- 1Bristol Heart Institute, School of Clinical Sciences, University of Bristol, Bristol, UK
- 2Department of Microbiology and Molecular Genetics, University of Pittsburgh, Pittsburgh, PA, USA
Introduction
Macrophages are involved in inflammation from induction to resolution. Polarization of macrophages along the M1 (classical) or M2 (alternative) axis occurs during inflammation and can be at least partly categorized by the route of arginine metabolism within the macrophage, balancing the activities of the arginase and nitric oxide synthase (NOS) enzyme families (1, 2). Arginase activity is associated with tissue repair responses (via ornithine production and pro-proliferative effects). In contrast, NOS2 generates nitric oxide (NO) species with anti-proliferative effects that is necessary for protection against pathogens and aberrant cells (2, 3). Other NOS enzymes produce NO that acts in the regulation of smooth muscle tone and other cellular processes (4). Macrophages preferentially expressing the arginase or NOS2 pathways enzymes also influence T-cell activation, proliferation, signaling, and apoptosis in different ways (1).
While arginase and NOS enzymes can be used to ascertain the pathway of macrophage activation in rodents, there has been debate as to whether they are present in macrophages from humans and other mammals. The arginase and NOS enzymes are extensively conserved, and the NOS forms found in mammals are similar to those in cnidarians, mollusks, and other chordates (5, 6). These arginine-metabolizing enzymes are present in some human leukocytes, and there is evidence that they are also present in macrophages from other vertebrates, including chickens, rabbits, cows, and primates (7–12). However, comparisons of tissue macrophages of different species are lacking, which limits our understanding (13). Many studies in humans have principally focused on blood monocytes, leading some researchers to question the suitability of rodents as model of macrophage activation, as there is not always a direct correlation with human cells. Was Robert Koch correct when he said “Gentlemen, never forget that mice are not humans,” or can the differing results between species be explained, in part, by differences in the types of monocyte or macrophage studied? Our purpose here is to examine this question.
Arginine Metabolism in Mammalian Cells
Many mammalian cells, including neutrophils, granulocytes, erythrocytes, hepatocytes, cardiac myocytes, dendritic cells, myeloid-derived suppressor cells, foam cells, natural killer cells, endothelial cells, and smooth muscle cells, have arginase (12, 14–16) or NOS activity (8, 17–19), albeit to different degrees. Macrophages are the primary circulating cells that can express either of these enzymes, depending on the inflammatory circumstance. Experiments that detect NO, ornithine, or urea production (via NOS2 or arginase) have most often been performed on rodent macrophages. Macrophages from some mouse strains (e.g., the M1-biased C57BL/6 strain) can be stimulated by lipopolysaccharide (LPS) to produce considerable quantities of NO. Macrophages from others strains (e.g., M2-skewed BALB/c mice) produce much less NO (20) and produce more ornithine instead. Some researchers did not detect any NO production in macrophages from humans, pigs, and rabbits (8, 11, 14, 21–23), but others (including ourselves) have observed NOS or arginase activity in macrophages from rabbits, humans, and other primates (4, 7, 10, 12, 17, 24–26).
Why is There Controversy?
One main difference between the studies from laboratories is that some use monocyte-derived macrophages (MDM), while others study tissue macrophages directly. A number of groups have detected NOS or arginase activity in human monocytes or macrophages (3, 27–29); but others have not. Why is this so? Part of the explanation lies in the fact that in vitro-derived macrophages can generate different responses from macrophages obtained in vivo as discussed below (and shown in Table 1). Another explanation is that many groups use the identification of enzyme protein rather than detection of enzyme activity as evidence of enzyme expression. Failure to detect the presence of a protein is not definitive evidence for absence of expression (especially when considering potentially different detection thresholds of antibodies or the high Vmax of arginase, i.e., very little enzyme is required for ornithine production).
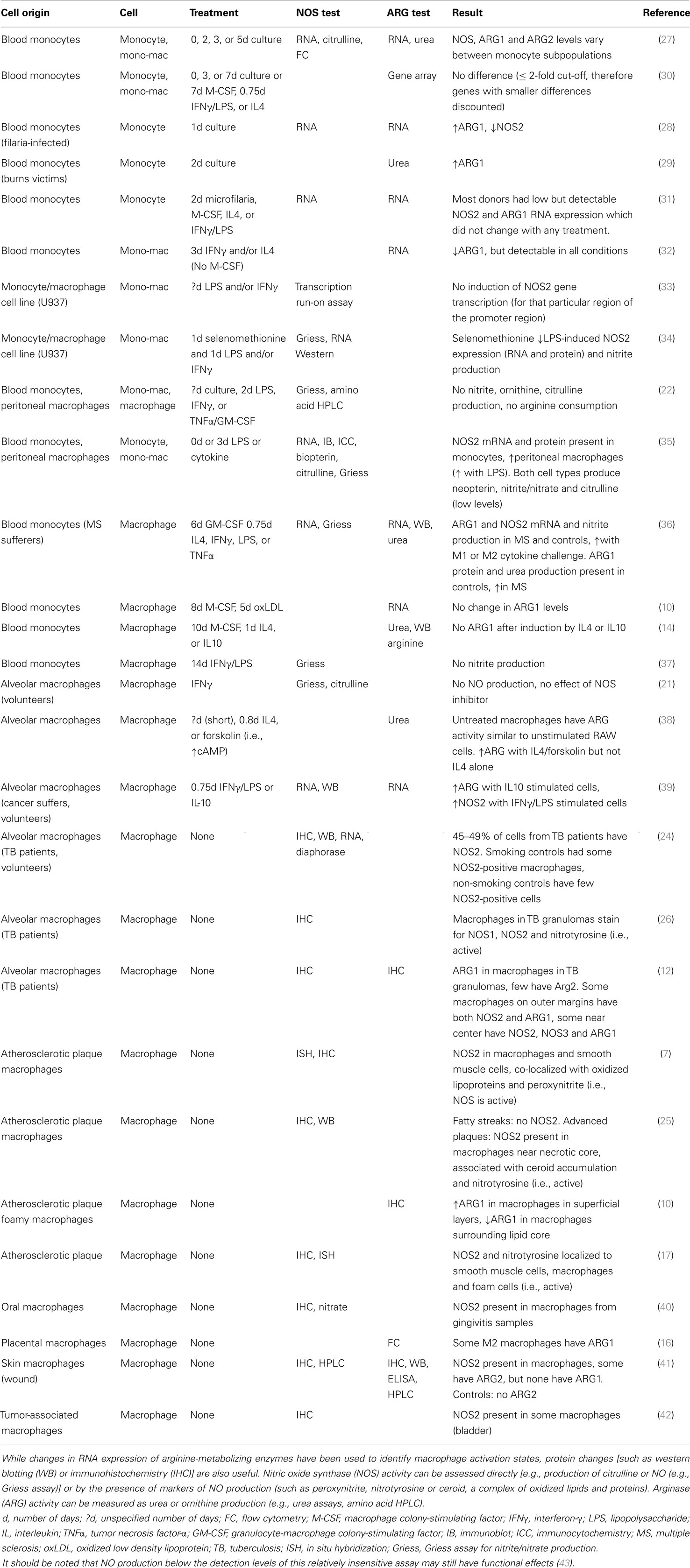
Table 1. The presence of arginine-metabolizing enzymes in human monocytes and macrophages varies with cell source, treatment and health status/stress level of the individual.
Macrophages Produced in vitro
Macrophages have been produced in vitro in a number of ways. Cells from bone marrow have been isolated and “differentiated” in culture medium containing high levels of cytokines (such as colony stimulating factors, CSFs) to produce bone marrow-derived macrophages (BMDM) (13, 23, 44–46). Macrophages have also been produced by isolating and culturing monocytes from blood, to produce MDM (10, 13, 22, 30, 37, 47, 48). Production of these in vitro-derived macrophages is cheap, simple, and reproducible, but they may not be a full representative of tissue macrophages, as the preparation and culture procedures may not be sufficient to induce cell activation (4). The differences between tissue macrophages and in vitro-derived macrophages are at least partly dependent on cell source, time in culture, and the degree of manipulation in culture. Each research group will use different types and sources of culture media and sera, which vary greatly in the concentrations of factors that influence NOS2 or arginase expression, such as transforming growth factor β (TGFβ) (4, 20, 49). Another confounding issue is that circulating monocytes and tissue macrophages arise from different stem cell populations (50), although some macrophages found at sites of infection or inflammation may derive from infiltrating monocytes (51). Together, these factors may account for many of the differences observed in NO and urea production in these macrophages (8, 20).
Monocyte-derived macrophages or BMDM from different strains of mice can differ in their response to interferon-γ (IFNγ), LPS, and tumor necrosis factor-α (TNFα) (4, 8), and differences in the rodent background can result in differences in macrophage gene expression (13, 20, 49). Human in vitro-derived macrophages also show variability in their responses to LPS (4, 22, 46). It may be that the same stimulus is able to generate quite different responses in genetically diverse individuals, as it does between mouse strains (38, 49, 52). In general, human macrophages are not as responsive to LPS as mouse macrophages, possibly because of the lower environmental exposure of humans to LPS. It is also possible that human monocytes may be more effectively stimulated to become M1-activated macrophages by cytokines other than IFNγ and LPS/TNFα (e.g., IFNα) (4, 18, 43). Human macrophages take longer time to respond to the stimulatory factors in vitro than mouse macrophages, and some experiments using human MDM may have ended before a response was detected (48). There are other indications that the timing and length of the exposure of the cells to varying cytokines in vitro are important. For example, when M1-polarizing cytokines were removed from the culture medium, NOS2 levels in mouse BMDM were reduced and NO production (measured as nitrite) ceased (45). In addition, whichever arginase or NOS enzyme was induced earliest, the alternative enzyme decreased in expression and activity, unless arginine was present in excess (15, 45, 53). Macrophages require the local environment to continuously give appropriate activation cues. Changes in environmental cues can stimulate macrophage populations in vitro to express varying percentages of M1 or M2 dominant activity (54). When activation cues are reduced or removed, macrophages may become deactivated (e.g., M2c) or indeterminate (e.g., have features of M1 and M2).
Macrophages Obtained in vivo
Macrophages can be identified in whole tissues and organs or isolated in large numbers from in vivo sources such as the peritoneum or granulomas, and either examined immediately or used ex vivo. Macrophages obtained in vivo or made from monocytes can respond differently to the same stimulus (35, 47). In one study, monocytes and tissue macrophages were obtained from patients with an inflammatory disease (either rheumatoid or psoriatic arthritis). Compared with tissue macrophages, the MDM had a blunted response to the M2 cytokines interleukin-4 (IL-4) and IL-13, at least partly due to a reduction in some of the receptor elements for these cytokines (47). These results suggest that the response of the macrophages to M2 cytokines may be source specific, but it is possible that these cytokines alone were not sufficient to fully stimulate the MDM (38). Several lines of evidence suggest that macrophages in vivo express functional NOS2. Blood monocytes and peritoneal macrophages obtained from women during laparoscopic procedures contained NOS2 mRNA and protein. The macrophages had higher NOS levels than the monocytes, and this could be increased by treatment with LPS. The monocytes and macrophages also produced neopterin, nitrite/nitrate, and citrulline (suggesting that the enzyme was active). Although the production of NO from these macrophages was low, it would probably have been sufficient to cause functional changes (35).
Macrophages can also be obtained from alveolar aspirates, skin, and the placenta (10, 16, 21, 38, 39, 55, 56). For example, sponges placed subcutaneously into mice, rats, or rabbits attract large numbers of macrophages. The sponges can be removed from the animal and the macrophages were isolated and purified (10, 55, 56). It is a little more difficult to obtain and purify macrophages from other tissues, such as atherosclerotic vessels (44), but intact biopsy, surgical, or cadaveric specimens can also be investigated. It should be noted that resident macrophages from different tissues observed at different times (and different health states) may not necessarily have identical properties (51, 57).
In order to perform their full range of functions, macrophage populations exhibit “plasticity” of phenotype (52, 58), regardless of whether they are found in vivo or derived in vitro. As macrophages adapt or change their functions, they can simultaneously express markers of M1 and M2 activation, including NOS2 and arginase-1 (12, 59, 60). For example, tissue macrophages (and MDM) from Mycobacterium tuberculosis-infected cynomolgus macaques have been observed to co-express functional NOS and arginase enzymes (12). We suggest that macrophages display a spectrum of activation phenotypes, and it is the relative (and not absolute) proportion of M1 or M2 markers that we can use as a ‘handle’ to determine the type of activation state.
Effect of Disease and Trauma on Macrophage Activation
Blood monocytes from healthy volunteers do not usually need to produce NOS or arginase, so it is not surprising that many studies have not detected NOS or arginase in these cells (10, 14, 21, 22, 29, 30, 37). However, studies performed on tissue or cells from people undergoing stress, trauma [e.g., burns (29)], pregnancy (16), or disease {such as infection [e.g., tuberculosis (12, 24, 26) or filarial infection (28)], atherosclerosis (7, 10, 17, 25), autoimmune diseases (27, 36) and cancer (42, 61)} demonstrate that human macrophages (and sometimes monocytes) can produce active forms of the arginine-metabolizing enzymes (Table 1).
Trauma results in a pattern of gene expression in macrophages that is consistent with a wound-healing response, with an initial increase in NOS followed by decreased NOS production and activity, elevated IL-4, IL-10, and TGFβ levels, and increased arginase expression and activity, resulting in decreased plasma arginine levels (28, 29, 62).
Disease, however, causes different patterns of gene expression. For example, monocytes from multiple sclerosis sufferers not only have higher levels of arginase-1 and increased urea production, but also have increased NOS2 mRNA and nitrite production (particularly when stimulated by M1 cytokines or LPS) (36). Macrophages from patients with inflammatory diseases, such as tuberculosis, malaria, or rheumatoid arthritis, have increased levels of NOS2 mRNA and active protein (4, 8, 24, 26, 63), which may contribute to elevated plasma NO levels (64). Atherosclerosis is another inflammatory disease with a considerable macrophage contribution, with oxidized low-density lipoproteins taken up by macrophages during their transformation into foam cells. Plaque macrophages express NOS2 RNA and protein, as well as markers of NOS activity (including the presence of nitrotyrosine or ceroid) (4, 7, 17, 25). Plaque macrophages and foam cells express arginase-1 (10), and macrophages laser-dissected from plaque have upregulated levels of arginase-2 and NOS2 (65). Macrophages present in some neoplastic diseases also produce active NOS2 (4, 42, 66). Reducing the local levels of arginine has been proposed as a treatment for these diseases, by reducing inflammation-triggered immune dysfunction, tumor escape, fibrosis, and immunosuppression (61). Possible pharmacological interventions include treatment with arginine degrading enzymes, NOS competitors and inhibitors, asymmetric dimethylarginine, NO-releasing aspirins, cyclooxygenase, and phosphodiesterase or arginase inhibitors (8, 61). These studies suggest that an inflammatory environment is necessary in order to observe NOS or arginase in human monocytes and macrophages. The in vitro experiments that do not demonstrate arginase or NOS expression may simply be lacking the additional cues needed for expression rather than demonstrating an inability to actually express these factors.
Conclusion
The modulation of macrophages to express NOS or arginase has clear benefits for treating disease in humans (and other species). To do this, one needs to either determine suitable signals to stimulate these pathways or obtain a sufficient number of human macrophages (e.g., by tissue culture) that function like tissue macrophages.
Because macrophages from different inbred strains of mice vary greatly in their macrophage NOS and arginase balance, one would predict similar variability to be found in humans as well. In addition, the source of the macrophages being studied has been found to be important. Several groups have reported that human monocytes from healthy volunteers that have been differentiated or manipulated in vitro using current protocols tend not to have detectable levels of arginase and NOS enzymes, whereas MDM from diseased or stressed individuals or tissue macrophages obtained from normal, diseased, or stressed individuals do express NOS and/or arginase. Together these observations suggest that the current system of differentiating macrophages from human peripheral monocytes in vitro needs further refinement before it can be considered to be an accurate model of human macrophage behavior in vivo (63). In turn, we need to understand the differences and similarities between the different species and the cells being studied to develop experimental models that will answer some of the outstanding questions regarding macrophage M1/M2 or other activation states: What regulates macrophage activation in tissues? What mechanisms regulate macrophage plasticity and stability? How does plasticity of phenotype affect tissue macrophages? What are the full in vivo ramifications of the M1/M2 paradigm?
Further work is important to be sure that our observations of the human system in vitro are real, and not due to our cell source, measurements, or manipulations. We suggest that macrophages obtained from mice remain useful for investigating aspects of these questions in humans/human macrophages. So, although mice are not men (as Robert Koch observed), we agree with Rudolf Virchow that “Between animal and human medicine there is no dividing line – nor should there be. The object is different but the experience obtained constitutes the basis of all medicine” [Rudolph Virchow, 1821–1902].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Anita C. Thomas is supported by funding from the British Heart Foundation. Joshua T. Mattila is supported in part by NIH RO1A103785 and Bill and Melinda Gates Foundation grants to JoAnne Flynn (University of Pittsburgh).
References
1. Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest (2006) 116:2777–90. doi: 10.1172/JCI28828
2. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest (2007) 117:175–84. doi:10.1172/JCI29881
3. Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol (2012) 32:463–88. doi:10.1615/CritRevImmunol.v32.i6.10
4. Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med (1998) 4:557–91.
5. Perozich J, Hempel J, Morris J. Roles of conserved residues in the arginase family. Biochim Biophys Acta (1998) 1382:23–37.
6. Dzik JM. The ancestry and cumulative evolution of immune reactions. Acta Biochim Pol (2010) 57:443–66.
7. Luoma JS, Stralin P, Marklund SL, Hiltunen TP, Sarkioja T, Yla-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler Thromb Vasc Biol (1998) 18:157–67. doi:10.1161/01.ATV.18.2.157
8. Bogdan C. Nitric oxide and the immune response. Nat Immunol (2001) 2:907–16. doi:10.1038/ni1001-907
9. Djeraba A, Musset E, van RN, Quere P. Resistance and susceptibility to Marek’s disease: nitric oxide synthase/arginase activity balance. Vet Microbiol (2002) 86:229–44. doi:10.1016/S0378-1135(02)00010-X
10. Thomas AC, Sala-Newby GB, Ismail Y, Johnson JL, Pasterkamp G, Newby AC. Genomics of foam cells and nonfoamy macrophages from rabbits identifies arginase-I as a differential regulator of nitric oxide production. Arterioscler Thromb Vasc Biol (2007) 27:571–7. doi:10.1161/01.ATV.0000256470.23842.94
11. Zelnickova P, Matiasovic J, Pavlova B, Kudlackova H, Kovaru F, Faldyna M. Quantitative nitric oxide production by rat, bovine and porcine macrophages. Nitric Oxide (2008) 19:36–41. doi:10.1016/j.niox.2008.04.001
12. Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol (2013) 191:773–84. doi:10.4049/jimmunol.1300113
13. Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol (2011) 89:557–63. doi:10.1189/jlb.0710409
14. Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood (2005) 105:2549–56. doi:10.1182/blood-2004-07-2521
16. Kropf P, Baud D, Marshall SE, Munder M, Mosley A, Fuentes JM, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol (2007) 37:935–45. doi:10.1002/eji.200636542
17. Buttery LD, Springall DR, Chester AH, Evans TJ, Standfield EN, Parums DV, et al. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab Invest (1996) 75:77–85.
18. Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol (2005) 5:641–54. doi:10.1038/nri1668
19. Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med (2011) 9:90. doi:10.1186/1479-5876-9-90
20. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164:6166–73. doi:10.4049/jimmunol.164.12.6166
21. Cameron ML, Granger DL, Weinberg JB, Kozumbo WJ, Koren HS. Human alveolar and peritoneal macrophages mediate fungistasis independently of l-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis (1990) 142:1313–9. doi:10.1164/ajrccm/142.6_Pt_1.1313
22. Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis (1993) 167:1358–63. doi:10.1093/infdis/167.6.1358
23. Kapetanovic R, Fairbairn L, Beraldi D, Sester DP, Archibald AL, Tuggle CK, et al. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J Immunol (2012) 188:3382–94. doi:10.4049/jimmunol.1102649
24. Nicholson S, Bonecini-Almeida MG, Lapa e Silva JL, Nathan C, Xie QW, Mumford R, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med (1996) 183:2293–302. doi:10.1084/jem.183.5.2293
25. Cromheeke KM, Kockx MM, De Meyer GRY, Bosmans JM, Bult H, Beelaerts WJF, et al. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc Res (1999) 43:744–54. doi:10.1016/S0008-6363(99)00148-0
26. Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med (2002) 166:178–86. doi:10.1164/rccm.2201023
27. Rouzaut A, Subira ML, de Miguel C, Domingo-de-Miguel E, Gonzalez A, Santiago E, et al. Co-expression of inducible nitric oxide synthase and arginases in different human monocyte subsets. Apoptosis regulated by endogenous NO. Biochim Biophys Acta (1999) 1451:319–33. doi:10.1016/S0167-4889(99)00106-8
28. Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis (2009) 199:1827–37. doi:10.1086/599090
29. Kobayashi M, Jeschke MG, Shigematsu K, Asai A, Yoshida S, Herndon DN, et al. M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol (2010) 185:7174–9. doi:10.4049/jimmunol.0903935
30. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol (2006) 177:7303–11. doi:10.4049/jimmunol.177.10.7303
31. Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun (2011) 79:3957–65. doi:10.1128/IAI.05191-11
32. Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol (2005) 77:321–7. doi:10.1189/jlb.0304212
33. Zhang X, Laubach VE, Alley EW, Edwards KA, Sherman PA, Russell SW, et al. Transcriptional basis for hyporesponsiveness of the human inducible nitric oxide synthase gene to lipopolysaccharide/interferon-gamma. J Leukoc Biol (1996) 59:575–85.
34. Shen Y, Yang S, Shi Z, Lin T, Zhu H, Bi F, et al. SeMet mediates anti-inflammation in LPS-induced U937 cells targeting NF-kB signaling pathway. Inflammation (2014). doi:10.1007/s10753-014-9984-0
35. Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood (1995) 86:1184–95.
36. Christophi GP, Panos M, Hudson CA, Christophi RL, Gruber RC, Mersich AT, et al. Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Invest (2009) 89:742–59. doi:10.1038/labinvest.2009.32
37. Padgett EL, Pruett SB. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun (1992) 186:775–81. doi:10.1016/0006-291X(92)90813-Z
38. Erdely A, Kepka-Lenhart D, Clark M, Zeidler-Erdely P, Poljakovic M, Calhoun WJ, et al. Inhibition of phosphodiesterase 4 amplifies cytokine-dependent induction of arginase in macrophages. Am J Physiol Lung Cell Mol Physiol (2006) 290:L534–9. doi:10.1152/ajplung.00326.2005
39. Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of l-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol (2010) 136:35–45. doi:10.1007/s00432-009-0634-0
40. Ozer L, Elgun S, Ozdemir B, Pervane B, Ozmeric N. Arginine-nitric oxide-polyamine metabolism in periodontal disease. J Periodontol (2011) 82:320–8. doi:10.1902/jop.2010.100199
41. Debats IB, Wolfs TG, Gotoh T, Cleutjens JP, Peutz-Kootstra CJ, van der Hulst RR. Role of arginine in superficial wound healing in man. Nitric Oxide (2009) 21:175–83. doi:10.1016/j.niox.2009.07.006
42. Suriano F, Santini D, Perrone G, Amato M, Vincenzi B, Tonini G, et al. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res (2013) 32:87. doi:10.1186/1756-9966-32-87
43. Bogdan C. Response to ‘Species differences in macrophage NO production are important’. Nat Immunol (2002) 3:102. doi:10.1038/ni0202-102a
44. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med (2006) 203:1273–82. doi:10.1084/jem.20052205
45. Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, et al. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol (2006) 176:5918–24. doi:10.4049/jimmunol.176.10.5918
46. Shalhoub J, Falck-Hansen MA, Davies AH, Monaco C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm (2011) 8:9. doi:10.1186/1476-9255-8-9
47. Hart PH, Bonder CS, Balogh J, Dickensheets HL, Donnelly RP, Finlay-Jones JJ. Differential responses of human monocytes and macrophages to IL-4 and IL-13. J Leukoc Biol (1999) 66:575–8.
48. Geelhaar-Karsch A, Schinnerling K, Conrad K, Friebel J, Allers K, Schneider T, et al. Evaluation of arginine metabolism for the analysis of M1/M2 macrophage activation in human clinical specimens. Inflamm Res (2013) 62:865–9. doi:10.1007/s00011-013-0642-z
49. Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol (2001) 21:399–425. doi:10.1615/CritRevImmunol.v21.i5.10
50. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity (2014) 41:21–35. doi:10.1016/j.immuni.2014.06.013
51. Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol (2014) 14:81–93. doi:10.1038/nri3600
52. Ravasi T, Wells CA, Hume DA. Systems biology of transcription control in macrophages. Bioessays (2007) 29:1215–26. doi:10.1002/bies.20683
53. El Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol (2003) 171:4561–8. doi:10.4049/jimmunol.171.9.4561
54. Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One (2010) 5:e8852. doi:10.1371/journal.pone.0008852
55. Albina JE, Mills CD, Barbul A, Thirkill CE, Henry WL, Mastrofrancesco B, et al. Arginine metabolism in wounds. Am J Physiol (1988) 254: E459–67.
56. Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol (2010) 87:59–67. doi:10.1189/jlb.0409236
57. Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol (2009) 20(5):370–8. doi:10.1097/MOL.0b013e3283309848
58. Hume DA. Plenary perspective: the complexity of constitutive and inducible gene expression in mononuclear phagocytes. J Leukoc Biol (2012) 92:433–44. doi:10.1189/jlb.0312166
59. Poljakovic M, Porter DW, Millecchia L, Kepka-Lenhart D, Beighley C, Wolfarth MG, et al. Cell- and isoform-specific increases in arginase expression in acute silica-induced pulmonary inflammation. J Toxicol Environ Health A (2007) 70:118–27. doi:10.1080/15287390600755075
60. Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun (2011) 79:1915–26. doi:10.1128/IAI.01270-10
61. Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol (2009) 158:638–51. doi:10.1111/j.1476-5381.2009.00291.x
62. Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME, Rockich AK, et al. Arginase I expression and activity in human mononuclear cells after injury. Ann Surg (2001) 233:393–9. doi:10.1097/00000658-200103000-00014
63. Nathan C. Role of iNOS in human host defense. Science (2006) 312:1874–5. doi:10.1126/science.312.5782.1874b
64. Boutlis CS, Tjitra E, Maniboey H, Misukonis MA, Saunders JR, Suprianto S, et al. Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant papuan adults. Infect Immun (2003) 71:3682–9. doi:10.1128/IAI.71.7.3682-3689.2003
65. Tuomisto TT, Korkeela A, Rutanen J, Viita H, Brasen JH, Riekkinen MS, et al. Gene expression in macrophage-rich inflammatory cell infiltrates in human atherosclerotic lesions as studied by laser microdissection and DNA array: overexpression of HMG-CoA reductase, colony stimulating factor receptors, CD11A/CD18 integrins, and interleukin receptors. Arterioscler Thromb Vasc Biol (2003) 23:2235–40. doi:10.1161/01.ATV.0000102551.91154.96
Keywords: arginase, human macrophage, macrophage polarization, nitric oxide synthase, macrophage activation
Citation: Thomas AC and Mattila JT (2014) “Of mice and men”: arginine metabolism in macrophages. Front. Immunol. 5:479. doi: 10.3389/fimmu.2014.00479
Received: 01 August 2014; Accepted: 19 September 2014;
Published online: 07 October 2014.
Edited by:
Charles Dudley Mills, BioMedical Consultants, USAReviewed by:
Charles Dudley Mills, BioMedical Consultants, USASidney Morris, University of Pittsburgh, USA
Copyright: © 2014 Thomas and Mattila. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:YS50aG9tYXNAYnJpc3RvbC5hYy51aw==
 Anita C. Thomas
Anita C. Thomas Joshua T. Mattila
Joshua T. Mattila