- 1Department of Radiation Oncology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Biostatistics and Epidemiology, University of Massachusetts, Amherst, MA, United States
- 3Department of Oncology, The Fifth Hospital of Wuhan, Wuhan, China
- 4Department of Radiology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Purpose: Dosimetric parameters (e.g., mean lung dose (MLD), V20, and V5) can predict radiation pneumonitis (RP). Constraints thereof were formulated before the era of combined immune checkpoint inhibitors (ICIs) and radiotherapy, which could amplify the RP risk. Dosimetric predictors of acute RP (aRP) in the context of ICIs are urgently needed because no data exist thus far.
Methods and Materials: All included patients underwent thoracic intensity-modulated radiotherapy, previously received ICIs, and followed-up at least once. Logistic regression models examined predictors of aRP (including a priori evaluation of MLD, V20, and V5), and their discriminative capacity was assessed by receiver operating characteristic analysis.
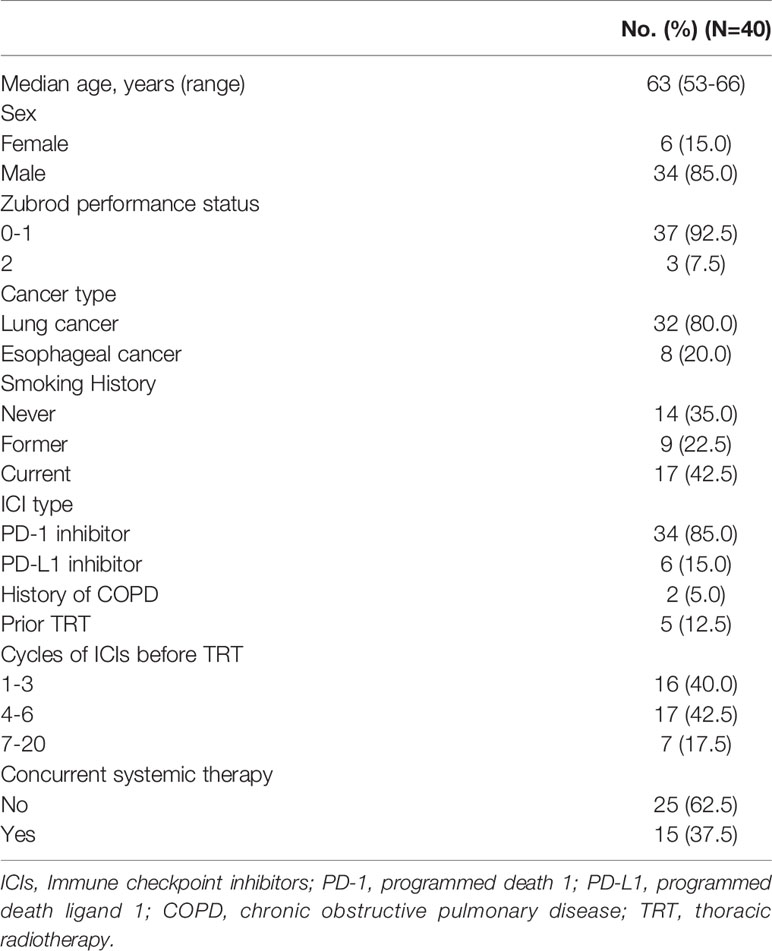
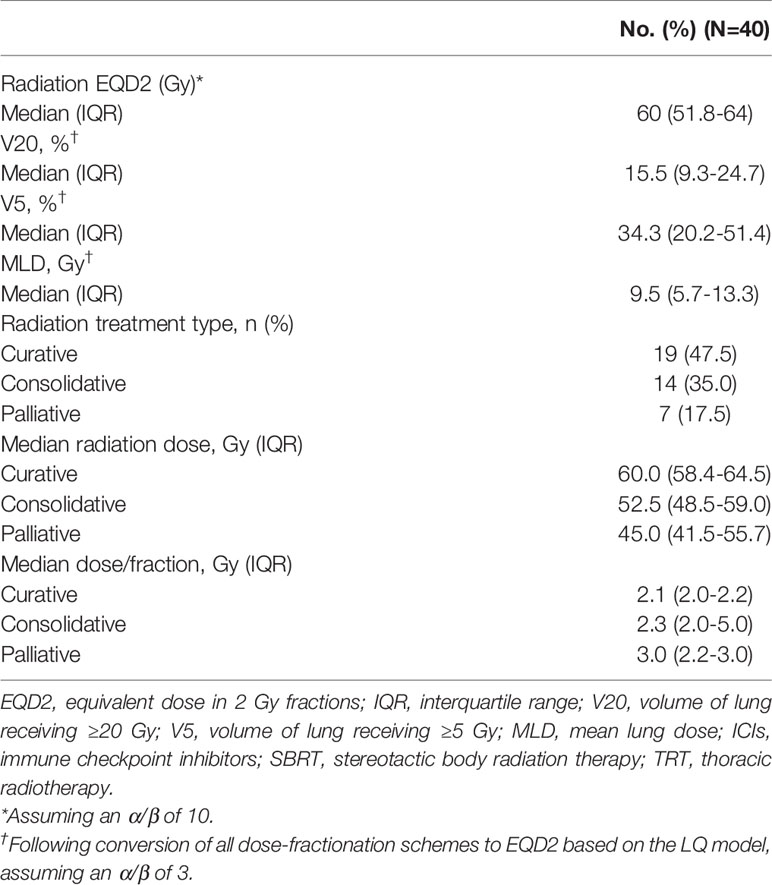
Results: Median follow-up of the 40 patients was 5.3 months. Cancers were lung (80%) or esophageal (20%). ICIs were PD-1 (85%) or PD-L1 (15%) inhibitors (median 4 cycles). Patients underwent definitive (n=19), consolidative (n=14), or palliative (n=7) radiotherapy; the median equivalent dose in 2 Gy fractions (EQD2) was 60 Gy (IQR, 51.8-64 Gy). Grades 1-5 aRP occurred in 25%, 17.5%, 15%, 2.5%, and 5%, respectively. The only variables associated with any-grade aRP were V20 (p=0.014) and MLD (p=0.026), and only V20 with grade ≥2 aRP (p=0.035). Neither the number of prior ICI cycles nor the delivery of concurrent systemic therapy significantly associated with aRP risk. Graphs were constructed showing the incrementally increasing risk of aRP based on V20 and MLD (continuous variables).
Conclusions: This is the first study illustrating that V20 and MLD may impact aRP in the setting of prior ICIs. However, these data should not be extrapolated to patients without pre-radiotherapy receipt of prior ICIs, or to evaluate the risk of chronic pulmonary effects. If these results are validated by larger studies with more homogeneous populations, the commonly accepted V20/MLD dose constraints could require revision if utilized in the setting of ICIs.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized oncologic care throughout the world and now represent the standard of care for many metastatic or locally advanced cancers. Combining ICIs and radiation therapy (RT) represents a major area of ongoing active investigation aimed to promote synergy between modalities in efforts to potentially improve outcomes (1).
However, it is well recognized that ICIs can cause adverse events that may be additive with those caused by RT. One such example is pneumonitis, which can be caused independently by ICIs as well as from RT (2–4). Some reports have observed relatively high rates of radiation pneumonitis (RP) in patients with combined ICI and RT, including fatal events (5, 6). However, the safety of combined therapy remains poorly understood owing to 1) the relatively recent adoption of ICIs as well as 2) the rise in RT delivered to suprapalliative doses for metastatic cancers (e.g. for oligometastatic or oligoprogressive disease).
It has long been known that radiation dosimetry is a powerful predictive factor for RP (7), especially the mean lung dose (MLD), the volume of lungs receiving ≥20 Gy (V20) (8), and potentially also the volume of lungs receiving ≥5 Gy (V5) (9). However, those data were not in context of ICIs, which makes their applicability to the ICI setting uncertain.
To our knowledge, there are no dosimetric analyses of MLD, V20, and V5 as possible predictors for acute RP (aRP) in the context of ICI therapy. These analyses are urgently needed because the previously recommended thresholds for these dosimetric parameters (8) may not be applicable to the combined RT-ICI setting and could result in an excess aRP risk. The present study was designed to address this knowledge gap in efforts to question whether the commonly accepted dose constraints for MLD, V20, and V5 may require revision in the future as the combined usage of RT and ICIs continues to expand.
Methods and Materials
Patients and Treatment
This investigation was approved by the institutional review and ethics board. We conducted a retrospective review of all patients who received thoracic intensity-modulated radiotherapy (IMRT) at our institution from March 2020 to July 2021. From this institutional dataset, patients who had previously received ICIs were included (concurrent therapy was allowed but not used as an exclusion criterion in order to avoid selection biases). Because the study’s aim was to investigate aRP, patients who did not follow-up (with imaging assessment) were excluded.
Patients with a variety of thoracic cancers were included, so workup and follow-up were individualized, but pre-RT workup always involved chest computed tomography (CT) or positron emission tomography (PET)-CT imaging, and follow-up after RT generally consisted of follow-up chest CT (or PET-CT) one month after RT and every 3 months thereafter.
In efforts to reduce selection biases from the intent of RT, this study included patients who underwent definitive RT, palliative RT (i.e., for symptomatology), or consolidative RT (e.g., for oligoprogression). RT was planned and conducted according to the fundamental principles of each paradigm including with dose constraints put forth by QUANTEC and the AAPM Task Group 101 (8, 10). Image guidance was used for all cases, thrice per week in the first week and twice per week in the remaining weeks.
Toxicity Assessments
The definition of aRP was based on the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. All suspected diagnoses of pneumonitis (regardless of grade) were centrally reviewed by a multidisciplinary committee consisting of at least one radiologist, pulmonologist, and oncologist. CT (or PET-CT) imaging was reviewed for each patient, which was then compared to the radiation treatment plan; other causes such as infection or tumor progression had to be ruled out using the appropriate workup.
Statistical Analysis
This study was specifically designed to (a priori) evaluate MLD, V20, and V5 (defined as both lungs minus the planning target volume) as candidate dosimetric parameters for aRP; its goal was not to examine other dose-volume metrics because the three aforementioned parameters represent a “common language” to study RP and have been previously validated and/or widely propagated. Despite the use of a variety of dose-fractionation schemes herein, the aforementioned dosimetric parameters are expressed in 2 Gy equivalent doses (EQD2); based on the linear-quadratic model, MiM software was used to convert the physical dose distribution into EQD2 assuming α/β of 10 and 3 for the target and the normal lung, respectively (11). Logistic regression models were used to assess the association between patient characteristics and the aRP risk. The discriminative capacity of models was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). All data analyses were conducted using R software (version 4.1.0) and used 2-sided tests with p<0.05 indicating statistical significance.
Results
Characteristics of Patients
Of 3,276 patients who received thoracic irradiation, 52 patients had previously received ICIs. Of these 52 patients, 12 did not have adequate follow-up in order to evaluate for aRP; therefore, 40 patients were included herein. The median follow-up from thoracic irradiation was 5.3 months [interquartile range (IQR), 3.6-7.7 months].
Salient characteristics of the population are shown in Table 1. All patients had lung (80%) or esophageal (20%) cancer, and all patients previously received PD-1 (85%) or PD-L1 (15%) inhibitors. The median number of previous ICI cycles was 4 (interquartile range (IQR), 3-6), and thoracic RT commenced at a median of 26 days (IQR, 18-42) thereafter. During thoracic RT, 15 patients received concurrent systemic therapy (n=8 concurrent anti-PD-1/PD-L1 with chemotherapy, n=4 anti-PD-1/PD-L1 monotherapy, n=3 chemotherapy or targeted agents).
Table 2 displays RT-related characteristics of the population. Nineteen patients received definitive RT, 14 patients underwent consolidative RT for oligoprogression while on ICI therapy, and the remainder (n=7) received palliative RT. The median EQD2 for all patients was 60 Gy (IQR, 51.8-64 Gy). Given the heterogeneity in dose/fractionation schemas, all plans were standardized using EQD2 doses; following this action, the median (IQR) MLD, V20, and V5 were 9.5 Gy (5.7-13.3), 15.5% (9.3-24.7), and 34.3% (20.2-51.4), respectively.
Incidence and Characteristics of aRP
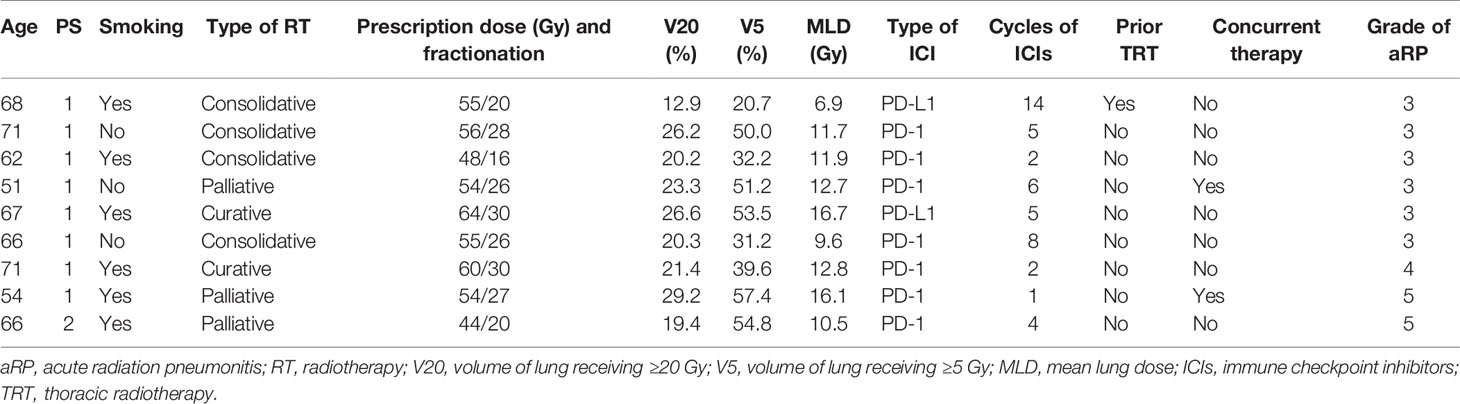
At the time of last follow-up, 14 (35%) of patients had not developed aRP. Grade 1 (asymptomatic) RP was detected in 10 (25%) patients. Symptomatic grade ≥2 aRP was present in 16 (40%) patients, of which there were 7 (17.5%), 6 (15%), 1 (2.5%), and 2 (5%) cases of grades 2, 3, 4, and 5 aRP, respectively. Table 3 displays characteristics of the nine patients who developed grade ≥3 aRP. The median time to aRP was 67 days (IQR, 50-88 days); the most common symptoms included fever (n=12), nonproductive cough (n=10), dyspnea (n=8), and wheezing (n=7). Methylprednisolone was delivered to all patients who developed grade ≥2 aRP.
Of note, two patients developed symptomatic aRP (both grade 2) during the course of RT (the remainder occurred after RT completed) (Figure 1). The first had received 50.6 Gy in 23 fractions over 33 days, and the other had received just 9.6 Gy in 2 fractions over 2 days. Both of them were treated with PD-1 inhibitors and had RT interruptions of 10 and 5 days, respectively. Both received methylprednisolone, following which they became asymptomatic and completed the prescribed RT course thereafter.
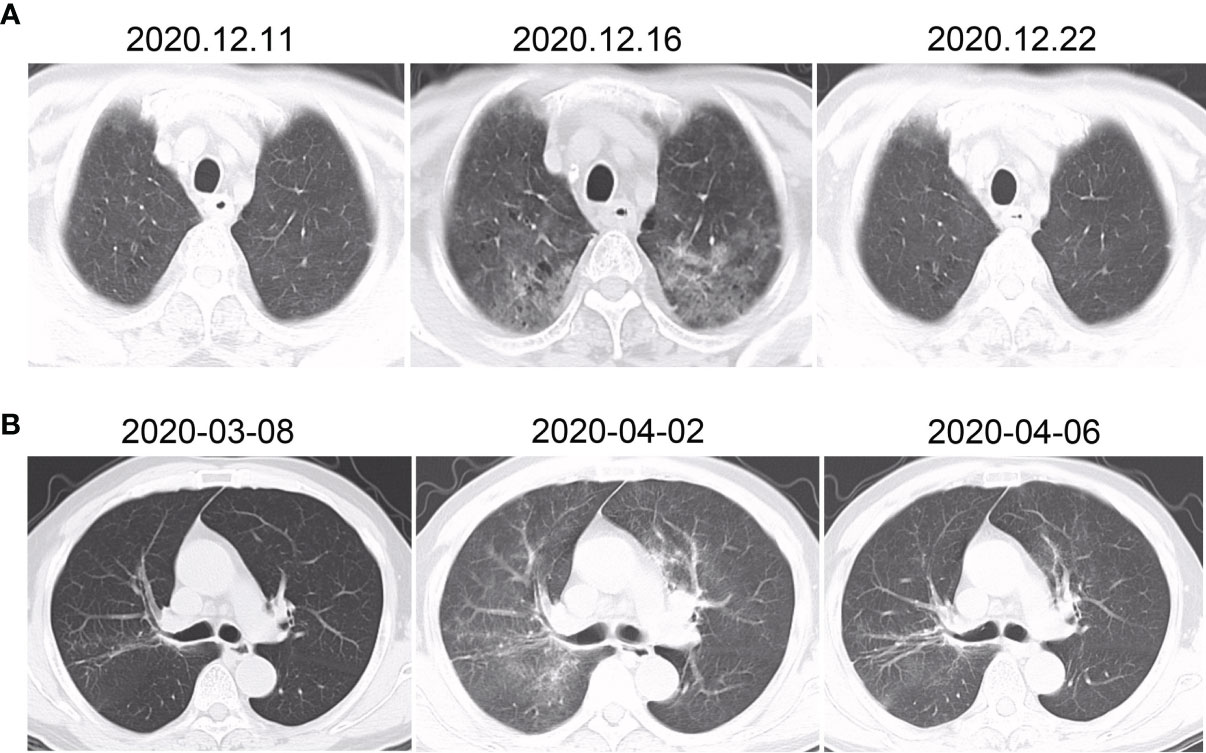
Figure 1 CT changes of the two patients who developed pneumonitis during the course of radiotherapy (both grade 2). The first patient (A) developed fever and cough after 23 fractions (50.6 Gy total dose), with no abnormalities initially (left). Five days later, chest CT showed ground-glass changes (center). One week after methylprednisolone commenced, the inflammation had substantially dissipated (right). The second patient (B) developed fever after two fractions (9.6 Gy total dose). Initially there were no major findings (left), but patchy infiltrates were soon found in the radiation field (center). After 4 days of methylprednisolone, the findings had significantly reduced.
Predictors of aRP
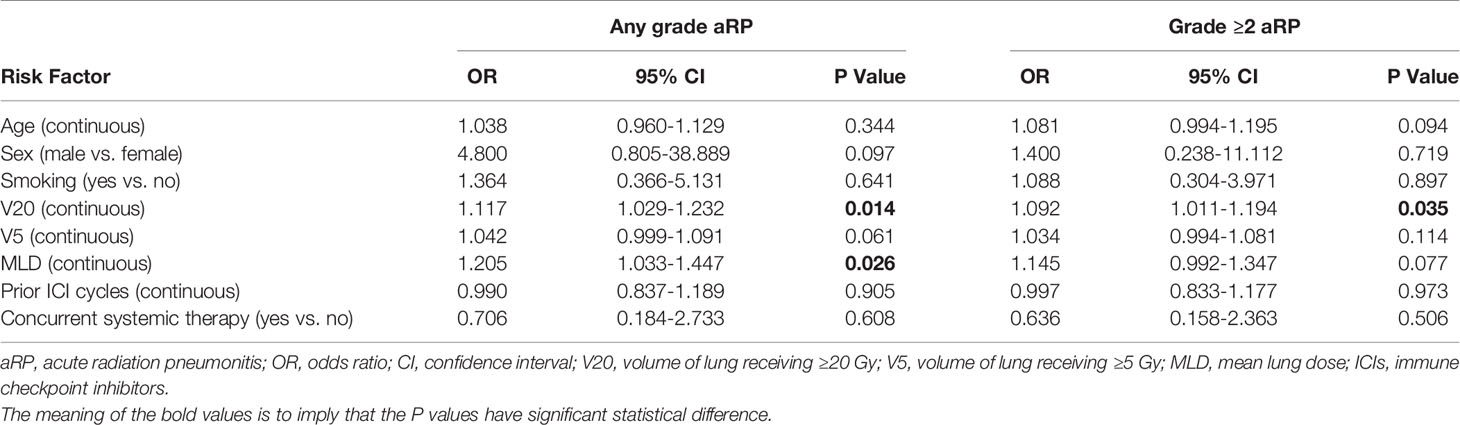
Table 4 illustrates factors associated with the risk of aRP. Regarding any-grade aRP, there were only two associated variables: V20 (odds ratio (OR) 1.117, 95% confidence interval (CI) 1.029-1.232, p=0.014) and MLD (OR 1.205, 95% CI 1.033-1.447, p=0.026). For grade ≥2 aRP, only V20 (OR 1.092, 95% CI 1.011-1.194, p=0.035) was associated. Of note, neither the number of prior ICI cycles nor the delivery of concurrent systemic therapy significantly associated with aRP risk.
ROC analysis is shown in Figure 2, and revealed an AUC of 0.762 for the relationship between V20 and any-grade aRP and 0.707 for MLD. The AUC of V20 for grade ≥2 aRP was 0.703. Based on this analysis, graphs were constructed to pictorially present the incrementally increasing risk of aRP based on V20 and MLD as continuous variables (Figure 2). According to these models, V20 = 8.98% or MLD=5.55Gy would predict for a 50% risk of ≥ grade 1 pneumonitis (Figures 2A, B), and V20 = 21.1% would predict for a 50% risk of ≥ grade 2 pneumonitis (Figure 2C). Of note, the y-intercept (corresponding to a V20 or MLD of 0) in these graphs is not 0 because ICIs carry an independent pneumonitis risk.
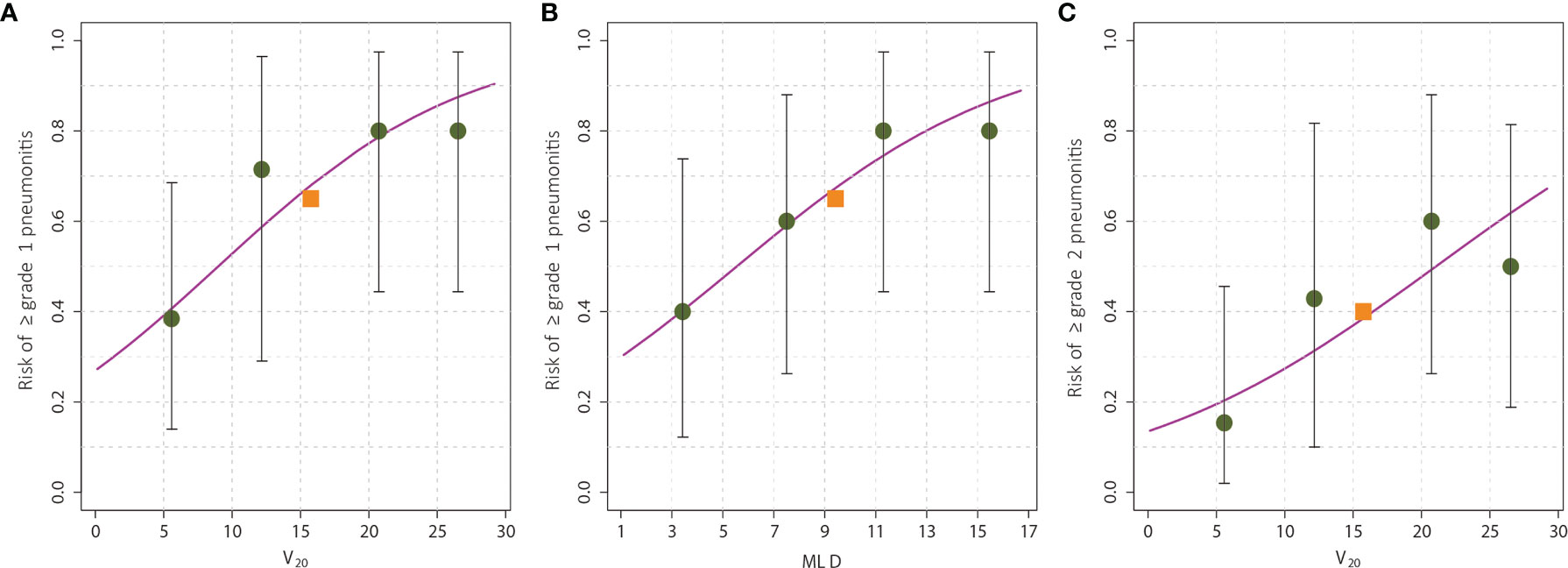
Figure 2 The risk of radiation pneumonitis based on dose-volume parameters. The risk of any-grade radiation pneumonitis was associated with V20 (A) and mean lung dose (B), whereas the risk of grade ≥2 pneumonitis was associated with only V20 (C). The observed pneumonitis rate among all patients is denoted by the red square, and that for each quartile of the particular dose-volume parameter is denoted by the black circles. The error bars on the circles represent the exact binomial 95% confidence intervals. Of note, the y-intercept (corresponding to a V20 or MLD of 0) in these graphs is not 0 because immune checkpoint inhibitors carry an independent pneumonitis risk.
Discussion
The use of combined ICIs and RT is rapidly expanding for metastatic (12, 13) and locally advanced cancers (14), but the risk of potentially additive toxicities – especially higher grade events – remains a concern. It is an urgent necessity to report experiences of modifiable variables (e.g., dosimetric parameters) which may alter the risk of toxicities such as aRP. To our knowledge, this is the first study that has observed a relationship between radiation dosimetric variables and aRP in the context of combined RT-ICI therapy. These results undoubtedly require validation, but suggest that the well-recognized dose-volume constraints for thoracic RT may require revision in the future if used in the context of ICI therapy.
The 22.5% rate of grade ≥3 aRP herein is somewhat higher than existing data, which have generally reported figures around 11-15% (15–17). This could be due to two major reasons. First, patients herein received full courses of ICIs prior to RT initiation, whereas in other studies (11–16) most patients did not have as high of a degree of immune system galvanization prior to commencing RT. KEYNOTE-799 (which delivered ICIs before RT) observed a <10% rate of grade ≥3 pneumonitis, but that trial delivered a median of 1 cycle of pembrolizumab/chemotherapy prior to RT (as compared to a median of 4 cycles herein) (18). The higher level of immune activation prior to starting RT in this cohort could have predisposed these patients to develop RP more frequently and with greater severity than patients in the aforementioned publications, but this notion requires further corroboration. Second, a much higher proportion of patients in two other publications (15, 16) received SBRT than those of this investigation, which presumes that irradiated volumes were smaller and more conformally treated than those of the present study. These potential explanations notwithstanding, it is essential for further research to examine whether prior receipt of full-course ICIs are causatively linked to a higher rate of RP, especially because the optimal sequencing of ICIs and RP for metastatic disease remains unknown.
To our knowledge, this is the first investigation which defines dosimetric relationships between lung dose-volume parameters and RP in the context of combined RT and ICIs. Two studies attempted similar goals, but neither could elucidate any such dosimetric factors (15, 16). This is likely related to two major reasons. First, those studies had similarly small sample sizes as this investigation and lower event rates. The publication from MD Anderson Cancer Center observed nine grade ≥3 events in 60 patients (15), and the publication from Emory University documented 19 cases of any-grade RP in 56 patients (16). Conversely, these data are considerably better equipped to detect predictors of RP because it involved a much higher event rate (26 cases of any-grade RP and nine grade ≥3 events in 40 patients). Second, owing to the dearth of available data, all existing studies suffer from heterogeneous patient populations. To that extent, an important strength of this work was that all patients received ICIs targeting the PD-1—PD-L1 axis, whereas the aforementioned publications utilized a mixture of anti-PD-1/PD-L1 agents alone, anti-CTLA-4 compounds alone, and dual immune checkpoint blockade [although it has been suggested that PD-1 and PD-L1 inhibitors have differential rates of pneumonitis (19)].
Owing to similar sample size and heterogeneity concerns as other existing data, as well as the a priori nature of dosimetric examination herein, our study also cannot rule out finer differences in other parameters potentially associated with RP that could have gone undetected. That being said, the main message from this study is that V20 and MLD can – and should – continue to be used as important modifiable factors during RT planning. Our study suggests that accepted V20 and MLD constraints (8) may require revision in the future if planning is done in the context of prior ICIs. To this extent, we encourage a more generous utilization of a variety of techniques that could reduce dose exposure to the normal lung, such as deep-inspiration breath hold technique, intensity-modulated or proton therapy, smaller target margins, and high-quality volumetric image guidance.
Importantly, our findings suggest that V5 may not be as robust of a marker with which to predict RP in the context of ICI therapy. This is consistent with its general lack of validation in the non-ICI setting (20), and is potentially reassuring given that full-course ICI therapy prior to RT could activate the immune system to such an extent that even the “low dose bath” could cause RP. However, as mentioned above, finer differences cannot be excluded from any study with smaller sample sizes. Additionally, one case of grade 2 aRP during the RT course herein occurred after just 9.6 Gy in 2 fractions. Even though this patient received a high fractional dose (which in itself could have been the cause of aRP), it suggests that there can indeed be cases of RP at lower doses than would be predicted in the non-ICI setting.
Limitations of this investigation must be contextualized with the fact that the available data on this topic are very scant at the present time. First, as mentioned above, all known data aiming to address dosimetric predictors of RP in the context of ICIs (15–17) are retrospective, with small sample sizes, and short follow-up; along with heterogeneous populations, treatment paradigms, and workup/follow-up. For this reason, this study is not equipped to examine whether concurrent RT & systemic therapy after prior ICIs increases the RP risk over prior ICI therapy alone. Similarly, the contribution of fractional dose (i.e., conventional vs. various degrees of hypofractionation) to RP also cannot be ascertained. It is also unknown whether a greater number of ICI cycles prior to RT increases the risk of RP. Therefore, as the use of combined ICIs and RT increases over time, validating this study and those publications with larger sample sizes is essential. Second, this study should not be extrapolated to situations that do not involve prior ICI therapy. As mentioned above, receipt of full-course prior ICIs could pose a very different RP-related risk than up-front concurrent therapy, RT followed by ICIs, or a short course of ICIs followed by RT. Third, this study only aimed to examine acute RP and should not be used to estimate chronic RP or pulmonary fibrosis. It is possible that this study underestimates the rate of overall RP for this reason, along with the fact that death is a competing risk for RP in this population. Lastly, not all variables can be input into the logistic regression model, especially those with small subgroup sample sizes. We also did not evaluate several other candidate variables potentially associated with RP such as baseline pulmonary function, prior pneumonitis or other adverse events from pre-RT ICI therapy, and size of the planning target volume.
Conclusions
It is an urgent necessity to report experiences of modifiable variables (e.g., dosimetric parameters) which may alter the risk of toxicities such as aRP. To our knowledge, this is the first investigation that has observed a relationship between radiation dosimetric variables and aRP in the context of combined RT-ICI therapy. We demonstrate that the lung V20 and MLD was independently associated with any-grade RP, and the V20 was associated with grade ≥2 aRP. This study is only applicable for aRP (not chronic RP or pulmonary fibrosis) and in the setting of prior full-course ICI therapy. These results require validation from studies with larger and more homogeneous populations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional ethics board of Hubei Cancer Hospital of Huazhong University of Science and Technology in Wuhan, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JB, GH, and VV performed the literature search and study design. JB, JQ, DY, LS, SL, YL, XX, and TN collected the data, processed statistical data, performed and data interpretation. JB, JQ, GH, and VV drafted the manuscript. GH and VV revised the final manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Cancer Center Climbing Foundation (No. NCC201917B03) and Hubei Provincial Health Commission (No. ZY2021M008). JQ is supported by University of Massachusetts Amherst.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab With or Without Radiotherapy for Metastatic Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomised Trials. Lancet Respir Med (2021) 9:467–75. doi: 10.1016/S2213-2600(20)30391-X
2. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol (2019) 10:108. doi: 10.3389/fimmu.2019.00108
3. Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer. Clin Lung Cancer (2020) 21(5):e435–44. doi: 10.1016/j.cllc.2020.02.025
4. Thomas R, Chen YH, Hatabu H, Mak RH, Nishino M. Radiographic Patterns of Symptomatic Radiation Pneumonitis in Lung Cancer Patients: Imaging Predictors for Clinical Severity and Outcome. Lung Cancer (2020) 145:132–9. doi: 10.1016/j.lungcan.2020.03.023
5. Lu CS, Liu JH. Pneumonitis in Cancer Patients Receiving Anti-PD-1 and Radiotherapies: Three Case Reports. Med (Baltimore) (2017) 96:e5747. doi: 10.1097/MD.0000000000005747
6. Kroeze SG, Fritz C, Hoyer M, Lo SS, Ricardi U, Sahgal A, et al. Toxicity of Concurrent Stereotactic Radiotherapy and Targeted Therapy or Immunotherapy: A Systematic Review. Cancer Treat Rev (2017) 53:25–37. doi: 10.1016/j.ctrv.2016.11.013
7. Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J. Predicting Risk of Radiation-Induced Lung Injury. J Thorac Oncol (2007) 2:864–74. doi: 10.1097/JTO.0b013e318145b2c6
8. Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation Dose-Volume Effects in the Lung. Int J Radiat Oncol Biol Phys (2010) 76:S70–76. doi: 10.1016/j.ijrobp.2009.06.091
9. Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, et al. Analysis of Clinical and Dosimetric Factors Associated With Treatment-Related Pneumonitis (TRP) in Patients With Non-Small-Cell Lung Cancer (NSCLC) Treated With Concurrent Chemotherapy and Three-Dimensional Conformal Radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys (2006) 66:399–407. doi: 10.1016/j.ijrobp.2006.07.1337
10. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic Body Radiation Therapy: The Report of AAPM Task Group 101. Med Phys (2010) 37:4078–101. doi: 10.1118/1.3438081
11. Schröder C, Stiefel I, Tanadini-Lang S, Pytko I, Vu E, Guckenberger M, et al. Re-Irradiation in the Thorax - An Analysis of Efficacy and Safety Based on Accumulated EQD2 Doses. Radiother Oncol (2020) 152:56–62. doi: 10.1016/j.radonc.2020.07.033
12. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab Alone or With Chemotherapy Versus Cetuximab With Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
13. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38(14):1505–17. doi: 10.1200/JCO.19.03136
14. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-An Update From the PACIFIC Trial. J Thorac Oncol (2021) 16(5):860–7. doi: 10.1016/j.jtho.2020.12.015
15. Verma V, Cushman TR, Selek U, Tang C, Welsh JW. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int J Radiat Oncol Biol Phys (2018) 101:1141–8. doi: 10.1016/j.ijrobp.2018.04.054
16. Tian S, Switchenko JM, Buchwald ZS, Patel PR, Shelton JW, Kahn SE, et al. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis. Int J Radiat Oncol Biol Phys (2020) 108:304–13. doi: 10.1016/j.ijrobp.2019.12.030
17. Cousin F, Desir C, Ben Mustapha S, Mievis C, Coucke P, Hustinx R. Incidence, Risk Factors, and CT Characteristics of Radiation Recall Pneumonitis Induced by Immune Checkpoint Inhibitor in Lung Cancer. Radiother Oncol (2021) 157:47–55. doi: 10.1016/j.radonc.2021.01.001
18. Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol (2021) 7(9):1–9. doi: 10.1001/jamaoncol.2021.2301
19. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest (2017) 152:271–81. doi: 10.1016/j.chest.2017.04.177
20. Chun S, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol (2017) 35:56–62. doi: 10.1200/JCO.2016.69.1378
Keywords: radiotherapy, immune checkpoint inhibitor, pneumonitis, dosimetry, risk factors
Citation: Bi J, Qian J, Yang D, Sun L, Lin S, Li Y, Xue X, Nie T, Verma V and Han G (2022) Dosimetric Risk Factors for Acute Radiation Pneumonitis in Patients With Prior Receipt of Immune Checkpoint Inhibitors. Front. Immunol. 12:828858. doi: 10.3389/fimmu.2021.828858
Received: 04 December 2021; Accepted: 27 December 2021;
Published: 13 January 2022.
Edited by:
Dawei Chen, Shandong Cancer Hospital, ChinaReviewed by:
Yujin Xu, University of Chinese Academy of Sciences, ChinaZhigang Liu, The Fifth Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2022 Bi, Qian, Yang, Sun, Lin, Li, Xue, Nie, Verma and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Han, aGc3OTEzQGhvdG1haWwuY29t; Vivek Verma, dml2ZWszMzNAZ21haWwuY29t
†These authors have contributed equally to this work
 Jianping Bi
Jianping Bi Jing Qian
Jing Qian Dongqin Yang3†
Dongqin Yang3† Lu Sun
Lu Sun Shouyu Lin
Shouyu Lin Ying Li
Ying Li Xudong Xue
Xudong Xue Tingting Nie
Tingting Nie Vivek Verma
Vivek Verma Guang Han
Guang Han