Abstract
Adoptive cell therapy has emerged as a powerful treatment for advanced cancers resistant to conventional agents. Most notable are the remarkable responses seen in patients receiving autologous CD19-redirected chimeric antigen receptor (CAR) T cells for the treatment of B lymphoid malignancies; however, the generation of autologous products for each patient is logistically cumbersome and has restricted widespread clinical use. A banked allogeneic product has the potential to overcome these limitations, yet allogeneic T-cells (even if human leukocyte antigen-matched) carry a major risk of graft-versus-host disease (GVHD). Natural killer (NK) cells are bone marrow-derived innate lymphocytes that can eliminate tumors directly, with their activity governed by the integration of signals from activating and inhibitory receptors and from cytokines including IL-15, IL-12, and IL-18. NK cells do not cause GVHD or other alloimmune or autoimmune toxicities and thus, can provide a potential source of allogeneic “off-the-shelf” cellular therapy, mediating major anti-tumor effects without inducing potentially lethal alloreactivity such as GVHD. Given the multiple unique advantages of NK cells, researchers are now exploring the use of CAR-engineered NK cells for the treatment of various hematological and non-hematological malignancies. Herein, we review preclinical data on the development of CAR-NK cells, advantages, disadvantages, and current obstacles to their clinical use.
Introduction
Chimeric antigen receptor (CAR) T cells have gained enormous clinical recognition with remarkable responses reported in patients receiving autologous CD19 (a B cell-specific antigen)-redirected T cells for the treatment of patients with relapsed or refractory B-cell malignancies (1–9). CAR T cells are genetically engineered to express a single chain variable fragment (scFv) derived from an antibody on their surface, which is coupled to a T-cell signaling domain, thus rendering them highly antigen-specific in a non-human leukocyte antigen (HLA)-restricted manner (2, 10, 11). Thus far, the clinical application of CAR T cells has been largely restricted to CD19-expressing B cell malignancies (1–9); however, ongoing studies are testing its applications in other hematological malignancies such as Hodgkin and non-Hodgkin lymphoma, multiple myeloma, and acute myeloid leukemia (12–14). The US Food and Drug Administration recently approved two autologous CD19 CAR T cell products for the treatment of acute lymphoblastic leukemia and certain types of relapsed or refractory large B-cell lymphoma. However, CAR T-cells have several limitations: (i) it is logistically cumbersome to generate an autologous product from patients; (ii) it takes several weeks before CAR T cells are generated—making it impractical for patients with aggressive disease; and (iii) generation of clinically relevant doses of CAR T-cells can be unfeasible from heavily pretreated lymphopenic patients. An alternative approach is to use previously collected T cells from an allogeneic source; however, even if HLA-matched, T cells pose a risk of serious graft-versus-host disease (GVHD) (15). In contrast to the popularity of CAR T cells, the generation and clinical application of CAR natural killer (NK) cells has lagged behind for various reasons, despite the multiple advantages of NK cells. Herein, we describe these barriers and discuss strategies to overcome them.
Advantages of NK Cells for CAR Therapy
Among cytolytic lymphocytes, NK cells represent on a per cell basis the most efficient effectors against tumors with a distinct mechanism of action (Figure 1) and provide an attractive source of cells for cancer immunotherapy (16, 17). In contrast to other lymphocytes such as T or B cells, NK cells do not express rearranged, antigen-specific receptors. Instead, NK cells express germline-encoded receptors, which are either activating or inhibitory. Upon interaction with their ligands on target cells, the receptors induce a positive or a negative signal, respectively (18). The balance of these signals ultimately govern NK effector function (16, 19) Among the most heavily studied NK cells receptors are the killer-cell immunoglobulin-like receptors (KIRs) are that recognize classical HLA class-I molecules (HLA- A, -B, and -C). Other receptors belong to the C-type lectin family (CD94 and NKG2s, such as NKG2A, -B, -C, -D, -E, and -F) that recognize non-classical HLA class-I molecules (HLA-E and stress-induced MHC-I-related chains—MICA and MICB) [reviewed in Ref. (20–23)]. Healthy cells are protected from NK mediated lysis by the recognition of “self” HLA molecules on their surface by inhibitory NK receptors protects (24–27). On the other hand, tumor or viral infected cells often downregulate or lose their HLA molecules as an escape mechanism against T-cells (28, 29). Loss of HLA class I expression makes them susceptible to lysis by the NK cells due to loss of the inhibitory signal (21, 30–45). Indeed, the clinical significance of NK cell alloreactivity has been demonstrated in multiple studies in the setting of hematopoietic stem cell transplant (HSCT), where patients that received a graft containing alloreactive NK cells had a significantly lower risk of relapse and improved survival (46–55). Adoptive transfer of alloreactive NK cells as a stand-alone therapy (independent of HSCT) also demonstrated encouraging outcomes in a variety of malignancies (56–62). In contrast, several studies of autologous NK cell adoptive therapy showed rather disappointing results (63–71).
Figure 1
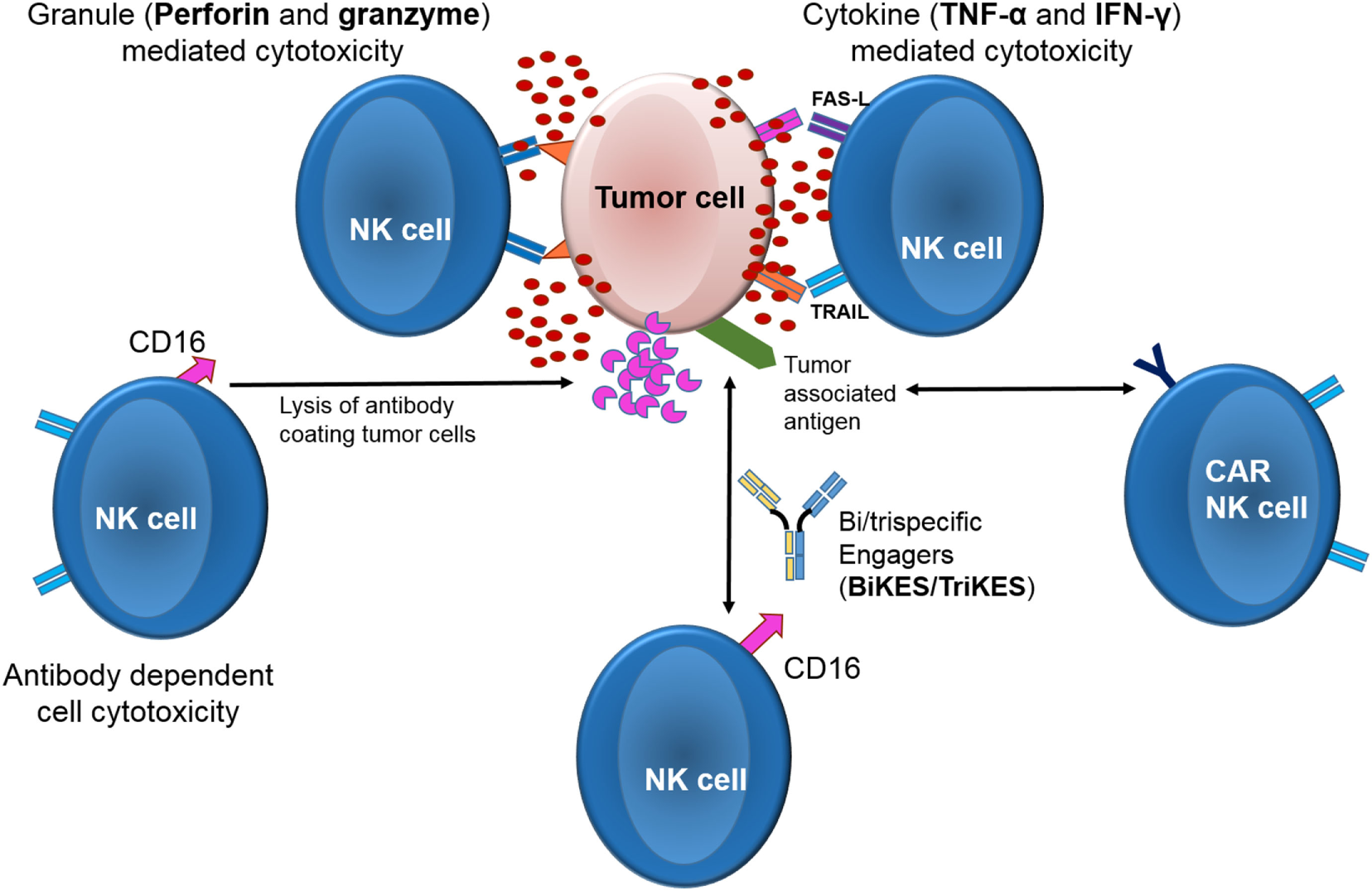
Mechanisms of action of natural killer cell cytotoxicity.
Thus, NK cells offer an attractive alternative to T-cells for CAR engineering for a number of reasons: (i) allogeneic NK cells should not cause GVHD, as predicted by observations in murine models (72, 73), as well as clinical studies of haploidentical and cord blood (CB)-derived NK cell infusions in patients with hematologic or solid malignancies (56, 59); (ii) mature NK cells have a relatively limited life-span, permitting effective antitumor activity while reducing the probability of long-term adverse events, such as prolonged cytopenias due to on-target/off-tumor toxicity to normal tissues such as B cell aplasia (in the case of CD19 CARs), which can last up to 3 years (74); and (iii) CAR-NK cells retain their intrinsic capacity to recognize and target tumor cells through their native receptors; therefore when compared with the CAR T cells, it is theoretically less likely for tumor cells to escape NK immunosurveillance even if they downregulate the CAR target antigen (75). This unique property of NK cells could be further exploited for the generation of NK-CARs by selecting donors based on the donor-recipient KIR-ligand mismatch, or based on donor haplotype B KIR gene content, as both have been shown to be beneficial in the setting of allogeneic HSCT (48, 50, 55, 76). Thus, allogeneic NK cells offer the potential for an off-the-shelf cellular product for immunotherapy that could be readily available for immediate clinical use, in contrast to the current shortage of CAR T-cell products at many centers (77).
Source of NK Cells for Adoptive Immunotherapy
Functional NK cells can be generated from numerous sources. Although autologous NK cells can be utilized for adoptive therapy, their efficacy against autologous cancer cells is rather limited (63–71, 78, 79), which we have shown may not be easily overcome by CAR engineering (80). Allogeneic NK cell sources include peripheral blood (PB), bone marrow (BM), human embryonic stem cells (hESCs), induced pluripotent stem cells (iPSCs) (81–83), umbilical CB, or readily available NK cell lines (84). Obtaining NK cells from the PB by apheresis or from BM by harvesting are both cumbersome and are associated with potential risks to the healthy donors (85–87). NK cell derivation from hESCs or iPSCs (81–83) is a complex process and the field is still evolving. In contrast, NK cell lines such as NK-92 (88–93), KHYG-1 (94), NKL, NKG, and YT, to name a few, provide an easily accessible and homogeneous source of cells for the generation of large numbers of CAR-transduced NK cells. NK-92 is a highly cytotoxic NK cell line that was derived from a patient with NK lymphoma (95) and is characterized as CD56brightCD16neg/lowNKG2Apositive and KIRnegative (except for KIR2DL4) (96, 97). Phase I clinical studies demonstrated the safety of NK-92 cell infusion in cancer patients, even up to doses of 1010 cells/m2 (98–100). Based on these data, there is great interest in CAR-engineered NK-92 cells for clinical use (Table 1) (88–92, 101–115). However, NK-92 cells have a number of disadvantages that need to be taken into account. First and foremost, NK-92 cells are derived from a patient with NK lymphoma (95) and thus have the potential for tumor engraftment following infusion. Moreover, they are EBV-positive and carry multiple cytogenetic abnormalities resembling those of NK lymphoma (116). Thus, as a safety measure, NK-92 cells must be irradiated before infusion into patients to prevent permanent engraftment. This can negatively impact their in vivo proliferation and persistence, both factors shown to be crucial for the success of cellular therapy in studies with infusion of tumor-infiltrating lymphocytes (117–119) as well as CAR-T cells (3). Indeed, in a study of NK-92 cells engineered with ErbB2/HER2-CAR, while irradiation had no effect on the in vitro cytotoxicity of CAR-transduced NK92 cells, it negatively impacted their in vivo replication and persistence, with the cells no longer detectable within 7 days of adoptive infusion (109). Of note, NK-92 cells are CD16 (FCRIIIγ) negative and cannot mediate antibody-dependent cell cytotoxicity (ADCC), unless genetically modified to express CD16 (120).
Table 1
| Clinical trial identifier | NK cell source | Target antigen | Disease | Study location |
|---|---|---|---|---|
| NCT02944162 | NK-92 cell line | CD33 | AML | China |
| NCT02892695 | NK-92 cell line | CD19 | CD19 positive B cell malignancies | China |
| NCT02742727 | NK-92 cell line | CD7 | CD7 positive leukemia or lymphoma | China |
| NCT02839954 | NK-92 cell line | MUC1 | MUC1 positive solid tumors (colorectal, gastric, pancreatic, NSCLC, breast, glioma) | China |
| NCT03056339 | Cord blood | CD19 | CD19 positive leukemia or lymphoma | MDACC, USA |
Clinical trials with NK CAR.
AML, acute myeloid leukemia; MDACC, MD Anderson Cancer Center; NK, natural killer; NSCLC, non-small cell lung cancer.
Cord blood, on the other hand, is a readily available source of allogeneic NK cells with distinct benefits over related or unrelated adult donors, including the speed of availability (especially since it is available as an off-the-shelf frozen product) and tolerance of HLA mismatches, the latter of which expands the donor pool. The frequencies of NK cells in CB (~15–20%) are similar to PB (~10–15%) (121–124). However, until recently the small volume of blood in a CB unit made it challenging to obtain adequate numbers of NK cells for clinical use. Moreover, resting CB NK cells are phenotypically and functionally immature, with higher expression of the inhibitory receptor NKG2A and lower expression of activating and maturation receptors such as NKp46, NKG2C, DNAM-1 (124), and CD57 (124–127). To overcome these limitations, our group has developed a Good Manufacturing Practice (GMP)-compliant procedure, using GMP-grade K562-based artificial antigen-presenting cells (aAPCs) expressing membrane bound IL-21 and 4-1BB ligand, which reliably generates clinically relevant doses of GMP-grade NK cells from a CB unit for adoptive immunotherapy (128). Following ex vivo activation and expansion, CB-derived NK cells display the full array of activating and inhibitory receptors, strongly express eomesodermin (Eomes) and T-bet, two factors necessary for NK cell maturation, and exert similar cytotoxicity to PB-NK cells (129, 130). Taken together, these studies support the use of NK cells as a source of cellular therapy in cancer.
Constituents of CAR
A CAR construct consists of three components: an extracellular antigen-recognition part, a transmembrane domain and an intracellular signaling domain. The extracellular domain is the antigen-recognition site and is generally composed of an scFv derived from the variable regions of both the heavy and light chains of a monoclonal antibody, fused together via a flexible linker. Most scFvs studied to date are of murine origin, with the potential to induce a human antimouse antibody (HAMA) or an anti-idiotype immune response. A number of investigators are exploring strategies to humanize scFVs (131–136) to circumvent induction of HAMA; however, this approach will not prevent the development of anti-idiotype antibodies. The antigen binding domain of a CAR is linked to a “hinge” which imparts flexibility for adequate orientation and binding to the antigen. The hinge binds the extracellular component to a transmembrane domain, which is the link to the intracellular signaling component (137–139). The size of the hinge region has been shown to affect CAR-T cell function, with some studies reporting superior anti-tumor activity of CAR T-cells expressing a shorter hinge (140, 141). The transmembrane domain lies between the hinge and the signaling endodomains. Different types of transmembrane domains have been studied, including the CD3-ζ chain of the T-cell receptor, CD4, CD8, or CD28. The type of transmembrane domain has also been shown to affect the function and stability of the CAR molecule in T cells (142). The endodomain then transmits activation signals to T cells. The “first-generation” CARs used a single intracellular signaling domain (CD3-ζ chain alone) while the second- and third-generation CARs incorporate one or more additional costimulatory signaling domains, such as CD28, CD137, or OX40 to render them more potent (143, 144).
CD3ζ is critical for signaling and activation of both T and NK cells (145). In NK cells, CD3ζ homodimer transmits signals from FcγRIII (CD16), thus aiding in ADCC (146). Although CD28 is one of the most commonly employed costimulatory domains in CAR T cells, except in certain cell lines (147), its role in NK cell function is less clearly defined (148). Nonetheless, its addition to CD3ζ in a second generation ErbB2-specific NK-92 CAR led to improved function compared to a CD3ζ construct alone and was similar to that of CD137-CD3ζ CAR against ErbB2-expressing tumor cells (109). Another study showed that NK-92 cells transduced with a CD19-CAR expressing CD28-CD3ζ had superior cytotoxicity against CD19-positive targets compared to cells expressing a CD137-CD3ζ containing CAR (88). DNAX-activation protein 12 (DAP12) is transmembrane protein involved in signal transduction of several NK cell activating receptors including NKG2C, NKp44, and the activating KIRs (149). One study tested if a CAR against prostate stem cell antigen (PSCA) that used DAP12 as an intracellular signaling domain can provide sufficient signaling to induce NK cell activation when compared to a CD3ζ-containing CAR. The authors transduced YTS-NK cells and primary NK cells with PSCA-DAP12 CAR and noted superior cytotoxicity when compared to NK cells expressing a CD3ζ-based CAR (150). While the importance of incorporating costimulatory molecules in the CAR construct has been clearly shown for CAR T cells (4, 5, 151), additional studies are needed to define the optimal costimulatory molecule and signaling endodomain for NK cells.
CAR Transduction
The incorporation of a foreign gene into a cell requires the use of a vector, which can be based on viral or non-viral systems. The most commonly used tools for CAR gene delivery include genetically engineered retroviruses [lentiviral (152) and gamma-retroviral (153) vectors]. Lentiviruses have the advantage that they are capable of infecting both dividing and non-dividing cells, while retroviruses only infect dividing cells. Therefore, lentiviral vectors can be used for transduction of a wider variety of cell types including quiescent stem cells (154–156). In addition, lentiviral vectors can accommodate larger transgenes when compared with retroviral vectors (157). Insertional mutagenesis, although extremely rare, remains a concern with viral vectors although its likelihood is influenced by a number of factors such as the specific type of vector used and the site of integration (158). For instance, in earlier trials of gene therapy with CD34+ hematopoietic cells for X-linked severe combined immunodeficiency (SCID), 2 of the 10 treated children developed acute leukemia in one study (159) and 1 of the 4 children in another study (160). However, none of the subsequent trials of gene-modified hematopoietic stem cells in SCID children (161) or studies of adoptive immunotherapy with CAR T-cells (2, 3, 5, 9, 162) have witnessed adverse events related to insertional mutagenesis to date. Yet, to mitigate any theoretical concerns, various non-viral techniques such as the transposon/transposase system (82, 163) or mRNA transfection (113, 115) have also been tested. The transduction efficiency using these techniques varies remarkably from study to study (82, 113, 115, 163, 164) and depends on a number of factors, including the cell source (82, 113, 115, 163–167). In general, the transduction efficiency for CAR T cells is about 50% but can range up to 90% or higher (152, 164, 168).
Challenges
Despite the many advantages of NK cells, there are several impediments to the successful generation of CAR NK cells for clinical use. Until recently, the genetic engineering of NK cells, even with viral methods, had proved challenging, with reports of <10% transduction efficiency for primary CB or PB derived NK cells (113, 165). However, recent optimization in protocols for viral transduction and electroporation (166, 167) has revived enthusiasm for the genetic engineering of NK cells. While viral methods appear to be largely ineffective for inducing CAR expression in freshly isolated PB NK cells, significantly better transduction efficiency can be achieved when NK cells from PB (12–73%) (113) or CB are activated and expanded (median 69%; range 43–93%) (169) in one study and 80% (range 67–96%) (170) in another study. In contrast to studies with primary NK cells, NK92 cells are easier to transduce with mRNA electroporation (113, 115), with efficiencies averaging from 25 to 50% (171). However, as the mRNA transcript is not incorporated into the genome, expression of the CAR molecule is often short-lived and detectable for only a few days, which may negatively impact the efficacy of the engineered cells following adoptive transfer (115, 166, 167).
Another concern with using allogeneic NK cells is the possibility of infusing contaminating T or B cells in the expanded NK cell product, which can theoretically cause GVHD or posttransplant lymphoproliferative disease, respectively. As some degree of HLA-mismatch after CB transplantation is well tolerated, the risk of clinically significant GVHD may be less with CB-derived CAR-NK cells compared to PB. Plus, with the exception of one study reporting GVHD following adoptive transfer of donor-derived IL-15/4-1BBL-activated NK cells in recipients of HLA-matched, T-cell-depleted PB HSCT (72), clinical studies of haploidentical and CB NK cell infusions in hundreds of patients with both hematologic and solid malignancies have not reported a higher risk of GVHD (56, 58, 128, 172–174). Rather, experimental evidence obtained in mice have reported reduced risk of GVHD with NK cells via multiple mechanisms, including depletion of host antigen-presenting cells and activated alloreactive T cells (46, 72, 175, 176). Another potential limitation of NK cells for immunotherapy is that in contrast to T cells, they are highly sensitive to the freeze and thaw process and they lose activity after thawing. A number of groups are exploring strategies to optimally cryopreserve NK cells and have shown that the activity of frozen NK cells can be restored by overnight incubation with cytokines such as IL-2 (177–182). It is not yet known if a similar strategy can be used to restore function of frozen CAR NK cells for adoptive therapy.
Another characteristic of NK is that they do not persist after adoptive transfer without cytokine support (183). While the shorter life-span of NK cells may be advantageous, allowing for antitumor activity while reducing the probability of long-term adverse events such as prolonged cytopenias caused by on-target/off-tumor toxicity to normal tissues, it may also limit their efficacy. For in vivo survival and proliferation, NK cells require continuous cytokine support, without which they are detectable in the circulation for only 1–2 weeks (183). The two most commonly used cytokines to support the persistence of adoptively transferred NK cells are IL-2 and IL-15 (184, 185). The infusion of IL-2 has substantial side effects including fevers, chills, myalgias and capillary leak syndrome (186), and can promote expansion of regulatory T cells (Tregs) which are suppressive to NK cells (187). IL-15, on the other hand, does not support Treg (188) expansion but when administered as an exogenous bolus to patients with metastatic melanoma and renal carcinoma can result in dose-dependent toxicity, including neutropenia (189). An alternative approach to exogenous administration of cytokines is to treat patients with lymphodepleting chemotherapy such as cyclophosphamide and fludarabine prior to infusion of NK cells, which provides a favorable environment for NK cell expansion by depleting mature lymphocytes (which consume IL-15), resulting in a marked increase in endogenous IL-15 levels (56). Another novel technique is to incorporate genes for IL-2 (104, 190–192) or IL-15 (80, 193–195) within the CAR construct to constantly provide cytokine support to the CAR-transduced cells. We recently showed the feasibility and efficacy of this approach in a mouse model of Raji lymphoma. Although a single infusion of 1 × 107 CAR.19+ (without IL15) or CAR.19/IL15+ CB-NK cells both improved tumor control and prolonged survival compared to non-transduced CB-NK, CAR.19/IL15+ CB-NK cells controlled tumor expansion and prolonged survival significantly better than the CAR.CD19 construct lacking the IL-15 gene, which underscores the critical influence of IL-15 in enhancing antitumor activity in vivo (80).
Suicide Genes
Given the recent safety concerns such as cytokine release syndrome and neurotoxicity associated with infusion of CAR-modified T cells (196, 197) (and possibly NK cells), careful consideration of whether a suicide system should be incorporated into the construct as a safety measure is needed. One of the most extensively tested safety switches include the herpes simplex virus thymidine kinase gene (198, 199). While a number of studies have tested this approach, the highly immunogenic virus-derived protein can lead to the rejection of cells expressing it, plus it requires administration of ganciclovir—which takes several days to work and leads to cytopenias (200–202). Because of these disadvantages, inducible caspase-9 (iCasp9) has emerged as one of the most commonly used suicide genes in adoptive cell therapy trials (80, 193, 203–206). When exposed to a synthetic bioinert small-molecule dimerizing drug, the iCasp9 becomes activated and leads to rapid apoptosis of cells expressing it. Another suicide gene under investigation is the truncated epidermal growth factor receptor (EGFR), which lacks intracellular tyrosine kinase activity while expressing an intact binding epitope that can be targeted with the anti-EGFR monoclonal antibody cetuximab for the rapid elimination of the transgenic cells (207, 208).
Preclinical Studies of CAR-NK Cells
Building on the knowledge gained with CAR T-cells, a multitude of preclinical studies have tested the efficacy of CAR NK cells against a variety of target antigens for hematological malignancies such as CD19 (167, 169), CD20 (209, 210), CD138 (211), CS1 (111), CD3 (212), CD5 (101), CD123 (213), as well as solid tumors such as HER-2/Erb-2 (109, 214–216), GD2 (114), EpCAM (195), EGFR and mutant EGFRvIII (89), WT1 (217), and ROR-1 (218) to name a few. An alternative and non-antigen specific approach to engineering NK cells was tested by Chang et al. (170), where the authors induced supra-physiologic expression of NKG2D, a key receptor for NK cell activation and signal transduction via DNAX-activating protein 10 (DAP10). Ex vivo expanded PB NK cells transduced with a retroviral vector encoding NKG2D-DAP10-CD3ζ showed impressive in vivo cytotoxicity in xenogeneic mouse models of hematologic and solid tumors but showed no activity against non-transformed blood or mesenchymal cells (170).
Despite these impressive preclinical data, there are currently only five registered clinical trials testing the safety and efficacy of CAR-NK cells in cancer patients (Table 1). Four of these trials are being conducted in China using the CAR-engineered NK92 cells. The only trial using primary NK cells (CB NK cells) is being conducted in the United States by our group at the MD Anderson Cancer Center (NCT03056339). Patients with relapsed or refractory CD19+ B cell lymphoid malignancies are eligible for this trial. All patients receive lymphodepleting chemotherapy with fludarabine and cyclophosphamide, followed by the infusion of allogeneic CB-derived NK cells that are genetically modified with a retroviral vector, iC9-2A-CAR.CD19-CD28-CD3zeta-2A-OhIL-15 (iC9/CAR.19/IL15) (80), which (i) includes CAR.19 gene to redirect specificity to CD19; (ii) produces IL-15 ectopically—a cytokine crucial for NK cell survival and proliferation (219); and (iii) incorporates inducible caspase-9 (iCasp9)—a suicide gene, which can be activated pharmacologically to eliminate CAR cells, as needed (203, 219).
Conclusion
We are in an exciting era in the field of cellular therapy. NK cells hold great promise and offer the potential for an off-the-shelf cellular product for immunotherapy that could be readily available for immediate clinical use. Yet, before NK cells can be extended to larger cohorts of patients a number of scientific questions and regulatory hurdles must be addressed. What is the ideal vector, signaling endodomain and costimulatory molecule for NK cells—one with the best response and safety profile? Will CAR NK cells have a different safety and efficacy profile to CAR T cells, given their distinct mechanism of action? Will “off-the-shelf” CAR NK cells be able to sustain the clinical demand, give the shortage of CAR-T cells at many centers and the uncertainty regarding the health economics of this treatment? Can CAR NK cells lead to durable responses, considering their limited in vivo life-span or will they be used as a “bridge” to more aggressive treatment such as HSCT? Based on the results of haploidentical and unrelated donor HSCT, should NK cells for CAR modification be selected based on KIR-KIR ligand mismatch or KIR haplotype to harness their native NK cell activity and will this approach reduce the risk of disease escape through downregulation of the CAR target antigen? With the plethora of preclinical studies and clinical research that are underway, it is expected that engineered NK cells will make a significant contribution to the recent paradigm shift in cancer treatment.
Statements
Author contributions
RM and KR reviewed the literature, analyzed data, and wrote the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Kochenderfer JN Wilson WH Janik JE Dudley ME Stetler-Stevenson M Feldman SA et al Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood (2010) 116(20):4099–102.10.1182/blood-2010-04-281931
2
Porter DL Levine BL Kalos M Bagg A June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med (2011) 365(8):725–33.10.1056/NEJMoa1103849
3
Porter DL Hwang WT Frey NV Lacey SF Shaw PA Loren AW et al Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med (2015) 7(303):303ra139.10.1126/scitranslmed.aac5415
4
Lee DW Kochenderfer JN Stetler-Stevenson M Cui YK Delbrook C Feldman SA et al T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (2015) 385(9967):517–28.10.1016/S0140-6736(14)61403-3
5
Maude SL Frey N Shaw PA Aplenc R Barrett DM Bunin NJ et al Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371(16):1507–17.10.1056/NEJMoa1407222
6
Davila ML Riviere I Wang X Bartido S Park J Curran K et al Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med (2014) 6(224):224ra25.10.1126/scitranslmed.3008226
7
Li Z Chen L Rubinstein MP . Cancer immunotherapy: are we there yet?Exp Hematol Oncol (2013) 2(1):33.10.1186/2162-3619-2-33
8
Brentjens RJ Davila ML Riviere I Park J Wang X Cowell LG et al CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med (2013) 5(177):177ra38.10.1126/scitranslmed.3005930
9
Grupp SA Kalos M Barrett D Aplenc R Porter DL Rheingold SR et al Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med (2013) 368(16):1509–18.10.1056/NEJMoa1215134
10
Irving BA Weiss A . The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell (1991) 64(5):891–901.10.1016/0092-8674(91)90314-O
11
Gross G Waks T Eshhar Z . Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A (1989) 86(24):10024–8.10.1073/pnas.86.24.10024
12
Luo Y Chang L-J Hu Y Dong L Wei G Huang H . First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood (2015) 126:3778.
13
Ritchie DS Neeson PJ Khot A Peinert S Tai T Tainton K et al Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther (2013) 21(11):2122–9.10.1038/mt.2013.154
14
Wang QS Wang Y Lv HY Han QW Fan H Guo B et al Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther (2015) 23(1):184–91.10.1038/mt.2014.164
15
Goulmy E . Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev (1997) 157:125–40.10.1111/j.1600-065X.1997.tb00978.x
16
Davies JOJ Stringaris K Barrett AJ Rezvani K . Opportunities and limitations of natural killer cells as adoptive therapy for malignant disease. Cytotherapy (2014) 16(11):1453–66.10.1016/j.jcyt.2014.03.009
17
Vivier E Ugolini S Blaise D Chabannon C Brossay L . Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol (2012) 12(4):239–52.10.1038/nri3174
18
Lanier LL . NK cell receptors. Annu Rev Immunol (1998) 16:359–93.10.1146/annurev.immunol.16.1.359
19
Caligiuri MA . Human natural killer cells. Blood (2008) 112(3):461–9.10.1182/blood-2007-09-077438
20
Parham P . MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol (2005) 5(3):201–14.10.1038/nri1570
21
Moretta L Moretta A . Killer immunoglobulin-like receptors. Curr Opin Immunol (2004) 16(5):626–33.10.1016/j.coi.2004.07.010
22
Farag SS Fehniger TA Ruggeri L Velardi A Caligiuri MA . Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood (2002) 100(6):1935–47.10.1182/blood-2002-02-0350
23
Moretta A Bottino C Vitale M Pende D Cantoni C Mingari MC et al Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol (2001) 19:197–223.10.1146/annurev.immunol.19.1.197
24
Lanier LL . Face off – the interplay between activating and inhibitory immune receptors. Curr Opin Immunol (2001) 13(3):326–31.10.1016/S0952-7915(00)00222-3
25
Yokoyama WM . Natural killer cell receptors. Curr Opin Immunol (1998) 10(3):298–305.10.1016/S0952-7915(98)80168-4
26
Ljunggren HG Karre K . In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today (1990) 11(7):237–44.10.1016/0167-5699(90)90097-S
27
Harel-Bellan A Quillet A Marchiol C DeMars R Tursz T Fradelizi D . Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6. Proc Natl Acad Sci U S A (1986) 83(15):5688–92.10.1073/pnas.83.15.5688
28
Algarra I Garcia-Lora A Cabrera T Ruiz-Cabello F Garrido F . The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother (2004) 53(10):904–10.10.1007/s00262-004-0517-9
29
Costello RT Gastaut JA Olive D . Tumor escape from immune surveillance. Arch Immunol Ther Exp (Warsz) (1999) 47(2):83–8.
30
Robertson MJ Ritz J . Biology and clinical relevance of human natural killer cells. Blood (1990) 76(12):2421–38.
31
Cooper MA Fehniger TA Caligiuri MA . The biology of human natural killer-cell subsets. Trends Immunol (2001) 22(11):633–40.10.1016/S1471-4906(01)02060-9
32
Fehniger TA Cooper MA Nuovo GJ Cella M Facchetti F Colonna M et al CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood (2003) 101(8):3052–7.10.1182/blood-2002-09-2876
33
Yokoyama WM . Natural killer cell receptors. Curr Opin Immunol (1995) 7(1):110–20.10.1016/0952-7915(95)80036-0
34
Brandt CS Baratin M Yi EC Kennedy J Gao Z Fox B et al The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med (2009) 206(7):1495–503.10.1084/jem.20090681
35
Pogge von Strandmann E Simhadri VR von Tresckow B Sasse S Reiners KS Hansen HP et al Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity (2007) 27(6):965–74.10.1016/j.immuni.2007.10.010
36
Bloushtain N Qimron U Bar-Ilan A Hershkovitz O Gazit R Fima E et al Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol (2004) 173(4):2392–401.10.4049/jimmunol.173.4.2392
37
Jinushi M Takehara T Tatsumi T Kanto T Groh V Spies T et al Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer (2003) 104(3):354–61.10.1002/ijc.10966
38
Boyington JC Sun PD . A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol Immunol (2002) 38(14):1007–21.10.1016/S0161-5890(02)00030-5
39
Boyington JC Brooks AG Sun PD . Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol Rev (2001) 181:66–78.10.1034/j.1600-065X.2001.1810105.x
40
Steinle A Li P Morris DL Groh V Lanier LL Strong RK et al Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics (2001) 53(4):279–87.10.1007/s002510100325
41
Das H Groh V Kuijl C Sugita M Morita CT Spies T et al MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity (2001) 15(1):83–93.10.1016/S1074-7613(01)00168-6
42
Cosman D Müllberg J Sutherland CL Chin W Armitage R Fanslow W et al ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity (2001) 14(2):123–33.10.1016/S1074-7613(01)00095-4
43
Bauer S Groh V Wu J Steinle A Phillips JH Lanier LL et al Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science (1999) 285(5428):727–9.10.1126/science.285.5428.727
44
Gumperz JE Barber LD Valiante NM Percival L Phillips JH Lanier LL et al Conserved and variable residues within the Bw4 motif of HLA-B make separable contributions to recognition by the NKB1 killer cell-inhibitory receptor. J Immunol (1997) 158(11):5237–41.
45
Mandelboim O Reyburn HT Sheu EG Vales-Gomez M Davis DM Pazmany L et al The binding site of NK receptors on HLA-C molecules. Immunity (1997) 6(3):341–50.10.1016/S1074-7613(00)80336-2
46
Ruggeri L Capanni M Urbani E Perruccio K Shlomchik WD Tosti A et al Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295(5562):2097–100.10.1126/science.1068440
47
Ruggeri L Capanni M Casucci M Volpi I Tosti A Perruccio K et al Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood (1999) 94(1):333–9.
48
Cooley S Trachtenberg E Bergemann TL Saeteurn K Klein J Le CT et al Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood (2009) 113(3):726–32.10.1182/blood-2008-07-171926
49
Oevermann L Michaelis SU Mezger M Lang P Toporski J Bertaina A et al KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood (2014) 124(17):2744–7.10.1182/blood-2014-03-565069
50
Cooley S Weisdorf DJ Guethlein LA Klein JP Wang T Le CT et al Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood (2010) 116(14):2411–9.10.1182/blood-2010-05-283051
51
Cooley S Weisdorf DJ Guethlein LA Klein JP Wang T Marsh SG et al Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol (2014) 192(10):4592–600.10.4049/jimmunol.1302517
52
Stringaris K Adams S Uribe M Eniafe R Wu CO Savani BN et al Donor KIR genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant (2010) 16(9):1257–64.10.1016/j.bbmt.2010.03.004
53
Willemze R Ruggeri A Purtill D Rodrigues CA Gluckman E Rocha V et al Is there an impact of killer cell immunoglobulin-like receptors and KIR-ligand incompatibilities on outcomes after unrelated cord blood stem cell transplantation? Best Pract Res Clin Haematol (2010) 23(2):283–90.10.1016/j.beha.2010.05.005
54
Willemze R Rodrigues CA Labopin M Sanz G Michel G Socié G et al KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia (2009) 23(3):492–500.10.1038/leu.2008.365
55
Sekine T Marin D Cao K Li L Mehta P Shaim H et al Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood (2016) 128(2):297–312.10.1182/blood-2016-03-706317
56
Miller JS Soignier Y Panoskaltsis-Mortari A McNearney SA Yun GH Fautsch SK et al Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood (2005) 105(8):3051–7.10.1182/blood-2004-07-2974
57
Iliopoulou EG Kountourakis P Karamouzis MV Doufexis D Ardavanis A Baxevanis CN et al A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother (2010) 59(12):1781–9.10.1007/s00262-010-0904-3
58
Rubnitz JE Inaba H Ribeiro RC Pounds S Rooney B Bell T et al NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol (2010) 28(6):955–9.10.1200/JCO.2009.24.4590
59
Curti A Ruggeri L D’Addio A Bontadini A Dan E Motta MR et al Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood (2011) 118(12):3273–9.10.1182/blood-2011-01-329508
60
Geller MA Cooley S Judson PL Ghebre R Carson LF Argenta PA et al A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy (2011) 13(1):98–107.10.3109/14653249.2010.515582
61
Bachanova V Cooley S Defor TE Verneris MR Zhang B McKenna DH et al Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood (2014) 123(25):3855–63.10.1182/blood-2013-10-532531
62
Shaffer BC Le Luduec JB Forlenza C Jakubowski AA Perales MA Young JW et al Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant (2016) 22(4):705–9.10.1016/j.bbmt.2015.12.028
63
Soiffer RJ Murray C Cochran K Cameron C Wang E Schow PW et al Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood (1992) 79(2):517–26.
64
Caligiuri MA Murray C Robertson MJ Wang E Cochran K Cameron C et al Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest (1993) 91(1):123–32.10.1172/JCI116161
65
Weisdorf DJ Anderson PM Blazar BR Uckun FM Kersey JH Ramsay NK . Interleukin 2 immediately after autologous bone marrow transplantation for acute lymphoblastic leukemia – a phase I study. Transplantation (1993) 55(1):61–6.10.1097/00007890-199301000-00012
66
Lister J Rybka WB Donnenberg AD deMagalhaes-Silverman M Pincus SM Bloom EJ et al Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res (1995) 1(6):607–14.
67
Meropol NJ Porter M Blumenson LE Lindemann MJ Perez RP Vaickus L et al Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res (1996) 2(4):669–77.
68
Blaise D Attal M Pico JL Reiffers J Stoppa AM Bellanger C et al The use of a sequential high dose recombinant interleukin 2 regimen after autologous bone marrow transplantation does not improve the disease free survival of patients with acute leukemia transplanted in first complete remission. Leuk Lymphoma (1997) 25(5–6):469–78.10.3109/10428199709039034
69
Miller JS Tessmer-Tuck J Pierson BA Weisdorf D McGlave P Blazar BR et al Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant (1997) 3(1):34–44.
70
deMagalhaes-Silverman M Donnenberg A Lembersky B Elder E Lister J Rybka W et al Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother (2000) 23(1):154–60.10.1097/00002371-200001000-00018
71
Burns LJ Weisdorf DJ DeFor TE Vesole DH Repka TL Blazar BR et al IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant (2003) 32(2):177–86.10.1038/sj.bmt.1704086
72
Olson JA Leveson-Gower DB Gill S Baker J Beilhack A Negrin RS . NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood (2010) 115(21):4293–301.10.1182/blood-2009-05-222190
73
Ruggeri L Mancusi A Burchielli E Capanni M Carotti A Aloisi T et al NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis (2008) 40(1):84–90.10.1016/j.bcmd.2007.06.029
74
Kalos M Nazimuddin F Finklestein JM Gupta M Kulikovskaya I Ambrose DE et al Long-term functional persistence, B cell aplasia and anti-leukemia efficacy in refractory B cell malignancies following T cell immunotherapy using CAR-redirected T cells targeting CD19. Blood (2013) 122:163.
75
Sotillo E Barrett DM Black KL Bagashev A Oldridge D Wu G et al Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov (2015) 5(12):1282–95.10.1158/2159-8290.CD-15-1020
76
Venstrom JM Pittari G Gooley TA Chewning JH Spellman S Haagenson M et al HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med (2012) 367(9):805–16.10.1056/NEJMoa1200503
77
Couzin-Frankel J . Supply of promising T cell therapy is strained. Science (2017) 356(6343):1112–3.10.1126/science.356.6343.1112
78
Rouce RH Shaim H Sekine T Weber G Ballard B Ku S et al The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia (2016) 30(4):800–11.10.1038/leu.2015.327
79
Stringaris K Sekine T Khoder A Alsuliman A Razzaghi B Sargeant R et al Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica (2014) 99(5):836–47.10.3324/haematol.2013.087536
80
Liu E Tong Y Dotti G Shaim H Savoldo B Mukherjee M et al Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia (2018) 32(2):520–31.10.1038/leu.2017.226
81
Ni Z Knorr DA Bendzick L Allred J Kaufman DS . Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells (2014) 32(4):1021–31.10.1002/stem.1611
82
Wilber A Linehan JL Tian X Woll PS Morris JK Belur LR et al Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells (2007) 25(11):2919–27.10.1634/stemcells.2007-0026
83
Knorr DA Ni Z Hermanson D Hexum MK Bendzick L Cooper LJ et al Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med (2013) 2(4):274–83.10.5966/sctm.2012-0084
84
Matsuo Y Drexler HG . Immunoprofiling of cell lines derived from natural killer-cell and natural killer-like T-cell leukemia-lymphoma. Leuk Res (2003) 27(10):935–45.10.1016/S0145-2126(03)00024-9
85
Yuan S Ziman A Smeltzer B Lu Q Goldfinger D . Moderate and severe adverse events associated with apheresis donations: incidences and risk factors. Transfusion (2010) 50(2):478–86.10.1111/j.1537-2995.2009.02443.x
86
Miller JP Perry EH Price TH Bolan CD Jr Karanes C Boyd TM et al Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant (2008) 14(9 Suppl):29–36.10.1016/j.bbmt.2008.05.018
87
Winters JL . Complications of donor apheresis. J Clin Apher (2006) 21(2):132–41.10.1002/jca.20039
88
Oelsner S Friede ME Zhang C Wagner J Badura S Bader P et al Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy (2017) 19(2):235–49.10.1016/j.jcyt.2016.10.009
89
Genssler S Burger MC Zhang C Oelsner S Mildenberger I Wagner M et al Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology (2016) 5(4):e1119354.10.1080/2162402X.2015.1119354
90
Chen X Han J Chu J Zhang L Zhang J Chen C et al A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget (2016) 7(19):27764–77.10.18632/oncotarget.8526
91
Romanski A Uherek C Bug G Seifried E Klingemann H Wels WS et al CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med (2016) 20(7):1287–94.10.1111/jcmm.12810
92
Zhang C Burger MC Jennewein L Genßler S Schönfeld K Zeiner P et al ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst (2016) 108(5).10.1093/jnci/djv375
93
Suck G Odendahl M Nowakowska P Seidl C Wels WS Klingemann HG et al NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother (2016) 65(4):485–92.10.1007/s00262-015-1761-x
94
Kobayashi E Kishi H Ozawa T Hamana H Nakagawa H Jin A et al A chimeric antigen receptor for TRAIL-receptor 1 induces apoptosis in various types of tumor cells. Biochem Biophys Res Commun (2014) 453(4):798–803.10.1016/j.bbrc.2014.10.024
95
Gong JH Maki G Klingemann HG . Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia (1994) 8(4):652–8.
96
Tonn T Becker S Esser R Schwabe D Seifried E . Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res (2001) 10(4):535–44.10.1089/15258160152509145
97
Maki G Klingemann HG Martinson JA Tam YK . Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res (2001) 10(3):369–83.10.1089/152581601750288975
98
Tonn T Schwabe D Klingemann HG Becker S Esser R Koehl U et al Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy (2013) 15(12):1563–70.10.1016/j.jcyt.2013.06.017
99
Arai S Meagher R Swearingen M Myint H Rich E Martinson J et al Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy (2008) 10(6):625–32.10.1080/14653240802301872
100
Boyiadzis M Agha M Redner RL Sehgal A Im A Hou JZ et al Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy (2017) 19(10):1225–32.10.1016/j.jcyt.2017.07.008
101
Chen KH Wada M Pinz KG Liu H Lin KW Jares A et al Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia (2017) 31(10):2151–60.10.1038/leu.2017.8
102
Wang Z Guo L Song Y Zhang Y Lin D Hu B et al Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-betaR II and NKG2D. Cancer Immunol Immunother (2017) 66(4):537–48.10.1007/s00262-017-1959-1
103
Hsieh YT Aggarwal P Cirelli D Gu L Surowy T Mozier NM . Characterization of FcgammaRIIIA effector cells used in in vitro ADCC bioassay: comparison of primary NK cells with engineered NK-92 and Jurkat T cells. J Immunol Methods (2017) 441:56–66.10.1016/j.jim.2016.12.002
104
Jochems C Hodge JW Fantini M Fujii R Morillon YM II Greiner JW et al An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget (2016) 7(52):86359–73.10.18632/oncotarget.13411
105
Samara P Skopeliti M Tsiatas ML Georgaki S Gouloumis C Voelter W et al A cytokine cocktail augments the efficacy of adoptive NK-92 cell therapy against mouse xenografts of human cancer. Anticancer Res (2016) 36(7):3373–82.
106
Alkins R Burgess A Kerbel R Wels WS Hynynen K . Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol (2016) 18(7):974–81.10.1093/neuonc/nov318
107
Clémenceau B Valsesia-Wittmann S Jallas AC Vivien R Rousseau R Marabelle A et al In vitro and in vivo comparison of lymphocytes transduced with a human CD16 or with a chimeric antigen receptor reveals potential off-target interactions due to the IgG2 CH2-CH3 CAR-spacer. J Immunol Res (2015) 2015:482089.10.1155/2015/482089
108
Han J Chu J Keung Chan W Zhang J Wang Y Cohen JB et al CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep (2015) 5:11483.10.1038/srep11483
109
Schönfeld K Sahm C Zhang C Naundorf S Brendel C Odendahl M et al Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther (2015) 23(2):330–8.10.1038/mt.2014.219
110
Boissel L Betancur-Boissel M Lu W Krause DS Van Etten RA Wels WS et al Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology (2013) 2(10):e26527.10.4161/onci.26527
111
Chu J Deng Y Benson DM He S Hughes T Zhang J et al CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia (2014) 28(4):917–27.10.1038/leu.2013.279
112
Li J Liu H Li L Wu H Wang C Yan Z et al The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma. Oncol Rep (2013) 29(3):895–902.10.3892/or.2012.2217
113
Boissel L Betancur M Lu W Wels WS Marino T Van Etten RA et al Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma (2012) 53(5):958–65.10.3109/10428194.2011.634048
114
Esser R Müller T Stefes D Kloess S Seidel D Gillies SD et al NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med (2012) 16(3):569–81.10.1111/j.1582-4934.2011.01343.x
115
Boissel L Betancur M Wels WS Tuncer H Klingemann H . Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res (2009) 33(9):1255–9.10.1016/j.leukres.2008.11.024
116
MacLeod RA Nagel S Kaufmann M Greulich-Bode K Drexler HG . Multicolor-FISH analysis of a natural killer cell line (NK-92). Leuk Res (2002) 26(11):1027–33.10.1016/S0145-2126(02)00055-3
117
Rosenberg SA Dudley ME . Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol (2009) 21(2):233–40.10.1016/j.coi.2009.03.002
118
Besser MJ Shapira-Frommer R Treves AJ Zippel D Itzhaki O Hershkovitz L et al Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res (2010) 16(9):2646–55.10.1158/1078-0432.CCR-10-0041
119
Yee C Thompson JA Byrd D Riddell SR Roche P Celis E et al Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A (2002) 99(25):16168–73.10.1073/pnas.242600099
120
Chen Y You F Jiang L Li J Zhu X Bao Y et al Gene-modified NK-92MI cells expressing a chimeric CD16-BB-zeta or CD64-BB-zeta receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget (2017) 8(23):37128–39.10.18632/oncotarget.16201
121
Tanaka H Kai S Yamaguchi M Misawa M Fujimori Y Yamamoto M et al Analysis of natural killer (NK) cell activity and adhesion molecules on NK cells from umbilical cord blood. Eur J Haematol (2003) 71(1):29–38.10.1034/j.1600-0609.2003.00081.x
122
Dalle JH Menezes J Wagner E Blagdon M Champagne J Champagne MA et al Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res (2005) 57(5 Pt 1):649–55.10.1203/01.PDR.0000156501.55431.20
123
Wang Y Xu H Zheng X Wei H Sun R Tian Z . High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol (2007) 4(5):377–82.
124
Luevano M Daryouzeh M Alnabhan R Querol S Khakoo S Madrigal A et al The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol (2012) 73(3):248–57.10.1016/j.humimm.2011.12.015
125
Björkström NK Riese P Heuts F Andersson S Fauriat C Ivarsson MA et al Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood (2010) 116(19):3853–64.10.1182/blood-2010-04-281675
126
Abo T Miller CA Balch CM . Characterization of human granular lymphocyte subpopulations expressing HNK-1 (Leu-7) and Leu-11 antigens in the blood and lymphoid tissues from fetuses, neonates and adults. Eur J Immunol (1984) 14(7):616–23.10.1002/eji.1830140707
127
Bradstock KF Luxford C Grimsley PG . Functional and phenotypic assessment of neonatal human leucocytes expressing natural killer cell-associated antigens. Immunol Cell Biol (1993) 71(Pt 6):535–42.10.1038/icb.1993.59
128
Shah N Martin-Antonio B Yang H Ku S Lee DA Cooper LJ et al Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One (2013) 8(10):e76781.10.1371/journal.pone.0076781
129
Gill S Vasey AE De Souza A Baker J Smith AT Kohrt HE et al Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood (2012) 119(24):5758–68.10.1182/blood-2012-03-415364
130
Intlekofer AM Takemoto N Wherry EJ Longworth SA Northrup JT Palanivel VR et al Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol (2005) 6(12):1236–44.10.1038/ni1268
131
Sommermeyer D Hill T Shamah SM Salter AI Chen Y Mohler KM et al Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia (2017) 31(10):2191–9.10.1038/leu.2017.57
132
Song DG Ye Q Poussin M Liu L Figini M Powell DJ Jr . A fully human chimeric antigen receptor with potent activity against cancer cells but reduced risk for off-tumor toxicity. Oncotarget (2015) 6(25):21533–46.10.18632/oncotarget.4071
133
Sun M Shi H Liu C Liu J Liu X Sun Y . Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res (2014) 16(3):R61.10.1186/bcr3674
134
Alonso-Camino V Sánchez-Martín D Compte M Nuñez-Prado N Diaz RM Vile R et al CARbodies: human antibodies against cell surface tumor antigens selected from repertoires displayed on T cell chimeric antigen receptors. Mol Ther Nucleic Acids (2013) 2:e93.10.1038/mtna.2013.19
135
Lanitis E Poussin M Hagemann IS Coukos G Sandaltzopoulos R Scholler N et al Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther (2012) 20(3):633–43.10.1038/mt.2011.256
136
Zhao Y Wang QJ Yang S Kochenderfer JN Zheng Z Zhong X et al A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol (2009) 183(9):5563–74.10.4049/jimmunol.0900447
137
Call ME Schnell JR Xu C Lutz RA Chou JJ Wucherpfennig KW . The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell (2006) 127(2):355–68.10.1016/j.cell.2006.08.044
138
Gosse JA Wagenknecht-Wiesner A Holowka D Baird B . Transmembrane sequences are determinants of immunoreceptor signaling. J Immunol (2005) 175(4):2123–31.10.4049/jimmunol.175.4.2123
139
Yamasaki S Ishikawa E Kohno M Saito T . The quantity and duration of FcRgamma signals determine mast cell degranulation and survival. Blood (2004) 103(8):3093–101.10.1182/blood-2003-08-2944
140
Hudecek M Lupo-Stanghellini MT Kosasih PL Sommermeyer D Jensen MC Rader C et al Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res (2013) 19(12):3153–64.10.1158/1078-0432.CCR-13-0330
141
Hombach A Heuser C Gerken M Fischer B Lewalter K Diehl V et al T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Ther (2000) 7(12):1067–75.10.1038/sj.gt.3301195
142
Dotti G Gottschalk S Savoldo B Brenner MK . Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev (2014) 257(1):107–26.10.1111/imr.12131
143
Finney HM Akbar AN Lawson AD . Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol (2004) 172(1):104–13.10.4049/jimmunol.172.1.104
144
Finney HM Lawson AD Bebbington CR Weir AN . Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol (1998) 161(6):2791–7.
145
Moingeon P Lucich JL McConkey DJ Letourneur F Malissen B Kochan J et al CD3 zeta dependence of the CD2 pathway of activation in T lymphocytes and natural killer cells. Proc Natl Acad Sci U S A (1992) 89(4):1492–6.10.1073/pnas.89.4.1492
146
Vivier E Ackerly M Rochet N Anderson P . Structure and function of the CD16:zeta:gamma complex expressed on human natural-killer cells. Int J Cancer Suppl (1992) 7:11–4.
147
Chen X Allan DS Krzewski K Ge B Kopcow H Strominger JL . CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A (2006) 103(27):10346–51.10.1073/pnas.0604236103
148
Lang S Vujanovic NL Wollenberg B Whiteside TL . Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur J Immunol (1998) 28(3):780–6.10.1002/(SICI)1521-4141(199803)28:03<780::AID-IMMU780>3.0.CO;2-8
149
Campbell KS Colonna M . DAP12: a key accessory protein for relaying signals by natural killer cell receptors. Int J Biochem Cell Biol (1999) 31(6):631–6.10.1016/S1357-2725(99)00022-9
150
Töpfer K Cartellieri M Michen S Wiedemuth R Müller N Lindemann D et al DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol (2015) 194(7):3201–12.10.4049/jimmunol.1400330
151
Savoldo B Ramos CA Liu E Mims MP Keating MJ Carrum G et al CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest (2011) 121(5):1822–6.10.1172/JCI46110
152
Tumaini B Lee DW Lin T Castiello L Stroncek DF Mackall C et al Simplified process for the production of anti-CD19-CAR-engineered T cells. Cytotherapy (2013) 15(11):1406–15.10.1016/j.jcyt.2013.06.003
153
Miller DG Adam MA Miller AD . Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol (1990) 10(8):4239–42.10.1128/MCB.10.8.4239
154
van Til NP Wagemaker G . Lentiviral gene transduction of mouse and human hematopoietic stem cells. Methods Mol Biol (2014) 1185:311–9.10.1007/978-1-4939-1133-2_21
155
Lin P Lin Y Lennon DP Correa D Schluchter M Caplan AI . Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl Med (2012) 1(12):886–97.10.5966/sctm.2012-0086
156
Ye Z Yu X Cheng L . Lentiviral gene transduction of mouse and human stem cells. Methods Mol Biol (2008) 430:243–53.10.1007/978-1-59745-182-6_17
157
De Meyer SF Vanhoorelbeke K Chuah MK Pareyn I Gillijns V Hebbel RP et al Phenotypic correction of von Willebrand disease type 3 blood-derived endothelial cells with lentiviral vectors expressing von Willebrand factor. Blood (2006) 107(12):4728–36.10.1182/blood-2005-09-3605
158
Sadelain M . Insertional oncogenesis in gene therapy: how much of a risk?Gene Ther (2004) 11(7):569–73.10.1038/sj.gt.3302243
159
Hacein-Bey-Abina S Von Kalle C Schmidt M McCormack MP Wulffraat N Leboulch P et al LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science (2003) 302(5644):415–9.10.1126/science.1088547
160
Hacein-Bey-Abina S von Kalle C Schmidt M Le Deist F Wulffraat N McIntyre E et al A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med (2003) 348(3):255–6.10.1056/NEJM200301163480314
161
Aiuti A Slavin S Aker M Ficara F Deola S Mortellaro A et al Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science (2002) 296(5577):2410–3.10.1126/science.1070104
162
Brudno JN Somerville RP Shi V Rose JJ Halverson DC Fowler DH et al Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol (2016) 34(10):1112–21.10.1200/JCO.2015.64.5929
163
Kebriaei P Singh H Huls MH Figliola MJ Bassett R Olivares S et al Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest (2016) 126(9):3363–76.10.1172/JCI86721
164
Johnson LA June CH . Driving gene-engineered T cell immunotherapy of cancer. Cell Res (2017) 27(1):38–58.10.1038/cr.2016.154
165
Sutlu T Nystrom S Gilljam M Stellan B Applequist SE Alici E . Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: implications for gene therapy. Hum Gene Ther (2012) 23(10):1090–100.10.1089/hum.2012.080
166
Li L Liu LN Feller S Allen C Shivakumar R Fratantoni J et al Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther (2010) 17(3):147–54.10.1038/cgt.2009.61
167
Shimasaki N Fujisaki H Cho D Masselli M Lockey T Eldridge P et al A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy (2012) 14(7):830–40.10.3109/14653249.2012.671519
168
Anurathapan U Chan RC Hindi HF Mucharla R Bajgain P Hayes BC et al Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol Ther (2014) 22(3):623–33.10.1038/mt.2013.262
169
Imai C Iwamoto S Campana D . Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood (2005) 106(1):376–83.10.1182/blood-2004-12-4797
170
Chang YH Connolly J Shimasaki N Mimura K Kono K Campana D . A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res (2013) 73(6):1777–86.10.1158/0008-5472.CAN-12-3558
171
Tassev DV Cheng M Cheung NK . Retargeting NK92 cells using an HLA-A2-restricted, EBNA3C-specific chimeric antigen receptor. Cancer Gene Ther (2012) 19(2):84–100.10.1038/cgt.2011.66
172
Locatelli F Moretta F Brescia L Merli P . Natural killer cells in the treatment of high-risk leukemia. Semin Immunol (2014) 26(2):173–9.10.1016/j.smim.2014.02.004
173
Yoon SR Lee YS Yang SH Ahn KH Lee JH Lee JH et al Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant (2010) 45(6):1038–46.10.1038/bmt.2009.304
174
Rezvani K Rouce RH . The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol (2015) 6:578.10.3389/fimmu.2015.00578
175
Asai O Longo DL Tian ZG Hornung RL Taub DD Ruscetti FW et al Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest (1998) 101(9):1835–42.10.1172/JCI1268
176
Shlomchik WD Couzens MS Tang CB McNiff J Robert ME Liu J et al Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science (1999) 285(5426):412–5.10.1126/science.285.5426.412
177
Fujiwara S Akiyama M Yamakido M Seyama T Kobuke K Hakoda M et al Cryopreservation of human lymphocytes for assessment of lymphocyte subsets and natural killer cytotoxicity. J Immunol Methods (1986) 90(2):265–73.10.1016/0022-1759(86)90084-0
178
Dominguez E Lowdell MW Perez-Cruz I Madrigal A Cohen SB . Natural killer cell function is altered by freezing in DMSO. Biochem Soc Trans (1997) 25(2):175S.10.1042/bst025175s
179
Voshol H Dullens HF Den Otter W Vliegenthart JF . Human natural killer cells: a convenient purification procedure and the influence of cryopreservation on cytotoxic activity. J Immunol Methods (1993) 165(1):21–30.10.1016/0022-1759(93)90102-D
180
Domogala A Madrigal JA Saudemont A . Cryopreservation has no effect on function of natural killer cells differentiated in vitro from umbilical cord blood CD34(+) cells. Cytotherapy (2016) 18(6):754–9.10.1016/j.jcyt.2016.02.008
181
Koehl U Brehm C Huenecke S Zimmermann SY Kloess S Bremm M et al Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol (2013) 3:118.10.3389/fonc.2013.00118
182
Lapteva N Durett AG Sun J Rollins LA Huye LL Fang J et al Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy (2012) 14(9):1131–43.10.3109/14653249.2012.700767
183
Fujisaki H Kakuda H Shimasaki N Imai C Ma J Lockey T et al Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res (2009) 69(9):4010–7.10.1158/0008-5472.CAN-08-3712
184
Davis ZB Felices M Verneris MR Miller JS . Natural killer cell adoptive transfer therapy: exploiting the first line of defense against cancer. Cancer J (2015) 21(6):486–91.10.1097/PPO.0000000000000156
185
Becknell B Caligiuri MA . Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol (2005) 86:209–39.10.1016/S0065-2776(04)86006-1
186
Antony GK Dudek AZ . Interleukin 2 in cancer therapy. Curr Med Chem (2010) 17(29):3297–302.10.2174/092986710793176410
187
Pedroza-Pacheco I Madrigal A Saudemont A . Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol (2013) 10(3):222–9.10.1038/cmi.2013.2
188
Waldmann TA Lugli E Roederer M Perera LP Smedley JV Macallister RP et al Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood (2011) 117(18):4787–95.10.1182/blood-2010-10-311456
189
Conlon KC Lugli E Welles HC Rosenberg SA Fojo AT Morris JC et al Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol (2015) 33(1):74–82.10.1200/JCO.2014.57.3329
190
Tam YK Maki G Miyagawa B Hennemann B Tonn T Klingemann HG . Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther (1999) 10(8):1359–73.10.1089/10430349950018030
191
Nagashima S Mailliard R Kashii Y Reichert TE Herberman RB Robbins P et al Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood (1998) 91(10):3850–61.
192
Konstantinidis KV Alici E Aints A Christensson B Ljunggren HG Dilber MS . Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp Hematol (2005) 33(2):159–64.10.1016/j.exphem.2004.11.003
193
Liu E Tong Y Dotti G Savoldo B Muftuoglu M Kondo K et al Cord blood derived natural killer cells engineered with a chimeric antigen receptor targeting CD19 and expressing IL-15 have long term persistence and exert potent anti-leukemia activity. Blood (2015) 126:3091.
194
Imamura M Shook D Kamiya T Shimasaki N Chai SM Coustan-Smith E et al Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood (2014) 124(7):1081–8.10.1182/blood-2014-02-556837
195
Sahm C Schonfeld K Wels WS . Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother (2012) 61(9):1451–61.10.1007/s00262-012-1212-x
196
Hay KA Hanafi LA Li D Gust J Liles WC Wurfel MM et al Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T cell therapy. Blood (2017) 130(21):2295–306.10.1182/blood-2017-06-793141
197
Fitzgerald JC Weiss SL Maude SL Barrett DM Lacey SF Melenhorst JJ et al Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med (2017) 45(2):e124–31.10.1097/CCM.0000000000002053
198
Greco R Oliveira G Stanghellini MT Vago L Bondanza A Peccatori J et al Improving the safety of cell therapy with the TK-suicide gene. Front Pharmacol (2015) 6:95.10.3389/fphar.2015.00095
199
Fillat C Carrio M Cascante A Sangro B . Suicide gene therapy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: fifteen years of application. Curr Gene Ther (2003) 3(1):13–26.10.2174/1566523033347426
200
Ciceri F Bonini C Stanghellini MT Bondanza A Traversari C Salomoni M et al Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol (2009) 10(5):489–500.10.1016/S1470-2045(09)70074-9
201
Tiberghien P Ferrand C Lioure B Milpied N Angonin R Deconinck E et al Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood (2001) 97(1):63–72.10.1182/blood.V97.1.63
202
Bonini C Ferrari G Verzeletti S Servida P Zappone E Ruggieri L et al HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science (1997) 276(5319):1719–24.10.1126/science.276.5319.1719
203
Di Stasi A Tey SK Dotti G Fujita Y Kennedy-Nasser A Martinez C et al Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med (2011) 365(18):1673–83.10.1056/NEJMoa1106152
204
Budde LE Berger C Lin Y Wang J Lin X Frayo SE et al Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One (2013) 8(12):e82742.10.1371/journal.pone.0082742
205
Hoyos V Savoldo B Quintarelli C Mahendravada A Zhang M Vera J et al Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia (2010) 24(6):1160–70.10.1038/leu.2010.75
206
Straathof KC Pulè MA Yotnda P Dotti G Vanin EF Brenner MK et al An inducible caspase 9 safety switch for T-cell therapy. Blood (2005) 105(11):4247–54.10.1182/blood-2004-11-4564
207
Wang X Chang WC Wong CW Colcher D Sherman M Ostberg JR et al A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood (2011) 118(5):1255–63.10.1182/blood-2011-02-337360
208
Busch DH . Choice of better T-cells for adoptive immunotherapy. Blood (2015) 126:SCI–22.
209
Chu Y Hochberg J Yahr A Ayello J van de Ven C Barth M et al Targeting CD20+ aggressive B-cell non-Hodgkin lymphoma by anti-CD20 CAR mRNA-Modified expanded natural killer cells in vitro and in NSG mice. Cancer Immunol Res (2015) 3(4):333–44.10.1158/2326-6066.CIR-14-0114
210
Müller T Uherek C Maki G Chow KU Schimpf A Klingemann HG et al Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother (2008) 57(3):411–23.10.1007/s00262-007-0383-3
211
Jiang H Zhang W Shang P Zhang H Fu W Ye F et al Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol (2014) 8(2):297–310.10.1016/j.molonc.2013.12.001
212
Chen KH Wada M Firor AE Pinz KG Jares A Liu H et al Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget (2016) 7(35):56219–32.10.18632/oncotarget.11019
213
Sinha C Seth A Kahali B Cunningham L . Development and evaluation of NK-CD123 CAR against high risk acute myeloid leukemia. Biol Blood Marrow Transplant (2017) 23(3):S253.10.1016/j.bbmt.2016.12.423
214
Kruschinski A Moosmann A Poschke I Norell H Chmielewski M Seliger B et al Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A (2008) 105(45):17481–6.10.1073/pnas.0804788105
215
Liu H Yang B Sun T Lin L Hu Y Deng M et al Specific growth inhibition of ErbB2expressing human breast cancer cells by genetically modified NK92 cells. Oncol Rep (2015) 33(1):95–102.
216
Uherek C Tonn T Uherek B Becker S Schnierle B Klingemann HG et al Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood (2002) 100(4):1265–73.
217
Zhao Q Ahmed M Tassev DV Hasan A Kuo TY Guo HF et al Affinity maturation of T-cell receptor-like antibodies for Wilms tumor 1 peptide greatly enhances therapeutic potential. Leukemia (2015) 29(11):2238–47.10.1038/leu.2015.125
218
Park H Awasthi A Ayello J Chu Y Riddell S Rosenblum J et al ROR1-specific chimeric antigen receptor (CAR) NK cell immunotherapy for high risk neuroblastomas and sarcomas. Biol Blood Marrow Transplant (2017) 23(3 Suppl):S136–7.10.1016/j.bbmt.2017.01.056
219
Tagaya Y Bamford RN DeFilippis AP Waldmann TA . IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity (1996) 4(4):329–36.10.1016/S1074-7613(00)80246-0
Summary
Keywords
natural killer cells, chimeric antigen receptor, chimeric antigen receptor, chimeric antigen receptor T, chimeric antigen receptor natural killer, cancer, hematopoietic stem cell transplant
Citation
Mehta RS and Rezvani K (2018) Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 9:283. doi: 10.3389/fimmu.2018.00283
Received
15 November 2017
Accepted
31 January 2018
Published
15 February 2018
Volume
9 - 2018
Edited by
Ignacio Melero, Centro de Investigación Médica Aplicada (CIMA), Spain
Reviewed by
Daniel Olive, Institut National de la Santé et de la Recherche Médicale (INSERM), France; Manel Juan, Hospital Clínic, Spain; Michael R. Verneris, University of Colorado Denver, United States
Updates
Copyright
© 2018 Mehta and Rezvani.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rohtesh S. Mehta, rmehta1@mdanderson.org; Katayoun Rezvani, krezvani@mdanderson.org
Specialty section: This article was submitted to Cancer Immunity and Immunotherapy, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.