- Department of Chemistry, University of California, Davis, Davis, CA, United States
Sialic acids constitute a family of negatively charged structurally diverse monosaccharides that are commonly presented on the termini of glycans in higher animals and some microorganisms. In addition to N-acetylneuraminic acid (Neu5Ac), N-glycolyl neuraminic acid (Neu5Gc) is among the most common sialic acid forms in nature. Nevertheless, unlike most animals, human cells loss the ability to synthesize Neu5Gc although Neu5Gc-containing glycoconjugates have been found on human cancer cells and in various human tissues due to dietary incorporation of Neu5Gc. Some pathogenic bacteria also produce Neu5Ac and the corresponding glycoconjugates but Neu5Gc-producing bacteria have yet to be found. In addition to Neu5Gc, more than 20 Neu5Gc derivatives have been found in non-human vertebrates. To explore the biological roles of Neu5Gc and its naturally occurring derivatives as well as the corresponding glycans and glycoconjugates, various chemical and enzymatic synthetic methods have been developed to obtain a vast array of glycosides containing Neu5Gc and/or its derivatives. Here we provide an overview on various synthetic methods that have been developed. Among these, the application of highly efficient one-pot multienzyme (OPME) sialylation systems in synthesizing compounds containing Neu5Gc and derivatives has been proven as a powerful strategy.
Graphical Abstract. Chemical and chemoenzymatic synthetic methods for Neu5Gc and Neu5Gc-sialosides are reviewed. One-pot multienzyme (OPME) chemoenzymatic strategy has advantages in accessing a large number of Neu5Gc-sialosides and their derivatives.
Introduction
Sialic acids (Sias) are a family of negatively charged monosaccharides with a nine carbon backbone. More than 50 structurally distinct Sias have been found in nature (1–3), out of which more than 15 have been identified in human (4–6). They are commonly presented as the terminal monosaccharides of the carbohydrate moieties of glycoproteins and glycolipids on cell surface of deuterostome animals, in secreted glycans and glycoconjugates including those in the milk of mammals (2, 7–9). Some microorganisms including pathogenic bacteria also produce sialic acid and sialic acid-containing structures (7, 10). Three basic forms of sialic acids are N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and 2-keto-3-deoxynonulosonic acid (Kdn) (Figure 1A) (1–3). In nature, sialic acid-containing oligosaccharides and glycoconjugates are formed mainly by sialyltransferase-catalyzed reactions transferring sialic acid from its activated sugar nucleotide, cytidine 5′-monophosphate-sialic acid (CMP-Sia), to suitable acceptors (11) although trans-sialidases have also been used by parasites and bacteria to harvest sialic acids from the hosts to decorate their own surface (12, 13). Neu5Ac is the most common form of sialic acids. Compared to Neu5Ac, Neu5Gc has an extra oxygen, presented as the hydroxyl in the N-glycolyl group at C-5.
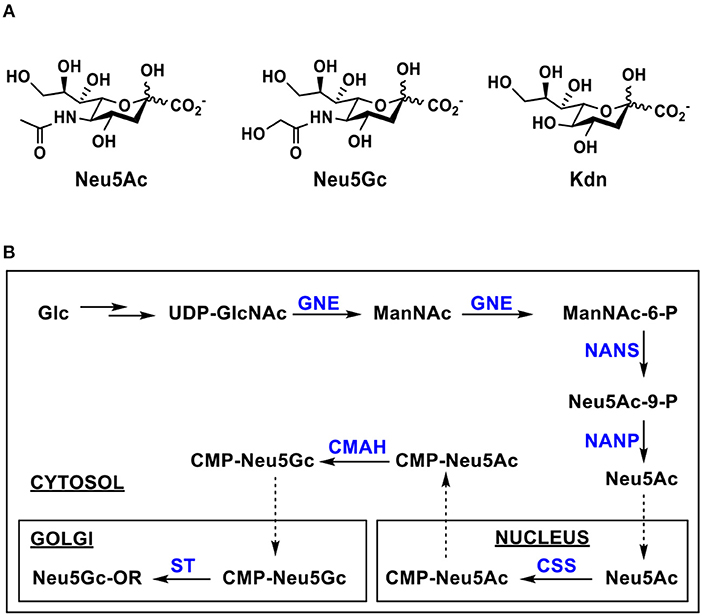
Figure 1. (A) Three basic forms of sialic acids including N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and 3-deoxy-D-glycero-D-galacto-2-nonulosonic acid (Kdn); (B) Biosynthesis of Neu5Gc and its sialosides in eukaryotic cells. Enzymes and abbreviations: GNE, UDP-GlcNAc 2-epimerase/ManNAc-6-kinase; NANS, Neu5Ac-9-P synthetase; NANP, Neu5Ac-9-P phosphatase; CSS, CMP-sialic acid synthetase; CMAH, cytidine 5′-monophosphate-N-acetylneuraminic acid hydroxylase.
The biosynthesis of Neu5Ac from uridine 5′-diphosphate N-acetylglucosamine (UDP-GlcNAc) in eukaryotic cells takes place in the cytosol by three enzymes. The first two committed steps are hydrolytic epimerization of UDP-GlcNAc to form N-acetylmannosamine (ManNAc) followed by phosphorylation to form ManNAc-6-P catalyzed by a single bifunctional enzyme UDP-GlcNAc 2-epimerase/ManNAc-6-kinase (GNE). Phosphoenolpyruvate is then condensed with ManNAc-6-P by Neu5Ac 9-phosphate synthase (NAPS) to produce Neu5Ac-9-P which is dephosphorylated to form Neu5Ac by Neu5Ac-9-phosphate phosphatase (NANP). The Neu5Ac synthesized in the cytosol is transferred into nucleus and used to form CMP-Neu5Ac, the activated form of Neu5Ac, by CMP-sialic acid synthetase (CSS). CMP-Neu5Gc formed in the cytosol from CMP-Neu5Ac by CMP-Neu5Ac hydroxylase (CMAH)-catalyzed reaction (11, 14–17) is transferred into Golgi and used by various sialyltransferases to form glycoconjugates which are secreted or expressed on cell surfaces (Figure 1B) (10, 18, 19). The CMAH gene is inactive in humans. Therefore, humans do not biosynthesize Neu5Gc-containing structures themselves (20, 21). New World monkeys were also shown to loss the function of Neu5Gc production due to an independent CMAH inactivation (22).
Regardless of CMAH inactivation in human, Neu5Gc has been found on the cell surface of human tumors and even in normal human tissues although at a lower amount (23, 24). Neu5Gc in human glycoconjugates comes likely from the consumption of animal-derived diets, such as red meat and animal milk (25–27). On the other hand, during infancy (around the age of 6 months) humans develop varying levels of polyclonal antibodies of IgG (28, 29), IgM, and IgA (30, 31) types against a diverse array of Neu5Gc-containing glycans (32–35). The mechanism of developing such anti-Neu5Gc antibodies early in the human life is unclear although incorporating dietary Neu5Gc by bacteria colonized in humans, such as non-typeable Haemophilus influenzae (NTHi) to form Neu5Gc-containing epitopes, such as cell surface lipooligosaccharides (LOS) is a likely source of the corresponding immunogens (32, 36, 37). So far, de novo synthesis of Neu5Gc and Neu5Gc-containing structures has not been demonstrated in bacteria. The presence of CMAH-like sequences has been found in the genomes of some bacteria but the activities of the corresponding enzymes have not been confirmed (38–40).
The presence of Neu5Gc-containing xeno-auto-antigens and anti-Neu5Gc xeno-autoantibodies in human (24) may lead to potential complications, such as chronic inflammation namely “xenosialitis” (41), atherosclerotic cardiovascular diseases, cancers, and autoimmune diseases (34, 38, 42–45). In addition, exposure to clinically used Neu5Gc-presenting animal-derived biotherapeutics (such as immunosuppressant rabbit anti-human thymocyte globulin, ATG) elicited anti-Neu5Gc antibodies (46, 47) with a profile that may be different from the “pre-existing” ones (28, 48). The biological consequences of this have not been revealed. A recent analysis showed that treating kidney transplant patients with ATG did not increase the risk of colon cancer (49). Neu5Gc has also been found on biodevices (such as bioprosthetic heart valves) which may affect their duration of function due to interaction with anti-Neu5Gc antibodies which can lead to calcification (50, 51). Furthermore, Neu5Gc in addition to α-Gal epitopes presented on animal tissues causes barriers for animal-to-human xenotransplantation (such as porcine skin xenografting and organ xenotransplantation) (52, 53).
Neu5Gc and its glycosides are important tools for profiling anti-Neu5Gc antibodies and sialic acid-binding proteins, understanding Neu5Gc-related immune responses, and designing potential therapeutics. To better understand their important roles, it is critical to obtain structurally defined glycans and glycoconjugates containing Neu5Gc or its derivatives.
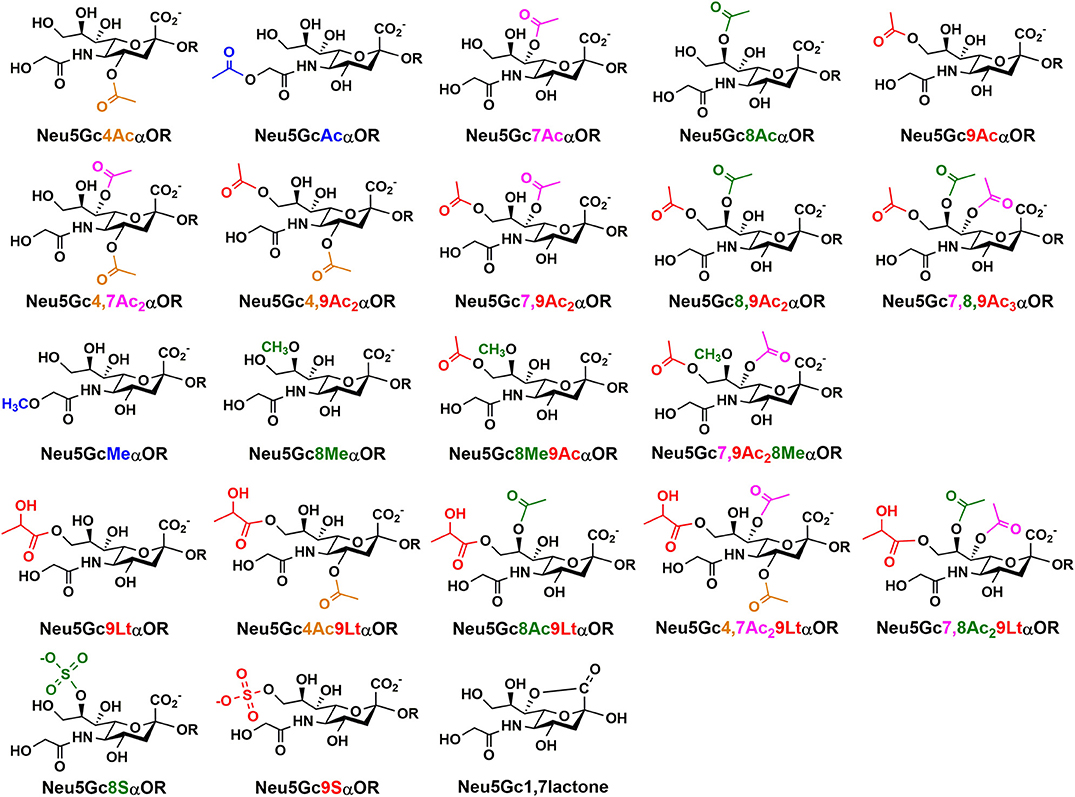
Neu5Gc and derivatives have been found and can be isolated from natural sources including non-human mammals, some higher invertebrates, such as sea urchin, sea cucumber, and starfish (11, 23, 54, 55), as well as the surface of salmonid fish eggs (56). For example, Neu5Gc has been extracted from sea cucumber Cucumaria echinate in 99% purity. It constitutes about 85% of the total sialic acids in dry weight of Gumi (sea cucumber), and 23.6 mg was obtained from 135 g of fresh body weight (57). Neu5Gc-containing oligosaccharides have been reported in the milk of primates, domestic herbivores, pigs, lion, and leopard (58). So far, twenty-two Neu5Gc derivatives (Figure 2) have been reported (1, 3). These include mono-, di-, and tri-O-acetylation at C4, C5, C7, C8, and/or C9 positions in Neu5Gc as well as other modifications including O-methylation at C5- or C8-, O-lactylation at C9, or O-sulfation at C8 or C9 of Neu5Gc with or without O-acetylation. Neu5Gc1,7lactone has also been identified.
Neu5Gc and derivatives can link to other carbohydrate moieties with different sialyl linkages including α2–3- and α2–6-linked to galactose; α2–6-linked to N-acetylgalactosamine, N-acetylglucosamine, galactose or glucose; α2–8- and α2–9-linked to another Sia molecule; and α2–5-linked between polymers of Neu5Gc (7, 10, 59–61), adding diversity to sialic acid-containing compounds. The modification and linkage patterns of Sia play a pivotal role in many biochemical processes, such as cell signaling, cell-cell interaction, cellular adhesion, inflammation, fertilization, viral infection and malignancies, and regulation of apoptosis and proliferation (62, 63).
Numerous outstanding reports have been published describing the synthesis of sialic acids and sialoside. The focus, however, has been on Neu5Ac-containing compounds. The synthesis of Neu5Gc-containing glycans is attracting an increasing attention in recent years. This review provides an overview of various chemical and chemoenzymatic synthetic methods developed for the production of Neu5Gc and derivatives as well as the corresponding sialosides.
Chemical and Chemoenzymatic Synthesis of Neu5Gc and Derivatives
Only a limited number of naturally occurring and non-natural Neu5Gc derivatives have been chemically or chemoenzymatically synthesized.
Neu5Gc was chemically synthesized from D-arabinose by the Wong group. The C5-acylamino group of Neu5Gc and a vinyl group were simultaneously introduced to D-arabinose by a modified Petasis coupling reaction. The vinyl group was then converted to γ-hydroxy-α-keto acid by a 1,3-dipolar cycloaddition reaction with N-tert-butyl nitrone followed by a base-catalyzed β elimination and hydrolysis to produce Neu5Gc in 22% overall yield (64).
O-Acetylation is the most frequent modification of Neu5Gc in nature. 9-O-Acetyl-Neu5Gc (Neu5Gc9Ac) has been found in bovine submandibular gland glycoprotein (65, 66). On the other hand, 4-O-acetyl-Neu5Gc (Neu4Ac5Gc) has been found in horse glycoproteins (61), α2–8-linked polysialic acids on glycoproteins from unfertilized kokanee salmon egg (67), the serum of guinea pigs (68), and gangliosides in human colon cancer tissues (69). Both Neu5Gc9Ac and Neu4Ac5Gc have been successfully synthesized. The use of orthoester intermediates is a very efficient method for producing various 9-O-acyl derivatives of Neu5Gc. Highly regioselective acylation at C9-hydroxyl of Neu5Gc was achieved by the treatment of Neu5Gc with a trimethyl orthoacetate in the presence of a catalytic amount of p-toluenesulfonic acid (p-TsOH) to form Neu5Gc9Ac in 90% yield. A similar strategy was applied for the synthesis of non-natural derivatives of Neu5Gc including 9-O-butyroyl-Neu5Gc and 9-O-benzoyl-Neu5Gc in 88 and 70% yields, respectively (70). On the other hand, Neu4Ac5Gc was synthesized using an efficient chemoenzymatic approach involving a sialic acid aldolase-catalyzed reaction from D-mannosamine (ManNH2) acylated with a benzyl protected N-glycolyl group. The obtained N-(2-benzyloxyacetyl)-D-mannosamine was enzymatically converted to a Neu5Gc derivative in a quantitative yield by recombinant Pasteurella multocida sialic acid aldolase (PmNanA) (71). Following a number of selective protection strategies, 4-hydroxyl group was selectively acetylated. The desired 4-OAc-Neu5Gc was obtained in an overall yield of 46% after de-protection of other hydroxyl groups (72).
In nature, the major function of sialic acid aldolases is to break down sialic acids, such as Neu5Ac to form 6-carbon amino sugar N-acetylmannosamine (ManNAc) and a three-carbon metabolite pyruvic acid. Nevertheless, they are capable of catalyzing the reversed reaction and have been used as synthetically useful enzymes for the formation of sialic acids and derivatives. Sialic acid aldolase-catalyzed reactions can be a general and highly efficient approach for chemoenzymatic synthesis of a diverse array of Neu5Gc and derivatives from the corresponding N-glycolylmannosamine (ManNGc) and derivatives. PmNanA was found to have a better expression level and more promiscuous substrate specificity than the more commonly used Escherichia coli sialic acid aldolase (EcNanA) in catalyzing the formation of sialic acids and derivatives (71). Both enzymes have been used for chemoenzymatic synthesis of Neu5Gc and derivatives. For the synthesis of Neu5Gc from ManNGc by sialic acid aldolase-catalyzed reaction, ManNGc could be obtained by chemical synthesis from D-mannosamine (ManNH2) (73, 74) or D-glucose (75), or by alkaline epimerization of N-acetylglucosamine (GlcNAc) (76). For the synthesis of ManNGc from ManNH2, the N-glycol group could be installed using commercially available inexpensive acetoxyacetyl chloride followed by de-O-acetylation by hydrolysis under a basic condition. However, it was found that ManNGc could be epimerized to form N-glycolylglucosamine (GlcNGc) under even mild basic conditions. Aspergillus niger lipase (Amano A) was found to be efficient in de-O-acetylation without the problem of epimerization (76). Installing the N-glycol group using N-succinimidyl glycolate (74) or 2-(benzyloxy)acetyl chloride followed by hydrogenation (77) could also avoid the complication of epimerization. The Neu5Gc formed went through additional chemical reactions for the synthesis of N-glycolyl-2,3-dehydro-2-deoxyneuraminic acid (Neu5Gc2en) (78), a transition state analog inhibitor of some sialidases. Together with N-acetyl-2,3-dehydro-2-deoxyneuraminic acid (Neu5Ac2en), they have been found to be effective in protecting mice from bacteria sepsis in a CD24/SiglecG-dependent manner (79) in a cecal ligation and puncture (CLP) mouse model (80). The protection was improved by combining the use of Neu5Ac2en and Neu5Gc2en with antibiotic treatment (79). In addition, Neu5Gc2en alone was effective in protecting mice from endotoxemia by inhibiting mouse sialidase NEU1 expressed on cell surface upon lipopolysaccharide (LPS) stimulation (81).
The sialic acid aldolase-catalyzed reactions can also be used to synthesize naturally occurring and non-natural derivatives of Neu5Gc. As shown in Figure 3A, Neu5Gc derivatives with C5 and/or C9-modifications have been synthesized by sialic acid aldolase-catalyzed reactions from C2- and/or C6-substituted ManNGc derivatives as their 6-carbon sugar precursors (74, 82–87). Naturally occurring 8-O-methyl Neu5Gc (Neu5Gc8Me) (Figure 3B) was also synthesized from chemically synthesized 5-O-methyl ManNGc (ManNGc5Me) by a PmNanA-catalyzed reaction. A good yield of 86% was achieved using five equivalents of sodium pyruvate in Tris-HCl buffer (100 mM, pH 7.5) at 37°C for 24 h followed by the combination of anion exchange chromatography and gel filtration column purification (88).
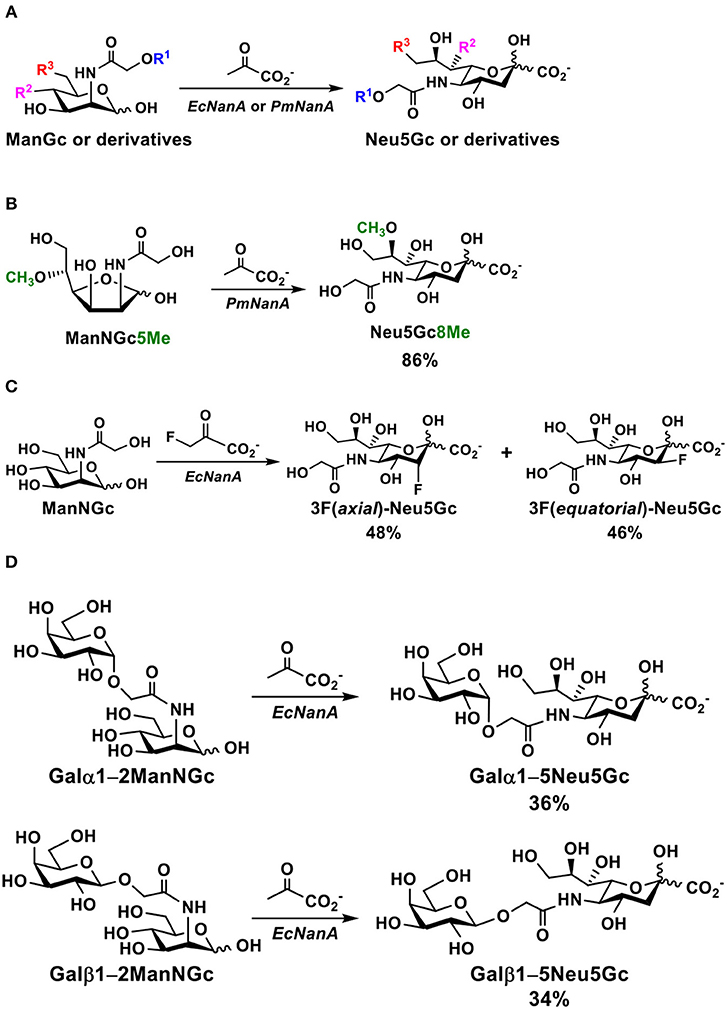
Figure 3. (A) A general chemoenzymatic synthetic strategy of sialic acid aldolase (EcNanA or PmNanA)-catalyzed synthesis of Neu5Gc and derivatives containing modifications at C5, C7, and/or C9 from ManNGc and derivatives, (B) PmNanA-catalyzed synthesis of Neu5Gc8Me, (C) EcNanA-catalyzed synthesis of 3-fluoro-Neu5Gc, and (D) EcNanA-catalyzed synthesis of disaccharides containing Neu5Gc at the reducing end.
EcNanA-catalyzed aldol addition of ManNGc and 3-fluoro-pyruvate resulted in a mixture of 3F(equatorial)Neu5Gc and 3F(axial)Neu5Gc with a ratio of close to 1:1. They were readily separated by a simple flash chromatography (Figure 3C) (89).
Disaccharides with a ManNGc at the reducing end could also be suitable substrates for EcNanA. Two chemically synthesized disaccharides Galα1–2ManNGc and Galβ1–2ManNGc were used as the substrates for EcNanA for the synthesis of the corresponding disaccharides Galα1–5Neu5Gc and Galβ1–5Neu5Gc in 36 and 34% yields, respectively (Figure 3D) (85).
Synthesis of Simple Glycosides of Neu5Gc
Simple glycosides of Neu5Gc have been synthesized from the corresponding Neu5Ac derivatives by directly de-N-acetylating the N-acetyl group of Neu5Ac under a strong basic condition followed by acylation and deprotection. For example, as shown in Figure 4A, the N-acetyl group in the carboxyl protected allyl α-Neu5Ac-glycoside was removed to produce the free amino group in 80% yield by refluxing in tetramethylammonium hydroxide. Acylation with acetoxyacetyl chloride followed by hydrolysis of the ester produced the desired allyl α-Neu5Gc-glycoside (90). An improved microwave-assisted de-N-acetylation process was also reported (91). In this case, fully protected methyl α-Neu5Ac glycoside was treated with 2.0 M of NaOH under an optimized microwave irradiation condition (15 min at 120°C at a maximum power of 100 W) produced the desired 5-amino derivative in 91% yield. The resulting compound was then converted to the target methyl α-Neu5Gc glycoside (Neu5GcαOMe) by reacting with acetoxyacetyl chloride, followed by de-O-acetylation (Figure 4B). The same method was applied successfully for the formation of Neu5Gc2en from per-acetylated Neu5Ac2en methyl carboxylate as well as the production of poly-Neu5Gc from the corresponding α2–8-linked homopolymer of Neu5Ac (91).
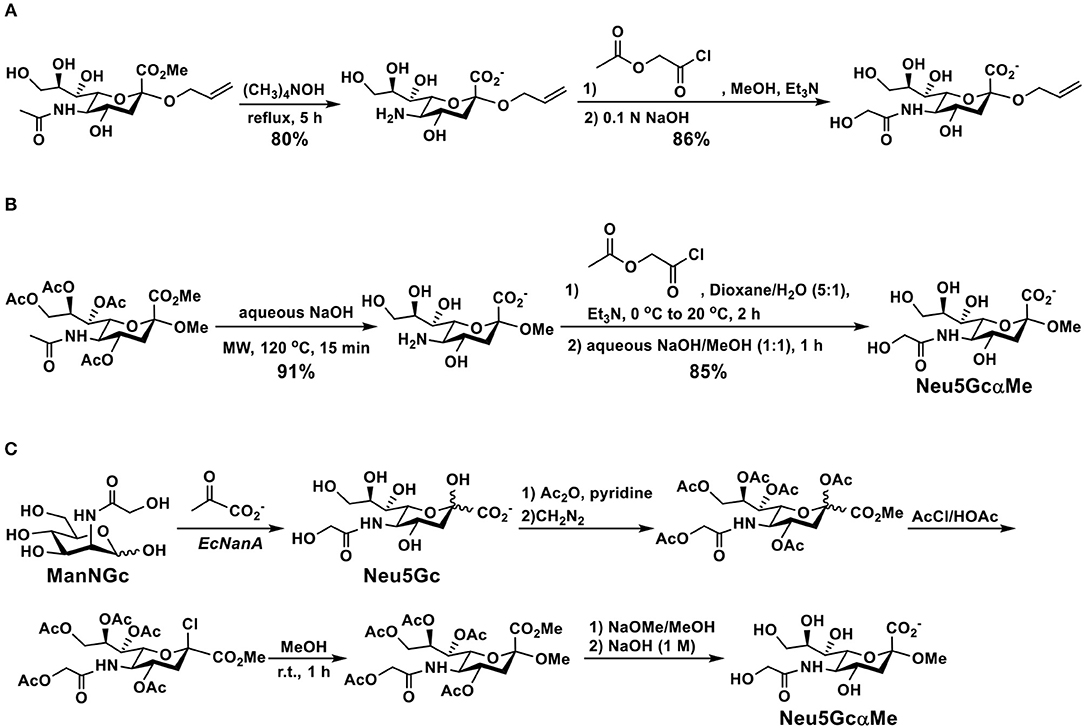
Figure 4. Synthesis of Neu5Gc-glycosides by de-N-acetylation of Neu5Ac-glycoside derivatives without (A) or with (B) a microwave-assisted process and (C) by glycosylation of protected Neu5Gc formed enzymatically from ManNGc and pyruvate.
An 9-azido derivative of Neu5Gc2en (Neu5Gc9N32en) was also chemically synthesized from Neu5Ac9N32en by substituting the 9-hydroxyl group with an azido group followed by replacing the -NHAc moiety with N-glycolyl group (92).
An alternative strategy for the synthesis of Neu5GcαOMe (Figure 4C) involved enzymatic formation of Neu5Gc from ManNGc using an EcNanA-catalyzed reaction. Protection of Neu5Gc, followed by activation, glycosylation, and deprotection led to the formation of the desired Neu5GcαOMe which was used for ELISA inhibition assays and for purifying anti-Neu5Gc antibodies from human sera (33).
Chemical Synthesis of Neu5Gc-Containing Oligosaccharides
Several chemical glycosylation methods have been developed for the synthesis of Neu5Gc-containing oligosaccharides. The following discussion will be focused on different types of glycosyl donors used.
Glycosyl Chloride Donors
A glycosyl chloride donor was used for synthesizing Neu5Acα2–5Neu5Gc disaccharide which contained a sialyl α2–5-Neu5Gc linkage similar to that found in the poly(-5Neu5Gcα2-) structure on the jelly coat of sea urchin eggs (93). The strategy involved the formation of an allyl glycoside of protected Neu5Ac as an important intermediate which went through oxidative cleavage of the C = C double bond in the allyl group (94) to form a protected Neu5Ac glycoside with a carboxymethoxy aglycone. Most recently, a similar strategy using protected Neu5Gc allyl glycoside donor was applied in the synthesis of Neu5Gcα2–5Neu5Gc disaccharide building block for the formation of a tetrasaccharide capped with 9-O-sulfo-Neu5Gc (Neu5Gc9S) found on sea urchin egg surface proteins (95).
The same Neu5Ac glycosyl chloride donor was used for glycosylation with methyl glycolate. The glycosylated product was deprotected and de-N-acetylated to form an amino-containing intermediate which can be either protected by a fluorenylmethyloxycarbony (Fmoc) group at the amino group or by a methyl group on the carboxyl groups. The resulting compounds were coupled to form the amide bond, linking two sialic acid units together to produce the desired disaccharide containing a Neu5Gc residue (96). α2–5-Linked Neu5Gc oligomers for up to octasaccharide were also synthesized using a similar strategy by coupling carboxyl and amine protecting groups of sialic acid building blocks by amide formation (97).
An O-acetyl protected Neu5Gc glycosyl chloride donor was also used for the synthesis of Neu5Gcα2–3Galβ1–4Glc trisaccharide building block for the formation of Neu5Gc-GM3 ganglioside although with a low yield and a poor stereo-selectivity (98).
Thioglycoside Donors
Sialyl thioglycoside donors have been widely applied in chemically formation of sialyl glycosidic bonds. A thioglycoside donor of Neu5Ac was used for the synthesis of Neu5Acα2–5Neu5Gc disaccharide found as the structural component of the jelly coat of sea urchin eggs. The strategy relied on the formation of a protected Neu5Ac glycoside with a carboxymethoxy aglycone which was readily coupled with the amino group of the protected neuraminic acid to form the desired amide bond in the disaccharide (93).
Thioglycoside donors of Neu5Gc have also been used for the synthesis of more complex Neu5Gc-containing sialosides. The Kiso group reported the synthesis of protected Neu5Gcα2–3GalβOMP disaccharide using N-2,2,2-trichloroethoxycarbonyl (Troc)-protected thiophenyl sialoside donor which was readily obtained from its corresponding N-acetyl derivative. Sialylation of a selectively protected galactoside acceptor led to the formation of sialyl disaccharide. Removal of the N-Troc group by zinc in acetic acid formed a free amino group which can be acylated with acetoxyacetyl chloride to produce the desired protected Neu5Gc-containing disaccharide (99). A similar strategy was used for the synthesis of a Neu5Gc8Me-containing tetrasaccharide building block of the pentasaccharide component, Neu5Gc8Meα2–3(Neu5Gc8Meα2–6)GalNAcβ1–3Galβ1–4Glc, in GAA-7 ganglioside (100). In addition to the use of acetoxyacetyl chloride as a reagent for introducing a protected glycolyl group to the amino group on neuraminic acid (Neu) residue for the formation of Neu5Gc, 1,3-dioxolan-2,4-dione (101) prepared from glycolic acid was also used for the formation of Neu5Gc-GM1 ganglioside directly from naturally more abundant Neu5Ac version of GM1 (102).
The Sato group used a N-Troc-protected thiophenyl sialoside donor for the synthesis of sialyllactoside component of ganglioside LL3 tetrasaccharide. The removal of the N-Troc group followed by conjugation with a protected Neu5Ac glycoside with a carboxymethoxy aglycone and deprotection steps formed the desired LL3 tetrasaccharide (103, 104).
The amino intermediate of the protected sialoside formed after the removal of the N-Troc group could be converted directly (99) to a 1,5-lactamized bicycle structure. Alternatively, N-trifluoroacetyl (N-TFA)-protected thiophenyl sialoside donor can also be used similarly for the formation of sialyl glycosides. The N-TFA group could be readily removed and the resulting amino group-containing intermediate could be converted to a 1,5-lactamized bicycle structure under mild basic conditions. The resulting intermediate could be selectively protected at C-9 of the sialic acid and used as a well-suited sialylation acceptor. A similar N-Troc and 8,9-acetal-protected thiotoluene sialoside donor was used for the synthesis of protected Neuα2–6GalαSer as the sialyl Tn disaccharide building block that was coupled with the pre-formed Neu5Gcα2–5Neu5Gc disaccharide component for the formation of the sea urchin egg surface Neu5Gc9S-capped tetrasaccharide (95).
Trisaccharide Neu5Gcα2–4Neu5Acα2–6Glc, a structural component of ganglioside HLG-2, was synthesized by the Kiso group by stereoselective coupling of N-Troc-protected thiophenyl Neu5Gc-sialoside donor with the pre-formed Siaα2–6Glc 1,5-lactamized disaccharide acceptor (105, 106). A similar strategy was used for the synthesis of Fucα1–4Neu5Acα2–5Neu5Gcα2–4Neu5Acα2–6Glc, a pentasaccharide component of HPG-7 ganglioside (107) and Fucα1–8Neu5Gcα2–4Neu5Acα2–6Glc, a tetrasaccharide components of ganglioside HPG-1 (108).
The Crich group reported the synthesis of Neu5Gc-containing oligosaccharides in high stereoselectivity by iterative one-pot route. A series of four trisaccharides were synthesized in one pot by coupling of a 5N-acetoxyacetimide-5N, 4O-oxazolidinone-protected adamantanyl thiosialoside donor with the first thiogalactosyl acceptor followed by addition of the second acceptor after 20 min (109).
The Nifant'ev group used an N-tert-butyloxycarbonyl (N-Boc) and N-acetyl (N-Ac) protected thiophenyl sialoside donor for the synthesis of 3-aminopropyl glycoside of Neu5Gcα2–6LacNAc from N-acetyllactosamine (LacNAc) 4′,6′-diol acceptor (30). A glycosylation yield of 84% with 1.3:1 (α:β) selectivity was achieved. Removal of the N-acetyl and N-Boc groups followed by N-acylation and subsequent deprotection steps formed the desired trisaccharide. A similar strategy was used for the synthesis of 3-aminopropyl glycoside of Neu5Gcα2–3LacNAc using LacNAc 2′,3′,4′-triol acceptor (110).
Instead of installing N-glycolyl group after the formation of sialyl glycosidic bond, properly protected Neu5Gc thioglycoside donors could be directly used for glycosylation. For example, an acetyl-protected thiophenyl Neu5Gc-glycoside donor was used directly with α-selectivity and good sialylation yields for the synthesis of Neu5Gc-containing glycosides including sialyl Lewis × pentasaccharyl ganglioside analog (111) and α2–3-sialyl lactotetraose and neolactotetraose derivatives (112). A thiophenyl Neu5Gc-glycoside donor was also successfully used for the synthesis of Neu5Gcα2–6GalOMP disaccharide and its derivative Neu5Gc9N3α2–6GalOMP containing a 9-azido-9-deoxy-Neu5Gc residue. The 9-azido group of the latter was converted to an amino group and the resulting compound was used to generate a library of 9-N-acylated derivatives of Neu5Gc-sialosides. Some of the compounds were low-micromolar inhibitors of CD22 (or Siglec-2), a well-known B cell-specific sialic acid-binding immunoglobulin-like lectin (113). The same strategy was used to synthesize a similar class of sialosides with different aglycons as improved CD22 inhibitors with up to nanomolar potency (114, 115). In addition to protected thiophenyl Neu5Gc-glycoside donors, a benzyl-protected thiomethyl Neu5Gc-glycoside donor was developed and used for the synthesis of Neu5Gc-containing trisaccharides with 55–63% yields with α-selectivity (116) and a sea cucumber disaccharyl ganglioside analog (117).
Phosphite Donors
Phosphite donors of Neu5Gc are considered to be more reactive than thioglycoside donors. They were used for the synthesis of Neu5Gc-glycosides in propionitrile at −78°C in good yields and α-selectivity (Figure 5) (118).
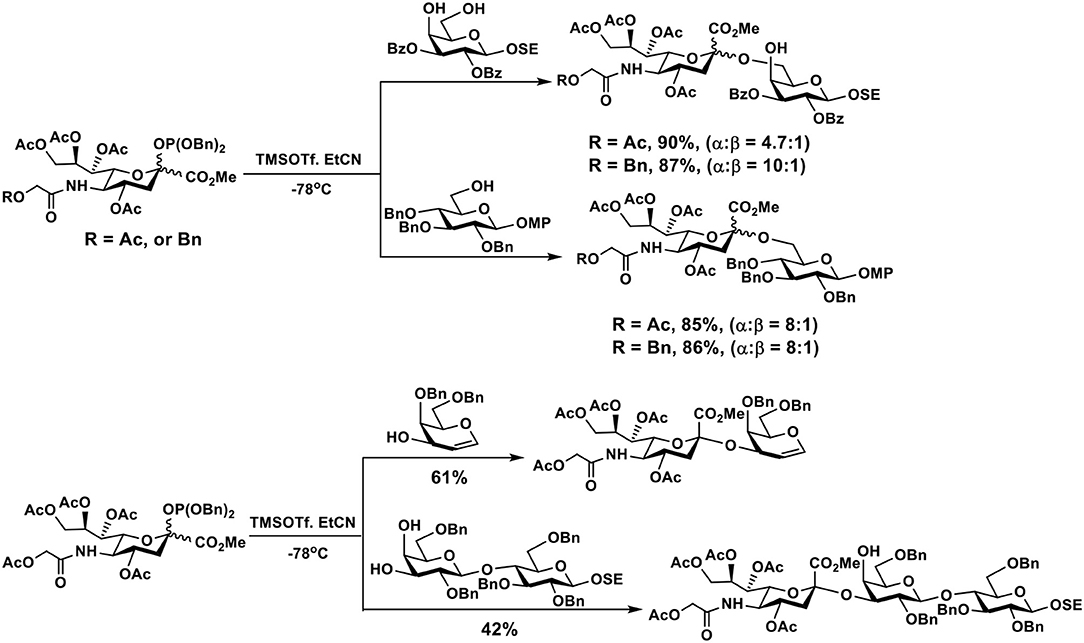
Figure 5. Efficient synthesis of Neu5Gc-glycosides using phosphite donors in good yields and α-selectivity.
Trichloroacetimidate Donors
Trichloroacetimidate donors are the most commonly used glycosyl donors. They have been used for the synthesis of complex Neu5Gc-containing sialosides. The Kiso group reported the first total synthesis of Neu5Gc8Me-containing ganglioside GAA-7 which showed neuritogenic activity. The strategy involved the assembly of the ceramide moiety by Witting, Grignard, and amide formation reactions. Stereoselective β-glycosylation with a glucosyl trichloroacetimidate donor produced a glucosyl ceramide (GlcβCer) cassette which was readily coupled with the protected Neu5Gc-containing tetrasaccharyl trichloroacetimidate donor to form the protected ganglioside. Global deprotection produced GAA-7, a pentasaccharyl β-ceramide Neu5Gc8Meα2–3(Neu5Gc8Meα2–6)GalNAcβ1–3Galβ1–4GlcβCer (119). Protected Neu5Gc-containing disaccharyl trichloroacetimidate donors have also been used for the synthesis of Neu5Gc-containing glycans of lacto- and neolacto-series gangliosides. The reducing ends of these oligosaccharides were further modified by 2-(tetradecyl)hexadecanol to form glycolipid mimics of ceramide-containing gangliosides (120).
N-Phenyltrifluoroacetimidate Donors
N-Phenyltrifluoroacetimidate (121) sialyl donors were designed to improve their reactivity for glycosylation. The feature was combined with 5-N-phthaloyl group protection of the sialyl donors to favor α-sialyl isomer formation (122, 123) and allow their suitability for one-pot procedures (124). As shown in Figure 6, desired α-sialoside was stereoselectively synthesized using this donor. The synthesized α-sialoside was further coupled to another acceptor in one-pot to synthesize trisaccharides with various internal disaccharide units. The 5-N-phthaloyl group on sialic acid of trisaccharides was readily removed, acylated, and deprotected to form the N-glycolyl group in Neu5Gc (124).
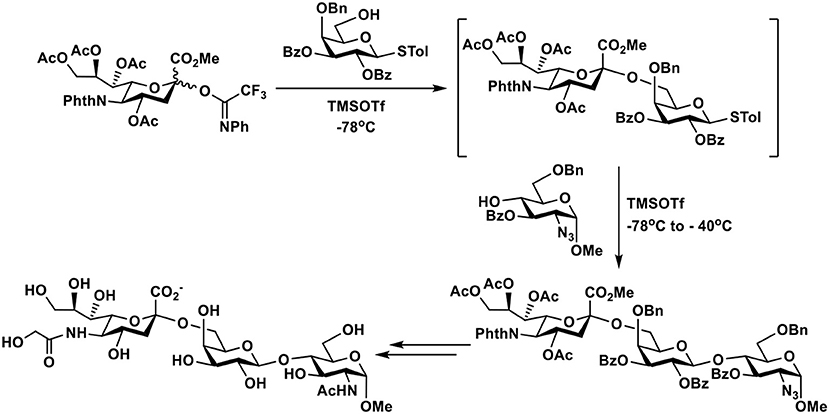
Figure 6. An example of one-pot chemical synthesis of Neu5Gc-containing trisaccharides using 5-N-phthaloyl group protected N-phenyltrifluoroacetimidate sialyl donor.
Chemoenzymatic Synthesis of Neu5Gc-Containing Oligosaccharides
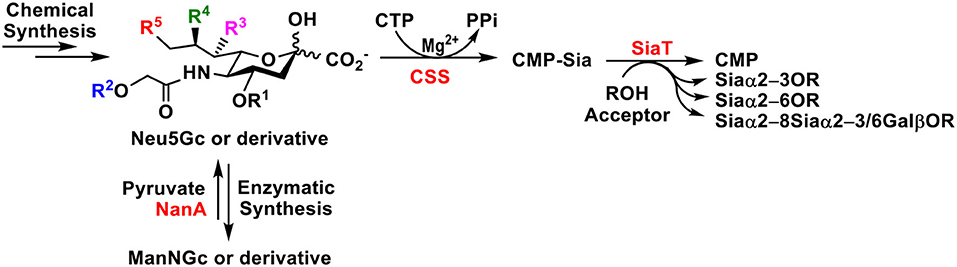
Sialyltransferase-catalyzed glycosylation can be considered as the most efficient approach for the production of sialic acid-containing structures. The strategy offers great advantages, including high regioselectivity and stereoselectivity for the formation of sialyl linkages as well as mild reaction condition in aqueous solutions, etc. (2, 10). The increasing availability of substrate promiscuous sialyltransferases in large amounts makes the strategy practical even for large-scale synthesis. As the sugar nucleotide donor, CMP-Neu5Gc, for sialyltransferase-catalyzed synthesis of Neu5Gc-glycosides is not commercially available, additional enzymes including CMP-sialic acid synthetases (CSSs) with or without sialic acid aldolases are commonly used. Although biosynthetically CMP-Neu5Gc is directly synthesized from CMP-Neu5Ac by CMAH-catalyzed hydroxylation, Neu5Gc is a well-tolerated substrate for CSSs from bacterial sources including those from Neisseria meningitidis (NmCSS), Escherichia coli (EcCSS), Streptococcus agalactiae serotype V (SaVCSS), Pasteurella multocida strain P-1059 (PmCSS), Haemophillus ducreyi (HdCSS), and Clostridium thermocellum (CtCSS) (74, 125, 126). Among these, NmCSS with a high expression level, a high specific activity, and substrate promiscuity is an excellent choice for chemoenzymatic synthesis of sialosides with or without sialic acid modifications (125).
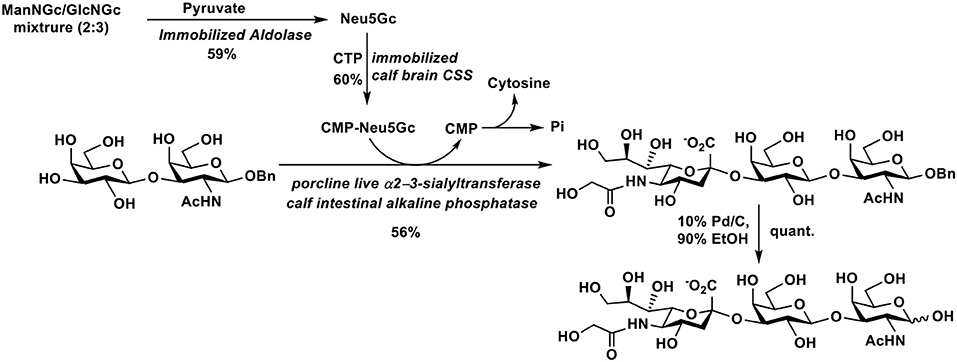
Starting from pyruvate and a mixture of ManNGc and GlcNGc, chemoenzymatic synthesis of trisaccharide Neu5Gcα2–3Galβ1–3GalNAc, which has been found in porcine submaxillary mucin, was achieved (127). As shown in Figure 7, Neu5Gc was synthesized in 59% yield using an immobilized sialic acid aldolase. It was used for the formation of CMP-Neu5Gc using an immobilized calf brain CMP-sialic acid synthetase in 60% yield. Sialylation of Galβ1–3GalNAcβOBn was carried out by a porcine liver α2–3-sialyltransferase-catalyzed reaction using CMP-Neu5Gc as donor. Deprotection by catalytic hydrogenation produced the target trisaccharide Neu5Gcα2–3Galβ1–3GalNAc in 56% yield.
As reaction conditions for sialic acid aldolase, CSS, and sialyltransferase are compatible, they can be mixed together in one-pot with ManNGc, pyruvate, CTP, and a sialyltransferase acceptor for the synthesis of target Neu5Gc-glycosides. Such one-pot multienzyme (OPME) sialylation reactions (82, 84, 86, 128) are highly efficient for chemoenzymatic synthesis of a large library of Neu5Gc-glycosides containing different sialyl linkages and various internal glycans. Sialosides containing modified Neu5Gc forms can also be produced by this strategy.
As shown in Figure 8A, in the OPME reaction containing a sialic acid aldolase, a CSS, and a sialyltransferase, chemically synthesized ManNGc or derivative is enzymatically converted to Neu5Gc or derivative by the sialic acid aldolase. Activation of the formed Neu5Gc or derivative to CMP-Neu5Gc or derivative by CSS followed by sialylation led to the production of the desired sialoside containing Neu5Gc or derivative. Both sialic acid and CMP-sialic acid are generated in situ and do not need to be purified.
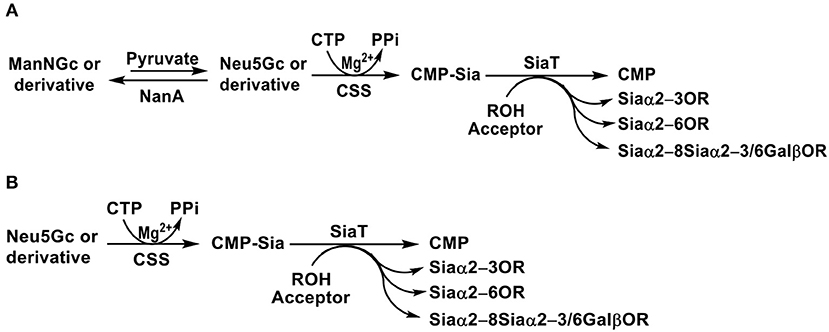
Figure 8. Synthesis of sialosides containing Neu5Gc or derivative using one-pot multienzyme (OPME) sialylation systems containing (A) three enzymes including sialic acid aldolase (NanA), CMP-sialic acid synthetase (CSS), and sialyltransferase (SiaT) or (B) two enzymes including CSS and SiaT.
If Neu5Gc and derivatives are available, OPME reaction containing a CSS and a sialyltransferase without the presence of a sialic acid aldolase (Figure 8B) is sufficient to produce sialosides containing Neu5Gc or derivatives. The strategy is particularly suited for sialosides containing a Neu5Gc derivative that cannot be directly obtained by a sialic acid aldolase-catalyzed reaction, such as Neu4Ac5Gc (72). The method was also used for synthesizing sialosides containing 3F(equatorial)-Neu5Gc or 3F(axial)-Neu5Gc. In this case, 3F(equatorial)-Neu5Gc, and 3F(axial)-Neu5Gc were pre-synthesized from ManNGc and 3-fluoro-pyruvate by EcNanA-catalyzed reaction and purified before being subjected to OPME sialylation reactions.
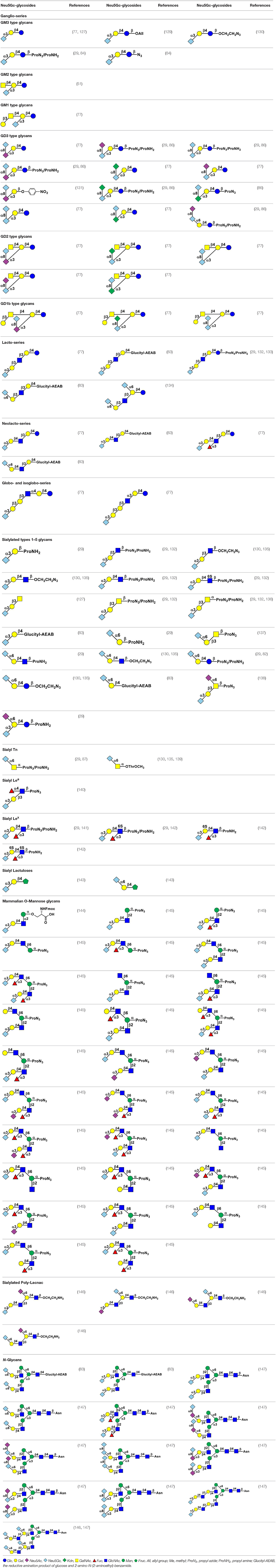
Using efficient OPME sialyltransferase systems with two- or three-enzymes (Figure 8), a diverse array of sialosides including glycosphingolipid glycans, sialylated types 1–5 glycans, and sialyl Tn, containing Neu5Gc (Table 1) as well as sialosides containing different Neu5Gc derivatives including 3F-Neu5Gc, Neu4Ac5Gc, Neu5Gc9Ac, Neu5GcMe, or Neu5GcAc (Table 2) have been synthesized. The obtained compounds have been used to construct sialyl glycan microarrays (28, 33, 34, 42, 83, 150–156), sialoside-protein conjugates (148), and sialidase substrate specificity studies (131, 157–159). Among bacterial sialyltransferases used, Pasteurella multocida sialyltransferase 1 (PmST1) (84) and its single mutant PmST1 M144D with decreased donor hydrolysis and sialidase activities (141) were broadly applied for the synthesis α2–3-linked sialyl oligosaccharides containing Neu5Gc, 3F-Neu5Gc, Neu5Gc9Ac, Neu5GcMe, or Neu5GcAc (77, 83, 84, 89, 157, 160). For synthesizing α2–3-sialyl oligosaccharides containing Neu4Ac5Gc, however, only Pasteurella multocida sialyltransferase 3 (PmST3) (161) was found to be a suitable enzyme (72). PmST3 was also well-suited for the synthesis of α2–3-linked Neu5Gc-containing sialyl glycopeptides (162). Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST) (82), Photobacterium species α2–6-sialyltransferase (Psp2,6ST) (87) and its single mutant with improved expression level and slightly enhanced activity Psp2,6ST A366G (163) were used for synthesizing α2–6-linked sialosides containing Neu5Gc, 3F-Neu5Gc, Neu5Gc9Ac, Neu5GcMe, or Neu5GcAc (82, 83, 89, 157, 160). Psp2,6ST was well-suited for the synthesis of sialyl Tn-antigens (Sia α2–6GalNAcαOR) (87). Campylobacter jejuni sialyltransferase CstII (CjCstII) (164) was found to be an efficient sialyltransferase for the synthesis of a diverse array of Neu5Gc-containing α2–8-linked sialosides (77, 86, 131). For synthesizing sialosides containing Neu5Gc or its stable analogs, such as 3F-Neu5Gc and Neu5GcMe, the pH of the OPME reactions was controlled at 8.5 to allow highly efficient catalysis by all enzymes involved in the reactions. For synthesizing sialosides containing base-labile groups, such as Neu4Ac5Gc, Neu5GcAc, or Neu5Gc9Ac, the pH of the OPME reactions was controlled at 7.0 to minimize de-O-acetylation during the reaction.
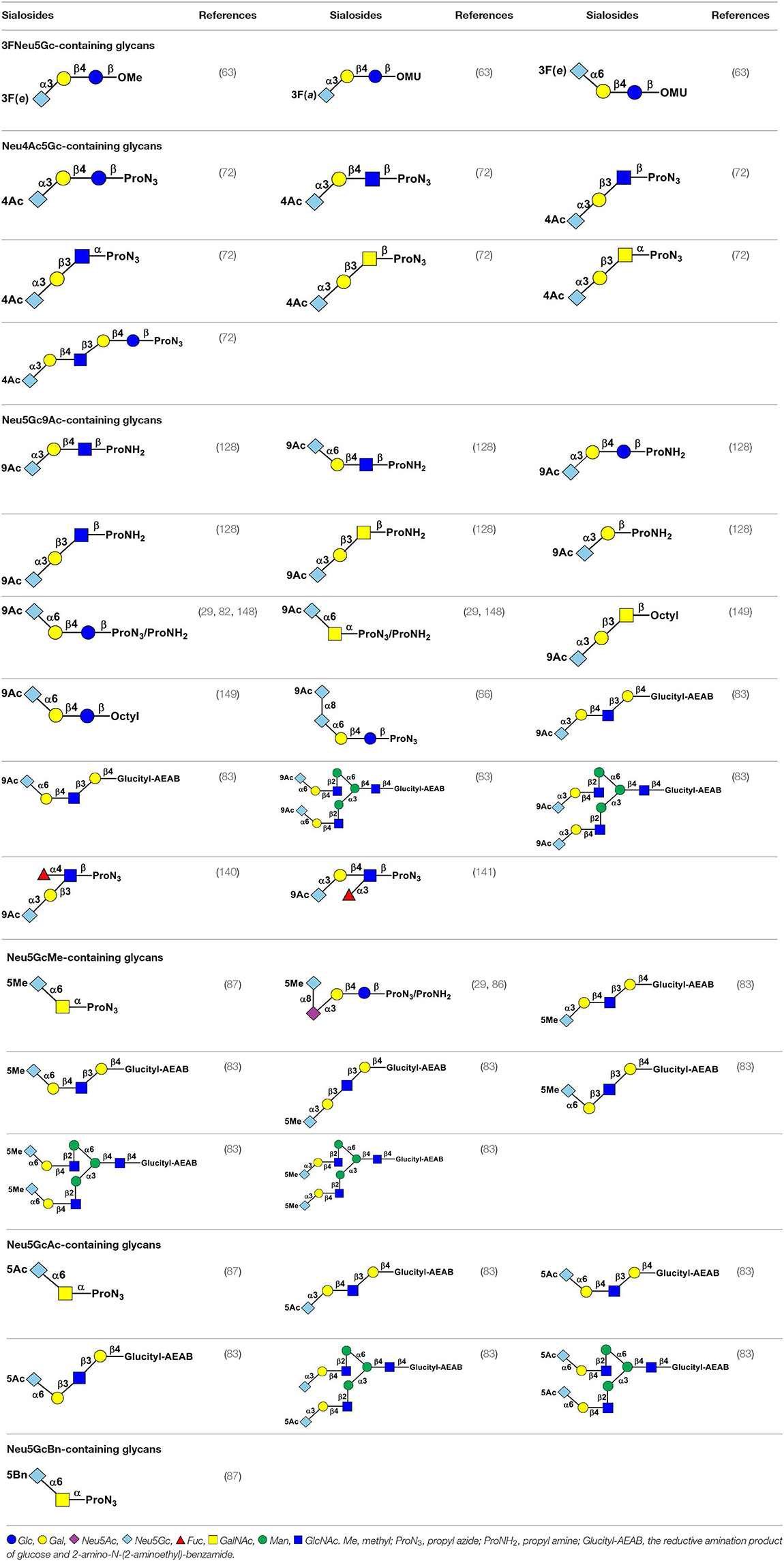
Table 2. Chemoenzymatically synthesized sialosides containing 3FNeu5Gc, Neu4Ac5Gc, Neu5Gc9Ac, Neu5GcMe, Neu5GcAc, or Neu5GcBn.
Recently, the OPME α2–3-sialylation system containing PmNanA, NmCSS, and PmST1 M144D was coupled with Streptococcus pneumoniae sialidase SpNanC-catalyzed reaction for the formation of Neu5Gc2en from ManNGc, pyruvate, CTP, and lactose (165).
Chemoenzymatic Synthesis of Neu5Gc-Containing Glycoconjugates
The alkyl azido aglycone in chemoenzymatically synthesized Neu5Gc-containing sialosides can be readily converted to an alkyl amino group by catalytic hydrogenation to allow convenient conjugation with N-hydroxysuccinimide-activated or epoxide-activated slide surface for generating glycan microarrays (34). It was also used to react with adipic acid p-nitrophenyl diester to form half-esters which were coupled to the amino group (e.g., in lysine residues) of biotinylated human (STn) antigens Neu5Gc/Neu5Gc9Acα2–6GalNAcαOR (Figure 9A) and sialyl lactosides Neu5Gcα2–6Galβ1–4GlcβOR (Figure 9B) containing Neu5Gc or Neu5Gc9Ac were successfully synthesized and used for ELISA inhibition studies (33, 148).
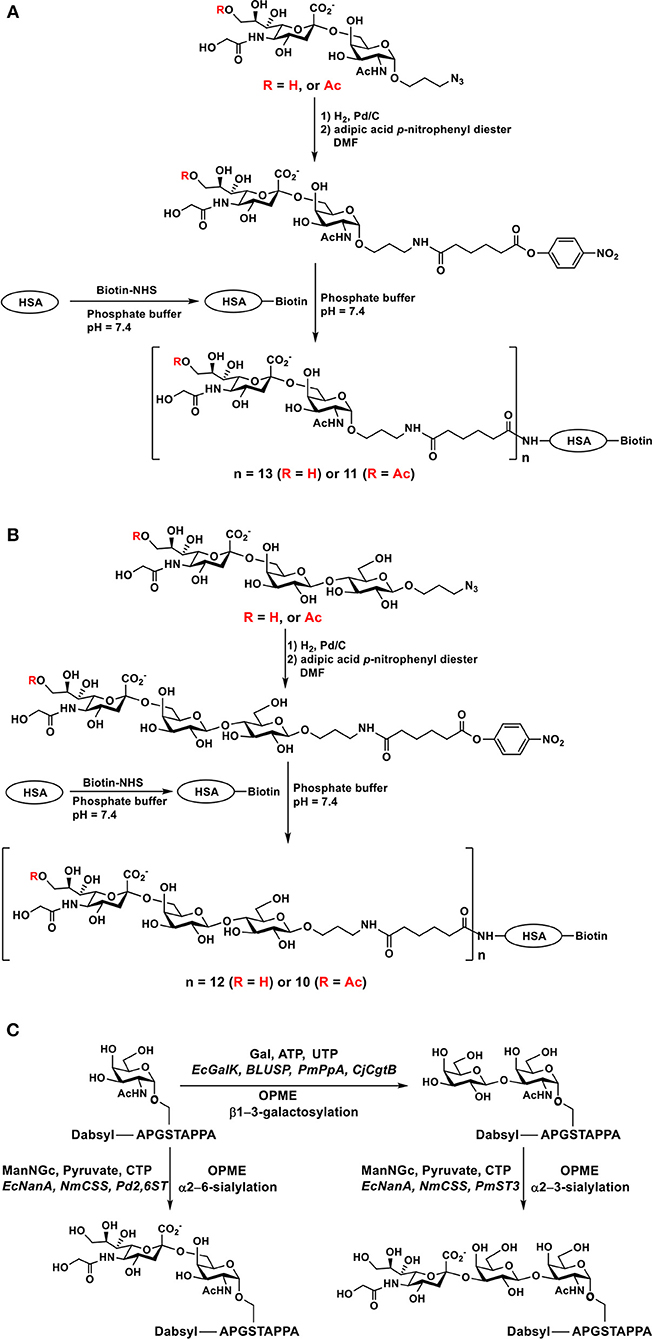
Figure 9. Synthesis of biotinylated human serum albumin-sialoglycoside conjugates containing Neu5Gc or Neu5Gc9Ac including (A) sTn epitopes, (B) sialyl lactoside, and (C) chemoenzymatic synthesis of dabsyl fluorophore-tagged glycopeptides including sTn, T, and ST-antigens containing Neu5Gc. Adopted and modified from Yu et al. (148) and Malekan et al. (162) with permission.
OPME chemoenzymatic sialylation reactions have also been used in the synthesis of sialyl-Tn-MUC1 and sialyl-T-MUC1 glycopeptides containing Neu5Gc (Figure 9C). Pasteurella multocida α2–3-sialyltransferase (PmST3), Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST) Neisseria meningitidis CMP-sialic acid synthetase (NmCSS) and E. coli sialic acid aldolase are the enzymes used for OPME sialylation of glycoproteins (162).
Hidari et al. recently reported the synthesis of multivalent Neu5Gc-containing sialoglycopolypeptides. Treating the chemically synthesized Lac or LacNAc-carrying peptides as acceptors and CMP-Neu5Gc as the donor substrate, sialoglycopolypeptides with α2–3- and α2–6-sialyl linkages were obtained in the presence of ST3Gal III or ST6Gal I, respectively. They found that multivalent α2–3-linked Neu5Gc-ligands selectively inhibited hemagglutination mediated by influenza viruses with a strong inhibitory activity (166). Hernaiz et al. also reported that the enzymatic approach could be directly applied to sialylating lactose-carrying glycoclusters using α2–6-sialyltransferase from rat liver and CMP-Neu5Gc as the donor to produce Neu5Gc-containing glycoclusters (167).
Conclusions and Perspective
Significant advances have been made in the synthesis of sialosides although the focus has been on those containing Neu5Ac, the most common sialic acid form. With the increasing recognition of the presence and the important functions of Neu5Gc and human anti-Neu5Gc xeno-autoantibodies, more attention has been and will be paid to the synthesis of sialosides containing Neu5Gc and its derivatives. Chemical synthetic methods developed for the formation of Neu5Ac-containing molecules can be extended to Neu5Gc counterparts with modifications. Chemoenzymatic methods using sialyltransferases have been recognized as efficient strategies for accessing challenging sialic acid-containing molecules including those containing Neu5Gc and derivatives. Among these, one-pot multienzyme (OPME) systems have been proven powerful tools. Large library of sialosides containing Neu5Gc and derivatives will become available for elucidating their biological roles and exploring their potential applications. These will be indispensable probes for profiling anti-Neu5Gc antibodies and investigating other Neu5Gc-binding proteins. Such information will help us to better understand the physiological and pathological roles of Neu5Gc and its binding partners. Combining sialidase-treatment and sialyltransferase-catalyzed re-sialylation with Neu5Gc or Neu5Ac will be a potentially efficient approach for generating glycoconjugates with a desired sialic acid form for improved therapeutic applications.
Author Contributions
AK, HY, and XC searched the literature, read the papers, and wrote the manuscript.
Funding
The authors would like to acknowledge the financial support from United States National Institutes of Health (NIH) Grants under Award Numbers U01GM120419, U01GM125288, and R01AI130684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors XC.
References
1. Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. (2002) 102:439–69. doi: 10.1021/cr000407m
2. Chen X, Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem Biol. (2010) 5:163–76. doi: 10.1021/cb900266r
3. Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. (2000) 17:485–99. doi: 10.1023/A:1011062223612
4. Bulai T, Bratosin D, Pons A, Montreuil J, Zanetta JP. Diversity of the human erythrocyte membrane sialic acids in relation with blood groups. FEBS Lett. (2003) 534:185–9. doi: 10.1016/S0014-5793(02)03838-3
5. Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta JP, et al. Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the intestinal tract. J Biol Chem. (2003) 278:46337–48. doi: 10.1074/jbc.M302529200
6. Zanetta JP, Pons A, Iwersen M, Mariller C, Leroy Y, Timmerman P, et al. Diversity of sialic acids revealed using gas chromatography/mass spectrometry of heptafluorobutyrate derivatives. Glycobiology. (2001) 11:663–76. doi: 10.1093/glycob/11.8.663
7. Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. (2009) 19:507–14. doi: 10.1016/j.sbi.2009.06.003
8. Nakano T, Sugawara M, Kawakami H. Sialic acid in human milk: composition and functions. Acta Paediatr Taiwan. (2001) 42:11–7. doi: 10.7097/APT.200102.0011
9. Chen X. Human milk oligosaccharides (HMOS): structure, function, and enzyme-catalyzed synthesis. Adv Carbohydr Chem Biochem. (2015) 72:113–90. doi: 10.1016/bs.accb.2015.08.002
10. Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol. (2012) 94:887–905. doi: 10.1007/s00253-012-4040-1
11. Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. (2001) 33:54–69. doi: 10.1002/ajpa.10018
12. Colli W. Trans-sialidase: a unique enzyme activity discovered in the protozoan Trypanosoma cruzi. FASEB J. (1993) 7:1257–64. doi: 10.1096/fasebj.7.13.8405811
13. Varki A, Schnaar RL, Schauer R. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of Glycobiology. 3rd ed. New York, NY: Cold Spring Harbor; Laboratory Press (2015). p. 179–95.
14. Kawano T, Koyama S, Takematsu H, Kozutsumi Y, Kawasaki H, Kawashima S, et al. Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J Biol Chem. (1995) 270:16458–63. doi: 10.1074/jbc.270.27.16458
15. Shaw L, Schneckenburger P, Carlsen J, Christiansen K, Schauer R. Mouse liver cytidine-5′-monophosphate-N-acetylneuraminic acid hydroxylase. Catalytic function and regulation. Eur J Biochem. (1992) 206:269–77. doi: 10.1111/j.1432-1033.1992.tb16925.x
16. Samraj AN, Laubli H, Varki N, Varki A. Involvement of a non-human sialic acid in human cancer. Front Oncol. (2014) 4:33. doi: 10.3389/fonc.2014.00033
17. Kozutsumi Y, Kawano T, Kawasaki H, Suzuki K, Yamakawa T, Suzuki A. Reconstitution of CMP-N-acetylneuraminic acid hydroxylation activity using a mouse liver cytosol fraction and soluble cytochrome b5 purified from horse erythrocytes. J Biochem. (1991) 110:429–35. doi: 10.1093/oxfordjournals.jbchem.a123598
18. Kean EL, Munster-Kuhnel AK, Gerardy-Schahn R. CMP-sialic acid synthetase of the nucleus. Biochim Biophys Acta. (2004) 1673:56–65. doi: 10.1016/j.bbagen.2004.04.006
19. Altheide TK, Hayakawa T, Mikkelsen TS, Diaz S, Varki N, Varki A. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: evidence for two modes of rapid evolution. J Biol Chem. (2006) 281:25689–702. doi: 10.1074/jbc.M604221200
20. Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. (1998) 95:11751–6. doi: 10.1073/pnas.95.20.11751
21. Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. (1998) 273:15866–71. doi: 10.1074/jbc.273.25.15866
22. Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. (2014) 66:671–4. doi: 10.1007/s00251-014-0795-0
23. Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, et al. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. (2009) 4:e4241. doi: 10.1371/journal.pone.0004241
24. Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. (2009) 114:5225–35. doi: 10.1182/blood-2009-05-220400
25. Inoue S, Sato C, Kitajima K. Extensive enrichment of N-glycolylneuraminic acid in extracellular sialoglycoproteins abundantly synthesized and secreted by human cancer cells. Glycobiology. (2010) 20:752–62. doi: 10.1093/glycob/cwq030
26. Labrada M, Clavell M, Bebelagua Y, Leon J, Alonso DF, Gabri MR, et al. Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy. Expert Opin Biol Ther. (2010) 10:153–62. doi: 10.1517/14712590903443084
27. Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. (2005) 280:4228–37. doi: 10.1074/jbc.M412040200
28. Amon R, Ben-Arye SL, Engler L, Yu H, Lim N, Berre LL, et al. Glycan microarray reveal induced IgGs repertoire shift against a dietary carbohydrate in response to rabbit anti-human thymocyte therapy. Oncotarget. (2017) 8:112236–44. doi: 10.18632/oncotarget.23096
29. Bashir S, Leviatan Ben Arye S, Reuven EM, Yu H, Costa C, Galiñanes M, et al. Presentation mode of glycans affect recognition of human serum anti-Neu5Gc IgG antibodies. Bioconjug Chem. (2019) 30:161–8. doi: 10.1021/acs.bioconjchem.8b00817
30. Sherman AA, Yudina ON, Shashkov AS, Menshov VM, Nifant'ev NE. Synthesis of Neu5Ac- and Neu5Gc-α-(2 → 6′)-lactosamine 3-aminopropyl glycosides. Carbohydr Res. (2001) 330:445–58. doi: 10.1016/S0008-6215(01)00002-7
31. Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. (2012) 28:147–76. doi: 10.5661/bger-28-147
32. Dhar C, Sasmal A, Varki A. From “Serum Sickness” to “Xenosialitis”: past, present, and future significance of the non-human sialic acid Neu5Gc. Front Immunol. (2019) 10:807. doi: 10.3389/fimmu.2019.00807
33. Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. (2008) 18:818–30. doi: 10.1093/glycob/cwn072
34. Padler-Karavani V, Tremoulet AH, Yu H, Chen X, Burns JC, Varki A. A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease. PLoS ONE. (2013) 8:e58443. doi: 10.1371/journal.pone.0058443
35. Amon R, Reuven EM, Leviatan Ben-Arye S, Padler-Karavani V. Glycans in immune recognition and response. Carbohydr Res. (2014) 389:115–22. doi: 10.1016/j.carres.2014.02.004
36. Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. (2010) 207:1637–46. doi: 10.1084/jem.20100575
37. Apicella M. Nontypeable Haemophilus influenzae: The role of N-acetyl-5-neuraminic acid in biology. Front Cell Infect Microbiol. (2012) 2:1–7. doi: 10.3389/fcimb.2012.00019
38. Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J. (2009) 26:231–45. doi: 10.1007/s10719-008-9183-z
39. Peri S, Kulkarni A, Feyertag F, Berninsone PM, Alvarez-Ponce D. Phylogenetic distribution of CMP-Neu5Ac hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biol Evol. (2018) 10:207–19. doi: 10.1093/gbe/evx251
40. Angata T. Possible influences of endogenous and exogenous ligands on the evolution of human siglecs. Front Immunol. (2018) 9:2885. doi: 10.3389/fimmu.2018.02885
41. Varki NM, Strobert E, Dick EJ Jr, Benirschke K, Varki A. Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu Rev Pathol. (2011) 6:365–93. doi: 10.1146/annurev-pathol-011110-130315
42. Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. (2011) 71:3352–63. doi: 10.1158/0008-5472.CAN-10-4102
43. Eleftheriou P, Kynigopoulos S, Giovou A, Mazmanidi A, Yovos J, Skepastianos P, et al. Prevalence of anti-Neu5Gc antibodies in patients with hypothyroidism. Biomed Res Int. (2014) 2014:963230. doi: 10.1155/2014/963230
44. Okerblom J, Varki A. Biochemical, cellular, physiological, and pathological consequences of human loss of N-glycolylneuraminic acid. ChemBioChem. (2017) 18:1155–71. doi: 10.1002/cbic.201700077
45. Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. (2015) 112:542–7. doi: 10.1073/pnas.1417508112
46. Couvrat-Desvergnes G, Salama A, Le Berre L, Evanno G, Viklicky O, Hruba P, et al. Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J Clin Invest. (2015) 125:4655–65. doi: 10.1172/JCI82267
47. Salama A, Evanno G, Lim N, Rousse J, Le Berre L, Nicot A, et al. Anti-Gal and anti-Neu5Gc responses in nonimmunosuppressed patients after treatment with rabbit antithymocyte polyclonal IgGs. Transplantation. (2017) 101:2501–7. doi: 10.1097/TP.0000000000001686
48. Rousse J, Salama A, Leviatan Ben-Arye S, Hruba P, Slatinska J, Evanno G, et al. Quantitative and qualitative changes in anti-Neu5Gc antibody response following rabbit anti-thymocyte IgG induction in kidney allograft recipients. Eur J Clin Invest. (2019) 49:e13069. doi: 10.1111/eci.13069
49. Soulillou JP, Susal C, Dohler B, Opelz G. No increase in colon cancer risk following induction with Neu5Gc-bearing rabbit anti-T cell IgG (ATG) in recipients of kidney transplants. Cancers. (2018) 10:324. doi: 10.3390/cancers10090324
50. Reuven EM, Leviatan Ben-Arye S, Marshanski T, Breimer ME, Yu H, Fellah-Hebia I, et al. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation. (2016) 23:381–92. doi: 10.1111/xen.12260
51. Zhang R, Wang Y, Chen L, Wang R, Li C, Li X, et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/beta4GalNT2/CMAH. Acta Biomater. (2018) 72:196–205. doi: 10.1016/j.actbio.2018.03.055
52. Scobie L, Padler-Karavani V, Le Bas-Bernardet S, Crossan C, Blaha J, Matouskova M, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. (2013) 191:2907–15. doi: 10.4049/jimmunol.1301195
53. Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. (2010) 17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x
54. Bergwerff AA, Hulleman SH, Kamerling JP, Vliegenthart JF, Shaw L, Reuter G, et al. Nature and biosynthesis of sialic acids in the starfish Asterias rubens. Identification of sialo-oligomers and detection of S-adenosyl-L-methionine: N-acylneuraminate 8-O-methyltransferase and CMP-N-acetylneuraminate monooxygenase activities. Biochimie. (1992) 74:25–37. doi: 10.1016/0300-9084(92)90181-D
55. Muralikrishna G, Reuter G, Peter-Katalinic J, Egge H, Hanisch FG, Siebert HC, et al. Identification of a new ganglioside from the starfish Asterias rubens. Carbohydr Res. (1992) 236:321–6.
56. Sato C, Kitajima K, Tazawa I, Inoue Y, Inoue S, Troy FA II. Structural diversity in the alpha 2–>8-linked polysialic acid chains in salmonid fish egg glycoproteins. Occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J Biol Chem. (1993) 268:23675–84.
57. Sumi T, Sallay I, Asakawa M, Park SS, Miyazaki M, Ohba H. Purification and identification of N-glycolylneuraminic acid (Neu5Gc) from the holothuroidea Gumi, Cucumaria echinata. Preparative Biochem Biotechnol. (2001) 31:135–46. doi: 10.1081/pb-100103379
58. Urashima T, Messer M, Oftedal OT. Chapter 3–oligosaccharides in the milk of other mammals. In: McGuire MK, McGuire MA, Bode L, editors. Prebiotics and Probiotics in Human Milk. San Diego, CA: Academic Press (2017). p. 45–139.
59. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. (1993) 3:97–130. doi: 10.1093/glycob/3.2.97
60. Corfield T. Bacterial sialidases–roles in pathogenicity and nutrition. Glycobiology. (1992) 2:509–21. doi: 10.1093/glycob/2.6.509
61. Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. (1982) 40:131–234. doi: 10.1016/S0065-2318(08)60109-2
62. Kiefel MJ, von Itzstein M. Recent advances in the synthesis of sialic acid derivatives and sialylmimetics as biological probes. Chem Rev. (2002) 102:471–90. doi: 10.1021/cr000414a
63. Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. (2012) 1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x
64. Hong Z, Liu L, Hsu CC, Wong CH. Three-step synthesis of sialic acids and derivatives. Angew Chem Int Ed Engl. (2006) 45:7417–21. doi: 10.1002/anie.200601555
65. Reuter G, Pfeil R, Stoll S, Schauer R, Kamerling JP, Versluis C, et al. Identification of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur J Biochem. (1983) 134:139–43. doi: 10.1111/j.1432-1033.1983.tb07542.x
66. Stehling P, Gohlke M, Fitzner R, Reutter W. Rapid analysis of O-acetylated neuraminic acids by matrix assisted laser desorption/ionization time-of-flight mass spectrometry. Glycoconj J. (1998) 15:339–44. doi: 10.1023/A:1006965600322
67. Iwasaki M, Inoue S, Troy FA. A new sialic acid analogue, 9-O-acetyl-deaminated neuraminic acid, and alpha-2,8-linked O-acetylated poly(N-glycolylneuraminyl) chains in a novel polysialoglycoprotein from salmon eggs. J Biol Chem. (1990) 265:2596–602. doi: 10.4052/tigg.2.271
68. Iwersen M, Vandamme-Feldhaus V, Schauer R. Enzymatic 4-O-acetylation of N-acetylneuraminic acid in guinea-pig liver. Glycoconj J. (1998) 15:895–904. doi: 10.1023/A:1006911100081
69. Miyoshi I, Higashi H, Hirabayashi Y, Kato S, Naiki M. Detection of 4-O-acetyl-N-glycolylneuraminyl lactosylceramide as one of tumor-associated antigens in human colon cancer tissues by specific antibody. Mol Immunol. (1986) 23:631–8. doi: 10.1016/0161-5890(86)90100-8
70. Ogura H, Furuhata K, Sato S, Anazawa K, Itoh M, Shitori Y. Synthesis of 9-O-acyl- and 4-O-acetyl-sialic acids. Carbohydr Res. (1987) 167:77–86. doi: 10.1016/0008-6215(87)80269-0
71. Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, et al. Pasteurella multocida sialic acid aldolase: a promising biocatalyst. Appl Microbiol Biotechnol. (2008) 79:963–70. doi: 10.1007/s00253-008-1506-2
72. Yu H, Zeng J, Li Y, Thon V, Shi B, Chen X. Effective one-pot multienzyme (OPME) synthesis of monotreme milk oligosaccharides and other sialosides containing 4-O-acetyl sialic acid. Org Biomol Chem. (2016) 14:8586–97. doi: 10.1039/c6ob01706a
73. Choi SK, Lee S, Whitesides GM. Synthesis of C-5 analogs of N-acetylneuraminic acid via indium-mediated allylation of N-substituted 2-amino-2-deoxymannoses. J Org Chem. (1996) 61:8739–45. doi: 10.1021/jo9614856
74. Yu H, Yu H, Karpel R, Chen X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg Med Chem. (2004) 12:6427–35. doi: 10.1016/j.bmc.2004.09.030
75. Pearce OMT, Varki A. Chemo-enzymatic synthesis of the carbohydrate antigen N-glycolylneuraminic acid from glucose. Carbohydr Res. (2010) 345:1225–9. doi: 10.1016/j.carres.2010.04.003
76. Kuboki A, Okazaki H, Sugai T, Ohta H. An expeditious route to N-glycolylneuraminic acid based on enzyme-catalyzed reaction. Tetrahedron. (1997) 53:2387–400. doi: 10.1016/S0040-4020(96)01189-1
77. Yu H, Li Y, Zeng J, Thon V, Nguyen DM, Ly T, et al. Sequential one-pot multienzyme chemoenzymatic synthesis of glycosphingolipid glycans. J Org Chem. (2016) 81:10809–24. doi: 10.1021/acs.joc.6b01905
78. Li Y, Cao H, Yu H, Chen Y, Lau K, Qu J, et al. Identifying selective inhibitors against the human cytosolic sialidase NEU2 by substrate specificity studies. Mol Biosyst. (2011) 7:1060–72. doi: 10.1039/c0mb00244e
79. Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. (2011) 29:428–35. doi: 10.1038/nbt.1846
80. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. (2009) 4:31–6. doi: 10.1038/nprot.2008.214
81. Chen GY, Brown NK, Wu W, Khedri Z, Yu H, Chen X, et al. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife. (2014) 3:e04066. doi: 10.7554/eLife.04066
82. Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew Chem Int Ed Engl. (2006) 45:3938–44. doi: 10.1002/anie.200600572
83. Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, et al. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J Biol Chem. (2011) 286:31610–22. doi: 10.1074/jbc.M111.274217
84. Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, et al. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc. (2005) 127:17618–9. doi: 10.1021/ja0561690
85. Huang S, Yu H, Chen X. Disaccharides as sialic acid aldolase substrates: synthesis of disaccharides containing a sialic acid at the reducing end. Angew Chem Int Ed Engl. (2007) 46:2249–53. doi: 10.1002/anie.200604799
86. Yu H, Cheng J, Ding L, Khedri Z, Chen Y, Chin S, et al. Chemoenzymatic synthesis of GD3 oligosaccharides and other disialyl glycans containing natural and non-natural sialic acids. J Am Chem Soc. (2009) 131:18467–77. doi: 10.1021/ja907750r
87. Ding L, Yu H, Lau K, Li Y, Muthana S, Wang J, et al. Efficient chemoenzymatic synthesis of sialyl Tn-antigens and derivatives. Chem Commun. (2011) 47:8691–3. doi: 10.1039/c1cc12732b
88. Yu H, Cao H, Tiwari VK, Li Y, Chen X. Chemoenzymatic synthesis of C8-modified sialic acids and related alpha2-3- and alpha2-6-linked sialosides. Bioorg Med Chem Lett. (2011) 21:5037–40. doi: 10.1016/j.bmcl.2011.04.083
89. Chokhawala HA, Cao H, Yu H, Chen X. Enzymatic synthesis of fluorinated mechanistic probes for sialidases and sialyltransferases. J Am Chem Soc. (2007) 129:10630–1. doi: 10.1021/ja072687u
90. Roy R, Laferrière CA. Synthesis of protein conjugates and analogues of N-acetylneuraminic acid. Can J Chem. (1990) 68:2045–54. doi: 10.1139/v90-313
91. Chopra P, Madge PD, Thomson RJ, Grice ID, von Itzstein M. Microwave-assisted synthesis of N-glycolylneuraminic acid derivatives. Tetrahedron Lett. (2013) 54:5558–61. doi: 10.1016/j.tetlet.2013.07.107
92. Khedri Z, Li Y, Cao H, Qu J, Yu H, Muthana MM, et al. Synthesis of selective inhibitors against V. cholerae sialidase and human cytosolic sialidase NEU2. Org Biomol Chem. (2012) 10:6112–20. doi: 10.1039/c2ob25335f
93. Ren C-T, Chen C-S, Wu S-H. Synthesis of a sialic acid dimer derivative, 2′α-O-benzyl Neu5Ac-α-(2 → 5)Neu5Gc. J Org Chem. (2002) 67:1376–9. doi: 10.1021/jo015930v
94. Fan G-T, Lee C-C, Lin C-C, Fang J-M. Stereoselective synthesis of Neu5Acα(2 → 5)Neu5Gc: the building block of oligo/poly(→5-O-glycolylNeu5Gcα2→) chains in sea urchin egg cell surface glycoprotein. J Org Chem. (2002) 67:7565–8. doi: 10.1021/jo025988p
95. Das A, Li P-J, Adak AK, Wu H-R, Anwar MT, Chiang P-Y, et al. Stereoselective synthesis of a 9-O-sulfo Neu5Gc-capped O-linked oligosaccharide found on the sea urchin egg receptor. Org Chem Front. (2019) 6:54–61. doi: 10.1039/C8QO00996A
96. McAuliffe JC, Rabuka D, Hindsgaul O. Synthesis of O-glycolyl-linked neuraminic acids through a spirocyclic intermediate. Org Lett. (2002) 4:3067–9. doi: 10.1021/ol026317g
97. Ren CT, Chen CS, Yu YP, Tsai YF, Lin PY, Chen YJ, et al. Synthesis of alpha-(2–>5)Neu5Gc oligomers. Eur J Org Chem. (2003) 9:1085–95. doi: 10.1002/chem.200390101
98. Numata M, Sugimoto M, Shibayama S, Ogawa T. A total synthesis of hematoside, α-NeuGc-(2 → 3)-β-Gal-(1 → 4)-β-Glc-(1 → 1)-Cer. Carbohydr Res. (1988) 174:73–85. doi: 10.1016/0008-6215(88)85082-1
99. Ando H, Koike Y, Ishida H, Kiso M. Extending the possibility of an N-Troc-protected sialic acid donor toward variant sialo-glycoside synthesis. Tetrahedron Lett. (2003) 44:6883–6. doi: 10.1016/S0040-4039(03)01707-6
100. Tamai H, Ando H, Ishida H, Kiso M. First synthesis of a pentasaccharide moiety of ganglioside GAA-7 containing unusually modified sialic acids through the use of N-Troc-sialic acid derivative as a key unit. Org Lett. (2012) 14:6342–5. doi: 10.1021/ol303122w
101. Davies WH. Anhydrocarboxy-derivatives of hydroxy- and mercapto-acids. J Chem Soc. (1951) 0:1357–9. doi: 10.1039/JR9510001357
102. Sonnino S, Acquotti D, Fronza G, Cantu L, Chigorno V, Pitto M, et al. Semisynthetic preparation of N-glycolylneuraminic acid containing GM1 ganglioside: chemical characterization, physico-chemical properties and some biochemical features. Chem Phys Lipids. (1988) 46:181–91. doi: 10.1016/0009-3084(88)90020-5
103. Hanashima S, Ishikawa D, Akai S, Sato K-i. Synthesis of the starfish ganglioside LLG-3 tetrasaccharide. Carbohydr Res. (2009) 344:747–52. doi: 10.1016/j.carres.2009.02.003
104. Tamai H, Ando H, Tanaka HN, Hosoda-Yabe R, Yabe T, Ishida H, et al. The total synthesis of the neurogenic ganglioside LLG-3 isolated from the starfish Linckia laevigata. Angew Chem Int Ed Engl. (2011) 50:2330–3. doi: 10.1002/anie.201006035
105. Ando H, Koike Y, Koizumi S, Ishida H, Kiso M. 1,5-Lactamized sialyl acceptors for various disialoside syntheses: novel method for the synthesis of glycan portions of Hp-s6 and HLG-2 gangliosides. Angew Chem Int Ed Engl. (2005) 44:6759–63. doi: 10.1002/anie.200501608
106. Iwayama Y, Ando H, Ishida H, Kiso M. A first total synthesis of ganglioside HLG-2. Eur J Org Chem. (2009) 15:4637–48. doi: 10.1002/chem.200802706
107. Iwayama Y, Ando H, Tanaka HN, Ishida H, Kiso M. Synthesis of the glycan moiety of ganglioside HPG-7 with an unusual trimer of sialic acid as the inner sugar residue. Chem Comm. (2011) 47:9726–8. doi: 10.1039/c1cc13200h
108. Shimizu H, Iwayama Y, Imamura A, Ando H, Ishida H, Kiso M. Synthesis of the disialic acid-embedded glycan part of ganglioside HPG-1. Biosci Biotechnol Biochem. (2011) 75:2079–82. doi: 10.1271/bbb.110619
109. Crich D, Wu B. Stereoselective iterative one-pot synthesis of N-glycolylneuraminic acid-containing oligosaccharides. Org lett. (2008) 10:4033–5. doi: 10.1021/ol801548k
110. Sherman AA, Yudina ON, Shashkov AS, Menshov VM, Nifantiev NE. Preparative route to N-glycolylneuraminic acid phenyl 2-thioglycoside donor and synthesis of Neu5Gc-α-(2 → 3′)-lactosamine 3-aminopropyl glycoside. Carbohydr Res. (2002) 337:451–7. doi: 10.1016/S0008-6215(02)00003-4
111. Hasegawa A, Uchimura A, Ishida H, Kiso M. Synthesis of a sialyl Lewis X ganglioside analogue containing N-glycolyl in place of the N-acetyl group in the N-acetylneuraminic acid residue. Biosci Biotechnol Biochem. (1995) 59:1091–4. doi: 10.1271/bbb.59.1091
112. Tanahashi E, Fukunaga K, Ozawa Y, Toyoda T, Ishida H, Kiso M. Synthesis of sialyl-α-(2 → 3)-neolactotetraose derivatives containing different sialic acids: Molecular probes for elucidation of substrate specificity of human α1,3-fucosyltransferases. J Carbohydr Chem. (2000) 19:747–68. doi: 10.1080/07328300008544114
113. Abdu-Allah HHM, Tamanaka T, Yu J, Zhuoyuan L, Sadagopan M, Adachi T, et al. Design, synthesis, and structure–affinity relationships of novel series of sialosides as CD22-specific inhibitors. J Med Chem. (2008) 51:6665–81. doi: 10.1021/jm8000696
114. Abdu-Allah HHM, Watanabe K, Hayashizaki K, Takaku C, Tamanaka T, Takematsu H, et al. Potent small molecule mouse CD22-inhibitors: Exploring the interaction of the residue at C-2 of sialic acid scaffold. Bioorg Med Chem Lett. (2009) 19:5573–5. doi: 10.1016/j.bmcl.2009.08.044
115. Abdu-Allah HHM, Watanabe K, Completo GC, Sadagopan M, Hayashizaki K, Takaku C, et al. CD22-Antagonists with nanomolar potency: the synergistic effect of hydrophobic groups at C-2 and C-9 of sialic acid scaffold. Bioorg Med Chem. (2011) 19:1966–71. doi: 10.1016/j.bmc.2011.01.060
116. Sugata T, Higuchi R. A facile preparation of the methyl 2-thioglycoside of N-glycolylneuraminic acid, an efficient donor of NeuGc. Tetrahedron Lett. (1996) 37:2613–4. doi: 10.1016/0040-4039(96)00383-8
117. Higuchi R, Mori T, Sugata T, Yamada K, Miyamoto T. Partial synthesis of a sea cucumber ganglioside analogue from a starfish cerebroside. Eur J Org Chem. (1999) 1999:145–7. doi: 10.1002/(SICI)1099-0690(199901)1999:1<145::AID-EJOC145>3.0.CO;2-E
118. Hanashima S, Tomiya T, Ishikawa D, Akai S, Sato K. Sialylation using N-glycolylneuraminyl phosphite donors to synthesize Neu5Gc-containing glycans. Carbohydr Res. (2009) 344:959–65. doi: 10.1016/j.carres.2009.03.004
119. Tamai H, Imamura A, Ogawa J, Ando H, Ishida H, Kiso M. First total synthesis of ganglioside GAA-7 from starfish Asterias amurensis versi-color. Eur J Org Chem. (2015) 2015:5199–211. doi: 10.1002/ejoc.201500606
120. Fukunaga K, Toyoda T, Ishida H, Kiso M. Synthesis of lacto- and neolacto-series ganglioside analogs containing N-glycolylneuraminic acid: Probes for investigation of specific receptor structures recognized by influenza A viruses. J Carbohydr Chem. (2003) 22:919–37. doi: 10.1081/CAR-120026602
121. Yu B, Tao H. Glycosyl trifluoroacetimidates. Part 1: preparation and application as new glycosyl donors. Tetrahedron Lett. (2001) 42:2405–7. doi: 10.1016/S0040-4039(01)00157-5
122. Tanaka K, Goi T, Fukase K. Highly efficient sialylation towards α(2-3)- and α(2-6)-Neu5Ac-Gal synthesis: significant ‘fixed dipole effect’ of N-phthalyl group on α-selectivity. Synlett. (2005) 2005:2958–62. doi: 10.1055/s-2005-921889
123. Tanaka Si, Goi T, Tanaka K, Fukase K. Highly efficient α-sialylation by virtue of fixed dipole effects of N-phthalyl group: Application to continuous flow synthesis of α(2-3)-and α(2-6)-Neu5Ac-Gal motifs by microreactor. J Carbohydr Chem. (2007) 26:369–94. doi: 10.1080/07328300701634796
124. Hsu Y, Ma HH, Lico LS, Jan JT, Fukase K, Uchinashi Y, et al. One-pot synthesis of N-acetyl- and N-glycolylneuraminic acid capped trisaccharides and evaluation of their influenza A(H1 N1) inhibition. Angew Chem Int Ed Engl. (2014) 53:2413–6. doi: 10.1002/anie.201309646
125. Li Y, Yu H, Cao H, Muthana S, Chen X. Pasteurella multocida CMP-sialic acid synthetase and mutants of Neisseria meningitidis CMP-sialic acid synthetase with improved substrate promiscuity. Appl Microbiol Biotechnol. (2012) 93:2411–23. doi: 10.1007/s00253-011-3579-6
126. Mizanur RM, Pohl NL. Cloning and characterization of a heat-stable CMP-N-acylneuraminic acid synthetase from Clostridium thermocellum. Appl Microbiol Biotechnol. (2007) 76:827–34. doi: 10.1007/s00253-007-1053-2
127. Lubineau A, Augé C, Narvor CG-L, Ginet J-C. Combined chemical and enzymatic synthesis of the sialylated non reducing terminal sequence of GM1b glycolylated ganglioside, a potential human tumor marker. Bioorg Med Chem. (1994) 2:669–74. doi: 10.1016/0968-0896(94)85016-X
128. Yu H, Chokhawala HA, Huang S, Chen X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat Protoc. (2006) 1:2485–92. doi: 10.1038/nprot.2006.401
129. Chappell MD, Halcomb RL. Enzyme-catalyzed synthesis of oligosaccharides that contain functionalized sialic acids. J Am Chem Soc. (1997) 119:3393–4. doi: 10.1021/ja963894p
130. Blixt O, Paulson JC. Biocatalytic preparation of N-glycolylneuraminic acid, deaminoneuraminic acid (KDN) and 9-azido-9-deoxysialic acid oligosaccharides. Adv Synth Catal. (2003) 345:687–90. doi: 10.1002/adsc.200303032
131. Tasnima N, Yu H, Li Y, Santra A, Chen X. Chemoenzymatic synthesis of para-nitrophenol (pNP)-tagged alpha2-8-sialosides and high-throughput substrate specificity studies of alpha2-8-sialidases. Org Biomol Chem. (2016) 15:160–7. doi: 10.1039/c6ob02240e
132. Huang S, Yu H, Chen X. Chemoenzymatic synthesis of alpha2-3-sialylated carbohydrate epitopes. Sci China Chem. (2011) 54:117–28. doi: 10.1007/s11426-010-4175-9
133. Yao W, Yan J, Chen X, Wang F, Cao H. Chemoenzymatic synthesis of lacto-N-tetrasaccharide and sialyl lacto-N-tetrasaccharides. Carbohydr Res. (2015) 401:5–10. doi: 10.1016/j.carres.2014.10.017
134. Yu H, Yan X, Autran CA, Li Y, Etzold S, Latasiewicz J, et al. Enzymatic and chemoenzymatic syntheses of disialyl glycans and their necrotizing enterocolitis preventing effects. J Org Chem. (2017) 82:13152–60. doi: 10.1021/acs.joc.7b02167
135. Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. (2003) 278:31007–19. doi: 10.1074/jbc.M304331200
136. Lau K, Yu H, Thon V, Khedri Z, Leon ME, Tran BK, et al. Sequential two-step multienzyme synthesis of tumor-associated sialyl T-antigens and derivatives. Org Biomol Chem. (2011) 9:2784–9. doi: 10.1039/c0ob01269f
137. Xu Y, Fan Y, Ye J, Wang F, Nie Q, Wang L, et al. Successfully engineering a bacterial sialyltransferase for regioselective α2,6-sialylation. ACS Catal. (2018) 8:7222–7. doi: 10.1021/acscatal.8b01993
138. Meng X, Yao W, Cheng J, Zhang X, Jin L, Yu H, et al. Regioselective chemoenzymatic synthesis of ganglioside disialyl tetrasaccharide epitopes. J Am Chem Soc. (2014) 136:5205–8. doi: 10.1021/ja5000609
139. Blixt O, Allin K, Pereira L, Datta A, Paulson JC. Efficient chemoenzymatic synthesis of O-linked sialyl oligosaccharides. J Am Chem Soc. (2002) 124:5739–46. doi: 10.1021/ja017881+
140. Tasnima N, Yu H, Yan X, Li W, Xiao A, Chen X. Facile chemoenzymatic synthesis of Lewis a (Le(a)) antigen in gram-scale and sialyl Lewis a (sLe(a)) antigens containing diverse sialic acid forms. Carbohydr Res. (2018) 472:115–21. doi: 10.1016/j.carres.2018.12.004
141. Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, et al. A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating LewisX. ACS Chem Biol. (2012) 7:1232–40. doi: 10.1021/cb300125k
142. Santra A, Yu H, Tasnima N, Muthana MM, Li Y, Zeng J, et al. Systematic chemoenzymatic synthesis of O-sulfated sialyl Lewis × antigens. Chem Sci. (2016) 7:2827–31. doi: 10.1039/c5sc04104j
143. Yu H, Li Y, Wu Z, Li L, Zeng J, Zhao C, et al. H. pylori alpha1-3/4-fucosyltransferase (Hp3/4FT)-catalyzed one-pot multienzyme (OPME) synthesis of Lewis antigens and human milk fucosides. Chem Commun. (2017) 53:11012–5. doi: 10.1039/c7cc05403c
144. Zhang Y, Meng C, Jin L, Chen X, Wang F, Cao H. Chemoenzymatic synthesis of α-dystroglycan core M1 O-mannose glycans. Chem Comm. (2015) 51:11654–7. doi: 10.1039/c5cc02913a
145. Meng C, Sasmal A, Zhang Y, Gao T, Liu CC, Khan N, et al. Chemoenzymatic assembly of mammalian O-mannose glycans. Angew Chem Int Ed Engl. (2018) 57:9003–7. doi: 10.1002/anie.201804373
146. Nycholat CM, Peng W, McBride R, Antonopoulos A, de Vries RP, Polonskaya Z, et al. Synthesis of biologically active N- and O-linked glycans with multisialylated poly-N-acetyllactosamine extensions using P. damsela alpha2-6 sialyltransferase. J Am Chem Soc. (2013) 135:18280–3. doi: 10.1021/ja409781c
147. Wu Z, Liu Y, Ma C, Li L, Bai J, Byrd-Leotis L, et al. Identification of the binding roles of terminal and internal glycan epitopes using enzymatically synthesized N-glycans containing tandem epitopes. Org Biomol Chem. (2016) 14:11106–16. doi: 10.1039/c6ob01982j
148. Yu H, Chokhawala HA, Varki A, Chen X. Efficient chemoenzymatic synthesis of biotinylated human serum albumin-sialoglycoside conjugates containing O-acetylated sialic acids. Org Biomol Chem. (2007) 5:2458–63. doi: 10.1039/b706507h
149. Hunter CD, Khanna N, Richards MR, Rezaei Darestani R, Zou C, Klassen JS, et al. Human neuraminidase isoenzymes show variable activities for 9-O-acetyl-sialoside substrates. ACS Chem Biol. (2018) 13:922–32. doi: 10.1021/acschembio.7b00952
150. Samraj AN, Bertrand KA, Luben R, Khedri Z, Yu H, Nguyen D, et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS ONE. (2018) 13:e0197464. doi: 10.1371/journal.pone.0197464
151. Lu Q, Padler-Karavani V, Yu H, Chen X, Wu SL, Varki A, et al. LC-MS analysis of polyclonal human anti-Neu5Gc xeno-autoantibodies immunoglobulin G Subclass and partial sequence using multistep intravenous immunoglobulin affinity purification and multienzymatic digestion. Anal Chem. (2012) 84:2761–8. doi: 10.1021/ac2030893
152. Leviatan Ben-Arye S, Yu H, Chen X, Padler-Karavani V. Profiling anti-Neu5Gc IgG in human sera with a sialoglycan microarray assay. J Vis Exp. (2017) 125:e56094. doi: 10.3791/56094
153. Fei Y, Sun YS, Li Y, Yu H, Lau K, Landry JP, et al. Characterization of receptor binding profiles of influenza A viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules. (2015) 5:1480–98. doi: 10.3390/biom5031480
154. Deng L, Bensing BA, Thamadilok S, Yu H, Lau K, Chen X, et al. Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood. PLoS Pathog. (2014) 10:e1004540. doi: 10.1371/journal.ppat.1004540
155. Bradley KC, Galloway SE, Lasanajak Y, Song X, Heimburg-Molinaro J, Yu H, et al. Analysis of influenza virus hemagglutinin receptor binding mutants with limited receptor recognition properties and conditional replication characteristics. J Virol. (2011) 85:12387–98. doi: 10.1128/jvi.05570-11
156. Amon R, Grant OC, Leviatan Ben-Arye S, Makeneni S, Nivedha AK, Marshanski T, et al. A combined computational-experimental approach to define the structural origin of antibody recognition of sialyl-Tn, a tumor-associated carbohydrate antigen. Sci Rep. (2018) 8:10786. doi: 10.1038/s41598-018-29209-9
157. Chokhawala HA, Yu H, Chen X. High-throughput substrate specificity studies of sialidases by using chemoenzymatically synthesized sialoside libraries. Chembiochem. (2007) 8:194–201. doi: 10.1002/cbic.200600410
158. Cao H, Li Y, Lau K, Muthana S, Yu H, Cheng J, et al. Sialidase substrate specificity studies using chemoenzymatically synthesized sialosides containing C5-modified sialic acids. Org Biomol Chem. (2009) 7:5137–45. doi: 10.1039/b916305k
159. Khedri Z, Muthana MM, Li Y, Muthana SM, Yu H, Cao H, et al. Probe sialidase substrate specificity using chemoenzymatically synthesized sialosides containing C9-modified sialic acid. Chem Commun. (2012) 48:3357–9. doi: 10.1039/c2cc17393j
160. Chokhawala HA, Huang S, Lau K, Yu H, Cheng J, Thon V, et al. Combinatorial chemoenzymatic synthesis and high-throughput screening of sialosides. ACS Chem Biol. (2008) 3:567–76. doi: 10.1021/cb800127n
161. Thon V, Li Y, Yu H, Lau K, Chen X. PmST3 from Pasteurella multocida encoded by Pm1174 gene is a monofunctional alpha2-3-sialyltransferase. Appl Microbiol Biotechnol. (2012) 94:977–85. doi: 10.1007/s00253-011-3676-6
162. Malekan H, Fung G, Thon V, Khedri Z, Yu H, Qu J, et al. One-pot multi-enzyme (OPME) chemoenzymatic synthesis of sialyl-Tn-MUC1 and sialyl-T-MUC1 glycopeptides containing natural or non-natural sialic acid. Bioorg Med Chem. (2013) 21:4778–85. doi: 10.1016/j.bmc.2013.02.040
163. Ding L, Zhao C, Qu J, Li Y, Sugiarto G, Yu H, et al. A Photobacterium sp. alpha2-6-sialyltransferase (Psp2,6ST) mutant with an increased expression level and improved activities in sialylating Tn antigens. Carbohydr Res. (2015) 408:127–33. doi: 10.1016/j.carres.2014.12.007
164. Cheng J, Yu H, Lau K, Huang S, Chokhawala HA, Li Y, et al. Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities. Glycobiology. (2008) 18:686–97. doi: 10.1093/glycob/cwn047
165. Xiao A, Li Y, Li X, Santra A, Yu H, Li W, et al. Sialidase-catalyzed one-pot multienzyme (OPME) synthesis of sialidase transition-state analogue inhibitors. ACS Catal. (2018) 8:43–7. doi: 10.1021/acscatal.7b03257
166. Ogata M, Koizumi A, Otsubo T, Ikeda K, Sakamoto M, Aita R, et al. Chemoenzymatic synthesis and characterization of N-glycolylneuraminic acid-carrying sialoglycopolypeptides as effective inhibitors against equine influenza virus hemagglutination. Biosci Biotechnol Biochem. (2017) 81:1520–8. doi: 10.1080/09168451.2017.1325315
Keywords: sialic acid, sialoside, Neu5Gc, chemical synthesis, chemoenzymatic synthesis
Citation: Kooner AS, Yu H and Chen X (2019) Synthesis of N-Glycolylneuraminic Acid (Neu5Gc) and Its Glycosides. Front. Immunol. 10:2004. doi: 10.3389/fimmu.2019.02004
Received: 31 January 2019; Accepted: 07 August 2019;
Published: 28 August 2019.
Edited by:
Jean Paul Soulillou, Université de Nantes, FranceReviewed by:
Hongzhi Cao, Shandong University, ChinaRichard D. Cummings, Emory University, United States
Copyright © 2019 Kooner, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Chen, eGlpY2hlbkB1Y2RhdmlzLmVkdQ==
 Anoopjit Singh Kooner
Anoopjit Singh Kooner Hai Yu
Hai Yu Xi Chen
Xi Chen