- 1CRCINA, INSERM, CNRS, Université d’Angers, Université de Nantes, Nantes, France
- 2Sanofi R&D, Biologics Research, Centre de Recherche Vitry Alfortville, Paris, France
- 3LabEx IGO “Immunotherapy, Graft, Oncology”, Nantes, France
Vγ9Vδ2 T cells represent a major unconventional γδ T cell subset located in the peripheral blood of adults in humans and several non-human primates. Lymphocytes that constitute this transitional subset can sense subtle level changes of intracellular phosphorylated intermediates of the isoprenoid biosynthesis pathway (phosphoantigens, pAg), such as isopentenyl pyrophosphate, during cell stress events. This unique antigenic activation process operates in a rigorous framework that requires the expression of butyrophilin 3A1 (BTN3A1/CD277) molecules, which are type I glycoproteins that belong to the B7 family. Several studies have further shown that pAg specifically bind to the intracellular B30.2 domain of BTN3A1 linked to the antigenic activation of Vγ9Vδ2 T cells. Here, we highlight the recent advances in BTN3A1 dynamics induced upon the binding of pAg and the contribution of the different subunits to this activation process. Recent reports support that conformational modifications of BTN3A1 might represent a key step in the detection of infection or tumorigenesis by Vγ9Vδ2 T cells. A better understanding of this mechanism will help optimize novel immunotherapeutical approaches that target defined functions of this unique γδ T cell subset.
1. γδ T Cells Compose a Special Immunological Unit
Discovered in the mid-1980s, gamma delta (γδ) T lymphocytes still puzzle and fascinate by their unconventional features. During thymic ontogeny, γδ T cell subsets originating from common lymphoid precursor cells emerge before αβ T cells to represent the predominant CD3+ population at the fetal development. Their relative frequency then decreases after birth, while αβ T cells progressively predominate. Importantly, for yet unclear reasons, the expression of particular TCR Vγ and Vδ regions is associated with preferential tissue locations. Hence, the major human peripheral γδ T cell subset (frequency >80%) in healthy adult expresses a heterodimeric TCR composed of Vγ9and Vδ2 chains, and represents about 5% of total lymphoïd cells (1, 2). By contrast, Vδ1 and Vδ3 subsets are mainly detected in epithelial tissues, liver, spleen, tonsils, lymph nodes, and thymus (3). Interestingly, γδ T cells compose the majority of circulating T lymphocytes in some non-primate species (i.e., cattle, sheep, pigs, and birds), which raises questions about evolutionary processes and the biology of this subset (4).
From a functional point of view, γδ T cells are involved in the control of microbial infections (e.g., bacteria, virus, and parasite), cell transformation, homeostasis, and tissue repair already reviewed in Ref. (5, 6). Their activation during these physio-pathological contexts induces the release of cytotoxic and bacteriostatic molecules, such as perforin, granzymes, granulysin, and defensins, death-inducing receptor, and TNF-related apoptosis-inducing ligand receptor (TRAIL). Activated γδ T cells also regulate immune responses by secreting a large panel of soluble molecules, such as cytokines, that can promote the clearance of either intracellular pathogens (e.g., TNFα, IFNγ), extracellular bacteria, fungi (IL-17) or parasites (e.g., IL-4, IL-5, and IL-13); inflammatory (e.g., TNFα, IFNγ) or anti-inflammatory responses (e.g., TGFβ, IL-10); tissue healing, epithelium repair, and cell survival. Interestingly, complementary studies have shown that activated γδ T cells, through type I IFN sensitizations, also promote dendritic cells (DC) maturation and, therefore, could represent adjuvant cells (7–9). Moreover, some γδ T cell subset, like human Vγ9Vδ2 T cells, can acquire an antigen-presenting cell (APC)-like phenotype and regulate conventional CD4+/CD8+ αβ T cell responses (10).
In contrast to most conventional αβ T cells which directly recognize antigenic structures composed of proteasome-generated peptides and polymorphic presenting molecules, that are related to the major histocompatibility complex (MHC) family (e.g., MHC class I/II molecules), the antigenic activation of γδ T cells is mostly MHC-independent, which strengthens their therapeutical interest (i.e., lack of alloreactivity) (11). The antigenic activation of γδ T cells is linked with their tissue residency and the Vδ chain expressed (12, 13). Interestingly, several studies have now reported that γδ T lymphocyte subsets can be activated by various native or modified molecules that mainly derive from a Self origin, including MHC-like molecules in mice (e.g., T10–T22) and in humans (e.g., MICA/B, CD1c, CD1d, and EPCR) (14–18) and yet unrelated native molecules, such as F0–F1 ATP synthase, phycoerythrin, and apolipoprotein A-I (19). More recently, Annexin-A2, which is expressed in tumor cell(s) upon oxidative stress, has been shown to be directly recognized by human Vγ8Vδ3 T lymphocytes (20). TLRs, dectins, and NLRs may act as γδ TCR costimulator (21). Of note, in most cases, the γδ TCR-dependent activation is also tightly regulated by a set of various molecules, including TLRs, dectins, and NLRs, killer Ig-like receptors (e.g., KIR2D, KIR3D), C-type lectins (CD94/NKG2A-C, NKG2D), and several costimulatory molecules shared with αβ T cells (e.g., LFA1, CD2, CD27, and CD28) (22). In this review, we focus our analysis on the γδ TCR-dependent activation modalities of the major peripheral Vγ9Vδ2 T cell subset.
2. Human Vγ9Vδ2 T Cells are Specifically Activated by Phosphoantigens
In healthy adult primates, the major peripheral γδ T cell subset, which expresses a TCR composed of Vγ9 and Vδ2 chains, does not account for more than 10% of the total peripheral T cell pool. Interestingly, this lymphocyte subset expands upon microbial infections (e.g., Mycobacterium leprae, Mycobacterium tuberculosis) (23, 24). In vitro assays that rely on the incubation of peripheral lymphoid cells with mycobacterial lysates have evidenced Vγ9Vδ2 T cell expansion mediated by protease-resistant and phosphatase-sensitive components, hereafter called phosphoantigens (pAg) (25). These low molecular weight agonists are constituted of alkyl esters associated with a diphosphate moiety that carries their bioactivity (26–28). Isopentenyl PyroPhosphate (IPP), which was the first natural pAg identified from the mycobacteria M. smegmatis, is also synthesized in eukaryotic cells where it is an intermediate metabolite of the isoprenoid mevalonate (MVA) pathway leading to cholesterol synthesis (29). Several natural pAg have been further identified and characterized from vertebrates (e.g., DMAPP, dimethylallyl pyrophosphate) and microbes (e.g., HDMAPP/HMBPP, 4-hydroxy-3-dimethylallyl pyrophosphate). These microbial metabolites, produced from the DOXP/MEP (1-deoxy-d-xylulose-5-phosphate/2-C-methyl-d-erythritol-4-phosphate) pathway (30–32), are much more efficient to activate Vγ9Vδ2 T cells than MVA-derived IPP. This property could explain the strong reactivity displayed by Vγ9Vδ2 T cells in infectious contexts (33). Dysregulation of the eukaryotic MVA pathway, which leads to an intracellular accumulation of IPP, has been reported in various types of tumor cells (34). For example, the over-expression of HMG-CoA reductase, in non-Hodgkin B cell lymphoma cell-line Daudi or breast adenocarcinoma cells, induces their spontaneous recognition by γ9Vδ2 T cells (35). Accordingly, pharmacological MVA pathway inhibitors that target upstream (e.g., statins) or downstream (e.g., aminobisphosphonates) IPP synthesis, respectively, suppress or trigger pAg-induced Vγ9Vδ2 T cell activation (36).
Primate Vγ9Vδ2 T cells can specifically sense weak modifications of the expression of Self molecules, such as pAg, in a contact- and TCR-dependent manner. However, the mechanisms and pathways involved in this peculiar antigenic activation process remain ill-defined. Despite several attempts, direct interactions between pAg and Vγ9Vδ2 TCR have never been clearly evidenced. While the contribution of additional molecules to this species-specific process has been suggested by various complementary studies [e.g., implication of TCR CDRs (37)], this had not been shown until the groundbreaking evidence that butyrophilins could represent a first group of key molecules.
3. The Butyrophilin BTN3A1 Orchestrates Vγ9Vδ2 T Cell Antigenic Activation Induced by Phosphoantigens
Following the key identification of pAg as potent and specific agonist compounds, the clear evidence that butyrophilin-3A (BTN3A/CD277) molecules also play a mandatory role in the antigenic activation of primate Vγ9Vδ2 T cells was a groundbreaking step to better understand this peculiar and mysterious immunological process (38). Phylogenetically, ubiquitously expressed type I glycoprotein butyrophilin (BTN) molecules share a common ancestor with other members of the B7-CD28 superfamily, which thus suggests that they display immunological functions (39). Indeed, various studies suggest that BTN, as well as BTN-like (BTNL) molecules are involved in some regulatory processes by triggering yet unclear pathways (40). In humans, BTN genes (>10) are located in the telomeric part close to HLA class I region of the chromosome 6p. BTN molecules, which are structurally highly homologous, are divided in three subfamilies (BTN1, BTN2, and BTN3). The BTN3A (CD277) subfamily, then contains three isoforms BTN3A1, -A2, and -A3, belonging to the immunoglobulin (Ig) superfamily and sharing a high structural homology for the extracellular domain composed of an Ig-like IgV and an IgC domain (39) (Figure 1). Of note, the sequences of both B7 and BTN receptors are sufficiently distinct to prevent the latter ones from binding to known costimulatory T ligands, such as CD28 or CTLA-4 (41, 42). While the ectodomain of the three BTN3A isoforms have a very high homology (>95%), only BTN3A1 and BTN3A3 isoforms express an intracellular portion which is composed of a poorly conserved PRY/SPRY B30.2 (hereafter called B30.2) (43). The B30.2 domain, described as a key region for mediating protein–protein interactions, is shared by other BTN family members, as well as various “immunological” proteins, such as TRIM (TRIpartite Motif) and pyrin families (44). The human genome contains four identified btnl genes, with the designations of BTNL2, -3, -8, and -9. btnl2, the best characterized family member, is clustered with the btn genes on chromosome 6, but near to human MHC class II region, whereas the least explored family members btnl3, btnl8, and btnl9 are localized on chromosome 5 (45). Interestingly, BTNL molecules share an homology with the murine Skint family molecules and more particulary with Skint1, which drives the intrathymic differentiation of murine Vγ5Vδ1 T cells (46). The biological function of BTN3A1 molecules is elusive. A recent report shows that BTN3A1 is a positive regulator of the nucleic acid-mediated type I IFN signaling pathway. Upon nucleic acid stimulation, BTN3A1 moves along microtubules toward the perinuclear region, where it directs the interaction of TBK1 with IRF3, thereby facilitating the phosphorylation of IRF3. This process is controlled by microtubule-associated protein 4 (MAP4) (47). Our group described the specific and mandatory contribution of BTN3A1, expressed at the membrane of cellular targets, to the pAg-induced reactivity of primate Vγ9Vδ2 T cells (38). BTN3A1 is ubiquitously expressed in primates, which seems associated with the presence of pAg-reactive γδ T cells in these species (48, 49). Accordingly, BTN3A1 orthologs are not expressed in the rodent lineage that lacks Vγ9Vδ2 T cell counterparts specific for pAg. The emergence of Vγ9Vδ2 TCR and BTN3 molecules with eutherian placental mammals has been reported, suggesting a strong co-evolutionary link (50).
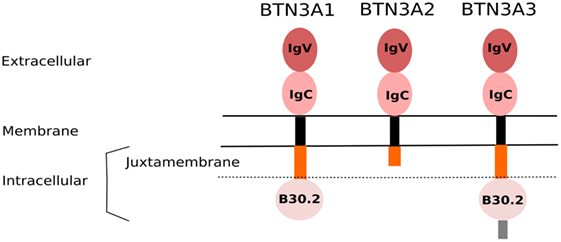
Figure 1. Domain organization of BTN3A isoforms. The BTN3A family proteins share a high structural homology, mainly through their extracellular domain comprising a membrane-proximal IgC and a N-terminal IgV domains. They are linked to a poorly conserved intracellular part via a single transmembrane structure (black). The submembrane region (orange) represents the juxtamembrane domain (JTM). BTN3A1 and BTN3A3, but not BTN3A2, contain an intracellular B30.2 domain. The BTN3A3 isoform is further composed of an additional C-terminal extension (gray).
The combined mode of action of BTN3A1 and pAg molecules for triggering a strong and specific antigenic activation of primate Vγ9Vδ2 T cells remains unclear and controversial. BTN3A1 has been first proposed as a “classical” antigen-presenting molecule for pyrophosphate compounds. In this model, pAg would bind a shallow groove within the distal IgV extracellular domain, which induces the formation of stable complexes that then directly interact and stimulate the Vγ9Vδ2 TCR, similarly to the peptide-MHC molecules and αβ TCR system (51). This model, which implies cognate physical interactions between a conserved portion of the IgV domain of BTN3A1, pAg, and the Vγ9Vδ2 TCR, diverges from the data published in several studies that clearly show the key specific requirement for the BTN3A1 isoform in the pAg-mediated activation of Vγ9Vδ2 T cells. In line with these observations, our model proposes that the intracellular B30.2 domain of the BTN3A1 isoform, but not BTN3A3, drives Vγ9Vδ2 T cell antigenic activation through a direct binding of pAg to a charged groove. This model, based on the intracellular sensing of pAg by BTN3A1 molecules, has been supported by several complementary observations. Depletion, domain swapping, and mutation experiments indicate that BTN3A1 lacking its intracellular domain B30.2, or expressing a BTN3A3 B30.2 domain, or at least mutated on some of its critical pAg-binding residues, fail to trigger an efficient pAg-induced Vγ9Vδ2 T cell stimulation. Conversely, chimeric BTN3A3 molecules that expressed the B30.2 domain of BTN3A1 efficiently trigger a pAg-mediated activation (52, 53). Together, these results support the mandatory role played by the intracellular BTN3A1 B30.2 domain in the pAg-binding and sensing by Vγ9Vδ2 T cells.
4. B30.2, The Lock/Key System of Intracellular Phosphoantigen Sensing
While the B30.2 domains of BTN3A1 and BTN3A3 display a strong homology (approximately 87% amino acid identity), the domain of BTN3A3 fails to efficiently bind pAg and to trigger a significant antigenic activation of Vγ9Vδ2 T cells. The crystal structure of the B30.2 domain of BTN3A1 gave key information about pAg binding site. Importantly, specificity of the BTN3A1 B30.2 domain is a highly positive charged pocket which is constituted by basic residues, including arginines (R442, R448, and R499), histidines (H381 and H408), and lysine (K423). Positively charged B30.2 domain represents an ideal pocket candidate for binding negatively charged pAg. Accordingly, the mutation from basic to (negatively charged) acidic residues completely abrogates pAg-binding and Vγ9Vδ2 T cell activation (53, 54). However, these results did not entirely explain the differences between the capacity of BTN3A1 and BTN3A3 to bind pAg. Close examination of the amino acid differences between these isoforms revealed a single amino acid difference in position 381 within the binding pocket: a histidine in BTN3A1 and an arginine in BTN3A3 (Figure 2). Swapping this single amino acid between the domains of each isoform (i.e., mutating the H into R in BNT3A1 and R into H in BTN3A3) transferred both binding and functional abilities to stimulate Vγ9Vδ2 T cells. Affinity differences have been measured between endogenous and exogenous pAg by a technique called Isothermal Titration Calorimetry (ITC): KD ≃1 mM for endogenous and KD ≃1 mM for exogenous pAg (55). These results also confirmed that the functional potency of those compounds in mediating activation of Vγ9Vδ2 T cells despite is not directly proportional to the affinity (56). The endogenous IPP is typically 100,000-fold weaker potency than the exogenous HMBPP (57).

Figure 2. Alignment of the intracellular B30.2 domain sequences of BTN3A1 and BTN3A3 isoforms. Amino acid sequence alignment of the B30.2 domains of BTN3A1 (top line) and BTN3A3 (bottom line). Dashes indicate absent residues in BTN3A1. Amino acids are shown in the single letter designations and numbered according full length nomenclature. Gray boxes indicate residues which constitute the pAg-binding positively charged pocket. Red font highlights the single amino acid difference between pAg-binding pocket, in position 381, H in BTN3A1, and R in BTN3A3 isoforms adapted from Ref. (53).
Adams and colleagues have investigated the effects of pAg binding to the intracellular domain B30.2 of BTN3A1 by NMR spectrometry and molecular dynamics simulation. Using a BTN3A1 full-length intracellular domain model, they have shown that pAg binding induces conformational changes of the BTN3A1 B30.2 domain. The Y382 residue, close to the positive pocket, has been identified as critical by showing the largest perturbation induced by pAg binding. ABP-treated Y352A mutants are less capable of mediating Vγ9Vδ2 T cell activation than the wild-type B30.2-containing protein (58). These conformational changes could represent the first signals delivered to distinguish activating or non-activating molecules. In fact, the BTN3A1 B30.2 domain is able to bind additional negatively charged small molecules, like malonate, citrate, adenosine-diphosphate (ADP), and nucleotidic pAg (59). Exogenous pAg (e.g., HMBPP/HDMAPP) induce a chemical shift into the B30.2 domain that extended to the binding site. IPP binding, a less affine pAg, induces similar shift perturbations but qualitatively smaller in magnitude. These results confirmed the antigenic potential difference between exogenous and endogenous pAg. In contrast, titration of the B30.2 domain with malonate and citrate revealed only few chemical shifts with a small magnitude. Both of these nonantigenic molecules failed to induce perturbations in residues more distal to the pAg-binding site. Strikingly, the conformational changes induced by ADP occur in a different direction than those with pAg (60). NMR and crystallography studies suggest that a precise conformation of BTN3A1 B30.2 domain is required to induce Vγ9Vδ2 T cell activation.
Studies from Massaia’s group provided further mechanistic inputs about the contribution of BTN3A1 in pAg-induced Vγ9Vδ2 T lymphocyte activation. They showed that ABP-treated dendritic cells (DC) release extracellular IPP that can induce a significant Vγ9Vδ2 T cell proliferation (61). They identified the ATP-binding cassette transporter 1 (ABCA1) as a major complex involved in this extracellular release of IPP by ABP-treated DCs, with the physical cooperation of BTN3A1 and apolipoprotein A-I (ApoA-I) molecules (62). BTN3A1 is physically linked to ABCA1 but not associated with ApoA-I. Gene silencing of BTN3A1 in ABP-treated DCs slightly decreased the amounts of IPP released. This important study highlighted the existence of pAg membrane transporter complexes that are involved in the export of these compounds. Conversely, the ways by which external charged pAg could cross the plasmic membrane to reach intracellular butyrophilins and then induce the reactivity of Vγ9Vδ2 T cells remain unclear and will need to be further defined.
5. The Juxtamembrane Domain of BTN3A1, Another Key Player in the Sensing of Phosphoantigens
The role played by the extracellular and intracellular B30.2 domains of BTN3A1 in the antigenic activation of human Vγ9Vδ2 T cells has been extensively studied. Moreover, the contribution of additional portions of these molecules, such as the juxtamembrane (JTM) domain, has also been carefully analyzed. The JTM domain of many transmembrane receptors, such as growth factor receptors, has been shown to be involved in signaling processes (63). The intracellular JTM region of BTN3A, which is a rather flexible structure, connects the transmembrane domain to the B30.2 one. Our functional activation assays performed with BTN3A1 chimeras swapped for their JTM region support that this intracellular part is a strong regulator of the Vγ9Vδ2 T cell activation. Indeed, BTN3A1 chimeras that express the JTM domains from BTN1A1, BTN2A2, BTNL3, or BTNL9 fail to trigger the antigenic activation of Vγ9Vδ2 T cells. Interestingly, chimeric BTN3A1 molecules expressing the JTM domain of BTN3A3 induce a massive antigenic activation of Vγ9Vδ2 T cells more efficiently than wild-type BTN3A1 (64) (Figure 3). Accordingly, a very recent report further supports these observations by showing that the binding of pAg, such as HMBPP, to the B30.2 domain perturbs residues within the JTM region, suggesting ligand-induced conformational changes. Interestingly, HMBPP could interact with residues within both the B30.2 and the JTM region at different contact points. Furthermore, this report also indicates that both key residues Ser/Thr296/297 and Thr304 fall within a critical functional BTN3A1 JTM region (65).

Figure 3. Alignment of sequences encoded by the intracellular juxtamembrane (JTM) domain from BTN3A1 and BTN3A3 isoforms. Amino acids sequence alignment of the JTM domains of BTN3A1 (top line) and BTN3A3 (bottom line). Amino acids are shown in the single letter designation and numbered according to full length nomenclature. Gray boxes show key residues, Ser/Thr296/297 and Thr304, involved in pAg binding in the JTM region due to the folding of the B30.2 domain in Vγ9Vδ2 T cell antigenic activation adapted from Ref. (64).
The binding of pAg to the intracellular B30.2 domain has been shown to induce conformational changes of the JTM that could have important functional consequences (55). For example, these modifications could either spread to the extracellular domain of BTN3A molecules or alter its membrane topology and dynamics, leading to their recognition by γδ T cells.
6. The Holy Grail: Understanding the Cryptic Mechanism of Antigenic Activation of Vγ9Vδ2 T Cells
Despite representing significant advances by suggesting that butyrophilins do not operate as “classical” antigen-presenting partners, such as MHC and MHC-like molecules, these recent observations rather complicated the poor understanding of this peculiar antigenic activation process. Ultimately, γδ T lymphocyte immunologists will need to answer to the challenging question about the mechanism(s) by which the Vγ9Vδ2 TCR exquisitely and specifically sense them, following the increase of pAg levels and their association(s) to butyrophilins, to deliver strong and rapid activation signals. From a fundamental point of view, these future analyses should provide evidences about the intracellular trafficking, the dynamics of these molecules (e.g., intracellular/membrane multimeric complexes), the regulation of this process in normal vs. pathological contexts (e.g., tumor cells). Basically, these results should bring some important information about the still unclear biological functions displayed by these molecules. On a more evolutionary side, these results should help to finally provide an unified overview of the antigenic activation process of human and murine γδ T lymphocyte subsets, some of the latter ones being also regulated by non-BTN3A1 butyrophilin-related molecules (e.g., Skint or BTNL8) (66, 67).
To assist these complex deciphering steps, novel elements have been recently brought, such as the recruitment of BTN3A1 partners and the contribution of other isoforms and conformational changes. As BTN3A1 isoforms have not been shown to directly interact with the Vγ9Vδ2 TCR, various independent studies have been conducted to identify and to characterize extra- and intracellular partner molecules. Consequently, different groups first confirmed that the expression of human BTN3A1 molecules in rodent cells may not be sufficient to simply induce the reactivity of primate Vγ9Vδ2 T cells (68). The transfer of human chromosome 6 in those cells, which triggers this species-specific activation, then suggested that partner molecule(s) are encoded by gene(s) located within this chromosome (69). Two studies recently reported that molecular partners, such as RhoB or periplakin, cognately interact with BTN3A molecules (70). RhoB is a small G protein of the Rho GTPase family that regulates actin reorganization, vesicles transport, and apoptosis in transformed cells following DNA damage. RhoB contributes to various cellular events, including cancer progression through multiple pathways by regulating DNA damage responses, apoptosis, cell cycle progression, migration, and invasion (71). Lipid modifications affect main subcellular localizations (i.e., endosomes, Golgi vesicles, and nucleus) of RhoB and its levels are acutely regulated in response to a variety of stimuli. Using a biolayer interferometry approach, Kuball’s group demonstrated that RhoB binds to the full-length BTN3A1 intracellular domain, while binding was significantly reduced to the B30.2 domain alone. However, the precise contribution of RhoB, which is conserved between humans and rodents and encoded by a gene located in chromosome 2, to the activation of Vγ9Vδ2 T lymphocytes is yet unclear and will require a deeper analysis.
The plakin family member, cytoskeleton adaptor protein periplakin (PPL), whose gene is located in chromosome 16, has also been shown to bind to the BTN3A1 JTM (72). PPL might contribute to the formation of responsive and dynamic structures which could implicate both cytoskeleton components (e.g., intermediate filaments, actin) and pAg. Accordingly, our results from fluorescence recovery after photobleaching (FRAP) experiments have shown an immobilization of BTN3A1 molecules linked to pAg sensitization (53). This suggests that the antigenic activation of Vγ9Vδ2 T lymphocytes is linked to the recruitment and containment of BTN3A1 proteins in selected subcellular domains which are located in the vicinity of the plasma membrane and focal adhesions (LB & ES, unpublished observations). However, PPL knockdown using siRNAs had no clearly interpretable effects on BTN3A1-mediated activation of Vγ9Vδ2 T cells. In this work, the main evidence for a functional contribution of these interactions is a correlation between loss of PPL binding and loss of activation, induced by a deletion of either the VKLLEEL JTM stretch (located in exon 5 of BTN3A1) or only of its di-leucine motif. In so far as PPL does not bind to BTN3A3, while active BTN3A3 carrying the R351H mutation efficiently activates Vγ9Vδ2 T lymphocytes, it seems unlikely that PPL is required for the Vγ9Vδ2 T lymphocyte antigenic activation process.
Initial experiments evidenced that agonist #20.1 BNT3A-specific mAbs bind the IgV ectodomain of BTN3A glycoproteins, which leads to the activation of Vγ9Vδ2 T lymphocytes (38, 73). Among various hypotheses, the possibility of mAb-induced conformational changes, which could mimic pAg-induced modifications, deserves attention. The functional impact of pAg-induced changes of the intracellular B30.2 and JTM domains, that could be then transduced to the extracellular domain and trigger the sensing of these modified Self complexes by Vγ9Vδ2 T lymphocytes, has been investigated. Accordingly, independent studies have shown that the conformation of both the B30.2 domain and its upstream JTM region vary upon pAg binding (58, 60). An important study has shown that pAg bind the B30.2 pocket and weakly interact with some residues constituting the JTM region. Based on both length and flexibility characteristics, the authors propose that the B30.2 domain of BTN3A1 is moved toward the JTM region and closer to the membrane upon ligand binding. Such intracellular changes would be sensed by γδ T cells through modifications of either the extracellular domain or interactions with other molecular partners (65). Non-BTN3A proteins composed of an intracellular B30.2 domain have been shown to naturally multimerize and this status is important to fulfill their functions (74, 75).
While first studies proposed that the ectodomain of BTN3A exist at the surface into either V-shaped or head-to-tail conformations (49, 76), recent experiments suggest that only the ectodomain adopts a V-shaped conformation (58). Strikingly, this study indicates that the expression of BTN3A1–BTN3A2 heterodimers in lipid nanodiscs is more stable than BTN3A1 homodimers, which suggest a role for the BTN3A2 isoform (which contains no intracellular B30.2 domain). A growing set of studies from various laboratories confirmed that BTN3A1 molecules are mandatory for pAg-dependent activation of Vγ9Vδ2 T lymphocytes. The contribution of BTN3A2 and BTN3A3 isoforms to this process remained to be understood and was analyzed. Initial functional studies, using global, and likely incomplete, BTN3A-knockdown (shRNA delivered by lentivirus) combined to a forced expression of selected isoforms (transfection), first showed that the expression of BTN3A2 and BTN3A3 isoforms is not sufficient to induce the activation of Vγ9Vδ2 T lymphocytes by pAg. So far, the results failed to demonstrate any inhibitory or activatory role played by non-BTN3A1 isoforms. A work from Hayday’s group proposes that BTN3A1 and BTN3A2 heterodimers would contribute to this process according to this model. The ectodomain and the B30.2 domain of BTN3A1 would represent active entities in Vγ9Vδ2 T lymphocyte stimulation, while BTN3A2 isoforms would rather participate by regulating the appropriate routing (e.g., ER trafficking), kinetics, and/or stability of BTN3A1 (77).
Despite these major breakthroughs, the main question remains yet unsolved: how could such conformational changes and heterodimeric associations be specifically sensed by the TCR of Vγ9Vδ2 T lymphocytes. This issue represents major future research tracks in this field (Figure 4). To summarize, complementary research issues can be identified: (i) the subcellular localization for the interactions of pAg with BTN3A molecules; (ii) the role played by additional intra- vs. extracellular partners (e.g., cargo ?); (iii) the characterization of BTN3A antigenic complexes and their dynamics, linked to the antigenic activation status; (iv) the conformation(s)/multimerization of integral BTN3A molecules; and (v) the specific role played by BTN3A2 and BTN3A3 isoforms in these processes.
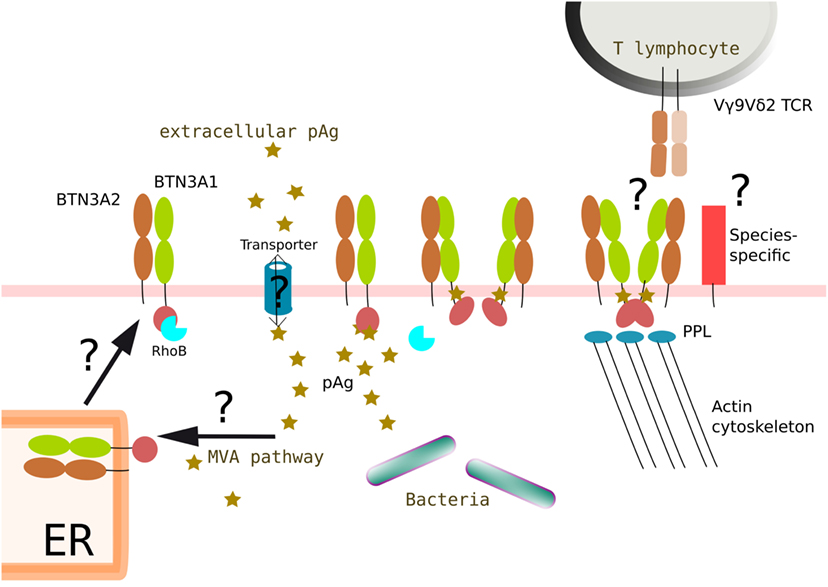
Figure 4. Proposed integrated model of BTN3A1 modifications induced by phosphoantigens and leading to the antigenic activation of Vγ9Vδ2 T lymphocytes. Intracellular accumulation of pAg could originate from a dysregulation of the Self mevalonate (MVA) pathway in pathological situations (e.g., cancer, infections) and/or be from exogenous origin (e.g., pathogens). In some situations, negatively charged pAg would need to be routed (e.g., import/export) via active processes (e.g., ABC transporters). pAg would then bind to intracellular parts of butyrophilin molecules (e.g., B30.2 ± juxtamembrane domains) at different sub-cellular locations during synthesis and routing steps of the molecules (e.g., ER, cell membrane). The binding of pAg to butyrophilins induces structural modifications that affect the dynamics of the molecules (e.g., membrane diffusion) and the immunological visibility of these molecules. Accordingly, the transition from resting to activatory state of these molecular complexes might also be linked to the nature of the multimerization of BTN3A1 glycoproteins (e.g., homodimers, heterodimers). The contribution of additional partner molecules, some of them being species-specific, regulating actin cytoskeleton modifications (e.g., RhoB, PPL) might also be important. The mechanisms that drive this unique antigenic activation process of human Vγ9Vδ2 T lymphocytes sensing these subtle molecular changes though a specific, contact-, and Vγ9Vδ2 TCR-dependent process remain a major conundrum. The question marks (?) refer to unsolved or yet unclear issues.
Author Contributions
Both authors contributed to the writing process, prepared the manuscript, and approved the final version.
Conflict of Interest Statement
LB was employed by company Sanofi-Aventis R&D. All other authors declare no competing interests.
Acknowledgments
The authors thank the staff of the cellular and tissular imaging core (MicroPICell) and the cytometry facility (Cytocell) from Nantes CRCINA, SFRF. Bonamy and Université de Nantes for their expert assistance. The authors thank Quentin Ayoul-Guilmard for critical reading of the manuscript.
Funding
This work was supported by funds from INSERM, CNRS, Université de Nantes, Institut National du Cancer (INCa, PLBio2014), Agence Nationale de la Recherche (ANR, GDSTRESS), Ligue Nationale contre le Cancer (AO InterRégional 2017), Fondation pour la Recherche Médicale (FRM Equipe 2017), Cancéropôle Grand-Ouest, and SANOFI. The work was realized in the context of the LabEX IGO and the IHU-Cesti programs, supported by the National Research Agency Investissements d’Avenir via the programs ANR-11-LABX-0016-01 and ANR-10-IBHU-005, respectively. The IHU-Cesti project is also supported by Nantes Métropole and Région Pays de la Loire.
References
1. Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol (2000) 18:975–1026. doi:10.1146/annurev.immunol.18.1.975
2. Chien Y-H, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol (2014) 32:121–55. doi:10.1146/annurev-immunol-032713-120216
3. Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med (1989) 169(4):1277–94. doi:10.1084/jem.169.4.1277
4. Davis WC, Brown WC, Hamilton MJ, Wyatt CR, Orden JA, Khalid AM, et al. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet Immunol Immunopathol (1996) 52(4):275–83. doi:10.1016/0165-2427(96)05578-X
5. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity (2009) 31(2):184–96. doi:10.1016/j.immuni.2009.08.006
6. Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol (2010) 10(7):467–78. doi:10.1038/nri2781
7. Devilder M-C, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated V gamma 9v delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol (2006) 176(3):1386–93. doi:10.4049/jimmunol.176.3.1386
8. Devilder M-C, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol (2009) 183(6):3625–33. doi:10.4049/jimmunol.0901571
9. Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol (2005) 174(1):252–60. doi:10.4049/jimmunol.174.1.252
10. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science (2005) 309(5732):264–8. doi:10.1126/science.1110267
11. Allison TJ, Winter CC, Fournié JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature (2001) 411(6839):820–4. doi:10.1038/35081115
12. Kisielow J, Kopf M. The origin and fate of γδ T cell subsets. Curr Opin Immunol (2013) 25(2):181–8. doi:10.1016/j.coi.2013.03.002
13. Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol (2013) 13(2):88–100. doi:10.1038/nri3384
14. Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol (2012) 13(9):872–9. doi:10.1038/ni.2394
15. Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med (2000) 191(6):937–48. doi:10.1084/jem.191.6.937
16. Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity (2013) 39(6):1032–42. doi:10.1016/j.immuni.2013.11.001
17. Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of gammadelta T cell receptors. Science (2005) 308(5719):252–5. doi:10.1126/science.1106480
18. Adams EJ, Chien Y-H, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science (2005) 308(5719):227–31. doi:10.1126/science.1106885
19. Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, et al. Tumor recognition following Vgamma9vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity (2005) 22(1):71–80. doi:10.1016/j.immuni.2004.11.012
20. Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci U S A (2017) 114(12):3163–8. doi:10.1073/pnas.1621052114
21. Wesch D, Peters C, Oberg H-H, Pietschmann K, Kabelitz D. Modulation of γδ T cell responses by TLR ligands. Cell Mol Life Sci (2011) 68(14):2357–70. doi:10.1007/s00018-011-0699-1
22. Ribot JC, debarros A, Silva-Santos B. Searching for “signal 2”: costimulation requirements of γδ T cells. Cell Mol Life Sci (2011) 68(14):2345–55. doi:10.1007/s00018-011-0698-2
23. Balbi B, Valle MT, Oddera S, Giunti D, Manca F, Rossi GA, et al. T-lymphocytes with gamma delta+ V delta 2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis (1993) 148(6 Pt 1):1685–90. doi:10.1164/ajrccm/148.6_Pt_1.1685
24. Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol (2000) 22(3):191–217. doi:10.1007/s002810000042
25. Pfeffer K, Schoel B, Gulle H, Kaufmann SH, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol (1990) 20(5):1175–9. doi:10.1002/eji.1830200534
26. Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science (1994) 264(5156):267–70. doi:10.1126/science.8146660
27. Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, et al. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem (2001) 276(21):18337–44. doi:10.1074/jbc.M100495200
28. Zhang Y, Song Y, Yin F, Broderick E, Siegel K, Goddard A, et al. Structural studies of Vgamma2vdelta2 T cell phosphoantigens. Chem Biol (2006) 13(9):985–92. doi:10.1016/j.chembiol.2006.08.007
29. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature (1995) 375(6527):155–8. doi:10.1038/375155a0
30. Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J (1993) 295(Pt 2):517–24. doi:10.1042/bj2950517
31. Jomaa H, Feurle J, Lühs K, Kunzmann V, Tony HP, Herderich M, et al. Vgamma9/Vdelta2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-D-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol (1999) 25(4):371–8. doi:10.1016/S0928-8244(99)00110-8
32. Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, et al. 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human gammadelta T cells. J Biol Chem (1999) 274(45):32079–84. doi:10.1074/jbc.274.45.32079
33. Eberl M, Altincicek B, Kollas A-K, Sanderbrand S, Bahr U, Reichenberg A, et al. Accumulation of a potent gammadelta T-cell stimulator after deletion of the lytB gene in Escherichia coli. Immunology (2002) 106(2):200–11. doi:10.1046/j.1365-2567.2002.01414.x
34. Gober H-J, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med (2003) 197(2):163–8. doi:10.1084/jem.20021500
35. Asslan R, Pradines A, Pratx C, Allal C, Favre G, Le Gaillard F. Epidermal growth factor stimulates 3-hydroxy-3-methylglutaryl-coenzyme A reductase expression via the ErbB-2 pathway in human breast adenocarcinoma cells. Biochem Biophys Res Commun (1999) 260(3):699–706. doi:10.1006/bbrc.1999.0945
36. Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med (1999) 340(9):737–8. doi:10.1056/NEJM199903043400914
37. Wang H, Fang Z, Morita CT. Vgamma2vdelta2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol (2010) 184(11):6209–22. doi:10.4049/jimmunol.1000231
38. Harly C, Guillaume Y, Nedellec S, Peigné C-M, Mönkkönen H, Mönkkönen J, et al. Key implication of CD277/butyrophilin-3 (BTN3a) in cellular stress sensing by a major human γδ T-cell subset. Blood (2012) 120(11):2269–79. doi:10.1182/blood-2012-05-430470
39. Arnett HA, Escobar SS, Viney JL. Regulation of costimulation in the era of butyrophilins. Cytokine (2009) 46(3):370–5. doi:10.1016/j.cyto.2009.03.009
40. Rhodes DA, Reith W, Trowsdale J. Regulation of immunity by butyrophilins. Annu Rev Immunol (2016) 34:151–72. doi:10.1146/annurev-immunol-041015-055435
41. Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature (2001) 410(6828):604–8. doi:10.1038/35069112
42. Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature (2001) 410(6828):608–11. doi:10.1038/35069118
43. D’Cruz AA, Babon JJ, Norton RS, Nicola NA, Nicholson SE. Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity. Protein Sci (2013) 22(1):1–10. doi:10.1002/pro.2185
44. Rhodes DA, de Bono B, Trowsdale J. Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology (2005) 116(4):411–7. doi:10.1111/j.1365-2567.2005.02248.x
45. Abeler-Dörner L, Swamy M, Williams G, Hayday AC, Bas A. Butyrophilins: an emerging family of immune regulators. Trends Immunol (2012) 33(1):34–41. doi:10.1016/j.it.2011.09.007
46. Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet (2008) 40(5):656–62. doi:10.1038/ng.108
47. Seo M, Lee S-O, Kim J-H, Hong Y, Kim S, Kim Y, et al. MAP4-regulated dynein-dependent trafficking of BTN3a1 controls the TBK1-IRF3 signaling axis. Proc Natl Acad Sci U S A (2016) 113(50):14390–5. doi:10.1073/pnas.1615287113
48. Compte E, Pontarotti P, Collette Y, Lopez M, Olive D. Frontline: characterization of BT3 molecules belonging to the B7 family expressed on immune cells. Eur J Immunol (2004) 34(8):2089–99. doi:10.1002/eji.200425227
49. Palakodeti A, Sandstrom A, Sundaresan L, Harly C, Nedellec S, Olive D, et al. The molecular basis for modulation of human Vγ9vδ2 T cell responses by CD277/butyrophilin-3 (BTN3a)-specific antibodies. J Biol Chem (2012) 287(39):32780–90. doi:10.1074/jbc.M112.384354
50. Karunakaran MM, Göbel TW, Starick L, Walter L, Herrmann T. Vγ9 and Vδ2 T cell antigen receptor genes and butyrophilin 3 (BTN3) emerged with placental mammals and are concomitantly preserved in selected species like alpaca (Vicugna pacos). Immunogenetics (2014) 66(4):243–54. doi:10.1007/s00251-014-0763-8
51. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3a1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol (2013) 14(9):908–16. doi:10.1038/ni.2665
52. Wang H, Henry O, Distefano MD, Wang Y-C, Räikkönen J, Mönkkönen J, et al. Butyrophilin 3a1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2vδ2 T cells. J Immunol (2013) 191(3):1029–42. doi:10.4049/jimmunol.1300658
53. Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, et al. The intracellular B30.2 domain of butyrophilin 3a1 binds phosphoantigens to mediate activation of human Vγ9vδ2 T cells. Immunity (2014) 40(4):490–500. doi:10.1016/j.immuni.2014.03.003
54. Wang H, Morita CT. Sensor function for butyrophilin 3a1 in prenyl pyrophosphate stimulation of human Vγ2vδ2 T cells. J Immunol (2015) 195(10):4583–94. doi:10.4049/jimmunol.1500314
55. Hsiao C-HC, Lin X, Barney RJ, Shippy RR, Li J, Vinogradova O, et al. Synthesis of a phosphoantigen prodrug that potently activates Vγ9δ2 T-lymphocytes. Chem Biol (2014) 21(8):945–54. doi:10.1016/j.chembiol.2014.06.006
56. Shippy RR, Lin X, Agabiti SS, Li J, Zangari BM, Foust BJ, et al. Phosphinophosphonates and their tris-pivaloyloxymethyl prodrugs reveal a negatively cooperative butyrophilin activation mechanism. J Med Chem (2017) 60(6):2373–82. doi:10.1021/acs.jmedchem.6b00965
57. Altincicek B, Moll J, Campos N, Foerster G, Beck E, Hoeffler JF, et al. Cutting edge: human gamma delta T cells are activated by intermediates of the 2-C-methyl-D-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol (2001) 166(6):3655–8. doi:10.4049/jimmunol.166.6.3655
58. Gu S, Sachleben JR, Boughter CT, Nawrocka WI, Borowska MT, Tarrasch JT, et al. Phosphoantigen-induced conformational change of butyrophilin 3a1 (BTN3a1) and its implication on Vγ9vδ2 T cell activation. Proc Natl Acad Sci U S A (2017) 114(35):E7311–20. doi:10.1073/pnas.1707547114
59. Moulin M, Alguacil J, Gu S, Mehtougui A, Adams EJ, Peyrottes S, et al. Vγ9vδ2 T cell activation by strongly agonistic nucleotidic phosphoantigens. Cell Mol Life Sci (2017) 74(23):4353–67. doi:10.1007/s00018-017-2583-0
60. Salim M, Knowles TJ, Baker AT, Davey MS, Jeeves M, Sridhar P, et al. BTN3a1 discriminates γδ T cell phosphoantigens from nonantigenic small molecules via a conformational sensor in its B30.2 domain. ACS Chem Biol (2017) 12(10):2631–43. doi:10.1021/acschembio.7b00694
61. Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9vδ2 T cells, αβ CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol (2011) 187(4):1578–90. doi:10.4049/jimmunol.1002514
62. Castella B, Kopecka J, Sciancalepore P, Mandili G, Foglietta M, Mitro N, et al. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9vδ2 T cell activation by dendritic cells. Nat Commun (2017) 8:15663. doi:10.1038/ncomms15663
63. Kovacs E, Das R, Wang Q, Collier TS, Cantor A, Huang Y, et al. Analysis of the role of the C-terminal tail in the regulation of the epidermal growth factor receptor. Mol Cell Biol (2015) 35(17):3083–102. doi:10.1128/MCB.00248-15
64. Peigné C-M, Léger A, Gesnel M-C, Konczak F, Olive D, Bonneville M, et al. The juxtamembrane domain of butyrophilin BTN3a1 controls phosphoantigen-mediated activation of human Vγ9vδ2 T cells. J Immunol (2017) 198(11):4228–34. doi:10.4049/jimmunol.1601910
65. Nguyen K, Li J, Puthenveetil R, Lin X, Poe MM, Hsiao C-HC, et al. The butyrophilin 3a1 intracellular domain undergoes a conformational change involving the juxtamembrane region. FASEB J (2017) 31(11):4697–706. doi:10.1096/fj.201601370RR
66. Barbee SD, Woodward MJ, Turchinovich G, Mention J-J, Lewis JM, Boyden LM, et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A (2011) 108(8):3330–5. doi:10.1073/pnas.1010890108
67. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell (2016) 167(1):203–18.e17. doi:10.1016/j.cell.2016.08.030
68. Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev (2007) 215:59–76. doi:10.1111/j.1600-065X.2006.00479.x
69. Riaño F, Karunakaran MM, Starick L, Li J, Scholz CJ, Kunzmann V, et al. Vγ9vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3a1) and additional genes on human chromosome 6. Eur J Immunol (2014) 44(9):2571–6. doi:10.1002/eji.201444712
70. Sebestyen Z, Scheper W, Vyborova A, Gu S, Rychnavska Z, Schiffler M, et al. RhoB mediates phosphoantigen recognition by Vγ9vδ2â T cell receptor. Cell Rep (2016) 15(9):1973–85. doi:10.1016/j.celrep.2016.04.081
71. Vega FM, Ridley AJ. The RhoB small GTPase in physiology and disease. Small GTPases (2016):1–10. doi:10.1080/21541248.2016.1253528
72. Rhodes DA, Chen H-C, Price AJ, Keeble AH, Davey MS, James LC, et al. Activation of human γδ T cells by cytosolic interactions of BTN3a1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol (2015) 194(5):2390–8. doi:10.4049/jimmunol.1401064
73. Starick L, Riano F, Karunakaran MM, Kunzmann V, Li J, Kreiss M, et al. Butyrophilin 3a (BTN3a, CD277)-specific antibody 20.1 differentially activates Vγ9vδ2 TCR clonotypes and interferes with phosphoantigen activation. Eur J Immunol (2017) 47(6):982–92. doi:10.1002/eji.201646818
74. Jeong J, Rao AU, Xu J, Ogg SL, Hathout Y, Fenselau C, et al. The PRY/SPRY/B30.2 domain of butyrophilin 1a1 (BTN1a1) binds to xanthine oxidoreductase: implications for the function of BTN1a1 in the mammary gland and other tissues. J Biol Chem (2009) 284(33):22444–56. doi:10.1074/jbc.M109.020446
75. Nepveu-Traversy M-E, Bérubé J, Berthoux L. TRIM5alpha and TRIMCyp form apparent hexamers and their multimeric state is not affected by exposure to restriction-sensitive viruses or by treatment with pharmacological inhibitors. Retrovirology (2009) 6:100. doi:10.1186/1742-4690-6-100
76. Gu S, Nawrocka W, Adams EJ. Sensing of pyrophosphate metabolites by Vγ9vδ2 T cells. Front Immunol (2014) 5:688. doi:10.3389/fimmu.2014.00688
Keywords: human γδ T cell, T cell receptor, antigenic activation, phosphoantigens, butyrophilin, B30.2
Citation: Boutin L and Scotet E (2018) Towards Deciphering the Hidden Mechanisms That Contribute to the Antigenic Activation Process of Human Vγ9Vδ2 T Cells. Front. Immunol. 9:828. doi: 10.3389/fimmu.2018.00828
Received: 15 February 2018; Accepted: 05 April 2018;
Published: 20 April 2018
Edited by:
Pierre Vantourout, King’s College London, United KingdomReviewed by:
Eric Champagne, UMR5282 Centre de Physiopathologie de Toulouse Purpan (CPTP), FranceAndrew Wiemer, University of Connecticut, United States
Jean Jacques Fournie, ERL5294 Centre de Recherches en Cancérologie de Toulouse, France
Copyright: © 2018 Boutin and Scotet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lola Boutin, bG9sYS5ib3V0aW5AdW5pdi1uYW50ZXMuZnI=;
Emmanuel Scotet, ZW1tYW51ZWwuc2NvdGV0QGluc2VybS5mcg==
 Lola Boutin1,2*
Lola Boutin1,2* Emmanuel Scotet
Emmanuel Scotet