- Infectious Diseases and Immunology Division, CSIR-Indian Institute of Chemical Biology, Kolkata, India
Leishmaniasis is a neglected protozoan disease that mainly affects the tropical as well as subtropical countries of the world. The primary option to control the disease still relies on chemotherapy. However, a hindrance to treatments owing to the emergence of drug-resistant parasites, enormous side effects of the drugs, their high cost, and requirement of long course hospitalization has added to the existing problems of leishmaniasis containment program. This review highlights the prospects of immunotherapy and/or immunochemotherapy to address the limitations for current treatment measures for leishmaniasis. In addition to the progress in alternate therapeutic strategies, the possibility and advances in developing preventive measures against the disease have been pointed. The review highlights our recent understandings of the protective immunology that can be exploited to develop an effective vaccine against leishmaniasis. Moreover, an update on the approaches that have evolved over the recent years are predominantly focused to overcome the current challenges in developing immunotherapeutic as well as prophylactic antileishmanial vaccines is discussed.
Introduction
Leishmania, a protozoan parasite, causes a complex form of disease called leishmaniasis. This disease is prevalent in 98 countries with major occurrence in the developing world (India, Bangladesh, Nepal, Ethiopia, Sudan, and Brazil) (1). Approximately 20,000–30,000 deaths and 0.7–1 million new cases of leishmaniasis occur per annum. The clinical forms include visceral (the most serious form of the disease, also known as kala-azar), cutaneous and mucocutaneous leishmaniasis (ML) (WHO, 2017). Leishmania parasites are transmitted in the mammalian host by infected sandfly. These flies mostly belong to the genus Phlebotomus and Lutzomyia (2). The control of leishmaniasis mainly relies on chemotherapy while other measures include sleeping under nets treated with insecticide, and spraying insecticides to kill sandflies. The current therapy against leishmaniasis depends on the use of the drugs such as pentavalent antimonials and amphotericin B. Other treatment options include miltefosine and paromomycin. The lacunae suffered by these drugs are inherent toxicity and requirement of long-term treatment. In addition, the expansion of human immunodeficiency virus (HIV) has influenced the epidemiology of the leishmaniasis. 35 out of the 70 countries, endemic for visceral leishmaniasis (VL), have documented cases of Leishmania–HIV coinfection (3). One of the unfavorable complications amalgamated with HIV coinfection is that it lowers the plausibility of a therapeutic response to treatment against Leishmania infantum and it also greatly boosts the possibility of a relapse (4). Furthermore, the problem of high cost and the emergence of resistant parasites to these drugs have frustrated the situation of leishmaniasis control (5). These issues necessitate alternative to chemotherapy like developing new non-toxic antileishmanials or different interventions like prophylactic and therapeutic vaccine. Interestingly, development of resistance to reinfection in individuals cured of Leishmania encourages the feasibility of protective vaccine. Moreover, application of cytokines and immunomodulators as immunotherapeutic agents that can direct curative immune response provides promising approach to immunotherapy against leishmaniasis. Since the sequencing of complete Leishmania genome has been achieved, advancement toward understanding the disease pathogenesis along with its defense by the host has paved new opportunities in the way for Leishmania vaccine and immunotherapy research. This review will discuss the development in the field of prophylactic and therapeutic vaccine and the challenges encountered as alternatives to chemotherapy against leishmaniasis.
Immunobiology of Leishmaniasis: Insights of the Disease
The basic necessity for developing any form of interventions against the disease is the better understanding of the host–pathogen interaction. The characteristic that allows the parasite to establish chronic infection lies in its ability to dampen as well as evade both the innate and adaptive machinery of the host’s immune system. The major innate immune cells that play a significant role in defense against Leishmania are neutrophils, macrophages, and dendritic cells (DCs). When the female sandfly sucks blood meal from the vertebrate hosts, flagellate metacyclic forms of Leishmania are delivered along with sandfly salivary ingredients into the skin of the hosts (6). Initially, the promastigotes are taken up by the neutrophils at the site of infection. Following apoptosis in these infected neutrophils, the released parasites infect neighboring macrophages (7, 8). These macrophages are recruited by the chemotactic properties of the proteophosphoglycans, delivered to the infection site by the vector at the time of its blood meal (9, 10). Binding of Leishmania parasites to C3b accelerates phagocytosis after which promastigotes get converted to the amastigote form (11). Following phagocytosis in macrophages, establishment of infection is determined by several survival strategies of the parasites, most prominent of which is the modulation and attenuation of immune responses. Leishmania parasite suppresses the release of Th1 associated cytokines like interleukin (IL)-12 from these cells. This, in turn, restrains DCs to present the parasite-specific antigens to the T cells. Thus preventing the activation of the acquired immunity, this is very crucial for the containment of the disease (12, 13). Influence of Th1/Th2 cytokine has been observed to vary in the progression of disease in VL compared with cutaneous leishmaniasis (CL). Although the classical Th1/Th2 paradigm of resistance/susceptibility appears to be valid during CL, a mixed Th1/Th2 response is required for disease control during VL (14). However, it is yet to establish a clear Th1/Th2 paradigm for curative and preventive response against both CL and VL. Moreover, for ML, the disease manifestation is largely due to inflammatory response than due to parasite burden. Therefore, conventional Th1/Th2 paradigm does not apply to ML. It has been found that Treg (CD4+CD25+ regulatory T cells) as well as Th17 (other subsets of T cells) cells, play a significant role in disease outcome in both CL and VL, their role in ML is much more complicated. Studies with Leishmania major and L. infantum have shown a protective role of IL-17 as well as IL-22 (Th17 cytokines) against intracellular parasites (15, 16). Recently it was shown that when recombinant IL-17 or IL-23 was administered to mice it caused a considerable containment of parasite load in infected organs with significant production of factors such as IFN-γ, nitric oxide, etc. Thus, this study demonstrated the association of Th17-based cytokines in providing protection against the disease (17). As an important constituent of the immune system, Treg cells are known to regulate immune response of other cells. These cells were observed to be present in human cutaneous lesions (18). Increased expression of lesional FoxP3 and IL-10 during progressive L. major infection in a murine model and similarly during Leishmania braziliensis infection in human patients suggest the disease-promoting role of these regulatory cytokines (19). The preliminary data suggest that despite Th1 polarization production of IL-10 and Treg cells is associated with delayed healing of CL (20). Apart from T-cell subsets (Treg and Th17) other than conventional T cells, the role of innate immune response has been essentially linked to disease outcome. In fact, engagement of the macrophage toll-like receptors (TLRs) by the parasites has not only shown to improve phagocytosis but also lead to the killing of the parasites due to triggering of NF-κB transactivation and concomitant production of the downstream mediators including pro-inflammatory cytokines. For instance, TLR9 activation has been found to be beneficial for the host against these parasites. But this situation may not be true for all the TLRs. Lipophosphoglycan, a TLR2 agonist has been shown to have antagonizing effect on TLR9 mediated signal cascade in host macrophage, which in turn facilitates parasite survival (21, 22). Studies showed that treatment with TLR4 and TLR9 agonists decreased the disease severity following challenge infection with L. major in BALB/c mice (23). However, in human VL, comparison of mRNA expression levels between pretreatment and posttreatment splenic aspirate samples showed considerably more TLR2 and TLR4 expression but no change in TLR9 expression during Leishmania donovani infection (24). Despite advances, achieving a comprehensive and clear picture of the immunobiology of leishmaniasis is still required to develop effective interventions such as type-specific vaccine and immunotherapy for leishmaniasis.
What Necessitates a Vaccine?
Chemotherapy is the key intervention to control leishmaniasis. The existing drugs for the treatment of leishmaniasis in the market are pentavalent antimonials, amphotericin B, miltefosine, paromomycin, and amphotericin B in liposomal forms. But the major setback of these drugs include toxicity, cost, route of injection, treatment duration, and the predominant one being blooming of drug-resistant parasites (5). Pentavalent antimonials are the first line course of medication for leishmaniasis worldwide except Indian subcontinent. In India, about 90% of all infections are resistant to pentavalent antimonials (25). Accordingly, amphotericin B has been used as the leading drug to treat patients infected with Leishmania. But high toxicity and reports of drug-resistant parasites have narrowed down their use (26). Miltefosine, an oral medication, came up with promising results in the beginning but there is increasing occurrence of relapses in cases prescribed with this drug (27–29). Lately, in a multicentric clinical trial, it was found that Ambisome (liposomal amphotericin B) was effective enough in a single-dose treatment with a lesser degree of toxicity compared with mainstream treatments (30). However, this line of medication protocol raises the possibility of advancement in the development of drug-resistant parasites. Consequently, there is a development of combinatorial drug therapy for use in endemic regions (31, 32). Nevertheless, mouse model studies imply that even the combinatorial drug treatment can develop drug resistance L. donovani (33). Regardless of the advances in antileishmanial chemotherapeutics, it is implausible that chemotherapy solely will facilitate disease eradication. Since leishmaniasis is predominantly a disease of the poverty-stricken community, chemotherapy proves inadequate and less acceptable. This socioeconomic concern calls for a preventive and/or immunotherapeutic alternative to chemotherapy. Hence there is an imperative need for an effective prophylactic and therapeutic vaccine if enduring ambition of managing and eliminating this disease are to be accomplished.
Prospects of Vaccine Development
Even though the global share of leishmaniasis is limited to selective parts of the world, the number of individuals being affected and are at risk is noteworthy. The ongoing scope to deal with this concern includes vector control, development of technologies for easy and quick diagnosis, refinement of drugs for improved treatment and developing vaccine approaches both prophylactically and therapeutically.
Prophylactic Vaccine Approaches
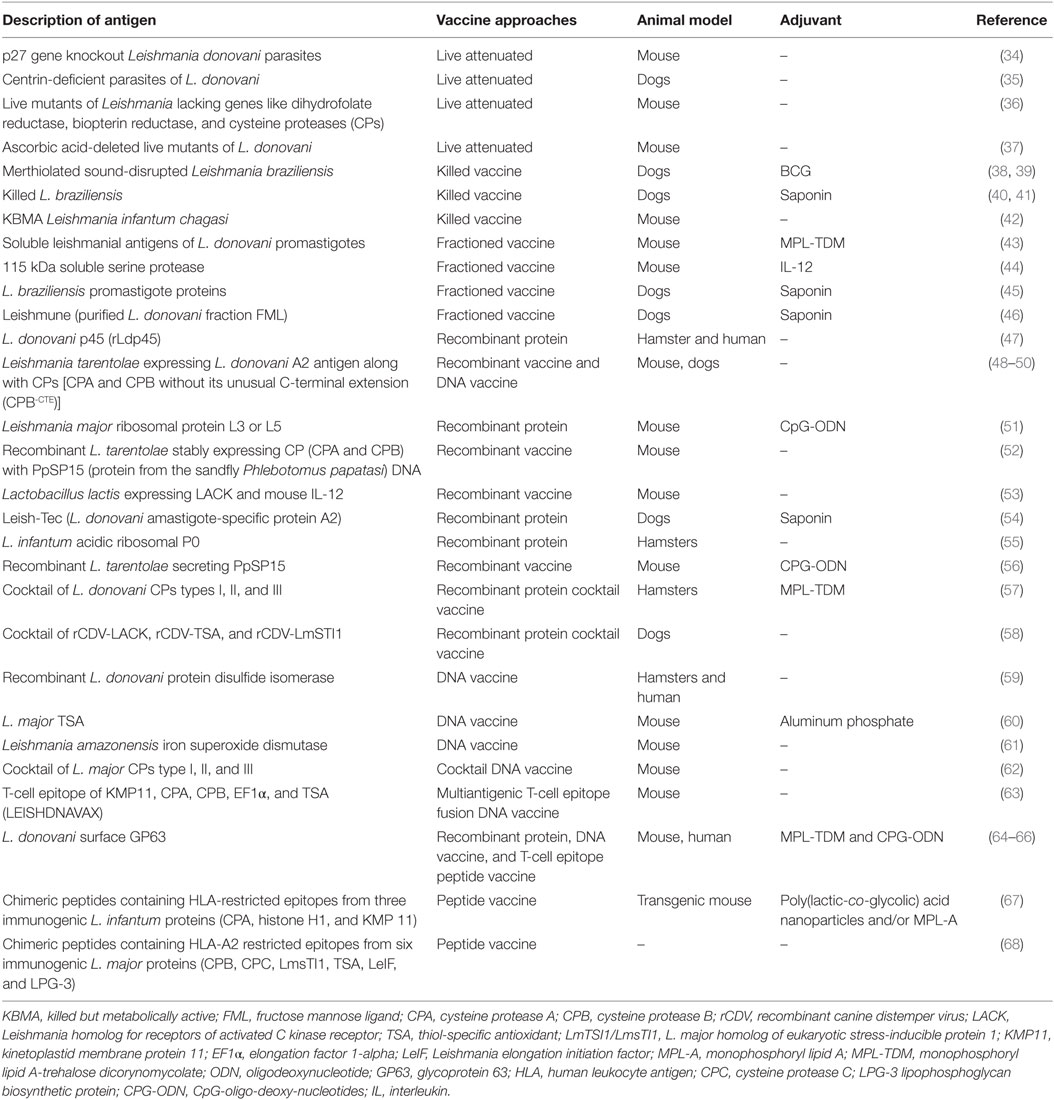
Admittedly, at present, there is no human administrable vaccine against leishmaniasis. However, development is in progress. At present, the vaccine designing approaches (Table 1) are broadly grouped as follows: (a) genetically manipulated live vaccines; (b) preparation of whole killed parasites or their fractions; and (c) vaccines based on defined molecules, which include recombinant protein vaccines and/or DNA vaccines as single or multiantigen combinations.
Live but Attenuated Leishmania Parasites As Vaccine
Vaccination with live parasites has always been an appealing approach as it mimics the natural infection. An effective vaccine comprising of live and virulent parasites termed as leishmanization has existed in the past. However, development of nonhealing lesions in some individuals led to the issue of questionable safety of this approach and it was discontinued in most of the countries except Uzbekistan (6). More recently, the aptness in expertise to edit the Leishmania genome to design genetically engineered parasites by deleting essential virulent genes rejuvenates the dormant utility of live attenuated Leishmania vaccine. Recently, in an approach to test the feasibility of live attenuated vaccine, researchers attenuated live L. donovani parasites by deleting centrin which on immunization exhibited protective immunity against L. infantum infection in dogs (35). In another study, mutant L. donovani obtained by deleting gene of ascorbic acid was shown to confer long-term protection against VL (37). Similarly, attenuated Leishmania parasites derived by deleting promising genes including cysteine proteases (CPs), biopterin reductase, and dihydrofolate reductase manifested significant protection in vaccinated mice against challenge infection with virulent parasites (36, 69). Studies on p27 gene knocked out live attenuated L. donovani parasites in BALB/c shows induction of long-term protective immunity (34). Recently, growth arrested live attenuated amastigotes of Leishmania have been explored as an encouraging technique for vaccine development against VL (35). Even though genetically attenuated live vaccines have been shown to be efficacious in experimental model, the safety of these mutants following mass vaccination cannot be affirmed because in immunocompromised individuals and HIV-positive persons the possibility of vaccine-induced leishmaniasis still remains.
Killed or Avirulent Leishmania Parasites As Vaccine
Pioneering work of the late 1930s by Brazilian scientists has shown the therapeutic and prophylactic efficacy of killed parasite vaccines against CL and VL. From then, it was realized that if the biochemical composition including antigenicity of these killed Leishmania parasites remains unperturbed it could be used as a promising vaccine candidate. In 1990s, Mayrink and coworkers, using merthiolated sound-disrupted L. braziliensis and BCG vaccine, showed 90% protection in phase I and II clinical trial against experimental canine VL challenged with Leishmania chagasi. However, in a well-designed field Phase III trial, this vaccine formulation failed to show any significant difference in dogs as compared with placebos (38, 39). The authors speculated that the difference between the artificial and the natural challenge could account for this failure in the field assay. Giunchetti et al. experimented with killed L. braziliensis vaccine along with saponin and/or sandfly saliva extract and got some quite significant results (40, 41). However, these speculations were little hindered because the whole-parasite based vaccine failed to confer significant protection to humans against leishmaniasis (70). Recently, an attempt was made to improve the efficacy of whole-cell vaccination. For this first promastigote of L. chagasi was exposed to low dose of UV radiation to generate Leishmania organism termed killed but metabolically active. Further using this processed cells in combination with amotosalen, S-59; a psoralen compound, this vaccine conjugate was shown to have promising results comparable to vaccination with the virulent live organisms (42). However, to develop vaccines out of the whole cell is still a challenge that limits its widespread use. Therefore, despite being a safe and dependable option, killed parasite vaccines demand further in-depth investigation for a stable alternative.
Purified Fractions of Leishmanial Lysate
Purified fractions, as well as subfractions, of the Leishmania parasites have shown significant immunoprotective profile when used in several vaccine models. For example, cationic liposomes encapsulating soluble antigens isolated from L. donovani promastigotes (SLA) when mixed with the adjuvant [monophosphoryl lipid A-trehalose dicorynomycolate (MPL-TDM)] and given subcutaneously to BALB/c mice conferred long-term protection against experimental VL (43). Similarly, fructose mannose ligand (FML), isolated from L. donovani, when used in combination with saponin (adjuvant) conferred significant protection against canine VL (46). Moreover, the vaccine not only provides a promising tool to prevent canine VL but is also advantageous in controlling transmission of zoonotic VL (71). Recently, the LiESP/QA-21 vaccine was licensed for commercialization under the name of CaniLeish® in Europe. It is composed of purified excreted-secreted proteins of L. infantum (LiESP) adjuvanted with QA-21 (saponin) (72). However, problems associated with purification, as well as the large-scale production of these fractioned vaccines, are some of the limiting factors in their extensive use. Therefore, development of alternatives having vaccine potential including recombinant proteins, polyproteins, DNA vaccines are in the process as discussed in the following sections.
Immunogenic Recombinant Antigen Based Vaccine
The advent of the recombinant DNA technology has boosted enormously the vaccine development program against leishmaniasis. It has allowed generating leishmanial recombinant proteins as desired. These proteins owing to their high purity and yield provide an advantage of developing promising vaccine candidates. For example, recombinant GP63 expressed in bacteria was used as encapsulated form in cationic liposomes to immunize BALB/c mice in combination with TLR4 agonist-MPL-TDM. This combinatorial vaccine formulation was found to confer significant protection against murine VL through activation of both CD4 and CD8 T cell-mediated immune responses (64). Similarly, combination of CpG-oligo-deoxy-nucleotides, a known TLR9 agonist with recombinant ribosomal antigen L3 or L5 from L. major, improved the protection in two different murine models against homologous challenge infection (51). In addition, recently a new approach was taken to improve vaccine potential of the recombinant proteins. For instance, Katebi et al. genetically engineered (56) the non-pathogenic Leishmania tarentolae species to express and deliver a specific sandfly salivary antigen, PpSP15. Use of this recombinant L. tarentolae-PpSP15 with CpG conferred a significant protection against infection to L. major. Similarly, Saljoughian et al. developed (48) L. tarentolae that expresses L. donovani A2 antigen. When they used this genetically engineered cell along with CPs for immunization of BALB/c mice in a prime-boost manner, it showed significant protection against L. infantum challenge. Another group evaluated the immunogenicity and protective efficacy of L. tarentolae expressing a trifusion protein containing L. donovani A2 antigen along with CPs A and B without its unusual C-terminal extension (CPB-CTE) against L. infantum infectious challenge with prime-boost regimen in dogs. Vaccinated dogs showed higher levels of Th1 immune response with a strong DTH response and low parasite burden representing a promising candidate against canine VL (49). The strongest defensive efficacy in the mice models (C57BL/6 and BALB/c) against infection to L. major was observed by Zahedifard et al., when they primed these animals with PpSP15 DNA and boosted them with the combination of PpSP15 DNA and L. tarentolae (live) that was engineered to stably express genes for CPs (52). Recently, Lactobacillus lactis (alr-) strains are being used as an expression and delivery vehicles of biological compounds, such as cytokines and antigens, in mice and humans. In one such study, live L. lactis solely expressing the Leishmania antigen, Leishmania homolog for receptors of activated C kinase receptor (LACK) and mouse IL-12 was generated for orally immunizing BALB/c mice against L. major challenge. Immunization with the L. lactis expressing both LACK and IL-12 in secretory form induced LACK-specific Th1 immune response demonstrating the use of L. lactis as a live oral vaccine against leishmaniasis (53).
After a lot of effort in developing a vaccine against leishmaniasis, Leish-Tec, an amastigote-specific A2 recombinant protein vaccine against canine VL, is now commercially available in Brazil. Leish-Tec® was found to be immunogenic in different breeds of canine population (54). Despite that these above vaccine formulations exhibited significant protection against various Leishmania models, scientists believe that a cocktail of different conserved antigens could provide better protection. In lieu of that a cocktail of recombinant canine distemper virus (rCDV)-LACK, rCDV-thiol-specific antioxidant (TSA), and rCDV-LmSTI1 was used to immunize dogs. This vaccine formulation provided significant protection to dogs against parasite challenge (58). Further, the use of cocktails of CPs in a hamster model of VL was found to be more effective when encapsulated in a liposome and delivered along with MPL-TDM (57). It is challenging to ensure persistent delivery of protein based antigens in intracellular milieu as a mimic of pathogenesis, therefore alternatively DNA-based vaccination strategy for cell-mediated immunity against leishmaniasis is sought to be explored.
DNA-Based Vaccines
DNA-based vaccines use bacterial plasmids, which are genetically engineered to encode antigens of interest. The advantage of these vaccines compared with the conventional live virus or protein subunit vaccines is that these are flexible, can be manufactured rapidly, are cost effective, and are able to induce cellular immunity (73). Immunization with DNA vaccine for expression of iron superoxide dismutase from Leishmania amazonensis is shown to protect BALB/c mice partially against challenge with the parasite (61). Tabatabaie et al. (60), however, showed that in addition with aluminum phosphate, TSA-based DNA vaccine confers significant protection against L. major infection in murine model. Shahbazi et al. did immunological comparison of electroporation and cationic solid–lipid nanoparticle delivery systems to administer a trifusion DNA vaccine A2-CPA-CPB-CTE in dogs demonstrating both the systems as equally efficient vaccine delivery systems against canine VL (50). Interestingly, efficacy of CPs cocktail DNA vaccine was found to be enhanced following delivery in cationic lipid nanoparticles against murine model of CL exhibiting robust protective T-cell response (62). Besides these cocktail and fusion DNA vaccines, multiantigenic T-cell epitope-enriched DNA vaccine, LEISHDNAVAX has also developed showing significant protection. Its preclinical safety and tolerability studies have shown promising results. Moreover, distribution of the DNA vectors was systemic with no accumulation upon repeated injections. These results prompted initiation of clinical trials with the aim to use it for preventive as well as therapeutic applications (63). Heterologous immunization with DNA vaccine for priming followed by protein boosters using model antigen gp63 with CpG was shown to induce strong protective immune responses in mice. Moreover, it confers a long-term immunity to fight against the intracellular pathogens. The findings indicate that DNA-prime/protein-boost vaccination modality is superior to other possible combinations (65). Overall, these observations suggest that DNA vaccines are promising alternatives to conventional protein vaccines for controlling leishmaniasis. However, though DNA vaccines have proven their efficacy in animal models, they are strictly regulated for human use. Therefore, to potentially exploit DNA vaccines for the human use further effort are needed to make DNA vaccines safer avoiding autoimmune and cancer-related adverse effects.
Peptide-Based Vaccines
The peptide-based vaccine designing combines the benefits of a computational prediction with defined experimental validation to identify immunogenic epitopes within protein antigens. The boon of peptide-based vaccine design relies on their capability to trigger immune response solely dedicated to relevant epitopes overriding other irrelevant responses or unwanted side effects. Although the host defense mechanisms against leishmaniasis is not raveled completely, till date studies decipher the requirement of T cell-mediated response in controlling parasite infection. In silico approach of mining the proteome of parasites and analysis of the candidate antigens have helped in identifying both MHC class I- and class II-restricted T-cell epitope against L. donovani and L. major, which may serve as highly promiscuous peptides for developing subunit vaccine (74–76). Seyed et al. used in silico prediction to screen six L. major antigens for potential CD8+ T cell-activating epitopes presented by HLA-A*0201 (68). In another in silico approach, researchers refined 10 epitopes after screening thousands of epitopes derived from 8,000 proteins conserved in different Leishmania species. They tested the immunogenicity of these epitopes by stimulating the PBMCs of cured CL patients and only 50% of them were able to stimulate the proliferation of lymphocytes (77). Therefore, developing a plausible peptide-based vaccine needs to overcome quite a few considerable adversities. These include curbing the low immunogenicity and poor population coverage of individual peptide due to MHC restriction by combining multiple epitopes along with some immune response boosting adjuvant to target the adaptive immune response. Athanasiou et al. designed a chimeric peptide encapsulated in nanoparticle with monophosphoryl lipid A (MPL-A) or surface modification targeting TNF receptor II aimed to study their capability of stimulating the immunomodulatory properties of DCs. Chimeric peptide from three antigens incorporated in PLGA nanoparticle along with MPL-A were shown to induce maturation and activation of DCs imparting strong protective immunity against L. infantum infection in HLA A2.1 transgenic mice (67). Thus incorporation of multiple peptide-based epitopes in immunomodulatory delivery vehicles particles is a promising strategy for vaccine development against leishmaniasis.
Immunotherapy
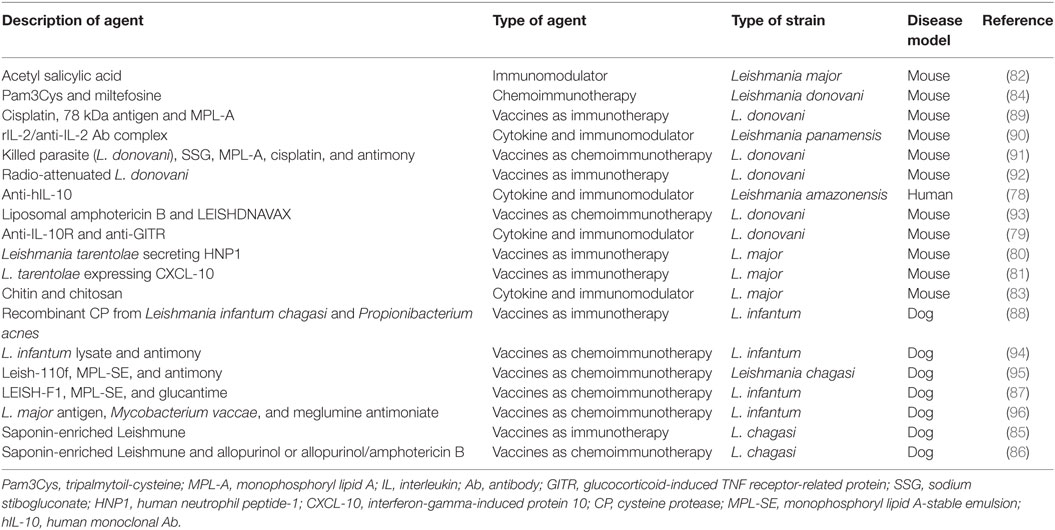
Immunotherapy comprises the use of biological and/or synthetic substances to modulate immune responses to that of cure. Strategies for immunotherapy include cytokine or chemokine treatment, antibody (Ab) blocking, immune modulation by vaccine antigens or adjuvants alone or in combination with chemotherapy. Different immunotherapeutic approaches against leishmaniasis have been listed in Table 2. In one such study, Castellano et al. administered antihuman monoclonal Ab in L. braziliensis SLA (soluble leishmanial antigens) stimulated cells from CL patients of endemic area with active or healed lesions to block IL-10 production, which showed decreased levels of IL-10, IL-4, and TNF-α in most of the patients except with active lesions. Moreover, there was limited alteration in production of an IFN-γ dependent chemokine, CXCL-10 (78). Another study examined the therapeutic efficacies of anti-glucocorticoid-induced TNF receptor-related protein and anti-IL-10R in L. donovani-infected C57BL/6 mice. Blocking IL-10 controlled parasite burden but combinatorial therapy using both the Abs could not suppress parasite proliferation in both liver and spleen even if there was low dose challenge. In addition, there was significant increase in TNF-α and IFN-γ in combinatorial therapy in comparison with single Abs (79). Abdossamadi et al. and Montakhab-Yeganeh et al. checked the suitability of transgenic L. tarentolae expressing leishmanial antigens such as human neutrophil peptide-1, and chemokines such as interferon-gamma-induced protein 10 or CXCL-10, respectively, as immunotherapeutic tools against L. major-infected BALB/c mice (80, 81). Both the approaches represented a promising immunotherapeutic strategy to improve treatment of CL. Immunomodulators, depending on their properties, can direct immune system response both negatively and positively. These immunomodulators can be exploited in a manner that can modulate immune response to control the infection. In an approach, researchers orally administered acetyl salicylic acid (ASA) as immunomodulator in L. major-infected BALB/c mice. ASA induced NO production, reduced proliferation of amastigote in macrophages, lesion size, and visceralization of parasites (82). Hoseini et al. used chitin and chitosan microparticles (MPs) as immunomodulators against L. major infection in BALB/c mice. Chitosan, acetylated form of chitin is a homopolymer extracted from shells of shrimp. Both the chitinous MPs showed reduced lesions and parasite load, induced cell proliferation and chitin but not chitosan induced TNF-α and IL-10 production (83). There are attempts to see the synergistic effect of various immunomodulators with chemotherapeutic agents as a combinatorial therapy against leishmaniasis. In one such attempt, studies were conducted to determine the lower dose effect of an antileishmanial drug, miltefosine, in combination with a single dose of tripalmytoil-cysteine (Pam3Cys) in BALB/c mice infected with L. donovani. The authors found this combination to be significantly effective in comparison with groups receiving Pam3Cys or miltefosine due to enhanced production of ROS, Th1 cytokines, and increase in phagocytosis index (84). Vaccines that can elicit cell-mediated immune response can be considered as potential candidate for immunotherapy, and there are various studies that are considering vaccine components with or without chemotherapy as immunotherapeutic tool. In one such study, Santos and coworkers found that Leishmune (saponin enriched) could significantly reduce the clinical symptoms and parasite load in the liver, spleen, bone marrow, and blood in seropositive and symptomatic dogs infected by L. chagasi (85). Borja-Cabrera et al. continued the study to compare the Leishmune (saponin enriched) with immunochemotherapy (saponin-enriched Leishmune in combination with allopurinol or AmB/allopurinol). They saw that immunochemotherapy cleared all the disease symptoms along with reduced infection and increased survival of the dogs infected with L. donovani (86). In an another canine VL study, glucantime treatment was compared with human trial candidate LEISH-F1 + monophosphoryl lipid A-stable emulsion (MPL-SE) in an Open Trial and a Blinded Trial. Glucantime alone failed to treat most of the cases whereas LEISH-F1 + MPL-SE was found to be effective not only for mild cases also but reduced the symptoms of severe canine VL as well (87). Recently, a study showed recombinant CP from L. infantum chagasi (rLdccys1) to be an effective immunotherapeutic agent against naturally infected dogs from Teresina, Piauí, a region of high incidence of VL in Brazil (88). Both murine and dogs along with human studies indicate immunotherapy to be a favorable alternative to conventional chemotherapy. However, there is lack of standardized immunotherapeutic protocols for use in treatment of leishmaniasis.
Status of Field Trials
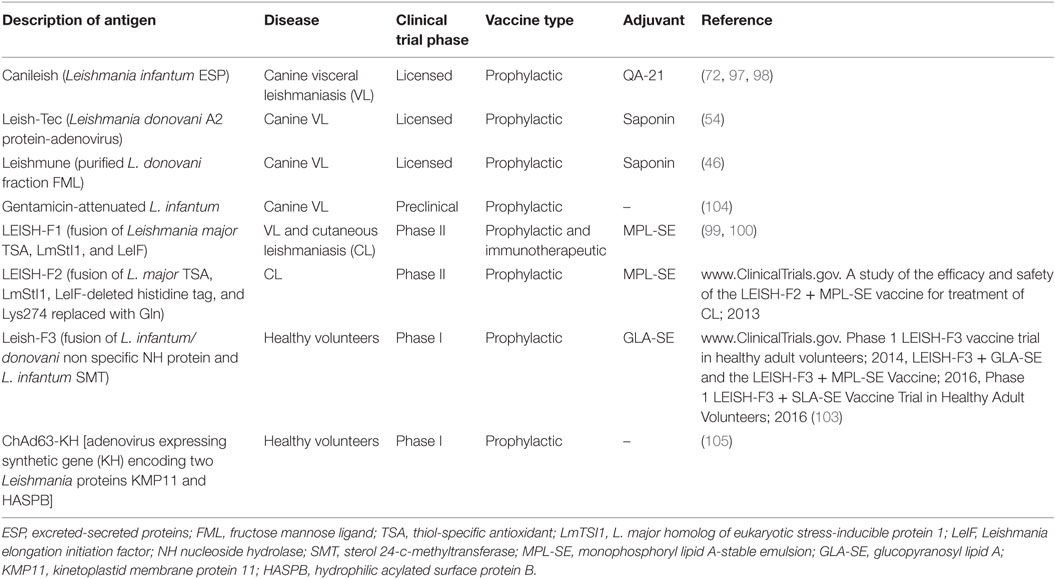
Following success of a number of vaccine candidates at laboratory evaluation, a few have been tested in the field and are listed in Table 3. Notably, Canileish, recently licensed for prophylaxis against canine VL has demonstrated Th1 biased persistent antileishmanial immunity (72, 97, 98). The success of other two licensed canine vaccines: Leish-Tec (L. donovani A2 protein-adenovirus) and Leishmune (FML-saponin formulation) have persuaded researchers to develop human leishmaniasis vaccine. LEISH-F1 (formerly Leish-111F), comprising of a fusion of three relatively conserved L. major antigens (TSA, LmStI1, and Leishmania elongation initiation factor) formulated as stable emulsions of MPL-A in squalene oil, is the first among defined vaccines for leishmaniasis to be clinically evaluated (99, 100). It has been reported that in animal models LEISH-F1 + MPL-SE stimulated partial protection against VL. However, Phase III trial of LEISH-F1 + MPL-SE showed unsuccessful results in defending dogs against infection (101). Human phase I trials of LEISH-F1 + MPL-SE targeting VL and CL were conducted in Colombia (2007), Brazil (2007), Peru (2007), and India (2008) demonstrating the formulation to be safe, immunogenic, and well tolerated in people irrespective of their serostatus (102). Moreover, the LEISH-F1 + MPL-SE was also found to have therapeutic significance when used with chemotherapy in patients with ML (100). The noteworthy early success of the LEISH-F1 + MPL-SE has encouraged IDRI researchers to redesign this vaccine candidate into a new construct LEISH-F2. The new candidate has a deleted N-terminal histidine tag so as to keep the recombinant molecule close to its original form with replacement of a residue Lys274, a potential site for proteolytic activity, with Gln (102). Promising phase I trial of LEISH-F2 + MPL-SE has lead to a phase II trial where the safety, immunogenicity, and efficacy were studied in evaluation with standard chemotherapy in adolescent and adults participants infected with CL [www.ClinicalTrials.gov. A study of the efficacy and safety of the LEISH-F2 + MPL-SE vaccine for treatment of CL; 2013]. A third candidate LEISH-F3 under investigation by IDRI, a tandemly fused polypeptide of open reading frame of two Leishmania proteins: L. infantum/donovani nonspecific nucleoside hydrolase protein (aa 1–314) and L. infantum sterol 24-c-methyltransferase protein (aa 2–353), is in a phase I trials in USA. These trials aims to assess the safety and immunogenicity of unadjuvanted LEISH-F3 with variety of adjuvants likely to be glucopyranosyl lipid A formulated as stable emulsion (GLA-SE), MPL-SE, second generation lipid adjuvant stable emulsion (SLA-SE) in different studies [www.ClinicalTrials.gov. Phase 1 LEISH-F3 vaccine trial in healthy adult volunteers; 2014, LEISH-F3 + GLA-SE and the LEISH-F3 + MPL-SE Vaccine; 2016, Phase 1 LEISH-F3 + SLA-SE Vaccine Trial in Healthy Adult Volunteers; 2016] (103).
Other group has explored the safety, tolerability, and immunogenicity of vaccine candidate ChAd63-KH for human VL and post kala azar dermal leishmaniasis (PKDL) in Phase I clinical trials. ChAd63-KH is a simian adenoviral vaccine with defective replication, which expresses a novel synthetic gene (KH) encoding two Leishmania proteins kinetoplastid membrane protein 11 and hydrophilic acylated surface protein B. Phase I trial showed induced innate response characterized by activation of DCs and production of IFN-γ along with robust CD8+ T-cell response, which suggests the further development of ChAd63-KH as vaccine for VL and PKDL (105).
Challenges
For the effective control and complete eradication of any infectious disease, the potential approach that can be exploited as an economical means is vaccination. Over the past two decades, immunotherapy, either alone or in combination with chemotherapy, has been developed as an additional approach to combat leishmaniasis. Lifetime immunity against reinfection manifests possibility of developing an effective vaccine (prophylactic and therapeutic) against leishmaniasis. Nevertheless, an antileishmanial vaccine for human administration is still unavailable. Therefore, the present scenario augments questions concerning the issues or limitations in the advancement of effective interventions against eradication of leishmaniasis. Some of the major challenges that need to be addressed are as follows:
1. Vaccines against leishmaniasis, malaria, schistosomiasis and a number of other bacterial and viral diseases are unappealing to the industry considering limits of financial benefits (300–800 million US dollars) (1). According to the G-Finder, over US$ 66 million has been granted for research and development of vaccine, preventative, and therapeutic, against leishmaniasis largely from chief public sector and charitable trusts (from the year 2007 to 2013). Some of the major funding sources are Carlos Slim Foundation, Bill & Melinda Gates Foundation, Wellcome Trust, Indian Council of Medical Research, European Commission, Institute Pasteur, German Federal Ministry of Education and Research (BMBF), and the U.S. National Institutes of Health (Policy Cures. G-Finder; 2015). In addition, new and enhanced funding from public–private joint ventures and pharmaceutical companies will be encouraging to boost vaccine (preventive and therapeutic) research.
2. Leishmania-infected individuals gain considerable lifelong immunity to reinfection, suggesting the feasibility of vaccination. However, regardless of many potential vaccine candidates, translation of these to develop a human administrable antileishmaniasis vaccine is still arduous. Selection of promising vaccine candidates has persistently been a complex issue. As reviewed in this article, a number of antigens (Table 1) have been tested with varied success based on the animal model and the vaccine formulation. The difference in opinion regarding the choice of antigens has resulted in a never-ending argument where some proclaim for a molecularly outlined formulation whereas others postulate for a live attenuated vaccine. For a human administrable vaccine and/or immunotherapy, there is an imperative need to imply a coordinated approach that minimizes the toxicity of live vaccine and maximizes the immunogenicity and efficacy of defined vaccines and immunotherapeutic agents.
3. An appealing approach to maximize the immunogenicity of the defined vaccine is the use of a suitable adjuvant (immunomodulators). The factors considerably determining the suitability of an adjuvant are the route of administration, the nature of antigens like solubility, the course of immunization, and the nature of immune response required. Development of an efficient antigen-adjuvant formulation primarily requires an in-depth knowledge of the mode of action, toxicity, and human administrability of the adjuvant. Chemically defined and licensed adjuvants that can elicit cell-mediated immune responses seem to be encouraging candidates for development of antileishmanial vaccines and immunotherapy.
4. Leishmania infection follows a complex clinical outcome varying from the cutaneous to visceral form as the parasite is equipped for generating an extensive assortment of atypical and uncommon variations. The virulence factors as well as in the immune responses induced by the different strains and species of Leishmania is not fully understood. Improved understanding of the immunobiology and vaccine (prophylactic and therapeutic) development prerequisites for the different forms of leishmaniasis will provide tools that can be exploited to overcome the virulence dynamics of Leishmania species.
5. Suitability of mouse and hamsters as disease model for leishmaniasis is questionable as it imitates only some facets of the leishmaniasis in human. For example, the elicited immune response and the resultant disease are governed by the choice of mouse strains, route and mode of challenge infection. The immune responses leading to protection in humans have not been fully elucidated due to lack in correlation to the immune response in the animal model. In order reduce the mismatch between laboratory disease models and human trials, genetically modified animal model expressing human leukocyte antigen (HLA) molecules in mice were generated (106), but the immune responses generated in these preliminary models were more restricted toward mouse MHC than human HLA. Replacement technique of MHC with HLA can be potentially exploited to generate optimized humanized animals for preclinical studies.
Conclusion
Most conventional treatment option for leishmaniasis is highly expensive and toxic drugs. Vaccination (prophylactic and/or therapeutic), if possible, can be considered to be the most efficient strategy to control this infectious disease. Leishmaniasis is not an exception as patients cured of the disease become impervious to further infection. A plethora of vaccine candidates against leishmaniasis has been explored ranging from live vaccine to recombinant polyprotein and multiantigenic T-cell epitope-based vaccines. Moreover, chemotherapy along with immunotherapy that can elicit protective immune response can clear infection more effectively providing better possibility of recovery in patients. Subunit vaccine candidates—Leish-F1, Leish-F2, Leish-F3, and ChAd63-KH—are currently in different stages of clinical trials have kept alive the optimism for a licensed human vaccine (prophylactic and/or therapeutic) in near future. Although, licensed vaccines for canine leishmaniasis are available, the scope for improvement with newer approaches remains undaunted. With the rapid progress in understanding the propagation of protective immunity during leishmaniasis, development of better correlates of immunity to evaluate vaccines, novel delivery systems, immunotherapy with or without drugs, immunomodulators and adjuvant together with updated revelation in genetic information about the parasite have opened up opportunities for advanced research in the vaccine field. Moreover, if funding trust can lead the long road of vaccine development, the unmet goal of alternative approaches to chemotherapy will be achieved very soon.
Author Contributions
ND collected literature and drafted the manuscript. MS, AS, and NA edited the manuscript. NA finalized the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Md Asad and Rudra Chhajer for giving valuable suggestions during preparation of manuscript.
Funding
Research in the authors’ laboratory is funded by the DAE-BRNS Raja Rammana Fellowship DAE Order no. 10/3(23)/2016/RRF/R&D-II/293 dated 05.01.2017, Council of Scientific and Industrial Research and University Grants Commission, Government of India.
References
1. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One (2012) 7(5):e35671. doi:10.1371/journal.pone.0035671
2. Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol (2007) 37(10):1097–106. doi:10.1016/j.ijpara.2007.04.003
3. Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev (2008) 21(2):334–59, tableofcontents. doi:10.1128/CMR.00061-07
4. Pintado V, Martin-Rabadan P, Rivera ML, Moreno S, Bouza E. Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients. A comparative study. Medicine (Baltimore) (2001) 80(1):54–73. doi:10.1097/00005792-200101000-00006
5. Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis (2010) 2(2):167–76. doi:10.4103/0974-777X.62887
6. Khamesipour A. Therapeutic vaccines for leishmaniasis. Expert Opin Biol Ther (2014) 14(11):1641–9. doi:10.1517/14712598.2014.945415
7. Mollinedo F, Janssen H, de la Iglesia-Vicente J, Villa-Pulgarin JA, Calafat J. Selective fusion of azurophilic granules with Leishmania-containing phagosomes in human neutrophils. J Biol Chem (2010) 285(45):34528–36. doi:10.1074/jbc.M110.125302
8. Beattie L, Kaye PM. Leishmania-host interactions: what has imaging taught us? Cell Microbiol (2011) 13(11):1659–67. doi:10.1111/j.1462-5822.2011.01658.x
9. Rogers M, Kropf P, Choi BS, Dillon R, Podinovskaia M, Bates P, et al. Proteophosophoglycans regurgitated by Leishmania-infected sand flies target the l-arginine metabolism of host macrophages to promote parasite survival. PLoS Pathog (2009) 5(8):e1000555. doi:10.1371/journal.ppat.1000555
10. Rogers ME, Corware K, Muller I, Bates PA. Leishmania infantum proteophosphoglycans regurgitated by the bite of its natural sand fly vector, Lutzomyia longipalpis, promote parasite establishment in mouse skin and skin-distant tissues. Microbes Infect (2010) 12(11):875–9. doi:10.1016/j.micinf.2010.05.014
11. Ueno N, Wilson ME. Receptor-mediated phagocytosis of Leishmania: implications for intracellular survival. Trends Parasitol (2012) 28(8):335–44. doi:10.1016/j.pt.2012.05.002
12. Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol (2012) 2:83. doi:10.3389/fcimb.2012.00083
13. Kedzierski L. Leishmaniasis vaccine: where are we today? J Glob Infect Dis (2010) 2(2):177–85. doi:10.4103/0974-777X.62881
14. Mansueto P, Vitale G, Di Lorenzo G, Rini GB, Mansueto S, Cillari E. Immunopathology of leishmaniasis: an update. Int J Immunopathol Pharmacol (2007) 20(3):435–45. doi:10.1177/039463200702000302
15. Nascimento MS, Carregaro V, Lima-Junior DS, Costa DL, Ryffel B, Duthie MS, et al. Interleukin 17A acts synergistically with interferon gamma to promote protection against Leishmania infantum infection. J Infect Dis (2015) 211(6):1015–26. doi:10.1093/infdis/jiu531
16. Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, et al. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog (2013) 9(3):e1003243. doi:10.1371/journal.ppat.1003243
17. Ghosh K, Sharma G, Saha A, Kar S, Das PK, Ukil A. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J Infect Dis (2013) 207(6):1016–25. doi:10.1093/infdis/jis771
18. Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis (2006) 193(9):1313–22. doi:10.1086/502980
19. Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol (2007) 28(9):378–84. doi:10.1016/j.it.2007.07.004
20. Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol (2005) 174(5):2934–41. doi:10.4049/jimmunol.174.5.2934
21. Weinkopff T, Mariotto A, Simon G, Hauyon-La Torre Y, Auderset F, Schuster S, et al. Role of Toll-like receptor 9 signaling in experimental Leishmania braziliensis infection. Infect Immun (2013) 81(5):1575–84. doi:10.1128/IAI.01401-12
22. Srivastava S, Pandey SP, Jha MK, Chandel HS, Saha B. Leishmania expressed lipophosphoglycan interacts with toll-like receptor (TLR)-2 to decrease TLR-9 expression and reduce anti-leishmanial responses. Clin Exp Immunol (2013) 172(3):403–9. doi:10.1111/cei.12074
23. Raman VS, Bhatia A, Picone A, Whittle J, Bailor HR, O’Donnell J, et al. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol (2010) 185(3):1701–10. doi:10.4049/jimmunol.1000238
24. Kumar R, Singh OP, Gautam S, Nylen S, Sundar S. Enhanced expression of toll-like receptors 2 and 4, but not 9, in spleen tissue from patients with visceral leishmaniasis. Parasite Immunol (2014) 36(12):721–5. doi:10.1111/pim.12145
25. Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull (2012) 104:175–96. doi:10.1093/bmb/lds031
26. Srivastava P, Prajapati VK, Rai M, Sundar S. Unusual case of resistance to amphotericin B in visceral leishmaniasis in a region in India where leishmaniasis is not endemic. J Clin Microbiol (2011) 49(8):3088–91. doi:10.1128/JCM.00173-11
27. Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, et al. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis (2012) 6(5):e1657. doi:10.1371/journal.pntd.0001657
28. Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis (2013) 56(11):1530–8. doi:10.1093/cid/cit102
29. Sundar S, Singh A. What steps can be taken to counter the increasing failure of miltefosine to treat visceral leishmaniasis? Expert Rev Anti Infect Ther (2013) 11(2):117–9. doi:10.1586/eri.12.170
30. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med (2010) 362(6):504–12. doi:10.1056/NEJMoa0903627
31. Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet (2011) 377(9764):477–86. doi:10.1016/S0140-6736(10)62050-8
32. Sundar S, Chakravarty J. Leishmaniasis: an update of current pharmacotherapy. Expert Opin Pharmacother (2013) 14(1):53–63. doi:10.1517/14656566.2013.755515
33. Garcia-Hernandez R, Manzano JI, Castanys S, Gamarro F. Leishmania donovani develops resistance to drug combinations. PLoS Negl Trop Dis (2012) 6(12):e1974. doi:10.1371/journal.pntd.0001974
34. Dey R, Dagur PK, Selvapandiyan A, McCoy JP, Salotra P, Duncan R, et al. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J Immunol (2013) 190(5):2138–49. doi:10.4049/jimmunol.1202801
35. Fiuza JA, Gannavaram S, Santiago Hda C, Selvapandiyan A, Souza DM, Passos LS, et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine (2015) 33(2):280–8. doi:10.1016/j.vaccine.2014.11.039
36. Bhattacharya P, Dey R, Dagur PK, Kruhlak M, Ismail N, Debrabant A, et al. Genetically modified live attenuated Leishmania donovani parasites induce innate immunity through classical activation of macrophages that direct the Th1 response in mice. Infect Immun (2015) 83(10):3800–15. doi:10.1128/IAI.00184-15
37. Anand S, Madhubala R. Genetically engineered ascorbic acid-deficient live mutants of Leishmania donovani induce long lasting protective immunity against visceral leishmaniasis. Sci Rep (2015) 5:10706. doi:10.1038/srep10706
38. Mayrink W, Genaro O, Silva JC, da Costa RT, Tafuri WL, Toledo VP, et al. Phase I and II open clinical trials of a vaccine against Leishmania chagasi infections in dogs. Mem Inst Oswaldo Cruz (1996) 91(6):695–7. doi:10.1590/S0074-02761996000600006
39. Foroughi-Parvar F, Hatam G. Vaccines for canine leishmaniasis. Adv Prev Med (2014) 2014:569193. doi:10.1155/2014/569193
40. Giunchetti RC, Correa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Roatt BM, de Oliveira Aguiar-Soares RD, et al. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine (2007) 25(44):7674–86. doi:10.1016/j.vaccine.2007.08.009
41. Giunchetti RC, Correa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Roatt BM, de Oliveira Aguiar-Soares RD, et al. A killed Leishmania vaccine with sand fly saliva extract and saponin adjuvant displays immunogenicity in dogs. Vaccine (2008) 26(5):623–38. doi:10.1016/j.vaccine.2007.11.057
42. Bruhn KW, Birnbaum R, Haskell J, Vanchinathan V, Greger S, Narayan R, et al. Killed but metabolically active Leishmania infantum as a novel whole-cell vaccine for visceral leishmaniasis. Clin Vaccine Immunol (2012) 19(4):490–8. doi:10.1128/CVI.05660-11
43. Ravindran R, Maji M, Ali N. Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid-trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route. Mol Pharm (2012) 9(1):59–70. doi:10.1021/mp2002494
44. Choudhury R, Das P, De T, Chakraborti T. 115 kDa serine protease confers sustained protection to visceral leishmaniasis caused by Leishmania donovani via IFN-gamma induced down-regulation of TNF-alpha mediated MMP-9 activity. Immunobiology (2013) 218(1):114–26. doi:10.1016/j.imbio.2012.02.008
45. Resende LA, Roatt BM, Aguiar-Soares RD, Viana KF, Mendonca LZ, Lanna MF, et al. Cytokine and nitric oxide patterns in dogs immunized with LBSap vaccine, before and after experimental challenge with Leishmania chagasi plus saliva of Lutzomyia longipalpis. Vet Parasitol (2013) 198(3–4):371–81. doi:10.1016/j.vetpar.2013.09.011
46. Fernandes CB, Junior JT, de Jesus C, Souza BM, Larangeira DF, Fraga DB, et al. Comparison of two commercial vaccines against visceral leishmaniasis in dogs from endemic areas: IgG, and subclasses, parasitism, and parasite transmission by xenodiagnosis. Vaccine (2014) 32(11):1287–95. doi:10.1016/j.vaccine.2013.12.046
47. Gupta R, Kushawaha PK, Tripathi CD, Sundar S, Dube A. A novel recombinant Leishmania donovani p45, a partial coding region of methionine aminopeptidase, generates protective immunity by inducing a Th1 stimulatory response against experimental visceral leishmaniasis. Int J Parasitol (2012) 42(5):429–35. doi:10.1016/j.ijpara.2012.02.013
48. Saljoughian N, Taheri T, Zahedifard F, Taslimi Y, Doustdari F, Bolhassani A, et al. Development of novel prime-boost strategies based on a tri-gene fusion recombinant L. tarentolae vaccine against experimental murine visceral leishmaniasis. PLoS Negl Trop Dis (2013) 7(4):e2174. doi:10.1371/journal.pntd.0002174
49. Shahbazi M, Zahedifard F, Taheri T, Taslimi Y, Jamshidi S, Shirian S, et al. Evaluation of live recombinant nonpathogenic Leishmania tarentolae expressing cysteine proteinase and A2 genes as a candidate vaccine against experimental canine visceral leishmaniasis. PLoS One (2015) 10(7):e0132794. doi:10.1371/journal.pone.0132794
50. Shahbazi M, Zahedifard F, Saljoughian N, Doroud D, Jamshidi S, Mahdavi N, et al. Immunological comparison of DNA vaccination using two delivery systems against canine leishmaniasis. Vet Parasitol (2015) 212(3–4):130–9. doi:10.1016/j.vetpar.2015.07.005
51. Ramirez L, Santos DM, Souza AP, Coelho EA, Barral A, Alonso C, et al. Evaluation of immune responses and analysis of the effect of vaccination of the Leishmania major recombinant ribosomal proteins L3 or L5 in two different murine models of cutaneous leishmaniasis. Vaccine (2013) 31(9):1312–9. doi:10.1016/j.vaccine.2012.12.071
52. Zahedifard F, Gholami E, Taheri T, Taslimi Y, Doustdari F, Seyed N, et al. Enhanced protective efficacy of nonpathogenic recombinant Leishmania tarentolae expressing cysteine proteinases combined with a sand fly salivary antigen. PLoS Negl Trop Dis (2014) 8(3):e2751. doi:10.1371/journal.pntd.0002751
53. Hugentobler F, Di Roberto RB, Gillard J, Cousineau B. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine (2012) 30(39):5726–32. doi:10.1016/j.vaccine.2012.07.004
54. Testasicca MC, dos Santos MS, Machado LM, Serufo AV, Doro D, Avelar D, et al. Antibody responses induced by Leish-Tec(R), an A2-based vaccine for visceral leishmaniasis, in a heterogeneous canine population. Vet Parasitol (2014) 204(3–4):169–76. doi:10.1016/j.vetpar.2014.04.025
55. Pereira L, Abbehusen M, Teixeira C, Cunha J, Nascimento IP, Fukutani K, et al. Vaccination with Leishmania infantum acidic ribosomal P0 but not with nucleosomal histones proteins controls Leishmania infantum infection in hamsters. PLoS Negl Trop Dis (2015) 9(2):e0003490. doi:10.1371/journal.pntd.0003490
56. Katebi A, Gholami E, Taheri T, Zahedifard F, Habibzadeh S, Taslimi Y, et al. Leishmania tarentolae secreting the sand fly salivary antigen PpSP15 confers protection against Leishmania major infection in a susceptible BALB/c mice model. Mol Immunol (2015) 67(2 Pt B):501–11. doi:10.1016/j.molimm.2015.08.001
57. Das A, Ali N. Combining cationic liposomal delivery with MPL-TDM for cysteine protease cocktail vaccination against Leishmania donovani: evidence for antigen synergy and protection. PLoS Negl Trop Dis (2014) 8(8):e3091. doi:10.1371/journal.pntd.0003091
58. Miura R, Kooriyama T, Yoneda M, Takenaka A, Doki M, Goto Y, et al. Efficacy of recombinant canine distemper virus expressing Leishmania antigen against Leishmania challenge in dogs. PLoS Negl Trop Dis (2015) 9(7):e0003914. doi:10.1371/journal.pntd.0003914
59. Kushawaha PK, Gupta R, Tripathi CD, Sundar S, Dube A. Evaluation of Leishmania donovani protein disulfide isomerase as a potential immunogenic protein/vaccine candidate against visceral Leishmaniasis. PLoS One (2012) 7(4):e35670. doi:10.1371/journal.pone.0035670
60. Tabatabaie F, Mahdavi M, Faezi S, Dalimi A, Sharifi Z, Akhlaghi L, et al. Th1 Platform immune responses against Leishmania major induced by thiol-specific antioxidant-based DNA vaccines. Jundishapur J Microbiol (2014) 7(2):e8974. doi:10.5812/jjm.8974
61. Campos BL, Silva TN, Ribeiro SP, Carvalho KI, Kallas EG, Laurenti MD, et al. Analysis of iron superoxide dismutase-encoding DNA vaccine on the evolution of the Leishmania amazonensis experimental infection. Parasite Immunol (2015) 37(8):407–16. doi:10.1111/pim.12206
62. Doroud D, Zahedifard F, Vatanara A, Najafabadi AR, Taslimi Y, Vahabpour R, et al. Delivery of a cocktail DNA vaccine encoding cysteine proteinases type I, II and III with solid lipid nanoparticles potentiate protective immunity against Leishmania major infection. J Control Release (2011) 153(2):154–62. doi:10.1016/j.jconrel.2011.04.011
63. Riede O, Seifert K, Oswald D, Endmann A, Hock C, Winkler A, et al. Preclinical safety and tolerability of a repeatedly administered human leishmaniasis DNA vaccine. Gene Ther (2015) 22(8):628–35. doi:10.1038/gt.2015.35
64. Mazumder S, Maji M, Ali N. Potentiating effects of MPL on DSPC bearing cationic liposomes promote recombinant GP63 vaccine efficacy: high immunogenicity and protection. PLoS Negl Trop Dis (2011) 5(12):e1429. doi:10.1371/journal.pntd.0001429
65. Mazumder S, Maji M, Das A, Ali N. Potency, efficacy and durability of DNA/DNA, DNA/protein and protein/protein based vaccination using gp63 against Leishmania donovani in BALB/c mice. PLoS One (2011) 6(2):e14644. doi:10.1371/journal.pone.0014644
66. Elfaki ME, Khalil EA, De Groot AS, Musa AM, Gutierrez A, Younis BM, et al. Immunogenicity and immune modulatory effects of in silico predicted L. donovani candidate peptide vaccines. Hum Vaccin Immunother (2012) 8(12):1769–74. doi:10.4161/hv.21881
67. Athanasiou E, Agallou M, Tastsoglou S, Kammona O, Hatzigeorgiou A, Kiparissides C, et al. A poly(lactic-co-glycolic) acid nanovaccine based on chimeric peptides from different Leishmania infantum proteins induces dendritic cells maturation and promotes peptide-specific IFNγ-producing CD8+ T cells essential for the protection against experimental visceral leishmaniasis. Front Immunol (2017) 8:684. doi:10.3389/fimmu.2017.00684
68. Seyed N, Zahedifard F, Safaiyan S, Gholami E, Doustdari F, Azadmanesh K, et al. In silico analysis of six known Leishmania major antigens and in vitro evaluation of specific epitopes eliciting HLA-A2 restricted CD8 T cell response. PLoS Negl Trop Dis (2011) 5(9):e1295. doi:10.1371/journal.pntd.0001295
69. Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunol (2014) 3(3):e13. doi:10.1038/cti.2014.4
70. Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, et al. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine (2008) 26(52):6759–67. doi:10.1016/j.vaccine.2008.09.085
71. Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol (2006) 141(1–2):1–8. doi:10.1016/j.vetpar.2006.05.001
72. Moreno J, Vouldoukis I, Schreiber P, Martin V, McGahie D, Gueguen S, et al. Primary vaccination with the LiESP/QA-21 vaccine (CaniLeish) produces a cell-mediated immune response which is still present 1 year later. Vet Immunol Immunopathol (2014) 158(3–4):199–207. doi:10.1016/j.vetimm.2014.01.011
73. Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines (2012) 11(2):189–209. doi:10.1586/erv.11.188
74. Dikhit MR, Kumar A, Amit A, Dehury B, Nathsharma YP, Ansari MY, et al. Mining the proteome of Leishmania donovani for the development of novel MHC class I restricted epitope for the control of visceral leishmaniasis. J Cell Biochem (2018) 119(1):378–91. doi:10.1002/jcb.26190
75. Kashyap M, Jaiswal V, Farooq U. Prediction and analysis of promiscuous T cell-epitopes derived from the vaccine candidate antigens of Leishmania donovani binding to MHC class-II alleles using in silico approach. Infect Genet Evol (2017) 53:107–15. doi:10.1016/j.meegid.2017.05.022
76. Dietze-Schwonberg K, Grewe B, Brosch S, Kuharev J, van Zandbergen G, Rammensee HG, et al. In silico prediction of Leishmania major-specific CD8+ epitopes. Exp Dermatol (2017) 26(9):838–40. doi:10.1111/exd.13295
77. Silva Rde FE, Ferreira LF, Hernandes MZ, de Brito ME, de Oliveira BC, da Silva AA, et al. Combination of in silico methods in the search for potential CD4(+) and CD8(+) T cell epitopes in the proteome of Leishmania braziliensis. Front Immunol (2016) 7:327. doi:10.3389/fimmu.2016.00327
78. Castellano LR, Argiro L, Dessein H, Dessein A, da Silva MV, Correia D, et al. Potential use of interleukin-10 blockade as a therapeutic strategy in human cutaneous leishmaniasis. J Immunol Res (2015) 2015:152741. doi:10.1155/2015/152741
79. Faleiro RJ, Kumar R, Bunn PT, Singh N, Chauhan SB, Sheel M, et al. Combined immune therapy for the treatment of visceral leishmaniasis. PLoS Negl Trop Dis (2016) 10(2):e0004415. doi:10.1371/journal.pntd.0004415
80. Abdossamadi Z, Taheri T, Seyed N, Montakhab-Yeganeh H, Zahedifard F, Taslimi Y, et al. Live Leishmania tarentolae secreting HNP1 as an immunotherapeutic tool against Leishmania infection in BALB/c mice. Immunotherapy (2017) 9(13):1089–102. doi:10.2217/imt-2017-0076
81. Montakhab-Yeganeh H, Abdossamadi Z, Zahedifard F, Taslimi Y, Badirzadeh A, Saljoughian N, et al. Leishmania tarentolae expressing CXCL-10 as an efficient immunotherapy approach against Leishmania major-infected BALB/c mice. Parasite Immunol (2017) 39(10). doi:10.1111/pim.12461
82. Nahrevanian H, Jalalian M, Farahmand M, Assmar M, Rastaghi AE, Sayyah M. Inhibition of murine systemic leishmaniasis by acetyl salicylic acid via nitric oxide immunomodulation. Iran J Parasitol (2012) 7(2):21–8.
83. Hoseini MH, Moradi M, Alimohammadian MH, Shahgoli VK, Darabi H, Rostami A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol Int (2016) 65(2):99–104. doi:10.1016/j.parint.2015.10.007
84. Shakya N, Sane SA, Vishwakarma P, Gupta S. Enhancement in therapeutic efficacy of miltefosine in combination with synthetic bacterial lipopeptide, Pam3Cys against experimental visceral leishmaniasis. Exp Parasitol (2012) 131(3):377–82. doi:10.1016/j.exppara.2012.05.007
85. Santos FN, Borja-Cabrera GP, Miyashiro LM, Grechi J, Reis AB, Moreira MA, et al. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine (2007) 25(33):6176–90. doi:10.1016/j.vaccine.2007.06.005
86. Borja-Cabrera GP, Santos FN, Santos FB, Trivellato FA, Kawasaki JK, Costa AC, et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine (2010) 28(3):597–603. doi:10.1016/j.vaccine.2009.09.071
87. Trigo J, Abbehusen M, Netto EM, Nakatani M, Pedral-Sampaio G, de Jesus RS, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine (2010) 28(19):3333–40. doi:10.1016/j.vaccine.2010.02.089
88. Ferreira JH, Silva Ldos S, Longo-Maugeri IM, Katz S, Barbieri CL. Use of a recombinant cysteine proteinase from Leishmania (Leishmania) infantum chagasi for the immunotherapy of canine visceral leishmaniasis. PLoS Negl Trop Dis (2014) 8(3):e2729. doi:10.1371/journal.pntd.0002729
89. Joshi J, Kaur S. To investigate the therapeutic potential of immunochemotherapy with cisplatin + 78 kDa + MPL-A against Leishmania donovani in BALB/c mice. Parasite Immunol (2014) 36(1):3–12. doi:10.1111/pim.12071
90. Ehrlich A, Castilho TM, Goldsmith-Pestana K, Chae WJ, Bothwell AL, Sparwasser T, et al. The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection. J Immunol (2014) 193(6):2961–70. doi:10.4049/jimmunol.1400728
91. Joshi J, Malla N, Kaur S. A comparative evaluation of efficacy of chemotherapy, immunotherapy and immunochemotherapy in visceral leishmaniasis-an experimental study. Parasitol Int (2014) 63(4):612–20. doi:10.1016/j.parint.2014.04.002
92. Datta S, Roy S, Manna M. Therapy with radio-attenuated vaccine in experimental murine visceral leishmaniasis showed enhanced T cell and inducible nitric oxide synthase levels, suppressed tumor growth factor-beta production with higher expression of some signaling molecules. Braz J Infect Dis (2015) 19(1):36–42. doi:10.1016/j.bjid.2014.10.009
93. Seifert K, Juhls C, Salguero FJ, Croft SL. Sequential chemoimmunotherapy of experimental visceral leishmaniasis using a single low dose of liposomal amphotericin B and a novel DNA vaccine candidate. Antimicrob Agents Chemother (2015) 59(9):5819–23. doi:10.1128/AAC.00273-15
94. Guarga JL, Moreno J, Lucientes J, Gracia MJ, Peribanez MA, Castillo JA. Evaluation of a specific immunochemotherapy for the treatment of canine visceral leishmaniasis. Vet Immunol Immunopathol (2002) 88(1–2):13–20. doi:10.1016/S0165-2427(02)00128-9
95. Miret J, Nascimento E, Sampaio W, Franca JC, Fujiwara RT, Vale A, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine (2008) 26(12):1585–94. doi:10.1016/j.vaccine.2008.01.026
96. Jamshidi S, Avizeh R, Mohebali M, Bokaie S. Immunotherapy using autoclaved L. major antigens and M. vaccae with meglumine antimoniate, for the treatment of experimental canine visceral leishmaniasis. Iran J Parasitol (2011) 6(3):26–34.
97. Bongiorno G, Paparcone R, Foglia Manzillo V, Oliva G, Cuisinier AM, Gradoni L. Vaccination with LiESP/QA-21 (CaniLeish(R)) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs – a preliminary xenodiagnosis study. Vet Parasitol (2013) 197(3–4):691–5. doi:10.1016/j.vetpar.2013.05.008
98. Gradoni L. Canine Leishmania vaccines: still a long way to go. Vet Parasitol (2015) 208(1–2):94–100. doi:10.1016/j.vetpar.2015.01.003
99. Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine (2011) 29(19):3531–7. doi:10.1016/j.vaccine.2011.02.096
100. Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine (2010) 28(40):6581–7. doi:10.1016/j.vaccine.2010.07.063
101. Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine (2005) 23(45):5245–51. doi:10.1016/j.vaccine.2005.07.001
102. Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine (2016) 34(26):2992–5. doi:10.1016/j.vaccine.2015.12.071
103. Carter D, Fox CB, Day TA, Guderian JA, Liang H, Rolf T, et al. A structure-function approach to optimizing TLR4 ligands for human vaccines. Clin Transl Immunology (2016) 5(11):e108. doi:10.1038/cti.2016.63
104. Daneshvar H, Namazi MJ, Kamiabi H, Burchmore R, Cleaveland S, Phillips S. Gentamicin-attenuated Leishmania infantum vaccine: protection of dogs against canine visceral leishmaniosis in endemic area of southeast of Iran. PLoS Negl Trop Dis (2014) 8(4):e2757. doi:10.1371/journal.pntd.0002757
105. Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis (2017) 11(5):e0005527. doi:10.1371/journal.pntd.0005527
Keywords: leishmaniasis, vaccine, immunotherapy, chemotherapy, immunology
Citation: Didwania N, Shadab M, Sabur A and Ali N (2017) Alternative to Chemotherapy—The Unmet Demand against Leishmaniasis. Front. Immunol. 8:1779. doi: 10.3389/fimmu.2017.01779
Received: 09 October 2017; Accepted: 28 November 2017;
Published: 21 December 2017
Edited by:
Alexandre Barbosa Reis, Universidade Federal de Ouro Preto, BrazilReviewed by:
Sima Rafati, Pasteur Institute of Iran, IranBruno Mendes Roatt, Universidade Federal de Minas Gerais – UFMG, Brazil
Copyright: © 2017 Didwania, Shadab, Sabur and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nahid Ali, bmFsaUBpaWNiLnJlcy5pbg==
†Present address: Md. Shadab, Department of Dermatology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States
 Nicky Didwania
Nicky Didwania Md. Shadab
Md. Shadab Abdus Sabur
Abdus Sabur Nahid Ali
Nahid Ali