- 1Department of Dermatology, Chang Gung Memorial Hospital, Linkou, Taiwan
- 2School of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Vitiligo Center and Pigment Research Center, Chang Gung Memorial Hospital, Linkou, Taiwan
- 4Graduate Institute of Clinical Medical Sciences, Chang Gung University, Taoyuan, Taiwan
- 5Department of Physical Medicine and Rehabilitation, Lotung Poh-Ai Hospital, Lo-Hsu Medical Foundation, Inc., Yilan, Taiwan
- 6Department of Dermatology, Jen-Ai Hospital, Tai Chung, Taiwan
Interleukin (IL)-17 inhibitor is a biological therapy approved for moderate to severe psoriasis and psoriatic arthritis. The common adverse events of IL-17 inhibitor include injection site reaction, infections, nasopharyngitis, and headache. However, vitiligo associated with the use of IL-17 inhibitors was rarely reported in the previous literature. Here we described a woman who developed de novo vitiligo after 4 months of IL-17A inhibitor treatment for psoriasis and psoriatic arthritis. Upon discontinuation of IL-17A inhibitor and shifting to a broader T cell inhibitor—cyclosporine, our patient had control of both psoriasis and vitiligo and achieved 75% repigmentation after 3 months of oral cyclosporine without phototherapy. Due to the increasing use of anti-IL-17 biologics in psoriasis patients, clinicians should inquire about vitiligo’s history before treatment and inform patients of the possible adverse effects.
Introduction
Interleukin (IL)-17 inhibitor is a biologic therapy approved for moderate to severe psoriasis and psoriatic arthritis (1). The common adverse events of IL-17 inhibitor include injection site reaction, infections, nasopharyngitis, and headache (1). However, vitiligo associated with the use of IL-17 inhibitor was rarely reported in the previous literature (2–4). Here we described a woman who developed vitiligo after IL-17A inhibitor treatment for psoriasis and psoriatic arthritis.
Case report
A 72-year-old woman presented with chronic plaque psoriasis and psoriatic arthritis since she was 16 years old. She received methotrexate and TNF-α inhibitors for psoriatic arthritis and psoriasis, but it was unsatisfactory. Her psoriatic plaques cleared, and her arthritis improved under the treatment of an IL-17A inhibitor (ixekizumab). However, she experienced skin erythema, burning sensation, and itchiness in the eighth week of treatment, followed by the rapid development of depigmented patches on the face in the fourth month of treatment (Figure 1A). Wood’s lamp examination showed bluish–whitish fluorescence on the depigmented patches, confirming the diagnosis of vitiligo (Figure 1B). The laboratory examination for lupus and thyroid profile was unremarkable [anti-nuclear antibody (≤1:80; range: ≤1:80), complement 3 (119 mg/dl; range: 90–180), complement 4 (23.4 mg/dl; degree: 10–40), rheumatic factor IgM (<5 units; range: ≤6), normal anti-thyroid peroxidase antibody (anti-TPO; <3 IU/ml; range: <5.6), normal free T4 (0.95 ng/dl; range: 0.70–1.48), and thyroid-stimulating hormone (1.656 uIU/ml; range: 0.35–4.94)]. We discontinued the IL-17A inhibitor, and her psoriasis worsened gradually with no signs of repigmentation of the face. We treated her with oral methotrexate, topical steroid, and topical calcineurin inhibitor for 2 months, but her response was poor. Subsequently, we shifted to oral cyclosporine (3 mg/kg/day) to control psoriasis and vitiligo. Her psoriasis and arthritis were well controlled under cyclosporine. Interestingly, her facial vitiligo also repigmented and achieved 75% repigmentation in the third month of treatment (Figures 1C, D). She did not receive phototherapy during the treatment period due to inconvenience.
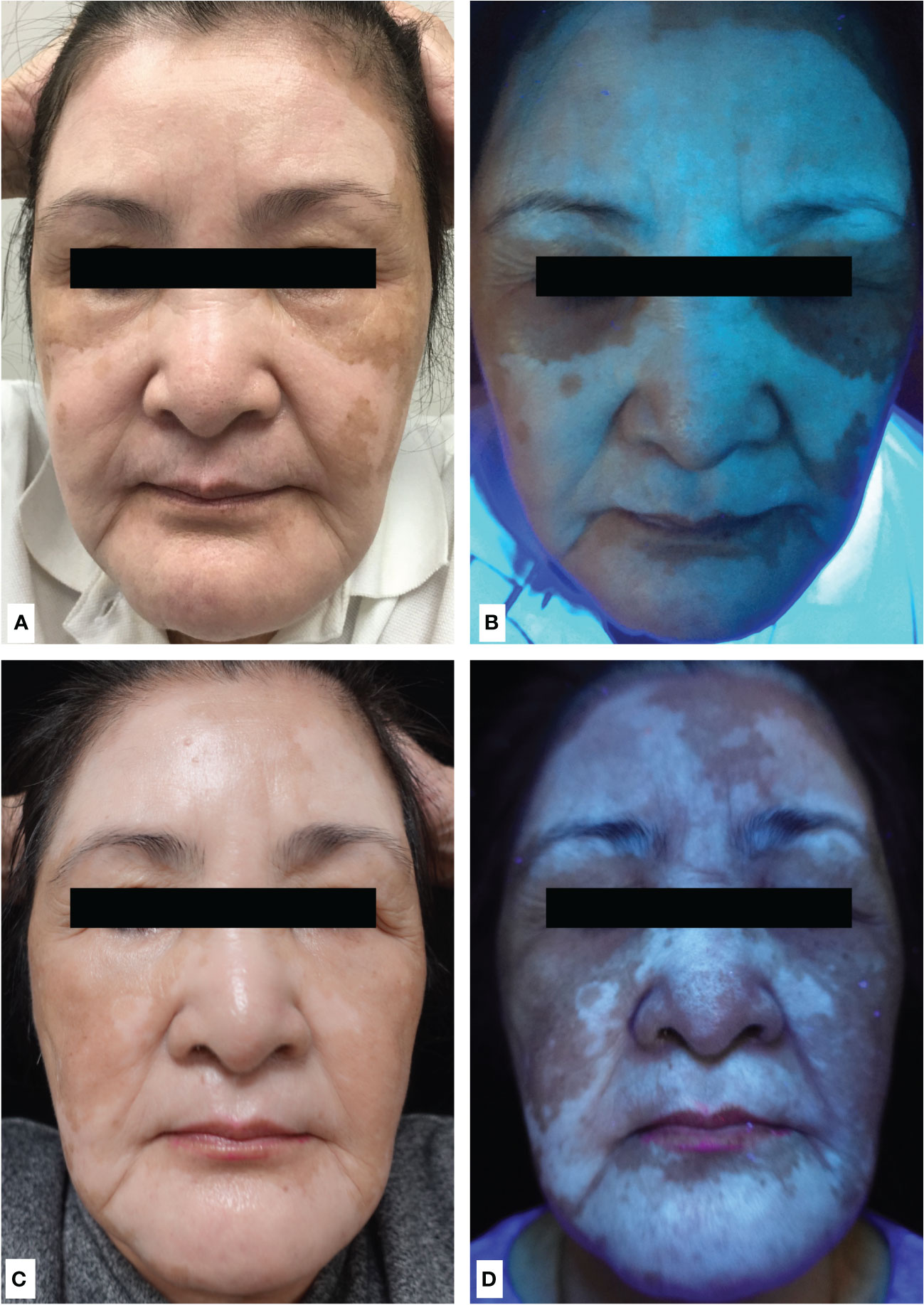
Figure 1 (A) Multiple depigmented facial patches sparing the periorbital areas after 4 months of treatment with anti-IL-17A antibody (ixekizumab). (B) Wood’s lamp of depigmented patches on the face showed bluish–white fluorescence, confirming the diagnosis of vitiligo. (C) Achieving 75% repigmentation of vitiligo under normal light and (D) under Wood’s lamp after discontinuation of ixekizumab and 3 months of cyclosporine.
Discussion
Vitiligo is an autoimmune disease with progressive destruction of melanocytes in the skin, resulting in patchy disfiguring depigmentation (5). Recent studies implicate the autoreactive CD8+ T cell-mediated destruction of melanocytes, and IFN-γ is the critical driver of autoimmunity in vitiligo (6). Studies have shown that IFN-γ may contribute to the polarization of T cells to a T helper type 1 (Th1) phenotype (7). Although circulating IL-17 levels and T helper type 17 (Th17) numbers have been shown to increase in vitiligo patients, the pathogenic role of IL-17 and Th17 in vitiligo is debatable (4).
IL-17A inhibitor-associated de novo vitiligo is a rare adverse event. Our case developed facial skin redness and itchiness followed by rapid depigmentation after IL-17A inhibitor therapy for chronic plaque psoriasis and psoriatic arthritis. A pilot study using IL-17 inhibitors to treat active non-segmental vitiligo has shown the progression of the disease, thus limiting further enrollment (4). Speeckart et al. found that patients with progression of vitiligo have significantly increased Th17.1 and Th1 lymphocytes, but not Th17 lymphocytes (4). We postulate that targeting IL-17 inhibits Th17, skewing towards a predominantly Th1 response that exacerbates vitiligo. On the contrary, a broad-spectrum T-cell calcineurin inhibitor does not influence the Th1/Th17 balance and therefore controls both psoriasis and vitiligo.
A nationwide cohort study reported a few isolated cases of de novo vitiligo following biologic therapy (2). The most commonly reported were TNF-α inhibitors, followed by IL-12/23 inhibitors. However, IL-17A inhibitor was rarely reported. Table 1 shows a summary of the published cases of IL-17 inhibitor-associated vitiligo in the literature (2–4, 8). There has been primarily reported cases in association with secukinumab (2, 4), one case with ixekizumab (3), and no case with brodalumab. A paradoxical reaction resulting in the appearance or worsening of chronic immune-mediated disease that usually responds to this drug class is not uncommon during biologic therapy (9). A delicate balance in the Th1/Th17 immune response is vital to prevent paradoxical and adverse reactions. Studies have demonstrated that Th1 and Th17 regulate one another through a delicate balance. The lack of one of these corresponding effector cytokines can promote a response dominated by the other. In an animal study, IL-17 deficiency contributes to increased IFN-γ+Th1 cells and an elevated Th1 response (10).
Upon the discontinuation of IL-17A inhibitor and shifting to a broader T cell inhibitor—cyclosporine, our patient had control of both psoriasis and vitiligo and managed to achieve 75% repigmentation of facial vitiligo 3 months later without phototherapy. This leaves the question of whether the patient would benefit from a broad-spectrum immunosuppressant or a specific targeted biologic therapy. Considering the balance of Th1/Th17 immune response in patients with concomitant psoriasis and vitiligo, the selection of steroid-sparing immunosuppressants with broader immunosuppressive properties—such as cyclosporine, JAK inhibitors, and methotrexate—may be beneficial (11).
Both vitiligo and psoriasis are common chronic autoimmune skin disorders. Studies have shown that the frequency of vitiligo among psoriasis patients is 2%, and patients with vitiligo showed a 2.29-fold risk of concomitant psoriasis (12). Therefore, a concurrent diagnosis of psoriasis and vitiligo is not uncommon. In contrast to psoriasis, vitiligo is more difficult to treat and irreversible if the melanocyte destruction is too advanced, resulting in permanent “color scarring” that significantly affects the patients’ quality of life and causing social stigma. De novo vitiligo or worsening preexisting vitiligo may develop during biologic therapy for inflammatory disorders such as psoriasis. Due to the increasing use of anti-IL-17 biologics in psoriasis patients, when both diseases coexist, clinicians should be cautious when selecting a targeted biologic therapy, and the history of vitiligo should be taken into consideration.
Furthermore, clinicians should inquire about vitiligo’s history before treatment and inform patients of the possible adverse effects. The early recognition of this adverse effect and prompt treatment can also prevent overt melanocyte destruction. Further studies should be conducted for factors predicting the risk of this paradoxical adverse effect. It is worth noting that, due to the complexity of each patient with varied cytokine responses to biologics, immunophenotyping of each patient may be an option to direct personalized biologic therapy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Chang Gung Memorial hospital, IRB no. 202100855B0. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
H-JS: manuscript drafting and literature review. Y-PC: literature review. P-CS: literature review. C-LK: Conceptualization. CN: conceptualization, manuscript revision, data acquisition, funding. All authors contributed to the article and approved the submitted version.
Funding
This study received financial assistance (CMRPGBJ0023 and MOST 110-2314-B-182A-155-MY3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Loft ND, Vaengebjerg S, Halling AS, Skov L, Egeberg A. Adverse events with IL-17 and IL-23 inhibitors for psoriasis and psoriatic arthritis: A systematic review and meta-analysis of phase III studies. J Eur Acad Dermatol Venereol (2020) 34:1151–60. doi: 10.1111/jdv.16073
2. Méry-Bossard L, Bagny K, Chaby G, Khemis A, Maccari F, Marotte H, et al. New-onset vitiligo and progression of pre-existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol (2017) 31:181–6. doi: 10.1111/jdv.13759
3. Pirro F, Caldarola G, De Simone C, Moretta G, Giovanardi G, Peris K. Multiple paradoxical reactions during ixekizumab therapy. Dermatol Ther (2019) 32:e12852. doi: 10.1111/dth.12852
4. Speeckaert R, Mylle S, Van Geel N. IL-17A is not a treatment target in progressive vitiligo. Pigment Cell Melanoma Res (2019) 32:842–7. doi: 10.1111/pcmr.12789
5. Picardo M, Dell'anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers (2015) 1:15011. doi: 10.1038/nrdp.2015.11
6. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol (2012) 132:1869–76. doi: 10.1038/jid.2011.463
7. Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology (2009) 126:386–93. doi: 10.1111/j.1365-2567.2008.02905.x
8. Giordano D, Magri F, Persechino F, Lepore A, Verde R, Capalbo A, et al. Vitiligo with progressive repigmentation during secukinumab treatment in a patient with psoriatic arthritis: A case report. Case Rep Dermatol (2021) 13:209–15. doi: 10.1159/000510831
9. Murphy MJ, Cohen JM, Vesely MD, Damsky W. Paradoxical eruptions to targeted therapies in dermatology: A systematic review and analysis. J Am Acad Dermatol (2020) 86(5):1080–91. doi: 10.1016j.jaad.2020.12.010
10. Das G, Sheridan S, Janeway CA Jr. The source of early IFN-gamma that plays a role in Th1 priming. J Immunol (2001) 167:2004–10. doi: 10.4049/jimmunol.167.4.2004
11. Bergqvist C, Ezzedine K. Vitiligo: A review. Dermatology (2020) 236:571–92. doi: 10.1159/000506103
Keywords: vitiligo, anti-IL17 agents, de novo, paradoxical reaction, psoriasis, Anti-IL17A-antibody
Citation: Su H-J, Chan Y-P, Shen P-C, Ku C-L and Ng CY (2023) Anti-IL-17A antibody-associated de novo vitiligo: Case report and review of literature. Front. Immunol. 13:1077681. doi: 10.3389/fimmu.2022.1077681
Received: 23 October 2022; Accepted: 23 December 2022;
Published: 18 January 2023.
Edited by:
Roopesh Singh, Vanderbilt University Medical Center, United StatesReviewed by:
Mary A Markiewicz, University of Kansas Medical Center, United StatesEfterpi Zafiriou, University of Thessaly, Greece
Copyright © 2023 Su, Chan, Shen, Ku and Ng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chau Yee Ng, bWRjaGFybGVuZUBnbWFpbC5jb20=; Y3luZzg3QGNnbWgub3JnLnR3
 Hsing-Jou Su
Hsing-Jou Su Yu-Pei Chan
Yu-Pei Chan Peng-Chieh Shen
Peng-Chieh Shen Cheng-Lung Ku
Cheng-Lung Ku Chau Yee Ng
Chau Yee Ng