- 1 Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, USA
- 2 Department of Pediatrics, College of Osteopathic Medicine, Michigan State University, East Lansing, MI, USA
Recombinant Adenovirus (Ad) based vectors have been utilized extensively as a gene transfer platform in multiple pre-clinical and clinical applications. These applications are numerous, and inclusive of both gene therapy and vaccine based approaches to human or animal diseases. The widespread utilization of these vectors in both animal models, as well as numerous human clinical trials (Ad-based vectors surpass all other gene transfer vectors relative to numbers of patients treated, as well as number of clinical trials overall), has shed light on how this virus vector interacts with both the innate and adaptive immune systems. The ability to generate and administer large amounts of this vector likely contributes not only to their ability to allow for highly efficient gene transfer, but also their elicitation of host immune responses to the vector and/or the transgene the vector expresses in vivo. These facts, coupled with utilization of several models that allow for full detection of these responses has predicted several observations made in human trials, an important point as lack of similar capabilities by other vector systems may prevent detection of such responses until only after human trials are initiated. Finally, induction of innate or adaptive immune responses by Ad vectors may be detrimental in one setting (i.e., gene therapy) and be entirely beneficial in another (i.e., prophylactic or therapeutic vaccine based applications). Herein, we review the current understanding of innate and adaptive immune responses to Ad vectors, as well some recent advances that attempt to capitalize on this understanding so as to further broaden the safe and efficient use of Ad-based gene transfer therapies in general.
Introduction
Viruses are obligate intracellular, metabolically inert particles composed of DNA or RNA and a protein coat. They are very efficient in transferring (transducing) their simple “genomes” into vulnerable cells of multicellular organisms.
Replication-deficient adenovirus based vectors (Ads) have been the focus of considerable interest in the last few years for their potential applications in both gene therapy and vaccine applications (Amalfitano and Parks, 2002; St George, 2003; Amalfitano, 2004; Waehler et al., 2007; Lasaro and Ertl, 2009; Russell, 2009; Barouch, 2010). Adenoviruses are a family of non-enveloped viruses containing an icosahedral protein capsid with a 30- to 40-kb linear double-stranded DNA (dsDNA) genome. In general, of the immunologically distinct human Ad serotypes, none are associated with any neoplastic disease, with most causing relatively mild, self-limiting respiratory illnesses in immunocompetent individuals (Lichtenstein and Wold, 2004; Russell, 2009). At least 51 serotypes of human Ad have been identified, and Ad serotypes 5 (Ad5) and Ad2, both belonging to subclass C, are the most extensively studied and characterized both relative to general Ad biology, as well in regard to utilization as a gene transfer vector.
Ad vectors continue to be the most widely utilized gene transfer vector in human clinical trials worldwide. More to the point, as of 2010, over 387 human clinical trials have administered Ad-based vectors by any number of routes to both normal human volunteers, as well as patients affected by a number of diseases potentially treatable by an Ad-based gene transfer approach1 (Seregin and Amalfitano, 2009; Liu, 2010). In China, Ad vectors are routinely administered for the treatment of some forms of cancer (Huang et al., 2009). These facts may be surprising to some, as it is in sharp contrast to the erroneous views widely held by many basic researchers within, as well as outside the field of gene therapy, that Ad vectors are not recommended for human usage. Much of this confusion stems from an incident in 1999 (the “ornithine transcarbamylase, OTC clinical trial”), in which the tragic death of a clinical trial subject occurred after intravascular administration of a large dose of an Ad-based vector (Raper et al., 2002, 2003). The OTC trial was further compromised by problems in the design and conduct of the trial, as acknowledged and fully detailed by the trial’s Principal Investigator (Wilson, 2009). To be clear, there have been subsequent human trials to the OTC trial, that have also intravascularly administered (in a similar fashion to the OTC trial) equivalent, or even higher doses of Ad-based vectors to large numbers of trial subjects, and not had the unfortunate outcome reported in the 1999 OTC trial (Atencio et al., 2006). The reasons for this dichotomy in responses are numerous, and likely may never be resolved (Wilson, 2009).
Why do Ad vectors continue to be so widely utilized? Ad vectors posses several important advantages, the most important of which is that they can be easily, and routinely produced to high titers in a good manufacturing practice (GMP) compliant fashion (up to 1 × 1013 vp/ml). Non-dependence upon transfection based packaging systems for vector production is a major reason for this efficiency, a feature that has likely limited the clinical use of other vector systems that have been available for as long a period of time as Ad vector systems. Additionally, Ad vectors allow for efficient transduction of various proliferating and quiescent cell types, they can allow for transfer of large segments of foreign DNA (up to 35 kb in some systems), and most importantly, Ad vectors do not integrate, and therefore are much less likely to cause insertional mutagenesis or germ line transmission associated problems, in contrast to integrating virus based vectors, such as retrovirus and lentivirus based gene transfer systems (Hacein-Bey-Abina et al., 2003; Modlich et al., 2005; Howe et al., 2008).
As a result of the ability to both easily concentrate and deliver large amounts of Ad vectors, coupled with high level transgene expression derived from the vector once successful transduction in vivo has occurred, it has become clear that the use of Ad vectors also rapidly activates innate immune responses as well induces potent cellular and humoral adaptive immune responses, against both the vector and transgene product being expressed. These events likely occur subsequent to gene transduction with use of any gene transfer vector, virus or non-virus based. However, an ability to only deliver very low particle titers of vector, or low overall transduction efficiencies, may alter or prevent detection of innate or adaptive immune responses subsequent to use of other (non-Ad based) gene transfer vectors.
As viruses have evolved to more efficiently transduce host cells, the mammalian immune system has co-evolved cellular and humoral immune responses to prevent or limit the growth of an invading virus or pathogen. The immune response to viruses is generally composed of two branches: a rapid and non-specific response mediated by the innate arm of the immune system, as well as a relatively slower, more highly specific adaptive immune response, the latter being endowed with memory of past infections to respond more efficiently upon repeat exposure to an infecting organism such as a virus. The innate immune response promotes initiation of the adaptive immune response, and can also orchestrate its overall progression.
While innate immune responses are primarily driven by virion components (capsid proteins, DNA, or RNA genomes) present upon initial administration of the Ad virus into a living animal, adaptive immune responses are mainly associated with the leaky expression of Ad derived genes in early generations of Ad vectors (so called E1 deleted Ads), or more importantly driven by whether or not the transgene being expressed by the Ad vector is perceived by the host as immunologically foreign (Tripathy et al., 1994, 1996; Ding et al., 2001; Kiang et al., 2006b). Regardless of the cause, activation of the innate and adaptive immune systems by Ad vectors can lead to tissue or organ inflammation, enhanced immune-mediated clearance of vector transduced cells, and reduced transgene expression (Amalfitano, 2004). The presence of memory T- and B-cells responses in individuals previously exposed to wild-type Ads further limits the potential for benefit when utilizing Ad-based vectors (Barouch, 2010).
Ad vectors with additional deletions in their genome (accommodated by use of newer generation, trans-complementing packaging cell lines) in the E2A, E2B, and E4 Ad genes have been generated (Engelhardt et al., 1994; Amalfitano et al., 1998; Raper et al., 1998; Amalfitano and Parks, 2002). These advanced generation Ad vectors produce fewer Ad derived gene products as compared to first generation Ads, and can minimize the induction of vector-specific adaptive immune responses (Engelhardt et al., 1994; Ding et al., 2001). These benefits are furthered by the use of helper-dependent (HD)-fully deleted Ad vectors, that have their entire genome deleted (thereby can accommodate up to a 35-kb transgene payload) and are propagated with high efficiency via use of a highly engineered helper virus (Parks et al., 1996; Brunetti-Pierri and Ng, 2009). Overall, newer generations of Ad vectors elicit lower immunogenicity (diminished adaptive immune responses to Ad antigens), and allow for longer transgene expression (Parks et al., 1996; Morral et al., 1998; Amalfitano and Parks, 2002; Everett et al., 2003).
Despite the improved features of multiply deleted or HD-Ad vectors, these vectors continue to elicit innate immune profiles in a pattern similar to that induced by wild-type or first generation Ad vectors (Brunetti-Pierri et al., 2004; McCaffrey et al., 2008; Seregin and Amalfitano, 2009). Furthermore, some advanced generation Ad vectors may still be subject to immunological neutralization or cell mediate clearance by the presence of anti-Ad neutralizing antibodies (NAbs) and/or Ad specific memory T cells present in individuals previously exposed to wild-type Ads (Sumida et al., 2004; Hutnick et al., 2010). Exploring and understanding the mechanism by which Ad vectors interact with and activate the innate and adaptive immune systems will not only allow for the safer use of these vectors, but may also allow for pre-emptive and specific modulation of these responses in efforts to allow for better utilization of these important gene transfer platforms. Lessons learned from the use of Ad vectors are also directly applicable to most, if not all gene transfer vectors (virus or non-virus based), as the mammalian innate and adaptive immune systems have evolved to detect and rapidly neutralize all manner of invading particle, especially those containing a DNA or RNA genome. We will review here much of the current understanding relative to Ad vector mediated induction of the innate and adaptive immune responses, and the impact these responses have on the safety and efficacy of Ad vector based therapies. We will also highlight some strategies proposed to either mitigate or harness Ad-induced innate and adaptive immune responses for the improved and broadened development of advanced, Ad-based therapies.
Innate Immune Responses to Viral Infection
The innate immune system is conserved across species and represents the first line of general defense against pathogenic infections, inclusive of viral infections specifically (Hoffmann et al., 1999). These, “pathogen associated molecular patterns” (PAMPs), are detected by the host’s deployment of a wide array of extracellular, cell surface or intracellular molecules, proteins, and receptors generally known as “pattern recognition receptors” (PRRs; Girardin et al., 2002; Kawai and Akira, 2010). The innate immune system is also composed of a network of different cell types expressing or reacting to PRR activation, including: monocytes/macrophages, dendritic cells (DCs), natural killer (NK) cells, NK T cells, neutrophils, gamma delta T cells, and mast cells. Each cell of the innate immune system expresses various types of PRRs. For example, a widely studied family of PRRs are the toll-like receptors (TLRs; Kawai and Akira, 2010). Emerging data also indicates an important role for other families of intracellular PRRs, including the nucleotide-binding oligomerization domain/leucine-rich repeat receptors (NOD–LRR) and the retinoic acid-inducible gene (RIG)-1-like receptors (RLRs; Takeuchi and Akira, 2010). Once activated by a specific ligand, these various PRRs trigger a series of signaling cascades that result in down-stream activation and transcription of immune response genes via the NFκB, mitogen-activated protein kinases (MAPKs), and/or interferon regulatory factors (IRFs) 3 and/or 7 signaling pathways, as but some examples. Activation of these pathways eventuates in the production and/or rapid release of pro-inflammatory cytokines and chemokines, initiation of type I interferon responses that limit the replication of invading pathogens, as well as the promotion and shaping of pathogen-specific B- and T cell adaptive immune responses (Medzhitov, 2007; O’Neill and Bowie, 2010).
Innate Immune Responses to Ad Vectors
Similar to any invading pathogen, gene transfer vectors in general, and Ad vectors in particular are recognized by both immune and non-immune cells, recognitions that activate robust innate immune responses in vivo (Schnell et al., 2001; Zhang et al., 2001; Hartman et al., 2007, 2008; Rhee et al., 2011). These responses are dose-dependent and can limit the efficacy of gene transfer mediated by these vectors due to the development of inflammation and the rapid activation of the humoral and/or cytolytic arms of the innate and adaptive immune system (Everett et al., 2003).
One must take note that the mammalian innate system has evolved to rapidly detect very low numbers of an invading pathogen such as a virus, and respond accordingly. Therefore, administration of most any gene transfer vector will result in activation of the innate immune system, and likely in an exaggerated manner since large numbers of gene transfer vectors (virus or non-virus based) need to be delivered before evidence of relevant levels of transgene expression can be confirmed. This caveat is in contrast to the generally low numbers of particles being present during the initial stages of a wild-type virus infection. To account for this, the immunological response is much like a rheostat, a rheostat that has a very large and multi-faceted “dynamic range.” For example, detection of low levels of pathogen numbers, or replication, by the innate immune system may result in undetectable, or low level cytokine or chemokine elevations resulting in undetectable or milder symptoms, such as arthralgia, malaise, or fever (the latter being much more difficult to ascertain in some animal models). Greater provocation of the innate immune system after exposure to greater numbers of virus particles induces greater levels of pro-inflammatory compounds and cellular activations. These responses can become exaggerated, and manifest as a cytokine “storm” that can cause serious medical complications, including the systemic inflammatory response syndrome, and even death as in OTC clinical trial (Raper et al., 2003; Matsuda and Hattori, 2006). Furthermore, these responses by the innate immune system are also likely occurring at the local level, and/or when lower levels of vector are administered, though these activations may not be easily detected.
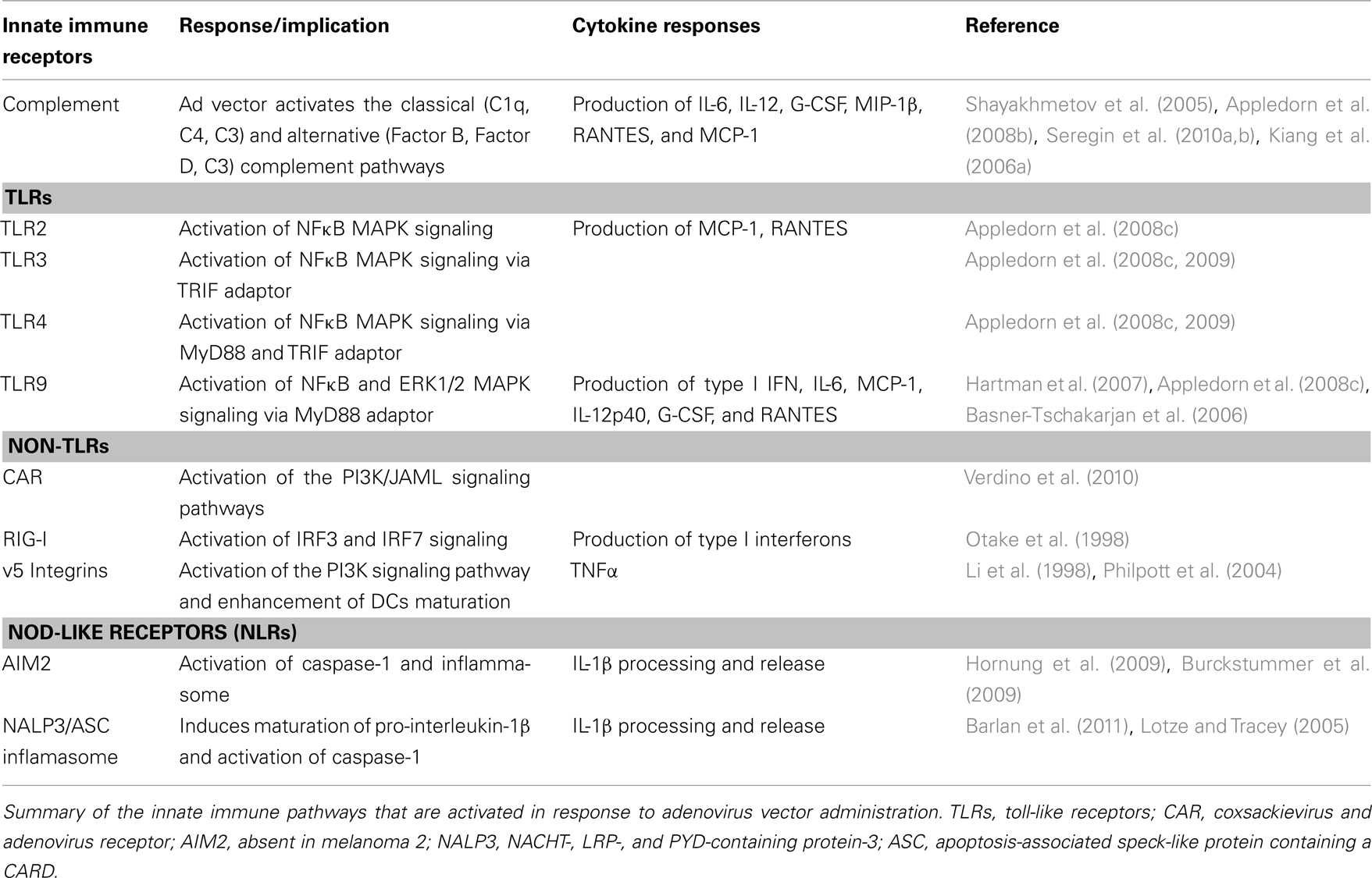
Upon initial introduction into a host, The innate immune response can be initiated following the binding or coating of the Ad vector capsid with several humoral (extracellular) factors including: surfactant-A (SP-A), lactoferrin, pre-existing immunoglobulin, and protein members of the complement pathways, both classical (C1q, C4) and alternative (Factor B, Factor D; Jiang et al., 2004; Shayakhmetov et al., 2005; Shifrin et al., 2005; Huarte et al., 2006; Kiang et al., 2006; Johansson et al., 2007; Zhu et al., 2007; Appledorn et al., 2008). These early interactions attempt to usher the virus away from vulnerable tissues or organs, and rather deliver the virus particles to cells of the reticulo-endothelial system (Jiang et al., 2004; Blom and Ram, 2008; Seregin et al., 2010a,b). It should be clear to the reader that these mechanisms are likely at play for any virus vector (or for that matter, complexes of DNA or RNA coupled with complex liposomes; i.e., containing targeting motifs), and may obviate attempts to retarget capsid modified vectors to various tissues beyond the reticulo-endothelial system (RES).
Adenovirus Vector Induction of Pro-Inflammatory Cytokine and Chemokine Responses
Despite the initial interactions with the several components of the innate immune system, Ad vector particles can and do infect target cells (primarily accomplished by administration of excessive doses of the vector), this many times accomplished by administering large doses of the vector that effectively overruns the capacity of the RES system to sequester virus particles (Bristol et al., 2000; Morral et al., 2002; Ziegler et al., 2002). However, this does not come without a serious cost. The administration of Ad vectors also results in the immediate production (1–6 h post injection) of various pro-inflammatory cytokines and chemokines, as well as type I interferons in mice, non-human, and human primates (Schnell et al., 2001; Basner-Tschakarjan et al., 2006; Hartman et al., 2007, 2008; Appledorn et al., 2008, 2010). Specifically, high dose, intravascular administrations of Ad vectors have been found to induce high levels of the cytokines tumor necrosis factor α (TNFα), IL-6, IL-12, interferon γ (IFNγ), IL-1α, and IL-1β and the chemokines RANTES (regulated on activation, normal T cell expressed and secreted), MCP-1(monocytes chemoattractant protein 1), KC, MIP-1α (macrophage inhibitory protein-1 alpha), MIP-1β, and IFNγ inducible protein 10 (IP-10; Hartman et al., 2007; Appledorn et al., 2008c, 2010; Di Paolo et al., 2009), (Table 1).
The origins of these pro-inflammatory mediators in vivo is not fully known but is likely from multiple sources inclusive of Kupffer cells, macrophages, endothelial cells as well as Ad transduced tissues, and organs themselves (Shifrin et al., 2005; Appledorn et al., 2008c). Some studies suggest that conventional DCs (cDCs) and macrophages are the main source of acute inflammatory cytokines in response to systemic Ad vectors administration (Zhang et al., 2001). Depletion of Kupffer cells in mice, by intravenous injection of gadolinium chloride (GdCl3), resulted in inhibition of Ad-mediated TNFα production. However, robust production of IL-6 has been observed in Kupffer cell depleted mice with no change in NFκB activity, suggesting that there might be additional cell types that contribute to Ad vector-mediate pro-inflammatory cytokine production (Lieber et al., 1997).
Furthermore, Ad vectors can either activate of directly transduce various immune cells types in the liver and spleen including: DCs (Lore et al., 2007), both plasmacytoid DCs (pDCs; Basner-Tschakarjan et al., 2006) and (cDCs; Lindsay et al., 2010), macrophages (Aldhamen et al., 2011), and to a lesser extent (NK) cells (Schroers et al., 2004; Aldhamen et al., 2011). The induction of type I interferon is critical for innate immune defense against Ad vectors in vivo (Zhu et al., 2008); the maturation of antigen presenting cells, both DCs and macrophages (Hensley et al., 2005); and the regulation of the induction of pro-inflammatory cytokines (Huarte et al., 2006; Zhu et al., 2007).
In addition to DCs and macrophages, we and others have shown that NK cells are another major group of innate immune effector cells that responds to the presence of Ad vectors. As early as 6 h post injection, NK cells accumulate in the liver and spleen producing high levels of IFNγ, which contributes significantly to the innate immune elimination of Ad vectors and the induction of T helper cell type 1 (TH1) adaptive immune responses (Peng et al., 2001; Ruzek et al., 2002; Zhu et al., 2008, 2010; Appledorn et al., 2010, 2011; Aldhamen et al., 2011). Furthermore, NK cell activation by Ad vectors contributes to liver injury, as NK cell depletion using anti-NK1.1 or anti-asialo GM1 (AsGM1) antibodies reduced Ad vector induced elevations of transaminases, as well as hepatocyte cell death (Liu et al., 2000).
Molecular Basis for Cellular Recognition of Adenovirus Vector Infection
Ad5 vectors interact with both the coxsackie-adenovirus receptor (CAR; via the Ad fiber knob domain) and with cellular integrins (via the Ad penton base RGD motifs) to initiate host cell penetration (Wickham et al., 1993; Bergelson et al., 1997). The penetration process itself will also simultaneously trigger cellular pro-inflammatory innate immune responses. Inductions of IP-10 by Ads have been detected after infecting kidney derived epithelial cells with WT capsid vectors, as well as CAR binding ablated fiber knob mutants (Tibbles et al., 2002). However, recent data has shown that binding of CAR by Ads also promotes the clustering of junctional adhesion molecule-like protein (JAML) and activation of the P13K signaling pathway, suggesting another role for CAR binding during the initiation of innate immune responses to Ads (Verdino et al., 2010). Various reports have also shown a significant role for αv-integrins for Ad vector induced innate immune responses (Huang et al., 1996; Li et al., 1998; Nemerow and Stewart, 1999). Specifically, Ad penton base interactions with αv-integrins have also been shown to activate the PI3K signaling pathway and induce DCs maturation via TNFα autocrine signaling (Li et al., 1998; Philpott et al., 2004). Inhibiting PI3K signaling also blocked Ad-induced DC maturation and reduced Ad-induced TNFα production (Philpott et al., 2004).
After internalization and upon endosomal escape, Ad vectors have been shown to activate MAPK and NFκB signaling pathways via both TLR dependent and TLR non-dependent mechanisms (Zhu et al., 2007; Appledorn et al., 2008c). We and others have shown that, Ad vectors activate TLR signaling and induce various cytokines and chemokines responses in a MyD88 and TLR9 dependent manner (Basner-Tschakarjan et al., 2006; Hartman et al., 2007; Appledorn et al., 2008c). However, the induction of some chemokines, for example KC and MCP-1, was not dependent on TLR9, but still required a functional MyD88 adaptor protein, suggesting either the involvement of other TLRs, or other MyD88 dependent signaling systems, for the complete induction of the innate immune response to Ad vectors (Cerullo et al., 2007). Studies in our laboratory have shown that the induction of several cytokines and chemokines, the expression of innate immune response genes, and the generation of antibodies to both Ad vectors (NAbs) as well as Ad expressed transgene products were also TLR2, TLR3, and TLR4 dependent, and required both functional MyD88 and TRIF (TIR-domain-containing adapter-inducing interferon-beta) adaptors proteins (Appledorn et al., 2008c, 2009). In addition, our studies also demonstrated a suppressive role for TLR4 signaling in some Ad-induced innate immune responses; suggesting a complex role for TLRs in Ad vectors-mediated immune responses (Appledorn et al., 2009).
Besides TLRs, significant evidence has been accumulated in recent years implicating a TLR9-independent mechanism for sensing Ad5 (dsDNA) genomes (Nociari et al., 2007; Zhu et al., 2007; Shayakhmetov et al., 2010). Takaoka et al. (2007) showed that a dsDNA sensor called DNA-dependent activator of interferon regulatory factors (DAI) activates type I interferon in response to DNA viruses in L-929 cells, but subsequent studies suggested the presence of other cytoplasmic DNA sensor(s) (Ishii and Akira, 2006; Ishii et al., 2008). Absent in melanoma 2 (AIM2) has been shown to respond to cytoplasmic dsDNA and activate the inflammasome, driving the activation of caspase-1 and IL-1β processing and release (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). AIM2 was suggested to be important for the induction of pro-inflammatory immune responses against several viruses that produce cytosolic DNA during their lifecycle, such as vaccinia virus, herpes simplex virus-1 (HSV-1), and adenovirus (Kanneganti, 2010; Rathinam et al., 2010). However, it is our understanding that there is as yet no direct evidence linking the activation of the inflammasome by Ads to AIM2.
The activation of the NALP3 inflammasome by Ad derived dsDNA leads to caspase-dependent activation of IL-1β and induction of pro-inflammatory cytokine and chemokine responses including elevations of IL-6, MIP-1β, IP-10, and MCP-1 (Muruve et al., 2008). In addition, Ad-mediated disruption of lysosomal membranes, and the release of cathepsin B into the cytoplasm, are required for Ad-induced NLRP3 inflammasome activation (Barlan et al., 2011). Furthermore, Ad5 activation of NLRP3 also induced necrotic cell death, resulting in the release of the pro-inflammatory molecule High-mobility group box 1 protein (HMGB1), a recently identified damage (or danger)-associated molecular pattern (DAMP) that mediates the response to infection, injury, and inflammation (Lotze and Tracey, 2005; Barlan et al., 2011). It is important to note that, only partial reductions of pro-inflammatory cytokines and chemokines have been observed in Ad treated mice deficient of TLR9, NALP3, or the ASC inflammasome; suggesting the involvement of other innate immune PRRs, or a synergy between the inflammasome and other innate immune receptors during the innate immune recognition of Ad vector derived DNA (Cerullo et al., 2007; Muruve et al., 2008; Hornung et al., 2009). In addition, it has been demonstrated that adenovirus virus-associated RNA (VA) is recognized by retinoic acid-inducible gene I (RIG-I), a cytosolic PRR, and activates RIG-I down-stream signaling, leading to the induction of type I interferons (IFNs; Minamitani et al., 2011).
Together, these findings confirm that Ad vectors activate a number of complex innate immune response networks (Table 1). These newly identified networks may also provide targets for the development of new approaches to further improve the safety and efficacy of Ad based, as well as other important gene transfer platforms.
Modulation of Innate Immune Responses to Ad Vectors
Various strategies have been attempted in efforts to manipulate Ad vector-inductions of the innate immune system to develop safe and efficacious Ad-based gene transfer therapies. For gene transfer based applications applied to genetic diseases, multiple approaches have been utilized to minimize innate immune responses to Ad vectors including the use of immunosuppressive agents and depletion of innate immune cells (i.e., DCs and macrophages) thought to be primarily responsible for cytokine and chemokine production following Ad vector administrations. For example, the modulation of Ad vector induced inflammation by prophylactic use of anti-inflammatory corticosteroids (such as Dexamethasone, DEX) was associated with a significantly reduced ability of Ad vectors to induce several acute inflammatory cytokine and chemokine responses including: TNFα, IL-6, IL-12, MCP-1, MIP-1α, G-CSF, in concert with sustained, as well in some cases, improved transgene expression subsequent to gene transduction (Otake et al., 1998; Seregin et al., 2009). These improvements were in some instances coupled with reductions in pro-inflammatory leukocyte infiltrations into the liver, as well reduced induction of pro-inflammatory gene expression in Ad transduced liver hepatocytes and spleen derived cells (Seregin et al., 2009). In addition, the use of more targeted strategies in mice, such as: TLR9 blockade with ODN-2088, administration of anti-TNFα monoclonal antibodies, and administration of Erk or p38 MAPK inhibitors (U0126 and SB203580, respectively) prior to Ad vector administration each significantly reduced Ad-triggered innate immune responses (Tibbles et al., 2002; Wilderman et al., 2006; Cerullo et al., 2007; Appledorn et al., 2008c).
Ad vectors have also been engineered to enhance innate immune responses subsequent to administration. In tumor immunotherapy based approaches, Ad vectors expressing pro-inflammatory mediators, such as IFNγ, IL-2, IL-12, IL-18, and IL-23, have been shown to possess potent anti-tumor activities in several pre-clinical and clinical investigations utilizing the vectors as tumor-lytics (Siddiqui et al., 2007; Urosevic et al., 2007; Reay et al., 2009; Choi et al., 2011). Administration of Ad vectors over-expressing MyD88 has been shown to improve the induction of antigen specific adaptive immune responses, and enhance tumor cell lysis in a mouse model in vivo (Hartman et al., 2010). Studies in our laboratory have shown that Ad vectors expressing a recombinant Eimeria tenella derived TLR agonist (rEA) induced potent innate immune responses that correlated with an improved induction of cellular immune responses to several target antigens (Appledorn et al., 2010). Analogous to use of MyD88 (Hartman et al., 2010), we have also recently shown that Ad vectors expressing EAT-2 (Ewing’s sarcoma-associated transcript-2), a signaling lymphocytic activation molecules (SLAM) derived adaptor protein, enhanced innate immune cell (NK-, DCs, and macrophages) activation and induced beneficial cytokine and chemokine responses. These responses also correlated with improved inductions of antigen specific adaptive immune responses (including induction of cytotoxic T cell responses) by Ads expressing EAT-2 in vivo (Aldhamen et al., 2011).
Adaptive Immune Responses Against Adenovirus Vectors
Development of neutralizing antibodies and CD8+ T Cell Responses to the Ad Vector
When attempting to summarize adaptive immune responses to Ad-based vectors, one must consider two primary issues, adaptive immune responses to Ad derived proteins (either Ad genes still expressed from early generation vectors, or the Ad capsid proteins themselves) as well the adaptive immune responses to the transgene expressed by a respective Ad vector.
Both gene therapy and vaccine applications utilizing Ad-based vector platforms may suffer from diminished efficacy (i.e., diminished antigen specific immune responses or blunted transient expression of therapeutic transgene) when pre-existing Ad5 immunity is present. A majority (over 60%) of the worldwide human population have had previous exposure to wild-type Ads in childhood or adulthood, these exposures result in the development of various forms of pre-existing immunity to the most common Ad serotypes (Abbink et al., 2007; Lasaro and Ertl, 2009; Seregin and Amalfitano, 2009). NABs to Ad vectors are primarily directed against the surface loops of the viral hexon protein, however, antibodies to the penton base or the fiber can also neutralize Ads (Sumida et al., 2005). Ad NABs affect the efficacy of Ad vector gene transfer by blocking cell transduction (Smith et al., 2008; Parker et al., 2009; Pichla-Gollon et al., 2009; Seregin and Amalfitano, 2009).
When utilizing early generation Ad5 based vectors that are deleted for only the E1 genes, or alternative serotype Ad vectors that are also only E1 deleted, this results in reduced transgene product expression and thus, reduced induction of transgene-specific CD8+ T cell immune responses in vaccine applications (Sumida et al., 2004; Thorner et al., 2006; McCoy et al., 2007; Liu et al., 2008; Gabitzsch et al., 2009b; Osada et al., 2009; Haut et al., 2011). Since the presence of high levels of NABs to the most commonly utilized Ad vector, Ad5, are highly prevalent in certain populations, including sub-Saharan Africa (Abbink et al., 2007; Barouch et al., 2011), recent efforts have focused on developing Ad vectors that have either had immunogenic portions of the Ad5 capsid replaced with homologous regions from alternative serotype Ads, or on Ad vectors entirely derived from rarer human, or chimp derived Ad serotypes (Abbink et al., 2007; Barouch, 2008; Seregin and Amalfitano, 2009; Geisbert et al., 2011). While the use of these alternative serotypes may allow for partial overcoming of transductional inefficiencies due to the presence of pre-existing Ad5 NABs, their use will also have several limitations, including altered biodistribution and biosafety profiles, as well they are also subject to neutralization upon their readministration (Barouch et al., 2004; Thorner et al., 2006; Appledorn et al., 2008a; Liu et al., 2009).
Additionally, several studies emphasize a significant role for Ad specific CD8+ T cells in pre-existing Ad immunity. Ad vector specific (mostly against hexon capsid protein) CD8+ T cells can be readily detected in PBMCs in a high percentage of healthy adults (Molinier-Frenkel et al., 2000, 2002; Tang et al., 2006). This latter point must also be considered when alternative, rarer Ad serotypes are utilized in attempts to circumvent pre-existing common Ad serotype specific antibodies, since pre-existing T cell responses to one Ad serotype can still be harnessed when a host is exposed to an alternative Ad serotype (Heemskerk et al., 2003; Leen et al., 2004; Hutnick et al., 2010). An important and recent study demonstrated that CD8+ T cells against the E2b encoded polymerase protein can be found at frequencies as high as found for CD8+ T cells against the major immunodominant Ad protein hexon in Ad immune individuals (Joshi et al., 2009). This feature may be responsible for the recent finding that administration of Ad5 vectors devoid of expression of the Ad polymerase (E2b) protein can induce beneficial adaptive immune responses to expressed antigens, even in the Ad5 immune host (Gabitzsch et al., 2009a,b, 2010; Weaver et al., 2009; Clarke et al., 2010).
Adaptive Immune Responses to Ad Vector Expressed Transgenes
A continued fallacy regarding the use of Ad-based gene transfer is the notion that Ads are incapable of allowing for long term transgene expression in the immune competent host. However, Ad vectors have been repeatedly shown to allow for long term transgene expression, especially in animals that do not perceive the transgene being delivered by the Ad vector as immunologically foreign. We and others have reviewed this topic extensively in the past, and refer the reader to those publications for full validation and understanding of these views (Amalfitano, 2004; Seregin and Amalfitano, 2009, 2010).
Conversely, Ad-based vectors have a potent ability to induce potent humoral, but more importantly, cellular immune responses to expressed foreign antigens, and have therefore recently received much attention for use in a number of vaccine based applications (Lasaro and Ertl, 2009; Barouch, 2010). Specifically, E1 deleted Ad5 vectors expressing the HIV-1 gag, pol, and nef genes have been utilized in human trial subjects (Buchbinder et al., 2008; Priddy et al., 2008). The results from the early-phase clinical trials demonstrated that the Ad5 vector-based vaccines elicited some of the most potent, HIV specific cellular immune responses in humans to date, however, the presence of pre-existing Ad5-specific NABs partially suppressed these responses (Buchbinder et al., 2008; Priddy et al., 2008).
Utilization of advanced generation Ad vectors have also recently been shown to allow for improved efficacy in several vaccine based applications (Gabitzsch et al., 2009b, 2010, 2011; Weaver et al., 2009). Specifically, [E1-, E2b-]Ad5 vectors were able to induce heightened gene specific T cell responses in mice and primates (Gabitzsch et al., 2009a,b, 2010). In contrast to E1 deleted Ad vectors, these types of Ad vaccines can show strong efficacy despite the existence of of pre-existing Ad immunity possibly by their avoidance of CD8+ T cell responses directed to the Ad polymerase gene (Gabitzsch et al., 2009a,b, 2010, 2011; Osada et al., 2009). [E1-, E2b-]Ad vectors, expressing tumor derived antigens were also able to induce beneficial, cytolytic T cell responses that promoted tumor regression in murine models (Gabitzsch et al., 2011). Based upon these improvements, a phase I/II clinical trial is currently underway, utilizing a CEA (Carcinoembryonic Antigen) expressing [E1-, E2b-]Ad5 vector in an attempt to safely induce beneficial, CEA specific adaptive immune responses in patients (both Ad5 naive and Ad5 immune) bearing CEA expressing tumors2.
Further Strategies for Evading Host Immune Responses after Ad Vector Mediated Gene Transfer
Several elegant approaches have been developed in various attempts to diminish Ad vector and transgene-triggered innate and/or adaptive immune responses, thereby, maximizing the efficiency and persistence of Ad-mediated gene transfer for a variety of applications. This chapter will briefly summarize the latest advances, and refer readers to several recent reviews summarizing earlier papers on these topics (Seregin and Amalfitano, 2010).
The approaches attempting to maximize the duration of transgene expression from an Ad vector and minimize potential side effects can be categorized as follows: (Waehler et al., 2007) pre-emptive transient immune modulation of the host; consisting of the use of immunosuppressive drugs or specific compounds to block important immune pathways, which are known to be induced by Ad vectors and (St George, 2003) selective modification of the Ad vector itself. The latter approaches includes several innovative strategies, with the most prominent being covalent modifications of the entire Ad vector capsid moiety; Ad capsid-display of specific inhibitors or ligands; the use of tissue specific promoters to drive transgene expression in selected tissues to minimize adaptive immune responses, and as alluded to previously, the use of genome modified Ads, chimeric Ads, and alternative Ad serotypes (Seregin and Amalfitano, 2010).
Transient, non-specific immunosuppression of the host with (Dexamethasone, FK506, cyclosporine A), or selective immunosuppression of the host (TLR9 or TNFα blockers, CTLA4-Ig, anti-CD40 antibodies), and/or attempts at induction of a generalized tolerance state (IL-10, TGFβ) have all been strategies that have been described in animal models to successfully improve outcomes of Ad-based gene transfer. Moreover, some of these immunosuppression approaches have been tested in clinical trials, as reviewed (Seregin and Amalfitano, 2010). In regards to the non-specific modification of the Ad vector capsid itself, Ad vectors have also been complexed with several polymers in a manner to shield the capsid from either innate or adaptive humoral immune components, such as NABs. These moieties included the use of polyethylene glycol (PEG; O’Riordan et al., 1999), polylactic glycolic acid (PLGA; Matthews et al., 1999) or other lipids (Lee et al., 2000), each of which have been reported to improve efficacy and/or safety of Ad-mediated gene transfer, when the vector is produced in a complex with these moieties.
The non-enveloped Ad virion is composed of a large capsid of about 90 nm in diameter, containing nine proteins (Rux and Burnett, 2004; Parks, 2005; Vellinga et al., 2005). Several novel and specific modifications to the Ad capsid have also been engineered in attempts to improve the efficacy of the basic Ad vector platform. The fiber, penton, protein IX, and hexon proteins have all been exploited for genetic insertion of foreign peptides, either as “in-frame” insertions within the proteins, or as “in-frame” C-terminal fusions.
In a vaccine targeted application, antigenic epitopes, derived from the hemagglutinin (HA) protein of the influenza A virus, were incorporated into, and displayed from the Ad capsid as hexon, penton base, fiber knob, or protein IX fusions (Krause et al., 2006). The fiber-displaying Ads induced the highest levels of HA-specific immunity, as determined by measuring production of HA-specific IgM and IgG humoral responses, as well as HA-specific IL-4 or IFNγ producing CD4+ T cellular immune responses (Krause et al., 2006). In another example, the immunodominant portions of the Bacillus anthracis protective antigen (PA) were genetically inserted and displayed from the HVR5 site of the Ad5 hexon (McConnell et al., 2006). Intramuscular injections of the novel vector into BALB/c mice resulted in generation of PA-specific IgG1 and IgG2a antibodies, possibly indicating that TH1 and TH2 immunity to the antigen was generated, surpassing the efficacy of synthetic peptide based vaccination strategies (McConnell et al., 2006). The HVR5 site of hexon has also been exploited for display of the Pseudomonas aeruginosa B cell epitope-encoding peptide. Footpad vaccinations with the novel vaccines induced antibody responses to the P. aeruginosa antigen (Worgall et al., 2005). Both IFNγ-positive CD4+ and CD8+ P. aeruginosa specific T cell responses were also generated. Most convincingly, mice vaccinated with the P. aeruginosa B cell epitope displaying Ad were protected against subsequent lethal pulmonary challenge with several P. aeruginosa strains (Worgall et al., 2005).
In contrast, Ad capsid-display of immuno-evasive proteins can also dramatically improve the efficacy and/or safety of Ad-based gene transfer (Seregin et al., 2010a, 2011). Specifically, the human decay-accelerating factor (DAF) natural complement inhibitor was shown to retain anti-complement activity when displayed from the surface of the Ad capsid in a retro-oriented fashion (Seregin et al., 2010). Subsequent studies have shown that mice injected with the “DAF-displaying” Ad5 vector, demonstrated significant reductions in pro-inflammatory cytokine release, avoidance of thrombocytopenia, reduced endothelial cell activation, minimized activation of pro-inflammatory genes expression, and reduced plasma ALT levels in mice as compared to unmodified Ad5 vectors. Moreover, these results correlated positively with a significantly decreased activation of DCs, NK cells, CD3+CD8+ T cells, and CD3+CD8− T cells (Seregin et al., 2010a, 2011). Importantly, this modulation of the complement dependent arm of the innate immune response resulted in significantly reduced induction of Ad neutralizing antibody responses, as well as in blunted T cell responses to the transgene (i.e., HIV-gag or GFP) by the DAF-displaying Ad (Seregin et al., 2011).
In addition, genetic modifications, or wholesale “swapping” of the Ad capsid hexon and fiber proteins from one serotype to another have been undertaken in efforts to avoid pre-existing Ad serotype specific neutralizing antibody responses, as previously described (Gall et al., 1998; Roy et al., 1998; Molinier-Frenkel et al., 2002; Ritter et al., 2002; Wu et al., 2002).
Conclusion and Future Perspectives
After full analysis of the several points covered in this review, the reader should understand that the interactions of Ad-based vectors with the host innate and adaptive immune systems are multi-faceted and complex. These complexities should make it clear that one cannot make simple assertions regarding the potential for use of Ad vectors in a specific gene therapy or vaccine based application, especially, if only limited information is provided, or general assumptions are being promulgated. It is also clear that despite this level of complexity and potential for several limitations, Ad vectors continue to be the platform of choice for an ever increasing number of clinical trials worldwide.
Utilization of Ad vectors (or any gene transfer vector) for implementing gene therapy for genetic disorders is very challenging. The induction of potent innate immune responses by the vector may limit its utility for this purpose in some instances. However, this same limitation may be of benefit when considering utilization of the vector in various vaccine based applications. It should also be clear that there have been a number of vector modifications attempted to improve the safety or efficacy of Ad-based gene transfer, some of these modified Ads may well be better utilized for some clinical applications but not for others. Despite this context-specific utility, it is obvious that the Ad-based gene transfer platform appears to be highly “plastic” and capable of tolerating a number of elegant molecular manipulations. Importantly, solely with use of first generation (E1 deleted) Ad vectors, the number of Ad vector utilizing clinical trials has doubled within the last 5 years. Bolstered by a clearer understanding as to the benefits, and more importantly, identified limitations of Ad-based vectors, we predict a continued expansion for clinical use of Ad vectors in general, an expansion that will be bolstered by the current and expanded future use of modified Ad vectors, as well inclusion of pharmacological and/or other supportive interventions to further enhance both the safety and efficacy of Ad-mediated gene transfer into humans.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Abbink, P., Lemckert, A. A., Ewald, B. A., Lynch, D. M., Denholtz, M., Smits, S., Holterman, L., Damen, I., Vogels, R., Thorner, A. R., O’Brien, K. L., Carville, A., Mansfield, K. G., Goudsmit, J., Havenga, M. J., and Barouch, D. H. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81, 4654–4663.
Aldhamen, Y. A., Appledorn, D. M., Seregin, S. S., Liu, C. J., Schuldt, N. J., Godbehere, S., and Amalfitano, A. (2011). Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J. Immunol. 186, 722–732.
Amalfitano, A. (2004). Utilization of adenovirus vectors for multiple gene transfer applications. Methods 33, 173–178.
Amalfitano, A., Hauser, M. A., Hu, H., Serra, D., Begy, C. R., and Chamberlain, J. S. (1998). Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J. Virol. 72, 926–933.
Amalfitano, A., and Parks, R. J. (2002). Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr. Gene Ther. 2, 111–133.
Appledorn, D. M., Aldhamen, Y. A., Depas, W., Seregin, S. S., Liu, C. J., Schuldt, N., Quach, D., Quiroga, D., Godbehere, S., Zlatkin, I., Kim, S., McCormick, J. J., and Amalfitano, A. (2010). A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS ONE 5, e9579
Appledorn, D. M., Aldhamen, Y. A., Godbehere, S., Seregin, S. S., and Amalfitano, A. (2011). Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin. Vaccine Immunol. 18, 150–160.
Appledorn, D. M., Kiang, A., McBride, A., Jiang, H., Seregin, S., Scott, J. M., Stringer, R., Kousa, Y., Hoban, M., Frank, M. M., and Amalfitano, A. (2008a). Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 5, 885–901.
Appledorn, D. M., McBride, A., Seregin, S., Scott, J. M., Schuldt, N., Kiang, A., Godbehere, S., and Amalfitano, A. (2008b). Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 15, 1606–1617.
Appledorn, D. M., Patial, S., McBride, A., Godbehere, S., Van Rooijen, N., Parameswaran, N., and Amalfitano, A. (2008c). Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181, 2134–2144.
Appledorn, D. M., Patial, S., Godbehere, S., Parameswaran, N., and Amalfitano, A. (2009). TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses. J. Innate Immun. 1, 376–388.
Atencio, I. A., Grace, M., Bordens, R., Fritz, M., Horowitz, J. A., Hutchins, B., Indelicato, S., Jacobs, S., Kolz, K., Maneval, D., Musco, M. L., Shinoda, J., Venook, A., Wen, S., and Warren, R. (2006). Biological activities of a recombinant adenovirus p53 (SCH 58500) administered by hepatic arterial infusion in a Phase 1 colorectal cancer trial. Cancer Gene Ther. 13, 169–181.
Barlan, A. U., Griffin, T. M., McGuire, K. A., and Wiethoff, C. M. (2011). Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85, 146–155.
Barouch, D. H. (2010). Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV AIDS 5, 386–390.
Barouch, D. H., Kik, S. V., Weverling, G. J., Dilan, R., King, S. L., Maxfield, L. F., Clark, S., Ng’ang’a, D., Brandariz, K. L., Abbink, P., Sinangil, F., de Bruyn, G., Gray, G. E., Roux, S., Bekker, L. G., Dilraj, A., Kibuuka, H., Robb, M. L., Michael, N. L., Anzala, O., Amornkul, P. N., Gilmour, J., Hural, J., Buchbinder, S. P., Seaman, M. S., Dolin, R., Baden, L. R., Carville, A., Mansfield, K. G., Pau, M. G., and Goudsmit, J. (2011). International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29, 5203–5209.
Barouch, D. H., Pau, M. G., Custers, J. H., Koudstaal, W., Kostense, S., Havenga, M. J., Truitt, D. M., Sumida, S. M., Kishko, M. G., Arthur, J. C., Korioth-Schmitz, B., Newberg, M. H., Gorgone, D. A., Lifton, M. A., Panicali, D. L., Nabel, G. J., Letvin, N. L., and Goudsmit, J. (2004). Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172, 6290–6297.
Basner-Tschakarjan, E., Gaffal, E., O’Keeffe, M., Tormo, D., Limmer, A., Wagner, H., Hochrein, H., and Tüting, T. (2006). Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8, 1300–1306.
Bergelson, J. M., Cunningham, J. A., Droguett, G., Kurt-Jones, E. A., Krithivas, A., Hong, J. S., Horwitz, M. S., Crowell, R. L., and Finberg, R. W. (1997). Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323.
Blom, A. M., and Ram, S. (2008). Contribution of interactions between complement inhibitor C4b-binding protein and pathogens to their ability to establish infection with particular emphasis on Neisseria gonorrhoeae. Vaccine 26(Suppl. 8), I49–I55.
Bristol, J. A., Shirley, P., Idamakanti, N., Kaleko, M., and Connelly, S. (2000). In vivo dose threshold effect of adenovirus-mediated factor VIII gene therapy in hemophiliac mice. Mol. Ther. 2, 223–232.
Brunetti-Pierri, N., and Ng, P. (2009). Progress towards liver and lung-directed gene therapy with helper-dependent adenoviral vectors. Curr. Gene Ther. 9, 329–340.
Brunetti-Pierri, N., Palmer, D. J., Beaudet, A. L., Carey, K. D., Finegold, M., and Ng, P. (2004). Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 15, 35–46.
Buchbinder, S. P., Mehrotra, D. V., Duerr, A., Fitzgerald, D. W., Mogg, R., Li, D., Gilbert, P. B., Lama, J. R., Marmor, M., Del Rio, C., McElrath, M. J., Casimiro, D. R., Gottesdiener, K. M., Chodakewitz, J. A., Corey, L., Robertson, M. N., and Step Study Protocol Team. (2008). Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893.
Burckstummer, T., Baumann, C., Blüml, S., Dixit, E., Dürnberger, G., Jahn, H., Planyavsky, M., Bilban, M., Colinge, J., Bennett, K. L., and Superti-Furga, G. (2009). An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272.
Cerullo, V., Seiler, M. P., Mane, V., Brunetti-Pierri, N., Clarke, C., Bertin, T. K., Rodgers, J. R., and Lee, B. (2007). Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15, 378–385.
Choi, I. K., Lee, J. S., Zhang, S. N., Park, J., Lee, K. M., Sonn, C. H., and Yun, C. O. (2011). Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. [Epub ahead of print].
Clarke, J. M., Morse, M. A., Lyerly, H. K., Clay, T., and Osada, T. (2010). Adenovirus vaccine immunotherapy targeting WT1-expressing tumors. Expert Opin. Biol. Ther. 10, 875–883.
Di Paolo, N. C., Miao, E. A., Iwakura, Y., Murali-Krishna, K., Aderem, A., Flavell, R. A., Papayannopoulou, T., and Shayakhmetov, D. M. (2009). Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31, 110–121.
Ding, E. Y., Hodges, B. L., Hu, H., McVie-Wylie, A. J., Serra, D., Migone, F. K., Pressley, D., Chen, Y. T., and Amalfitano, A. (2001). Long-term efficacy after [E1-, polymerase-] adenovirus-mediated transfer of human acid-alpha-glucosidase gene into glycogen storage disease type II knockout mice. Hum. Gene Ther. 12, 955–965.
Engelhardt, J. F., Ye, X., Doranz, B., and Wilson, J. M. (1994). Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc. Natl. Acad. Sci. U.S.A. 91, 6196–6200.
Everett, R. S., Hodges, B. L., Ding, E. Y., Xu, F., Serra, D., and Amalfitano, A. (2003). Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum. Gene Ther. 14, 1715–1726.
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J., and Alnemri, E. S. (2009). AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513.
Gabitzsch, E. S., Xu, Y., Balcaitis, S., Balint, J. P. Jr., and Jones, F. R. (2011). An Ad5[E1-, E2b-]-HER2/neu vector induces immune responses and inhibits HER2/neu expressing tumor progression in Ad5 immune mice. Cancer Gene Ther. 18, 326–335.
Gabitzsch, E. S., Xu, Y., Balint, J. P. Jr., Hartman, Z. C., Lyerly, H. K., and Jones, F. R. (2010). Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol. Immunother. 59, 1131–1135.
Gabitzsch, E. S., Xu, Y., Yoshida, L. H., Balint, J., Amalfitano, A., and Jones, F. R. (2009a). Novel Adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine 27, 6394–6398.
Gabitzsch, E. S., Xu, Y., Yoshida, L. H., Balint, J., Gayle, R. B., Amalfitano, A., and Jones, F. R. (2009b). A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses. Immunol. Lett. 122, 44–51.
Gall, J. G., Crystal, R. G., and Falck-Pedersen, E. (1998). Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 72, 10260–10264.
Geisbert, T. W., Bailey, M., Hensley, L., Asiedu, C., Geisbert, J., Stanley, D., Honko, A., Johnson, J., Mulangu, S., Pau, M. G., Custers, J., Vellinga, J., Hendriks, J., Jahrling, P., Roederer, M., Goudsmit, J., Koup, R., and Sullivan, N. J. (2011). Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J. Virol. 85, 4222–4233.
Girardin, S. E., Sansonetti, P. J., and Philpott, D. J. (2002). Intracellular vs extracellular recognition of pathogens – common concepts in mammals and flies. Trends Microbiol. 10, 193–199.
Hacein-Bey-Abina, S., Von Kalle, C., Schmidt, M., McCormack, M. P., Wulffraat, N., Leboulch, P., Lim, A., Osborne, C. S., Pawliuk, R., Morillon, E., Sorensen, R., Forster, A., Fraser, P., Cohen, J. I., de Saint Basile, G., Alexander, I., Wintergerst, U., Frebourg, T., Aurias, A., Stoppa-Lyonnet, D., Romana, S., Radford-Weiss, I., Gross, F., Valensi, F., Delabesse, E., Macintyre, E., Sigaux, F., Soulier, J., Leiva, L. E., Wissler, M., Prinz, C., Rabbitts, T. H., Le Deist, F., Fischer, A., and Cavazzana-Calvo, M. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419.
Hartman, Z. C., Appledorn, D. M., and Amalfitano, A. (2008). Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 132, 1–14.
Hartman, Z. C., Kiang, A., Everett, R. S., Serra, D., Yang, X. Y., Clay, T. M., and Amalfitano, A. (2007). Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 81, 1796–1812.
Hartman, Z. C., Osada, T., Glass, O., Yang, X. Y., Lei, G. J., Lyerly, H. K., and Clay, T. M. (2010). Ligand-independent toll-like receptor signals generated by ectopic overexpression of MyD88 generate local and systemic antitumor immunity. Cancer Res. 70, 7209–7220.
Haut, L. H., Ratcliffe, S., Pinto, A. R., and Ertl, H. (2011). Effect of preexisting immunity to adenovirus on transgene product-specific genital T cell responses on vaccination of mice with a homologous vector. J. Infect. Dis. 203, 1073–1081.
Heemskerk, B., Veltrop-Duits, L. A., van Vreeswijk, T., ten Dam, M. M., Heidt, S., Toes, R. E., van Tol, M. J., and Schilham, M. W. (2003). Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J. Virol. 77, 6562–6566.
Hensley, S. E., Giles-Davis, W., McCoy, K. C., Weninger, W., and Ertl, H. C. (2005). Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 175, 6032–6041.
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A., and Ezekowitz, R. A. (1999). Phylogenetic perspectives in innate immunity. Science 284, 1313–1318.
Hornung, V., Ablasser, A., Charrel-Dennis, M., Bauernfeind, F., Horvath, G., Caffrey, D. R., Latz, E., and Fitzgerald, K. A. (2009). AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518.
Howe, S. J., Mansour, M. R., Schwarzwaelder, K., Bartholomae, C., Hubank, M., Kempski, H., Brugman, M. H., Pike-Overzet, K., Chatters, S. J., de Ridder, D., Gilmour, K. C., Adams, S., Thornhill, S. I., Parsley, K. L., Staal, F. J., Gale, R. E., Linch, D. C., Bayford, J., Brown, L., Quaye, M., Kinnon, C., Ancliff, P., Webb, D. K., Schmidt, M., von Kalle, C., Gaspar, H. B., and Thrasher, A. J. (2008). Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118, 3143–3150.
Huang, P. I., Chang, J. F., Kirn, D. H., and Liu, T. C. (2009). Targeted genetic and viral therapy for advanced head and neck cancers. Drug Discov. Today 14, 570–578.
Huang, S., Kamata, T., Takada, Y., Ruggeri, Z. M., and Nemerow, G. R. (1996). Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70, 4502–4508.
Huarte, E., Larrea, E., Hernández-Alcoceba, R., Alfaro, C., Murillo, O., Arina, A., Tirapu, I., Azpilicueta, A., Hervás-Stubbs, S., Bortolanza, S., Pérez-Gracia, J. L., Civeira, M. P., Prieto, J., Riezu-Boj, J. I., and Melero, I. (2006). Recombinant adenoviral vectors turn on the type I interferon system without inhibition of transgene expression and viral replication. Mol. Ther. 14, 129–138.
Hutnick, N. A., Carnathan, D., Demers, K., Makedonas, G., Ertl, H. C., and Betts, M. R. (2010). Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 28, 1932–1941.
Ishii, K. J., and Akira, S. (2006). Innate immune recognition of, and regulation by, DNA. Trends Immunol. 27, 525–532.
Ishii, K. J., Kawagoe, T., Koyama, S., Matsui, K., Kumar, H., Kawai, T., Uematsu, S., Takeuchi, O., Takeshita, F., Coban, C., and Akira, S. (2008). TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729.
Jiang, H., Wang, Z., Serra, D., Frank, M. M., and Amalfitano, A. (2004). Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol. Ther. 10, 1140–1142.
Johansson, C., Jonsson, M., Marttila, M., Persson, D., Fan, X. L., Skog, J., Frängsmyr, L., Wadell, G., and Arnberg, N. (2007). Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 81, 954–963.
Joshi, A., Tang, J., Kuzma, M., Wagner, J., Mookerjee, B., Filicko, J., Carabasi, M., Flomenberg, N., and Flomenberg, P. (2009). Adenovirus DNA polymerase is recognized by human CD8+ T cells. J. Gen. Virol. 90(Pt 1), 84–94.
Kanneganti, T. D. (2010). Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698.
Kawai, T., and Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 11, 373–384.
Kiang, A., Hartman, Z. C., Everett, R. S., Serra, D., Jiang, H., Frank, M. M., and Amalfitano, A. (2006a). Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 14, 588–598.
Kiang, A., Hartman, Z. C., Liao, S., Xu, F., Serra, D., Palmer, D. J., Ng, P., and Amalfitano, A. (2006b). Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol. Ther. 13, 127–134.
Krause, A., Joh, J. H., Hackett, N. R., Roelvink, P. W., Bruder, J. T., Wickham, T. J., Kovesdi, I., Crystal, R. G., and Worgall, S. (2006). Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J. Virol. 80, 5523–5530.
Lasaro, M. O., and Ertl, H. C. (2009). New insights on adenovirus as vaccine vectors. Mol. Ther. 17, 1333–1339.
Lee, S. G., Yoon, S. J., Kim, C. D., Kim, K., Lim, D. S., Yeom, Y. I., Sung, M. W., Heo, D. S., and Kim, N. K. (2000). Enhancement of adenoviral transduction with polycationic liposomes in vivo. Cancer Gene Ther. 7, 1329–1335.
Leen, A. M., Sili, U., Vanin, E. F., Jewell, A. M., Xie, W., Vignali, D., Piedra, P. A., Brenner, M. K., and Rooney, C. M. (2004). Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 104, 2432–2440.
Li, E., Stupack, D., Klemke, R., Cheresh, D. A., and Nemerow, G. R. (1998). Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 72, 2055–2061.
Lichtenstein, D. L., and Wold, W. S. (2004). Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 11, 819–829.
Lieber, A., He, C. Y., Meuse, L., Schowalter, D., Kirillova, I., Winther, B., and Kay, M. A. (1997). The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71, 8798–8807.
Lindsay, R. W., Darrah, P. A., Quinn, K. M., Wille-Reece, U., Mattei, L. M., Iwasaki, A., Kasturi, S. P., Pulendran, B., Gall, J. G., Spies, A. G., and Seder, R. A. (2010). CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 185, 1513–1521.
Liu, J., Ewald, B. A., Lynch, D. M., Denholtz, M., Abbink, P., Lemckert, A. A., Carville, A., Mansfield, K. G., Havenga, M. J., Goudsmit, J., and Barouch, D. H. (2008). Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82, 4844–4852.
Liu, J., O’Brien, K. L., Lynch, D. M., Simmons, N. L., La Porte, A., Riggs, A. M., Abbink, P., Coffey, R. T., Grandpre, L. E., Seaman, M. S., Landucci, G., Forthal, D. N., Montefiori, D. C., Carville, A., Mansfield, K. G., Havenga, M. J., Pau, M. G., Goudsmit, J., and Barouch, D. H. (2009). Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457, 87–91.
Liu, Z. X., Govindarajan, S., Okamoto, S., and Dennert, G. (2000). NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 164, 6480–6486.
Lore, K., Adams, W. C., Havenga, M. J., Precopio, M. L., Holterman, L., Goudsmit, J., and Koup, R. A. (2007). Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J. Immunol. 179, 1721–1729.
Lotze, M. T., and Tracey, K. J. (2005). High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342.
Matsuda, N., and Hattori, Y. (2006). Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J. Pharmacol. Sci. 101, 189–198.
Matthews, C., Jenkins, G., Hilfinger, J., and Davidson, B. (1999). Poly-L-lysine improves gene transfer with adenovirus formulated in PLGA microspheres. Gene Ther. 6, 1558–1564.
McCaffrey, A. P., Fawcett, P., Nakai, H., McCaffrey, R. L., Ehrhardt, A., Pham, T. T., Pandey, K., Xu, H., Feuss, S., Storm, T. A., and Kay, M. A. (2008). The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol. Ther. 16, 931–941.
McConnell, M. J., Hanna, P. C., and Imperiale, M. J. (2006). Cytokine response and survival of mice immunized with an adenovirus expressing Bacillus anthracis protective antigen domain 4. Infect. Immun. 74, 1009–1015.
McCoy, K., Tatsis, N., Korioth-Schmitz, B., Lasaro, M. O., Hensley, S. E., Lin, S. W., Li, Y., Giles-Davis, W., Cun, A., Zhou, D., Xiang, Z., Letvin, N. L., and Ertl, H. C. (2007). Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81, 6594–6604.
Medzhitov, R. (2007). Recognition of microorganisms and activation of the immune response. Nature 449, 819–826.
Minamitani, T., Iwakiri, D., and Takada, K. (2011). Adenovirus virus-associated RNAs induce type I interferon expression through a RIG-I-mediated pathway. J. Virol. 85, 4035–4040.
Modlich, U. Kustikova, O. S., Schmidt, M., Rudolph, C., Meyer, J., Li, Z., Kamino, K., von Neuhoff, N., Schlegelberger, B., Kuehlcke, K., Bunting, K. D., Schmidt, S., Deichmann, A., von Kalle, C., Fehse, B., and Baum, C. (2005). Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood 105, 4235–4246.
Molinier-Frenkel, V., Gahery-Segard, H., Mehtali, M., Le Boulaire, C., Ribault, S., Boulanger, P., Tursz, T., Guillet, J. G., and Farace, F. (2000). Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74, 7678–7682.
Molinier-Frenkel, V., Lengagne, R., Gaden, F., Hong, S. S., Choppin, J., Gahery-Ségard, H., Boulanger, P., and Guillet, J. G. (2002). Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 76, 127–135.
Morral, N., O’Neal, W. K., Rice, K., Leland, M. M., Piedra, P. A., Aguilar-Córdova, E., Carey, K. D., Beaudet, A. L., and Langston, C. (2002). Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 13, 143–154.
Morral, N., Parks, R. J., Zhou, H., Langston, C., Schiedner, G., Quinones, J., Graham, F. L., Kochanek, S., and Beaudet, A. L. (1998). High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum. Gene Ther. 9, 2709–2716.
Muruve, D. A., Pétrilli, V., Zaiss, A. K., White, L. R., Clark, S. A., Ross, P. J., Parks, R. J., and Tschopp, J. (2008). The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107.
Nemerow, G. R., and Stewart, P. L. (1999). Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63, 725–734.
Nociari, M., Ocheretina, O., Schoggins, J. W., and Falck-Pedersen, E. (2007). Sensing infection by adenovirus: toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81, 4145–4157.
O’Neill, L. A., and Bowie, A. G. (2010). Sensing and signaling in antiviral innate immunity. Curr. Biol. 20, R328–R333.
O’Riordan, C. R., Lachapelle, A., Delgado, C., Parkes, V., Wadsworth, S. C., Smith, A. E., and Francis, G. E. (1999). PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 10, 1349–1358.
Osada, T., Yang, X. Y., Hartman, Z. C., Glass, O., Hodges, B. L., Niedzwiecki, D., Morse, M. A., Lyerly, H. K., Amalfitano, A., and Clay, T. M. (2009). Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther. 16, 673–682.
Otake, K., Ennist, D. L., Harrod, K., and Trapnell, B. C. (1998). Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum. Gene Ther. 9, 2207–2222.
Parker, A. L., Waddington, S. N., Buckley, S. M., Custers, J., Havenga, M. J., van Rooijen, N., Goudsmit, J., McVey, J. H., Nicklin, S. A., and Baker, A. H. (2009). Effect of neutralizing sera on factor x-mediated adenovirus serotype 5 gene transfer. J. Virol. 83, 479–483.
Parks, R. J., Chen, L., Anton, M., Sankar, U., Rudnicki, M. A., and Graham, F. L. (1996). A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U.S.A. 93, 13565–13570.
Peng, Y., Falck-Pedersen, E., and Elkon, K. B. (2001). Variation in adenovirus transgene expression between BALB/c and C57BL/6 mice is associated with differences in interleukin-12 and gamma interferon production and NK cell activation. J. Virol. 75, 4540–4550.
Philpott, N. J., Nociari, M., Elkon, K. B., and Falck-Pedersen, E. (2004). Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. U.S.A. 101, 6200–6205.
Pichla-Gollon, S. L., Lin, S. W., Hensley, S. E., Lasaro, M. O., Herkenhoff-Haut, L., Drinker, M., Tatsis, N., Gao, G. P., Wilson, J. M., Ertl, H. C., and Bergelson, J. M. (2009). Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J. Virol. 83, 5567–5573.
Priddy, F. H., Brown, D., Kublin, J., Monahan, K., Wright, D. P., Lalezari, J., Santiago, S., Marmor, M., Lally, M., Novak, R. M., Brown, S. J., Kulkarni, P., Dubey, S. A., Kierstead, L. S., Casimiro, D. R., Mogg, R., DiNubile, M. J., Shiver, J.W., Leavitt, R. Y., Robertson, M. N., Mehrotra, D. V., Quirk, E., and Merck V520-016 Study Group. (2008). Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46, 1769–1781.
Raper, S. E., Chirmule, N., Lee, F. S., Wivel, N. A., Bagg, A., Gao, G. P., Wilson, J. M., and Batshaw, M. L. (2003). Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158.
Raper, S. E., Haskal, Z. J., Ye, X., Pugh, C., Furth, E. E., Gao, G. P., and Wilson, J. M. (1998). Selective gene transfer into the liver of non-human primates with E1-deleted, E2A-defective, or E1-E4 deleted recombinant adenoviruses. Hum. Gene Ther. 9, 671–679.
Raper, S. E., Yudkoff, M., Chirmule, N., Gao, G. P., Nunes, F., Haskal, Z. J., Furth, E. E., Propert, K. J., Robinson, M. B., Magosin, S., Simoes, H., Speicher, L., Hughes, J., Tazelaar, J., Wivel, N. A., Wilson, J. M., and Batshaw, M. L. (2002). A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 13, 163–175.
Rathinam, V. A., Jiang, Z., Waggoner, S. N., Sharma, S., Cole, L. E., Waggoner, L., Vanaja, S. K., Monks, B. G., Ganesan, S., Latz, E., Hornung, V., Vogel, S. N., Szomolanyi-Tsuda, E., and Fitzgerald, K. A. (2010). The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402.
Reay, J., Kim, S. H., Lockhart, E., Kolls, J., and Robbins, P. D. (2009). Adenoviral-mediated, intratumor gene transfer of interleukin 23 induces a therapeutic antitumor response. Cancer Gene Ther. 16, 776–785.
Rhee, E. G., Blattman, J. N., Kasturi, S. P., Kelley, R. P., Kaufman, D. R., Lynch, D. M., La Porte, A., Simmons, N. L., Clark, S. L., Pulendran, B., Greenberg, P. D., and Barouch, D. H. (2011). Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J. Virol. 85, 315–323.
Ritter, T., Lehmann, M., and Volk, H. D. (2002). Improvements in gene therapy: averting the immune response to adenoviral vectors. BioDrugs 16, 3–10.
Roy, S., Shirley, P. S., McClelland, A., and Kaleko, M. (1998). Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 72, 6875–6879.
Russell, W. C. (2009). Adenoviruses: update on structure and function. J. Gen. Virol. 90(Pt 1), 1–20.
Ruzek, M. C., Kavanagh, B. F., Scaria, A., Richards, S. M., and Garman, R. D. (2002). Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol. Ther. 5, 115–124.
Schnell, M. A., Zhang, Y., Tazelaar, J., Gao, G. P., Yu, Q. C., Qian, R., Chen, S. J., Varnavski, A. N., LeClair, C., Raper, S. E., and Wilson, J. M. (2001). Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3(Pt 1), 708–722.
Schroers, R., Hildebrandt, Y., Hasenkamp, J., Glass, B., Lieber, A., Wulf, G., and Piesche, M. (2004). Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp. Hematol. 32, 536–546.
Seregin, S. S., Aldhamen, Y. A., Appledorn, D. M., Hartman, Z. C., Schuldt, N. J., Scott, J., Godbehere, S., Jiang, H., Frank, M. M., and Amalfitano, A. (2010a). Adenovirus capsid-display of the retro-oriented human complement inhibitor DAF reduces Ad vector-triggered immune responses in vitro and in vivo. Blood 116, 1669–1677.
Seregin, S. S., Hartman, Z. C., Appledorn, D. M., Godbehere, S., Jiang, H., Frank, M. M., and Amalfitano, A. (2010b). Novel adenovirus vectors “capsid-displaying” a human complement inhibitor. J. Innate Immun. 2, 353–359.
Seregin, S. S., Aldhamen, Y. A., Appledorn, D. M., Zehnder, J., Voss, T., Godbehere, S., and Amalfitano, A. (2011). Use of DAF-displaying adenovirus vectors reduces induction of transgene- and vector-specific adaptive immune responses in mice. Hum. Gene Ther.. [Epub ahead of print].
Seregin, S. S., and Amalfitano, A. (2010). Improving Adenovirus based gene transfer: strategies to accomplish immune evasion. Viruses 2, 2013–2036.
Seregin, S. S., and Amalfitano, A. (2009). Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin. Biol. Ther. 9, 1521–1531.
Seregin, S. S., Appledorn, D. M., McBride, A. J., Schuldt, N. J., Aldhamen, Y. A., Voss, T., Wei, J., Bujold, M., Nance, W., Godbehere, S., and Amalfitano, A. (2009). Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 17, 685–696.
Shayakhmetov, D. M., Di Paolo, N. C., and Mossman, K. L. (2010). Recognition of virus infection and innate host responses to viral gene therapy vectors. Mol. Ther. 18, 1422–1429.
Shayakhmetov, D. M., Gaggar, A., Ni, S., Li, Z. Y., and Lieber, A. (2005). Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79, 7478–7491.
Shifrin, A. L., Chirmule, N., Zhang, Y., and Raper, S. E. (2005). Macrophage ablation attenuates adenoviral vector-induced pancreatitis. Surgery 137, 545–551.
Siddiqui, F., Li, C. Y., Larue, S. M., Poulson, J. M., Avery, P. R., Pruitt, A. F., Zhang, X., Ullrich, R. L., Thrall, D. E., Dewhirst, M. W., and Hauck, M. L. (2007). A phase I trial of hyperthermia-induced interleukin-12 gene therapy in spontaneously arising feline soft tissue sarcomas. Mol. Cancer Ther. 6, 380–389.
Smith, J. G., Cassany, A., Gerace, L., Ralston, R., and Nemerow, G. R. (2008). Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport. J. Virol. 82, 6492–6500.
St George, J. A. (2003). Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 10, 1135–1141.
Sumida, S. M., Truitt, D. M., Kishko, M. G., Arthur, J. C., Jackson, S. S., Gorgone, D. A., Lifton, M. A., Koudstaal, W., Pau, M. G., Kostense, S., Havenga, M. J., Goudsmit, J., Letvin, N. L., and Barouch, D. H. (2004). Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 78, 2666–2673.
Sumida, S. M., Truitt, D. M., Lemckert, A. A., Vogels, R., Custers, J. H., Addo, M. M., Lockman, S., Peter, T., Peyerl, F. W., Kishko, M. G., Jackson, S. S., Gorgone, D. A., Lifton, M. A., Essex, M., Walker, B. D., Goudsmit, J., Havenga, M. J., and Barouch, D. H. (2005). Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174, 7179–7185.
Takaoka, A., Wang, Z., Choi, M. K., Yanai, H., Negishi, H., Ban, T., Lu, Y., Miyagishi, M., Kodama, T., Honda, K., Ohba, Y., and Taniguchi, T. (2007). DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505.
Takeuchi, O., and Akira, S. (2010). Pattern recognition receptors and inflammation. Cell 140, 805–820.
Tang, J., Lengagne, R., Gaden, F., Hong, S. S., Choppin, J., Gahery-Ségard, H., Boulanger, P., and Guillet, J. G. (2006). Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 350, 312–322.
Thorner, A. R., Lemckert, A. A., Goudsmit, J., Lynch, D. M., Ewald, B. A., Denholtz, M., Havenga, M. J., and Barouch, D. H. (2006). Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J. Virol. 80, 12009–12016.
Tibbles, L. A., Spurrell, J. C., Bowen, G. P., Liu, Q., Lam, M., Zaiss, A. K., Robbins, S. M., Hollenberg, M. D., Wickham, T. J., and Muruve, D. A. (2002). Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76, 1559–1568.
Tripathy, S. K., Black, H. B., Goldwasser, E., and Leiden, J. M. (1996). Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2, 545–550.
Tripathy, S. K., Goldwasser, E., Lu, M. M., Barr, E., and Leiden, J. M. (1994). Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc. Natl. Acad. Sci. U.S.A. 91, 11557–11561.
Urosevic, M., Fujii, K., Calmels, B., Laine, E., Kobert, N., Acres, B., and Dummer, R. (2007). Type I IFN innate immune response to adenovirus-mediated IFN-gamma gene transfer contributes to the regression of cutaneous lymphomas. J. Clin. Invest. 117, 2834–2846.
Vellinga, J., Van der Heijdt, S., and Hoeben, R. C. (2005). The adenovirus capsid: major progress in minor proteins. J. Gen. Virol. 86(Pt 6), 1581–1588.
Verdino, P., Witherden, D. A., Havran, W. L., and Wilson, I. A. (2010). The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science 329, 1210–1214.
Waehler, R., Russell, S. J., and Curiel, D. T. (2007). Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587.
Weaver, E. A., Nehete, P. N., Buchl, S. S., Senac, J. S., Palmer, D., Ng, P., Sastry, K. J., and Barry, M. A. (2009). Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 4, e5059.
Wickham, T. J., Mathias, P., Cheresh, D. A., and Nemerow, G. R. (1993). Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73, 309–319.
Wilderman, M. J., Kim, S., Gillespie, C. T., Sun, J., Kapoor, V., Vachani, A., Sterman, D. H., Kaiser, L. R., and Albelda, S. M. (2006). Blockade of TNF-alpha decreases both inflammation and efficacy of intrapulmonary Ad.IFNbeta immunotherapy in an orthotopic model of bronchogenic lung cancer. Mol. Ther. 13, 910–917.
Wilson, J. M. (2009). Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 96, 151–157.
Worgall, S., Krause, A., Rivara, M., Hee, K. K., Vintayen, E. V., Hackett, N. R., Roelvink, P. W., Bruder, J. T., Wickham, T. J., Kovesdi, I., and Crystal, R. G. (2005). Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Invest. 115, 1281–1289.
Wu, H., Dmitriev, I., Kashentseva, E., Seki, T., Wang, M., and Curiel, D. T. (2002). Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J. Virol. 76, 12775–12782.
Zhang, Y., Chirmule, N., Gao, G. P., Qian, R., Croyle, M., Joshi, B., Tazelaar, J., and Wilson, J. M. (2001). Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3(Pt 1), 697–707.
Zhu, J., Huang, X., and Yang, Y. (2007). Innate immune response to adenoviral vectors is mediated by both toll-like receptor-dependent and -independent pathways. J. Virol. 81, 3170–3180.
Zhu, J., Huang, X., and Yang, Y. (2008). A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. 16, 1300–1307.
Zhu, J., Huang, X., and Yang, Y. (2010). NKG2D is required for NK cell activation and function in response to E1-deleted adenovirus. J. Immunol. 185, 7480–7486.
Ziegler, R. J., Li, C., Cherry, M., Zhu, Y., Hempel, D., van Rooijen, N., Ioannou, Y. A., Desnick, R. J., Goldberg, M. A., Yew, N. S., and Cheng, S. H. (2002). Correction of the nonlinear dose response improves the viability of adenoviral vectors for gene therapy of Fabry disease. Hum. Gene Ther. 13, 935–945.
Keywords: adenovirus, innate immunity, adaptive immunity, cellular responses, humoral responses, vaccines
Citation: Aldhamen YA, Seregin SS and Amalfitano A (2011) Immune recognition of gene transfer vectors: focus on adenovirus as a paradigm. Front. Immun. 2:40. doi: 10.3389/fimmu.2011.00040
Received: 22 June 2011; Paper pending published: 14 July 2011;
Accepted: 18 August 2011; Published online: 06 September 2011.
Edited by:
Roland W. Herzog, University of Florida, USAReviewed by:
Dirk Homann, University of Colorado at Denver, USAPhyllis Flomenberg, Thomas Jefferson University, USA
Copyright: © 2011 Aldhamen, Seregin and Amalfitano. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Andrea Amalfitano, Michigan State University, 4194 Biomedical and Physical Sciences Building, East Lansing, MI 48823, USA. e-mail:YW1hbGZpdDFAbXN1LmVkdQ==
 Yasser Ali Aldhamen1
Yasser Ali Aldhamen1