- 1Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Rio de Janeiro, Brazil
- 2Orygen Biotecnologia, São Paulo, São Paulo, Brazil
Data herein reported and discussed refer to vaccination with the recombinant fatty acid binding protein (FABP) family member of the schistosomes, called Sm14. This antigen was discovered and developed under a Brazilian platform led by the Oswaldo Cruz Foundation, from the Health Ministry in Brazil, and was assessed for safety and immunogenicity in healthy volunteers. This paper reviews past and recent outcomes of developmental phases of the Sm14-based anti schistosomiasis vaccine addressed to, ultimately, impact transmission of the second most prevalent parasitic endemic disease worldwide.
Introduction
Schistosomiasis is considered by the World Health Organization to be 1 of 17 neglected tropical diseases (1). With 800 million people at risk, and 200 million infected in 74 countries, schistosomiasis is the second most prevalent human, parasitic disease in the world after malaria. Some 7.1 million people are infected with Schistosoma mansoni in the Americas, of whom 95% are in Brazil (1). It is estimated that 25 million people are exposed to the risk of schistosomiasis in the Americas (2). The WHO estimated that the morbidity of schistosomiasis resulted in the annual loss of 1.7 million disability-adjusted life years (DALYs), while mortality was estimated to be 41,000 deaths per year (3). Control measures aim to reduce morbidity through treatment with praziquantel, improved sewerage, access to potable water, and snail control (1–4). Vaccination, even if not 100% effective, could contribute to the long-term reduction of egg-excretion from the host, and thus controls transmission. An effective vaccine would also contribute to a positive trade-off regarding the aggressive inflammatory response that has been observed following interrupted chemotherapy in children living in high-transmission areas (5–7). The underlying reason for this “rebound morbidity” is unclear, but is thought to be due to an interruption of the natural down-regulating process of specific immunological mechanisms typical for this disease. This outcome results from the typically high-level re-infection after chemotherapy and is a direct result of chemotherapy being primarily directed against morbidity and less against transmission of the disease. This effect needs to be taken seriously, as the observed aggravated gross symptoms reflect long-term pathology, which is difficult to remedy (8).
There have been initiatives in several countries to develop a vaccine against schistosomiasis. The Brazilian Sm14-based anti schistosomiasis vaccine is the sole technology that emerged from an endemic country, and that is at an advanced stage of development toward a safe highly innovative product.
In this review, we present the evolution of the Sm14 anti-schistosome vaccine from the initial gene cloning to the results of the recently completed phase I clinical trials.
Demonstration That Adult Schistosome Saline Extracts Contain Protective Antigens
The experimental background for the development of anti-schistosome vaccines lies with the use of animal models of infection that showed that an initial parasite infection resulted in partial immunity against re-infection (9–11). Levels of resistance achievable in laboratory models ranged from 60% in mice up to 90% in rabbits. In an attempt to establish whether vaccine development was feasible, extracts of adult parasites were utilized to immunize experimental hosts to investigate whether such antigen preparations also possessed the capacity to protect against infections. In two independent lines of investigation, it was demonstrated that simple saline extracts of live adult worms, which are enriched in surface associated molecules, were indeed capable of inducing protection comparable to that achieved with live infection (12–14).
Adult Worm Antigen Gene Cloning
Once gene-cloning technology became incorporated into the vaccine research field, the genes for a number of major antigens released by adult schistosomes briefly cultured in saline were cloned and sequenced (15–17). One of these proved to be a fatty acid binding protein (FABP) termed Sm14, which subsequently became the basis of the experimental vaccine herein discussed. The protein derived from the cloned gene exhibited significant homologies with a family of related polypeptides, which bind hydrophobic ligands, and purified recombinant protein exhibited an affinity to fatty acids. Antibodies to the purified protein were shown to bind to tubercles, which are structures located on the dorsal surface of adult male schistosome and known to contain lipids (17). In addition, the protein was localized to the muscle layers as well as in the body of the parasite. As the schistosome cannot synthesize fatty acids de novo, and is dependent on the uptake of lipids from serum, the available data supported a role for Sm14 in the transport of fatty acids. Following transfer of the Sm14 gene to a high level expression vector, subsequent experiments demonstrated that the recombinant rSm14 was able to protect outbred Swiss mice by up to 66% and New Zealand White rabbits by up to 89% against challenge with S. mansoni cercariae. It was thus demonstrated that rSm14 could provide the basis of an anti-schistosome vaccine (18).
Vaccine Development
The Sm14 project has been mostly funded by public funds at Oswaldo Cruz Institute/FIOCRUZ, belonging to the Brazilian Ministry of Health. Since the early stage of the development, there was a critical concern to reduce the cost of production at its lowest level and to ensure the use of non-proprietary components in the production process in order to get a low final price for the vaccine. The strategy adopted to reduce the cost of production of the human Sm14 vaccine involved the following steps:
i. Scaling up steps of production process: this started in 2003 with the primary target being the investigation of Sm14 stability. New constructs were developed with highly stable novel molecular design (19), beginning thus to pave the way for the ultimate goal of achieving large-scale production of highly purified vaccine at both high yield and low cost.
ii. Replacement of more expensive reagents seeking a royalty and proprietary free route of components: as presented in Figures 1 and 2, two substitutions were successfully achieved, which were the replacement of IPTG for lactose or salt (NaCl), for the steps of induction of protein expression in culture. Expression vectors were also constructed to avoid commercial ones. Furthermore, the Sm14 vaccine is based only on two highly purified and well-characterized components: the protein itself and the glucopyranosyl lipid adjuvant stable emulsion (GLA-SE) adjuvant produced and supplied by the Infectious Disease Research Institute (IDRI, Seattle). Our partner, IDRI, is a non-profit organization fully committed to the support of the development of technologies for the control of the so-called neglected diseases.
iii. Production process of the protein in large-scale is presently in place at Ourofino, the partner for the veterinary vaccine. There is currently pilot scale production of the vaccine in 5 L fermentor, which is being scaled-up to 50 and 100 L fermentors, with final cost already estimated to be approximately US $1,00) for one 50 μg dose.
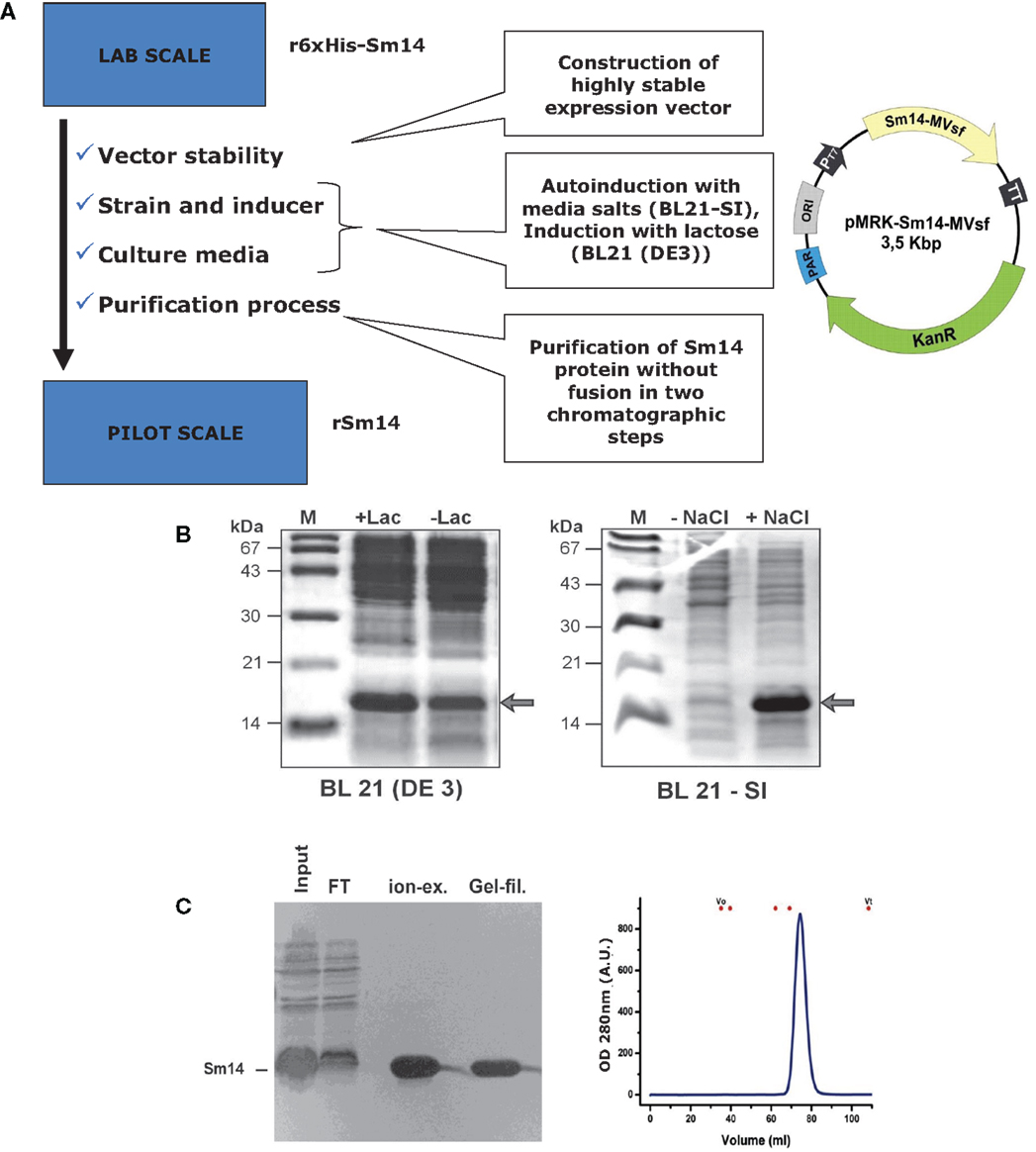
Figure 1. Expression of Sm14 in Escherichia coli. (A) Steps taken to improve the production process of Sm14 in E. coli. (B) Induction/expression systems with lactose (left) and salt (right). (C) Final purification steps involving ion exchange chromatography or gel filtration.
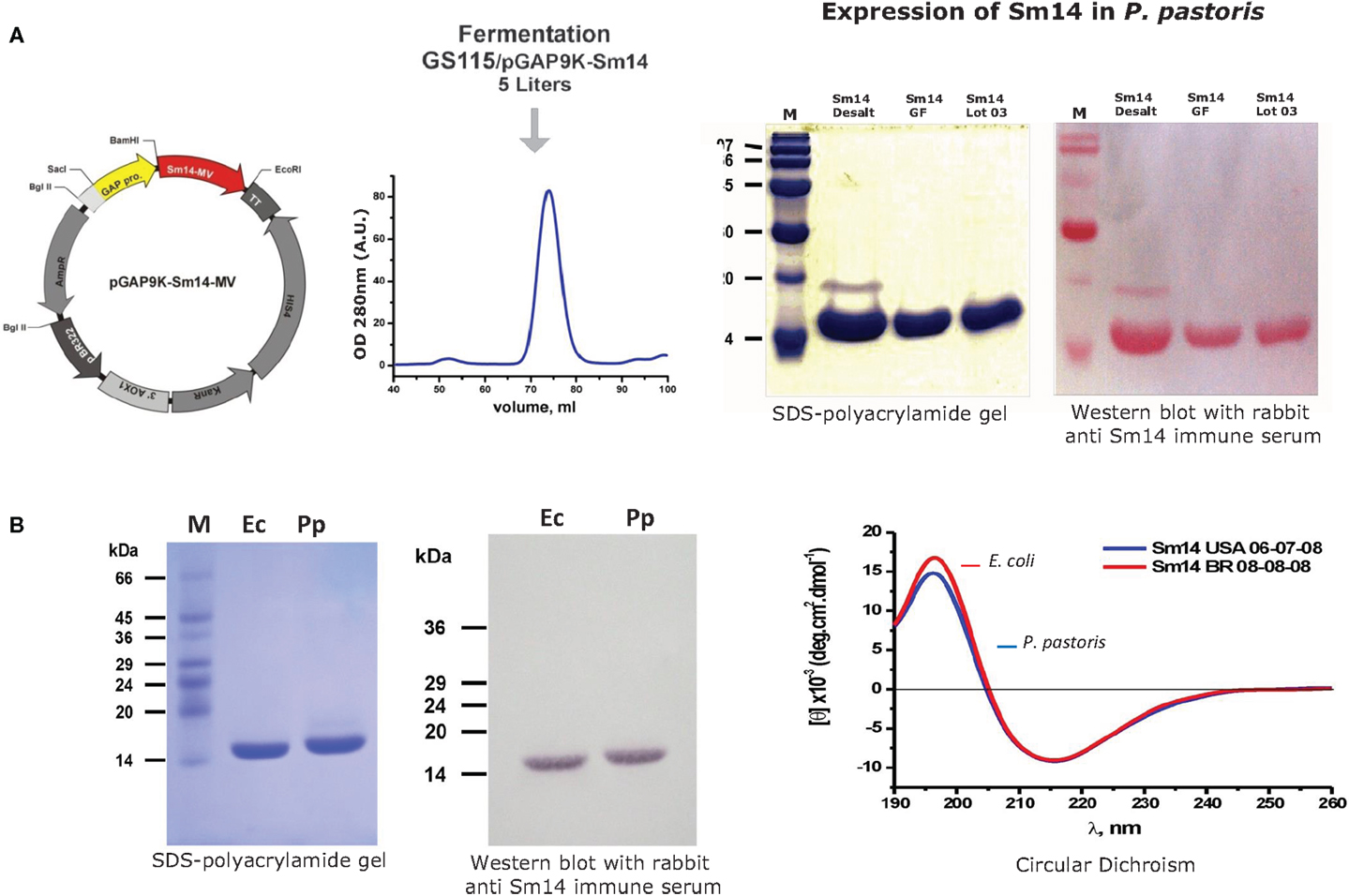
Figure 2. Expression of Sm14 in Pichia pastoris. (A) Purification and detection of Sm14 expressed in P. pastoris. (B) Comparison between Sm14 protein batches purified using both platforms; E. coli (Ec) versus P. pastoris (Pp).
A series of modifications of the prototypic experimental Sm14 vaccine, which consisted of a fusion protein presented with RIBI adjuvants (oil-in-water emulsions derived from bacterial and mycobacterial cell wall components), were undertaken to gradually convert the original laboratory-bench protein into a clinical product. First, it was demonstrated that rSm14 could be produced in a non-fused form while retaining its protective immunogenicity (20). A genomic polymorphism was identified in the Sm14 gene whereby the conserved methionine at position 20 is polymorphic, being exchangeable with threonine (M20T) (21). Both forms were found to be protectively immunogenic to adopt the same three dimensional structure in solution and to be functional in that they were able to bind fatty acids. The M20 isoform was found to exhibit superior stability, however, and was adopted for further vaccine development. A variety of approaches to vaccine formulation were explored in which it was demonstrated that short peptides derived from the C-terminal of sm14 were capable of conferring equivalent levels of protective immunity to experimental animals as intact rSm14 (22). rSm14 was found to be protective when presented in a live vaccine form within recombinant Mycobacterium bovis BCG (23, 24), and that rSm14 expressed as a fusion with tetanus toxin fragment C induced immunoprotection against schistosomiasis in mice (25). Last, a modified version of the protein with improved stability due to the avoidance of dimerization and subsequent aggregation was engineered by Cys62 replacement. The latter version was adopted as the lead compound for vaccine development (19).
The final steps toward a clinically applicable formulation of rSm14 were taken by developing a Pichia pastoris based expression system for the protein (26), and further process development resulted in modifications that avoided any proprietary genetic structures or media requirements enabling low-cost manufacturing. In addition, the synthetic adjuvant GLA-SE was selected for incorporation into the final product, which has now been utilized in Phase I clinical trials since this adjuvant enhances the Th-1 type responses such as gamma-interferon production that have been identified as representing the basis of Sm-14 mediated protective immunity both in human patients and animal models (see below) (27). The good manufacturing practice (GMP) production of rSm14 for clinical trials was undertaken at the LICR protein production facility at Cornell University in Ithaca, NY.
Immune Response to Sm14
The collaborative research group based at the Fundação Oswaldo Cruz has focused exclusively on the stepwise development of schistosomiasis vaccine guided solely by molecular considerations together with assays of protective immunity in animal models. On the other hand, other groups have investigated the nature of the immune response elicited by the antigen particularly in the context of natural human infection and the development of resistance to infection. As a first step, it was demonstrated that the sera of schistosomasis patients contain significant amounts of IgG1 and IgG3 subclass antibodies, whereas low levels of IgM, IgA, or IgE were measurable (28, 29). Specifically, the cellular immune responses to rSm14 were examined in chronic, treated patients and uninfected individuals living in an endemic area for schistosomiasis. Lymphocyte proliferative responses to rSm14 were detectable in the peripheral blood mononuclear cells of all groups studied with the highest proliferation index to rSm14 being detected in uninfected endemic normal (EN) individuals who are naturally resistant to schistosomiasis (28). This result provides direct evidence that the immune response to Sm14 may contribute to protective immunity in man. Moreover, it was determined that lymphocyte proliferation in the uninfected group was dependent on IFN-gamma suggesting that the Th1 might be associated with resistance to infection. Furthermore, analyses in mice suggested that the same immune effector mechanism may be responsible for the protective immunity stimulated by rSm14 vaccination, i.e., that the schistosome vaccine based on Sm14 may reproduce naturally occurring protective immunity in man (30). Interestingly, the strong parallel relationship between naturally occurring immunity and rSm14 vaccination could be extrapolated to the molecular level. T-cell epitopes were identified within the molecule that are recognized by T-cells producing gamma interferon from resistant individuals. Furthermore, the peptide epitopes from Sm14, but not from another schistosome antigen (paramyosin), stimulated protective immunity and gamma-interferon producing T-cells in vaccinated mice (31, 32).
Phase I Clinical Trials
Following approval by the local Ethics Committee and the Brazilian Regulatory Agency, ANVISA, two separate Phase I clinical trials of the rSm14/GLA-SE vaccine have been undertaken with healthy volunteers. The first trial involved 20 males and the second involved 10 females (www.clinicaltrials.gov) (Number NCT01154049). These trials demonstrated that the vaccine is safe and immunogenic. The vaccine was administered intramuscularly in three 0.5 mL doses, each containing 50 μg of both rSm14 and GLA-SE. The second dose was administered 8 weeks after the first one, while the third dose was given 1–2 months later. There were no serious adverse events reported with the only side effects being mild local pain at the site of vaccination in some individuals. With the support of Infectious Disease Research (IDRI, Seattle, USA) clinical trial team, cells and sera from Brazilian volunteers were shipped to Seattle and extensively screened for the immune response generated by vaccination with Sm14 + GLA-SE toward the identification of the immunological signature of human immunization. Vaccination stimulated anti-Sm14 IgG antibodies as well as a Th1 T-cell response, resulting in gamma-interferon production in the vaccinated individuals. (manuscript submitted to Vaccine).
rSm14 as a Multi-Specific Anti-Helminth Vaccine
It has long been known that there is cross reactive protective immunity between the animal parasite Fasciola hepatica and schistosomes. Analysis of the molecular basis of this protective response identified a cross reactive antigen present in F. hepatica, termed Fh15 (33). The cloning and sequencing of Sm14 revealed this to be the corresponding protein in S. mansoni. Molecular models showed that Fh15 and Sm14 adopt the same basic three-dimensional structure, consisting of a barrel-shaped molecule, and also identified shared discontinuous epitopes principally derived from amino acids in the C-terminal portions of the molecules. Moreover, rSm14 provided complete protection against challenge with F. hepatica metacercariae in a mouse model, suggesting that it may be possible to produce a single vaccine that would be effective against at least two parasites, F. hepatica and S. mansoni, of veterinary and human importance, respectively (18). Further analysis confirmed these initial findings and also demonstrated that vaccination with rSm14 can protect the natural host of F. hepatica, the sheep, against experimental parasite challenge resulting in complete abolition of liver pathology (34). In independent experiments undertaken in Spain, protection against fasciola infection was later also achieved in goats immunized with rSm14, where again significantly reduced liver damage was recorded (35). Several groups around the world have been undertaking studies to evaluate the potential of FABPs homologous to Sm14 derived from various organisms as vaccines against a number of different helminth diseases. Those include diseases caused by F. hepatica (18, 36–41), S. mansoni (18, 42), Schistosoma japonicum (43, 44), Echinoccus granulosus (45), and Clonorchis sinensis (46) in both experimental and natural veterinary hosts. The published reports from these groups provide a robust dataset, indicating the widespread potential efficacy of vaccines based on Sm14. Although experimental work has been undertaken with the FABPs from many parasites, only Sm14 has reached the stage of GMP production and clinical trials.
Future Perspectives
To conclude all pre-clinical stages, Sm14 project has overcome bottlenecks of a vaccine development, scaled-up the production, formulated the product, and fulfilled all regulatory requirements to start clinical study phase. The national regulatory authority (ANVISA) approved the results of phase 1 clinical studies, and Fiocruz has licensed Sm14 technology to a Brazilian company, Ourofino, for final development and commercialization of Sm14 vaccine for use in cattle herds against fasciola.
Field based immunogenicity and safety phase 2 trials of the Brazilian Sm14 + GLA-SE anti schistosomiasis vaccine are planned to start in 2015 in endemic areas (Brazil and Africa).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our research team from Experimental Schistosomiasis Laboratory/Fiocruz and nurses from staff study from INI/Fiocruz for conducting the study; Donald De Roo from LICR for technical support for setting up the Fill Finish procedures at Florida Biologics and Quality Control Panel at PPD Inc; Flavia Parra for the organization of Data Bank; Tom Vedvick, from Infectious Disease Research Institute, for most valuable advices and support for formulation of Sm14with GLA; Tatiane dos Santos for support with preparation of Brochure of Investigator and Toxicology Tests; Carlos Henrique Henrique, from Ourofino Animal Health, for support at all levels with operational steps and preclinical animal tests; Jorge Bermudez, Vice President of Innovation and Production at Fiocruz and Robert Bergquist and PHI/WHO(World Health Organization), for strategic support. Funding: This study was funded by FINEP (Financiadora de Estudos e Projetos – Governmental Funding Agency); FAPERJ(Funding Agency from State of Rio de Janeiro); IOC/IOCRUZ(Oswaldo Cruz Institute/Oswaldo Cruz Foundation, Brazilian Health Ministry); and OUROFINO Saude Animal, SP, Brazil.
References
1. World Health Organization. Report of an Informal Consultation on Schistosomiasis Control. (2010). Available from: http://www.who.int/schistosomiasis/epidemiology/PZQ_WHO_report_meeting.pdf?ua=1
2. Pan-American Health Organization. Prevention and Control of Communicable Diseases. (2009). Available from: http://new.paho.org/hq/dmdocuments/2009/nds-epi-profiles.pdf
3. World Health Organization. Action against Worms. (2008). Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/PCTNewsletter12_En.pdf?ua=1
4. World Health Organization. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. (2012). Available from: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf
5. Olveda RM, Daniel BL, Ramirez BD, Aligui GD, Acosta LP, Fevidal P, et al. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis (1996) 174(1):163–72. doi: 10.1093/infdis/174.1.163
6. Richards FO Jr, Eigege A, Miri ES, Jinadu MY, Hopkins DR. Integration of mass drug administration programmes in Nigeria: the challenge of schistosomiasis. Bull World Health Organ (2006) 84:673–6. doi:10.2471/BLT.06.029652
7. Reimert CM, Tukahebwa EM, Kabatereine NB, Dunne DW, Vennervald BJ. Assessment of Schistosoma mansoni induced intestina inflation by means of eosinophil cationic protein, eosinophil protein X and myeloperoxidase before and after treatment with praziquentel. Acta Trop (2008) 105(3):253–9. doi:10.1016/j.actatropica.2007.11.004
8. Olds GR, Olveda R, Wu G, Wiest P, McGarvey S, Aligui G, et al. Immunity and morbidity in Schistosomiasis japonicum infection. Am J Trop Med Hyg (1996) 55(Suppl 5):121–6.
9. Smithers SR, Terry RJ. Acquired resistance to experimental infections of Schistosoma mansoni in the albino rat. Parasitology (1965) 55(4):711–7.
10. Smithers SR, Terry RJ. The stimulation of acquired resistance to schistosome infection. Proc R Soc Med (1967) 60(2):169–70.
11. Almeida MS, Pinto RM, Noronha D, Katz N, Tendler M. Curative and protective activity in rabbits after reinfection with Schistosoma mansoni. A new model of immunity? J Parasitol (1989) 75:308–10. doi:10.2307/3282780
12. Smithers SR, Hackett F, Ali PO, Simpson AJ. Protective immunization of mice against Schistosoma mansoni with purified adult worm surface membranes. Parasite Immunol (1989) 11(4):301–18. doi:10.1111/j.1365-3024.1989.tb00669.x
13. Tendler M, Almeida MS, Pinto RM, Noronha D, Katz N. Schistosoma mansoni-new zealand rabbit model: resistance induced by infection followed by active immunization with protective antigen. J Parasitol (1991) 77(1):138–41. doi:10.2307/3282571
14. Tendler M, Pinto RM, Lima Ade O, Savino W, Katz N. Vaccination in murine schistosomiasis with adult worm-derived antigens: variables influencing protection outbred mice. Int J Parasitol (1991) 21(3):299–306. doi:10.1016/0020-7519(91)90031-2
15. Jeffs SA, Hagan P, Allen R, Correa-Oliveira R, Smithers SR, Simpson AJ. Molecular cloning and characterisation of the 22-kilodalton adult Schistosoma mansoni antigen recognised by antibodies from mice protectively vaccinated with isolated tegumental surface membranes. Mol Biochem Parasitol (1991) 46(1):159–67. doi:10.1016/0166-6851(91)90209-O
16. Knight M, Kelly C, Rodrigues V, Yi X, Wamachi A, Smithers SR, et al. A cDNA clone encoding part of the major 25,000-dalton surface membrane antigen of adult Schistosoma mansoni. Parasitol Res (1989) 75(4):280–6. doi:10.1007/BF00931812
17. Moser D, Tendler M, Griffiths G, Klinkert MQ. A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid binding proteins. J Biol Chem (1991) 266(13):8447–54.
18. Tendler M, Brito CA, Vilar MM, Serra-Freire N, Diogo CM, Almeida MS, et al. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci U S A (1996) 93(1):269–73. doi:10.1073/pnas.93.1.269
19. Ramos CR, Spisni A, Oyama S Jr, Sforça ML, Ramos HR, Vilar MM, et al. Stability improvement of the fatty acid binding protein Sm14 from S. mansoni by Cys replacement: Structural and functional characterization of a vaccine candidate. Biochim Biophys Acta (2009) 1794(4):655–62. doi:10.1016/j.bbapap.2008.12.010
20. Ramos CR, Vilar MM, Nascimento AL, Ho PL, Thaumaturgo N, Edelenyi R. r-Sm14-pRSETA efficacy in experimental animals. Mem Inst Oswaldo Cruz (2001) 96(Suppl):131–5. doi:10.1590/S0074-02762001000900019
21. Ramos CR, Figueredo RC, Pertinhez TA, Vilar MM, do Nascimento AL, Tendler M, et al. Gene structure and M20T polymorphism of the Schistosoma mansoni Sm14 fatty acid-binding protein: molecular, functional and immunoprotection analysis. J Biol Chem (2003) 278(15):12745–51. doi:10.1016/j.bbapap.2008.12.010
22. Vilar MM, Barrientos F, Almeida M, Thaumaturgo N, Simpson A, Garratt R, et al. An experimental bivalent peptide vaccine against schistosomiasis and fascioliasis. Vaccine (2003) 22(1):137–44. doi:10.1016/S0264-410X(03)00300-1
23. Varaldo PB, Leite LC, Dias WO, Miyaji EN, Torres FI, Gebara VC, et al. Recombinant Mycobacterium bovis BCG expressing the Sm14 antigen of Schistosoma mansoni protects mice from cercarial challenge. Infect Immun (2004) 72(6):3336–43. doi:10.1128/IAI.72.6.3336-3343.2004
24. Varaldo PB, Miyaji EN, Vilar MM, Campos AS, Dias WO, Armôa GR, et al. Mycobacterial codon optimization of the gene encoding the Sm14 antigen of Schistosoma mansoni in recombinant Mycobacterium bovis Bacille Calmette-Guérin enhances protein expression but not protection against cercarial challenge in mice. FEMS Immunol Med Microbiol (2006) 48(1):132–9. doi:10.1111/j.1574-695X.2006.00133.x
25. Abreu PA, Miyasato PA, Vilar MM, Dias WO, Ho PL, Tendler M, et al. Sm14 of Schistosoma mansoni in fusion with tetanus toxin fragment C induces immunoprotection against tetanus and schistosomiasis in mice. Infect Immun (2004) 72(10):5931–7. doi:10.1128/IAI.72.10.5931-5937.2004
26. Huang CJ, Damasceno LM, Anderson KA, Zhang S, Old LJ, Batt CA. A proteomic analysis of the Pichia pastoris secretome in methanol-induced cultures. Appl Microbiol Biotechnol (2011) 90(1):235–47. doi:10.1007/s00253-011-3118-5
27. Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One (2010) 5(10):e13677. doi:10.1371/journal.pone.0013677
28. Brito CF, Fonseca CT, Goes AM, Azevedo V, Simpson AJ, Oliveira SC. Human IgG1 and IgG3 recognition of Schistosoma mansoni 14kDa fatty acid-binding recombinant protein. Parasite Immunol (2000) 22(1):41–8.
29. Brito CF, Caldas IR, Coura Filho P, Correa-Oliveira R, Oliveira SC. CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon-γ and tumor necrosis factor-α in response to the recombinant 14kda Schistosoma mansoni fatty acid-binding protein. Scand J Immunol (2000) 51(6):595–601. doi:10.1046/j.1365-3083.2000.00710.x
30. Fonseca CT, Brito CF, Alves JB, Oliveira SC. l-12 enhances protective immunity in mice engendered by immunization with recombinant 14 kDa Schistosoma mansoni fatty acid-binding protein through an IFN-γ and TNF-α dependent pathway. Vaccine (2004) 22(3–4):503–10. doi:10.1016/j.vaccine.2003.07.010
31. Fonseca CT, Cunha-Neto E, Goldberg AC, Kalil J, de Jesus AR, Carvalho EM, et al. Identification of paramyosin T cell epitopes associated with human resistance to Schistosoma mansoni reinfection. Microbes Infect (2005) 7(2):204–12. doi:10.1016/j.micinf.2004.10.012
32. Garcia TC, Fonseca CT, Pacifico LG, Durães Fdo V, Marinho FA, Penido ML, et al. Peptides containing T cell epitopes, derived from Sm14, but not from paramyosin, induce a Th1 type of immune response, reduction in liver pathology and partial protection against Schistosoma mansoni infection in mice. Acta Trop (2008) 106(3):162–7. doi:10.1016/j.actatropica.2008.03.003
33. Rodríguez-Pérez J, Rodríguez-Medina JR, García-Blanco MA, Hillyer GV. Fasciola hepatica: molecular cloning, nucleotide sequence, and expression of a gene encoding a polypeptide homologous to a Schistosoma mansoni fatty acid-binding protein. Exp Parasitol (1992) 74(4):400–7. doi:10.1016/0014-4894(92)90202-L
34. Almeida MS, Torloni H, Lee-Ho P, Vilar MM, Thaumaturgo N, Simpson AJ, et al. Vaccination against Fasciola hepatica infection using a Schistosoma mansoni defined recombinant antigen, Sm14. Parasite Immunol (2003) 25(3):135–7. doi:10.1046/j.1365-3024.2003.00619.x
35. Mendes RE, Zafra R, Pérez-Ecija RA, Buffoni L, Martínez-Moreno A, Tendler M, et al. Evaluation of local immune response to Fasciola hepatica experimental infection in the liver and hepatic lymph nodes of goats immunized with Sm14 vaccine antigen. Mem Inst Oswaldo Cruz (2010) 105(5):698–705. doi:10.1590/S0074-02762010000500017
36. Muro A, Ramajo V, López J, Simón F, Hillyer GV. Fasciola hepatica: vaccination of rabbits with native and recombinant antigens related to fatty acid binding proteins. Vet Parasitol (1997) 69(3–4):219–29. doi:10.1016/S0304-4017(96)01131-4
37. López-Abán J, Casanueva P, Nogal J, Arias M, Morrondo P, Diez-Baños P, et al. Progress in the development of Fasciola hepatica vaccine using recombinant fatty acid binding protein with the adjuvant adaptation system ADAD. Vet Parasitol (2007) 145(3–4):287–96. doi:10.1016/j.vetpar.2006.12.017
38. Estuningsih SE, Smooker PM, Wiedosari E, Widjajanti S, Vaiano S, Partoutomo S, et al. Evaluation of antigens of Fasciola gigantic as vaccines against tropical fasciolosis in cattle. Int J Parasitol (1997) 27(11):1419–28. doi:10.1016/S0020-7519(97)00096-9
39. Nambi PA, Yadav SC, Raina OK, Sriveny D, Saini M. Vaccination of buffaloes with Fasciola gigantic recombinant fatty acid binding protein. Parasitol Res (2005) 97(2):129–35. doi:10.1007/s00436-005-1397-4
40. Kumar N, Anju V, Gaurav N, Chandra D, Samanta S, Gupta SC, et al. Vaccination of buffaloes with Fasciola gigantic recombinant glutathione S-transferase and fatty acid binding protein. Parasitol Res (2012) 110(1):419–26. doi:10.1007/s00436-011-2507-0
41. Vicente B, López-Abán J, Rojas-Caraballo J, Pérez Del Villar L, Hillyer GV, Martínez-Fernández AR, et al. A Fasciola hepatica-derived fatty acid binding protein induces protection against schistosomiasis caused by Schistosoma bovis using the adjuvant adaptation (ADAD) vaccination system. Exp Parasitol (2014) 145:145–51. doi:10.1016/j.exppara.2014.08.007
42. Rabia Aly I, Diab M, El-Amir AM, Hendawy M, Kadry S. Fasciola gigantic fatty acid binding protein (FABP) as a prophylactic agent against Schistosoma mansoni infection in CD1 mice. Korean J Parasitol (2012) 50(1):37–43. doi:10.3347/kjp.2012.50.1.37
43. Becker MM, Kalinna BH, Waine GJ, McManus DP. Gene cloning, overproduction and purification of a functionally active cytoplasmic fatty acid-binding protein (Sj-FABPC) from the human blood fluke Schistosoma japonicum. Gene (1994) 148(2):321–5. doi:10.1016/0378-1119(94)90706-4
44. Liu JM, Cai XZ, Lin JJ, Fu ZQ, Yang GZ, Shi FH, et al. Gene cloning, expression and vaccine testing of Schistosoma japonicum SjFABP. Parasite Immunol (2004) 26(8–9):351–8. doi:10.1111/j.0141-9838.2004.00720.x
45. Chabalgoity JA, Harrison JA, Esteves A, Demarco de Hormaeche R, Ehrlich R, Khan CM, et al. Expression and immunogenicity of an Echinococcus granulosus fatty acid-binding protein in live attenuated Salmonella vaccine strains. Infect Immun (1997) 65(6):2402–12.
Keywords: vaccine, schistosomiasis, Sm14, disease of poverty, FABP, Brazil
Citation: Tendler M, Almeida M and Simpson A (2015) Development of the Brazilian anti Schistosomiasis vaccine based on the recombinant fatty acid binding protein Sm14 plus GLA-SE adjuvant. Front. Immunol. 6:218. doi: 10.3389/fimmu.2015.00218
Received: 11 February 2015; Accepted: 21 April 2015;
Published: 12 May 2015
Edited by:
Rashika El Ridi, Cairo University, EgyptReviewed by:
Sheila Donnelly, University of Technology Sydney, AustraliaRamaswamy Kalyanasundaram, University of Illinois, USA
Mohammed Bahey-El-Din, Alexandria University, Egypt
Copyright: © 2015 Tendler, Almeida and Simpson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miriam Tendler, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Av. Brasil 4365, Manguinhos, Rio de Janeiro, Rio de Janeiro 21040-900, Brazil,bXRlbmRsZXJAaW9jLmZpb2NydXouYnI=
 Miriam Tendler
Miriam Tendler Marilia Almeida1
Marilia Almeida1