- 1Division of General Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 2Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Ferrara, Italy
- 3Department of Obstetrics, Gynecology and Reproductive Biology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Human herpesviruses 6A (HHV-6A) and human herpesvirus 6B (HHV-6B)—collectively, HHV-6A/B—are recently-discovered but ancient human viruses. The vast majority of people acquire one or both viruses, typically very early in life, producing an ineradicable lifelong infection. The viruses have been linked to several neurological, pulmonary and hematological diseases. In early human history, the viruses on multiple occasions infected a germ cell, and integrated their DNA into a human chromosome. As a result, about 1% of humans are born with the full viral genome present in every cell, with uncertain consequences for health. HHV-6A may play a role in 43% of cases of primary unexplained infertility. Both the inherited and acquired viruses may occasionally trigger several of the factors that are important in the pathogenesis of preeclampsia. Transplacental infection occurs in 1-2% of pregnancies, with some evidence suggesting adverse health consequences for the child. While emerging knowledge about these viruses in reproductive diseases is not sufficient to suggest any changes in current practice, we write this review to indicate the need for further research that could prove practice-changing.
Introduction
Human herpesvirus-6A (HHV-6A) and human herpesvirus-6B (HHV-6B)—collectively, HHV-6A/B—are ancient human viruses that were discovered only about 30 years ago (1, 2). An expanding group of human illnesses have been definitively or provisionally linked to the viruses (3, 4).
Recently, studies have indicated that HHV-6A may be one cause of unexplained primary infertility and that both HHV-6A and HHV-6B may, in some cases, contribute to the pathogenesis of preeclampsia (PE). If the viruses play a role in these conditions, it may be due to their ability to infect endometrial epithelial cells, placental cells, natural killer (NK) cells and the endothelial cells of myometrial spiral arteries—and due to the immune response to infection. Both viruses also can cause transplacental infection of the newborn.
We will briefly describe the biology of these viruses. Then, we will describe what is known about their relationship to primary infertility and to PE, as well as what is known about transplacental congenital infection of newborns and its possible health consequences.
Biology of HHV-6A and -6B
HHV-6A and HHV-6B are betaherpesviruses, members of the Roseolovirus genus, as described in more detail elsewhere (3, 5–7). Initial infection with HHV-6B occurs in early childhood, and somewhat later for HHV-6A. Infection of the respiratory tract, including the tonsils and olfactory-ensheathing cells of the nasal cavity, is the primary route of infection (5, 8). Horizontal transmission, particularly from adult to child, is likely (7). Primary infection from breast feeding or blood transfusions has not been reported (3).
HHV-6A/B infects a wide variety of cells and tissues including: 1) multiple immune system cells—including CD4+ T cells, CD8+ T cells and NK cells; 2) multiple cells of the nervous system—astrocytes, microglial cells, oligodendrocytes and neuronal cells; 3) and cells of other tissues—liver cells, human fibroblasts, epithelial cells and endothelial cells (4, 6). The viruses also can infect multiple cells of the reproductive tract (9), as will be discussed shortly.
Over 95% of adults are infected with HHV-6B. A smaller but substantial fraction are infected with HHV-6A. As with all herpesviruses, the infections are permanent: the viruses establish latency—a state in which they cannot be eradicated and from which they can periodically reactivate (7).
Infection with HHV-6B in early childhood can cause roseola infantum (exanthem subitem) (10), cause minimal symptoms or be asymptomatic. HHV-6B causes encephalitis/encephalopathy and delirium in people undergoing hematopoietic stem cell transplantation (11–14). The viruses are a common trigger for febrile seizures in young children, including febrile status epilepticus (15–21). As summarized in detail elsewhere, they may be one trigger of mesial temporal lobe epilepsy; multiple sclerosis; drug rash with eosinophilia and systemic symptoms (DRESS); and Hashimoto’s thyroiditis (4).
When the viruses are acquired by a person early in life, they permanently infect a small number of somatic cells, inserting their full genomes into the telomeric region of a host cell’s chromosomes, through molecular mechanisms recently identified (22–24).
Remarkably, and of potential importance in reproductive disease, on multiple occasions in human history, both viruses inserted their genomes into a human germ cell. The earliest known occurrence of this appears to have been between 85,000-342,000 years ago, in Africa (25). Consequently, about 1% of the human race is born with the entire viral genome inside every cell—a condition called inherited chromosomally-integrated HHV-6A/B (iciHHV-6A/B) (22, 26, 27). This inherited viral genome can be transcriptionally active, producing viral proteins and even full virions. Diagnosis of iciHHV-6A/B can be made by viral load studies in whole blood that exceed 5.5 log10 copies/ml (26). Investigators have begun to explore the health consequences of iciHHV-6A/B (28).
The infection of somatic cells by acquired virus, and the ancient infection of a germ cell leading to an inherited viral genome, are summarized in Figure 1.
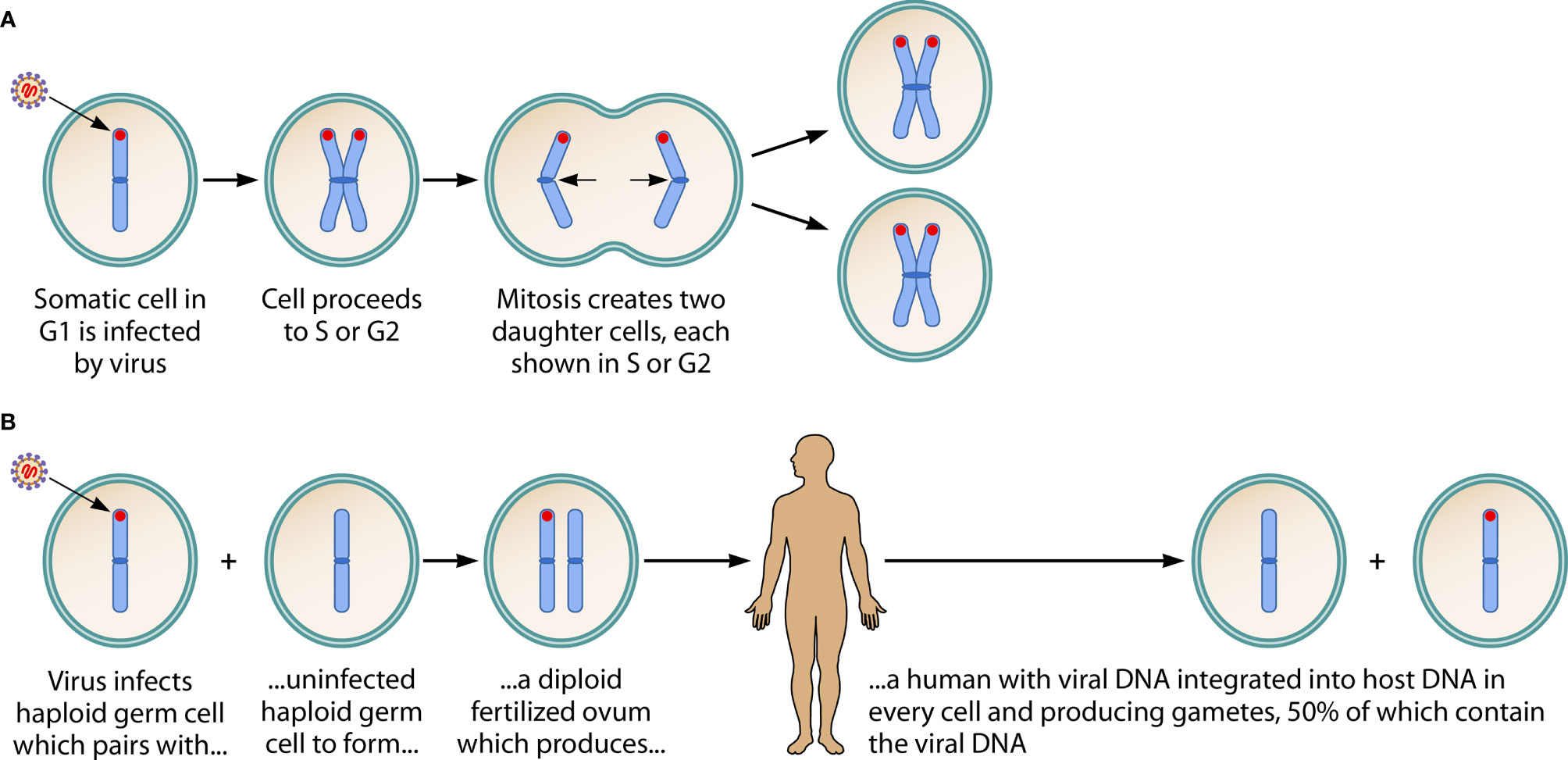
Figure 1 Consequences of HHV-6A/B DNA integration into a chromosome of a somatic cell, and into the host chromosome of a germ cell. (A) Shows acquired virus integrating its DNA (in red) into the telomere of a chromosome of a somatic cell. It has been hypothesized that this is a mechanism by which HHV-6A/B achieves latency. Since the viral genome is not integrated into the DNA of sperm or ova, no vertical transmission of the viral genome occurs. (Note: This figure shows only one of the 23 chromosomes, in early metaphase, and assumes viral infection and integration occurs in the G1 phase, then is replicated in the S phase and passed to both of the daughter cells during mitosis. When the integration event occurs during S or G2 phases—not shown in the figure—the daughter cells create a mosaic, since one contains the viral genome and the other does not.) (B) Describes an ancient event: on several occasions in human history, the viral genome integrated into the DNA of a haploid germ cell chromosome. This led to a fertilized ovum containing the viral genome and, hence, to a human with the viral genome integrated into a chromosome in every cell: inherited chromosomally-integrated HHV-6 (iciHHV-6). In Mendelian fashion, the integrated viral DNA is present in 50% of gametes (whether sperm or ova). About 1% of humans are born with iciHHV-6. The inherited presence of the viral genome in every cell, including germ cells, contrasts with the integration of acquired virus into only a small fraction of target somatic cells. Adapted from, and reprinted with permission of the publisher, from: Komaroff AL, Pellett PE, Jacobson S. Human herpesvirus 6A and 6B in brain diseases: Association vs. causation. Clinical Microbiology Reviews. 2021; 34:e00143-20. https://doi.org/10.1128/CMR.00143-20.
HHV-6A/B in Reproductive Organs
Prevalence of HHV-6A/B
HHV-6A and -6B can be found in the vaginal canal, cervix, and the uterus (29, 30). HHV-6A/B DNA has been detected in genital tract secretions from about 4% of non-pregnant women (30–32) and about 2-18% of pregnant women (29, 32–34), with higher viral loads in pregnant women (32, 34). It also is detected in about 10% of women attending a sexually transmitted disease clinic (31). In the great majority of cases, the infection is acquired rather than inherited.
Cellular Targets of HHV-6A/B
Infection of Endometrial, Cervical and Placental Cells
Most pertinent to their possible role in reproductive diseases, HHV-6A can infect endometrial cells (35) and syncytiotrophoblasts (35, 36). The virus reaches the reproductive organs via the circulation (primarily, infected lymphocytes). It is unclear if the virus can be transmitted sexually. The viruses also can infect cervical carcinoma cell lines, and have been identified along with human papillomavirus as a coinfecting agent in some cervical carcinoma tissue (37).
Infection of Endometrial NK Cells
HHV-6A/B infection of immune system cells can lead to the production of various chemokines (38) and chemokine receptors (6) as well as pro-inflammatory cytokines including IL-1β, TNF-α, IF-α, IF-γ and IL-6 (39–44). It also can decrease the expression of anti-inflammatory cytokines such as IL-10 (45).
The NK cell, in particular, plays a potentially important role in both primary infertility and PE. NK cells are the dominant lymphocytes in the endometrium. HHV-6A (and -6B) can infect both an NK cell line (46) as well as primary NK cells (47).
In a non-pregnant woman, when endometrial epithelial cells are infected by HHV-6A, the NK cytotoxic attack is severe, leading to the production of proinflammatory cytokines that inhibit implantation (47). Thus, viral infection of endometrial NK cells is a plausible contributor to the pathogenesis of primary infertility.
In a pregnant woman, in contrast, NK cells are less prone to attack “foreign” antigens. When NK cell receptors recognize human leucocyte antigen-E (HLA-E) and HLA-G antigens on cytotrophoblasts, the NK cells do not attack cells containing paternal antigens, facilitating implantation (48). Theoretically, HHV-6A/B infection of NK cells could disrupt the down-regulation of the NK cell attack on cytotrophoblasts, contributing to defective implantation and to the pathogenesis of both primary unexplained infertility and PE.
Infection of Endothelial Cells
Finally, HHV-6A/B also can infect endothelial cells (49–51), presumably including the endothelial cells of the myometrial spiral arteries, impairing implantation. It is important that this possibility be investigated.
Primary Unexplained Infertility
Evidence Linking HHV-6A to Infertility
Investigators examined endometrial biopsy tissues from 30 women with unexplained primary infertility (no evidence of endometriosis, endometritis, recurrent miscarriage, ovulatory dysfunction or anatomical uterine pathologies) and 36 fertile women with at least one previous successful pregnancy. The two groups were similar with regard to age, length of menstrual cycle, smoking habits, and levels of FSH, LH, TSH, FT4 and progesterone (9).
HHV-6A (but not HHV-6B) DNA was found in the endometrial epithelial cells of 43% of women with primary unexplained infertility vs. none of the fertile controls, a highly significant difference (P<0.00001). In vitro studies demonstrated that endometrial NK cells in the infertile, infected women attacked endometrial epithelial cells that were infected with the virus (9).
Pathobiology of Infertility
One likely cause of primary unexplained infertility is defective endometrial receptivity (52, 53). Inflammatory changes in the endometrium and/or in the placenta, triggered by infection, theoretically could inhibit implantation. Indeed, viral agents long have been postulated as possible environmental factors in infertility (54, 55).
Endometrial HHV-6A Infection
HHV-6A infection of endometrial epithelial cells, endometrial NK cells (46, 47) and trophoblast cells (35, 36) promotes changes that can impair implantation. Indeed, women with primary unexplained infertility and endometrial HHV-6A infection have higher levels of pro-inflammatory cytokines in uterine washings (56).
HHV-6A infection of the endometrium reduces levels of two decidualization markers, soluble human leucocyte antigen-G (sHLA-G) and mucin1 (48, 57), possibly augmenting the maternal immune response against paternally-derived fetal antigens (56). Infection of endometrial epithelial cells also generates a pattern of microRNA expression that has been linked to implantation failure (35).
HHV-6A infection of the endometrium in women with primary unexplained infertility is seen more often in women with a particular polymorphism for an ATP-gated ion channel, P2X7R. An antagonist of P2X7R has been shown to reduce the infectability of target cells by HHV-6A, and to reduce its replication, in vitro (56).
HHV-6A infection of endometrial epithelial cells inhibited the ability of a human choriocarcinoma trophoblast cell line to attach to endometrial cells (35). Although HHV-6A also can infect syncytiotrophoblast cells in vitro (35, 36), it has not been demonstrated that such infection occurs in vivo, and influences implantation.
Summary
HHV-6A infection of both endometrial epithelial cells, endometrial NK cells and, possibly, trophoblasts may be one trigger of primary unexplained infertility, through multiple different mechanisms that may impair implantation. Further research is required to determine if therapeutic interventions directed at blocking the pathobiology produced by endometrial HHV-6A infection—for example, antiviral therapy—improve prognosis in women with primary unexplained infertility, particularly in those with documented endometrial infection. If antiviral therapy improved prognosis, it would strongly suggest an etiologic role for HHV-6A.
HHV-6A/B and Preeclampsia (PE)
Evidence Linking HHV-6A/B to Some Cases of Preeclampsia
Investigators from the University of Cambridge looked for non-human messenger RNA (any RNA virus or replicating DNA virus) in placental samples from 99 cases of PE [according to ACOG 2013 criteria (58)], 48 cases of fetal growth retardation and 132 healthy pregnancy controls (59). Since the study involved placentas obtained at term, it could not assess whether active viral infection earlier in the course of a pregnancy might contribute to the etiology of PE. Importantly, the investigators had no prior hypothesis about any particular virus that might be linked to PE: they used nucleic acid sequencing to detect any known human virus present.
The analysis revealed only two viruses to be correlated with PE: HHV-6A/B. Quantitation of the viral DNA, and study of the DNA from both parents, confirmed that in 70% of the positive cases the viral genes had been inherited from either the mother or father (iciHHV-6A/B), and that in 30% of cases the virus had been acquired from the mother. Analysis of the inherited DNA sequence and the mRNA sequences found in the placenta confirmed that the inherited viral DNA was transcribing the viral mRNA found in the placenta (59).
The investigators then sought to replicate their findings in two larger datasets. In the first, the presence of iciHHV-6A/B was determined in cord blood DNA from 368 pregnancies complicated by PE and 3,674 pregnancies without PE. When these subjects were combined with the original cases and controls, iciHHV-6A/B was found in 2.1% of cases vs. 0.8% of controls (OR 2.8, P=0.008) (59).
The investigators then studied cord blood from 740 new cases of PE and compared the incidence of iciHHV-6A/B in these cases to the incidence in a meta-analysis of several large-scale population studies involving 61,549 subjects. The incidence in cases and controls was 1.6% vs. 0.7% (OR 2.5, P=0.001) (59).
If iciHHV-6A/B increases the probability of PE, one might expect it also to increase the probability of spontaneous abortion—through some of the mechanisms discussed below. Indeed, a recent report of 23 women with iciHHV-6A/B compared to 285 matched controls without iciHHV-6A/B finds a significantly increased odds ratio of spontaneous abortion (OR 6.41, 95%CI 1.10-37.4) (60). Theoretically, iciHHV-6A/B also might increase the probability of intrauterine growth retardation, a possibility that requires investigation.
Hence, iciHHV-6A/B—and, possibly, acquired infection with HHV-6A/B—appear over-represented among cases of PE, and may therefore predispose to PE. At the same time, HHV-6A/B are found only in a small fraction of cases—at least as determined by tissue (placentas, cord blood) obtained at term. Since studies of the virus in endometrial tissue earlier in pregnancy might conclude the viruses are linked to a larger fraction of cases, this possibility should be investigated.
Pathobiology of PE
If acquired or inherited HHV-6A/B increases the risk of PE, they most likely do so through one or more of the many well-established risk factors for PE. Indeed, it is plausible that HHV-6A/B could affect several of those risk factors.
Abnormal Placentation
Whereas implantation is prevented or ended prematurely in infertility, it is allowed but distorted in miscarriage and PE (53). While abnormal placentation may not be a necessary first step in the pathogenesis of PE, it may well be a contributing factor in women with other risk factors for PE—particularly hypertension, diabetes or obesity (61).
Defective placentation may lead to placental ischemia due to a failure of proliferating cytotrophoblasts to penetrate the decidua, invade the myometrium, and remodel the myometrial spiral arteries into the wide, low-pressure vascular channels necessary for a healthy pregnancy. While HHV-6A/B can infect syncytiotrophoblasts (35, 36), it is unclear if they can infect cytotrophoblasts [as can another betaherpesvirus, cytomegalovirus (62)], and thereby inhibit the capacity of cytotrophoblasts to form a robust villous architecture and to remodel myometrial spiral arteries.
Finally, as discussed earlier, HHV-6A/B infection of NK cells could also lead to defective implantation and consequent placental ischemia, contributing to the pathogenesis of PE.
Endothelial Dysfunction, Atherosclerosis, Thrombosis
There is systemic and placental dysfunction of the vascular endothelium in PE, manifested by impaired vasodilation (63–65), and increased reactivity to angiotensin II (66). Endothelial dysfunction, in turn, likely contributes to several key features of PE: hypertension, proteinuria, edema and coagulopathy. HHV-6A/B can infect endothelial cells, provoking endothelial dysfunction, atherosclerosis (49–51, 67) and the intravascular inflammation (increased immune cellular infiltrates and cytokine levels) seen in PE (68). The relevance of endothelial infection by HHV-6A/B to PE requires further study.
Defective Angiogenesis
An excess of antiangiogenic forces relative to angiogenic forces is seen in PE. This is reflected by increased plasma levels of soluble endoglin (sEng) plus an increased ratio of soluble tms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PIGF), which are strongly predictive of PE (68–70). As of now, it is not clear whether and how HHV-6A/B might contribute to defective angiogenesis, beyond its apparent ability to promote endothelial dysfunction.
Genetic Factors in PE
Twin studies find that PE has a heritability range from 22% to 47% (68), and that the inherited vulnerability may derive either from the maternal or paternal side.
For decades, the literature on PE has spoken of “dangerous fathers”: 1) men associated with one previous pre-eclamptic pregnancy who are more likely to be associated with a future pre-eclamptic pregnancy with a new partner (61); and 2) men born from a PE pregnancy, whose future spouse(s) are at higher risk of developing PE (61). Some of the risk from “dangerous fathers” comes from fathers with thrombophilias or polymorphisms in the IGF2 gene; however, these factors are not found in all “dangerous fathers”, indicating that other factors also may be involved (61).
Thus, the University of Cambridge study raises the question of whether one of those as-yet unidentified factors producing “dangerous fathers” may be iciHHV-6A/B: the greatly increased risk of PE seen in that study was the same whether the carrier of iciHHV-6A/B was the mother or the father (59).
HHV-6A/B Congenital Infection
HHV-6A/B can cause “congenital HHV-6A/B infection”, as defined by the presence of HHV-6A/B DNA in cord blood or placental tissues (71). About 1-2% of normal neonates have congenital HHV-6A/B infections; in 10% of congenital HHV-6A/B infections there are elevated viral loads in the infant’s blood; one third are due to HHV-6A (30, 72–74).
Congenital HHV-6A/B infection can occur in three ways: 1) iciHHV-6A/B in a parent is passed to the infant, with the infants inheriting the viral genome in every cell—which is the cause of 86% of cases of congenital infection; 2) in a mother with iciHHV-6A/B, the inherited viral genome produces viruses that are passed transplacentally to the infant—even though the infant does not have iciHHV-6A/B (i.e., the haploid oocyte that produced the infant did not contain the integrated viral genome)—which is the cause of about 10% of cases of congenital infection; 3) the mother has acquired (not inherited) the virus and then passed it transplacentally to the infant—which is the cause of about 4% of cases of congenital infection, as occurs with cytomegalovirus (74, 75).
While it now is well established that congenital infection with HHV-6A/B occurs, it is uncommon. More important, it is unclear whether congenital HHV-6A/B infection affects the health of the baby (or mother) (74). One study did find that children with transplacental infection subsequently have significantly lower scores on the Bayley Scale of Infant Development MDI instrument (76), but additional studies of developmental and other health outcomes are needed.
Conclusion
Human herpesviruses-6A and -6B (HHV-6A/B) are capable of infecting a remarkably wide range of cells and tissues, and are being linked to an increasing number of diseases (4). We summarize evidence that they may be linked to reproductive diseases, as are multiple other viruses (e.g., rubella virus, cytomegalovirus (54, 62).
One study has found endometrial infection by HHV-6A in 43% of cases of primary unexplained infertility vs. 0% of fertile, well-matched control subjects, a highly significant difference (9). The effects of the virus on the endometrial epithelium, on endometrial NK cells and possibly trophoblasts make the observed association plausible, and suggest the viruses could plausibly play a role in spontaneous abortion and intrauterine growth retardation.
The inherited form of infection (iciHHV-6A/B), and possibly the acquired form, are associated with PE, although only in a small fraction of cases. Effects of the viruses on placentation and endothelial function that could contribute to PE require further study, as well as whether the inherited form of the virus explains some cases of “dangerous fathers” more likely to be associated with cases of PE.
Finally, we have summarized how congenital infection can occur in 1-2% of neonates: the possible consequences for the health of the baby or mother warrant further study.
In short, we seek to alert readers to the possibility that these viruses can sometimes contribute to the pathogenesis of several reproductive diseases. Our emerging knowledge of the viruses does not justify any changes in current diagnostic testing and treatment practices for primary unexplained infertility, preeclampsia or congenital infection. However, the available evidence is provocative enough to justify further research.
Author Contributions
RR reviewed all of the information (text and references) about the virology of HHV-6A/B and the information about endometrial infection and primary unexplained infertility. JE reviewed all the information about preeclampsia (text and references). AK drafted the manuscript, reviewed all the references, and integrated the editorial contributions of RR and JE. All authors contributed to the article and approved the submitted version.
Funding
The HHV-6 Foundation supported the costs of publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Jill Mazzetta for her invaluable help in identifying, retrieving, and cataloguing the large literature we reviewed. We are grateful to Kristin Loomis and Dharam Ablashi, DVM: through the HHV-6 Foundation, they have stimulated research on human herpesviruses 6A and 6B, and have supported publication of this work. Patrick Lane, Sceyence Studios, created Figure 1.
References
1. Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science (1986) 234:596–601. doi: 10.1126/science.2876520
2. Lopez C, Pellett P, Stewart J, Goldsmith C, Sanderlin K, Black J, et al. Characteristics of human herpesvirus-6. J Infect Dis (1988) 157:1271–3. doi: 10.1093/infdis/157.6.1271
3. Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev (2015) 28(2):313–35. doi: 10.1128/CMR.00122-14
4. Komaroff AL, Pellett PE, Jacobson S. Human herpesviruses 6A and 6B in brain diseases: Association vs. causation. Clin Microbiol Rev (2021) 34(1):e00143–20. doi: 10.1128/CMR.00143-20
5. Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clin Microbiol Rev (1997) 10(3):521–67. doi: 10.1128/CMR.10.3.521
6. De Bolle L, Naesens L, de Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev (2005) 18(1):217–45. doi: 10.1128/CMR.18.1.217-245.2005
7. Yamanishi K, Mori Y, Pellett PE. Human herpesviruses 6 and 7. In: Knipe DM, Howley PM, editors. Fields Virology, 6th Edition, vol. 2 . Philadelphia: Wolters Klewer Health/Lippincott Williams & Wilkins (2013). p. 2059–111.
8. Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, et al. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci U S A (2011) 108(33):13734–9. doi: 10.1073/pnas.1105143108
9. Marci R, Gentili V, Bortolotti D, Lo Monte G, Caselli E, Bolzani S, et al. Presence of HHV-6A in endometrial epithelial cells from women with primary unexplained infertility. PloS One (2016) 11(7):e0158304. doi: 10.1371/journal.pone.0158304
10. Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet (1988) 1:1065–7. doi: 10.1016/S0140-6736(88)91893-4
11. Zerr DM, Gooley TA, Corey L, Anasetti C, Huang ML, Carpenter P, et al. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin Infect Dis (2001) 33(6):763–71. doi: 10.1086/322642
12. Hill JA, Venna N. Human herpesvirus 6 and the nervous system. In: Tselis AC, Booss J, editors. Neurovirology. Handbook of Clinical Neurology, vol. 123. Amsterdam: Elsevier (2014). p. 327–55.
13. Zerr DM, Komaroff AL. Cognitive dysfunction from HHV-6A and HHV-6B. In: Flamand L, Lautenschlager I, Krueger GRF, Ablashi DV, editors. Human Herpesviruses HHV-6A, HHV-6B and HHV-7: Diagnosis and Clinical Management, 3rd ed. Oxford, U. K: Elsevier (2014). p. 99–143.
14. Eliassen E, Hemond CC, Santoro JD. HHV-6-associated neurological disease in children: epidemiologic, clinical, diagnostic, and treatment considerations. Pediatr Neurol (2020) 105:10–20. doi: 10.1016/j.pediatrneurol.2019.10.004
15. Kondo K, Nagafuji H, Hata A, Tomomori C, Yamanishi K. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis (1993) 167:1197–200. doi: 10.1093/infdis/167.5.1197
16. Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med (1994) 331:432–8. doi: 10.1056/NEJM199408183310703
17. Barone SR, Kaplan MH, Krilov LR. Human herpesvirus-6 infection in children with first febrile seizures. J Pediatr (1995) 127(1):95–7. doi: 10.1016/S0022-3476(95)70263-6
18. Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child (2005) 90(6):619–23. doi: 10.1136/adc.2004.062216
19. Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol (2006) 35(3):165–72. doi: 10.1016/j.pediatrneurol.2006.06.004
20. Laina I, Syriopoulou VP, Daikos GL, Roma ES, Papageorgiou F, Kakourou T, et al. Febrile seizures and primary human herpesvirus 6 infection. Pediatr Neurol (2010) 42(1):28–31. doi: 10.1016/j.pediatrneurol.2009.07.016
21. You SJ. Human Herpesvirus-6 may be neurologically injurious in some immunocompetent children. J Child Neurol (2020) 35(2):132–6. doi: 10.1177/0883073819879284
22. Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A (2010) 107:5563–8. doi: 10.1073/pnas.0913586107
23. Gilbert-Girard S, Gravel A, Collin V, Wight DJ, Kaufer BB, Lazzerini-Denchi E, et al. Role for the shelterin protein TRF2 in human herpesvirus 6A/B chromosomal integration. PloS Pathog (2020) 16(4):e1008496. doi: 10.1371/journal.ppat.1008496
24. Wight DJ, Aimola G, Aswad A, Jill Lai CY, Bahamon C, Hong K, et al. Unbiased optical mapping of telomere-integrated endogenous human herpesvirus 6. Proc Natl Acad Sci U S A (2020) 117(49):31410–6. doi: 10.1073/pnas.2011872117
25. Aswad A, Aimola G, Wight D, Roychoudhury P, Zimmermann C, Hill J, et al. Evolutionary history of endogenous human herpesvirus 6 reflects human migration out of Africa. Mol Biol Evol (2020) 38:96–107. doi: 10.1093/molbev/msaa190
26. Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, et al. Chromsomally integrated human herpesvirus 6: questions and answers. Rev Med Virol (2012) 22(3):144–55. doi: 10.1002/rmv.715
27. Kaufer BB, Flamand L. Chromosomally integrated HHV-6: impact on virus, cell and organismal biology. Curr Opin Virol (2014) 9C:111–8. doi: 10.1016/j.coviro.2014.09.010
28. Gravel A, Dubuc I, Morissette G, Sedlak RH, Jerome KR, Flamand L. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc Natl Acad Sci U S A (2015) 112(26):8058–63. doi: 10.1073/pnas.1502741112
29. Okuno T, Oishi H, Hayashi K, Nonogaki M, Tanaka K, Yamanishi K. Human herpesviruses 6 and 7 in cervixes of pregnant women. J Clin Microbiol (1995) 33(7):1968–70. doi: 10.1128/JCM.33.7.1968-1970.1995
30. Caserta MT, Hall CB, Schnabel K, Lofthus G, McDermott MP. Human herpesvirus (HHV)-6 and HHV-7 infections in pregnant women. J Infect Dis (2007) 196(9):1296–303. doi: 10.1086/522430
31. Leach CT, Newton ER, McParlin S, Jenson HB. Human herpesvirus 6 infection of the female genital tract. J Infect Dis (1994) 169(6):1281–3. doi: 10.1093/infdis/169.6.1281
32. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol (2000) 16(3):149–57. doi: 10.1016/S1386-6532(99)00086-4
33. Maeda T, Okuno T, Hayashi K, Nagata M, Ueda M, Terashima K, et al. Outcomes of infants whose mothers are positive for human herpesvirus-6 DNA within the genital tract in early gestation. Acta Paediatr Jpn (1997) 39(6):653–7. doi: 10.1111/j.1442-200X.1997.tb03662.x
34. Ohashi M, Yoshikawa T, Ihira M, Suzuki K, Suga S, Tada S, et al. Reactivation of human herpesvirus 6 and 7 in pregnant women. J Med Virol (2002) 67(3):354–8. doi: 10.1002/jmv.10083
35. Bortolotti D, Soffritti I, D’Accolti M, Gentili V, Di Luca D, Rizzo R, et al. HHV-6A infection of endometrial epithelial cells affects miRNA expression and trophoblast cell attachment. Reprod Sci (2020) 27(3):779–86. doi: 10.1007/s43032-019-00102-8
36. Csoma E, Bácsi A, Liu X, Szabó J, Ebbesen P, Beck Z, et al. Human herpesvirus 6 variant A infects human term syncytiotrophoblasts in vitro and induces replication of human immunodeficiency virus type 1 in dually infected cells. J Med Virol (2002) 67(1):67–87. doi: 10.1002/jmv.2194
37. Chen M, Wang H, Woodworth CD, Lusso P, Berneman Z, Kingma D, et al. Detection of human herpesvirus 6 and human papillomavirus 16 in cervical carcinoma. Am J Pathol (1994) 145(6):1509–16.
38. Reynaud JM, Jegou JF, Welsch J, Horvat B. Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via TLR9. J Virol (2014) 88(10):5421–36. doi: 10.1128/JVI.03763-13
39. Flamand L, Gosselin J, D’Addario M, Hiscott J, Ablashi DV, Gallo RC, et al. Human herpesvirus 6 induces interleukin-1ß and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol (1991) 65:5105–10. doi: 10.1128/JVI.65.9.5105-5110.1991
40. Takahashi K, Segal E, Kondo T, Mukai T, Moriyama M, Takahashi M, et al. Interferon and natural killer cell activity in patients with exanthem subitum. Pediatr Infect Dis J (1992) 11(5):369–73. doi: 10.1097/00006454-199205000-00006
41. Arena A, Capozza AB, Di Luca D. Role of IFN gamma on TNF alpha, IL-1 and IL-6 release during HHV-6 infection. New Microbiol (1996) 19:183–91.
42. Mayne M, Cheadle C, Soldan SS, Cermelli C, Yamano Y, Akhyani N, et al. Gene expression profile of herpesvirus-infected T cells obtained using immunomicroarrays: induction of proinflammatory mechanisms. J Virol (2001) 75(23):11641–50. doi: 10.1128/JVI.75.23.11641-11650.2001
43. Tallóczy Z, Virgin HW,4, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy (2006) 2(1):24–9. doi: 10.4161/auto.2176
44. Flamand L, Stefanescu I, Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest (1996) 97(6):1373–81. doi: 10.1172/JCI118557
45. Meeuwsen S, Persoon-Deen C, Bsibsi M, Bajramovic JJ, Ravid R, De Bolle L, et al. Modulation of the cytokine network in human adult astrocytes by human herpesvirus-6A. J Neuroimmunol (2005) 164(1-2):37–47. doi: 10.1016/j.jneuroim.2005.03.013
46. Rizzo R, Soffritti I, D’Accolti M, Bortolotti D, Di Luca D, Caselli E. HHV-6A/6B infection of NK cells modulates the expression of miRNAs and transcription factors potentially associated to impaired NK activity. Front Microbiol (2017) 8:2143. doi: 10.3389/fmicb.2017.02143
47. Bortolotti D, Gentili V, Caselli E, Sicolo M, Soffritti I, D’Accolti M, et al. DNA sensors’ signaling in NK cells during HHV-6A, HHV-6B and HHV-7 infection. Front Microbiol (2020) 11:226. doi: 10.3389/fmicb.2020.00226
48. Rizzo R, Di Luca D. Human herpesvirus 6A and 6B and NK cells. Acta Microbiol Immunol Hung (2018) 65(2):119–25. doi: 10.1556/030.65.2018.010
49. Rotola A, Di Luca D, Cassai E, Ricotta D, Giulio A, Turano A, et al. Human herpesvirus 6 infects and replicates in aortic endothelium. J Clin Microbiol (2000) 38(8):3135–6. doi: 10.1128/JCM.38.8.3135-3136.2000
50. Caruso A, Rotola A, Comar M, Favilli F, Galvan M, Tosetti M, et al. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory cytokines. J Med Virol (2002) 67(4):528–33. doi: 10.1002/jmv.10133
51. Caruso A, Caselli E, Fiorentini S, Rotola A, Prandini A, Garrafa E, et al. U94 of human herpesvirus 6 inhibits in vitro angiogenesis and lymphangiogenesis. Proc Natl Acad Sci U S A (2009) 106(48):20446–51. doi: 10.1073/pnas.0905535106
52. Lessey BA. Assessment of endometrial receptivity. Fertil Steril (2011) 96(3):522–9. doi: 10.1016/j.fertnstert.2011.07.1095
53. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril (2019) 111(4):611–7. doi: 10.1016/j.fertnstert.2019.02.009
54. Baecher-Lind LE, Miller WC, Wilcox AJ. Infectious disease and reproductive health: a review. Obstet Gynecol Surv (2010) 65(1):53–65. doi: 10.1097/OGX.0b013e3181c9e7a1
55. Drago F, Broccolo F, Javor S, Drago F, Rebora A, Parodi A. Evidence of human herpesvirus-6 and -7 reactivation in miscarrying women with pityriasis rosea. J Am Acad Dermatol (2014) 71(1):198–9. doi: 10.1016/j.jaad.2014.02.023
56. Pegoraro A, Bortolotti D, Marci R, Caselli E, Falzoni S, De Marchi E, et al. The P2X7 receptor 489C>T gain of function polymorphism favors HHV-6A infection and associates with female idiopathic infertility. Front Pharmacol (2020) 11:96. doi: 10.3389/fphar.2020.00096
57. Rizzo R, Vercammen M, van de Velde H, Horn PA, Rebmann V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci (2011) 68(3):341–52. doi: 10.1007/s00018-010-0578-1
58. ACOG. Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol (2013) 122(5):1122–31.
59. Gaccioli F, Lager S, de Goffau MC, Sovio U, Dopierala J, Gong S, et al. Fetal inheritance of chromosomally integrated human herpesvirus 6 predisposes the mother to pre-eclampsia. Nat Microbiol (2020) 5(7):901–8. doi: 10.1038/s41564-020-0711-3
60. Miura H, Kawamura Y, Ohye T, Hattori F, Kozawa K, Ihira M, et al. Inherited chromosomally integrated human herpesvirus 6 is a risk factor for spontaneous abortion. J Infect Dis (2020) 132:104656. doi: 10.1093/infdis/jiaa606
61. Dekker G, Robillard PY, Roberts C. The etiology of preeclampsia: the role of the father. J Reprod Immunol (2011) 89(2):126–32. doi: 10.1016/j.jri.2010.12.010
62. Gibson CS, Goldwater PN, MacLennan AH, Haan EA, Priest K, Dekker GA. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. BJOG (2008) 115(4):492–500. doi: 10.1111/j.1471-0528.2007.01653.x
63. Taylor RN, Crombleholme WR, Friedman SA, Jones LA, Casal DC, Roberts JM. High plasma cellular fibronectin levels correlate with biochemical and clinical features of preeclampsia but cannot be attributed to hypertension alone. Am J Obstet Gynecol (1991) 165(4):895–901. doi: 10.1016/0002-9378(91)90435-T
64. McCarthy AL, Woolfson RG, Raju SK, Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol (1993) 168(4):1323–30. doi: 10.1016/0002-9378(93)90389-Z
65. Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol (2008) 198(1):7–22. doi: 10.1016/j.ajog.2007.07.040
66. Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens (2001) 14(6 Pt 2):1785–855. doi: 10.1016/s0895-7061(01)02086-6
67. Caruso A, Favilli F, Rotola A, Comar M, Horejsh D, Alessandri G, et al. Human herpesvirus-6 modulates RANTES production in primary human endothelial cell cultures. J Med Virol (2003) 70(3):451–8. doi: 10.1002/jmv.10416
68. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol (2014) 10(8):466–80. doi: 10.1038/nrneph.2014.102
69. Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol (2014) 10(9):531–40. doi: 10.1038/nrneph.2014.103
70. Rana S, Salahuddin S, Mueller A, Berg AH, Thadhani RI, Karumanchi SA. Angiogenic biomarkers in triage and risk for preeclampsia with severe features. Pregnancy Hypertens (2018) 13:100–6. doi: 10.1016/j.preghy.2018.05.008
71. Aubin JT, Poirel L, Agut H, Huraux JM, Bignozzi C, Brossard Y, et al. Intrauterine transmission of human herpesvirus 6. Lancet (1992) 340(8817):482–3. doi: 10.1016/0140-6736(92)91801-E
72. Adams O, Krempe C, Kögler G, Wernet P, Scheid A. Congenital infections with human herpesvirus 6. J Infect Dis (1998) 178(2):544–6. doi: 10.1086/517470
73. Hall CB, Caserta MT, Schnabel KC, Boettrich C, McDermott MP, Lofthus GK, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr (2004) 145(4):472–7. doi: 10.1016/j.jpeds.2004.06.017
74. Hall CB, Caserta MT, Schnabel K, Shelley LM, Marino AS, Carnahan JA, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics (2008) 122(3):513–20. doi: 10.1542/peds.2007-2838
75. Hall CB, Caserta MT, Schnabel KC, Shelley LM, Carnahan JA, Marino AS, et al. Transplacental congenital human herpesvirus 6 infection caused by maternal chromosomally integrated virus. J Infect Dis (2010) 201(4):505–7. doi: 10.1086/650495
Keywords: congenital infection, human herpesvirus-6A, human herpesvirus-6B, inherited chromosomally integrated human herpesvirus 6, preeclampsia, primary unexplained infertility, spontaneous abortion, intrauterine growth retardation (IUGR)
Citation: Komaroff AL, Rizzo R and Ecker JL (2021) Human Herpesviruses 6A and 6B in Reproductive Diseases. Front. Immunol. 12:648945. doi: 10.3389/fimmu.2021.648945
Received: 11 February 2021; Accepted: 09 March 2021;
Published: 25 March 2021.
Edited by:
Linda F. Van Dyk, University of Colorado Denver, United StatesReviewed by:
Benedikt B. Kaufer, Freie Universität Berlin, GermanyYasuko Mori, Kobe University, Japan
Copyright © 2021 Komaroff, Rizzo and Ecker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony L. Komaroff, a29tYXJvZmZAaG1zLmhhcnZhcmQuZWR1
 Anthony L. Komaroff
Anthony L. Komaroff Roberta Rizzo
Roberta Rizzo Jeffrey L. Ecker
Jeffrey L. Ecker