- 1Institute for Life Sciences, Faculty of Medicine, University of Southampton, University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom
- 2Institute of Developmental Science, Human Development and Health, Faculty of Medicine, University of Southampton, Southampton, United Kingdom
The success of prevention of mother to child transmission programs over the last two decades has led to an increasing number of infants who are exposed to human immunodeficiency virus (HIV), but who are not themselves infected (HIV-exposed, uninfected infants). Although the morbidity and mortality among HIV-exposed, uninfected infants is considerably lower than that among HIV-infected infants, they may remain at increased risk of infections in the first 2 years of life compared with their HIV-unexposed peers, especially in the absence of breastfeeding. There is some evidence of immunological differences in HIV-exposed, uninfected infants, which could play a role in susceptibility to infection. Cytomegalovirus (CMV) may contribute to the increased immune activation observed in HIV-exposed, uninfected infants. Infants born to HIV-infected women are at increased risk of congenital CMV infection, as well as early acquisition of postnatal CMV infection. In infants with HIV infection, CMV co-infection in early life is associated with higher morbidity and mortality. This review considers how HIV infection, HIV exposure, and CMV infection affect infant responses to vaccination, and explores possible immunological and other explanations for these findings. HIV-infected infants have lower vaccine-induced antibody concentrations following tetanus, diphtheria, pertussis, hepatitis B, and pneumococcal vaccination, although the clinical relevance of this difference is not known. Despite lower concentrations of maternal-specific antibody at birth, HIV-exposed, uninfected infants respond to vaccination at least as well as their HIV-unexposed uninfected peers. CMV infection leads to an increase in activation and differentiation of the whole T-cell population, but there is limited data on the effects of CMV infection on infant vaccine responses. In light of growing evidence of poor clinical outcomes associated with CMV infection in HIV-exposed, uninfected infants, further studies are particularly important in this group. A clearer understanding of the mechanisms by which maternal viral infections influence the developing infant immune system is critical to the success of maternal and infant vaccination strategies.
Introduction
Immunization is essential to global strategies to reduce infant mortality, especially in low-resource settings where infectious morbidity and mortality remain high (1, 2). In regions of the world where the burden of infectious diseases is high, even a small reduction in vaccine efficacy might have important clinical implications for young infants. Factors affecting infant vaccine responses can be divided into those relating to the infant, mother and environment; an important and potentially modifiable maternal factor is antenatal viral infections. To date, studies of the effects of maternal antenatal viral infections on infant vaccine responses have focused on two important viral infections: human immunodeficiency virus (HIV) and cytomegalovirus (CMV).
In 2015, there were 1.4 million pregnant women living with HIV (3), and an estimated 24% of those did not receive antiretroviral therapy (ART) for prevention of mother-to-child transmission (PMTCT) (4). In infants born with HIV infection, early commencement of combination ART significantly reduces mortality (5, 6) and is associated with increased magnitude and quality of infant antibody responses to vaccines (7–10), but there is some evidence of reduced humoral responses to vaccines even in those children starting ART at 6–8 weeks of age, compared with HIV-unexposed infants (9).
The expansion of PMTCT programs in the last two decades, and continued high numbers of HIV-infected women who become pregnant, have led to an increase in the number of HIV-exposed, uninfected infants, who now represent up to 30% of births in some parts of Southern Africa (11). Compared with HIV-unexposed infants, HIV-exposed, uninfected infants are at increased risk of hospitalization, pneumonia and mortality at 6 and 24 months in some settings, although the risk is reduced when they are breastfed and when infected children and their mothers receive ART (12). Increasing evidence shows immunological differences in HIV-exposed, uninfected infants, who have changes in T-cell populations, lower CD4 counts and increased T-cell differentiation by 10 weeks of age compared with HIV-unexposed infants (13). While these immunological differences may play a part in the increased morbidity which has been observed, factors such as socioeconomic status, maternal health, breastfeeding duration, exposure to ART, and exposure to co-infections are also likely to be important.
Cytomegalovirus is the most common congenital infection worldwide, affecting up to 1.2% of live births in developing countries. Although CMV infection is thought to lead to immune senescence and a poor response to the influenza vaccine in the elderly (14), it is not fully understood how CMV might affect infant responses to vaccines. Congenital CMV infection leads to changes in the infant T-cell population as a whole and is associated with increased morbidity in HIV-infected and HIV-exposed, uninfected infants. In HIV-infected infants who are not receiving ART, those with congenital CMV infection have an increased rate of HIV disease progression (15, 16). HIV-exposed infants may be at higher risk of congenital CMV than HIV-unexposed infants (17, 18). In HIV-exposed, uninfected infants, CMV infection may contribute to immune activation (11, 19, 20) and increase the risk of postnatal HIV infection (21, 22).
This review summarizes the evidence for alterations in infant vaccine responses associated with exposure to maternal HIV and CMV infection, and explores possible immunological and other explanations for these findings.
Methods
A literature search of English language publications was performed using Medline. Key search terms included: maternal, fetal, neonate, HIV, CMV, vaccination, and immunization. The full search strategy is detailed in Table S1 in Supplementary Material. A formal systematic review was not undertaken; how-ever, we sought to carry out a comprehensive review of the published literature. We screened titles and abstracts, selecting all articles with outcomes that included infant immune responses to routine Expanded Program on Immunizations vaccinations following antenatal exposure to either maternal HIV or CMV infection, or both.
Studies of HIV-infected infants in which the majority of participants received ART were selected when possible, for three main reasons: (1) ART is associated with increased magnitude and quality of infant antibody responses to vaccines (7–10); (2) studies in which infants do not receive ART are prone to survival bias because there is often a high mortality rate in the HIV-infected group (7, 9, 23); and (3) this review is intended to be relevant to current and future practice, looking toward universal ART in HIV-infected children. Since 2015, the World Health Organization guidelines have recommended that all children infected with HIV receive ART. The proportion of children with HIV receiving ART worldwide was 49% in 2015, and ART coverage in children and adults with HIV has been increasing year on year (24). Studies of vaccine responses in HIV-infected infants were excluded if they did not include a HIV-unexposed control group. Papers including a comparison between vaccine responses in HIV-exposed, uninfected infants with HIV-unexposed, uninfected infants were included. This review aimed to review the contemporary literature; there were no studies comparing vaccine responses of HIV-infected infants treated with ART and HIV-exposed, uninfected infants, therefore this comparison is not made in this review.
Vaccine Responses in HIV-Infected Infants
Humoral Responses
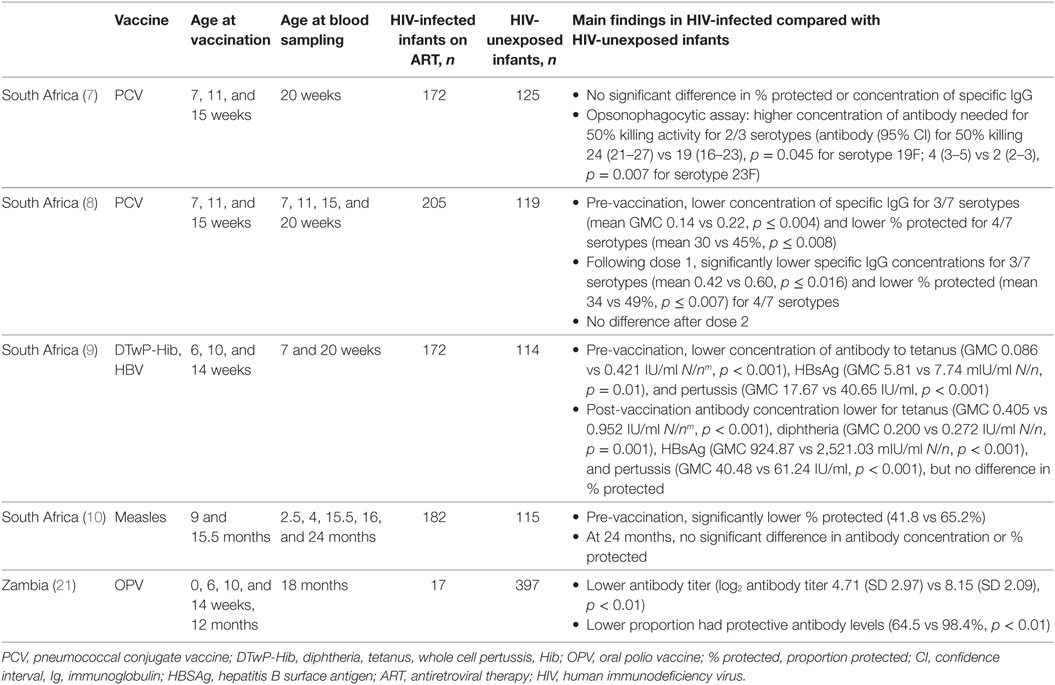
The published literature shows some heterogeneity in the differences in concentrations of specific immunoglobulin (Ig) G and proportion of infants protected following primary and booster doses of routine vaccines in HIV-infected infants receiving ART compared with HIV-unexposed infants (Table 1).
Following primary immunization, HIV-infected infants aged 20 weeks had significantly lower specific antibody concentrations to tetanus, diphtheria, pertussis and hepatitis B surface antigen (HBSAg), than HIV-unexposed infants in a South African study (9). However, the clinical significance of this is unclear, as high proportions (92–100%) of infants from both groups had concentrations of antibody to tetanus, diphtheria, and HBsAg that are deemed protective (9).
Pneumococcal conjugate vaccine (PCV) antibody concentrations were lower in HIV-infected infants after the first and second PCV doses (8), but after the third dose the antibody concentration and proportion of infants protected were similar in HIV-infected and HIV-unexposed infants (7). However, an op-sonophagocytic assay showed that antibody against two out of three PCV serotypes tested in HIV-infected infants receiving ART had 26–50% lower killing activity than that of HIV-unexposed infants. This suggests that HIV-infected infants may not mount as effective an antibody response against pneumococcal disease as HIV-uninfected infants, despite producing a similar concentration of antibody following vaccination (7). A UK study of older children (mean age 12.8 years, range 1–17.4 years) showed that a lower proportion of HIV-infected individuals were protected against three of 13 PCV serotypes, compared with HIV-unexposed children and adults. This was despite an equal or larger proportion of HIV-infected children having previously received the 7-valent or polysaccharide pneumococcal vaccines (25).
In a Zambian cohort in which mothers received short-course intrapartum nevirapine to prevent mother-to-child transmission during labor and delivery, but infants did not receive ART, HIV-infected infants who received oral polio vaccine (OPV) had 42% lower neutralizing antibody responses at 18 months (p < 0.01), and a lower proportion had protective antibody levels than HIV-unexposed infants (64.5 vs 98.4%, p < 0.01) (26).
Following measles vaccination at age 9 months, there was no significant difference in measles-specific IgG levels at 24 months in HIV-infected infants compared with HIV-unexposed infants in a study from South Africa, and no difference in the proportion of infants with protective antibody levels (10).
Human immunodeficiency virus-infected newborns are potentially more susceptible to vaccine-preventable diseases for a longer period than HIV-unexposed infants (8, 10). As well as having lower responses to the first two doses of vaccine (as seen with PCV and measles), compared with HIV-unexposed infants, HIV-infected infants had lower pre-vaccination concentrations of antibody to three of seven PCV serotypes (8), tetanus (GMC 0.086 vs 0.421, p < 0.001), HBsAg (GMC 5.81 vs 7.74, p = 0.01), and pertussis (GMC 17.67 vs 40.65, p < 0.001) (9, 10). Similarly, before vaccination a lower proportion of HIV-infected than HIV-unexposed infants had protective levels of antibody to PCV (for four serotypes, mean 30 vs 45% protected, p ≤ 0.008) and measles (9, 10).
Cellular Responses
In a study in South Africa, in which mothers received PMTCT and infected infants (diagnosed at 6 weeks) were not breastfed and did not receive ART, T-cell responses to BCG in HIV-infected infants were compared with those in HIV-unexposed infants (27). After BCG vaccine on day 1 of life, HIV-infected infants had severely impaired T-cell responses at 3 months, and by 9–12 months the response was almost absent (27). Both the magnitude of the CD4 and CD8 T-cell responses, and the polyfunctionality of the CD4 response were markedly reduced (27). Secreted cytokines interferon (IFN)-γ and interleukin-2 were also present in significantly lower concentrations in HIV-infected than HIV-unexposed infants at 3 months, although tumor necrosis factor (TNF)-α concentration was not significantly different (27).
Mechanisms of Altered Vaccine Responses in HIV-Infected Infants
Both maternal and infant factors are likely to be involved in the observed differences in antibody and cellular responses to vaccines seen in HIV-infected compared with HIV-unexposed infants (8). In HIV-infected infants, lower CD4 count may impair the mechanisms leading to induction and maintenance of immunological memory to vaccine antigens (26). The observation that specific antibody concentrations are lower in HIV-infected infants before vaccination may suggest reduced transfer of antibody across the placenta (8, 10). Both reduced antibody concentration and impaired placental function in HIV-infected mothers may contribute to this (28, 29).
There are inherent difficulties in comparing vaccine responses in HIV-infected with HIV-unexposed infant populations, as the two groups are likely to differ in duration of breastfeeding, exposure to ART, socioeconomic status, exposure to co-infections, nutritional status, and survival. In a multivariable analysis of factors associated with response to OPV (primary course and booster at age 12 months) at 18 months of age, increasing breast-feeding duration was associated with increasing poliovirus antibody level (26). In this study, median breastfeeding duration was 6 months in HIV-infected mother-infant pairs compared with 15 months in HIV-uninfected pairs (p < 0.01). Differences in breastfeeding duration in HIV-infected and unexposed groups were not stated in the other studies described earlier, although in four of the studies, infants were co-enrolled in the CHER trial in South Africa, a randomized controlled trial evaluating antiretroviral treatment strategies, in which only 14% of infants were breastfed (30). Short duration or refraining from breastfeeding in low and middle-income settings, including for HIV-infected infants, is associated with increased infectious morbidity, stunting, and wasting (31–33).
A recent review of the effects of maternal nutritional status on infant vaccine responses concluded that maternal macro- and micronutrient deficiency during pregnancy is likely to impair infant responses to vaccines, even in the presence of nutrient supplementation (34).
Increased exposure to opportunistic infections through breastfeeding (for example, CMV infection) or close contact with HIV-infected mothers who may have co-infection may affect immune responses in HIV-infected infants. In HIV and CMV co-infection, infants have accelerated HIV progression, increased mortality, growth delay and cognitive impairment, compared with HIV-infected infants without CMV (35). In Malawi, breastmilk CMV load had a stronger negative association with infant growth than breastmilk HIV load (36).
In summary, HIV-infected infants receiving ART may have impaired ability to mount quantitatively and qualitatively ade-quate antibody responses to vaccines compared with HIV-unexposed infants. The clinical effect of impaired vaccine responses on morbidity from vaccine-preventable disease is not known. Shorter breastfeeding duration, poorer nutritional status and increased exposure to co-infections in HIV-infected infants may be contributing factors, and their impact requires further investigation to fully understand the mechanisms underlying the changes in vaccine responses in HIV-infected infants. CMV infection is almost ubiquitous in low-resource settings, and its clinical effects on infants with HIV suggest it is having an important effect on the immune system, and could be an important modifiable factor in reducing morbidity and mortality of HIV-infected infants.
Vaccine Responses in HIV-Exposed, Uninfected Infants
Humoral Responses
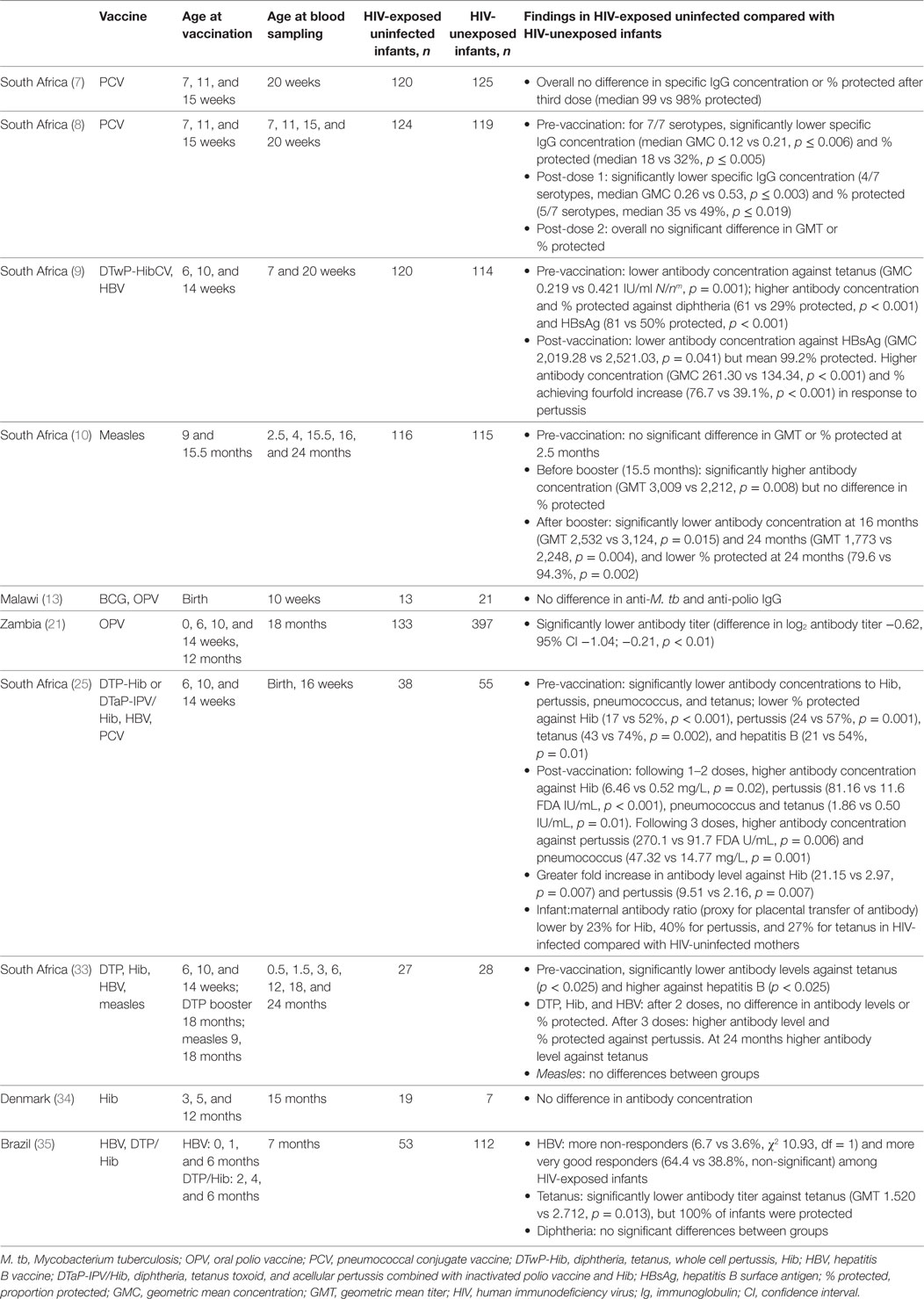
Detailed studies in HIV-exposed, uninfected infants have demonstrated differing patterns of antibody responses to vaccines compared with HIV-unexposed infants (Table 2), and revealed likely underlying mechanisms.
After three doses of pertussis-containing vaccine, antibody levels in HIV-exposed, uninfected infants were two to seven times higher than in unexposed infants (9, 29, 37). However, following the booster dose at 18 months, the proportion of children with protective antibody levels was non-significantly higher in HIV-exposed, uninfected infants than in HIV-unexposed children (37).
Following the first one to two doses of Hib vaccine, concentration of specific antibody was 12 times higher at 16 weeks in HIV-exposed, uninfected than unexposed infants (29), but there was no significant difference when infants had received all three doses (9, 29, 37, 38). Similarly, tetanus antibody concentration was higher after one to two doses, but not significantly different at 4, 5, or 6 months following three doses (9, 29, 37). Six months after the fourth (booster) dose, at 24 months, the antibody concentration was significantly higher in the HIV-exposed, uninfected children than in the HIV-unexposed children (p < 0.05) (37). One study found that at 7 months tetanus antibody concentration was significantly lower in HIV-exposed, uninfected infants than unexposed infants, but neither study found any significant difference in the proportion of infants protected (37, 39).
In studies in South Africa, HIV-exposed, uninfected infants who received all three doses of PCV had significantly higher antibody concentrations at 16 and 20 weeks (7, 29), although opsonophagocytic activity was reduced compared with unexposed infants for 1 out of 3 serotypes (7).
The increased antibody responses to the initial doses of Hib, tetanus, and other vaccines can be explained by reduced interference from maternally derived antibody in HIV-exposed, uninfected infants. Before vaccination, HIV-exposed, uninfected infants consistently had lower antibody concentrations against PCV (seven serotypes), pertussis, Hib, and tetanus in a number of studies (8, 9, 29, 37). For each of these specific antibodies, there was significantly reduced placental transfer, with reductions of 15–40% in the ratio of maternal antibody to infant antibody concentrations at birth (29). Individual infants with lower antibody levels at birth had larger antibody responses at 16 weeks, and HIV-exposed, uninfected infants had a significantly larger fold increase than unexposed infants following vaccination against PCV, pertussis, and Hib (29).
Two South African studies have compared measles vaccine responses in HIV-exposed, uninfected, and unexposed infants. One study found antibody concentrations were 36% higher at 16 months, but after the booster dose, both the antibody titer and proportion of infants protected were lower at 24 months (79.6 vs 94.3% protected, p = 0.002) (10). The other study found the opposite; however, in this study antibody responses were lower in all groups, especially HIV-unexposed infants, for whom only 50% had antibody concentrations associated with protection at 2 years (37). Pre-vaccination levels of measles antibody did not differ significantly in HIV-exposed and unexposed infants in either study (10, 37).
Antibody responses to hepatitis B vaccine in HIV-exposed, uninfected infants were heterogeneous, with higher proportions of both non-responders (6.7 vs 3.6%, χ2 10.93, df = 1) and very good responders (64.4 vs 38.4%, non-significant) at 7 months, compared with HIV-unexposed infants in Brazil (39). At time points between 3 and 24 months, no significant differences were found in the overall proportion of infants with protective antibody levels in HIV-exposed, uninfected, and unexposed groups in Brazil and South Africa (9, 29, 37, 39). Before vaccination, the proportion of infants with protective antibody levels was higher in HIV-exposed than unexposed infants in two studies from South Africa and lower in one study in the same country (9, 29, 37).
In two studies of responses to diphtheria vaccine, antibody responses in HIV-exposed, uninfected, and HIV-unexposed infants did not differ, with more than 98% protected following the primary course (9, 39). Pre-vaccination anti-diphtheria toxin antibody levels were significantly higher in HIV-exposed, uninfected infants (GMC 0.136 vs 0.078, p < 0.001) (9). There were no differences found in IgG concentrations against OPV at 10 weeks (13). The response to OPV at 18 months was lower in HIV-exposed uninfected infants than unexposed infants, but this was no longer significant after adjusting for breastfeeding duration (26).
In summary, typically vaccines for which the antibody concentration is lower before vaccination result in higher concentrations after vaccination. This is true for only the first one to two doses of Hib and tetanus vaccines, but persists to the end of the course of PCV and pertussis. For all four vaccines there is reduced maternal trans-placental transfer of antibody (29). There are less clear trends for measles and hepatitis B vaccines, which may be more dependent on population transmission and prevalence. HIV exposure did not appear to have any effect on antibody responses to diphtheria or OPV. Overall, HIV-exposed, uninfected infants respond at least as well to vaccines as their unexposed peers.
Cellular Responses
Human immunodeficiency virus-exposed, uninfected infants produce strong T-cell responses to BCG vaccine. In South Africa, BCG-specific CD4 and CD8 T-cell proliferation increased significantly after vaccination in HIV-exposed, uninfected, and unexposed infants at 14 weeks (40). In another study, all 94 HIV-exposed, uninfected infants formed a scar (41). T-cell proliferation and cytokine secretion were not affected by maternal HIV infection or Mycobacterium tuberculosis (M. tb) sensitization at time points between 6 weeks and 12 months (13, 27, 40, 42, 43).
Differences in the frequencies of specific T-cell subpopulations have been found between HIV-exposed, uninfected, and unexposed infants before and after BCG vaccination (40, 42). In HIV-exposed and uninfected infants, the CD4 and CD8 T-cell response at 14 weeks was less polyfunctional, indicating a less effective response (42). However, this may simply reflect immaturity, as infants were vaccinated within 3 days after birth, and another study in which the infants were vaccinated at 6 weeks found very little difference in T-cell subpopulations at 16 weeks, compared with HIV-unexposed infants (40).
At birth, no differences in BCG-specific T-cell proliferation or functionality are seen between HIV-exposed, uninfected, and unexposed infants (40). However, there are differences in the frequencies of some T-cell subsets, some of which correlate between mother-infant pairs, with the strongest associations between HIV-infected, M. tb sensitized mothers, and their infants (40). Secretion of TNF-α and IFN-γ in response to BCG antigens was increased at birth in HIV-exposed uninfected infants compared with HIV-unexposed infants, but only when their mothers had evidence of latent tuberculosis infection (40). These findings support the idea that HIV-exposed uninfected infants are able to mount just as robust a response to BCG vaccine as unexposed infants, but that the immune system may be primed by antenatal exposure to maternal HIV and tuberculosis infection (40).
Two studies have investigated the cellular response to other vaccine antigens in HIV-exposed, uninfected infants compared with unexposed infants. In response to pertussis vaccine, one study found no significant differences in T-cell proliferation at 14 weeks in HIV-exposed, uninfected, and unexposed infants, but HIV-exposed infants showed reduced polyfunctionality in CD4 and CD8 responses (42). Similarly, tetanus vaccine-specific T-cell responses showed no differences at 3 months, but at 12 months HIV-exposed uninfected infants had reduced polyfunctionality, and a lower proportion of effector memory T-cells compared with HIV-uninfected infants (43).
A similar pattern was seen in response to stimulation with staphylococcal enterotoxin B (SEB) in one study, even after adjusting for differences in birthweight, breastfeeding, and gestational age (42). However, another study found that cytokine production and polyfunctionality were increased overall at 3 months but reduced at 12 months (43).
Mechanisms of Altered Vaccine Responses in HIV-Exposed, Uninfected Infants
Human immunodeficiency virus-exposed, uninfected infants are exposed to antenatal factors that might affect both their antibody and T-cell responses to vaccines. There is compelling evidence that in mothers with HIV infection, less IgG is transferred across the placenta than in HIV-uninfected mothers, resulting in lower pre-vaccination levels of IgG specific to several vaccines (8–10, 29, 37). Results from analysis adjusting for maternal age, gravidity, and socioeconomic status show that maternal HIV infection is associated with the concentration of specific IgG following Hib, pertussis, PCV, and tetanus vaccines in exposed, uninfected infants (26, 29). In this study, mothers received ART during and after pregnancy, infants received zidovudine for the first month after birth, and no HIV-exposed, uninfected infants were exclusively breastfed. This finding is likely to be a result of lower vaccine-specific antibody levels in HIV-infected mothers, which correlate with CD4 count (29), and placental dysfunction resulting in reduced placental transfer of antibody (8, 9, 29, 37). Maternally derived antibody present in infants pre-vaccination may inhibit the infants’ own IgG responses, leading to the observation that infants with the highest pre-vaccine levels of anti-body had the lowest fold increase following vaccination (29). Although the mechanisms for this are incompletely understood in humans, animal models have shown that this inhibition is mediated by maternally derived antibody binding to vaccine antigens, which then form cross-linkage between the B cell receptor (which binds vaccine antigen) and the FcγIIB receptor (which recognizes the Fc portion of IgG). This results in inhibitory signals, reduced proliferation of B cells and decreased secretion of vaccine-specific IgG (44, 45).
Infants of mothers with HIV infection may be exposed antenatally to HIV proteins and/or maternal immune factors that have a wider effect on the development of the immune system in utero and early infancy. HIV-exposed, uninfected infants may have a smaller thymus, which has been associated with immune abnormalities in early infancy (38). There is some evidence that T-cells in HIV-exposed, uninfected infants show changes in proliferation and phenotype compared with HIV-unexposed infants (13). CD4 count may be significantly lower and represent a smaller proportion of total lymphocytes, and some studies have found an association between infant and maternal CD4 count (13, 46). The reduction in T-lymphocytes occurs mainly in less differentiated subsets, and cells expressing markers of replicative senescence (CD57 and PD-1) are more frequent (13). At birth and 6 weeks, the background concentration of IFNγ was reported to be significantly higher in HIV-exposed, uninfected infants than unexposed infants in one study in South Africa (41). These early changes could represent priming of some aspects of the immune response in utero, leading the alterations in proliferation and function of T-cell subsets in response to vaccinations in HIV-exposed infants (13, 39–41).
Another antenatal factor that may affect HIV-exposed, uninfected infants is exposure to ART. Nevirapine has been associated with slightly increased markers of immune activation in cord blood (47), and maternal ART was associated with reduced neutrophil and lymphocyte counts in HIV-exposed, uninfected infants, with the largest difference in infants of mothers on combination therapy (46). However, an association between maternal ART and infant vaccine-specific T-cell responses has not so far been demonstrated (42, 43).
Increasing maternal age is associated with higher infant levels of pertussis antibody at birth (29). This might be influenced by differing maternal exposure to circulating pertussis or to different vaccine coverage with pertussis vaccines at different times. Other maternal infections during pregnancy are likely to be important in determining infant antibody concentrations pre-vaccination and therefore potentially post-vaccination too, for example, high variability in infant hepatitis B antibody response is likely to be a result of higher prevalence of hepatitis B infection in HIV-infected mothers in some settings (37).
Postnatally both maternal and environmental factors probably have important effects on infant vaccine responses. Breastfeeding is an important conduit for transfer of IgA from mother to infant and is associated with larger thymic size, phenotypic changes to lymphocyte subpopulations and improved immune function (48). In studies of HIV-exposed, uninfected, and unexposed infants, there are often large differences in breastfeeding practices between groups (26, 29, 37), and many studies do not report data on breastfeeding (7–10, 13, 38–40, 43). One study reported that the reduction in neutralizing antibody response to OPV in HIV-exposed, uninfected infants could be accounted for by reduced breastfeeding duration (26). This could be because of reduced antibody transfer, or increased exposure to maternal infections such as CMV which are transmitted in breast milk (26).
Postnatal exposure to other infections may also affect specific antibody responses to vaccines. The large differences between studies in the proportion of infants protected against measles following vaccination raises the possibility that differences in transmission rates of measles infection may have affected the proportions of infants protected (49). Differences in nasopharyngeal colonization with pneumococcus among HIV-exposed, uninfected infants, and unexposed infants has also been suggested to contribute to differences in their vaccine responses (7). In low-resource settings HIV-exposed, uninfected infants have poorer nutritional status than unexposed infants (50), although a trial of nutritional supplementation between age 6 and 18 months had no effect on antibody responses to OPV in HIV-exposed, uninfected infants (26).
We conclude that there is convincing evidence that reduced antibody transfer across the placenta is associated with changes in antibody responses to the initial doses of PCV, tetanus, pertussis, and Hib vaccines in HIV-exposed, uninfected infants. The effect of HIV exposure on responses to hepatitis B and measles vaccines appears more variable between populations, and prevalence of these infections may be an important factor. The functional quality of vaccine-specific antibody produced by HIV-exposed, uninfected infants requires further investigation (29, 37), but overall it is encouraging that HIV-exposed, uninfected infants do not appear to have significantly reduced levels of protection from routine infant vaccines. Breastfeeding is likely to affect responses to other vaccines besides OPV, and further studies are now more feasible following changes in WHO recommendations to support breastfeeding in HIV-infected mothers in a wider range of settings (51).
Vaccine Responses in Infants with Congenital and Postnatal CMV Infection
Effect of CMV Infection on T-Cell Populations
Congenital and postnatal CMV infection leads to a series of changes in infant CMV-specific CD4 and CD8 T-cells, as well as having an effect on the whole T-cell population. There is an initial increase in activation of the whole CD8 T-cell population, which returns to normal over 12–24 months (52–55). CMV-specific CD8 T-cells remain highly activated for at least 24 months following postnatal infection, but in congenital infection, activation may diminish more rapidly (53, 54). There is a shift toward more differentiated CD4 T-cells, but CMV-specific CD4 T-cells are infrequently found in infected infants (55, 56). In adults with CMV, these cells are common and are associated with effective control of viral replication, less severe disease, and lower risk of mother-to-child transmission (57, 58).Infant T-cells are less polyfunctional than those seen in adults, and polyfunctionality is also thought to be associated with improved control of CMV infection (55, 58–60). Therefore, congenital and postnatal CMV infection affects the whole T-cell population, and the effect is different in infants compared with adults. Infants have a longer duration of viremia than adults (55, 61), and their vaccine responses may be affected differently by CMV infection.
Effect of CMV Infection on Humoral and Cellular Vaccine Responses
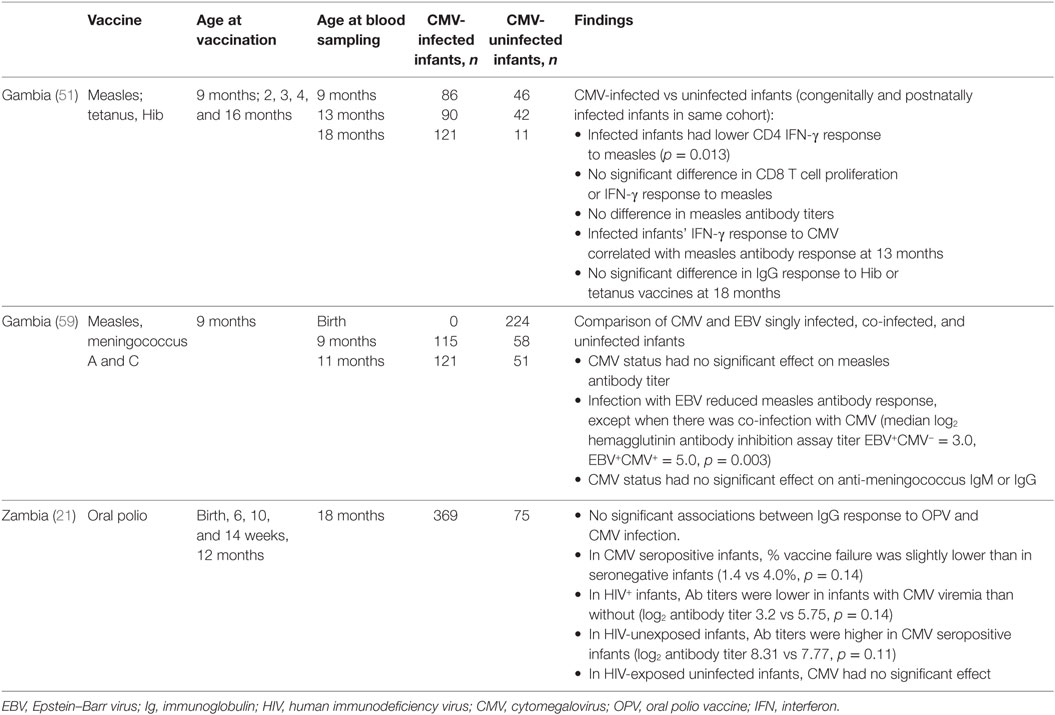
Studies in elderly adults have shown that latent CMV infection leads to the expansion of CD8+CD28− T-cells, which are thought to suppress immune responses to influenza vaccine and contribute to generalized immunosenescence in older adults (14). Few studies have evaluated the effects of CMV infection on infant vaccine responses (Table 3).
In a study of measles vaccine in Gambian infants, 1 week after vaccination infant CD8 T-cell responses did not vary with CMV infection acquired congenitally or postnatally, but CD4 T-cell IFN-γ responses were lower in CMV-infected infants than in infants without CMV infection (54). At 13 months of age, there were no differences in memory T-cell responses between CMV-infected and uninfected infants. However, CMV-infected infants showed significantly higher CD4 and CD8 T-cell IFN-γ responses to SEB, indicating that immune activation is present in CMV-infected infants. Furthermore, there was a positive correlation between the magnitudes of the responses to SEB and CMV (54).
A study of CMV and EBV co-infection supports the idea that the effect of CMV infection on antibody responses to measles is dependent on changes to the T-cell population. Epstein–Barr virus (EBV) infects B-cells, and EBV-infected infant IgG responses to measles vaccine and meningococcus A and C polysaccharide vaccine are reduced by approximately one third (62). In infants co-infected with CMV and EBV, the measles-specific IgG vaccine response is similar to uninfected infants. However, CMV co-infection does not have a significant effect on the IgG response to meningococcus (a T-cell independent response) in EBV-infected infants, and the vaccine response is still lower than in EBV uninfected infants (62).
Gambian infants who acquired CMV antenatal or postnatally had no significant differences in anti-Hib or anti-tetanus toxoid IgG concentration measured compared with uninfected infants at 18 months (54). A study of antibody response to OPV in Zambian infants found that neither CMV seropositivity nor viremia had a significant effect on OPV neutralizing antibody titers or frequency of vaccine failure at 18 months of age (26). However, trends in the data suggested that co-infection with HIV and CMV may have negative synergistic effects on the antibody response to OPV. CMV seropositivity at 18 months was associated with a trend toward a small decrease in vaccine failures in HIV-unexposed infants (0.4 vs 4.3% vaccine failure, p = 0.06), but not in HIV-exposed, uninfected infants. In HIV-infected infants antibody responses were reduced in CMV seropositive compared with CMV seronegative infants, although these results did not reach statistical significance (26).
Human immunodeficiency virus-infected mothers in this study had a mean breastfeeding duration of 6 months compared with 15 months in HIV-uninfected mothers, and longer breastfeeding duration was associated with increased mean poliovirus antibody titers in infants (26). The authors do not state whether mean breastfeeding duration differed between CMV seropositive and seronegative groups, but this is important because breastfeeding is the main route of transmission of postnatal CMV infection, and in HIV-exposed, uninfected infants in this study, the reduction in neutralizing antibody response to OPV could be accounted for by reduced breastfeeding duration (26).
Cytomegalovirus-infected infants show alterations in responses to measles and possibly polio vaccines, which are live vaccines, and no significant differences have been found in the responses to Hib or tetanus vaccines. Overall the ability to mount effective and lasting responses is preserved in otherwise well infants, at least in the short term, and responses to SEB indicate that some T-cell responses are increased in CMV infection. Interactions may occur between CMV and HIV or EBV to produce further alterations in vaccine responses, but there is still no significant impairment compared with CMV-uninfected infants. There is limited data on the effects of CMV infection on infant vaccine responses, and in light of growing evidence of poor clinical outcomes associated with CMV infection in HIV-exposed, uninfected infants, further studies are particularly important in this group.
Conclusion
Human immunodeficiency virus-infected infants have some impairment in their humoral and cellular responses to routine immunizations. However, as many of the infants in the studies reviewed were born to mothers who started ART a short time before delivery as part of PMTCT programs, and were not exclusively breastfed, future studies will be needed to determine whether the same changes in immune responses are present when mothers and infants undertake optimal HIV treatment, PMTCT, and feeding practices. The clinical importance of these findings is unknown, as the risk of vaccine-preventable infection in HIV-infected infants compared with HIV-unexposed infants has not been determined.
Human immunodeficiency virus-exposed, uninfected infants and those with CMV have alterations in their vaccine responses, but the evidence does not support changes to the vaccine schedule in these groups. Protecting infants from infection before their first vaccines, for example, by maternal immunization, is important in all infants, even more so in HIV-exposed, uninfected, and HIV-infected infants, who are less likely to be protected than HIV-unexposed infants. Maternal immunization is a key part of global efforts to reduce neonatal and infant infectious morbidity and mortality. A better understanding of the mechanisms by which maternal infection and immune responses influence the developing infant immune system are critical to ensuring the success of new vaccines.
Author Contributions
The theme and concept were designed by M-LN and CJ. OF undertook the literature review and wrote the original draft of the paper, which was reviewed and revised by CJ and M-LN.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
OF is funded by the National Institute for Health Research. CJ has received funding from the Immunizing PRegnant women and INfants neTwork (IMPRINT), funded by the GCRF Networks in Vaccines Research and Development, which was co-funded by the MRC and BBSRC, the National Vaccine Program Office (NVPO), and Bill & Melinda Gates Foundation, Grant OPP1119788, Global Alignment of Immunization Safety Assessment in pregnancy (GAIA). CJ is an investigator for clinical trials performed on behalf of the University of Southampton and University Hospital Southampton NHS Trust, UK, sponsored by vaccine manufacturers, including Novavax, GSK, and Janssen. She has received no personal funding for these activities.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fimmu.2018.00328/full#supplementary-material.
References
1. Lee J-W. Child survival: a global health challenge. Lancet (2003) 362:262. doi:10.1016/S0140-6736(03)14006-8
2. Victora CG, Requejo JH, Barros AJD, Berman P, Bhutta Z, Boerma T, et al. Countdown to 2015: a decade of tracking progress for maternal, newborn, and child survival. Lancet (2016) 387:2049–59. doi:10.1016/S0140-6736(15)00519-X
3. UNAIDS. AIDSInfo Data Sheet. (2017). Available from: aidsinfo.unaids.org.
4. UNAIDS. Fact Sheet, People Living With HIV, HIV, Antiretroviral Therapy, New HIV Infections, AIDS, Tuberculosis, Facts. (2016). p. 1–8. Available from: www.unaids.org/en/resources/fact-sheet
5. Abrams EJ, Woldesenbet S, Soares Silva J, Coovadia A, Black V, Technau K-G, et al. Despite access to antiretrovirals for prevention and treatment, high rates of mortality persist among HIV-infected infants and young children. Pediatr Infect Dis J (2017) 36:595–601. doi:10.1097/INF.0000000000001507
6. Wagner A, Slyker J, Langat A, Inwani I, Adhiambo J, Benki-Nugent S, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr (2015) 15:10. doi:10.1186/s12887-015-0325-8
7. Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis (2010) 202:355–61. doi:10.1086/653704
8. Madhi SA, Izu A, Violari A, Cotton MF, Panchia R, Dobbels E, et al. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine (2013) 31:777–83. doi:10.1016/j.vaccine.2012.11.076
9. Simani OE, Izu A, Violari A, Cotton MF, van Niekerk N, Adrian PV, et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS (2014) 28:531–41. doi:10.1097/QAD.0000000000000127
10. Simani OE, Adrian PV, Violari A, Kuwanda L, Otwombe K, Nunes MC, et al. Effect of in-utero HIV exposure and antiretroviral treatment strategies on measles susceptibility and immunogenicity of measles vaccine. AIDS (2013) 27:1583–91. doi:10.1097/QAD.0b013e32835fae26
11. Filteau S, Rowland-Jones S. Cytomegalovirus infection may contribute to the reduced immune function, growth, development, and health of HIV-exposed uninfected African children. Front Immunol (2016) 7:257. doi:10.3389/fimmu.2016.00257
12. Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis (2007) 196:562–9. doi:10.1086/519847
13. Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology (2010) 129:446–54. doi:10.1111/j.1365-2567.2009.03186.x
14. Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol (2004) 25:406–10. doi:10.1016/j.it.2004.05.006
15. Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric pulmonary and cardiovascular complications of vertically transmitted hiv infection study group. N Engl J Med (1999) 341:77–84. doi:10.1056/NEJM199907083410203
16. Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J (1996) 15:1102–6. doi:10.1097/00006454-199612000-00010
17. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol (2007) 17:355–63. doi:10.1002/rmv.544
18. Mwaanza N, Chilukutu L, Tembo J, Kabwe M, Musonda K, Kapasa M, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-Saharan Africa. Clin Infect Dis (2014) 58:728–35. doi:10.1093/cid/cit766
19. Evans C, Chasekwa B, Rukobo S, Govha M, Mutasa K, Ntozini R, et al. Cytomegalovirus acquisition and inflammation in human immunodeficiency virus-exposed uninfected Zimbabwean infants. J Infect Dis (2017) 215:698–702. doi:10.1093/infdis/jiw630
20. Garcia-Knight MA, Nduati E, Hassan AS, Nkumama I, Etyang TJ, Hajj NJ, et al. Cytomegalovirus viraemia is associated with poor growth and T-cell activation with an increased burden in HIV-exposed uninfected infants. AIDS (2017) 31:1809–18. doi:10.1097/QAD.0000000000001568
21. Slyker JA, Lohman-Payne BL, Rowland-Jones SL, Otieno P, Maleche-Obimbo E, Richardson B, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS (2009) 23:117–24. doi:10.1097/QAD.0b013e32831c8abd
22. Chang TS, Wiener J, Dollard SC, Amin MM, Ellington S, Chasela C, et al. Effect of cytomegalovirus infection on breastfeeding transmission of HIV and on the health of infants born to HIV-infected mothers. AIDS (2015) 29:831–6. doi:10.1097/QAD.0000000000000617
23. Moss WJ, Scott S, Mugala N, Ndhlovu Z, Beeler JA, Audet SA, et al. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis (2007) 196:347–55. doi:10.1086/519169
24. UNAIDS. AIDS Data 2016. (2017). p. 1–84. Available from: www.unaids.org/sites/default/files/media_asset/2016-AIDS-data_en.pdf
25. Bamford A, Kelleher P, Lyall H, Haston M, Zancolli M, Goldblatt D, et al. Serological response to 13-valent pneumococcal conjugate vaccine in children and adolescents with perinatally acquired HIV infection. AIDS (2014) 28:2033–43. doi:10.1097/QAD.0000000000000385
26. Sanz-Ramos M, Manno D, Kapambwe M, Ndumba I, Musonda KG, Bates M, et al. Reduced poliovirus vaccine neutralising-antibody titres in infants with maternal HIV-exposure. Vaccine (2013) 31:2042–9. doi:10.1016/j.vaccine.2013.02.044
27. Mansoor N, Scriba TJ, de Kock M, Tameris M, Abel B, Keyser A, et al. HIV-1 infection in infants severely impairs the immune response induced by bacille Calmette-Guerin vaccine. J Infect Dis (2009) 199:982–90. doi:10.1086/597304
28. Vermaak A, Theron GB, Schubert PT, Kidd M, Rabie U, Adjiba BM, et al. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int J Gynaecol Obstet (2012) 119:239–43. doi:10.1016/j.ijgo.2012.06.016
29. Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC.Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA (2011) 305:576–84. doi:10.1001/jama.2011.100
30. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. CHER study team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med (2008) 359:2233–44. doi:10.1056/NEJMoa0800971
31. Taha T, Nour S, Li Q, Kumwenda N, Kafulafula G, Nkhoma C, et al. The effect of human immunodeficiency virus and breastfeeding on the nutritional status of African children. Pediatr Infect Dis J (2010) 29:514–8. doi:10.1097/INF.0b013e3181cda531
32. Creek TL, Kim A, Lu L, Bowen A, Masunge J, Arvelo W, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr (2010) 53:14–9. doi:10.1097/QAI.0b013e3181bdf676
33. Obimbo EM, Mbori-Ngacha DA, Ochieng JO, Richardson BA, Otieno PA, Bosire R, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected African children. Pediatr Infect Dis J (2004) 23:536–43. doi:10.1097/01.inf.0000129692.42964.30
34. Obanewa O, Newell M-L. Maternal nutritional status during pregnancy and infant immune response to routine childhood vaccinations. Future Virol (2017) 12:525–36. doi:10.2217/fvl-2017-0021
35. Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antire-troviral therapy era. Front Microbiol (2015) 6:1016. doi:10.3389/fmicb.2015.01016
36. Meyer SA, Westreich DJ, Patel E, Ehlinger EP, Kalilani L, Lovingood RV, et al. Postnatal cytomegalovirus exposure in infants of antiretroviral-treated and untreated HIV-infected mothers. Infect Dis Obstet Gynecol (2014) 2014:989721–8. doi:10.1155/2014/989721
37. Reikie BA, Naidoo S, Ruck CE, Slogrove AL, de Beer C, la Grange H, et al. Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin Vaccine Immunol (2013) 20:33–8. doi:10.1128/CVI.00557-12
38. Kolte L, Rosenfeldt V, Vang L, Jeppesen D, Karlsson I, Ryder LP, et al. Reduced thymic size but no evidence of impaired thymic function in uninfected children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J (2011) 30:325–30. doi:10.1097/INF.0b013e3182019bc3
39. Abramczuk BM, Mazzola TN, Moreno YM, Zorzeto TQ, Quintilio W, Wolf PS, et al. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin Vaccine Immunol (2011) 18:1406–9. doi:10.1128/CVI.05065-11
40. Jones CE, Hesseling AC, Tena-Coki NG, Scriba TJ, Chegou NN, Kidd M, et al. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination. AIDS (2015) 29:155–65. doi:10.1097/QAD.0000000000000536
41. Van Rie A, Madhi SA, Heera JR, Meddows-Taylor S, Wendelboe AM, Anthony F, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol (2006) 13:246–52. doi:10.1128/CVI.13.2.246-252.2006
42. Kidzeru EB, Hesseling AC, Passmore JA, Myer L, Gamieldien H, Tchakoute CT, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS (2014) 28:1421–30. doi:10.1097/QAD.0000000000000292
43. Garcia-Knight MA, Nduati E, Hassan AS, Gambo F, Odera D, Etyang TJ, et al. Altered memory T-cell responses to bacillus Calmette-Guerin and tetanus toxoid vaccination and altered cytokine responses to polyclonal stimulation in HIV-exposed uninfected Kenyan infants. PLoS One (2015) 10:e0143043. doi:10.1371/journal.pone.0143043
44. Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood (2011) 117:6143–51. doi:10.1182/blood-2010-11-320317
45. Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol (2014) 5:446. doi:10.3389/fimmu.2014.00446
46. Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the women and infants transmission study. J Infect Dis (2006) 194:1089–97. doi:10.1086/507645
47. Schramm DB, Kuhn L, Gray GE, Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr (2006) 42:545–53. doi:10.1097/01.qai.0000225009.30698.ce
48. Palmer AC. Nutritionally mediated programming of the developing immune system. Adv Nutr (2011) 2:377–95. doi:10.3945/an.111.000570
49. Helfand RF, Witte D, Fowlkes A, Garcia P, Yang C, Fudzulani R, et al. Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi. J Infect Dis (2008) 198:1457–65. doi:10.1086/592756
50. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis (2016) 16:e92–107. doi:10.1016/S1473-3099(16)00055-4
51. World Health Organization. Fund UNC. Guideline: Updates on HIV and Infant Feeding. Geneva: World Health Organization (2016).
52. Marchant A, Appay V, van der Sande M, Dulphy N, Liesnard C, Kidd M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest (2003) 111:1747–55. doi:10.1172/JCI200317470
53. Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol (2007) 81:5766–76. doi:10.1128/JVI.00052-07
54. Miles DJ, Sanneh M, Holder B, Crozier S, Nyamweya S, Touray ES, et al. Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology (2008) 124:388–400. doi:10.1111/j.1365-2567.2007.02787.x
55. Gibson L, Barysauskas CM, McManus M, Dooley S, Lilleri D, Fisher D, et al. Reduced frequencies of polyfunctional CMV-specific T cell responses in infants with congenital CMV infection. J Clin Immunol (2015) 35:289–301. doi:10.1007/s10875-015-0139-3
56. Lidehäll AK, Engman M-L, Sund F, Malm G, Lewensohn-Fuchs I, Ewald U, et al. Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol (2013) 77:135–43. doi:10.1111/sji.12013
57. Lilleri D, Fornara C, Revello MG, Gerna G. Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J Infect Dis (2008) 198:536–43. doi:10.1086/590118
58. Gamadia LE, Remmerswaal EBM, Weel JF, Bemelman F, van Lier RAW, Berge Ten IJM. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood (2003) 101:2686–92. doi:10.1182/blood-2002-08-2502
59. Harari A, Dutoit V, Cellerai C, Bart P-A, Pasquier Du RA, Pantaleo G.Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev (2006) 211:236–54. doi:10.1111/j.0105-2896.2006.00395.x
60. Nebbia G, Mattes FM, Smith C, Hainsworth E, Kopycinski J, Burroughs A, et al. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant (2008) 8:2590–9. doi:10.1111/j.1600-6143.2008.02425.x
61. Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol (2011) 21:240–55. doi:10.1002/rmv.695
Keywords: human immunodeficiency virus, cytomegalovirus, vaccines, immune responses, infant
Citation: Falconer O, Newell M-L and Jones CE (2018) The Effect of Human Immunodeficiency Virus and Cytomegalovirus Infection on Infant Responses to Vaccines: A Review. Front. Immunol. 9:328. doi: 10.3389/fimmu.2018.00328
Received: 16 October 2017; Accepted: 06 February 2018;
Published: 02 March 2018
Edited by:
Rashika El Ridi, Cairo University, EgyptReviewed by:
Pietro Speziale, Università Degli Studi di Pavia, ItalyAnita S. Iyer, Harvard Medical School, United States
Copyright: © 2018 Falconer, Newell and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivia Falconer, by5mYWxjb25lckBzb3Rvbi5hYy51aw==
 Olivia Falconer
Olivia Falconer Marie-Louise Newell2
Marie-Louise Newell2