- 1Centre for Immunology and Vaccinology, Imperial College London, London, United Kingdom
- 2Department of HIV/GU Medicine, Chelsea and Westminster Hospital, London, United Kingdom
HIV-1 controllers (HIC) are extremely rare patients with the ability to control viral replication, maintain unchanging CD4 T-cell count, and evade disease progression for extensive periods of time, in the absence of antiretroviral therapy. In order to establish the representation of key genetic correlates of atypical disease progression within a cohort of HIV-1+ individuals who control viral replication, we examine four-digit resolution HLA type and single-nucleotide polymorphisms (SNP) previously identified to be correlated to non-progressive infection, in strictly defined HIC. Clinical histories were examined to identify patients exhibiting HIC status. Genomic DNA was extracted, and high definition HLA typing and genome-wide SNP analysis was performed. Data were compared with frequencies of SNP in European long-term non-progressors (LTNP) and primary infection cohorts. HLA-B alleles associated with atypical disease progression were at very high frequencies in the group of five HIC studied. All four HIC of European ancestry were HLA-B*57+ and half were also HLA-B*27+. All HIC, including one of self-reported African ethnicity, had the HLA-Cw*0602 allele, and the HLA-DQ9 allele was present only in HIC of European ancestry. A median 95% of the top 19 SNP known to be associated with LTNP status was observed in European HIC (range 78–100%); 17/19 of the SNP considered mapped to chromosome 6 in the HLA region, whereas 2/19 mapped to chromosome 8. The HIC investigated here demonstrated high enrichment of HLA types and SNP previously associated with long-term non-progression. These findings suggest that the extreme non-progressive phenotype considered here is associated with a genetic signature characterized by a single-genetic unit centered around the HLA-B*57 haplotype and the possible additive effect of HLA-B*27.
Introduction
A very small proportion of over 7,000 HIV-1+ patients, currently attending the Chelsea and Westminster Hospital have been identified by our group as HIV-1 controllers (HIC) (1, 2). These individuals meet the following strictly defined criteria: (i) infected with HIV-1 for ≥7 years, (ii) maintain stable CD4+ T-cell counts within the normal healthy range (450–1,650 cells/μl blood; slope ≥0 cells/μl blood) throughout clinical follow up, (iii) suppress HIV-1 plasma RNA levels to below detectable limit (<50 copies/ml plasma), and (iv) no history of opportunistic infection despite never receiving antiretroviral therapy (1–3). These unusual patients provide an opportunity to establish objectives for immunotherapy in HIV-1+ individuals, who exhibit irreversible decline of immune function, despite otherwise successful suppressive antiretroviral therapy (4).
HLA types have long been associated with varying rates of disease progression (5), and differing effects have been reported between subtypes of HLA alleles (6), indicating that HLA typing to a high resolution (i.e., four digits) that defines the antigen-binding site is required to provide relevant distinguishing information. This is particularly important as specificity at the amino acid level within the MHC class I molecule-binding groove affects peptide presentation and is a major determinant of clinical phenotype (7, 8).
Genome-wide association studies (GWAS) enable the exploration of various genetic factors on HIV-1 susceptibility, control, and pathogenesis (9). GWAS allow investigation of single-nucleotide polymorphism (SNP) profiles of interesting individuals. Furthermore, genetic correlates of phenotype can be deciphered through comparison with a control group (10). We previously performed a GWAS on a cohort of individuals defined as long-term non-progressors (LTNP) (11). However, plasma viral load was not specified in the inclusion criteria for this LTNP cohort, and the HLA class I and II types of the patients had not been studied in this main GWAS study.
Here, we report a substantial enrichment of both HLA types and SNP, previously identified as associated with non-progression, in the group of rare HIC identified in the Chelsea and Westminster Hospital cohort using consistently undetectable viral load of <50 copies/ml plasma at every visit over the >7 year period follow-up as one of the inclusion criteria.
Materials and Methods
Five patients who formed a subset of participants studied in a larger GWAS were identified within the Chelsea and Westminster Hospital cohort who met the HIC criteria previously defined (1, 2). The group of five HIC had a median CD4 T-cell count 882 cells/μl blood (IQR: 688–985), from a total of 74 visits over a period of 72.7 patient years of follow-up. The CD4 T-cell count slopes for each of these individuals were not significantly different from 0 (i.e., non-declining) over the entire period of clinical follow-up. All individuals investigated were male, clade B infected, with a median age of 42 years (IQR 40–45). Of the five HIC, one was of self-reported African ethnicity, while the other four were European Caucasian. Following ethical approval from the National Research Ethics Committee, written informed consent was obtained prior to blood sample collection. Genomic DNA was isolated as previously described (2). HLA typing at four-digit resolution was performed (HLA class I by reference strand conformation analysis and class II by single-specific primer PCR; Department of Clinical Immunology, Histocompatibility and Immunogenetics Laboratory, Hammersmith Hospital). GWAS was carried out as part of the previously described collaborative study (11). For each HIC, high definition HLA type, ethnicity, and length of time since first diagnosis as HIV-1+, are detailed in Table 1.
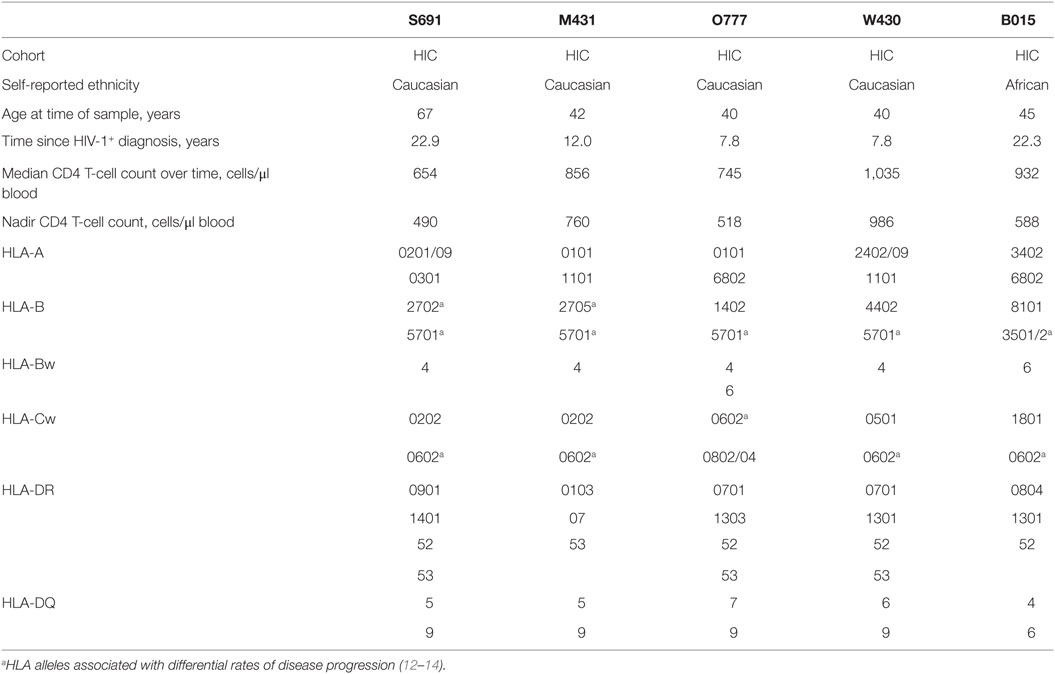
Table 1. Patient characteristics and the high definition HLA types for the HIV-1 controllers (HIC) investigated.
Results
HLA typing revealed a high representation of HLA-B*5701 (100% of Caucasian HIC; 4/4; Table 1), found to be 38.5 times more frequent in these four Caucasian HIC than in a control group of 9,510 Europeans (www.ncbi.nlm.nih.gov/projects/gv/mhc). Two HIC were HLA-B*27+ (Table 1). The unusual HLA-B*2702 allele, found to be present in one of four Caucasian HIC, was found at a very low frequency of 0.002 in the European control group. The more frequent B*2705 allele (f[European control group] = 0.016), was also found to be present in one of the four Caucasian HIC. HLA-Cw*0602 was present in 100% of the HIC investigated here, whereas the allelic frequency in the European population is 0.091 (n = 293; www.ncbi.nlm.nih.gov/projects/gv/mhc). All four Caucasian HIC in this study group had the HLA-DQ9 allele.
Genome-wide association studies were performed on the HIC included in this study as part of the collaborative GISHEAL study, along with 139 others who fulfilled LTNP criteria from the French asymptomatic long term, Italian evaluation of LTNP viro-immunologic study (ELVIS), and additional patients from the Chelsea and Westminster cohort (11, 15, 16), in which viral loads were not taken into account. The group of HIC described here, however, was stringently defined using undetectable viral load (<50 copies/ml plasma at every visit over the >7 year period follow up) as one of the inclusion criteria. SNP of interest, recently shown to correlate with non-progressive HIV-1 infection (11), were identified by comparing profiles of atypical progressors with the control French PRIMO cohort (11, 17). We focused our analysis on the selected SNP that were significantly over—or under—represented in the LTNP cohort. The minor allele of these SNP occurred at increased frequency in the five HIC investigated (Table 2). The four Caucasian HIC carried the minor allele (either homozygous or heterozygous) in 78–100% (median 95%) of the top 19 SNP over-represented in LTNP (11). Conversely, the minor alleles for the five SNP under-represented in LTNP were observed only in 0–60% (median 20%) of the four Caucasian HIC (Table 2). The median frequency of the minor allele in the top 19 over-represented SNP was 0.50 (range 0.25–0.88) among all five HIC investigated, with 80% (range 40–100) of HIC carrying the minor allele in the SNP of interest. When the HIC of African ancestry was excluded and the four Caucasian HIC were analyzed, the median allelic frequency of the top 19 over-represented SNP increased to 0.63 (range 0.33–0.88), with 100% (range 67–100) of HIC carrying the minor allele in the SNP of interest (Table 2).
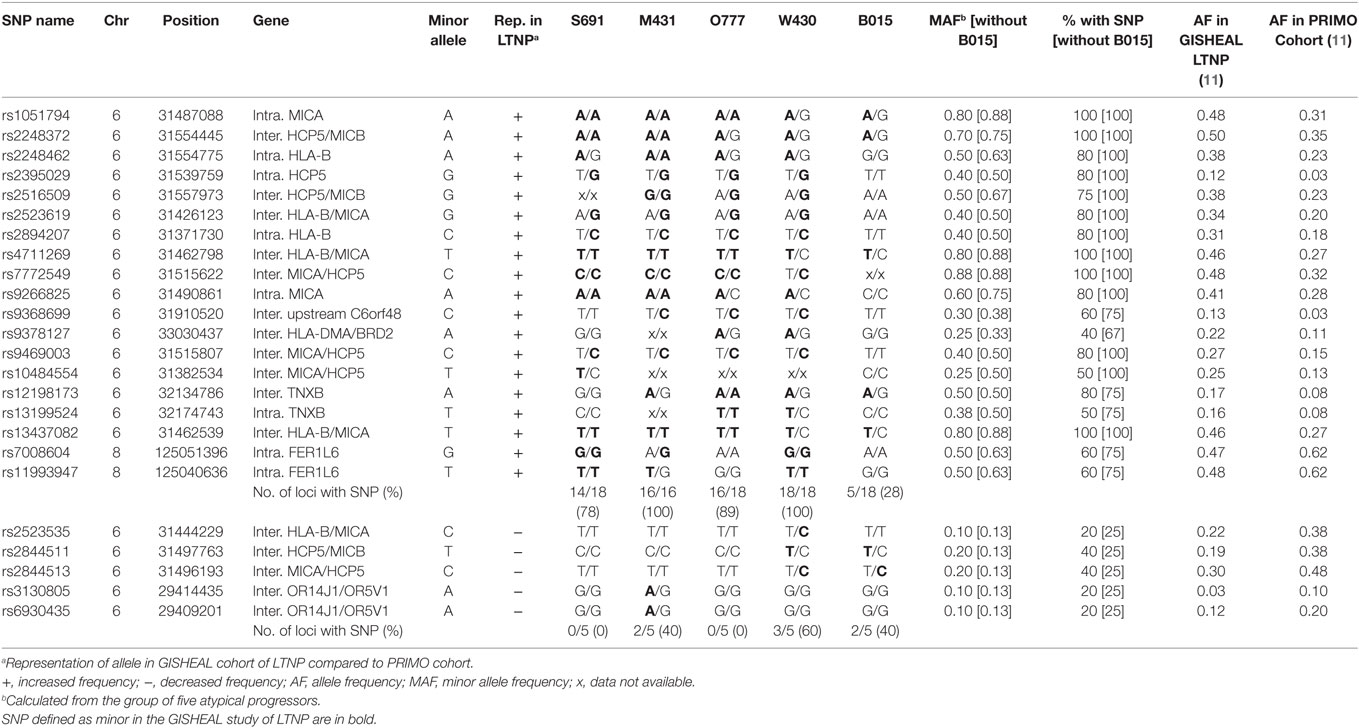
Table 2. Representation of single-nucleotide polymorphism (SNP) found at a significantly higher or lower frequency in the GISHEAL long-term non-progressors (LTNP) cohort (11), in the five atypical progressors.
Of note, 17 of the 19 SNP considered map to chromosome 6 within the HLA region (i.e., between positions 28,510,120 and 33,480,577), raising the possibility that the SNP are in linkage with each other and with the HLA-B*57 haplotype.
Five SNP under-represented in LTNP, all mapping to chromosome 6, had a median allelic frequency of 0.10 (range 0.10–0.20) in the five HIC investigated, with 20% (range 20–40) of HIC carrying the minor allele in the SNP of interest. When the four Caucasian HIC were analyzed, the frequency of the minor allele was 0.13, with 25% of HIC carrying the minor allele in the SNP of interest.
The immunogenetics of elite control of HIV-1 infection in five patients revealed that HLA-B*5701, HLA-Cw*0602, HLA-DQ9, and multiple SNP present in or near MHC genes on chromosome 6 are strongly associated with elite control and that these alleles of the various MHC genes are present on an MHC ancestral haplotype.
Discussion
The association between HLA-B*57 and -B*27 alleles and a slower rate of HIV-1 disease progression is strongly supported by host immunogenetics (7, 12, 18). HLA-B*57 is associated with protection from disease progression at early stages, epitomized by a delay in the HIV-1-induced CD4+ T-cell decline. HLA-B*57 was present in all the Caucasian HIC considered in our study and was greatly enriched compared to a control HIV-1-negative European population. In contrast, HLA-B*27, which is known to delay presentation of AIDS-defining illnesses once the CD4+ T-cell count drops below 200 cells/μl blood (19), was observed in 50% of Caucasian HIC studied. This temporal difference suggests that the anti-HIV-1 functions associated with these HLA types are distinct from one another. When both these alleles are present, as in two of the HIC in our study, the effects may be cumulative.
Natural killer (NK) cells exert a crucial antiviral function during the innate immune response against viral infections. The defined functional and phenotypic features of NK cells in HIC have underlined the contribution of innate immunity in control of viral replication (16). Recent studies have shown that NK cell subset distribution and function are affected by distinct levels of HLA-C (20). Hence, HLA-C expression levels by influencing both the innate and adaptive CD8 T-cell immunity impact both HIV-1 load and disease progression. We found that all five HIC in our study carried the HLA-Cw*0602 allele. HLA-Cw*0602 is an MHC class I allele that has been linked to enhanced HLA-expression and presentation of peptides (21). Additionally, it is associated with psoriasis, exhibits non-responsiveness to upregulation by key pro-inflammatory cytokines, including IFN-α (22), and is associated with delayed disease progression in HIV-1 infection (23). All four Caucasian HIC showed the HLA-B*57 and HLA-Cw*0602 allelic combination, which is also associated with HIV-1 non-progression (14). Moreover, HLA-Cw*6 has associations with the activating KIR 2DS1, the combination representing a major risk factor for psoriasis (24). HLA-C Cw*0602 has not been previously shown to have an independent effect in HIV-1 infection, although the interaction of its gene product with its appropriate cognate KIR molecule most probably plays a role. KIR-typing was not performed on these individuals; however, the interaction between MHC class I and KIR is likely to have implications in HIV-1 control and should be considered in future studies. Recent reports indicate that such studies are warranted which assess the consequences of distinct HLA-C expression, co-infections and, resulting interactions with KIR (20).
HLA-DQ9 was present in all Caucasian HIC and has also been associated with psoriasis (25), as well as other autoimmune disorders, including vitiligo (26) and juvenile diabetes (27). In generalized vitiligo, peripheral regulatory T cells exhibit impaired suppressive activity on autologous CD8 T cells (28), which react to self-peptide. A similar mechanism may operate in HLA-DQ9+ HIV-1-infected individuals, leading to increased activity of HIV-1-specific CD8 T cells, and favoring effective viral control.
Single-nucleotide polymorphisms identified from a large study of LTNP were present in a small cohort of HIC at a greatly enriched frequency and in combination with multiple other SNP of interest. The majority (22/24) of the SNP which were enriched in the HIC, map to the HLA region of chromosome 6, suggesting that HLA-Cw*0602, HLA-DQ9, and most of the SNP are in relatively strong genetic linkage with each other on the extended HLA-B*57 haplotype. Two of the SNP considered map on chromosome 8 and may not be in direct genetic linkage with HLA-B*57. However, we cannot exclude that even these two SNP may be in relatively strong linkage disequilibrium with the HLA region, irrespective of chromosomal separation. Thus, it is possible that all these markers represent the same single-genetic unit in the Caucasian HIC. Unfortunately, HLA type was not defined in LTNP from the GISHEAL and PRIMO cohorts, which prevented us from comparing the allelic frequencies of the SNP between the HIC in our study and a population of subjects carrying HLA-B*57 without the other variants. Consequently, we cannot determine whether the combination of HLA-B*57 with HLA-Cw*0602, HLA-DQ9, and the SNP is simply due to linkage disequilibrium or whether they represent multiple genetic factors with a cumulative beneficial effect on disease progression.
The rate of HIV-1 disease progression is a continuous variable (2), in that within a group of LTNP some individuals are able to control viral replication to higher or lower levels than others. Although HIC are exceedingly rare among HIV-1+ individuals, our data suggest that genetic profiles associated with non-progression may be observed at an even higher frequency in the subgroup of LTNP who, in addition to delayed disease progression, also control viral replication (1). As the HLA class I and II types of the patients had not been studied in the aforementioned GWAS study, the main new information reported is the class I and II types of the patients. Furthermore, the study describes the HLA associations of these exceptional HIC which show a strong enrichment of HLA-B*57 and B*27. Regardless of limitations, the goal was to demonstrate that extreme viral control, albeit rare, is associated with a strong genetic predisposition and that such genetic background is in turn a defining feature of HIC. Recognizing the processes behind this outermost long-term non-progressive phenotype is essential for gaining insight into the pathogenesis of HIV-1 disease and for the identification of an effective functional cure.
The group of patients studied is indeed unique emphasizing the value and rarity of the work presented herein, which results from the identification of HIC from more than 10 years of clinical follow-up, among one of the largest single center cohorts of people living with HIV in Europe. Findings imply a strictly defined group of HIC may be characterized by a “genetic signature,” representative of multiple determinants of resistance from disease progression. Future work into understanding the mechanisms behind the distinct immunological and virological outcome of these HIC profiles is required to develop potent immunotherapeutic strategies.
Ethics Statement
This study was carried out in accordance with the recommendations of “NRES Directorate Guidelines, Riverside Research Ethics Committee” with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “Riverside Research Ethics Committee.”
Author Contributions
All authors conceived, designed, performed and analyzed the experiments, and contributed to the interpretation of results and writing of the manuscript. All authors contributed significantly to the work and have seen and agreed to all content in the manuscript, including the data as presented.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank the patients and staff of the St Stephen’s Center. The authors also thank Ruhena Sargeant and Paul Brookes for assistance with HLA typing, Julien Guergnon and Ioannis Theodorou for help with genome-wide SNP analysis and interpretation, and Sundhiya Mandalia for help with statistical analysis.
Funding
The work presented herein was supported by European Commission initiative GISHEAL EU Project (LSHP-CP-2007-037616), Westminster Medical School Research Trust, St Stephen’s AIDS Trust and the Medical Research Council (grant number G0501957).
References
1. Mandalia S, Westrop SJ, Beck EJ, Nelson M, Gazzard BG, Imami N. Are long-term non-progressors very slow progressors? Insights from the Chelsea and Westminster HIV cohort, 1988–2010. PLoS One (2012) 7:e29844. doi:10.1371/journal.pone.0029844
2. Westrop SJ, Qazi NA, Pido-Lopez J, Nelson MR, Gazzard B, Gotch FM, et al. Transient nature of long-term nonprogression and broad virus-specific proliferative T-cell responses with sustained thymic output in HIV-1 controllers. PLoS One (2009) 4:e5474. doi:10.1371/journal.pone.0005474
3. Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity (2007) 27:406–16. doi:10.1016/j.immuni.2007.08.010
4. Imami N, Westrop SJ, Grageda N, Herasimtschuk AA. Long-term non-progression and broad HIV-1-specific proliferative T-cell responses. Front Immunol (2013) 4:58. doi:10.3389/fimmu.2013.00058
5. Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A (2000) 97:2709–14. doi:10.1073/pnas.050567397
6. Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, et al. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med (2001) 344:1668–75. doi:10.1056/NEJM200105313442203
7. Carrington M, Walker BD. Immunogenetics of spontaneous control of HIV. Annu Rev Med (2012) 63:131–45. doi:10.1146/annurev-med-062909-130018
8. Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science (2010) 330:1551–7. doi:10.1126/science.1195271
9. van Manen D, van ’t Wout AB, Schuitemaker H. Genome-wide association studies on HIV susceptibility, pathogenesis and pharmacogenomics. Retrovirology (2012) 9:70. doi:10.1186/1742-4690-9-70
10. Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, et al. A whole-genome association study of major determinants for host control of HIV-1. Science (2007) 317:944–7. doi:10.1126/science.1143767
11. Guergnon J, Dalmasso C, Broet P, Meyer L, Westrop SJ, Imami N, et al. Single-nucleotide polymorphism-defined class I and class III major histocompatibility complex genetic subregions contribute to natural long-term nonprogression in HIV infection. J Infect Dis (2012) 205:718–24. doi:10.1093/infdis/jir833
12. Hendel H, Caillat-Zucman S, Lebuanec H, Carrington M, O’Brien S, Andrieu JM, et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J Immunol (1999) 162:6942–6.
13. Jin X, Gao X, Ramanathan M Jr, Deschenes GR, Nelson GW, O’Brien SJ, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+-T-cell responses for groups of HIV-1-infected individuals with different HLA-B*35 genotypes. J Virol (2002) 76:12603–10. doi:10.1128/JVI.76.24.12603-12610.2002
14. Salgado M, Simon A, Sanz-Minguela B, Rallon NI, Lopez M, Vicario JL, et al. An additive effect of protective host genetic factors correlates with HIV nonprogression status. J Acquir Immune Defic Syndr (2011) 56:300–5. doi:10.1097/QAI.0b013e3182036f14
15. Marchetti G, Riva A, Cesari M, Bellistri GM, Gianelli E, Casabianca A, et al. HIV-infected long-term nonprogressors display a unique correlative pattern between the interleukin-7/interleukin-7 receptor circuit and T-cell homeostasis. HIV Med (2009) 10:422–31. doi:10.1111/j.1468-1293.2009.00710.x
16. Vieillard V, Fausther-Bovendo H, Samri A, Debre P; French Asymptomatiques à Long Terme (ALT) ANRS-CO15 Study Group. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr (2010) 53:564–73. doi:10.1097/QAI.0b013e3181d0c5b4
17. Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, et al. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS One (2008) 3:e3907. doi:10.1371/journal.pone.0003907
18. Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A (2007) 104:6776–81. doi:10.1073/pnas.0611244104
19. Gao X, Bashirova A, Iversen AK, Phair J, Goedert JJ, Buchbinder S, et al. AIDS restriction HLA allotypes target distinct intervals of HIV-1 pathogenesis. Nat Med (2005) 11:1290–2. doi:10.1038/nm1333
20. Sips M, Liu Q, Draghi M, Ghebremichael M, Berger CT, Suscovich TJ, et al. HLA-C levels impact natural killer cell subset distribution and function. Hum Immunol (2016) 77:1147–53. doi:10.1016/j.humimm.2016.08.004
21. Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet (2010) 42:985–90. doi:10.1038/ng.694
22. Hundhausen C, Bertoni A, Mak RK, Botti E, Di Meglio P, Clop A, et al. Allele-specific cytokine responses at the HLA-C locus: implications for psoriasis. J Invest Dermatol (2012) 132:635–41. doi:10.1038/jid.2011.378
23. Blais ME, Zhang Y, Rostron T, Griffin H, Taylor S, Xu K, et al. High frequency of HIV mutations associated with HLA-C suggests enhanced HLA-C-restricted CTL selective pressure associated with an AIDS-protective polymorphism. J Immunol (2012) 188:4663–70. doi:10.4049/jimmunol.1103472
24. Hayley M, Bourbigot S, Booth V. Self-association of an activating natural killer cell receptor, KIR2DS1. PLoS One (2011) 6:e23052. doi:10.1371/journal.pone.0023052
25. Schmitt-Egenolf M, Boehncke WH, Stander M, Eiermann TH, Sterry W. Oligonucleotide typing reveals association of type I psoriasis with the HLA-DRB1*0701/2, -DQA1*0201, -DQB1*0303 extended haplotype. J Invest Dermatol (1993) 100:749–52. doi:10.1111/1523-1747.ep12476080
26. Xia Q, Zhou WM, Liang YH, Ge HS, Liu HS, Wang JY, et al. MHC haplotypic association in Chinese Han patients with vitiligo. J Eur Acad Dermatol Venereol (2006) 20:941–6. doi:10.1111/j.1468-3083.2006.01686.x
27. Ikegami H, Kawaguchi Y, Yamato E, Kuwata S, Tokunaga K, Noma Y, et al. Analysis by the polymerase chain reaction of histocompatibility leucocyte antigen-DR9-linked susceptibility to insulin-dependent diabetes mellitus. J Clin Endocrinol Metab (1992) 75:1381–5. doi:10.1210/jc.75.5.1381
Keywords: HIV-1, disease progression, elite controllers, HLA antigens, single-nucleotide polymorphism
Citation: Westrop SJ, Cocker ATH, Boasso A, Sullivan AK, Nelson MR and Imami N (2017) Enrichment of HLA Types and Single-Nucleotide Polymorphism Associated With Non-progression in a Strictly Defined Cohort of HIV-1 Controllers. Front. Immunol. 8:746. doi: 10.3389/fimmu.2017.00746
Received: 09 February 2017; Accepted: 12 June 2017;
Published: 27 June 2017
Edited by:
Aurelio Cafaro, Istituto Superiore di Sanità, ItalyReviewed by:
Antonella Caputo, University of Padua, ItalySarah Rowland-Jones, Oxford University, United Kingdom
Copyright: © 2017 Westrop, Cocker, Boasso, Sullivan, Nelson and Imami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nesrina Imami, bi5pbWFtaUBpbXBlcmlhbC5hYy51aw==
 Samantha J. Westrop
Samantha J. Westrop Alexander T. H. Cocker
Alexander T. H. Cocker Adriano Boasso
Adriano Boasso Ann K. Sullivan2
Ann K. Sullivan2 Nesrina Imami
Nesrina Imami