower potential payoff from novel stimuli, and may be more neophobic than lower-ranking or less socially-integrated individuals (Hegner, 1985; Greenberg-Cohen et al., 1994; Lahti, 1998; Wolf et al., 2007; An et al., 2011). Furthermore, in some species the presence of a conspecific may facilitate the acceptance of novel food or objects (birds: Coleman and Mellgren, 1994; Huber et al., 2001; rodents: Forkman, 1991; canids: Moretti et al., 2015; primates: Visalberghi and Addessi, 2000; Voelkl et al., 2006; Hardus et al., 2015). The latter findings may result from very different processes. On the one hand, social facilitation may reduce neophobia (Addessi and Visalberghi, 2001). On the other hand, especially in a foraging context, novel resources may be accepted as a means to reduce competition (Amici et al., 2020b).
Beyond age, sex and sociality, other factors like previous experience and risk attitude might also explain individual variation in response to novelty, although these factors have been usually overlooked in previous studies. Differences in neophobia may partly result from the complex interactions that individuals have with the environment during their life (Greenberg, 2003; Biondi et al., 2010). For instance, response to novelty may depend on how much the novel stimulus differs from other familiar stimuli, and thus on how easily familiar features can be generalized to novel stimuli. Generalizations to novel stimuli have been well documented both in the context of foraging (e.g., Greenberg, 1990; Greenberg and Mettke-Hofmann, 2001) and in the context of predation (e.g., Brown et al., 2013). In lambs, for example, early life experiences with novel food reduces neophobia when individuals are exposed to other kinds of novel food (Catanese et al., 2012). In whitetail damselfish (Pomacentrus chrysurus), previous experiences with predators predict a decrease in neophobia toward predators (Crane and Ferrari, 2017), with higher neophobia (and higher survival rate) in individuals with no previous experiences of escaping from predators (Ferrari et al., 2015). However, the link between previous experiences and neophobia may be context-specific, as shown by a recent study finding that early predator experience did not affect the latency of the daffodil cichlid (Neolamprologus pulcher) to feed near a novel object (Bannier et al., 2017).
Neophobia may also reflect how animals generally assess uncertainty. Animals face uncertainty because they do not always know the outcome of their decisions. Studies with non-human primates have assessed how animals react to uncertainty by testing their risk attitude (Heilbronner et al., 2008; MacLean et al., 2012; Rosati and Hare, 2012; De Petrillo et al., 2015). In these tasks, individuals are usually presented with a series of choices between two options, one yielding a reward that is constant in amount (safe option) and one yielding a reward that varies probabilistically around the mean (risky option), with the two options leading on average to the same payoff. Individuals with a preference for the risky option are considered risk-prone, whereas those with a preference for the safe option are considered risk-averse (Kacelnik and Bateson, 1996). While these studies consider risk as the failure to receive a reward, animals in their own environment also face the risk of losing valuable resources or being physically injured (Paglieri et al., 2014). Neophobic behaviors may represent an individual’s reaction to uncertainty due to a lack of information associated with a novel stimulus (Crane et al., 2020). Risk-prone individuals may focus on the potentially valuable payoff that novelty offers rather than on its risky outcome. Thus, it should be possible that risk proneness and neophobia are two related traits and that the individual attitude to face risk shapes the response to novelty (Greenberg, 2003).
In the present study, we investigated inter-individual variation in neophobia toward novel food and, in particular, the role of previous experience and risk attitude on how individuals respond to novelty in a social setting. We did so in an unconstrained social setting, where all individuals in a group could freely participate in the experiment on a voluntary basis, thus mimicking the social context in which individuals usually interact with novel stimuli in their daily life, and allowing more meaningful comparisons with data collected in the wild. We used capuchin monkeys as a model species, assessing their relative preference for novel and familiar food and their latency to retrieve it under two different conditions. Capuchin monkeys have been largely studied both in the wild and in captivity for their complex foraging strategies (e.g., tool use, Visalberghi and Limongelli, 1994; Fujita et al., 2011; Falótico and Ottoni, 2016; processing of toxic foods, Sirianni and Visalberghi, 2013) and their neophobic response to novel food and objects (e.g., Addessi et al., 2004; Addessi and Visalberghi, 2006). We expected inter-individual differences in how capuchin monkeys react to novel food. In particular, we predicted that more experienced individuals (i.e., having been more frequently exposed to novel food in previous studies) would be less neophobic than less experienced ones. Moreover, we predicted that more risk-prone individuals (i.e., those that preferred a risky option over a safe one when confronted with two options yielding on average the same payoff; De Petrillo et al., 2015) would be less neophobic than less risk-prone ones. Finally, we predicted that the presence of higher-ranking individuals would affect the choice of lower-ranking ones, so that the latter would prefer familiar food to novel food in the absence of higher-ranking individuals, but would reverse their preference in the presence of higher-ranking individuals. We tested for our predictions while controlling for age, sex and rank, which were found to be significant predictors of neophobia in previous studies. We did not explicitly control for reproductive status and past reproductive experience because the last pregnancies in the colony occurred 10 years ago and because reproductive experience is correlated to sex and age, already included in the analysis.
Materials and Methods
Ethics
This study complied with protocols approved by the Italian Health Ministry (DM 633/2020-PR to the last author). All procedures were performed in full accordance with the ethics requirements of the Directive 2010/63/EU on the protection of animals used for scientific purposes and conformed to the “Guidelines for the treatment of animals in behavioral research and teaching” (Bee et al., 2020).
Subjects
The study involved 23 subjects belonging to four social groups (Group 1 = 5 subjects; Group 2 = 5 subjects; Group 3 = 4 subjects; Group 4 = 9 subjects; Table 1), hosted at the Primate Center of the Unit of Cognitive Primatology, Institute of Cognitive Science and Technologies, National Research Council of Italy, Rome, Italy. In the colony we tested some changes in group composition occurred in the past years. However, from 2004 the composition of our groups was rather stable. Seventeen out of the 23 subjects had previously participated in studies in which they were presented with novel food (Table 1). Eight out of the 23 subjects had previously participated in a risky decision-making task (Table 1). All groups were housed in enclosures with indoor and outdoor areas. The indoor area measured 25.4 m3 for all groups, and was divided in two sections; the outdoor area measured 53.2–374.0 m3 depending on the study group. All enclosures were furnished with wooden perches, tree trunks and branches. Testing occurred in the morning, between 09:30 and 13:30 h, before the main meal, and water was available ad libitum. Individuals participated on a voluntary basis. Data were collected in November and December 2020.
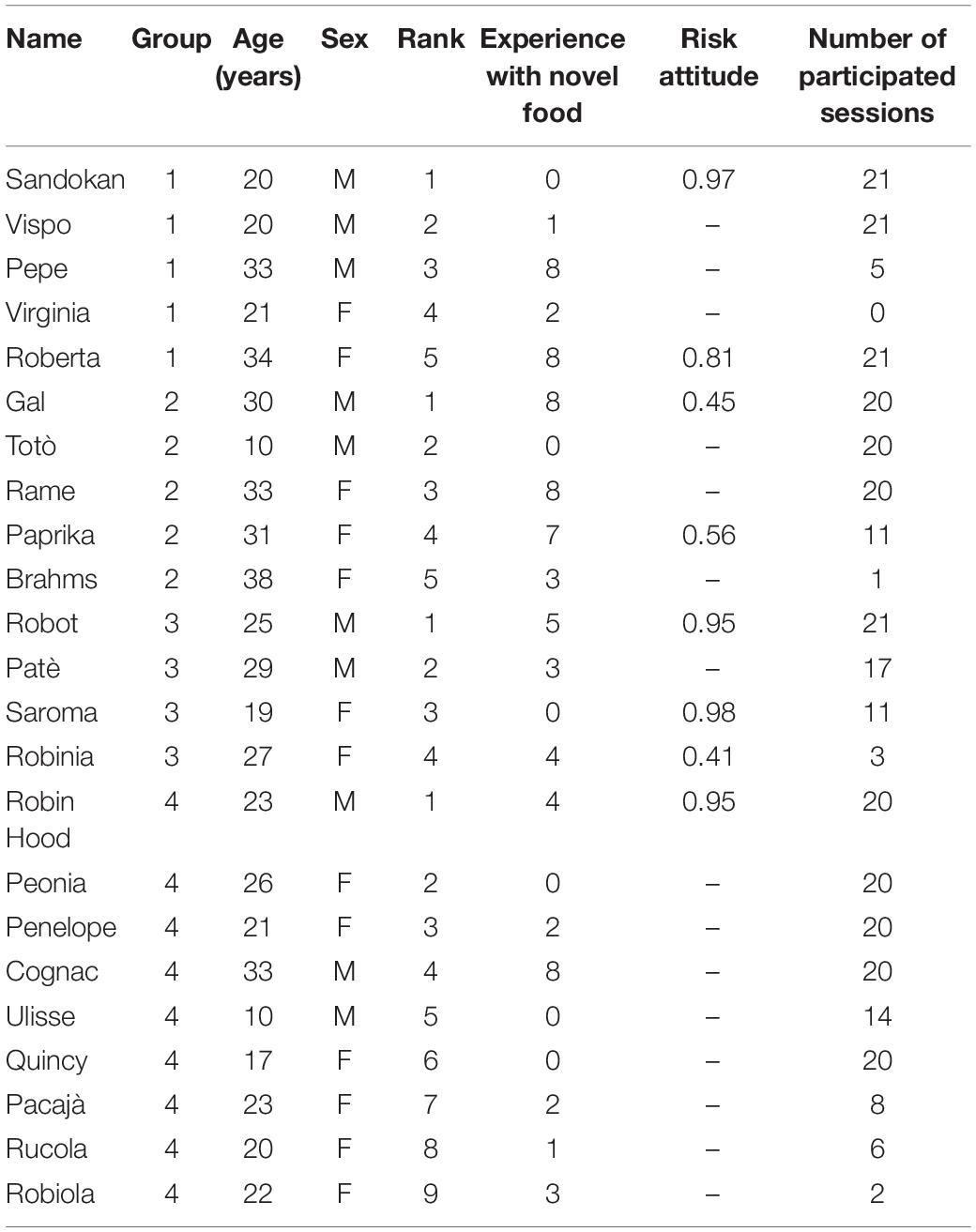
Table 1. Individual characteristics of the study subjects, including their name, group, age (in years), sex, rank (with 1 referring to the highest-ranking individual in each group), experience with novel food (as the number of experiments on neophobia in which each subject had participated before being tested), risk attitude (as the mean proportion of trials in which the subject had chosen the risky over the safe option in a decision-making task; De Petrillo et al., 2015) and number of total sessions of conditions A and B in which each subject participated.
Experience, Risk Attitude and Social Context
Subjects participated to a variable extent in previous experiments testing their reaction to novel food (Supplementary Table 1). Previous experiments using novel food were carried out for about 10 years since 1992 and used both fresh fruits and vegetables (e.g., pineapple, kiwi, tomato, celery, broccoli) and processed food (e.g., mashed canned “Spagna” beans, colored blue; mashed boiled skinned lentils, colored red; mashed boiled aubergine colored green; mashed canned “borlotti” beans, colored violet; semolina cooked with water and sugar). As a proxy of experience with novel or unfamiliar food, we used the number of such experiments in which each subject had participated before being tested in the present study. Some subjects (N = 8; Table 1) had also participated in a decision-making task requiring them to choose between a safe option (i.e., yielding a constant reward) and a risky option (i.e., yielding a reward that probabilistically varied around the mean; De Petrillo et al., 2015). We used the mean proportion of trials in which each subject had chosen the risky option as a proxy of his/her risk attitude. Although this experiment was performed a few years before the current study, relative risk attitude among subjects seems to be a stable trait in humans (Straznicka, 2012; Josef et al., 2016) and, while detailed information for capuchin monkeys is still lacking, new data collected in 2019 on our study subjects (which we did not employ in the present study as they have not been published yet) showed no significant difference from those collected in 2014 by De Petrillo et al. (2015). Since our study subjects were tested in a social context, we also tested whether reaction to novelty by lower-ranking subjects was affected by the presence of higher-ranking individuals. To evaluate this effect, every time we observed a monkey retrieving a food item, we measured the difference between the rank of the subject retrieving the food item and the rank of the highest-ranking individual present in the testing area at the time of retrieval. The dominance hierarchy was obtained from the observation of the direction of dyadic unidirectional agonistic interactions among group members. Since capuchins’ societies are characterized by a high degree of tolerance, aggressions are very rare and rank determination within capuchin groups held at ISTC-CNR Primate Center is updated continuously based on opportunistic observations. Moreover, before the start of the experiments, we used the “peanut test” to resolve ambiguous cases (i.e., where the hierarchical position of two individuals was not well defined). The test consisted in placing a peanut in the middle of a cage, equidistant from the two individuals and observing which subject retrieved it, and it was administered four times for each of the three “ambiguous” dyads. In all cases, the same subject retrieved all of the peanuts.
Procedure
We exposed subjects to novel and familiar food in a social setting, which better mirrors the natural context in which captive and wild individuals usually interact with novel stimuli. During the sessions, subjects could freely move between the indoor and outdoor areas of their enclosure. Food items (banana slices) were distributed on the floor of two adjacent sections of the indoor area (hereafter, “testing areas”), connected through sliding doors to the outdoor area of the enclosure (Supplementary Figure 1). We distributed food items on the testing area floor so that they were as evenly distributed as possible while ensuring visibility from the point of view of recording cameras (section “Coding”). In each testing area, we distributed a number of food items which was proportional to the number of individuals in the group (i.e., four food items per individual, with the only exception of two groups who received a lower number of food items in the first session, i.e., two food items per individual). Half of the food items were familiar banana slices, and the other half were “novel” banana slices, which had been dyed with odorless and tasteless food coloring (Lo Conte, decorì). Since novelty has been shown to increase with stimulus complexity (Greggor et al., 2015), we also added a novel texture to the “novel” banana slices, covering those with seeds that subjects had never eaten before. We administered two conditions, which were identical except for the kind of novel stimuli used: blue banana slices with sesame seeds (condition A), and red banana slices with poppy seeds (condition B). The order of the two conditions was counterbalanced across the study groups to control for order effects (i.e., Groups 1 and 4 received condition A before condition B, whereas the order was reversed for Groups 2 and 3). We administered 10 sessions (with the exception of two groups who received a lower number of food items in the first session and to which we administered 11 sessions) for each study group and condition (total number of sessions: 82). To maintain motivation high, we only administered one session per day.
Coding
We videotaped each session, and also verbally announced on video the identity of every individual entering the testing areas. Each session started when the sliding doors connecting the indoor and outdoor areas opened so that individuals were able to enter into the testing areas, and it ended when all the banana slices had been eaten (mean session duration: 216 s; min-max 81–511 s). By using the software BORIS (Friard and Gamba, 2016), we later coded from the videoclips, for each episode of food retrieval (i.e., an observed event of one monkey retrieving one banana slice, hereafter, “retrieval episode”), the identity of the individual retrieving the food, and the type of food retrieved (i.e., familiar or novel). Subjects ate almost all of the food items they retrieved. In only 39 instances out of a total of 1,936 observed episodes of food retrieval, the food item was dropped. Moreover, for each session and individual, we coded the latency to retrieve familiar food and the latency to retrieve novel food. Latency to retrieve familiar food was defined as the time elapsed from the first entrance of a subject in one of the two testing areas and the first retrieval of a familiar food item in the session. Similarly, latency to retrieve novel food was the length of the time interval between the first entrance of the subject and the first retrieval of a novel food item. Videos were independently coded by two observers (MV and GSg). Inter-observer reliability was assessed on 20% of recordings for familiar food retrieval (ICC = 0.996), novel food retrieval (ICC = 0.998), latency to retrieve familiar food (ICC = 0.997), and latency to retrieve novel food (ICC = 0.997).
Statistical Analyses
We performed all statistical analyses and plotting using R v. 4.1.0 (R Core Team, 2021). We used the glmmTMB package (version 1.1.2.3; Brooks et al., 2017) to fit (Generalized) Linear Mixed Models (GLMM, Baayen et al., 2008).
In our experimental design, an equal number of familiar and novel food items was presented to the monkeys at the beginning of each session (section “Procedure”). However, as the session proceeded and food items were consumed by the monkeys, the absolute number of available food items decreased, and the relative number of familiar and novel items changed erratically. We operationally defined preference for retrieving familiar over novel food as the marginal probability to retrieve a familiar food item estimated by a binomial model, where the response was whether each retrieved food item was familiar (1) or novel (0) and which included a term to account for the relative availability of familiar over novel food items (i.e., the proportion of familiar to novel food available when each food item was retrieved).
To test if each individual’s preference for retrieving familiar or novel food (i.e., the probability to retrieve one type of food or the other, accounting for their availability) depended on his/her experience and the presence of higher-ranking individuals, we fitted a GLMM (M1) with binomial error structure and logit link function, where the binary dependent variable was whether, for each retrieval episode, the food retrieved was familiar or novel (food type). As test predictors, we entered the subject’s experience (experience, i.e., number of previous experiments involving the presentation of novel foods in which she/he had participated), the difference between the subject’s rank and the rank of the highest-ranking individual in the testing area at the moment of food retrieval (rank difference), the cumulative number of food items retrieved by the subject in the course of the experiment for each condition before the current retrieval episode (n. retrieved items, which allowed to control for the decreasing novelty of the “novel” food in the course of the experiment), the subject’s rank, and the 2-way interactions of experience, rank difference and rank with n. retrieved items. The three interactions model the predictions that the effect of experience, rank difference and rank may change as the novel items become increasingly familiar (in particular, the effect of rank difference on food preference may change as the higher-ranking individuals change their preference across sessions, which affects their potential monopolization of preferred food). We note that our definition of the term controlling for decreasing novelty of the “novel” food as the number of retrieved food items formally implies the assumption that monkeys only acquire experience when they do retrieve food items, rather than by just standing by while the experimental sessions unfold. While this assumption appears reasonable to us, we appreciate that, at least when they enter the cages, monkeys may acquire some familiarity with the novel food by just standing by and looking. However, we remark that, by including a term for the interaction of rank and n. retrieved items our analysis is in fact flexible enough to adjust to either of these assumptions or to a combination of them (of course, with potential changes in the interpretation given to significant terms for rank, n. retrieved items and their interaction). As controls, we included the individuals’ age, sex and group, the condition (A or B), the number of food items which was proportional to the number of individuals in the group (food per subject) and, importantly, the proportion of familiar vs. novel food available when food was retrieved (proportion familiar, which ensured that our analysis actually modeled retrieval preference, rather than choices dictated by availability). Individual identity (subject) was included as a random intercept. As a binomial model only allows for probabilities (in this case representing retrieval preferences) to “plateau” at 0 or 1, we aimed at avoiding episodes of food retrieval happening after complete disappearance of “novelty” effect (i.e., when the plateau had been reached) by only including the first 10 retrieval episodes for each individual, condition and food type. We fitted model M1 on 383 observations from 22 individuals.
In a second model (M2), we further tested whether individuals preferentially retrieved familiar or novel food depending on their risk attitude, as measured in De Petrillo et al. (2015). As we did not have risk-attitude data for all individuals, we only used a subset of 8 individuals. Model M2 was similar to M1 and considered the same test predictors as M1, plus the individual risk-attitude score (risk attitude, see section “Materials and Methods”) and the 2-way interaction between risk attitude and n. retrieved items. In order to overcome collinearity, we had to remove group (only one subject in group 4), age (collinear with experience) and sex (collinear with rank) from the controls. In M2, therefore, we included, as controls, proportion familiar, food per subject and condition, as well as subject, modeled as a random intercept. Again, only the first 10 retrieval episodes for each individual, condition and food type were considered (total number of observations = 148).
In a third model, we assessed which factors predicted participation in the experiment by fitting a GLMM (M3) with binomial error structure and logit link, where the binary response variable was whether the subject entered the testing area at least once during each session (participation). As test predictors, we included the 2-way interaction of subject’s experience with session number (the cumulative number of sessions for each group and condition), and the 2-way interaction of subject’s rank with session number (for a rationale, see M1). As, in this model, each observation represented a combination of one individual and one whole session, in which all members of its group could, potentially, participate, it would not make sense to consider rank difference as a predictor. As controls, we included individuals’ age and sex, and condition. Group was not included as a control factor in this model because we detected high collinearity of group and rank (VIF = 12.6 and 11.5, respectively). Individual identity (subject) was included as a random intercept. We fitted model M3 on 469 observations of 23 individuals.
To test whether experience and rank of individuals affected their latency to retrieve familiar or novel food, we fitted a LMM (M4) where the (log-transformed) latency time was the response variable (see section “Materials and Methods” for latency definition). As test predictors, we included the 3-way interaction of subject’s experience with session number and food type (familiar or novel), and the 3-way interaction of subject’s rank with session number and food type, plus all the 2-way interactions and main effects included in them. We did not include rank difference in this model because observations referred to time intervals that could extend up to more than 2 min (max latency time was 139 s), so that it was not possible to determine a single value for the rank difference which would be valid for the whole observation. The rationale for this model structure was that the difference in latency times between novel and familiar food is expected to decrease according to the subject’s experience and rank, but this effect may be weaker in higher-ranking individuals, and might decrease through sessions. As controls, we included individuals’ age, sex, and group, the condition, plus the interaction of sex and food type (modeling sex differences in neophobia). We included random intercepts for subject and session identity, as more than one data point was entered for each individual in each session. As not all individuals participated in all sessions, latency times were not available for all individuals in all sessions (number of observations = 549, number of subjects = 22).
Last, we fitted a model similar to M4 which also included risk attitude among the predictors and a term for the 3-way interaction of risk attitude with session number and food type (M5). We fitted M5 on a subset of 219 observations from the 8 individuals for which risk-attitude scores were available. As for M2, we had to remove group, age and sex from the controls so to avoid collinearity issues. Moreover, we could not include a random intercept for session identity as this would have resulted in over parameterization and lack of convergence.
In all models, we z-transformed all quantitative predictors. We compared full models (containing test predictors, controls, and random factors) to null models (only containing controls and random factors) by Likelihood Ratio Test, using the R function “ANOVA.” We obtained the p-values for each term using the R function “drop1” (Dobson and Barnett, 2018). If full-null model comparison was not significant (α = 0.05), we only computed p-values for control terms. If an interaction was not significant, we re-run the model after downgrading it. We used the DHARMa R package v. 0.4.4 (Hartig, 2021) to assess the distribution of residuals and check for violation assumptions. Collinearity among predictors was measured by Variance Inflation Factors (VIF) estimated using the function “check_collinearity” (R package performance, Lüdecke et al., 2021). We considered the thresholds suggested by the performance package. VIF < 5: low collinearity; 5–10: moderate collinearity; > 10: high collinearity. We found no convergence or over/under-dispersion issues in the models presented. We checked for the robustness of model results to influential cases by re-fitting each of the final models after removing observations from one subject at a time. Except where noted, we did not detect any important effect of influential cases.
As the four social groups involved in our study comprise different numbers of subjects (section “Experience, Risk Attitude and Social Context,” Table 1), we also considered employing a standardized measure of rank (and rank differences), so as to eliminate correlation between absolute rank and belonging to a particular group. We refitted all of our models after standardizing rank and rank difference within each group to a [0,1] interval. This procedure allowed to eliminate the collinearity between group and rank in model M3 allowing to keep group among the control terms in M3. However, the results of none of the models changed appreciably.
Data tables and the complete R script including all steps of data analysis are available as Supplementary Data Sheet 1.
Results
In M1 and M2, we assessed which factors predicted individual preferences to retrieve familiar over novel food. For M1, the full-null comparison was not significant (χ2 = 8.85 df = 7, p = 0.264). Among the controls, besides the trivial effect of the proportion of familiar food items available (proportion familiar), only condition was significant (p < 0.001; Table 2), with monkeys retrieving novel items more likely in condition A (i.e., blue banana slices with sesame poppy seeds) than in condition B (i.e., red banana slices with poppy seeds), indicating that the different treatment of “novel” food items significantly affected the reaction of monkeys. In particular, by taking the estimated values from model M1 for the intercept, proportion of familiar items available and condition (and keeping all other, non-significant, coefficients at their sample mean), the overall probability for a monkey to retrieve the novel item when both familiar and novel items were equally available, throughout the first 10 retrieval episodes for each subject and condition, could be calculated at 0.63 and 0.37, respectively, for condition A and B (Figure 1), indicating that, as far as the preference for novel over familiar food is concerned, the neophobic effect—if there was any—was generally low in our experiment, and definitely weak in condition A. In model M1, we detected moderate collinearity of experience and age (VIF = 6.7 and 6.5, respectively). We, thus, re-fitted the model after excluding age from the predictors, but the results did not change appreciably.
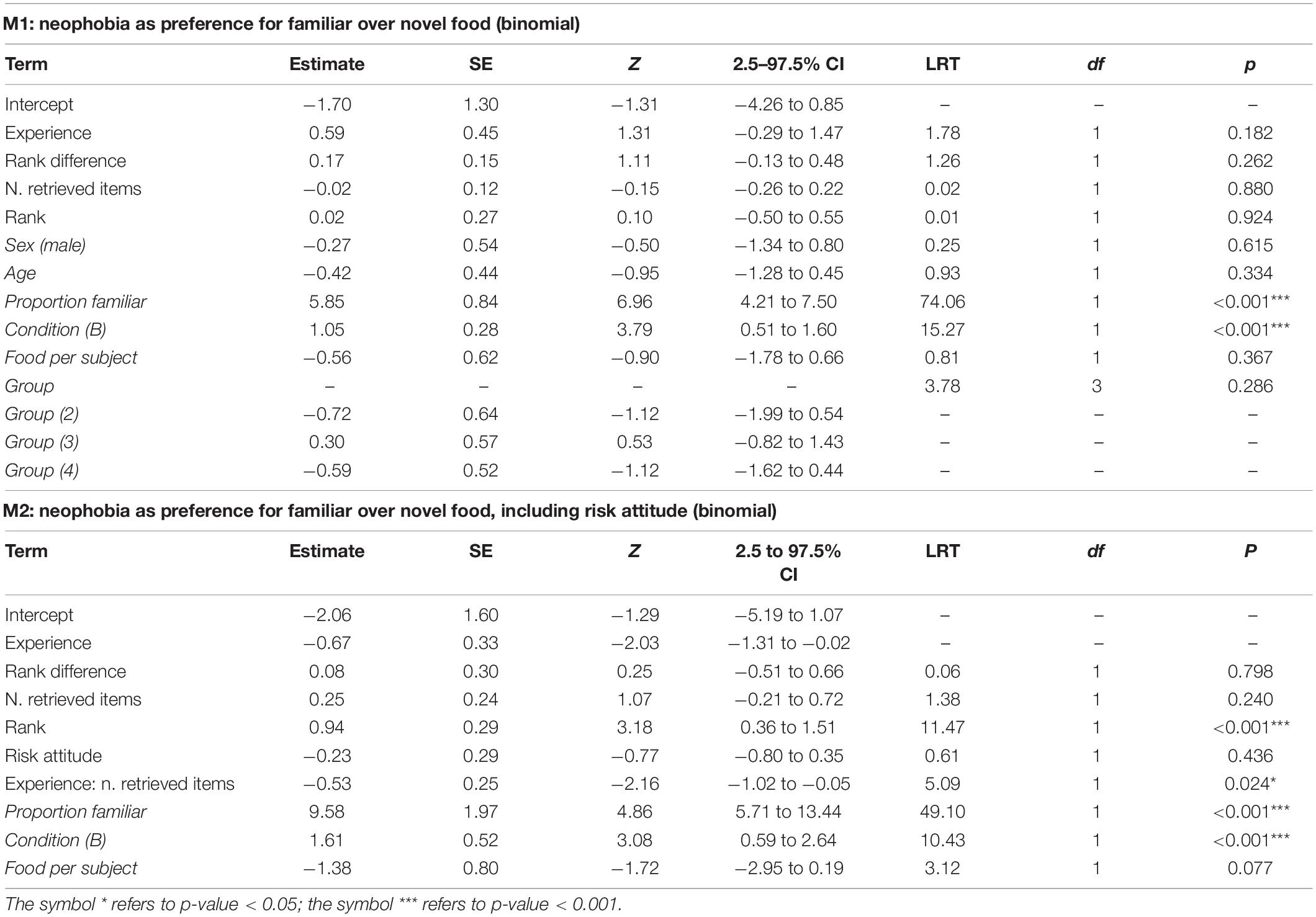
Table 2. For models M1 and M2, test predictors and controls (in italics) included in the final model, estimates, standard errors (SE), z-values (z), confidence intervals (CIs), likelihood ratio test’s χ2 (LRT), degrees of freedom (df) and p-values.
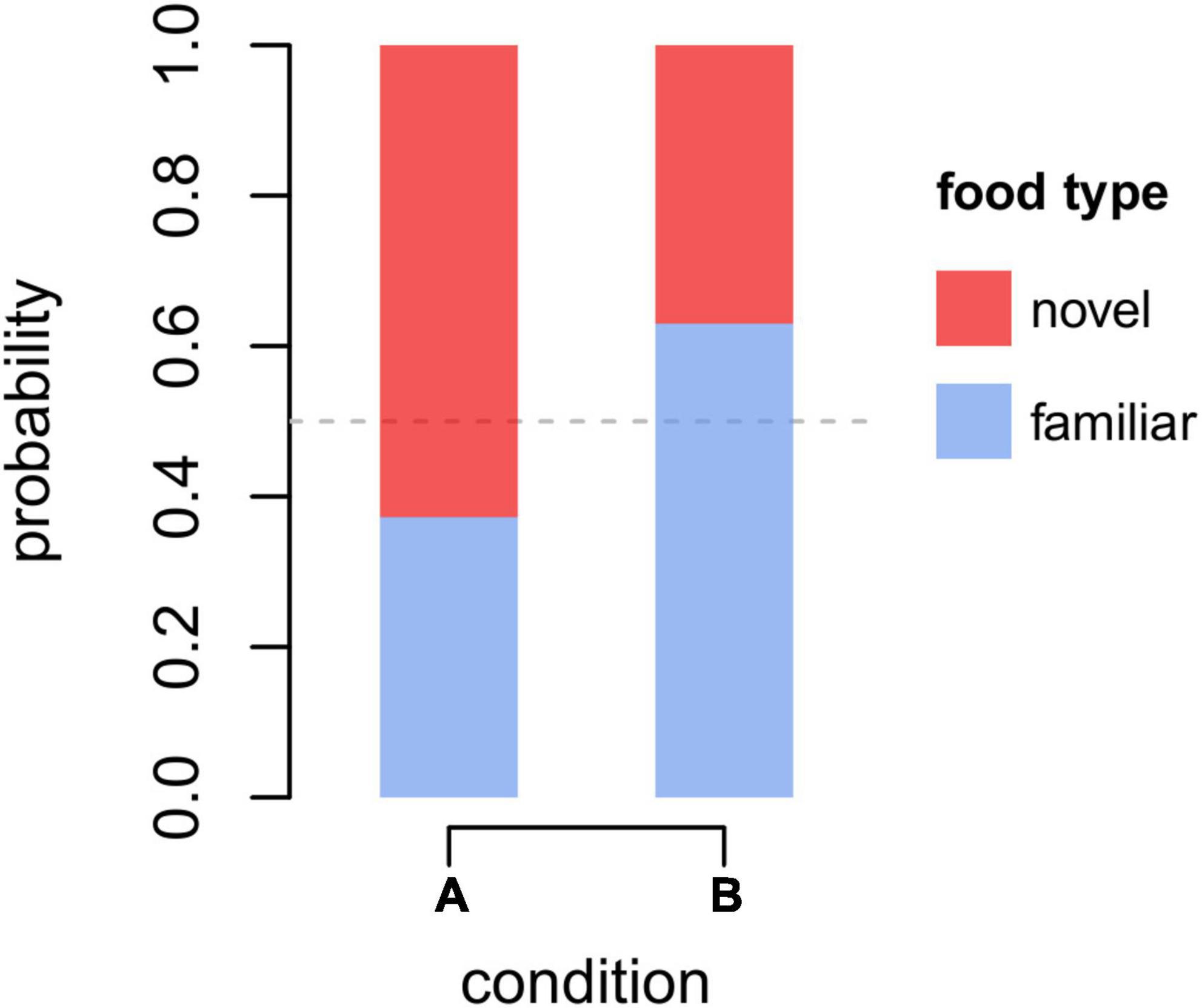
Figure 1. Overall probability to retrieve the familiar (blue) or novel (red) food when both types of food were equally available in conditions A and B in the first (up to) 10 retrieval episodes for each subject and condition. Probabilities were estimated according to coefficients of model M1 (see Table 2), keeping all non-significant predictors at their sample mean.
The full model M2 was a significantly better fit than the corresponding null model (χ2 = 22.18, df = 9, p = 0.008) to the reduced data set (including data from 8 individuals with risk-attitude score). The final M2 model revealed a significant effect of rank, with higher rank corresponding to a lower preference for the familiar food (p < 0.001, Table 2), and the interaction of experience and n. retrieved items, whereby more experienced individuals would decrease their preference for the familiar food with more retrieval episodes accumulated, while less experienced individuals would do the opposite (p = 0.024, Table 2). While the effect of rank may be interpreted as a higher neophobic attitude of lower-ranking subjects, the effect of the interaction is less interpretable. Moreover, that the same effect was not found to be significant in the whole dataset and was significant in an analysis of a subset of data was definitely unexpected and required deeper investigation. Model M2 differs from model M1 under three aspects: (i) risk attitude is a predictor in M2 but not in M1; (ii) group, age and sex are controls in M1 but not in M2; (iii) M1 was fitted on 383 observations from 22 subjects while M2 was fitted on a subset of 148 observations from 8 subjects. To check whether the inclusion of risk attitude as a predictor was responsible for the significant interaction of experience and food type in M2, we fitted a reduced version of the (final) M2 model where risk attitude was removed on the subset of 8 subjects (M2a). To check whether the absence of group, age and sex as controls was responsible for the significant interaction of experience and food type in M2, we fitted a model with the same structure as M2a to the complete data set (M2b). Both rank and the interaction of experience and n. retrieved items were still significant in M2a (rank: χ2 = 11.76, df = 1, p < 0.001, experience:n. retrieved items : χ2 = 5.53, df = 1, p = 0.019), but obviously non-significant in M2b (rank: χ2 = 0.007, df = 1, p = 0.93, experience:n. retrieved items : χ2 = 0.203, df = 1, p = 0.65), thus indicating that neither the inclusion of risk attitude nor the removal of group, age and sex were responsible for the differences between results of M1 and M2 and that the statistically significant effects of rank and the interaction of experience and n. retrieved items is an idiosyncratic feature of the smaller dataset used to fit M2.
The full model M3 significantly differed from the corresponding null model (χ2 = 29.83, df = 5, p < 0.001). All subjects, with the exception of one individual, participated at least once in the experimental session (Table 1). The final model revealed a significant effect of the 2-way interaction of rank and session number (p < 0.001; Table 3). In particular, higher-ranking individuals participated more than lower-ranking ones (indeed, rank 1 individuals participated in all sessions) and participation of low-ranking individuals decreased through sessions (Figure 2). We signal that the p-value for the interaction between experience and session number was 0.054 (participation would be higher for more experienced individuals, which would also show more marked change between early and late sessions, not shown). Among the controls, only condition was significant (p = 0.021; Table 3), with monkeys participating more often in condition A.
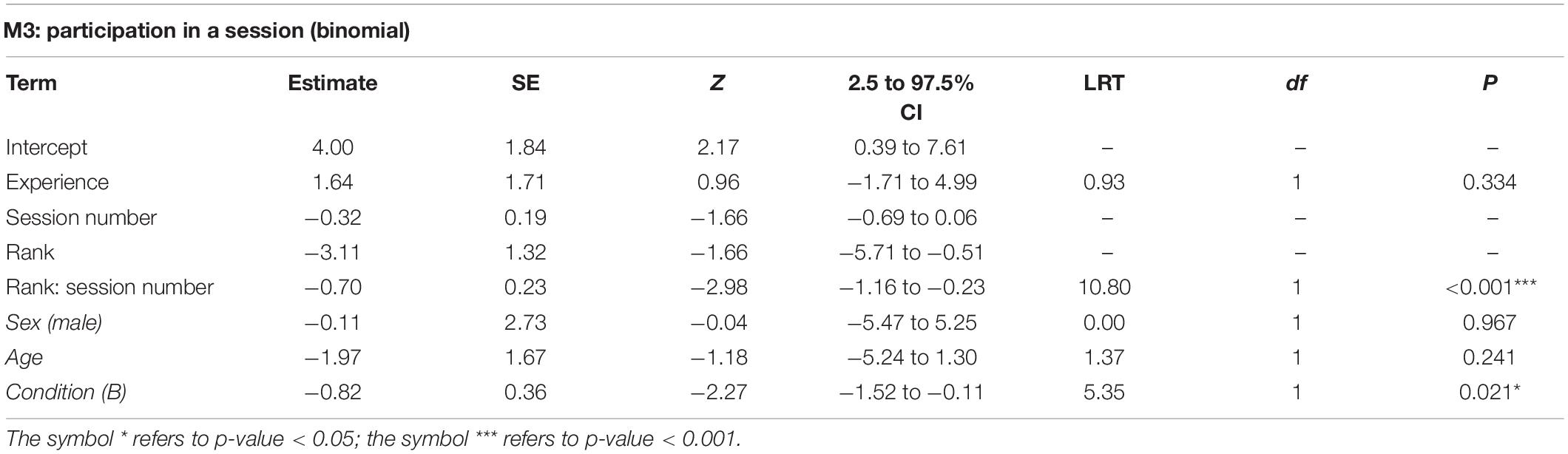
Table 3. For model M3, test predictors and controls (in italics) included in the final model, estimates, standard errors (SE), z-values (z), confidence intervals (CIs), likelihood ratio test’s χ2 (LRT), degrees of freedom (df) and p-values.
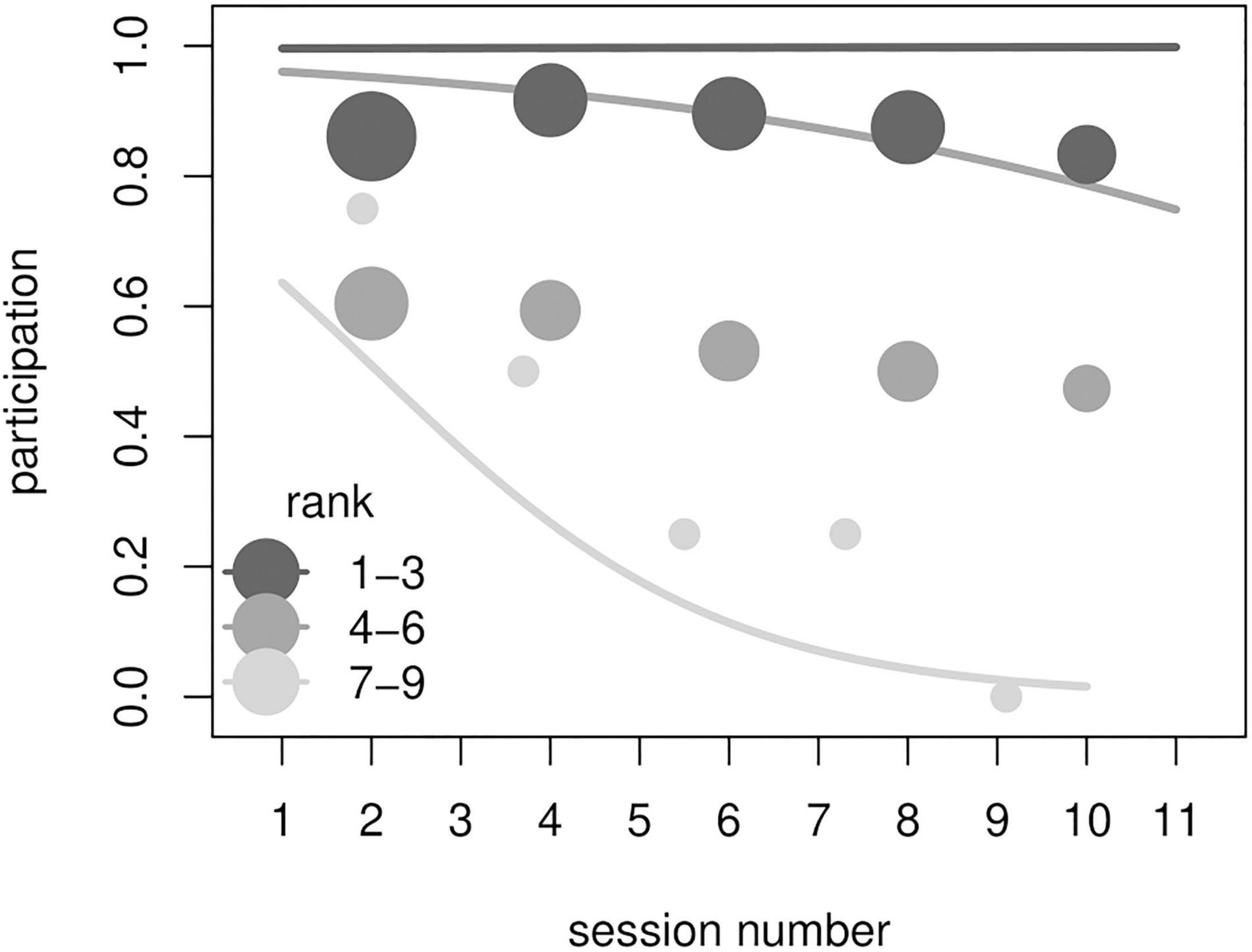
Figure 2. Results from model M3. Participation in an experimental session as a function of the interaction between rank and session number. Lines show probabilities to participate in a session according to the (binned) session number and (binned) ranks as estimated by model M3 (all other predictors were kept at their sample mean). Circles show the observed frequency of participation for each rank and session bin. Shade of lines and circles represents rank (darker = higher ranking, see legend). Circles size is proportional to the number of sessions for each rank and session number bin. High-ranking individuals participated more often overall, and participation of lower-ranking individuals decreased markedly throughout the course of the experiment.
In M4, the full-null model comparison was significant (χ2 = 54.05, df = 10, p < 0.001), with the final model revealing a significant effect of the 2-way interactions of rank and food type (p < 0.001; Table 4) and session number and food type (p = 0.043; Table 4). In particular, Figures 3, 4 show that latency to retrieve food was overall higher for higher-ranking than for lower-ranking individuals and that, while latency times for familiar food did not change much during the course of the experiment, latency times for novel food items decreased markedly throughout the sessions, reflecting the decreasing novelty of modified bananas. Among the controls, the interaction of sex and food type was highly significant (p < 0.001; Table 4), with females showing longer latency times than males, but only markedly so for familiar foods (Figures 3, 4). Interestingly, while low-ranking individuals initially displayed longer latencies for the novel food than for the familiar food, and ended up with more similar latencies for the two types of food, the behavior of higher-ranking subjects differed among sexes, with males starting the experiment with similar latencies for the two types of food and ending up with shorter latencies for the novel food than for the familiar food, and females showing shorter latencies for the novel food throughout the whole experiment (Figure 4). Group was another significant control (p = 0.020; Table 4), with the longest latencies observed in group 4. In model M4, we detected moderate collinearity of experience and age (VIF = 6.6 and 6.4, respectively). We, thus, re-fitted the model after excluding age from the predictors, but the results did not change appreciably.
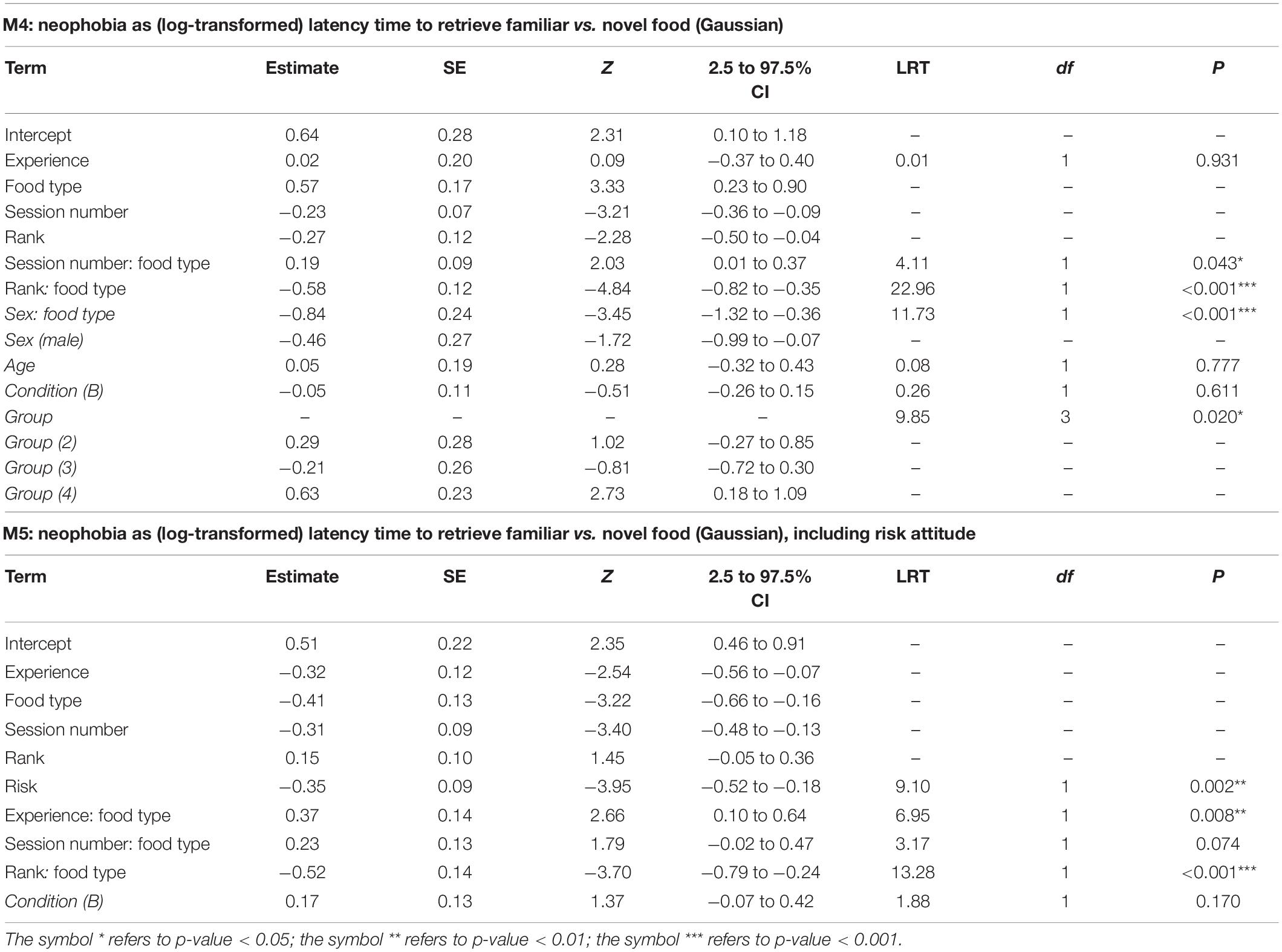
Table 4. For models M4 and M5, test predictors and controls (in italics) included in the final model, estimates, standard errors (SE), z-values (Z), confidence intervals (CIs), likelihood ratio test’s χ2 (LRT), degrees of freedom (df) and p-values.
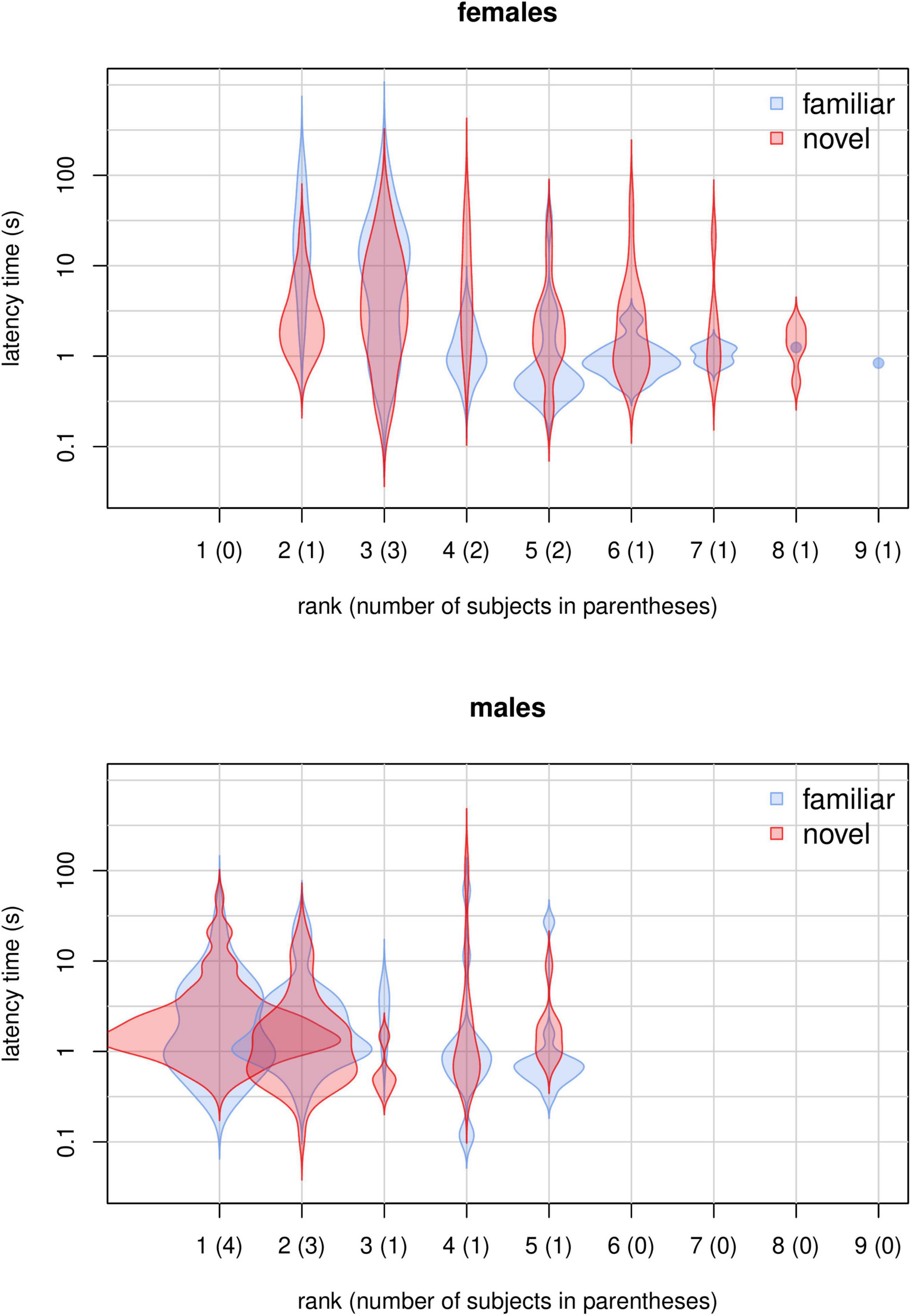
Figure 3. Violin plots showing the distribution of latency times for novel and familiar food items for each rank and sex.
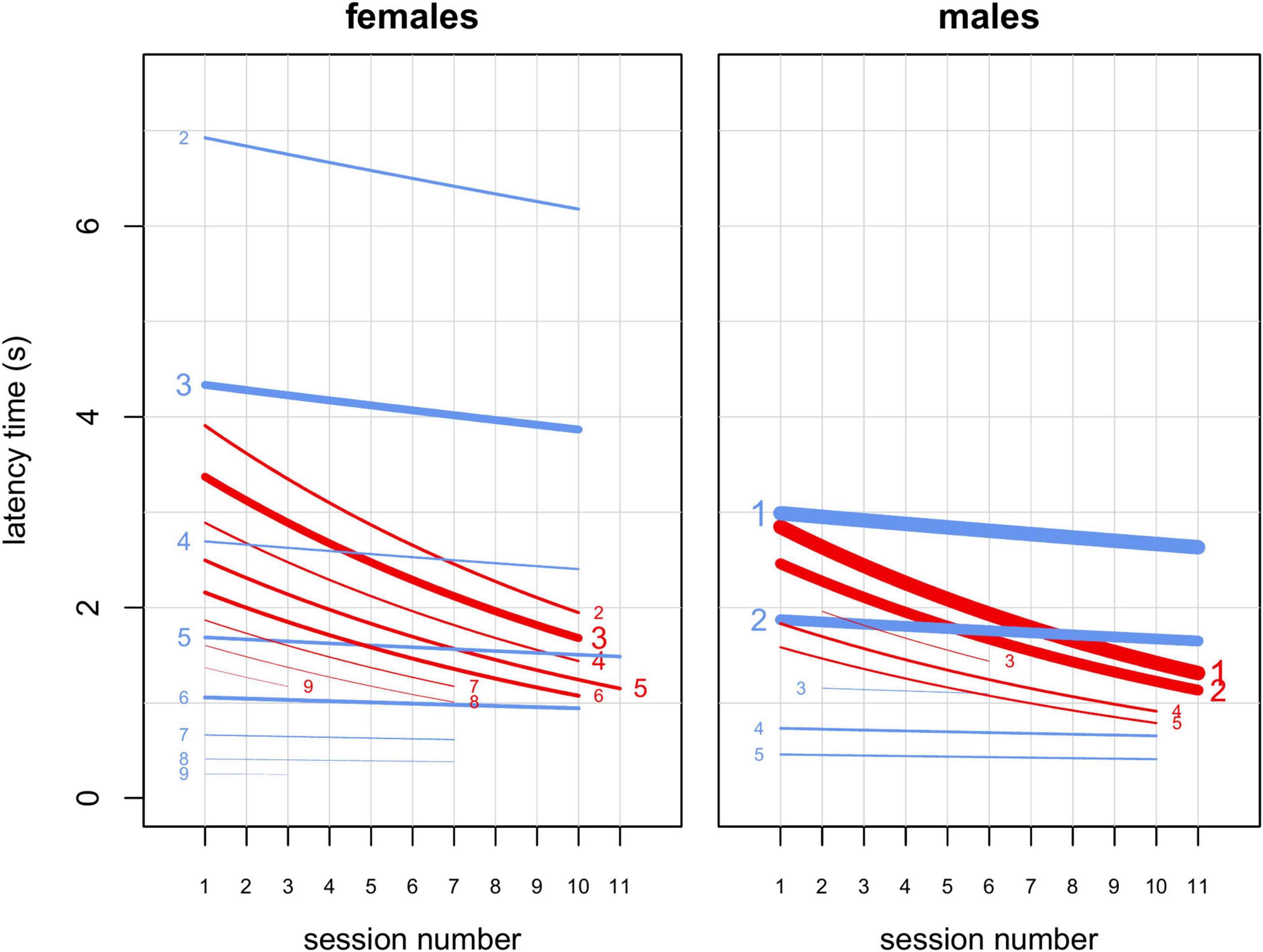
Figure 4. Results from model M4. Interactions of sex, rank and session number with food type (familiar or novel). Lines represent estimated latency times as a function of session number for novel (red) and familiar (blue) foods and different ranks, indicated by numbers on the right (for novel) or left (for familiar) of each line. The plot on the left concerns females, while the plot on the right concerns males. All other predictors were kept at their sample mean. Line width is proportional to the number of observations for each combination of food type, rank and sex. Lines extend from the minimum to the maximum session number for each combination of food type, rank and sex. Font size of numbers indicating rank is proportional to the number of subjects in each rank in the study sample. Latency time is generally shorter for low-ranking individuals and latency time for novel food decreases markedly from early to late sessions.
The full-null model comparison for M5 was highly significant (χ2 = 55.11, df = 15, p < 0.001). None of the 3-way interactions tested was significant. Similar to M4, the final model M5 contained a significant term for the interaction of rank and food type (p < 0.001; Table 4). To enhance comparison with M4, we retained the interaction of session number and food type despite it being non-significant at α = 0.05 (p = 0.074), but all of the following also applied when this interaction was downgraded (not shown). Risk attitude was found to be a significant predictor of latency times (p = 0.002), which was shorter for more risk-prone subjects (Table 4), but this was not different for novel and familiar food (non-significant interaction of risk attitude and food type). However, in contrast to M4, M5 revealed a significant effect of the interaction of experience and food type (p = 0.008; Table 4), corresponding to higher neophobia in less experienced subjects. To check whether the differences between results of M4 and M5 were due to the inclusion of risk attitude as a predictor or the absence of group, age and sex as controls in M5, we proceeded as with M2 and M1. Again, the interaction of experience and food type was still significant in a model equivalent to (final) M5 without risk attitude among predictors (M5a: χ2 = 6.700, df = 1, p = 0.010), but obviously non-significant in a similar model applied to the complete data set (M5b: χ2 = 0.424, df = 1, p = 0.516), thus indicating that neither the inclusion of risk attitude nor the removal of group, age and sex were responsible for the difference between results of M4 and M5 and that the statistically significant interaction of experience and food type is an idiosyncratic feature of the smaller dataset used to fit M5. Indeed, checking for influential cases (see section “Materials and Methods”) showed that the final model fitted on a subset of data excluding observations from Sandokan (Table 1) revealed a clearly outlier (much lower than the estimate for the final model) and a non-significant value for the coefficient of the interaction of experience and food type.
Discussion
In this study, we tested neophobic response toward novel food in 23 captive capuchin monkeys. When neophobia was measured in terms of the preference (relative probability after controlling for availability) to retrieve familiar over novel food, we did not observe a clear bias in favor of the familiar food items in the first 10 retrieval episodes for each individual and condition (model M1; Table 2 and Figure 1). Actually, a neophobic response does not necessarily imply an overall preference for retrieving familiar food items rather than novel food items. The relative preference for retrieving one or the other type of food may be seen as resulting from the combination of the overall attractiveness of each of the food items and the potential neophobic response against the novelty. As neophobia concerns a bias against novelty per se, the presence of a neophobic response can straightforwardly be revealed by a decrease in the relative preferences for familiar food over novel food as the subjects become acquainted with the novel food, which we modeled by an interaction of food type (familiar or novel) and number of retrieved items in models M1 and M2. Our analyses, however, did not reveal this interaction to be significant. Therefore, as long as the relative preference for retrieving familiar over novel food is concerned, we did not find clear evidence of a neophobic response. Conversely, in terms of latency to retrieve food, we observed the kind of significant interaction (in that case, between food type and session number) predicted by a neophobic response, with latency to retrieve novel food decreasing throughout the experiment, and latency to retrieve familiar food approximately constant over time (Figure 4). The better performance of analyses of latency over those of preference to retrieve food in revealing neophobia may, at least in part, depend on the more straightforward statistical modeling that can be applied to (log-transformed) latency times, which may be effectively modeled as a normally-distributed response in a linear (mixed) model. Preference for familiar over novel food, conversely, is better modeled as a probability that asymptotically converges to an arbitrary value (reflecting the relative palatability of each food type) and approximating it by a binomial GLMMs may not be ideal (despite we attempted to overcome this limitations by only analyzing pre-convergence observations, see section “Materials and Methods”).
Our results provided no clear support to the hypothesis that previous experience generally decreases the neophobic response toward novel food. In fact, we did not find evidence that previous experience with experiments involving the presentation of unfamiliar food was related to a lower neophobia toward novel food items, in terms of either the probability or in the latency to retrieve familiar over novel food. These results are in contrast with findings showing that individuals with previous experience with novel stimuli, food or predators, have lower levels of neophobia when exposed to other types of novel food or predators (food: Catanese et al., 2012; predators: Ferrari et al., 2015; Crane and Ferrari, 2017). Indeed, it has been suggested that reduction of neophobia associated with previous experience with novelty is limited to very specific contexts (e.g., Bannier et al., 2017). However, we deem unlikely that our results may depend on a lack of context-specificity, because we measured “experience” specifically in terms of past exposure to experiments involving novel food (some of which with rather similar stimuli, Supplementary Table 1). On the contrary, we might speculate that generalization in capuchins is actually less context-specific than suggested by Bannier et al. (2017) in a fish model. In this view, as the captive capuchins involved in this study were all exposed to a variety of experimental conditions in the course of their lives, they could have all accumulated a “saturated” level of experience with novelty, making our measure actually irrelevant. Moreover, all the experiments with unfamiliar food, that we considered as part of subjects’ experience, were performed many years before the current study; thus, their effect may have been diluted over time. Future studies should include more detailed measures of individuals’ previous experience with novelty, and better assess how such experience might affect response to novelty in a variety of different contexts (Ferrari et al., 2015; Bannier et al., 2017; Crane and Ferrari, 2017). However, it is possible that the stimuli we presented were not novel enough to the study subjects, reducing our ability to detect the effect of previous exposure to novelty on neophobia, or that the capuchins’ long habituation to being fed in captivity made them perceive any food presented as essentially harmless. In other words, regardless of their specific previous experience with novel food, capuchins may have perceived the “novel” bananas as being rather familiar or, anyway, benign, thus showing overall little neophobia. This interpretation is consistent with the small (if any) bias in favor of familiar food that we have observed (Figure 1). Finally, we must note that our analysis of the reduced dataset including the 8 individuals with risk-attitude data did actually reveal statistical significance for the effect of the interaction between experience and food type on latency times (model M5, Table 4), suggesting that neophobia was lower in subjects with more previous experience. As the same result was not confirmed by the analysis of the whole data set, we cannot consider this as sufficient evidence, but it suggests that the relation of primates’ neophobia with past experience in a feeding context deserves further investigation. We also found no support to the hypothesis that risk-proneness is linked to lower neophobia, as we did not find differences in the relative preference for familiar over novel food associated with previous measures of individuals’ risk attitude (model M1, Table 2), nor a significant effect of the interaction between risk attitude and food type on latency to retrieve food (model M5, Table 4). Since novelty is associated with uncertainty, it is reasonable to expect risk-proneness (i.e., the preference for riskier over safer options; De Petrillo et al., 2015) to be associated with low neophobia. Most of the studies that found an association of neophobia with risk, however, have investigated neophobic responses in dangerous situations, as predation (fishes: Brown et al., 2013; Brown et al., 2016; review in Crane and Ferrari, 2017) or anthropogenic disturbance (birds: Bókony et al., 2012; Greggor et al., 2016), suggesting that being exposed to a risky situation induces neophobia (e.g., predator avoidance; Brown et al., 2013). It is possible that the way in which we operationalized risk attitude (i.e., propensity to seek a randomly varying reward over a more predictable one) simply does not mirror the uncertainty that is instead linked to novelty, when subjects lack any information about the possible payoffs associated with novel stimuli (see Paglieri et al., 2014). However, as risk-attitude scores were available for only 8 out of our 23 subjects, low sample size certainly limited our power to detect a link between risk attitude and neophobia. Future studies should investigate the relationship between risk attitude and neophobia using different measures of risk proneness and including a larger sample of individuals.
We did not find that individuals reversed their food preferences in the presence of higher-ranking group members, nor that the presence of other conspecifics affected individuals’ preference for familiar over novel food (model M1, full-null not significant, Table 2). This result seems to be in contrast with other studies showing that the presence of a conspecific facilitates the acceptance of novel food in primates (Visalberghi and Addessi, 2000, 2001; Addessi and Visalberghi, 2001; Addessi et al., 2007; Hardus et al., 2015; Englerova et al., 2019). In these studies, however, conspecifics were not directly in contact with the focal subject (i.e., they had only visual but not physical contact). Therefore, the presence of conspecifics may decrease neophobic response, although only in a non-competitive context, in which individuals do not have access to the same food resources (but see Visalberghi and Fragaszy, 1995).
We found that several aspects of sociality were linked to neophobia. First, in agreement with the literature (Ellis, 1995; Altmann, 1998; Hohmann et al., 2006), higher-ranking monkeys had priority of access to resources, participating more than lower-ranking individuals in our experiment (model M3; Table 3 and Figure 2). Moreover, participation decreased for lower-ranking individuals (Figure 2), suggesting that, as all subjects familiarized with the experimental setup, higher-ranking individuals strengthened their control over the provided food. Then, in contrast to what reported by Crane et al. (2020), our data indicated separate detectable effects of rank and sex on latency times (model M4; Table 4 and Figure 4). Higher-ranking individuals showed generally longer latency to retrieve food (both familiar and novel) than lower-ranking ones, probably because the former do not need to be quick in order to outcompete conspecifics (Figures 3, 4). Notably, lower-ranking individuals typically retrieved their first food item in about one second or less (Figures 3, 4). Moreover, higher-ranking individuals appeared generally less neophobic than lower-ranking ones, showing a stronger bias toward a quicker retrieval of the familiar food than the novel food. Based on results from model M4, we estimated that, in the earliest sessions of the experiment, lower-ranking subjects (females ranking ≥ 5 and males ranking ≥ 2) retrieved the familiar food more quickly than the novel food (Figure 4), while, for higher-ranking individuals, there was no such a clear trend (higher-ranking females tended to retrieve the familiar food more slowly than the novel food, and higher-ranking males had similar times for both types). Toward the end of the experiment, higher-ranking monkeys (both females and males) retrieved the novel food more quickly than the familiar one and it seems reasonable that they learned to appreciate the texture and small added nutritional value provided by the seeds covering the “novel” banana slices (Addessi et al., 2004). Rank and session number being equal, latency times for females were significantly longer than for males when both retrieved familiar foods, while the effect of sex on latency times to retrieve novel food was much smaller (Figure 4). This observation may indicate that, at least in this captive population, female capuchins are actually less neophobic than males (in line with a study on wild capuchin monkeys; Visalberghi et al., 2003a; but in contrast to Crane et al., 2020). However, given that this effect seems to be driven by the longer times female took to retrieve familiar items, rather than by their quicker retrieval of novel items, it is still possible that this sex difference actually depends on other social aspects of the feeding behavior of males and females which could not be revealed by our measures. Overall, these results suggest that individuals flexibly adjust their behavior when tested in a social context, while it does not clearly support the idea that lower-ranking individuals preferentially retrieve the food that reduces occasions for competitive interactions (Gomez-Melara et al., 2021). On the one hand, we did not observe a significant effect of rank difference (presence of higher ranking individuals) on preference for familiar over novel food (model M1, Table 2). On the other hand, if differences in latency to food retrieval can be taken as an alternative measure of preference, we would expect that, as higher-ranking individuals shifted more toward novel food over the course of the experiment, lower-ranking individuals did the opposite, which was not the case (Figure 4).
An arguably odd feature of our study was the observation of statistically significant effects of interactions involving previous experience with novel food in the reduced data set comprising those monkeys with risk-attitude scores available. None of these effects could be confirmed in the complete data set, and our checks seem to indicate that they were indeed idiosyncratic features of the subsets of data used to fit models M2 and M5, comprising observations from eight subjects. Those eight individuals belonged to all four social groups and included all of the dominant males plus four females that are known to be particularly willing to participate in experiments, and may represent a sample that is not entirely representative of the whole colony. Interestingly, the distribution of experience with novel food in this data set is relatively unbalanced, with two subjects that have not been involved in previous experiments (experience = 0) and the remaining six individuals having performed from four to eight experiments. At least in the case of model M5, the influence of a single individual was clearly decisive while, for model M2, there was no such an obvious explanation. While it is difficult to determine if and how the peculiar features of each data set may drive to statistically significant signals that cannot be straightforwardly generalized, this finding highlights the difficulty to generate robust and general conclusions about inter-individual as well as inter-and intraspecific behavioral patterns. We, therefore, strongly embrace the call for investigating individual differences and its causes in animal cognition possibly through large, collaborative open-science projects (e.g., Thornton and Lukas, 2012; Many Primates et al., 2019).
Although our study failed to confirm our main predictions, its findings contribute to our understanding of individual variation in primate response to novelty. Future research should extend the study of neophobia in social settings, by using a larger variety of social contexts with different levels of competition over food, in order to monitor how the presence of conspecifics affects changes in the individual response to novelty. Moreover, it would be interesting to use a larger variety of novel stimuli, to systematically address how primates generalize previous experience and novelty depending on the specific characteristics of the stimuli they are exposed to. A better understanding of how animals respond to novelty will provide us with crucial information about their ability to respond to novel socio-ecological challenges and, importantly, it will have ethical implications, by contributing to improve the welfare of captive animals (Buchanan-Smith, 2011), to increase the effectiveness of reintroduction programs (Hardus et al., 2015), and to carry out more effective conservation programs in habitats where rapid environmental changes arise (e.g., in deforested or urbanized environments; McLennan et al., 2017) resulting in animals facing more frequently the necessity to switch over novel food sources for their survival.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Italian Health Ministry (DM 633/2020-PR to GSi).
Author Contributions
MV, GSa, EA, and GSi contributed to the design of the study. MV, GSa, and GSg contributed to data collection. MV organized the database. PG performed the statistical analyses. MV and PG drafted the manuscript. All authors contributed to the final writing of the manuscript and approved the submitted version.
Funding
This project has received fundings from the Sapienza University of Rome (Ph.D. program XXXIV in Environmental and Evolutionart Biology, MV) and the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement 839363 to GSi. We would also like to thank Marco Ramazzotti and the project Grandi Scavi 2019 and 2020 (Sapienza University of Rome) for the financial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Federica Amici for help with statistical analyses and insightful comments on the manuscript. We would also like to thank Arianna Manciocco, Massimiliano Bianchi and Simone Catarinacci for assistance with capuchins, and Roma Capitale-Museo Civico di Zoologia and the Fondazione Bioparco for hosting the ISTC-CNR Unit of Cognitive Primatology and Primate Center.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.820323/full#supplementary-material
Supplementary Figure 1 | Structure of the enclosure and experimental setting. Food items (banana slices) were provided in both indoor areas, connected through sliding doors to the outdoor area of the enclosure. Yellow circles indicate the familiar bananas, blue circles indicate the novel bananas in condition A (i.e., blue banana slices with sesame seeds). Food items were as evenly distributed as possible while ensuring visibility from the point of view of recording cameras.
Supplementary Table 1 | List of studies on individual reaction to novel food and, in the last line, on risk attitude, which have been carried out at the Primate Centre of the ISTC-CNR, Rome, Italy.
References
Addessi, E., Chiarotti, F., Visalberghi, E., and Anzenberger, G. (2007). Response to novel food and the role of social influences in common marmosets (Callithrix jacchus) and Goeldi’s monkeys (Callimico goeldii). Am. J. Primatol. 69, 1210–1222. doi: 10.1002/ajp.20429
Addessi, E., Galloway, A. T., Birch, L., and Visalberghi, E. (2004). Taste perception and food choices in capuchin monkeys and human children. Primatologie 6, 101–128.
Addessi, E., and Visalberghi, E. (2001). Social facilitation of eating novel food in tufted capuchin monkeys (Cebus apella): input provided by group members and responses affected in the observer. Anim.Cogn. 4, 297–303. doi: 10.1007/s100710100113
Addessi, E., and Visalberghi, E. (2006). “How social influences affect food neophobia in captive chimpanzees: a comparative approach,” in Cognitive Development in Chimpanzees, eds T. Matsuzawa, M. Tomonaga, and M. Tanaka (Tokyo: Springer), 246–264. doi: 10.1007/4-431-30248-4_16
Altmann, S. A. (1998). Foraging for Survival: Yearling Baboons in Africa. Chicago, IL: University of Chicago Press.
Amici, F., Caicoya, A. L., Majolo, B., and Widdig, A. (2020a). Innovation in wild Barbary macaques (Macaca sylvanus). Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-61558-2
Amici, F., Widdig, A., MacIntosh, A. J., Francés, V. B., Castellano-Navarro, A., Caicoya, A. L., et al. (2020b). Dominance style only partially predicts differences in neophobia and social tolerance over food in four macaque species. Sci. Rep. 10, 1–10. doi: 10.1038/s41598-020-79246-6
An, Y. S., Kriengwatana, B., Newman, A. E., MacDougall-Shackleton, E. A., and MacDougall-Shackleton, S. A. (2011). Social rank, neophobia and observational learning in black-capped chickadees. Behaviour 148, 55–69. doi: 10.1163/000579510X54582
Baayen, R. H., Davidson, D. J., and Bates, D. M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 59, 390–412. doi: 10.1016/j.jml.2007.12.005
Bannier, F., Tebbich, S., and Taborsky, B. (2017). Early experience affects learning performance and neophobia in a cooperatively breeding cichlid. Ethology 123, 712–723. doi: 10.1111/eth.12646
Bee, M., Bernal, X., Calisi, R., Carere, C., Carter, T., Fuertbauer, I., et al. (2020). Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 159, 1–10. doi: 10.1016/j.anbehav.2019.11.002
Bergman, T. J., and Kitchen, D. M. (2009). Comparing responses to novel objects in wild baboons (Papio ursinus) and geladas (Theropithecus gelada). Anim. Cogn. 12, 63–73. doi: 10.1007/s10071-008-0171-2
Biondi, L. M., Bó, M. S., and Vassallo, A. I. (2010). Inter-individual and age differences in exploration, neophobia and problem-solving ability in a Neotropical raptor (Milvago chimango). Anim. Cogn. 13, 701–710. doi: 10.1007/s10071-010-0319-8
Bókony, V., Kulcsár, A., Tóth, Z., and Liker, A. (2012). Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS One 7:e36639. doi: 10.1371/journal.pone.0036639
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.3929/ethz-b-000240890
Brown, G. E., Ferrari, M. C. O., Elvidge, C. K., Ramnarine, I., and Chivers, D. P. (2013). Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B 280:20122712. doi: 10.1098/rspb.2012.2712
Brown, G. E., Jackson, C. D., Joyce, B. J., Chivers, D. P., and Ferrari, M. C. (2016). Risk-induced neophobia: does sensory modality matter? Anim. Cogn. 19, 1143–1150. doi: 10.1007/s10071-016-1021-2
Buchanan-Smith, H. M. (2011). Environmental enrichment for primates in laboratories. Adv. Sci. Res. 5, 41–56. doi: 10.5194/asr-5-41-2010
Catanese, F., Distel, R. A., Provenza, F. D., and Villalba, J. J. (2012). Early experience with diverse foods increases intake of nonfamiliar flavors and feeds in sheep. J. Anim. Sci. 90, 2763–2773. doi: 10.2527/jas.2011-4703
Coleman, S. L., and Mellgren, R. L. (1994). Neophobia when feeding alone or in flocks in zebra finches, Taeniopygia guttata. Anim. Behav. 48, 903–907. doi: 10.1006/anbe.1994.1315
Crane, A. L., Brown, G. E., Chivers, D. P., and Ferrari, M. C. (2020). An ecological framework of neophobia: from cells to organisms to populations. Biol. Rev. 95, 218–231. doi: 10.1111/brv.12560
Crane, A. L., and Ferrari, M. C. (2017). Patterns of predator neophobia: a meta-analytic review. Proc. R. Soc. B 284:20170583. doi: 10.1098/rspb.2017.0583
Day, R. L., Coe, R. L., Kendal, J. R., and Laland, K. N. (2003). Neophilia, innovation and social learning: a study of intergeneric differences in callitrichid monkeys. Anim. Behav. 65, 559–571. doi: 10.1006/anbe.2003.2074
De Petrillo, F., Ventricelli, M., Ponsi, G., and Addessi, E. (2015). Do tufted capuchin monkeys play the odds? Flexible risk preferences in Sapajus spp. Anim. Cogn. 18, 119–130. doi: 10.1007/s10071-014-0783-7
Dell’Anna, F., Llorente, M., Weiß, B. M., von Fersen, L., and Amici, F. (2020). The effect of individual and food characteristics on food retrieval and food sharing in captive Guinea baboons (Papio papio). Am. J. Primatol. 82:e23078. doi: 10.1002/ajp.23078
Dobson, A. J., and Barnett, A. G. (2018). An Introduction to Generalized Linear Models. Boca Raton, FL: CRC press.
Ellis, L. (1995). Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257–333. doi: 10.1016/0162-3095(95)00050-U
Englerova, K., Klement, D., Frynta, D., Rokyta, R., and Nekovarova, T. (2019). Reactions to novel objects in monkeys: what does it mean to be neophobic? Primates 60, 347–353. doi: 10.1007/s10329-019-00731-2
Ensminger, A. L., and Westneat, D. F. (2012). Individual and sex differences in habituation and neophobia in house sparrows (Passer domesticus). Ethology 118, 1085–1095. doi: 10.1111/eth.12009
Falótico, T., and Ottoni, E. B. (2016). The manifold use of pounding stone tools by wild capuchin monkeys of Serra da Capivara National Park, Brazil. Behaviour 153, 421–442. doi: 10.1163/1568539X-00003357
Ferrari, M. C., McCormick, M. I., Meekan, M. G., and Chivers, D. P. (2015). Background level of risk and the survival of predator-naive prey: can neophobia compensate for predator naivety in juvenile coral reef fishes? Proc. R. Soc. B 282:20142197. doi: 10.1098/rspb.2014.2197
Forkman, B. A. (1991). Social facilitation is shown by gerbils when presented with novel but not with familiar food. Anim. Behav. 42, 860–861. doi: 10.1016/S0003-3472(05)80132-0
Forss, S. I., Koski, S. E., and van Schaik, C. P. (2017). Explaining the paradox of neophobic explorers: the social information hypothesis. Int. J. Primatol. 38, 799–822. doi: 10.1007/s10764-017-9984-7
Forss, S. I. F., Motes Rodrigo, A., Hrubesch, C., and Tennie, C. (2019). Differences in novel food response between pongo and pan. Am. J. Primatol. 81:e22945. doi: 10.1002/ajp.22945
Fox, R. A., and Millam, J. R. (2007). Novelty and individual differences influence neophobia in orange-winged Amazon parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 104, 107–115. doi: 10.1016/j.applanim.2006.04.033
Friard, O., and Gamba, M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Fujita, K., Sato, Y., and Kuroshima, H. (2011). Learning and generalization of tool use by tufted capuchin monkeys (Cebus apella) in tasks involving three factors: reward, tool, and hindrance. J. Exp. Psychol. Anim. Behav. Process. 37, 10–19. doi: 10.1037/a0020274
Gomez-Melara, J. L., Acosta-Naranjo, R., Castellano-Navarro, A., Francés, V. B., Caicoya, A. L., MacIntosh, A. J., et al. (2021). Dominance style predicts differences in food retrieval strategies. Sci. Rep. 11:2726. doi: 10.1038/s41598-021-82198-0
Greenberg, R. (1990). Feeding neophobia and ecological plasticity: a test of the hypothesis with captive sparrows. Anim. Behav. 39, 375–379. doi: 10.1016/S0003-3472(05)80884-X
Greenberg, R., and Mettke-Hofmann, C. (2001). “Ecological aspects of neophobia and neophilia in birds,” in Current Ornithology, eds V. Nolan and C. F. Thompson (Boston, MA: Springer), 119–178. doi: 10.1007/978-1-4615-1211-0_3
Greenberg, R. S. (2003). “The role of neophobia and neophilia in the development of innovative behaviour of birds,” in Animal Innovation, eds K. N. Laland and S. M. Reader (New York, NY: Oxfrod University Press), 175–196. doi: 10.1093/acprof:oso/9780198526223.003.0008
Greenberg-Cohen, D., Alkon, P. U., and Yom-Tov, Y. (1994). A linear dominance hierarchy in female Nubian ibex. Ethology 98, 210–220. doi: 10.1111/j.1439-0310.1994.tb01072.x
Greggor, A. L., Clayton, N. S., Fulford, A. J., and Thornton, A. (2016). Street smart: faster approach towards litter in urban areas by highly neophobic corvids and less fearful birds. Anim. Behav. 117, 123–133. doi: 10.1016/j.anbehav.2016.03.029
Greggor, A. L., Thornton, A., and Clayton, N. S. (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 6, 82–89. doi: 10.1016/j.cobeha.2015.10.007
Griffin, A. S., and Diquelou, M. C. (2015). Innovative problem solving in birds: a cross-species comparison of two highly successful passerines. Anim. Behav. 100, 84–94. doi: 10.1016/j.anbehav.2014.11.012
Gustafsson, E., Saint Jalme, M., Bomsel, M. C., and Krief, S. (2014). Food neophobia and social learning opportunities in great apes. Int. J. Primatol. 35, 1037–1071. doi: 10.1007/s10764-014-9796-y
Hardus, M. E., Lameira, A. R., Wich, S. A., de Vries, H., Wahyudi, R., Shumaker, R. W., et al. (2015). Effect of repeated exposures and sociality on novel food acceptance and consumption by orangutans. Primates 56, 21–27. doi: 10.1007/s10329-014-0441-3
Hartig, F. (2021). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.4.
Hegner, R. E. (1985). Dominance and anti-predator behaviour in blue tits (Parus caeruleus). Anim. Behav. 33, 762–768. doi: 10.1016/S0003-3472(85)80008-7
Heilbronner, S. R., Rosati, A. G., Stevens, J. R., Hare, B., and Hauser, M. D. (2008). A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biol. Lett. 4, 246–249. doi: 10.1098/rsbl.2008.0081
Hohmann, G., Robbins, M. M., and Boesch, C. (eds) (2006). Feeding Ecology in Apes and Other Primates. Cambridge, MA: Cambridge University Press.
Huber, L., Rechberger, S., and Taborsky, M. (2001). Social learning affects object exploration and manipulation in keas, Nestor notabilis. Anim. Behav. 62, 945–954. doi: 10.1006/anbe.2001.1822
Hughes, R. N. (2007). Neotic preferences in laboratory rodents: issues, assessment and substrates. Neurosci. Biobehav. Rev. 31, 441–464. doi: 10.1016/j.neubiorev.2006.11.004
Josef, A. K., Richter, D., Samanez-Larkin, G. R., Wagner, G. G., Hertwig, R., and Mata, R. (2016). Stability and change in risk-taking propensity across the adult life span. J. Pers. Soc. Psychol. 111:430. doi: 10.1037/pspp0000090
Kacelnik, A., and Bateson, M. (1996). Risky theories: the effects of variance on foraging decisions. Am. Zool. 36, 402–434. doi: 10.1093/icb/36.4.402
Lahti, K. (1998). Social dominance and survival in flocking passerine birds: a review with an emphasis on the willow tit Parus montanus. Ornis Fennica 75, 1–17.
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P., and Makowski, D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Sour. Softw. 6:3139. doi: 10.21105/joss.0313
MacLean, E. L., Mandalaywala, T. M., and Brannon, E. M. (2012). Variance-sensitive choice in lemurs: constancy trumps quantity. Anim. Cogn. 15, 15–25. doi: 10.1007/s10071-011-0425-2
Many Primates, Altschul, D. M., Beran, M. J., Bohn, M., Call, J., DeTroy, S., et al. (2019). Establishing an infrastructure for collaboration in primate cognition research. PLoS One 14:e0223675. doi: 10.1371/journal.pone.0223675
McLennan, M. R., Spagnoletti, N., and Hockings, K. J. (2017). The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int. J. Primatol. 38, 105–121. doi: 10.1007/s10764-017-9962-0
Mettke-Hofmann, C. (2014). Cognitive ecology: ecological factors, life-styles, and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5, 345–360. doi: 10.1002/wcs.1289
Mettke-Hofmann, C., Ebert, C., Schmidt, T., Steiger, S., and Stieb, S. (2005). Personality traits in resident and migratory warbler species. Behaviour 142, 1357–1375. doi: 10.1163/156853905774539427
Mettke-Hofmann, C., Winkler, H., and Leisler, B. (2002). The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249–272. doi: 10.1046/j.1439-0310.2002.00773.x
Miller, R., Lambert, M. L., Frohnwieser, A., Brecht, K. F., Bugnyar, T., Crampton, I., et al. (2022). Socio-ecological correlates in neophobia in corvids. Curr. Biol. 32, 74.e4–85.e4. doi: 10.1016/j.cub.2021.10.045
Moretti, L., Hentrup, M., Kotrschal, K., and Range, F. (2015). The influence of relationships on neophobia and exploration in wolves and dogs. Anim. Behav. 107, 159–173. doi: 10.1016/j.anbehav.2015.06.008
Overington, S. E., Cauchard, L., Côté, K. A., and Lefebvre, L. (2011). Innovative foraging behaviour in birds: what characterizes an innovator? Behav. Process. 87, 274–285. doi: 10.1016/j.beproc.2011.06.002
Paglieri, F., Addessi, E., De Petrillo, F., Laviola, G., Mirolli, M., Parisi, D., et al. (2014). Nonhuman gamblers: lessons from rodents, primates, and robots. Front. Behav. Neurosci. 8:33. doi: 10.3389/fnbeh.2014.00033
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rosati, A. G., and Hare, B. (2012). Decision making across social contexts: competition increases preferences for risk in chimpanzees and bonobos. Anim. Behav. 84, 869–879. doi: 10.1016/j.anbehav.2012.07.010
Russell, P. A. (1973). Relationships between exploratory behaviour and fear: a review. Br. J. Psychol. 64, 417–433. doi: 10.1111/j.2044-8295.1973.tb01369.x
Sabbatini, G., Stammati, M., Tavares, M. C. H., and Visalberghi, E. (2007). Response toward novel stimuli in a group of tufted capuchins (Cebus libidinosus) in Brasilia National Park, Brazil. Am. J. Primatol. 69, 457–470. doi: 10.1002/ajp.20365
Schaffer, A., Caicoya, A. L., Colell, M., Holland, R., von Fersen, L., Widdig, A., et al. (2021). Neophobia in 10 ungulate species—a comparative approach. Behav. Ecol. Sociobiol. 75, 1–12. doi: 10.1007/s00265-021-03041-0
Schuett, W., Tregenza, T., and Dall, S. R. X. (2010). Sexual selection and animal personality. Biol. Rev. 85, 217–246. doi: 10.1111/J.1469-185X.2009.00101.X
Sirianni, G., and Visalberghi, E. (2013). Wild bearded capuchins process cashew nuts without contacting caustic compounds. Am. J. Primatol. 75, 387–393. doi: 10.1002/ajp.22119
Sol, D., Griffin, A. S., Bartomeus, I., and Boyce, H. (2011). Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS One 6:e19535. doi: 10.1371/journal.pone.0019535
Straznicka, K. (2012). “Temporal stability of risk preference measures,” in Proceedings of the GATE-Groupe d’Analyse et de Théorie Économique Lyon-St Étienne Working Paper, (Lyon: Université de Lyon).
Thornton, A., and Lukas, D. (2012). Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2773–2783. doi: 10.1098/rstb.2012.0214
Visalberghi, E., and Addessi, E. (2000). Seeing group members eating a familiar food enhances the acceptance of novel foods in capuchin monkeys. Anim. Behav. 60, 69–76. doi: 10.1006/anbe.2000.1425
Visalberghi, E., and Addessi, E. (2001). Acceptance of novel foods in capuchin monkeys: do specific social facilitation and visual stimulus enhancement play a role? Anim. Behav. 62, 567–576. doi: 10.1006/anbe.2001.1787
Visalberghi, E., and Fragaszy, D. (1995). The behaviour of capuchin monkeys, Cebus apella, with novel food: the role of social context. Anim. Behav. 49, 1089–1095. doi: 10.1006/anbe.1995.0137
Visalberghi, E., Janson, C. H., and Agostini, I. (2003a). Response toward novel foods and novel objects in wild Cebus apella. Int. J. Primatol. 24, 653–675. doi: 10.1023/A:1023700800113
Visalberghi, E., Sabbatini, G., Stammati, M., and Addessi, E. (2003b). Preferences towards novel foods in Cebus apella: the role of nutrients and social influences. Physiol. Behav. 80, 341–349. doi: 10.1016/j.physbeh.2003.08.004
Visalberghi, E., and Limongelli, L. (1994). Lack of comprehension of cause-effect relations in tool-using capuchin monkeys (Cebus apella). J. Comp. Psychol. 108, 15–22. doi: 10.1037/0735-7036.108.1.15
Voelkl, B., Schrauf, C., and Huber, L. (2006). Social contact influences the response of infant marmosets towards novel food. Anim. Behav. 72, 365–372. doi: 10.1016/j.anbehav.2005.10.013
Webster, S. J., and Lefebvre, L. (2001). Problem solving and neophobia in a columbiform-passeriform assemblage in Barbados. Anim. Behav. 62, 23–32. doi: 10.1006/anbe.2000.1725
Wolf, M., van Doorn, G. S., Leimar, O., and Weissing, F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. doi: 10.1038/nature05835
Keywords: novel food, neophobia, primates, capuchin monkeys, individual variation, previous experience, risk attitude, social context
Citation: Ventricelli M, Gratton P, Sabbatini G, Addessi E, Sgaraglia G, Rufo F and Sirianni G (2022) Individual Variation in Response to Novel Food in Captive Capuchin Monkeys (Sapajus spp.). Front. Ecol. Evol. 10:820323. doi: 10.3389/fevo.2022.820323
Received: 22 November 2021; Accepted: 07 March 2022;
Published: 28 April 2022.
Edited by:
Karline Janmaat, University of Amsterdam, NetherlandsReviewed by:
Breanna N. Harris, Texas Tech University, United StatesSang-im Lee, Daegu Gyeongbuk Institute of Science and Technology (DGIST), South Korea
Copyright © 2022 Ventricelli, Gratton, Sabbatini, Addessi, Sgaraglia, Rufo and Sirianni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marialba Ventricelli, marialba.ventricelli@uniroma1.it; marialbaventricelli@gmail.com
 Marialba Ventricelli
Marialba Ventricelli Paolo Gratton3
Paolo Gratton3 Gloria Sabbatini
Gloria Sabbatini Elsa Addessi
Elsa Addessi Giulia Sgaraglia
Giulia Sgaraglia Fabrizio Rufo
Fabrizio Rufo