- 1Unité de Recherche en Biologie des Microorganismes, Laboratoire d’Immunologie et de Microbiologie, NAmur Research Institute for Life Sciences (NARILIS), Université de Namur, Namur, Belgium
- 2Institute for Medical Immunology, Université Libre de Bruxelles, Brussels, Belgium
- 3Department of Molecular and Cellular Interactions, Vlaams Interuniversitair Instituut voor Biotechnologie, Vrije Universiteit Brussel, Brussels, Belgium
- 4Laboratory of Cellular and Molecular Immunology, GIGA- Research & WELBIO, University of Liège, Liège, Belgium
- 5Immunology Unit, Scientific Institute for Public Health (WIV-ISP), Brussels, Belgium
- 6Laboratory of Neurophysiology, Université Libre de Bruxelles, Brussels, Belgium
- 7Laboratoire de Parasitologie, Faculté de Médecine, Université Libre de Bruxelles, Brussels, Belgium
Allergic asthma is a chronic Th2 inflammatory disease of the lower airways affecting a growing number of people worldwide. The impact of infections and microbiota composition on allergic asthma has been investigated frequently. Until now, however, there have been few attempts to investigate the impact of asthma on the control of infectious microorganisms and the underlying mechanisms. In this work, we characterize the consequences of allergic asthma on intranasal (i.n.) infection by Brucella bacteria in mice. We observed that i.n. sensitization with extracts of the house dust mite Dermatophagoides farinae or the mold Alternaria alternata (Alt) significantly increased the number of Brucella melitensis, Brucella suis, and Brucella abortus in the lungs of infected mice. Microscopic analysis showed dense aggregates of infected cells composed mainly of alveolar macrophages (CD11c+ F4/80+ MHCII+) surrounded by neutrophils (Ly-6G+). Asthma-induced Brucella susceptibility appears to be dependent on CD4+ T cells, the IL-4/STAT6 signaling pathway and IL-10, and is maintained in IL-12- and IFN-γR-deficient mice. The effects of the Alt sensitization protocol were also tested on Streptococcus pneumoniae and Mycobacterium tuberculosis pulmonary infections. Surprisingly, we observed that Alt sensitization strongly increases the survival of S. pneumoniae infected mice by a T cell and STAT6 independent signaling pathway. In contrast, the course of M. tuberculosis infection is not affected in the lungs of sensitized mice. Our work demonstrates that the impact of the same allergic sensitization protocol can be neutral, negative, or positive with regard to the resistance of mice to bacterial infection, depending on the bacterial species.
Introduction
One striking feature of infectious diseases is the marked interindividual variation in susceptibility/resistance. Interestingly, individuals with the highest level of infection are also often the major disseminators (termed super-spreaders) of an epidemic among a population (1). Thus, with a better understanding of the factors predisposing individuals to susceptibility to a particular infectious agent, we may be able to both anticipate and better treat individual infections and more efficiently control the dissemination of infection among populations.
Allergic asthma is one of the most common lung diseases. It affects an estimated 300 million people worldwide (2) and its prevalence continues to increase in many parts of the world (3). It is characterized by recurring symptoms of reversible airflow obstruction, bronchial hyperresponsiveness and lower airway inflammation. The lungs of atopic individuals display increased levels of IL-4-mediated (Th2) inflammation and eosinophilia (4). The impact of infections and host microbiota composition on allergic asthma has been investigated frequently [reviewed in Ref. (5)]. However, despite the significant proportion of people worldwide affected by asthma and though some epidemiological studies (6–8) report increasingly severe bacterial and viral infections in asthma patients, little effort has been made to investigate the impact of asthma on the control of infectious microorganisms and identify the underlying mechanisms.
Brucella (alpha-proteobacteria) are facultative intracellular Gram-negative coccobacilli that infect wild and domestic mammals and cause brucellosis. Human brucellosis is a zoonotic infection transmitted mainly through ingestion and inhalation (9). Airborne transmission is a major cause of outbreaks of human brucellosis in bovine and porcine slaughterhouses, vaccine production laboratories, research laboratories, and rural areas (10–13). Due to its easy aerosolization, high infectivity, and airborne transmission, Brucella species are considered potential biological weapons (14, 15) and are classified as category B bioterrorism agents. Four species of Brucella can cause human disease: Brucella melitensis, Brucella abortus, Brucella suis, and Brucella canis. The vast majority of cases worldwide are attributed to B. melitensis [reviewed in Ref. (16)]. Although it is rarely fatal, Brucella can cause a devastating multi-organ disease in humans with serious health complications in the absence of prolonged antibiotic treatment (16, 17). Despite significant progress, the incidence of human brucellosis remains very high in endemic areas (18) and is considered to be largely underestimated (19, 20). Currently, there are no effective vaccines available to protect humans. All commercially available animal vaccines are live vaccines that would cause disease in humans (21, 22). In a mouse intranasal (i.n.) infection model, we demonstrated previously that IFN-γ-producing CD4+ T cells (Th1) are key actors in the control of Brucella multiplication and persistence in lungs (23).
In the current study, we developed an original experimental model to analyze the impact of allergic asthma sensitization on the course of i.n. Brucella infection in mice. Extracts of the house dust mite Dermatophagoides farinae (HDM) (24) and the mold Alternaria alternata (Alt) (25), which are both recognized as important causes of respiratory allergies, were used to sensitize mice before and during infection. Our results showed that allergic asthma increases the susceptibility of mice to Brucella infection. However, this phenomenon cannot be generalized to other bacterial infections as we observed that the same protocol led to better control of Streptococcus pneumoniae and had no effect on the course of Mycobacterium tuberculosis infections in a murine model.
Materials and Methods
Ethics Statement
The procedures used in this study and the handling of the mice complied with current European legislation (directive 86/609/EEC) and the corresponding Belgian law “Arrêté royal relatif à la protection des animaux d’expérience du 6 avril 2010 publié le 14 mai 2010.” The Animal Welfare Committee of the Université de Namur (UNamur, Belgium) reviewed and approved the complete protocol for Brucella infections (Permit Number: UN-LE-14/220). The Animal Welfare Committee of the Université Libre de Bruxelles (ULB, Belgium) reviewed and approved the complete protocol for S. pneumoniae infections (Permit Number: ULB-IBMM-2016-21-88). The Ethics committee of the WIV-ISP and CODA-CERVA approved the complete protocol for M. tuberculosis infections (ethics agreement number 201405-14-01).
Mice and Reagents
Wild-type BALB/c and C57BL/6 mice were acquired from Harlan (Bicester, UK). STAT6−/− BALB/c mice, IL4−/− BALB/c mice, IL12p40−/− BALB/c mice, TCR-δ−/− C57BL/6 and IL-10GFP transgenic [B6(Cg)-Il10tm1.1Karp/J] C57BL/6 mice were all purchased from The Jackson Laboratory (Bar Harbor, ME, USA). IFN-γR−/− (26) and IL-12p35−/− C57BL/6 mice (27) were acquired from Dr. B. Ryffel (University of Orleans, France). TAP1−/− C57BL/6 mice (28), MHCII−/− C57BL/6 mice (29) were acquired from Jörg Reimann (University of Ulm, Ulm, Germany). IL-10−/− BALB/c mice were obtained from Guillaume Holdenhove (Université Libre de Bruxelles, Gosselies, Belgium). All wild-type and deficient mice used in this study were bred in the animal facility of the Gosselies campus of the Université Libre de Bruxelles (ULB, Belgium). We used wild-type strains of B. melitensis 16M and B. suis 1330. We also used B. melitensis 16M and B. abortus 2308 strains stably expressing a rapidly maturing variant of the red fluorescent protein DsRed (30), the mCherry protein (mCherry-Br), under the control of the strong Brucella spp. promoter, PsojA. Construction of the mCherry-Br strains has been described previously in detail (31). All Brucella were handled under BSL-3 containment according to Council Directive 98/81/EC of 26 October 1998 and a law of the Walloon government of 4 July 2002.
Cultures were grown overnight with shaking at 37°C in 2YT medium (Luria-Bertani brothwith double quantity of yeast extract) and were washed twice in RPMI 1640 (Gibco Laboratories) (3,500 × g, 10 min) before inoculation of the mice.
Allergens and Allergic Asthma Sensitization Protocol
Lyophilized house dust mite D. farinae (abbreviated HDM) extracts were from Greer Laboratories (Lenoir, NC, USA). A. alternata (strain18586) (abbreviated Alt) was obtained from the BCCM™/IHEM (Institute of Public Health, WIV-ISP, Brussels, Belgium) and cultured for 3 weeks at 27°C in flasks containing 250 ml of Czapek medium. Mold pellicles were harvested and homogenized in 0.4% NH4HCO3 + polyvinyl polypyrrolidone (Sigma) with an ultra-thurax. The homogenates were then shaken for 3 h at 4°C. Extracts were centrifuged twice for 30 min at 20,000 g, dialyzed against phosphate-buffered saline (PBS) and stored at −20°C in 50% glycerol.
For asthma sensitization, mice were lightly anesthetized with isoflurane [from Abbott laboratories (# No. B506)]. Once the mice were unresponsive but breathing comfortably, a solution of HDM (100 µg of D. farinae extract in 50 µl of PBS) or Alt (5 µg of A. alternata extract in 100 µl of PBS) was applied directly to the nostrils. The animals were allowed to slowly inhale the liquid and were then allowed to recover in a supine position. For the HDM model, mice were instilled intranasally (i.n.) once per week throughout the experiment (32) and for the Alt model mice received the extract twice per week throughout the experiment (25). Mice were infected 17 days after the first instillation.
Brucella Infection
Mice were anesthetized with a cocktail of Xylasine (9 mg/kg) and Ketamine (36 mg/kg) in PBS before being inoculated i.n. with 2 × 103 or 2 × 104 CFU of B. melitensis, as indicated, 2 × 103 CFU of B. abortus, or 2 × 103 CFU of B. suis. We used wild-type or mCherry-expressing Brucella in 30 µl of PBS [described in Ref. (31)]. Control animals were inoculated with the same volume of PBS. The infectious doses were validated by plating serial dilutions of the inoculums. At the selected time after infection, mice were sacrificed by cervical dislocation. Immediately after sacrifice, spleen, liver, and lung cells were collected for bacterial count, flow cytometry, and/or microscopic analyses. All infections were performed in an Animal Biosafety Level 3 facility.
For bacterial counting, organs were crushed and transferred to PBS/0.1% X-100 Triton (Sigma-Aldrich). We performed successive serial dilutions in RPMI to obtain the most accurate bacterial count and plated them on 2YT medium. The CFU were counted after 5 days of culture at 37°C.
S. pneumoniae Infection
Streptococcus pneumoniae serotype 1 (clinical isolate E1586) were grown in Todd Hewitt Yeast Broth (Sigma-Aldrich) as described previously (33). For infection, frozen working stocks were washed and diluted in PBS. Mice were anesthetized by intraperitoneal injection of ketamine–xylazine and 20 µl of the lethal inoculum (2 × 107 CFU) were administered by i.n. route. Mouse survival was recorded every 24 h.
M. tuberculosis Infection
BALB/c mice were infected with 50–100 CFU of virulent M. tuberculosis H37Rv using a nose-only inhalation exposure system (CH Technologies, Inc. Westwood, NJ, USA). The M. tuberculosis H37Rv strain used was grown for 2 weeks as a surface pellicle on Sauton medium and stored frozen in aliquots at −80°C. For bacterial counting, serial threefold total lung homogenate dilutions were plated on 7H11 Middlebrook agar supplemented with oleic acid-albumin-dextrose-catalase. Colonies were counted visually after 4 weeks. CFU counts obtained from two or three dilutions were used to calculate the total number of CFU/lung/mouse. Data are expressed as log10 CFU per organ per mouse. All M. tuberculosis infections were performed in a BSL3 facility at the Scientific Institute of Public Health (WIV-ISP) according to rules established by the ethics committee of the WIV-ISP and CODA-CERVA (ethics agreement number 201405-14-01).
Bronchoalveolar Lavages (BALs)
Mice were euthanized by cervical dislocation. The trachea was exposed and incised. A needle (1.2 mm × 40 mm) was inserted into the trachea and bronchoalveolar fluid was harvested by washing the lungs twice with 1 ml of PBS. Total cell counts were determined with a hemacytometer. Differential cell counts were obtained by counting at least 500 cells on cytospin slides stained with Diff-Quick (Dade Behring).
Determination of Serum Levels of Total IgE
Serum IgE levels in sera were determined using a sandwich ELISA. Plates were coated with a rat antimouse IgE mAb (LO-ME-2, IMEX, UCL, Brussels, Belgium) and saturated. Serial twofold dilutions of serum or purified monoclonal mouse IgE (LB-4, IMEX, UCL, Brussels, Belgium) were applied for 2 h. Then, peroxidase labeled rat antimouse IgE (LO-ME-3) was used. After 1 h of incubation at room temperature (RT), plates were washed four times in PBS, and 100 µl substrate solution (BD OptEiA; BD Biosciences) was added to each well. After 10 min of incubation at RT in the dark, the enzyme reaction was stopped by adding 25 μl/well 2 N H2SO4, and absorbance was measured at 450 nm.
Quantitative PCR for IL-4
Total RNA was extracted from homogenized lung cells with Tri-reagent (Sigma-Aldrich), according to the manufacturer’s instructions 24 h after the last instillation. cDNA was synthesized using the Promega GoScript Reverse Transcription System. RT-qPCR was performed on a Stratagene Mx 3000P using a qPCR Go Taq master mix with Bryt Green (Promega). Each RT-qPCR amplification was performed in duplicate under the following conditions: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The forward and reverse primers used for IL-4 were 5-GTGCAGCTTATCGATGAATCC-3′ and 5-AGCCATATCCACGGATGCGAC-3, Hydroxymethylbilane synthase (HmBS, forward 5′-GAAACTCTGCTTCGCTGCATT-3′, reverse 5′-TGCCCATCTTTCATCACTGTATG-3′) mRNA was used as the reference housekeeping gene for normalization. For each sample (x), the normalization factor was calculated using the formula, mean of Ct of ref(i) − Ct of ref(x), where i represents all samples (Ct is on the threshold cycle-value shown as the mean of three different RT-qPCR reactions), as described in Ref. (34). The level of target mRNA, relative to the mean of the reference housekeeping gene, was calculated by raising 2 to the power of {40 − [Ct of target + mean of (Ct of norm. HmBS)]}.
Determination of Arginase Activity
Arginase activity was determined by measuring the total production of urea from lung homogenates. Individual lungs were crushed and transferred to 1 ml of PBS. The homogenates were centrifuged, and urea levels were determined in the supernatant using an Arginase Activity Assay kit (Sigma MAK112). Arginase activity is expressed as units per liter of sample, where 1 unit of arginase converts 1 μmole of l-arginine to ornithine and urea per min at pH 9.5 and 37°C.
Arginase Inhibition
Mice were injected i.n. with 200 µg of nor-NOHA (N-hydroxy-nor-l-arginine, Enzolifesciences) in 50 µl of PBS on days 8, 15, 18, 21, 24, and 27 post first mold instillation. In order to verify the effectiveness of the inhibitor, we measured the arginase activity using the Arginase Activity Assay kit (Sigma MAK112) on naive and asthmatic lung extracts in the presence or absence of nor-NOHA (2 mg/ml) during the arginase reaction.
Cytofluorometric Analysis
As described previously (23), lungs were harvested, cut into small pieces, and incubated for 1 h at 37°C with a mix of DNAse I fraction IX (Sigma-Aldrich) (100 µg/ml) and 1.6 mg/ml of collagenase (400 Mandl U/ml). Lung cells were washed and filtered, and then incubated with saturating doses of purified 2.4G2 (anti-mouse Fc receptor, ATCC) in 200 µl PBS, 0.2% BSA, 0.02% NaN3 (FACS buffer) for 20 min at 4°C to prevent antibody (Ab) binding to the Fc receptor. Various fluorescent mAb combinations in FACS buffer were used to stain 3–5 × 106 cells. We acquired the following mAbs from BD Biosciences: Fluorescein (FITC)-coupled HL3 (anti-CD11c), FITC-coupled 145-2C11 (anti-CD3ε), FITC-coupled M1/70 (anti-CD11b), FITC-coupled 1A8 (anti-Ly6G), phycoerythrin (PE)-coupled RM4-5 (anti-CD4), PE-coupled 53-6.7 (anti-CD8α), PE-coupled PK136 (anti-NK1.1), PE-coupled GL3 (anti–TCR-γ/δ), PE-coupled E50-2440 (anti-SIGLEC-F), allophycocyanin (APC)-coupled BM8 (anti-F4/80), biotin-coupled M5/114.15.2 (anti-MHCII, I-A/I-E), and biotin-coupled 307707 (anti-CD101). Incubation with a streptavidin-coupled APC for 30 min was necessary for the biotin-coupled Ab. The cells were analyzed on a FACScalibur cytofluorometer. Dead cells and debris were eliminated from the analysis according to size and scatter.
Immunofluorescence Microscopy
Lungs were fixed for 2 h at RT in 2% paraformaldehyde (pH 7.4), washed in PBS, and incubated overnight at RT in a 20% PBS-sucrose solution under a vacuum. Tissues were then embedded in the Tissue-Tek OCT compound (Sakura), frozen in liquid nitrogen, and cryostat sections (5 µm) were prepared. For staining, tissue sections were rehydrated in PBS and incubated in a PBS solution containing 1% blocking reagent (Boeringer) (PBS-BR 1%) for 20 min before incubation overnight in PBS-BR 1% containing any of the following mAbs or reagents: DAPI nucleic acid stain Alexa Fluor 350, 488 phalloidin (Molecular Probes), biotin-coupled 1A8 (anti-Ly6G), biotin-coupled 53-2.1 (anti-CD90.2), biotin-coupled polyclonal anti mouse Major Binding Protein (MBP, MyBioSource), Alexa Fluor® 647-coupled M5/114.15.2 (anti-I-A/I-E, MHCII), APC-coupled BM8 (anti-F4/80, Abcam), Alexa Fluor 647-coupled HL3 (anti-CD11c, BD Biosciences). Incubation with a streptavidin-coupled APC for 2 h was necessary for the biotin-coupled Ab. iNOS and arginase-1 were detected using IgG H-52 (anti-Arg1, Santa Cruz Biotechnology) and rabbit polyclonal anti-NOS2 Ab (Calbiochem), respectively and were stained with a secondary Ab, Alexa Fluor 647-coupled goat anti-rabbit IgG (Molecular Probes). Slides were mounted in Fluoro-Gel medium (Electron Microscopy Sciences, Hatfield, PA, USA). Labeled tissue sections were visualized with an Axiovert M200 inverted microscope (Zeiss, Iena, Germany) equipped with a high-resolution monochrome camera (AxioCam HR, Zeiss). Images (1,384 × 1,036 pixels, 0.16 μm/pixel) were acquired sequentially for each fluorochrome with A-Plan 10×/0.25 N.A. and LD-Plan-NeoFluar 63×/0.75 N.A. dry objectives and recorded as eight-bit gray-level *.zvi files. At least three slides were analyzed per organ from three different animals and the results are representative of two independent experiments.
Confocal Microscopy
Confocal analyses were performed using the LSM780 confocal system fitted on an Observer Z 1 inverted microscope equipped with an alpha Plan Apochromat 63×/1.46 NA oil immersion objective (Zeiss, Iena, Germany). Hoechst/DAPI was excited using a 405 nm blue diode, and emission was detected using a band-pass filter (410–480 nm). The 488 nm excitation wavelength of the Argon/2 laser was used in combination with a band-pass emission filter (BP500–535 nm) to detect Alexa Fluor 488 phalloidin. The 543 nm excitation wavelength of the HeNe1 laser, and a band-pass emission filter (BP580–640 nm) were used for the red fluorochrome mCherry. The 633 excitation wavelength of the HeNe2 laser, and a band-pass emission filter (BP660–695 nm) were used for far-red fluorochromes such as APC. To ensure optimal separation of the fluorochromes, blue and red signals were acquired simultaneously in one track and green and far-red signals were acquired in a second track. The electronic zoom factor and stack depth were adjusted to the region of interest while keeping image scaling constant (x–y: 0.066 µm, z: 0.287 µm). A line average of 4 was used and datasets were stored as 8-bit proprietary *.czi files. The images were displayed using Zen2012 software (Zeiss) with linear manual contrast adjustment and exported as 8-bit uncompressed *.TIF images. The Figures, representing single optical sections across the region of interest, were prepared using the Canvas program.
Hematoxylin-Eosin-Safran (HES) Histology
Lungs were fixed in 10% formalin. After fixation overnight, the lungs were embedded in paraffin. Tissues were sliced and 5 µm sections were stained with HES for light microscopy examination of the lung inflammation.
Statistical Analysis
We used a (Wilcoxon-)Mann–Whitney test provided by the GraphPad Prism software to statistically analyze our results. Each group of deficient mice was compared to the wild-type mice. We also compared each group with the others and displayed the results when required. Values of p < 0.05 were considered to represent a significant difference. *, **, *** denote p < 0.05, p < 0.01, p < 0.001, respectively.
Results
Allergic Asthma Favors the Multiplication of Brucella in the Lungs
Repeated intranasal (i.n.) sensitization with an extract from the house dust mite D. farinae (HDM) (24) or an extract of the mold A. alternata (Alt) (25) has been described to induce allergic asthma in murine models. Under our experimental conditions, BALB/c mice sensitized during 17 days according to standard protocols (see Materials and Methods) display readily detectable cellular infiltration in the lungs (Figure S1A in Supplementary Material). BALs showed an increased number of lymphocytes, macrophages, neutrophils, and eosinophils (Figure S1B in Supplementary Material). As expected, this phenomenon was associated with increased IL-4 mRNA expression in the lungs (Figure S1C in Supplementary Material) and increased total IgE in the blood (Figure S1D in Supplementary Material). Alt induced stronger cellular recruitment in the lungs, but closely similar IL-4 and IgE levels compared to HDM.
IFN-γ producing CD4+ T (Th1) cells play a key role in the control of B. melitensis infection (23). As chronic IL-4-dominated Th2 response induced by helminthes is well known to negatively affect the Th1 response (35–41), we compared the ability of groups of mice subjected to i.n. sensitization with PBS (cont), HDM, or Alt for 17 days to control i.n. B. melitensis infection. We chose a dose of Brucella infection similar to that used in our previous characterization of the B. melitensis intranasal infection model (23): 2 × 104 CFU, as described in the Section “Materials and Methods.” This was a compromise to limit the individual variability resulting from the use of a low dose of infection (102–103 CFU) and the pulmonary inflammation resulting from a high dose of infection (106–107 CFU). It is important to note that the sensitization treatments were continued throughout the course of infection in all of our experiments to mimic chronic asthma. We observed that HDM and Alt sensitization negatively affect the control of B. melitensis infection in the lungs but have a weak or no effect in the spleen and liver (Figure 1A). Asthmatic mice display strongly enhanced bacterial loads in the lungs, with mean bacteria CFU/gr that were 416 times higher in the HDM group and 26,302 times higher in the Alt group compared to the control group at 12 days post infection.
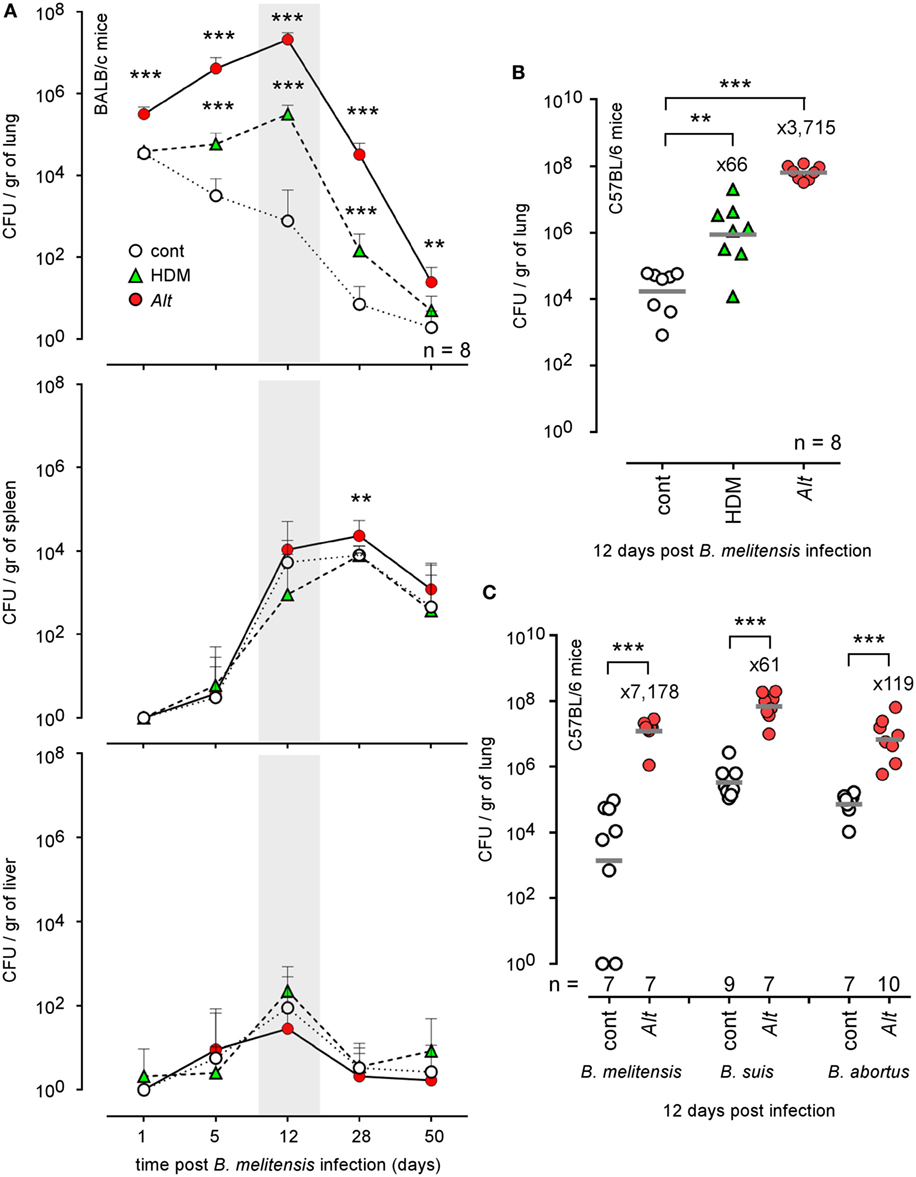
Figure 1. Impact of allergic asthma sensitization on the course of Brucella infection in wild-type mice. Wild-type BALB/c and C57BL/6 mice received repeated i.n. administration of phosphate-buffered saline, HDM, or Alt before i.n. infection with 2 × 104 CFU of mCherry-Brucella melitensis (A,B) or 2 × 103 CFU of B. melitensis, B. abortus, or Brucella suis (C), as indicated in the figure and in the Section “Materials and Methods.” The mice were sacrificed at the selected time post infection. The data represent the number of CFU/gr of lung, spleen, and liver. n denotes the number of mice used for each lineage. These results are representative of at least two independent experiments. **p < 0.01, ***p < 0.001.
Genetic background is important to the Th1/Th2 balance in mice. C57BL/6 mice preferentially mount a Th1 response with high IFN-γ and low IL-4, whereas that in BALB/c mice is a Th2 response with low IFN-γ and high IL-4 in vitro (42) and in vivo (43). Thus, C57BL/6 is regarded as a prototypic Th1-biased mouse strain. Despite this Th1 bias, C57BL/6 mice sensitized with HDM or Alt also display enhanced bacterial growth in the lungs (Figure 1B), demonstrating that this phenomenon is not restricted to Th2-biased BALB/c mice. Finally, to generalize our observation, we also tested the impact of Alt sensitization on the ability of BALB/c mice to control B. suis and B. abortus infection, the two other classical Brucella species causing human brucellosis. At 12 days post infection, we observed that asthmatic mice display enhanced levels of both Brucella strain in the lungs (Figure 1C). Note that we used a lower dose (2 × 103 CFU) of B. melitensis, B. suis, and B. abortus to infect the mice, because B. suis and B. abortus already display a higher level of growth and persistence in the lungs of control mice compared to B. melitensis.
Using mCherry-expressing B. melitensis (31), we performed a microscopic fluorescent analysis to identify infected cells in lung tissues from control HDM- and Alt-sensitized BALB/c mice. Infected lungs from asthmatic mice display large and dense aggregates of Ly-6G+ cells, presumably neutrophils, surrounding highly infected cells (Figures 2A,B). These aggregates are not observed in the other groups of mice. Due to a low CFU level, infected cells could not be detected in the control mice.
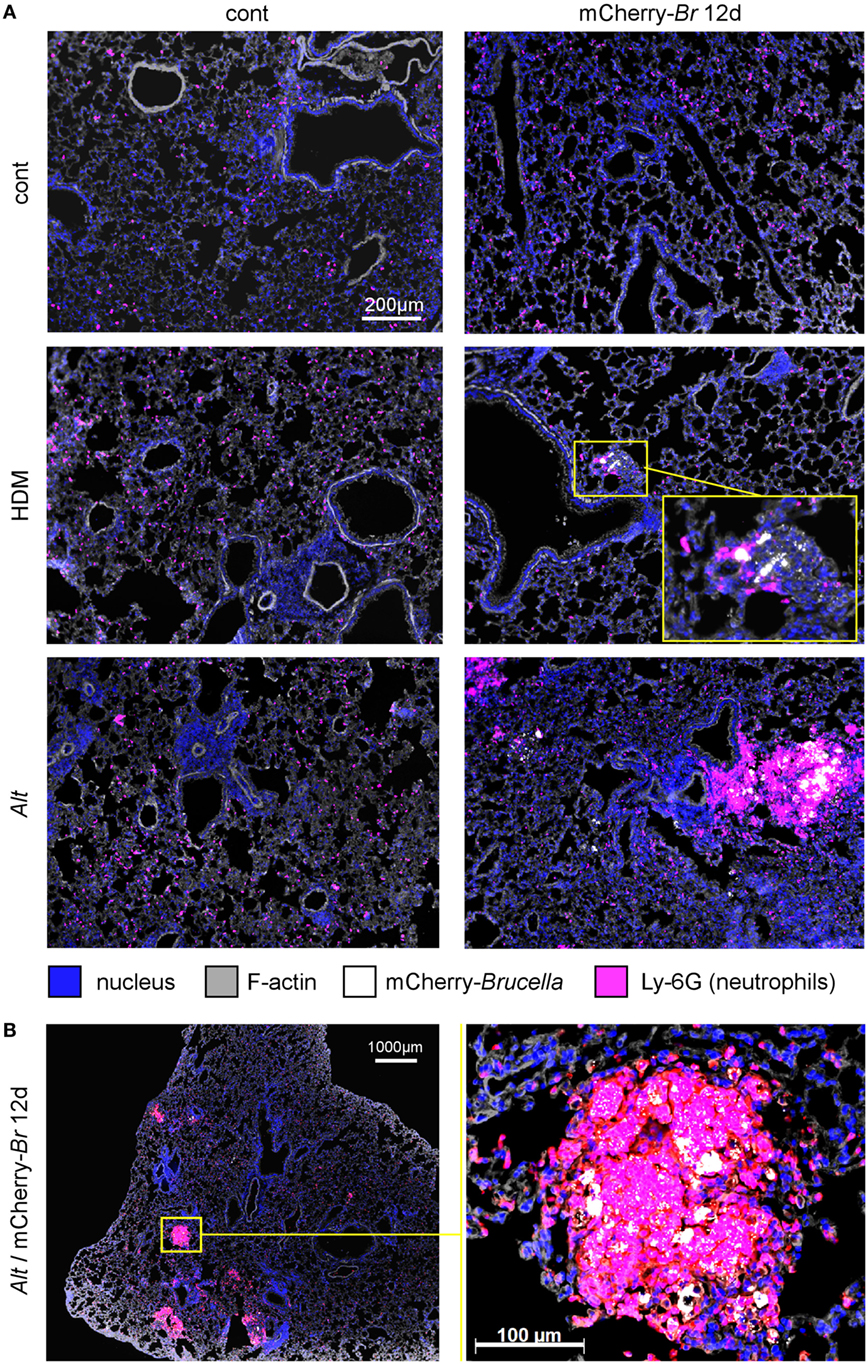
Figure 2. Microscopic analysis of lungs from control, HDM and Alt sensitized BALB/c mice. Wild-type BALB/c mice received repeated i.n. administration of phosphate-buffered saline (PBS) or Alt before i.n. inoculation of PBS or 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection. The lungs were harvested and fixed. Frozen sections were examined by immunohistofluorescence for bacteria (mCherry signal) and Ly-6G-expressing cells. (A) Comparison of control (cont), HDM, and Alt sensitized mice, infected or not. (B) High magnification representative view from Alt sensitized infected mice. The panels are color-coded by Ag as indicated. The data are representative of two independent experiments.
In asthmatic mice, mCherry+ infected cells are heterogeneous in phenotype and morphology (Figure 3A). A large fraction of the mCherry signal colocalizes with CD11c, F4/80, MHCII, and Ly-6G markers but rarely with major basic protein (MBP) or CD90 (Figure 3B), suggesting that infected cells are predominantly composed of alveolar macrophages (CD11c+ F4/80+ MHCII+) and neutrophils (Ly-6G+), but not of eosinophils (MBP+) or T cells (CD90+). The more highly infected cells are large cells with a typical alveolar macrophage morphology and colocalizing with the CD11c, F4/80, and MHCII staining. In contrast, the infected cells are small cells displaying fewer bacteria and are mainly observed in dense aggregates surrounding highly infected alveolar macrophages (Figure 2B). Infection of the Ly-6G+ neutrophils (Figure 4A) and CD11c+ alveolar macrophages (Figure 4B) was confirmed by confocal microscopy. We also observed the presence of highly infected multinucleated giant cells in the infected asthmatic mice (Figure 4C). These cells express low levels of CD11c, suggesting that they could result from the fusion of infected alveolar macrophages.
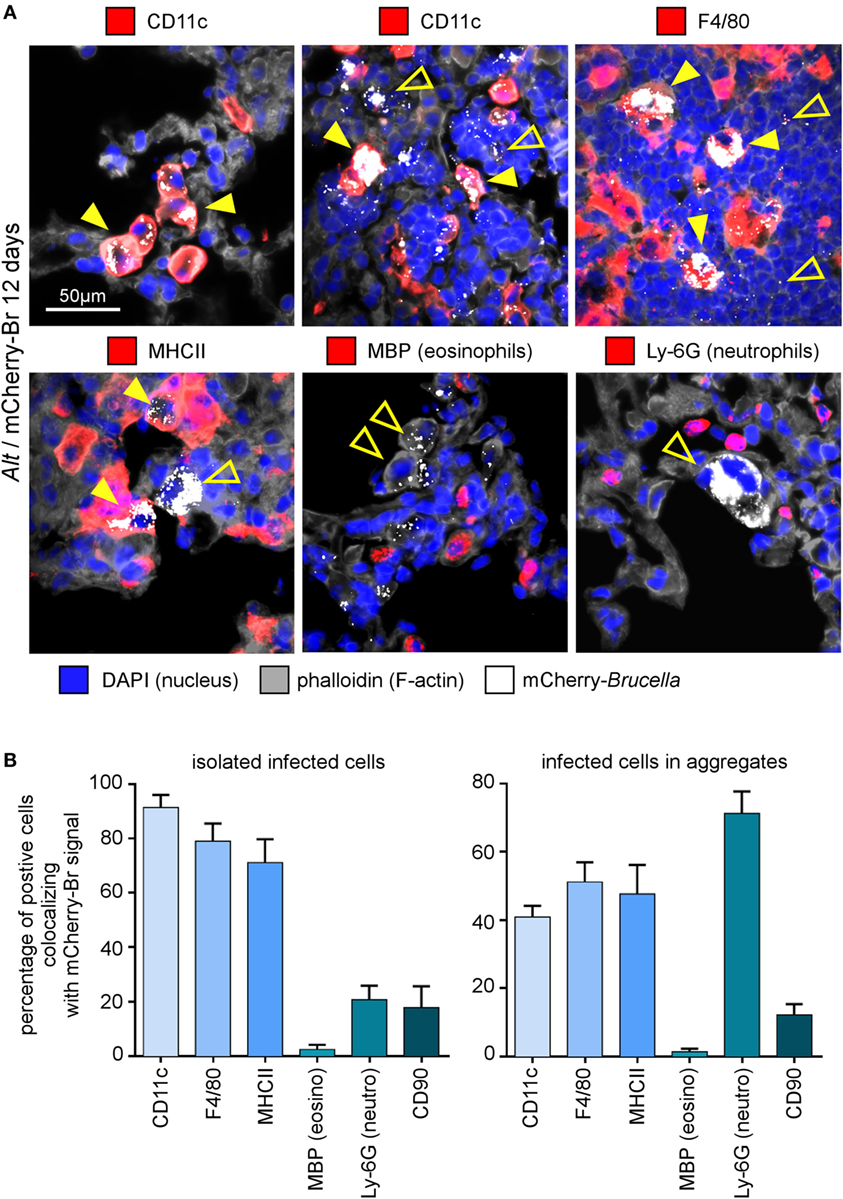
Figure 3. Cell surface phenotype of Brucella melitensis infected cells in asthmatic lungs. Wild-type BALB/c mice received repeated i.n. administration of Alt before i.n. infection with 2 × 104 CFU of mCherry-B. melitensis. The mice were sacrificed at 12 days post infection. The lungs were harvested and fixed. Frozen sections were examined by immunohistofluorescence for mCherry (Brucella), CD11c, F4/80, MHCII, major basic protein (MBP), Ly-6G, and CD90 signals. The panels are color-coded by antigen as indicated. (A) High magnification representative view of infected cells in the lungs. (B) The data represent the percentage of mCherry-Br signal that co-localizes with CD11c, F4/80, MHCII, MBP, Ly-6G, and CD90 markers. When >12 infected cells are observed in the same observation field (approximately 700 μm × 500 μm), they are considered as “aggregated,” and when <12 are observed, they are considered as “isolated.” At least 200 infected cells from three different infected mice were analyzed for each staining. These data are taken from two independent experiments.
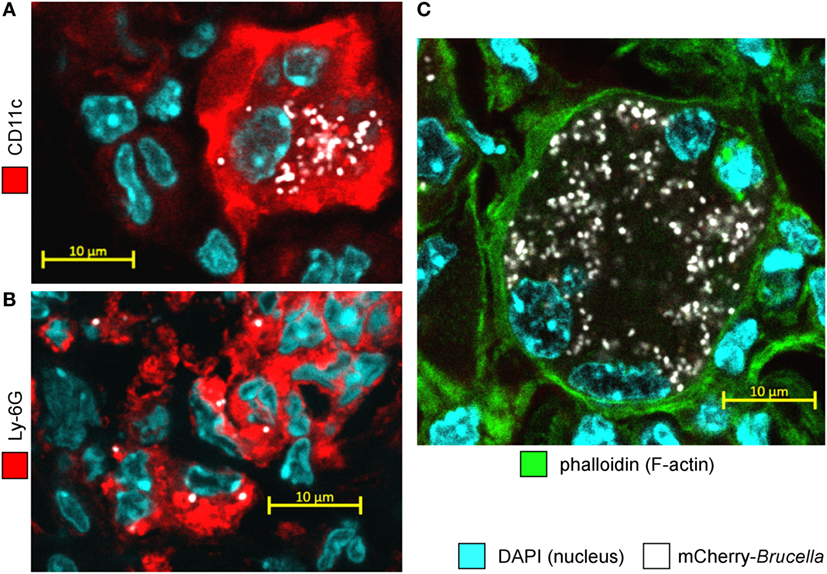
Figure 4. Alveolar macrophages and neutrophils are both infected with Brucella melitensis in the lungs of Alt-sensitized BALB/c mice. Wild-type BALB/c mice received repeated i.n. administration of Alt before i.n. infection with 2 × 104 CFU of mCherry-B. melitensis. The mice were sacrificed at 12 days post infection. The lungs were harvested, fixed, and frozen sections were immunostained and examined by confocal microscopy. (A,B) Representative confocal images of single optical sections of mCherry-Br infected CD11c+ cells and Ly-6G+ cells stained with DAPI from Alt sensitized wild-type BALB/c mice. (C) Representative infected multinucleated giant cells stained with DAPI and phalloidin. The panels are color-coded for DAPI, phalloidin, the cell surface antigen examined or mCherry-Brucella. Scale bars = 10 µm, as indicated.
Allergic Asthma-Induced Susceptibility to Brucella Is Dependent on the IL-4/STAT6 Signaling Pathway and CD4+ T Cells
Mice deficient for IL-4, IL-13, or STAT6 develop attenuation of certain features of asthma, including eosinophil recruitment and airway hyperresponsiveness (44, 45). However, DNA microarray profile analysis of wild-type and STAT6−/− asthmatic mice showed that a large portion of the transcriptional asthma signature is STAT6 independent (46). Comparative analysis of wild-type and STAT6−/− asthmatic lungs showed that sensitized STAT6−/− mice displayed reduced IgE levels in the blood (Figure S2A in Supplementary Material) and reduced infiltration of lymphocytes and eosinophils in the lung airways (Figure S2B in Supplementary Material). In contrast, macrophage and neutrophil infiltration remains much higher in sensitized STAT6−/− mice than in control STAT6−/− mice. As neutrophils have been reported to exert a suppressive effect on the protective response against Brucella (47), we thought first to determine whether enhanced susceptibility to B. melitensis lung infection induced by HDM and Alt treatment is dependent or independent of the IL-4/STAT6 signaling pathway. We, therefore, compared the impact of HDM and Alt sensitization on B. melitensis infection in wild-type, IL-4−/− and STAT6−/− BALB/c mice. We observed that neutralization of the IL-4/STAT6 signaling pathway completely abrogates the enhanced susceptibility to B. melitensis infection due to both HDM and Alt sensitization (Figure 5), thus demonstrating that enhanced susceptibility to Brucella observed in asthmatic wild-type mice is strictly dependent on the Th2 response. Note that we have previously published (48) that the absence of STAT-6 signaling does not affect the course of B. melitensis infection in non-asthmatic BALB/c mice.
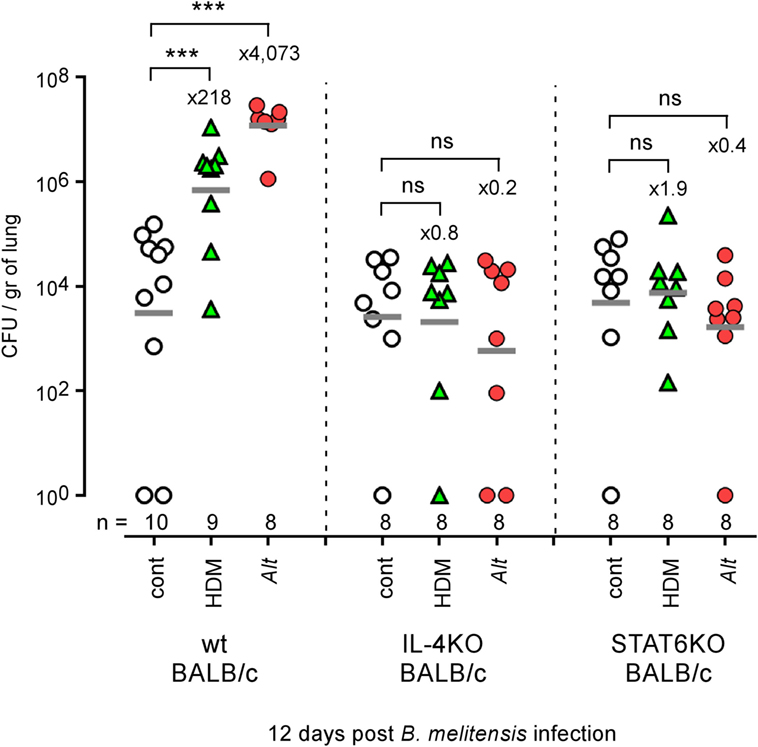
Figure 5. Impact of IL-4 and STAT6 deficiency on asthma-induced Brucella susceptibility in BALB/c mice. Wild-type, IL4−/− and STAT6−/− BALB/c mice received repeated i.n. administration of phosphate-buffered saline, HDM, or Alt before i.n. infection with 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection. The data represent the number of CFU/gr of lung at 12 days post infection from each group. n denotes the number of mice used for each lineage. These results are representative of at least two independent experiments. ***p < 0.001.
As both αβ+CD4+ T cells (4) and γδ+ T cells (49) have been implicated in the development of allergic asthma in mice models, we next compared the ability of wild-type, γδ−/−, TAP1−/− (CD8+ T cells deficient), and MHCII−/− (CD4+ T cells deficient) C57BL/6 mice sensitized with Alt to control i.n. B. melitensis infection. Flow cytometry analysis (Figure 6A) showed that CD4+ T cell deficiency completely abrogated inflammatory eosinophil recruitment [SIGLEC-F+ CD11c− CD101+ cells (50)] in infected asthmatic mice. The absence of γδ+ T cells and CD8+ T cells also significantly reduces eosinophil recruitment, though more moderately. However, we observed that only CD4+ T cell deficiency impaired Alt-induced susceptibility to Brucella infection significantly (Figure 6B).
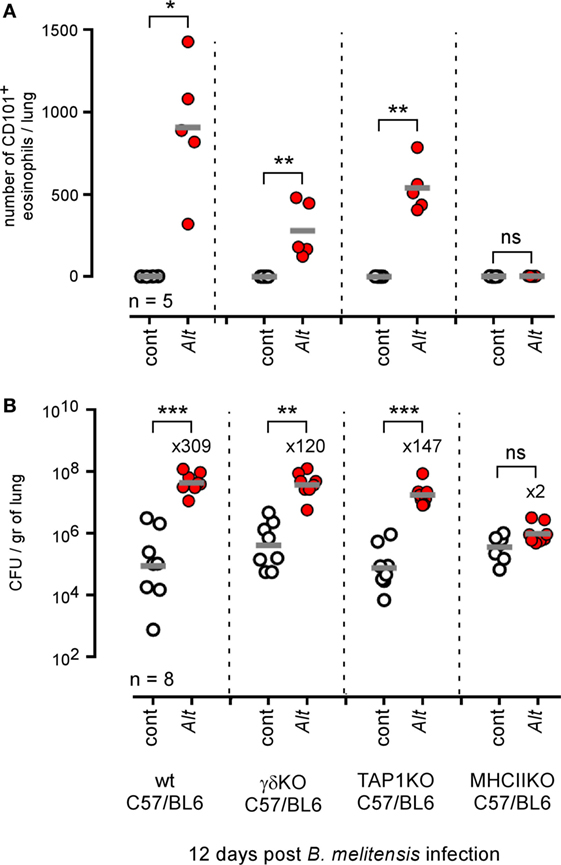
Figure 6. Impact of T lymphocyte deficiency on Alt-induced Brucella susceptibility. Wild-type, γδTCR−/−, TAP1−/−, and MHCII−/− C57BL/6 mice received repeated i.n. administration of phosphate-buffered saline or Alt before i.n. infection with 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection. (A) The data represent the number of eosinophils recruited (SIGLEC-F+ CD11c− CD101+) × 105 per lung from each group as determined by flow cytometry. (B) The data represent the number of CFU/gr of lung at 12 days post infection from each group. Horizontal gray lines represent the medians. n denotes the number of mice used for each lineage. These results are representative of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Allergic Asthma-Induced Susceptibility to Brucella Is not Associated With Preferential Multiplication in M2 Macrophages and Is Independent of Arginase-1 Activity
Allergic asthma is associated with increased polarization of alveolar macrophages toward an M2 phenotype characterized by high arginase-1 expression levels [reviewed in Ref. (51)]. It has been demonstrated that, in vitro, B. abortus multiply more actively in arginase-1+ M2 macrophages than in iNOS+ M1 macrophages (52), and we have reported that B. melitensis splenic reservoir cells from infected IL-12−/− mice express high levels of arginase-1 (48). Thus, we tried to determine whether asthma-induced susceptibility to Brucella infection could be the consequence of the preferential invasion by Brucella of more abundant M2 macrophages present in asthmatic lungs. As expected, Alt sensitization significantly increases arginase activity in the lungs (Figure 7A) and microscopic analysis showed that infected asthmatic mice display an increase number of arginase-1+ cells (Figure 7B). However, these mice also display much higher numbers of iNOS+ cells (Figure 7B) compared to infected control mice. Microscopic analysis showed that infected cells colocalize with arginase-1 and iNOS staining at an almost equivalent frequency (Figure 7C), demonstrating that Brucella multiplication is no more associated with the M1 profile than with the M2 profile in our experimental model. We also observed that neutralization of arginase activity by repeated administration of nor-NOHA, a specific arginase inhibitor known to have the capacity to attenuate allergic airway inflammation (53) and neutralize arginase activity of asthmatic lungs in vitro (Figure S3 in Supplementary Material), does not reduce susceptibility to Brucella infection induced by Alt-sensitization (Figure 7D). On the whole, these data suggest that asthma-induced susceptibility to Brucella is not dependent on preferential invasion of M2 macrophages or arginase activity.
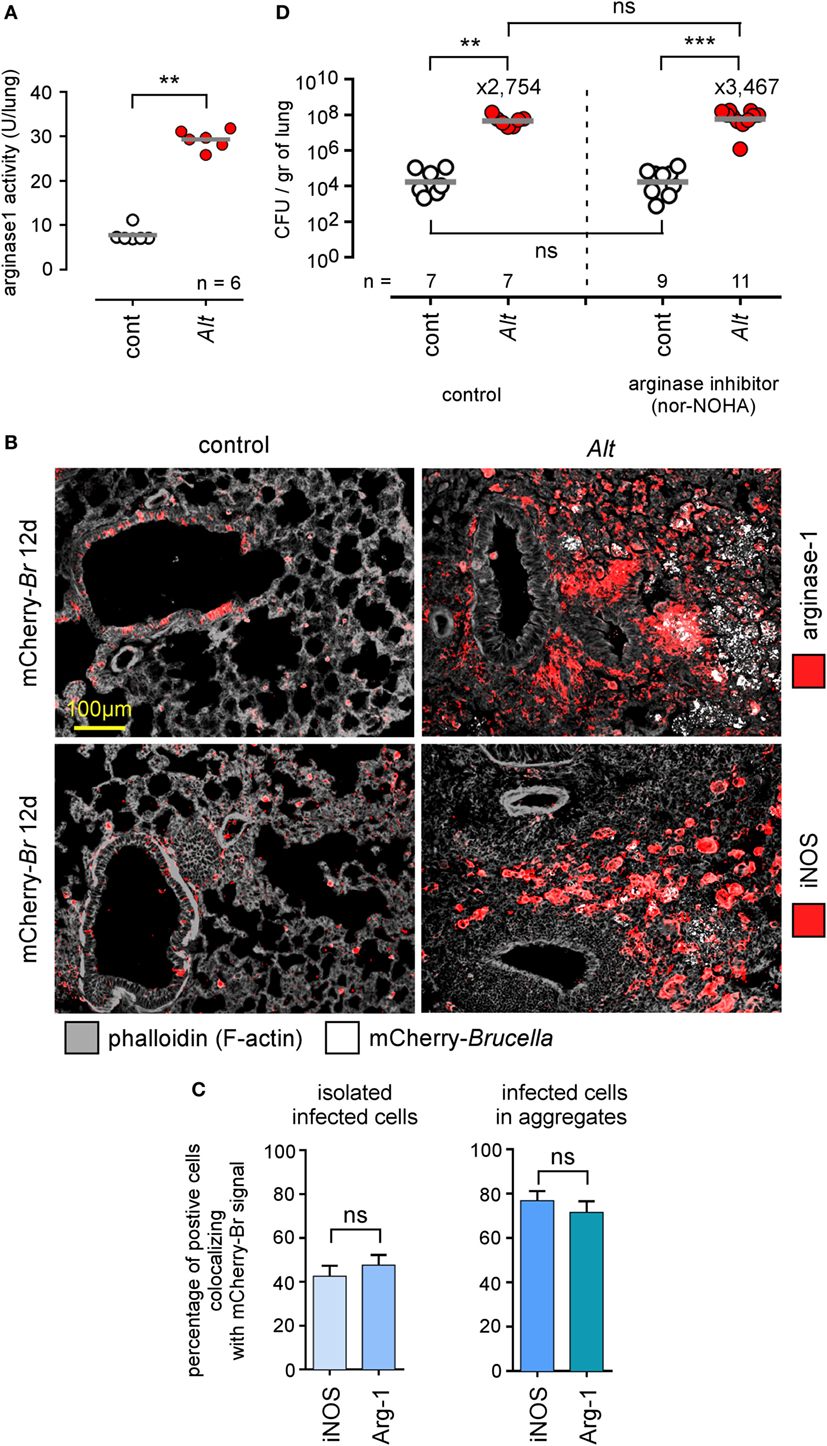
Figure 7. Asthma-induced Brucella susceptibility is independent of arginase activity. Wild-type BALB/c mice received repeated i.n. administration of phosphate-buffered saline (PBS) (control) or Alt before i.n. administration of PBS or 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection. The lungs were harvested and fixed. Frozen sections were examined by immunohistofluorescence for mCherry (Brucella), arginase-1 and iNOS signals. During the sensitization and infection process, the mice received repeated administration of PBS (control group) or the arginase inhibitor nor-NOHA. (A) The panel represents the arginase activity/lung homogenate from control and Alt sensitized wild-type BALB/c mice. (B) Representative view of mCherry+, arginase+, and iNOS+ cells in the lungs of control and Alt sensitized mice. The panels are color-coded by antigen as indicated. (C) The data represent the percentage of mCherry-Br signal that co-localizes with arginase-1 and iNOS staining. When >12 infected cells are observed in the same observation field (approximately 700 μm × 500 μm), they are considered as “aggregated,” and when <12 are observed, they are considered as “isolated.” At least 200 infected cells from three different infected mice were analyzed for each staining. (D) The panel represents the number of CFU/gr of lung at 12 days post infection from each group of mice. Horizontal gray lines represent the medians. n denotes the number of mice used for each lineage. These results are representative of at least two independent experiments. **p < 0.01, ***p < 0.001.
Allergic Asthma-Induced Susceptibility to Brucella Is Partially Dependent on IL-10 but Independent of IL-12
IL-10 is an anti-inflammatory cytokine notably produced by T cells (54) and B cells (55) during the asthma reaction. It is a master negative regulator of the Th1 response (56, 57) able to reduce the protective immune response against Brucella (58). We compared the ability of HDM and Alt sensitized wild-type, IL-10−/−, and IL-12p40−/− BALB/c mice to control B. melitensis infection. While IL-10 deficiency in BALB/c mice strongly reduces asthma-induced susceptibility (158- to 1.7-fold increase for HDM and 1,148- to 75-fold increase for Alt), this is not the case of IL-12p40 deficiency (158- to 1,324-fold increase for HDM and 1,148- to 74,131-fold increase for Alt) (Figure 8A). A similar result was observed in C57BL/6 mice, thus demonstrating that this result is not dependent on the mouse strain used. The absence of IL12p35 and IFN-γR in C57BL/6 mice does not reduce (204- to 812-fold increase) or affects weakly (204- to 89-fold increase) the impact of asthma sensitization on Brucella levels, respectively (Figure 8B). Flow cytometry analysis of lung cells from control, HDM, and Alt sensitized infected transgenic mice expressing GFP under the control of the IL-10 promoter (IL-10GFP mice) showed that IL-10 is mainly produced by B cells in control mice and by B cells, CD4+ T cells, and to a lesser extent eosinophils, CD8+ T and NK1.1+ cells following asthma sensitization (Figure 9). Unfortunately, alveolar macrophages display high levels of autofluorescence in the GFP channel (59), thus rendering analysis of their IL-10 expression in IL-10GFP mice impossible. Taken together, these results suggest that IL-10 is a major mediator of HDM and Alt-induced susceptibility to B. melitensis lung infection, but that this phenomenon is independent of neutralization of the IL-12-dependent Th1 signaling pathway.
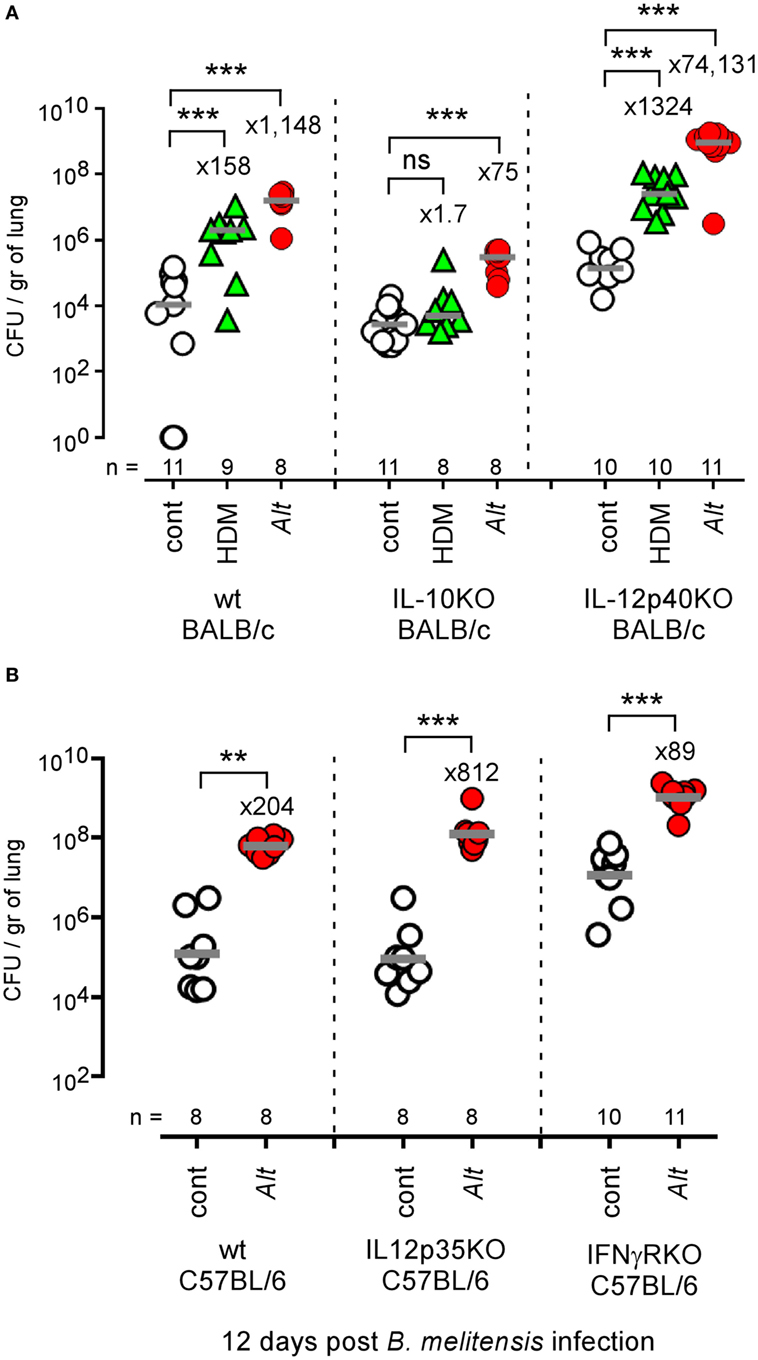
Figure 8. Impact of IL-10 and IL-12 deficiency on asthma-induced Brucella susceptibility. (A) Wild-type, IL-10−/−, and IL-12p40−/− BALB/c mice received repeated i.n. administration of phosphate-buffered saline (PBS) or Alt before i.n. infection with 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection. The data represent the CFUs per gram of lung. (B) Wild-type, IL-12p35−/−, and IFN-γR−/− C57BL/6 mice received repeated i.n. administration of PBS or Alt before i.n. infection with 2 × 104 CFU of mCherry-B. melitensis. The mice were sacrificed at 12 days post infection. The data represent the CFU/gr of lung. Horizontal gray lines represent the medians. n denotes the number of mice used for each lineage. These results are representative of at least three independent experiments. **p < 0.01, ***p < 0.001.
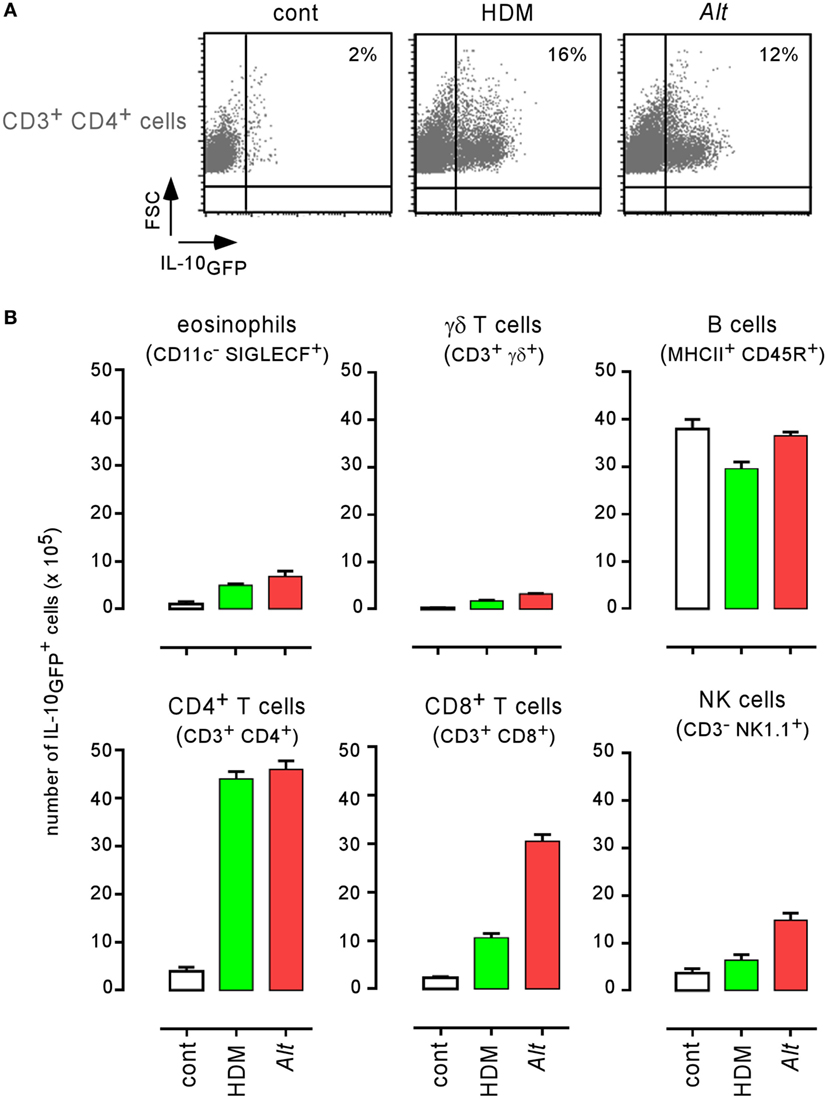
Figure 9. Characterization of IL-10 producing cells in lungs from infected asthmatic mice. IL-10GFP transgenic C57BL/6 mice were sensitized with phosphate-buffered saline (PBS) (control group), HDM, or Alt before i.n. infection with 2 × 104 CFU of Brucella melitensis. The mice were sacrificed at 12 days post infection, and the spleen cells were analyzed by flow cytometry. (A) Cells from PBS, HDM, and Alt infected mice were analyzed for IL-10GFP, CD3, and CD4 expression. The figure shows representative dot plots from individual lungs in each group, as indicated in the figure. Numbers represent the percentage of IL-10GFP+ cells in 50,000 acquired events. (B) The data represent the number of IL-10GFP+ eosinophils, γδ+ T cells, B cells, CD4+ T cells, CD8+ T cells, and natural killer cells per 105 lung cells from control, HDM, and Alt infected mice. The data are representative of two independent experiments.
Allergic Asthma Does not Affect Protective Immune Memory Against Brucella
As both HDM and Alt sensitization strongly favor the growth of B. melitensis in the lungs, we tried to determine whether they also affect the development of protective memory. We compared the ability of groups of wild-type and STAT-6−/− BALB/c mice sensitized with PBS, HDM, or Alt during the course of a primary i.n. Brucella infection to control a secondary i.n. infection. As described in Figure 10A, mice were sensitized for 17 days with PBS (control), HDM, or Alt. before receiving 2 × 104 CFU of wild-type B. melitensis i.n. Fifty days later, the mice were challenged with mCherry-expressing B. melitensis and sacrificed 28 days post challenge. Note that the sensitization treatment was continued during both primary infection and secondary infection in order to mimic chronic asthma inflammation. As illustrated in Figures 10B,C, we observed that all groups of wild-type and STAT-6−/− BALB/c mice, whether sensitized or not, displayed a similar ability to control secondary Brucella infection in the spleen or lungs, suggesting that chronic asthma does not significantly affect the development of protective memory against i.n. B. melitensis infection.
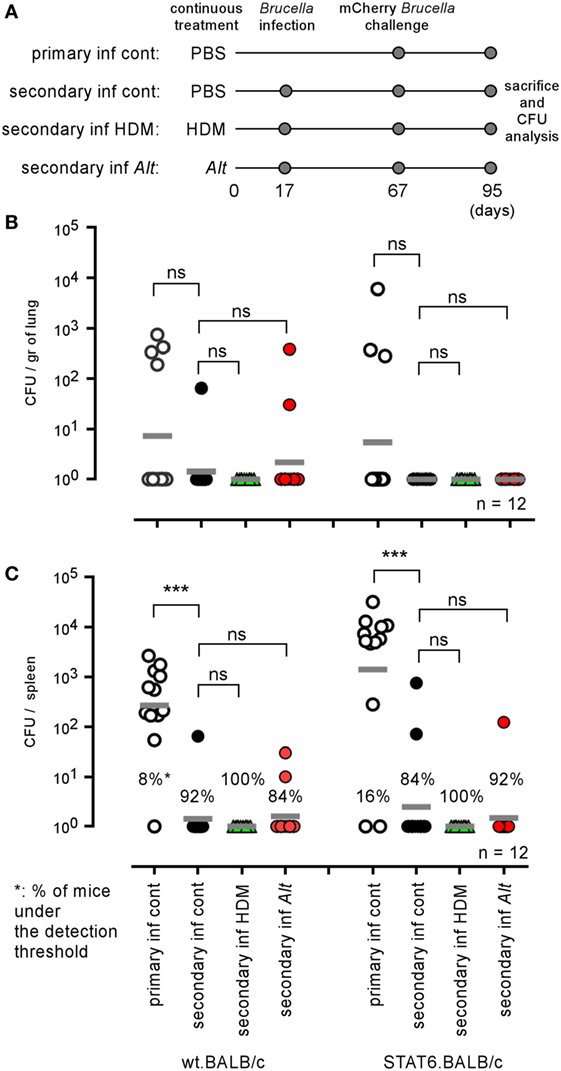
Figure 10. Influence of allergic asthma on the development of protective memory against Brucella melitensis. Wild-type and STAT-6−/− BALB/c mice were sensitized with phosphate-buffered saline (PBS), HDM, or Alt extracts, as described in the Section “Materials and Methods,” throughout the entire experiment. Primary and secondary groups received i.n. administration of PBS or 2 × 104 CFU of wild-type B. melitensis at 17 days, respectively. All groups were infected i.n. with 2 × 104 CFU of mCherry-B. melitensis at 67 days, were sacrificed at 95 days, and the spleens were harvested and the CFUs were counted. (A) Is a schematic representation of the protocol. (B,C) Represent the number of CFU/gr of lung (B) and spleen (C). Horizontal gray lines represent the medians. n denotes the number of mice used for each lineage. These results are representative of at least two independent experiments. ***p < 0.001.
Allergic Asthma Increases Resistance to S. pneumoniae Infection in Mice
As the Alt sensitization protocol strongly increases the susceptibility of mice to pulmonary Brucella infection, we tried to determine whether this effect could be generalized to other lung infections with extracellular or intracellular bacteria. Therefore, we analyzed the impact of Alt sensitization on S. pneumoniae and M. tuberculosis infection in mice, two common serious human pulmonary pathogens.
Control and Alt sensitized wild-type C57BL/6 (Figures 11A,B) and BALB/c (Figure 11C) mice were infected i.n. with 2 × 107 CFU of S. pneumoniae. This bacterial dose is 95–100% lethal in control C57BL/6 and BALB/c mice, but appears to be well tolerated by asthmatic mice. Only 10–15% of the latter died after 10 days of infection. As expected, asthma-induced resistance is associated to a drastic reduction of CFU count in lung and spleen 48 h post infection (Figure 11B). Surprisingly, asthma-induced resistance to S. pneumoniae is also observed in sensitized CD3−/− C57BL/6 mice (Figure 11A) and STAT-6−/− BALB/c mice (Figure 11C), demonstrating that the protective effect of asthma is independent of Th2 CD4+ T cells.
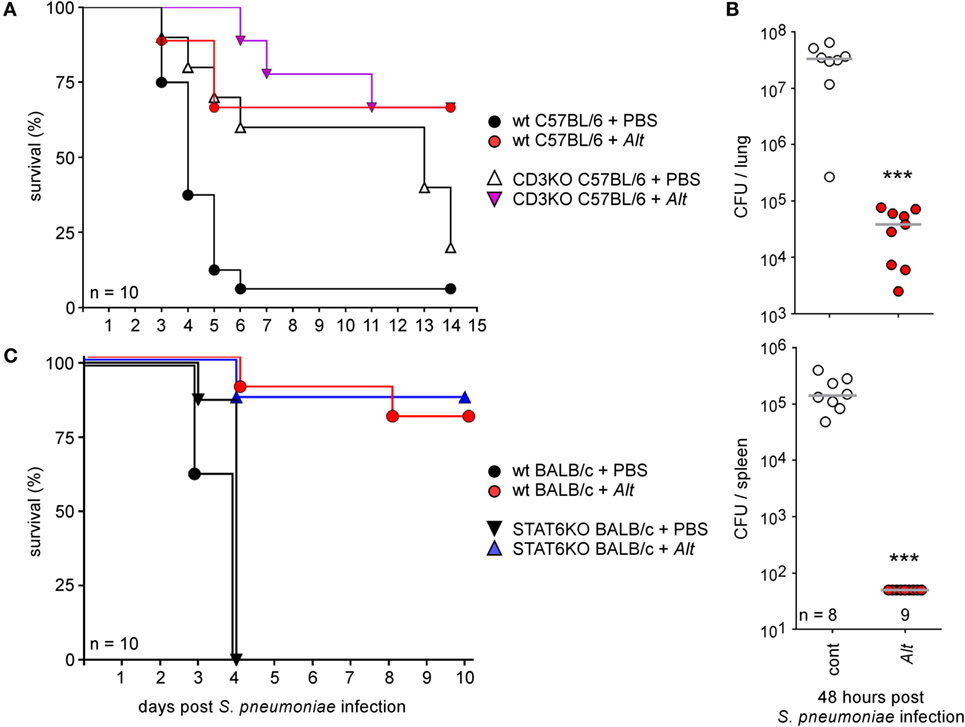
Figure 11. Asthma sensitization increases resistance against intranasal Streptococcus pneumoniae infection. Wild-type and CD3−/− C57BL/6 (A,B) and wild-type and STAT-6−/− BALB/c mice (C) were sensitized with phosphate-buffered saline (cont) or Alt before i.n. infection with a lethal CFU dose (2 × 107 CFU) of S. pneumonia, as indicated in the legend of each graph. (A,C) The data shown are the percentage of mice surviving infection at a given time. (B) The data represent the number of CFU per lung or spleen, as indicated. n indicates the number of mice per group. These results are representative of at least two independent experiments.
Control and Alt sensitized wild-type BALB/c mice (Figure 12) were also infected by aerosol with 100 CFU of M. tuberculosis. We observed that control and asthmatic mice display similar CFU counts in the lungs at 12 and 28 days post infection, suggesting that Alt sensitization does not significantly affect the course of M. tuberculosis infection in the lungs.
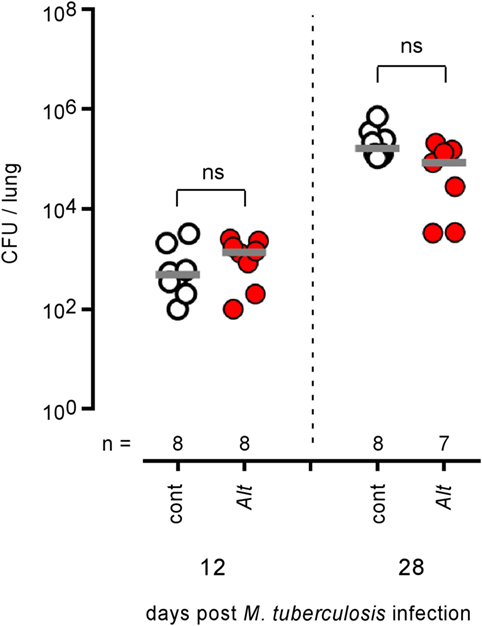
Figure 12. Asthma sensitization does not affect the course of Mycobacterium tuberculosis infection in the lungs of mice. Wild-type BALB/c mice were sensitized with phosphate-buffered saline (cont) or Alt before aerosol infection with 102 CFU of M. tuberculosis. Mice were sacrificed at the indicated time post M. tuberculosis infection. The data show the CFU/lung. n indicates the number of mice per group. These results are representative of at least two independent experiments.
On the whole, these results demonstrate that the same asthma sensitization protocol can affect the ability of the immune system to control lung bacterial infections in very different and unpredictable ways.
Discussion
Allergic asthma is one of the most common chronic inflammatory lung diseases affecting humans (2, 3). The risk factors for developing asthma are a combination of genetic predisposition along with environmental exposure to inhaled substances. The most common indoor allergens are derived from dust mites, mammals (including wild rodents and pets), and fungi (60). Infection also plays a well-documented role in the development of asthma (5). Conversely, the impact of asthma as a predisposing or aggravating condition to infection has been documented [for a review, see Ref. (61)] but has rarely been clearly addressed experimentally. Allergic asthma is dominated by the IL-4 (Th2) immune response that is well known since the pioneering work of Mossman and Coffman (35) to counter-regulate the IFN-γ dominated (Th1) immune response that controls viral and bacterial infection. For example, the chronic Th2 response induced by helminth infections has been associated in numerous experimental models with an impaired Th1 response to intracellular (36–38) and extracellular (39) bacteria as well as viruses (40, 41) and can reduce the efficacy of vaccines (62–64). However, contradictory results have been also reported, showing no effect (65) or even boosting effect (66) of helminth infections on the Th1 response.
In the present study, we analyzed the impact of chronic i.n. sensitization with house dust mite D. farinae (HDM) or mold A. alternata (Alt) extracts on the course of Brucella spp., S. pneumoniae, and M. tuberculosis lung infections. We observed that both asthma sensitization protocols strongly enhanced the growth of Brucella melitensis in the lungs, but not in other organs such as the spleen or liver following i.n. infection. In particular, at 12 days post infection, Alt sensitization induced a 2 and 3 log CFU increase in the lungs of C57BL/6 and BALB/c mice, respectively. This increase is very surprising by comparison with the impact of IFNγ-R or αβTCR deficiency in C57BL/6 that only leads to a 1 to 1.5 log increase of Brucella in the lungs of C57BL/6 (23). The lungs of infected asthmatic mice display large aggregates of Ly-6G+ cells, presumably neutrophils, surrounding Brucella infected alveolar macrophages (CD11c+ F4/80+ cells). Infection is concentrated in these areas and large fractions of lung tissue seem uninfected and healthy. In keeping with this observation, mortality was not increased in the infected asthmatic mice, even after 100 days of chronic sensitization and infection (data not shown). Asthma sensitization also favors the growth of B. abortus and B. suis infection in the lungs, demonstrating that this phenomenon can be observed with the three main Brucella species described to infect humans.
Comparison of various mice strains displaying selective deficiencies for key elements of the immune response demonstrated that the impact of asthma on Brucella lung infection is strictly dependent on CD4+ T cells and IL-4/STAT6 pathways, which suggests that IL-4-producing Th2 CD4+ T cells are key actors of this phenomenon. IL-10 deficiency partially restores the control of Brucella multiplication in the lungs of asthmatic mice. Flow cytometry analysis of IL-10GFP transgenic mice showed that asthma sensitization increases the frequency of IL-10-producing cells in the lungs. Taken together, these data suggest that asthma-induced susceptibility is partially due to the inhibition by IL-10 of immune effector mechanisms controlling Brucella multiplication in the lungs. However, asthma-induced susceptibility is also observed in IL-12−/− and IFN-γR−/− mice, suggesting that asthma-induced IL-10 affects IL-12 independent effector mechanisms controlling Brucella in the lungs. As the non Th1 immune effectors controlling Brucella growth in the lungs remain largely unknown, we failed to identify the immune effectors suppressed by IL-10 in our model.
Brucella abortus has been reported to survive and replicate more efficiently in arginase-1+ M2 macrophages than in iNOS+ M1 macrophages in vitro, notably because M2 macrophages display increased intracellular glucose availability, which promotes Brucella replication (52). As higher M2 alveolar macrophage counts have been documented during allergic asthma (51), we tried to determine whether asthma-induced increased Brucella susceptibility could be the result of the preferential invasion of M2 macrophages by Brucella. Microscopic analysis showed a strong increase in the frequency of both iNOS+ and arginase-1+ cells in the lungs of Alt sensitized mice. However, we did not observe preferential co-localization of Brucella with iNOS or arginase-1 staining in the lungs of asthmatic mice. Moreover, arginase activity neutralization by repeated injection of nor-NOHA did not reduce Brucella susceptibility induced by Alt sensitization. These results suggest that the positive impact of asthma on Brucella multiplication in our experimental model was not dependent on invasion of the M2 macrophages. However, M2 macrophages have been described to actively suppress the Th1 response in several likely redundant ways such as via the chitinase-like 3 protein (Ym1) (67) and programmed death ligand 2 (PD-L2) (68). We, therefore, cannot exclude their implication in increased susceptibility to Brucella infection in asthmatic mice. We also cannot exclude that the increased multiplication of Brucella in the macrophages of asthmatic mice was not partially due to the fact that these cells are enriched in nutrients necessary for the growth of Brucella.
The extracellular, Gram-positive bacterium S. pneumoniae is a serious human pathogen that causes more than 50% of cases of community-acquired bacterial pneumonia and is the most common cause of death from infection in developed countries [for a review, see Ref. (69, 70)]. In mice models, innate immunity is crucial during the early phase of natural anti-pneumococcal host defenses, and alveolar macrophages and neutrophils play a key role in the control of bacteria. While clinical studies have identified asthma as a significant risk factor for invasive pneumococcal disease (7, 71–73), we observed that our Alt sensitization protocol dramatically increased the resistance of mice to S. pneumoniae infection, demonstrating that asthma-induced susceptibility to Brucella cannot be generalized to all bacterial infections in our experimental model and suggesting that asthma can also, in some cases, improve resistance to bacterial infection. Wildly conflicting results have been published on the impact of asthma on S. pneumoniae infection in mice. Intraperitoneally, OVA-sensitized mice have been reported to display reduced (74), similar (75), or enhanced (76, 77) susceptibility to infection. The discrepancies between these results may be connected with the serotype of S. pneumoniae used, the sanitary level of the mice, or a delay between infection and asthma challenge. Surprisingly, in our model, CD3 and STAT-6 deficiency did not impair resistance to S. pneumoniae infection following asthma sensitization, demonstrating that this phenomenon is induced by a route that is completely different from that of asthma-induced Brucella susceptibility. Stimulation of innate lung immunity with an aerosolized lysate of non-typeable Haemophilus influenzae confers a high level of protection against a challenge with otherwise lethal inocula of S. pneumoniae (78). Intranasal administration of flagellin from Salmonella enterica serovar Typhimurium protects from S. pneumoniae infection in wild-type and B- and T-cell-deficient SCID mice, but not in neutrophil-depleted mice (33). This suggests that strong neutrophilia induced by asthma sensitization in STAT-6−/− mice (Figure S2 in Supplementary Material) could be sufficient to increase resistance to S. pneumoniae in our model. Interestingly, a recent study (79) in a mouse model reported that allergic asthma also decreases lung infection with Klebsiella pneumoniae in a neutrophil-dependent and IL-4- and IL-17-independent manner.
Finally, we also tested the impact of Alt sensitization on M. tuberculosis lung infection. Although the course of M. tuberculosis infection, like Brucella infection, is mainly controlled by IFN-γ-producing CD4+ T cells (80–82) in mice, we observed that asthma sensitization does not affect M. tuberculosis multiplication in the lungs. It is noteworthy that while different studies indicate that BCG vaccination or infection with M. tuberculosis is associated with a decreased risk of allergic diseases (83–85) and that active tuberculosis disease is associated with increased allergic sensitization (86) or protection against allergy (84), no clear association between asthma and increased susceptibility to M. tuberculosis infection has been reported to the best of our knowledge. Overall, this result indicates again that the impact of Alt sensitization on Brucella infection cannot be generalized to all other intracellular bacterial infections.
We observed recently (87) that the strong Th1 response induced by Trypanosoma brucei infection significantly reduced Brucella growth but did not affect the course of M. tuberculosis infection in mice. Taken together, the absence of an impact of the Th1 response induced by T. brucei and the Th2 response induced by asthmatic sensitization on M. tuberculosis infection in our mice models is very surprising and suggests that control of M. tuberculosis is not simply dependent on a balance between the Th1 and Th2 responses.
On the whole, our results demonstrated that the same protocol for allergic asthma sensitization might have no effect or dramatically reduce or increase resistance to bacterial infection in mice, depending on the infectious agent. These results open up new areas for investigation of immune effectors controlling Brucella and S. pneumoniae growth in the lungs, and suggest that it could be interesting to perform further clinical studies on the impact of asthma on Brucella and S. pneumoniae infections in humans.
Ethics Statement
The procedures used in this study and the handling of the mice complied with current European legislation (directive 86/609/EEC) and the corresponding Belgian law “Arrêté royal relatifà la protection des animaux d’expérience du 6 avril 2010 publié le 14 mai 2010.” The Animal Welfare Committee of the Université de Namur (UNamur, Belgium) reviewed and approved the complete protocol for Brucella infections (Permit Number: UN-LE-14/220). The Animal Welfare Committee of the Université Libre de Bruxelles (ULB, Belgium) reviewed and approved the complete protocol for Streptococcus pneumoniae infections (Permit Number: ULB-IBMM-2016-21-88). The Ethics committee of the WIV-ISP and CODA-CERVA approved the complete protocol for Mycobacterium tuberculosis infections (ethics agreement number 201405-14-01).
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Manon Merckx, Margaux Van Vyve, Aurore Lison, and Daniel Van Vlaender (UNamur) for their helpful and kind assistance.
Funding
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) (convention FRSM FNRS 3.4.600.06.F, Belgium), and by the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office. EM is a Senior Research Associate from the FRS-FNRS (Belgium). AM and GP hold FRIA Ph.D. grants from the FRS-FNRS (Belgium).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01856/full#supplementary-material.
Figure S1. Comparison of allergic asthma models induced by intranasal sensitization with HDM or Alt extracts. Wild-type BALB/c mice were instilled i.n. with phosphate-buffered saline (control), HDM, or Alt extracts for 17 days to induce allergic asthma. Three days after the last sensitization, the mice were sacrificed and the lungs were collected to evaluate the asthma severity. (A) Hematoxylin and eosin (HE) staining of lung paraffin sections (scale bar: 200 µm). (B) Differential cell counts in bronchoalveolar lavages fluid. (C) Q-PCR analysis of IL-4 mRNA expression levels in the lungs. (D) Total blood IgE Ab concentration determined by ELISA. n denotes the number of mice used for each lineage. These results are representative of at least two independent experiments. *p < 0.05, **p < 0.01.
Figure S2. Impact of IL-4 and STAT6 deficiency on asthma-induced cell recruitment in the lungs of BALB/c mice. Wild-type, IL4−/− and STAT6−/− BALB/c mice received repeated i.n. administration of phosphate-buffered saline, HDM, or Alt before i.n. infection with 2 × 104 CFU of mCherry-Brucella melitensis. The mice were sacrificed at 12 days post infection to evaluate the severity of the allergic phenotype. (A) Circulating blood non-specific IgE Ab concentration determined by ELISA. (B) Number of cell counts in bronchoalveolar lavage fluid from control, HDM, and Alt infected mice. These results are representative of at least two independent experiments. **p < 0.01, ***p < 0.001.
Figure S3. Treatment with nor-NOHA inhibitor neutralizes arginase activity in lung homogenates from Alt sensitized BALB/c mice. Wild-type BALB/c mice received repeated i.n. administration of phosphate-buffered saline (PBS) (control) or Alt for 2 weeks. The mice were sacrificed and the lungs were harvested and homogenized. The panel represents the arginase activity/lung homogenate from control and Alt sensitized wild-type BALB/c mice incubated in vitro with PBS or nor-NONA inhibitor (2 mg/ml). These results are representative of at least two independent experiments. **p < 0.01, ***p < 0.001.
References
1. Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis (2011) 15:e510–3. doi:10.1016/j.ijid.2010.06.020
2. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy (2004) 59:469–78. doi:10.1111/j.1398-9995.2004.00526.x
3. Anandan C, Nurmatov U, Van Schayck OCP, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy (2010) 65:152–67. doi:10.1111/j.1398-9995.2009.02244.x
4. Fahy JV. Type 2 inflammation in asthma — present in most, absent in many. Nat Immunol (2015) 15:57–65. doi:10.1038/nri3786
5. Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther (2015) 4:143–57. doi:10.2147/ITT.S61528
6. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet (2002) 359:831–4. doi:10.1016/S0140-6736(02)07953-9
7. Klemets P, Lyytika O, Ruutu P, Ollgren J, Kaijalainen T, Leinonen M, et al. Risk of invasive pneumococcal infections among working age adults with asthma. Thorax (2010) 65:698–703. doi:10.1136/thx.2009.132670
8. Juhn YJ. Influence of asthma epidemiology on the risk for other diseases. Allergy Asthma Immunol Res (2012) 4:122–31. doi:10.4168/aair.2012.4.3.122
9. Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res (2005) 36:313–26. doi:10.1051/vetres:2005003
10. Kaufmann AF, Fox MD, Boyce JM, Anderson DC, Potter ME, Martone WJ, et al. Airborne spread of brucellosis. Ann N Y Acad Sci (1980) 353:105–14. doi:10.1111/j.1749-6632.1980.tb18912.x
11. Hendricks SL, Borts IH, Heeren RH, Hausler WJ, Held JR. Brucellosis outbreak in an Iowa packing house. Am J Public Health (1962) 52(7):1166–78. doi:10.2105/AJPH.52.7.1166
12. Olle-Goig JE, Canela-Soler J. An outbreak of Brucella melitensis infection by airborne transmission among laboratory workers. Am J Public Health (1987) 77(3):335–8. doi:10.2105/AJPH.77.3.335
13. Wallach JC, Samartino LE, Efron A, Baldi PC. Human infection by Brucella melitensis: an outbreak attributed to contact with infected goats. FEMS Immunol Med Microbiol (1997) 19(4):315–21. doi:10.1016/S0928-8244(97)00098-9
14. Pappas G, Panagopoulou P, Christou L, Akritidis N. Brucella as a biological weapon. Cell Mol Life Sci (2006) 63:2229–36. doi:10.1007/s00018-006-6311-4
15. Yagupsky P, Baron EJ. Laboratory exposures to Brucellae and implications for bioterrorism. Emerg Infect Dis (2005) 11(8):1180–5. doi:10.3201/eid1108.041197
16. Pappas G, Akritidis N, Bosilkovski M. Brucellosis. N Engl J Med (2005) 352:130–4. doi:10.1056/NEJMra050570
17. Colmenero JD, Reguera JM, Martos F, Sánchez-De-Mora D, Delgado M, Causse M, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) (1996) 75(4):195–211.
18. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis (2006) 6:91–9.
19. Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol (2010) 140:392–8. doi:10.1016/j.vetmic.2009.06.021
20. Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis (2012) 6(1–9):e1865. doi:10.1371/journal.pntd.0001865
21. Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM, Rice-Ficht AC. Brucellosis: the case for live, attenuated vaccines. Vaccine (2009) 27:D40–3. doi:10.1016/j.vaccine.2009.08.058
22. Oliveira SC, Giambartolomei GH, Cassataro J. Confronting the barriers to develop novel vaccines against brucellosis. Expert Rev Vaccines (2011) 10:1291–305. doi:10.1586/erv.11.110
23. Hanot Mambres D, Machelart A, Potemberg G, De Trez C, Ryffel B, Letesson J-J, et al. Identification of immune effectors essential to the control of primary and secondary intranasal infection with Brucella melitensis in mice. J Immunol (2016) 196:3780–93. doi:10.4049/jimmunol.1502265
24. Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, et al. Inflammatory dendritic cells — not basophils — are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med (2010) 207:2097–112. doi:10.1084/jem.20101563
25. Denis O, Van Den Brûle S, Heymans J, Havaux X, Rochard C, Huaux F, et al. Chronic intranasal administration of mould spores or extracts to unsensitized mice leads to lung allergic inflammation, hyper-reactivity and remodelling. Immunology (2007) 122:268–78. doi:10.1111/j.1365-2567.2007.02636.x
26. Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, et al. Immune response in mice that lack the interferon-gamma receptor. Science (1993) 259:1742–5. doi:10.1126/science.8456301
27. Carrera L, Gazzinelli RT, Badolato R, Hieny S, Muller W, Kuhn R, et al. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med (1996) 183:515–26. doi:10.1084/jem.183.2.515
28. Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell (1992) 71:1205–14. doi:10.1016/S0092-8674(05)80068-6
29. Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, et al. Mice lacking MHC class II molecules. Cell (1991) 66:1051–66. doi:10.1016/0092-8674(91)90448-8
30. Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol (2004) 22:1567–72. doi:10.1038/nbt1037
31. Copin R, Vitry M-A, Hanot Mambres D, Machelart A, De Trez C, Vanderwinden J-M, et al. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog (2012) 8:e1002575. doi:10.1371/journal.ppat.1002575
32. Marichal T, Bedoret D, Mesnil C, Pichavant M, Goriely S, Trottein F, et al. Interferon response factor 3 is essential for house dust mite-induced airway allergy. J Allergy Clin Immunol (2010) 126:836–44.e13. doi:10.1016/j.jaci.2010.06.009
33. Muñoz N, Van Maele L, Marqués JM, Rial A, Sirard JC, Chabalgoity JA. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun (2010) 78:4226–33. doi:10.1128/IAI.00224-10
34. Korf JE, Pynaert G, Tournoy K, Boonefaes T, Van Oosterhout A, Ginneberge D, et al. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med (2006) 174:152–60. doi:10.1164/rccm.200507-1175OC
35. Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, et al. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev (1992) 127:183–204. doi:10.1111/j.1600-065X.1992.tb01414.x
36. Dias AT, de Castro SBR, Alves CCS, Rezende AB, Rodrigues MF, Machado RRP, et al. Lower production of IL-17A and increased susceptibility to Mycobacterium bovis in mice coinfected with Strongyloides venezuelensis. Mem Inst Oswaldo Cruz (2011) 106:617–9. doi:10.1590/S0074-02762011000500015
37. Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med (2011) 208:1863–74. doi:10.1084/jem.20091473
38. Monin L, Griffiths KL, Lam WY, Gopal R, Kang DD, Ahmed M, et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest (2015) 125:4699–713. doi:10.1172/JCI77378
39. Apiwattanakul N, Thomas PG, Kuhn RE, Herbert DBR, McCullers JA. Helminth infections predispose mice to pneumococcal pneumonia but not to other pneumonic pathogens. Med Microbiol Immunol (2014) 203:357–64. doi:10.1007/s00430-014-0344-3
40. Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci U S A (1993s) 90:948–52. doi:10.1073/pnas.90.3.948
41. Actor JK, Marshall MA, Eltoum IA, Buller RML, Berzofsky JA, Sher A. Increased susceptibility of mice infected with Schistosoma mansoni to recombinant vaccinia virus: association of viral persistence with egg granuloma formation. Eur J Immunol (1994) 24:3050–6.
42. Hsieh C, Macatonia SE, Garra AO, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med (1995) 181:713–21. doi:10.1084/jem.181.2.713
43. Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med (1989) 169:59–72. doi:10.1084/jem.169.1.59
44. Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, et al. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med (1998) 187:1537–42. doi:10.1084/jem.187.9.1537
45. Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol (2000) 165:108–13. doi:10.4049/jimmunol.165.1.108
46. Zimmermann N, Mishra A, King NE, Fulkerson PC, Doepker MP, Nikolaidis NM, et al. Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. J Immunol (2004) 172:1815–24. doi:10.4049/jimmunol.172.3.1815
47. Barquero-Calvo E, Martirosyan A, Ordoñez-Rueda D, Arce-Gorvel V, Alfaro-Alarcón A, Lepidi H, et al. Neutrophils exert a suppressive effect on Th1 responses to intracellular pathogen Brucella abortus. PLoS Pathog (2013) 9:e1003167. doi:10.1371/journal.ppat.1003167
48. Mambres DH, Machelart A, Vanderwinden JM, De Trez C, Ryffel B, Letesson JJ, et al. In situ characterization of splenic Brucella melitensis reservoir cells during the chronic phase of infection in susceptible mice. PLoS One (2015) 10:e0137835. doi:10.1371/journal.pone.0137835
49. Mathews JA, Kasahara DI, Ribeiro L, Wurmbrand AP, Ninin FMC, Shore SA. γδ T cells are required for M2 macrophage polarization and resolution of ozone-induced pulmonary inflammation in mice. PLoS One (2015) 10:e0131236. doi:10.1371/journal.pone.0131236
50. Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest (2016) 126:3279–95. doi:10.1172/JCI85664
51. Moreira AP, Hogaboam CM. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J Interfon cytokine Res (2011) 31:485–91. doi:10.1089/jir.2011.0027
52. Xavier MN, Winter MG, Spees AM, Den Hartigh AB, Nguyen K, Roux CM, et al. PPARγ-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages. Cell Host Microbe (2013) 14:159–70. doi:10.1016/j.chom.2013.07.009
53. Takahashi N, Ogino K, Takemoto K, Hamanishi S, Wang D, Takigawa T, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae -induced NC/ Nga mouse model. Am J Physiol Lung Cell Mol Physiol (2010) 299:17–24. doi:10.1152/ajplung.00216.2009
54. Leech MD, Benson RA, Fitch M, Howie SEM, Cd CD, Regulatory F, et al. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4 + CD25 + Foxp3 + regulatory cells. J Immunol (2007) 179:7050–8. doi:10.4049/jimmunol.179.10.7050
55. Braza F, Chesne J, Durand M, Dirou S, Brosseau C. A regulatory CD9 + B-cell subset inhibits HDM-induced allergic airway inflammation. Allergy (2015) 70:1421–31. doi:10.1111/all.12697
56. Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med (2007) 204(2):239–43. doi:10.1084/jem.20070104
57. Couper K, Blount D, Riley E. IL-10: the master regulator of immunity to infection. J Immunol (2008) 180:5771–7. doi:10.4049/jimmunol.180.9.5771
58. Corsetti PP, de Almeida LA, Carvalho NB, Azevedo V, Silva TMA, Teixeira HC, et al. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS One (2013) 8:e74729. doi:10.1371/journal.pone.0074729
59. Misharin A, Morales-Nebreda L, Mutlu G, Budinger G, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol (2013) 49:503–10. doi:10.1165/rcmb.2013-0086MA
60. Pomés A, Chapman MD, Wünschmann S. Indoor allergens and allergic respiratory disease. Curr Allergy Asthma Rep (2016) 16:43. doi:10.1007/s11882-016-0622-9
61. Patella V, Bocchino M, Steinhilber G. Asthma is associated with increased susceptibility to infection. Minerva Med (2015) 106:1–7.
62. Elias D, Akuffo H, Pawlowski A, Haile M, Schön T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine (2005) 23:1326–34. doi:10.1016/j.vaccine.2004.09.038
63. Noland GS, Chowdhury DR, Urban JF Jr, Zavala F, Kumar N. Helminth infection impairs the immunogenicity of a Plasmodium falciparum DNA vaccine, but not irradiated sporozoites, in mice. Vaccine (2010) 28:2917–23. doi:10.1016/j.vaccine.2010.02.055
64. Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis (1996) 173:269–72. doi:10.1093/infdis/173.1.269
65. Rafi W, Bhatt K, Gause WC, Salgame P. Neither primary nor memory immunity to Mycobacterium tuberculosis infection is compromised in mice with chronic enteric helminth infection. Infect Immun (2015) 83:1217–23. doi:10.1128/IAI.03004-14
66. Scheer S, Krempl C, Kallfass C, Frey S, Jakob T, Mouahid G, et al. S. mansoni bolsters anti-viral immunity in the murine respiratory tract. PLoS One (2014) 9:e112469. doi:10.1371/journal.pone.0112469
67. Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science (2014) 345:578–83. doi:10.1126/science.1256942
68. Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood (2017) 116:3311–21. doi:10.1182/blood-2010-02-271981
69. Klugman KP, Feldman C. Streptococcus pneumoniae respiratory tract infections. Curr Opin Infect Dis (2001) 14:173–9. doi:10.1097/00001432-200104000-00011
70. van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet (2009) 374:1543–56. doi:10.1016/S0140-6736(09)61114-4
71. Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol (2008) 122:719–23. doi:10.1016/j.jaci.2008.07.029
72. Jounio U, Juvonen R, Bloigu A, Silvennoinen-Kassinen S, Kaijalainen T, Kauma H, et al. Pneumococcal carriage is more common in asthmatic than in non-asthmatic young men. Clin Respir J (2010) 4:222–9. doi:10.1111/j.1752-699X.2009.00179.x
73. Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. Pediatr Infect Dis J (2005) 24:854–63. doi:10.1097/01.inf.0000178067.63225.e8
74. Sanfilippo AM, Furuya Y, Roberts S, Salmon SL, Metzger DW. Allergic lung inflammation reduces tissue invasion and enhances survival from pulmonary pneumococcal infection in mice: correlation with increased expression of TGF-β1 and SiglecFlow alveolar macrophages. Infect Immun (2015) 83:2976–83. doi:10.1128/IAI.00142-15
75. Clement CG, Tuvim MJ, Evans CM, Tuvin DM, Dickey BF, Evans SE. Allergic lung inflammation alters neither susceptibility to Streptococcus pneumoniae infection nor inducibility of innate resistance in mice. Respir Res (2009) 10:70. doi:10.1186/1465-9921-10-70
76. Guo S, Wu LX, Jones CX, Chen L, Hao CL, He L, et al. Allergic airway inflammation disrupts interleukin-17 mediated host defense against streptococcus pneumoniae infection. Int Immunopharmacol (2016) 31:32–8. doi:10.1016/j.intimp.2015.12.010
77. Kang CI, Rouse MS, Patel R, Kita H, Juhn YJ. Allergic airway inflammation and susceptibility to pneumococcal pneumonia in a murine model with real-time in vivo evaluation. Clin Exp Immunol (2009) 156:552–61. doi:10.1111/j.1365-2249.2009.03925.x
78. Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med (2008) 177:1322–30. doi:10.1164/rccm.200607-1038OC
79. Dulek DE, Newcomb DC, Goleniewska K, Cephus J, Zhou W, Reiss S, et al. Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect Immun (2014) 82:3723–39. doi:10.1128/IAI.00035-14
80. Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol (2005) 174:4185–92. doi:10.4049/jimmunol.174.7.4185
81. Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted Interferon-g genes. Science (1993) 259:1739–42. doi:10.1126/science.8456300
82. Caruso AM, Serbina N, Klein E, Bloom BR, Flynn JL, Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN- γ, Yet succumb to tuberculosis. J Immunol (1999) 162:5407–16.
83. Freyne B, Curtis N. Does neonatal BCG vaccination prevent allergic disease in later life? Arch Dis Child (2014) 99:182–4. doi:10.1136/archdischild-2013-305655
84. Obihara CC, Kimpen JLL, Gie RP, Van Lill SW, Hoekstra MO, Marais BJ, et al. Mycobacterium tuberculosis infection may protect against allergy in a tuberculosis endemic area. Clin Exp Allergy (2006) 36:70–6. doi:10.1111/j.1365-2222.2005.02408.x
85. Steenhuis TJ, Van Aalderen WMC, Bloksma N, Nijkamp FP, Van Der Laag J, Van Loveren H, et al. Bacille-Calmette-Guerin vaccination and the development of allergic disease in children: a randomized, prospective, single-blind study. Clin Exp Allergy (2008) 38:79–85. doi:10.1111/j.1365-2222.2007.02859.x
86. Ellertsen LK, Storla DG, Diep LM, Brokstad KA, Wiker HG, Hetland G. Allergic sensitisation in tuberculosis patients at the time of diagnosis and following chemotherapy. BMC Infect Dis (2009) 9:100. doi:10.1186/1471-2334-9-100
Keywords: allergic asthma, infection, Brucella melitensis, Streptococcus pneumoniae, brucellosis, Mycobacterium tuberculosis
Citation: Machelart A, Potemberg G, Van Maele L, Demars A, Lagneaux M, De Trez C, Sabatel C, Bureau F, De Prins S, Percier P, Denis O, Jurion F, Romano M, Vanderwinden J-M, Letesson J-J and Muraille E (2018) Allergic Asthma Favors Brucella Growth in the Lungs of Infected Mice. Front. Immunol. 9:1856. doi: 10.3389/fimmu.2018.01856
Received: 24 January 2018; Accepted: 27 July 2018;
Published: 10 August 2018
Edited by:
Amy Rasley, United States Department of Energy (DOE), United StatesReviewed by:
M. Victoria Delpino, CONICET Instituto de Inmunología, ArgentinaCynthia Baldwin, University of Massachusetts Amherst, United States
Copyright: © 2018 Machelart, Potemberg, Van Maele, Demars, Lagneaux, De Trez, Sabatel, Bureau, De Prins, Percier, Denis, Jurion, Romano, Vanderwinden, Letesson and Muraille. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Muraille, ZW11cmFpbGxlQGhvdG1haWwuY29t
†These authors should be considered as equal first authors.
 Arnaud Machelart
Arnaud Machelart Georges Potemberg
Georges Potemberg Laurye Van Maele2
Laurye Van Maele2 Aurore Demars
Aurore Demars Maxime Lagneaux
Maxime Lagneaux Carl De Trez
Carl De Trez Marta Romano
Marta Romano Jean-Marie Vanderwinden
Jean-Marie Vanderwinden Eric Muraille
Eric Muraille