Abstract
Purpose: The investigation of anti-inflammatory and immunosuppressive functions of Kynurenic acid (KYNA) is now in focus. There is also substantial evidence that TSG-6 has an anti-inflammatory activity. Therefore, in the present study, we compared the effects of newly synthetized KYNA analogs on the TNF-α production in U-937 monocytic cells in correlation with the effects on the TSG-6 expression.
Methods: TNF-α production was measured by ELISA, the TSG-6 expression was determined by RTqPCR method. As cytokine inducers Staphylococcus aureus and Chlamydia pneumoniae were used.
Results: KYNA and KYNA analogs attenuated TNF-α production and increased TSG-6 mRNA expression in U-937 cells stimulated by heat inactivated Staphylococcus aureus. In contrast, KYNA and some of the KYNA analogs increased the TNF-α production of C. pneumoniae infected U-937 cells; however, the newly synthetized analogs (SZR104, SZR 105, and SZR 109) exerted significant inhibitory effects on the TNF-α synthesis. The inhibitory and stimulatory effects correlated inversely with the TSG-6 expression.
Conclusions: TSG-6 expression following activation with bacterial components could participate in the suppression of inflammatory cytokines, such as TNF-α, We suppose that the elevation of the TSG-6 expression by KYNA and especially by new KYNA analogs might be one of the mechanisms that are responsible for their suppressive effect on TNF-α production as a feedback mechanism. KYNA and KYNA analogs have an important role in influencing TSG-6 expression, and there is a possible benefit of targeting TSG-6 expression by kynurenines in inflammatory conditions following infections.
Introduction
There is an increasing interest in the role of kynurenines in the immune function. The kynurenine pathway is a regulator of both innate and adaptive immune responses, and the tryptophan metabolism kynurenine and production reflect a crucial interface between the immune and nervous systems (1, 2). Kynurenic acid (KYNA) is one of the products of the kynurenine pathway of tryptophan metabolism (3–5). KYNA as an antagonist of ionotropic glutamate receptors N-methyl-D-aspartate (NMDA) and the α7 nicotinic acetylcholine receptor (α7nAchR) exert neuroprotective effects (2, 4–10). KYNA acts both as a blocker of the glycine co-agonistic site of the NMDA receptor and as a non-competitive inhibitor of the α7 nicotinic acetylcholine receptor (11). The investigation of anti-inflammatory and immunosuppressive functions of KYNA is now in focus. It has been proved that these immunomodulatory properties are based on the signaling by G-protein-coupled receptor 35 (GP35) and aryl hydrocarbon receptor (AHR)-mediated pathways ys (2, 12–14).
Several studies have revealed that KYNA can attenuate inflammation induced by different stimuli (2, 15, 16). Previously, we demonstrated that KYNA and a KYNA analog reduced the TNF-α secretion from human mononuclear cells (17). In the present study, we compared the effects of newly synthetized KYNA analogs on the α TNF-α production in U-937 monocytic cell line. We focused on the potential correlation between the effects on the TSG-6 (TNFα- stimulated gene 6) expression and the influence, i.e., the suppression, of TNF-α production by different KYNA analogs.
Tumor necrosis factor -stimulated gene-6 (TSG-6) product is an 35-kDa hyaluronan(HA)-binding protein (18, 19) that is secreted by a wide range of cell types in response to inflammatory mediators. TSG-6 expression has been shown to be induced in fibroblasts, chondrocytes, monocytes, mesenchymal stem cells, vascular smooth muscle cells upon stimulation by proinflammatory signals (20). Moreover, TSG-6 is expressed by astrocytes in the brain (21). A substantial number of studies have shown that TSG-6 has anti-inflammatory activity (18, 20, 22–27).
TSG-6 has been reported to inhibit the association of TLR4 with MyD88, thereby suppressing NF-κB activation (26). TSG-6 has also prevented the expression of proinflammatory proteins (iNOS, IL-6, TNFα, IL-1β). TSG-6 functions by converting macrophages from a proinflammatory to an anti-inflammatory phenotype by suppression of TLR4/NF-κB signaling and STAT1 and STAT3 activation (26).The inhibition of the TLR2 pathway has also been reported (28).
Therefore, the aim of the present study is to evaluate a possible connection between the capacity of KYNA and KYNA analogs on the TSG-6 expression and the inhibition of TNF-α production first of all in U-937 monocytic cells. Our hypothesis was that activation of TSG-6 expression might be at least partially responsible for the TNF-α inhibitory effect of KYNA. TNF-α induction in U-937 cells was performed with heat killed Staphylococcus aureus, and the effects were compared with Chlamydia pneumoniae (C. pneumoniae). Staphylococcus aureus is a Gram-positive pyogenic coccus and a good inducer of TNF in mononuclear cells, and it mimics natural conditions (29, 30). Chlamydia pneumoniae is a Gram-negative bacterium, growing intracellularly, and it is responsible for different inflammatry conditions, especially in the lungs and in atherosclerosis. Chlamydia pneumoniae attach monocytes and multiply in them (31).The main question was, whether the production of TNF-α, and TSG-6 could be induced by these criteriae in U-937 cells. It was demonstrated in a previous study, that C.pneumoniae upregulated. numerous inflammatory genes in U-937 cells (32).
Materials and Methods
Reagents
KYNA (Kynurenic acid) was purchased from Sigma-Aldrich (Steinheim, Germany). Compounds SZR-72, SZR-73, and SZR-81 were synthesized by direct amidation of KYNA (33). In case of SZR-104, SZR-105, and SZR-109, the syntheses were achieved starting from the corresponding amides followed by C-3 aminoalkylation with morpholine or with diethylamine in the presence of formaldehyde (34, 35) (Table 1). KYNA and the analogs were dissolved in phosphate buffered saline (PBS) and added in increasing concentration in the μM range to the cell cultures.
Table 1
| Code | Structure | Chemical name | Empirical formula and Mw |
|---|---|---|---|
| KYNA | 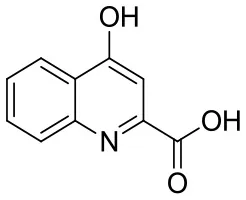 | 4-hydroxyquinolin-2-carboxylic acid | C10H7NO3 189.17 |
| SzR-72 | 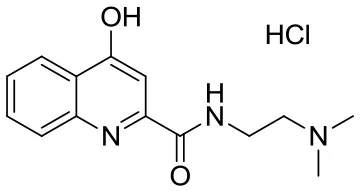 | N-(2-(dimethylamino)ethyl)-4-hydroxyquinoline-2-carboxamide hydrochloride | C14H18ClN3O2 295.76 |
| SzR-73 | 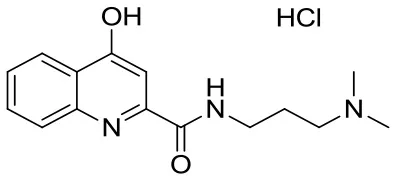 | N-(3-(dimethylamino)propyl)-4-hydroxyquinoline-2-carboxamide hydrochloride | C15H20ClN3O2 309.79 |
| SzR-81 | 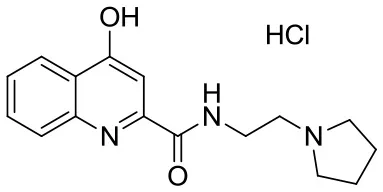 | N-(2-(pyrrolidin-1-yl)ethyl)-4-hydroxyquinoline-2-carboxamide hydrochloride | C16H20ClN3O2 321.80 |
| SzR-104 | 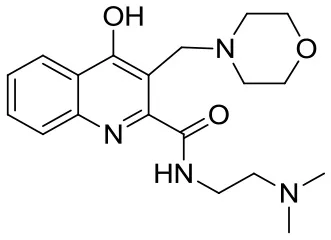 | N-(2-(dimethylamino)ethyl)-3-(morpholinomethyl)-4-hydroxyquinoline-2-carboxamide | C19H26N4O3 358.43 |
| SzR-105 | 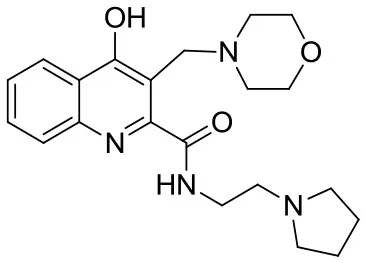 | N-(2-(pyrrolidin-1-yl)ethyl)-3-(morpholinomethyl)-4-hydroxyquinoline-2-carboxamide | C21H28N4O3 384.47 |
| SzR-109 | 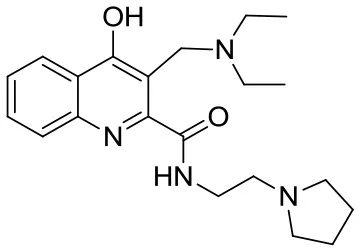 | N-(2-(pyrrolidin-1-yl)ethyl)-3-((diethylamino)methyl)-4-hydroxyquinoline-2-carboxamide | C21H30N4O2 370.49 |
KYNA and KYNA analogs used in the experiments.
Cell Lines and Infection
U-937 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (Biowest, Nuaille, France), 2 mmol/L L-glutamine, 1x nonessential amino acids, HEPES 4 mmol/L, 25 μg/mL gentamicin, and 0.5 μg/mL fungizone. HEp-2 cells were maintained in minimal essential medium (MEM) with Earle's salts completed with 10% FBS, 2 mmol/L L-glutamine, 1x nonessential amino acids, 25 μg/mL gentamicin, and 0.5 μg/mL fungizone. All reagents were purchased from SIGMA, St. Louis, MO, USA, unless otherwise indicated. The cell lines were purchased from ATCC. For TNF-α and TSG-6 induction, 5 × 105 U-937 cells/mL were infected with 107 heat inactivated Staphylococcus aureus (S.aureus), or with 5 MOI (multiplicity of infection) Chlamydia pneumoniae. Cell supernatants were tested for TNF-α content by ELISA and cell lysates for TSG-6 mRNA by RT qPCR.
Bacterial Strains
Staphylococcus aureus (S. aureus, SA1) 108 /mL, were heat inactivated (29) and were used as a TNF-α inducer (30).
Chlamydia pneumoniae (C. pneumoniae) CWL029 strain from American Types Culture Collection (ATCC) was propagated in HEp-2 cells. Infective chlamydiae were quantitated by indirect immunofluorescent method applying anti-Chlamydia lipopolysaccharide (cLPS) monoclonal antibody (AbD Serotec, Oxford, United Kingdom) and FITC-labeled anti-mouse IgG (Sigma-Aldrich, St. Louis, MO). The concentration of infective elementary bodies (EB)-s was expressed as inclusion forming units/mL (IFU/mL).
Stimulation of U 937 Cells by Bacteria Infection
(a) U-937 cells (5 × 105 cells/mL) were stimulated with 107 heat inactivated S. aureus (29) as a TNF inducer (30) and were incubated for 24 h in CO2 incubator at 37°C in complete RPMI. In parallel experiments, the cell cultures were pretreated for 30 min with KYNA and KYNA analoques at a concentration of 250–500 μM. In our prevous experiments (17), these concentrations proved to be optimal in reducing cytokine production. Cell supernatants were tested for TNF-α and TSG-6 content by ELISA and cell lysates for TSG-6 mRNA by RT qPCR.
(b) U-937 cells were seeded in 24-well plates (5 × 105 cells/well), and the cells were then infected with C. pneumoniae at a multiplicity of infection (MOI) of 5 in complete RPMI with 0.5% glucose and centrifuged at 800 × g for 1 h RT. The growth medium was replaced in the wells with a medium containing KYNA analogs at a concentration of 250–500 μM. The culture plates were incubated for 24 h in CO2 incubator at 37°C. Cell supernatants were tested for TNF-α and TSG-6 content by ELISA and cell lysates for TSG-6 mRNA by RT qPCR.
Chlamydial DNA Quantitation
For the quantitative assessment of chlamydial replication, a direct DNA quantitation method was used (36). The cells in the 96-well plates were infected with C. pneumoniae at a multiplicity of infection (MOI) of 5. After 24 and 48 h, the infected cells in 3 parallel wells were washed in the plates twice with 200 μL/well phosphate buffered saline (PBS). Then 100 μL Milli-Q water was added to the wells, and the plates were stored at −80°C. In order to free the DNA from the cells, two freeze-thaw cycles were applied. Thoroughly mixed lysates were used as templates directly for quantitative PCR (qPCR) using SsoFast™ EvaGreen® Supermix (BioRad). For the detection of C. pneumoniae DNA, the following primers were used: ompA F: 5′ TGCGACGCTATTAGCTTACGT 3′ and ompA R: 5′ TAGTTTGCAGCAGCGGATCCA 3′. A BLAST search was performed to check the specificity of the product target sequence of the primer sets. The primers were synthetized by Integrated DNA Technologies Inc. (Montreal, Quebec, Canada). During qPCR reaction, after the 10 min at 95°C polymerase activation step, 40 PCR cycles of 20 s at 95°C, and 1 min at 64°C were performed. The fluorescence intensity was measured at the end of the annealing–extension step. The specificity of amplification was confirmed by the melting curve analysis. For each PCR, the cycle threshold (Ct) corresponding to the cycle, where the amplification curve crossed the base line, was determined. The difference in Ct values detected in the samples incubated with KYNA and the analogs at a concentration of 250 and 500 μM compared to that of the untreated samples was calculated.
TNF-α ELISA
The TNF-α concentrations in the supernatants were quantified by using the TNF-α ELISA kit (Legend Max BioLegend San Diego) according to the instructions of the manufacturer.
TSG-6 ELISA
The TSG-6 concentrations in the supernatants were quantified by using the TSG-6 ELISA kit (SIGMA U.S.A. St. Louis) according to the instructions of the manufacturer.
TSG-6 mRNA Quantification by Reverse Transcription Quantitative PCR (RT qPCR)
Total RNA was extracted from the samples by using TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's protocol. The quality and the quantity of the extracted RNA were assessed by a NanoDrop Lite spectrophotometer (Thermo Scientific, Waltham, MA, USA). First-strand cDNA was synthesized by using 2 μg of total RNA with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) strictly adhering to the manufacturer's recommendations. The qPCR was conducted with cDNA, 1 μL of primers (10 μM) and SensiFast SYBR® No-ROX Mix (Bioline GmbH, Luckenwalde, Germany) in a total volume of 10 μL. The primers used in the assay were the following: TSG-6 sense 5′- ACT CAA GTA TGG TCA GCG TAT TC−3′, TSG-6 antisense 5′- GCC ATG GAC ATC ATC GTA ACT−3′; β-actin sense 5′- TTC TAC AAT GAG CTG CGT GTG GCT−3′, and β-actin antisense 5′- TAG CAC AGC CTG GAT AGC AAC GTA−3′. All primers were synthetized by Integrated DNA Technologies Inc. (Montreal, Quebec, Canada). The RT-qPCR was performed in a CFX96 Touch PCR detection system (Bio-Rad, Hercules, CA, USA). Thermal cycling was initiated with a denaturation step of 2 min at 95 °C followed by 40 cycles each of 10 s at 95°C and 1 min at 60°C. The fluorescence intensity was detected at the end of the annealing–extension steps. The specificity of amplification was confirmed by carrying out a melting curve analysis. The cycle threshold (Ct) corresponding to the cycle, where the amplification curve crossed the base line, was determined. The Ct of target transcripts was compared with that of β-actin, the difference being referred to as ΔCt. The relative expression level was given as 2−(ΔΔCt), where ΔΔCt = ΔCt for the experimental sample minus ΔCt for the control sample. Increases in transcripts >2-fold compared to the control samples were considered to be significant. Uninfected cells were used as controls. All of the measurements were performed in duplicate from 3 biological repetitions.
Human Blood Samples
EDTA-anticoagulated peripheral blood samples from 10 healthy volunteers were obtained.
Samples (1 mL each) were incubated in the presence of heat inactivated S, aureus for 18 hr. Parallel blood samples were pretreated for 30 min with KYNA and KYNA analogs at a concentration of 500 μM. Following the incubation period, the blood samples were centrifuged at 300 × g, and the supernatants were tested for TNF-α and TSG-6 content by ELISA.
For the experiments performed with the human blood we have the approval of the ethics commitee of the Medical Faculty of the University. of Szeged (ETT-TUKEB 905/PI/09). This study was conducted in full accordance with the tenets of Declaration of Helsinki (1964).
Statistical Analysis
Data are expressed as means ± SD. Differences between group means were determined by the unpaired Student t-test. p-values < 0.05 were considered significant. Data of box and whiskers analysis were evaluated by Mann-Whitney test. The correlation between the TNF-α production and expression of TSG-6 was evaluated by correlation analysis. All statistical calculations were performed with the Graph-Pad Prism 5 statistical program (GraphPad Software Inc., San Diego, CA, USA).
Results
KYNA and KYNA Analogs Attenuate TNF-α Production in U-937 Human Monocytic Cells Stimulated by Heat Inactivated Staphylococcus aureus
The maximum TNF-α concentrations in the supernatants in SA1-induced cultures of U-937 cells without pretreatment of KYNA and derivates were 95. ± 8.5 pg/mL. At a concentration of 500 μM, all KYNA analogs suppressed the TNF-α level significantly, except SZR 73 (Figure 1). The new analogs SZR 104, 105, and 109 exerted the most potent inhibitory effects (p < 0.001) in equimolar (500 μM) concentration. Results obtained with 500 μM of the chemicals are demonstrated in Figure 1. In our previous experiments (17), 25 μM KYNA and SZR72 proved to be ineffective. At increasing concentrations (125, 250, and 500 μM), KYNA and SZR72 exhibited increasing inhibitory effects on TNF-α production. Similar results were obtained in the present experiments (data not shown), but only the result with the most effective concentration (500 μM) is demonstrated in this paper (Figure 1).
Figure 1
KYNA and KYNA Analogs Increase TSG-6 mRNA Expression in U-937 Cells
To gain further insight into the connection between the inhibition of TNF-α production and the induction of TSG-6 expression exerted by KYNA analoques, we determined the effects of KYNA analoques on TSG-6 mRNA expression. Both KYNA and KYNA analogs increased the TSG-6 relative expression at equimolar concentrations of 500 μM (Figure 2) significantly. SZR 73 was not effective in this respect, similarly as it was observed in the experiments with TNF-α production. Thus, we suspect that there is a connection between the attenuation of SA1-induced TNF protein synthesis and the TSG-6 gene transcription, which is elevated by KYNA and KYNA analoques.
Figure 2
KYNA and the KYNA Analogs Differently Influence TNF-α Production Induced by C. pneumoniae in U-937 Human Monocytic Cells
We wanted to compare the effects of KYNA and KYNA derivates on TNF-α production when the inducer is a Gram-negative, intracellular bacterium, i.e., Chlamydia pneumoniae (C. pneumoniae). Our results were unexpected; instead of having inhibitory effects, KYNA and some of the KYNA analoques increased the TNF-α production of C. pneumoniae infected U-937 cells. In contrast, the newly synthetized analogs (SZR104, SZR 105, and SZR 109) exerted a significant inhibitory effect on the cytokine synthesis (Figure 3).
Figure 3
KYNA and KYNA Analogs Differently Influence TSG-6 mRNA Expression in U-937 Cells Infected With Chlamydia pneumoniae
C. pneumoniae induced a considerable TSG-6 expression in U-937 cells. KYNA, SZR72, and SZR81 inhibited the rate of expression (Figure 4A). Interestingly, the same chemicals enhanced the TNF-α production of C. pneumoniae-induced U-937 cells (Figure 3). On the other hand, further KYNA analoques (SZR 104, SZR 105, and SZR 109) with different chemical structure (see Table 1) stimulated TSG-6 expression (Figure 4B). It is also noteworthy that only these analoques inhibited significantly the TNF-α production of C. pneumoniae-induced U-937 cells (Figure 3). Considering the variable effects of KYNA analogs on the TSG-6 expression and also on the TNF-α production, we checked the correlation between the two effects. As it was expected, a significant inverse correlation was found between the effects on the TNF-α secretion and the TSG-6 expression exerted by different KYNA analogs (Figure 5). KYNA, SZR72, and SZR81 induced higher TNF-α secretion by U-937 cells after C. pneumoniae infection, but they decreased the TSG-6 expression compared to the cells that were infected only with C. peumomiae, without any of the compounds (i.e., Cpn in Figure 5). In contrast, in the case of the highest rate of TSG-6 expression (SZR 105), a maximal rate of inhibition of TNF-α production was observed. Therefore, we suppose that the different effects of KYNA analoques on the TSG-6 expression in C. pneumoniae infected cells might explain the difference in their effects on the secretion of TNF-α.
Figure 4
Figure 5
Altogether, from these data, it seems that inhibition of TNF-α is not only in correlation with the antiinflammatory effect of TSG-6, but in this situations, KYNA analogs are able to increase or even decrease the expression of TSG-6.
Effects of KYNA Analoques on the Quantity of C. pneumoniae
To ascertain that the effects of KYNA analoques on the TNF-α or TSG-6 induction is not simply due to their effects on the replication of C. pneumoniae, we performed experiments for quantitative assessment of chlamydial replication by a direct quantitative PCR method (36). C. pneumoniae ompA gene was detected in the lysate of U-937 cells infected with C. pneumoniae at a MOI of 5 in the presence or absence of KYNA analoques at a concentration of 250 or 500 μM, respectively. Direct detection of C. pneumoniae DNA in the lysate of infected cells was done at 24 and 48 h postinfection. There was no significant inhibition or even elevation in the quantity of chlamydial DNA in the presence of different KYNA analoques after the 24 h (open bars) or 48 h (filled bars) incubation period. The results of the samples tested at 24 and 48 h of incubation are presented in Figure 6. Therefore, we assume that KYNA analoques do not influence the replication or the quantity of C. pneumoniae.
Figure 6
Effects of KYNA Analogs on TGS-6 Protein Production in U-937 Human Monocytic Cells Stimulated With Heat Inactivated S. aureus or by Chlamydia pneumoniae
To ascertain whether the effects of KYNA and analogs on the TSG-6 expression influence parallelly the protein level, the TSG-6 concentrations in the supernatants of U-937 cells were determined.
At a concentration of 500 μM, KYNA and KYNA analogs increased the TGF-6 level significantly, except SZR 73 in SA1 induced cells (Figure 7A). The new analogs SZR 104, 105, and 109 exerted the most potent stimulatory effects (p < 0.001) in equimolar (500 μM) concentration. C. pneumoniae induced also TSG-6 production in U-937 cells, but KYNA, SZR72, and SZR81 decreased the level of TSG-6 protein expression (Figure 7B). On the other hand, further KYNA analoques (SZR 104, SZR 105, and SZR 109) increased the TSG-6 concentration in the supernatants (7b). These experiments obtained with 500 μM of KYNA and KYNA analogs support the results obtained with RT PCR data demonstrating the effects of the chemicals on the TSG-6 RNA expression.
Figure 7
KYNA Analogs SZR 72 and SZR 105 Attenuate TNF-α Production and Increase TSG-6 Secretion in Human Whole Blood Cells Stimulated by Heat Inactivated Staphylococcus aureus
Some of the results obtained by in vitro experiments with U-937 monocytic cells were repeated by “ex vivo” experimets investigating the effects of two KYNA analogs in human peripheral blood.
There was big individual differences in the TNF-α concentrations and in TSG-6 concentrations in the supernatants in SA1-induced blood cultures (Figure 8), between 179 pg/ml and 850 pg/ml, and between 150 and 750 pg/ml, respectively. At a concentration of 500 μM, both SZR 72 and SZR 105 suppressed the TNF-α level significantly in the S. aureus induced blood cultures. Again, the new analog SZR 105 exerted more potent inhibitory effect (p = 0.001) in equimolar (500 μM) concentration. Similarly to the effects on U-937 cells, the KYNA analogs SZR72 and SZR 105 significantly increased the TSG-6 concentrations in SA1 induced blood samples (Figure 8).
Figure 8
Discussion
In our experiments, KYNA and different KYNA derivates inhibited the TNF-α production of U-937 cells stimulated with heat inactivated Staphylococcus aureus. The rate of the inhibition was variable according the structure of the analoques (Figure 1). The effect of the analogs were compared in equimolar concentration on the TNF-α production when the inducer was Chlamydia pneumoniae, a Gram negative, intracellular bacterium. In these experiments, however, not all KYNA derivates inhibited TNF-α production by U-937 monocytic cells; moreover, KYNA itself, and SZR72 and SZR81 increased it (Figure 3). We hypothesized that the difference in the influence on the TNF-α production might be connected with the difference in the TSG-6 expression (Figure 4).
The production of TNF-α in C. pneumoniae infected cells was inhibited only by the KYNA derivates (SZR 104, SZR105, SZR109) that upregulated the expression of TSG-6 (Figures 4, 5).
It is noteworthy, that TSG-6 itself does not only exert an antiinflammatory effect (20, 26, 27), but its expression might be under the influence of KYNA (37). It has been published that kynurenic acid controls TSG-6-mediated immunosuppression of the human mesenchymal stem cells (MSCs). In elegant experiments, it has been demonstrated that KYNA specifically regulates TSG-6 production by activating aryl hydrocarbon receptor (AHR). KYNA activates AHR, which directly binds to the TSG-6 promoter to enhance TSG-6 expression. Moreover, KYNA-pretreated MSCs can further boost TSG-6 production, and thus enhance the therapeutic capacity of human MSCs against lipopolysaccharide (LPS)-induced acute lung injury (37).
We found that in most experiments, TSG-6 expression was up-regulated in U-937 monocytic cells stimulated with bacterial components, and KYNA and KYNA analogs were able to influence the rate of expression of TSG-6. The elevation of the TSG-6 expression might be one of the mechanisms that are responsible for the suppression of TNF-α production as a feedback effect. This effect was clearly demonstrated in our experiments using heat inactivated S. aureus as a cytokine inducer. In the case of C. pneumoniae infection, however, KYNA and KYNA analoques did not exert this effect uniformly. Some of them increased TSG-6 expression with a concomitant inhibition of the production of TNF-α, but several compounds (KYNA, SZR72, and SZR 81) rather decreased the expression of TSG-6, and it is very likely that this could lead to an elevated TNF-α production compared to the TNF-α production of U-937 cells infected with C. pneumoniae without any KYNA analoque. We hypothesized that the explanation of the difference in the results might be due to the different chemical structure of the analoques (see Table 1). The examined substrates (SZR-72, SZR-73 SZR-81, SZR-104, SZR-105, and SZR-109) can be classified into two classes of compounds: the first are amide derivatives (SZR-72, SZR-73, SZR-81) containing cationic center at the amide side chain. The second class of compounds (SZR-104, SZR-105, and SZR-109) are the C-3 aminoalkylated amides, therefore they can be interpreted as derivatives with dual cationic centers.
They could differently influence the binding of C. pneumoniae to the Toll-like receptor 2 (TLR2), and especially, differently activate AHR in the presence of C. pneumoniae. It has to be highlighted that the newly synthetized analogs, SZR 105 and SZR 109, were the most potent inducers of TSG-6 expression, and the highest inhibitors of TNF-α production in both types of bacterial inducers. The study of the exact effect of Chlamydia pneumoniae on the interaction between AHR and some KYNA analogs needs to be further investigated and proved.
Whatever the explanation is, our results indicate that there is a close connection between TNF production and TSG-6 expression, and there is an inverse correlation between the TSG-6 expression and TNF-α production in the presence of KYNA and KYNA analogs.
This negative correlation was further demonstrated at the protein level of TSG-6 measured in the supernatants of U-937 cells. and also in unseparated human peripheral blood samples
The stimulation of TSG-6 expression by KYNA and KYNA derivates might be one of the mechanisms that have an important role in their suppressive effect on TNF-α production. TSG-6 expression following activation with bacterial components could participate in the suppression of inflammatory cytokines, such as TNF-α, and it is noteworthy that KYNA and especially KYNA analogs are able to enhance this effect. Further studies are required to elucidate the different effects of KYNA derivates in the case of different bacterial inducers and the possible benefits of targeting TSG-6 expression by kynurenines in inflammatory conditions following infections.
Statements
Data availability statement
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Ethics statement
For the experiments performed with the human blood we have the approval of the ethics commitee of the Medical Faculty of the University of Szeged (ETT-TUKEB 905/PI/09). This study was conducted in full accordance with the tenets of Declaration of Helsinki (1964).
Author contributions
YM designed research and wrote the manuscript. VE, KB, and IL conducted experiments with Chlamydia. TM performed experiments with RT-PCR. FF, IS, and BL contributed new reagents. AB provided the blood samples, YM and VE analyzed data. LV organized research for neurological project. All authors read and approved the manuscript.
Funding
This work was supported by GINOP 2.3.2-2015-16-00034 and Ministry of Human Capacities, Hungary Grant, 20391-3/2018/FEKUSTRAT.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
MándiYVécseiL. The kynurenine system and immunoregulation. J Neural Transm Vienna Austr. (2012) 119:197–209. 10.1007/s00702-011-0681-y
2.
WirthgenEHoeflichAReblAGüntherJ. Kynurenic Acid: the janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. (2017) 8:1957. 10.3389/fimmu.2017.01957
3.
SwartzKJDuringMJFreeseABealMF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci Off J Soc Neurosci. (1990) 10:2965–73.
4.
StoneTW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. (2000) 21:149–54. 10.1016/S0165-6147(00)01451-6
5.
StoneTW. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci. (2007) 25:2656–65. 10.1111/j.1460-9568.2007.05540.x
6.
VécseiLMillerJMacGarveyUBealMF. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res Bull. (1992) 28:233–8.
7.
VécseiLSzalárdyLFülöpFToldiJ. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. (2013) 12:64–82. 10.1038/nrd3793
8.
RobotkaHToldiJVécseiL. L-kynurenine: metabolism and mechanism of neuroprotection. Future Neurol. (2008) 3:169–88. 10.2217/14796708.3.2.169
9.
VamosEPardutzAKlivenyiPToldiJVecseiL. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J Neurol Sci. (2009) 283:21–27. 10.1016/j.jns.2009.02.326
10.
SchwarczRBrunoJPMuchowskiPJWuH-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. (2012) 13:465–77. 10.1038/nrn3257
11.
HilmasCPereiraEFAlkondonMRassoulpourASchwarczRAlbuquerqueEX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci Off J Soc Neurosci. (2001) 21:7463–73. 10.1523/JNEUROSCI.21-19-07463.2001
12.
WangJSimonaviciusNWuXSwaminathGReaganJTianHet al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. (2006) 281:22021–8. 10.1074/jbc.M603503200
13.
JulliardWFechnerJHMezrichJD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. (2014) 5:458. 10.3389/fimmu.2014.00458
14.
WirthgenEHoeflichA. Endotoxin-induced tryptophan degradation along the kynurenine pathway: the role of indolamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J Amino Acids. (2015) 2015:973548. 10.1155/2015/973548
15.
KiankCZedenJ-PDrudeSDomanskaGFuschGOttenWet al. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE. (2010) 5:e11825. 10.1371/journal.pone.0011825
16.
MałaczewskaJSiwickiAKWójcikRMTurskiWAKaczorekE. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes - in vitro and ex vivo studies. Cent Eur J Immunol. (2016) 41:39–46. 10.5114/ceji.2016.58815
17.
TiszlaviczZNémethBFülöpFVécseiLTápaiKOcsovszkyIet al. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch Pharmacol. (2011) 383:447–55. 10.1007/s00210-011-0605-2
18.
LeeTHWisniewskiHGVilcekJ. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. (1992) 116:545–57.
19.
LesleyJGálIMahoneyDJCordellMRRuggMSHymanRet al. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J Biol Chem. (2004) 279:25745–54. 10.1074/jbc.M313319200
20.
MilnerCMDayAJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. (2003) 116:1863–73. 10.1242/jcs.00407
21.
Coulson-ThomasVJLauerMESolemanSZhaoCHascallVCDayAJet al. Tumor necrosis factor-stimulated gene-6 (TSG-6) is constitutively expressed in adult central nervous system (CNS) and associated with astrocyte-mediated glial scar formation following spinal cord injury. J Biol Chem. (2016) 291:19939–52. 10.1074/jbc.M115.710673
22.
LeeRHPulinAASeoMJKotaDJYlostaloJLarsonBLet al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. (2009) 5:54–63. 10.1016/j.stem.2009.05.003
23.
WisniewskiHGMaierRLotzMLeeSKlampferLLeeTHet al. TSG-6: a TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J Immunol Baltim Md. (1993) 151:6593–01.
24.
OhJYRoddyGWChoiHLeeRHYlöstaloJHRosaRHet al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci USA. (2010) 107:16875–80. 10.1073/pnas.1012451107
25.
DanchukSYlostaloJHHossainFSorgeRRamseyABonvillainRWet al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res Ther. (2011) 2:27. 10.1186/scrt68
26.
MittalMTiruppathiCNepalSZhaoY-YGrzychDSoniDet al. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc Natl Acad Sci USA. (2016) 113:E8151–8. 10.1073/pnas.1614935113
27.
DayAJMilnerCM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. (2019) 78–79:60–83. 10.1016/j.matbio.2018.01.011
28.
ChoiHLeeRHBazhanovNOhJYProckopDJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. (2011) 118:330–8. 10.1182/blood-2010-12-327353
29.
MegyeriKMándiYDegréMRosztóczyI. Induction of cytokine production by different Staphylococcal strains. Cytokine. (2002) 19:206–12. 10.1006/cyto.2002.0876
30.
WangJEJørgensenPFAlmlöfMThiemermannCFosterSJAasenAOet al. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infect Immun. (2000) 68:3965–70. 10.1128/iai.68.7.3965-3970.2000
31.
BlasiFTarsiaPAlibertiS. Chlamydophila pneumoniae. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. (2009) 15:29–35. 10.1111/j.1469-0691.2008.02130.x
32.
VirokDLobodaAKariLNebozhynMChangCNicholsCet al. Infection of U937 monocytic cells with Chlamydia pneumoniae induces extensive changes in host cell gene expression. J Infect Dis. (2003) 188:1310–21. 10.1086/379047
33.
FülöpFSzatmáriIVámosEZádoriDToldiJVécseiL. Syntheses, transformations and pharmaceutical applications of kynurenic acid derivatives. Curr Med Chem. (2009) 16:4828–42. 10.2174/092986709789909602
34.
FülöpFSzatmáriIToldiJVécseiL. Modifications on the carboxylic function of kynurenic acid. J Neural Transm Vienna Austr. (2012) 119:109–14. 10.1007/s00702-011-0721-7
35.
FülöpFSzatmáriIToldiJVécseiL. Novel Types of c-3 Substituted Kinurenic Acid Derivatives With Improved Neuroprotective Activity. (2017) Available online at: https://patents.google.com/patent/WO2017149333A1/en (accessed February 10, 2019).
36.
EszikILantosIÖnderKSomogyváriFBuriánKEndrészVet al. High dynamic range detection of Chlamydia trachomatis growth by direct quantitative PCR of the infected cells. J Microbiol Methods. (2016) 120:15–22. 10.1016/j.mimet.2015.11.010
37.
WangGCaoKLiuKXueYRobertsAILiFet al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. (2018) 25:1209–23. 10.1038/s41418-017-0006-2
Summary
Keywords
kynurenic acid, TNF-α, TSG-6, U-937, Staphylococcus, Chlamydia pneumoniae
Citation
Mándi Y, Endrész V, Mosolygó T, Burián K, Lantos I, Fülöp F, Szatmári I, Lőrinczi B, Balog A and Vécsei L (2019) The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front. Immunol. 10:1406. doi: 10.3389/fimmu.2019.01406
Received
26 March 2019
Accepted
04 June 2019
Published
21 June 2019
Volume
10 - 2019
Edited by
Elisa Wirthgen, University Hospital Rostock, Germany
Reviewed by
Chai K. Lim, Macquarie University, Australia; Gaurav K. Gupta, National Institutes of Health (NIH), United States
Updates
Copyright
© 2019 Mándi, Endrész, Mosolygó, Burián, Lantos, Fülöp, Szatmári, Lőrinczi, Balog and Vécsei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yvette Mándi mandi.yvette@med.u-szeged.hu
This article was submitted to Inflammation, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.