- Department of Medicine, Clínica Girassol, Luanda, Angola
Cirrhosis is a common final pathway for most chronic liver diseases; representing an increasing burden worldwide and is associated with increased morbidity and mortality. Current evidence has shown that, after an initial injury, the immune response has a significant participation in the ongoing damage, and progression from chronic viral hepatitis (CVH) to cirrhosis, driving the activation and maintenance of main fibrogenic pathways. Among immune deregulations, those related to the subtype 17 of T helper lymphocytes (Th17)/interleukin-17 (IL-17) axis have been recognized as key immunopathological and prognostic elements in patients with CVH. The Th17/IL-17 axis has been found involved in several points of fibrogenesis chain from the activation of stellate cells, increased expression of profibrotic factors as TGF-β, promotion of the myofibroblastic or epithelial–mesenchymal transition, stimulation of the synthesis of collagen, and induction of imbalance between matrix metalloproteinases and tissue inhibitors of metalloproteinases (TIMPs). It also promotes the recruitment of inflammatory cells and increases the expression of proinflammatory cytokines such as IL-6 and IL-23. So, the Th17/IL-17 axis is simultaneously the fuel and the flame of a sustained proinflammatory and profibrotic environment. This work aims to present the immunopathologic and prognostic role of the Th17/IL-17 axis and related pathways in fibrogenesis and progression to cirrhosis in patients with liver disease due to hepatitis B virus (HBV) and hepatitis C virus (HCV).
Introduction
Liver cirrhosis is a common final pathway for most chronic liver diseases; and is increasingly becoming a major cause of global health burden, being responsive for high morbidity and mortality worldwide. Chronic viral hepatitises (CVHs) are the leading cause of cirrhosis, also with increasing burden worldwide (1). According to the report of Global Burden of Disease Study 2013, between 1990 and 2013, occurred a 63% increase in the global viral hepatitis deaths, passing from the tenth (in 1990) to seventh (in 2013) leading cause of death worldwide (1). There was also an increase in attributable years of life lost, years living with a disability (34% for each), and in the absolute burden of the disease (1). In parallel, despite significant progress in the treatment of CVH during the last decades, the number of deaths from cirrhosis and hepatocellular carcinoma (HCC) increased in the last 20 years (2, 3).
Currently, it is known that after the initial injury, before cirrhosis is established, multiple pathways of fibrogenesis are activated as a result of continuous interaction between pathogen-related factors (4–6), the host genetic such as certain HLA haplotypes and cytokines gene polymorphisms (7–11), liver resident cells, and the immune system (9–13) (Figure 1). Indeed, cirrhosis is a reflection of sustained injuries and constant and exaggerated attempts of tissue repair, in which the immune system has crucial participation (14–16). The inappropriate immune response have an influence on the activation and maintenance of fibrogenic pathways and progression from CVH to cirrhosis (17–20). Multiple imbalances in immune response, either in cellular or soluble factors, have been associated with the evolution to cirrhosis in viral hepatitis (21, 22). In addition, the immune response influences on viral persistence and response to treatment (11, 23). Thus, efforts have been made in research and clinical practice, aiming to get a better comprehension of the immunological mechanisms underlying these pathways, their exploration as immune biomarkers to predict outcomes, response to treatment, as well as explore their potential as targets for adjuvant therapeutics (24–26).
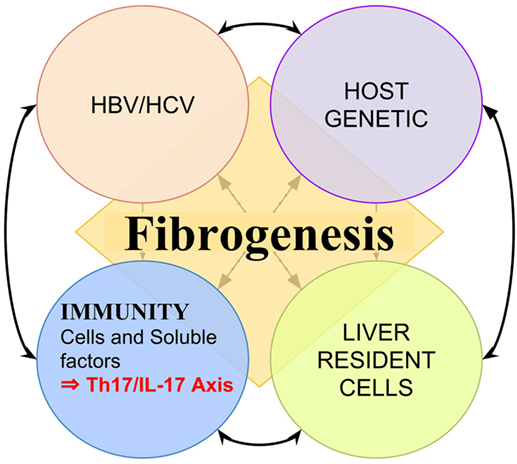
Figure 1. Representation of multiple factors influencing liver fibrogenesis. Fibrogenesis is a result of continuous interaction between pathogen-related factors such as hepatitis B virus (HBV) protein X and hepatitis C virus (HCV) core antigen (4–6, 27), the host genetic such as certain HLA haplotypes (7, 8), liver resident cells, and the immune system such as certain interleukin polymorphisms (9–13, 15).
Among the elements of the immune response, the subtype 17 of T helper lymphocytes (Th17) has gained space as a biomarker with noteworthy performance in the prediction of progression to cirrhosis in liver diseases, particularly in CVH (20, 28). Therefore, the cytokines secreted by Th17, particularly the interleukin-17 (IL-17), also have been implicated in the activation of fibrogenic pathways and progression to cirrhosis (13, 17). Th17 cells have a predominantly effector functional profile, being responsible for the immune surveillance, but are also involved in the pathogenesis of many autoimmune diseases and in the mechanisms of fibrosis in many organs, after the initial injury (29–31).
In CVH, the Th17/IL-17 axis expresses a sustained aggression, and especially a proinflammatory and profibrotic environment with recruitment and activation of other cells, promoting this way, more tissue injury and dysfunctional reparative responses (13, 17, 32, 33). This review aims to summarize the existing knowledge on the immunopathologic and prognostic role of the Th17/IL-17 axis and related pathways in fibrogenesis and progression to cirrhosis in patients with liver disease due to hepatitis B virus (HBV) and hepatitis C virus (HCV).
An Overview of General Inflammatory Biomarkers as Outcome Predictors in CVH
General Inflammatory biomarkers, such as C-reactive protein (CRP), and interleukin-6, have been associated with poor outcomes on viral hepatitis (11, 26, 34). High levels of interleukin-6 are related to adverse outcomes in the CVH, including greater severity (35, 36), worse response to treatment and viral persistence (11, 26), and evolution to cirrhosis, HCC, or death (35, 36). In a study including 149 patients with CHC (and 17 controls) who underwent 12 weeks’ treatment with pegylated IFN-α2b and ribavirin, serum IL-6 levels were significantly higher in CHC patients than in controls, and a high pretreatment IL-6 was associated with lower rates of sustained virologic response (SVR) (52 vs. 79%; P = 0.012) (26). In addition, SVR was accompanied by a significant decrease in IL-6 levels (from 2.7 to 1.5 pg/ml; P = 0.029) at 4 weeks of treatment, remaining significantly lower in responder than in non-responder (26). In other studies, it was observed that certain polymorphisms in IL-6 or its promoter are associated with lower rate of spontaneous clearance (11), and increased risk of HCC in patients with CVH-related cirrhosis (10, 37). Many other proinflammatory cytokines, including interleukin IL-1β, IL-18, and TNF-α have been associated with increased risk of progression to cirrhosis (38–40). Certain polymorphisms in TNF-α are also associated with higher risk of becoming chronic carriers of viral infection, progression to cirrhosis and HCC (15, 40).
In addition to the soluble factors, cells of the inflammatory response as neutrophils (19, 41) and monocytes have been found associated with worse outcomes in viral hepatitis. All these elements (cells and soluble factors) are part of a context in which the Th17/IL-17 axis imbalances work as crucial elements to feed and keep this proinflammatory and profibrotic microenvironment (20, 22, 42–44), as described in the next sections.
The Th17/IL-17 Axis and Outcome Prediction in CVH
The inappropriate immune response at the level of the Th17/IL-17 axis exerts influence in maintaining the fibrogenic pathways and progression from CVH to cirrhosis (9, 44). The fibrogenic role of IL-17, the main cytokine of Th17, has been appointed in increasing number of publications in recent years (13, 45, 46). In addition to its direct effect on fibrogenesis, the Th17/IL-17 axis is the fuel to sustain the proinflammatory environment, with the recruitment of the other cells and stimulation of the synthesis of other soluble factors (29, 33, 47–50). At the same time, Th17/IL-17 axis expresses itself, a response to a sustained proinflammatory stimulus (51, 52). So, we can understand the broad scope of the elements of this axis on the CVH, predicting outcomes in different points of the disease, from spontaneous healing (53), response to treatment (23, 54, 55), occurrence of acute decompensations (56), progression to cirrhosis (9, 28), and HCC (53), including post-transplant recurrence (57), as detailed in this section.
The Th17/IL-17 Axis and Severity of CVH
The Th17/IL-17 axis is associated with high disease severity in CVHs, as found in a study where patients with chronic hepatitis B (CHB) had higher percentage of Th17 cells in peripheral blood than healthy controls (HCs) (1.53 vs. 0.92%); and among patients there was a positive correlation between peripheral Th17 cells and serum alanine aminotransferase (ALT) (58). In another study, including 96 patients with HBV-related conditions, the serum IL-17 concentration, intrahepatic, and peripheral Th17 cells were significantly higher in both CHB and acute-on-chronic liver failure (ACLF) patients than in asymptomatic surface antigen carriers (AsC) or HCs (59). Th17 cells increased progressively as aggravated the immune inflammation from AsC, CHB, to ACLF, with a positive correlation with severity markers (INR and MELD score) (59). Compared with HCs, patients with CHC had also higher proportions of Th17 cells either circulating (1.56 vs. 0.96%) or infiltrating the liver (16.08 vs. 0.82/hpf), associated with higher serum IL-17 levels (84.86 vs. 60.52 pg/mL) (42). In these patients, both peripheral and intrahepatic Th17 cells correlated with the severity of liver inflammation and damage (28, 42).
The Th17/IL-17 Axis and Progression to Cirrhosis in CVH
Concerning the progression to cirrhosis, in a study including 173 patients with chronic liver diseases, there was a significant increase in serum IL-17 protein and IL -17 mRNA levels in chronic HBV-related conditions than in HCs (P < 0.001); and patients with cirrhosis exhibited the highest serum IL-17 and IL-17 mRNA in peripheral blood mononuclear cells (PBMCs) (44). In another study with 101 patients with HBV-related LC or CHB; peripheral Th17 cells increased significantly in patients with cirrhosis as increased disease severity (mean: 3.51, 3.94, and 4.46; for Child–Pugh A, B, and C, respectively) (28). In intrahepatic tissue, a study conducted among 91 patients with chronic liver diseases, there was a significant increase intrahepatic expression of IL-17 in chronic HBV-related conditions than in HCs; furthermore, intrahepatic IL-17 was mainly localized in the fibrosis region, and its level correlated strongly with the degree of fibrosis (60). Other studies have also found increased intrahepatic IL-17+ cells or IL-17 levels, which correlate positively with fibrotic staging scores and clinical progression from CHB to cirrhosis, and most IL-17+ cells located in the fibrotic area (28, 44). In addition, intrahepatic IL-17 accompanied the higher serum IL-17 protein and mRNA levels in PBMCs, being higher among patients with cirrhosis than those with CHB, or HBsAg carriers (P < 0.01, for both) (44). Among HCV patients, a study evaluating the effect of HCV recurrence after orthotopic liver transplantation (OLT) showed that recipients with significant HCV-induced allograft fibrosis/cirrhosis and inflammation, presented higher frequency of HCV-specific Th17 cells, as well as proinflammatory mediators (IL-17, IL-1β, IL-6, IL-8, and MCP-1) (57).
In genetic studies, it has been noted that certain polymorphisms in IL-17 genes are more often present among those who progress to LC than in CHB patients (9, 14, 53); and are associated with a significant increase in LC risk either among monozygotic patients [OR 4.1, 95% CI: 1.4–11.84 or single allele carriers (OR 1.8, 95% CI: 1.16–2.9)] (14).
The Th17/IL-17 Axis and the Response to Treatment in CVH
In CVH, the Th17/IL-17 axis status is associated with response to available treatments (23), as found in a study with 30 CHB patients, in which treatment with entecavir was associated with a significant decrease in the Th17 and Treg cell frequencies and related cytokines, in parallel to the reduction of HBV DNA load (61, 62). In other studies in CHB, the treatment either with telbivudine or with interferon-α resulted in the normalization of serum ALT and reduction or suppression of viral replication, associated with a significant decline in circulating Th17 cells and IL-17 levels (23, 54). Studies in HCV also found the same effect, as shown in a study including 27 HCV-infected patients, in which combined treatment with pegylated IFN-α and ribavirin resulted in a significant decrease in factors related to Th17 (IL-6 and IL-17), Th1 (IFN-α and MIP-1) responses, and profibrotic factors (FGF-b, VEGF); and this impact was principally in responder patients (62). In another study, Th17-related gene polymorphisms were associated with sustained responses to PEG-IFNa-2α (55).
In line with these findings, in an interventional study involving 56 cirrhotic patients allocated to autologous bone marrow mesenchymal stem cells (ABMSCs) transplantation or control group; transplantation significantly improved the liver function, accompanied by a marked decrease in Th17 cells and serum levels of proinflammatory cytokines (IL-17, TNF-α, and IL-6) (63).
The Th17/IL-17 Axis and Mortality Prediction in CVH
Beyond its effect in predicting disease severity, the Th17/IL-17 axis elements are also predictors of mortality (56). In a study including 60 HBV-infected patients (30 with CHB and 30 with ACLF), the disproportionate increase of Th17 (compared to Treg) was associated with low survival among those with ACLF (64). These results have also been found in another study including 98 patients with HBV-related conditions (70 with CHB and 28 with LC), where the low Treg/Th17 ratio was associated with low survival among patients with cirrhosis and was associated with worse Child–Pugh and MELD scores (65). In another study, including 80 HBV-infected patients (40 with ACLF and 40 with CHB), the frequency of Th17 cells in peripheral blood, as well as IL-17 mRNA level in PBMCs, was significantly higher among ACLF patients who died than among survivors; and correlated positively with serum total bilirubin (r = 0.392, P = 0.012) and MELD score (r = 0.383, P = 0.015) (56). These findings suggest a significant contribution of this immune imbalance in disease severity and mortality.
The Role of Other IL-17 Sources and Related Imbalances in CVH Outcomes
Besides the Th17 cells, recent investigations have found other immune cells, such as mast cells, and neutrophils as important sources of IL-17 in CVH, especially in late fibrosis stages (66–68). These findings emerge in a context after many studies have shown the domain of neutrophils as a predictor of outcomes in viral hepatitis and related diseases (19, 41). Therefore, these research together bring to light the importance of the interleukin-17 in pathogenesis and progression of CVH and can be one of the ways by which neutrophils, and other immune cell populations, exercise their pathogenic and predictive effect in CVH. Other Th17-related cytokines, such as IL-6 and IL-23, are involved in the evolution of CVH, regardless of stimulation of Th17 (11, 37, 69). The IL-23 and its receptor, in particular, have been found higher among HCV-infected patients than in control (mean 24.6 vs. 20.2 pg/mL; P = 0.005), with a positive correlation with ALT in HCV patients (69). Concerning the IL-6, its role was described in previous sections.
Besides increased Th17 cells, CVH has been associated with decreased or disproportionate count of regulatory T lymphocytes (Treg), another T helper subset, which is the functional counterbalance of Th17 cells (70); configuring a Treg/Th17 imbalance (13, 42, 64, 71). The balance between these two T helper lymphocytes subpopulations is fundamental (21, 22), and influenced by various factors (72–74). There is a plasticity and reciprocity between the two cell subpopulations that depends on environmental factors (70, 75, 76), being the proinflammatory environment, like that of the CVH, favorable to the polarization to Th17 cells (77, 78). The Treg/Th17 imbalance has been associated with greater hepatocellular damage in CVHs (21, 71, 79) and is related to advanced stages of cirrhosis and HCC (22, 65, 79), correlating inversely with severity scores and mortality (22, 64, 65). The Treg/Th17 imbalance has also been observed in other fibrosing liver diseases, such as autoimmune liver diseases (ALD) (80), biliary atresia (81), primary biliary cirrhosis (PBC) (82–84), non-alcoholic steatohepatitis (85, 86), schistosoma-induced hepatitis (87–89), and drug-induced hepatitis (90, 91).
Table 1 summarizes the clinical studies that evaluated the role of the Th17/IL-17 axis and related imbalances as drivers and predictors of outcomes in CVH.
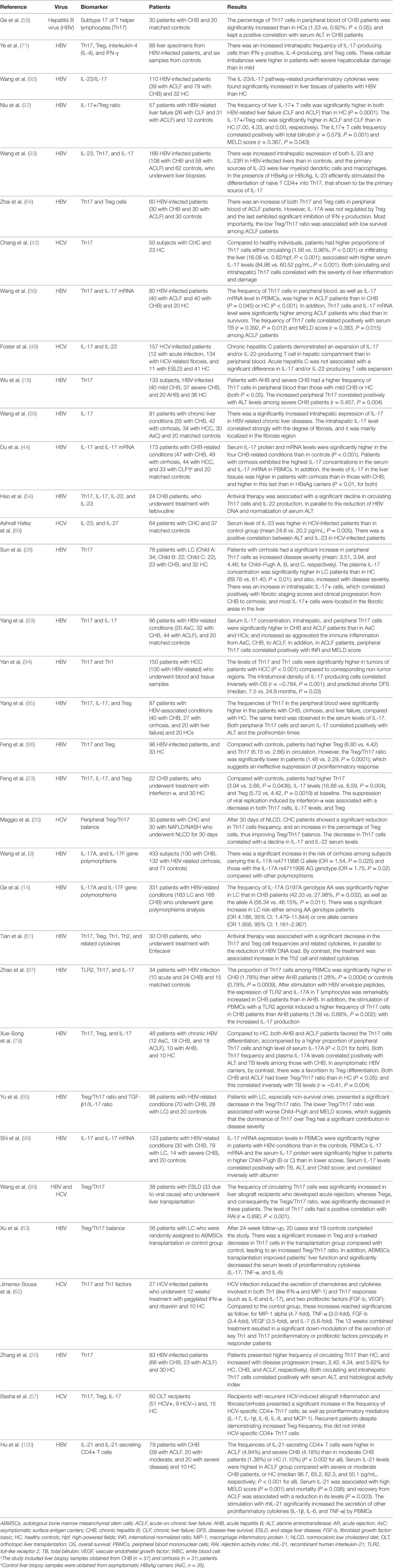
Table 1. Clinical studies on the role of the Th17/IL-17 axis and associated imbalances in predicting outcomes in chronic viral hepatitis (CVH) and cirrhosis.
Mechanisms and Pathways Linking the Th17/IL-17 Axis to Fibrogenesis and Cirrhosis in CVH
The Th17 Differentiation and Th17-Secreted Cytokines
Overall, Th17 cells differentiate from naive T helper cells, in response to a variety of stimuli, in the presence of key cytokines, namely IL-1β, IL-6, IL-21, IL-23, and TGF-β (101–105). In viral hepatitis, the virus particles are recognized by toll-like receptor (TLR2 and TLR4) present on the surface of the antigen-presenting cells (dendritic cells, macrophages, and monocytes) that result in their activation (97, 106). These activated cells, using the nuclear factor kappa B (NF-κB) and/or mitogen-activated protein kinase (MAPK) signaling pathways, produce the proinflammatory cytokines IL-1, IL-6, IL-21, and IL-23 (38, 93, 107) that drives the Th17 differentiation (93, 104, 107, 108). In the particular case of HCV, there are two additional pathways: the first one consists in the production of the thymic stromal lymphopoietin (TSLP) by HCV-infected hepatocytes, in an NF-κB-dependent process, and is this hepatocyte-derived TSLP that enhances activated APCs to produce the IL-1, IL-6, IL-21, and IL-23 (109). The second consists in particular evidence that HCV core protein exerts a function of a toll-like receptor 2 ligand, promoting, by itself, the activation of the APCs, the production of inflammatory cytokines that favor Th17 differentiation, and the evasion of the immune system (4, 12, 27). After being differentiated, the Th17 cells secrete its cytokines (IL-17, IL-21, and IL-22), being the IL-17 the main driver of a chain of events that have in common the favoritism of the proinflammatory and profibrotic pathways (13, 47, 49).
The Role of IL-17 Axis, and Associated Signaling Pathways, in Liver Fibrogenesis and Cirrhosis
The subtype 17 of T helper lymphocyte cells is increased in almost all chronic and fibrosing liver diseases, including ALD, such as autoimmune hepatitis (AIH) (50, 110, 111), primary sclerosing cholangitis (PSC) (112, 113), PBC (16, 83, 114, 115); biliary atresia (29, 81, 116), non-alcoholic steatohepatitis (85, 117, 118), and viral hepatitis (42, 44, 58, 109). These findings reveal the pivotal role of the Th17/IL-17 axis in liver fibrogenesis. However, the stimuli that attract Th17 cells to the liver are not completely elucidated. What is known is that injured liver cells secrete a variety of chemokines, such as CXCL9, CXCL10, and CCL20 (119, 120), that drive the recruitment of Th17 cells to the liver, binding to their receptors (CXCR3 and CCR6) expressed in Th17 cells (16, 119–123). This aggregate of chemokines and their receptors seems to determine, the differential cellular recruitment (32, 123, 124), the disposition of the Th17 cells within fibrosis areas (16, 60, 119, 120); and CXCL10, in particular, has itself a profibrotic effect influencing in the number and activity of HSCs, and participating in the cross talk between hepatocytes, HSCs, and immune cells (124–126).
In the liver, Th17 cells produce their cytokines (93), with IL-17 being the most associated with the progression of cirrhosis (13, 28, 49). There are receptors for IL-17 expressed in hepatocytes, in the liver sinusoids endothelial cells, in hepatic stellate cells (HSCs), and Kupffer cells (KC) (13). The functional implication of IL-17 in liver tissue is well characterized in the activation and/or stimulation of HSCs and KC (13, 17, 127) and cholangiocytes (115, 116).
Hepatic Stellate Cells
As already said, stellate cells express receptors for IL-17 (IL-17RA and IL-17RC) on their surface (13). The stimulation with IL-17 induces the rapid translocation of transcription factors NF-κB and Stat3 to the cellular nucleus (13), where activate the gene transcription of proinflammatory cytokines (IL-6 and TNF-α) and profibrotic factors (TGF-β1) (17, 50). In addition, IL-17 promotes the proliferation of HSCs, the upregulation of TGF-β receptor, IL-17RA, and IL-17RC (13, 16, 127). So, the IL-17 has the property to induce the activation of HSCs and fibrogenesis, and this effect seems to be synergistic with that of IL-6 and TNF-α (13, 128).
In the liver tissue, particularly in HSCs, the IL-17 also presents the following effects: increases the genic expression of type I collagen and induces its production through TGF-β, or Stat3/SMAD2/3 signaling pathways (13, 127). In addition, IL-17 upregulates matrix metalloproteinases (MMP2, MMP3, and MMP9) expression via NF-κB and Stat3 signaling pathways (13, 127, 129) and increases the expression of tissue inhibitor of matrix metalloproteinase I (TIMP1) and the production of related proteins (127). The combination of these effects results in increased production of extracellular matrix and changes in its degradation. This role of IL-17 has been reinforced in experimental model where liver fibrosis was inhibited or attenuated in IL-17RA−/− mice exposed to carbon tetrachloride (CCl4) or subjected to bile duct ligation (BDL), associated with reduced mRNA expression of fibrogenic genes (collagen-α1, MMP3, TIMP1, and TGF-β1) (13, 130). The role of IL-17 on MMPs and related signaling pathway has also been found in other organs such as the heart (131).
IL-17, TGF-β, and Induction of Cellular Transition/Transdifferentiation
In addition to the direct effects, described above, the IL-17 cooperates with TGF-β1 to induce the activation of HSCs and their transition into a proliferative, contractile, and fibrogenic phenotype—the myofibroblast (13, 132, 133). These events also lead to an excessive synthesis of ECM and the contractility of myofibroblasts resulting in changes in the hepatic microarchitecture and microcirculation (134–136). The IL-17 also has the effect of inducing epithelial–mesenchymal transition (EMT) in hepatic tissue as observed in hepatocytes of patients with HCC (137) and biliary epithelial cells as seen in PBC (115). It is important to emphasize that the exposure of the hepatic tissue to IL-17 increased the expression of TGF-β in almost all liver resident cells (13, 127). Moreover, the TGF-β is well documented that induces the EMT of the hepatocytes (138–140). Therefore, we can deduce that the IL-17, through the induction of TGF-β production, is an indirect promoter of EMT in the liver (13, 132, 137, 139). This effect of IL-17 in EMT is well described in many other organs (and clinical conditions), as in the respiratory epithelium (141, 142) and prostate (143); and has been proven to be a process dependent on TGF-β or NF-κB pathways (115, 141, 142, 144).
The activation of HSCs is associated with two other Th17/IL-17 axis-related changes. The first results from the rarefaction of retinoic acid in HSCs. Under normal conditions, the quiescent HSCs have many granules containing retinoic acid (135), which acts inhibiting the Th17 and favoring the Treg cells differentiation, with a protective effect on the liver (145, 146). However, when activated HSCs undergo changes in their metabolism and retinoic acid content, which affects the differentiation of Treg cells and, consequently, the loss of the protective effect (135, 147, 148). The second results from the evidence that activated HSCs exacerbate liver fibrosis by enhancing IL-17A production by T cells, in a TLR3-dependent manner (45), that in combination with the rarefaction of retinoic acid results promoting further Treg/Th17 imbalance and fibrogenesis (45, 149).
Kupffer Cells
In KC, as well as in HSCs, stimulation with IL-17 led to the production of proinflammatory cytokines and TGF-β1, through NF-κB and Stat3 pathways (13, 150–153). It also upregulates the receptors of TGF-β, IL-17A, and IL-17C and promotes the further production of IL-17A, IL-17F (13, 127). In addition, under stress conditions, KC are involved in increased differentiation of Th17 and decreased Treg, through an IL-6-dependent mechanism, promoting further Treg/Th17 imbalance and perpetuating the proinflammatory and fibrogenic consequences of this axis (43, 154).
The Role of IL-17 in the Systemic Circulation and Its Repercussions in the Liver Tissue
In the systemic circulation, the IL-17 sustains the proinflammatory environment, stimulating the granulopoiesis through the release of granulocyte-macrophage colony-stimulating factor (GM-CSF) (33, 155–158). On inflammatory cells (either in systemic circulation or infiltrating the liver), the IL-17 induces the release of the IL-6 (159). The elevation of the IL-6 contributes to further Th17 responses because it promotes its differentiation (104), as IL-6 receptors are expressed on the surface of CD4+ T cells (43, 51). This process also occurs using the STAT3, NF-κB, and MAPK signaling (43, 151).
The repercussion at the organic level is the liver infiltration by inflammatory cells that occurs because IL-17 promotes endothelial activation (32, 33, 47), and release of attracting chemokines such as CXCL5 and CXCL8/IL-8 (156, 160, 161) whose receptors (CXCR1 and CXCR2) are abundantly expressed neutrophils and monocytes (32, 125, 161–164). Once in the liver, neutrophils are involved in various points of the fibrogenesis chain, including the release MMP9 (165–167) and seem to affect the functioning of the TIMP-1 (168). Recent studies have shown that neutrophils released itself the IL-17, mainly in the advanced stages of fibrosis (66); and there is a sustained cross talk between neutrophils and Th17 cells (32, 164). Figure 2 represents the chain of events from viral injury, Th17/IL-17 axis activation, to liver fibrogenesis and cirrhosis.
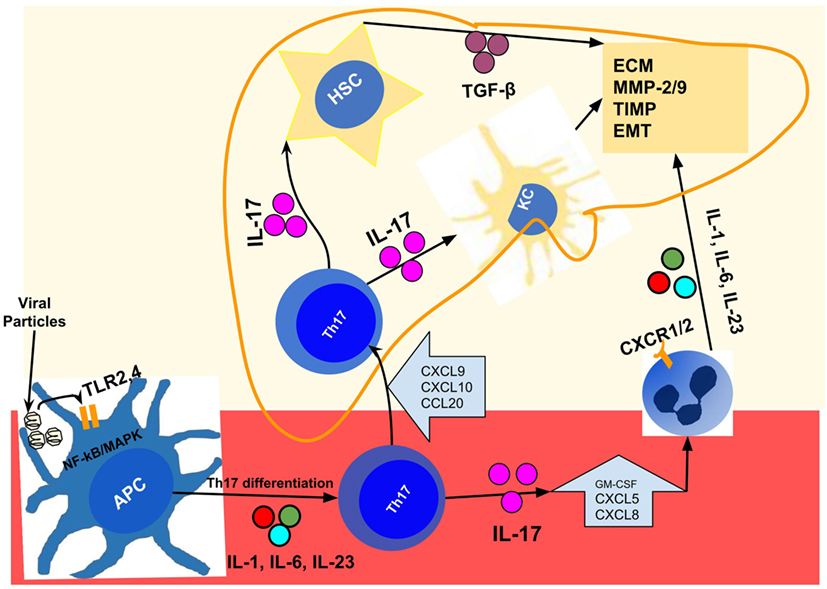
Figure 2. Representation of events chain from viral injury to Th17/IL-17 axis activation, liver fibrogenesis, and cirrhosis in chronic viral hepatitis. After infection by hepatitis B virus/hepatitis C virus; distinct viral particles are recognized byTLR2 and TLR4 present on the surface of the APCs (dendritic cells, macrophages, and monocytes), which result in their activation (97, 106). These activated cells, using the NF-κB and MAPK signaling pathways, produce the proinflammatory cytokines IL-1, IL-6, IL-21, and IL-23 (38, 93, 107) that drive the Th17 differentiation and IL-17 production in peripheral blood (93, 104, 107, 108). In the liver, injured cells secrete a variety of chemokines like CXCL9, CXCL10, and CCL20 (119, 120) that drive the recruitment of Th17 cells to the liver, through binding to their receptors (CXCR3 and CCR6) expressed in Th17 cells (120–123). Intrahepatic Th17 cells and IL-17 are responsible for hepatic stellate cell activation (13, 17), increased expression of TGF-β (127), MMP (13, 127, 130), collagen synthesis (13, 127), and induction of EMT (115, 137, 142). In addition, it promotes the recruitment of other inflammatory cells (32, 160) through the release of chemokines such as CXCL5 and CXCL8/IL-8 (156, 160, 161) whose receptors (CXCR1 and CXCR2) are abundantly expressed in neutrophils, monocytes, and macrophages (32, 125, 161–164). APCs, antigen-presenting cells (dendritic cells, macrophages, and monocytes); EMT, epithelial–mesenchymal transition; HSC, hepatic stellate cell; IL-1, interleukin-1; IL-17, interleukin-17; IL-21, interleukin-21; IL-23, interleukin-23; IL-6, interleukin-6; KC, Kupffer cells; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinases; NF-κB, nuclear factor-kappa B; TGF-β, transforming growth factor beta; Th17, T helper lymphocytes, subtype 17; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4.
The Role of IL-17 Axis in Other Liver Diseases and Other Organs Fibrosis
The role of Th17/IL-17 axis in hepatic fibrosis has been found in various liver diseases such as NASH (117, 118), obstructive cholestasis (169), PSC (112), PBC (16, 114, 115), biliary atresia (29, 116), drug-induced (91, 170), protozoa-associated cirrhosis (87, 171, 172), and viral hepatitis (20, 30, 44, 173). Moreover, the role of Th17/IL-17 axis extends to related diseases such as HCC (94, 174, 175), which suggests a continuous, or at least related parts of a whole, in the pathogenesis of these conditions (53, 94, 174, 175). Additional evidence comes from observational studies that have found an increased occurrence of hepatic inflammation associated with dysregulated protein/lipid metabolism, and progression to cirrhosis in clinical conditions such as psoriasis that have the Th17/IL-17 axis hyperactivation as the crucial point in their pathogenesis (48, 176).
It is worth noting that HCV infection induces the development of autoantibodies to liver autoantigens, in up to 10% of patients, in addition to previously described mechanisms, leading to an overlap between CVH and AIH (177–180). Certainly, the Th17/IL-17 axis plays a pivotal role in the progression to cirrhosis regardless of the dominant mechanism in the pathogenesis (20, 49, 111, 181, 182).
The role of the Th17/IL-17 axis has been found in other diseases with a fibrosing behavior in various organs such as intestine (31, 183), lung (184, 185), and peritoneum or kidney (46, 186); and its block has shown to prevent/mitigate the fibrosis in many pathological conditions (187–189).
Hepatoprotective Agents Targeting the Th17/IL-17 Axis, Associated Cytokines, and Signaling Pathways
As we have seen the Th7/IL-17 axis is involved in several known points of fibrogenesis chain, including the activation of HSCs (17), recruitment of inflammatory cells (32, 160), expression of proinflammatory and profibrotic cytokines (127), in stimulus to collagen synthesis, and in the imbalance between MMP and TIMPs (13, 127, 133). Added to this is the parallel evidence of being a promoter of EMT or myofibroblast transition (115, 137, 142). Therefore, the inhibition of Th17/IL-17 axis (and its signaling pathways) represents a promising strategy in the treatment of organic fibrosis.
Agents That Downregulate the Th17/IL-17 Axis or Restore the Treg/Th17 Balance
There are a variety of agents targeting these immune pathways, with the potential to slow down the CVH-induced cirrhosis. From those that downregulates the Th17/IL-17 axis to those that restore the Treg/Th17 balance. Brief citations are made about some of these agents that have shown benefit in preliminary studies, such as cannabinoid receptor 2 agonists, whose action is to counteract immune and fibrogenic responses induced by interleukin-17 (190). Another treatment that ameliorates hepatic fibrosis by regulation of Treg/Th17 cells, and downregulating the IL-17, is bone marrow-derived stem cells transplantation (63, 191). It is also worth to cite the vitamin D and analogs that inhibit the development of liver fibrosis by reducing the Th17 differentiation and IL-17 production, and activate the Treg differentiation (186, 192–194). The rapamycin demonstrated to improve hepatic fibrosis that was associated with a decrease of Th17 and expansion of Treg (91, 195). In another study, the administration of an mTOR inhibitor decreased the IL-17 production induced by IL-6; and this effect occurred through decreasing mTOR/STAT3 activation (43). Halofuginone, an inhibitor of Th17 cells differentiation (196); has shown to be a promising antifibrotic drug, and its impact in reducing liver fibrosis severity is associated with a significant reduction in Th17 cells and related cytokines (197). Another compound with protective effects in liver fibrosis is the polyphenolic molecule mongol that inhibits Th17 cells differentiation and suppresses HSCs activation (198).
Still, about Treg/Th17 balance restoration, adoptive transfer of Tregs ameliorated the severity of liver injury, accompanied by increased levels of hepatic Treg and IL-10 as shown in a model of Triptolide-induced liver injury (90). On the other hand, some drugs with use and efficiency established in viral hepatitis, such as interferon, appear to have effects that involve this axis, among its various mechanisms (24, 62, 187).
Agents Targeting the Receptors and Signaling Pathways Involved in Th17/IL-17 Axis Effects
The inhibition of the activation of HSCs is, undoubtedly, one of the most attractive strategies to slow down fibrogenesis (199). Multiple receptors and signaling pathways are involved in the chain of events of Th17/IL-17 axis-mediated HSCs activation, and can also be therapeutic targets. So, agents such as resveratrol, curcumin, Dioscin, the flavonoid quercetin, and other agents have emerged as inhibitors of the HSCs activation targeting the TLR3, TLR2/4, STAT3, and/or MAPK/NF-κB, the main receptors and pathways in Th17/IL-17 axis-mediated HSCs activation (200–207). Agents such as rosiglitazone and rapamycin demonstrated the potential to interfere with the fibrogenic pathways by reducing the expression of TGF-β (195, 208). Others such as the ruxolitinib have also shown the potential to inhibit the hepatotoxicity and fibrogenesis, after initial injury by multiple agents, by inhibiting the JAK/STAT pathway (207, 209, 210).
Conclusion and Future Directions
The imbalanced immunity at Th17/IL-17 axis level plays a significant role in liver fibrogenesis after initial HCV or HBV injury. The Th17/IL-17 axis drives of a chain of events that promote a proinflammatory and profibrotic environment by recruiting neutrophils and monocytes and by inducing the expression and production of interleukin-23 and IL-6 either in the liver or peripheral cells. All resident liver cells express receptors for IL-17; and liver cells respond to the IL-17 exposition by increasing the expression of profibrotic and proinflammatory factors such as TGF-β, MMPs, and TIMP. In addition, IL-17 induces the transformation of HSCs to myofibroblasts and the EMT of the hepatocytes, promoting the synthesis of extracellular matrix, cell contractility, and all changes in the liver microstructure and microcirculation.
Author Contributions
FCP: prepared the manuscript text and figures.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet (2016) 388:1081–8. doi:10.1016/S0140-6736(16)30579-7
2. Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med (2014) 12:145. doi:10.1186/s12916-014-0145-y
3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2095–128. doi:10.1016/S0140-6736(12)61728-0
4. Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology (2004) 127:1513–24. doi:10.1053/j.gastro.2004.08.067
5. Xiang WQ, Feng WF, Ke W, Sun Z, Chen Z, Liu W. Hepatitis B virus X protein stimulates IL-6 expression in hepatocytes via a MyD88-dependent pathway. J Hepatol (2011) 54:26–33. doi:10.1016/j.jhep.2010.08.006
6. Liu L, Liang H, Chen X, Zhang W, Yang S, Xu T, et al. The role of NF-kappaB in hepatitis b virus X protein-mediated upregulation of VEGF and MMPs. Cancer Invest (2010) 28:443–51. doi:10.3109/07357900903405959
7. Ali L, Mansoor A, Ahmad N, Siddiqi S, Mazhar K, Muazzam AG, et al. Patient HLA-DRB1* and -DQB1* allele and haplotype association with hepatitis C virus persistence and clearance. J Gen Virol (2010) 91:1931–8. doi:10.1099/vir.0.018119-0
8. Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet (2009) 41:591–5. doi:10.1038/ng.348
9. Wang J, Liu Y, Xie L, Li S, Qin X. Association of IL-17A and IL-17F gene polymorphisms with chronic hepatitis B and hepatitis B virus-related liver cirrhosis in a Chinese population: a case-control study. Clin Res Hepatol Gastroenterol (2016) 40:288–96. doi:10.1016/j.clinre.2015.10.004
10. Falleti E, Fabris C, Toniutto P, Fontanini E, Cussigh A, Bitetto D, et al. Interleukin-6 polymorphisms and gender: relationship with the occurrence of hepatocellular carcinoma in patients with end-stage liver disease. Oncology (2009) 77:304–13. doi:10.1159/000260057
11. Barrett S, Collins M, Kenny C, Ryan E, Keane CO, Crowe J. Polymorphisms in tumour necrosis factor-alpha, transforming growth factor-beta, interleukin-10, interleukin-6, interferon-gamma, and outcome of hepatitis C virus infection. J Med Virol (2003) 71:212–8. doi:10.1002/jmv.10472
12. Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology (2007) 133:1627–36. doi:10.1053/j.gastro.2007.08.003
13. Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology (2012) 143:765–76. doi:10.1053/j.gastro.2012.05.049
14. Ge J, Yu Y-Z, Li T, Guo Z-Y, Wu H, Tang S-C, et al. IL-17A G197A gene polymorphism contributes to susceptibility for liver cirrhosis development from patients with chronic hepatitis B infection in Chinese population. Int J Clin Exp Med (2015) 8:9793–8.
15. Radwan MI, Pasha HF, Mohamed RH, Hussien HI, El-Khshab MN. Influence of transforming growth factor-β1 and tumor necrosis factor-α genes polymorphisms on the development of cirrhosis and hepatocellular carcinoma in chronic hepatitis C patients. Cytokine (2012) 60:271–6. doi:10.1016/j.cyto.2012.05.010
16. Shi T, Zhang T, Zhang L, Yang Y, Zhang H, Zhang F. The distribution and the fibrotic role of elevated inflammatory Th17 cells in patients with primary biliary cirrhosis. Medicine (Baltimore) (2015) 94:e1888. doi:10.1097/MD.0000000000001888
17. Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, et al. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol (2013) 191:1835–44. doi:10.4049/jimmunol.1203013
18. Wu W, Li J, Chen F, Zhu H, Peng G, Chen Z. Circulating Th17 cells frequency is associated with the disease progression in HBV infected patients. J Gastroenterol Hepatol (2010) 25:750–7. doi:10.1111/j.1440-1746.2009.06154.x
19. Liu H, Zhang H, Wan G, Sang Y, Chang Y, Wang X, et al. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat (2014) 21:499–507. doi:10.1111/jvh.12160
20. Zhang J-Y, Zhang Z, Lin F, Zou Z-S, Xu R-N, Jin L, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology (2010) 51:81–91. doi:10.1002/hep.23273
21. Li J, Qiu S-J, She W-M, Wang F-P, Gao H, Li L, et al. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One (2012) 7:e39307. doi:10.1371/journal.pone.0039307
22. Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, et al. The balance between intrahepatic IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol (2011) 12:47. doi:10.1186/1471-2172-12-47
23. Feng H, Yin J, Han Y-P, Zhou X-Y, Chen S, Yang L, et al. Sustained changes of Treg and Th17 cells during interferon-α therapy in patients with chronic hepatitis B. Viral Immunol (2015) 28:412–7. doi:10.1089/vim.2015.0024
24. Cui F, Meng J, Luo P, Chen P. IFN-alpha blocks IL-17 production by peripheral blood mononuclear cells in patients with chronic active hepatitis B infection. BMC Infect Dis (2014) 14:55. doi:10.1186/1471-2334-14-55
25. Maggio R, Viscomi C, Andreozzi P, D’Ettorre G, Viscogliosi G, Barbaro B, et al. Normocaloric low cholesterol diet modulates Th17/Treg balance in patients with chronic hepatitis C virus infection. PLoS One (2014) 9:e112346. doi:10.1371/journal.pone.0112346
26. Ueyama M, Nakagawa M, Sakamoto N, Onozuka I, Funaoka Y, Watanabe T, et al. Serum interleukin-6 levels correlate with resistance to treatment of chronic hepatitis C infection with pegylated-interferon-α2b plus ribavirin. Antivir Ther (2011) 16:1081–91. doi:10.3851/IMP1864
27. Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to toll-like receptor ligands by activation of toll-like receptor 2. J Infect Dis (2010) 202:853–61. doi:10.1086/655812
28. Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat (2012) 19:396–403. doi:10.1111/j.1365-2893.2011.01561.x
29. Klemann C, Schröder A, Dreier A, Möhn N, Dippel S, Winterberg T, et al. Interleukin 17, produced by γδ T cells, contributes to hepatic inflammation in a mouse model of biliary atresia and is increased in livers of patients. Gastroenterology (2016) 150:229–41.e5. doi:10.1053/j.gastro.2015.09.008
30. Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol (2011) 300:G723–8. doi:10.1152/ajpgi.00414.2010
31. Ray S, De Salvo C, Pizarro TT. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr Opin Gastroenterol (2014) 30:531–8. doi:10.1097/MOG.0000000000000119
32. Griffin GK, Newton G, Tarrio ML, Bu D, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol (2012) 188:6287–99. doi:10.4049/jimmunol.1200385
33. Yuan S, Zhang S, Zhuang Y, Zhang H, Bai J, Hou Q. Interleukin-17 stimulates STAT3-mediated endothelial cell activation for neutrophil recruitment. Cell Physiol Biochem (2015) 36:2340–56. doi:10.1159/000430197
34. Huang C-F, Hsieh M-Y, Yang J-F, Chen W-C, Yeh M-L, Huang C-I, et al. Serum hs-CRP was correlated with treatment response to pegylated interferon and ribavirin combination therapy in chronic hepatitis C patients. Hepatol Int (2010) 4:621–7. doi:10.1007/s12072-010-9200-8
35. Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer (2009) 124:2766–70. doi:10.1002/ijc.24281
36. Kao J-T, Lai H-C, Tsai S-M, Lin P-C, Chuang P-H, Yu C-J, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naïve hepatitis B infection patients. Liver Int (2012) 32:928–36. doi:10.1111/j.1478-3231.2011.02742.x
37. Sghaier I, Mouelhi L, Rabia NA, Alsaleh BR, Ghazoueni E, Almawi WY, et al. Genetic variants in IL-6 and IL-10 genes and susceptibility to hepatocellular carcinoma in HCV infected patients. Cytokine (2017) 89:62–7. doi:10.1016/j.cyto.2016.10.004
38. Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol (2013) 87:12284–90. doi:10.1128/JVI.01962-13
39. Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog (2013) 9:e1003330. doi:10.1371/journal.ppat.1003330
40. Gusatti Cde S, Costi C, de Medeiros RM, Halon ML, Grandi T, Medeiros AF, et al. Association between cytokine gene polymorphisms and outcome of hepatitis B virus infection in southern Brazil. J Med Virol (2016) 88:1759–66. doi:10.1002/jmv.24518
41. Biyik M, Ucar R, Solak Y, Gungor G, Polat I, Gaipov A, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol (2013) 25:435–41. doi:10.1097/MEG.0b013e32835c2af3
42. Chang Q, Wang Y-K, Zhao Q, Wang C-Z, Hu Y-Z, Wu B-Y. Th17 cells are increased with severity of liver inflammation in patients with chronic hepatitis C. J Gastroenterol Hepatol (2012) 27:273–8. doi:10.1111/j.1440-1746.2011.06782.x
43. Kim HY, Jhun JY, Cho M-L, Choi JY, Byun JK, Kim E-K, et al. Interleukin-6 upregulates Th17 response via mTOR/STAT3 pathway in acute-on-chronic hepatitis B liver failure. J Gastroenterol (2014) 49:1264–73. doi:10.1007/s00535-013-0891-1
44. Du WJ, Zhen JH, Zeng ZQ, Zheng ZM, Xu Y, Qin LY, et al. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection: IL-17 in HBV infection. Diagn Pathol (2013) 8:40. doi:10.1186/1746-1596-8-40
45. Seo W, Eun HS, Kim SY, Yi H-S, Lee Y-S, Park S-H, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology (2016) 64:616–31. doi:10.1002/hep.28644
46. Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol (2015) 235:79–89. doi:10.1002/path.4430
47. Roussel L, Houle F, Chan C, Yao Y, Bérubé J, Olivenstein R, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol (2010) 184:4531–7. doi:10.4049/jimmunol.0903162
48. Al-Harbi NO, Nadeem A, Al-Harbi MM, Zoheir KM, Ansari MA, El-Sherbeeny AM, et al. Psoriatic inflammation causes hepatic inflammation with concomitant dysregulation in hepatic metabolism via IL-17A/IL-17 receptor signaling in a murine model. Immunobiology (2017) 222:128–36. doi:10.1016/j.imbio.2016.10.013
49. Foster RG, Golden-Mason L, Rutebemberwa A, Rosen HR. Interleukin (IL)-17/IL-22-producing T cells enriched within the liver of patients with chronic hepatitis C viral (HCV) infection. Dig Dis Sci (2012) 57:381–9. doi:10.1007/s10620-011-1997-z
50. Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One (2011) 6:e18909. doi:10.1371/journal.pone.0018909
51. Zhang F, Yao S, Yuan J, Zhang M, He Q, Yang G, et al. Elevated IL-6 receptor expression on CD4+ T cells contributes to the increased Th17 responses in patients with chronic hepatitis B. Virol J (2011) 8:270. doi:10.1186/1743-422X-8-270
52. Zhang F, Yao S, Zhang M, Yuan J, Chen X, Zhou B. Roles of circulating soluble interleukin (IL)-6 receptor and IL-6 receptor expression on CD4+ T cells in patients with chronic hepatitis B. Int J Infect Dis (2011) 15:e267–71. doi:10.1016/j.ijid.2010.12.008
53. Li N, Zhu Q, Li Z, Han Q, Zhang G, Chen J. IL17A gene polymorphisms, serum IL-17A and IgE levels, and hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. Mol Carcinog (2014) 53(6):447–57. doi:10.1002/mc.21992
54. Hao C, Wang J, Kang W, Xie Y, Zhou Y, Ma L, et al. Kinetics of Th17 cytokines during telbivudine therapy in patients with chronic hepatitis B. Viral Immunol (2013) 26:336–42. doi:10.1089/vim.2013.0032
55. Ju H, Liu H, Tian Z, Jiang Y, Zhang C, Liu X. Association of polymorphisms in key Th-17 immune response genes with HBeAg-positive chronic hepatitis B susceptibility and response to PEG-IFNa-2α. Virology (2017) 509:35–41. doi:10.1016/j.virol.2017.05.011
56. Wang L-Y, Meng Q-H, Zou Z-Q, Fan Y-C, Han J, Qi Z-X, et al. Increased frequency of circulating Th17 cells in acute-on-chronic hepatitis B liver failure. Dig Dis Sci (2012) 57:667–74. doi:10.1007/s10620-011-1930-5
57. Basha HI, Subramanian V, Seetharam A, Nath DS, Ramachandran S, Anderson CD, et al. Characterization of HCV-specific CD4+Th17 immunity in recurrent hepatitis C-induced liver allograft fibrosis. Am J Transplant (2011) 11:775–85. doi:10.1111/j.1600-6143.2011.03458.x
58. Ge J, Wang K, Meng Q-H, Qi Z-X, Meng F-L, Fan Y-C. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol (2010) 30:60–7. doi:10.1007/s10875-009-9328-2
59. Yang B, Wang Y, Zhao C, Yan W, Che H, Shen C, et al. Increased Th17 cells and interleukin-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunol Lett (2013) 149:41–9. doi:10.1016/j.imlet.2012.12.001
60. Wang L, Chen S, Xu K. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int J Mol Med (2011) 27:385–92. doi:10.3892/ijmm.2011.594
61. Tian Z-F, You Z-L, Yi H, Kuang X-M, Wang Y-M. Effect of entecavir on CD4+ T-cell subpopulations in patients with chronic hepatitis B. Ann Hepatol (2016) 15:174–82. doi:10.5604/16652681.1193705
62. Jimenez-Sousa MA, Almansa R, de la Fuente C, Caro-Paton A, Ruiz L, Sanchez-Antolín G, et al. Increased Th1, Th17 and pro-fibrotic responses in hepatitis C-infected patients are down-regulated after 12 weeks of treatment with pegylated interferon plus ribavirin. Eur Cytokine Netw (2010) 21:84–91. doi:10.1684/ecn.2010.0191
63. Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L, et al. A randomized trial of autologous bone marrow mesenchymal stem cells transplantation for HBV cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol (2014) 29:1620–8. doi:10.1111/jgh.12653
64. Zhai S, Zhang L, Dang S, Yu Y, Zhao Z, Zhao W, et al. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol (2011) 24:303–10. doi:10.1089/vim.2010.0135
65. Yu X, Guo R, Ming D, Su M, Lin C, Deng Y, et al. Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-β1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis. J Gastroenterol Hepatol (2014) 29:1065–72. doi:10.1111/jgh.12459
66. Macek Jilkova Z, Afzal S, Marche H, Decaens T, Sturm N, Jouvin-Marche E, et al. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int (2016) 36:1116–24. doi:10.1111/liv.13060
67. Taylor PR, Bonfield TL, Chmiel JF, Pearlman E. Neutrophils from F508del cystic fibrosis patients produce IL-17A and express IL-23 – dependent IL-17RC. Clin Immunol (2016) 170:53–60. doi:10.1016/j.clim.2016.03.016
68. Tu J-F, Pan H-Y, Ying X-H, Lou J, Ji J-S, Zou H. Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine (Baltimore) (2016) 95:e3220. doi:10.1097/MD.0000000000003220
69. Ashrafi Hafez A, Ahmadi Vasmehjani A, Baharlou R, Mousavi Nasab SD, Davami MH, Najfi A, et al. Analytical assessment of interleukin- 23 and -27 cytokines in healthy people and patients with hepatitis C virus infection (genotypes 1 and 3a). Hepat Mon (2014) 14:e21000. doi:10.5812/hepatmon.21000
70. Zhao L, Qiu DK, Ma X. Th17 cells: the emerging reciprocal partner of regulatory T cells in the liver. J Dig Dis (2010) 11:126–33. doi:10.1111/j.1751-2980.2010.00428.x
71. Ye Y, Xie X, Yu J, Zhou L, Xie H, Jiang G, et al. Involvement of Th17 and Th1 effector responses in patients with hepatitis B. J Clin Immunol (2010) 30:546–55. doi:10.1007/s10875-010-9416-3
72. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev (2014) 13:668–77. doi:10.1016/j.autrev.2013.12.004
73. O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science (2010) 327:1098–102. doi:10.1126/science.1178334
74. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol (2010) 40:1830–5. doi:10.1002/eji.201040391
75. Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol (2013) 25:305–12. doi:10.1016/j.smim.2013.10.009
76. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity (2009) 30:646–55. doi:10.1016/j.immuni.2009.05.001
77. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity (2006) 24:179–89. doi:10.1016/j.immuni.2006.01.001
78. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol (2012) 181:8–18. doi:10.1016/j.ajpath.2012.03.044
79. Xue-Song L, Cheng-Zhong L, Ying Z, Mo-Bin W. Changes of Treg and Th17 cells balance in the development of acute and chronic hepatitis B virus infection. BMC Gastroenterol (2012) 12:43. doi:10.1186/1471-230X-12-43
80. Mitra S, Anand S, Das A, Thapa B, Chawla YK, Minz RW. A molecular marker of disease activity in autoimmune liver diseases with histopathological correlation; FoXp3/RORγt ratio. APMIS (2015) 123:935–44. doi:10.1111/apm.12457
81. Yang Y, Liu YJ, Tang ST, Yang L, Yang J, Cao GQ, et al. Elevated Th17 cells accompanied by decreased regulatory T cells and cytokine environment in infants with biliary atresia. Pediatr Surg Int (2013) 29:1249–60. doi:10.1007/s00383-013-3421-6
82. Rong G, Zhou Y, Xiong Y, Zhou L, Geng H, Jiang T, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol (2009) 156:217–25. doi:10.1111/j.1365-2249.2009.03898.x
83. Fenoglio D, Bernuzzi F, Battaglia F, Parodi A, Kalli F, Negrini S, et al. Th17 and regulatory T lymphocytes in primary biliary cirrhosis and systemic sclerosis as models of autoimmune fibrotic diseases. Autoimmun Rev (2012) 12:300–4. doi:10.1016/j.autrev.2012.05.004
84. Zhong YM, Wu XR, Wang Q, Yu MF, Lu T, Zhao MY. [Changes in peripheral blood 25 – hydroxyvitamin D3, Th17 cells, and CD4(+) regulatory T cells and their clinical significance in patients with primary biliary cirrhosis]. Zhonghua Gan Zang Bing Za Zhi (2016) 24:829–33. doi:10.3760/cma.j.issn.1007-3418.2016.11.007
85. Rau M, Schilling A-K, Meertens J, Hering I, Weiss J, Jurowich C, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol (2016) 196:97–105. doi:10.4049/jimmunol.1501175
86. Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond) (2016) 130:193–203. doi:10.1042/CS20150405
87. Chen D, Luo X, Xie H, Gao Z, Fang H, Huang J. Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology (2013) 139:523–32. doi:10.1111/imm.12105
88. Tian F, Hu X, Xian K, Zong D, Liu H, Wei H, et al. B10 cells induced by Schistosoma japonicum soluble egg antigens modulated regulatory T cells and cytokine production of T cells. Parasitol Res (2015) 114:3827–34. doi:10.1007/s00436-015-4613-x
89. Shainheit MG, Lasocki KW, Finger E, Larkin BM, Smith PM, Sharpe AH, et al. The pathogenic Th17 cell response to major schistosome egg antigen is sequentially dependent on IL-23 and IL-1β. J Immunol (2011) 187:5328–35. doi:10.4049/jimmunol.1101445
90. Wang X, Sun L, Zhang L, Jiang Z. Effect of adoptive transfer or depletion of regulatory T cells on triptolide-induced liver injury. Front Pharmacol (2016) 7:99. doi:10.3389/fphar.2016.00099
91. Gu L, Deng W-S, Sun X-F, Zhou H, Xu Q. Rapamycin ameliorates CCl4-induced liver fibrosis in mice through reciprocal regulation of the Th17/Treg cell balance. Mol Med Rep (2016) 14:1153–61. doi:10.3892/mmr.2016.5392
92. Wang Q, Zheng Y, Huang Z, Tian Y, Zhou J, Mao Q, et al. Activated IL-23/IL-17 pathway closely correlates with increased Foxp3 expression in livers of chronic hepatitis B patients. BMC Immunol (2011) 12:25. doi:10.1186/1471-2172-12-25
93. Wang Q, Zhou J, Zhang B, Tian Z, Tang J, Zheng Y, et al. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog (2013) 9:e1003410. doi:10.1371/journal.ppat.1003410
94. Yan J, Liu X-L, Xiao G, Li N-L, Deng Y-N, Han L-Z, et al. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One (2014) 9:e96080. doi:10.1371/journal.pone.0096080
95. Yang C, Cui F, Chen L, Gong X, Qin B. Correlation between Th17 and nTreg cell frequencies and the stages of progression in chronic hepatitis B. Mol Med Rep (2015) 13:853–9. doi:10.3892/mmr.2015.4618
96. Feng H, Yin J, Han Y-P, Zhou X-Y, Chen S, Yang L, et al. Regulatory T cells and IL-17(+) T helper cells enhanced in patients with chronic hepatitis B virus infection. Int J Clin Exp Med (2015) 8:8674–85.
97. Zhao R-R, Yang X-F, Dong J, Zhao Y-Y, Wei X, Huang C-X, et al. Toll-like receptor 2 promotes T helper 17 cells response in hepatitis B virus infection. Int J Clin Exp Med (2015) 8:7315–23.
98. Shi M, Wei J, Dong J, Meng W, Ma J, Wang T, et al. Function of interleukin-17 and -35 in the blood of patients with hepatitis B-related liver cirrhosis. Mol Med Rep (2015) 11:121–6. doi:10.3892/mmr.2014.2681
99. Wang Y, Zhang M, Liu Z-W, Ren W-G, Shi Y-C, Sun Y-L, et al. The ratio of circulating regulatory T cells (Tregs)/Th17 cells is associated with acute allograft rejection in liver transplantation. PLoS One (2014) 9:e112135. doi:10.1371/journal.pone.0112135
100. Hu X, Ma S, Huang X, Jiang X, Zhu X, Gao H, et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J Viral Hepat (2011) 18:458–67. doi:10.1111/j.1365-2893.2011.01475.x
101. Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature (2008) 454:350–2. doi:10.1038/nature07021
102. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity (2009) 30:576–87. doi:10.1016/j.immuni.2009.02.007
103. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity (2009) 31:331–41. doi:10.1016/j.immuni.2009.08.001
104. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol (2007) 8:967–74. doi:10.1038/ni1488
105. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol (2007) 8:942–9. doi:10.1038/ni1496
106. Lu X, Xu Q, Bu X, Ma X, Zhang F, Deng Q, et al. Relationship between expression of toll-like receptors 2/4 in dendritic cells and chronic hepatitis B virus infection. Int J Clin Exp Pathol (2014) 7:6048–55.
107. Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol (2010) 134:178–87. doi:10.1016/j.clim.2009.09.013
108. Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature (2007) 448:484–7. doi:10.1038/nature05970
109. Lee H-C, Sung S-SJ, Krueger PD, Jo Y-A, Rosen HR, Ziegler SF, et al. Hepatitis C virus promotes t-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology (2013) 57:1314–24. doi:10.1002/hep.26128
110. Yu H, Huang J, Liu Y, Ai G, Yan W, Wang X, et al. IL-17 contributes to autoimmune hepatitis. J Huazhong Univ Sci Technolog Med Sci (2010) 30:443–6. doi:10.1007/s11596-010-0446-0
111. Longhi MS, Liberal R, Holder B, Robson SC, Ma Y, Mieli-Vergani G, et al. Inhibition of interleukin-17 promotes differentiation of CD25− cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology (2012) 142:1526–35.e6. doi:10.1053/j.gastro.2012.02.041
112. Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology (2013) 58:1084–93. doi:10.1002/hep.26447
113. Kunzmann L, Schoknecht T, Stein S, Ehlken H, Hartl J, Pannicke N, et al. Increased in vivo and in vitro TH17 differentiation in patients with primary sclerosing cholangitis. Z Gastroenterol (2016) 54:1343–404. doi:10.1055/s-0036-1597393
114. Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology (2014) 59:1944–53. doi:10.1002/hep.26979
115. Huang Q, Chu S, Yin X, Yu X, Kang C, Li X, et al. Interleukin-17A-induced epithelial-mesenchymal transition of human intrahepatic biliary epithelial cells: implications for primary biliary cirrhosis. Tohoku J Exp Med (2016) 240:269–75. doi:10.1620/tjem.240.269
116. Lages CS, Simmons J, Maddox A, Jones K, Karns R, Sheridan R, et al. The dendritic cell-T helper 17-macrophage axis controls cholangiocyte injury and disease progression in murine and human biliary atresia. Hepatology (2017) 65:174–88. doi:10.1002/hep.28851
117. Paquissi FC. Immune imbalances in non-alcoholic fatty liver disease: from general biomarkers and neutrophils to interleukin-17 axis activation and new therapeutic targets. Front Immunol (2016) 7:490. doi:10.3389/fimmu.2016.00490
118. Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Cappelletti M, Huppert SS, Iwakura Y, et al. Regulation of inflammation by IL-17A and IL-17F modulates non-alcoholic fatty liver disease pathogenesis. PLoS One (2016) 11:e0149783. doi:10.1371/journal.pone.0149783
119. Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology (2014) 59:1331–42. doi:10.1002/hep.26916
120. Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, et al. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol (2012) 57:1044–51. doi:10.1016/j.jhep.2012.07.008
121. Xu Z, Zhang X, Lau J, Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert Rev Mol Med (2016) 18:e16. doi:10.1017/erm.2016.16
122. Zhang X, Han J, Man K, Li X, Du J, Chu ES, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol (2016) 64:160–70. doi:10.1016/j.jhep.2015.09.005
123. Arsent’eva NA, Semenov AV, Lyubimova NE, Ostankov YV, Elezo DS, Kudryavtsev IV, et al. Chemokine receptors CXCR3 and CCR6 and their ligands in the liver and blood of patients with chronic hepatitis C. Bull Exp Biol Med (2015) 160:252–5. doi:10.1007/s10517-015-3142-z
124. Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun (2010) 35:424–35. doi:10.1016/j.jaut.2010.09.003
125. Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology (2014) 147:577–94.e1. doi:10.1053/j.gastro.2014.06.043
126. Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology (2015) 61:1066–79. doi:10.1002/hep.27332
127. Fabre T, Kared H, Friedman SL, Shoukry NH. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol (2014) 193:3925–33. doi:10.4049/jimmunol.1400861
128. Amara S, Lopez K, Banan B, Brown S-K, Whalen M, Myles E, et al. Synergistic effect of pro-inflammatory TNFα and IL-17 in periostin mediated collagen deposition: potential role in liver fibrosis. Mol Immunol (2015) 64:26–35. doi:10.1016/j.molimm.2014.10.021
129. Li XL, Dou YC, Liu Y, Shi CW, Cao LL, Zhang XQ, et al. Atorvastatin ameliorates experimental autoimmune neuritis by decreased Th1/Th17 cytokines and up-regulated T regulatory cells. Cell Immunol (2011) 271:455–61. doi:10.1016/j.cellimm.2011.08.015
130. Li J, Lau GK-K, Chen L, Dong S, Lan H-Y, Huang X-R, et al. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One (2011) 6:e21816. doi:10.1371/journal.pone.0021816
131. Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol (2007) 293:H3356–65. doi:10.1152/ajpheart.00928.2007
132. Bi W-R, Yang C-Q, Shi Q. Transforming growth factor-β1 induced epithelial-mesenchymal transition in hepatic fibrosis. Hepatogastroenterology (2012) 59:1960–3. doi:10.5754/hge11750
133. Hara M, Kono H, Furuya S, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J Surg Res (2013) 183:574–82. doi:10.1016/j.jss.2013.03.025
134. Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver sinusoidal endothelial cells. Compr Physiol (2015) 5:1751–74. doi:10.1002/cphy.c140078
135. Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol (2013) 3:1473–92. doi:10.1002/cphy.c120035
136. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol (2011) 6:425–56. doi:10.1146/annurev-pathol-011110-130246
137. Xu J, Wang J, Hu Y, Cheng L, Yu H. [Associations between interleukin-17A expression and epithelial-mesenchymal transition in patients with hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi (2015) 37:585–90.
138. Kaimori A, Potter J, Kaimori J, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem (2007) 282:22089–101. doi:10.1074/jbc.M700998200
139. Kaimori A, Potter JJ, Choti M, Ding Z, Mezey E, Koteish AA. Histone deacetylase inhibition suppresses the transforming growth factor β1-induced epithelial-to-mesenchymal transition in hepatocytes. Hepatology (2010) 52:1033–45. doi:10.1002/hep.23765
140. Chen Y-L, Lv J, Ye X-L, Sun M-Y, Xu Q, Liu C-H, et al. Sorafenib inhibits transforming growth factor β1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology (2011) 53:1708–18. doi:10.1002/hep.24254
141. Ji X, Li J, Xu L, Wang W, Luo M, Luo S, et al. IL4 and IL-17A provide a Th2/Th17-polarized inflammatory milieu in favor of TGF-β1 to induce bronchial epithelial-mesenchymal transition (EMT). Int J Clin Exp Pathol (2013) 6:1481–92.
142. Vittal R, Fan L, Greenspan DS, Mickler EA, Gopalakrishnan B, Gu H, et al. IL-17 induces type V collagen overexpression and EMT via TGF-β-dependent pathways in obliterative bronchiolitis. Am J Physiol Lung Cell Mol Physiol (2013) 304:L401–14. doi:10.1152/ajplung.00080.2012
143. Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z, et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene (2017) 36:687–99. doi:10.1038/onc.2016.240
144. Gu K, Li M-M, Shen J, Liu F, Cao J-Y, Jin S, et al. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer Res (2015) 5:1169–79.
145. Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol (2008) 181:2277–84. doi:10.4049/jimmunol.181.4.2277
146. Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol (2013) 190:2009–16. doi:10.4049/jimmunol.1201937
147. Lee Y-S, Jeong W-I. Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol (2012) 27:75–9. doi:10.1111/j.1440-1746.2011.07007.x
148. Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H. Hepatic stellate cells function as regulatory bystanders. J Immunol (2011) 186:5549–55. doi:10.4049/jimmunol.1003917
149. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med (2007) 13:1324–32. doi:10.1038/nm1663
150. Xu R, Tao A, Zhang S, Zhang M. Neutralization of interleukin-17 attenuates high fat diet-induced non-alcoholic fatty liver disease in mice. Acta Biochim Biophys Sin (Shanghai) (2013) 45:726–33. doi:10.1093/abbs/gmt065
151. Chen J, Liao M, Gao X, Zhong Q, Tang T, Yu X, et al. IL-17A induces pro-inflammatory cytokines production in macrophages via MAPKinases, NF-κB and AP-1. Cell Physiol Biochem (2013) 32:1265–74. doi:10.1159/000354525
152. Kono H, Fujii H, Ogiku M, Hosomura N, Amemiya H, Tsuchiya M, et al. Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. J Immunol (2011) 187:4818–25. doi:10.4049/jimmunol.1100490
153. Luedde T, Schwabe RF. NF-κB in the liver – linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2011) 8:108–18. doi:10.1038/nrgastro.2010.213
154. Gao J, Jiang Z, Wang S, Zhou Y, Shi X, Feng M. Endoplasmic reticulum stress of Kupffer cells involved in the conversion of natural regulatory T cells to Th17 cells in liver ischemia-reperfusion injury. J Gastroenterol Hepatol (2016) 31:883–9. doi:10.1111/jgh.13163
155. McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol (2005) 175:404–12. doi:10.4049/jimmunol.175.1.404
156. Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol (2002) 26:748–53. doi:10.1165/ajrcmb.26.6.4757
157. Liu B, Tan W, Barsoum A, Gu X, Chen K, Huang W, et al. IL-17 is a potent synergistic factor with GM-CSF in mice in stimulating myelopoiesis, dendritic cell expansion, proliferation, and functional enhancement. Exp Hematol (2010) 38:877–84.e1. doi:10.1016/j.exphem.2010.06.004
158. Ko H-J, Brady JL, Ryg-Cornejo V, Hansen DS, Vremec D, Shortman K, et al. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J Immunol (2014) 192:2202–9. doi:10.4049/jimmunol.1302040
159. Gu Y, Hu X, Liu C, Qv X, Xu C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol (2008) 142:109–14. doi:10.1111/j.1365-2141.2008.07161.x
160. Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol (2006) 292:L1023–9. doi:10.1152/ajplung.00306.2006
161. Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, et al. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest (2012) 122:974–86. doi:10.1172/JCI60588
162. Khanam A, Trehanpati N, Riese P, Rastogi A, Guzman CA, Sarin SK. Blockade of neutrophil’s chemokine receptors CXCR1/2 abrogate liver damage in acute-on-chronic liver failure. Front Immunol (2017) 8:464. doi:10.3389/fimmu.2017.00464
163. Mizutani N, Nabe T, Yoshino S. IL-17A promotes the exacerbation of IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation via CXCR2 signaling in mice. J Immunol (2014) 192:1372–84. doi:10.4049/jimmunol.1301538
164. Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood (2010) 115:335–43. doi:10.1182/blood-2009-04-216085
165. Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol (2005) 78:279–88. doi:10.1189/jlb.1004612
166. Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol (2006) 79:214–22. doi:10.1189/jlb.0605353
167. Ohashi N, Hori T, Chen F, Jermanus S, Nakao A, Uemoto S, et al. Matrix metalloproteinase-9 in the initial injury after hepatectomy in mice. World J Gastroenterol (2013) 19:3027–42. doi:10.3748/wjg.v19.i20.3027
168. Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology (2015) 61:238–48. doi:10.1002/hep.27378
169. O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol (2013) 183:1498–507. doi:10.1016/j.ajpath.2013.07.019
170. Liao C-C, Day Y-J, Lee H-C, Liou J-T, Chou A-H, Liu F-C. Baicalin attenuates IL-17-mediated acetaminophen-induced liver injury in a mouse model. PLoS One (2016) 11:e0166856. doi:10.1371/journal.pone.0166856
171. Zhang Y, Huang D, Gao W, Yan J, Zhou W, Hou X, et al. Lack of IL-17 signaling decreases liver fibrosis in murine schistosomiasis japonica. Int Immunol (2015) 27:317–25. doi:10.1093/intimm/dxv017
172. Zhang Y, Chen L, Gao W, Hou X, Gu Y, Gui L, et al. IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur J Immunol (2012) 42:1523–35. doi:10.1002/eji.201141933
173. Xu L, Chen S, Xu K. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int J Mol Med (2011) 27:385–92. doi:10.3892/ijmm.2011.594
174. Liao R, Sun J, Wu H, Yi Y, Wang J-X, He H-W, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res (2013) 32:3. doi:10.1186/1756-9966-32-3
175. Zhang J-P, Yan J, Xu J, Pang X-H, Chen M-S, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol (2009) 50:980–9. doi:10.1016/j.jhep.2008.12.033
176. Xu X, Su L, Gao Y, Ding Y. The prevalence of nonalcoholic fatty liver disease and related metabolic comorbidities was associated with age at onset of moderate to severe plaque psoriasis: a cross-sectional study. PLoS One (2017) 12:e0169952. doi:10.1371/journal.pone.0169952
177. Dalekos GN, Obermayer-Straub P, Bartels M, Maeda T, Kayser A, Braun S, et al. Cytochrome P450 2A6: a new hepatic autoantigen in patients with chronic hepatitis C virus infection. J Hepatol (2003) 39:800–6. doi:10.1016/S0168-8278(03)00356-8
178. Dalekos GN, Makri E, Loges S, Obermayer-Straub P, Zachou K, Tsikrikas T, et al. Increased incidence of anti-LKM autoantibodies in a consecutive cohort of hepatitis C patients from central Greece. Eur J Gastroenterol Hepatol (2002) 14:35–42. doi:10.1097/00042737-200201000-00007
179. Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med (2008) 205:1409–22. doi:10.1084/jem.20071859
180. Kitazawa E, Igarashi T, Kawaguchi N, Matsushima H, Kawashima Y, Hankins RW, et al. Differences in anti-LKM-1 autoantibody immunoreactivity to CYP2D6 antigenic sites between hepatitis C virus-negative and -positive patients. J Autoimmun (2001) 17:243–9. doi:10.1006/jaut.2001.0565
181. Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol (2011) 2011:345803. doi:10.1155/2011/345803
182. Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology (2014) 59:1007–15. doi:10.1002/hep.26583
183. Biancheri P, Pender SL, Ammoscato F, Giuffrida P, Sampietro G, Ardizzone S, et al. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair (2013) 6:13. doi:10.1186/1755-1536-6-13
184. Geng J, Zhang K, Chen L, Miao J, Yao M, Ren Y, et al. Enhancement of CD147 on M1 macrophages induces differentiation of Th17 cells in the lung interstitial fibrosis. Biochim Biophys Acta (2014) 1842:1770–82. doi:10.1016/j.bbadis.2014.06.008
185. Bozinovski S, Seow HJ, Chan SP, Anthony D, McQualter J, Hansen M, et al. Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette-smoke-induced lung inflammation in mice. Clin Sci (Lond) (2015) 129:785–96. doi:10.1042/CS20140703
186. González-Mateo GT, Fernández-Míllara V, Bellón T, Liappas G, Ruiz-Ortega M, López-Cabrera M, et al. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One (2014) 9:e108477. doi:10.1371/journal.pone.0108477
187. Lee J, Lee J, Park M-K, Lim M-A, Park E-M, Kim E-K, et al. Interferon gamma suppresses collagen-induced arthritis by regulation of Th17 through the induction of indoleamine-2,3-deoxygenase. PLoS One (2013) 8:e60900. doi:10.1371/journal.pone.0060900
188. Zhang S, Huang D, Weng J, Huang Y, Liu S, Zhang Q, et al. Neutralization of interleukin-17 attenuates cholestatic liver fibrosis in mice. Scand J Immunol (2016) 83:102–8. doi:10.1111/sji.12395
189. Mi S, Li Z, Yang H-Z, Liu H-Z, Wang J-P, Ma Y-G, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol (2011) 187:3003–14. doi:10.4049/jimmunol.1004081
190. Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani E-S, et al. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology (2014) 59:296–306. doi:10.1002/hep.26598
191. Zheng L, Chu J, Shi Y, Zhou X, Tan L, Li Q, et al. Bone marrow-derived stem cells ameliorate hepatic fibrosis by down-regulating interleukin-17. Cell Biosci (2013) 3:46. doi:10.1186/2045-3701-3-46
192. Tian A, Ma H, Cao X, Zhang R, Wang X, Wu B. Vitamin D improves cognitive function and modulates Th17/T reg cell balance after hepatectomy in mice. Inflammation (2015) 38:500–9. doi:10.1007/s10753-014-9956-4
193. Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, et al. 1α,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett (2010) 134:7–16. doi:10.1016/j.imlet.2010.07.002
194. Abramovitch S, Sharvit E, Weisman Y, Bentov A, Brazowski E, Cohen G, et al. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am J Physiol Gastrointest Liver Physiol (2015) 308:G112–20. doi:10.1152/ajpgi.00132.2013
195. Li WW, Sun P, Chen DD, Wang WQ, Jiao GH, Wang YJ, et al. [Preventive and therapeutic effects of rapamycin against autoimmune hepatitis and liver fibrosis and possible mechanisms]. Zhonghua Gan Zang Bing Za Zhi (2016) 24:368–74. doi:10.3760/cma.j.issn.1007-3418.2016.05.011
196. Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science (2009) 324:1334–8. doi:10.1126/science.1172638
197. Liang J, Zhang B, Shen R-W, Liu J-B, Gao M-H, Geng X, et al. The effect of antifibrotic drug halofugine on Th17 cells in concanavalin A-induced liver fibrosis. Scand J Immunol (2014) 79:163–72. doi:10.1111/sji.12144
198. Zhang H, Ju B, Zhang X, Zhu Y, Nie Y, Xu Y, et al. Magnolol attenuates concanavalin A-induced hepatic fibrosis, inhibits CD4(+) T helper 17 (Th17) cell differentiation and suppresses hepatic stellate cell activation: blockade of Smad3/Smad4 signalling. Basic Clin Pharmacol Toxicol (2017) 120:560–70. doi:10.1111/bcpt.12749
199. Schon H-T, Bartneck M, Borkham-Kamphorst E, Nattermann J, Lammers T, Tacke F, et al. Pharmacological intervention in hepatic stellate cell activation and hepatic fibrosis. Front Pharmacol (2016) 7:33. doi:10.3389/fphar.2016.00033
200. Li X, Jin Q, Yao Q, Xu B, Li Z, Tu C. Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways. Toxicol Lett (2016) 261:1–12. doi:10.1016/j.toxlet.2016.09.002
201. Ma J-Q, Li Z, Xie W-R, Liu C-M, Liu S-S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int Immunopharmacol (2015) 28:531–9. doi:10.1016/j.intimp.2015.06.036
202. Shu J-C, He Y-J, Lv X, Ye G-R, Wang L-X. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J Nat Med (2009) 63:415–20. doi:10.1007/s11418-009-0347-3
203. Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y, et al. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-κB signaling pathway. Sci Rep (2015) 5:18038. doi:10.1038/srep18038
204. Samuhasaneeto S, Thong-Ngam D, Kulaputana O, Suyasunanont D, Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol (2009) 2009:981963. doi:10.1155/2009/981963
205. Zhang H, Sun Q, Xu T, Hong L, Fu R, Wu J, et al. Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor-κB pathways. Mol Med Rep (2015) 13:224–30. doi:10.3892/mmr.2015.4497
206. Nunez Lopez O, Bohanon FJ, Wang X, Ye N, Corsello T, Rojas-Khalil Y, et al. STAT3 inhibition suppresses hepatic stellate cell fibrogenesis: HJC0123, a potential therapeutic agent for liver fibrosis. RSC Adv (2016) 6:100652–63. doi:10.1039/C6RA17459K
207. Gu Y-J, Sun W-Y, Zhang S, Li X-R, Wei W. Targeted blockade of JAK/STAT3 signaling inhibits proliferation, migration and collagen production as well as inducing the apoptosis of hepatic stellate cells. Int J Mol Med (2016) 38:903–11. doi:10.3892/ijmm.2016.2692
208. Lee SJ, Yang EK, Kim SG. Peroxisome proliferator-activated receptor-gamma and retinoic acid X receptor alpha represses the TGFbeta1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation. Mol Pharmacol (2006) 70:415–25. doi:10.1124/mol.106.022954
209. Hazem SH, Shaker ME, Ashamallah SA, Ibrahim TM. The novel Janus kinase inhibitor ruxolitinib confers protection against carbon tetrachloride-induced hepatotoxicity via multiple mechanisms. Chem Biol Interact (2014) 220:116–27. doi:10.1016/j.cbi.2014.06.017
Keywords: Th17 Cells, interleukin-17, TGF-β, fibrogenesis, cirrhosis, chronic viral hepatitis, hepatitis C virus, hepatitis B virus
Citation: Paquissi FC (2017) Immunity and Fibrogenesis: The Role of Th17/IL-17 Axis in HBV and HCV-induced Chronic Hepatitis and Progression to Cirrhosis. Front. Immunol. 8:1195. doi: 10.3389/fimmu.2017.01195
Received: 05 July 2017; Accepted: 11 September 2017;
Published: 28 September 2017
Edited by:
Guixiu Shi, Xiamen University, ChinaReviewed by:
Rui Li, University of Pennsylvania, United StatesUrs Christen, Goethe University Frankfurt, Germany
Ankit Saxena, National Institutes of Health (NIH), United States
Copyright: © 2017 Paquissi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feliciano Chanana Paquissi, ZmVwYXF1aXNzaUBnbWFpbC5jb20=
 Feliciano Chanana Paquissi
Feliciano Chanana Paquissi