- 1Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
- 2Department of Genetics, Medical Science Division, University of Oxford, Oxford, United Kingdom
The retinoid X receptor agonist bexarotene promotes remyelination in patients with multiple sclerosis. Murine studies have also demonstrated that RXR agonists have anti-inflammatory effects by enhancing the ability of all-trans-retinoic acid (atRA) to promote T-regulatory cell (Treg) induction and reduce Th17 differentiation in vitro. By stimulating human naïve CD4 T-cells in the presence of Treg or Th17 skewing cytokines, we show that bexarotene also tips the human Treg/Th17 axis in favor of Treg induction, but unlike murine cells this occurs independently of atRA and retinoic acid receptor signaling. Tregs induced in the presence of bexarotene express canonical markers of T-regulation and are functionally suppressive in vitro. Circulating Treg numbers did not increase in the blood of trial patients receiving bexarotene; we believe this is because Treg induction is likely to occur within tissues. These findings lend support to developing RXR agonists as treatments of autoimmune diseases, in particular multiple sclerosis.
Introduction
Bexarotene, a pan retinoid X receptor (RXR) agonist licensed for use in humans as a treatment of cutaneous T-cell lymphoma, has recently been shown to promote remyelination in multiple sclerosis (MS). Although the Cambridge Centre for Myelin Repair (CCMR)-one trial failed to meet its primary endpoint pre-planned analyses demonstrated that bexarotene promoted the remyelination of the most demyelinated lesions, particularly in grey matter (as determined radiologically). In addition, conduction in the visual pathway improved in patients randomized to bexarotene, providing further evidence of its pro-remyelinating effect (Reference Lancet Neurology manuscript).
The CCMR-One clinical trial largely came about because of work done in rodents, which demonstrated that oligodendrocytes express RXR (specifically RXR-γ) during active remyelination, and that remyelination can be promoted, in vitro and in vivo, following administration of RXR agonists (1).
Murine studies have also demonstrated that RXR agonists can enhance the ability of all-trans-retinoic acid (atRA), the primary active metabolite of vitamin A, to promote Treg induction (iTregs), and reduce Th17 differentiation in vitro, following stimulation of naïve CD4 cells in the presence of TGF- β (2). At physiological concentrations, atRA binds to the retinoic acid receptor (RAR) which is bound to DNA as a heterodimer with RXR in regions called retinoic acid response elements (RAREs). Binding of atRA to the RAR/RXR heterodimer leads to a conformational change, which in turn promotes down-stream transcription of hundreds of genes (3), including those involved in the induction of Tregs.
In contrast to atRA, RXR ligands are unable to promote the differentiation of murine naïve T cells to iTregs directly. Instead, they act synergistically with atRA to promote FOXP3 expression (2). In this way RXR is said to act as a conditionally permissive binding partner to RAR – that is, whilst RXR ligand binding is unable to trigger down-stream signaling, maximal receptor signaling and transcriptional activity is achieved when RAR and RXR ligands bind simultaneously (2, 3).
Here we sought to determine if the RXR agonist bexarotene, licensed for use in humans and recently shown to have pro-remyelinating effects in MS, has similar immunoregulatory effects on the human iTreg/Th17 axis.
Methods and Materials
Cell Preparation
Human PBMCs were isolated from the fresh buffy coats of whole blood of healthy donors by Ficoll-Paque Plus centrifugation (GE Healthcare, Sweden). PBMCs were counted by trypan blue exclusion and resuspended in PBS. Naive CD4+ T cells were either isolated through magnetic activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS). With MACS, naive T cells were isolated through negative selection from total PBMC using the naive CD4+ T Cell Isolation Kit, human (Miltenyi, 130-094-131), according to the manufacturer’s instructions. With FACS, naive CD3+CD4+CD127+CD25lowCD45RA+CD62L+CD27+CCR7+ were isolated from CD4+ enriched PBMCs following negative isolation using CD4+ T cell Isolation Kit, human (Miltenyi, 130-096-533), using a BD Influx (Supplementary Figure 1). Staining for FACS was done on cell surface markers, live/dead, CD3, CD4, CD127, CD25, CD62L, CD45RA, CD27 and CCR7 with relevant mAbs (BioLegend and BD). CD25high “nTregs” and CD3+CD4+CD127+CD45RA-CD62L- “Teffs” were also isolated from the same CD4+ enriched population and were cryopreserved in 10% DMSO (Sigma-Aldrich) and 90% FBS (Life Technologies, Thermo Fisher Scientific), for later use in suppression assays. Naive CD4+ T cells were cultured at 5% CO2/37°C in serum-free X-Vivo 15 medium (Lonza).
iTreg Differentiation
Naive CD4+ T cells were plated under iTreg differentiation conditions at 1.0x105 cells/well in 96 U-bottom well plates (Falcon). For cell stimulation, plates were coated at least 5 hours prior to use with 5 ug/ml plate-bound anti-CD3 antibody (clone OKT3; BioLegend, LEAF grade), which is then washed off with PBS, with 1 ug/ml soluble anti-CD28 antibody (BioLegend, LEAF grade) and 5 ng/ml IL-2 (carrier-free; Tocris R&D Systems) added with cells. Cells treated with only these functioned as the mock control. For iTreg induction, TGF- β (5ng/ml; carrier-free; Tocris R&D Systems), anti-IFN γ antibody (2 ug/ml; clone MD-1, Biolegend), bexarotene (1 µM, Abcam) or atRA (100 nM; Sigma-Aldrich) were added additionally. The DMSO control (for bexarotene, atRA and AGN190) had no effect on FOXP3 expression. Cells were incubated for 7 days unless otherwise stated.
Th17 Cell Differentiation
After either MACS or FACS isolation, naive cells were plated under Th17 differentiation conditions at 5x105 cells/well in 96 U-bottom well plates (Falcon). For cell stimulation, plates were coated at least 5 hours prior to use with 3 ug/ml plate-bound anti-CD3 antibody (clone OKT3; BioLegend, LEAF grade), 1 ug/ml soluble anti-CD28 antibody (BioLegend, LEAF grade) and 2 ng/ml IL-2 (carrier-free; Tocris R&D Systems). Cells treated with only these functioned as the mock control. For Th17 induction, TGF- β (30 ng/ml; carrier-free; Tocris R&D Systems), IL-6 (30 ng/ml; carrier-free; Tocris R&D Systems), IL-1 β (10 ng/ml; carrier-free; Tocris R&D Systems), IL-23 (50 ng/ml; carrier-free; Tocris R&D Systems), anti-IFN γ antibody (2 ug/ml; clone MD-1, Biolegend), anti-IL-4 antibody (2 ug/ml; clone MD-1, Biolegend), bexarotene (1 µM, Abcam), or atRA (100 nM; Sigma-Aldrich) were added additionally. The DMSO control (for bexarotene, atRA and AGN190) had no effect on IL-17A cytokine expression. Cells were incubated for 7 days unless otherwise stated.
In Vitro Suppression Assays
For use in suppression assays, on day 7 iTregs were washed twice in PBS and their viability and count assessed with trypan blue exclusion. Cells were then allowed to rest for 3 hours in fresh, serum free X-Vivo 15 media prior to addition into suppression assay cell culture at 5% CO2 at 37°C. The mock IL-2 treated control cells were also washed, counted, and rested in the same manner as for iTregs. Cryopreserved Teffs from donors were thawed quickly within a water bath at 37°C, washed and rested for 4 hours at 5% CO2 37°C. To discriminate between Teffs and Tregs in the suppression assay, Teffs were labelled with 5 mM cell proliferation dye EFV450, and iTregs were labelled with 5 mM cell proliferation dye BV670 (both from eBioscience). Teffs were plated at 5x104 cells per well in duplicate, with and without iTregs, in serum free X-Vivo 15 media alone. Cells were cultured for 3 days. For the final analysis, dead cells were excluded using dead cell exclusion staining (Zombie NIR; eBioscience), and cells were counterstained with Abs to CD4. Due to unknown suppressive capacity, iTregs were titrated up so that the iTreg/Teff ratio was 1:1 to 3:1 and 6:1. Duplicate control wells of CD4+ T cells without stimulus, and Tregs, at various dilutions, with and without stimulus, were also cultured. Treg Suppression Inspector beads (Miltenyi Biotec) were used to stimulate the assay, according to the manufacturer’s instructions.
Flow Cytometry and Antibodies
Viability Staining
Cells taken from culture were washed once with 1 mL PBS and resuspended in 100 ul PBS. Cells were then stained with LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) for 30 minutes, in the dark at 4°C. Cells were then washed twice with 1 mL of FACS buffer (0.5% BSA/PBS) and taken for subsequent cell surface marker staining.
Surface Staining
Cell surface staining was performed in the dark at 4°C in antibody dilutions in FACS buffer for 30 minutes, with a final staining volume of 100 ul. Cells were then washed twice with 1 mL PBS and either taken for flow cytometry analysis or used for subsequent intracellular staining
Cell Permeabilization and Intracellular Staining
Cell permeabilization and fixation was performed using the Foxp3 Staining Buffer Set (eBioscience), according to the manufacturer’s instructions at room temperature for 40 minutes. Intracellular staining was then performed, according to manufacturer’s instructions in the dark at 4°C. To measure intracellular cytokines, cells were stimulated for 4h prior to staining with 1x Cell Activation Cocktail (PMA, ionomycin, and Brefeldin A; 500X).
Antibodies (Anti-Human)
The complete list of antibodies used is as follows and were purchased from eBioscience, BD Biosciences or BioLegend, unless otherwise indicated. Brackets indicate (fluorochrome, antibody clone).
Sorting Panel
CD45RA (AF488; HI100), CCR7 (PerCP-Cy5.5; G043H7), Zombie Aqua (live/dead stain), CD27 (BV605; 323), CD3 (BV650; UCHT1), CD62L (BV786; DREG-56), FOXP3 (PE; 259D, 259D/C7, 206D), CD127 (PE-Cy7; eBioRDR5), CD25 (APC; M-A251) and CD4 (APC-R700; RPA-T4), and for bisulfite sequencing only - FOXP3 (PE; 259D, 259D/C7, 206D).
iTreg Staining
FOXP3 (PE; 259D), CD25 (APC, 2A3), CD3 (BV650; UCHT1), CD4 (APC-R700, RPA-T4), CD127 (PE-Cy7; eBioRDR5).
Th17 Staining
CD3 (BV650, UCHT1), CD4 (APC-R700, RPA-T4), CD25 (APC, 2A3), CD127 (PE-Cy7, eBioRDR5), IFN-y (BUV395; B27), IL-17 (PE, SCPL1362) IL-21 (BV421; 3A3-N2.1).
CCMR-One
FOXP3 (PE; 259D), CD25 (BV421, 2A3 and MA251), CCR6 (BV650, 11A9), CCR4 (BB700, 1G1), CD3 (BV605, SK7), CD45RA (BV785, HI100), HELIOS (FITC, 22F6), CD8 (PE-Cy5, RPA-T8), CD4 (APC, RPA-T4), HLA-DR (AFR-700, L243), T.S. monocyte blocker, CD127 (PE-Cy7, PCH101), CD27 (APC-eFluor 780, 0323), CCR4 (PerCP eFluor 710, D8SEE).
Acquisition
Acquisition was performed on a BD LSR Fortessa and compensation was performed manually using single stained color controls. Cell sorting was performed on BD Influx under sterile conditions. FACS data was analyzed using FlowJo (V10.1) and sort reports were collected for each cell sort experiment.
Enzyme-Linked Immunosorbent Assay (ELISA) Measurements
IL-17a ELISAs were performed using Human IL-17A (homodimer) ELISA Ready-SET-Go!™ Kit (Invitrogen™; eBioscience™), according to the manufacturer’s instructions. Plates were coated the day prior to beginning the ELISA and kept covered at 4°C.
Analysis of TSDR Methylation
CD4+ CD25+ Foxp3+ iTregs and CD4+ CD25+ Foxp3− non-Tregs were sorted by FACS from “bulk iTreg cultures” from 4 male and 4 female donors, then stored immediately as a dry frozen pellet for bisulfite sequencing. DNA of FACS sorted iTregs and control cells from female and male donors was isolated using the DNeasy Blood and Tissue Kit (Qiagen), according to the manufacturer’s instructions. Bisulfite sequencing was performed in house, as previously described (Rainbow et al., 2015). Six replicates of 3000 cells per replicate (5 ng DNA) were analyzed from a single donor and the median (with range) reads with eight or nine sites demethylated at FOXP3 for the replicates, is reported.
Statistical Analysis
All flow cytometry data were analyzed in-house with FlowJo (v10.1); all other data analyses and statistical fitting were performed in-house with GraphPad (Prism). Where appropriate, ANOVA and two-tailed t-tests were performed to assess for differences in Foxp3 protein expression between wells and experiments. One-way ANOVAs were corrected using Tukey’s multiple comparisons test. Repeated measures one-way ANOVAs and two-tailed t-tests were corrected using Šidák’s correction. The test and corresponding correction performed is indicated in each figure legend.
Study Approval
Healthy Controls
Peripheral blood mononuclear cells (PBMCs) were isolated from anonymous healthy donor buffy coats, which were obtained from the Addenbrooke’s Hospital NHS blood and transplant service (Cambridge, United Kingdom). All individuals gave written consent, and the study was approved by a local ethical review committee (REC: 11/EE/0007).
CCMR-One Patients
Individuals aged 18-50 years with relapsing remitting multiple sclerosis, stable on dimethyl fumarate, who were randomized on the CCMR-One study to bexarotene (300mg/m2 body surface area per day, given orally for 6 months) gave blood for research under REC 15/LO/0108. For full details of the CCMR-One protocol please refer to the published trial manuscript (Ref).
Results
Bexarotene Promotes the Induction of Human Tregs Independent of RAR Activation
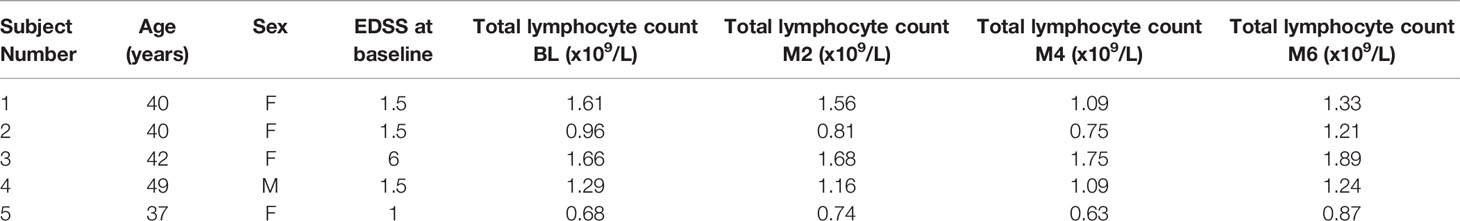
To determine the effect of bexarotene on human iTreg induction FACS-sorted naïve CD4 T conventional (Tconv) cells (CD3+CD4+CD127+CD25lowCD45RA+CD62L+CD27+CCR7+; Supplementary Figure 1) were cultured for 7 days in serum-free, and therefore atRA free media, under iTreg inducing conditions (anti-CD3/28 stimulation plus IL-2 and TGF-β) with and without the addition of bexarotene (1 µM), atRA (100nM) or both. As a negative control, naïve cells were also stimulated with anti-CD3/28 beads in the presence of IL-2 only (“IL-2 control”).
In contrast to what was expected from murine studies, bexarotene alone was sufficient to increase Treg induction as measured by percent FOXP3 expression (Figure 1A, from 21.52% to 39.25% p=0.0038). This increase was equivalent to that seen following the addition of atRA (41.29% p=0.0013). No synergistic effect between bexarotene and atRA was observed (Figure 1B). FOXP3 expression within the iTreg population was unaffected by culture conditions (Supplementary Figure 2). Cell viability was high (>90% for FOXP3+ cells, and >70% for FOXP3- cells) in the presence of bexarotene (1 µM) and/or atRA (100nM) and was equivalent to that seen in control iTreg conditions - confirming increased iTreg differentiation rather than selective cell loss (Supplementary Figure 3A).
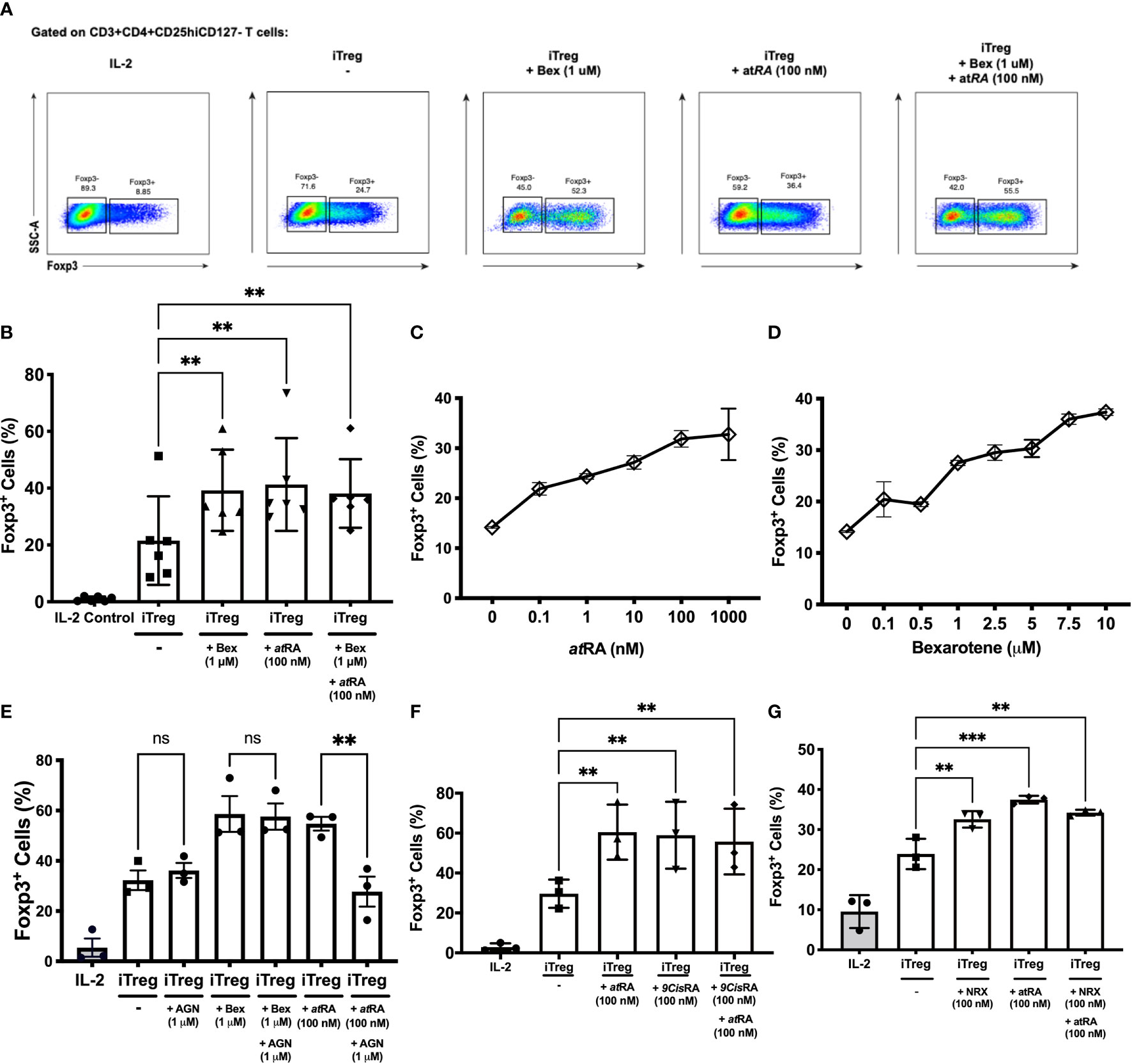
Figure 1 RXR agonists promote human iTreg differentiation independent of RAR signaling. (A) Gating strategy and example dot plots showing iTreg induction following culture of naïve T cells for 7 days in iTreg skewing conditions +/- bexarotene (1 μM), +/- atRA (100 nM) or both (B) Summary data for n=6 experiments (C, D) percent iTreg induction over a range of atRA and bexarotene concentrations. (E) Co-culture with the RAR antagonist AGN reverses increased iTreg induction seen in the presence of atRA, but not Bexarotene. (F, G) The RXR agonists 9CisRA and NRX194204 also promote human iTreg differentiation in vitro. Shown are mean values ± SEM. Significance calculated using a repeated measures one-way ANOVA. Šidák’s correction was performed. *p < 0.05, **p < 0.01, ***p < 0.001 ns, not significant.
Next a dose ranging experiment was performed which demonstrated that bexarotene and atRA caused a similar dose-dependent increase in iTreg induction, resulting in up to ~2.5 fold more FOXP3+ cells (Figures 1C, D). At concentrations above 20µM bexarotene inhibited iTreg differentiation and resulted in significant cell death (>95% dead; data not shown).
Although all assays were performed in serum-free media, and therefore atRA free conditions; to be certain that bexarotene was not driving Treg differentiation via a RAR dependent mechanism, the above assays were repeated following pre-incubation with AGN 193109, a potent and selective RAR antagonist (Figure 1E). Pre-incubation with AGN fully blocked the pro-iTreg inducing effects of atRA (% iTregs 54.78% vs 22.75%, p=0.0066, compared to iTreg conditions alone = 32.27%). In keeping with its RAR-independent mode of action, the addition of AGN had no effect on increased iTreg differentiation in the presence of bexarotene (% iTregs 58.63% vs 57.60%). AGN did not affect cell viability at the concentrations used (data not shown).
9-cisRA and the Synthetic RXR Agonist, NRX 194204 Also Promote Human iTreg Differentiation In Vitro
To determine if the effects seen were specific to bexarotene, or a generic effect of RXR agonism, FACS sorted naïve T-cells were cultured in serum-free media under iTreg inducing conditions (IL-2 and TGF-β) with and without the addition of a synthetic RXR agonist (NRX 194294) or 9-cisRA (a metabolite of Vitamin A, and the primary endogenous RXR agonist).
As seen with bexarotene, both 9-cisRA and NRX enhanced to the same degree naïve T-cell differentiation to iTregs independently of RAR agonism, and neither had a synergistic effect with atRA (% iTregs with 9-cisRA= 58.93, % iTregs with NRX= 32.56%; Figures 1F, G).
Bexarotene Induced Tregs Are Suppressive In Vitro
In addition to expressing FOXP3, the regulatory phenotype of the iTregs was confirmed through suppression assays. In brief, naïve CD4+ T cells were isolated from healthy donors and following 7 days of iTreg induction protocol with and without bexarotene, cells were co-cultured with Teffectors for 3 days and proliferation measured.
Tregs induced in the presence of bexarotene were as suppressive as Tregs induced in standard Treg induction conditions (59.2% suppression at a ratio of 1:6, Teff:iTregs) (Figure 2A). This suppressive effect was demonstrated to be titratable. At ratios of 1:1, 3:1 and 6:1, there was a non-statistically significant trend for iTregs induced in the presence of bexarotene to be more suppressive than iTregs induced without bexarotene (+9.7%, +13.1% and +7.1% respectively). However, this is likely to reflect increased percentage of FOXP3+ cells within the iTregs in bexarotene-induced conditions, as shown in Fig. 1B. Overall, these data confirm that iTregs induced in the presence of bexarotene are functionally suppressive.
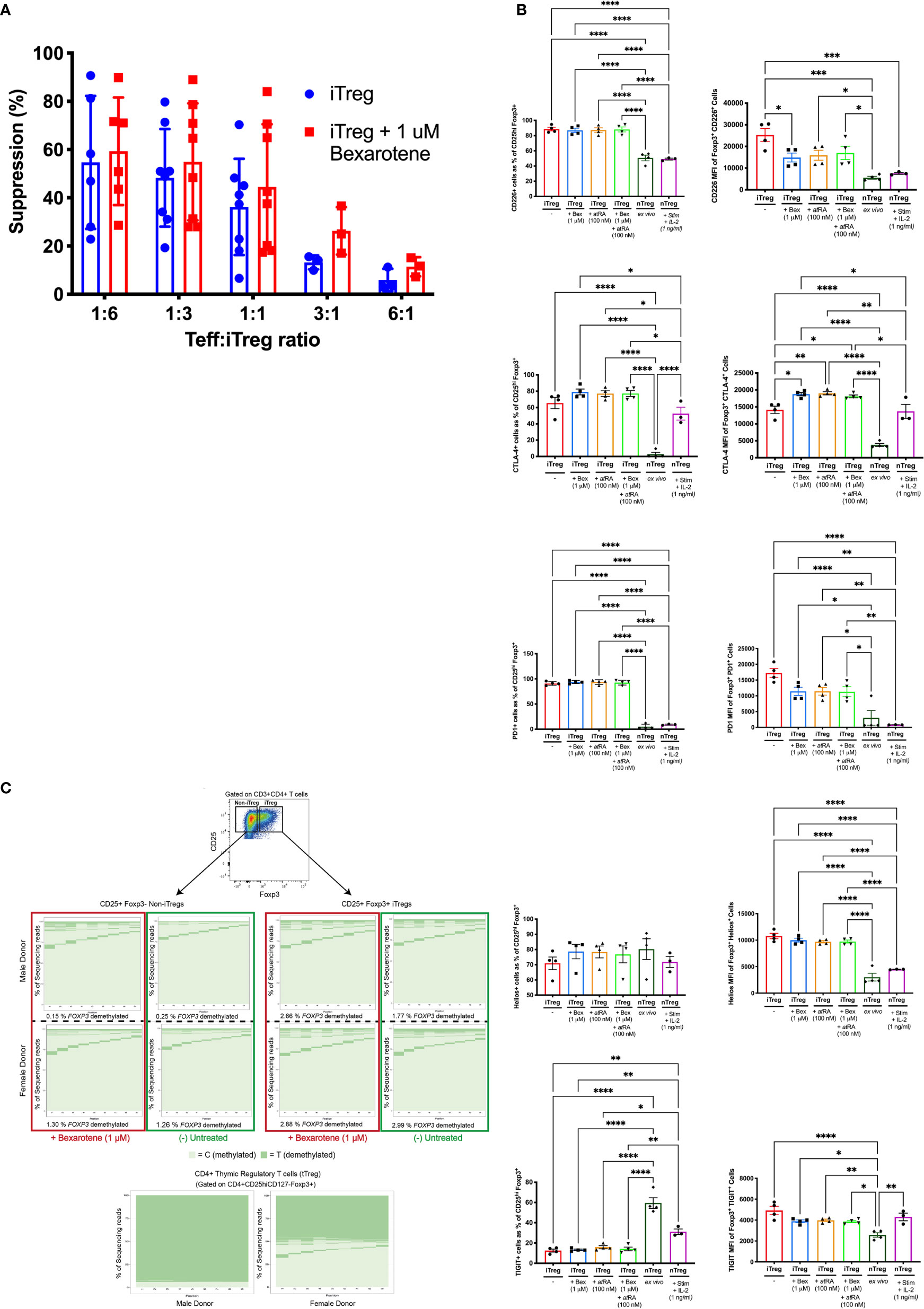
Figure 2 Comparison of iTregs vs donor-matched nTregs. (A) Percent suppression of CD3/CD28 stimulated Teff in the presence of iTregs or iTregs induced in the presence of bexarotene (two-tailed ANOVA, ns). (B) Phenotypic characteristics of iTregs, induced +/-bexarotene, +/-atRA or both vs. donor-matched nTregs stained immediately (ex-vivo) or following culture for 7 days (5 μM CD3, 1 μM CD28, 1 ng/ml IL-2). Shown are mean values ± SEM. iTreg, n = 4. ex vivo iTreg, n=4. nTreg + stim, n = 3. Significance was calculated with one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (Tukey’s correction). (C) FOXP3 TSDR methylation, determined by bisulfite sequencing, of iTregs and iTregs induced in the presence of bexarotene vs. donor-matched nTregs. Owing to X inactivation FOXP3 TSDR demethylation is ∼50% lower in females compared to males. Plots from two representative donors (one male, one female) are shown; total donors n = 6.
Phenotypic Differences Between nTregs and iTregs Induced in Different Conditions
To explore any phenotypic differences between human nTregs and iTregs induced under different conditions, the expression of several Treg signature molecules was analyzed in iTregs, induced in the presence of atRA or bexarotene versus nTregs (ex vivo, unstimulated and stimulated) (Figure 2B).
The proportion of cells expressing the inhibitory receptors CTLA4 and PD1 was higher in iTregs compared to ex vivo nTregs (74.72% vs 2.76% and 92.71% vs 16.56% respectively, Fig 2A). Furthermore, density of CTLA4 and PD1 expression was more than four-fold higher in iTregs (MFI: 17,512 vs 3805.5 and 12,857 vs. 3005.0 respectively). Following nTregs stimulation surface CLTA4 expression increased significantly but remained slightly lower than iTregs induced in the presence of bexarotene and/or atRA. In contrast, nTreg PD1 expression remained very low post-stimulation.
Comparing between iTreg conditions, both bexarotene and atRA increased CTLA4 expression (MFI of positive cells) but had no effect on PD 1 expression.
Compared to nTregs (ex vivo and stimulated), fewer iTregs expressed the inhibitory molecule TIGIT (59.48%, 41.11% and 19.42%, respectively), but those expressing TIGIT did so at a higher density (MFI 4914.8 vs 2575.25; p=<0.0001). However, this difference was lost when nTregs were stimulated. In contrast, expression of the T-cell activating molecule CD226, which competes against TIGIT for binding to their shared ligands (CD155 and CD122) was found to be increased on iTregs compared to ex vivo and activated nTregs, both in terms of percent expression and MFI. Treg induction in the presence of bexarotene, atRA or both reduced TIGIT (p=0.034, p=0.048, p=0.040) and CD226 (p=0.002, p=0.004, p=0.008) expression on a per-cell basis but did not affect the percent positive.
A higher percentage of bexarotene or atRA induced iTregs expressed HELIOS, 83.68% and 81.40%, respectively versus 70.975% in untreated iTregs, this increase was not observed in cells cultured with both compounds, or DMSO control (as both bexarotene and atRA are reconstituted in DMSO). bexarotene and atRA had no effect upon the per-cell expression of HELIOS.
Bexarotene Does Not Alter the FOXP3 TSDR of iTregs
To address the question of iTreg lineage stability, we assessed methylation status of the FOXP3 Treg specific demethylation region (TSDR) within intron 1 of FOXP3 using bisulfite sequencing (4). Tregs with a highly demethylated TSDR are known to exhibit stable FOXP3 expression whereas cells that transiently express FOXP3 are highly methylated within this region and may lose Treg associated activity. As FOXP3 is on the X-chromosome, X-chromosome inactivation approximately halves the level of TSDR demethylation seen within female donors.
As expected, flow sorted nTregs were fully demethylated whereas iTregs displayed a methylated FOXP3 TSDR, comparable to that of FOXP3- cells from the same culture. The addition of 1 μM bexarotene had no effect on the TSDR methylation of iTregs (Figure 2C).
Bexarotene Suppresses the Induction of Th17 Cells
In addition to enhancing the conversion of FOXP3+ iTregs in vitro from naive CD4+ T cells, RXR agonists have been reported to inhibit murine Th17 development in vitro and in vivo in an RAR independent manner. Here we sought to determine whether bexarotene was capable of suppressing human Th17 induction in a similar manner.
Briefly, MACS separated naive CD4+ lymphocytes stimulated in serum free media, with or without the addition of bexarotene and/or atRA, under Th17 skewing conditions (namely in the presence of TGF-β, IL-6, IL-1B, IL-23 and with neutralizing antibodies against IL-4 and IFN-γ).
Both bexarotene and atRA were equally able to suppress human Th17 cell differentiation in vitro, as measured by intracellular IL-17A cytokine staining (Figures 3A, B) and IL-17A secretion (through ELISA of supernatant) (Figures 3B, C). No synergistic effect was observed, with bexarotene and atRA together suppressing Th17 cell differentiation to the same degree as found with either bexarotene or atRA alone. Cell viability was high (>90% for IL-17A+ cells, and >65% for IL-17A- cells) in the presence of bexarotene (1 µM) and/or atRA (100nM) and was not significantly different than that seen in Th17 control conditions - confirming reduced Th17 differentiation rather than selective cell loss (Supplementary Figure 3B).
Figure 3 Bexarotene significantly reduces the differentiation of CD4+ naive lymphocytes into Th17 IL-17a+ cells in vitro. (A) Representative dot plots showing intracellular Th17 and IFN-γ following culture of human naiïve CD4 cells in Th17 skewing conditions +/- bexarotene, +/-atRA or both. (B) Summary data showing percent IL-17+ cells as a percent of total live CD4 cells at the end of culture (n = 7). (C) Supernatant IL-17a as measured at day 7 (n = 7). Shown are mean values ± SEM. Significance was calculated with repeated measures one-way ANOVA. Šidaák’s correction was performed. **p < 0.01, ****p < 0.0001.
These findings demonstrate for the first time that an RXR specific agonist can actively inhibit human Th17 differentiation and the secretion of IL-17A following stimulation in vitro.
No Differences Are Observed in the Peripheral Blood of Individuals Treated With Bexarotene on the CCMR-One Study
Patients enrolled within the CCMR One trial (reference Lancet Neurology manuscript) receiving bexarotene, in addition to standard MS treatment with dimethyl fumarate, were immunophenotyped to examine if the immunomodulatory effects of bexarotene could be replicated in vivo - see Table 1 patient for demographic information. PBMCs were collected from patients randomized to receive bexarotene pre- and at months 2, 4 and 6 of treatment. Serum samples were collected at the same time points for measurement of IL-17A.
The frequency of CD4+ T cells and absolute CD4 counts were unchanged by bexarotene treatment (Figure 4A, Supplementary Figure 4). In keeping with treatment with the MS disease modifying drug dimethyl fumarate 2 of the 5 patients had lymphocyte counts below the lower limit of normal (LLN - 1.10x109/L). No difference was found in the frequency or absolute numbers of circulating CD4+Tregs (Figures 4B–E) or Th17 cells (Figures 4G, I, J) (identified by intracellular flow cytometry) following treatment with bexarotene, and serum IL-17A levels were also unchanged (Figure 4H). Furthermore, in line with our in vitro data, no difference was found in the methylation status of Tregs from both male and female donors (Figure 4F).
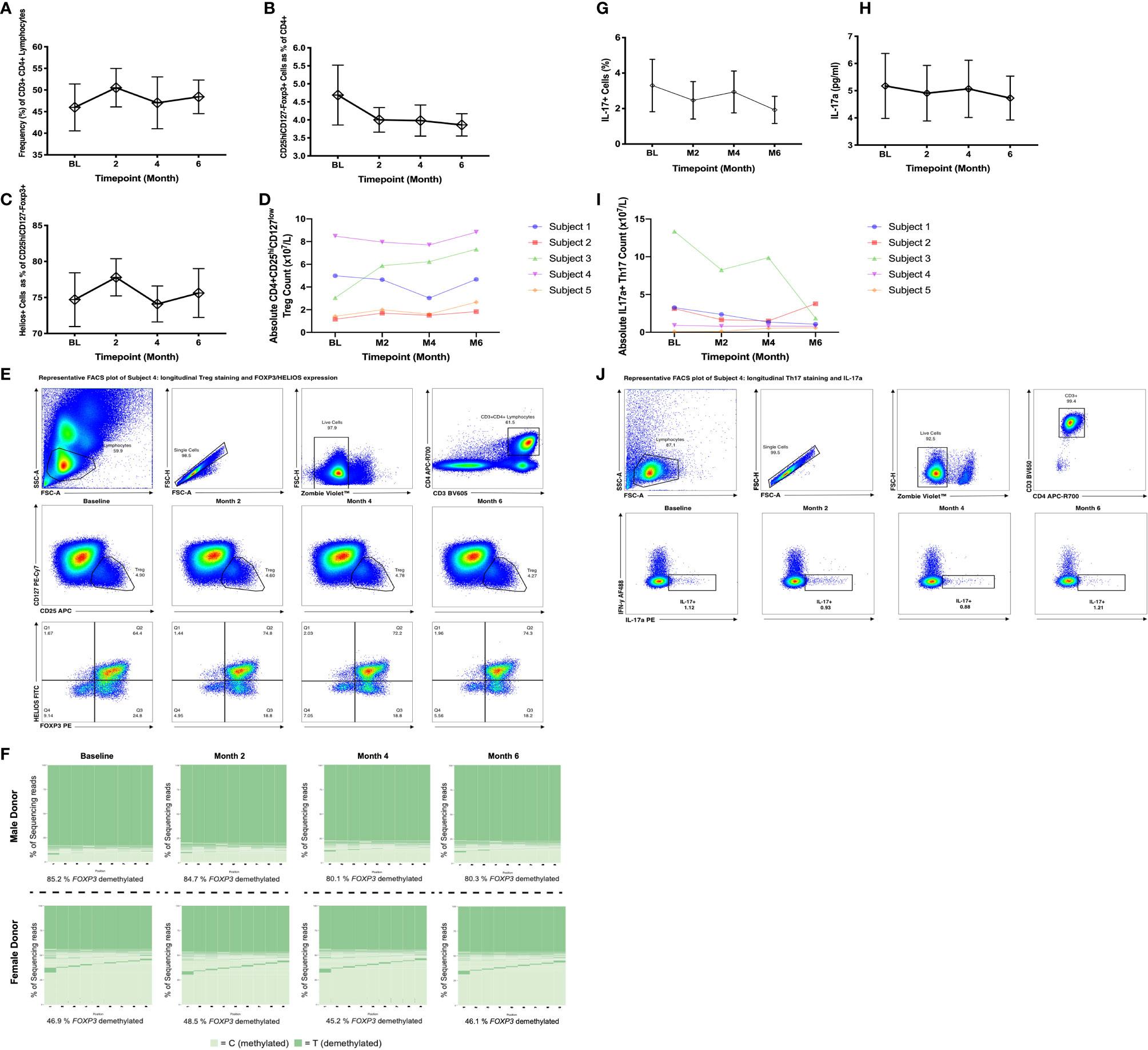
Figure 4 Peripheral blood immune phenotyping of patients randomized to receive bexarotene on the CCMR-One trial. Samples were analyzed at baseline, then at months 2, 4 and 6 following treatment. (A) Frequency of CD3+CD4+ cells as a percent of total lymphocytes. (B) Frequency of CD25hiCD127−FOXP3+ Tregs as percent of CD4+ cells. (C) Frequency of Helios+ cells as a percent of CD25hiCD127−FOXP3+ Tregs. Shown are mean values ± SEM, n=5. (D) Absolute Treg counts as defined by CD4+CD25hiCD127low surface phenotype. (E) Representative longitudinal plots of Treg gating and quantification. FOXP3 and HELIOS expression for these populations are also shown. (F) FOXP3 TSDR methylation of flow sorted, circulating CD4+CD25hi CD127low Tregs pre- and post-bexarotene (n=5; two representative donors are shown). (G) Frequency IL-17a+ cells, (H) IL-17a supernatant concentration and (I) absolute Th17 cell counts (as measured by IL-17a positivity). (J) Representative longitudinal plots of intracellular IL17 and IFN-γ in CD3+CD4+ cells, from patients pre- and post-bexarotene, following stimulation with PMA + Ionomycin + Brefeldin A. Each sample was run in duplicate. For further information regarding CCMR-One subject demographics, refer to Table 1.
Discussion
We have demonstrated that the RXR agonist bexarotene, which has recently been shown to promote remyelination in individuals with MS has immunomodulatory effects on human cells - promoting naive CD4+ T-cell differentiation into Tregs and suppressing their differentiation into Th17 cells. Our findings lend support to the development of RXR agonists as a therapy for MS.
Despite opposing functions, Th17 cells and iTregs share a closely related developmental pathway mediated by TGF-β. In the presence of TGF-β and IL-6 or IL-21 (produced by activated T-cells and DCs) naive CD4+ cells differentiate into Th17 cells. However, in the absence of proinflammatory cytokines, and in the presence of TGF-β and IL-2, naive cells differentiate into Tregs. TGF-β induces SMAD2 and SMAD3 phosphorylation which in turn activate FOXP3, which drives cells towards the Treg lineage (in contrast IL-6 inhibits SMAD signaling). In addition, IL-2 phosphorylates STAT5 which activates FOXP3. iTregs induced in vivo are termed “pTregs” (for peripherally induced), to distinguish them from thymically derived Tregs (also known as naturally occurring or “nTregs”). It is thought that pTregs may constitute a significant proportion of Tregs in the periphery particularly at certain sites such as the lamina propria and maternal placenta where they may maintain tolerance against commensal bacteria and the developing fetus (5). In addition to their shared dependency on TGF-β, many studies have reported plasticity between iTregs and Th17 cells under certain conditions. For example, studies tracing the fate of IL-17+ cells in the gut, reveal that many at some point express FOXP3 (6). Given this, it is not surprising that the Th17/Treg balance has been shown to play a critically important role in many autoimmune diseases, including MS (7).
In contrast to published murine studies, we have shown that bexarotene promotes human iTreg differentiation independent of RAR signaling. Therefore, RXR supplementation alone should be sufficient to promote iTreg induction in patients. Independence from RAR signaling was shown both by culturing cells in serum-free, and therefore atRA free media, and by using the pan-RAR antagonist AGN193109. Also counter to murine data is our observation that bexarotene and atRA do not work synergistically to enhance human iTreg differentiation in vitro. One possibility is that atRA was used at concentrations reaching the maximum threshold for atRA enhancement of human iTregs differentiation. atRA is thought to promote iTreg differentiation through enhancing TGF-β driven SMAD3 signaling and inhibiting the activity of IL-6 and IL-23 (8), meaning it is possible that these mechanisms were saturated leaving bexarotene unable to promote further atRA-mediated enhancement. In addition to RXR forming a heterodimer with RAR, it can also form a homodimer or dimerize with alternative binding partners - including the 1a,25- (OH)2 vitamin D3 receptor (VDR), peroxisome proliferator-activated receptor (PPAR), liver X receptors (LXR), thyroid hormone receptors (TR), pregnane X receptors (PXR) and farnesoid X receptor (FXR) (9).
We have not determined through which receptor-dimer complex bexarotene promotes iTreg differentiation. However, as it occurs independently of RAR engagement it must do so either through RXR/RXR homodimers, or via permissive nuclear receptors, such as PPAR or LXR. Evidence in support of PPAR engagement comes from studies showing that PPAR-α and PPAR-γ agonists also promote FOXP3 expression and TSDR demethylation in CD4+CD25− T cells upon TCR stimulation in the presence of TGF-β and IL-2 (10).
Compared to thymically derived nTregs (ex vivo or activated), iTregs (induced in all conditions, including bexarotene) expressed higher surface levels of the inhibitory molecules CTLA-4 and PD-1 (both in terms of percentage and on a per-cell basis). Per-cell surface CTLA-4 expression was further increased by exposure to bexarotene or atRA. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a crucial inhibitory molecule and homolog to the co-stimulatory molecule CD28, with which it shares ligands (CD80 and CD86 expressed on APCs). Binding of CD80/86 (on antigen presenting cells) to CD28 on T-cells provides a costimulatory signal, whereas CTLA-4 captures CD80/86 from the surface of APCs by transendocytosis, preventing CD28 engagement and T-cell activation (11). PD-1 interactions with PD-L1 and PD-L2 also down-modulate T-cell immune responses (12). In contrast to CTLA-4 and PD-1, significantly fewer iTregs expressed the co-inhibitory molecule TIGIT (T cell Ig and ITIM domain) compared to nTregs, although iTregs expressing TIGIT did so at a higher level. Expression of the co-stimulatory molecules CD226 was also increased on iTregs (percent and per-cell expression) compared to nTregs, although per-cell expression was slightly lower when Tregs were induced in the presence of bexarotene and/or atRA. Similar to CD28 and CTLA-4, CD226 and TIGIT share and compete for ligands expressed on APCs (CD112 and CD155) promoting or inhibiting T-cell activation respectively (13, 14). Despite high CD226:TIGIT expression, and perhaps as a consequence of high PD-1 and CTLA-4 expression, iTregs induced in the presence of bexarotene were found to be at least as suppressive in vitro as iTregs induced in standard conditions.
In addition to promoting iTreg differentiation, we have shown that bexarotene is also capable of suppressing human Th17 development in vitro, as measured through intracellular IL-17A staining and changes in supernatant IL-17A. This is the first report using human cells and is keeping with murine studies where RXR ligands have been shown to suppress Th17 development. Two such studies report that RXR ligands are able to suppress Th17 development, attenuate active and Th17 mediated passive EAE and suppress the numbers of CD4+ lymphocytes capable of secreting pro-inflammatory cytokines (3, 15). Furthermore, RXR ligands have been shown to reduce the expression of cell activation markers Ki-67 and CCR6 on Th17 cells which is required for entry into the central nervous system (CNS). These results are encouraging for diseases such as MS, thought to be driven by aberrant regulation of Th17 cell responses that are found with increased frequency in the periphery and the CNS of patients (16, 17).
In this study, we took advantage of access to blood samples from individuals with MS receiving bexarotene as participants on the CCMR-One phase 2a trial, to explore the effects of bexarotene in vivo. Disappointingly our in vitro observations were not mirrored in the blood of individuals receiving bexarotene. This may be because, at the dose given (300mg/m2 body surface area per day, for 6 months) bexarotene is unable to promote iTregs in vivo. However alternatively this may be due to the fact that bexarotene is likely to exert its effects at sites of CD4 naive cell activation and differentiation (i.e. within lymph nodes and tissues) and therefore would be unlikely to affect circulating Treg/Th17 numbers (18). Our results are also in keeping with findings with use of bexarotene in cutaneous T cell lymphoma (CTCL), where no change in the number of circulating Tregs was found in peripheral blood (19), although in this study “Tregs” were defined purely on the basis of CD25hi expression.
Bexarotene was poorly tolerated in CCMR-One (reference Lancet Neurology manuscript). Of the 32 patients dosed in Cambridge, 16 developed central hypothyroidism and 14 developed elevated triglycerides, both well recognized side-effects of bexarotene. Given this, it is unlikely that bexarotene will progress further into clinical development as a therapeutic strategy in MS. However, these results do provide hope and momentum behind the development of other RXR-isoform specific agonists.
In summary we suggest that RXR agonism may be an effective therapeutic approach for the treatment autoimmune disorders particularly those such as MS where there is an imbalance in the Treg/Th17 axis. MS is a particularly attractive target as RXR agonists have also been reported to promote remyelination.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by - for healthy controls REC: 11/EE/0007; for patients treated with Bexarotene REC: 15/LO/0180. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CG, LJ, and JJ designed the research. CG, DR, RM, HM, NC, ZG, JB, AC, and JJ performed the research. CG, DR, and JJ analysed the data. CG, DR and JJ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded in part, by the Wellcome Trust (Grant number RG79413). For the purpose of open access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. The work was also supported by the Cambridge NIHR BRC Cell Phenotyping Hub and NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed here are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. CG was funded by a BBSRC CASE studentship. LJ and JJ were funded by the WellcomeTrust (RG79413).
Author Disclaimer
The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.712241/full#supplementary-material
References
1. Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, et al. Retinoid X Receptor Gamma Signaling Accelerates CNS Remyelination. Nat Neurosci (2011) 14:45–53. doi: 10.1038/nn.2702
2. Takeuchi H, Yokota-Nakatsuma A, Ohoka Y, Kagechika H, Kato C, Song S-Y, et al. Retinoid X Receptor Agonists Modulate Foxp3+ Regulatory T Cell and Th17 Cell Differentiation With Differential Dependence on Retinoic Acid Receptor Activation. J Immunol (2013) 191:3725–33. doi: 10.4049/jimmunol.1300032
3. Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell (2014) 157:255–66. doi: 10.1016/j.cell.2014.03.012
4. Rainbow DB, Yang X, Burren O, Pekalski ML, Smyth DJ, Klarqvist DR, et al. Epigenetic Analysis of Regulatory T Cells Using Multiplex Bisulfite Sequencing. Eur J Immunol (2015) 45:3200–3. doi: 10.1002/eji.201545646
5. Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol (2015) 6:639. doi: 10.3389/fimmu.2015.00639
6. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the Transcription Factor Foxp3 Leads to the Generation of Pathogenic Memory T Cells. vivo Nat Immunol (2009) 10:1000–7. doi: 10.1038/ni.1774
7. Lee GR. The Balance of Th17 Versus Treg Cells in Autoimmunity. Int J Mol Sci (2018) 19:1–14. doi: 10.3390/ijms19030730
8. Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic Acid Increases Foxp3+ Regulatory T Cells and Inhibits Development of Th17 Cells by Enhancing TGF-β -Driven Smad3 Signaling and Inhibiting IL-6 and IL-23. J Immunol (2008) 154:450–8. doi: 10.1016/j.clim.2008.03.149
9. Fields AL, Soprano DR, Soprano KJ. Retinoids in Biological Control and Cancer. J Cell Biochem (2007) 102:886–98. doi: 10.1002/jcb.21530
10. Jin Lei Y, Hasegawa H, Matsumoto T. Peroxisome Proliferator Activated Receptor Alpha and Gamma Agonists Together With TGFb Convert Human CD4 Positive CD25 Negative T Cells Into Functional Foxp3+ Regulatory T Cells. J Immunol (2010) 185:7186–98. doi: 10.4049/jimmunol.1001437
11. Ovcinnikovs V, Ross EM, Petersone L, Edner NM, Heuts F, Ntavli E, et al. CTLA-4-Mediated Transendocytosis of Costimulatory Molecules Primarily Targets Migratory Dendritic Cells. Sci Immunol (2019) 4:31. doi: 10.1126/sciimmunol.aaw0902
12. Francisco LM, Sage PT, Sharpe AH. The PD-1 Pathway in Tolerance and Autoimmunity. Immunological Rev (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
13. Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 Axis Regulates Human T Cell Function. J Immunol (2012) 188:3869–75. doi: 10.4049/jimmunol.1103627
14. Fuhrman CA, Yeh W-I, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, et al. Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226. J Immunol (2015) 195:145–55. doi: 10.4049/jimmunol.1402381
15. Chandraratna RA, Noelle RJ, Nowak EC. Treatment With Retinoid X Receptor Agonist IRX4204 Ameliorates Experimental Autoimmune Encephalomyelitis. Am J Transl Res (2016) 8:1016–26.
16. Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple Sclerosis. Neuromethods (2018) 138:263–95. doi: 10.1007/978-1-4939-7880-9_8
17. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple Sclerosis. Lancet (London England) (2018) 391:1622–36. doi: 10.1016/S0140-6736(18)30481-1
18. Whibley N, Tucci A, Powrie F. Regulatory T Cell Adaptation in the Intestine and Skin. Nat Immunol (2019) 20:1. doi: 10.1038/s41590-019-0351-z
Keywords: T cells, Th17 & Tregs cells, multiple sclerosis, autoimmunity, retinoid X receptor (RXR), retinoids
Citation: Gaunt CM, Rainbow DB, Mackenzie RJ, Jarvis LB, Mousa HS, Cunniffe N, Georgieva Z, Brown JW, Coles AJ and Jones JL (2021) The MS Remyelinating Drug Bexarotene (an RXR Agonist) Promotes Induction of Human Tregs and Suppresses Th17 Differentiation In Vitro. Front. Immunol. 12:712241. doi: 10.3389/fimmu.2021.712241
Received: 20 May 2021; Accepted: 16 July 2021;
Published: 10 August 2021.
Edited by:
Isabel Merida, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Graham J. Britton, Icahn School of Medicine at Mount Sinai, United StatesIris Caramalho, Gulbenkian Institute of Science (IGC), Portugal
Copyright © 2021 Gaunt, Rainbow, Mackenzie, Jarvis, Mousa, Cunniffe, Georgieva, Brown, Coles and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanne L. Jones, amxzNTNAbWVkc2NobC5jYW0uYWMudWs=
 Christopher M. Gaunt
Christopher M. Gaunt Daniel B. Rainbow
Daniel B. Rainbow Ruairi J. Mackenzie
Ruairi J. Mackenzie Lorna B. Jarvis
Lorna B. Jarvis Hani S. Mousa
Hani S. Mousa Nicholas Cunniffe1
Nicholas Cunniffe1 Alasdair J. Coles
Alasdair J. Coles Joanne L. Jones
Joanne L. Jones