- 1Department of Immunology, Jiangsu Key Laboratory of Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, China
- 2Department of Laboratory Medicine, Affiliated Hospital of Jiangsu University, Zhenjiang, China
With the ability to induce T cell activation and elicit humoral responses, B cells are generally considered as effectors of the immune system. However, the emergence of regulatory B cells (Bregs) has given new insight into the role of B cells in immune responses. Bregs exhibit immunosuppressive functions via diverse mechanisms, including the secretion of anti-inflammatory cytokines and direct cell contact. The balance between Bregs and effector B cells is important for the immune tolerance. In this review, we focus on recent advances in the characteristics of Bregs and their functional roles in autoimmunity.
Introduction
Regulatory B cells (Bregs) are immunosuppressive cells that downregulate immune responses and support immunological tolerance (1). The roles of Bregs in AIDs have been widely reported, such as type 1 diabetes (T1D), rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE), and inflammatory bowel disease (IBD) (2–5). Moreover, Bregs could be biomarkers of treatment responses, including methotrexate and rituximab treatments (6, 7).
Unlike natural Tregs, specific transcriptional factors of Bregs have not been discovered because of the diversity of suppressive mechanisms and signals for induction. The identification of Bregs is dependent on their immunomodulatory effects, such as inhibition of T cell activation and cytokine secretion (1, 8). Evidence that B cells could regulate immune responses was firstly demonstrated in 1974, and progress has been made in the past few decades, including the phenotypes, functional molecules, in vitro induction, as well as expanding number of diseases implicated (9). There are three models that explain the generation of Bregs: (1) multi-lineage Bregs, suggesting that subsets of Bregs can generate from B cells at different stages, such as IL-10+ B cells; (2) single-lineage Bregs, meaning that subsets of Bregs derive from a specific progenitor (B1 or B2 cells), such as CD5+CD1d+ B cells; and (3) induced Bregs, indicating that Bregs can differentiate from any B cell upon stimulation with specific stimuli, such as BAFF or IL-1β/IL-6-induced IL-10+ B cells (3, 10–13). The suppresssive activities of Bregs are mainly related to the secretion of anti-inflammatory cytokines (by IL-10, IL-35, etc.) and/or the expression of inhibitory molecules (PD-1/PD-L1 and FasL). In this review, we focus on recent advances in the characteristics of Bregs and their functions in AIDs.
Characteristics of Bregs
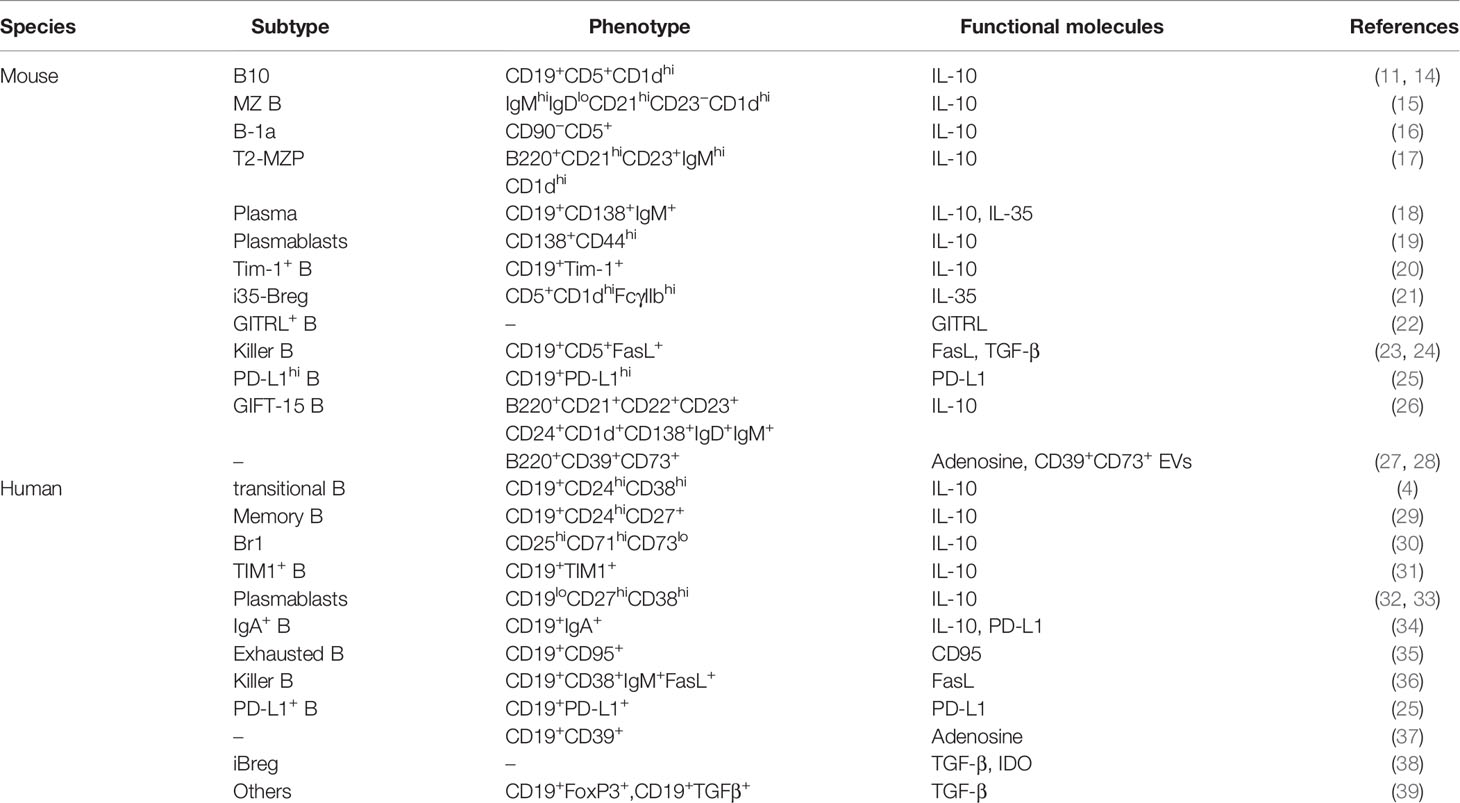
The suppressive activities of Bregs and the molecules that carry out their suppressive functions have been partially described, including the inhibition of T cell activation, induction of Tregs, the expression of IL-10, IL-35, TGF-β, and PD-1/PD-L1. Some specific markers used for the identification of Bregs have been elucidated, but there are some phenotypic overlaps among Breg subsets (Table 1).
IL-10-producing B cells are regulatory B cell subsets with the capacity to downregulate immune responses via IL-10. IL-10 production is the most studied suppressive mechanism that most investigated, there are some phenotypes within IL-10+ B cells. Several studies have confirmed that human CD19+CD24hiCD38hi B cells, a phenotype that has been related to transitional B cells, comprise the highest fraction of IL-10+ B cells in human peripheral blood upon stimulation with CD40L, CpG, Brefeldin A, phorbol 12-myristate 13-acetate and ionomycin (2, 40). Similar to human transitional B cells, mouse CD19+CD21hiCD24hiCD23hi transitional 2 marginal zone precursor (T2-MZP) B cells are also capable of producing IL-10. The inhibitory function of T2-MZP B cells depends on IL-10 production because anti-IL-10 or anti-IL-10R treatments eliminate the inhibitory effect of B cells on IFN-γ secretion by CD4+ T cells (17). Human CD24+CD27+ B cells, a phenotype reminiscent of memory B cells, also have been characterized as the major source of IL-10+ B cells upon stimulation with CpG and CD40L (3). Also, CD24low/negCD38hi plasmablast-like regulatory B cells are the members of IL-10+ B cells in human (41). Mouse CD138+CD44hi plasmablasts and plasma cells cell-derived IL-10 inhibited the generation of CD4+IFN-γ+ and CD4+IL-17+ T cells (19). In mice, CD1dhiCD5+ B cells are the main subsets of IL-10+ B cells. Adoptive transfer of CD1dhiCD5+ B cells to mice could prevent experimental autoimmune myasthenia gravis associated with downregulation of mature dendritic cell markers and expansion of Tregs (42). CD21hiCD23− marginal zone (MZ) B cells were producers of IL-10 upon stimulation with inflammatory stimuli including TLR9 and TLR4, and adoptive cell transfer experiments in which the absence of IL-10-producing B cells conferred the host a greater capability to induce Th1 responses and clear the infection (15, 43, 44). Besides, mouse B-1 cells, Tim-1+ B cells in both human and mice have been revealed to exert their functions in an IL-10-dependent manner (16, 20, 31).
In addition to IL-10-producing B cells, Breg subsets function through other mechanisms also have been widely reported. PD-L1+ B cells limited the expansion of human Tfh cells and the proliferation of mouse CD8+ T cells, these cells functioned through the interaction between PD-1 and PD-L1 (25, 45). FasL is expressed on both human and mouse B cells, these B cells are termed as killer B cells and have been confirmed to induce immune tolerance via FasL (36, 46, 47). As a receptor of FasL, CD95 (also called Fas) is expressed on CD24highCD38high and CD5+ B cells, these B cells are termed as CD95+ exhausted Bregs and are positively associated with severe colitis in human (35). Interleukin-35 was a novel anti-inflammatory cytokine of the IL-12 family cytokines and was found to be produced by human B cells and mouse CD138+ plasma cells (48, 49). IL-35 was shown to induce the expression of itself by B cells, as well as IL-10 (48, 50). Adenosine-producing B cells are also the subsets of Bregs, including human CD19+CD39+ B cells and murine B220+CD39+CD73+ cells, and CD39 and CD73 hydrolyze ATP to produce adenosine (27, 51). Also, TGF-β-producing B cells are non-negligible subgroups in Bregs, inhibiting T cell proliferation and inducing the generation of Tregs (38).
As described above, we can find that Bregs contain diverse subsets, and IL-10+ B cells are the major subsets. Most Bregs exert their functions via producing anti-inflammatory cytokines and expressing inhibitory molecules, and the importance of these cytokines and inhibitory molecules has been evidenced with experiments in vivo and in vitro.
Functions of Bregs in autoimmune diseases
B cells are critical members of humoral immunity with the ability to produce autoantibodies and to present antigens, traditionally thought to play a pathogenic role in AIDs. However, numerous studies have characterized the immunoregulatory functions of Bregs in AIDs (52). Here, Bregs have been reported to exert inhibitory effects through IL-10, IL-35, TGF-β, and PD-1/PD-L1 in both patients with AIDs and murine models. In the following sections, we will discuss the role of Bregs in inflammatory bowel disease (IBD), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), primary sjögren’s syndrome (pSS), type 1 diabetes (T1D), thyroid autoimmune disorders, multiple sclerosis (MS), and other AIDs.
Bregs in Inflammatory Bowel Disease
Inflammatory bowel disease is a chronic inflammatory disease, including ulcerative colitis (UC) and Crohn’s disease (CD). The etiology of IBD is related to microbiota, genetics, environmental factors, and imblanced Th17/Treg responses (53, 54). Transfer of microbiotas from IBD patients into germfree mice increased numbers of intestinal Th17 cells and Th2 cells and decreased numbers of RORγt+ Tregs, indicating a pathogenic role of Th cells caused by microbiotas (55). In patients with CD, the ability of B cells to produce IL-10 was impaired, and the frequency of CD19+CD1d+IL-10+ B cells was decreased in PBMCs. In this study, the presence of CD was related to the decreased production of IL-10 by peripheral blood B cells (5). Patients with UC also exhibited decreased frequencies of CD24hiCD38hi and CD5+ Bregs in the peripheral blood and intestinal tissues. Besides, mayo clinic scores, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) in UC patients were negatively correlated with the frequency of Bregs in PBMCs (35). In patients with UC, serum vasoactive intestinal peptide (VIP) levels were positively correlated with IL-10 mRNA expression in CD19+CD73−CD25+CD71+ Bregs, indicating that VIP might regulate the function of Bregs and the further exploration has confirmed it in murine models (56). Suppressive role of B cells in chronic colitis were demonstrated by Mizoguchi et al., they found that B cells and Igs could suppress colitis induced by the transfer of mesenteric lymph node (MLN) cells from TCR-α−/− × Igμ−/− mice, presumably by affecting the clearance of apoptotic cells (57). A later study conducted by Mizoguchi et al. revealed that CD1d-expressing B cells suppressed the progression of intestinal inflammation, which was associated with the enhanced IL-10 expression in MLN CD1d+ B cells (58). Dextran sulfate sodium (DSS) induced chronic colitis is a murine model of human CD. In this model, Wang et al. reported that B cells could suppress DSS-induced colitis in an IL-10 independent manner because an adoptive transfer of Il-10−/− B cells also attenuated colitis. In this study, B cells contributed to the maintenance of gut-associated lymphoid tissues (GALT) Tregs that in turn promoted B-cell differentiation into IgA-producing plasma cells, then prevented excessive immune responses that can lead to colitis (59). IL-33, IL-35, bacterial immunogenicity, and endometrial regenerative cells have been revealed to maintain/expand Bregs and ameliorate colitis, indicating a protective role of Bregs in IBD (60–64).
Bregs in Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease that occurs more frequently in females and is characterized by the breakdown of immune tolerance, high levels of autoantibody production, and multiple organ damage (65). Patients with SLE exhibit deficiencies in the function of Bregs. CD19+CD24hiCD38hi B cells isolated from healthy individuals exerted regulatory capacity, but these cells derived from SLE patients lost the ability to inhibit the expression of IFN-γ and TNF-α by CD4+ T cells (4). Heinemann and colleagues identified that the percentage of CD19+CD24hiCD38hi B cells in SLE patients was similar to that of healthy individuals (66). In contrast, another report described an increase of CD19+CD24hiCD38hi B cells in patients with SLE (67). Such discrepancies may be attributed to the complexity of the disease, different stages of diseases, and physiological environments within individuals. Plasmacytoid dendritic cells (pDCs) promoted the differentiation of immature B cells into Bregs via IFN-α and CD40-CD40L in healthy individuals. This form of immune tolerance was deficient in SLE patients because pDCs derived from SLE patients promoted plasmablast differentiation by producing relatively higher levels of IFN-α. Notably, newly repopulated immature B cells in SLE patients responding to rituximab showed normalized expression of STAT1 and STAT3 and could differentiate into CD24+CD38hi Bregs (68). The iNKT cell number and function were rescued in SLE patients responding to rituximab upon normalization of CD1d expression in repopulated immature B cells, indicating an important role of immature B cells in mataining the homeostasis of iNKT cells (69). MRL-Faslpr/lpr mice and NZB/NZW (NZB/W) F1 mice are murine models used to investigate SLE. BAFF is a cytokine generally thought to be crucial to B cell maturation and survival, it could induce IL-35 production by CD5+CD1dhiFcγRIIbhi Bregs in MRL-Faslpr/lpr mice. These IL-35-producing Bregs suppressed inflammatory cytokines (including TNF-α and IFN-γ) production by conventional CD4+ T cells and promoted the expansion of Tregs (21). Intriguingly, treatment of mice with IL-35 enriched IL-10+ Bregs in mild and moderate SLE mice as well as peripheral blood cells in severe SLE mice, which was accompanied by the expansion of Tregs (70). Taken together, the microenvironment within SLE patients could likely interfere with the generation of Bregs and impair their functions. Moreover, recovering the function of Bregs may be a method to ameliorate SLE.
Bregs in Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by synovial hyperplasia and bone destruction of the joints, affecting about 0.5–1.0% of adults in developed countries (71). Collagen-induced arthritis (CIA) is induced by immunization bovine or chicken collagen in a susceptible strain of DBA/1 mice or C57/BL6 mice, and CIA mice have become useful animal models of RA (72, 73). The involvement of B cells in RA has been well recognized, such as the precence of anti-citrullinated protein antibodies (ACPAs), rheumatoid factor (RF), higher total serum IgA, and elevated level of unmutated IgG+ B cells compared to healthy controls (74, 75). As important immune regulators, Bregs have been revealed in RA patients with impaired functions (76). In addition, IL-10+ B cells were inversely related to DAS28 and the levels of RF (77). As members of Bregs, CD24hiCD38hi and CD24hiCD27+ B cells from RA patients lost the ability to convert CD4+CD25− T cells into regulatory T cells (77). Furthermore, circulating CD19+CD24hiCD38hi Bregs are biomarkers of response to methotrexate in early rheumatoid arthritis patients (6). Besides, the frequency of CD19+CD24hiCD27+ regulatory B10 cells was increased in patients treated with a TNF inhibitor (78). PD-L1+ Bregs with CD8+ T cell suppressive capacity activity were decreased in untreated RA patients compared to healthy dornors. With successful treatment (methotrexate, TNF inhibitors, or JAK inhibitors), PD-L1+ B cells were increased in patients (45). Similarly, several Bregs inhibit the development of the CIA have been reported. Evans et al. found that T2-MZP B cells suppressed arthritis through the inhibition of type II collagen (C II) specific T cell activation and Th1 response. This process was dependent on IL-10 because T2-MZP B cells purified from IL-10 knockout mice failed to alleviate arthritis (17). Also, CD1d+ T2-MZP Bregs induced suppressive invariant natural killer (iNKT) cells via CD1d-lipid presentation, then secreted IFN-γ by iNKT cells resulted in the downregulation of Th1 and Th17 immune responses and amelioration of antigen-induced arthritis (79). Yang and colleagues reported that BAFF-induced IL-10-producing CD5+CD1dhi B10 cells inhibited the proliferation of naïve T cells, accompanied by decreased expression of RORγt (a key transcriptional factor for Th17 cells) and the differentiation of Th17 cells, consequently resulted in the amelioration of CIA (13, 80). Besides, FoxP3-expressing B cells ameliorated autoimmune arthritis via regulating the balance of Treg/Th17 cells (81). Thus, Bregs inhibit the development of RA through the reduction of Th responses and enhanced Tregs responses, and expansion of Bregs in vitro can be a promising treatment of arthritis.
Bregs in Thyroid Autoimmune Disorders
Graves’ disease and Hashimoto’s thyroiditis (HT) are two common thyroid autoimmune disorders (AITD), characterized by the presence of circulating anti-thyroid antibodies (including pathognomonic activating autoantibodies, autoantibodies to the thyroid self-antigens thyroglobulin and thyroid peroxidase), and lymphocytic infiltration into the thyroid. T cells, B cells, and DCs are actively involved in the pathogenesis of diseases (82). A study of both types of patients (Graves’ disease and HT) showed a tendency for decreased numbers of CD19+CD24+CD27+IL-10+ and CD19+IL-10+ B cells, which could be responsible for immune imbalance and AITDs (83). Co-occurrence of AIDs within a patient is a common phenomenon in rheumatic diseases, AITD are also the most frequent diseases associated with polyautoimmunity (84, 85). HT is often associated with other non-endocrine autoimmune diseases (NEAD), such as celiac diseases and chronic atrophic gastritis. Markedly higher percentages of CD24hiCD38hi unstimulated Bregs and Th17 cells were observed in patients with HT+NEAD, but unstimulated Bregs with a memory phenotype (CD24hiCD38− and CD24hiCD27+) were dramatically reduced. After CpG stimulation, IL-10+CD24hiCD38hi Bregs were similar in patients with HT+NEAD and healthy controls (86, 87). However, it is unknown whether such changes within subsets are accompanied by functional changes. Thyroid-associated ophthalmopathy (TAO) is an autoimmune disease that threatens vision. When stimulated with CD40L and CpG, PBMCs from patients with TAO showed a decreased frequency of IL-10+ B cells compared to healthy controls (88). Another study conducted by the same laboratory reported that active TAO patients had higher baseline levels of IL-10+ B cells in their peripheral blood than inactive patients and healthy controls, though their functions were impaired (89). Above all, there are limited studies that focus on the function of Bregs in patients with AITD, functional studies will provide more information about the treatment of AITD.
Bregs in Type 1 Diabetes
Impaired functions of IL-10+ B cells in type 1 diabetes (T1D) patients have been confirmed (2). The percentages of CD24hiCD38hi B cells in PBMCs of patients with T1D were significantly lower compared to healthy controls, and these cells produced less IL-10 upon stimulation with Brefeldin A together with phorbol 12-myristate 13-acetate and ionomycin. CD24hiCD38hi B cells in patients with T1D lacked regulatory capacity, which was related to the enhanced CD4+IFN-γ+ T cell and CD4+TNF-α+ T cell responses (2). In mice, intravenous injection of activated B cells into young NOD mice delayed disease onset and protected mice from diabetes. The therapeutic effect was related to reduced IFN-γ secretion and increased IL-4 and IL-10 production by splenocytes and T cells. However, this treatment only delayed the disease onset in old NOD mice. IL-10 was indispensable to this process because the effect disappeared when the mice were transferred with Il-10−/− B cells (90). FasL-expressing B cells were involved in the protection of diabetes by producing TGF-β, down-regulating Th1 responses and inhibiting antigen-presenting cells (APCs) to stimulate responder T cell proliferation (23). Taken together, regulatory B cells inhibit the development of T1D by reducing the pathogenic T cell-mediated tissue inflammation, then recover the function of islet β cells.
Bregs in Multiple Sclerosis
Multiple sclerosis (MS) is characterized by chronic inflammation of the central nervous system (CNS) and axonal damage (91). Knippenberg et al. revealed that IL-10 production by Bregs from relapsing-remitting MS (RRMS) patients during relapse and RRMS patients in remission was impaired. In this study, RRMS patients in remission also had a reduced naïve (CD3−CD19+CD27−)/memory (CD3−CD19+CD27+) IL-10+ Bregs ratio in PBMCs (92). Partial reversal of MS was achieved with Fingolimod and Siponimod targeting sphingosine 1-phosphate (S1PR), these treatments were accompanied with increased levels of Bregs, including CD24hiCD38hi Bregs, CD43+CD27+ Bregs, and TGF-β+ Bregs (93, 94). Experimental autoimmune encephalomyelitis (EAE) is one of the original models that Wolf et al. observed the suppressive effect of B cells in autoimmune models (95). Fillatreau et al. showed that toll-like receptor 9 generate proB cells (CpG-proBs) could home to reactive lymph nodes, and limited immunopathogenesis through IL-10 in EAE mice (96). Another study reported that transfusion of IL-10+ Bregs reversed the established clinical EAE, accompanied with CNS resident CD11b+CD45intLy6C− microglia, and infiltrating CD11b+CD45high monocytes/macrophages content reverts to normal and polarize to a M2-like phenotype (97). IL-35-producing B cells were shown to be in the protection of EAE. Mice in which only B cells did not express IL-35 (p35 or Ebi3) lost their ability to recover from EAE (49). TGF-β1 expression in B cell was also important for the amelioration of EAE. Mice deficient for TGF-β1 expression in B cells showed an earlier onset of neurologic impairment compared to their littermate controls, associated with augmented CNS Th 1/17 responses (98). Integrin α4 was required for the immunosuppressive function of B cells in the EAE, because deletion of Itga4 in B cells leads to EAE exacerbation and Itga4-sufficient B cells can control EAE severity in CD19CreItga4fl/fl mice (99). Interestingly, natalizumab (targeting integrin α4) has been used to treat MS via inhibiting aggregation and inflammatory activity of activated immune cells, which seems to be contrary to the effect of integrin α4 on B cells in EAE (99, 100). Probably, natalizumab functions mainly through preventing the aggregation of effector cells in CNS rather than Bregs.
Bregs in Other AIDs
Studies of Bregs in patients with other AIDs are relatively limited in comparison with SLE and RA. The frequency of CD24hiCD38hi B cells was increased in patients with pSS, but these B cells were deficient in inhibiting IFN-γ andTNF-α production by CD4+ T cells (101). Tfh cells are involved in the GC formation and B cell terminal differentiation. Lin and colleagues demonstrated that Tfh cells were positively related to pSS disease severity and were negatively related to the number of IL10+CD24+CD38hi B cells in pSS patients (102). Another report demonstrated the effect of Bregs on Tfh responses, in which the differentiation of CD4+ Tconv into Tfh cells was inhibited. In a Tfh-B cell co-culture system, the addition of CD40/TLR9 stimulated B cells dampened the secretion of immunoglobulins and promoted the expansion of FoxP3+CXCR5+PD-1+ follicular regulatory T cells (Tfr) (103). In patients with systemic sclerosis, Tim-1+ B cells lost the ability to inhibit autologous CD4+ T cell responses, including the proliferation of CD4+ T cells and the production of IFN-γ, TNF-α, and IL-17 (104). Patients with anti-neutrophil cytoplasmic antibodies-associated vasculitis (AAV) often have a diminished number of IL-10-producing B cells, which were correlated with the increased levels of Th1 and Th17 cells (105, 106) (Figure 1).
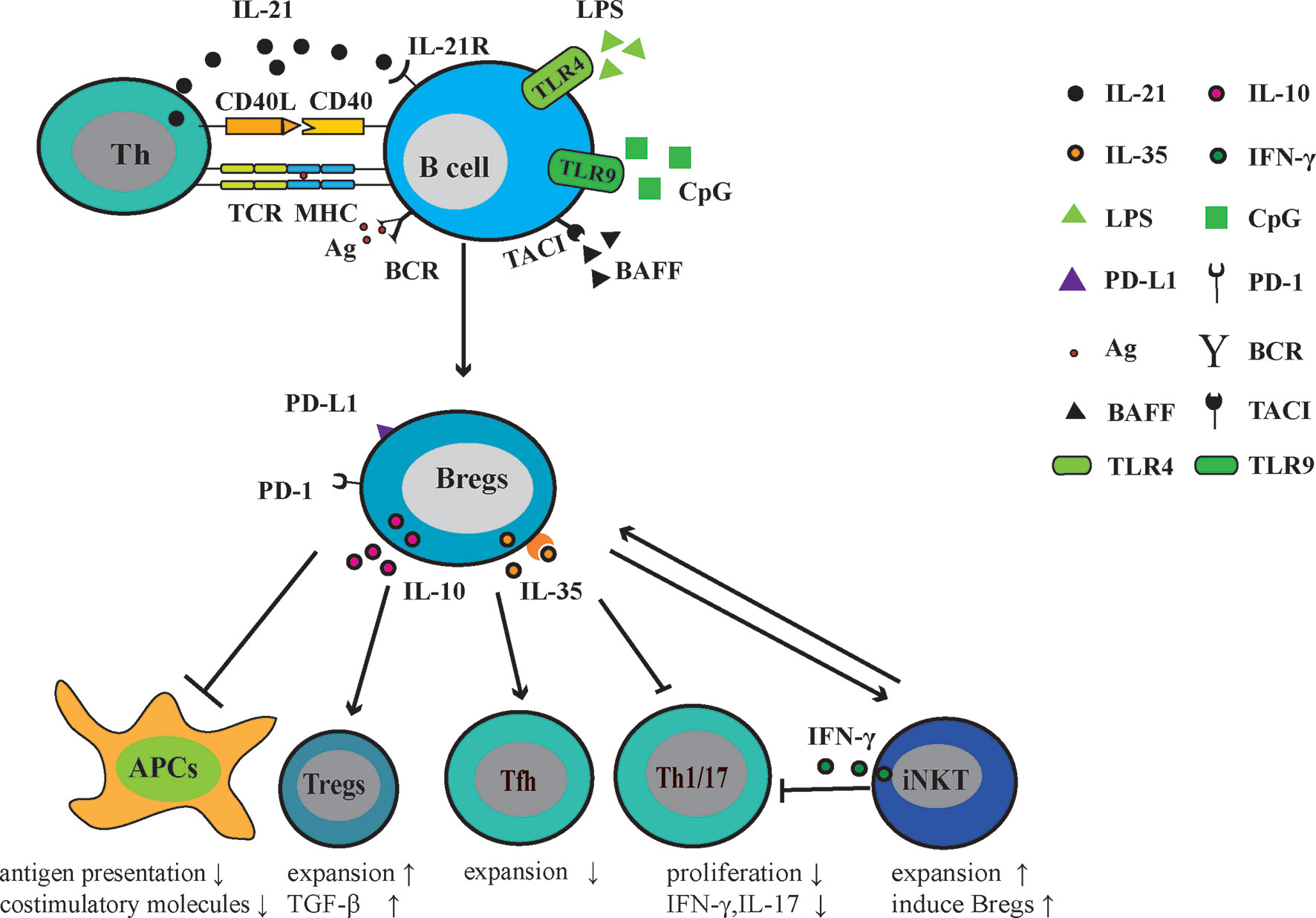
Figure 1 Regulatory function of Bregs in AIDs. Following exposure to the autoantigen, (presented to T cells via MHC molecules) CD40 signaling, Toll-like receptor (TLR) agonists (LPS or CpG), BAFF, IL-21, and/or IL-35, B cells mature into Bregs that can actively express and secrete immuno-modulatory molecules, including IL-10, IL-35, PD-1, and its ligand-PD-L1. Through these molecules, Bregs negatively regulate the antigen presentation and expression of costimulatory molecules. In Th response, Bregs restrict Tfh, Th1, and Th17 responses. In addition, Bregs promote the generation of Tregs and induce suppressive natural killer T cells. Thus, Bregs play a protective role in the development of AIDs.
Negative relationships among disease activity, pro-inflammatory responses, and Bregs suggest that Bregs play a protective role in AIDs. Murine Bregs inhibit APCs to stimulate T cell proliferation and cytokine production (IFN-γ, TNF-α, and IL-17). Similar to murine models, human Bregs always suppress the proliferation of effector T cells and their pro-inflammatory activity. Also, human Bregs have been confirmed to indirectly dampen humoral responses via Tfh cells. Thus, enhancing the activity of Bregs might directly or indirectly reduce cellular/humoral responses and strengthen the function of Tregs, ameliorating inflammatory responses within AIDs.
Potential Targets for Therapy
The majority of current treatments for AIDs are immunosuppressive drugs, such as glucocorticoids, which can result in complications in some patients during long-term use. For example, long-term prednisolone use increases the risk of osteoporosis, hypertension, and diabetes (107). Recently, cell-based therapies have been successfully used for the treatment of AIDs. These treatments include Treg-based, hematopoietic stem cell-based, and mesenchymal stem cell-based therapies (108–110).
From the above description, Bregs often play a protective role in AIDs. Therefore, it is worth considering Bregs as therapeutic targets for AIDs. Interestingly, microbiota-derived 5 Hydroxyindole-3-acetic acid (5-HIAA) suppressed murine arthritis by expanding AhR+IL-10+CD19+CD21hiCD24hi Bregs, suggesting the importance of microbes in the maintenance of Bregs (111). Gut microbiota-driven IL-1β and IL-6 induced the differentiation of mouse IL-10–producing B cells with the support of CD40 signaling (12). Murine B cells stimulated with E. coli led to an increased production of IL-10, these B cells were capable of efficiently inhibiting the maturation and function of dendritic cells (DCs), preventing the proliferation and polarization of Th1 and Th17 cells (63). Upon exposure to inflammatory cytokines, such as BAFF, IL-1β, IL-6, IL-21, IL-33, IL-35 alone or combine with other stimuli, Bregs numerically expand or functionally enhance (12, 21, 50, 60, 112). For example, culturing B cells with BAFF can induce the differentiation of IL-10-producing B cells in mice (13). IL-35 mediated the expansion of murine Bregs that produced both IL-35 and IL-10 (50). However, the administration of cytokines may also evoke unexpected inflammatory responses in vivo. For example, IL-21 and CD40L could synergistically promote B cells from human tonsils into plasma cells (113), BAFF could promote B cell activation in pSS patients (114). Therefore, it might be better to transfer ex vivo expanded Bregs for treatment rather than administering cytokines. Mesenchymal stem cells (MSCs) are promising biological agents, and mouse CD23+CD43+ Bregs generated from B cells co-cultured with MSC for 48 h inhibited T cell proliferation via IL-10 (115). For some AIDs with physiologic barriers, migration is limited for circulating Bregs into inflammatory sites. Exosomes are extracellular vesicles with a size range of ~40 to 160 nm (average ~100 nm) in diameter and they can cross the physiologic barriers (116). Recently, IL-35-carrying exosomes from ex-vivo-generated IL-35+ Bregs have been reported to cross the blood-retinal barrier and suppressed experimental autoimmune uveitis via suppressing Th17 responses as well as inducing expansion of Tregs, with minimal toxicity and alloreactivity (117). Thus, the exosomes-mediated regulatory function of Bregs may be a promising treatment of AIDs with physiologic barriers, but it still needs further investigation. Therefore, a detailed methodology for the expansion of Bregs should be explored, which will provide better material (such as extracellular vesicles from engineered Bregs) for AIDs treatments. In this manner, the morbidity associated with AIDs will reduce and the life quality of patients will get improved.
Conclusion
B cells are essential components of the adaptive immune system and display important roles in the pathogenesis of AIDs. With the stimulation of inflammatory cytokines and chemokines, B cells migrate into tissues and exert functions. In contrast, the identification of Bregs provides new insight into the role of B cells in AIDs. Based on the phenotypes described above, we can find that the phenotype of Bregs are varied including the phenotypes related to transitional B cells as well as highly differentiated plasma (blasts) cells. Without specific transcriptional factors or unique markers, the characterization of Bregs is usually based on their ability to secrete anti-inflammatory cytokines and express inhibitory molecules, suppressing the activity of effector cells and inducing the generation of Tregs. Considering the excessive immune responses in AIDs, Bregs act as protectors from AIDs with their immunomodulatory functions. Antigen signals (self or foreign antigens) appear to drive the development of Bregs, with many AIDs associated molecules, activators and supporters of Bregs. Thus, the adoptive transfer of ex vivo expanded Bregs might be a therapeutic strategy for AIDs, especially tissue-specific AIDs (such as T1D) due to the limited inflammation in the local tissues. However, there remain significant unknowns about Bregs, including specific surface markers and transcriptional factors that could be used to identify these cells, and how to maintain functional stability in vivo. Further investigations should focus on these unknowns, for a better understanding of Bregs and their practical application to AIDs treatments.
Author Contributions
QZ drafted the manuscript. KR and SW discussed and revised the manuscript. JT conceived the topic and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81971542, 81701612), Natural Science Foundation of Jiangsu (Grant No. BK20170563), Summit of the Six Top Talents Program of Jiangsu Province (Grant No. 2017-YY-006), the Primary Research and Development Plan of Zhenjiang (Grant SH2020041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity (2015) 42:607–12. doi: 10.1016/j.immuni.2015.04.005
2. Wang Y, Qin Y, Wang X, Zhang L, Wang J, Xu X, et al. Decrease in the proportion of CD24(hi) CD38(hi) B cells and impairment of their regulatory capacity in type 1 diabetes patients. Clin Exp Immunol (2020) 200:22–32. doi: 10.1111/cei.13408
3. Bankó Z, Pozsgay J, Szili D, Tóth M, Gáti T, Nagy G, et al. Induction and Differentiation of IL-10-Producing Regulatory B Cells from Healthy Blood Donors and Rheumatoid Arthritis Patients. J Immunol (2017) 198:1512–20. doi: 10.4049/jimmunol.1600218
4. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity (2010) 32:129–40. doi: 10.1016/j.immuni.2009.11.009
5. Oka A, Ishihara S, Mishima Y, Tada Y, Kusunoki R, Fukuba N, et al. Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis (2014) 20:315–28. doi: 10.1097/01.MIB.0000437983.14544.d5
6. Fortea-Gordo P, Villalba A, Nuno L, Santos-Bornez MJ, Peiteado D, Monjo I, et al. Circulating CD19+CD24hiCD38hi regulatory B cells as biomarkers of response to methotrexate in early rheumatoid arthritis. Rheumatol (Oxford) (2020) 59:3081–91. doi: 10.1093/rheumatology/keaa186
7. Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, et al. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve (2014) 49:487–94. doi: 10.1002/mus.23951
8. Savage PA, Klawon DEJ, Miller CH. Regulatory T Cell Development. Annu Rev Immunol (2020) 38:421–53. doi: 10.1146/annurev-immunol-100219-020937
9. Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature (1974) 251:550–1. doi: 10.1038/251550a0
10. von Borstel A, et al. Circulating CD24hiCD38hi regulatory B cells correlate inversely with the ThEM17 cell frequency in granulomatosis with polyangiitis patients. Rheumatol (Oxford) (2019) 58:1361–6. doi: 10.1093/rheumatology/key412
11. Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol (2009) 182:7459–72. doi: 10.4049/jimmunol.0900270
12. Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med (2014) 20:1334–9. doi: 10.1038/nm.3680
13. Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol (2010) 184:3321–5. doi: 10.4049/jimmunol.0902551
14. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity (2008) 28:639–50. doi: 10.1016/j.immuni.2008.03.017
15. Huber K, Sarmay G, Kovesdi D. MZ B cells migrate in a T-bet dependent manner and might contribute to the remission of collagen-induced arthritis by the secretion of IL-10. Eur J Immunol (2016) 46:2239–46. doi: 10.1002/eji.201546248
16. Hsu LH, Li KP, Chu KH, Chiang BL. A B-1a cell subset induces Foxp3(-) T cells with regulatory activity through an IL-10-independent pathway. Cell Mol Immunol (2015) 12:354–65. doi: 10.1038/cmi.2014.56
17. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel Suppressive Function of Transitional 2 B Cells in Experimental Arthritis. J Immunol (2007) 178:7868–78. doi: 10.4049/jimmunol.178.12.7868
18. Fillatreau S. Natural regulatory plasma cells. Curr Opin Immunol (2018) 55:62–6. doi: 10.1016/j.coi.2018.09.012
19. Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity (2014) 41:1040–51. doi: 10.1016/j.immuni.2014.10.016
20. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest (2011) 121:3645–56. doi: 10.1172/JCI46274
21. Zhang Y, Li J, Zhou N, Zhang Y, Wu M, Xu J, et al. The Unknown Aspect of BAFF: Inducing IL-35 Production by a CD5 + CD1d hi FcγRIIb hi Regulatory B-Cell Subset in Lupus. J Invest Dermatol (2017) 137:2532–43. doi: 10.1016/j.jid.2017.07.843
22. Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol (2012) 188:3188–98. doi: 10.4049/jimmunol.1103354
23. Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol (2001) 167:1081–9. doi: 10.4049/jimmunol.167.2.1081
24. Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res (2009) 58:345–57. doi: 10.1007/s00011-009-0014-x
25. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun (2015) 6:5997. doi: 10.1038/ncomms6997
26. Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, et al. A granulocyte-macrophage colony–stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med (2009) 15:1038. doi: 10.1038/nm.2003
27. Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A Novel Mechanism of B Cell–Mediated Immune Suppression through CD73 Expression and Adenosine Production. J Immunol (2014) 193:5904–13. doi: 10.4049/jimmunol.1400336
28. Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu C, et al. Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8+ T Cell Responses. Immunity (2019)50:547–9. doi: 10.1016/j.immuni.2019.01.010
29. Jin L, Weiqian C, Lihuan Y. Peripheral CD24hi CD27+ CD19+ B cells subset as a potential biomarker in naive systemic lupus erythematosus. Int J Rheum Dis (2013) 16:698–708. doi: 10.1111/1756-185X.12229
30. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol (2013) 131:1204–12. doi: 10.1016/j.jaci.2013.01.014
31. Gu XL, He H, Lin L, Luo GX, Wen YF, Xiang DC, et al. Tim-1(+) B cells suppress T cell interferon-gamma production and promote Foxp3 expression, but have impaired regulatory function in coronary artery disease. APMIS (2017) 125:872–9. doi: 10.1111/apm.12729
32. Mao H, Pan F, Wu Z, Wang Z, Zhou Y, Zhang P, et al. CD19(lo)CD27(hi) Plasmablasts Suppress Harmful Th17 Inflammation Through Interleukin 10 Pathway in Colorectal Cancer. DNA Cell Biol (2017) 36:870–7. doi: 10.1089/dna.2017.3814
33. de Masson A, Bouaziz JD, Le Buanec H, Robin M, O'Meara A, Parquet N, et al. CD24(hi)CD27(+) and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood (2015) 125:1830–9. doi: 10.1182/blood-2014-09-599159
34. Fehres CM, van Uden NO, Yeremenko NG, Fernandez L, Franco Salinas G, van Duivenvoorde LM, et al. APRIL Induces a Novel Subset of IgA(+) Regulatory B Cells That Suppress Inflammation via Expression of IL-10 and PD-L1. Front Immunol (2019) 10:1368. doi: 10.3389/fimmu.2019.01368
35. Wang X, Zhu Y, Zhang M, Wang H, Jiang Y, Gao P. Ulcerative Colitis Is Characterized by a Decrease in Regulatory B Cells. J Crohns Colitis (2016) 10:1212–23. doi: 10.1093/ecco-jcc/jjw074
36. van Rensburg IC, Kleynhans L, Keyser A, Walzl G, Loxton AG. B-cells with a FasL expressing regulatory phenotype are induced following successful anti-tuberculosis treatment. Immun Inflamm Dis (2017) 5:57–67. doi: 10.1002/iid3.140
37. Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL. Phenotypic and functional characteristics of CD39highhuman regulatory B cells (Breg). Oncoimmunology (2016) 5:2. doi: 10.1080/2162402X.2015.1082703
38. Nouel A, Pochard P, Simon Q, Segalen I, Le Meur Y, Pers JO, et al. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. J Autoimmun (2015) 59:53–60. doi: 10.1016/j.jaut.2015.02.004
39. Guo Y, Zhang X, Qin M, Wang X. Changes in peripheral CD19(+)Foxp3(+) and CD19(+)TGFbeta(+) regulatory B cell populations in rheumatoid arthritis patients with interstitial lung disease. J Thorac Dis (2015) 7:471–7. doi: 10.3978/j.issn.2072-1439.2015.02.11
40. Wang H, Liu C, Chen W, Ding G. The skewed frequency of B-cell subpopulation CD19(+) CD24 (hi) CD38 (hi) cells in peripheral blood mononuclear cells is correlated with the elevated serum sCD40L in patients with active systemic lupus erythematosus. J Cell Biochem (2019) 120:11490–7. doi: 10.1002/jcb.28427
41. Giacomini E, Rizzo F, Etna MP, Cruciani M, Mechelli R, Buscarinu MC, et al. Thymosin-alpha1 expands deficient IL-10-producing regulatory B cell subsets in relapsing-remitting multiple sclerosis patients. Mult Scler (2018) 24:127–39. doi: 10.1177/1352458517695892
42. Sheng JR, Quan S, Soliven B. CD1d(hi)CD5+ B cells expanded by GM-CSF in vivo suppress experimental autoimmune myasthenia gravis. J Immunol (2014) 193:2669–77. doi: 10.4049/jimmunol.1303397
43. Sanchez LR, Godoy GJ, Gorosito Serrán M, Breser ML, Fiocca Vernengo F, Engel P, et al. IL-10 Producing B Cells Dampen Protective T Cell Response and Allow Chlamydia muridarum Infection of the Male Genital Tract. Front Immunol (2019) 10:356. doi: 10.3389/fimmu.2019.00356
44. Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol (2005) 25:29–40. doi: 10.1007/s10875-005-0355-6
45. Zacca ER, Onofrio LI, Acosta CDV, Ferrero PV, Alonso SM, Ramello MC, et al. PD-L1(+) Regulatory B Cells Are Significantly Decreased in Rheumatoid Arthritis Patients and Increase After Successful Treatment. Front Immunol (2018) 9:2241. doi: 10.3389/fimmu.2018.02241
46. Kummari E, Rushing E, Nicaise A, McDonald A, Kaplan BLF. TCDD attenuates EAE through induction of FasL on B cells and inhibition of IgG production. Toxicology (2021) 448:152646. doi: 10.1016/j.tox.2020.152646
47. Lundy SK, Klinker MW, Fox DA. Killer B lymphocytes and their fas ligand positive exosomes as inducers of immune tolerance. Front Immunol (2015) 6:122. doi: 10.3389/fimmu.2015.00122
48. Ye Z, Jiang Y, Sun D, Zhong W, Zhao L, Jiang Z. The Plasma Interleukin (IL)-35 Level and Frequency of Circulating IL-35+ Regulatory B Cells are Decreased in a Cohort of Chinese Patients with New-onset Systemic Lupus Erythematosus. Sci Rep (2019) 9:13210. doi: 10.1038/s41598-019-49748-z
49. Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature (2014) 507:366–70. doi: 10.1038/nature12979
50. Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med (2014) 20:633–41. doi: 10.1038/nm.3554
51. Zacca ER, Amezcua Vesely MC, Ferrero PV, Acosta CDV, Ponce NE, Bossio SN, et al. B cells from Patients with Rheumatoid Arthritis Show Conserved CD39-Mediated Regulatory Function and increased CD39 Expression After Positive Response to Therapy. J Mol Biol (2021) 433:166687. doi: 10.1016/j.jmb.2020.10.021
52. Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol (2013) 10:122–32. doi: 10.1038/cmi.2012.60
53. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol (2018) 15:39–49. doi: 10.1038/nrgastro.2017.136
54. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol (2016) 13:13–27. doi: 10.1038/nrgastro.2015.186
55. Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, et al. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity (2019) 50:212–24.e214. doi: 10.1016/j.immuni.2018.12.015
56. Sun X, Guo C, Zhao F, Zhu J, Xu Y, Liu ZQ, et al. Vasoactive intestinal peptide stabilizes intestinal immune homeostasis through maintaining interleukin-10 expression in regulatory B cells. Theranostics (2019) 9:2800–11. doi: 10.7150/thno.34414
57. Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive Role of B Cells in Chronic Colitis of T Cell Receptor α Mutant Mice. J Exp Med (1997) 186:1749–56. doi: 10.1084/jem.186.10.1749
58. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity (2002) 16:219–30. doi: 10.1016/S1074-7613(02)00274-1
59. Wang L, Ray A, Jiang X, Wang JY, Basu S, Liu X, et al. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol (2015) 8:1297–312. doi: 10.1038/mi.2015.20
60. Zhu J-f, Xu Y, Zhao J, Li X, Meng X, Wang T-q, et al. IL-33 Protects Mice against DSS-Induced Chronic Colitis by Increasing Both Regulatory B Cell and Regulatory T Cell Responses as Well as Decreasing Th17 Cell Response. J Immunol Res (2018) 2018:1–12. doi: 10.1155/2018/1827901
61. Zhu J, Xu Y, Zhu C, Zhao J, Meng X, Chen S, et al. IL-33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium-induced colitis. Int Immunopharmacol (2017) 46:38–47. doi: 10.1016/j.intimp.2017.02.006
62. Wang S, Qin C. Interleukin 35 Rescues Regulatory B Cell Function, but the Effect Is Dysregulated in Ulcerative Colitis. DNA Cell Biol (2017) 36:413–21. doi: 10.1089/dna.2016.3570
63. Maerz JK, Trostel C, Lange A, Parusel R, Michaelis L, Schäfer A, et al. Bacterial Immunogenicity Is Critical for the Induction of Regulatory B Cells in Suppressing Inflammatory Immune Responses. Front Immunol (2019) 10:3093. doi: 10.3389/fimmu.2019.03093
64. Xu X, Wang Y, Zhang B, Lan X, Lu S, Sun P, et al. Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res Ther (2018) 9:146. doi: 10.1186/s13287-018-0874-5
65. Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol (2020)21:605–14. doi: 10.1038/s41590-020-0677-6
66. Heinemann K, Wilde B, Hoerning A, Tebbe B, Kribben A, Witzke O, et al. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol (2016) 45:312–6. doi: 10.3109/03009742.2015.1126346
67. Wang T, Li Z, Li X, Chen L, Zhao H, Jiang C, et al. Expression of CD19(+)CD24(high)CD38(high) B cells, IL-10 and IL-10R in peripheral blood from patients with systemic lupus erythematosus. Mol Med Rep (2017) 16:6326–33. doi: 10.3892/mmr.2017.7381
68. Menon M, Blair PA, Isenberg DA, Mauri C, Regulatory Feedback between Plasmacytoid Dendritic Cells A. and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity (2016) 44:683–97. doi: 10.1016/j.immuni.2016.02.012
69. Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity (2012) 36:477–90. doi: 10.1016/j.immuni.2012.02.008
70. Cai Z, Wong CK, Dong J, Chu M, Jiao D, Kam NW, et al. Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice. Clin Exp Immunol (2015) 181:253–66. doi: 10.1111/cei.12639
71. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers (2018) 4(1):18002. doi: 10.1038/nrdp.2018.1
72. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc (2007) 2:1269–75. doi: 10.1038/nprot.2007.173
73. Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol (2018) 40:193–200. doi: 10.1080/08923973.2018.1434793
74. Wang Y, Lloyd KA, Melas I, Zhou D, Thyagarajan R, Lindqvist J, et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci Rep (2019) 9:19995. doi: 10.1038/s41598-019-56279-0
75. Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis (2015) 74:2151–6. doi: 10.1136/annrheumdis-2014-205428
76. Ummarino D. Rheumatoid arthritis: Defective IL-10-producing Breg cells. Nat Rev Rheumatol (2017) 13:132. doi: 10.1038/nrrheum.2017.10
77. Daien CI, Gailhac S, Mura T, Audo R, Combe B, Hahne M, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol (2014) 66:2037–46. doi: 10.1002/art.38666
78. Shi L, Hu F, Zhu L, Xu C, Zhu H, Li Y, et al. CD70-mediated CD27 expression downregulation contributed to the regulatory B10 cell impairment in rheumatoid arthritis. Mol Immunol (2020) 119:92–100. doi: 10.1016/j.molimm.2020.01.016
79. Oleinika K, Rosser EC, Matei DE, Nistala K, Bosma A, Drozdov I, et al. CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat Commun (2018) 9:684. doi: 10.1038/s41467-018-02911-y
80. Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol (2012) 180:2375–85. doi: 10.1016/j.ajpath.2012.03.010
81. Park MK, Jung YO, Lee SY, Lee SH, Heo YJ, Kim EK, et al. Amelioration of autoimmune arthritis by adoptive transfer of Foxp3-expressing regulatory B cells is associated with the Treg/Th17 cell balance. J Transl Med (2016) 14:191. doi: 10.1186/s12967-016-0940-7
82. Ramos-Levi AM, Marazuela M. Pathogenesis of thyroid autoimmune disease: the role of cellular mechanisms. Endocrinol Nutr (2016) 63:421–9. doi: 10.1016/j.endoen.2016.09.005
83. Stozek K, Grubczak K, Marolda V, Eljaszewicz A, Moniuszko M, Bossowski A. Lower proportion of CD19(+)IL-10(+) and CD19(+)CD24(+)CD27(+) but not CD1d(+)CD5(+)CD19(+)CD24(+)CD27(+) IL-10(+) B cells in children with autoimmune thyroid diseases. Autoimmunity (2020) 53:46–55. doi: 10.1080/08916934.2019.1697690
84. Botello A, Herrán M, Salcedo V, Rodríguez Y, Anaya J-M, Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: A systematic review and meta-analysis. Clin Endocrinol (Oxf) (2020)93:375–89. doi: 10.1111/cen.14304
85. Matusiewicz A, Stróżyńska-Byrska J, Olesińska M. Polyautoimmunity in rheumatological conditions. Int J Rheum Dis (2019) 22:386–91. doi: 10.1111/1756-185X.13454
86. Santaguida MG, Gatto I, Mangino G, Virili C, Stramazzo I, Fallahi P, et al. BREG cells in Hashimoto’s thyroiditis isolated or associated to further organ-specific autoimmune diseases. Clin Immunol (2017) 184:42–7. doi: 10.1016/j.clim.2017.04.012
87. Santaguida MG, Gatto I, Mangino G, Virili C, Stramazzo I, Fallahi P, et al. Breg Cells in Celiac Disease Isolated or Associated to Hashimoto’s Thyroiditis. Int J Endocrinol (2018) 2018:5290865. doi: 10.1155/2018/5290865
88. Ding YG, Chen G, Li Q, Wen XF, Wei L, Yang HS. Frequency of IL-10-producing regulatory B cells associated with disease activity in thyroid-associated orbitopathy. Int J Ophthalmol (2018) 11:1458–62. doi: 10.18240/ijo.2018.09.05
89. Chen G, Ding Y, Li Q, Li Y, Wen X, Ji X, et al. Defective Regulatory B Cells Are Associated with Thyroid Associated Ophthalmopathy. J Clin Endocrinol Metab (2019)104:4067–77. doi: 10.1210/jc.2018-01812
90. Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol (2007) 179:7225–32. doi: 10.4049/jimmunol.179.11.7225
92. Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol (2011) 239:80–6. doi: 10.1016/j.jneuroim.2011.08.019
93. Wu Q, Mills EA, Wang Q, Dowling CA, Fisher C, Kirch B, et al. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight (2020) 5:e134251. doi: 10.1172/jci.insight.134251
94. Blumenfeld-Kan S, Staun-Ram E, Miller A. Fingolimod reduces CXCR4-mediated B cell migration and induces regulatory B cells-mediated anti-inflammatory immune repertoire. Mult Scler Relat Disord (2019) 34:29–37. doi: 10.1016/j.msard.2019.06.016
95. Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med (1996) 184:2271–8. doi: 10.1084/jem.184.6.2271
96. Korniotis S, Gras C, Letscher H, Montandon R, Mégret J, Siegert S, et al. Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells. Nat Commun (2016) 7:12134. doi: 10.1038/ncomms12134
97. Pennati A, Nylen EA, Duncan ID, Galipeau J. Regulatory B Cells Normalize CNS Myeloid Cell Content in a Mouse Model of Multiple Sclerosis and Promote Oligodendrogenesis and Remyelination. J Neurosci (2020) 40:5105–15. doi: 10.1523/JNEUROSCI.2840-19.2020
98. Bjarnadottir K, Benkhoucha M, Merkler D, Weber MS, Payne NL, Bernard CCA, et al. B cell-derived transforming growth factor-beta1 expression limits the induction phase of autoimmune neuroinflammation. Sci Rep (2016) 6:34594. doi: 10.1038/srep34594
99. Glatigny S, Wagner CA, Bettelli E. Cutting Edge: Integrin alpha4 Is Required for Regulatory B Cell Control of Experimental Autoimmune Encephalomyelitis. J Immunol (2016) 196:3542–6. doi: 10.4049/jimmunol.1502614
100. Sellner J, Rommer PS. A review of the evidence for a natalizumab exit strategy for patients with multiple sclerosis. Autoimmun Rev (2019) 18:255–61. doi: 10.1016/j.autrev.2018.09.012
101. Lin W, Jin L, Chen H, Wu Q, Fei Y, Zheng W, et al. B cell subsets and dysfunction of regulatory B cells in IgG4-related diseases and primary Sjogren’s syndrome: the similarities and differences. Arthritis Res Ther (2014) 16:R118. doi: 10.1186/ar4571
102. Lin X, Wang X, Xiao F, Ma K, Liu L, Wang X, et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjogren’s syndrome. Cell Mol Immunol (2019) 16:921–31. doi: 10.1038/s41423-019-0227-z
103. Achour A, Simon Q, Mohr A, Séité JF, Youinou P, Bendaoud B, et al. Human regulatory B cells control the T(FH) cell response. J Allergy Clin Immunol (2017) 140:215–22. doi: 10.1016/j.jaci.2016.09.042
104. Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, et al. TIM-1 defines a human regulatory B cell population that is altered in frequency and function in systemic sclerosis patients. Arthritis Res Ther (2017) 19:8. doi: 10.1186/s13075-016-1213-9
105. Dolff S, Witzke O, Wilde B. Th17 cells: do regulatory B-cells (Breg) take control in ANCA-vasculitis? Rheumatol (Oxford) (2019) 58:1329–30. doi: 10.1093/rheumatology/kez133
106. Wilde B, Thewissen M, Damoiseaux J, Knippenberg S, Hilhorst M, van Paassen P, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis (2013) 72:1416. doi: 10.1136/annrheumdis-2012-202986
107. Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Controlled Release (2002) 79:103–12. doi: 10.1016/S0168-3659(01)00532-6
108. Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discovery (2019) 18:749–69. doi: 10.1038/s41573-019-0041-4
109. Rad F, Ghorbani M, Mohammadi Roushandeh A, Habibi Roudkenar M. Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Mol Biol Rep (2019) 46:1533–49. doi: 10.1007/s11033-019-04588-y
110. Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA. Hematopoietic stem cell therapy for autoimmune diseases - Clinical experience and mechanisms. J Autoimmun (2018) 92:35–46. doi: 10.1016/j.jaut.2018.06.002
111. Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab (2020) 31:837–51.e810. doi: 10.1016/j.cmet.2020.03.003
112. Hu Y, Yu P, Yu X, Hu X, Kawai T, Han X. IL-21/anti-Tim1/CD40 ligand promotes B10 activity in vitro and alleviates bone loss in experimental periodontitis in vivo. Biochim Biophys Acta Mol Basis Dis (2017) 1863:2149–57. doi: 10.1016/j.bbadis.2017.06.001
113. Ding BB, Bi E, Chen H, Yu JJ, Ye BH. IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J Immunol (2013) 190:1827–36. doi: 10.4049/jimmunol.1201678
114. Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren’s syndrome. Arthritis Res Ther (2006) 8:R51. doi: doi: 10.1186/ar1912
115. Chen X, Cai C, Xu D, Liu Q, Zheng S, Liu L, et al. Human Mesenchymal Stem Cell-Treated Regulatory CD23(+)CD43(+) B Cells Alleviate Intestinal Inflammation. Theranostics (2019) 9:4633–47. doi: 10.7150/thno.32260
116. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367:eaau6977. doi: 10.1126/science.aau6977
Keywords: B cells, regulatory B cells, IL-10+ B cells, immunomodulation, autoimmune diseases
Citation: Zhu Q, Rui K, Wang S and Tian J (2021) Advances of Regulatory B Cells in Autoimmune Diseases. Front. Immunol. 12:592914. doi: 10.3389/fimmu.2021.592914
Received: 08 August 2020; Accepted: 22 March 2021;
Published: 15 April 2021.
Edited by:
Linda L. Kusner, George Washington University, United StatesReviewed by:
Marco Centanni, Sapienza University of Rome, ItalyJan Damoiseaux, Maastricht University Medical Centre, Netherlands
Copyright © 2021 Zhu, Rui, Wang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Tian, MTAwMDAwNDQxNUB1anMuZWR1LmNu
 Qiugang Zhu
Qiugang Zhu Ke Rui
Ke Rui Shengjun Wang
Shengjun Wang Jie Tian
Jie Tian