Abstract
Honey bees can be found all around the world and fulfill key pollination roles within their natural ecosystems, as well as in agriculture. Most species are typically docile, and most interactions between humans and bees are unproblematic, despite their ability to inject a complex venom into their victims as a defensive mechanism. Nevertheless, incidences of bee stings have been on the rise since the accidental release of Africanized bees to Brazil in 1956 and their subsequent spread across the Americas. These bee hybrids are more aggressive and are prone to attack, presenting a significant healthcare burden to the countries they have colonized. To date, treatment of such stings typically focuses on controlling potential allergic reactions, as no specific antivenoms against bee venom currently exist. Researchers have investigated the possibility of developing bee antivenoms, but this has been complicated by the very low immunogenicity of the key bee toxins, which fail to induce a strong antibody response in the immunized animals. However, with current cutting-edge technologies, such as phage display, alongside the rise of monoclonal antibody therapeutics, the development of a recombinant bee antivenom is achievable, and promising results towards this goal have been reported in recent years. Here, current knowledge on the venom biology of Africanized bees and current treatment options against bee envenoming are reviewed. Additionally, recent developments within next-generation bee antivenoms are presented and discussed.
Introduction
Bees are economically beneficial insects whose existence dates back to the Cretaceous period during the Mesozoic era (1). Bees have provided several products to humans, such as honey, beeswax, pollen, royal jelly, and propolis (2). They also pollinate a wide variety of agricultural crops (3). Although bees are extremely beneficial to crops and humans, they do present a danger due to their ability to inflict painful and toxic stings (4). Fortunately, most honey bees are not aggressive towards humans and only attack when they feel threatened. However, due to the human introduction of the Africanized bee, a hybrid with highly aggressive behavior, massive bee sting attacks have markedly increased and are now endemic in most of the Americas (excluding Chile and Canada) (5). Standardized medical approaches exist for handling cases, where victims allergic to venom components are stung by bees, or where milder envenomings are caused by only a few bee stings. Yet, no antivenom exists for treating severe bee envenomings. The underlying reason for this derives from the low immunogenicity of bee venom proteins (e.g., melittin), which hinders successful immunization of production animals to yield high antibody titers in their plasma and, consequently, complicates the development of a bee antivenom significantly (6). To develop a treatment against severe bee envenoming, the design of an effective antivenom is a necessity. Here, current knowledge on bee biology, spreading of Africanized bee hybrids in the Americas, and the bee venom apparatus and toxins are reviewed, and a discussion on current and next-generation treatments of bee envenomings is provided.
Bee Species, Behavior, and Epidemiology
Honey bees (Apis species) are social insects that live in well-organized communities and are very important to a significant proportion of the world economy due to the key role they fulfill as pollinators in agriculture (7). However, over the past decade, they have received increasing attention due to another physiological feature: their ability to deliver a venomous sting (8). The bee species predominantly responsible for human envenomings are Apis mellifera mellifera (A. m. mellifera) and A. m. ligustica in Europe, and A. m. scutellata in Africa (8).
Bee stings are not a novel phenomenon. In fact, significant exposure of humans to bee stings dates back over 7,000 years, when humans started to manage bee populations by providing them with artificial hives to enable an efficient harvest of their honey and wax, or for pollination purposes (9, 10). Despite significant breeding efforts, honey bees remain to be successfully domesticated, and a reduction in additive genetic variance, fixation of alleles associated with traits of economic importance, increased tameness, and the development of breed-specific characteristics amongst other properties have not been reported (11). In fact, targeted breeding appears to have increased rather than decreased genetic diversity (12).
The majority of commercial honey bee populations are derived from Europe, although they from an evolutionary perspective originated from Africa and were introduced to Europe through two independent migration events (13). In the 1620s, European honey bees (A. m. mellifera) were successfully introduced to North America for pollination and honey production. Later, in 1822, they were introduced to Australia (14). Attempts to replicate the original successes from North America and Australia failed in 1839 in Brazil and in other tropical regions (15, 16). This failure was believed to stem from the very different climates on both continents. New attempts were made in 1955 involving the African honey bee (A. m. scutellata), which was crossbred with honey bees of European descent to create a hybrid species that would better thrive in tropical environments and would produce large quantities of high quality honey (15–18). In the subsequent year, however, 26 queens and their swarms of Africanized (hybrid) honey bees escaped the laboratory and invaded large parts of the Americas, expanding 300–500 km per year (Figure 1) (15–17). The bees reached Mexico in 1986, the USA in 1990 (Texas), and have since spread into many states, including California, Arizona, Utah, New Mexico, Oklahoma, Louisiana, Arkansas, Alabama, and Florida (16, 19–22). Although climate limitations, particularly cold winters, have significantly slowed down the spread of these hybrid bees and currently restrict the range of their habitat, they are still believed to be able to colonize North America, where the harsh winter will be their only natural barrier. This range is likely to expand with global increases in temperatures (16, 23).
Figure 1
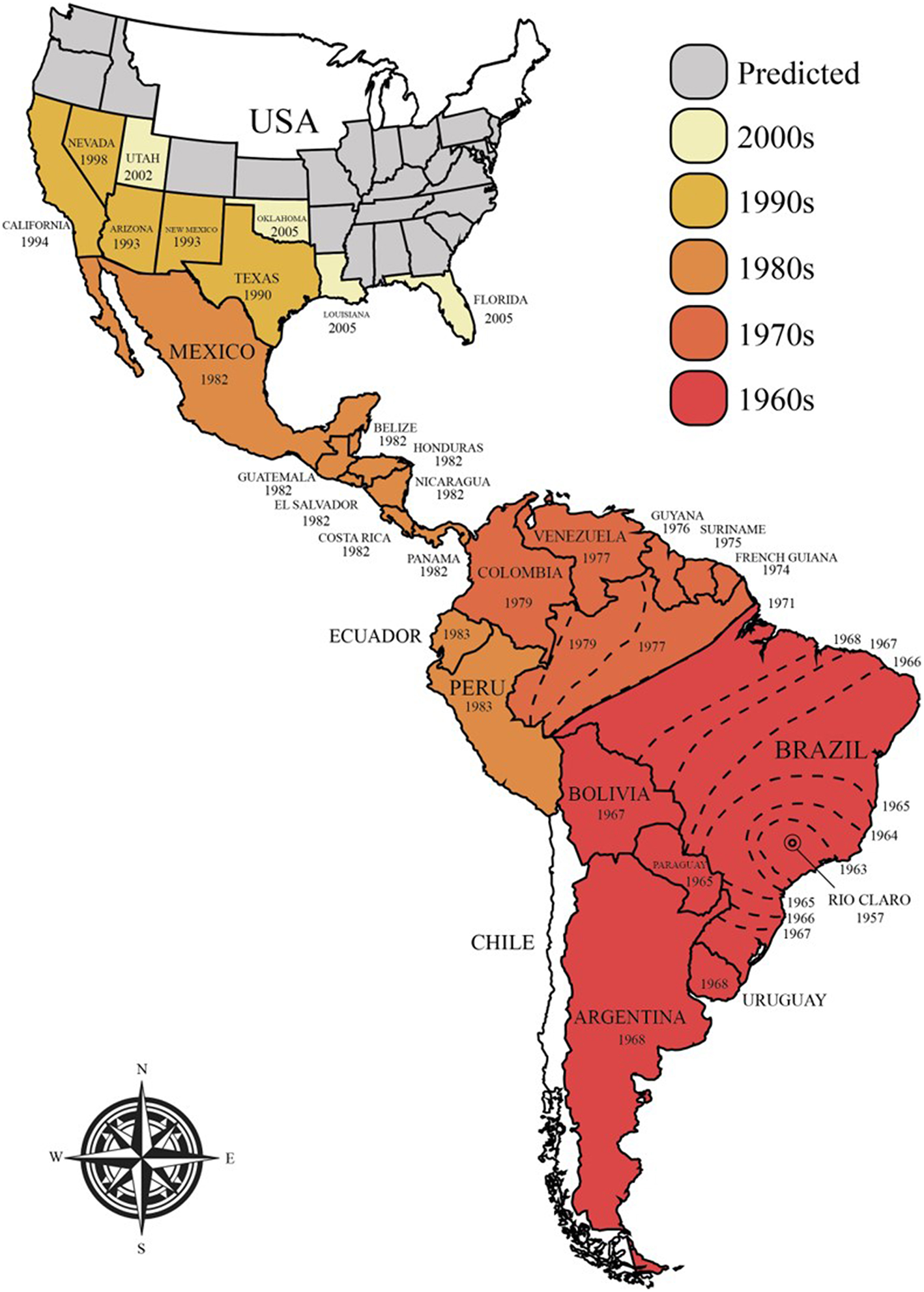
Current and predicted future spread of Africanized honey bees in the Americas.
The success of Africanized honey bees in the Americas has been attributed to a combination of ecological and genetic factors that have provided them with increased fitness compared to the resident pollinators (15, 16). Examples include higher reproductive rates, a shorter developmental cycle (i.e., the worker bees take 19–20 days, instead of 21, and queens take 14 days, instead of 16 to mature), higher drone production/abundance, higher absconding rates (i.e., forced colony relocation in case of food scarcity) and higher swarming rates (natural colony expansion and reproduction; 6–12 times per year in case of food abundance), lower honey-storing needs, disease resistance, and decreased selectivity when choosing nest sites (15, 24–26). Furthermore, Africanized bees are significantly more defensive than other bees. This is manifested in their propensity to attack with little stimulation, increased numbers of bees that co-attack at a greater distance to the hive than usual, their pronounced insistence to chase intruders for a longer period of time, and their release of putatively larger volumes of venom (15, 16). These characteristics have led to them being commonly known as “killer bees”.
The increased aggression of these bee hybrids is thought to cause significant ongoing livestock losses and human health issues, yet there is a scarcity of reliable information on the frequency of massive stinging events and severe envenomings (8, 27, 28). This lack of data is likely because the majority of bee stings are of minor medical importance and the stung individuals do not seek medical care (8, 27). Furthermore, few governmental agencies collect data on sting frequencies (8), and often group all animal bites and stings together, in the medical records from the emergency departments (29). In the US, for instance, the annual report of the American Association of Poison Control Centers stated that 41,850 animal bites/stings and four deaths occurred in 2017, yet the lack of specificity of the data makes it impossible to attribute a certain number of cases to bee envenomings (29). Indeed, although the recent report from the Centers for Disease Control and Prevention (CDC) shows that accidents and deaths by stinging insects increased over the last 5 years (annual average of 62 deaths), the report combines accidents within hornets, wasps, and bees together (30). However, though one exemplary report for envenomings by terrestrial animals in Brazil exists, where data was collected over the course of 12 years (8, 27). The study found that a total of 1,192,667 envenomings were recorded between 2001 and 2012, of which 66,283 (5.6%) could be attributed to bees. Notably, bee stings had the second highest case fatality rate (0.33%; 216 deaths), with only snakebites exceeding them (0.43%; 3,394 deaths) (27). The study showed that case fatality rates did not appear to undergo any significant fluctuations between 2001 and 2012 (27). Due to the significant incidence of bee stings in Brazil, it is likely that similar public health issues exist in other countries in the Americas with large numbers of Africanized honey bee colonies (27, 28). In fact, since their arrival in the USA, there have been several reports of deaths after Africanized bee swarm attacks (31). Taken together, the significant number of bee stings and the relatively high fatality rates of these stings suggest that there is a growing medical need for innovative treatment options, such as specific bee antivenoms to address severe envenomings. However, the financial prospects of developing antivenom products for the market are currently unknown and difficult to predict.
Bee Sting and Venom
The bee sting apparatus exhibits three functionally distinct parts; the motor part, the piercing part, and the venom-related part (Figure 2A) (32–34). In the piercing part, stylet, and lancets have important roles. They are covered by tetrahedron-shaped barbs, which are distributed in a spiral right-handed manner. This specific type of distribution plays a fundamental role in the helically clockwise rotation of the sting during the penetration of the stinger into the wound and reduces the penetration force (35). These barbs make it almost impossible for the bee to retract its stinger from the elastic flesh of mammals when escaping (Figure 2B). This situation may easily lead to sting autotomy, where the sting apparatus and its associated muscles are separated from the rest of the abdomen upon the bee's escape from the victim (36).
Figure 2
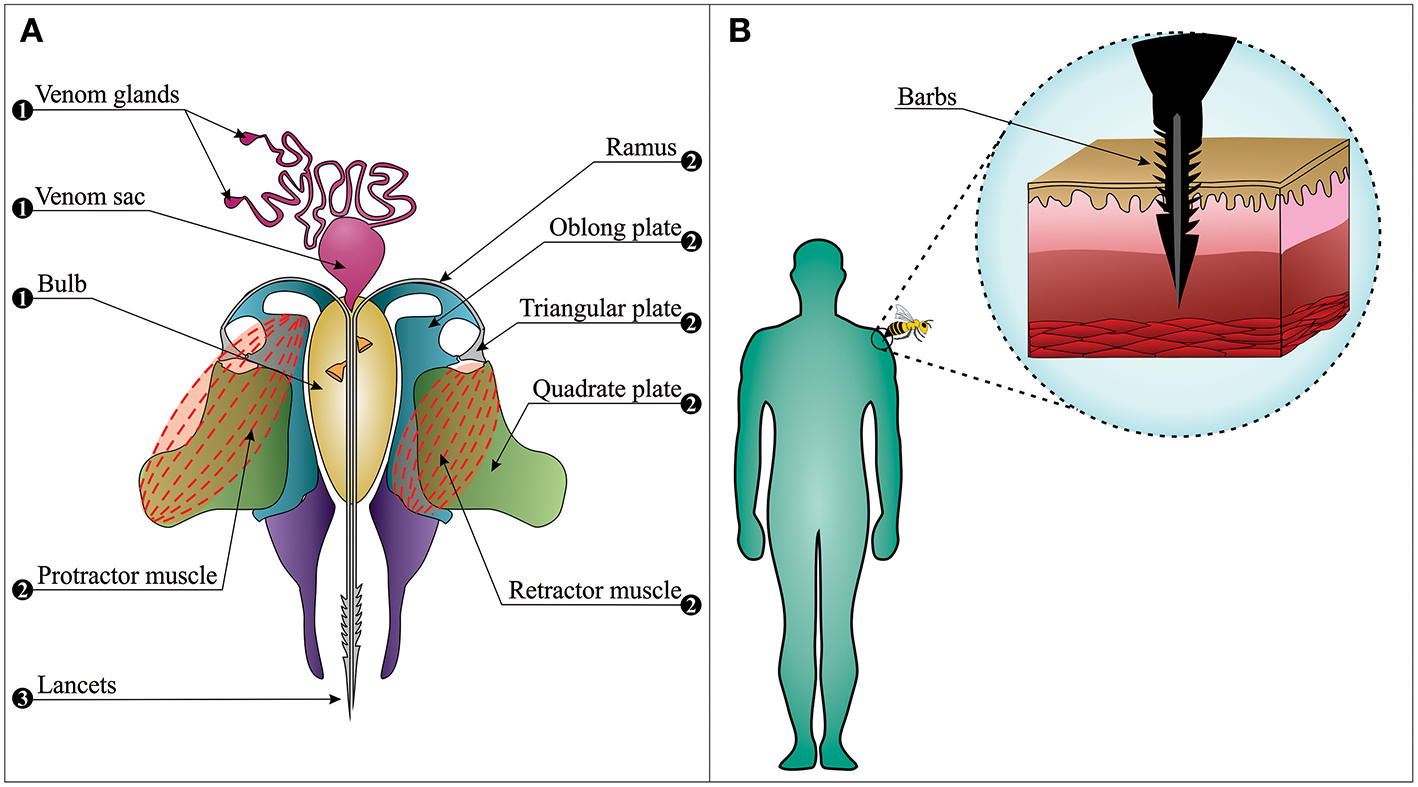
The bee sting apparatus. (A) The venom apparatus consists of three functionally distinct parts: (1) The venom-related part is composed of a venom sac, two venom glands, and a bulb. (2) The motor part is composed of muscles, plates, and ramus on each side. (3) The piercing part is composed of two lancets and a stylet (note: the stylet cannot be observed in this figure since the longitudinal section has passed from the middle of the venom canal that leaves the stylet on the upper section). (B) Barbs anchor the stinger into the skin, from where the stinger cannot be retracted when the bee escapes (i.e., sting autotomy).
Contrary to popular belief, worker bees stay alive for 18–114 h after the sting autotomization and continue playing their role as defenders (37). When the bee escapes, its autotomized sting continues to embed itself into the wound over a period of ~30 s (38), and venom can still be delivered. It is noteworthy that at least 90% of the venom sac content is delivered within the first 20 s after the stinging event (38), and removal of the stinger (see section Bee Envenomings: Clinical Manifestations) 1 min after this event is unlikely to reduce venom-induced toxicity. On average, 140–150 μg of venom is delivered in a stinging event, and the median lethal dose (LD50) of bee venom varies between 2.8 and 3.5 mg of venom per kg of human body weight (38–41). It can thus be speculated that a non-allergic person weighing 60–70 kg has a 50% chance of death upon being stung by 1,000–1,500 bees, although deaths caused by only 200–500 stings have also been reported (38, 42). Indeed, the severity of the envenoming is determined by victim age, body weight, number of stings, and individual characteristics of the victim (immune status, comorbidities, and previous sensitization) (43).
Bee venom is a complex mixture of compounds, which include proteins, peptides, amino acids, phospholipids, sugars, biogenic amines, volatile compounds, pheromones, and a high quantity of water (>80%) (44–46). The composition of bee venom has already been elucidated by omics techniques (47–49) and by fractionation of the venom (50–53). In this review, only components with important clinical and therapeutic effects, and with enough literature support, will be detailed, while other bee venom compounds are only listed in Table 1. It is important to emphasize that bees are insects from the Hymenoptera order, which includes wasps (80). Therefore, bee venoms contain some of the same compounds as wasp venoms, such as adrenaline, dopamine, histamine, hyaluronidase, noradrenaline, phospholipases A2 (PLA2s), phospholipases B (PLBs), and serotonin (81), while only bee venoms contain apamin (82), melittin (50), and mast cell-degranulating peptide (MCD) (83, 84).
Table 1
| Name (others) | Mass (Da) | Access (uniprot) | % of dryed venom# | References |
|---|---|---|---|---|
| α-Glucosidase | 65,565 | Q17058 | 0.6 | (54, 55) |
| Acid phosphatase (Api m 3) | 45,389 | Q5BLY5 | 1 | (56) |
| Adolapin | 11,500 11,092 |
– | 0.1–0.8 | (57) |
| Apamin | 5,223 | P01500 | 1–3 | (58) |
| Api m 6.01 | 7,190 | P83563* | – | (59) |
| Api m 6.02 | 7,400 | |||
| Api m 6.03 | 7,598 | |||
| Api m 6.04 | 7,808 | |||
| Cardiopep | 1,940 | – | 0.7 | (60) |
| Dipeptidylpeptidase IV (Api m 5) | 87,937 | B2D0J4 | – | (61) |
| Hyaluronidase (Api m 2) | 44,260 | Q08169 | 1–3 | (62) |
| Icarapin (Api m 10) | 24,819 | Q5EF78 | – | (63, 64) |
| MRJP (1–5) | 49,000 | O18330 | – | (65) |
| 87,000 | ||||
| O77061 | ||||
| Q17060 | ||||
| Q17061 | ||||
| O97432 | ||||
| MRJP9 (Api m 11.0101) | 48,518 | Q4ZJX1 | – | (66) |
| MCD (Peptide 401) | 5,781 | P01499 | 1–3 | (67) |
| Melittin | 2,846 | P01501* | 50–60 | (68–70) |
| Melittin-S | 2,830 | 1–2 | ||
| Synthetic melittin | – | – | ||
| Melittin-F | 2,208 | – | 0.01 | (71) |
| Minimine | 6,000 | – | 2–3 | (72) |
| PLA2 (Api m 1) | 19,058 | P00630 | 10–12 | (73, 74) |
| PLB (Lysophospholipase) | – | – | – | (75) |
| Procamine | <1,000 | – | 1.4 | (44) |
| Secapin | 8,664 | P02852 | 1–2 | (71) |
| Secapin-1 | 2,822 | – | 1 | (76) |
| Secapin-2 | 2,872 | – | – | (77) |
| Serine proteases (Api m 7) | 39,000 | – | – | (78) |
| Tertiapin | 2,459 | P56587 | 0.1 | (79) |
Bee venom compounds.
MRJPs, Major Royal Jelly Proteins; MCD, Mast Cell-Degranulating peptide; PLA2, phospholipase A2; PLB, phospholipase B.
Isoforms are represented by the same entry in the UniprotKB due to the small differences in their amino acid sequence.
Dried venom excludes volatile compounds.
Melittin is the main and most toxic compound in bee venom, constituting 50–60% of the whole venom (85). Melittin only induces minor allergic reactions (86), but causes the majority of the pain associated with bee stings (4), which is induced through direct and indirect actions on primary nociceptor cells. The direct action is caused by melittin activation of thermal nociceptor transient receptor potential vanilloid 1 (TRPV1) via the PLA2 cascade pathway, resulting in sensitization of the primary nociceptors (87–89). The indirect action is based on the pore-forming actions of melittin (Figure 3), which allows for the release of pain-inducing substances such as H+, adenosine triphosphate (ATP), and 5-hydroxytryptamine (5-HT) from mast cells, as well as melittin causes tissue damage, resulting in activation of the pain receptors. Pore formation induced by melittin can also release mediators, such as histamine, bradykinin, and ATP, which activate G-protein-coupled receptors (GPCRs), resulting in the phosphorylation of phospholipase C (PLC). PLC cleaves phosphatidylinositol 4,5-bisphosphateintodiacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG is an endogenous activator of transient receptor potential canonical (TRPC) channels, resulting in the indirect excitation of primary nociceptive neurons (i.e., pain). A more detailed overview of the pain-inducing mechanism of melittin can be found elsewhere (4). Melittin is classified as a lytic peptide that is able to destroy membrane phospholipids, which include erythrocytes, leading to hemolysis (85, 91). This action may derive from conformational modification, where melittin molecules have been proposed to bind perpendicularly to membranes forming a pore (90) (Figure 3). Furthermore, melittin can increase the activity of PLA2s (92). As an example, PLA2 activity was tested with and without melittin on lecithin liposomes, and it was observed that PLA2 activity was 5-fold higher in the presence of melittin (92). Different isoforms of melittin can be found in bee venom, such as melittin-S and melittin-F. However, these exist only in low abundance (68, 71). Melittin has been demonstrated to have antimicrobial activity in vitro and in vivo (91), anti-inflammatory effects in vitro (93), antiviral activity in vitro (94), and anti-cancer effects in vitro and in vivo (95). Finally, it is worth mentioning that melittin can be chemically synthesized to obtain high amounts of the peptide (69, 96, 97).
Figure 3
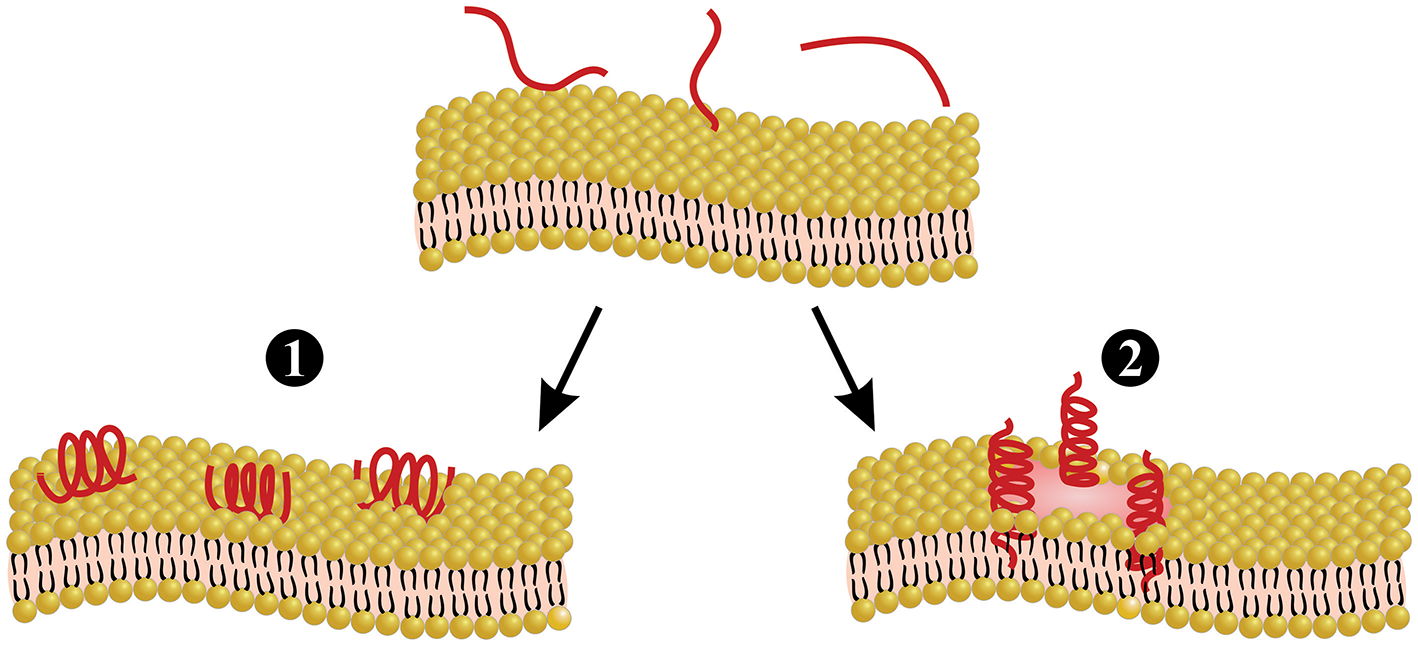
Melittin-induced pore formation model. Melittin can bind to the membrane either in a parallel orientation (1) or a perpendicular orientation (2). The perpendicular orientation induces pore formation, whereas the parallel orientation is inactive. Parallel orientation has also been hypothesized to protect the membrane, since this prevents other melittin molecules from forming pores. Figure adapted from van den Bogaart et al. (90).
Phospholipase A2 (PLA2) is the second most abundant compound (10–12%) and the most allergenic and immunogenic protein in bee venom (83). Alone, PLA2 is a non-toxic protein (44, 83), but when PLA2 forms a complex with melittin, called bee hemolytic factor, it cleaves cellular membrane phospholipids (98). In vitro, bee PLA2 possesses several activities, such as trypanocidal and antibacterial effects (99, 100), neuronal protection caused by prion proteins (101), and anti-tumor properties (102). Moreover, bee PLA2 was able to decrease hepatotoxicity caused by acetaminophen in mice (103). Phospholipase B (PLB) has also been reported to be present in bee venom (75). PLB exhibits both PLA1 and PLA2 activity, being responsible for cleaving phospholipids on sn-1 and sn-2 position of acyl chains (104), which enhances PLA2 activity (46). Notably, PLBs are also important components of snake venoms (46, 75, 105, 106).
Apamin is another important peptide in bee venom, which comprises 1–3% of crude venom and is able to allosterically and selectively inhibit Ca2+-dependent K+ channels (SK channels), found in the central nervous system (CNS) (81, 107, 108). Only SK2 and SK3 channels are known to be sensitive to apamin, and when blocked, there is a decrease of the delayed hyperpolarization of cells, which results in increased continuous firing of neurons in the mesencephalon and cerebellum, elevating cell sensitivity to excitatory inputs (107, 109). Moreover, apamin is able to activate inhibitory muscarinic receptors of motor nerve terminals (i.e., reducing neuromuscular transmission), which has been experimentally explored as a potential treatment against diseases presenting high muscle excitability (110), such as Parkinson's disease (111), learning deficit disorder (112), and other disabilities (81, 113).
Hyaluronidase is an enzyme found in bee venom (1–3%), as well as many other animal venoms (114–117). Hyaluronidase is responsible for fast distribution of toxins, also known as the “spreading factor,” as this enzyme cleaves hyaluronic acid from the extracellular matrix (ECM) (83, 118, 119), leading to a faster and systemic envenoming by disrupting tissues (120). In addition, hyaluronidase is considered a potent allergen in bee venom (121).
Mast cell-degranulating (MCD) peptide is also considered an important component in bee venom based on its capability to induce histamine release from mast cells, which exhibit a central role on inflammation and allergy (122). In high quantities, however, MCD presents an opposite action, where it inhibits mast cell degranulation (i.e., by inhibiting histamine release). Thus, MCD can also act as an anti-allergic molecule (123). Indeed, studies have demonstrated that MCD peptide presents anti-inflammatory activity in vitro and in vivo (124, 125).
Beside the above mentioned components, bee venom also contains amines, such as histamine and catecholamines (81). Histamine is able to increase capillary permeability, contributing to the inflammatory response, while catecholamines (i.e., noradrenaline and dopamine) enhance bee venom distribution, since they, among other functions, increase cardiac output (122).
As for other venoms (105, 126, 127), bee venom is very susceptible to variability, depending on bee age, species, social condition, geographic localization, amongst other factors (44). For instance, young worker bees (foragers/guards/nurses) have higher levels of apamin and lower levels of melittin compared to old workers (foragers/guards). In contrast, queen bees present lower levels of melittin and apamin (128) and higher levels of histamine (129). Furthermore, young bees have low levels of histamine, while, at 35-days-old, they present high levels of this molecule. Melittin reaches maximum concentration when the bee is 4-weeks-old, and then decreases during bee aging; while promelittin is most prevalent when the bees are 8–10-days-old (130). Hyaluronidase levels also vary in bee venoms. Although hyaluronidase can be detected immediately after the pupae emerge from the eggs as adult bees (i.e., eclosion), the enzyme levels increase with bee aging (131). Whilst low concentrations of PLA2 are found during bee eclosion, they increase gradually and reach the highest levels when the bees are 7–10-days-old (51).
Regarding venom variations among different bee species, African bees release a low amount of venom when stinging, with lower quantities of melittin and hyaluronidase, and increased amounts of PLA2, which can be explained by the fact that these bees possess smaller venom glands than the European bees (132–135). Additionally, seasonal changes may have an impact on bee venom content, since the seasons affect flowers and fruits, and therefore also bee feeding (104). Melittin production changes during the summer (136), while melittin-S production increases during winter, allowing mellitin-S to reach an abundance of 10% of whole venom (68).
Venom milking methods can also affect bee venom composition. Bee venom can be collected by extraction of glandular venom or by electrical stimulation, and venoms collected by these methods present differences on chromatographic profiles. Volatile components such as histamine can disappear when bee venom is collected by electrical stimulation (44, 137). Moreover, through proteomic analysis, bee venom obtained by gland extraction may have contamination of proteins from the gland tissue, so that down to only 40% of the obtained material is actual bee venom proteins. However, generally when electrical stimulation is used, more than 80% of the obtained material is venom proteins (48).
Bee Envenomings: Clinical Manifestations
Bee envenomings can result in mild to severe clinical manifestations depending mainly on the number of stings that the victim has received. Patient age, weight, co-morbidities, and medical care can also influence the severity of an envenoming (28). Moreover, atopic individuals (e.g., individuals with asthma or allergic rhinitis) and a family history of bee sting allergy are associated with higher incidence of severe reactions (138). Typically, the clinical manifestations of bee envenoming can be divided into local inflammatory reactions (1), allergic manifestations (2), anaphylactic shock (3), and systemic toxic reactions (4) (43, 139). (1) Local inflammatory reactions are characterized by pain, swelling (edema and erythema), itching, and pruritus at the sting site. These reactions are experienced by most non-allergic individuals and are normally resolved within 24 h (39). (2) Bee sting allergic reactions are IgE-dependent and are classified as hypersensitivity type I reactions. These reactions occur about 10 min after the sting, and the symptoms can vary in severity. PLA2 is considered the main compound that induces IgE-sensitization of mast cells, although hyaluronidases and melittin are also considered allergens (140) (see section Bee Sting and Venom). Allergic patients can develop systemic urticaria, pruritus, angioedema, vomiting, and diarrhea (28). (3) In some cases, the allergic reactions can evolve to an anaphylactic reaction, resulting in bronchoconstriction and anaphylactic shock (39). Between 25% and 70% of patients with insect allergies exhibit systemic reactions when challenged with the allergen (i.e., bee venom) (140). Interestingly, some non-allergic individuals can also develop bee anaphylaxis due to systemic mastocytosis (141–143). Systemic mastocytosis is a heterogeneous disorder characterized by proliferation of mast cells and the extent of granulation, which is caused by mutations in the c-Kit gene (a growth factor for mast cells) (144, 145). (4) Systemic toxic reactions are characterized by direct toxic effects of the bee venom, independent of immune mechanisms, which are also known as venom volume-dependent reactions. Systemic toxic reactions are always considered severe and are caused by multiple stings (about 50 simultaneous stings). Patients suffering from systemic toxic reactions may present fatigue, dizziness, nausea, vomiting, and diarrhea, which can evolve into myocardial injury, hypertension, hepatic injury, rhabdomyolysis, hemolysis, comatose, and acute renal failure (43, 146, 147). Deaths are likely to occur when the victim has received about 500 stings, which are considered necessary to cause death by direct toxicity (42), although fewer stings (30–50) have proven fatal in children (148).
Other rare clinical manifestations have also been reported for bee stings, including peripheral neuritis (149), Fisher's syndrome (150), acute inflammatory polyradiculoneuropathy (Guillain-Barré syndrome) (151), optic neuropathy (152), septicemia (153), bilateral empyema (154), and even urticaria for a baby (12-day-old) being breastfed by its mother who had been stung by a bee (155).
Current Treatment
There are generally three different bee sting scenarios that require treatment. (1) Few stings on a non-sensitized person; (2) one or more stings on a hypersensitive person; and (3) massive bee envenoming by multiple stings (Figure 4).
Figure 4
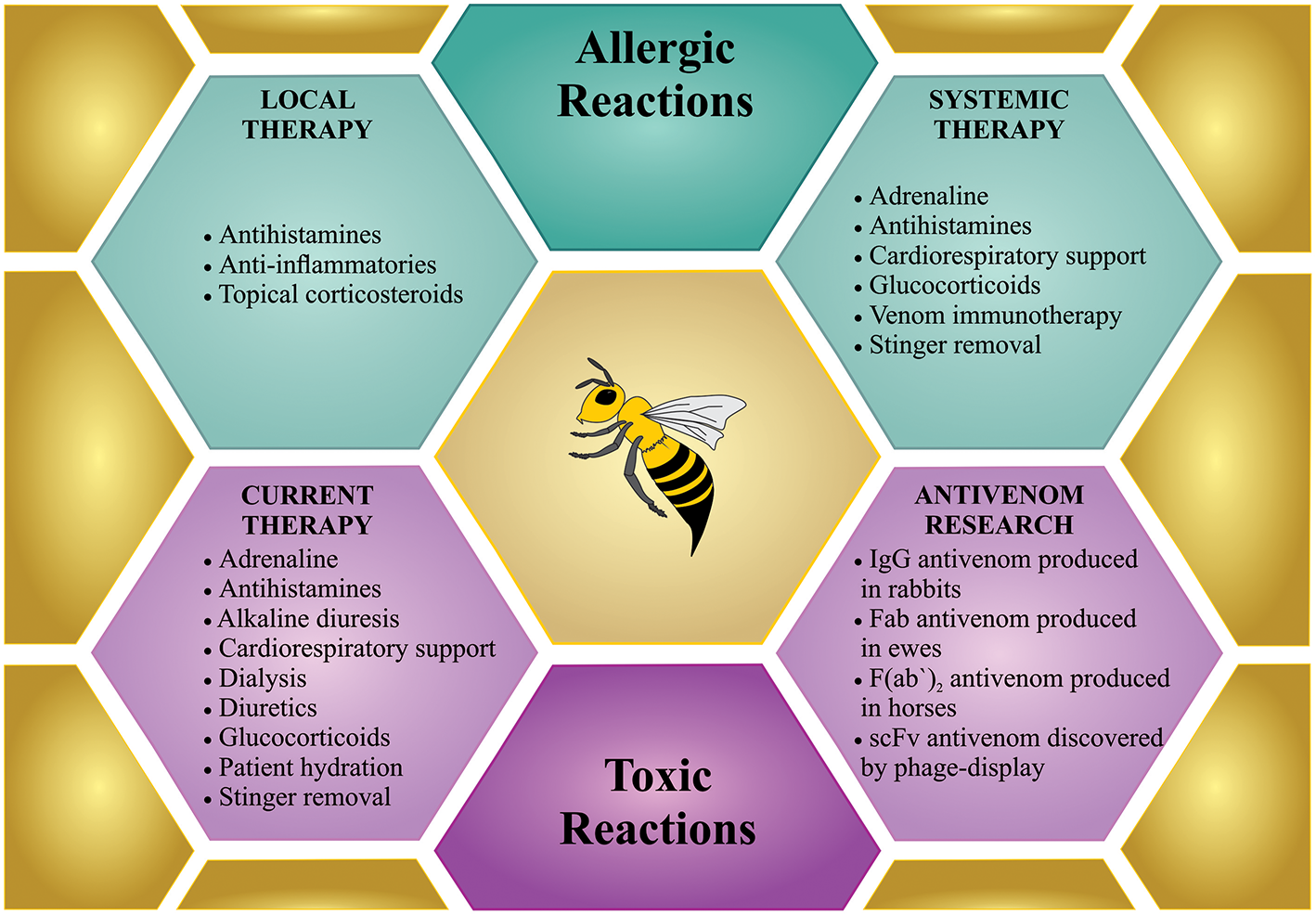
Treatment for bee sting(s). Bees incidents can involve few stings, which can cause local reactions or anaphylactic shock, which request a treatment similar to any allergic reactions (in green). However, mass stinging events can prove life-threatening via the toxic action of the venom when injected in large amounts, which demands intensive treatment (in purple). Although specific treatment is not available so far, only few antivenom researchers are working on developing new therapies against bee envenoming.
Treatment of a Few Stings on a Non-sensitized Person
Non-sensitized people present only a localized reaction to a bee sting. In the normal reaction to a bee sting, the skin manifests itself as an area of pain, redness, and swelling that is generally <10 cm in diameter and normally disappears within 24 h (156). Experiments conducted in guinea-pigs stung by bees, which had their tissues removed and subjected to histologic analysis, demonstrated only local marked inflammation (i.e., edema, high cellular infiltration, and necrosis) during 24 h after the sting (157). Medical assistance is unnecessary in these cases, although the use of topical corticosteroids is recommended, which produce anti-inflammatory, immunosuppressive, and anti-mitogenic effects (158). However, in the so-called large local reactions to an insect sting, the area of swelling and redness may be larger than 10 cm in diameter, and pain can persists for several days. Although this reaction is presumably of allergic origin, it is not necessarily mediated by IgE. In this situation, oral antihistamines can be prescribed (156).
Treatment of Hypersensitivity
If a person who is hypersensitive to bee venom (i.e., an allergic person) is stung by at least one bee, immediate medical attention is required, since anaphylaxis might occur. The majority of the deaths that occur in this case are due to allergic individuals not reaching medical care fast enough (159). The preferred first line of action against anaphylaxis differs from study to study. Some studies report as first line of action to scrape off the bee stingers carefully (by avoiding to pull or squeeze the stingers, which could lead to injection of more venom) (160), although this action is mainly relevant if performed within 60 s of the stinging event, in which period the stinger ejects all its venom (39). Other studies state that the first-line of action should be to administer intramuscular adrenaline (also known as epinephrine), and only then remove the stinger (161). While these studies might disagree on whether stinger removal should be the first action followed by adrenaline, or the opposite, they do agree that the first drug to be used is intramuscular adrenaline (39, 160–162). Even a few minutes delay in the administration of adrenaline can lead to hypoxia or death. Indeed, the lack of access to emergent adrenaline plays a critical role in the mortality and morbidity for allergic patients. Thus, there has been an increased awareness of the need for adrenaline auto-injectors in public locations including schools, parks, airports, and shopping malls (163, 164).
Adrenaline acts as an α and β-agonist. Through its α-1 agonistic effect, it works as a vasoconstrictor, which prevents and relieves airway edema, hypotension, and shock. The β-1 agonistic effects of adrenaline are chronotropic and inotropic and thus increase the rate and force of cardiac contractions, while the β-2 agonistic effects of adrenaline lead to bronchodilation (162). Furthermore, the β2-adrenergic agonistic effects of adrenaline also increase the intracellular levels of cyclic adenosine monophosphate in mast cells, which inhibits further release of inflammatory mediators, such as histamine, leukotrienes, and prostaglandin D2 (165). Following the administration of adrenaline, other first-line treatments include oxygen, intravenous fluid resuscitation, and inhaled short-acting β2 agonists (161, 166, 167). Second-line treatment usually consists of H1-antihistamines, H2-antihistamines, and glucocorticoids, which are given as adjuvant therapy and are considered optional. The antihistamines are only recommended for the relief of cutaneous symptoms, while glucocorticoids may be effective for treating airway edema and could prevent protracted anaphylaxis symptoms (161, 166).
A preventive treatment available to allergic individuals is venom immunotherapy (VIT). VIT consists of inoculating small increasing amounts of purified venom extracts in the allergic individual over a period of time. Venom extracts were introduced in the 1970s, and since then, VIT has become increasingly popular. Several different administration regimens have been developed to shorten the time required to reach the maintenance period and to minimize side effects (168). According to the European Academy of Allergy and Clinical Immunology (EAACI), VIT can be performed using different venom products (purified and non-purified, aqueous or depot) and different treatment protocols (conventional, cluster, rush, and ultra-rush), administered by the subcutaneous or sublingual routes (169).
Effective VIT restores immunotolerance to allergens by different mechanisms: (1) desensitization of mast cells and basophils; (2) suppression of innate lymphoid cells (ILC2); (3) activation of regulatory T cells (Tregs), which increase the levels of interleukin 10 (IL-10) and transforming growth factor-β (TGF-β); (4) and immunoglobulin cell-switch to IgG4 and IgA induced by Treg cytokines (170). The decision whether to start VIT depends on an accurate diagnosis, an assessment of the person's risk of having another allergic reaction, the degree to which the allergy affects their quality of life, the person's age and comorbid medical conditions, as well as whether the person suffers from concurrent mast cell disorder. Moreover, the allergen preparation (see EAACI guidelines) and the administered dose should be taken into account to avoid adverse effects as well as to ensure therapeutic success (169). In any event, VIT needs to be performed under medical supervision due to the risk of an allergic reaction. A study found that almost one in 10 people treated with VIT had an allergic reaction to the treatment (171). However, adverse events are normally mild and, although it is recommended to reduce the allergen dose in case of systemic adverse reactions, patients should not discontinue the therapy, since VIT is effective in reducing the risk of a subsequent systemic reaction to a bee sting in 77–84% of the treated patients (169).
Therapy Against Massive Bee Envenoming
The initial treatment against massive bee envenoming follows the same course as for a case of hypersensitivity. Allergic and systemic toxic reactions are difficult to differentiate, especially in the first minutes, and anaphylactic shock is the most immediate danger to the patient (172). However, once it is established that a hypersensitive event is not (or not only) occurring, specific treatment for massive bee envenoming is initiated. The ideal treatment against the severe toxic effects of bee venom would likely be antivenom. However, there are no specific antivenoms available, although major efforts are being made (see section Next-Generation Antivenom Therapy) (139). Patients who have more than 50 stings should be monitored, since the circulating venom toxins may persist in their body for hours or days and may have the potential to cause delayed reactions. Initially, the stung victim may be stable. Hours later though, the victim's conditions may deteriorate (28, 172, 173). Clinical monitoring should focus on levels of creatinine, serum urea nitrogen, electrolytes, and myoglobin to asses renal function and the risk of rhabdomyolysis (172, 174). Furthermore, to check for the development of acute respiratory distress syndrome and acidosis, blood pH, and oxygen levels should be monitored. If a patient shows signs of myoglobinuria, intravenous injection of sodium bicarbonate can be performed for alkalization of urine (i.e., to accelerate renal excretion). Alkaline diuresis can prevent the crystallization of myoglobin in kidney tubules, which may eventually lead to acute renal failure (172). Additionally, aggressive hydration and diuretics are often administered (139, 174). The patient can be started on either hemo or peritoneal dialysis, exchange transfusion, or plasmapheresis, to eliminate low molecular weight components of the venom, such as melittin or PLA2, or if acute renal failure develops (5, 172).
Next-Generation Antivenom Therapy
One of the obstacles for producing antibodies by immunization procedures for bee envenoming therapies is the lack of immunogenicity of several of the key bee venom toxins, such as melittin. As earlier mentioned (see section Bee Sting and Venom), melittin is a cell membrane lytic factor (85, 175) with a small molecular size (5, 176, 177), random conformation (178), and very hydrophobic regions (177), resulting in low immunogenicity (6), which highly complicates the production of effective bee antivenoms, as melittin fails to induce a strong antibody response in immunized animals.
Over the past decades, several attempts to develop an effective bee envenoming therapy have been reported. In 1996, Schumacher et al. reported the first attempt to produce heterologous antibody-based bee antivenom. Here, a polyclonal mixture of immunoglobulin G (IgG) antibodies was produced by successive immunizations of rabbits with purified PLA2, melittin, or crude bee venom, and neutralization capacities were further assessed in mice. It was observed that the specific anti-PLA2 antibodies clearly reduced PLA2-associated toxicity, when the toxin was administrated alone to mice. In contrast, it had no significant effect on lethality once crude venom was employed. Even when a combination of anti-PLA2 and anti-melittin antibodies was used, crude venom lethality did not decrease in mice, although authors identified melittin-binding antibodies in the rabbit serum (176).
In 1999, Jones et al. described a different approach based on Fab (fragment antigen binding) antibody fragments. In their study, Welsh ewes were successively immunized with bee venom for the production of IgGs, which were further digested with papain to obtain Fab fragments. Using an enzyme-linked immunosorbent assay (ELISA), researchers demonstrated that the Fab-based antivenom was able to recognize melittin. In addition, using standard efficacy (ED50) tests in vivo, the researchers determined that 20.5 mg of the ovine Fab-based antiserum was required to neutralize the toxic effects and to prevent lethality in mice when the antivenom was pre-incubated with 1 mg of bee venom (179). Later, a horse antibody fragment F(ab')2-based antivenom, described by Santos et al. (180), was demonstrated to be efficient in neutralizing the toxic activities of bee venom in vitro and in vivo. In vitro, the hemolytic activity of 1 mg of bee venom was neutralized by ~50 mg of the antivenom. In vivo, the horse antivenom was able to completely neutralize the myotoxic effects of bee venom with an effective dose (ED50) of 1.11 mg/mL (mg of bee venom/mL of antivenom) (180). Also, using horse immunization, Barraviera and co-authors recently developed a horse F(ab')2-based antivenom with an ED50 of 1.25 mg/mL (181). This study also developed a protocol for phase I/II clinical trials using the generated equine antivenom (181).
The current methods for producing antivenoms are based on successive immunizations of different animals, followed by low-cost purification of the animal plasma-derived IgGs. In spite of the historical clinical success achieved with animal plasma-derived antivenoms, these envenoming therapies have a propensity to cause adverse effects due to their heterologous nature (182, 183). It has been observed that 6–59% of the snakebite patients treated with plasma-derived antivenoms experienced early-onset adverse reactions after the administration of the antivenom, whereas 5–23% of patients experienced some delayed-onset (serum sickness) reactions, with symptoms such as fever, rash, and urticaria (182, 184). These antivenom-related adverse reactions are mainly a result of the composition and quality of the antivenom, the antibody format, and/or the total amount of protein administrated to the patient (185). Plasma-derived antivenoms are the only commercially available option for envenoming therapy. However, current progress made within the fields of biotechnology and monoclonal antibodies has positively contributed to the development of experimental antivenoms based on mixtures of specific recombinant monoclonal antibodies (186–188). Although these experimental antivenoms are yet to enter the clinical setting, envenoming therapies based on recombinant monoclonal antibodies and antibody fragments are predicted to one day be brought to the market and to be economically feasible to manufacture in the future (189).
In the field of recombinant bee antivenoms, Barbosa et al. were the first to report the discovery of fully human single-chain variable fragment (scFv)-based antibodies obtained via phage display technology against melittin and PLA2. These toxins act synergistically, and the combination of monoclonal antibodies against these toxins may therefore find its utility in treating severe bee envenomings. Specific monoclonal antibodies against melittin and PLA2 were selected from a phage display library and further selected for toxin specificity via ELISA. Two different scFv clones, named A7 and C12, against PLA2 and melittin, respectively, were discovered. Neutralization studies demonstrated that these two clones were able to neutralize the hemolytic activity of bee venom in vitro at a mass to mass ratio of 3:1 (scFv:bee venom). Moreover, the same monoclonal scFvs inhibited myotoxicity and delayed mortality in mice challenged with 1.5 LD50 of bee venom at the same ratio (190). Later, the same researchers selected two new monoclonal scFv-based antibodies against melittin and PLA2 using phage display technology, named Afribumab 1 and Afribumab 2, respectively. Afribumab 1 and 2 presented the capacity to inhibit bee venom hemolysis (0.5 μg) in vitro at a mass to mass ratio of 1:1:1 (bee venom:Afribumab 1:Afribumab 2). Using mice challenged with 2 LD50s of bee venom (corresponding to 9.484 μg/g of bee venom), the combination of Afribumabs 1 and 2 with the same ratio of 1:1:1 was demonstrated to reduce edema and prolong mouse survival for more than 400 min (compared to around 100 min when the mice were only challenged with 2 LD50s of bee venom) (191). Combined, these studies demonstrated that phage display technology can be an effective methodology for selecting antibodies with specificity against non-immunogenic components of bee venom (e.g., melittin). Such antibodies could not have easily been generated by more traditional antibody discovery approaches relaying on animal immunization. Potentially, such monoclonal antibodies against key toxins from bee venom could be formulated into a recombinant antivenom for treating severe bee envenoming. Although the precise timing for efficacious administration of such a bee antivenom cannot be predicted due to limited knowledge on the toxicokinetics of bee venom components in human subjects, it is likely that antivenom administration should occur within 24 h, since kinetic studies in mice have demonstrated that bee venom can be detected in different organs, such as the kidneys, during this period (192).
As effective therapeutic intervention is essential for the most severe cases of massive bee envenoming, and as scientific prior art demonstrates the applicability of different biotechnological techniques and antibody discovery methodologies in this field, it is likely that significant advances within the development of recombinant antivenoms against bee envenoming will occur in the next few decades. It seems eminent that antivenom design and development approaches from the neighboring field of snakebite envenoming may be adopted in the development of next-generation bee envenoming therapies. Particularly, the investigation of the utility of different monoclonal antibody formats (including nanobodies) and possibly non-antibody-based binding proteins (such as DARPins and other emerging scaffold proteins) is warranted, as this may enable the design of recombinant antivenom products with beneficial pharmacokinetic properties, such as rapid distribution and the ability to penetrate and target toxins deep within tissues (182, 193, 194). Such investigations may also prompt the exploration of low-cost manufacturing strategies for oligoclonal antibodies (189) or formulation strategies for improved stability and extended shelf-life. Also, the use of low-cost small molecule inhibitors may be an area relevant for further research. Finally, it is even possible that efforts within the development of improved bee envenoming therapeutics may encourage research and development in the field of bee envenoming diagnostics, which may aid stratification of patients and clinical decision making.
Final Remarks
Africanized bee attacks are considered a public health concern in Brazil, where they originated from. Other American countries have also noticed the effects of this serious threat, as these bee hybrids are currently spreading across the Americas. As a consequence, bee stings and envenomings will likely increase. The solution to this severe problem requires well-prepared medical emergency services and specific treatments against bee envenoming, such as antivenoms. To this date, only a few reports demonstrating positive results using animal immunization exist in the scientific literature, and no antivenom for treating severe bee envenomings is so far available to treating physicians. A possible explanation for the lack of commercial bee antivenoms is the difficulty of obtaining specific antibodies against key components of the bee venom, as these have low immunogenicity. Traditional methods based on successive animal immunizations therefore fail to generate high enough antibody titres for therapeutic utility. In contrast, phage display technology has proven to be a promising methodology for generating antibodies against key bee toxins with low immunogenicity. This technology may thus enable the development of effective recombinant bee antivenoms in the future (186–188). However, this technology is still a quite recent addition to the field of antivenom development, and many efforts are still needed before an effective antivenom for the treatment of severe bee envenomings will see the light of day.
Statements
Author contributions
FC, IO, TJ, LA, CS, and SA wrote part of the review and provided critical feedback. FC and TJ prepared figures. MP and AL designed the review, wrote part of the manuscript, and provided revisions. JB gave his valuable and professional suggestions. All authors read and approved the final manuscript.
Funding
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, The National Council for Scientific and Technological Development, grant no. 307155/2017-0); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation, grant no. 2017/04724-4, scholarship to FC no. 2017/14035-1 and 2018/14158-9, and scholarship to ISO 2017/03580-9 and 2018/21233-7); the Villum Foundation (grant no. 00025302).
Acknowledgments
JB (1949–2019) passed away during the final stages of the preparation of this article. This work is dedicated to his memory in gratitude for all his discoveries within bee antivenom and for his mentorship. We thank Cecilie Mullerup Kiel for proofreading the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Engel MS . A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). Novi. (2000) 2000:1–11. 10.1206/0003-0082(2000)3296<0001:ANIOTO>2.0.CO;2
2.
Mizrahi A Lensky Y . Bee Products: Properties, Applications, and Apitherapy. Tel Aviv: Springer Science & Business Media (2013).
3.
Hoover SE Ovinge LP . Pollen collection, honey production, and pollination services: managing honey bees in an agricultural setting. J Econ Entomol. (2018) 111:1509–16. 10.1093/jee/toy125
4.
Chen J Guan S-M Sun W Fu H . Melittin, the major pain-producing substance of bee venom. Neurosci Bull. (2016) 32:265–72. 10.1007/s12264-016-0024-y
5.
Schumacher MJ Egen NB . Significance of Africanized bees for public health: a review. Arch Intern Med. (1995) 155:2038–43. 10.1001/archinte.155.19.2038
6.
King TP Coscia MR Kochoumian L . Structure-immunogenicity relationship of melittin and its N-terminal truncated analogs. Biochemistry. (1993) 32:3506–10. 10.1021/bi00064a039
7.
Gallai N Salles J-M Settele J Vaissière BE . Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ. (2009) 68:810–21. 10.1016/j.ecolecon.2008.06.014
8.
Schmidt JO . Clinical consequences of toxic envenomations by Hymenoptera. Toxicon. (2018) 150:96–104. 10.1016/j.toxicon.2018.05.013
9.
Crane E . The World History of Beekeeping and Honey Hunting. New York, NY: Routledge (1999).
10.
Bloch G Francoy TM Wachtel I Panitz-Cohen N Fuchs S Mazar A . Industrial apiculture in the Jordan valley during Biblical times with Anatolian honeybees. Proc Natl Acad Sci USA. (2010) 107:11240–4. 10.1073/pnas.1003265107
11.
Oxley PR Oldroyd BP . Chapter 3–the genetic architecture of honeybee breeding. Adv Insect Physiol.39:83–118. 10.1016/B978-0-12-381387-9.00003-8
12.
Harpur BA Minaei S Kent CF Zayed A . Management increases genetic diversity of honey bees via admixture. Mol Ecol. (2012) 21:4414–21. 10.1111/j.1365-294X.2012.05614.x
13.
Whitfield CW Behura SK Berlocher SH Clark AG Johnston JS Sheppard WS et al . Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science. (2006) 314:642–5. 10.1126/science.1132772
14.
Crosby AW . Ecological Imperialism: The Biological Expansion of Europe, 900–1900. Cambridge: Cambridge University Press (2004).
15.
Scott Schneider S DeGrandi-Hoffman G Smith DR . The African honey bee: factors contributing to a successful biological invasion. Ann Rev Entomol. (2004) 49:351–76. 10.1146/annurev.ento.49.061802.123359
16.
Ferreira RS Jr Almeida RAMB Barraviera SRCS Barraviera B . Historical perspective and human consequences of Africanized bee stings in the Americas. J Toxicol Environ Health B. (2012) 15:97–108. 10.1080/10937404.2012.645141
17.
Kumschick S Devenish A Kenis M Rabitsch W Richardson DM Wilson JRU . Intentionally introduced terrestrial invertebrates: patterns, risks, and options for management. Biol Invasions. (2016) 18:1077–88. 10.1007/s10530-016-1086-5
18.
Kerr WE . The history of the introduction of African bees in Brazil. South African Bee J. (1967) 39:33–5.
19.
Rinderer TE Oldroyd BP Sheppard WS . Africanized bees in the US. Sci Am. (1993) 269:84–90. 10.1038/scientificamerican1293-84
20.
Lin W McBroome J Rehman M Johnson BR . Africanized bees extend their distribution in California. PLoS ONE. (2018) 13:e0190604. 10.1371/journal.pone.0190604
21.
Rangel J Giresi M Pinto MA Baum KA Rubink WL Coulson RN et al . Africanization of a feral honey bee (Apis mellifera) population in South Texas: does a decade make a difference?Ecol Evol. (2016) 6:2158–69. 10.1002/ece3.1974
22.
Portman ZM Tepedino VJ Tripodi AD Szalanski AL Durham SL . Local extinction of a rare plant pollinator in Southern Utah (USA) associated with invasion by Africanized honey bees. Biol Invasions. (2018) 20:593–606. 10.1007/s10530-017-1559-1
23.
Harrison JF Fewell JH Anderson KE Loper GM . Environmental physiology of the invasion of the Americas by Africanized honeybees. Integr Comp Biol. (2006) 46:1110–22. 10.1093/icb/icl046
24.
Caron DM . Africanized Honey Bees in the Americas. Medina: AI Root Co. (2001).
25.
Schmidt JO Hurley R . Selection of nest cavities by Africanized and European honey bees. Apidologie. (1995) 26:467–75. 10.1051/apido:19950603
26.
Winston ML . The biology and management of Africanized honey bees. Ann Rev Entomol. (1992) 37:173–93. 10.1146/annurev.en.37.010192.001133
27.
Chippaux J-P . Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. J Venom Anim Toxins. (2015) 21:13. 10.1186/s40409-015-0011-1
28.
Fan HW Kalil J . Massive bee envenomation. In: Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R, White J, editors. Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient. Cham: Springer (2017). p. 2627–36.
29.
Gummin DD Mowry JB Spyker DA Brooks DE Osterthaler KM Banner W . 2017 Annual report of the american association of poison control centers' national poison data system (NPDS): 35th annual report. Clin Toxicol. (2018) 56:1213–415. 10.1080/15563650.2018.1533727
30.
CDC . QuickStats: Number of deaths from hornet, wasp, and bee stings, among males and females—National vital statistics system, United States, 2000–2017. MMWR Morb Mortal Wkly Rep. (2019) 68:649. 10.15585/mmwr.mm6829a5
31.
Hughes RL . A fatal case of acute renal failure from envenoming syndrome after massive bee attack: a case report and literature review. Am J Foren Med Pathol. (2019) 40:52–7. 10.1097/PAF.0000000000000451
32.
Bücherl W Buckley EE Deulofeu V . Venomous Animals and Their Venoms. Academic Press (1968). Available online at: https://www.elsevier.com/books/venomous-animals-and-their-venoms/bucherl/978-1-4832-2949-2 (accessed April 8, 2019).
33.
Zhao Z-L Zhao H-P Ma G-J Wu C-W Yang K Feng X-Q . Structures, properties, and functions of the stings of honey bees and paper wasps: a comparative study. Biol Open. (2015) 4:921–8. 10.1242/bio.012195
34.
Bridges AR Owen MD . The morphology of the honey bee (Apis mellifera L.) venom gland and reservoir. J Morphol. (1984) 181:69–86. 10.1002/jmor.1051810107
35.
Wu J Yan S Zhao J Ye Y . Barbs Facilitate the Helical Penetration of Honeybee (Apis mellifera Ligustica) Stingers. PLoS ONE. (2014) 9:e103823. 10.1371/journal.pone.0103823
36.
Hermann HR Blum MS . Defensive mechanisms in the social hymenoptera. Soc Insects. (1981) 2:77–197. 10.1016/B978-0-12-342202-6.50009-5
37.
Nouvian M Reinhard J Giurfa M . The defensive response of the honeybee Apis mellifera. J Exp Biol. (2016) 219:3505–17. 10.1242/jeb.143016
38.
Schumacher MJ Tveten MS Egen NB . Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol. (1994) 93:831–5. 10.1016/0091-6749(94)90373-5
39.
Fitzgerald KT Flood AA . Hymenoptera Stings. Clin Tech Small Anim Pract. (2006) 21:194–204. 10.1053/j.ctsap.2006.10.002
40.
Schumacher MJ Schmidt JO Egen NB . Lethality of “killer” bee stings. Nature. (1989) 337:413. 10.1038/337413a0
41.
Ali MAA-SM . Studies on bee venom and its medical uses. Int J Adv Res Technol. (2012) 1:69–83.
42.
França FO Benvenuti LA Fan HW Dos Santos DR Hain SH Picchi-Martins FR et al . Severe and fatal mass attacks by “killer” bees (Africanized honey bees–Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. Q J Med. (1994) 87:269–82.
43.
Toledo LFM de Moore DCBC Caixeta DM da L Salú MDS Farias CVB Azevedo ZMA de . Multiple bee stings, multiple organs involved: a case report. Rev Soc Bras Med Trop. (2018) 51:560–2. 10.1590/0037-8682-0341-2017
44.
Abd El-Wahed AA Khalifa SAM Sheikh BY Farag MA Saeed A Larik FA et al . Chapter 13–bee venom composition: from Chemistry to biological activity. In Atta-ur-Rahman editor. Studies in Natural Products Chemistry.Oxford: Elsevier (2017). p. 459–84.
45.
Szókán G Horváth J Almás M Saftics G Palócz A . Liquid chromatographic analysis and separation of polypeptide components from honey bee venoms. J Liq Chromatogr. (1994) 17:3333–49. 10.1080/10826079408013516
46.
Hossen MS Shapla UM Gan SH Khalil MI . Impact of bee venom enzymes on diseases and immune responses. Molecules. (2016) 22:1–16. 10.3390/molecules22010025
47.
Danneels EL Van Vaerenbergh M Debyser G Devreese B de Graaf DC . Honeybee venom proteome profile of queens and winter bees as determined by a mass spectrometric approach. Toxins. (2015) 7:4468–83. 10.3390/toxins7114468
48.
Li R Zhang L Fang Y Han B Lu X Zhou T et al . Proteome and phosphoproteome analysis of honeybee (Apis mellifera) venom collected from electrical stimulation and manual extraction of the venom gland. BMC Genom. (2013) 14:766. 10.1186/1471-2164-14-766
49.
Resende VMF Vasilj A Santos KS Palma MS Shevchenko A . Proteome and phosphoproteome of Africanized and European honeybee venoms. Proteomics. (2013) 13:2638–48. 10.1002/pmic.201300038
50.
Teoh ACL Ryu K-H Lee EG . One-step purification of melittin derived from Apis mellifera bee venom. J Microbiol Biotechnol. (2017) 27:84–91. 10.4014/jmb.1608.08042
51.
Owen MD Pfaff LA Reisman RE Wypych J . Phospholipase A2 in venom extracts from honey bees (Apis mellifera L.) of different ages. Toxicon. (1990) 28:813–20. 10.1016/S0041-0101(09)80004-4
52.
Dotimas EM Hamid KR Hider RC Ragnarsson U . Isolation and structure analysis of bee venom mast cell degranulating peptide. Biochim Biophys Acta. (1987) 911:285–93. 10.1016/0167-4838(87)90069-0
53.
Banks BE Dempsey CE Pearce FL Vernon CA Wholley TE . New methods of isolating been venom peptides. Anal Biochem. (1981) 116:48–52. 10.1016/0003-2697(81)90320-1
54.
Ohashi K Sawata M Takeuchi H Natori S Kubo T . Molecular cloning of cDNA and analysis of expression of the gene for alpha-glucosidase from the hypopharyngeal gland of the honeybee Apis mellifera L. Biochem Biophys Res Commun. (1996) 221:380–5. 10.1006/bbrc.1996.0604
55.
Kubo T Sasaki M Nakamura J Sasagawa H Ohashi K Takeuchi H et al . Change in the expression of hypopharyngeal-gland proteins of the worker honeybees (Apis mellifera L.) with age and/or role. J Biochem. (1996) 119:291–5. 10.1093/oxfordjournals.jbchem.a021237
56.
Hoffman DR Weimer ET Sakell RH Schmidt M . Sequence and characterization of honeybee venom acid phosphatase. J Allergy Clin Immunol. (2005) 115:S107. 10.1016/j.jaci.2004.12.442
57.
Shkenderov S Koburova K . Adolapin–a newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon. (1982) 20:317–21. 10.1016/0041-0101(82)90234-3
58.
Haux P Sawerthal H Habermann E . Sequence analysis of bee venom neurotoxin (apamine) from its tryptic and chymotryptic cleavage products. Hoppe-Seyler's Z Physiol Chem. (1967) 348:737–8.
59.
Kettner A Hughes GJ Frutiger S Astori M Roggero M Spertini F et al . Api m 6: a new bee venom allergen. J Allergy Clin Immunol. (2001) 107:914–20. 10.1067/mai.2001.113867
60.
Vick JA Shipman WH Brooks R . Beta adrenergic and anti-arrhythmic effects of cardiopep, a newly isolated substance from whole bee venom. Toxicon. (1974) 12:139–44. 10.1016/0041-0101(74)90237-2
61.
Blank S Seismann H Bockisch B Braren I Cifuentes L McIntyre M et al . Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. (2010) 184:5403–13. 10.4049/jimmunol.0803709
62.
Gmachl M Kreil G . Bee venom hyaluronidase is homologous to a membrane protein of mammalian sperm. Proc Natl Acad Sci USA. (1993) 90:3569. 10.1073/pnas.90.8.3569
63.
Peiren N de Graaf DC Brunain M Bridts CH Ebo DG Stevens WJ et al . Molecular cloning and expression of icarapin, a novel IgE-binding bee venom protein. FEBS Lett. (2006) 580:4895–9. 10.1016/j.febslet.2006.08.005
64.
Peiren N Vanrobaeys F de Graaf DC Devreese B Van Beeumen J Jacobs FJ . The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim Biophys Acta. (2005) 1752:1–5. 10.1016/j.bbapap.2005.07.017
65.
Schmitzová J Klaudiny J Albert S Schröder W Schreckengost W Hanes J et al . A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell Mol Life Sci. (1998) 54:1020–30. 10.1007/s000180050229
66.
Klaudiny J . MRJP9, an ancient protein of the honeybee MRJP family with non-nutritional function. J Apicult Res. (2007) 46:99–104. 10.3896/IBRA.1.46.2.06
67.
Haux P . Amino acid sequence of MCD-peptide, a specific mast cell-degranulating peptide from bee venom. Hoppe-Seyler's Z Physiol Chem. (1969) 350:536–46. 10.1515/bchm2.1969.350.1.536
68.
Sciani JM Marques-Porto R Lourenço Junior A Orsi R de O Ferreira Junior RS Barraviera B et al . Identification of a novel melittin isoform from Africanized Apis mellifera venom. Peptides. (2010) 31:1473–9. 10.1016/j.peptides.2010.05.001
69.
Tosteson MT Levy JJ Caporale LH Rosenblatt M Tosteson DC . Solid-phase synthesis of melittin: purification and functional characterization. Biochemistry. (1987) 26:6627–31. 10.1021/bi00395a010
70.
Schröder E Lübke K Lehmann M Beetz I . Haemolytic activity and action on the surface tension of aqueous solutions of synthetic melittins and their derivatives. Experientia. (1971) 27:764–5. 10.1007/BF02136851
71.
Gauldie J Hanson JM Shipolini RA Vernon CA . The structures of some peptides from bee venom. Eur J Biochem. (1978) 83:405–10. 10.1111/j.1432-1033.1978.tb12106.x
72.
Lowy PH Sarmiento L Mitchell HK . Polypeptides minimine and melittin from bee venom: effects on Drosophila. Arch Biochem Biophys. (1971) 145:338–43. 10.1016/0003-9861(71)90044-0
73.
Shipolini RA Callewaert GL Cottrell RC Vernon CA . The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur J Biochem. (1974) 48:465–76. 10.1111/j.1432-1033.1974.tb03787.x
74.
Kuchler K Gmachl M Sippl MJ Kreil G . Analysis of the cDNA for phospholipase A2 from honeybee venom glands. The deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur J Biochem. (1989) 184:249–54. 10.1111/j.1432-1033.1989.tb15014.x
75.
Doery HM Pearson JE . Phospholipase B in snake venoms and bee venom. Biochem J. (1964) 92:599–602. 10.1042/bj0920599
76.
Meng Y Yang XX Sheng YX Zhang JL Yu DQ . A novel peptide from Apis mellifera and solid-phase synthesis of its analogue. Chin Chem Lett. (2012) 23:1161–4. 10.1016/j.cclet.2012.09.003
77.
Mourelle D Brigatte P Bringanti LDB De Souza BM Arcuri HA Gomes PC et al . Hyperalgesic and edematogenic effects of Secapin-2, a peptide isolated from Africanized honeybee (Apis mellifera) venom. Peptides. (2014) 59:42–52. 10.1016/j.peptides.2014.07.004
78.
Georgieva D Greunke K Betzel C . Three-dimensional model of the honeybee venom allergen Api m 7: structural and functional insights. Mol BioSyst. (2010) 6:1056. 10.1039/b923127g
79.
Ovchinnikov YA Miroshnikov AI Kudelin AB Kostina MB Boikov VA Magazanik LG et al . Structure and presynaptic activity of tertiapin, a neurotoxin from honeybee venom. Bioorganicheskaya Khimiya. (1980) 6:359–65.
80.
Vetter RS Visscher PK . Bites and stings of medically important venomous arthropods. Int J Dermatol. (1998) 37:481–96. 10.1046/j.1365-4362.1998.00455.x
81.
Moreno M Giralt E . Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. (2015) 7:1126–50. 10.3390/toxins7041126
82.
Ramalingam K Snyder GH . Selective disulfide formation in truncated apamin and sarafotoxin. Biochemistry. (1993) 32:11155–61. 10.1021/bi00092a027
83.
Elieh Ali Komi D Shafaghat F Zwiener RD . Immunology of bee venom. Clin Rev Allergy Immunol. (2018) 54:386–96. 10.1007/s12016-017-8597-4
84.
Buku A . Mast cell degranulating (MCD) peptide: a prototypic peptide in allergy and inflammation. Peptides. (1999) 20:415–20. 10.1016/S0196-9781(98)00167-3
85.
Raghuraman H Chattopadhyay A . Melittin: a membrane-active peptide with diverse functions. Biosci Rep. (2007) 27:189–223. 10.1007/s10540-006-9030-z
86.
Paull BR Yunginger JW Gleich GJ . Melittin: an allergen of honeybee venom. J Allergy Clin Immunol. (1977) 59:334–8. 10.1016/0091-6749(77)90056-2
87.
Ding J Xiao Y Lu D Du Y-R Cui X-Y Chen J . Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca2+ rise in primary sensory cells. Neurosci Bull. (2011) 27:135–42. 10.1007/s12264-011-1018-4
88.
Hao J Liu M-G Yu Y-Q Cao F-L Li Z Lu Z-M et al . Roles of peripheral mitogen-activated protein kinases in melittin-induced nociception and hyperalgesia. Neuroscience. (2008) 152:1067–75. 10.1016/j.neuroscience.2007.12.038
89.
Ding J Zhang J-R Wang Y Li C-L Lu D Guan S-M et al . Effects of a non-selective TRPC channel blocker, SKF-96365, on melittininduced spontaneous persistent nociception and inflammatory pain hypersensitivity. Neurosci Bull. (2012) 28:173–81. 10.1007/s12264-012-1213-y
90.
van den Bogaart G Guzmán JV Mika JT Poolman B . On the mechanism of pore formation by melittin. J Biol Chem. (2008) 283:33854–7. 10.1074/jbc.M805171200
91.
Memariani H Memariani M Shahidi-Dadras M Nasiri S Akhavan MM Moravvej H . Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol. (2019) 103:3265–76. 10.1007/s00253-019-09698-y
92.
Mollay C Kreil G . Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. (1974) 46:141–4. 10.1016/0014-5793(74)80354-6
93.
Moon D-O Park S-Y Lee K-J Heo M-S Kim K-C Kim M-O et al . Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. (2007) 7:1092–101. 10.1016/j.intimp.2007.04.005
94.
Baghian A Jaynes J Enright F Kousoulas KG . An amphipathic alpha-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1. (HSV-1)-induced cell fusion and virus spread. Peptides. (1997) 18:177–83. 10.1016/S0196-9781(96)00290-2
95.
Oršolić N . Bee venom in cancer therapy. Cancer Metastasis Rev. (2012) 31:173–94. 10.1007/s10555-011-9339-3
96.
Juvvadi P Vunnam S Merrifield RB . Synthetic melittin, its enantio, retro, and retroenantio isomers, and selected chimeric analogs: their antibacterial, hemolytic, and lipid bilayer action. J Am Chem Soc. (1996) 118:8989–97. 10.1021/ja9542911
97.
Sun X Chen S Li S Yan H Fan Y Mi H . Deletion of two C-terminal Gln residues of 12-26-residue fragment of melittin improves its antimicrobial activity. Peptides. (2005) 26:369–75. 10.1016/j.peptides.2004.10.004
98.
Mingarro I Pérez-Payá E Pinilla C Appel JR Houghten RA Blondelle SE . Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett. (1995) 372:131–4. 10.1016/0014-5793(95)00964-B
99.
Boutrin M-CF Foster HA Pentreath VW . The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp Parasitol. (2008) 119:246–51. 10.1016/j.exppara.2008.02.002
100.
Leandro LF Mendes CA Casemiro LA Vinholis AHC Cunha WR de Almeida R et al . Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An Acad Bras Cienc. (2015) 87:147–55. 10.1590/0001-3765201520130511
101.
Jeong J-K Moon M-H Bae B-C Lee Y-J Seol J-W Park S-Y . Bee venom phospholipase A2 prevents prion peptide induced-cell death in neuronal cells. Int J Mol Med. (2011) 28:867–73. 10.3892/ijmm.2011.730
102.
Son DJ Lee JW Lee YH Song HS Lee CK Hong JT . Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. (2007) 115:246–70. 10.1016/j.pharmthera.2007.04.004
103.
Kim H Keum DJ Kwak J won Chung H-S Bae H . Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE. (2014) 9:e114726. 10.1371/journal.pone.0114726
104.
Abusabbah M Lau WH Mahmoud ME Salih AM Omar D . Prospects of using carbohydrates as supplemented-diets and protein rich mixture as alternative-diet to improve the quality of venom produced by Apis cerana L. J Entomol Zool Stud. (2016) 4:23–6.
105.
Oliveira IS de Cardoso IA Bordon K de CF Carone SEI Boldrini-França J Pucca MB et al . Global proteomic and functional analysis of Crotalus durissus collilineatus individual venom variation and its impact on envenoming. J Proteomics. (2019) 191:153–65. 10.1016/j.jprot.2018.02.020
106.
Wiezel GA Shibao PYT Cologna CT Morandi Filho R Ueira-Vieira C De Pauw E et al . In-depth venome of the Brazilian rattlesnake Crotalus durissus terrificus: an integrative approach combining its venom gland transcriptome and venom proteome. J Proteome Res. (2018) 17:3941–58. 10.1021/acs.jproteome.8b00610
107.
Lamy C Goodchild SJ Weatherall KL Jane DE Liégeois J-F Seutin V et al . Allosteric block of KCa2 channels by apamin. J Biol Chem. (2010) 285:27067–77. 10.1074/jbc.M110.110072
108.
Adelman JP Maylie J Sah P . Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. (2012) 74:245–69. 10.1146/annurev-physiol-020911-153336
109.
Liegeois J Mercier F Graulich A Graulich-Lorge F Scuvee-Moreau J Seutin V . Modulation of small conductance calcium-activated potassium (SK) channels: a new challenge in medicinal chemistry. Curr Med Chem. (2003) 10:625–47. 10.2174/0929867033457908
110.
de Matos Silva LFC de Paula Ramos ER Ambiel CR Correia-de-SáP Alves-Do-Prado W . Apamin reduces neuromuscular transmission by activating inhibitory muscarinic M(2) receptors on motor nerve terminals. Eur J Pharmacol. (2010) 626:239–43. 10.1016/j.ejphar.2009.09.064
111.
Alvarez-Fischer D Noelker C Vulinović F Grünewald A Chevarin C Klein C et al . Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE. (2013) 8:e61700. 10.1371/journal.pone.0061700
112.
Messier C Mourre C Bontempi B Sif J Lazdunski M Destrade C . Effect of apamin, a toxin that inhibits Ca(2+)-dependent K+ channels, on learning and memory processes. Brain Res. (1991) 551:322–6. 10.1016/0006-8993(91)90950-Z
113.
Chase TN Oh-Lee JD . Composition for Treating Parkinson's Disease (2013). Available online at: https://patents.google.com/patent/WO2013083574A1/en?oq=Thomas%2c+N.C.;+Justin%2c+D.O.L.+Composition+for+Treatins+Parkinsin%E2%80%99s+Disease.+WO2013083574+A1%2c+13+June+2013+ (accessed April 14, 2019).
114.
Fox JW . A brief review of the scientific history of several lesser-known snake venom proteins: l-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon. (2013) 62:75–82. 10.1016/j.toxicon.2012.09.009
115.
Boldrini-França J Cologna CT Pucca MB Bordon K de CF Amorim FG Anjolette FAP et al . Minor snake venom proteins: Structure, function and potential applications. Biochim Biophys Acta Gen Subj. (2017) 1861:824–38. 10.1016/j.bbagen.2016.12.022
116.
Bordon KCF Perino MG Giglio JR Arantes EC . Isolation, enzymatic characterization and antiedematogenic activity of the first reported rattlesnake hyaluronidase from Crotalus durissus terrificus venom. Biochimie. (2012) 94:2740–8. 10.1016/j.biochi.2012.08.014
117.
Horta CCR Magalhães B de F Oliveira-Mendes BBR do Carmo AO Duarte CG Felicori LF et al . Molecular, immunological, and biological characterization of Tityus serrulatus venom hyaluronidase: new insights into its role in envenomation. PLoS Negl Trop Dis. (2014) 8:e2693. 10.1371/journal.pntd.0002693
118.
Kreil G . Hyaluronidases–a group of neglected enzymes. Protein Sci. (1995) 4:1666–9. 10.1002/pro.5560040902
119.
Marković-Housley Z Miglierini G Soldatova L Rizkallah PJ Müller U Schirmer T . Crystal structure of hyaluronidase, a major allergen of bee venom. Structure. (2000) 8:1025–35. 10.1016/S0969-2126(00)00511-6
120.
Girish KS Kemparaju K . The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. (2007) 80:1921–43. 10.1016/j.lfs.2007.02.037
121.
Clinton PM Kemeny DM Youlten LJ Lessof MH . Histamine release from peripheral blood leukocytes with purified bee venom allergens: effect of hyperimmune beekeeper plasma. Int Arch Allergy Appl Immunol. (1989) 89:43–8. 10.1159/000234921
122.
Habermann E . Bee and wasp venoms. Science. (1972) 177:314–22. 10.1126/science.177.4046.314
123.
Birr C Wengert-Müller M . Molecular perspectives of synthetic mast cell-degranulating peptide. In: Eberle AN, Wieland T, Geiger R, editors. Perspectives in Peptide Chemistry.Basel: Karger (1981). p. 372–80.
124.
Banks BE Dempsey CE Vernon CA Warner JA Yamey J . Anti-inflammatory activity of bee venom peptide 401 (mast cell degranulating peptide) and compound 48/80 results from mast cell degranulation in vivo. Br J Pharmacol. (1990) 99:350–4. 10.1111/j.1476-5381.1990.tb14707.x
125.
Ziai MR Russek S Wang H-C Beer B Blume AJ . Mast cell degranulating peptide: a multi-functional neurotoxin. J Pharm Pharmacol. (1990) 42:457–61. 10.1111/j.2042-7158.1990.tb06595.x
126.
Jin A-H Dutertre S Dutt M Lavergne V Jones A Lewis RJ et al . Transcriptomic-proteomic correlation in the predation-evoked venom of the cone snail, Conus imperialis. Mar Drugs. (2019) 17:177. 10.3390/md17030177
127.
Pucca MB Amorim FG Cerni FA Bordon K de CF Cardoso IA Anjolette FAP et al . Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity. Toxicon. (2014) 90:326–6. 10.1016/j.toxicon.2014.08.064
128.
Baracchi D Turillazzi S . Differences in venom and cuticular peptides in individuals of Apis mellifera (Hymenoptera: Apidae) determined by MALDI-TOF MS. J Insect Physiol. (2010) 56:366–75. 10.1016/j.jinsphys.2009.11.013
129.
Owen MD Braidwood JL Bridges AR . Age dependent changes in histamine content of venom of queen and worker honey bees. J Insect Physiol. (1977) 23:1031–5. 10.1016/0022-1910(77)90131-7
130.
Bachmayer H Kreil G Suchanek G . Synthesis of promelittin and melittin in the venom gland of queen and worker bees: patterns observed during maturation. J Insect Physiol. (1972) 18:1515–21. 10.1016/0022-1910(72)90230-2
131.
Owen MD . Relationship between age and hyaluronidase activity in the venom of queen and worker honey bees (Apis mellifera L.). Toxicon. (1979) 17:94–8. 10.1016/0041-0101(79)90260-5
132.
Schumacher MJ Schmidt JO Egen NB Dillon KA . Biochemical variability of venoms from individual European and Africanized honeybees (Apis mellifera). J Allergy Clin Immunol. (1992) 90:59–65. 10.1016/S0091-6749(06)80011-4
133.
Owen MD . The venom system and venom hyaluronidase of the African honeybee (Apis mellifera Adansonii). Toxicon. (1983) 21:171–4. 10.1016/0041-0101(83)90061-2
134.
Funari SRC Zeidler PR Rocha HC Sforcin JM . Venom production by Africanized honeybees (Apis mellifera) and Africanized-European hybrids. J Venom Anim Toxins. (2001) 7:190–8. 10.1590/S0104-79302001000200005
135.
Hoffman DR Jacobson RS . Allergens in hymenoptera venom XII: how much protein is in a sting?Ann Allergy. (1984) 52:276–8.
136.
Owen MD Pfaff LA . Melittin synthesis in the venom system of the honey bee (Apis mellifera L.). Toxicon. (1995) 33:1181–8. 10.1016/0041-0101(95)00054-P
137.
Ferreira Junior RS Sciani JM Marques-Porto R Junior AL Orsi R de O Barraviera B et al . Africanized honey bee (Apis mellifera) venom profiling: seasonal variation of melittin and phospholipase A(2) levels. Toxicon. (2010) 56:355–62. 10.1016/j.toxicon.2010.03.023
138.
Ediger D Terzioglu K Ozturk RT . Venom allergy, risk factors for systemic reactions and the knowledge levels among Turkish beekeepers. Asia Pac. Allergy. (2018) 8:e15. 10.5415/apallergy.2018.8.e15
139.
Almeida RAM de B Olivo TET Mendes RP Barraviera SRCS Souza L do R Martins JG et al . Africanized honeybee stings: how to treat them. Rev Soc Bras Med Trop. (2011) 44:755–61. 10.1590/S0037-86822011000600020
140.
Hymenoptera Allergy Committee of the SEAIC Alfaya Arias T Soriano Gómis V Soto Mera T Vega Castro A Vega Gutiérrez J et al . Key issues in hymenoptera venom allergy: an update. J Invest Allergol Clin Immunol. (2017) 27:19–31. 10.18176/jiaci.0123
141.
Jimenez-Rodriguez TW Garcia-Neuer M Alenazy LA Castells M . Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. (2018) 11:121–42. 10.2147/JAA.S159411
142.
Castells MC Hornick JL Akin C . Anaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both?J Allergy Clin Immunol Pract. (2015) 3:350–5. 10.1016/j.jaip.2015.03.015
143.
Greenhawt M Akin C . Mastocytosis and allergy. Curr Opin Allergy Clin Immunol. (2007) 7:387–92. 10.1097/ACI.0b013e3282a6443e
144.
Bonadonna P Zanotti R Müller U . Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. (2010) 10:347–53. 10.1097/ACI.0b013e32833b280c
145.
Piao X Bernstein A . A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood. (1996) 87:3117–23.
146.
Dart RC . Medical Toxicology. Philadelphia, PA: Lippincott Williams and Wilkins (2004).
147.
Nisahan B Selvaratnam G Kumanan T . Myocardial injury following multiple bee stings. Trop Doct. (2014) 44:233–4. 10.1177/0049475514525606
148.
James ES Walker WG . ACTH in wasp stings. Med Assoc J. (1952) 67:50–1.
149.
Ross AT . Peripheral neuritis: allergy to honeybee stings. J Allergy. (1939) 10:382–4. 10.1016/S0021-8707(39)90154-1
150.
Marks HG Augustyn P Allen RJ . Fisher's syndrome in children. Pediatrics. (1977) 60:726–9.
151.
Bachman DS Paulson GW Mendell JR . Acute inflammatory polyradiculoneuropathy following hymenoptera stings. JAMA. (1982) 247:1443–5. 10.1001/jama.247.10.1443
152.
Maltzman JS Lee AG Miller NR . Optic neuropathy occurring after bee and wasp sting. Ophthalmology. (2000) 107:193–5. 10.1016/S0161-6420(99)00020-2
153.
Truskinovsky AM Dick JD Hutchins GM . Fatal infection after a bee sting. Clin Infect Dis. (2001) 32:E36–8. 10.1086/318451
154.
Venkataramanappa SK Gowda A Raju S Harihar V . An unusual case of bilateral empyema associated with bee sting. Case Rep Med. (2014) 2014:985720. 10.1155/2014/985720
155.
Kaya A Okur M . Bee sting in mother and urticarial rash in her baby. Indian Pediatr. (2012) 49:499.
156.
Przybilla B Ruëff F . Insect Stings. Minich: Deutsches Aerzteblatt (2012).
157.
Gordon RM . Reactions produced by arthropods. BMJ. (1950) 2:316–8. 10.1136/bmj.2.4674.316
158.
Kragballe K . Topical corticosteroids: mechanisms of action. Acta Derm Venereol Suppl. (1989) 151:7–10.
159.
Sherman RA . What physicians should know about Africanized honeybees. West J Med. (1995) 163:541–6.
160.
da Silva GB Vasconcelos AG Rocha AMT de Vasconcelos VR de Barros J Fujishima JS et al . Acute kidney injury complicating bee stings–a review. Rev Inst Med Trop São Paulo. (2017) 59:e25. 10.1590/s1678-9946201759025
161.
Muraro A Roberts G Worm M Bilò MB Brockow K Rivas MF et al . Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. (2014) 69:1026–45. 10.1111/all.12437
162.
Simons FER Ebisawa M Sanchez-Borges M Thong BY Worm M Tanno LK et al . 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. (2015) 8:32. 10.1186/s40413-015-0080-1
163.
Dudley LS Mansour MI Merlin MA . Epinephrine for anaphylaxis: underutilized and unavailable. West J Emerg Med. (2015) 16:385–7. 10.5811/westjem.2015.3.25337
164.
Campbell RL Manivannan V Hartz MF Sadosty AT . Epinephrine auto-injector pandemic. Pediatr Emerg Care. (2012) 28:938–42. 10.1097/PEC.0b013e318267f689
165.
Brown AF . Therapeutic controversies in the management of acute anaphylaxis. Emerg Med J. (1998) 15:89–95. 10.1136/emj.15.2.89
166.
Lieberman P Nicklas RA Randolph C Oppenheimer J Bernstein D Bernstein J et al . Anaphylaxis—a practice parameter update 2015. Annal Allergy Asthma Immunol. (2015) 115:341–84. 10.1016/j.anai.2015.07.019
167.
Simons FER Ardusso LRF Bilò MB El-Gamal YM Ledford DK Ring J et al . World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. (2011) 127:587–93.e22. 10.1016/j.jaci.2011.01.038
168.
Incorvaia C Frati F Dell'Albani I Robino A Cattaneo E Mauro M et al . Safety of hymenoptera venom immunotherapy: a systematic review. Expert Opin Pharmacother. (2011) 12:2527–32. 10.1517/14656566.2011.616494
169.
Sturm GJ Varga E-M Roberts G Mosbech H Bilò MB Akdis CA et al . EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. (2018) 73:744–64. 10.1111/all.13262
170.
Głobińska A Boonpiyathad T Satitsuksanoa P Kleuskens M van de Veen W Sokolowska M et al . Mechanisms of allergen-specific immunotherapy: diverse mechanisms of immune tolerance to allergens. Ann Allergy Asthma Immunol. (2018) 121:306–12. 10.1016/j.anai.2018.06.026
171.
Boyle RJ Elremeli M Hockenhull J Cherry MG Bulsara MK Daniels M et al . Venom immunotherapy for preventing allergic reactions to insect stings. Coch Datab Syst Rev. (2012) 10:CD008838. 10.1002/14651858.CD008838.pub2
172.
Mitchell A . Africanized killer bees A case study. Crit Care Nurse. (2006) 26:23–31.
173.
Tunget CL Clark RF . Invasion of the “killer” bees. Separating fact from fiction. Postgrad Med. (1993) 94:92–4. 10.1080/00325481.1993.11945694
174.
Bresolin NL Carvalho FC Goes JC Fernandes V Barotto AM . Acute renal failure following massive attack by Africanized bee stings. Pediatr Nephrol. (2002) 17:625–7. 10.1007/s00467-002-0888-0
175.
Abbas AK Lichtman AH Pillai S . Basic Immunology: Functions and Disorders of the Immune System. 5th ed. St. Louis, MO: Elsevier (2016).
176.
Schumacher MJ Egen NB Tanner D . Neutralization of bee venom lethality by immune serum antibodies. Am J Trop Med Hyg. (1996) 55:197–201. 10.4269/ajtmh.1996.55.197
177.
King TP Sobotka AK Kochoumian L Lichtenstein LM . Allergens of honey bee venom. Arch Biochem Biophys. (1976) 172:661–71. 10.1016/0003-9861(76)90121-1
178.
Terra RMS . Análise Conformacional da Melitina por Dinâmica Molecular e Caracterização dos Efeitos do Peptídeo na Função Plaquetária. Universidade do Rio Grande do Sul, Porto Alegre, Brazil (2006). Available online at: https://lume.ufrgs.br/handle/10183/10943
179.
Jones RG Bhogal G Corteling RL Landon J . A novel Fab-based antivenom for the treatment of mass bee attacks. Am J Trop Med Hyg. (1999) 61:361–6. 10.4269/ajtmh.1999.61.361
180.
Santos KS Stephano MA Marcelino JR Ferreira VMR Rocha T Caricati C et al . Production of the first effective hyperimmune equine serum antivenom against Africanized bees. PLoS ONE. (2013) 8:e79971. 10.1371/journal.pone.0079971
181.
Barbosa AN Boyer L Chippaux J-P Medolago NB Caramori CA Paixão AG et al . A clinical trial protocol to treat massive Africanized honeybee (Apis mellifera) attack with a new apilic antivenom. J Venom Anim Toxins Incl Trop Dis. (2017) 23:14. 10.1186/s40409-017-0106-y
182.
Laustsen AH Gutiérrez JM Knudsen C Johansen KH Bermúdez-Méndez E Cerni FA et al . Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon. (2018) 146:151–75. 10.1016/j.toxicon.2018.03.004
183.
Pucca MB Cerni FA Janke R Bermúdez-Méndez E Ledsgaard L Barbosa JE et al . History of envenoming therapy and current perspectives. Front Immunol. (2019) 10:1598. 10.3389/fimmu.2019.01598
184.
LoVecchio F Klemens J Roundy EB Klemens A . Serum sickness following administration of Antivenin (Crotalidae) Polyvalent in 181 cases of presumed rattlesnake envenomation. Wilderness Environ Med. (2003) 14:220–1. 10.1580/1080-6032(2003)14[220:SSFAOA]2.0.CO;2
185.
León G Herrera M Segura Á Villalta M Vargas M Gutiérrez JM . Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. (2013) 76:63–76. 10.1016/j.toxicon.2013.09.010
186.
Laustsen AH Karatt-Vellatt A Masters EW Arias AS Pus U Knudsen C et al . In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat Commun. (2018) 9:3928. 10.1038/s41467-018-06086-4
187.
Pucca MB Cerni FA Peigneur S Arantes EC Tytgat J Barbosa JE . Serrumab: a novel human single chain-fragment antibody with multiple scorpion toxin-neutralizing capacities. J Immunotoxicol. (2014) 11:133–40. 10.3109/1547691X.2013.809175
188.
Silva LC Pucca MB Pessenda G Campos LB Martinez EZ Cerni FA et al . Discovery of human scFvs that cross-neutralize the toxic effects of B. jararacussu and C. d. terrificus venoms. Acta Tropica. (2018) 177:66–73. 10.1016/j.actatropica.2017.09.001
189.
Laustsen AH Johansen KH Engmark M Andersen MR . Recombinant snakebite antivenoms: a cost-competitive solution to a neglected tropical disease?PLoS Negl Trop Dis. (2017) 11:e0005361. 10.1371/journal.pntd.0005361
190.
Funayama JC Pucca MB Roncolato EC Bertolini TB Campos LB Barbosa JE . Production of human antibody fragments binding to melittin and phospholipase A2 in Africanised bee venom: minimising venom toxicity. Basic Clin Pharmacol Toxicol. (2012) 110:290–7. 10.1111/j.1742-7843.2011.00821.x
191.
Pessenda G Silva LC Campos LB Pacello EM Pucca MB Martinez EZ et al . Human scFv antibodies (Afribumabs) against Africanized bee venom: advances in melittin recognition. Toxicon. (2016) 112:59–67. 10.1016/j.toxicon.2016.01.062
192.
Yonamine CM Costa H Silva J a. A, Muramoto E Rogero JR Troncone LRP . Biodistribution studies of bee venom and spider toxin using radiotracers. J Venom Anim Toxins Incl Trop Dis. (2005) 11:39–50. 10.1590/S1678-91992005000100006
193.
Jenkins T Fryer T Dehli R Jürgensen J Fuglsang-Madsen A Føns S et al . Toxin neutralization using alternative binding proteins. Toxins. (2019) 11:53. 10.3390/toxins11010053
194.
Laustsen AH . Guiding recombinant antivenom development by omics technologies. N Biotechnol. (2018) 45:19–27. 10.1016/j.nbt.2017.05.005
Summary
Keywords
bee antivenom, bee allergy, bee envenoming, bee therapy, bee toxins, bee venom
Citation
Pucca MB, Cerni FA, Oliveira IS, Jenkins TP, Argemí L, Sørensen CV, Ahmadi S, Barbosa JE and Laustsen AH (2019) Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 10:2090. doi: 10.3389/fimmu.2019.02090
Received
16 June 2019
Accepted
19 August 2019
Published
06 September 2019
Volume
10 - 2019
Edited by
Sandip D. Kamath, James Cook University, Australia
Reviewed by
Wayne Robert Thomas, University of Western Australia, Australia; Sarita Patil, Massachusetts General Hospital and Harvard Medical School, United States
Updates
Copyright
© 2019 Pucca, Cerni, Oliveira, Jenkins, Argemí, Sørensen, Ahmadi, Barbosa and Laustsen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuela B. Pucca manu.pucca@ufrr.brAndreas H. Laustsen ahola@bio.dtu.dk
This article was submitted to Vaccines and Molecular Therapeutics, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.