- Department of Neurology, Thomas Jefferson University, Philadelphia, PA, United States
Interleukin-27 (IL-27) plays an important role in regulation of anti-inflammatory responses and autoimmunity; however, the molecular mechanisms of IL-27 in modulation of immune tolerance and autoimmunity have not been fully elucidated. Dendritic cells (DCs) play a central role in regulating immune responses mediated by innate and adaptive immune systems, but regulatory mechanisms of DCs in CD4+ T cell-mediated immune responses have not yet been elucidated. Here we show that IL-27 treated mature DCs induced by LPS inhibit immune tolerance mediated by LPS-stimulated DCs. IL-27 treatment facilitates development of the CD4+ CD127+3G11+ regulatory T cell subset in vitro and in vivo. By contrast, IL-27 treated immature DCs fail to modulate development of the CD4+CD127+3G11+ regulatory T cell sub-population in vitro and in vivo. Our results suggest that IL-27 may break immune tolerance induced by LPS-stimulated mature DCs through modulating development of a specific CD4+ regulatory T cell subset mediated by 3G11 and CD127. Our data reveal a new cellular regulatory mechanism of IL-27 that targets DC-mediated immune responses in autoimmune diseases such as multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE).
Introduction
Interleukin-27 (IL-27) is an important cytokine that plays a critical role in regulation of immune responses in vivo (1). Previous research has shown that IL-27 is an anti-inflammatory cytokine. For example, it blocks Th17-mediated immune responses (2). However, recent data also indicate that IL-27 plays the role of an inflammatory cytokine that facilitates T cell-mediated immune responses (3–5). These contradictory results suggest the complex regulatory mechanisms of IL-27 in vivo(1).
CD127 is a subunit of interleukin-7 (IL-7) receptor. It is composed of 459 amino acids and is expressed in mature T cells, monocytes and macrophages. In particular, CD127 is a biomarker of regulatory T cells (Tregs) (6, 7). Several sub-populations of Tregs have been defined according to the importance of CD127 expression on CD4+ T cells (8).
3G11 is a membrane antigen expressed on murine CD4+ T cells. 3G11 is a ganglioside with mobility between GD1a and GD1b complexes in the human brain. 3G11 antigen is identified as the disialoganglioside IV3(NeuAc)2-GgOse4Cer. The immune function of 3G11 on CD4+ T cells has not been fully elucidated. Recent research demonstrated that 3G11 may be a biomarker of Treg subsets (8–13).
Dendritic cells (DCs) are important immune regulatory cells that play a central role in development of T cells such as CD4+ T helper cells. DCs modulate development and differentiation of T cells through production of multiple cytokines such as IL-27 (5). It is not clear whether IL-27 can affect DC-mediated CD4+ T cell immune responses. The effect of IL-27 on development of CD4+ regulatory T cells was examined in this project. Our data show that IL-27 modulates development of mature DC-mediated differentiation of Treg subsets.
Regulatory T cells are important immune cells in vivo and they play a central role in induction of immune tolerance and anti-inflammatory responses (14, 15). Multiple subsets of Tregs have been reported. For example, our previous data show that there are two subpopulations of Tregs including CD4+CD25+FoxP3+GITR+CD127+3G11+ and CD4+CD25+FoxP3+GITR+CD127+3G11− Treg subsets in vivo. Although the immune function of these two new subsets of Tregs is unclear, the number of Treg sub-populations mediated by 3G11 and CD127 is different in mice with experimental autoimmune encephalomyelitis (EAE) development and those with immune tolerance. However, the regulatory mechanisms of CD4+CD127+3G11+ Tregs are still unclear (8). Our project is focused on whether or not IL-27 plays an important role in the development of CD4+CD127+3G11+ Tregs mediated by immature or mature DCs induced by LPS. Our results will show that IL-27 modulates development of CD4+CD127+3G11+ Tregs mediated by mature DCs, and they may help to reveal a new mechanism of IL-27 in mature DC-mediated immune responses.
Materials and Methods
Mice
Wild type C57 BL/6J female mice (8–12 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred in Thomas Jefferson Animal Care facilities and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Immunogen and Peptide
Mouse MOG35−55 peptide (MEVGWYRSPFSRVVHLYRNGK), an ingredient of myelin oligodendrocyte glycoprotein (MOG), was obtained from Invitrogen (Invitrogen, Carlsbad, CA, USA).
Isolation of Bone Marrow
As described previously, femurs and tibiae were isolated from muscle tissue of mice. The intact bones were then sterilized with 70% ethanol for 5 min and washed with phosphate-buffered saline (PBS). Bone ends were cut and the bone marrow was flushed with PBS. Cellular clusters within the bone marrow suspension were disintegrated and washed with PBS (8, 16–20).
Bone Marrow-Derived DC Culture
As described previously, leucocytes from bone marrow were fed in bacteriological 100 mm Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) at 2 × 106 cells per dish. Cells were cultured in RPMI1640 complete medium (Gibco-BRL, Eggenstein, Germany) including penicillin (100 U/ml, Sigma, St. Louis, MO, USA), streptomycin (100 U/ml, Sigma), L-glutamine (2 mM, Sigma), 2-mercaptoethanol (2-ME, 50 μM, Sigma), 10% heated, inactivated and filtered (0.22 μm, Milipore, Inc., Bedford, MA, USA) Fetal Calf Serum (FCS, Sigma) and granulocyte-macrophage colony-stimulating factor (GM-CSF, PeproTech, Rocky Hill, NJ, USA) at 20 ng/ml at day 0 (10 ml medium per dish) (8, 16–20).
At day 3, 10 ml fresh medium with GM-CSF (20 ng/ml) was added to each dish and, at day 6, half of the medium (about 10 ml supernatant) was collected and centrifuged at 300 g for 5 min. Subsequently, cells were re-suspended in 10 ml fresh medium with GM-CSF (20 ng/ml) and were then re-fed in the original dish. Only non-adherent cells (DCs) were harvested and seeded in a fresh dish; 10 ml fresh medium including GM-CSF (20 ng/ml) was added at day 8 (8, 16–20).
Cells were also treated with lipopolysaccharide (LPS, Sigma) for 24 h at 1 μg/ml. LPS was isolated from K. Pneumoniae. DCs or LPS-treated DCs were pulsed with 0.1 μM MOG peptide for 30 min and then washed twice with PBS at 300 g × 5 min before i.v. transfer to EAE mice. DCs were treated with IL-27 at 100 ng/ml for 72 h before conducting flow cytometry assay or i.v transfer experiments. Fresh non-adherent DCs were then collected and washed with PBS at 300 g for 5 min and characterized by flow cytometry or i.v. transferred to EAE mice. More than 90% of cells expressed DC marker CD11c (8, 16–20).
Flow Cytometry
MOG-primed T lymphocytes were isolated from EAE mice and incubated with anti-mouse CD4 (Pacific blue), CD25 (APC), CD127 (PerCP-Cy5.5), 3G11 (PE-Cy7) and GITR (APC-Cy7) antibodies (Biolegend). Cells were washed twice with 5% FCS in PBS at 300 g for 5 min, fixed with 5% formalin in PBS at 4°C for 2 h and then permeated for intracellular staining (8, 16–20).
For intracellular staining, spleen cells were stimulated by leukocyte activator (BD) for 6 h. Splenocytes were then washed twice with 5% FCS in PBS at 300 g for 5 min and fixed with 5% formalin (Sigma) in PBS at 4°C for 2 h. After cells were washed with permeabilization buffer (Biolegend) twice at 300 g × 10 min, anti-mouse FoxP3 (PE) antibody (Biolegend) was incubated with cells at 4°C for 24 h. Cells were then washed with permeabilization buffer twice at 300 g for 5 min, re-suspended in 0.5 ml cell staining buffer (Biolegend), and tested in a FACSAria (BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo software (Treestar, Ashland, OR, USA) (8, 16–20).
Generation of Effector T Cells in vitro
C57 BL/6J mice were immunized with MOG35−55 peptide (Invitrogen) 200 μg, QuilA (Sigma) 20 μg, and keyhole limpet hemocyanin (KLH, Sigma) 20 μg per mouse at day 0. Spleen cells were then isolated at day 10 after immunization. CD4+ T lymphocytes were purified with mouse CD4+ T cell subset column kit (R&D Systems). CD4+ T cells (1 × 106 cells/per well) were co-cultured with DCs at 10:1 (T cells: DCs) and pulsed with MOG 35−55 peptide at 0.1 μM in complete medium with mouse IL-2 at 1 ng/ml for 3 days. Cells were harvested and MOG-primed CD4+ T cells were gated and analyzed by flow cytometry (8, 16–20).
EAE Induction and Treatment
C57BL/6J mice (female, 8–12 week) were immunized with MOG35−55 peptide/complete Freund's adjuvant (CFA, Sigma) at 200 μg/200 μl/per mouse (subcutaneous injection, s.c.). Pertussis toxin (PT, Sigma) was simultaneously injected at 200 ng/per mouse (intraperitoneal injection) and the second PT injection was conducted after 48 h. EAE was assessed following standard clinical scores: 0.5: paralysis of half the tail, 1: paralysis of whole tail, 2: paralysis of tail and one leg, 3: paralysis of tail and two legs, 4: moribund, 5: death (8, 16–20).
Mice were divided into five groups. DCs were washed with PBS twice and were immediately injected via tail vein (3 × 105 cells/per mouse/per time) on days 11, 14, and 17 post-immunization (p.i): (1) injected with PBS only (EAE control); (2) injected with DCs pulsed with MOG peptide; (3) injected with IL-27-treated DCs pulsed with MOG peptide; (4) injected with LPS-DCs pulsed with MOG peptide; (5) injected with LPS and IL-27-treated DCs pulsed with MOG peptide (8, 16–20).
At day 25 p.i., splenocytes were isolated and stimulated with MOG peptide (0.1 μM) and mouse IL-2 (1 ng/ml) for 3 days. Cells were then harvested for flow cytometry assay (8, 16–20).
Statistical Analysis
Experimental data were analyzed using Prism software (GraphPad, La Jolla, CA, USA). A two-way ANOVA test was performed for analysis of clinical score of EAE; t tests were conducted for analysis of flow cytometry data. Error bars represent the mean and standard deviation (SD) or standard error of arithmetic mean (SEM). Results are considered to show a significant difference if the P value is less than 0.05 (8, 16–20).
Results
IL-27-Treated Immature DCs do not Affect Expression of Treg-Associated Molecules on CD4+ T Cells
Since CD25, CD127, FoxP3, GITR, and 3G11 are Treg-associated molecules and expressed on CD4+ T cells, we supposed that IL-27-treated DCs may affect expression of CD25, CD127, FoxP3, GITR, and 3G11 on CD4+ T cells and then regulate development of Tregs via modulating expression of Treg-associated molecules. To test whether IL-27-treated immature bone marrow-derived DCs can affect protein expression of Treg-associated molecules on MOG-primed CD4+ T cells, DCs (Thin line) or IL-27-treated DCs (Thick line) were pulsed with MOG peptide and co-cultured with MOG-primed CD4+ T cells. The expression of CD25, CD127, FoxP3, GITR, and 3G11 on CD4+ T cells co-cultured with MOG-loaded DC or MOG-pulsed DC (MOG-DCs) treated with IL-27 is shown (Figures 1A–E). The experimental data indicate that there is no significant difference in expression of Treg-associated molecules on CD4+ T cells incubated with MOG-DCs or MOG-DCs-treated with IL-27.
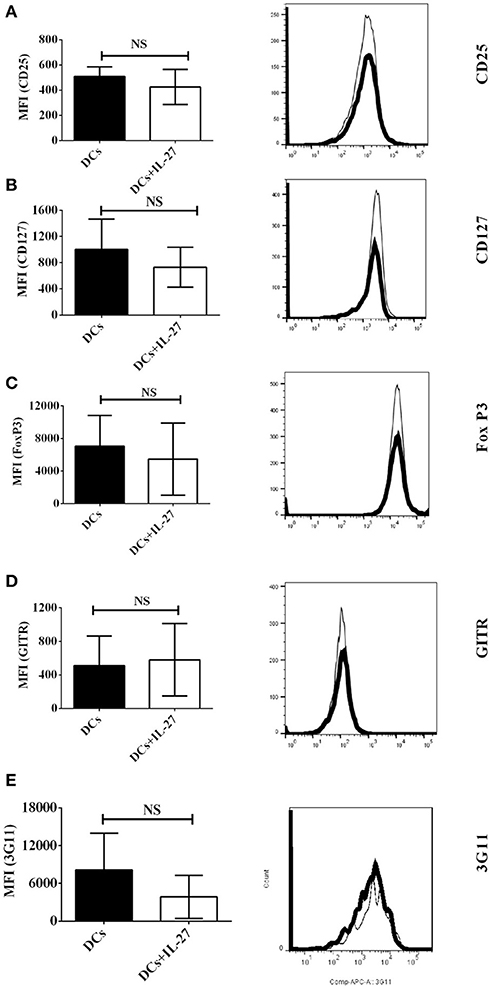
Figure 1. Expression of Treg-associated molecules on MOG-primed CD4+ T cells co-culture with immature DCs (Thin line) or IL-27-treated immature DCs (Thick line) pulsed with MOG peptide in vitro. C57 BL/6J mice were immunized with MOG (200 μg)/Quil A (20 μg) / KLH (20 μg)/per mouse at day 0. Splenocytes were harvested at day 10. CD4+ T cells were then isolated using mouse CD4+ T cell subset column kit (R and D Systems). CD4+ T Lymphocytes were re-stimulated with MOG peptide (0.1 μM) and IL-2 (1 ng/ml) for 72 h. Cells were then stained by anti-mouse CD25 (A), CD127 (B), FoxP3 (C), GITR (D), and 3G11 (E) antibodies. Protein expression of Treg-associated molecules on CD4+ T cells is shown. Error bars indicated in this figure represent mean and SD of triplicate determinations of mean fluorescence intensity (MFI) of Treg-associated molecule expression on CD4+ T cells (n = 3, t test, PA = 0.4198; PB = 0.4450; PC = 0.6047; PD = 0.8372; PE = 0.2523; NS, no significant difference).
Nor do IL-27-Treated Mature DCs Induced by LPS Affect Expression of Treg-Associated Molecules on MOG-Primed CD4+ T Cells
Although IL-27-treated immature DCs do not affect protein expression of Treg-associated molecules on CD4+ T cells (Figure 1), it is unclear whether or not mature DCs induced by LPS can do that. To detect whether or not IL-27 treatment can modulate mature DC-mediated expression of Treg-associated molecules on CD4+ T cells, MOG-pulsed mature bone marrow-derived DCs induced by LPS were treated with IL-27 (Thick line) or without IL-27 treatment (Dot line) and co-cultured with MOG-primed CD4+ T cells. The expression of CD25, CD127, FoxP3, GITR, and 3G11 on CD4+ T cells is demonstrated (Figures 2A–E). Our data indicate that expression of Treg-associated molecules on CD4+ T cells co-cultured with IL-27-treated mature DCs is similar to that on CD4+ T cells co-cultured with mature DCs without IL-27 treatment. It can be concluded that IL-27 treatment does not modulate either immature or mature DC-mediated expression of CD25, CD127, FoxP3, GITR, and 3G11 on MOG-primed CD4+ T cells.
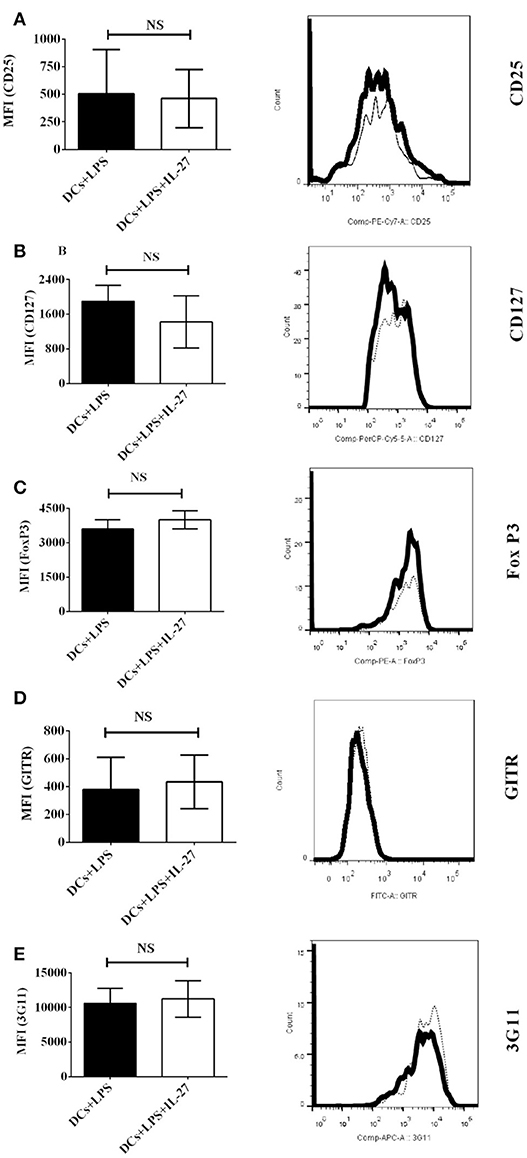
Figure 2. Protein expression of Treg-associated molecules on MOG-primed CD4+ T cells incubated with LPS-induced mature DCs or IL-27-treated mature DCs pulsed with MOG peptide in vitro. Bone marrow-derived dendritic cells were stimulated with LPS (1 μg/ml) for 24 hrs. LPS-stimulated DCs were also simultaneously incubated with IL-27 (20 ng/ml) (Thick line) for 72 hrs or had no IL-27 treatment (Dot line). DCs were then co-cultured with MOG-primed CD4+ T cells as shown in Figure 1. Protein expression of CD25 (A), CD127 (B), FoxP3 (C), GITR (D), and 3G11 (E) on CD4+ T cells is shown. Error bars indicated in this figure represent mean and SD of MFI of Treg-associated molecules expressing on CD4+ T cells in three independent experiments (n = 3, t test, PA = 0.8809; PB = 0.3012; PC = 0.2879; PD = 0.7744; PE = 0.7549; NS, no significant difference).
IL-27 Treatment Facilitates Development of CD4+CD127+3G11+ Tregs Mediated by LPS-Induced Mature DCs
Although immature and mature DCs treated with IL-27 do not affect expression of Treg-associated molecules on CD4+ T cells (Figures 1, 2), we assumed that IL-27-treated immature or mature DCs may still modulate development of Treg sub-populations. To test whether IL-27 can affect immature and mature DC-mediated development of CD4+ Treg subsets, immature, and mature DCs were incubated with or without IL-27 treatment. Immature and mature DCs were then pulsed with MOG peptide and co-cultured with MOG-primed CD4+ T cells. Phenotypes of CD4+ Tregs-mediated by CD127 and 3G11 are shown (Figure 3). The experimental results indicate that immature DCs treated with IL-27 cannot modulate development of CD4+CD127+3G11+ Treg subset; however, LPS-induced mature DCs treated with IL-27 can enhance development of the CD4+CD127+3G11+ Treg sub-population (Figure 3). This suggests that LPS may modulate mature DC-mediated development of CD4+ Treg subsets in vitro.
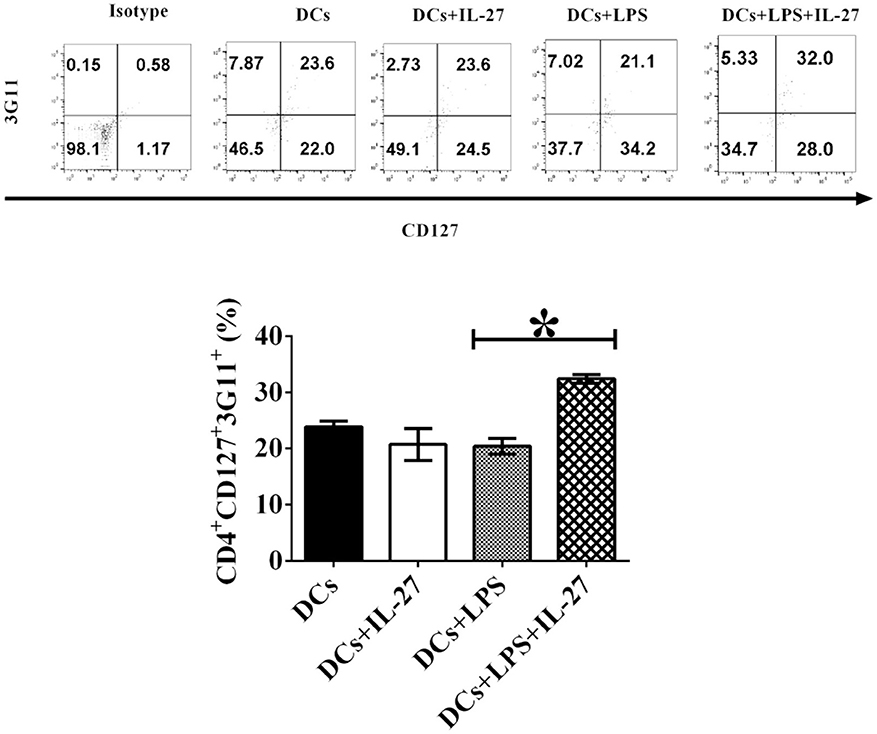
Figure 3. IL-27 treatment facilitates LPS-induced mature DCs-mediated development of CD4+CD127+3G11+ Treg subset in vitro. Bone marrow-derived DCs shown in Figures 1, 2 were treated with IL-27 (20 ng/ml, 72 h) or/and LPS (1 μg/ml, 24 h) or without LPS or IL-27 incubation. DCs were then co-cultured with MOG-primed CD4+ T cells indicated in Figures 1, 2 for 72 h. CD4+CD25+FoxP3+GITR+ cells were gated. The frequency of CD127+3G11+ Tregs is shown. Isotype control is CD4+ T cells incubated with isotype control antibodies. Error bars shown in this figure represent mean and SD of frequency of CD4+CD127+3G11+ Tregs in three independent experiments (n = 3, t test, P (DC, DC+IL−27) = 0.1357; P(DC+LPS, DC+LPS+IL−27) = 0.0002).
IL-27-Treated Mature DCs Block Immune Tolerance Induced by LPS-Stimulated DCs in vivo
We have investigated the effect of immature and mature DCs treated with IL-27 on development of Tregs in vitro (Figures 1–3). It is necessary for establishment of in vivo model to detect whether or not immature and mature DCs treated with IL-27 can regulate development of Tregs. To test whether or not IL-27 treated immature and mature DCs can affect MOG-primed CD4+ T cell-induced autoimmunity in vivo, immature and LPS-induced mature DCs were pulsed with MOG peptide and incubated with or without IL-27 treatment. Immature and mature DCs were then i.v transferred into C57BL/6J mice immunized with MOG peptide to induce EAE. Our data indicate that IL-27 treatment blocks immune tolerance mediated by LPS-induced mature DCs. By contrast, IL-27 treatment does not affect the development of EAE in mice that are i. v. transferred with immature DCs (Figure 4). The experimental results suggest that IL-27 may affect mature DC-mediated immune responses but that it does not affect immature DC-mediated immune responses.
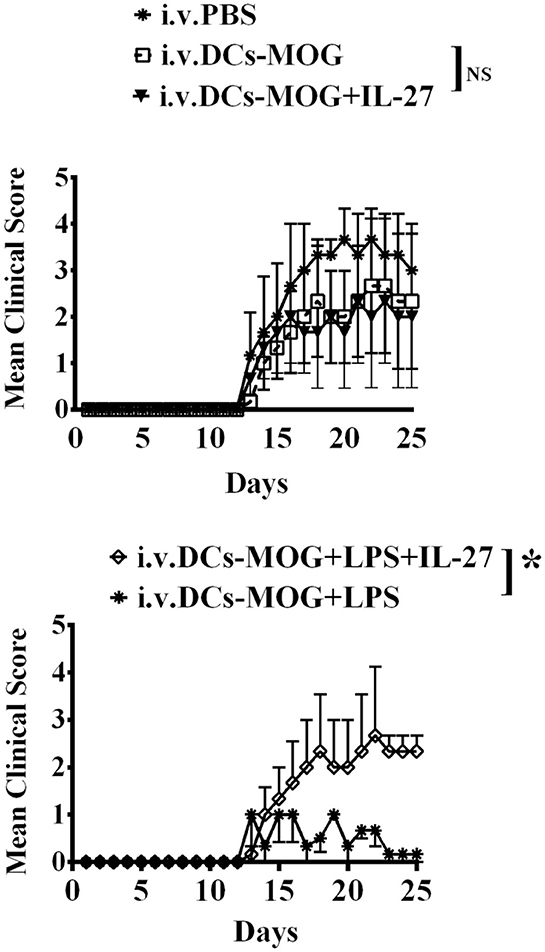
Figure 4. i.v. transfer of IL-27-treated mature DCs induced by LPS inhibits immune tolerance mediated by LPS-stimulated DCs in vivo. C57 BL/6J mice were immunized with MOG peptide (200 μg/per mouse) and CFA. Immature dendritic cells (DCs) were incubated with IL-27 (DCs+IL-27) or LPS (DCs+LPS) or both IL-27 and LPS (DCs+LPS+IL-27). Mice in control group were i.v. transferred with PBS. EAE was then induced and shown by clinical score. Error bars in this figure represent mean and SEM of triplicate determinations of EAE clinical score in one experiment (n = 3, two-way ANOVA test, P (DC, DC+IL−27) = 0.7960; P(DC+LPS, DC+LPS+IL−27) = 0.0001; NS, no significant difference).
IL-27 Treated Immature DCs do not Affect Expression of Treg-Associated Molecules on CD4+ T Cells ex vivo
Our data of in vitro assay have shown that IL-27-treated immature DCs do not affect expression of Treg-associated molecules on CD4+ T cells (Figure 1), however, it is still unknown whether or not IL-27-treated immature DCs can affect expression of Treg-associated molecules on CD4+ T cells in vivo. To test whether or not IL-27-treated immature DCs can modulate expression of Treg-associated molecules on CD4+ T cells in vivo, MOG peptide-pulsed immature DCs incubated with IL-27 or without IL-27 treatment were i.v. transferred into EAE mice shown in Figure 4 at day 11, 14, and 17 after immunization. Lymphocytes were isolated from mice which are i.v transferred with IL-27-treated DCs or immature DCs without IL-27 treatment shown in Figure 4 at day 25. Expression of Treg-associated molecules on CD4+ T cells was detected using flow cytometry. Our results demonstrated that there is no difference in expression of Treg -associated molecules, including CD25, CD127, FoxP3, GITR, and 3G11, on CD4+ T cells isolated from mice that are i.v. transferred with IL-27-treated immature DCs or DCs without IL-27 incubation (Figures 5A–E). Our data suggest that IL-27-treated immature DCs do not affect development of Tregs in vivo.
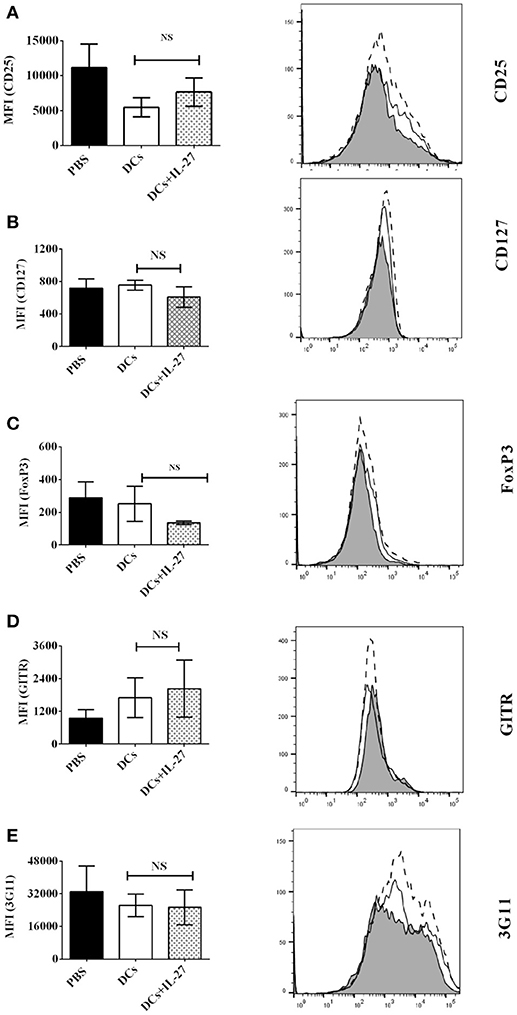
Figure 5. IL-27-treated immature DCs do not affect expression of Treg-associated molecules on CD4+ T cells ex vivo. Spleen cells were isolated from mice treated with PBS (Shade) or DCs (Dot line) or DCs+IL-27 (Thin line) indicated in Figure 4. T lymphocytes were re-stimulated by MOG peptide (0.1 μM) and IL-2 (1ng/ml) for 72 h. The expression of CD25 (A), CD127 (B), FoxP3 (C), GITR (D), and 3G11 (E) on CD4+ T cells is shown. Error bars demonstrated in this figure represent mean and SD of MFI of Treg-associated molecules present on CD4+ T cells in three independent experiments (n = 3, t test, PA = 0.1944; PB = 0.1476; PC = 0.2879; PD = 0.7744; PE = 0.7549; NS, no significant difference).
IL-27-Treated Mature DCs do not Affect Expression of Treg-Associated Molecules on CD4+ T Cells ex vivo
We have testified that IL-27-treated mature DCs do not affect expression of Treg-associated molecules on CD4+ T cells in vitro (Figure 2), however, it is still unclear that whether or not IL-27-treated mature DCs can modulate expression of Treg-associated molecules on CD4+ T cells in vivo. To determine whether IL-27-treated mature DCs can modulate expression of Treg-associated molecules on CD4+ T cells in vivo, IL-27-treated mature DCs induced by LPS or mature DCs without IL-27 treatment were i.v. transferred into EAE mice shown in Figure 4 at day 11, 14 and 17 after immunization. Protein expression of CD25, CD127, FoxP3, GITR, and 3G11 on CD4+ T cells was tested using flow cytometry. The experimental data show that i.v. transfer of IL-27-treated mature DCs or mature DCs without IL-27 treatment failed to modulate expression of Treg-associated molecules on CD4+ T cells (Figures 6A–E). Our results suggest that IL-27-treated mature DCs do not affect development of Tregs through regulating expression of Treg-associated molecules on CD4+ T cells in vivo.
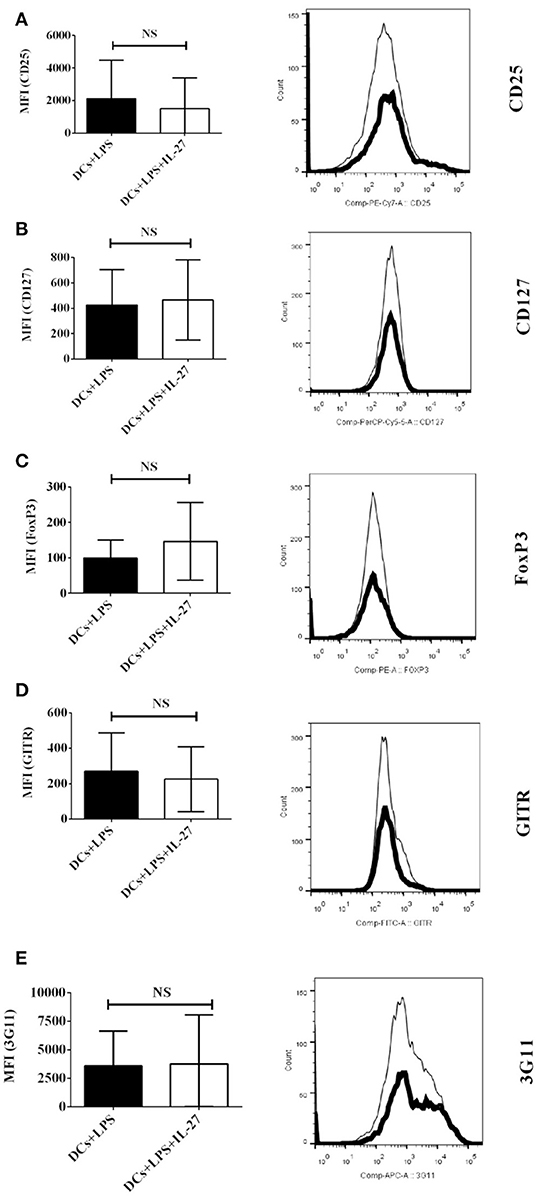
Figure 6. IL-27-treated mature DCs induced by LPS do not affect expression of Treg-associated molecules on CD4+ T cells ex vivo. LPS-stimulated DCs (Thin line) or IL-27-treated mature DCs induced by LPS (Thick line) were i.v. transferred into mice shown in Figure 4. Spleen cells were isolated and re-stimulated with MOG peptide (0.1 μM) and IL-2 (1 ng/ml) for 72 hrs. The expression of CD25 (A), CD127 (B), FoxP3 (C), GITR (D), and 3G11 (E) on CD4+ T cells is shown. Error bars indicated in this figure represent mean and SD of MFI of Treg-associated molecules expressing on CD4+ T cells in three independent experiments (n = 3, t test, PA = 0.7015; PB = 0.8555; PC = 0.4659; PD = 0.7642; PE = 0.9505; NS, no significant difference).
IL-27-Treated Mature DCs Facilitate Development of CD4+ CD127+3G11+ Treg Subset ex vivo
Our results of in vitro assay demonstrated that IL-27-treated mature DCs elicit development of CD4+ CD127+3G11+ Treg subset (Figure 3), however, it is unknown whether or not IL-27-treated mature DCs also can facilitate development of CD4+ CD127+3G11+ Treg subset in vivo. To detect whether or not IL-27-treated mature or immature DCs can regulate development of Treg sub-populations in vivo, LPS-induced mature DCs and immature DCs without LPS stimulation were pulsed with MOG peptide and incubated with or without IL-27 treatment. IL-27-treated immature/mature DCs or immature/mature DCs without incubation with IL-27 were i.v. transferred into EAE mice shown in Figure 4 at day 11, 14 and 17 after immunization. Lymphocytes were isolated from mice that had been i.v. transferred with IL-27-treated immature/mature DCs or immature/mature DCs without IL-27 treatment shown in Figure 4 at day 25. Our results indicate that immature DCs incubated with or without IL-27 treatment did not modulate development of CD4+3G11+CD127+ Treg subset but that mature DCs treated with IL-27 facilitated development of CD4+ CD127+3G11+ Treg sub-populations ex vivo (Figure 7). Our data suggest that IL-27-treated mature DCs may block autoimmunity (Figure 4) through upregulation of CD4+CD127+3G11+ Tregs development in vivo.
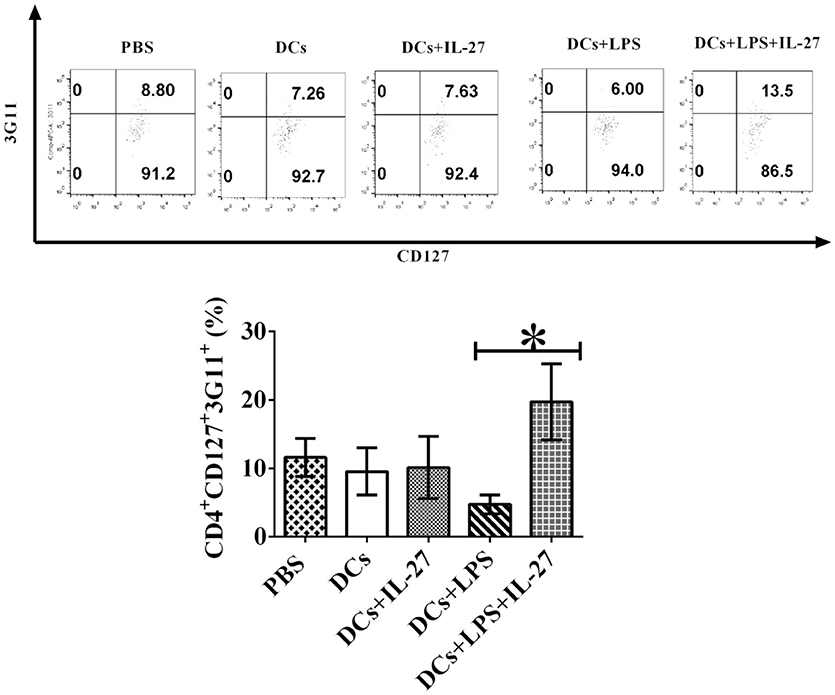
Figure 7. IL-27 facilitates LPS-stimulated mature DC-mediated development of CD4+CD127+3G11+ Treg subset ex vivo. Bone marrow-derived DCs were pulsed with MOG peptide and treated with IL-27 (20 ng/ml, 72 h) (DCs+IL-27) or LPS (DCs+LPS) (1 μg/ml, 24 h) or both LPS and IL-27 (DCs+LPS+IL-27). These DCs were then i.v. transferred into mice with EAE shown in Figure 4. Mice treated with PBS are control. Spleen cells were isolated and re-stimulated with MOG peptide (0.1 μM) and IL-2 (1 ng/ml) for 72 h. Cells were collected and CD4+CD25+FoxP3+GITR+ Tregs were gated. The frequency of CD127+3G11+ cells is demonstrated. T lymphocytes incubated with isotype control antibodies are isotype control. Error bars indicated in this figure represent mean and SD of frequency of CD4+CD127+3G11+ cells in three independent experiments [n = 3, t test, P(DC, DC+IL−27) = 0.8682; P(DC+LPS, DC+LPS+IL−27) = 0.0105].
Discussion
IL-27 is a novel cytokine whose immune function has not yet been fully elucidated. Chiyo et al. reported that tumor cells expressing IL-27 activate CD4+ T helper cells, CD8+ cytotoxic T lymphocytes and natural killer cells (21, 22). IL-27 shows an effect of anti-tumor immunity as a possible therapeutic target for cancer (21). IL-27 plays an important role in T cell differentiation and regulation of T cell-mediated immune responses. For example, IL-27 produced by dendritic cells facilitates the polarization of T helper 1 cells in Lewis rats (23). Harker et al. recently found that IL-27-mediated signaling is necessary for anti-viral immunity (24). Moreover, IL-27 promotes differentiation of T helper 17 cells in vivo (25). Our data also indicate that IL-27 inhibits LPS-induced mature DC-mediated immune tolerance. These data suggest that IL-27 is a pro-inflammatory cytokine and a positive regulator in T cell-mediated immune responses.
Interestingly, the experimental data also indicate that IL-27 is an anti-inflammatory cytokine and inhibits development of autoimmunity in vivo. For instance, Mascanfroni et al. reported that IL-27 induces expression of CD39 on DCs and blocks development of T helper 1 and 17 cells to inhibit EAE induction (26). Tsoumarkidou et al. also found that tolerogenic CD1c+ DCs regulate development of Tregs via IL-27/IL-10 inducible co-stimulatory ligands (27). Rostami et al. have published data showing that the induction of peripheral tolerance is dependent on IL-27-mediated signal transduction pathway in DCs (28). These data suggest that regulatory mechanisms of IL-27 in the immune system are extremely complex and that IL-27 may play a dual role in equilibrium between autoimmunity and immune tolerance. Our results demonstrate that IL-27 does not affect immature DC-mediated immune responses but that it facilitates mature DC-mediated CD4+CD127+3G11+ Treg development. This suggests that the immune function of IL-27 on DCs is dependent on their maturation.
The cellular and molecular regulatory mechanisms of IL-27 have been recently investigated. For example, it is known that IL-27 can modulate development and biological function of T helper 17 cells, dendritic cells, NK cells and neutrophils (22, 25, 29, 30). IL-27 produced by DCs is necessary for trafficking of Tregs to locate in tumor (31), and pulmonary CD1c+ DC-mediated development of Tregs is dependent on IL-27/IL-10/inducible costimulator ligand (27). IL-27 also facilitates development of Treg and induces immune tolerance in vivo (32). By contrast, our data indicate that IL-27-treated mature DCs elicit development of CD4+CD127+3G11+ Treg subset and inhibits mature DC-mediated immune tolerance in vivo (Figure 7).
The immunological significance of this study is that we find a new subset of CD4+ Tregs mediated by 3G11 and CD127. Biological function of CD4+CD127+3G11+ Tregs may be different from that of conventional CD4+ Tregs. Previous studies showed that CD4+ Tregs is a negative regulator of autoimmunity. By contrast, the frequency of CD4+CD127+3G11+ Tregs increases in mice in which LPS-stimulated DC-mediated immune tolerance is inhibited. This new sub-population of Tregs may be a positive regulator which facilitates T cell-mediated immune responses in vivo. This has never been reported.
IL-27 can act as both pro-and anti-inflammatory cytokine, however, molecular mechanisms of IL-27 to modulate autoimmunity and immune tolerance have not yet been fully elucidated. Our data indicated that IL-27 does not affect immature DC-mediated immune responses, however, IL-27 can block immune tolerance induced by LPS-stimulated mature DCs. IL-27 acts as a pro-inflammatory cytokine to inhibit immune function of LPS-treated mature DCs. Interestingly, IL-27 only elicits development of CD4+CD127+3G11+ Tregs mediated by mature DCs induced by LPS. IL-27 does not affect that of Treg subset mediated by immature DCs. It may be dependent on maturation of DCs whether IL-27 plays a role of pro- or anti-inflammatory cytokine in vivo.
The interesting question is how IL-27 regulates LPS-stimulated mature DC-mediated immune tolerance in vivo. There is little amount of data to reveal it. The molecular mechanisms of IL-27 to block immune tolerance mediated by LPS-treated mature DCs should be investigated in the future so that a new immune therapy using CD4+CD127+3G11+ Treg sub-population can be designed to treat human diseases.
In summary, Our results imply that CD4+CD127+3G11+ cells may be a type of positive Tregs which are different from conventional CD4+ Tregs which inhibit autoimmunity in vivo. This new subset of Tregs are CD127 positive cells and conventional CD4+ Tregs express CD127 with low level, although both of them are CD4+CD25+FoxP3+GITR+ cells. Biological function of CD4+CD127+3G11+ Tregs may be different from that of conventional CD4+CD25+CD127lowFoxP3+GITR+ Tregs. Immune functions of this new CD4+CD127+3G11+ Treg subset should be investigated in future studies.
Ethics Statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Author Contributions
FZ designed and conducted experiments for this research project. G-XZ and AR supervised the research and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Mrs. Katherine Regan for proofreading the article.
Abbreviations
APC, Allophycocyanin; CD, Cluster of differentiation; CFA, Complete Freund's adjuvant; DC, Dendritic cell; EAE, Experimental autoimmune encephalomyelitis; FCS, Fetal Calf Serum; Fig, Figure; GM –CSF, Granulocyte-macrophage colony-stimulating factor; IL, Interleukin; i. p., Intraperitoneal; i.v., Intravenous; KLH, Keyhole limpet hemocyanin; LPS, Lipopolysaccharide; MOG, Myelin oligodendrocyte glycoprotein; 2-ME, 2-mercaptoethanol; MS, Multiple sclerosis; PBS, Phosphate-buffered saline; pDCs, Plasmacytoid dendritic cells; PI, Propidium Iodide; PT, Pertussis toxin; s.c., Subcutaneous; SD, Standard deviation; SEM, Standard error of arithmetic mean; Th, T helper cells.
References
1. Jones GW, Hill DG, Cardus A, Jones SA. IL-27: a double agent in the IL-6 family. Clin Exp Immunol. (2018) 193:37–46. doi: 10.1111/cei.13116
2. Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. (2007) 13:711–8. doi: 10.1038/nm1585
3. Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol. (2018) 19:583–93. doi: 10.1038/s41590-018-0102-6
4. Peters A, Fowler KD, Chalmin F, Merkler D, Kuchroo VK, Pot C. IL-27 induces Th17 differentiation in the absence of STAT1 signaling. J Immunol. (2015) 195:4144–53. doi: 10.4049/jimmunol.1302246
5. Visperas A, Do JS, Bulek K, Li X, Min B. IL-27, targeting antigen-presenting cells, promotes Th17 differentiation and colitis in mice. Mucosal Immunol. (2014) 7:625–33. doi: 10.1038/mi.2013.82
6. Sharma S, Khosla R, David P, Rastogi A, Vyas A, Singh D, et al. CD4+ CD25+ CD127low regulatory T cells play predominant anti-tumor suppressive role in hepatitis B virus-associated hepatocellular carcinoma. Front Immunol. (2015) 6:49. doi: 10.3389/fimmu.2015.00049
7. Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, et al. Increased CD127 expression on activated FOXP3+ CD4+ regulatory T cells. Eur J Immunol. (2010) 40:2528–38. doi: 10.1002/eji.201040531
8. Zhou F, Zhang GX, Rostami A. LPS-treated bone marrow-derived dendritic cells induce immune tolerance through modulating differentiation of CD4+ regulatory T cell subpopulations mediated by 3G11 and CD127. Immunol Res. (2017) 65:630–8. doi: 10.1007/s12026-016-8881-z
9. Zhou F, Zhang GX, Rostami A. 3G11 expression in CD4+ T cell-mediated autoimmunity and immune tolerance. Int Immunopharmacol. (2011) 11:593–6. doi: 10.1016/j.intimp.2010.11.005
10. Zhao Z, Ciric B, Yu S, Zhang GX, Rostami A. Targeting ganglioside epitope 3G11 on the surface of CD4+ T cells suppresses EAE by altering the Treg/Th17 cell balance. Int Immunol. (2010) 22:817–26. doi: 10.1093/intimm/dxq432
11. Zhao Z, Ciric B, Yu S, Li H, Yang J, Kamoun M, et al. Expression of 3G11 epitope defines subpopulations of regulatory T cells with different suppressive potency. J Neurol Sci. (2010) 295:66–74. doi: 10.1016/j.jns.2010.04.019
12. Zhang GX, Yu S, Calida D, Zhao Z, Gran B, Kamoun M, et al. Loss of the surface antigen 3G11 characterizes a distinct population of anergic/regulatory T cells in experimental autoimmune encephalomyelitis. J Immunol. (2006) 176:3366–73. doi: 10.4049/jimmunol.176.6.3366
13. Greer JM, Koerner TA, Hayakawa K, Hardy RR, Kemp JD. The 3G11+ antigen, a marker for murine CD4+ TH1 lymphocytes, is a ganglioside. Glycobiology (1993) 3:391–401. doi: 10.1093/glycob/3.4.391
14. Schmetterer KG, Pickl WF. The IL-10/STAT3 axis: contributions to immune tolerance by thymus and peripherally derived regulatory T-cells. Eur J Immunol. (2017) 47:1256–65. doi: 10.1002/eji.201646710
15. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science (2015) 348:589–94. doi: 10.1126/science.aaa7017
16. Zhou F, Zhang GX, Rostami A. Apoptotic cell-treated dendritic cells induce immune tolerance by specifically inhibiting development of CD4+ effector memory T cells. Immunol Res. (2016) 64:73–81. doi: 10.1007/s12026-015-8676-7
17. Zhou F, Ciric B, Zhang GX, Rostami A. Immunotherapy using lipopolysaccharide-stimulated bone marrow-derived dendritic cells to treat experimental autoimmune encephalomyelitis. Clin Exp Immunol. (2014) 178:447–58. doi: 10.1111/cei.12440
18. Zhou F, Lauretti E, di Meco A, Ciric B, Gonnella P, Zhang GX, et al. Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology (2013) 218:1069–76. doi: 10.1016/j.imbio.2013.02.003
19. Zhou F, Ciric B, Zhang GX, Rostami A. Immune tolerance induced by intravenous transfer of immature dendritic cells via up-regulating numbers of suppressive IL-10+ IFN-gamma+-producing CD4+ T cells. Immunol Res. (2013) 56:1–8. doi: 10.1007/s12026-012-8382-7
20. Zhou F, Ciric B, Li H, Yan Y, Li K, Cullimore M, et al. IL-10 deficiency blocks the ability of LPS to regulate expression of tolerance-related molecules on dendritic cells. Eur J Immunol. (2012) 42:1449–58. doi: 10.1002/eji.201141733
21. Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer (2005) 115:437–42. doi: 10.1002/ijc.20848
22. Matsui M, Kishida T, Nakano H, Yoshimoto K, Shin-Ya M, Shimada T, et al. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. (2009) 69:2523–30. doi: 10.1158/0008-5472.CAN-08-2793
23. Saito F, Ohno Y, Morisawa K, Kamakura M, Fukushima A, Taniguchi T. Role of IL-27-producing dendritic [corrected] cells in Th1-immunity polarization in Lewis rats. Biochem Biophys Res Commun. (2005) 338:1773–8. doi: 10.1016/j.bbrc.2005.10.149
24. Harker JA, Wong KA, Dallari S, Bao P, Dolgoter A, Jo Y, et al. IL-27R signalling mediates early viral containment and impacts innate and adaptive immunity after chronic lymphocytic choriomeningitis virus infection. J Virol. (2018) 92:JVI.02196-17. doi: 10.1128/JVI.02196-17
25. Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity (2008) 29:68–78. doi: 10.1016/j.immuni.2008.05.008
26. Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. (2013) 14:1054–63. doi: 10.1038/ni.2695
27. Tsoumakidou M, Tousa S, Semitekolou M, Panagiotou P, Panagiotou A, Morianos I, et al. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. J Allergy Clin Immunol. (2014) 134:944–54.e948. doi: 10.1016/j.jaci.2014.05.045
28. Thome R, Moore JN, Mari ER, Rasouli J, Hwang D, Yoshimura S, et al. Induction of peripheral tolerance in ongoing autoimmune inflammation requires interleukin 27 signaling in dendritic cells. Front Immunol. (2017) 8:1392. doi: 10.3389/fimmu.2017.01392
29. Quirino GFS, Nascimento MSL, Davoli-Ferreira M, Sacramento LA, Lima MHF, Almeida RP, et al. Interleukin-27 (IL-27) mediates susceptibility to visceral leishmaniasis by suppressing the IL-17-neutrophil response. Infect Immun. (2016) 84:2289–98. doi: 10.1128/IAI.00283-16
30. Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, et al. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer (2009) 124:1372–8. doi: 10.1002/ijc.24107
31. Xia S, Wei J, Wang J, Sun H, Zheng W, Li Y, et al. A requirement of dendritic cell-derived interleukin-27 for the tumor infiltration of regulatory T cells. J Leukoc Biol. (2014) 95:733–42. doi: 10.1189/jlb.0713371
32. van Leeuwen MA, Costes LMM, van Berkel LA, Simons-Oosterhuis Y, du Pre MF, Kozijn AE, et al. Macrophage-mediated gliadin degradation and concomitant IL-27 production drive IL-10- and IFN-gamma-secreting Tr1-like-cell differentiation in a murine model for gluten tolerance. Mucosal Immunol. (2017) 10:635–49. doi: 10.1038/mi.2016.76
Keywords: dendritic cell, immune tolerance, immunotherapy, regulatory T cell, IL-27
Citation: Zhou F, Zhang G-X and Rostami A (2018) Distinct Role of IL-27 in Immature and LPS-Induced Mature Dendritic Cell-Mediated Development of CD4+ CD127+3G11+ Regulatory T Cell Subset. Front. Immunol. 9:2562. doi: 10.3389/fimmu.2018.02562
Received: 16 July 2018; Accepted: 17 October 2018;
Published: 13 November 2018.
Edited by:
Djordje Miljkovic, University of Belgrade, SerbiaReviewed by:
Bruce Milne Hall, University of New South Wales, AustraliaHarley Y. Tse, Wayne State University, United States
Copyright © 2018 Zhou, Zhang and Rostami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhou, emhvdV9mYW5nQGNhc2xhbXZhYy5jb20=
Abdolmohamad Rostami, YS5tLnJvc3RhbWlAamVmZmVyc29uLmVkdQ==
†Present Address: Fang Zhou, Department of Clinical and Experimental Immunology, CAS Lamvac Biotech Co., Ltd. Guangzhou, China
 Fang Zhou
Fang Zhou Guang-Xian Zhang
Guang-Xian Zhang