- 1Department of Food Safety and Biotechnology, Armenian National Agrarian University, Yerevan, Armenia
- 2International Association for Human and Animals Health Improvement, Yerevan, Armenia
- 3Yerevan State Medical University, Yerevan, Armenia
- 4Armenian National Agrarian University, Yerevan, Armenia
- 5Kyorin University School of Medicine, Tokyo, Japan
- 6Lawrence Berkeley National Laboratory, Berkeley, CA, United States
- 7Health Promoting Naturals Laboratory, School of Environmental and Biological Sciences, Rutgers State University, New Brunswick, NJ, United States
Intestinal microorganisms play a crucial role in health and disease. The disruption of host–microbiota homeostasis has been reported to occur not only during disease development but also as a result of medication. Familial Mediterranean fever (FMF) is an inflammatory genetic disease characterized by elevated systemic reactivity against the commensal gut microbiota and high levels of Candida albicans in the gut. This study’s major objective was to investigate the effects of commercial probiotic Narine on the relative abundance of gut bacteria (specifically, enterobacteria, lactobacilli, Staphylococcus aureus, and enterococci) of C. albicans carrier and non-carrier FMF patients in remission. Our main finding indicates that the probiotic reduces numbers of C. albicans and abundance of enterobacteria in male and female patients of C. albicans carriers and non-carriers. It has pivotal effect on Enterococcus faecalis: increase in male non-carriers and decrease in female ones regardless of C. albicans status. No effect was seen for Lactobacillus and S. aureus. Our data suggest that M694V/V726A pyrin inflammasome mutations leading to FMF disease may contribute to gender-specific differences in microbial community structure in FMF patients. The study’s secondary objective was to elucidate the gender-specific differences in the gut’s microbial community of FMF patients. The tendency was detected for higher counts of enterobacteria in female FMF subjects. However, the small number of patients of these groups preclude from conclusive statements, pointing at the need for additional investigations with appropriate for statistical analysis groups of subjects involved in the study.
Introduction
Candida albicans is an opportunistic pathogen, which often exists as a harmless human commensal microorganism (1). Mutually beneficial associations of C. albicans were reported with several members of the intestinal microbiota (2, 3). In contrast, cooperative interaction of C. albicans and Escherichia coli increased the probability of fungal urinary tract infections due to increased E. coli-induced adhesion of C. albicans to the bladder mucosa (4). Another possibly synergistic interaction was shown between Staphylococcus aureus and C. albicans (5).
The disruption of inflammasomes, intracellular protein complexes with an important role in the sensing of intracellular pathogen- and danger-associated molecular patterns, can lead to susceptibility to infection, gut auto-inflammation, and tumorigenesis (6–8). Disruption of the host immune system—intestinal microbiota’s homeostasis is the main cause of inflammatory bowel disease. In particular, Klebsiella pneumoniae and P. mirabilis were found to be associated with colitis in animals using the T-bet−/− × Rag2−/− ulcerative colitis model (9).
Separate from this is the gene responsible for another inflammatory disease: familial Mediterranean fever (FMF), designated MEFV, which encodes an inflammasome pyrin containing domain of purin (PYD), TRIM, and B30.2 and is activated by bacterial toxins like Clostridium difficile, toxin B (TcdB), and C3 toxins (10). MEFV, in addition to FMF, can cause pyrin-associated auto-inflammation with neutrophilic dermatosis (PAAND) (11). The PYD is detected in more than 20 human proteins related to inflammation and apoptosis, such as NLRP3 (12). A methionine residue at position 694, important for conservation of the pyrin’s structure, is associated with a severe form of FMF (prevalent among Armenian patients). Rowczenio and co-authors assumed that the location of p.M694 in the putative binding site of caspase-1 (the substitution/deletion of methionine) may promote IL-1β generation by inhibition of pyrin–caspase-1 interactions (13). The B30.2/SPRY domain may also interact with viral components (14).
Elevated systemic reactivity against commensal gut microbiota has been reported in Armenian FMF patients. In many patients, inflammation can persist during an attack-free period. It has been revealed that IgG antibodies against Bacteroides, Parabacteroides, Escherichia, and Enteroccocus antigens are significantly increased (15). It was also shown earlier that the presence of C. albicans above 103 CFU/g of fecal materials is a common problem for Armenian FMF patients. While no participant had used antibiotics in the 2–3 months prior to the investigation, 45.5% of patients carried C. albicans in relatively high numbers (more than 103 CFU/g fecal materials) (16). Culturing revealed high numbers of the bacterial genera Proteus, Klebsiella, Enterobacter, and Citrobacter at cell densities of more than 103 CFU/g fecal materials in FMF patients (17). Probiotic strains may have different clinical effects, and the efficacy of one strain does not indicate that the other strains will be similarly efficacious as described (18).
The probiotic Lactobacillus acidophilus INMIA 9602 Er strain 317/402 was isolated in 1963 from the gut microbiota of a healthy newborn infant and named Narine after the investigator’s daughter (19). Studies have revealed that the strain has antagonistic activities against a wide range of pathogenic Gram-positive and Gram-negative microorganisms, including several clinical isolates of Clostridium difficile (20), Bacillus subtilis, Pseudomonas aeruginosae, and Klebsiella pneumonia (21), and Cronobacter sakazakii (22). The normalization of erythrocyte sedimentation rate (ESR) and levels of C-reactive protein (CRP) in patients’ blood after consumption of a Narine probiotic formulation (Vitamax-E, Armenia) was observed in a double blind, partially randomized, placebo-controlled trial of 30 volunteer patients with FMF in remission (23). Moreover, regulatory action of this strain on gut commensal E. coli was shown in FMF patients (24). L. acidophilus INMIA 9602 Er 317/402 had no in vitro impact on C. albicans strains isolated from FMF patients (16).
This study examined the effect of L. acidophilus INMIA 9602 Er 317/402, isolated from the commercial probiotic formulation used by Armenian FMF patients, on the relative abundance of gut enteric bacteria, lactobacilli, S. aureus, and E. faecalis in C. albicans-carrier FMF patients with the MEFV pyrin inflammasome mutation M694V/V726A, which is the prevalent pattern in the Armenian cohort. Main research questions were to show (i) if the changes in gut microbiota of FMF patients, primarily associated with the M694V/V726A pyrin inflammasome mutations, could lead to overgrowth of gut C. albicans of the patients and (ii) if colchicine/probiotic could effect on gut microbiota of patients through the regulation of NLRP inflammasomes.
Materials and Methods
Forty healthy volunteers (20 male and 20 female) with less than baseline levels of C. albicans in the gut microbiota and without mutations in MEFV and 48 FMF volunteers (24 males and 24 females) were enrolled in a double-blind, partly randomized, placebo-controlled trial. Out of these participants, only 30 healthy (8 males and 22 females) and 31 (20 males and 11 females; 15 C. albicans non-carriers and 16 C. albicans carriers, see the Table 1) completed the trial. The probiotic Lactobacillus acidophilus INMIA 9602 Er 317/402 strain (Narine, Vitamax-E, Armenia) were prescribed to patients in each Narine FMF group. Remaining patients took placebo (capsule without the probiotic). The study participants took one capsule of placebo or probiotic preparation (150 mg of the probiotic strain contained no less than 1.5 × 108 of viable bacteria) twice a day for 30 days. The datasets generated for this study can be found here: https://www.ncbi.nlm.nih.gov/geo/info/linking.html (GSE111835 study at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE111835). Besides, another study on the effect of Narine probiotic on gut C. albicans of 12 FMF patients with C. albicans counts above 106 CFU/g fecal material (7 males and 5 females) were completed during these investigations.
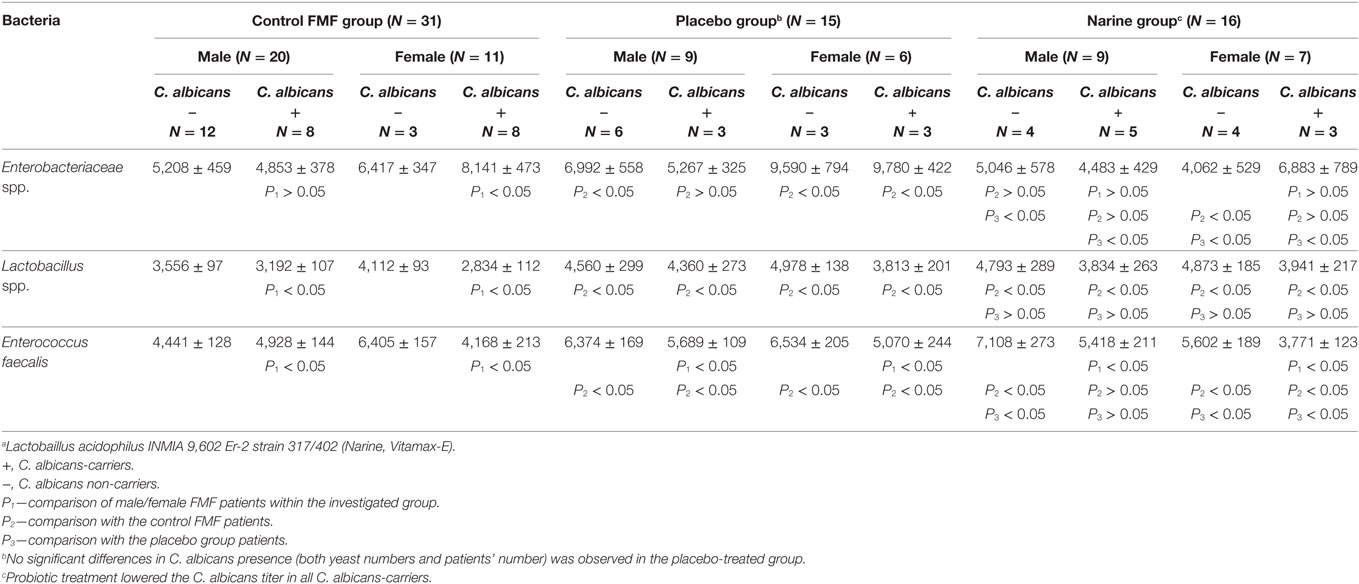
Table 1. Hybidization scores for gut bacteria in Candida albicans-carrier and non-carrier familial Mediterranean fever (FMF) patients after probioticaa therapy; average ± SD.
Fecal material was collected twice, before prescribing the probiotic and placebo and immediately after discontinuation of the treatment.
The age range of participants was 18–50 years. All patients’ diagnoses were confirmed by genetic analysis. None of the study participants had been treated with antibiotics, probiotics, hormones, or chemotherapeutic agents during the month leading up to the study. The duration of the colchicine treatment by patients was more than 7 year. During the 30-day period of study, patients with FMF used their regular colchicine medication (1 mg daily).
Study participants collected the fecal materials themselves in sterile plastic bags and transferred them to the laboratory not later than 2 h after collection.
The ZR Fecal DNA MiniPrep (Zymo Researc, Irvine, CA, USA) and the UltraClean® Tissue & Cells DNA Isolation Kit (QIAGEN, Germantown, MD, USA) were used for total genomic DNA isolation following the manufacturers’ recommendations.
The primer sequences used for microarrays and 16S rRNA clone libraries were: 27f.jgi (Bacteria-specific) 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492r.jgi (Bacteria/Archaea-specific) 5′-GGTTACCTTGTTACGACTT-3′.
The fecal bacterial communities were assessed by a third generation, culture-independent, high-density DNA microaray (PhyloChip™; Affymetrix, Santa Clara, CA, USA) analysis as previously described (25). This approach detects and measures the relative abundance of more than 50,000 individual microbial taxa. This approach is based on the analysis of the sequence of 16S ribosomal RNA genes. The PhyloChip™ relies on the analysis of all nine variable regions of the 16S gene and offers deeper taxonomic classification than other general approaches. With 1.2 million probes per chip, the microchip-based hybridization approach ensures that measurements on key low-abundance bacteria are not suppressed by dominant members of the microbial community. Nearly full-length 16S rRNA-gene fragments were amplified using universal bacterial primers. The amplicons were used for PhyloChip™ analysis, assessing the differences in hybridization intensity—reflective of differences in the relative abundance of bacterial taxa (25).
Candida albicans in fecal samples was quantified on Brilliance™ Candida agar (Thermo Scientific, Waltham, MA, USA) and confirmed by a C. albicans PCR kit (DNA-Technology LLC, Russia).
Statistical analyses were performed by the Multibase 2015 Excel Add-in program (NumericalDynamics, Tokyo, Japan).
Results
C. albicans In FMF Patients
Despite the prevalence of men among Armenian FMF patients (16, 26) and the indication of possible associations between C. albicans infections and gender (27), our investigations showed that the number of C. albicans-carrier male FMF patients did not differ from the number of female carriers. Eleven out of 24 male FMF volunteers and 11 out of 24 female FMF volunteers had C. albicans counts above baseline levels in the gut microbiota before the probiotic treatment.
Effects of Probiotic on Gut C. albicans in FMF Patients
Probiotic treatment lowered the C. albicans titer in the FMF patient community. Only one “non-trial” female FMF patient with 107 CFU/g C. albicans in fecal material and one “non-trial” male patient with 106 CFU/g C. albicans in fecal material carried C. albicans at levels 104 CFU/g fecal materials after probiotic treatment.
As expected, no significant differences in C. albicans presence (both yeast numbers and patients’ number) was observed in the placebo-treated group.
Culture-Independent Analysis of Enterobacteriaceae in the Gut Microbiota of FMF Patients
The relative abundance of enterobacteria in the gut microbiota of C. albicans-carrier patients is presented in Figure 1. The results show that female C. albicans-carrier FMF patients differ by their gut microbiota composition from that of male patients. Compared with diseased men, these women carried higher numbers of operational taxonomic units (OTUs) of the genera Salmonella, Escherichia, Averyella, Cronobacter, Klebsiella, and Serratia (P < 0.00021) (Figure 1A). Differences between the hybridization scores of male/female FMF patients show that the average intensity value of bacteria belonging to Enterobacteriaceae spp. is higher in the gut microbiota of C. albicans-carrier FMF women than in FMF men (8,141 ± 473 vs. 6,417 ± 347; P < 0.05) (Table 1). There was no detectable significant difference between OTUs in C. albicans-carrier male patients as compared to healthy people (P > 0.05) (Figure 1D).
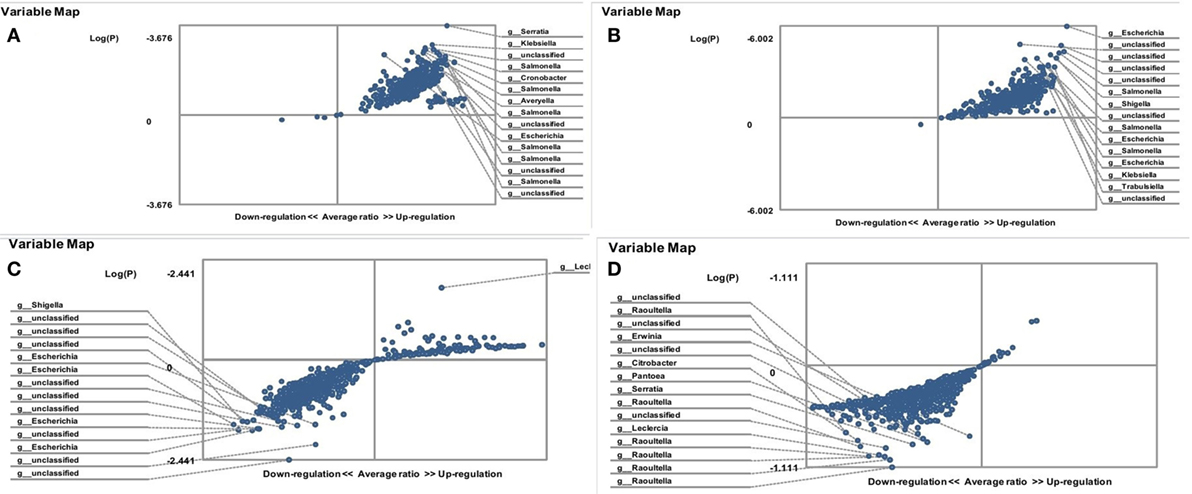
Figure 1. Distribution of gut Enterobacteriaceae in Candida albicans-carrier familial Mediterranean fever (FMF) patients. Contribution of variables to differentiation between two groups [X axis, average ratio between two groups; Y axis, −Log (P)]. The upper-right and lower-left corner are the most significant. (A) FMF women with gut C. albicans numbers above baseline level/FMF men with gut C. albicans numbers above baseline level; (B) FMF women with gut C. albicans numbers above baseline level before probiotic therapy/Healthy women; (C) FMF women with gut C. albicans numbers above baseline level after probiotic therapy/FMF women with gut C. albicans numbers above baseline level before probiotic therapy; (D) FMF men with gut C. albicans numbers above baseline level/Healthy men.
The comparison of OTUs of enteric bacteria in C. albicans-carrier and non-carrier female patients revealed a statistically significant increase in OTUs of Klebsiella and Erwinia spp. in C. albicans-carriers (P = 0.011). On the other hand, the diversity of enterobacteria of C. albicans-carrier female patients significantly differed from that of healthy people (Figure 1B). Compared with the healthy women, C. albicans-carriers contain a high OTUs for the genera Escherichia, Salmonella, Shigella, Klebsiella, and Trabulsiella (P < 10−5).
Effects of Probiotic on Gut Enterobacteriaceae
Treatment with probiotic Narine led to a decrease in OTUs of the fecal Enterobacteriaceae, especially in the genera Escherichia and Shigella, in C. albicans-carrier FMF women (P = 0.0036) (Figure 1C).
The placebo led to an increase in hybridization scores for gut Enterobacteriaceae, both in male [6,992 ± 558 (C. albicans non-carriers, placebo group) vs. 5,208 ± 459 (C. albicans non-carriers, control group); P < 0.05 and 5,267 ± 325 (C. albicans-carriers, placebo group) vs. 4,853 ± 378 (C. albicans-carriers, control group); P > 0.05] and female [9,590 ± 794 (C. albicans none-carriers, placebo group) vs. 6,417 ± 347 (C. albicans none-carriers, control group); and 9,780 ± 422 (C. albicans-carriers, placebo group) vs. 8,141 ± 473 (C. albicans-carriers, control group); P < 0.05] FMF patients, while the probiotic Narine decreased these scores for gut Enterobacteriaceae in FMF patients, overall (Table 1).
Culture-Independent Analysis of Lactobacilli in the Gut Microbiota of FMF Patients
A statistically significant decrease in lactobacilli was detected in the gut microbiota of FMF C. albicans-carriers (3,192 ± 107 vs. 3,556 ± 97; P < 0.05 and 2,834 ± 112 vs. 4,112 ± 93) (Table 1). The gut microbiota of FMF patients (both male and female) with C. albicans numbers below baseline levels contained a high OTU numbers of lactobacilli when compared with C. albicans-carriers (P < 10−5) (Figures 2A,D). Both male and female C. albicans-carrier FMF patients were closer to the healthy volunteers in the relative abundance of lactobacilli than to FMF patients overall. FMF patients with C. albicans below baseline levels showed a higher abundance of lactobacilli compared to healthy volunteers and C. albicans-carriers (P < 10−5) (Figures 2C,B).
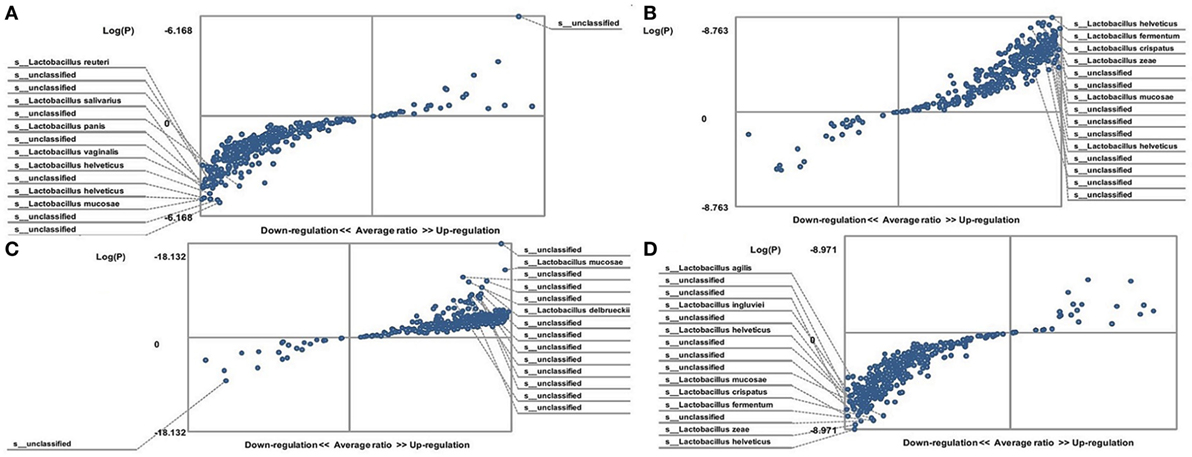
Figure 2. Distribution of gut lactobacilli in Candida albicans-carrier familial Mediterranean fever (FMF) patients. Contribution of variables to differentiation between two groups [X axis, average ratio between two groups; Y axis, −Log(P)]. The upper-right and left-down corner are the most significant. (A) FMF women with gut C. albicans numbers above baseline level/FMF women with C. albicans below baseline level; (B) FMF men with C. albicans below baseline level/healthy men; (C) FMF women with C. albicans below baseline level/healthy women; (D) FMF men with gut C. albicans numbers above baseline level/FMF men with C. albicans below baseline level.
We detected some changes in hybridization scores for a diversity of lactobacilli in FMF patients after taking placebo (4,560 ± 299 vs. 3,556 ± 97, 4,360 ± 173 vs. 3,192 ± 107, 4,978 ± 138 vs. 4,112 ± 93, and 3,813 ± 201 vs. 2,834 ± 112; P < 0.05). Probiotic uptake had no statistically significant effect on the gut lactobacilli of these patients (4,793 ± 289 vs. 4,560 ± 299, 3,834 ± 263 vs. 4,360 ± 273, 4,873 ± 185 vs. 4,978 ± 138, and 3,941 ± 217 vs. 3,813 ± 201; P > 0.05) (Table 1).
Culture-Independent Analysis of Staphylococcus aureus in the Gut Microbiota of FMF Patients
Changes were detected in S. aureus abundance between C. albicans-carrier and non-carrier FMF women. The female FMF cohort with C. albicans numbers above baseline levels had a low abundance of S. aureus (data not shown). There were no statistically significant changes in the abundance of S. aureus between C. albicans-carrier and non-carrier FMF men and between FMF and healthy men (P > 0.05).
Effects of Probiotic on Gut S. aureus
The investigation also explored if there were any differences in the abundance of gut S. aureus in FMF women before and after probiotic administrations. The results showed that the probiotic had no significant effect on S. aureus levels in FMF women (P > 0.05) (data not shown).
Culture-Independent Analysis of Enterococcus faecalis in the Gut Microbiota of FMF Patients
The hybridization scores for E. faecalis indicate differences between both male and female FMF C. albicans-carriers compared with non-carriers (P < 0.05) (Table 1). Despite this, these data show no significant differences in the relative abundance of E. faecalis between female FMF C. albicans-carriers compared with non-carriers (P > 0.08), while male FMF C. albicans-carriers have increased abundance of E. faecalis (P = 0.006) in their gut microbiota compared with that of male patients with C. albicans below baseline levels (Figures 3A,B).
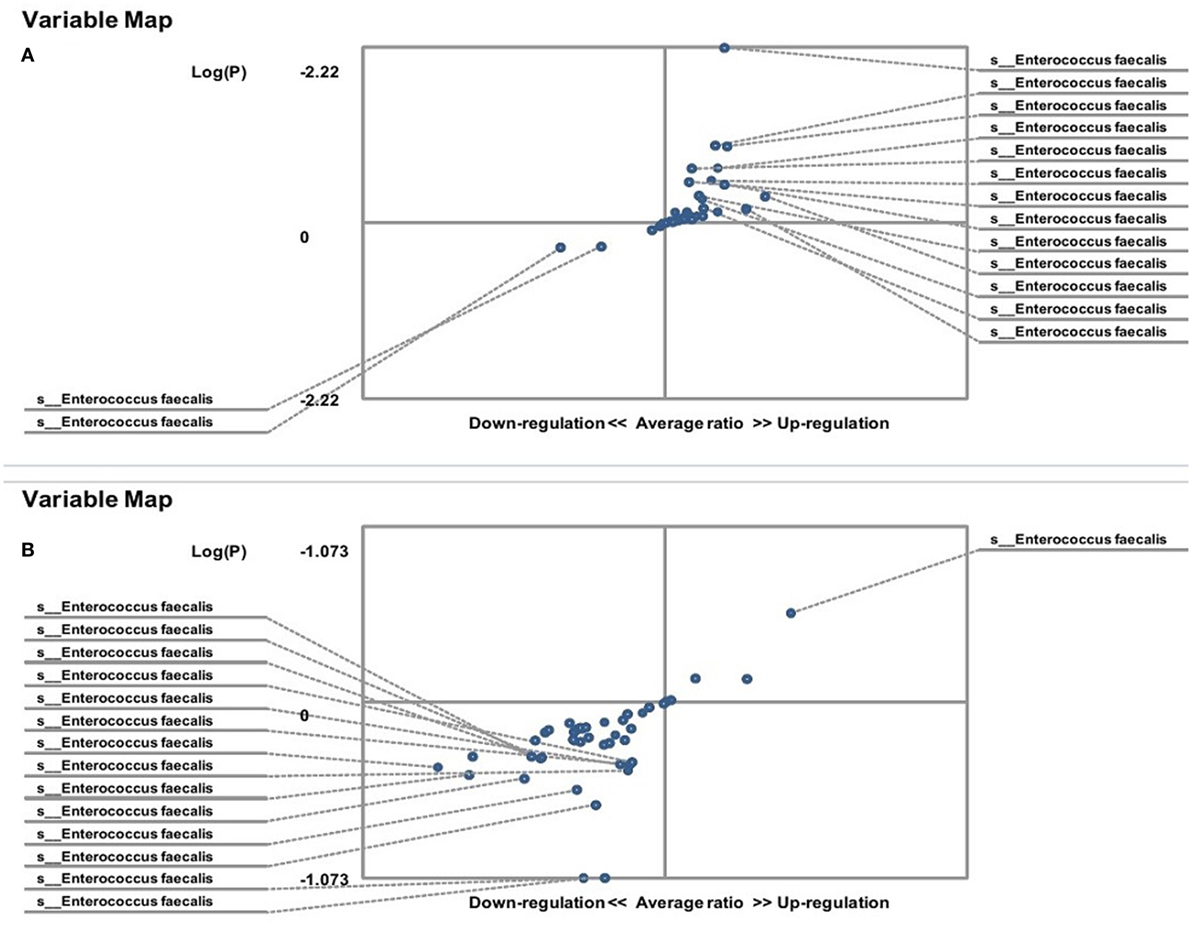
Figure 3. Distribution of gut Enterococcus faecalis in Candida albicans-carrier familial Mediterranean fever (FMF) patients. Contribution of variables to differentiation between two groups [X axis, average ratio between two groups; Y axis, −Log (P)]. The upper-right and left-down corner are the most significant. (A) FMF men with gut C. albicans numbers above baseline level/FMF men with C. albicans below baseline level; (B) FMF women with gut C. albicans numbers above baseline level/FMF women with C. albicans below baseline level.
On the other hand, the results showed that both groups of FMF women, C. albicans-carriers and non-carriers, differ from healthy volunteers in the abundance of E. faecalis in the gut microbiota (P < 0.05) (Figures 4A,B). There were no differences between healthy men and FMF men in terms of E. faecalis abundance in their gut microbiota (P > 0.05) (Figure 5A), while C. albicans-carrier FMF men differed from healthy men volunteers (P < 0.05) (Figure 5C).
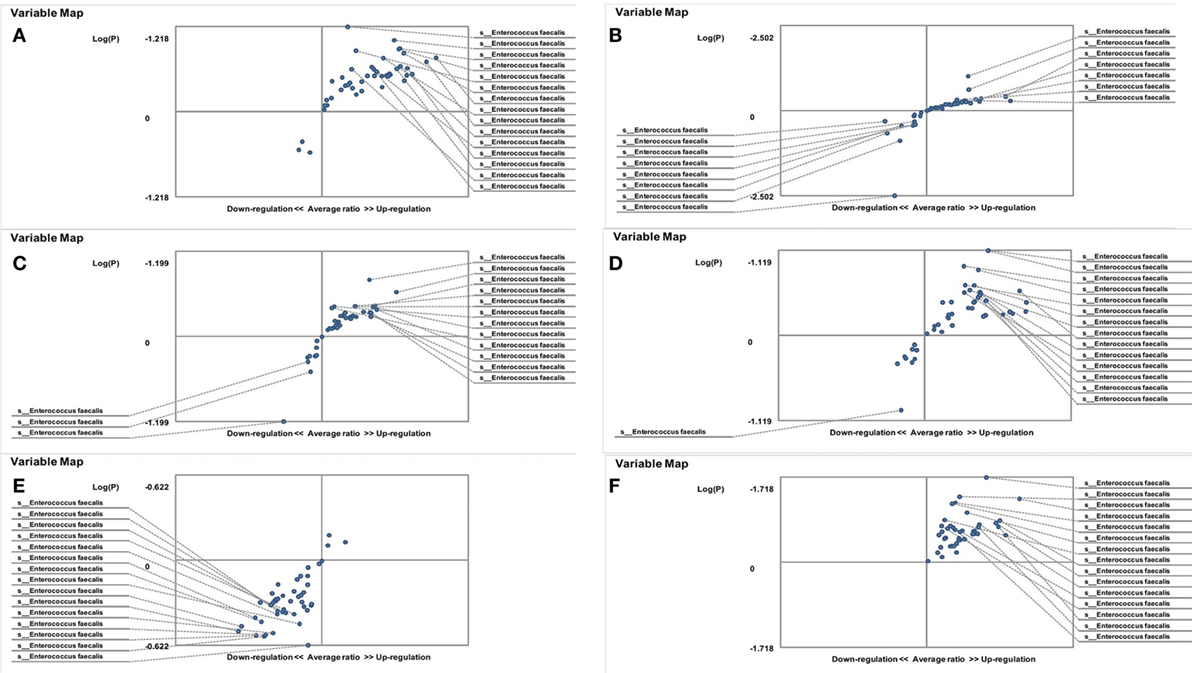
Figure 4. Distribution of gut Enterococcus faecalis in familial Mediterranean fever (FMF) carrier women. Contribution of variables to differentiation between two groups [X axis, average ratio between two groups; Y axis, −Log (P)]. The upper-right and lower-left corner are the most significant. (A) FMF women with Candida albicans below baseline level/healthy women; (B) FMF women with gut C. albicans numbers above baseline level/healthy women; (C) FMF women with C. albicans below baseline level after probiotic therapy/healthy women; (D) FMF women with gut C. albicans numbers above baseline level after probiotic therapy/healthy women; (E) FMF women with C. albicans below baseline level after probiotic therapy/FMF women with C. albicans below baseline level before probiotic therapy; (F) FMF women with gut C. albicans numbers above baseline level after probiotic therapy/FMF women with gut C. albicans numbers above baseline level before probiotic therapy.
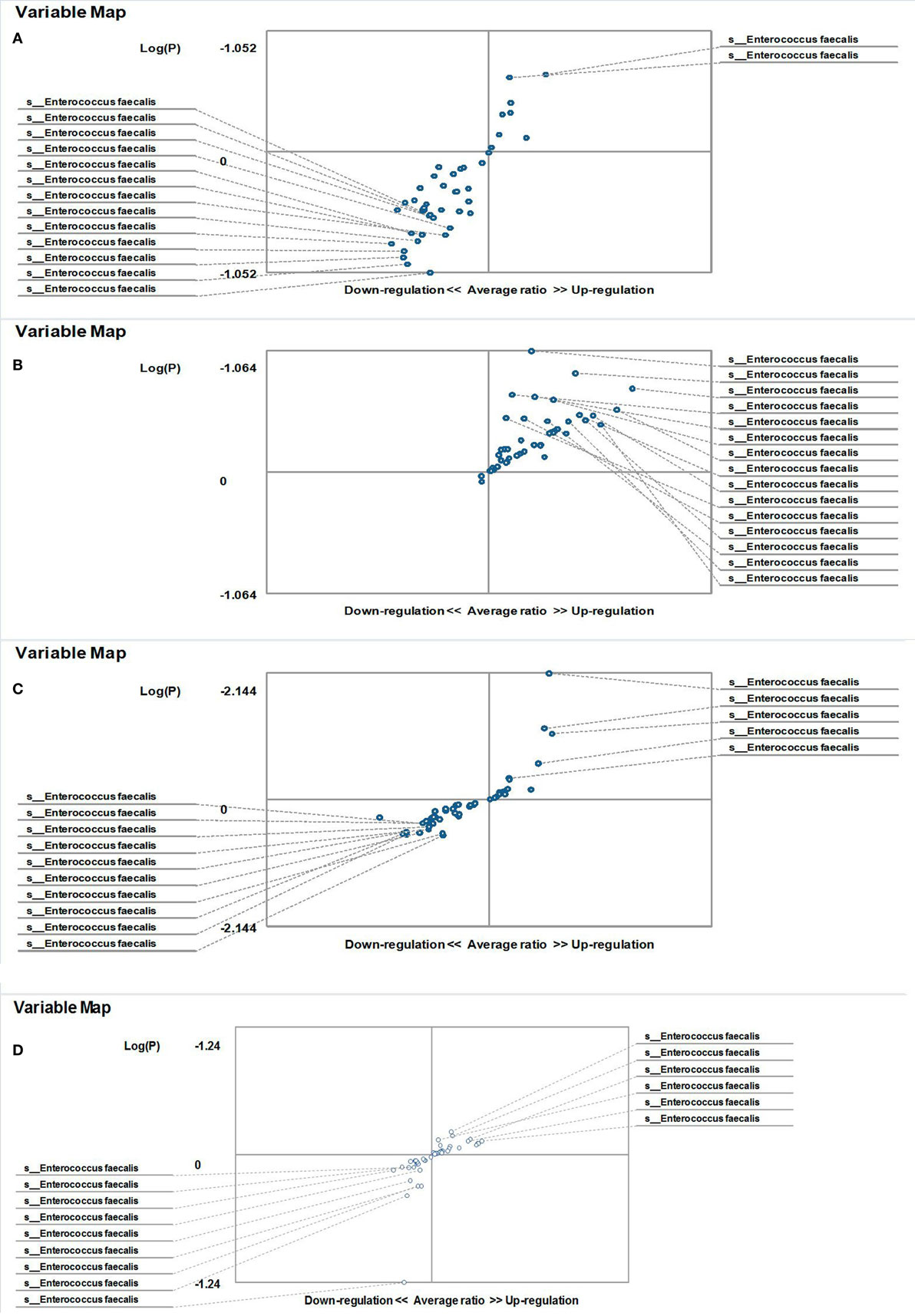
Figure 5. Distribution of gut Enterococcus faecalis in familial Mediterranean fever (FMF) men. Contribution of variables to differentiation between two groups [X axis, average ratio between two groups; Y axis, −Log (P)]. The upper-right and lower-left corner are the most significant. (A) FMF men with Candida albicans below baseline level/healthy men; (B) FMF men with gut C. albicans numbers above baseline level after probiotic therapy/healthy men; (C) FMF men with gut C. albicans numbers above baseline level/Healthy men; (D) FMF men with gut C. albicans numbers above baseline level before probiotic therapy/FMF men with gut C. albicans numbers above baseline level after probiotic therapy.
Effects of Probiotic Administration on Gut Enterococcus faecalis
Probiotic-therapy reduced the number of yeast in C. albicans-carrier women. However, there were no statistically significant differences between C. albicans-carrier/healthy women and C. albicans-non-carrier/healthy women in their gut E. faecalis abundances (P > 0.05) (Figures 4C,D) as well as differences between C. albicans- none carrier women before and after the probiotic administration (P > 0.05) (Figure 4E), in spite of this, there were statistically significant differences between C. albicans-carrier and C. albicans-non-carrier women in their gut E. faecalis abundances (P < 0.05) (Figure 4F). The placebo groups showed increase in hybridization scores of gut E. faecalis both in male (6,374 ± 169 vs. 4,441 ± 128; P < 0.05 and 5,689 ± 109 vs. 4,928 ± 144; P < 0.05) and female (6,534 ± 205 vs. 6,405 ± 157; and 5,070 ± 244 vs. 4,168 ± 213; P < 0.05) FMF patients. Compared to the placebo effect, the statistically significant increase in scores of gut E. faecalis was attributed to male FMF patients not carrying C. albicans treated with the probiotic (7,108 ± 273 vs. 5,689 ± 109; P < 0.05), while the probiotic-therapy decreased E. faecalis scores in C. albicans-carrier and non-carrier female patients (3,771 ± 123 vs. 5,070 ± 244 and 5,602 ± 189 vs. 6,534 ± 205; P < 0.05) (Table 1). After the treatment with commercial probiotic Narine, there were no statistically significant differences between healthy men and FMF men with C. albicans-carriers in gut E. faecalis (Figure 5B). Statistically significant differences between FMF men with C. albicans-carriers before and after the treatment with commercial probiotic Narine were observed (Figure 5D).
Discussion
The differences in the pathogenesis of infectious diseases in male and female patients were previously reported (28), and the significant influences of gender on the development of autoimmune disease have been described (28, 29). It was reported that a female steroid hormone, estrogen, probably playing a role in gut tight junction expression and permeability, might also be the reason for female autoimmune diseases (30). Moreover, the interaction between gender-specific immune differences and the specific immune response to individual microbes was reported previously (31). There are some indications of a possible association between C. albicans infections and gender in the literature as well (32, 33).
According to our investigations, the patients’ gender is one of the main factors affecting the composition of gut microbiota in FMF patients despite the lack of previous report on this difference. The male/female ratio of Armenian FMF patients with MEFV mutation patterns M694V/V726A, according to reported data, is 1.16–1.2:1 (16, 26, 34). In addition, our previous studies revealed gender differences in several blood parameters of FMF patients, such as ESR and CRP (35).
Gender-based differences in the relationship of IL-6 signaling and adrenocorticotropic hormone, which is usually produced in response to biological stress, was reported previously (36), and the relationships between triggering factors and FMF were reported for Armenian patients (37). The experiments included 177 male and 98 female FMF patients and showed that emotional stress was one of the most common triggering factors for FMF attacks with serositis (49.8%) after cold exposure (59.3%) (38). Moreover, the relationships between triggering factors and both M694V (with starvation) and V726A (with long-duration travel) alleles were revealed (38).
An important role for IL-6 in the immune response to E. coli and C. albicans was reported earlier using Il6 null mice (Il6−/−) (39, 40). Thus, Il-6 with its pro- and anti-inflammatory characteristics being responsible for the transition of innate-acquired immunity, could participate in “immune dimorphism” in male and female FMF patients. Generally, there should be differences between the specific bacterial OTUs (Enterobacteriaceae/lactobacilli/E. faecalis/S. aureus) of healthy people and C. albicans-carrier FMF patients, as well as in C. albicans-carrier and non-carrier FMF patients. These differences should be expected if the relationship between the investigated gut bacteria and C. albicans are critical for the yeast’s increased number in the gut microbiota of FMF patients.
According to our investigations, the diversity of enterobacteria in female C. albicans-carrier patients significantly differs from those in healthy participants and from those in female non-carrier patients. In addition, female C. albicans non-carrier patients were having a wider diversity of Enterobacteriaceae compared with the healthy women. We did not observe differences between the Enterobacteriaceae OTUs of healthy men and C. albicans-carrier FMF men despite the fact that probiotic treatment decreased the relative abundance of gut Enterobacteriaceae in C. albicans-carrier FMF men, too.
Possibly, the high diversity of Enterobacteriaceae in the gut microbiota of FMF patients may have an effect on the overgrowth of C. albicans in FMF women. The tendency of the simultaneous “normalization” of OTUs for enteric bacteria and C. albicans in the gut microbiota of female FMF patients after the probiotic Narine treatment supports this hypothesis. However, there was no such tendency detected in FMF male subjects.
Based on the effects of probiotic on the relative abundance of fecal-Enterobacteriaceae, we suggest that mechanisms of probiotic effects on gut C. albicans are different for male and female FMF patients. Similar analysis revealed a potential association between the OTUs of E. faecalis and C. albicans in the gut microbiota of male FMF patients. In contrast to fecal Enterobacteriaceae and E. faecalis OTUs, the gut microbiota of FMF patients (both male and female) with C. albicans below baseline levels contains a relatively high abundance of lactobacilli compared with C. albicans-carriers. However, the probiotic supplementation did not affect the abundance of lactobacilli in C. albicans-carrier FMF women and men. The primary comparative analysis on S. aureus OTUs in C. albicans-carrier/non-carrier FMF and healthy people does not support the hypothesis of potential strong synergistic interaction between S. aureus and C. albicans.
Although the probiotic L. acidophilus INMIA 9602 Er strain 317/402 does not have any effect on C. albicans in vitro (16), the number of FMF patients in remission (both male and female) who carried C. albicans above baseline levels, decreased after probiotic therapy in the double blind, partly randomized, placebo-controlled trial on 48 volunteer patients with FMF in remission. The abovementioned data show that greater relative abundances of enteric bacteria, especially Klebsiella, in C. albicans-carrier female FMF patients and E. faecalis in C. albicans-carrier male FMF patients may contribute to the increase in numbers of C. albicans in FMF patients. The report on antagonistic activity of the probiotic against Klebsiella in vitro supports this hypothesis (16).
Different inflammasomes are shown to be involved in the response to a C. albicans infection, including the NLRP3 inflammasome with a caspase recruitment domain and caspase-1, and the production of interleukin-1 beta (IL-1β) and IL-18 through the NLRP3 inflammasome is crucial for adaptive cellular protection against C. albicans (41). Another inflammasome, NLRC4, involved in the response to C. albicans infection (42), is able to recognize flagellin and components of the bacterial secretion systems providing host defense to a range of pathogens including K. pneumoniae (43) and Yersinia (44). NLRP3 and NLRC4 together allow for the recognition of different danger signals from the same pathogenic infection (45). Wild-type pyrin negatively modulates NLRP3 inflammasome-dependent IL-1β release (46).
Our investigations showed that the pyrin inflammasome mutation M694V/V726A, leading to FMF disease, effects the gut microbiota and contributes to differences between male and female patients. The number of C. albicans-carrier male FMF patients did not differ from the number of female carriers, indicating that there was no direct association between the host’s genetics and C. albicans-carriage. However, the considerable effects of other factors, such as comparatively high concentrations and/or long-term colchicine use (with other environmental factors) may outweigh the effects of the host’s genetic background.
The most effective long-term treatment for FMF is the administration of colchicine (47), which inhibits inflammasome activation within macrophages (48, 49). There is no indication of a different effect of the treatment between male and female patients. Most likely, colchicine inhibits NLRP3 inflammasome-dependent IL-1β release, thereby influencing the overgrowth of C. albicans in the gut microbiota of FMF patients.
Our investigations indicate that the uptake of L. acidophilus INMIA 9,602 Er 317/402 reduces the numbers of yeast in the gut microbiota of FMF patients.
Future microbiological and immunological studies will be aimed at clarification of the functional aspects and the detailed mechanisms of action of Lactobacillus acidophilus INMIA 9,602 Er 317/402 and its impact on human health in relation to FMF. This will assist in designing “individual diets” for FMF patients.
Ethics Statement
This study was performed in accordance with institutional ethical guidelines and was approved by the Ethics Committee at the Ministry of Education and Science of Armenia. All investigated patients gave written informed consent prior to the study.
Author Contributions
All authors have made a substantial, direct, and intellectual contribution to the work and have approved it for publication. AP and TT conceived and designed the experiments. MB, AM, SP, SPet, LG, and VT performed the experiments. AP analyzed the data and wrote the paper. TT, MC, and SK reviewed data analysis and the manuscript. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Piceno for supporting PhyloChip processing, Dr. Dilanyan for providing placebo capsules, and Drs. Mirzabekyan and Mirzoyan for their assistance in sampling and material processing.
Funding
The work was supported by the International Science and Technology Center (Project A-1980 and A-2134) and US Department of Energy’s Global Initiatives for Proliferation Prevention (GIPP) program.
References
1. Erb-Downward JR, Falkowski NR, Mason KL, Muraglia R, Huffnagle GB. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci Rep (2013) 3:2191. doi:10.1038/srep02191
2. Garsin DA, Lorenz MC. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microbes (2013) 13:409–15. doi:10.4161/gmic.26040
3. Pérez JC, Kumamoto CA, Johnson AD. Candida albicans commensalism and pathogenicity are intertwined traits directed by a tightly knit transcriptional regulatory circuit. PLoS Biol (2013) 11(3):e1001510. doi:10.1371/journal.pbio.1001510
4. Levison ME, Pitsakis PG. Susceptibility to experimental Candida albicans urinary tract infection in the rat. J Infect Dis (1987) 155:841–6. doi:10.1093/infdis/155.5.841
5. Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett (2009) 299:1–8. doi:10.1111/j.1574-6968.2009.01668.x
6. Fenini G, Contassot E, French LE. Potential of IL-1, IL-18 and inflammasome inhibition for the treatment of inflammatory skin diseases. Front Pharmacol (2017) 8:278. doi:10.3389/fphar.2017.00278
7. Elinav E, Henao-Mejia J, Flavell R. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol (2013) 6:4–13. doi:10.1038/mi.2012.115
8. Zmora N, Levy M, Pevsner-Fischer M, Elinav E. Inflammasomes and intestinal inflammation. Mucosal Immunol (2017) 10:865–83. doi:10.1038/mi.2017.19
9. Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe (2010) 8:292–300. doi:10.1016/j.chom.2010.08.004
10. Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol (2016) 17:914–21. doi:10.1038/ni.3457
11. Masters SL, Lagou V, Jéru I, Baker PJ, Van Eyck L, Parry DA, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med (2016) 8(332):332ra45. doi:10.1126/scitranslmed.aaf1471
12. Kohl A, Grutter MG. Fire and death: the pyrin domain joins the death-domain superfamily. C R Biol (2004) 327:1077–86. doi:10.1016/j.crvi.2004.08.006
13. Rowczenio D, Stanescu H, Iancu D. Autosomal dominant familial Mediterranean fever in Northern European Caucasians associated with deletion of p.M694 residue – a case series and genetic exploration. Rheumatology (2017) 56:209–13. doi:10.1093/rheumatology/kew058
14. Grandemange S, Aksentijevich I, Jeru I, Gul A, Touitou I. The regulation of MEFV expression and its role in health and familial Mediterranean fever. Genes Immun (2011) 12:497–503. doi:10.1038/gene.2011.53
15. Manukyan GP, Ghazaryan KA, Ktsoyan ZA, Khachatryan ZA, Arakelova KA, Kelly D, et al. Elevated systemic antibodies towards commensal gut microbiota in autoinflammatory condition. PLoS One (2008) 3(9):e3172. doi:10.1371/journal.pone.0003172
16. Harutyunyan N. Effects of Probiotics on Gut Microbiota of Periodic Disease Patients [Ph.D. Dissertation]. Yerevan, Armenia: Scientific and Production Center “Armbiotechnology” NAS RA (2014).
17. Harutyunyan NA, Pepoyan ES, Manvelyan AM, Malkhasyan LM, Pepoyan AZ, Hakopyan GS, et al. Quantitative and qualitative changes of commensal Enterobacteriaceae in gut microflora of patients with familial Mediterranean fever disease. Electron J Nat Sci (2007) 1:41–4.
18. Vieira AT, Teixeira MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol (2013) 4:445. doi:10.3389/fimmu.2013.00445
19. Yerzinkyan LA. Biological Properties of Some Isolates of Lactic Acid Bacteria. Yerevan: Academy of Sciences of Armenia (1971).
20. Mkrtchyan H, Gibbons S, Heidelberger S, Zloh M, Limaki KH. Purification, characterisation and identification of acidocin LCHV, an antimicrobial peptide produced by Lactobacillus acidophilus n.v. Er 317/402 strain Narine. Int J Antimicrob Agents (2010) 35:255–60. doi:10.1016/j.ijantimicag.2009.11.017
21. Harutyunyan KV. Adhesion of Probiotic Lactic Acid Bacteria as a Regulatory Factor in the Normal Gastrointestinal Microbiota. Yerevan, Armenia: Scientific and Production Center “Armbiotechnology” NAS RA (2016).
22. Charchoghlyan H, Kwon H, Hwang D-J, Lee JS, Lee J, Kim M. Inhibition of Cronobacter sakazakii by Lactobacillus acidophilus n.v. Er2 317/402. Korean J Food Sci Anim Resour (2016) 36:635–40. doi:10.5851/kosfa.2016.36.5.635
23. Balayan M, Manvelyan A, Marutyan S, Isajanyan M, Tsaturyan V, Pepoyan A, et al. Impact of Lactobacillus acidophilus INMIA 9602 Er-2 and Escherichia coli M-17 on some clinical blood characteristics of familial Mediterranean fever disease patients from the Armenian cohort. Int J Probiotics Prebiotics (2015) 10:91–5.
24. Pepoyan AZ, Balayan MH, Manvelyan AM, Mamikonyan V, Isajanyan M, Tsaturyan VV, et al. Lactobacillus acidophilus INMIA 9602 Er-2 strain 317/402 probiotic regulates growth of commensal Escherichia coli in gut microbiota of familial Mediterranean fever disease subjects. Lett Appl Microbiol (2017) 64:254–60. doi:10.1111/lam.12722
25. Kellogg CA, Piceno YM, Tom LM, DeSantis TZ, Gray MA, Zawada DG, et al. Comparing bacterial community composition between healthy and white plague-like disease states in Orbicella annularis using PhyloChip™ G3 microarrays. PLoS One (2013) 8(11):e79801. doi:10.1371/journal.pone.0079801
26. Sarkisian T, Ajrapetian H, Beglarian A, Shahsuvarian G, Egiazarian A. Familial Mediterranean fever in Armenian population. Georgian Med News (2008) 156:105–11.
27. Loster JE, Wieczorek A, Loster BW. Correlation between age and gender in Candida species infections of complete denture wearers: a retrospective analysis. Clin Interv Aging (2016) 11:1707–14. doi:10.2147/CIA.S116658
28. Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita (2016) 52:198–204. doi:10.4415/ANN_16_02_11
29. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol (2014) 35:347–69. doi:10.1016/j.yfrne.2014.04.004
30. Zhou Z, Zhang L, Ding M, Luo Z, Yuan S, Bansal MB, et al. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin Immunol (2017) 183:174–80. doi:10.1016/j.clim.2017.08.019
31. McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (2011) 59:203–13. doi:10.1007/s00005-011-0124-3
32. Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev (2000) 24:627–38. doi:10.1016/S0149-7634(00)00027-0
33. Najar MS, Saldanha CL, Banday KA. Approach to urinary tract infections. Indian J Nephrol (2009) 19:129–39. doi:10.4103/0971-4065.59333
34. Cazeneuve C, Sarkisian T, Pêcheux C, Dervichian M, Nédelec B, Reinert P, et al. MEFV analysis in Armenian FMF patients. Am J Hum Genet (1999) 65:88–97. doi:10.1086/302459
35. Pepoyan A, Harutyunyan N, Grigoryan A, Balayan M, Tsaturyan V, Manvelyan A, et al. Some clinical blood characteristics of patients with familial Mediterranean fever disease from an Armenian cohort. Klinicheskaya Laboratornaya Diagnostika (2015) 60:46–7.
36. Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, et al. Sex difference in link between IL-6 and stress. Endocrinology (2007) 148:3758–64. doi:10.1210/en.2006-1650
37. Yenokyan G, Armenian HK. Triggers for attacks in familial Mediterranean fever: application of the case-crossover design. Am J Epidemiol (2012) 15:1054–61. doi:10.1093/aje/kwr460
38. Karadag O, Tufan A, Yazisiz V, Ureten K, Yilmaz S, Cinar M, et al. The factors considered as trigger for the attacks in patients with familial Mediterranean fever. Rheumatol Int (2013) 33:893–7. doi:10.1007/s00296-012-2453-x
39. Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature (1994) 368:339–42. doi:10.1038/368339a0
40. Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun (1997) 65:4843–9.
41. Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence (2015) 6:327–37. doi:10.1080/21505594.2015.1004977
42. Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, et al. A novel role for the NLRC4 inflammasomes in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog (2011) 7(12):e1002379. doi:10.1371/journal.ppat.1002379
43. Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol (2012) 1188:5623–35. doi:10.4049/jimmunol.1200195
44. Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe (2010) 7:376–87. doi:10.1016/j.chom.2010.04.009
45. Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A (2014) 111:7391–6. doi:10.1073/pnas.1403477111
46. Omenetti A, Carta S, Delfino L, Martini A, Gattorno M, Rubartelli A. Increased NLRP3-dependent interleukin 1β secretion in patients with familial Mediterranean fever: correlation with MEFV genotype. Ann Rheum Dis (2014) 73:462–9. doi:10.1136/annrheumdis-2012-202774
47. Kalinich T, Haffer D, Niehues T. Colchicune use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics (2013) 119:474–83. doi:10.1542/peds.2006-1434
48. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature (2006) 440:237–41. doi:10.1038/nature04516
Keywords: familial Mediterranean fever, M694V/V726A mutations, gut microbiota, gender, Candida albicans, Lactobacillus acidophilus INMIA 9602 Er 317/402, Enteroccocus faecalis, Enterobacteriaceae
Citation: Pepoyan A, Balayan M, Manvelyan A, Galstyan L, Pepoyan S, Petrosyan S, Tsaturyan V, Kamiya S, Torok T and Chikindas M (2018) Probiotic Lactobacillus acidophilus Strain INMIA 9602 Er 317/402 Administration Reduces the Numbers of Candida albicans and Abundance of Enterobacteria in the Gut Microbiota of Familial Mediterranean Fever Patients. Front. Immunol. 9:1426. doi: 10.3389/fimmu.2018.01426
Received: 30 November 2017; Accepted: 08 June 2018;
Published: 26 June 2018
Edited by:
Haruki Kitazawa, Tohoku University, JapanReviewed by:
Yimin Miao, College of Staten Island, United StatesMarika Mikelsaar, University of Tartu, Estonia
Copyright: © 2018 Pepoyan, Balayan, Manvelyan, Galstyan, Pepoyan, Petrosyan, Tsaturyan, Kamiya, Torok and Chikindas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Astghik Pepoyan, YXBlcG95YW5AZ21haWwuY29t
 Astghik Pepoyan
Astghik Pepoyan Marine Balayan
Marine Balayan Anahit Manvelyan1
Anahit Manvelyan1 Sofi Pepoyan
Sofi Pepoyan Vardan Tsaturyan
Vardan Tsaturyan Shigeru Kamiya
Shigeru Kamiya Tamas Torok
Tamas Torok Michael Chikindas
Michael Chikindas