- Department of Infection Biology, Universitätsklinikum Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Erlangen, Germany
Lymphopenic conditions lead to expansion of memory-like T cells (TML), which develop from naïve T cells by spontaneous proliferation. TML cells are often increased in the elderly population, AIDS patients, and patients recovering from radio- or chemotherapy. At present, it is unclear whether TML cells can efficiently respond to foreign antigen and participate in antiviral immunity. To address this question, we analyzed the immune response during acute low-dose infection with lymphocytic choriomeningitis virus-WE in T cell lymphopenic CD4Cre/R-diphtheria toxin alpha (DTA) mice in which most peripheral T cells show a TML phenotype. On day 8 after infection, the total number of effector T cells and polyfunctional IFN-γ and TNF-α producing CD8 T cells were three- to fivefold reduced in CD4Cre/R-DTA mice as compared to controls. Viral clearance and the humoral immune response were severely impaired in CD4Cre/R-DTA mice although CTLs efficiently killed transferred target cells in vivo. Transfer of naïve CD4 T cells but not anti-PD-L1 blockade restored the expansion of antigen-specific polyfunctional CD8 T cells and resulted in lower viral titers. This finding indicates that under lymphopenic conditions endogenous CD4 TML cell lack the capacity to promote expansion of CTLs. However, CD8 TML cells retain sufficient functional plasticity to participate in antiviral immunity in the presence of appropriate help by fully functional CD4 T cells. This capacity might be exploited to develop treatments for improvement of CD8 T cell functions under various clinical settings of lymphopenia.
Introduction
T cell homeostasis is regulated by thymic output as well as proliferation and death of peripheral T cells (1). Naïve T cells require recognition of cognate MHC and cytokines like IL-7 or IL-15 for survival. Memory T cells (TM) are less dependent on cognate MHC but IL-7 and IL-15 mediated signals increase their lifespan (2). The total number of peripheral T cells and the ratio of naïve to TM remain remarkably stable until the seventh decade of life (3). Above that age T cell numbers decrease and certain T cell clones expand resulting in a lymphopenic immune system with an oligoclonal repertoire of TCR specificities and a relative increase in the pool of “memory phenotype” T cells (TMP). The pool of TMP cells consists of real antigen-experienced TM and memory-like T cells (TML) that converted from naïve T cells by spontaneous proliferation under lymphopenic conditions [lymphopenia-induced proliferation (LIP)] (4, 5). Increased numbers of TML cells are believed to make a substantial contribution to the TMP pool in AIDS patients or patients recovering from radio- or chemotherapy. However, it remains unclear whether TML cells can be engaged in mounting a primary immune response. TML cells may further compete with real TM for survival, which could lead to attrition of the TM pool under lymphopenic conditions (6–8).
Lymphopenia-induced proliferation requires recognition of self-peptide/MHC complexes but occurs independently of costimulatory signals (9, 10). For CD4 T cells, it could be shown that LIP can be subdivided in a fast, IL-7-independent and probably DC-dependent proliferative response (“spontaneous” or “endogenous” proliferation) and a slower, IL-7-dependent and probably DC-independent response (“homeostatic” proliferation) (11, 12). Lymphopenia is a risk factor for autoimmunity since self-reactive T cells can expand and initiate autoimmune reactions under lymphopenic conditions (13–15). Autoimmunity can develop in HIV patients under highly active anti-retroviral therapy due to expansion of autoreactive T cells (16). The elderly population often shows poor responses to vaccines and has a high frequency of TMP. Therefore, it is important to study the functionality of TML cells to develop more efficient vaccination strategies for the elderly population and to characterize peripheral tolerance mechanisms that control the onset of autoimmunity (17).
Although it remains difficult to estimate the contribution of TML cells to the total pool of memory T cells (TMP), it has recently been shown that the TMP population in germ-free mice contains TML cells, which demonstrates that TML cells are not dependent on the gut flora (18). For CD8 T cells, it has been shown that TML and antigen-experienced TM are basically indistinguishable by phenotype or gene expression profile (1, 19) although more recent data showed that CD8+ TML cells differ from true-memory cells by expression of different chemokine receptors (6). Some studies indicated that CD8+ TML cells are functional and provide protective immunity (6, 20). Furthermore, it was shown that protection against Listeria-OVA by OT-I TML cells that were generated by transfer into irradiated recipient mice required a low number of CD4 T cells (even antigen non-specific cells worked), irradiation-induced bacteremia, and IL-12 (21). Far less is known about TML cells within the CD4+ T cell compartment.
We previously generated mice in which most peripheral T cells show a memory phenotype (22). In this model, naïve T cells are deleted due to diphtheria toxin alpha (DTA) expression from the Rosa26 locus after Cre-mediated excision of a loxP-flanked stop cassette. These CD4Cre/R-DTA mice have severely reduced naïve (CD62L+CD44−) CD4 and CD8 T cells in peripheral lymphoid tissues due to DTA expression in developing T cells in the thymus (22). However, some T cells escape this toxin-mediated deletion, undergo homeostatic proliferation and develop into TML cells which fill the TMP niche to the same level as in control mice while naïve CD4 and CD8 T cells remain constitutively deleted.
Here, we used this mouse model to determine the functionality of TML cells in response to acute low-dose infection with lymphocytic choriomeningitis virus (LCMV)-WE. LCMV-infected CD4Cre/R-DTA mice efficiently killed transferred target cells in vivo in an antigen-specific manner. However, the infection-induced expansion of LCMV-specific T cells, viral clearance, and the humoral immune response were severely impaired in CD4Cre/R-DTA mice as compared to control mice. Transfer of polyclonal naïve CD4 T cells from wild-type mice but not anti-PD-L1 blockade restored the expansion and function of endogenous CD8 TML cells in CD4Cre/R-DTA mice.
Materials and Methods
Mice and Infection
Homozygous CD4Cre mice (23) were crossed to R-DTA mice (22) to generate lymphopenic CD4Cre+R-DTA+ mice (CD4Cre/R-DTA) and CD4Cre+R-DTA− control mice (CD4Cre). B6_CD45.1 congenic mice (B6.SJL-Ptprca Pepcb/BoyJ) were originally obtained from The Jackson Laboratory and crossed to normal C57BL/6J mice to generate heterozygous CD45.1+CD45.2+ congenic mice. C57BL/6J mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Mice were maintained in the Franz-Penzoldt-Zentrum in Erlangen under specific pathogen-free conditions. Mice were infected with 200 pfu of LCMV-WE intravenously under biosafety level 2 and analyzed at indicated points in time. All experiments were performed in accordance with German animal protection law and European Union guidelines 86/809 and were approved by the Federal Government of Lower Franconia.
Flow Cytometry
Single-cell suspensions of spleens were generated under biosafety level 2 by mechanical disruption and erythrocytes were lysed with ACK-buffer (0.15 M NH4Cl, 1 mM KHO3, 0.1 mM Na2EDTA). Cells were preincubated with anti-CD16/CD32 mAb (clone 2.4G2; BioXcell, West Lebanon, NH, USA) and stained with respective antibodies. The following antibodies were used for surface staining: PerCP-Cy5.5- or APCe780-labeled anti-CD4 (clone RM4-5), FITC-, PE-, or APC-labeled anti-CD8 (clone 53-6.7), PE-Cy7-labeled anti-CD62L (clone MEL-14), eFluor660-labeled anti-GL-7 (clone GL-7), FITC- or eFluor450-labeled anti-CD45R (clone RA3-6B2), FITC-labeled anti-CD44 (clone IM7), e450-labeled anti-KLRG1 (clone 2F1), PerCP-labeled anti-CD45.2 (clone 104), and PE- or e450-labeled anti-CD45.1 (clone A20) were purchased from eBioscience (San Diego, CA, USA). PE-Cy7-labeled anti-CD38 (clone 90), PE-Cy7-labeled anti-CD4 (clone RM4-5), and PE-Cy7-labeled or biotinylated anti-PD-1 (clone RMP1-30) were purchased from BioLegend (San Diego, CA, USA). Vioblue- or APC-labeled anti-CD44 (clone IM7.8.1) was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany), PE-labeled anti-CXCR5 (clone 2G8) and V500-labeled Streptavidin were from BD Biosciences (San Jose, CA, USA).
For dextramer stainings (gp33_H2-Db coupled to APC; Immudex, Copenhagen, Denmark), cells were washed with PBS containing 5% FCS, incubated with 5 µl dextramer per sample for 10 min at room temperature and then the antibody mixture for surface staining was added for an additional 20 min at 4°C. Tetramer staining (gp66_I-Ab coupled to PE, NIH tetramer core facility) was performed in RPMI1640 (PAN-Biotech, Aidenbach, Germany) containing 10% FCS. Cells were incubated with 0.3 ng tetramer for 2 h at 37°C, washed, and stained with respective antibodies.
FITC-labeled anti-mouse IFN-γ (clone XMG1.2; BioLegend) and PE-labeled anti-mouse TNF-α (clone MP6-XT22; eBioscience) were used for intracellular staining after cells had been fixed with 4% paraformaldehyde and permeabilized with the Intracellular Staining Perm Wash Buffer (BioLegend) according to the manufacturer’s protocol. Dead cells were excluded by staining with DAPI (Sigma-Aldrich, St. Louis, MO, USA), fixable viability dye APC-eFluor780, or fixable viability dye APC-eFluor506 (both from eBioscience). Samples were acquired with FACS Canto II (BD Bioscience) and MACSQuant (Miltenyi Biotec). Data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Restimulation of T Cells
Single-cell suspensions were either restimulated with 1 µg/ml gp33- (KAVYNFATM) or gp61- (GLKGPDIYKGVYQFKSVEFD) peptide (JPT, Berlin, Germany) for 4 h. After 2 h, 10 µg/ml Brefeldin A (Sigma-Aldrich, St. Louis, MO, USA) was added. IFN-γ and TNF-α production was measured by intracellular staining.
Quantitative RT-PCR
RNA was prepared from the indicated organs with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. To transcribe the viral RNA genome, 1 µg of total RNA was reversely transcribed into cDNA with the LCMV-gp-specific reverse primer. Quantitative PCR was performed with SYBR Select Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), and the following primer sequences: LCMV-gp forward primer 5′-CATTCACCTGGACTTTGACAGACTC-3′ and LCMV-gp reverse primer 5′-GCAACTGCTGTGTTCCCGAAAC-3′ under the following conditions: 95°C 30 s, 60°C 20 s, 72°C 20 s; 40 cycles. Copy numbers of LCMV genomes per gram organ were determined using a plasmid containing 115 bp of LCMV gp gene.
In Vivo Cytotoxicity Assay
Splenocytes from naïve B6 mice were stained with 5 µM CellTraceViolet (Thermo Fisher Scientific), loaded with 1 µM gp33 peptide (JPT), and mixed with splenocytes from CD45.1 congenic mice loaded with 1 µM m45 peptide of MCMV (JPT). The 4.5 × 106 cells per group were mixed and transferred into LCMV-infected or naïve mice at day 8 after infection. Analysis took place 90 min after cell transfer.
Adoptive T Cell Transfers
CD4 or CD8 T cells from spleens of CD45.1 congenic mice were isolated using the EasySep isolation Kit (STEMCELL Technologies, Vancouver, BC, Canada) according to manufacturer’s protocol. Then, 3 × 106 CD4 or CD8 T cells were transferred to recipient mice 1 day before LCMV infection. Mice were analyzed on indicated days after infection.
ELISA
Plates were coated with 1 µg/ml nucleoprotein of LCMV (Alpha Diagnostics, San Antonio, TX, USA), blocked with PBS + 1% BSA and incubated with sera overnight. Alkaline phosphatase-coupled anti-mouse IgM, anti-mouse IgG1, or anti-mouse IgG2c antibodies and para-nitrophenylphosphate substrate (both Southern Biotec, Birmingham, AL, USA) were used for detection. ELISA was measured at 405 nm with Multiskan FC multiplate photometer (Thermo Fisher Scientific).
Immunofluorescence Staining
To analyze the germinal centers, cryosections of spleens were dehydrated in −20°C cold acetone and rehydrated in PBS. Germinal centers were stained with biotinylated anti-mouse CD4 (clone GK1.5, eBioscience), APC-labeled anti-mouse CD45R (clone RA3-6B2, eBioscience), and FITC-labeled GL-7 (clone GL-7, eBioscience) followed. As secondary antibody we used Streptavidin-Cy3 (Jackson Immunoresearch, West Grove, PA, USA). Nuclei were counterstained with DAPI.
Statistics
Student’s t-test was used for normally distributed (Shapiro–Wilk test) data sets whereas Mann–Whitney U-test was used for non-normally distributed data sets. Analysis was performed with SigmaPlot (Systat Software Inc., San Jose, CA, USA) software, and P-values of less than 0.05 were considered statistically significant.
Results
Impaired T Cell and Humoral Response to LCMV in CD4Cre/R-DTA Mice
Expression of DTA in developing T cells of CD4Cre/R-DTA mice leads to almost complete deletion of naïve peripheral T cells despite normal numbers of T cells with a memory phenotype (TMP) [(22); Figure 1A]. This indicates that the niche for TMP cells had been completely filled by homeostatically proliferated TML cells that escaped deletion and retained the loxP-flanked stop cassette in front of DTA in the Rosa26 locus. To determine whether a TML-dominated immune system is able to mount a protective antiviral immune response, we infected CD4Cre/R-DTA mice and CD4Cre control mice with 200 pfu LCMV-WE and analyzed the T cell populations in the spleen on day 8 after infection. Both CD4Cre/R-DTA and control mice showed massive (~20- to 30-fold) expansion of effector CD8 T cells (CD44+CD62L−) in the spleen although the total number of effector CD8 T cells was fivefold lower in CD4Cre/R-DTA mice as compared to control mice (Figure 1; Figure S1 in Supplementary Material). By staining for antigen-specific T cells using gp33_H-2Db dextramers and gp66_I-Ab tetramers that cover immunodominant CD8 and CD4 T cell epitopes, respectively, we observed about 10-fold fewer CD8 and CD4 T cells specific for these epitopes in CD4Cre/R-DTA mice as compared to controls (Figure 1C). In vitro restimulation with LCMV peptides further revealed a significantly impaired production of IFN-γ and/or TNF-α in T cells of LCMV-infected CD4Cre/R-DTA mice (Figure 1D). The larger number of cytokine-producing as compared to gp33_H-2Db dextramer positive cells could be due to a contribution of CD8 T cells that recognized the gp33 peptide in H-2Kb. An alternative explanation would be that the dextramer staining did not detect all gp33_H-2Db-specific T cells due to downregulation of the TCR.
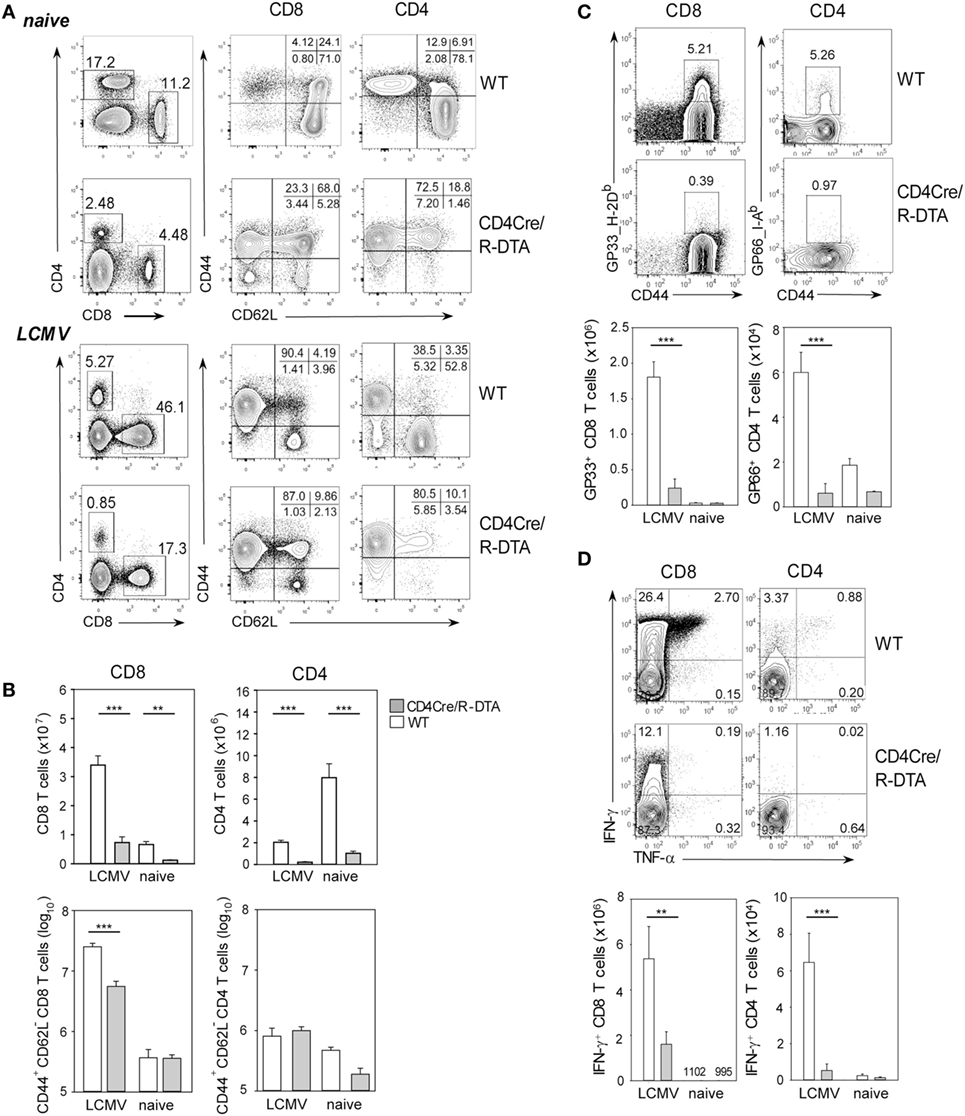
Figure 1. Impaired T cell response to LCMV in CD4Cre/R-diphtheria toxin alpha (DTA) mice. Spleens of CD4Cre/R-DTA mice and CD4Cre control mice were analyzed by flow cytometry before (naïve) or 8 days after (infected) lymphocytic choriomeningitis virus-WE infection. (A) Contour plots show representative stainings of the total CD8 and CD4 T cells and their activation status. (B) Bar graphs depict the mean number + SEM of activated (CD44+CD62L−) and total CD8 or CD4 T cells in CD4Cre (white) and CD4Cre/R-DTA mice (gray). (C) Contour plots show stainings with gp33 dextramer (left panel) on gated CD8 T cells or gp66 tetramer (right panel) on gated CD4 T cells. Bar graphs depict the mean number + SEM of gp33-specific CD8 T cells or gp66-specific CD4 T cells in LCMV-infected or naïve CD4Cre (white) and CD4Cre/R-DTA mice (gray). (D) Intracellular staining of IFN-γ and TNF-α in gated CD8 T cells restimulated with gp33 peptide (left panel) or in gated CD4 T cells restimulated with gp61 peptide (right panel). Bar graphs depict the mean number + SEM of IFN-γ producing CD8 or CD4 T cells in LCMV-infected or naïve CD4Cre (white) and CD4Cre/R-DTA mice (gray). A total of 2–15 naïve and at least 8 LCMV-infected mice per group from several independent experiments. **P < 0.01, ***P < 0.001 by Mann–Whitney U-test.
Although CD8 T cells are critical for control of acute LCMV infection, antibodies have been shown to contribute to long-term protection of LCMV infection by preventing viral spread and by maintaining functional CTL memory (24–26). We reasoned that in CD4Cre/R-DTA mice, the humoral immune response might be affected by the reduced number of CD4 T cells which are critical for providing help to B cells. Indeed, the formation of germinal centers in LCMV-infected CD4Cre/R-DTA mice was abolished (Figure 2A). This correlated with impaired expansion of T follicular helper (TFH) cells identified as CD4+PD-1+CXCR5+ cells and germinal center B cells identified as B220+CD38−GL-7+ cells (Figures 2B,C). Furthermore, the serum levels of LCMV-specific IgG2c antibodies were reduced in CD4Cre/R-DTA mice while IgM and IgG1 titers appeared comparable between both groups of mice (Figure 2D).
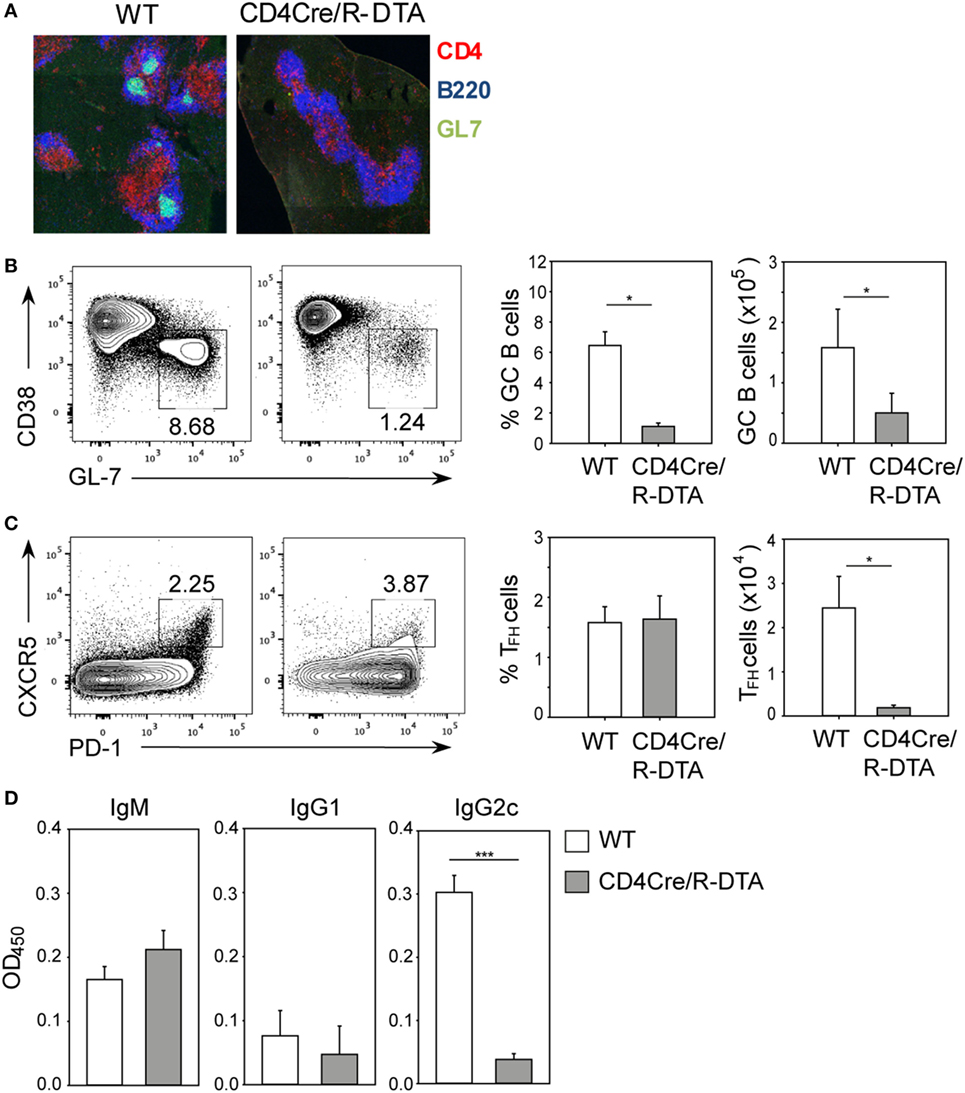
Figure 2. Impaired humoral immune response to lymphocytic choriomeningitis virus (LCMV) in CD4Cre/R-diphtheria toxin alpha (DTA) mice. CD4Cre and CD4Cre/R-DTA mice were infected with lymphocytic choriomeningitis virus-WE and spleens and sera were analyzed on day 14 after infection. (A) Spleen sections were stained with anti-CD4 (red), anti-B220 (blue), and GL-7 (green) to visualize the germinal centers. (B) Contour plots show the percentage of GC B cells (CD38loGL-7hi gated on B220+) in the spleen of indicated LCMV-infected mice. Bar graphs depict the mean percentage + SEM (left) or mean number + SEM (right) of GC B cells in LCMV-infected CD4Cre (white) and CD4Cre/R-DTA mice (gray). (C) Contour plots show the percentage of T follicular helper (TFH) cells (PD-1hiCXCR5hi gated on CD4+) in the spleen of indicated LCMV-infected mice. Bar graphs depict the mean percentage + SEM (left) or mean number + SEM of TFH cells in LCMV-infected CD4Cre (white) and CD4Cre/R-DTA mice (gray). A total of 4–17 mice per group from 1 to 4 experiments. (D) LCMV nucleoprotein-specific IgM, IgG1, and IgG2c antibody levels in sera from LCMV-infected CD4Cre (white) and CD4Cre/R-DTA mice (gray). Bar graphs show the mean + SEM of 9–12 mice per group from 3 experiments. *P < 0.05, ***P < 0.001 by Mann–Whitney U-test.
Lack of Viral Control Despite Efficient In Vivo Killing of Transferred Target Cells
With the next set of experiments we compared the in vivo killing capacity of CTLs, and the control of viral load in LCMV-infected CD4Cre/R-DTA and control mice. The in vivo killing capacity was determined by cotransfer of two differently marked target cells into day 8 LCMV-infected recipient mice. One set of target cells consisted of syngeneic splenocytes labeled with fluorescent CellTrace dye and loaded with LCMV-gp33 peptide while the other set of target cells consisted of MCMV-M45 control peptide-loaded splenocytes from congenic CD45.1 mice. The frequency of transferred target cells in the spleen was analyzed 90 min later by flow cytometry. We observed that the specific killing efficiency was comparable in both strains of mice (Figure 3A). Based on this result, we expected that CD4Cre/R-DTA mice would be able to control the infection. Therefore, we analyzed the copy numbers of LCMV genomes in spleen, liver, and kidney on day 4, 14, and 28 after infection by quantitative RT-PCR. Control mice eliminated the virus from liver and kidney within 14 days and from the spleen within 28 days. By contrast, CD4Cre/R-DTA mice were not able to control the infection and copy numbers of LCMV genomes even increased at 28 days after infection (Figure 3B).
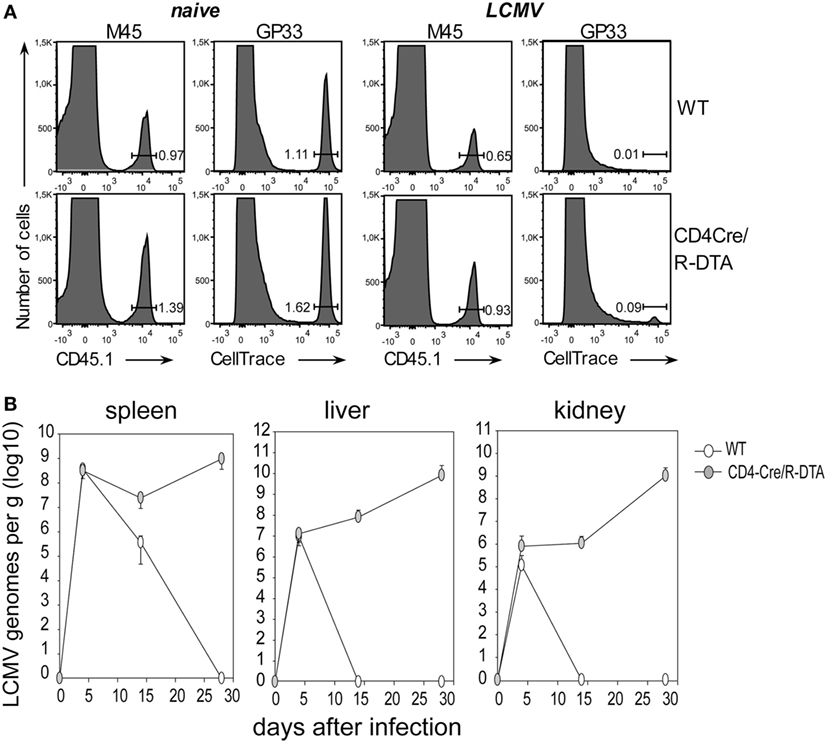
Figure 3. Lack of viral control despite efficient in vivo killing of transferred target cells. (A) In vivo killing assay. Lymphocytic choriomeningitis virus (LCMV)-infected or naïve CD4Cre and CD4Cre/R-diphtheria toxin alpha (DTA) mice received LCMV-gp33 peptide-loaded, CellTrace-labeled splenocytes together with control cells (CD45.1+) loaded with an irrelevant peptide (m45 from murine cytomegalovirus). Histograms show the frequency of indicated target cells at 90 min after cotransfer into day 8 LCMV-infected mice. Histograms are representative for three mice per group from two experiments. (B) CD4Cre (white) and CD4Cre/R-DTA mice (gray) were infected with LCMV and the copy numbers of viral genomes in spleen, liver, and kidney were determined by quantitative RT-PCR at the indicated days after infection. Data points show the mean copy number + SEM of three to five mice per group per timepoint.
Anti-PD-L1 Block Does Not Rescue the Functionality of CD8 TML Cells
The lack of viral control and impaired expansion of LCMV-specific effector T cells in CD4Cre/R-DTA mice raised the question whether expression of inhibitory receptors on TML cells might dampen their responsiveness as previously suggested by others (27). Indeed, a larger fraction of T cells expressed the inhibitory receptor PD-1 alone or in combination with the senescence marker KLRG1 in naïve CD4Cre/R-DTA mice as compared to control mice (Figure 4A). Expression of both markers increased after LCMV infection in both strains of mice (Figure 4B). The expression of PD-1 on CD8 T cells has also been observed in chronically LCMV-infected mice and correlated with a state of unresponsiveness or exhaustion which could be reversed by blocking the ligand PD-L1 (28). Therefore, we injected blocking anti-PD-L1 antibodies before and during LCMV infection to determine whether CD8 TML cell function could be restored. However, when mice were analyzed on day 10 after infection, we observed that the frequency and total number of polyfunctional IFN-γ and TNF-α producing CD8 T cells did not increase in comparison to isotype-treated animals (Figures 5A,B). This indicates that either other inhibitory checkpoints are involved in dampening the responsiveness of CD8 TML cells in CD4Cre/R-DTA mice or that the functionality of these cells cannot be restored by extrinsic signals. For reasons that remain unclear at this point we further observed increased viral loads in spleen, liver, and kidney of anti-PD-L1-treated as compared to isotype control-treated CD4Cre/R-DTA mice (Figure 5C).
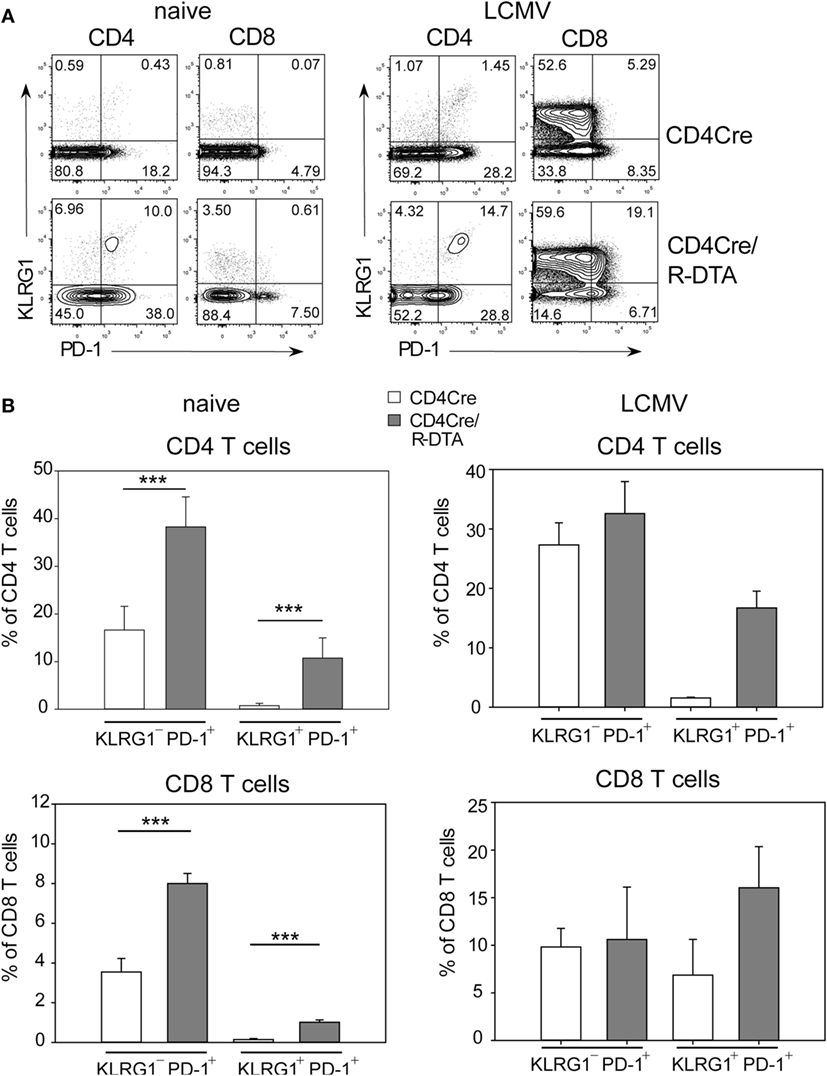
Figure 4. Expression of KLRG1 and PD-1 on T cells of naïve CD4Cre/R-diphtheria toxin alpha (DTA) mice. (A) Dot plots show the expression of KLRG1 and PD-1 on gated CD4 or CD8 T cells from naïve or lymphocytic choriomeningitis virus (LCMV)-infected CD4Cre/R-DTA and CD4Cre mice. (B) Bars show the mean + SEM frequency of KLRG1+PD-1+ and KLRG1−PD-1+ T cells among gated CD4 or CD8 T cells from naïve or LCMV-infected CD4Cre/R-DTA and CD4Cre mice. Naïve: 9–16 mice per group from three experiments. LCMV-infected: two to four mice per group from one experiment. ***P < 0.001 by Mann–Whitney U-test.
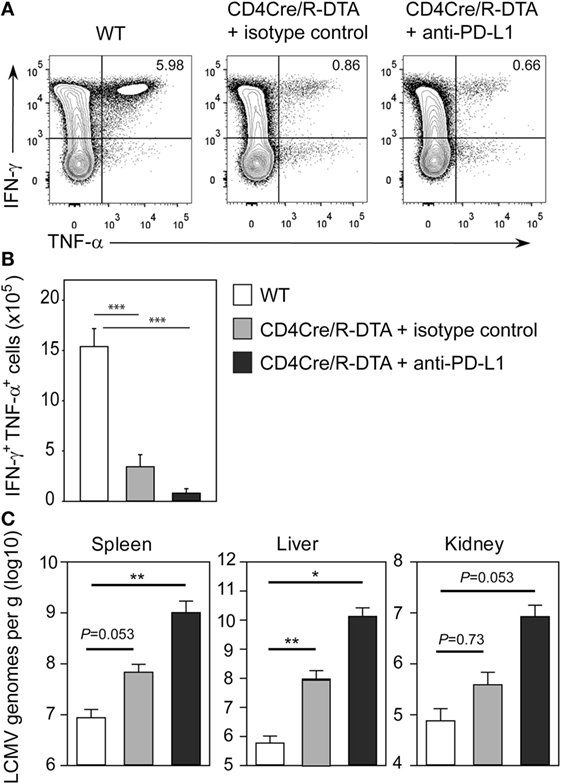
Figure 5. Anti-PD-L1 block does not restore the functionality of CD8 memory-like T cells. CD4Cre and CD4Cre/R-diphtheria toxin alpha (DTA) mice were infected with lymphocytic choriomeningitis virus (LCMV). CD4Cre/R-DTA mice were injected with 200 µg anti-PD-L1 antibody or isotype control i.p. at day 1, day 4, and day 7 after infection. Spleens were analyzed at day 10 after infection. (A) Contour plots show intracellular stainings of IFN-γ and TNF-α in gated CD8 T cells restimulated with gp33 peptide. (B,C) Bar graphs depict the mean number + SEM of IFN-γ and TNF-α producing CD8 T cells (B) or copy numbers of viral genomes in spleen, liver, and kidney determined by quantitative RT-PCR (C) in LCMV-infected CD4Cre mice (white), LCMV-infected isotype control-treated CD4Cre/R-DTA mice (gray), and LCMV-infected anti-PD-L1-treated CD4Cre/R-DTA mice (black). Six to seven mice per group from two experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by Mann–Whitney U-test.
Transfer of Naïve Polyclonal CD4 T Cells Is Sufficient to Restore CD8 TML Functionality in CD4Cre/R-DTA Mice
To further investigate whether functionality in CD8 TML cells can be restored in vivo, we transferred 3 × 106 purified polyclonal CD4 T cells from congenic CD45.1+CD45.2+ heterozygous mice into CD4Cre/R-DTA mice with the idea to provide “helper” cells that could mediate activation of endogenous CD8 TML cells. These reconstituted mice were infected with LCMV and analyzed on day 14 after infection. The transferred CD45.1+CD45.2+ CD4 T cells constituted about 60% of all CD4 T cells and 6% of them coexpressed IFN-γ and TNF-α after gp61 restimulation (Figure S2 in Supplementary Material). By contrast, only 0.8% of the endogenous CD4 TML cells produced both cytokines indicating a functional impairment of these cells. With regard to the CD8 T cell response, CD4Cre/R-DTA mice that had received CD4 T cells showed an increased frequency and total number of gp33_H-2Db dextramer positive and IFN-γ/TNF-α polyfunctional CD8 T cells as compared to non-reconstituted mice (Figures 6A,B). In addition, antiviral activity was partially restored (Figure 6C). This indicates that CD8 TML cells require help by CD4 T cells and endogenous CD4 TML cells in CD4Cre/R-DTA mice are not sufficient for this helper function. However, transferred CD4 cells from naïve wild-type mice could efficiently restore the functional capacity of CD8 TML cells in CD4Cre/R-DTA mice and thereby promote the antiviral immune responses.
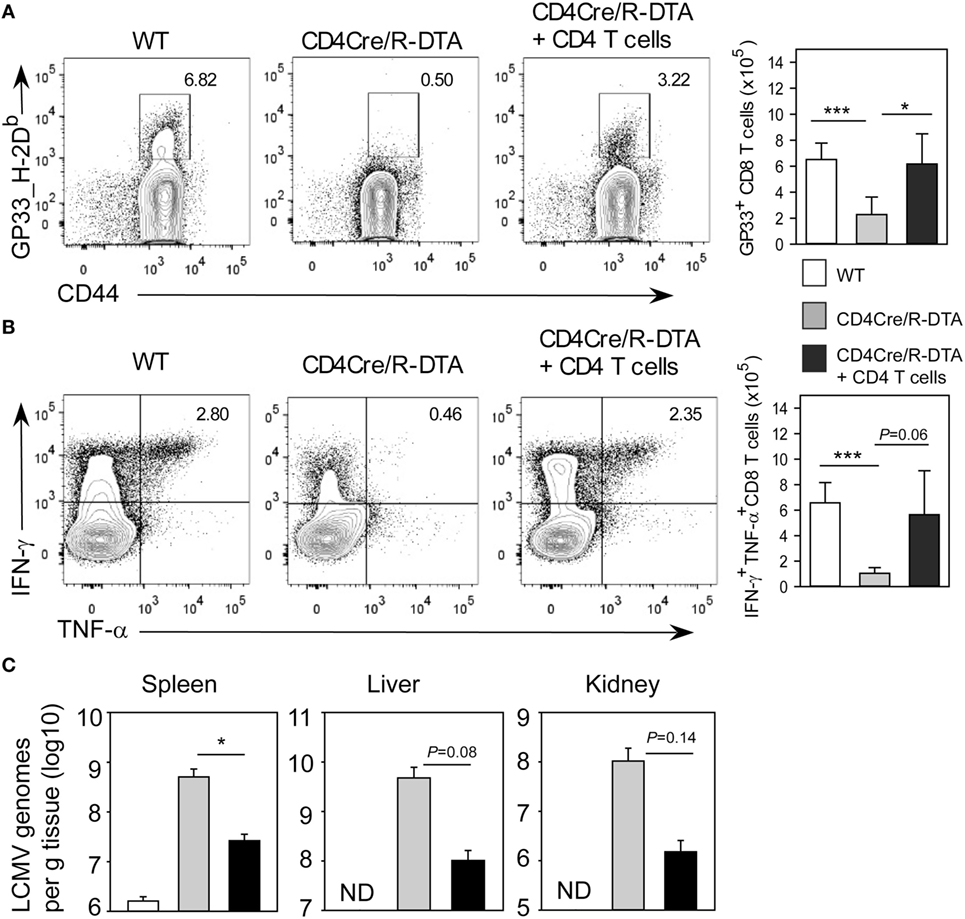
Figure 6. Transfer of naïve CD4 T cells is sufficient to restore CD8 memory-like T cells functionality in CD4Cre/R-diphtheria toxin alpha (DTA) mice. CD4Cre and CD4Cre/R-DTA mice were infected with LCMV. One group of CD4Cre/R-DTA mice received 3 × 106 polyclonal naïve CD4 T cells from normal CD45.1+CD45.2+ heterozygous congenic mice 1 day before infection. Analysis took place on day 14 after infection. (A) Contour plots show stainings with gp33_H2-Db dextramer on gated CD8 T cells. Bar graph depicts the mean number + SEM of gp33-specific CD8 T cells in lymphocytic choriomeningitis virus (LCMV)-infected CD4Cre (white), CD4Cre/R-DTA mice (gray), and CD4Cre/R-DTA mice with CD4 T cell transfer (black). (B) Intracellular staining of IFN-γ and TNF-α in gated CD8 T cells restimulated with gp33 peptide. Bar graph depicts the mean number + SEM of IFN-γ and TNF-α-producing CD8 T cells in LCMV-infected CD4Cre (white), CD4Cre/R-DTA mice (gray), and CD4Cre/R-DTA mice with CD4 T cell transfer (black). (C) Copy numbers of viral genomes in spleen, liver, and kidney of LCMV-infected CD4Cre (white), CD4Cre/R-DTA mice (gray), and CD4Cre/R-DTA mice with CD4 T cell transfer (black) were determined by quantitative RT-PCR. ND, not detectable. A total of 8–14 mice per group from 5 experiments. *P < 0.05; ***P < 0.001 by Mann–Whitney U-test (A,B) or Student’s t-test (C).
Discussion
The participation of lymphopenia-induced TML in protective immune responses remains unclear although the mechanisms of TML development are quite well understood (1). Functional characterization of TML cells in vivo is critical to elucidate mechanisms that could improve vaccinations of people where TML cells represent a dominant population such as the elderly population, AIDS patients, or people recovering from bone marrow transplantations.
Using constitutively T cell-lymphopenic CD4Cre/R-DTA mice we demonstrate here that CTLs developed from CD8 TML cells and efficiently killed transferred target cells but could not control viral replication during low-dose acute LCMV infection. In addition, expansion of TFH cells, germinal center formation, and antibody production was significantly impaired in CD4Cre/R-DTA mice. However, the impaired CTL functionality could be reversed by transfer of naïve polyclonal CD4 T cells from wild-type mice highlighting the functional plasticity of these cells which might be exploited for future vaccination strategies or therapeutic options in chronic viral infections.
It is important to note that the T cells which escape deletion in CD4Cre/R-DTA mice show a normal survival, turnover, and proliferation in vitro and in vivo excluding the possibility that they are short-lived or generally unresponsive cells (22). Yet, we observed a poor T cell response in CD4Cre/R-DTA mice during acute LCMV induction. By analysis of the Vβ chain usage we previously showed that the T cell receptor repertoire diversity is reduced in CD4Cre/R-DTA mice which indicates that only few thymic emigrants escaped the deletion and these cells retained the loxP-flanked STOP cassette in front of the DTA gene (22). The number of LCMV gp33_H-2Db-specific CD8 T cells in a naïve C57BL/6 mouse has been calculated to range between 300 and 1,000 (29, 30). Others provided evidence that more than 1,000 clonotypic TCRs for this particular peptide-MHC complex exist in the naïve repertoire of C57BL/6 mice (31). Assuming that lymphopenic conditions do neither inhibit nor favor expansion of gp33_H-2Db-specific CD8 T cells per se one can estimate that only about 100 of these cells are present in naïve CD4Cre/R-DTA mice and most of them probably acquired a TML phenotype due to homeostatic proliferation. Despite their low number and altered phenotype, they massively expanded to constitute about 2.5 × 105 cells in the spleen on day 8 after infection and they efficiently killed transferred target cells within 90 min in vivo. However, the viral titers remained high and the infection could not be controlled. This finding suggests that CTLs in CD4Cre/R-DTA mice were functionally impaired. On a single-cell level we observed fewer CTLs that expressed IFN-γ together with TNF-α after in vitro restimulation. It has been demonstrated that so-called polyfunctional T cells which express IFN-γ together with IL-2 and/or TNF-α are much more potent effector cells in mice and humans as compared to single cytokine producers (32, 33). However, mice that lack receptors for IFN-γ or TNF-α can still control acute LCMV infection indicating that other factors derived from polyfunctional CD8 T cells might be required to reduce viral titers in organs (34, 35).
The lower frequency of polyfunctional T cells could be linked to the expression of the senescence marker KLRG1 and the inhibitory receptor PD-1 on a large proportion of T cells in naïve CD4Cre/R-DTA mice. We have previously shown that KLRG1 is expressed on mouse and human T cells after activation when they reach replicative senescence (36, 37). It is well known that PD-1 is expressed on effector T cells but also on exhausted T cells (28). However, functional exhaustion of CTLs is also observed with cells from PD-1-deficient mice (38). PD-1 was further found to be expressed on the surface of dysfunctional homeostatically expanded T cells after adoptive transfer into lymphopenic hosts (27). Interestingly, the function of PD-1+ T cells could be restored by anti-PD-1 or anti-PD-L1 checkpoint inhibitors as demonstrated in a chronic LCMV clone 13 infection model (28). Further, blockade of another inhibitory receptor, Tim-3, or injection of IL-2 in combination with anti-PD-L1 showed improved reactivation of LCMV-specific CD8 T cells in the chronic LCMV clone 13 model (39, 40). However, as we show here PD-L1 blockade during acute LCMV-WE infection which becomes chronic in CD4Cre/R-DTA mice did not restore expansion of polyfunctional T cells and the infection could not be controlled.
Importantly, expansion, cytokine production, and antiviral activity of LCMV-specific CD8 T cells could be improved by transfer of 3 × 106 polyclonal CD4 T cells into CD4Cre/R-DTA mice. This indicates that LCMV-specific CD8 T cells are not per se missing in CD4Cre/R-DTA mice but they are functionally impaired and a relatively low number of transferred naïve CD4 T cells but not endogenous TML CD4 T cells were sufficient to revive these CD8 T cells. Future studies will have to identify the critical reviving factors provided by the transferred CD4 T cells to elucidate the mechanisms that regulate functional plasticity of TML cells in vivo. A better understanding of TML plasticity and effector functions will help to develop more efficient vaccines for the elderly population and perhaps boost antiviral CD8 T cell responses under lymphopenic conditions including AIDS patients or CMV patients after bone marrow transplantation.
Author Contributions
MS, SS, and DV designed experiments. MS and SS performed experiments. MS, SS, and DV wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank K. Castiglione and A. Matthies for technical assistance, the NIH tetramer core facility for providing gp66_I-Ab tetramers, and the members of the Voehringer lab for critical reading of the manuscript.
Funding
This work was funded in part by the Deutsche Forschungsgemeinschaft grant SFB643_B15 to DV.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2016.00622/full#supplementary-material.
References
1. Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol (2002) 2:547–56. doi:10.1038/nri853
2. Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity (2008) 29:848–62. doi:10.1016/j.immuni.2008.11.002
3. Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol (2005) 17:468–75. doi:10.1016/j.coi.2005.07.020
4. Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol (1993) 151:6657–69.
5. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol (2005) 174:7446–52. doi:10.4049/jimmunol.174.11.7446
6. Cheung KP, Yang E, Goldrath AW. Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J Immunol (2009) 183:3364–72. doi:10.4049/jimmunol.0900641
7. Dudani R, Murali-Krishna K, Krishnan L, Sad S. IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J Immunol (2008) 181:1700–9. doi:10.4049/jimmunol.181.3.1700
8. Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, et al. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity (1999) 11:733–42. doi:10.1016/S1074-7613(00)80147-8
9. Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity (1999) 11:183–90. doi:10.1016/S1074-7613(00)80093-X
10. Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol (2001) 167:5664–8. doi:10.4049/jimmunol.167.10.5664
11. Do JS, Min B. Differential requirements of MHC and of DCs for endogenous proliferation of different T-cell subsets in vivo. Proc Natl Acad Sci U S A (2009) 106:20394–8. doi:10.1073/pnas.0909954106
12. Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci U S A (2004) 101:3874–9. doi:10.1073/pnas.0400606101
13. King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell (2004) 117:265–77. doi:10.1016/S0092-8674(04)00335-6
14. Le Campion A, Gagnerault MC, Auffray C, Becourt C, Poitrasson-Riviere M, Lallemand E, et al. Lymphopenia-induced spontaneous T-cell proliferation as a cofactor for autoimmune disease development. Blood (2009) 114:1784–93. doi:10.1182/blood-2008-12-192120
15. Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci U S A (2008) 105:19414–9. doi:10.1073/pnas.0807743105
16. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev (2002) 1:329–37. doi:10.1016/S1568-9972(02)00086-1
17. Guy B. Strategies to improve the effect of vaccination in the elderly: the vaccine producer’s perspective. J Comp Pathol (2010) 142(Suppl 1):S133–7. doi:10.1016/j.jcpa.2009.09.009
18. Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med (2009) 206:435–48. doi:10.1084/jem.20081829
19. Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci U S A (2004) 101:16885–90. doi:10.1073/pnas.0407417101
20. Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol (2006) 7:475–81. doi:10.1038/ni1326
21. Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proc Natl Acad Sci U S A (2008) 105:18484–9. doi:10.1073/pnas.0806487105
22. Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol (2008) 180:4742–53. doi:10.4049/jimmunol.180.7.4742
23. Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity (2001) 15:763–74. doi:10.1016/S1074-7613(01)00227-8
24. Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur J Immunol (2004) 34:317–26. doi:10.1002/eji.200490005
25. Ciurea A, Hunziker L, Zinkernagel RM, Hengartner H. Viral escape from the neutralizing antibody response: the lymphocytic choriomeningitis virus model. Immunogenetics (2001) 53:185–9. doi:10.1007/s002510100314
26. Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol (2006) 6:231–43. doi:10.1038/nri1783
27. Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med (2007) 204:2321–33. doi:10.1084/jem.20062150
28. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature (2006) 439:682–7. doi:10.1038/nature04444
29. Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol (2008) 181:2124–33. doi:10.4049/jimmunol.181.3.2124
30. Seedhom MO, Jellison ER, Daniels KA, Welsh RM. High frequencies of virus-specific CD8+ T-cell precursors. J Virol (2009) 83:12907–16. doi:10.1128/JVI.01722-09
31. Pewe LL, Netland JM, Heard SB, Perlman S. Very diverse CD8 T cell clonotypic responses after virus infections. J Immunol (2004) 172:3151–6. doi:10.4049/jimmunol.172.5.3151
32. Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev (2006) 211:236–54. doi:10.1111/j.0105-2896.2006.00395.x
33. Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol (2006) 28:209–19. doi:10.1007/s00281-006-0025-4
34. Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature (1993) 364:798–802. doi:10.1038/364798a0
35. van den Broek MF, Muller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev (1995) 148:5–18. doi:10.1111/j.1600-065X.1995.tb00090.x
36. Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol (2001) 167:4838–43. doi:10.4049/jimmunol.167.9.4838
37. Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood (2002) 100:3698–702. doi:10.1182/blood-2002-02-0657
38. Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med (2015) 212:1125–37. doi:10.1084/jem.20142237
39. Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A (2010) 107:14733–8. doi:10.1073/pnas.1009731107
Keywords: lymphopenia, memory-like T cells, lymphocytic choriomeningitis virus, PD-1, KLRG1
Citation: Schmitt MER, Sitte S and Voehringer D (2016) CD4 T Helper Cells Instruct Lymphopenia-Induced Memory-Like CD8 T Cells for Control of Acute LCMV Infection. Front. Immunol. 7:622. doi: 10.3389/fimmu.2016.00622
Received: 27 August 2016; Accepted: 07 December 2016;
Published: 21 December 2016
Edited by:
Peter Katsikis, Erasmus University Rotterdam, NetherlandsReviewed by:
Kamal M. Khanna, University of Connecticut Health Center, USAJason Kyle Whitmire, University of North Carolina at Chapel Hill, USA
Stephen Philip Schoenberger, La Jolla Institute for Allergy and Immunology, USA
Copyright: © 2016 Schmitt, Sitte and Voehringer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Voehringer, ZGF2aWQudm9laHJpbmdlckB1ay1lcmxhbmdlbi5kZQ==
†These authors contributed equally to this work.
 Michaela E. R. Schmitt
Michaela E. R. Schmitt Selina Sitte
Selina Sitte David Voehringer
David Voehringer