- 1Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom
- 2National Institute for Health Research (NIHR), Leeds Biomedical Research Centre (BRC), Leeds Teaching Hospitals, Leeds, United Kingdom
- 3Department of Medicine ‘B’, Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel-Hashomer, Israel
- 4Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel
The term spondyloarthritis pertains to both axial and peripheral arthritis including ankylosing spondylitis (AS) and psoriatic arthritis (PsA), which is strongly linked to psoriasis and also the arthritis associated with inflammatory bowel disease. The argument supporting the role for IL-23 across the spectrum of SpA comes from 4 sources. First, genome wide associated studies (GWAS) have shown that all the aforementioned disorders exhibit IL-23R pathway SNPs, whereas HLA-B27 is not linked to all of these diseases-hence the IL-23 pathway represents the common genetic denominator. Secondly, experimental animal models have demonstrated a pivotal role for the IL-23/IL-17 axis in SpA related arthropathy that initially manifests as enthesitis, but also synovitis and axial inflammation and also associated aortic root and cutaneous inflammation. Thirdly, the emergent immunology of the human enthesis also supports the presence of IL-23 producing myeloid cells, not just at the enthesis but in other SpA associated sites including skin and gut. Finally, drugs that target the IL-23 pathway show excellent efficacy for skin disease, efficacy for IBD and also in peripheral arthropathy associated with SpA. The apparent failure of IL-23 blockade in the AS which is effectively a spinal polyenthesitis but evidence for efficacy of IL-23 inhibition for peripheral enthesitis in PsA and preliminary suggestions for benefit in axial PsA, raises many questions. Key amongst these is whether spinal inflammation may exhibit entheseal IL-17A production independent of IL-23 but peripheral enthesitis is largely dependent on IL-23 driven IL-17 production. Furthermore, IL-23 blocking strategies in animal models may prevent experimental SpA evolution but not prevent established disease, perhaps pointing towards a role for IL-23 in innate immune disease initiation whereas persistent disease is dependent on memory T-cell responses that drive IL-17A production independently of IL-23, but this needs further study. Furthermore, IL-12/23 posology in inflammatory bowel disease is substantially higher than that used in AS trials which merits consideration. Therefore, the IL-23 pathway is centrally involved in the SpA concept but the nuances and intricacies in axial inflammation that suggest non-response to IL-23 antagonism await formal definition. The absence of comparative immunology between the different skeletal sites renders explanations purely hypothetical at this juncture.
Introduction
The seminal clinical observations by Moll and Wright in the 1970s classified several diseases under the umbrella term of Spondyloarthorpathy (SpA) based on shared clinical and immunological features (1). These conditions included ankylosing spondylitis (AS), psoriatic arthritis (PsA) (and by extension of the psoriasis spectrum), inflammatory bowel disease (IBD) associated arthropathy including Crohn’s disease and ulcerative colitis, enterogenic and urethrogenic reactive arthritis and anterior uveitis which is also associated with these conditions (2, 3). The common theme across these disorders was axial inflammation, peripheral lower limb oligoarthritis, enthesitis in some cases, a link to infection or intestinal dysfunction and negativity for rheumatoid factor (4, 5). A unified pathological understanding for the SpA associated arthropathy was not proposed in the original iteration of the concept.
Following on shortly after the Moll and Wright’s SpA concept was the discovery of HLA-B27 that was associated with AS, PsA axial disease, IBD related axial arthritis, anterior uveitis and reactive arthritis (6–8). However, IBD itself or IBD related peripheral arthropathy was not associated with HLA-B27. The clinical features of Bechet’s disease (BD) resulted in the investigators also proposing this to represent a member of the SpA concept in a paper that has been cited highly over four decades (9). The absence of sacroiliitis and the lack of a strong association with HLA-B27 meant that BD was never widely adopted in this proposed classification scheme. However, alluded to in the following discussion, GWAS studies have shown that the IL-23 pathway related genetic polymorphisms occur along the entire SpA arthropathy spectrum including ankylosing spondylitis and psoriatic arthritis, in psoriasis and inflammatory bowel disease and indeed in BD, thus completely vindicating the entire concept alluded to by Moll and Wright (9, 10).
Current Therapy in AS
The current therapy options in AS include an anti-TNF agents for subjects that fail to respond to NSAIDS (Non-steroidal anti-inflammatory drugs). If anti-TNF is contraindicated or if there is loss of efficacy to anti-TNFs, one of two anti-IL-17A blockers can be considered with the provision that these agents should not be used for active associated IBD (11). The JAK inhibitors are likely to enter the clinical arena in AS in the coming years (12). Although guselkumab and ustekinumab may have some efficacy in PsA related axial disease (13, 14) there is no evidence for efficacy of this class of agent in AS from trials with ustekinumab and other p19 blocker risankizumab (15, 16).
IL-23/IL-17 Axis
When naïve T-cells encounter a cognate antigen in lymphoid tissues, they have the ability to differentiate into effector T-cells, depending on the local microenvironment. This involves MHC peptide presentation to the T-cell receptor (TCR) (signal 1) and then co-activation with CD80/86 binding to CD28 (signal 2) on T-cells (17). In humans, cytokine stimuli such as IL‐1β, IL‐6, IL‐21, and/or IL‐23 can drive IL‐17 production from T-cells, with the best described of these being CD4+ Th17 cells and CD8+ Tc17 cells (18). These IL-23 activated T-cells also secrete a range of other cytokines such as IL-17F, IL-22 and TNF (18).
The IL-23 Pathway Genetic Argument in SpA Spectrum Disorders
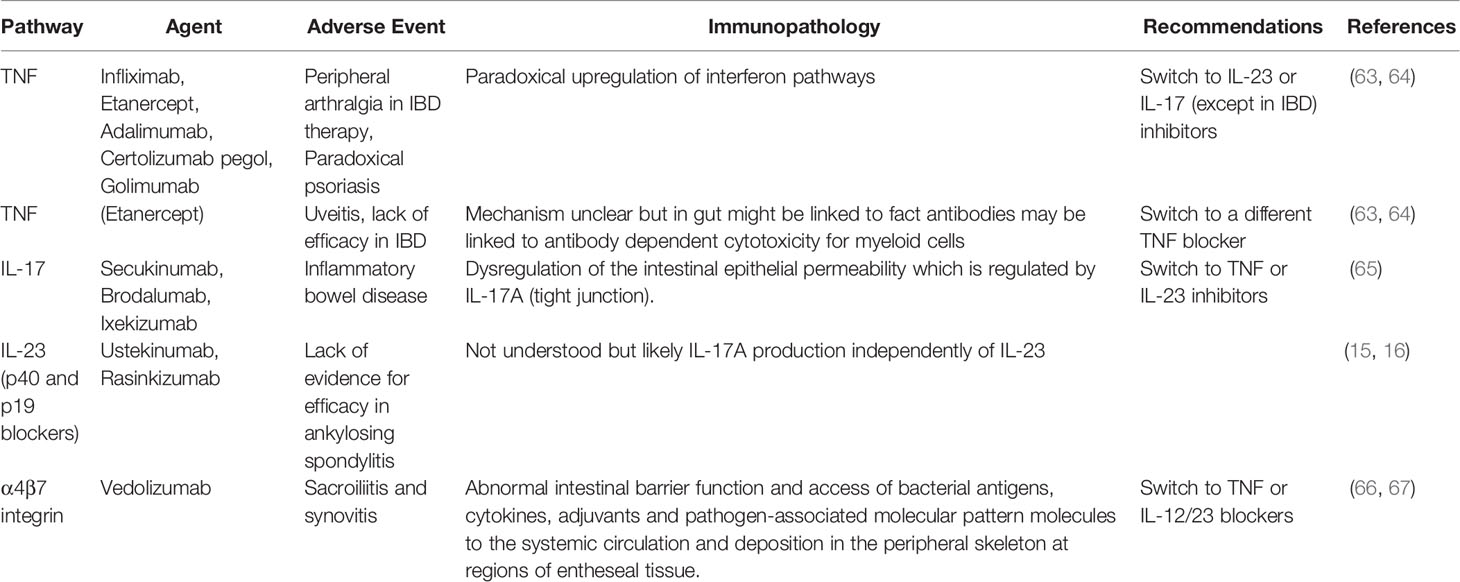
Remarkably, IL-23R polymorphisms have been reported across all of the aforementioned categories of disease but not in classical autoimmunity (Figure 1). Furthermore, several SNPs related to the IL-23 pathway including those in the IL-23 cytokine itself, downstream JAK2 and Tyk2, STAT3 and IL-17RA signalling have also been reported across all of these diseases (5, 9, 19). A wealth of other genetic polymorphism data has strongly vindicated these findings insofar that classical autoimmune diseases have a completely different non-IL-23 pathway related genetic architecture (20). The IL-23R pathway SNPs are also associated with IBD (21) and BD (22), thus reinvigorating the historical ties with SpA as suggested by Moll and Wright and colleagues. The SNP in the IL-23R (R381Q) confers protection from IBD, AS and psoriasis (23–25). At a functional level, it results in a loss of function and less STAT3 activation and thus less induced IL-17 from T-cells (26, 27). Thus, it appears that “completely normal” IL-23 pathway signalling and functioning, which is comparatively higher than in subjects with the R381Q polymorphism is linked to AS. It might be theorised that anti-IL-23 therapy would reduce this further and align it with production levels associated with the protective allele. However, this has not been corroborated from trials in AS. While IL-23 pathway is genetically implicated in all the aforementioned tissues, the difference in relative contribution of IL-23 and other cytokines to the different SpA associated diseases shows differential efficacy as demonstrated by clinical trials (Figure 2).
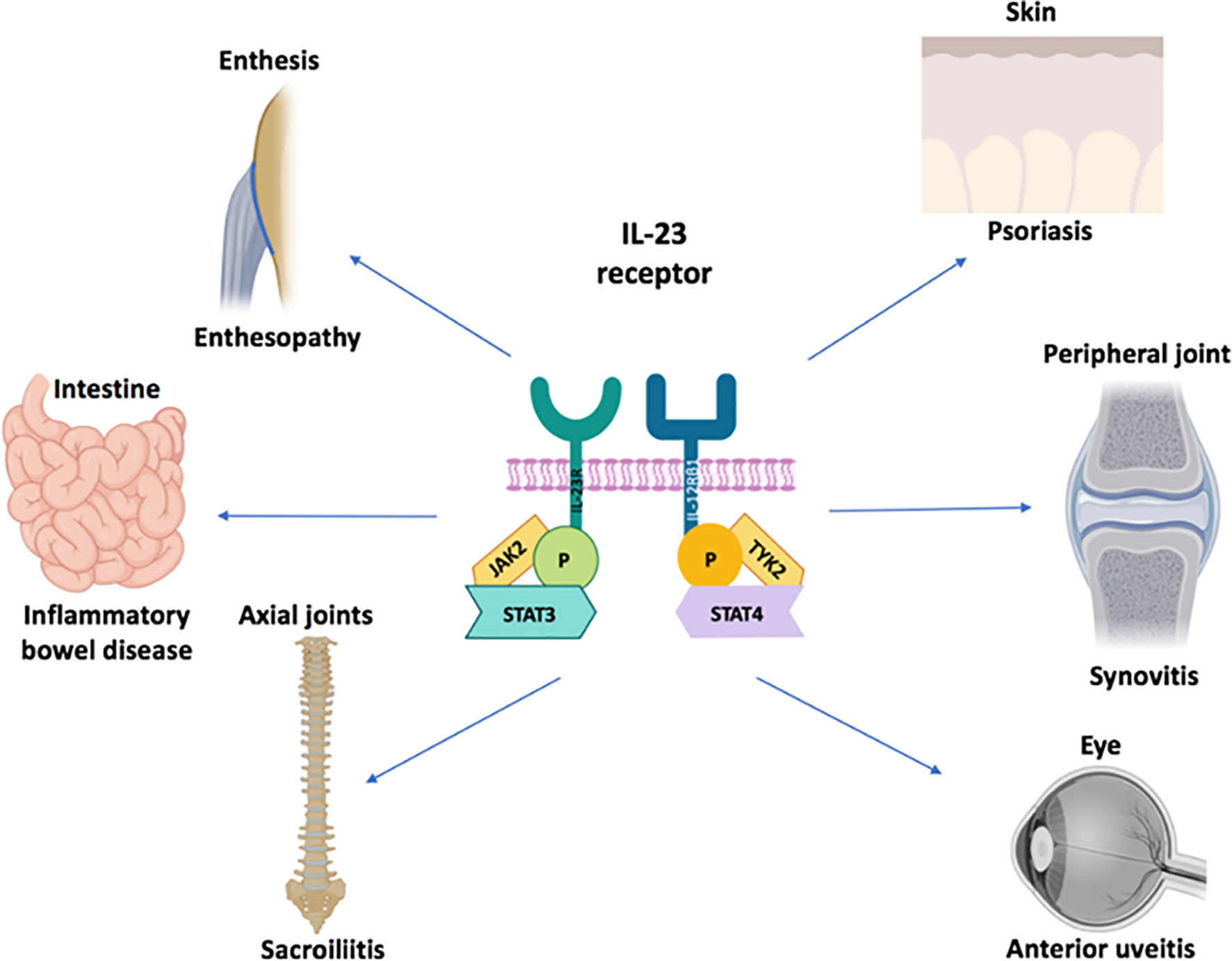
Figure 1 IL-23 receptor polymorphism associated disorders. The IL-23 cytokine pathway has been firmly linked to ocular, cutaneous, intestinal disease and arthropathy at the genetic and cellular immunology level. There is considerable immunological heterogeneity in different organ responses. For example, the TNF-Fc fusion protein, etanercept does not work for IBD and is generally not effective for anterior uveitis. The role of IL-23 blockers for anterior uveitis awaits further definition but it is generally considered that anti-IL-17A blockers are not effective in this SpA domain All the agents of that antagonise the IL-23/17 axis show remarkable efficacy for psoriasis compared to joint disease. With respect to gut disease, the IL-17 blockers have an important role in barrier function at this site which may explain exacerbations of IBD under anti-IL-17A therapy. All three cytokines including TNF, IL-17A and IL-23 may be equally important for peripheral arthritis in PsA and also for enthesitis in the peripheral skeleton because good responses are seen following therapeutic antagonism. The anti-p40 IL-12/23 may be less effective than the other three categories of drugs for Psoriatic arthritis although further studies are needed. Finally, only TNF and IL-17 blockers have shown efficacy in the axial skeleton where IL-23 blockade with either p40 or p19 blockers has not worked. The site-specific compartmentalisation of immunity has come into sharp focus in the past few years and likely reflects tissue specific factors and microbiota interactive factors shaping diverse immune responses.
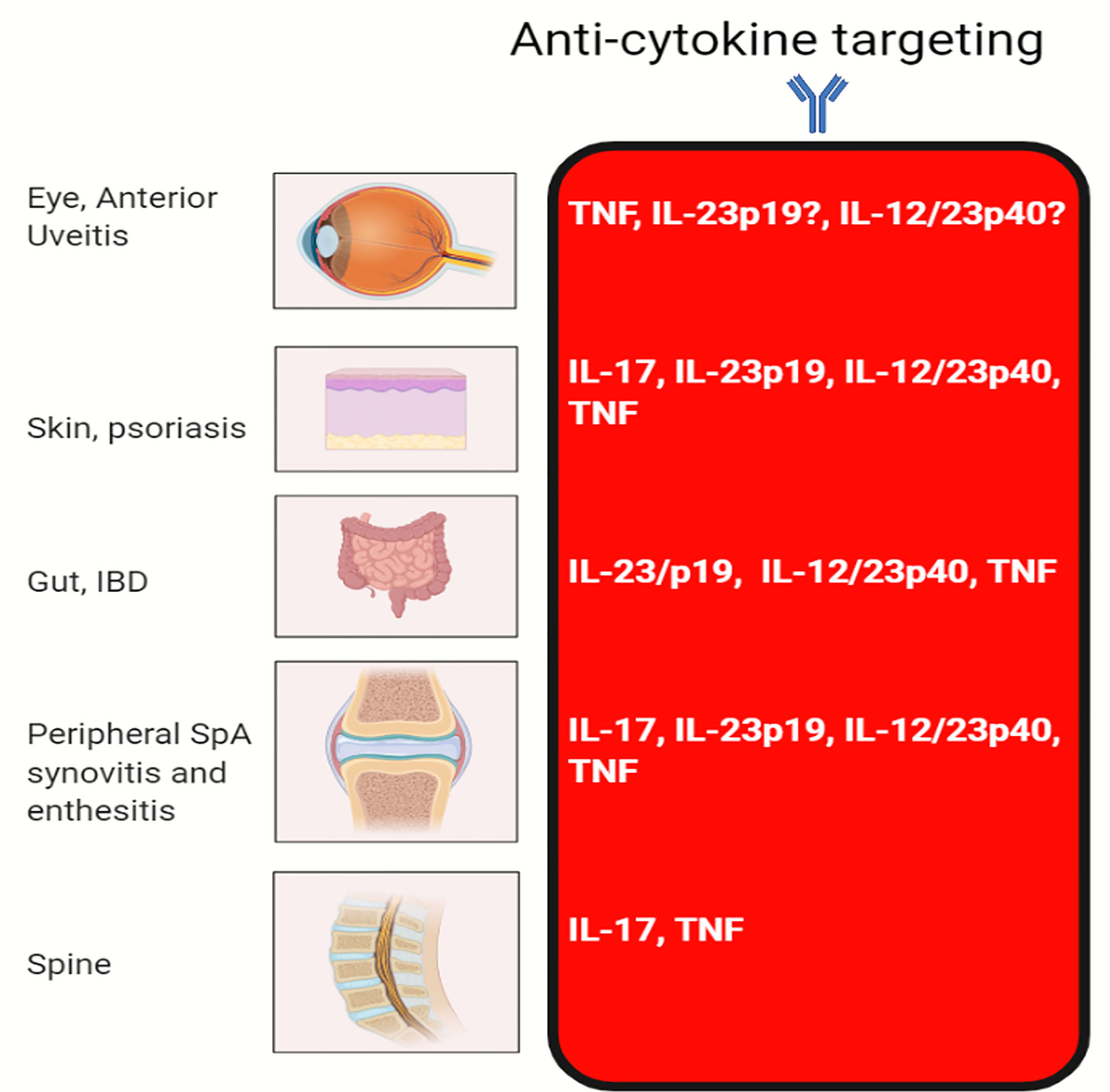
Figure 2 Efficacy of cytokine blocking in different organs. The classical MHC class II associated autoimmune diseases that are characterised by autoantibody production segregate in families and individuals and show a female preponderance. The SpA group of diseases do not show substantial sex differences, have MHC class 1 genetic associations, a lack of specific confirmed disease associated autoantibodies and disease localisation to sites of injury or physical stressing. It is into this mix that genetics and experimental immunology have firmly confirmed the key role for the IL-23 pathway. Given that IL-23 regulates both IL-22 and IL-17, we believe that in addition to immunity including anti-fungal immunity via IL-17 regulation that the IL-23 pathway fine tunes tissue repair at sites of injury and physical or chemical stressing as for example in the intestine. TNF-Fc fusion protein, etanercept does not work for IBD and generally not effective for anterior uveitis.
Tissue Microanatomy of IL-23 Pathway and Animal Models
It is well established that the synovium is the primary target of inflammation in RA with autoimmunity directed against citrullinated synovial proteins driving an inflammatory reaction culminating in periarticular joint destruction and erosion, with the well-recognised polyarticular joint destruction phenotype. In the mid-1990s, MRI studies showed that enthesitis was evident in both swollen small and large joints in PsA and SpA in general (28). This resulted in the enthesitis based model for SpA whereby it was proposed cytokine mediated primary inflammatory reactions at the enthesis triggered an adjacent synovitis, tenosynovitis and osteitis (29–31). It was subsequently shown in animal models that dysregulated TNF production at the enthesis triggered polyarticular joint destruction, which further validated enthesitis as a mechanism of disease (32, 33).
A seminal paper by Sherlock et al. demonstrated that the normal murine enthesis harboured an IL-23R expressing cell population (34). This model was later confirmed to be Tyk2 dependent (Tyk2 mediates IL-23 signalling) (35) while the same paper found Tyk2 SNPs correlate with human AS disease progression. In the IL-23 minicricle model, the distal over-expression of the IL-23 cytokine in the murine liver using DNA minicircle technology resulted in a rapid onset of peripheral enthesitis that subsequently spread to the synovium and bone leading to polyarticular joint destruction. In the Sherlock model of IL-23 dependent enthesitis (34), it was subsequently shown by Reinhardt et al. that the majority of IL-17A producing cells in the normal murine enthesis were IL-23R expressing γδ T-cells (36). This population of cells are heterogeneous and carry out diverse functions including early innate immune responses, priming of adaptive immunity as well as prominent roles in tissue repair (37). The role of IL-23 in the SpA concept was strongly cemented in this model by the induction of psoriasiform skin inflammation, aortic root inflammation and also the development of axial inflammation (34). Other investigators using the same minicircle technology emphasised the role of severe synovitis and bone erosion and rheumatoid arthritis like features (38).
Of course, there are several independent animal models showing the pivotal role of IL-23 in experimental gut inflammation and reactive type arthritis (39, 40). A body of emergent research has also linked intestinal microbiota to the IL-23/17 axis interdependence and cross-regulation with this being an area of active research (41–44). Another example of an IL-23 dependent SpA comes from the SKG mouse model that exhibits many of the SpA features in an IL-23/17 axis dependent pathway (45). The SKG contains a point mutation in the ZAP-70 gene, yielding reduced T-cell receptor signalling and following administration with fungal or bacterial adjuvants develops multi-organ inflammation and a SpA like disease (45). Collectively, these models support the idea that inflammation that is topographically enthesis centred drives disease (31).
Emerging Immunology of the IL-23 Pathway and IL-23/17 Axis in Human SpA
Clinical trials in man dissect human disease immunopathogenesis and it is important to turn to these, in order to better understand human enthesitis. First, IL-17A blockers have proven efficacy for both peripheral and axial SpA including evidence for efficacy for isolated enthesitis as a secondary outcome measure (46–50). Likewise, the published literature shows efficacy for IL-12/IL-23 p40 blockers for peripheral PsA and for isolated enthesitis (30, 51–53). Recent studies have also shown that IL-23p19 blockade is effective for peripheral synovitis and related enthesitis (54, 55). These findings alone point towards a biological role for IL-23 at the non-axial peripheral enthesis (56), but what is the biological basis for this?
Following on from the Sherlock et al. study (34), our group investigated the presence of IL-23/17 axis cytokines at the normal human spinal enthesis. We defined normal spinal enthesis bone and soft tissue resident IL-23R expressing group 3 innate lymphoid cells (57). IL-23/IL-1β stimulation of normal human enthesis tissue resulted in upregulation of IL-17A and IL-17F transcript (57). Moreover, in humans we previously reported the presence of macrophages in acute enthesitis (58). This raised the possibility that local IL-23 production may be possible at the human enthesis and it was subsequently shown that the normal enthesis contains IL-23 inducible protein production from CD14+ myeloid cell following bacterial or fungal stimulation (59). We also found that this IL-23 secretion could be attenuated by the addition of PDE4 blockers which may be relevant translationally since antagonism of this pathway shows efficacy for peripheral enthesitis in PsA in man (56). Both TNF and IL-17A are able to also induce osteogenesis in vitro in MSC from the spinal enthesis (60, 61).
Complexity of the IL-23 Pathway in the Spine and Other SpA Features
Since the failure of IL-23 blocking in the AS, there has been great scientific speculation into the reason why (62). Remarkably, although the SpA group of conditions are closely interlinked, they also exhibit a differential immunopathology between different sites that is best encapsulated in the non-efficacy of therapies in some domains (Table 1). For example, the TNF fusion protein etanercept shows efficacy for the skeleton but not in the gut (68). Likewise, IL-17A blockers show impressive efficacy in the skin and good efficacy in the skeleton but are ineffective in the gut and in some circumstances are associated with IBD exacerbation (69). Laboratory research following the failed human trials of anti-IL-17A in Crohn’s disease led to observations that γδ T-cell IL-17A production in the gut is produced independent of IL-23R signalling where IL -17 signalling was required for maintaining intestinal occludin junctions (70).
Given the aforementioned efficacy of IL-17 blockade in axial disease and the non-efficacy of IL-17A inhibition in the gut, the question arises as to whether there may be pathway for IL-17 production in the spine that is independent of IL-23 that may account for the curious reported lack of efficacy for IL-23 pathway inhibition in axial disease. Two trials of IL-23 pathway blockade including p40 and p19 blockade failed to show efficacy in AS, although marginal non-statistically significant improvements in CRP and subtle MRI improvements were evident under p40 antagonism (15, 16). There are two phase II trials of p19 blockers showing efficacy in psoriatic arthritis peripheral arthropathy including peripheral enthesitis (54, 55, 71). This has thrown up a new conundrum- how can a drug work for peripheral skeletal enthesitis but not axial enthesitis that underpins most of the AS pathology outside the sacroiliac joint. One important difference may be the presence of synovio-entheseal complexes in the peripheral skeleton but not in the spine (72).
Emergent Cellular Players in the Non-Linearity Between IL-23 and IL-17 Pathways in SpA
Human γδ T-cells are classified into two major groups- δ1 and δ2 (73). We have explored the concept that there may be heterogeneity in these populations in man. Both the normal spinal entheseal soft tissue and peri-entheseal bone have resident γδ T-cell populations with these being more numerous in the peri-entheseal bone (74). In the enthesis resident γδ T-cell populations, we found that the δ1 population lacked IL-23R expression but that the δ2 population expressed this receptor. Only the δ2 population upregulated IL-17A in response to IL-23 signalling. However, both populations could be induced to express IL-17A upon PMA or anti-CD3/CD28 stimulation (74). Hence, the complexity of the IL-23 pathway extends to the spine and our results indicate that IL-17A, a key cytokine in AS and spinal inflammation, may not depend exclusively on IL-23. Accordingly, the IL-23/IL-17A axis is a two-sided coin with IL-17A production independent of IL-23 having very different biological consequences for gut and skeletal inflammation with IL-17A blockade in the former being detrimental but potentially beneficial in the latter (75, 76). In recent times, other theories have emerged of IL-17 secretion independent of IL-23. Mucosal-associated invariant T (MAIT) cells, are specialised innate-like T-cells that serve to bridge innate and adaptive immunity. MAITs are activated by conserved bacterial ligands which are derived from vitamin B biosynthesis, which are presented by the MHC-class I like MR1 to the TCR (77). Following TCR activation and also stimulation with IL-12 and IL-18, MAIT cells have been shown to secrete IL-17 that is independent of IL-23 (78). The human enthesis also contains conventional T-cells, both CD4+ and CD8+. Both entheseal CD4+ and CD8+ are able to secrete IL-17A following TCR stimulation (anti-CD3/CD28) without the need for additional IL-23 stimulation (79).
IL-23 Blockade for the Prevention of SpA
The failed phase II trial of risankizumab in AS and the failed phase III ustekinumab trial in AS are responsible for these emergent immune insights (80, 81). This has been explored in the experimental SpA model induced in HLA-B27/Huβ2m transgenic rats that spontaneously develop SpA (82). These animals were either treated prophylactically with anti-IL-23R prior to disease onset or with control injections. Conversely the disease was allowed to fully manifest and then the animals were treated with anti-IL23R antibody or control. These experiments showed that IL-23 blockade was capable of preventing disease evolution but incapable of suppressing arthritis when fully established (82). How, exactly this relates to humans is unclear as the nuances of this rat model and its applicability to human SpA are not completely defined (4). For example, the findings might suggest a key role for memory T-cells that could produce IL-17A independent of IL-23 signalling. However, a role for CD8+ T-cells in HLA-B27 experimental SpA has never been substantiated (83), whereas the genetics of human SpA including HLA-B27, ERAP-1 and several other SNPs tends to incriminate this pathway (4).
There is some preliminary evidence supporting these animal models in humans. It has been recently shown that blocking of the IL-23 pathway with ustekinumab in psoriasis results in the regression of subclinical peripheral enthesopathy (84). Whether IL-23 blocker utilisation in psoriasis subjects will prevent axial inflammation evolution is an interesting and open question. It is worth pointing out that a secondary analysis of the pivotal phase III ustekinumab studies in PsA, showed efficacy in axial PsA including improvement in spinal domain pain (13).
Recent studies in abstract form have shown that patients with PsA enlisted in trials for polysynovitis, but also where 20% of patients had radiographic sacroilitis and back pain, that p19 blockade with guselkumab was associated with improvements in axial symptoms (14). These trials point to the possibility of inflammatory spinal disease immunological heterogeneity with some cases of PsA axial inflammation exhibiting a direct role for IL-23, which is stronger and different from that seen in AS.
Some Loose Ends With Respect to IL-23 in the Spine
It is unlikely that p19 blockade is interfering with the function of the poorly characterised cytokine IL-39, that also shares the p19 subunit (p19+EBI3) (85). Indeed, this cytokine remains a theoretical cytokine in humans with no evidence for either its formation or its function in vivo (85, 86). Hence, at this time it seems that the sole role of p19 blockade in main is on IL-23 and not another as yet ill-characterised cytokine, but further work is needed.
Most of the spinal inflammation in AS occurs in the peri-entheseal bone where disease localisation to this site is related to the HLA-B27 genetic status (58). Our work in human spinal entheses shows a much higher production or induction of IL-23 from the bone side of the enthesis (59). Whether this translates into therapeutics remains an open question and maybe higher doses of p19 blockers are needed to alleviate axial inflammation?
The failed trial of ustekinumab in AS used the 45mg and 90mg dosing regimen but the higher dose was associated with a non-significant CRP reduction and minor improvements in MRI lesions (81). The dose of ustekinumab used in Crohn’s disease includes and 6 mg/kg intravenous loading dose (87) which is potentially the equivalent of 18 months of ustekinumab at the 90mg sc regimen for AS in the failed study. Clearly there is room for dose escalation to formally evaluate these questions. Also, it has been suggested that p40 blockers may restrain the immunoregulatory effects of IL-12 in the skin (88) and likewise there is uncertainty about any negative impact that p40 blockers could be exerting outside of the IL-23 pathway. However, the negative p19 study in AS argues against this.
It must be clearly articulated that translational therapy in man, and not laboratory experimental science is leading the understanding of these pathways. It is noteworthy that p40 blockers were associated with efficacy for axial symptoms in PsA, but it must be acknowledged that HLA-B27 negative axial PsA might represent a different disease from AS (89). A clinical short cut to understanding the dosing issues around IL-23 blockers may come from an evaluation of Crohn’s disease therapy dosing on subjects with concomitant axial disease. Unfortunately, there is no comparative immunology between the spinal and peripheral entheses at this time so this is still largely conjectural.
Conclusions
For the purposes of this article the term SpA was taken to include the protean manifestations associated with axial inflammation including skin and gut involvement where it has clearly been shown that IL-23 SNPs are a common denominator across the different conditions. It is also clearly evident in experimental and human systems that the IL-23/IL-17 axis is involved in skin, gut and entheseal biology (90). A differential immunopathology exists within these disease domains reflecting the context dependent biology of different tissues that is currently best understood in terms of the barrier function role of IL-17A in the gut. The biological basis for IL-17 production in the spine that is seemingly independent of IL-23 needs verification, and if confirmed raises a vital question as to why IL-17A is so crucial to spinal immunobiology.
This non-linearity between IL-23 and IL-17 also appears to exist in the human spine but this knowledge is presently very rudimentary. Nevertheless, there is preliminary evidence suggesting that the downstream IL-17A pathway in axial biology is regulated in both IL-23 and IL-23 independent pathways. Further work is needed in man including IL-23 posology and careful assessment of disease subtypes and objective measures of inflammation including CRP and MRI appearances. The emergent biology of the IL-23/17 axis in the human skeleton strongly suggests that hidden within the current complexity is an IL-23 pathway, there may be a SpA subgroup with axial inflammation that might still be exploitable therapeutically with antagonism of this pathway.
Author Contributions
CB, AW, KS and DM all contributed to scientific discussion, writing and figure making for the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Moll J, Haslock I, Macrae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, the intestinal arthropathies, and Behcet’s syndrome. Med (Baltimore) (1974) 53:343–64. doi: 10.1097/00005792-197409000-00002
2. Generali E, Bose T, Selmi C, Voncken JW, Damoiseaux JG. Nature versus nurture in the spectrum of rheumatic diseases: Classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun Rev (2018) 17:935–41. doi: 10.1016/j.autrev.2018.04.002
3. Deodhar A, Miossec P, Baraliakos X. Is undifferentiated spondyloarthritis a discrete entity? A Debate Autoimmun Rev (2018) 17:29–32. doi: 10.1016/j.autrev.2017.11.006
4. Watad A, Bridgewood C, Russell T, Marzo-Ortega H, Cuthbert R, McGonagle D. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front Immunol (2018) 9:2668. doi: 10.3389/fimmu.2018.02668
5. Bridgewood C, Watad A, Cuthbert RJ, McGonagle D. Spondyloarthritis: new insights into clinical aspects, translational immunology and therapeutics. Curr Opin Rheumatol (2018) 30:526–32. doi: 10.1097/BOR.0000000000000529
6. Brewerton D, Hart FD, Nicholls A, Caffrey M, James D, Sturrock R. Ankylosing spondylitis and HL-A 27. Lancet (1973) 301:904–7. doi: 10.1016/S0140-6736(73)91360-3
7. McMichael A, Bowness P. HLA-B27: natural function and pathogenic role in spondyloarthritis. Arthritis Res Ther (2002) 4:S153. doi: 10.1186/ar571
8. Prajzlerova K, Grobelná K, Pavelka K, Šenolt L, Filkova M. An update on biomarkers in axial spondyloarthritis. Autoimmun Rev (2016) 15:501–9. doi: 10.1016/j.autrev.2016.02.002
9. McGonagle D, Aydin SZ, Gül A, Mahr A, Direskeneli H. ‘MHC-I-opathy’—unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol (2015) 11:731. doi: 10.1038/nrrheum.2015.147
10. Wang K, Zhang H, Kugathasan S, Annese V, Bradfield JP, Russell RK, et al. Diverse genome-wide association studies associate the IL12/IL23 pathway with Crohn Disease. Am J Hum Genet (2009) 84:399–405. doi: 10.1016/j.ajhg.2009.01.026
11. van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis (2016) 76(2017):978–91. doi: 10.1136/annrheumdis-2016-210770
12. Veale DJ, McGonagle D, McInnes IB, Krueger JG, Ritchlin CT, Elewaut D, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (2019) 58:197–205. doi: 10.1093/rheumatology/key070
13. Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, You Y, Li S, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis (2016) 75:1984–8. doi: 10.1136/annrheumdis-2015-209068
14. Helliwell P, Gladman D, Poddubnyy D, Mease P, Baraliakos X, Kollmeier A, et al. OP0054 Efficacy of Guselkumab, a Monoclonal Antibody That Specifically Binds to the P19-Subunit of Il-23, on Endpoints Related to Axial Involvement in Patients With Active Psa With Imaging-Confirmed Sacroiliitis: Week-24 Results From Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Studies. BMJ Publishing Group Ltd (2020). doi: 10.1136/annrheumdis-2020-eular.474
15. Baeten D, Østergaard M, Wei JC-C, Sieper J, Järvinen P, Tam L-S, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis (2018) 77:1295–302. doi: 10.1136/annrheumdis-2018-213328
16. Mease P. Ustekinumab fails to show efficacy in a phase III axial spondyloarthritis program: the importance of negative results. Arthritis Rheumatol (2019) 71:179–81. doi: 10.1002/art.40759
17. Purvis HA, Stoop JN, Mann J, Woods S, Kozijn AE, Hambleton S, et al. Low-strength T-cell activation promotes Th17 responses. Blood J Am Soc Hematol (2010) 116:4829–37. doi: 10.1182/blood-2010-03-272153
18. Boutet M-A, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci (2018) 19:530. doi: 10.3390/ijms19020530
19. Costantino F, Breban M, Garchon H-J. Genetics and functional genomics of spondyloarthritis. Front Immunol (2018) 9:2933. doi: 10.3389/fimmu.2018.02933
20. Brown MA, Kenna T, Wordsworth BP. Genetics of ankylosing spondylitis—insights into pathogenesis. Nat Rev Rheumatol (2016) 12:81. doi: 10.1038/nrrheum.2015.133
21. Kim SW, Kim ES, Moon CM, Park JJ, Kim TI, Kim WH, et al. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut (2011) 60:1527–36. doi: 10.1136/gut.2011.238477
22. Takeuchi M, Kastner DL, Remmers EF. The immunogenetics of Behçet’s disease: A comprehensive review. J Autoimmun (2015) 64:137–48. doi: 10.1016/j.jaut.2015.08.013
23. Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet (2007) 80:273–90. doi: 10.1086/511051
24. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science (2006) 314:1461–3. doi: 10.1126/science.1135245
25. W.T.C.C. Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature (2007) 447:661. doi: 10.1038/nature05911
26. Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci (2011) 108:9560–5. doi: 10.1073/pnas.1017854108
27. Di Meglio P, Di Cesare A, Laggner U, Chu C-C, Napolitano L, Villanova F, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PloS One (2011) 6:e17160. doi: 10.1371/journal.pone.0017160
28. McGonagle D, Gibbon W, O’Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondylarthropathy. Arthritis Rheumatism: Off J Am Coll Rheumatol (1998) 41:694–700. doi: 10.1002/1529-0131(199804)41:4<694::AID-ART17>3.0.CO;2-#
29. McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet (1998) 352:1137–40. doi: 10.1016/S0140-6736(97)12004-9
30. Schett G, Lories RJ, D’Agostino MA, Elewaut D, Kirkham B, Soriano ER, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol (2017) 13:731–41. doi: 10.1038/nrrheum.2017.188
31. Watad A, Cuthbert RJ, Amital H, McGonagle D. Enthesitis: much more than focal insertion point inflammation. Curr Rheumatol Rep (2018) 20:41. doi: 10.1007/s11926-018-0751-3
32. Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis (2014) 73:437–45. doi: 10.1136/annrheumdis-2013-203643
33. De Wilde K, Martens A, Lambrecht S, Jacques P, Drennan MB, Debusschere K, et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann Rheum Dis (2017) 76:585–92. doi: 10.1136/annrheumdis-2016-209454
34. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao C-C, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+ CD4– CD8– entheseal resident T cells. Nat Med (2012) 18:1069–76. doi: 10.1038/nm.2817
35. Gracey E, Hromadová D, Lim M, Qaiyum Z, Zeng M, Yao Y, et al. TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J Clin Invest (2020) 130(4):1863–78. doi: 10.1172/JCI126567
36. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol (2016) 68:2476–86. doi: 10.1002/art.39732
37. Lawand M, Déchanet-Merville J, Dieu-Nosjean M-C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol (2017) 8:761. doi: 10.3389/fimmu.2017.00761
38. Adamopoulos IE, Tessmer M, Chao C-C, Adda S, Gorman D, Petro M, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol (2011) 187:951–9. doi: 10.4049/jimmunol.1003986
39. Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflam Bowel Dis (2010) 16:1808–13. doi: 10.1002/ibd.21248
40. Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, et al. Interleukin-23 mediates the intestinal response to microbial β-1, 3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol (2014) 66:1755–67. doi: 10.1002/art.38638
41. Wilharm A, Tabib Y, Nassar M, Reinhardt A, Mizraji G, Sandrock I, et al. Mutual interplay between IL-17–producing γδT cells and microbiota orchestrates oral mucosal homeostasis. Proc Natl Acad Sci (2019) 116:2652–61. doi: 10.1073/pnas.1818812116
42. Chen L, He Z, Iuga AC, Martins Filho SN, Faith JJ, Clemente JC, et al. Diet modifies colonic microbiota and CD4+ T-cell repertoire to induce flares of colitis in mice with myeloid-cell expression of interleukin 23. Gastroenterology (2018) 155:1177–91.e16. doi: 10.1053/j.gastro.2018.06.034
43. Babaie F, Hasankhani M, Mohammadi H, Safarzadeh E, Rezaiemanesh A, Salimi R, et al. The role of gut microbiota and IL-23/IL-17 pathway in ankylosing spondylitis immunopathogenesis: New insights and updates. Immunol Lett (2018) 196:52–62. doi: 10.1016/j.imlet.2018.01.014
44. Ivanov II, de Llanos Frutos R, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe (2008) 4:337–49. doi: 10.1016/j.chom.2008.09.009
45. Rahman MA, Thomas R. The SKG model of spondyloarthritis. Best Pract Res Clin Rheumatol (2017) 31:895–909. doi: 10.1016/j.berh.2018.07.004
46. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2015) 386:1137–46. doi: 10.1016/S0140-6736(15)61134-5
47. Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, et al. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. J Rheumatol (2016) 43:1713–7. doi: 10.3899/jrheum.160275
48. McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis (2014) 73:349–56. doi: 10.1136/annrheumdis-2012-202646
49. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, Van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet (2013) 382:1705–13. doi: 10.1016/S0140-6736(13)61134-4
50. Dubash S, Bridgewood C, McGonagle D, Marzo-Ortega H. The advent of IL-17A blockade in ankylosing spondylitis: secukinumab, ixekizumab and beyond. Expert Rev Clin Immunol (2019) 15:123–34. doi: 10.1080/1744666X.2019.1561281
51. Gottlieb A, Narang K. Ustekinumab in the treatment of psoriatic arthritis: latest findings and clinical potential. Ther Adv Musculoskeletal Dis (2013) 5:277–85. doi: 10.1177/1759720X13501021
52. Kavanaugh A, Menter A, Mendelsohn A, Shen Y-K, Lee S, Gottlieb AB. Effect of ustekinumab on physical function and health-related quality of life in patients with psoriatic arthritis: a randomized, placebo-controlled, phase II trial. Curr Med Res Opin (2010) 26:2385–92. doi: 10.1185/03007995.2010.515804
53. McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet (2013) 382:780–9. doi: 10.1016/S0140-6736(13)60594-2
54. Deodhar A, Helliwell PS, Boehncke W-H, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1115–25. doi: 10.1016/S0140-6736(20)30265-8
55. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet (2020) 395(10227):912–20.
56. Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D. Interleukin-23 pathway at the enthesis: The emerging story of enthesitis in spondyloarthropathy. Immunol Rev (2020) 294:27–47. doi: 10.1111/imr.12840
57. Cuthbert RJ, Fragkakis EM, Dunsmuir R, Li Z, Coles M, Marzo-Ortega H, et al. Brief Report: Group 3 Innate Lymphoid Cells in Human Enthesis. Arthritis Rheumatol (2017) 69:1816–22. doi: 10.1002/art.40150
58. McGonagle D, Marzo-Ortega H, O’Connor P, Gibbon W, Pease C, Reece R, et al. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis Rheumatism (2002) 46:489–93. doi: 10.1002/art.10125
59. Bridgewood C, Watad A, Russell T, Palmer TM, Marzo-Ortega H, Khan A, et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann Rheum Dis (2019) 78:929–33. doi: 10.1136/annrheumdis-2018-214944
60. Russell T, Watad A, Bridgewood C, Rowe H, Khan A, Rao A, et al. IL-17A and TNF Modulate Normal Human Spinal Entheseal Bone and Soft Tissue Mesenchymal Stem Cell Osteogenesis, Adipogenesis, and Stromal Function. Cells (2021) 10:341. doi: 10.3390/cells10020341
61. Russell T, Bridgewood C, Rowe H, Altaie A, Jones E, McGonagle D. Cytokine “fine tuning” of enthesis tissue homeostasis as a pointer to spondyloarthritis pathogenesis with a focus on relevant TNF and IL-17 targeted therapies. Semin Immunopathol Springer (2021) 1–14. doi: 10.1007/s00281-021-00836-1
62. Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis (2019) 78:1015–8. doi: 10.1136/annrheumdis-2018-213654
63. Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open (2016) 2:e000239. doi: 10.1136/rmdopen-2015-000239
64. Garcovich S, De Simone C, Genovese G, Berti E, Cugno M, Marzano AV. Paradoxical skin reactions to biologics in patients with rheumatologic disorders. Front Pharmacol (2019) 10:282. doi: 10.3389/fphar.2019.00282
65. Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol (2016) 111:1599–607. doi: 10.1038/ajg.2016.298
66. Dubash S, Marianayagam T, Tinazzi I, Al-Araimi T, Pagnoux C, Weizman AV, et al. Emergence of severe spondyloarthropathy-related entheseal pathology following successful vedolizumab therapy for inflammatory bowel disease. Rheumatology (2019) 58:963–8. doi: 10.1093/rheumatology/key267
67. Fleisher M, Marsal J, Lee SD, Frado LE, Parian A, Korelitz BI, et al. Effects of vedolizumab therapy on extraintestinal manifestations in inflammatory bowel disease. Digest Dis Sci (2018) 63:825–33. doi: 10.1007/s10620-018-4971-1
68. O’Toole A, Lucci M, Korzenik J. Inflammatory bowel disease provoked by etanercept: report of 443 possible cases combined from an IBD referral center and the FDA. Digest Dis Sci (2016) 61:1772–4. doi: 10.1007/s10620-015-4007-z
69. Hohenberger M, Cardwell LA, Oussedik E, Feldman SR. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatol Treat (2018) 29:13–8. doi: 10.1080/09546634.2017.1329511
70. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity (2015) 43:727–38. doi: 10.1016/j.immuni.2015.09.003
71. Mease PJ, Kellner H, Morita A, Kivitz AJ, Papp KA, Aslanyan S, et al. OP0307 Efficacy and safety of risankizumab, a selective il-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: results from a phase 2 trial. Ann Rheum Dis (2018) 77:200–1. doi: 10.1136/annrheumdis-2018-eular.2140
72. McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheumatism (2007) 56:2482–91. doi: 10.1002/art.22758
73. Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol (2014) 32:121–55. doi: 10.1146/annurev-immunol-032713-120216
74. Cuthbert RJ, Watad A, Fragkakis EM, Dunsmuir R, Loughenbury P, Khan A, et al. Newton, Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis (2019) 78(11):1559–65. doi: 10.1136/annrheumdis-2019-215210
75. Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut (2012) 61:1693–700. doi: 10.1136/gutjnl-2011-301668
76. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med (2015) 373:2534–48. doi: 10.1056/NEJMoa1505066
77. Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat Rev Immunol (2019) 19:643–57. doi: 10.1038/s41577-019-0191-y
78. Cole S, Simpson C, Okoye R, Griffiths M, Baeten D, Shaw S, et al. OP0302 Mucosal-Associated Invariant T (MAIT)-Cell-Derived IL-17A and IL-17F Production IS IL-23-Independent and Biased TowardS IL-17F. BMJ Publishing Group Ltd (2019). doi: 10.1136/annrheumdis-2019-eular.1914
79. Watad A, Rowe H, Russell T, Zhou Q, Anderson LK, Khan A, et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis (2020) 79(8):1044–54. doi: 10.1136/annrheumdis-2020-219047
80. Baeten D, Østergaard M, Wei JC, Sieper J, Järvinen P, Tam LS, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis (2018) 77:1295–302. doi: 10.1136/annrheumdis-2018-213328
81. Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol (2019) 71:258–70. doi: 10.1002/art.40728
82. van Tok MN, Satumtira N, Dorris M, Pots D, Slobodin G, van de Sande MG, et al. Innate Immune Activation Can Trigger Experimental Spondyloarthritis in HLA-B27/Huβ2m Transgenic Rats. Front Immunol (2017) 8:920. doi: 10.3389/fimmu.2017.00920
83. Taurog JD, Dorris ML, Satumtira N, Tran TM, Sharma R, Dressel R, et al. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheumatism (2009) 60:1977–84. doi: 10.1002/art.24599
84. Savage L, Goodfield M, Horton L, Watad A, Hensor E, Emery P, et al. Regression of Peripheral Subclinical Enthesopathy in Therapy-Naive Patients Treated With Ustekinumab for Moderate-to-Severe Chronic Plaque Psoriasis: A Fifty-Two–Week, Prospective, Open-Label Feasibility Study. Arthritis Rheumatol (2019) 71:626–31. doi: 10.1002/art.40778
85. Bridgewood C, Alase A, Watad A, Wittmann M, Cuthbert R, McGonagle D. The IL-23p19/EBI3 heterodimeric cytokine termed IL-39 remains a theoretical cytokine in man. Inflamm Res (2019) 68:423–6. doi: 10.1007/s00011-019-01235-x
86. Detry S, Składanowska K, Vuylsteke M, Savvides SN, Bloch Y. Revisiting the combinatorial potential of cytokine subunits in the IL-12 family. Biochem Pharmacol (2019) 165:240–8. doi: 10.1016/j.bcp.2019.03.026
87. Khanna R, Afif W. Ustekinumab for Crohn’s Disease. Gastroenterology (2017) 152:1616–9. doi: 10.1053/j.gastro.2017.03.038
88. Kulig P, Musiol S, Freiberger SN, Schreiner B, Gyülveszi G, Russo G, et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun (2016) 7:13466. doi: 10.1038/ncomms13466
89. Castillo-Gallego C, Aydin SZ, Emery P, McGonagle DG, Marzo-Ortega H. Magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheumatism (2013) 65:2274–8. doi: 10.1002/art.38050
Keywords: IL-23, psoriatic arthritis, ankylosing spondylitis, enthesis, IL-17
Citation: McGonagle D, Watad A, Sharif K and Bridgewood C (2021) Why Inhibition of IL-23 Lacked Efficacy in Ankylosing Spondylitis. Front. Immunol. 12:614255. doi: 10.3389/fimmu.2021.614255
Received: 05 October 2020; Accepted: 18 January 2021;
Published: 19 March 2021.
Edited by:
Lars Rogge, Institut Pasteur, FranceReviewed by:
Daniel Wendling, Centre Hospitalier Universitaire de Besançon, FranceSaikat Majumder, University of Pittsburgh, United States
Copyright © 2021 McGonagle, Watad, Sharif and Bridgewood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dennis McGonagle, RC5HLk1jR29uYWdsZUBsZWVkcy5hYy51aw==
 Dennis McGonagle
Dennis McGonagle Abdulla Watad
Abdulla Watad Kassem Sharif
Kassem Sharif Charlie Bridgewood
Charlie Bridgewood