- 1National Center for Electron Beam Research, an International Atomic Energy Agency (IAEA) Collaborating Center for Electron Beam Technology, Texas A&M University, College Station, TX, United States
- 2Department of Food Science and Technology, Texas A&M University, College Station, TX, United States
Given the current pandemic the world is struggling with, there is an urgent need to continually improve vaccine technologies. Ionizing radiation technology has a long history in the development of vaccines, dating back to the mid-20th century. Ionizing radiation technology is a highly versatile technology that has a variety of commercial applications around the world. This brief review summarizes the core technology, the overall effects of ionizing radiation on bacterial cells and reviews vaccine development efforts using ionizing technologies, namely gamma radiation, electron beam, and X-rays.
Introduction
Vaccination is a cornerstone of public health measures. It promotes human and animal health as well as prevents the spread of communicable diseases in humans and animals. Over one hundred vaccines are currently licensed for human use in the United States (1). Despite this, many infectious diseases, such as Covid-19, HIV, Influenza, Malaria, and Tuberculosis continue to cause severe illness and death globally. In the feed and livestock animal industries, the use of antibiotic growth promoters has been substantially reduced due to fears of multi-drug resistant bacteria, (2–5). However, with the ban of antimicrobial usage, therapeutic usage of antimicrobials increased in Denmark by 33.6% (6) and mortality in weaning pigs increased by 1.5% (2). The resurgence of previously controlled infections and diseases have led to the intensive investigation and commercialization of multiple methods to control and improve animal health, with vaccinations being the most common (3, 7–9).
Current vaccine technologies have their advantages and disadvantages. Live vaccines often elicit strong immune responses, but a balance between attenuation, safety, and protection must be struck. Vaccination with attenuated strains has often been successful, although this option is not suitable for some diseases (10–13). A disadvantage of attenuated vaccines is the fear of regained virulence. Inactivated, or killed vaccines are inactivated using chemicals such as formalin, diethylpyrocarbonate and β-propiolactone. Although there are reduced safety risks associated with chemically inactivated vaccines, they often exhibit reduced immunogenicity due to damaged antigenic epitopes. Toxoids, recombinant vaccines, as well as subunit vaccines are typically considered safe because attenuation is induced by deletions preventing the strain from overgrowing and causing disease (14). The disadvantage of sub-unit vaccines is that only a singular antigen or at times multiple antigens are presented, generally limiting the cross-protective ability of such vaccines.
Given increased urbanization, climate change and close interaction of animals and humans, there is a continuous need to evaluate vaccine technologies to deal with epidemics, pandemics, and rapidly emerging infectious virus variants. The vaccine technologies should be robust and capable of dealing with multiple pathogens, their possible variants and host species (15). Ionizing radiation technology has benefitted society for over 65 years. Legacy nuclear technologies based on radioactive isotopes such as cobalt-60 and cesium-137 have resulted in significant benefits to human and animal health and agriculture. Besides radioactive isotope based ionizing radiation technology, electron beam (eBeam) and X-ray technologies have grown rapidly in the last decade and are now becoming widely used for a variety of commercial applications. The overall objective of this brief review is to summarize the history and the advances of using ionizing radiation technology for developing vaccines against infectious diseases.
Principles of Ionizing Radiation
Ionizing radiation is defined as energy capable of removing electrons from atoms and, thereby, causing ionization. The three main ionizing radiation technologies are gamma radiation technology (based on photons), electron beam (eBeam) technology (based on electrons), and X-rays (based on photons) (16). Gamma rays are electromagnetic radiation composed of photons emitted from the nucleus of a radioactive isotope. In most commercial settings, the isotope source is cobalt-60. In some instances, gamma rays are produced from cesium-137 as well. Electron beam (eBeam) technology is based on highly energetic electrons that are produced from regular electricity using industrial equipment called “eBeam accelerators”. X-rays are also electromagnetic radiation composed of photons. However, they are generated using energetic electrons from accelerators which are allowed to strike an extremely dense metal such as tantalum or tungsten resulting in the formation of X-ray photons. Cobalt-60 is a radioactive isotope and, therefore, it is of serious security concerns. Also, due to increasing cobalt-60 costs, its stringent safe-guarding requirements, and ultimate disposal needs and costs, this legacy technology is quickly becoming commercially unsustainable. Commercially, gamma radiation technology is being quickly replaced with accelerator-based technologies, namely eBeam and X-ray technologies (16, 17). From a commercial perspective, eBeam technology is an attractive technology because of its relatively overall lower costs and relative ease of adoption. One of the key attractive features of eBeam and X-ray technologies is that they are switch- on/switch-off technologies meaning that they can be switched off when not in use. This is in direct contrast to radioactive isotopes such as cobalt-60 where the emission of gamma ray photons cannot be switched off.
Today, eBeam and X-ray technologies are commercial off the shelf technologies with a diverse array of energy and beam power configurations. In commercial settings, eBeam irradiation is generated using accelerators. In these accelerators, electrons generated from commercial electricity are accelerated to approximately 99.999% of the speed of light resulting in electron energies up to 10 MeV (Mega electron volts) (18). These highly energetic electrons are then focused and pulsed uniformly over a material, solid or liquid (16, 18). When the electrons interact with a molecule leading to its ionization, the ejected electron becomes energized, going on to interact with and ionize an adjacent molecule. This chain reaction continues until the energy has fully dissipated (18). High energy eBeam technology is also currently used in the food and medical device industry for its ability to either pasteurize products or achieve complete sterility. In the food industry, this technology is regularly used for phytosanitary treatment, shelf-life elongation, pathogen inactivation, and occasionally terminal sterilization (16, 17). In the medical device industry, this technology is used to sterilize single-use medical devices and laboratory consumables (19).
Effect of Ionizing Radiation Exposure on Microorganisms
Ionizing radiation inactivates microorganisms through direct and indirect methods. Direct damage is caused as a result of interactions between energetic electrons or photons and the molecules within an organism, while indirect damage is caused as a result of interactions with products of water radiolysis (18, 20, 21). When an energized electron from an accelerator (or a gamma photon emitted from a radioactive isotope) interacts with a material, molecules are ionized, ejecting electrons from the outermost valence shells. These ejected electrons in turn cause a cascade of similar ionization events on adjoining atoms until all its energy is fully dissipated. In microorganisms, DNA is the largest molecule, therefore, resulting in it being the primary target of direct ionization events. The ionization of DNA results in the cleavage of the phosphodiester bonds along the DNA backbone. While single-stranded breaks are repairable, extensive double stranded breaks are much harder for an organism to repair and overcome. Due to excessive shearing of the nucleic acid, the microorganism is ultimately inactivated (21). The other major target of ionizing radiation in a microorganism is its cellular water content, leading to the production of radiolytic species. Radiolysis of water generates a diverse array of highly reactive, but short lived free radical species such as hydroxyl radicals, hydrogen peroxide, hydrogen, hydrated electrons, and hydrated protons. The summary equation for water radiolysis is presented below (Equation 1) with the quantity of each product per 100 eV of energy absorbed shown in parenthesis.
The damage to the cellular components often results indirectly from the interaction of these reactive species as opposed to the direct incident electrons. Hydroxyl radicals (*OH) are extremely short lived. However, during their short time, they can cause significant damage to molecules in their immediate surroundings (22). Superoxide radicals are also generated by the radiolysis of water, and it is hypothesized that these molecules accumulate within a microbial cell causing severe damage to proteins such as enzymes with exposed iron-sulfur clusters (23, 24). Additionally, methionine and cysteine have been shown to be especially susceptible to ionizing radiation (25). Superoxide radicals also react with endogenous nitric oxide within a cell, forming reactive nitrogen species (RNS) such as a peroxynitrite anion (ONOO-), nitrogen dioxide , and dinitrogen trioxide (N2O3), which cause further damage to the DNA and are the primary agents of damage to proteins within bacterial cells (26). This protein damage can have significant effects on the microorganism’s ability to function. Taken together, direct and indirect mechanisms of damage lead to the inactivation of microbial cells due to the high number of single and double strand breaks (21). Assuming a hypothetical genome size of 3.5 million base pairs, a dose of 1 kGy would cause approximately 200 single stranded breaks and 14 double stranded breaks, per copy of a bacteria’s genome (18, 27). This extent of DNA damage is irreparable in most microorganisms, resulting in their inactivation due to the inability of the DNA to replicate, thereby, resulting in the microbial population being unable to reproduce. This damage done to microorganisms is extremely rapid. Direct damage due to chemical bonds cleavage is estimated to occur within 10-14 – 10-12 seconds of exposure. Within one picosecond (10-12 s), superoxide and hydrogen peroxide radicals are formed. By about 1 millisecond after exposure, the reactions of most reactive species are hypothesized to be complete (25, 28).
While microbial cells cease to multiply due to damage to their nucleic acids, multiple studies have demonstrated that their cellular membrane remains intact even after exposure to ionizing radiation. It needs to be pointed out the how microbial cells respond to ionizing radiation can be extremely varied depending on the microorganism in question and the ionizing radiation dose applied to the cells. Studies conducted in our laboratory demonstrate that eBeam exposure even at lethal doses does not compromise the bacterial cellular membrane as observed using microscopy (29–33). Similarly, gamma irradiation has also been shown to cause no damage to bacterial cell membranes at lethal doses (34–36). Furthermore, there is now significant evidence that in cells treated with lethal doses of ionizing radiation, there is residual metabolic activity after treatment (33, 35, 37–41). For example, in Escherichia coli K-12 metabolic activity of E. coli was sustained for up to nine days following treatment, as demonstrated using AlamarBlue™ as well as ATP assays (33). Other studies have demonstrated that gamma radiation also does not significantly hinder cellular functions. Gamma irradiated cells maintained oxidative function and the ability to continue nucleic acid and protein synthesis (35, 38). Furthermore, metabolic activity persists, despite several double stranded breaks of the cell’s genome. Researchers hypothesize that there are portions of genomes which are still intact, enough to sustain cellular functions (35, 39, 42). Bacterial cells exposed to eBeam exposure exhibit similar features. Studies examining the metabolomic state of inactivated E. coli and Salmonella Typhimurium have shown that immediately after treatment, cells are metabolically active with metabolomic fluxes continuing even 24 hours after eBeam treatment (43). Nevertheless, the ability of microbial cells to continue their metabolic activity even after physical damage to their nucleic acids is a scientific conundrum that is worthy of deeper investigation. Taken together, this state in microbial cells where the cells cannot multiply yet remain metabolically active can be termed as a Metabolically Active, yet Non-Culturable (MAyNC) state. In vaccinology, the term that is often used especially with irradiated malarial sporozoites is “Metabolically active, non-replicating”. This state has potential broad applications in vaccine development. MAyNC cells are inactivated, but maintain cell membrane integrity, and therefore, function as a killed vaccine. The biological significance of residual metabolic activity on the potency of the vaccine is yet to be completely understood. Because ionizing radiation maintains membrane integrity, MAyNC cells may be specifically well-suited for vaccines against pathogens that require immune recognition of multiple antigenic epitopes. Furthermore, due to the growing availability of eBeam and X-ray technologies which can be installed inline to the manufacturing process, the ability to generate MAyNC cells of varying potency can be extremely valuable for vaccine development.
History of Vaccines Using Ionizing Radiation
The use of ionizing radiation as a method to attenuate or inactive microorganisms for the use as vaccines is not novel, with reports of gamma and x-ray-inactivated vaccine research dating back to the mid-20th century (44–50). The advantage of ionizing-radiation vaccines, or radio-vaccines, is that because they are inactivated, they are able to retain immunogenicity even when stored at non-refrigerated conditions potentially eliminating the need for cold-chain to preserve vaccine potency (31, 51, 52). The ability to store vaccines at ambient or refrigerated storage (as compared to frozen storage) can translate to significantly lower overall costs for vaccine transportation and distribution. The ability to distribute vaccines without the need for cold chain distribution also increases vaccine access in remote areas (53, 54). Importantly, eBeam and X-ray technologies are scalable, with the capability to inactivate large quantities of preparations (55).
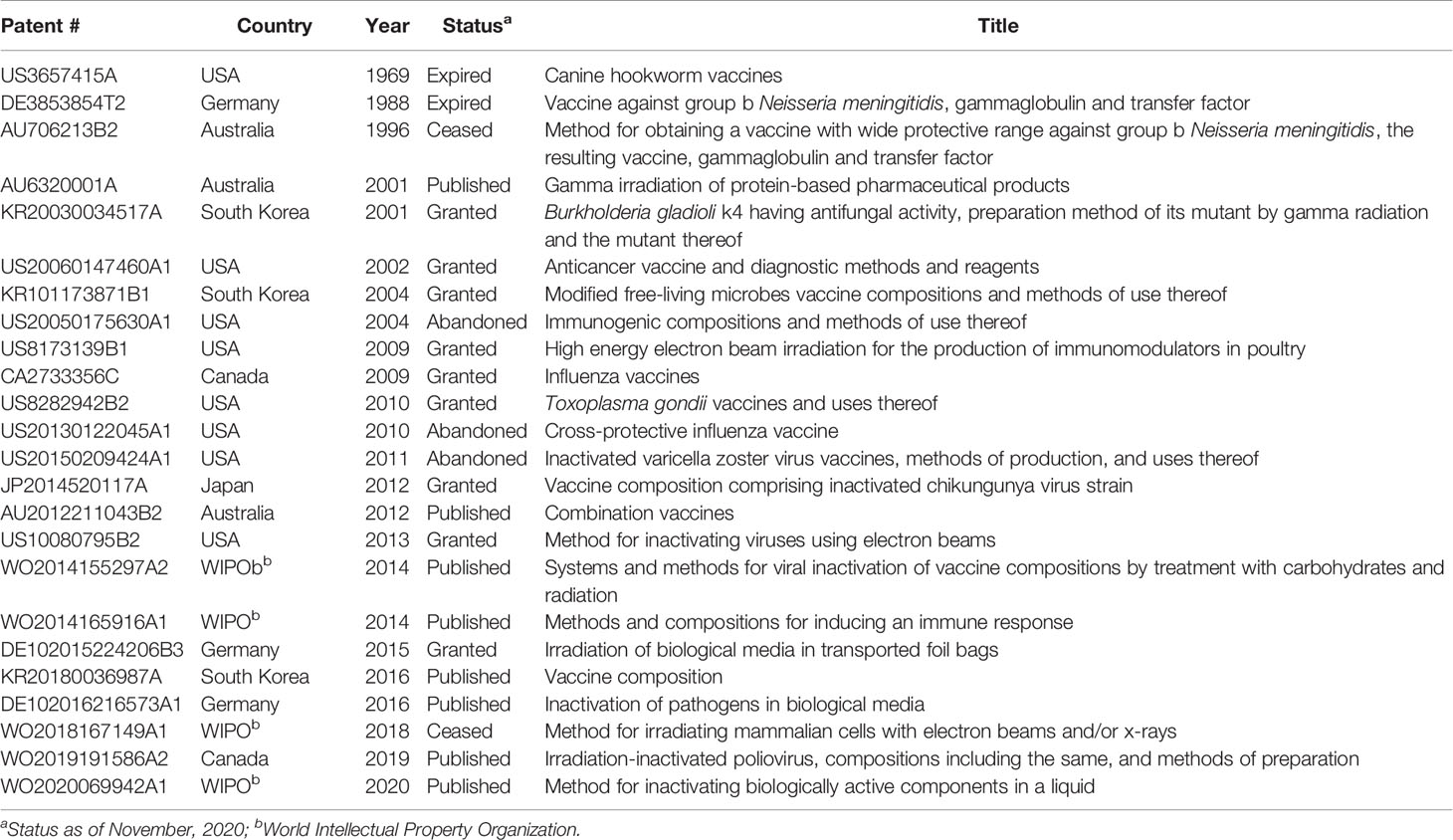
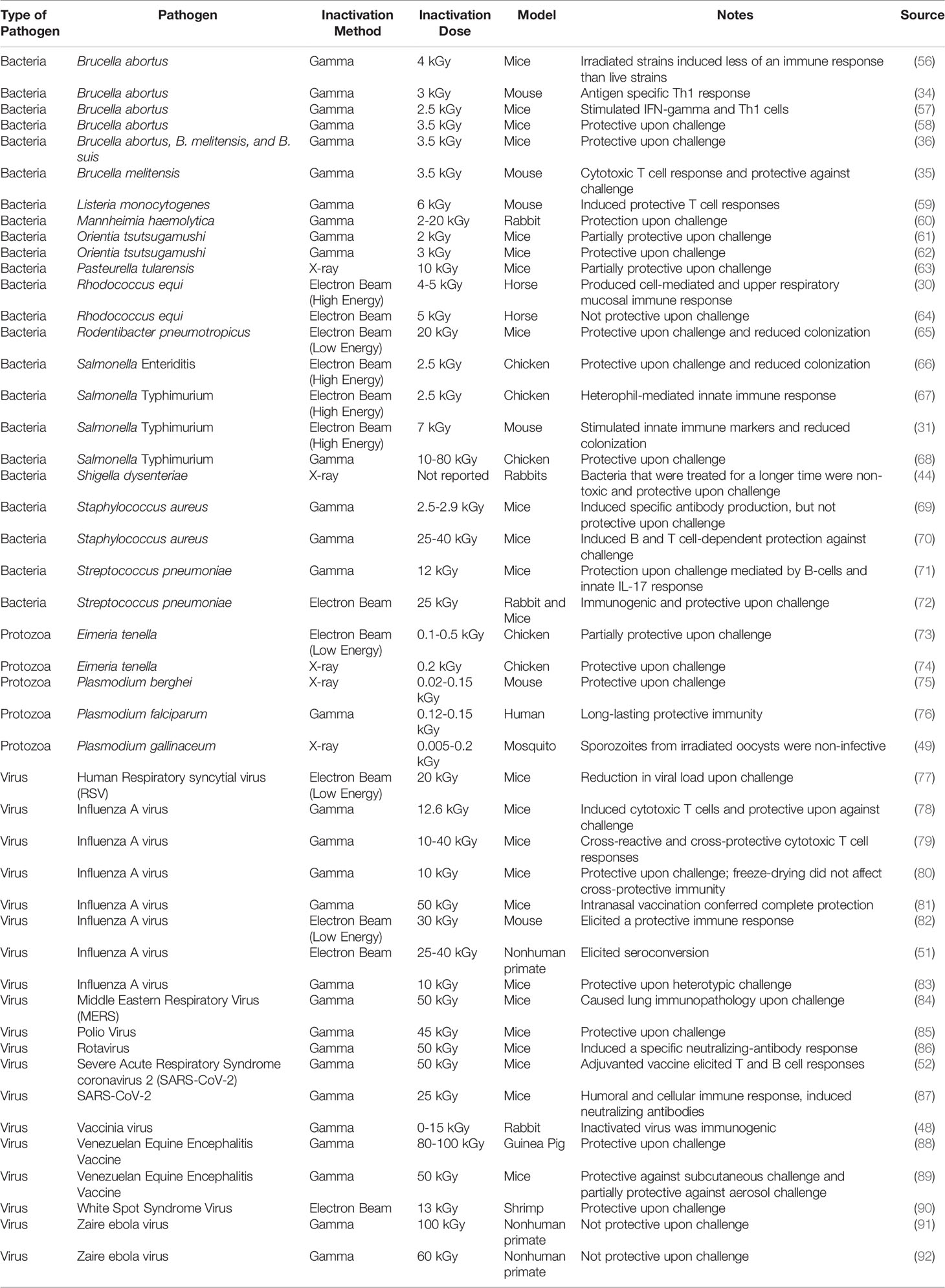
Due to the vast commercial capabilities, numerous patents related to “radio-vaccines” have already been filed (Table 1). Interest in radio- vaccines has increased significantly recently, with investigations into the creation of vaccines for bacterial, viral, and protozoan diseases (Table 2). While many of the researched vaccine candidates are based on gamma-irradiation, there is significantly less research conducted on eBeam or X-ray inactivated vaccines. This limited amount of information could be attributed to the relatively recent commercial availability of eBeam and X-ray technologies. Among all the research conducted on radio-vaccines, the most progress has been on Plasmodium sporozoites attenuated with irradiation to protect against malaria. First examined in 1967 using x-ray irradiation, this idea has evolved considerably over the last 50+ years to its current iteration in phase 2 clinical trials using gamma-attenuated sporozoites (75, 76, 93–97). Studies using gamma-irradiated Listeria monocytogenes have demonstrated that unlike other inactivation methods such as heat or formalin, irradiation better maintained antigenic properties and stimulated robust T cell responses (59).
Immune Responses to Radio-Vaccines
In multiple studies investigating the immune response to gamma-irradiated Brucella spp., investigators found that gamma-irradiated cells were metabolically active and inactivated cells were able to induce a significant cellular immune response and were protective when challenged (34–37, 56, 98). Furthermore, gamma-irradiated cells have even exhibited an ability to act as an adjuvant, increasing the immune response to co-administered antigens (71). A significant amount of research has been conducted on the development of a gamma-inactivated influenza vaccine, demonstrating that this vaccine is effective in eliciting a strong antigen-specific antibody response as well as protecting mice from challenge with heterologous influenza virus (27, 80, 83).
Electron beam (eBeam) technology has been investigated as a method to generate vaccine-like immunomodulators against Salmonella Typhimurium using a mice model (31). This concept has been expanded to demonstrating the immunomodulatory and protective effects of eBeam-inactivated Salmonella Enteriditis and Typhimurium in chickens and Rhodococcus equi in neonatal fouls (29–32, 40, 41, 64, 67). This concept is now been expanded to include the use of low energy eBeam as an inactivation technology for vaccine development with considerable success (73, 77, 82).
Role of Adjuvants
For a vaccine formulation to be effective upon challenge, it must be able to induce a prolonged and protective immune response. Live attenuated vaccines that retain their ability to replicate with a host, naturally eliciting a strong CD8+ and CD4+ T cell response, as well as a strong humoral response, while inactivated vaccines often require the assistance of an adjuvant to help the vaccine elicit a stronger immune response in the host. An adjuvant is technically defined as a component that is added to vaccine to enhance an immune response, and typically provides the benefits of increased antibody titers and an increased speed, breadth, and duration of an immune response. Because radio-vaccines are unable to replicate within a host, it has been proposed that their immunogenic potential has to be enhanced by the addition of an adjuvant. There are several reports about coupling radio-vaccines with experimental and commercially available adjuvants. Bayer et al. tested four different adjuvants in combination with Respiratory syncytial virus inactivated with low energy electron beam: Alhydrogel (alum based), MF59 (squalene based), QuilA (saponin based), and Poly IC : LC (synthetic double-stranded RNA based) (77). In their study, strong immune responses and significant reductions in viral loads were detected after immunization and subsequent challenge, although the poly IC : LC adjuvanted vaccine elicited lower titers of neutralizing antibodies than the other adjuvanted vaccines tested (77). Substantial humoral and cellular responses were observed when a gamma-inactivated polio vaccine candidate was combined with an alum adjuvant and when a gamma-irradiated HIN1 vaccine was co-administered with a plasmid encoding mouse interleukin-28B (99, 100). Gamma-inactivated SARS-CoV-2 also benefited from the addition of a GM-CSF adjuvant in order to induce a T cell response (52).
Conclusions
Though ionizing radiation has been researched as a vaccine technology for nearly a century, only recently have vaccines utilizing ionizing radiation reached commercial development. The general lack of interest in radio-vaccines could be attributed to advances in cloning technologies, mRNA vaccines and gene editing technologies. The recent availability of small footprint, low energy eBeam and X-ray equipment could, however, spur the development of radio-vaccines once again. Commercialization of eBeam and X-ray technologies for the medical device, food, and other industrial applications has led to a decrease in overall technology costs and an increase in technology availability (101). This review highlights the potential of ionizing radiation as a vaccine technology suitable against several pathogens causing diseases in various hosts species. This has been most recently demonstrated in the rapid development of vaccine candidates in response to the COVID-19 pandemic, caused by the virus SARS-CoV-2. Radio-vaccines have even been investigated as a response to previous outbreaks of SARS and MERS, and it was hypothesized that ionizing radiation could be used to rapidly produce a vaccine for SARS-CoV-2 (84, 102–104). Gamma-inactivated SARS-CoV-2 combined with GM-CSF as an adjuvant has demonstrated ability to induce neutralizing antibodies as well as a strong T and B cell response (87, 105).
Author Contributions
Major portions of this manuscript have been previously included in a doctoral dissertation by SB (106). SP was involved in writing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported funds from the USDA-NIFA program administered by Texas A&M AgriLife Research H-87080 as well as funds through contracts from the Pacific Northwest National Laboratories (PNNL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was prepared as part of the activities of the IAEA Collaborating Center for Electron Beam Technology.
References
1. FDA. Vaccines Licensed for Use in the United States (2021). FDA. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (Accessed September 19, 2021).
2. Wierup M. The Swedish Experience of the 1986 Year Ban of Antimicrobial Growth Promoters, With Special Reference to Animal Health, Disease Prevention, Productivity, and Usage of Antimicrobials. Microb Drug Resist (2001) 7:183–90. doi: 10.1089/10766290152045066
3. Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking Our Understanding of the Pathogenesis of Necrotic Enteritis in Chickens. Trends Microbiol (2009) 17:32–6. doi: 10.1016/j.tim.2008.09.005
4. Maron DF, Smith TJ, Nachman KE. Restrictions on Antimicrobial Use in Food Animal Production: An International Regulatory and Economic Survey. Global Health (2013) 9:48. doi: 10.1186/1744-8603-9-48
5. McEwen SA, Angulo FJ, Collignon PJ, Conly J. Potential Unintended Consequences Associated With Restrictions on Antimicrobial Use in Food-Producing Animals (2017). World Health Organization. Available at: https://www.ncbi.nlm.nih.gov/books/NBK487949/ (Accessed January 20, 2020).
6. DANMAP. DANMAP – Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria From Food Animals, Food and Humans in Denmark (2010). Available at: http://danmap.org/reports/older.
7. Cheng G, Hao H, Xie S, Wang X, Dai M, Huang L, et al. Antibiotic Alternatives: The Substitution of Antibiotics in Animal Husbandry? Front Microbiol (2014) 5:217. doi: 10.3389/fmicb.2014.00217
8. Caly DL, D’Inca R, Auclair E, Drider D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Front Microbiol (2015) 6:1336. doi: 10.3389/fmicb.2015.01336
9. Marquardt RR, Li S. Antimicrobial Resistance in Livestock: Advances and Alternatives to Antibiotics. Anim Fron (2018) 8:30–7. doi: 10.1093/af/vfy001
10. Thompson DR, Parreira VR, Kulkarni RR, Prescott JF. Live Attenuated Vaccine-Based Control of Necrotic Enteritis of Broiler Chickens. Vet Microbiol (2006) 113:25–34. doi: 10.1016/j.vetmic.2005.10.015
11. McCullers JA, Dunn JD. Advances in Vaccine Technology And Their Impact on Managed Care. P T (2008) 33:35–41.
12. Mishra N, Smyth JA. Oral Vaccination of Broiler Chickens Against Necrotic Enteritis Using a Non-Virulent NetB Positive Strain of Clostridium Perfringens Type A. Vaccine (2017) 35:6858–65. doi: 10.1016/j.vaccine.2017.10.030
13. Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging Concepts and Technologies in Vaccine Development. Front Immunol (2020) 11:583077. doi: 10.3389/fimmu.2020.583077
14. Spreng S, Dietrich G, Weidinger G. Rational Design of Salmonella-Based Vaccination Strategies. Methods (2006) 38:133–43. doi: 10.1016/j.ymeth.2005.09.012
15. Graham BS, Mascola JR, Fauci AS. Novel Vaccine Technologies: Essential Components of an Adequate Response to Emerging Viral Diseases. JAMA (2018) 319:1431–2. doi: 10.1001/jama.2018.0345
16. Pillai SD, Shayanfar S. Electron Beam Technology and Other Irradiation Technology Applications in the Food Industry, in: Applications of Radiation Chemistry in the Fields of Industry, Biotechnology and Environment Topics in Current Chemistry Collections (2017). Springer International Publishing (Accessed July 7, 2018).
17. Pillai SD, Bhatia SS. Electron Beam Technology: A Platform for Safe, Fresh, and Chemical-Free Food, in: Food Safety Magazine (2018). Available at: https://www.foodsafetymagazine.com/magazine-archive1/aprilmay-2018/electron-beam-technology-a-platform-for-safe-fresh-and-chemical-free-food/ (Accessed January 28, 2019).
18. Miller RB. Electronic Irradiation of Foods: An Introduction to the Technology. New York: Springer (2005).
19. IIA, GIPA. A Comparison of Gamma, E-Beam, X-Ray and Ethylene Oxide Technologies for the Industrial Sterilization of Medical Devices and Healthcare Products (2017). Available at: http://http://gipalliance.net/wp-content/uploads/2013/01/GIPA-WP-GIPA-iia-Sterilization-Modalities-FINAL-Version-2017-October-308772.pdf (Accessed January 23,2022).
20. Urbain WM. CHAPTER 4 - Biological Effects of Ionizing Radiation. In: Urbain WM, editor. Food Irradiation. Academic Press (1986). p. 83–117. doi: 10.1016/B978-0-12-709370-3.50010-5
21. Tahergorabi R, Matak KE, Jaczynski J. Application of Electron Beam to Inactivate Salmonella in Food: Recent Developments. Food Res Int (2012) 45:685–94. doi: 10.1016/j.foodres.2011.02.003
22. Mavragani IV, Nikitaki Z, Kalospyros SA, Georgakilas AG. Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers (Basel) (2019) 11:1789–818. doi: 10.3390/cancers11111789
23. Keyer K, Imlay JA. Superoxide Accelerates DNA Damage by Elevating Free-Iron Levels. PNAS (1996) 93:13635–40. doi: 10.1073/pnas.93.24.13635
24. Popović-Bijelić A, Mojović M, Stamenković S, Jovanović M, Selaković V, Andjus P, et al. Iron-Sulfur Cluster Damage by the Superoxide Radical in Neural Tissues of the SOD1(G93A) ALS Rat Model. Free Radic Biol Med (2016) 96:313–22. doi: 10.1016/j.freeradbiomed.2016.04.028
25. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid Redox Signal (2014) 21:260–92. doi: 10.1089/ars.2013.5489
26. Daly MJ. A New Perspective on Radiation Resistance Based on Deinococcus Radiodurans. Nat Rev Microbiol (2009) 7:237–45. doi: 10.1038/nrmicro2073
27. Alsharifi M, Müllbacher A. The γ-Irradiated Influenza Vaccine and the Prospect of Producing Safe Vaccines in General. Immunol Cell Biol (2010) 88:103–4. doi: 10.1038/icb.2009.81
28. Singh A, Singh H. Time-Scale and Nature of Radiation-Biological Damage: Approaches to Radiation Protection and Post-Irradiation Therapy. Prog Biophys Mol Biol (1982) 39:69–107. doi: 10.1016/0079-6107(83)90014-7
29. McReynolds JL, Pillai S, Jesudhasan PRR, Hernandez MLC. High Energy Electron Beam Irradiation for the Production of Immunomodulators in Poultry. (2012) US Patent US8173139B1.
30. Bordin AI, Pillai SD, Brake C, Bagley KB, Bourquin JR, Coleman M, et al. Immunogenicity of an Electron Beam Inactivated Rhodococcus Equi Vaccine in Neonatal Foals. PloS One (2014) 9:e105367. doi: 10.1371/journal.pone.0105367
31. Praveen C. Electron Beam as a Next Generation Vaccine Platform: Microbiological and Immunological Characterization of an Electron Beam Based Vaccine Against Salmonella Typhimurium. [Ph.D. Dissertation]. College Station (TX: Texas A&M University (2014).
32. Jesudhasan PR, McReynolds JL, Byrd AJ, He H, Genovese KJ, Droleskey R, et al. Electron-Beam–Inactivated Vaccine Against Salmonella Enteritidis Colonization in Molting Hens. Avian Dis (2015) 59:165–70. doi: 10.1637/10917-081014-ResNoteR
33. Hieke A-SC, Pillai SD. Escherichia Coli Cells Exposed to Lethal Doses of Electron Beam Irradiation Retain Their Ability to Propagate Bacteriophages and Are Metabolically Active. Front Microbiol (2018) 9:2138. doi: 10.3389/fmicb.2018.02138
34. Sanakkayala N, Sokolovska A, Gulani J, HogenEsch H, Sriranganathan N, Boyle SM, et al. Induction of Antigen-Specific Th1-Type Immune Responses by Gamma-Irradiated Recombinant Brucella Abortus RB51. Clin Diagn Lab Immunol (2005) 12:1429–36. doi: 10.1128/CDLI.12.12.1429-1436.2005
35. Magnani DM, Harms JS, Durward MA, Splitter GA. Nondividing But Metabolically Active Gamma-Irradiated Brucella Melitensis Is Protective Against Virulent B. Melitensis Challenge in Mice. Infect Immun (2009) 77:5181–9. doi: 10.1128/IAI.00231-09
36. Moustafa D, Garg VK, Jain N, Sriranganathan N, Vemulapalli R. Immunization of Mice With Gamma-Irradiated Brucella Neotomae and its Recombinant Strains Induces Protection Against Virulent B. Abortus, B. Melitensis and B. Suis Challenge. Vaccine (2011) 29:784–94. doi: 10.1016/j.vaccine.2010.11.018
37. Ahn TH, Nishihara H, Carpenter CM, Taplin GV. Respiration of Gamma Irradiated Brucella Abortus and Mycobacterium Tuberculosis. Proc Soc Exp Biol Med (1962) 111:771–3. doi: 10.3181/00379727-111-27917
38. Hiramoto RM, Galisteo AJ, do Nascimento N. And De Andrade, H200 Gy Sterilised Toxoplasma Gondii Tachyzoites Maintain Metabolic Functions and Mammalian Cell Invasion, Eliciting Cellular Immunity and Cytokine Response Similar to Natural Infection in Mice. F Vaccine (2002) 20:2072–81. doi: 10.1016/S0264-410X(02)00054-3
39. Secanella-Fandos S, Noguera-Ortega E, Olivares F, Luquin M, Julián E. Killed But Metabolically Active Mycobacterium Bovis Bacillus Calmette-Guérin Retains the Antitumor Ability of Live Bacillus Calmette-Guérin. J Urol (2014) 191:1422–8. doi: 10.1016/j.juro.2013.12.002
40. Jesudhasan PR, Bhatia SS, Sivakumar KK, Praveen C, Genovese KJ, He HL, et al. Controlling the Colonization of Clostridium Perfringens in Broiler Chickens by an Electron-Beam-Killed Vaccine. Animals (2021) 11:671. doi: 10.3390/ani11030671
41. Praveen C, Bhatia SS, Alaniz RC, Droleskey RE, Cohen ND, Jesudhasan PR, et al. Assessment of Microbiological Correlates and Immunostimulatory Potential of Electron Beam Inactivated Metabolically Active Yet Non Culturable (MAyNC) Salmonella Typhimurium. PloS One (2021) 16:e0243417. doi: 10.1371/journal.pone.0243417
42. Trampuz A. Effect of Gamma Irradiation on Viability and DNA of Staphylococcus Epidermidis and Escherichia Coli. J Med Microbiol (2006) 55:1271–5. doi: 10.1099/jmm.0.46488-0
43. Bhatia SS, Pillai SD. A Comparative Analysis of the Metabolomic Response of Electron Beam Inactivated E. Coli O26:H11 and Salmonella Typhimurium ATCC 13311. Front Microbiol (2019) 10:694. doi: 10.3389/fmicb.2019.00694
44. Moore HN, Kersten H. Preliminary Note on the Preparation of Non-Toxic Shiga Dysentery Vaccines by Irradiation With Soft X-Rays. J Bacteriol (1936) 31:581–4. doi: 10.1128/jb.31.6.581-584.1936
45. Jordan RT, Kempe LL. Inactivation of Some Animal Viruses With Gamma Radiation From Cobalt-60. Proc Soc Exp Biol Med (1956) 91:212–5. doi: 10.3181/00379727-91-22215
46. Tumanyan MA, Duplishcheva AP, Sedova TS. Influence of Massive Doses of $Gamma$-Rays on Immunological Properties of Bacteria of Intestinal Group. Zhur Mikrobiol Epidemiol i Immunobiol (1958) 4:3–10.
47. Carpenter CM. Preliminary Report on Vaccines Prepared From Gamma-Irradiated Mycobacterium Tuberculosis and Brucella Suis1, in: American Review of Tuberculosis and Pulmonary Diseases (1959). Available at: https://www.atsjournals.org/doi/pdf/10.1164/artpd.1959.79.3.374 (Accessed January 23, 2020).
48. Kaplan C. The Antigenicity of γ-Irradiated Vaccinia Virus. Epidemiol Infection (1960) 58:391–8. doi: 10.1017/S0022172400038535
49. Ward RA, Bell LH, Schneider RL. Effects of X-Irradiation on the Development of Malarial Parasites in Mosquitoes. Exp Parasitol (1960) 10:324–32. doi: 10.1016/0014-4894(60)90070-9
50. Kalenina EF, Abidov AZ. The Effect of Gamma Rays of Co-60 on Smallpox Vaccine Contaminating Microorganisms(1963). Available at: https://www.osti.gov/biblio/4687625 (Accessed January 23, 2020).
51. Scherließ R, Ajmera A, Dennis M, Carroll MW, Altrichter J, Silman NJ, et al. Induction of Protective Immunity Against H1N1 Influenza A(H1N1)pdm09 With Spray-Dried and Electron-Beam Sterilised Vaccines in Non-Human Primates. Vaccine (2014) 32:2231–40. doi: 10.1016/j.vaccine.2014.01.077
52. Sir Karakus G, Tastan C, Kancagi DD, Yurtsever B, Tumentemur G, Demir S, et al. Preliminary Report of Preclinical Efficacy and Safety Analysis of Gamma-Irradiated Inactivated SARS-CoV-2 Vaccine Candidates. (2020). doi: 10.1101/2020.09.04.277426
53. Orr MT, Kramer RM, Barnes LV, Dowling QM, Desbien AL, Beebe EA, et al. Elimination of the Cold-Chain Dependence of a Nanoemulsion Adjuvanted Vaccine Against Tuberculosis by Lyophilization. J Control Release (2014) 177:20–6. doi: 10.1016/j.jconrel.2013.12.025
54. Lloyd J, Cheyne J. The Origins of the Vaccine Cold Chain and a Glimpse of the Future. Vaccine (2017) 35:2115–20. doi: 10.1016/j.vaccine.2016.11.097
55. Fertey J, Thoma M, Beckmann J, Bayer L, Finkensieper J, Reißhauer S, et al. Automated Application of Low Energy Electron Irradiation Enables Inactivation of Pathogen- and Cell-Containing Liquids in Biomedical Research and Production Facilities. Sci Rep (2020) 10:12786. doi: 10.1038/s41598-020-69347-7
56. Surendran N, Hiltbold EM, Heid B, Sriranganathan N, Boyle SM, Zimmerman KL, et al. Heat-Killed and γ-Irradiated Brucella Strain RB51 Stimulates Enhanced Dendritic Cell Activation, But Not Function Compared With the Virulent Smooth Strain 2308. FEMS Immunol Med Microbiol (2010) 60:147–55. doi: 10.1111/j.1574-695X.2010.00729.x
57. Oliveira SC, Zhu Y, Splitter GA. Recombinant L7/L12 Ribosomal Protein and Gamma-Irradiated Brucella Abortus Induce a T-Helper 1 Subset Response From Murine CD4+ T Cells. Immunology (1994) 83:659–64.
58. Dabral N, Martha-Moreno-Lafont, Sriranganathan N, Vemulapalli R. Oral Immunization of Mice With Gamma-Irradiated Brucella Neotomae Induces Protection Against Intraperitoneal and Intranasal Challenge With Virulent B. Abortus 2308. PloS One (2014) 9(9):e107180. doi: 10.1371/journal.pone.0107180
59. Datta SK, Okamoto S, Hayashi T, Shin SS, Mihajlov I, Fermin A, et al. Vaccination With Irradiated Listeria Induces Protective T Cell Immunity. Immunity (2006) 25:143–52. doi: 10.1016/j.immuni.2006.05.013
60. Ahmed S, Ahmed BS, Mahmoud GI, Nemr W. And Rahim, E Comparative Study Between Formalin-Killed Vaccine and Developed Gammairradiation Vaccine Against Mannheimia haemolytica in Rabbits. Turk J Vet Anim Sci (2016) 40:19–224. doi: 10.3906/vet-1504-34
61. Jerrells TR, Palmer BA, Osterman JV. Gamma-Irradiated Scrub Typhus Immunogens: Development of Cell-Mediated Immunity After Vaccination of Inbred Mice. Infect Immun (1983) 39:262–9. doi: 10.1128/iai.39.1.262-269.1983
62. Eisenberg GHG, Osterman JV. Gamma-Irradiated Scrub Typhus Immunogens: Development and Duration of Immunity. Infect Immun (1978) 22:80–6. doi: 10.1128/iai.22.1.80-86.1978
63. Gordon M, Donaldson DM, Wright GG. Immunization of Mice With Irradiated Pasteurella Tularensis. J Infect Dis (1964) 114:435–40. doi: 10.1093/infdis/114.5.435
64. Rocha JN, Cohen ND, Bordin AI, Brake CN, Giguère S, Coleman MC, et al. Oral Administration of Electron-Beam Inactivated Rhodococcus Equi Failed to Protect Foals Against Intrabronchial Infection With Live, Virulent R. Equi. PloS One (2016) 11:1–18. doi: 10.1371/journal.pone.0148111
65. Fertey J, Bayer L, Kähl S, Haji RM, Burger-Kentischer A, Thoma M, et al. Low-Energy Electron Irradiation Efficiently Inactivates the Gram-Negative Pathogen Rodentibacter Pneumotropicus—A New Method for the Generation of Bacterial Vaccines With Increased Efficacy. Vaccines (Basel) (2020) 8:113–24. doi: 10.3390/vaccines8010113
66. Jesudhasan PR, McReynolds JL, Byrd AJ, He H, Genovese KJ, Droleskey R, et al. Electron-Beam-Inactivated Vaccine Against Salmonella Enteritidis Colonization in Molting Hens. Avian Dis (2015) 59:165–70. doi: 10.1637/10917-081014-ResNoteR
67. Kogut MH, McReynolds JL, He H, Genovese KJ, Jesudhasan PR, Davidson MA, et al. Electron-Beam Irradiation Inactivation of Salmonella: Effects on Innate Immunity and Induction of Protection Against Salmonella Enterica Serovar Typhimurium Challenge of Chickens. Proc Vaccinology (2012) 6:47–63. doi: 10.1016/j.provac.2012.04.008
68. Begum RH, Rahman H, Ahmed G. Development and Evaluation of Gamma Irradiated Toxoid Vaccine of Salmonella Enterica Var Typhimurium. Vet Microbiol (2011) 153:191–7. doi: 10.1016/j.vetmic.2011.06.013
69. van Diemen PM, Yamaguchi Y, Paterson GK, Rollier CS, Hill AVS, Wyllie DH. Irradiated Wild-Type and Spa Mutant Staphylococcus Aureus Induce Anti- S. Aureus Immune Responses in Mice Which do Not Protect Against Subsequent Intravenous Challenge. Pathog Dis (2013) 68:20–6. doi: 10.1111/2049-632X.12042
70. Gaidamakova EK, Myles IA, McDaniel DP, Fowler CJ, Valdez PA, Naik S, et al. Preserving Immunogenicity of Lethally Irradiated Viral and Bacterial Vaccine Epitopes Using a Radio- Protective Mn2+-Peptide Complex From Deinococcus. Cell Host Microbe (2012) 12:117–24. doi: 10.1016/j.chom.2012.05.011
71. Babb R, Chen A, Hirst TR, Kara EE, McColl SR, Ogunniyi AD, et al. Intranasal Vaccination With γ-Irradiated Streptococcus Pneumoniae Whole-Cell Vaccine Provides Serotype-Independent Protection Mediated by B-Cells and Innate IL-17 Responses. Clin Sci (2016) 130:697–710. doi: 10.1042/CS20150699
72. Pawlowski A, Svenson SB. Electron Beam Fragmentation of Bacterial Polysaccharides as a Method of Producing Oligosaccharides for the Preparation of Conjugate Vaccines. FEMS Microbiol Lett (1999) 174:255–63. doi: 10.1111/j.1574-6968.1999.tb13577.x
73. Thabet A, Schmäschke R, Fertey J, Bangoura B, Schönfelder J, Lendner M, et al. Eimeria Tenella Oocysts Attenuated by Low Energy Electron Irradiation (LEEI) Induce Protection Against Challenge Infection in Chickens. Vet Parasitol (2019) 266:18–26. doi: 10.1016/j.vetpar.2019.01.001
74. Jenkins MC, Augustine PC, Danforth HD, Barta JR. X-Irradiation of Eimeria Tenella Oocysts Provides Direct Evidence That Sporozoite Invasion and Early Schizont Development Induce a Protective Immune Response(s). Infect Immun (1991) 59:4042–8. doi: 10.1128/iai.59.11.4042-4048.1991
75. Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective Immunity Produced by the Injection of X-Irradiated Sporozoites of Plasmodium Berghei. Nature (1967) 216:160–2. doi: 10.1038/216160a0
76. Hoffman SL, Goh LML, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of Humans Against Malaria by Immunization With Radiation-Attenuated Plasmodium Falciparum Sporozoites. J Infect Dis (2002) 185:1155–64. doi: 10.1086/339409
77. Bayer L, Fertey J, Ulbert S, Grunwald T. Immunization With an Adjuvanted Low-Energy Electron Irradiation Inactivated Respiratory Syncytial Virus Vaccine Shows Immunoprotective Activity in Mice. Vaccine (2018) 36:1561–9. doi: 10.1016/j.vaccine.2018.02.014
78. Müllbacher A, Ada GL, Hla RT. Gamma-Irradiated Influenza A Virus can Prime for a Cross-Reactive and Cross-Protective Immune Response Against Influenza A Viruses. Immunol Cell Biol (1988) 66:153–7. doi: 10.1038/icb.1988.19
79. Furuya Y, Chan J, Wan E-C, Koskinen A, Diener KR, Hayball JD, et al. Gamma-Irradiated Influenza Virus Uniquely Induces IFN-I Mediated Lymphocyte Activation Independent of the TLR7/MyD88 Pathway. PloS One (2011) 6:e25765. doi: 10.1371/journal.pone.0025765
80. Furuya Y, Chan J, Regner M, Lobigs M, Koskinen A, Kok T, et al. Cytotoxic T Cells Are the Predominant Players Providing Cross-Protective Immunity Induced by -Irradiated Influenza A Viruses. J Virol (2010) 84:4212–21. doi: 10.1128/JVI.02508-09
81. David SC, Lau J, Singleton EV, Babb R, Davies J, Hirst TR, et al. The Effect of Gamma-Irradiation Conditions on the Immunogenicity of Whole-Inactivated Influenza A Virus Vaccine. Vaccine (2017) 35:1071–9. doi: 10.1016/j.vaccine.2016.12.044
82. Fertey J, Bayer L, Grunwald T, Pohl A, Beckmann J, Gotzmann G, et al. Pathogens Inactivated by Low-Energy-Electron Irradiation Maintain Antigenic Properties and Induce Protective Immune Responses. Viruses (2016) 8:319–33. doi: 10.3390/v8110319
83. Alsharifi M, Furuya Y, Bowden TR, Lobigs M, Koskinen A, Regner M, et al. Intranasal Flu Vaccine Protective Against Seasonal and H5N1 Avian Influenza Infections. PloS One (2009) 4:e5336. doi: 10.1371/journal.pone.0005336
84. Agrawal AS, Tao X, Algaissi A, Garron T, Narayanan K, Peng B-H, et al. Immunization With Inactivated Middle East Respiratory Syndrome Coronavirus Vaccine Leads to Lung Immunopathology on Challenge With Live Virus. Hum Vaccin Immunother (2016) 12:2351–6. doi: 10.1080/21645515.2016.1177688
85. Tobin GJ, Tobin JK, Gaidamakova EK, Wiggins TJ, Bushnell RV, Lee W-M, et al. A Novel Gamma Radiation-Inactivated Sabin-Based Polio Vaccine. PloS One (2020) 15:e0228006. doi: 10.1371/journal.pone.0228006
86. Shahrudin S, Chen C, David SC, Singleton EV, Davies J, Kirkwood CD, et al. Gamma-Irradiated Rotavirus: A Possible Whole Virus Inactivated Vaccine. PloS One (2018) 13:e0198182. doi: 10.1371/journal.pone.0198182
87. Turan RD, Tastan C, Kancagi DD, Yurtsever B, Karakus GS, Ozer S, et al. Gamma-Irradiated SARS-CoV-2 Vaccine Candidate, OZG-38.61.3, Confers Protection From SARS-CoV-2 Challenge in Human ACEII-Transgenic Mice. Sci Rep (2021) 11, 15799. doi: 10.1038/s41598-021-95086-4
88. Reitman M, Tribble HR, Green L. Gamma-Irradiated Venezuelan Equine Encephalitis Vaccines. Appl Microbiol (1970) 19:763–7. doi: 10.1128/am.19.5.763-767.1970
89. Martin SS, Bakken RR, Lind CM, Garcia P, Jenkins E, Glass PJ, et al. Comparison of the Immunological Responses and Efficacy of Gamma Irradiated V3526 Vaccine Formulations Against Subcutaneous and Aerosol Challenge With Venezuelan Equine Encephalitis Virus Subtype IAB. Vaccine (2010) 28:1031–40. doi: 10.1016/j.vaccine.2009.10.126
90. Motamedi-Sedeh F, Afsharnasab M, Heidarieh M, Tahami SM. Protection of Litopenaeus Vannamei Against White Spot Syndrome Virus by Electron-Irradiated Inactivated Vaccine and Prebiotic Immunogen. Radiat Phys Chem (2017) 130:421–5. doi: 10.1016/j.radphyschem.2016.09.020
91. Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, et al. An Ebola Whole-Virus Vaccine Is Protective in Nonhuman Primates. Science (2015) 348:439–42. doi: 10.1126/science.aaa4919
92. Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in Nonhuman Primates of Vaccines Against Ebola Virus. Emerg Infect Dis (2002) 8:503–7. doi: 10.3201/eid0805.010284
93. Clyde DF. Immunity to Falciparum and Vivax Malaria Induced by Irradiated Sporozoites: A Review of the University of Maryland Studie-75. Bull World Health Organ (1990) 68 Suppl:9–12.
94. Rieckmann KH. Human Immunization With Attenuated Sporozoites. Bull World Health Organ (1990) 68 Suppl:13–6.
95. Luke TC, Hoffman SL. Rationale and Plans for Developing a Non-Replicating, Metabolically Active, Radiation-Attenuated Plasmodium Falciparum Sporozoite Vaccine. J Exp Biol (2003) 206:3803–8. doi: 10.1242/jeb.00644
96. Seder RA, Chang L-J, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection Against Malaria by Intravenous Immunization With a Nonreplicating Sporozoite Vaccine. Science (2013) 341:1359–65. doi: 10.1126/science.1241800
97. Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M, Vallejo AF, Amado-Garavito AB, Céspedes N, et al. Protective Efficacy of Plasmodium Vivax Radiation-Attenuated Sporozoites in Colombian Volunteers: A Randomized Controlled Trial. PloS Negl Trop Dis (2016) 10(10):e0005070. doi: 10.1371/journal.pntd.0005070
98. Vemulapalli R, Cravero S, Calvert CL, Toth TE, Sriranganathan N, Boyle SM, et al. Characterization of Specific Immune Responses of Mice Inoculated With Recombinant Vaccinia Virus Expressing an 18-Kilodalton Outer Membrane Protein of Brucella Abortus. Clin Diagn Lab Immunol (2000) 7:114–8. doi: 10.1128/CDLI.7.1.114-118.2000
99. Alamuti MM, Ravanshad M, Motamedi-Sedeh F, Nabizadeh A, Ahmadi E, Hossieni SM. Immune Response of Gamma-Irradiated Inactivated Bivalent Polio Vaccine Prepared Plus Trehalose as a Protein Stabilizer in a Mouse Model. INT (2021) 64:140–6. doi: 10.1159/000515392
100. Sabbaghi A, Zargar M, Zolfaghari MR, Motamedi-Sedeh F, Ghaemi A. Protective Cellular and Mucosal Immune Responses Following Nasal Administration of a Whole Gamma-Irradiated Influenza A (Subtype H1N1) Vaccine Adjuvanted With Interleukin-28B in a Mouse Model. Arch Virol (2021) 166:545–57. doi: 10.1007/s00705-020-04900-3
101. Pillai SD, Pillai ET. Agriculture: Electron Beam Irradiation Technology Applications in the Food Industry. In: Greenspan E, editor. Encyclopedia of Nuclear Energy. Oxford: Elsevier (2021). p. 313–29. doi: 10.1016/B978-0-12-819725-7.00141-0
102. Beniac DR, deVarennes SL, Andonov A, He R, Booth TF. Conformational Reorganization of the SARS Coronavirus Spike Following Receptor Binding: Implications for Membrane Fusion. PloS One (2007) 2:e1082. doi: 10.1371/journal.pone.0001082
103. Durante M, Schulze K, Incerti S, Francis Z, Zein S, Guzmán CA. Virus Irradiation and COVID-19 Disease. Front Phys (2020) 8:565861. doi: 10.3389/fphy.2020.565861
104. Mullbacher A, Pardo J, Furuya Y. SARS-CoV-2 Vaccines: Inactivation by Gamma Irradiation for T and B Cell Immunity. Pathogens (2020) 9:928. doi: 10.3390/pathogens9110928
105. Sir Karakus G, Tastan C, Dilek Kancagi D, Yurtsever B, Tumentemur G, Demir S, et al. Preclinical Efficacy and Safety Analysis of Gamma-Irradiated Inactivated SARS-CoV-2 Vaccine Candidates. Sci Rep (2021) 11:5804. doi: 10.1038/s41598-021-83930-6
106. Bhatia SS. Investigations Into Metabolically Active Yet Non-Culturable (MAyNC) Clostridium Perfringens to Control Necrotic Enteritis in Broiler Chickens (2021). Available at: https://oaktrust.library.tamu.edu/handle/1969.1/193108 (Accessed November 9, 2021).
Keywords: ionizing radiation, vaccines, electron beam, gamma irradiation, killed vaccines
Citation: Bhatia SS and Pillai SD (2022) Ionizing Radiation Technologies for Vaccine Development - A Mini Review. Front. Immunol. 13:845514. doi: 10.3389/fimmu.2022.845514
Received: 29 December 2021; Accepted: 24 January 2022;
Published: 11 February 2022.
Edited by:
Viskam Wijewardana, International Atomic Energy Agency, AustriaCopyright © 2022 Bhatia and Pillai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suresh D. Pillai, c3VyZXNoLnBpbGxhaUBhZy50YW11LmVkdQ==
 Sohini S. Bhatia
Sohini S. Bhatia Suresh D. Pillai
Suresh D. Pillai