- Department of Dermatology, Inselspital-Bern University Hospital, University of Bern, Bern, Switzerland
Recent studies suggest an important role of immunoglobulin E (IgE) as an alternative pathogenic pathway in the development of bullous pemphigoid (BP), as the most frequent subepidermal blistering disease of the skin Use of IgE targeted therapies, such as omalizumab, has been shown promising in recent studies. The aim of this study was to assess the effect of omalizumab on FcεRI and IgE expression on circulating basophils and on lesional intradermal cells in BP to generate insight into the immunological effects of omalizumab in BP. We report two cases of BP patients treated with omalizumab. Efficacy of treatment was assessed clinically 4 months after initiation of the therapy. Lesional and non-lesional skin biopsies where taken before and 4 weeks after initiation of omalizumab therapy. In addition, FcεRI expression on circulating cells and IgE levels in serum and in the skin samples, as well as anti-BP180 and anti-BP230 in serum and eosinophils and basophils counts in blood were assessed before and during treatment. Both patients showed a marked improvement after 4 months, with no adverse effects. Down-regulation of FcεRI, IgE in lesional skin and on circulating basophils were observed in parallel with clinical improvement. The current case study supports the role of omalizumab in the treatment of a subset of BP patients. Our observations suggest that omalizumab represents a valuable therapeutic option in the management of BP patients. Its efficacy might be related to reduction in FcεRI+ and IgE+ basophils and intradermal cells.
Introduction
Bullous pemphigoid (BP) is a blistering antibody-mediated autoimmune skin disease. BP is associated with tissue-bound and circulating autoantibodies directed against two hemidesmosomal proteins, BP180 (also called BPAG2, type XVII collagen) and/or BP230 (also called BPAG1-e) (1, 2). For many decades, studies examining the pathogenic mechanisms of BP autoantibodies focused solely on IgG (3). In recent years, presence of IgE antibodies against the basement membrane zone components, elevated serum IgE and BP180-specific IgE in BP sera suggested that IgE has a role in BP pathogenesis (1–14). As a result, humanized monoclonal antibody directed to IgE, omalizumab, an approved treatment for severe asthma and chronic spontaneous urticaria, might represent an alternative drug for BP. Although the clinical effectiveness of omalizumab in treatment of BP has been already demonstrated (1, 2, 12, 15–18), the exact mechanism of action remains elusive. Furthermore, current data suggest that only a subset of BP patients respond favorably to omalizumab treatment. However, biomarkers to identify the subset of BP that profits from anti-IgE treatment have yet to be identified (19). In chronic spontaneous urticaria, changes in serum IgE levels and in FcεRI expression on basophils post anti-IgE therapy have been linked to therapy success. We aimed therefore in this study to assess the effect of omalizumab on FcεRI and IgE expression on circulating basophils and on lesional intradermal cells in BP to generate insight into the immunological effects of omalizumab in BP.
Report of the Cases
Two patients with severe recalcitrant BP treated with omalizumab at Department of Dermatology, Bern University Hospital were included after informed consent. Both patients have suffered from intense pruritus since long time. The previous standard local and systemic therapies could not improve their complaints.
Efficacy of treatment was assessed clinically 4 months after initiation of the therapy. Lesional and non-lesional skin biopsies where taken before and 4 weeks after initiation of omalizumab therapy. In addition, FcεRI expression on circulating cells and IgE levels in serum and in the skin samples, as well as anti-BP180 and -BP230 in serum, eosinophils and basophils counts in blood were assessed before and during treatment, as discussed before (20, 21).
After 4 months of therapy (300 mg subcutaneously every 4 weeks), the patients were clinically disease-free and the pruritus subsided completely. The patients did not report any adverse events (Table 1, Figure 1).
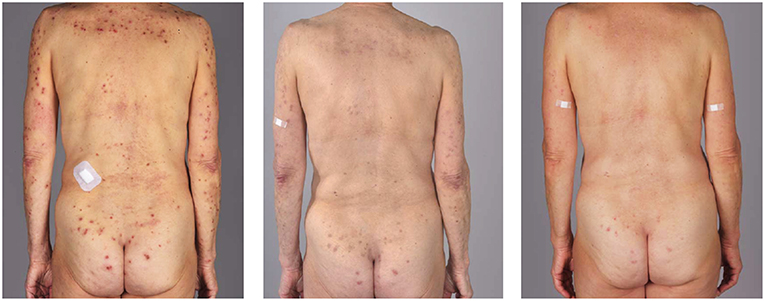
Figure 1. Clinical evaluation before (left), 10 days after (middle) and 4 months after the therapy in the first patient.
The eosinophils, basophils and IgE level in serum were variably affected and differed between the two patients. The BP-180/230 level decreased also slightly in our patients under Therapy. However, levels of FcεRI on circulating basophils decreased dramatically. Furthermore, a significant reduction of IgE+ and FcεRI+ cells in dermis were observed in the immunohistochemistry stainings after 4 weeks of omalizumab treatment (Figures 2, 3). To investigate the cellular source of FcεRI-carrying cells, we performed double immunofluorescence in lesional skin for markers of mast cells (tryptase) and antigen presenting cells (CD163) together with FcεRI staining. Both mast cells and APCs were found to express FcεRI in lesional skin (Figure 3).
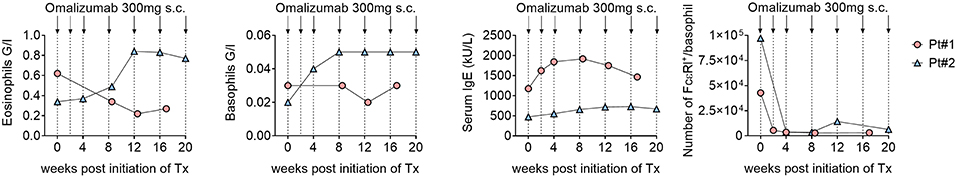
Figure 2. Despite therapy with omalizumab the eosinophils and basophils increased during therapy in the second patient. IgE level in serum did not also decrease. However, serum level of FcεRI decreased dramatically.
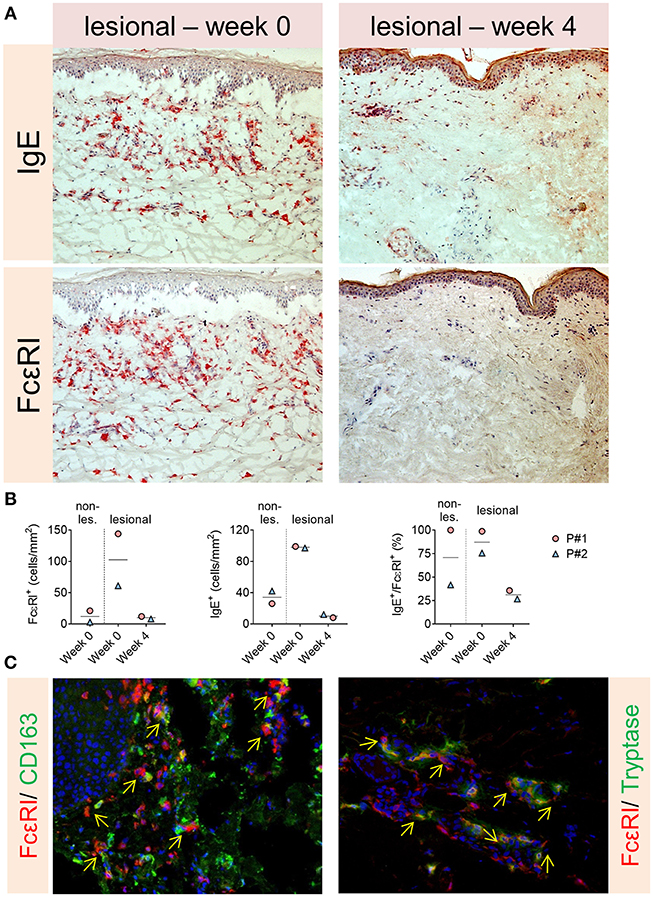
Figure 3. (A,B) Significant reduction of IgE+ and FcεRI+ cells in dermis in the immunohistochemistry stainings of the patients after 4 weeks of omalizumab treatment. (C) Both mast cells and APCs were found to express FcεRI in lesional skin (arrows show double positive cells).
Discussion
Our study in two patients with therapy-resistant BP in which omalizumab was used as rescue therapy has assessed the impact of omalizumab on levels of eosinophils, basophils, and IgE in the circulation, on FcεRI expression on circulating basophils, and on lesional expression of IgE and FcεRI in skin biopsy samples. Our observations suggest that changes in lesional IgE and FcεRI expression might be useful to distinguish the subset of BP patients who benefit from omalizumab therapy.
Frequent detection of high levels of IgE autoantibodies against BP180 and BP230, increased serum levels of IgE and the reported beneficial effect of anti-IgE therapy in small case series support the idea that IgE autoantibodies are involved in BP pathogenesis (1, 2, 5, 10, 12, 15–18, 22–24). However, the pathogenicity of IgE autoantibody in BP remains unclear (25). It is thought that IgE autoantibodies directed against the extracellular domain of BP180 are first bound to FcεRI on mast cells and eosinophils. This binding subsequently promotes degranulation and initiates an inflammatory reaction resulting in further tissue damage and blister formation (2, 12, 26). In addition, binding of specific IgE autoantibodies to the ectodomain of BP180 on basal keratinocytes may lead to internalization of BP180 and thereby contribute to cell-substrate dysadesion and blister formation (2, 12, 16).
Recent studies found that high serum levels of IgE correlate with disease severity in BP. In our two cases, however, there was no reduction of serum IgE levels 4 months after omalizumab therapy compared to baseline, despite clinical remission. It is conceivable that omalizumab, although it did not reduce the total IgE serum levels in our patients, is able to sequester free IgE and prevent its binding to its high-affinity IgE receptor, FcεRI (7, 27, 28). This process has been proposed to then down-regulate the expression of FcεRI on mast cells and basophils as well as antigen-presenting cells (7). In addition to its ability to neutralize the free IgE, omalizumab causes dissociation of IgE from the IgE-FcεRI complex (27, 28). IgE-free FcεRI is unstable and is then internalized for degradation. By this means, the activation of mast cells and basophils is reduced (7, 27–29). In line with these hypotheses, we found a sharp decrease of FcεRI expression on circulating basophils and a strong reduction of FcεRI+ cells in the skin of both patients after 4 weeks of omalizumab treatment (Figures 2, 3). These events might consequently lead to local depletion of IgE, as reflected by a strong reduction of IgE+ cells in dermis of both our patients after 4 weeks of omalizumab treatment. Interestingly, Metz et al. showed in a similar study that clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with decreases in FcεRI+ cells and IgE+ cells in lesional and non-lesional skin (dermis) of patients (to levels seen in healthy subjects) (30).
BP180 and BP230 ELISAs are highly sensitive methods for the diagnosis of BP, in particular BP180 ELISA, is a sensitive tool for monitoring disease activity (31–33). However, in our patients no decrease in the level of specific IgG BP180 and BP230 autoantibodies was observed after 4 months, despite marked clinical improvement. In line with this observation, Balakirski et al. reported no changes in circulating autoantibodies within 6 months after the treatment with omalizumab (16). Similarly, Yu et al. noticed only gradual decline in the level of these antibodies in 6 BP patients treated with omalizumab (23). It is thus possible that in a subset of BP patients, disease activity is not entirely driven by BP180/230-specific IgG antibodies but that IgE autoantibodies also substantially contribute to disease. It is tempting to speculate that these BP patients might represent the subset most likely to respond to anti-IgE treatment. Further studies are needed to investigate this hypothesis. Peripheral eosinophilia has been reported in 50–70% of BP patients (3, 22, 34–37). de Graauw et al. demonstrated that IL-5-activated eosinophils directly contribute to BP blister formation in the presence of BP autoantibodies. Dermal–epidermal separation by IL-5-activated eosinophils depends on adhesion and Fcγ receptor activation, requires elevated reactive oxygen species production, degranulation and involves eosinophil extracellular trap formation (38). It has recently been shown that peripheral eosinophilia correlated with the overall disease activity and the extent of erosions and blisters (3). Interestingly, our second patient did not have an elevated eosinophil count before treatment but showed rapid clinical improvement suggesting that peripheral eosinophilia might not serve as biomarker for response to omalizumab. In contrast, it has been claimed that asthma patients with a higher baseline peripheral eosinophil count show a better clinical outcome after omalizumab (39). To what extent the mechanisms of action of omalizumab is shared between BP, allergic asthma, and chronic spontaneous urticaria remains to be elucidated.
Our findings support the idea that IgE autoantibodies contribute to tissue damage. Prospective, randomized controlled trials are necessary to confirm that omalizumab represents a new treatment option for BP. However, the current literature suggests that only a subset of patients responds to omalizumab therapy. Therefore, it is paramount to identify biomarkers that predict the best treatment for the individual BP patient. Biomarker development typically relies on a solid understanding of the pathomechanisms at play. In this context, the exact mechanisms by which IgE contributes to pathogenesis in BP is not completely clear. Our data now suggests that optimal clinical responses to anti-IgE treatment in BP might be associated with dissociation of the IgE-FcεRI complex, consecutive down-regulation of FcεRI expression, and finally a decrease in lesional IgE-bearing immune cells. These observations provide novel insight into the immunomodulatory effects at the tissue level of anti-IgE treatment in BP and lay an important basis for future studies.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
Patients gave written informed consent for research use of their data.
Author Contributions
SS, KG, LF, LB, NY, and CS designed the study and performed acquisition, analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. SS, KG, and CS wrote the manuscript.
Funding
This work was supported by Department of Dermatology, Bern University, Bern, Switzerland. This study was supported by the Peter Hans Hofschneider Professorship for Molecular Medicine (to CS).
Conflict of Interest Statement
NY has received honoraria for consulting and advisory board attendance from Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ursula Läderach for her excellent technical assistance. We would also like to thank Werner Pichler, ADR-A GmbH for performing FcεRI density on basophils.
References
1. James T, Salman S, Stevenson B, Bundell C, Kelly G, Nolan D, et al. IgE blockade in autoimmunity: omalizumab induced remission of bullous pemphigoid. Clin Immunol. (2019) 198:54–6. doi: 10.1016/j.clim.2018.12.015
2. Vico-Alonso C, Calleja-Algarra A, Aragon-Miguel R, Sanchez-Velazquez A, Velasco-Tamariz V, Ortiz-Romero PL, et al. Omalizumab as an alternative therapeutic tool in the treatment of bullous pemphigoid: a case report. Dermatol Ther. (2019) 2019:e12829. doi: 10.1111/dth.12829
3. Messingham KN, Holahan HM, Frydman AS, Fullenkamp C, Srikantha R, Fairley JA. Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLoS ONE. (2014) 9:e107725. doi: 10.1371/journal.pone.0107725
4. Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, et al. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol. (2003) 120:784–8. doi: 10.1046/j.1523-1747.2003.12146.x
5. Fairley JA, Burnett CT, Fu C-L, Larson DL, Fleming MG, Giudice GJ. A Pathogenic role for IgE in autoimmunity: bullous pemphigoid ige reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. (2007) 127:2605–11. doi: 10.1038/sj.jid.5700958
6. Fania L, Caldarola G, Müller R, Brandt O, Pellicano R, Feliciani C, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. (2012) 143:236–45. doi: 10.1016/j.clim.2012.02.003
7. Kalowska M, Ciepiela O, Kowalewski C, Demkow U, Schwartz RA, Wozniak K. Enzyme-linked immunoassay index for Anti-NC16a IgG and IgE autoantibodies correlates with severity and activity of bullous pemphigoid. Acta Dermato Venereol. (2016) 96:191–6. doi: 10.2340/00015555-2101
8. Lin L, Hwang B-J, Culton DA, Li N, Burette S, Koller BH, et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Invest Dermatol. (2018) 138:1032–43. doi: 10.1016/j.jid.2017.11.031
9. Yayli S, Pelivani N, Beltraminelli H, Wirthmuller U, Beleznay Z, Horn M, et al. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: a useful clue for diagnosis. Br J Dermatol. (2011) 165:1133–7. doi: 10.1111/j.1365-2133.2011.10481.x
10. Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE Basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol. (2007) 127:1167–74. doi: 10.1038/sj.jid.5700681
11. Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. (2009) 123:704–5. doi: 10.1016/j.jaci.2008.11.035
12. Messingham K, Holahan H, Fairley J. Unraveling the significance of IgE autoantibodies in organ-specific autoimmunity: lessons learned from bullous pemphigoid. Immunol Res. (2014) 59:273–8. doi: 10.1007/s12026-014-8547-7
13. Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. (2008) 144:41–8. doi: 10.1001/archdermatol.2007.9
14. Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180–specific IgE production in bullous pemphigoid. J Immunol Methods. (2009) 346:18–25. doi: 10.1016/j.jim.2009.04.013
15. van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. (2016) 12:267–77. doi: 10.1586/1744666X.2016.1123092
16. Balakirski G, Alkhateeb A, Merk HF, Leverkus M, Megahed M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: report of two cases and review of literature. J Eur Acad Dermatol Venereol. (2016) 30:1778–82. doi: 10.1111/jdv.13758
17. Dufour C, Souillet AL, Chaneliere C, Jouen F, Bodemer C, Jullien D, et al. Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol. (2012) 166:1140–2. doi: 10.1111/j.1365-2133.2011.10748.x
18. Yalcin AD, Genc GE, Celik B, Gumuslu S. Anti-IgE monoclonal antibody (omalizumab) is effective in treating bullous pemphigoid and its effects on soluble CD200. Clin Lab. (2014) 60:523–4. doi: 10.7754/Clin.Lab.2013.130642
19. Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol. (2019) 33:918–24. doi: 10.1111/jdv.15350
20. Yan KX, Huang Q, Fang X, Zhang ZH, Han L, Gadaldi K, et al. IgE and FcepsilonRI are highly expressed on innate cells in psoriasis. Br J Dermatol. (2016) 175:122–33. doi: 10.1111/bjd.14459
21. Jorg L, Pecaric-Petkovic T, Reichenbach S, Coslovsky M, Stalder O, Pichler W, et al. Double-blind placebo-controlled trial of the effect of omalizumab on basophils in chronic urticaria patients. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2018) 48:196–204. doi: 10.1111/cea.13066
22. Cozzani E, Gasparini G, Di Zenzo G, Parodi A. Immunoglobulin E and bullous pemphigoid. Eur J Dermatol. (2018) 28:440–8. doi: 10.1684/ejd.2018.3366
23. Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. (2014) 71:468–74. doi: 10.1016/j.jaad.2014.04.053
24. Saniklidou AH, Tighe PJ, Fairclough LC, Todd I. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: a systematic review. Arch Dermatol Res. (2018) 310:11–28. doi: 10.1007/s00403-017-1789-1
25. Menzinger S, Kaya G, Schmidt E, Fontao L, Laffitte E. Biological and clinical response to omalizumab in a patient with bullous pemphigoid. Acta Dermato Venereol. (2018) 98:284–6. doi: 10.2340/00015555-2845
26. Ujiie H. IgE autoantibodies in bullous pemphigoid: supporting role, or leading player? J Dermatol Sci. (2015) 78:5–10. doi: 10.1016/j.jdermsci.2015.03.002
27. Navines-Ferrer A, Serrano-Candelas E, Molina-Molina GJ, Martin M. IgE-Related chronic diseases and anti-IgE-based treatments. J Immunol Res. (2016) 2016:8163803. doi: 10.1155/2016/8163803
28. Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gastaminza G, Goikoetxea MJ, et al. Comparable actions of omalizumab on mast cells and basophils. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2016) 46:92–102. doi: 10.1111/cea.12668
29. Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. (2001) 167:1290–6. doi: 10.4049/jimmunol.167.3.1290
30. Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcepsilonRI-positive cells in the skin. Theranostics. (2017) 7:1266–76. doi: 10.7150/thno.18304
31. Lee EH, Kim YH, Kim S, Kim S-E, Kim S-C. Usefulness of enzyme-linked immunosorbent assay using recombinant BP180 and BP230 for serodiagnosis and monitoring disease activity of bullous pemphigoid. Ann Dermatol. (2012) 24:45–55. doi: 10.5021/ad.2012.24.1.45
32. Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. (2017) 177:141–51. doi: 10.1111/bjd.15114
33. van Beek N, Luttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. (2017) 153:30–8. doi: 10.1001/jamadermatol.2016.3357
34. Mori O, Hachisuka H, Kusuhara M, Sasai Y, Fujiwara S. Bullous pemphigoid in a 19–year-old woman. A case with unusual target antigens. Br J Dermatol. (1994) 130:241–5. doi: 10.1111/j.1365-2133.1994.tb02909.x
35. Bernard P, Venot J, Constant F, Bonnetblane J-M. Blood eosinophilia as a severity marker for bullous pemphigoid. J Am Acad Dermatol. (1987) 16:879–81. doi: 10.1016/S0190-9622(87)80227-X
36. Bushkell LL, Jordon RE. Bullous pemphigoid: a cause of peripheral blood eosinophilia. J Am Acad Dermatol. (1983) 8:648–51. doi: 10.1016/S0190-9622(83)70073-3
37. Tanaka Y, Takenaka M, Matsunaga Y, Okada S, Anan S, Yoshida H, et al. High affinity IgE receptor (FcεRI) expression on eosinophils infiltrating the lesions and mite patch tested sites in atopic dermatitis. Arch Dermatol Res. (1995) 287:712–7. doi: 10.1007/BF01105794
38. de Graauw E, Sitaru C, Horn M, Borradori L, Yousefi S, Simon HU, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy. (2017) 72:1105–13. doi: 10.1111/all.13131
Keywords: bullous pemphigoid, FcεRI, IgE, omalizumab, skin
Citation: Seyed Jafari SM, Gadaldi K, Feldmeyer L, Yawalkar N, Borradori L and Schlapbach C (2019) Effects of Omalizumab on FcεRI and IgE Expression in Lesional Skin of Bullous Pemphigoid. Front. Immunol. 10:1919. doi: 10.3389/fimmu.2019.01919
Received: 29 May 2019; Accepted: 29 July 2019;
Published: 14 August 2019.
Edited by:
Ralf J. Ludwig, Universität zu Lübeck, GermanyReviewed by:
Cassian Sitaru, Freiburg University Medical Center, GermanyTakashi Hashimoto, Osaka City University, Japan
Frank Antonicelli, Université de Reims Champagne-Ardenne, France
Copyright © 2019 Seyed Jafari, Gadaldi, Feldmeyer, Yawalkar, Borradori and Schlapbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Schlapbach, Y2hyaXN0b3BoLnNjaGxhcGJhY2hAaW5zZWwuY2g=
†These authors have contributed equally to this work
 S. Morteza Seyed Jafari
S. Morteza Seyed Jafari Karolina Gadaldi†
Karolina Gadaldi† Nikhil Yawalkar
Nikhil Yawalkar Christoph Schlapbach
Christoph Schlapbach