- Department of Immunology and Microbiology, The Scripps Research Institute, La Jolla, CA, United States
Epithelial tissues line the body providing a protective barrier from the external environment. Maintenance of these epithelial barrier tissues critically relies on the presence of a functional resident T cell population. In some tissues, the resident T cell population is exclusively comprised of γδ T cells, while in others γδ T cells are found together with αβ T cells and other lymphocyte populations. Epithelial-resident γδ T cells function not only in the maintenance of the epithelium, but are also central to the repair process following damage from environmental and pathogenic insults. Key to their function is the crosstalk between γδ T cells and neighboring epithelial cells. This crosstalk relies on multiple receptor–ligand interactions through both the T cell receptor and accessory molecules leading to temporal and spatial regulation of cytokine, chemokine, growth factor, and extracellular matrix protein production. As antigens that activate epithelial γδ T cells are largely unknown and many classical costimulatory molecules and coreceptors are not used by these cells, efforts have focused on identification of novel coreceptors and ligands that mediate pivotal interactions between γδ T cells and their neighbors. In this review, we discuss recent advances in the understanding of functions for these coreceptors and their ligands in epithelial maintenance and repair processes.
Introduction
The epithelial tissues are home to populations of T cells that function to protect the body from environmental pathogens and other insults. A major portion of T cells in many of these tissues expresses the γδ T cell antigen receptor (TCR) (1). The importance of these cells to homeostasis and wound repair has been evident for several years and exemplified by studies in skin, intestine, and lung (2–9). An absence of epithelial-resident γδ T cells in these tissues results in dysregulation of the epithelium, more severe damage or disease, and a delay in repair processes (2, 6, 8, 10, 11). Constant communication between resident γδ T cells and their neighboring epithelia is crucial for homeostasis and repair processes following damage or disease. Recent studies have begun to define the role of distinct molecular interactions in the rapid and localized response of epithelial-resident γδ T cells to tissue injury, yet much of the triggers and sequence of events remain a mystery.
Epithelial Tissues
The resident T cell population in the epidermal layer of the murine skin is a highly dendritic γδ T cell termed dendritic epidermal T cell (DETC). DETC express a canonical Vγ3Vδ1 TCR [nomenclature according to Garman et al. (12); alternative nomenclature Vγ5Vδ1 (13)] and make numerous contacts with surrounding epithelial cells, in particular keratinocytes and Langerhans cells. Under homeostatic conditions, their individual dendrites extend between surrounding cells allowing for constant contact with numerous adjacent cells. This feature allows for regulated interactions between both cell surface receptors and soluble molecules facilitating homeostasis in the skin and allowing for rapid repair following damage or disease. Although differing in T cell composition, human epidermis also contains resident T cells that make crucial contributions to wound repair (9). As such, it is reasonable to suggest that similar mechanisms of communication exist in human epidermis.
The intestinal mucosal barrier is also occupied by resident T cells. These T cells are termed intraepithelial lymphocytes (IEL) that, as their name suggests, are found residing between epithelial cells and include subsets of both αβ and γδ T cells. The intestinal γδ T cell subset expresses predominantly a Vγ5 TCR [alternative nomenclature Vγ7 (13)] that is able to pair with a number of different Vδ chains. Although not dendritic like γδ T cells in the skin, γδ IEL are able to make contact with multiple epithelial cells through active migration within the intestinal epithelium. This gives a single γδ IEL the ability to surveil large areas of epithelium (14, 15) and defend against pathogenic assault (16). Although not as clearly defined, resistance to infection and repair from damage in the lung also relies on resident γδ T cells (3, 11, 17–20), again likely through numerous contacts with surrounding cells (21).
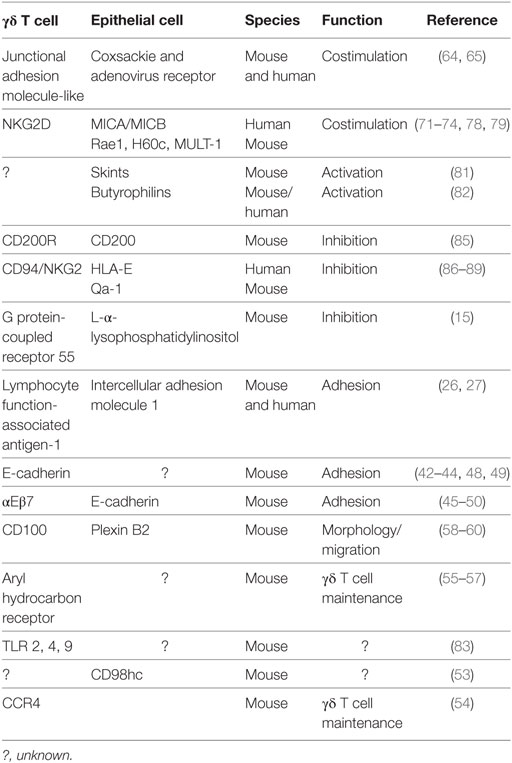
Continual interaction with neighboring epithelia is thus required for epithelial γδ T cells to perform their functions in homeostasis, resistance to infection, and damage repair. While the importance of the TCR is clear (22–25), it is becoming evident that additional distinct molecular interactions drive these functions of epithelial γδ T cells. Discussion of some of these interactions (Table 1) will be the focus of the remainder of this review.
Adhesion
The maintenance of epithelial-resident γδ T cells within the epithelium is known to involve adhesion through integrin and cadherin-mediated interactions. Expression of these molecules is also modulated in response to epithelial damage suggesting their functions may extend beyond maintenance to roles in repair processes as well.
Intercellular adhesion molecule 1 (ICAM-1), also known as CD54, is a membrane-bound adhesion molecule that is a ligand for leukocyte-expressed lymphocyte function-associated antigen-1 (LFA-1). This protein is well known to recruit leukocytes to sites of inflammation, but its interaction with tissue-resident γδ T cells is less understood. ICAM-1 is upregulated by the corneal epithelium following wounding and is required for γδ T cell recruitment to the site of damage in an LFA-1-dependent process (26). While ICAM-1 is also upregulated by endothelial cells and keratinocytes following wounding (27), and ICAM-1-deficient mice are known to exhibit delayed wound repair (27, 28), it is unknown whether the protein plays any role in DETC-mediated epithelial repair. ICAM-1 has also been shown to be important in shaping the gut lymphocyte populations, with ICAM-1-deficient mice displaying a relatively higher proportion of γδ T cells and a lower proportion of αβ T cells, though the biological effects of this population shift are unclear (29). Interestingly, the effect of ICAM-1/LFA-1 interactions on γδ T cells is not limited to leukocyte migration. Costimulation of peripheral mouse γδ T cells through TCR and LFA-1 was demonstrated to trigger apoptosis of these cells (30, 31), in contrast to the proliferative response observed in αβ T cells (30). However, ICAM-1/LFA-1 interaction has been shown to be involved in peripheral γδ T cell recognition of tumor cells and subsequent cytolytic response (32–36), so the outcome appears to be context dependent. While the majority of this work has focused on γδ T cell recognition of target cell-expressed ICAM-1, it should be noted that γδ T cells also express ICAM-1 (37). Blocking ICAM-1 expressed on the epithelial-associated Vδ1 T cell population has been reported to reduce cytotoxicity against myeloma cells (34). Studies in αβ T cells have shown ICAM-1 to be a costimulatory molecule promoting proliferation, IL-2 and IFNγ secretion, phosphatidylinositol-3 kinase activation, and a shift toward a memory phenotype (38–40). However, it remains to be seen whether epithelial-resident γδ T cells also have the ability to receive costimulatory signals through ICAM-1, and what the effects of LFA-1 engagement are in this population.
E-cadherin is an adhesion molecule that supports adhesion between keratinocytes (41). Interestingly, DETC also express E-cadherin as well as another E-cadherin ligand, αEβ7. Following wounding, DETC downregulate expression of E-cadherin, but maintain their level of expression of αEβ7 (42–44). In vitro and in vivo studies have demonstrated a role for αEβ7 in DETC activation with possible functions in adhesion and epidermal retention, dendrite anchoring, morphology and motility, cytotoxicity and costimulation (22, 45–47). In contrast, DETC-expressed E-cadherin functions as an inhibitory receptor for DETC activation (47). Murine intestinal IEL also express both E-cadherin and αEβ7 (48, 49), and αEβ7 is expressed on most γδ T cells in the bleomycin-induced mouse model of lung fibrosis (50), suggesting similar functions for these adhesion molecules on γδ T cells in other epithelial sites. Furthermore, the expression of both E-cadherin and αEβ7 on fetal thymic precursors of DETC (43, 44) indicates that inhibitory and costimulatory signals, respectively through these molecules may also influence thymic development and maturation of DETC precursors. This is further supported by the observation of diminished DETC numbers in the epidermis of αE deficient mice (46), although thymic populations were not directly analyzed in this study.
CD98hc is an amino acid transporter that associates with both cadherins and β1 integrins (51, 52). As such, it is perhaps not surprising that it too has been implicated in the regulation of skin homeostasis and wound healing (53), although it is unknown whether this involves direct interaction of CD98hc with DETC. In addition to adhesive interactions, the chemokine receptor CCR4 has been shown to be important for DETC retention in the epidermis (54). Additionally, the aryl hydrocarbon receptor (AhR) transcription factor is essential for maintaining both DETC and IEL in their respective tissues (55–57), although just how AhR signals lead to tissue retention of DETC and IEL, and whether AhR plays a role in epithelial γδ T cell activation and the wound repair process, is unknown.
Morphology and Migration
γδ IEL actively migrate within the intestinal epithelium and this migration is dependent on occludin expression in both IEL and the epithelium (14). In contrast, DETC in the epidermis are sessile under homeostatic conditions, communicating with surrounding keratinocytes through their numerous dendritic processes. Upon keratinocyte damage, DETC rapidly pull back these processes and adopt a more rounded morphology (6). Interestingly, downregulation of E-cadherin in keratinocytes can contribute to this rounding either through disruption of E-cadherin-mediated homophilic binding and/or αEβ7 integrin-mediated heterophilic binding (45).
In addition, binding of the semaphorin, CD100, to one of its ligands, Plexin B2, contributes to the DETC rounding response through activation of ERK kinase and cofilin (58). In the absence of CD100, the DETC rounding response to keratinocyte damage is delayed resulting in subsequent delayed wound closure (58). It has been suggested that the rounding of DETC permits them to migrate within the epidermis during wound repair, yet this remains to be demonstrated. Interestingly, in the intestinal epithelium, where IEL are in constant motion, CD100-plexin B2 interactions still play an important role as CD100-deficiency results in more severe damage as well as delayed repair in a mouse model of DSS-induced colitis (59). Similarly, a role for CD100 in lung allergic inflammation has been described (60). Whether CD100 is involved in γδ T cell migration in these epithelial tissues is yet to be determined.
Activation
To become fully activated, αβ T cells require engagement of molecules in addition to the TCR, such as CD4, CD8, and CD28 together with other costimulatory and adhesion molecules. Unlike αβ T cells, epithelial-resident γδ T cells do not express CD4, CD8 (although the CD8aa homodimer is expressed by some γδ IEL), or CD28 (61), however, a number of other molecules have recently been described to participate in the activation of these cells.
Striking similarities between CD28 and the junctional adhesion molecule-like (JAML) (62–64) suggest that JAML may play the role of primary costimulator for epithelial-resident γδ T cells through interaction with its ligand coxsackie and adenovirus receptor (CAR) (64, 65), expressed on epithelial cells. Like CD28 on αβ T cells, JAML is able to induce proliferation and cytokine production in epithelial γδ T cells. This response is mediated through PI3K which is recruited to JAML following CAR ligation (63). The PI3K binding motif in CD28 (66), similarly mediates delivery of a costimulatory signal. Although JAML expression has been demonstrated on activated peripheral γδ T cells, a population of activated CD8+ αβ T cells and other cell types of both the innate and adaptive arms of the immune system, including neutrophils, monocytes, and some memory T cells (64, 65, 67), the function of JAML as a costimulatory molecule appears confined to the epithelial subsets of γδ T cells.
Blocking of JAML-CAR costimulation in vivo impairs DETC activation and delays wound closure (64), demonstrating the importance of this interaction to DETC function. Just how this interaction might function in response to other perturbations to the skin, such as infection or malignancy, is unknown. A parallel role in IEL activation in the mouse intestine (64) is suggested by the similarity in expression patterns of JAML and CAR in the intestine. Whether this costimulatory pair also functions in human skin and intestinal T cell activation and tissue repair is still not known.
The NKG2D receptor (discussed in detail elsewhere) is an activating receptor expressed on NK, γδ, and CD8+ T cells (10, 68–70). In the mouse epidermis, NKG2D is expressed on DETC and ligation to its ligands Rae-1, H60, and MULT-1 on keratinocytes activates DETC (10). A reliance on PI3K signaling has been demonstrated, however, whether activation through NKG2D also requires simultaneous TCR stimulation or can stimulate DETC directly is somewhat controversial (71–75). Nevertheless, the importance of NKG2D signaling in epithelial γδ T cells has been demonstrated in models of wound healing, carcinogenesis, and contact hypersensitivity responses (72, 76, 77). Whether the difference in T cell receptor requirement for NKG2D-mediated DETC activation is due to differences in the induced ligand resulting from the type of damage elicited, is unclear at this time. In humans, there is evidence to suggest that recognition of MIC by Vδ1 expressing intestinal epithelial T cells (76, 78, 79) can either be direct, via the TCR, through NKG2D, or sequentially using both molecules (80). This idea, however, requires experimental confirmation.
It is increasingly evident that additional molecules are also important for the activation of epithelial-resident γδ T cells. A recent analysis of defective wound healing in aged mice highlighted the importance of Skint molecules (to be reviewed in detail elsewhere) in DETC activation and epidermal re-epithelialization (81). A role for the closely related butyrophilin (Btnl) molecules in the activation of intestinal γδ T cells in both mice and humans has recently been demonstrated (82). In addition, other molecules, such as toll-like receptors 2, 4, and 9 have been shown to be upregulated on γδ T cells following skin injury (83), suggesting a role in their activation, however, a precise function has yet to be defined.
Inhibition
The role of inhibitory signals in the control of αβ T cell activation is well established. Emerging evidence points to similar signals playing an important role in regulating the activation of epithelial-resident γδ T cells. The transmembrane glycoprotein CD200 expressed on keratinocytes has been implicated in the protection of hair follicles from autoimmune attack (84). Interestingly, resting DETC express low levels of the CD200-receptors 1, 2, and 3, but expression of CD200R1 is increased following activation in vitro. In functional assays, ligation of DETC-expressed CD200R with immobilized CD200 inhibits DETC proliferation and cytokine secretion highlighting an important role for CD200–CD200R interactions in the control of DETC activation (85). How this interaction may function during wound repair is unknown.
Inhibitory receptors expressed by NK cells are also found on γδ T cells, and appear to have similar inhibitory functions on these cells (86). The Ly49E and CD94/NKG2 receptors are expressed on mature fetal thymic DETC as well as those residing in the epidermis (86). DETC do not express other members of the Ly49 family. DETC cytotoxicity is inhibited by ligation of CD94/NKG2 and monoclonal antibody cross-linking of CD94/NKG2 prevents mature DETC thymocytes from killing FcγR+ target cells demonstrating a role for CD94/NKG2 as an inhibitory receptor on DETC (86). Just how these and other inhibitory interactions may function in epithelial wound repair processes warrants further investigation.
A recent report has identified an inhibitory role for G protein-coupled receptor 55 (GPR55) on intestinal IEL (15). GPR55 is highly expressed on γδ IEL and more modestly on αβ IEL and intestinal dendritic cells. Through interaction with its receptor L-α-lysophosphatidylinositol expressed on intestinal epithelial cells, GPR55 regulates the interaction between IEL and the epithelium and inhibits the accumulation of GPR55+ cells in the small intestine. Analysis of GPR55-deficient animals revealed increased γδ IEL migration within, and retention in, the small intestine, and enhanced IEL-epithelial cell crosstalk (15). Although the precise inhibitory role of GPR55 in the intestine is yet to be determined, Sumida et al. (15) propose that it may constrain IEL movement in the epithelium to allow normal epithelial cell functions to proceed.
Conclusion
By analogy with skin, gut, and lung, the existence of a resident γδ T cell population in all epithelial barrier tissues implies a crucial function for these cells throughout the body. An increasing number of receptor-ligand pairs are being identified as vital for the homeostasis and repair functions of these resident γδ T cells. An understanding of the precise mechanisms of action of these various molecules in the crosstalk between T cells and their adjacent epithelial cells will help to elucidate their roles throughout the epithelia.
Author Contributions
DW, MJ, and WH all contributed to the writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol (1991) 9:679–705. doi:10.1146/annurev.iy.09.040191.003335
2. Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci U S A (2002) 99(22):14338–43. doi:10.1073/pnas.212290499
3. Cheng M, Hu S. Lung-resident gammadelta T cells and their roles in lung diseases. Immunology (2017) 151(4):375–84. doi:10.1111/imm.12764
4. Dalton JE, Cruickshank SM, Egan CE, Mears R, Newton DJ, Andrew EM, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology (2006) 131(3):818–29. doi:10.1053/j.gastro.2006.06.003
5. Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, et al. Mucosal T cells bearing TCRγδ play a protective role in intestinal inflammation. J Immunol (2004) 173(2):1390–8. doi:10.4049/jimmunol.173.2.1390
6. Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin γδ T cells in wound repair. Science (2002) 296(5568):747–9. doi:10.1126/science.1069639
7. Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A (1995) 92(13):6147–51. doi:10.1073/pnas.92.13.6147
8. Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol (2005) 6(1):73–9. doi:10.1038/ni1152
9. Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, et al. A role for human skin-resident T cells in wound healing. J Exp Med (2009) 206(4):743–50. doi:10.1084/jem.20081787
10. Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by γδ T cells. Science (2001) 294(5542):605–9. doi:10.1126/science.1063916
11. King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, et al. Cutting edge: protective response to pulmonary injury requires γδ T lymphocytes. J Immunol (1999) 162(9):5033–6.
12. Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell (1986) 45(5):733–42. doi:10.1016/0092-8674(86)90787-7
13. Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature (1986) 322(6082):836–40. doi:10.1038/322836a0
14. Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, et al. Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A (2012) 109(18):7097–102. doi:10.1073/pnas.1112519109
15. Sumida H, Lu E, Chen H, Yang Q, Mackie K, Cyster JG. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol (2017) 2(18):eaao1135. doi:10.1126/sciimmunol.aao1135
16. Edelblum KL, Singh G, Odenwald MA, Lingaraju A, El Bissati K, McLeod R, et al. gammadelta intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology (2015) 148(7):1417–26. doi:10.1053/j.gastro.2015.02.053
17. Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O’Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol (1999) 71:77–144. doi:10.1016/S0065-2776(08)60400-9
18. Born WK, Lahn M, Takeda K, Kanehiro A, O’Brien RL, Gelfand EW. Role of γδ T cells in protecting normal airway function. Respir Res (2000) 1(3):151–8. doi:10.1186/rr26
19. Hahn YS, Taube C, Jin N, Takeda K, Park JW, Wands JM, et al. Vγ4+ γδ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol (2003) 171(6):3170–8. doi:10.4049/jimmunol.171.6.3170
20. Lahn M, Kanehiro A, Takeda K, Konowal A, O’Brien RL, Gelfand EW, et al. γδ T cells as regulators of airway hyperresponsiveness. Int Arch Allergy Immunol (2001) 125(3):203–10. doi:10.1159/000053817
21. Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, et al. Distribution and leukocyte contacts of γδ T cells in the lung. J Leukoc Biol (2005) 78(5):1086–96. doi:10.1189/jlb.0505244
22. Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol (2012) 13(3):272–82. doi:10.1038/ni.2240
23. Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol (2004) 172(6):3573–9. doi:10.4049/jimmunol.172.6.3573
24. Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal γδ cells with disrupted primary Vγ gene usage. Science (1998) 279(5357):1729–33. doi:10.1126/science.279.5357.1729
25. Zhang B, Wu J, Jiao Y, Bock C, Dai M, Chen B, et al. Differential requirements of TCR signaling in homeostatic maintenance and function of dendritic epidermal T cells. J Immunol (2015) 195(9):4282–91. doi:10.4049/jimmunol.1501220
26. Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol (2009) 175(2):571–9. doi:10.2353/ajpath.2009.090112
27. Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol (2000) 157(1):237–47. doi:10.1016/S0002-9440(10)64534-8
28. Gay AN, Mushin OP, Lazar DA, Naik-Mathuria BJ, Yu L, Gobin A, et al. Wound healing characteristics of ICAM-1 null mice devoid of all isoforms of ICAM-1. J Surg Res (2011) 171(1):e1–7. doi:10.1016/j.jss.2011.06.053
29. Steinhoff U, Klemm U, Greiner M, Bordasch K, Kaufmann SH. Altered intestinal immune system but normal antibacterial resistance in the absence of P-selectin and ICAM-1. J Immunol (1998) 160(12):6112–20.
30. Kobayashi N, Hiromatsu K, Matsuzaki G, Harada M, Matsumoto Y, Nomoto K, et al. A sustained increase of cytosolic Ca2+ in gammadelta T cells triggered by co-stimulation via TCR/CD3 and LFA-1. Cell Calcium (1997) 22(6):421–30. doi:10.1016/S0143-4160(97)90069-5
31. Matsumoto Y, Hiromatsu K, Sakai T, Kobayashi Y, Kimura Y, Usami J, et al. Co-stimulation with LFA-1 triggers apoptosis in gamma delta T cells on T cell receptor engagement. Eur J Immunol (1994) 24(10):2441–5. doi:10.1002/eji.1830241027
32. Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, et al. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol (2005) 175(8):5481–8. doi:10.4049/jimmunol.175.8.5481
33. Ensslin AS, Formby B. Comparison of cytolytic and proliferative activities of human gamma delta and alpha beta T cells from peripheral blood against various human tumor cell lines. J Natl Cancer Inst (1991) 83(21):1564–9. doi:10.1093/jnci/83.21.1564
34. Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy (2012) 14(9):1110–8. doi:10.3109/14653249.2012.700766
35. Liu Z, Guo B, Lopez RD. Expression of intercellular adhesion molecule (ICAM)-1 or ICAM-2 is critical in determining sensitivity of pancreatic cancer cells to cytolysis by human gammadelta-T cells: implications in the design of gammadelta-T-cell-based immunotherapies for pancreatic cancer. J Gastroenterol Hepatol (2009) 24(5):900–11. doi:10.1111/j.1440-1746.2008.05668.x
36. Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, et al. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun (2007) 354(2):613–8. doi:10.1016/j.bbrc.2007.01.031
37. Chao CC, Sandor M, Dailey MO. Expression and regulation of adhesion molecules by gamma delta T cells from lymphoid tissues and intestinal epithelium. Eur J Immunol (1994) 24(12):3180–7. doi:10.1002/eji.1830241240
38. Chirathaworn C, Kohlmeier JE, Tibbetts SA, Rumsey LM, Chan MA, Benedict SH. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J Immunol (2002) 168(11):5530–7. doi:10.4049/jimmunol.168.11.5530
39. Kohlmeier JE, Chan MA, Benedict SH. Costimulation of naive human CD4 T cells through intercellular adhesion molecule-1 promotes differentiation to a memory phenotype that is not strictly the result of multiple rounds of cell division. Immunology (2006) 118(4):549–58. doi:10.1111/j.1365-2567.2006.02396.x
40. Kohlmeier JE, Rumsey LM, Chan MA, Benedict SH. The outcome of T-cell costimulation through intercellular adhesion molecule-1 differs from costimulation through leucocyte function-associated antigen-1. Immunology (2003) 108(2):152–7. doi:10.1046/j.1365-2567.2003.01578.x
41. Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol (2005) 6(8):622–34. doi:10.1038/nrm1699
42. Aiba S, Nakagawa S, Ozawa H, Tagami H. Different expression of E-cadherin by two cutaneous gamma/delta TcR+ T-cell subsets, V gamma 5− and V gamma 5+ gamma/delta TcR+ T cells. J Invest Dermatol (1995) 105(3):379–82. doi:10.1111/1523-1747.ep12320959
43. Lee MG, Tang A, Sharrow SO, Udey MC. Murine dendritic epidermal T cells (DETC) express the homophilic adhesion molecule E-cadherin. Epithelial Cell Biol (1994) 3(4):149–55.
44. Lefrancois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol (1994) 24(3):635–40. doi:10.1002/eji.1830240322
45. Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw P, et al. Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood (2008) 112(3):619–25. doi:10.1182/blood-2008-01-134833
46. Schon MP, Schon M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol (2002) 119(1):190–3. doi:10.1046/j.1523-1747.2002.17973.x
47. Uchida Y, Kawai K, Ibusuki A, Kanekura T. Role for E-cadherin as an inhibitory receptor on epidermal γδ T cells. J Immunol (2011) 186(12):6945–54. doi:10.4049/jimmunol.1003853
48. Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature (1994) 372(6502):190–3. doi:10.1038/372190a0
49. Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, Nawa Y. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun (2005) 331(4):977–83. doi:10.1016/j.bbrc.2005.04.025
50. Braun RK, Sterner-Kock A, Kilshaw PJ, Ferrick DA, Giri SN. Integrin alpha E beta 7 expression on BAL CD4+, CD8+, and gamma delta T-cells in bleomycin-induced lung fibrosis in mouse. Eur Respir J (1996) 9(4):673–9. doi:10.1183/09031936.96.09040673
51. Lemaitre G, Stella A, Feteira J, Baldeschi C, Vaigot P, Martin MT, et al. CD98hc (SLC3A2) is a key regulator of keratinocyte adhesion. J Dermatol Sci (2011) 61(3):169–79. doi:10.1016/j.jdermsci.2010.12.007
52. Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, et al. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem (1999) 274(5):3009–16. doi:10.1074/jbc.274.5.3009
53. Boulter E, Estrach S, Errante A, Pons C, Cailleteau L, Tissot F, et al. CD98hc (SLC3A2) regulation of skin homeostasis wanes with age. J Exp Med (2013) 210(1):173–90. doi:10.1084/jem.20121651
54. Nakamura K, White AJ, Parnell SM, Lane PJ, Jenkinson EJ, Jenkinson WE, et al. Differential requirement for CCR4 in the maintenance but not establishment of the invariant Vgamma5(+) dendritic epidermal T-cell pool. PLoS One (2013) 8(9):e74019. doi:10.1371/journal.pone.0074019
55. Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol (2009) 30(9):447–54. doi:10.1016/j.it.2009.06.005
56. Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant γδ T cells in the murine epidermis. J Immunol (2011) 187(6):3104–10. doi:10.4049/jimmunol.1100912
57. Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell (2011) 147(3):629–40. doi:10.1016/j.cell.2011.09.025
58. Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity (2012) 37(2):314–25. doi:10.1016/j.immuni.2012.05.026
59. Meehan TF, Witherden DA, Kim CH, Sendaydiego K, Ye I, Garijo O, et al. Protection against colitis by CD100-dependent modulation of intraepithelial γδ T lymphocyte function. Mucosal Immunol (2014) 7(1):134–42. doi:10.1038/mi.2013.32
60. Shanks K, Nkyimbeng-Takwi EH, Smith E, Lipsky MM, DeTolla LJ, Scott DW, et al. Neuroimmune semaphorin 4D is necessary for optimal lung allergic inflammation. Mol Immunol (2013) 56(4):480–7. doi:10.1016/j.molimm.2013.05.228
61. Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immunol (2001) 2(11):997–1003. doi:10.1038/ni1101-997
62. Moog-Lutz C, Cave-Riant F, Guibal FC, Breau MA, Di Gioia Y, Couraud PO, et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood (2003) 102(9):3371–8. doi:10.1182/blood-2002-11-3462
63. Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science (2010) 329(5996):1210–4. doi:10.1126/science.1187996
64. Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial γδ T cell activation. Science (2010) 329(5996):1205–10. doi:10.1126/science.1192698
65. Zen K, Liu Y, McCall IC, Wu T, Lee W, Babbin BA, et al. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol Biol Cell (2005) 16(6):2694–703. doi:10.1091/mbc.E05-01-0036
66. Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol (2003) 3(7):544–56. doi:10.1038/nri1131
67. Luissint AC, Lutz PG, Calderwood DA, Couraud PO, Bourdoulous S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by α4β1 integrin activation. J Cell Biol (2008) 183(6):1159–73. doi:10.1083/jcb.200805061
68. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science (1999) 285(5428):727–9. doi:10.1126/science.285.5428.727
69. Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity (2002) 17(1):19–29. doi:10.1016/S1074-7613(02)00333-3
70. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol (2003) 3(10):781–90. doi:10.1038/nri1199
71. Whang MI, Guerra N, Raulet DH. Costimulation of dendritic epidermal γδ T cells by a new NKG2D ligand expressed specifically in the skin. J Immunol (2009) 182(8):4557–64. doi:10.4049/jimmunol.0802439
72. Yoshida S, Mohamed RH, Kajikawa M, Koizumi J, Tanaka M, Fugo K, et al. Involvement of an NKG2D ligand H60c in epidermal dendritic T cell-mediated wound repair. J Immunol (2012) 188(8):3972–9. doi:10.4049/jimmunol.1102886
73. Nitahara A, Shimura H, Ito A, Tomiyama K, Ito M, Kawai K. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol (2006) 126(5):1052–8. doi:10.1038/sj.jid.5700112
74. Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol (2008) 9(2):146–54. doi:10.1038/ni1556
75. Ibusuki A, Kawai K, Yoshida S, Uchida Y, Nitahara-Takeuchi A, Kuroki K, et al. NKG2D triggers cytotoxicity in murine epidermal gammadelta T cells via PI3K-dependent, Syk/ZAP70-independent signaling pathway. J Invest Dermatol (2014) 134(2):396–404. doi:10.1038/jid.2013.353
76. Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science (1998) 279(5357):1737–40. doi:10.1126/science.279.5357.1737
77. Nielsen MM, Dyring-Andersen B, Schmidt JD, Witherden D, Lovato P, Woetmann A, et al. NKG2D-dependent activation of dendritic epidermal T cells in contact hypersensitivity. J Invest Dermatol (2015) 135(5):1311–9. doi:10.1038/jid.2015.23
78. Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, et al. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity (2001) 15(1):83–93. doi:10.1016/S1074-7613(01)00168-6
79. Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A (1999) 96(12):6879–84. doi:10.1073/pnas.96.12.6879
80. Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a γδ T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A (2011) 108(6):2414–9. doi:10.1073/pnas.1015433108
81. Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, et al. Impaired epidermal to dendritic T cell signaling slows wound repair in aged skin. Cell (2016) 167(5):1323–38e14. doi:10.1016/j.cell.2016.10.052
82. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific gammadelta T cell compartments. Cell (2016) 167(1):203–18e17. doi:10.1016/j.cell.2016.08.030
83. Rani M, Zhang Q, Scherer MR, Cap AP, Schwacha MG. Activated skin gammadelta T-cells regulate T-cell infiltration of the wound site after burn. Innate Immun (2015) 21(2):140–50. doi:10.1177/1753425913519350
84. Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, et al. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol (2004) 123(5):880–7. doi:10.1111/j.0022-202X.2004.23461.x
85. Rosenblum MD, Woodliff JE, Madsen NA, McOlash LJ, Keller MR, Truitt RL. Characterization of CD 200-receptor expression in the murine epidermis. J Invest Dermatol (2005) 125(6):1130–8. doi:10.1111/j.0022-202X.2005.23948.x
86. Van Beneden K, De Creus A, Stevenaert F, Debacker V, Plum J, Leclercq G. Expression of inhibitory receptors Ly49E and CD94/NKG2 on fetal thymic and adult epidermal TCR V gamma 3 lymphocytes. J Immunol (2002) 168(7):3295–302. doi:10.4049/jimmunol.168.7.3295
87. Lanier LL. NK cell receptors. Annu Rev Immunol. (1998) 16:359–93. doi:10.1146/annurev.immunol.16.1.359
88. Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A (1998) 95(9):5199–204. doi:10.1073/pnas.95.9.5199
Keywords: epithelial, γδ T cell, activation, costimulation, inhibition, epidermis, intestine, lung
Citation: Witherden DA, Johnson MD and Havran WL (2018) Coreceptors and Their Ligands in Epithelial γδ T Cell Biology. Front. Immunol. 9:731. doi: 10.3389/fimmu.2018.00731
Received: 15 February 2018; Accepted: 23 March 2018;
Published: 09 April 2018
Edited by:
Pierre Vantourout, King’s College London, United KingdomReviewed by:
Tomasz Zal, University of Texas MD Anderson Cancer Center, United StatesJessica Strid, Imperial College London, United Kingdom
Nathalie Jacobs, University of Liège, Belgium
Copyright: © 2018 Witherden, Johnson and Havran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wendy L. Havran, aGF2cmFuQHNjcmlwcHMuZWR1
 Deborah A. Witherden
Deborah A. Witherden Margarete D. Johnson
Margarete D. Johnson Wendy L. Havran
Wendy L. Havran