- 1Department of Dermatology, Tokyo Medical University, Tokyo, Japan
- 2Graduate School of Integrated Sciences for Life, Hiroshima University, Hiroshima, Japan
- 3Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, Saitama, Japan
Mast cells are present in all vascularized tissues of the body. They are especially abundant in tissues that are in frequent contact with the surrounding environment and act as potential sources of inflammatory and/or regulatory mediators during development of various infections and diseases. Mature mast cells’ cytoplasm contains numerous granules that store a variety of chemical mediators, cytokines, proteoglycans, and proteases. Mast cells are activated via various cell surface receptors, including FcϵRI, toll-like receptors (TLR), Mas-related G-protein-coupled receptor X2 (MRGPRX2), and cytokine receptors. IgE-mediated mast cell activation results in release of histamine and other contents of their granules into the extracellular environment, contributing to host defense against pathogens. TLRs, play a crucial role in host defense against various types of pathogens by recognizing pathogen-associated molecular patterns. On the other hand, excessive/inappropriate mast cell activation can cause various disorders. Here, we review the published literature regarding the known and potential inflammatory and regulatory roles of mast cells in cutaneous inflammation, including atopic dermatitis, psoriasis, and contact dermatitis GVHD, as well as in host defense against pathogens.
Introduction
Mast cells are tissue-resident immune cells that are derived from hematopoietic stem cells (1). First, mast cell progenitors (MCPs) differentiate from hematopoietic stem cells in the bone marrow and/or spleen (shown in mice) and circulate via the vascular system (2, 3). The MCPs then infiltrate the local tissues from the blood, where they differentiate into functionally-mature mast cells under the control of the components of the cytokine milieu, such as stem cell factor (SCF), transforming growth factor (TGF)-β, nerve growth factor (NGF), interleukin (IL)-3, IL-4, IL-9, and IL-33 (4–6). Therefore, mast cells are present in all vascularized tissues of the body, and they are especially abundant in tissues that come into frequent contact with the surrounding environment, such as the gastrointestinal tract, skin, and respiratory epithelium (2).
Mature mast cells’ cytoplasm contains numerous granules that store a variety of chemical mediators (e.g., histamine), proteoglycans, and proteases. In both rodents and humans, mast cells have been categorized into two types based on their anatomical distribution and the kinds of proteases stored in their granules. In rodents, one type is the mucosal mast cell (MMC), which are located in the mucosa (mucosal epithelium) and whose granules contain tryptase (2). The second type is the connective tissue-type mast cell (CTMC), which are located in connective tissues such as the skin and submucosa and whose granules contain chymase, carboxypeptidases, and tryptase. In humans, one type is termed the TC mast cell (MCTC), whose granules contain tryptase and chymase, while the second type is termed the T mast cell (MCT), whose granules contain only tryptase (7).
In response to certain stimuli, mast cells release the contents of their granules into the extracellular environment this process is known as degranulation. In one major pathway, degranulation occurs immediately following crosslinking of antigens by antigen-specific immunoglobulin (Ig) E that is bound to FcϵRI on mast cells (8). Mobilization of Ca2+ is a key process that occurs during degranulation of mast cells after antigen/IgE/FcϵRI-crosslinking (9, 10). With or without degranulation, mast cells can also release de novo-synthesized inflammatory mediators. In addition, degranulated mast cells are able to replenish their granules, allowing them to undergo repeated degranulation in tissues (11).
Mast cells are able to mount a rapid immunological response by releasing prestored inflammatory mediators, and, owing to their location in the skin and mucosa, they are a part of the front line of defense against pathogens invading the body (12). The released inflammatory mediators bring about increased vascular permeability and fluid accumulation, as well as recruitment and activation of immune cells, including dendritic cells, macrophages, T cells, and B cells (2, 13). However, excessive mast cell activation can rapidly cause death via anaphylactic shock. Moreover, inappropriate mast cell activation can cause various diseases such as allergic and autoimmune diseases (14). On the other hand, mast cells play a suppressive role in certain diseases. Thus, they can act not only as pathogenic effector cells but also as suppressor cells in an immune response. Here, we review the published literature regarding the known and potential inflammatory and regulatory roles of mast cells in cutaneous inflammation as well as in host defense.
Mast Cells in Skin Inflammation During Infection
Toll-like receptors (TLRs), which are pattern-recognition receptors (PRRs), play a crucial role in host defense against various types of pathogens by recognizing pathogen-associated molecular patterns (15). Peptidoglycan (a TLR2 agonist) and lipopolysaccharide (LPS; a TLR4 agonist) are, respectively, components in the cell walls of gram-positive bacteria and mycobacteria, and of gram-negative bacteria. Poly (I:C) (a TLR3 agonist) is a mimic of viral dsRNA. These molecules have been shown to be able to induce cytokine and/or chemokine production by murine and/or human mast cells. In addition, various other peptidoglycans can induce degranulation of murine and/or human mast cells (16–18). Thus, mast cells are important for host defense against viruses and bacteria through PRRs such as TLRs. On the other hand, pathogen-derived antigens/components can promote excessive mast cell activation, resulting in exacerbation of inflammation (Figure 1).
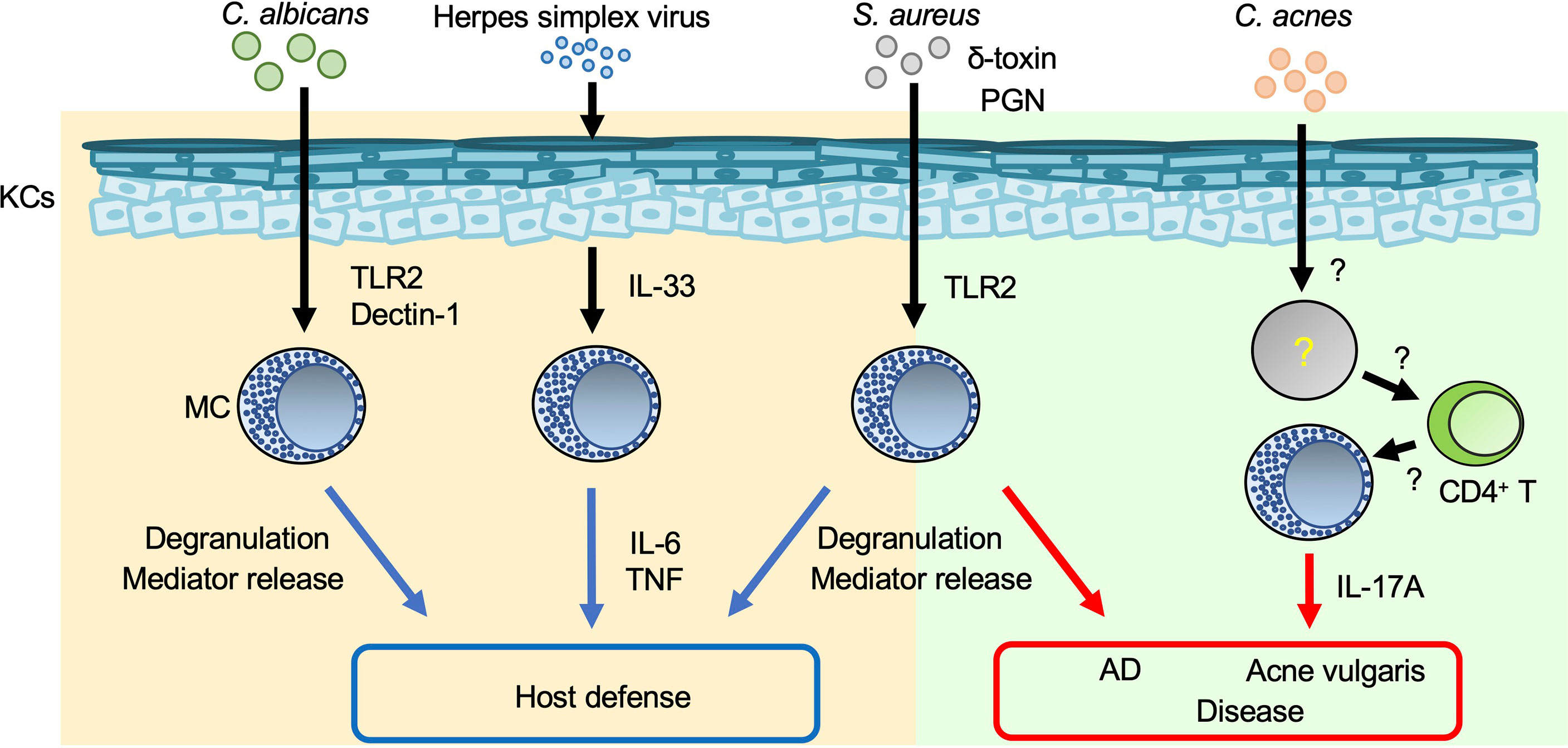
Figure 1 Mast cells in skin inflammation during infection. Mast cells (MCs) are important for host defense against various pathogens. On the other hand, inappropriate MC activation during infection results in aggravation of such skin diseases as AD and acne vulgaris. Blue arrows: appropriate MC activation; red arrows: excessive MC activation.
Staphylococcus (S.) aureus is a gram-positive bacterium that causes impetigo and staphylococcal scalded skin syndrome (SSSS), primarily in young children (19, 20). Impetigo is a superficial bacterial skin infection that occurs in bullous or non-bullous form. The exfoliative toxin of S. aureus cleaves desmoglein 1 and causes bullous impetigo (19). Hematogenous dissemination of exotoxins from the initial site of S. aureus infection leads to separation of epidermal keratinocytes and detachment of the superficial epidermis in SSSS (20). S. aureus invades and survives in human cord blood-derived mast cells (CBMCs) after internalization (21). S. aureus and S. aureus-derived peptidoglycan induce degranulation and cytokine production by human CBMCs and mouse bone marrow-derived cultured mast cells (BMCMCs) (16, 17, 21). Mast cells degranulate in response to S. aureus-derived δ-toxin, contributing to increased vascular permeability in mice (22). In addition, mast cells are responsible for development of S. aureus-mediated skin inflammation accompanied by spongiosis, parakeratosis, and neutrophil infiltration, suggesting that S. aureus-stimulated mast cells exacerbate dermatitis such as atopic dermatitis (AD) (22).
Acne vulgaris is a common cutaneous disorder characterized by chronic and recurrent development of multiple inflammatory papules, pustules and nodules, mainly on the face but also on the neck, chest and back. Hyperkeratotic plugs composed of corneocytes in the lower portion of the follicular infundibulum create a new environment that impacts the microbiota and fosters proliferation of a gram-positive bacillus, Cutibacterium acnes. IL-17A-producing mast cells were detected in the perifollicular area of acne vulgaris lesions (23). Activated memory/effector CD4+ T cells induced IL-17A production by human mast cells, implying a contribution of mast cells to development of acne vulgaris (23). However, their precise role remains unclear.
A fungus, Candida, is part of the normal flora of the gastrointestinal tract, oral/nasal cavity, and skin of humans, but it can cause disease when host immunity is compromised or there is an imbalance in the ecological niche. Mast cells reside in various tissues that can be colonized or infected by Candida spp., including Candida (C.) albicans (24). A human mast cell line (HMC-1) degranulated and produced IL-8 in response to C. albicans, contributing to enhanced migration of neutrophils (25). Rodent mast cells can phagocytose C. albicans and produce nitric oxide, a reactive oxygen species, cytokines and chemokines via Dectin-1 and/or TLR2 (26–29). These results suggest that mast cells play a critical role in host defense against C. albicans infection, but their precise role in the setting needs to be elucidated.
Dermatophytes are filamentous fungi of the genera Trichophyton, Microsporum, and Epidermophyton that infect the skin, hair, and nails. The yeast and mycelial forms of Malassezia are found in the skin scales of patients with pityriasis versicolor (30). MGL_1304, derived from Malassezia (M.) globosa, was identified in human sweat by mass-spectrometric analysis based on the histamine-releasing activity in basophils of patients with AD (31). Serum specific IgE against MGL_1304 was higher in patients with AD and cholinergic urticaria, which is a subtype of chronic urticaria whose symptoms are evoked by sweating, than in normal controls (32). To the best of our knowledge, there are no reports of other types of urticarias that involve MGL_1304. The level of degranulation of a human mast cell line, LAD2, sensitized with sera from patients with AD was greater than with healthy control sera after stimulation with MGL_1304 (32), suggesting that MGL_1304 is a major allergen involved in the exacerbation of AD and cholinergic urticaria via induction of mast cell degranulation.
Herpes simplex virus (HSV) is a double-stranded DNA virus and the cause of a common viral infection of epidermal cells that is typically transmitted via physical contact (33, 34). HSV can be transmitted even if the source is asymptomatic, but transmission is more likely if the source is symptomatic because the viral titer is much greater when lesions are present (33). Infections by HSV types 1 and 2 are characterized by recurrent, vesicular lesions that are accompanied by pain, tingling, pruritus, and/or burning. Lesions can develop anywhere on the body but occur mainly on the lips (HSV-1) and in the genital area (HSV-2) (33). HSV infections are associated with onset of eczema herpeticum (Kaposi’s varicelliform eruption) in patients with AD (35) or erythema multiforme (36). Mast-cell-deficient KitW/KitW-v mice were susceptible to HSV-2 compared with Kit+/+ mice (37). HSV-2 induces IL-33 production by keratinocytes, followed by activation of mast cells to produce IL-6 and tumor necrosis factor (TNF) (38), which are crucial for host defense against HSV-2 (37, 38).
Mast Cells in Contact Hypersensitivity
Allergic contact dermatitis/contact hypersensitivity (ACD/CHS) develops in response to repeated skin exposure to an allergen. Haptens are generally non-immunogenic, low-molecular-weight chemicals. When haptens are applied to the skin surface, they penetrate the stratum corneum barrier and form chemically-modified, immunogenic neo-antigens by binding with self-proteins (39). Haptens such as 2,4,6-trinitrochlorobenzene (TNCB), oxazalone, 1-fluoro-2,4-dinitrobenzene (DNFB), and fluorescein isothiocyanate (FITC) have long been used experimentally to study CHS in murine models (40). As noted in other reviews (1, 8, 41), the roles of mast cells in the development of acute and chronic CHS have differed with the experimental protocol. In some studies of acute CHS models using mast-cell-deficient and -depleted mice, mast cells (especially mast-cell-derived TNF) were responsible for development of CHS (1, 8, 41–43) (Figure 2). In addition, mast-cell-derived IL-25 promoted IL-1β production by dermal dendritic cells, which led to exacerbation of Th17-cell-mediated skin inflammation (44). On the other hand, mast cells were not essential for development of acute CHS in certain settings using mast-cell-deficient mice (1, 8, 41). Mast cells can also play a suppressive role in induction of acute CHS in some settings. For example, mast-cell-derived IL-5 was important for expansion of IL-10-producing regulatory B cells, which resulted in suppression of acute CHS (45). Similarly, mast-cell-derived IL-13 inhibited Th1-cell activation by suppressing IL-12 production by skin dendritic cells, which resulted in attenuation of acute CHS (46). Group III secreted phospholipase A2 (sPLA2-III; encoded by Pla2g3) released from immature mast cells is important for prostaglandin D2 (PGD2) production by fibroblasts (47). In turn, fibroblast-derived PGD2 causes immature mast cells to differentiate into mature mast cells (47). Mast-cell-derived sPLA2-III plays a suppressive role in development of acute CHS, but a promotive role in development of irritant-induced contact dermatitis (48).
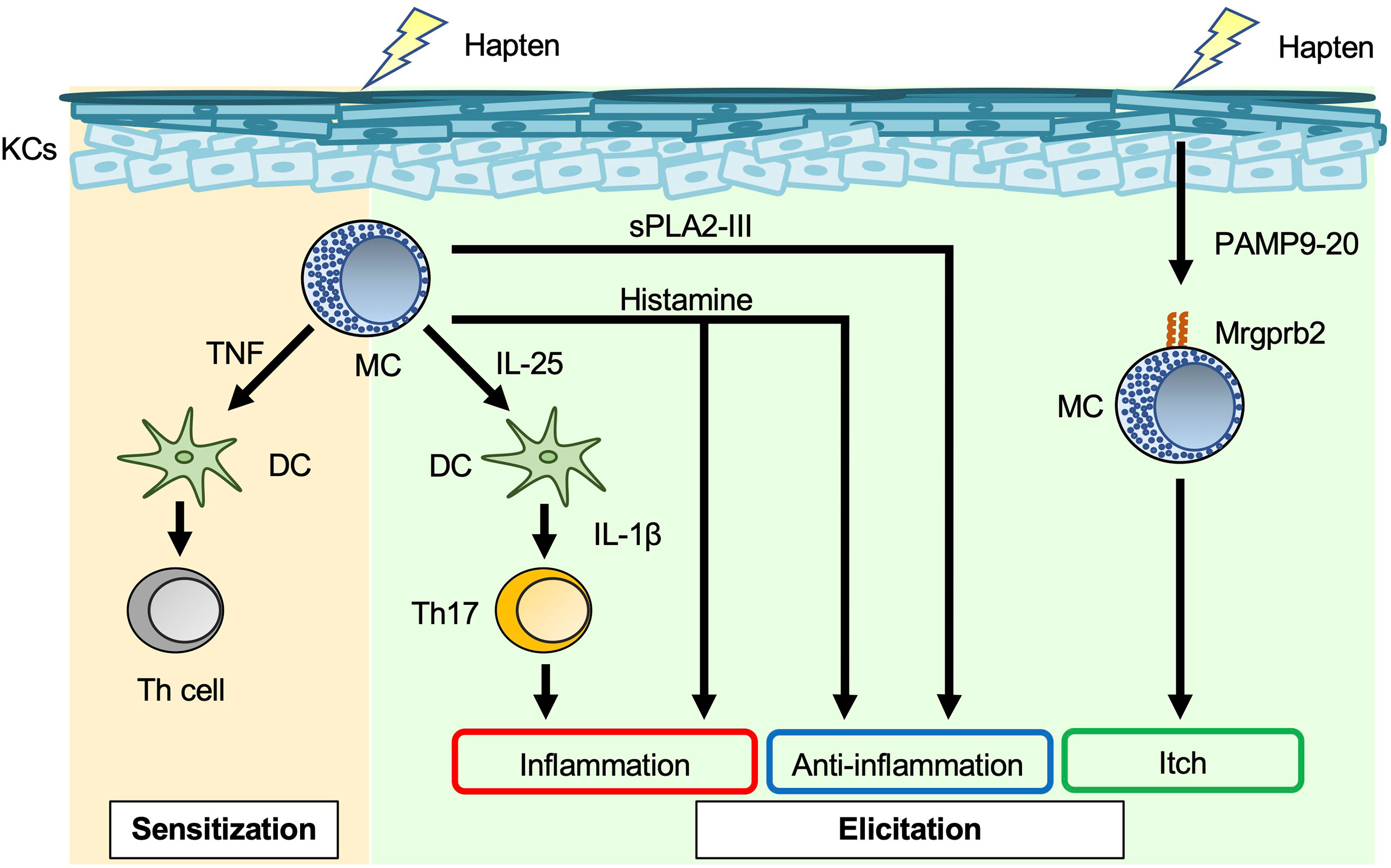
Figure 2 Mast cells in acute contact hypersensitivity. Mast cell (MC)-derived TNF enhances skin dendritic cell (DC) migration to draining LNs, leading to induction of hapten-specific Th-cell expansion in the sensitization phase of contact hypersensitivity (CHS). MC-derived IL-25 induces IL-1β production by dermal DCs, followed by promotion of IL-17 production by Th17 cells in the elicitation phase of CHS. Histamine has dual roles, i.e., inflammatory and anti-inflammatory, in the elicitation phase of CHS. Haptens stimulate keratinocytes (KCs) to produce PAMP9-20, a ligand for Mrgprb2. PAMP9-20 induces itch during CHS.
Mast cells are a major producer of histamine, which binds to H1R, H2R, H3R, and H4R. H1R and H4R play important roles in allergic diseases, such as urticaria and asthma; H2R stimulates gastric acid secretion; and H3R plays a crucial role in the control of sleep–wake behavior (49). H1R antagonists are widely used to treat pruritic skin inflammation, including urticaria and AD. Mice treated with H1R antagonists (chlorpheniramine, oxatomide, ketotifen, mequitazine, emedastine, terfenadine and azelastine) showed attenuated acute CHS (50), whereas mice treated with H1R antagonists (diphenhydramine, homochlorcyclizine, cyproheptadine and cetirizine) showed normal development of acute CHS (50, 51). Mice treated with an H2R antagonist (cimetidine) showed augmentation of acute CHS (52). In addition, mice deficient in histidine decarboxylase, which is an enzyme involved in histamine synthesis, showed exacerbation of acute CHS (53).
Mas-related G-protein-coupled receptor X2 (MRGPRX2) mRNA is most abundant in human skin, adipose tissue, the bladder, and the colon (54). MRGPRX2 is expressed on human mast cells, and its murine ortholog, Mrgprb2, is specifically expressed on murine CTMCs (55). A new technique based on near-infrared photoimmunotherapy was recently developed for ablation of cancer cells. In that technique, photosensitizer-conjugated monoclonal antibodies specific for a cell surface marker on cancer cells are delivered to the tumor, followed by activation of cytotoxicity (thermotoxicity) by illumination (56). As an extension of that technology, photosensitizer-conjugated monoclonal antibodies specific for MRGPRX2, a cell surface marker on mast cells, were employed to reduce the number of mast cells in the skin (56). MRGPRX2/Mrgprb2 is a promiscuous receptor for cationic ligands, including substance P, compound 48/80, and pro-adrenomedullin peptide 9–20 (PAMP9–20). These ligands induce degranulation of mast cells via MRGPRX2/Mrgprb2 (57, 58). PAMP9–20 expression is increased in the inflamed skin lesions of patients with ACD (58). Skin thickness was similarly increased in Mrgprb2-/- mice and wild-type mice during acute CHS (58), whereas scratching behavior and the number of inflammatory cells in the skin were significantly reduced in Mrgprb2-/- mice (58). Thus, Mrgprb2-mediated mast-cell-activation is somehow involved in induction of itch during acute CHS.
Meanwhile, chronic CHS was induced in rodents by repeated cutaneous exposure to haptens (59–63). Mast cell-deficient/depleted mice showed increased development of chronic CHS (Figure 3). In one setting, hapten/hapten-specific IgG1 complexes induced IL-10 production by mast cells by binding to FcγR on the cells, resulting in IL-10-mediated suppression of chronic CHS (59, 63). In another setting, hapten/hapten-specific IgE/FcϵR1 crosslinking induced IL-2 production by mast cells (61). Mast cell-derived IL-2 enhanced regulatory T cell (Treg) expansion, followed by suppression of inflammation during chronic CHS by Tregs (61). Therefore, mast cells have dual roles as both effector cells and regulatory cells in the development of acute and/or chronic CHS induced by certain haptens.
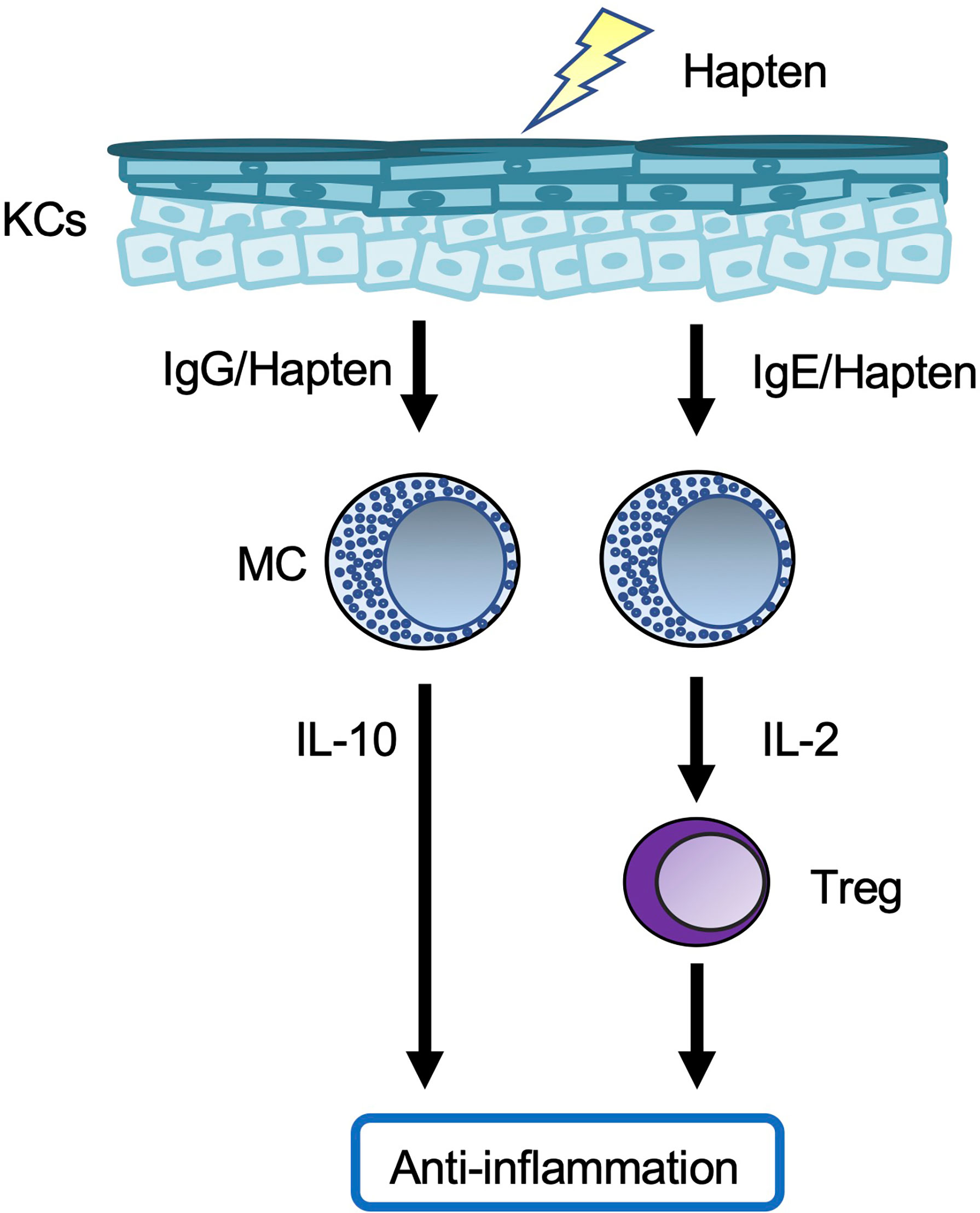
Figure 3 Mast cells in chronic contact hypersensitivity. In one chronic contact hypersensitivity (CHS) setting, crosslinked hapten/hapten-specific IgG complexes stimulate skin mast cells (MCs) to produce IL-10, which leads to suppression of the inflammation. In another chronic CHS setting, crosslinking of hapten/hapten-specific IgE/FcϵR1 results in production of IL-2 by splenic MCs, which leads to expansion of Tregs in the spleen. The Tregs migrate from the spleen to local skin lesions, where they suppress the inflammation.
Mast Cells in Urticaria
Mast cell degranulation was observed in the dermis immediately below wheals in various types of inducible urticaria (64). Histamine, which is released by mast cells during IgE-mediated degranulation, is known to be crucial for the pathogenesis of urticaria (65). Increased blood levels of histamine were noted following provocation of inducible urticaria (64). In patients with chronic spontaneous urticaria, more than 200 IgEs, which recognize autoantigens including IL-24, were detected in sera (66). In addition to H1R antagonists, an anti-human IgE monoclonal antibody (omalizumab) was reported to provide clinical benefit for chronic spontaneous urticaria (67). Moreover, it is known that there is IgE-independent pathogenesis of chronic urticaria (64). The number and proportion of MRGPRX2-positive skin mast cells are increased in the inflamed skin lesions of patients with chronic urticaria compared with the skin of healthy control subjects (68). Intradermal administration of substance P induced greater wheal reactions in patients with chronic spontaneous urticaria than in healthy subjects (69), suggesting that substance P/MRGPRX2-mediated mast cell degranulation is an alternative pathway for induction of chronic spontaneous urticaria. In addition, increased expression of IL-25 and IL-33 on mast cells was observed in lesional skin of patients with chronic spontaneous urticaria (70). IL-25 and IL-33 can modulate many aspects of mast cell function, including proliferation and production of a variety of Th2 cytokines in chronic spontaneous urticaria (64).
Mast Cells in Atopic Dermatitis
AD is a chronic, pruritic and inflammatory skin disease that occurs in 15–30% of children and approximately 5% of adults in industrialized nations (71). AD is characterized by barrier disruption, immunological dysfunction and elevated serum IgE. The symptoms of AD, such as recurrent dry, scaly and erythematous lesions and intense pruritus, can place an enormous burden on patients. A significant association was observed between the number of mast cells in AD skin lesions and the disease severity (assessed by the Eczema Area and Severity Index (EASI) score), although the number of mast cells was not changed by short-term treatment with topical tacrolimus (72).
Filaggrin, a filament-associated protein, is crucial for maintenance of the skin barrier (73). Mutations in the filaggrin gene are associated with increased prevalence of ichthyosis vulgaris and AD (73, 74). Mice with mutations in the filaggrin gene (Flgft mice; also called flaky tail mice) spontaneously develop dermatitis that is accompanied by increases in the serum IgE and number of dermal mast cells, thus resembling AD (75).
Topical application of a low-calcemic vitamin-D3-analog MC903 (calcipotriol), which is widely used in the treatment of psoriasis, resulted in development of AD-like dermatitis in mice. MC903-induced dermatitis was dependent on thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 in BALB/c mice (76), and on TSLP, but not IL-25 or IL-33, in C57BL/6 mice (77). Mast cells were involved in TSLP production and induction of skin inflammation in MC903-induced dermatitis (78).
Nc/Nga mice developed AD-like skin inflammation after topical application of an ointment containing Dermatophagoides farinae (Dfb) (79). The number of mast cells and histamine level were increased in the inflamed skin of Dfb-treated Nc/Nga mice (79). Although the frequency of scratching was decreased by application of an H4R antagonist (JNJ 7777120) to the skin of wild-type mice after intradermal histamine injection, that same antagonist was ineffective against itching and skin inflammation in Dfb-induced AD-like skin inflammation in NC/Nga mice (80).. Meanwhile, the EASI score was lower for inflammatory AD skin lesions in patients who were treated with an H4R antagonist (ZPL-3893787) than in those treated with a placebo (81).
Mast cells produce IL-4 and IL-13 (8). Treatment of AD patients with an anti-human IL-4Rα antibody (dupilumab) that inhibits binding of IL-4 and IL-13 to IL-4Rα improved the signs and symptoms of AD (including pruritus), anxiety and depression, as well as the quality of life, compared to placebo controls (82). However, to date, neither meta-analyses and systematic reviews of existing case series nor randomized controlled trials (RCTs) have generated concrete evidence of overall effectiveness of omalizumab for AD (83).
Mast Cells in Psoriasis
Psoriasis is a common chronic skin disease involving systemic inflammation that leads to formation of scaly patches on the skin. The number of mast cells in pruritic lesions was greater than in non-pruritic lesions in psoriasis (84). Activated mast cells were more abundant in psoriatic lesions than in non-lesional psoriatic skin and in healthy subjects, whereas resting mast cells were almost entirely absent in psoriatic skin lesions (85). Importantly, the proportion of resting mast cells gradually normalized in lesional psoriatic skin during etanercept (a TNF inhibitor) therapy (85). These results suggest that mast cells may be involved in the pathogenesis of psoriasis.
Topical application of imiquimod — a TLR7 agonist that is widely used to treat genital warts and actinic keratosis — resulted in development of psoriasis-like dermatitis in mice (86). However, it should be noted that the dermatitis induced in humans by topical imiquimod resembled contact dermatitis rather than psoriasis (87). Expression of Tlr7 mRNA was constitutively observed and increased in mouse BMCMCs in response to imiquimod, and TLR7 on mast cells was responsible for development of imiquimod-induced dermatitis in mice (88). In those same mice, mast cell activation by imiquimod via TLR7 led to TNF production, which in turn promoted skin dendritic cell migration (88) (Figure 4). Imiquimod can induce degranulation of mast cells in humans and mice dependent on human MRGPRX2 and mouse Mrgprb2 (89), although it remains unclear whether imiquimod can bind to MRGPRX2/Mrgprb2. Mrgprb2-dependent mast cell degranulation is crucial for development of imiquimod-induced dermatitis in mice (89).
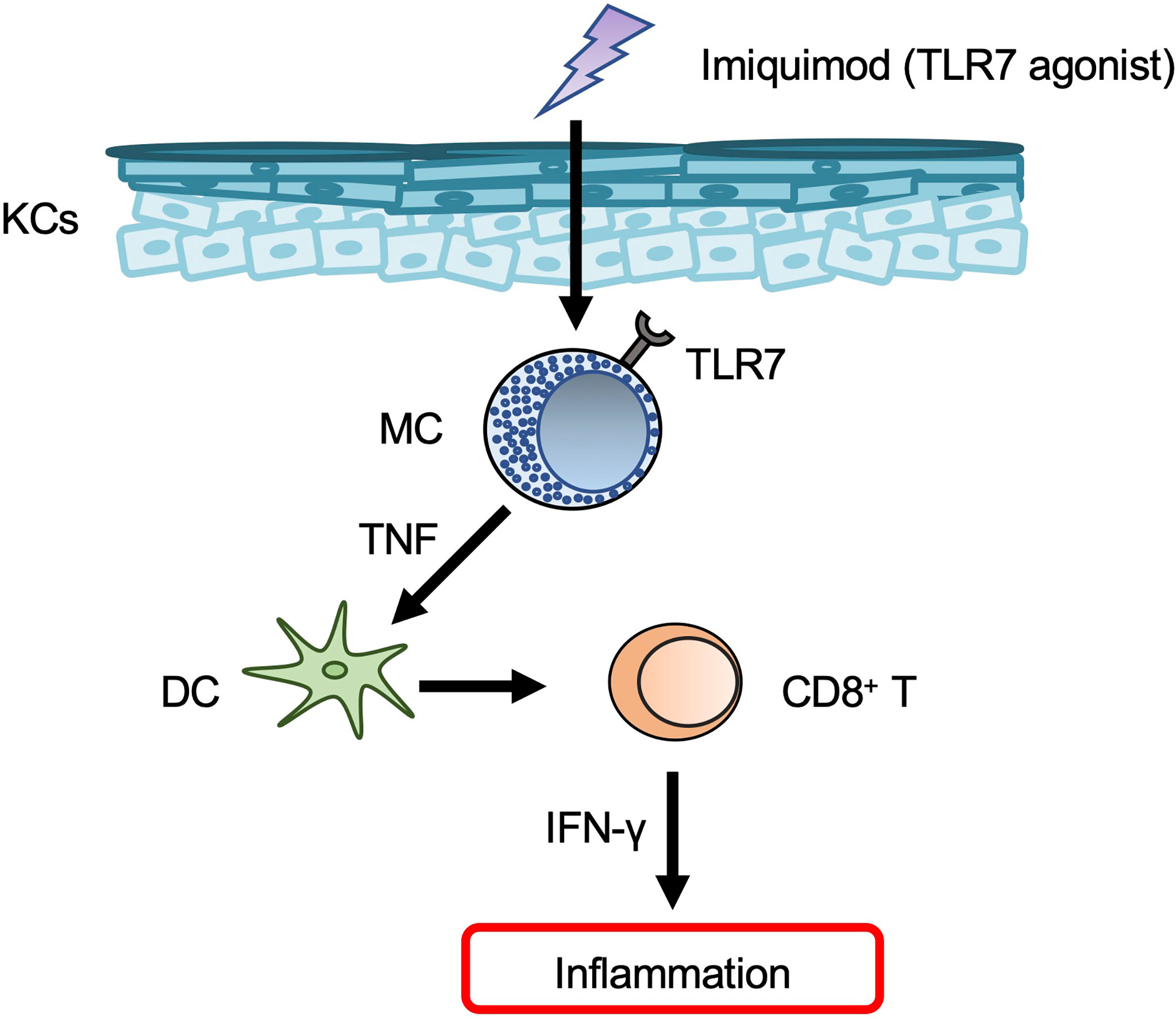
Figure 4 Mast cells in psoriasis. Mast cells (MCs) produce TNF in response to imiquimod via TLR7, and TNF then promotes dendritic cell (DC) migration from the skin to draining LNs. The migrated DCs induce CD8+ T-cell expansion in the peripheral blood.
Mast Cells in Collagen Synthesis (Wound Healing and Fibrosis)
Mast cells activated by tissue injury regulated various phases of skin repair (90). In mice 0.5~1 hour after wounding, the number of degranulated mast cells and the level of vascular permeability were most prominently increased in areas directly adjacent to the wounded skin (91). Moreover, mast cells were involved in neutrophil influx and wound healing in the wounded skin of mice at 12 hours and two to six days after wounding, respectively (91). Thus, mast cells appear to be important for induction of inflammation by increasing vascular permeability and recruiting inflammatory cells to wound sites. Scar width was significantly smaller in mast-cell-deficient KitW/W-v mice than in Kit+/+ mice at seven and 10 days after wounding (92). In addition, activated mast cells promoted fibroblast expansion (93). These data suggest that mast cells are involved in collagen deposition by activating fibroblasts during remodeling (Figure 5).
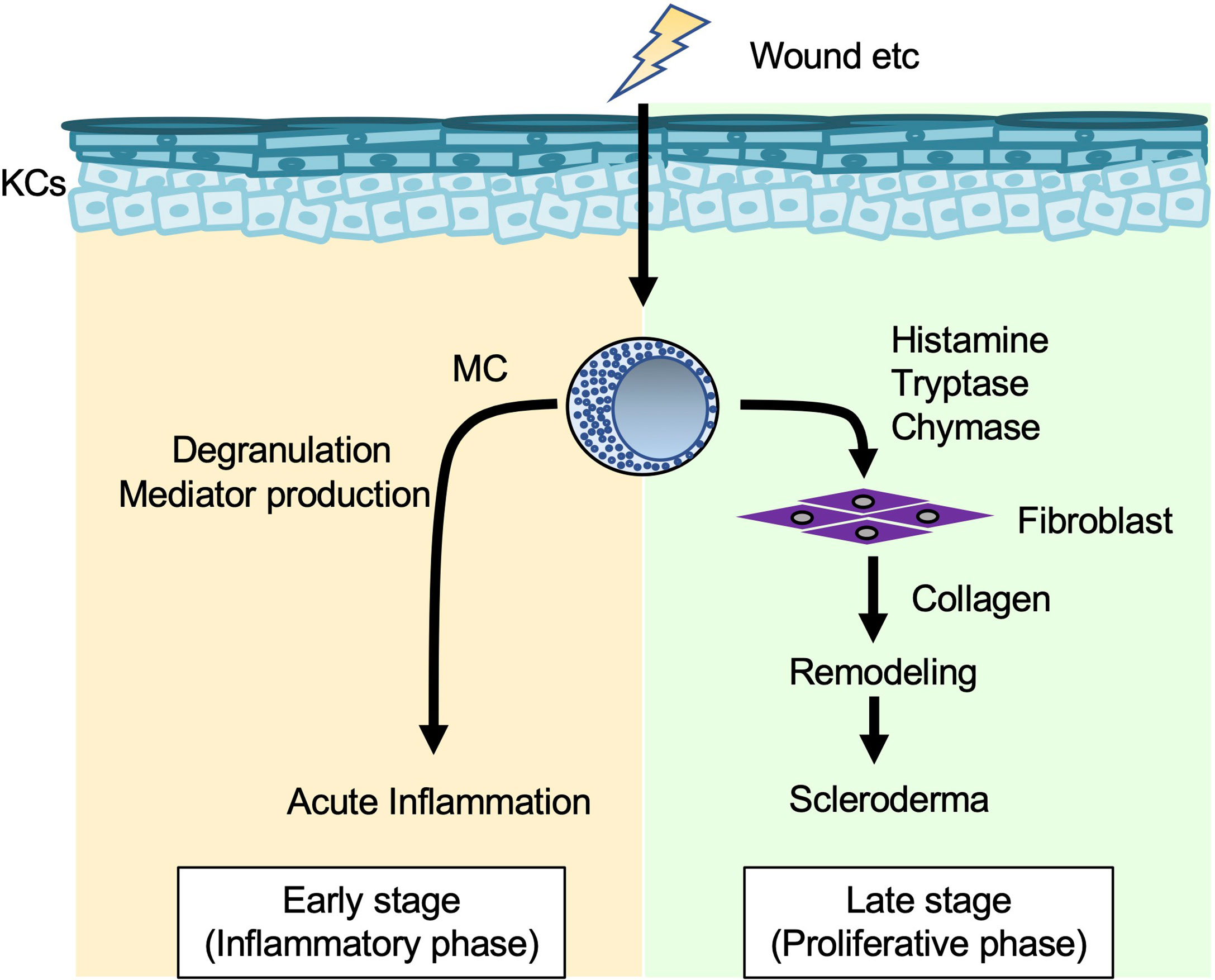
Figure 5 Mast cells in collagen synthesis (wound healing and fibrosis). When the skin is wounded, mast cells (MCs) degranulate and release various mediators that enhance vascular permeability and recruitment of inflammatory cells to injured sites in the early stage (inflammatory phase). In addition, MC-derived histamine, tryptase and chymase induce fibroblasts to produce collagen, which is involved in tissue remodeling and development of scleroderma in the late stage (proliferative phase).
Systemic sclerosis (scleroderma) is a systemic autoimmune connective tissue disorder characterized by vascular dysfunction and progressive fibrosis of the skin and internal organs, such as the lung and kidney. Mast cell density (mast cells/mm2) in the papillary and reticular dermis was significantly greater in patients with early progressive systemic sclerosis than in control subjects (94). Histamine and tryptase each enhanced proliferation and collagen synthesis in human skin fibroblasts (95). In aged tight-skin mice, which develop an inherited fibrotic disease resembling scleroderma, mast cells and chymase were responsible for augmentation of fibrosis (96, 97).
Mast cells were detected in lichen planopilaris (LPP), a type of scarring hair loss (cicatricial alopecia) characterized by lymphocytic infiltration in the upper portion of hair follicles (98). The number of IL-17A-positive mast cells was increased in LPP lesions compared with the normal scalp (98). IL-17R is expressed exclusively in follicular epithelial cells in LPP lesions. These observations suggest that mast-cell-derived IL-17A might somehow be involved in the pathogenesis of LPP via IL-17R on follicular epithelial cells.
Mast Cells in Skin Allografts and Graft-Versus-Host Disease (GVHD)
Long-term acceptance of skin allografts was enabled in mice by injection of anti-CD154 blocking antibody together with allogeneic cells (a process known as donor-specific transfusion; DST) (99). Tregs were important for tolerance to alloantigens in mice (100), and mast cells were increased in the skin allografts of DST-treated mice (99). In addition, in that DST model, Treg-derived IL-9 induced mast cell accumulation and activation in skin allografts, and the accumulated cells suppressed CD8+ T cell-mediated allograft rejection (99).
A major side effect of allogeneic hematopoietic stem cell transplantation is graft-versus-host disease (GVHD), in which donor lymphocytes attack the recipient’s body as non-self tissues. The number of mast cells was increased in the skin of patients with more severe acute GVHD (101). In GVHD induced by transplantation of CD8+ T cells and T cell-depleted bone marrow cells from C3H.SW mice, the survival rate was significantly higher for irradiated WBB6F1-KitW/W-v mice than for irradiated WBB6F1-Kit+/+ mice (102). The density of dyskeratotic cells was significantly lower in WBB6F1-KitW/W-v mice than in WBB6F1-Kit+/+ mice at 14, 21, and 28 days after transplantation, suggesting that mast cells act as effector cells in the development of acute GVHD (102) (Figure 6).
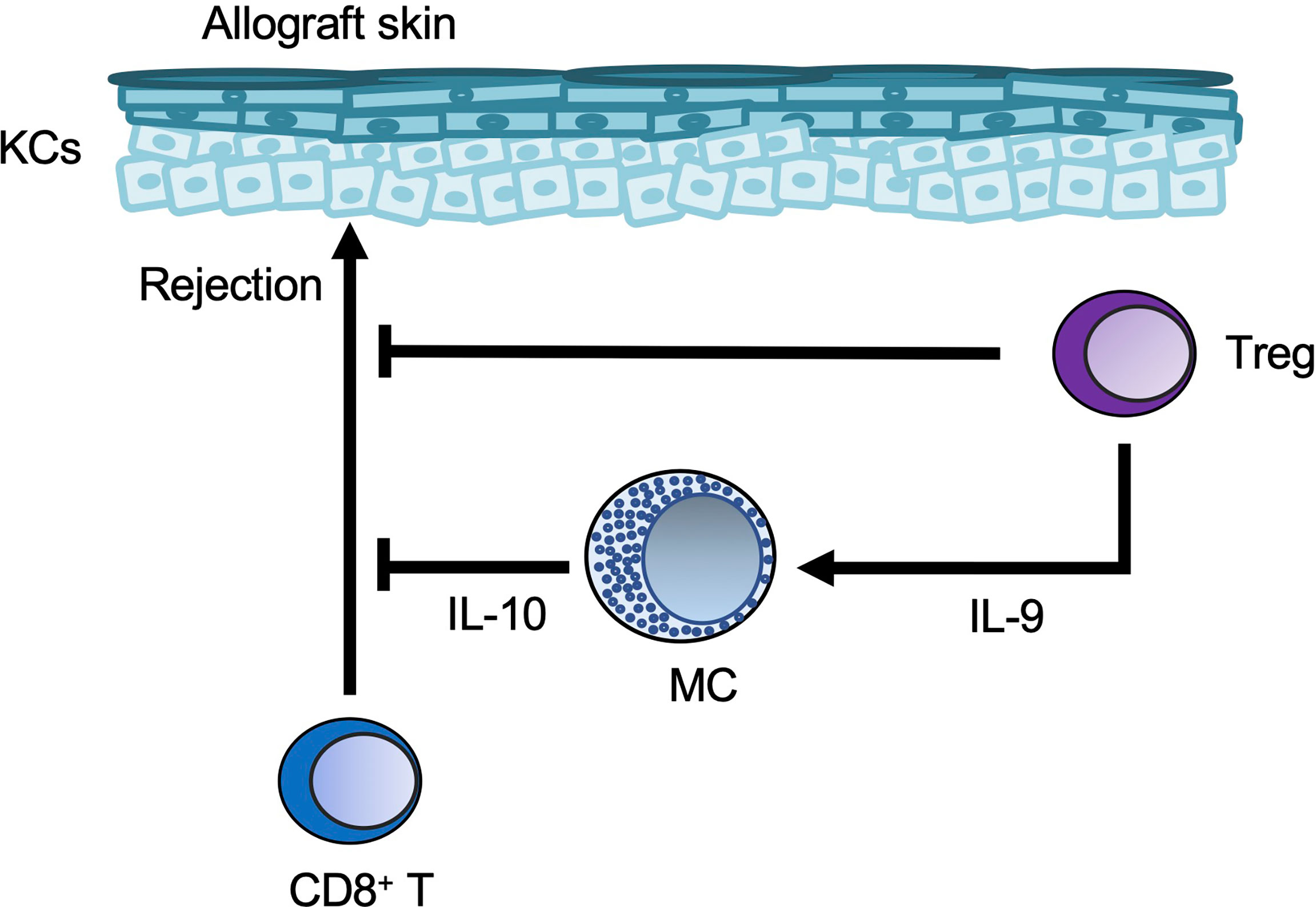
Figure 6 Mast cells in skin allografts. Regulatory T-cell (Treg-)derived IL-9 induces mast cell (MC) accumulation and activation in skin allografts. The Tregs and MCs then suppress CD8+ T-cell-mediated allograft rejection.
On the other hand, development of acute GVHD induced by intravenous injection of either T cell-depleted bone marrow from C57BL/6 mice or CD4+ and CD8+ T cells from FVB mice was significantly greater in irradiated C57BL/6-KitW-sh/W-sh mice than in irradiated C57BL/6 wild-type mice (103). These GVHD reactions were resolved in C57BL/6-KitW-sh/W-sh mice engrafted with wild-type BMCMCs, but not Il10-/- BMCMCs (103). These results indicate that mast-cell-derived IL-10 plays an important role in the inhibition of acute GVHD caused by MHC antigen mismatch.
Conclusion
Mast cells act as potential sources of inflammatory and/or regulatory mediators during development of various cutaneous infections and diseases (Figure 7). Considerable progress has been made in our understanding of these immune cells in recent years. Further elucidation of the complex interactions of mast cells will potentially lead to novel clinical approaches for various pathological conditions.
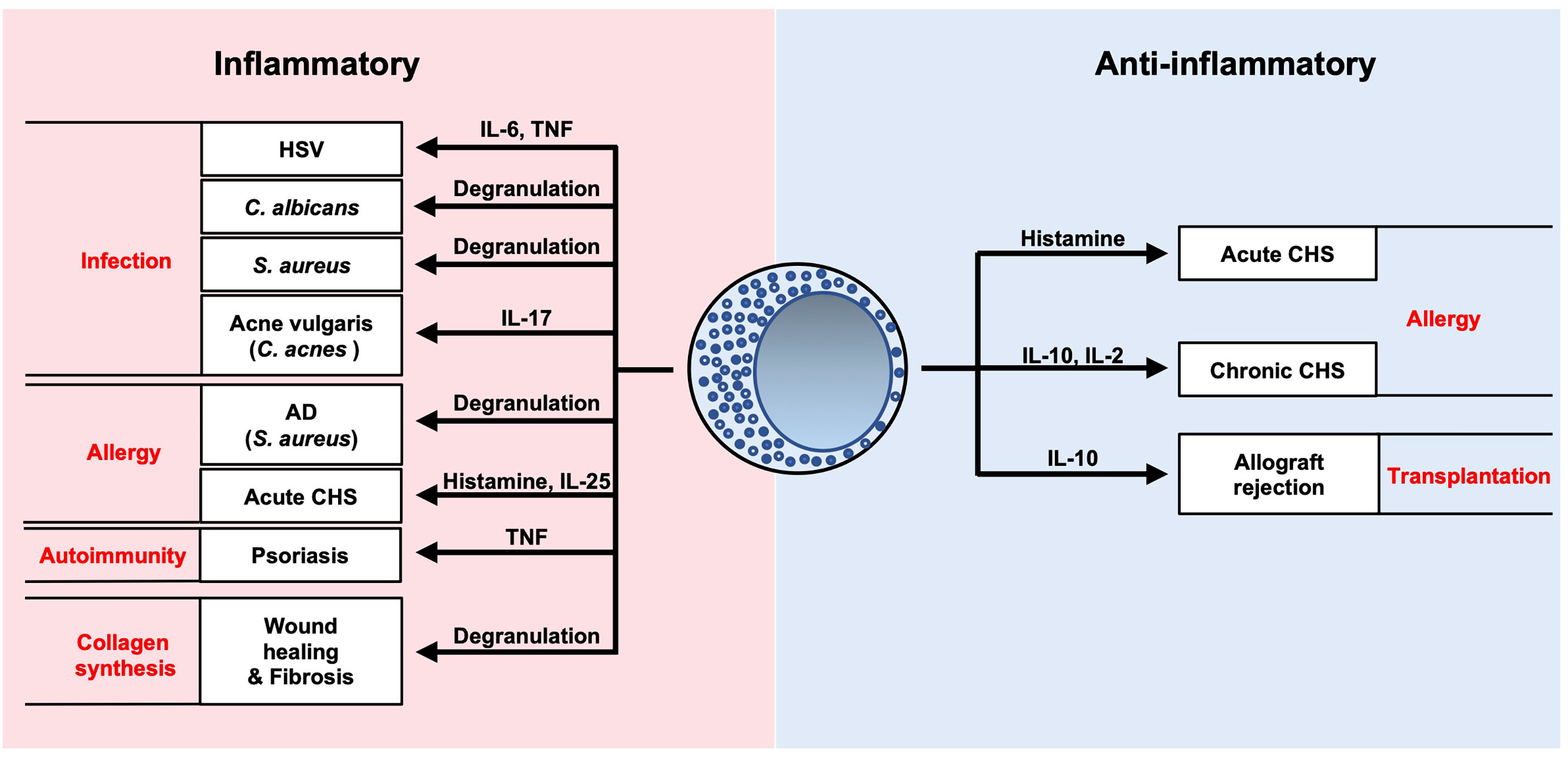
Figure 7 Summary of roles of mast cells in cutaneous diseases. Mast cells are potential sources of inflammatory and/or regulatory mediators during development of various cutaneous infections and diseases.
Author Contributions
TN designed and wrote the manuscript. KH and SN reviewed and revised the manuscript prior to submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B)(21H02963 to SN) from the Japan Society for the Promotion of Science, and Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency (JPMJPR18H6 to SN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACD, allergic contact dermatitis; AD, atopic dermatitis; BMCMCs, bone marrow-derived cultured mast cells; CBMCs, cord blood-derived mast cells; CHS, contact hypersensitivity; CTMC, connective tissue-type mast cell; Dfb, Dermatophagoides farina; DNFB, 1-fluoro-2,4-dinitrobenzene; DST, donor-specific transfusion; EASI, Eczema Area and Severity Index; FITC, fluorescein isothiocyanate; GVHD, graft-versus-host disease; HSV, herpes simplex virus; IL, interleukin; Ig, immunoglobulin; LPP, lichen planopilaris; LPS, lipopolysaccharide; MCPs, mast cell progenitors; MMC, mucosal mast cell; MRGPRX2, mas-related G-protein-coupled receptor X2; NGF, nerve growth factor; PAMP9–20, pro-adrenomedullin peptide 9–20; PGD2, prostaglandin D2; PRRs, pattern-recognition receptors; RCTs, randomized controlled trials; SCF, stem cell factor; sPLA2-III, group III secreted phospholipase A2; SSSS, staphylococcal scalded skin syndrome; TGF, transforming growth factor; TLR, toll-like receptors; TNCB, 2,4,6-trinitrochlorobenzene; TNF, tumor necrosis factor; Treg, regulatory T cell; TSLP, thymic stromal lymphopoietin.
References
1. Morita H, Saito H, Matsumoto K, Nakae S. Regulatory Roles of Mast Cells in Immune Responses. Semin Immunopathol (2016) 38(5):623–9. doi: 10.1007/s00281-016-0566-0
2. Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol (2015) 6:620. doi: 10.3389/fimmu.2015.00620
3. Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental Checkpoints of the Basophil/Mast Cell Lineages in Adult Murine Hematopoiesis. Proc Natl Acad Sci U.S.A. (2005) 102(50):18105–10. doi: 10.1073/pnas.0509148102
4. Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory Mast Cells: Negative, as Well as Positive, Regulators of Immunity. Nat Rev Immunol (2008) 8(6):478–86. doi: 10.1038/nri2327
5. Wang Y, Matsushita K, Jackson J, Numata T, Zhang Y, Zhou G, et al. Transcriptome Programming of Il-3-Dependent Bone Marrow-Derived Cultured Mast Cells by Stem Cell Factor (Scf). Allergy (2021) 76(7):2288–91. doi: 10.1111/all.14808
6. Ito T, Egusa C, Maeda T, Numata T, Nakano N, Nishiyama C, et al. Il-33 Promotes Mhc Class Ii Expression in Murine Mast Cells. Immun Inflammation Dis (2015) 3(3):196–208. doi: 10.1002/iid3.59
7. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two Types of Human Mast Cells That Have Distinct Neutral Protease Compositions. Proc Natl Acad Sci U.S.A. (1986) 83(12):4464–8. doi: 10.1073/pnas.83.12.4464
8. Mukai K, Tsai M, Saito H, Galli SJ. Mast Cells as Sources of Cytokines, Chemokines, and Growth Factors. Immunol Rev (2018) 282(1):121–50. doi: 10.1111/imr.12634
9. Turner H, Kinet JP. Signalling Through the High-Affinity Ige Receptor Fc Epsilonri. Nature (1999) 402(6760 Suppl):B24–30. doi: 10.1038/35037021
10. Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The Phospholipase C Gamma 1-Dependent Pathway of Fc Epsilon Ri-Mediated Mast Cell Activation Is Regulated Independently of Phosphatidylinositol 3-Kinase. J Biol Chem (2003) 278(48):48474–84. doi: 10.1074/jbc.M301350200
11. Burwen SJ. Recycling of Mast Cells Following Degranulation in Vitro: An Ultrastructural Study. Tissue Cell (1982) 14(1):125–34. doi: 10.1016/0040-8166(82)90012-x
12. Urb M, Sheppard DC. The Role of Mast Cells in the Defence Against Pathogens. PloS Pathog (2012) 8(4):e1002619. doi: 10.1371/journal.ppat.1002619
13. Kunder CA, St John AL, Abraham SN. Mast Cell Modulation of the Vascular and Lymphatic Endothelium. Blood (2011) 118(20):5383–93. doi: 10.1182/blood-2011-07-358432
14. Galli SJ, Tsai M. Ige and Mast Cells in Allergic Disease. Nat Med (2012) 18(5):693–704. doi: 10.1038/nm.2755
15. Kawasaki T, Kawai T. Toll-Like Receptor Signaling Pathways. Front Immunol (2014) 5:461. doi: 10.3389/fimmu.2014.00461
16. Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, et al. Differential Responses of Mast Cell Toll-Like Receptors 2 and 4 in Allergy and Innate Immunity. J Clin Invest (2002) 109(10):1351–9. doi: 10.1172/jci14704
17. Varadaradjalou S, Féger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, et al. Toll-Like Receptor 2 (Tlr2) and Tlr4 Differentially Activate Human Mast Cells. Eur J Immunol (2003) 33(4):899–906. doi: 10.1002/eji.200323830
18. Orinska Z, Bulanova E, Budagian V, Metz M, Maurer M, Bulfone-Paus S. Tlr3-Induced Activation of Mast Cells Modulates Cd8+ T-Cell Recruitment. Blood (2005) 106(3):978–87. doi: 10.1182/blood-2004-07-2656
19. Mannschreck D, Feig J, Selph J, Cohen B. Disseminated Bullous Impetigo and Atopic Dermatitis: Case Series and Literature Review. Pediatr Dermatol (2020) 37(1):103–8. doi: 10.1111/pde.14032
20. Liy-Wong C, Pope E, Weinstein M, Lara-Corrales I. Staphylococcal Scalded Skin Syndrome: An Epidemiological and Clinical Review of 84 Cases. Pediatr Dermatol (2021) 38(1):149–53. doi: 10.1111/pde.14470
21. Rocha-de-Souza CM, Berent-Maoz B, Mankuta D, Moses AE, Levi-Schaffer F. Human Mast Cell Activation by Staphylococcus Aureus: Interleukin-8 and Tumor Necrosis Factor Alpha Release and the Role of Toll-Like Receptor 2 and Cd48 Molecules. Infect Immun (2008) 76(10):4489–97. doi: 10.1128/iai.00270-08
22. Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Muñoz-Planillo R, Hasegawa M, et al. Staphylococcus Δ-Toxin Induces Allergic Skin Disease by Activating Mast Cells. Nature (2013) 503(7476):397–401. doi: 10.1038/nature12655
23. Eliasse Y, Leveque E, Garidou L, Battut L, McKenzie B, Nocera T, et al. Il-17(+) Mast Cell/T Helper Cell Axis in the Early Stages of Acne. Front Immunol (2021) 12:740540. doi: 10.3389/fimmu.2021.740540
24. Sakurai A, Yamaguchi N, Sonoyama K. Cell Wall Polysaccharides of Candida Albicans Induce Mast Cell Degranulation in the Gut. Biosci Microb Food Health (2012) 31(3):67–70. doi: 10.12938/bmfh.31.67
25. Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic Pathogen Candida Albicans Elicits a Temporal Response in Primary Human Mast Cells. Sci Rep (2015) 5:12287. doi: 10.1038/srep12287
26. Trevisan E, Vita F, Medic N, Soranzo MR, Zabucchi G, Borelli V. Mast Cells Kill Candida Albicans in the Extracellular Environment But Spare Ingested Fungi From Death. Inflammation (2014) 37(6):2174–89. doi: 10.1007/s10753-014-9951-9
27. De Zuani M, Paolicelli G, Zelante T, Renga G, Romani L, Arzese A, et al. Mast Cells Respond to Candida Albicans Infections and Modulate Macrophages Phagocytosis of the Fungus. Front Immunol (2018) 9:2829. doi: 10.3389/fimmu.2018.02829
28. Nieto-Patlán A, Campillo-Navarro M, Rodríguez-Cortés O, Muñoz-Cruz S, Wong-Baeza I, Estrada-Parra S, et al. Recognition of Candida Albicans by Dectin-1 Induces Mast Cell Activation. Immunobiology (2015) 220(9):1093–100. doi: 10.1016/j.imbio.2015.05.005
29. Pinke KH, Lima HG, Cunha FQ, Lara VS. Mast Cells Phagocyte Candida Albicans and Produce Nitric Oxide by Mechanisms Involving Tlr2 and Dectin-1. Immunobiology (2016) 221(2):220–7. doi: 10.1016/j.imbio.2015.09.004
30. Harada K, Saito M, Sugita T, Tsuboi R. Malassezia Species and Their Associated Skin Diseases. J Dermatol (2015) 42(3):250–7. doi: 10.1111/1346-8138.12700
31. Hiragun T, Ishii K, Hiragun M, Suzuki H, Kan T, Mihara S, et al. Fungal Protein Mgl_1304 in Sweat Is an Allergen for Atopic Dermatitis Patients. J Allergy Clin Immunol (2013) 132(3):608–15.e4. doi: 10.1016/j.jaci.2013.03.047
32. Hiragun M, Hiragun T, Ishii K, Suzuki H, Tanaka A, Yanase Y, et al. Elevated Serum Ige Against Mgl_1304 in Patients With Atopic Dermatitis and Cholinergic Urticaria. Allergol Int (2014) 63(1):83–93. doi: 10.2332/allergolint.13-OA-0611
33. Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. Jama (2022) 327(2):161–72. doi: 10.1001/jama.2021.23487
34. Wilkinson DE, Weller SK. The Role of DNA Recombination in Herpes Simplex Virus DNA Replication. IUBMB Life (2003) 55(8):451–8. doi: 10.1080/15216540310001612237
35. Micali G, Lacarrubba F. Eczema Herpeticum. N Engl J Med (2017) 377(7):e9. doi: 10.1056/NEJMicm1701668
36. Kokuba H, Aurelian L, Burnett J. Herpes Simplex Virus Associated Erythema Multiforme (Haem) Is Mechanistically Distinct From Drug-Induced Erythema Multiforme: Interferon-Gamma Is Expressed in Haem Lesions and Tumor Necrosis Factor-Alpha in Drug-Induced Erythema Multiforme Lesions. J Invest Dermatol (1999) 113(5):808–15. doi: 10.1046/j.1523-1747.1999.00754.x
37. Aoki R, Kawamura T, Goshima F, Ogawa Y, Nakae S, Nakao A, et al. Mast Cells Play a Key Role in Host Defense Against Herpes Simplex Virus Infection Through Tnf-α and Il-6 Production. J Invest Dermatol (2013) 133(9):2170–9. doi: 10.1038/jid.2013.150
38. Aoki R, Kawamura T, Goshima F, Ogawa Y, Nakae S, Moriishi K, et al. The Alarmin Il-33 Derived From Hsv-2-Infected Keratinocytes Triggers Mast Cell-Mediated Antiviral Innate Immunity. J Invest Dermatol (2016) 136(6):1290–2. doi: 10.1016/j.jid.2016.01.030
39. Gefen T, Vaya J, Khatib S, Rapoport I, Lupo M, Barnea E, et al. The Effect of Haptens on Protein-Carrier Immunogenicity. Immunology (2015) 144(1):116–26. doi: 10.1111/imm.12356
40. Gaspari AA, Katz SI, Martin SF. Contact Hypersensitivity. Curr Protoc Immunol (2016) 113:4.2.1–7. doi: 10.1002/0471142735.im0402s113
41. Gaudenzio N, Marichal T, Galli SJ, Reber LL. Genetic and Imaging Approaches Reveal Pro-Inflammatory and Immunoregulatory Roles of Mast Cells in Contact Hypersensitivity. Front Immunol (2018) 9:1275. doi: 10.3389/fimmu.2018.01275
42. Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast Cell-Associated Tnf Promotes Dendritic Cell Migration. J Immunol (Baltimore Md: 1950) (2006) 176(7):4102–12. doi: 10.4049/jimmunol.176.7.4102
43. Kakurai M, Monteforte R, Suto H, Tsai M, Nakae S, Galli SJ. Mast Cell-Derived Tumor Necrosis Factor Can Promote Nerve Fiber Elongation in the Skin During Contact Hypersensitivity in Mice. Am J Pathol (2006) 169(5):1713–21. doi: 10.2353/ajpath.2006.060602
44. Suto H, Nambu A, Morita H, Yamaguchi S, Numata T, Yoshizaki T, et al. Il-25 Enhances Th17 Cell-Mediated Contact Dermatitis by Promoting Il-1beta Production by Dermal Dendritic Cells. J Allergy Clin Immunol (2018) 142(5):1500–9.e10. doi: 10.1016/j.jaci.2017.12.1007
45. Kim HS, Lee MB, Lee D, Min KY, Koo J, Kim HW, et al. The Regulatory B Cell-Mediated Peripheral Tolerance Maintained by Mast Cell Il-5 Suppresses Oxazolone-Induced Contact Hypersensitivity. Sci Adv (2019) 5(7):eaav8152. doi: 10.1126/sciadv.aav8152
46. Leyva-Castillo JM, Das M, Artru E, Yoon J, Galand C, Geha RS. Mast Cell-Derived Il-13 Downregulates Il-12 Production by Skin Dendritic Cells to Inhibit the T(H)1 Cell Response to Cutaneous Antigen Exposure. J Allergy Clin Immunol (2021) 147(6):2305–15.e3. doi: 10.1016/j.jaci.2020.11.036
47. Taketomi Y, Ueno N, Kojima T, Sato H, Murase R, Yamamoto K, et al. Mast Cell Maturation Is Driven Via a Group Iii Phospholipase A2-Prostaglandin D2-Dp1 Receptor Paracrine Axis. Nat Immunol (2013) 14(6):554–63. doi: 10.1038/ni.2586
48. Taketomi Y, Endo Y, Higashi T, Murase R, Ono T, Taya C, et al. Mast Cell-Specific Deletion of Group Iii Secreted Phospholipase a(2) Impairs Mast Cell Maturation and Functions. Cells (2021) 10(7):1691. doi: 10.3390/cells10071691
49. Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The Histamine H3 Receptor as a Novel Therapeutic Target for Cognitive and Sleep Disorders. Trends Pharmacol Sci (2004) 25(12):618–25. doi: 10.1016/j.tips.2004.10.003
50. Inagaki N, Sakurai T, Abe T, Musoh K, Kawasaki H, Tsunematsu M, et al. Characterization of Antihistamines Using Biphasic Cutaneous Reaction in Balb/C Mice. Life Sci (1998) 63(11):Pl 145–50. doi: 10.1016/S0024-3205(98)00356-7
51. Griswold DE, Alessi S, Badger AM, Poste G, Hanna N. Differential Sensitivity of T Suppressor Cell Expression to Inhibition by Histamine Type 2 Receptor Antagonists. J Immunol (Baltimore Md: 1950) (1986) 137(6):1811–5.
52. Belsito DV, Kerdel FA, Potozkin J, Soter NA. Cimetidine-Induced Augmentation of Allergic Contact Hypersensitivity Reactions in Mice. J Invest Dermatol (1990) 94(4):441–5. doi: 10.1111/1523-1747.ep12874535
53. Garaczi E, Szell M, Janossy T, Koreck A, Pivarcsi A, Buzas E, et al. Negative Regulatory Effect of Histamine in Dnfb-Induced Contact Hypersensitivity. Int Immunol (2004) 16(12):1781–8. doi: 10.1093/intimm/dxh179
54. Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-Independent Activation of Mast Cell Is Mediated by Mrg Receptors. Biochem Biophys Res Commun (2006) 349(4):1322–8. doi: 10.1016/j.bbrc.2006.08.177
55. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a Mast-Cell-Specific Receptor Crucial for Pseudo-Allergic Drug Reactions. Nature (2015) 519(7542):237–41. doi: 10.1038/nature14022
56. Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity (2020) 52(2):404–16.e5. doi: 10.1016/j.immuni.2020.01.012
57. Babina M, Guhl S, Artuc M, Zuberbier T. Allergic Fcϵri- and Pseudo-Allergic Mrgprx2-Triggered Mast Cell Activation Routes Are Independent and Inversely Regulated by Scf. Allergy (2018) 73(1):256–60. doi: 10.1111/all.13301
58. Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-Histaminergic Itch. Immunity (2019) 50(5):1163–71.e5. doi: 10.1016/j.immuni.2019.03.013
59. Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast Cell-Derived Interleukin 10 Limits Skin Pathology in Contact Dermatitis and Chronic Irradiation With Ultraviolet B. Nat Immunol (2007) 8(10):1095–104. doi: 10.1038/ni1503
60. Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast Cells Are Key Promoters of Contact Allergy That Mediate the Adjuvant Effects of Haptens. Immunity (2011) 34(6):973–84. doi: 10.1016/j.immuni.2011.03.028
61. Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, et al. Mast Cell Interleukin-2 Production Contributes to Suppression of Chronic Allergic Dermatitis. Immunity (2011) 35(4):562–71. doi: 10.1016/j.immuni.2011.07.013
62. Gimenez-Rivera VA, Siebenhaar F, Zimmermann C, Siiskonen H, Metz M, Maurer M. Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J Immunol (Baltimore Md: 1950) (2016) 197(11):4240–6. doi: 10.4049/jimmunol.1600236
63. Reber LL, Sibilano R, Starkl P, Roers A, Grimbaldeston MA, Tsai M, et al. Imaging Protective Mast Cells in Living Mice During Severe Contact Hypersensitivity. JCI Insight (2017) 2(9):e92900. doi: 10.1172/jci.insight.92900
64. Church MK, Kolkhir P, Metz M, Maurer M. The Role and Relevance of Mast Cells in Urticaria. Immunol Rev (2018) 282(1):232–47. doi: 10.1111/imr.12632
65. Bracken SJ, Abraham S, MacLeod AS. Autoimmune Theories of Chronic Spontaneous Urticaria. Front Immunol (2019) 10:627. doi: 10.3389/fimmu.2019.00627
66. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. Il-24 Is a Common and Specific Autoantigen of Ige in Patients With Chronic Spontaneous Urticaria. J Allergy Clin Immunol (2018) 142(3):876–82. doi: 10.1016/j.jaci.2017.10.035
67. Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N Engl J Med (2013) 368(10):924–35. doi: 10.1056/NEJMoa1215372
68. Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-Related Gene X2 on Mast Cells Is Upregulated in the Skin of Patients With Severe Chronic Urticaria. J Allergy Clin Immunol (2014) 134(3):622–33.e9. doi: 10.1016/j.jaci.2014.05.004
69. Borici-Mazi R, Kouridakis S, Kontou-Fili K. Cutaneous Responses to Substance P and Calcitonin Gene-Related Peptide in Chronic Urticaria: The Effect of Cetirizine and Dimethindene. Allergy (1999) 54(1):46–56. doi: 10.1034/j.1398-9995.1999.00726.x
70. Kay AB, Clark P, Maurer M, Ying S. Elevations in T-Helper-2-Initiating Cytokines (Interleukin-33, Interleukin-25 and Thymic Stromal Lymphopoietin) in Lesional Skin From Chronic Spontaneous (‘Idiopathic’) Urticaria. Br J Dermatol (2015) 172(5):1294–302. doi: 10.1111/bjd.13621
71. Williams H, Flohr C. How Epidemiology Has Challenged 3 Prevailing Concepts About Atopic Dermatitis. J Allergy Clin Immunol (2006) 118(1):209–13. doi: 10.1016/j.jaci.2006.04.043
72. Simon D, Vassina E, Yousefi S, Kozlowski E, Braathen LR, Simon HU. Reduced Dermal Infiltration of Cytokine-Expressing Inflammatory Cells in Atopic Dermatitis After Short-Term Topical Tacrolimus Treatment. J Allergy Clin Immunol (2004) 114(4):887–95. doi: 10.1016/j.jaci.2004.05.066
73. Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, et al. Japanese Guidelines for Atopic Dermatitis 2020. Allergol Int (2020) 69(3):356–69. doi: 10.1016/j.alit.2020.02.006
74. Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin Mutations That Confer Risk of Atopic Dermatitis Confer Greater Risk for Eczema Herpeticum. J Allergy Clin Immunol (2009) 124(3):507–13, 13.e1-7. doi: 10.1016/j.jaci.2009.07.034
75. Moniaga CS, Egawa G, Kawasaki H, Hara-Chikuma M, Honda T, Tanizaki H, et al. Flaky Tail Mouse Denotes Human Atopic Dermatitis in the Steady State and by Topical Application With Dermatophagoides Pteronyssinus Extract. Am J Pathol (2010) 176(5):2385–93. doi: 10.2353/ajpath.2010.090957
76. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A Role for Il-25 and Il-33-Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J Exp Med (2013) 210(13):2939–50. doi: 10.1084/jem.20130351
77. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. Tslp Elicits Il-33-Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci Transl Med (2013) 5(170):170ra16. doi: 10.1126/scitranslmed.3005374
78. Svanberg S, Li Z, Öhlund P, Roy A, Åbrink M. Mast Cells Limit Ear Swelling Independently of the Chymase Mouse Mast Cell Protease 4 in an Mc903-Induced Atopic Dermatitis-Like Mouse Model. Int J Mol Sci (2020) 21(17):6311. doi: 10.3390/ijms21176311
79. Yamada Y, Ueda Y, Nakamura A, Kanayama S, Tamura R, Hashimoto K, et al. Immediate-Type Allergic and Protease-Mediated Reactions Are Involved in Scratching Behaviour Induced by Topical Application of Dermatophagoides Farinae Extract in Nc/Nga Mice. Exp Dermatol (2018) 27(4):418–26. doi: 10.1111/exd.13322
80. Kamo A, Negi O, Tengara S, Kamata Y, Noguchi A, Ogawa H, et al. Histamine H(4) Receptor Antagonists Ineffective Against Itch and Skin Inflammation in Atopic Dermatitis Mouse Model. J Invest Dermatol (2014) 134(2):546–8. doi: 10.1038/jid.2013.351
81. Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al. Efficacy and Safety of the Histamine H(4) Receptor Antagonist Zpl-3893787 in Patients With Atopic Dermatitis. J Allergy Clin Immunol (2019) 143(5):1830–7.e4. doi: 10.1016/j.jaci.2018.07.047
82. Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab Versus Placebo in Atopic Dermatitis. N Engl J Med (2016) 375(24):2335–48. doi: 10.1056/NEJMoa1610020
83. Wang HH, Li YC, Huang YC. Efficacy of Omalizumab in Patients With Atopic Dermatitis: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol (2016) 138(6):1719–22.e1. doi: 10.1016/j.jaci.2016.05.038
84. Nakamura M, Toyoda M, Morohashi M. Pruritogenic Mediators in Psoriasis Vulgaris: Comparative Evaluation of Itch-Associated Cutaneous Factors. Br J Dermatol (2003) 149(4):718–30. doi: 10.1046/j.1365-2133.2003.05586.x
85. Zhang Y, Shi Y, Lin J, Li X, Yang B, Zhou J. Immune Cell Infiltration Analysis Demonstrates Excessive Mast Cell Activation in Psoriasis. Front Immunol (2021) 12:773280. doi: 10.3389/fimmu.2021.773280
86. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated Via the Il-23/Il-17 Axis. J Immunol (Baltimore Md: 1950) (2009) 182(9):5836–45. doi: 10.4049/jimmunol.0802999
87. Garzorz-Stark N, Lauffer F, Krause L, Thomas J, Atenhan A, Franz R, et al. Toll-Like Receptor 7/8 Agonists Stimulate Plasmacytoid Dendritic Cells to Initiate T. J Allergy Clin Immunol (2018) 141(4):1320–33.e11. doi: 10.1016/j.jaci.2017.07.045
88. Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, et al. Mast Cells Are Crucial for Early Inflammation, Migration of Langerhans Cells, and Ctl Responses Following Topical Application of Tlr7 Ligand in Mice. Blood (2007) 110(3):946–53. doi: 10.1182/blood-2006-07-036889
89. Hao Y, Peng B, Che D, Zheng Y, Kong S, Liu R, et al. Imiquimod-Related Dermatitis Is Mainly Mediated by Mast Cell Degranulation Via Mas-Related G-Protein Coupled Receptor B2. Int Immunopharmacol (2020) 81:106258. doi: 10.1016/j.intimp.2020.106258
90. Wulff BC, Wilgus TA. Mast Cell Activity in the Healing Wound: More Than Meets the Eye? Exp Dermatol (2013) 22(8):507–10. doi: 10.1111/exd.12169
91. Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast Cells Are Required for Normal Healing of Skin Wounds in Mice. FASEB J: Off Publ Fed Am Soc Exp Biol (2006) 20(13):2366–8. doi: 10.1096/fj.06-5837fje
92. Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast Cells Contribute to Scar Formation During Fetal Wound Healing. J Invest Dermatol (2012) 132(2):458–65. doi: 10.1038/jid.2011.324
93. Levi-Schaffer F, Kupietzky A. Mast Cells Enhance Migration and Proliferation of Fibroblasts Into an in Vitro Wound. Exp Cell Res (1990) 188(1):42–9. doi: 10.1016/0014-4827(90)90275-f
94. Hawkins RA, Claman HN, Clark RA, Steigerwald JC. Increased Dermal Mast Cell Populations in Progressive Systemic Sclerosis: A Link in Chronic Fibrosis? Ann Intern Med (1985) 102(2):182–6. doi: 10.7326/0003-4819-102-2-182
95. Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, et al. Human Mast Cells Stimulate Fibroblast Proliferation, Collagen Synthesis and Lattice Contraction: A Direct Role for Mast Cells in Skin Fibrosis. Clin Exp Allergy: J Br Soc Allergy Clin Immunol (2002) 32(2):237–46. doi: 10.1046/j.1365-2222.2002.01293.x
96. Shiota N, Kakizoe E, Shimoura K, Tanaka T, Okunishi H. Effect of Mast Cell Chymase Inhibitor on the Development of Scleroderma in Tight-Skin Mice. Br J Pharmacol (2005) 145(4):424–31. doi: 10.1038/sj.bjp.0706209
97. Everett ET, Pablos JL, Harley RA, LeRoy EC, Norris JS. The Role of Mast Cells in the Development of Skin Fibrosis in Tight-Skin Mutant Mice. Comp Biochem Physiol A Physiol (1995) 110(2):159–65. doi: 10.1016/0300-9629(94)00127-f
98. Hobo A, Harada K, Maeda T, Uchiyama M, Irisawa R, Yamazaki M, et al. Il-17-Positive Mast Cell Infiltration in the Lesional Skin of Lichen Planopilaris: Possible Role of Mast Cells in Inducing Inflammation and Dermal Fibrosis in Cicatricial Alopecia. Exp Dermatol (2020) 29(3):273–7. doi: 10.1111/exd.13816
99. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast Cells Are Essential Intermediaries in Regulatory T-Cell Tolerance. Nature (2006) 442(7106):997–1002. doi: 10.1038/nature05010
100. Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. Il-10 Is Required for Regulatory T Cells to Mediate Tolerance to Alloantigens in Vivo. J Immunol (2001) 166(6):3789–96. doi: 10.4049/jimmunol.166.6.3789
101. Wu KN, Emmons RV, Lisanti MP, Farber JL, Witkiewicz AK. Foxp3-Expressing T Regulatory Cells and Mast Cells in Acute Graft-Versus-Host Disease of the Skin. Cell Cycle (2009) 8(21):3601–5. doi: 10.4161/cc.8.21.9999
102. Murphy GF, Sueki H, Teuscher C, Whitaker D, Korngold R. Role of Mast Cells in Early Epithelial Target Cell Injury in Experimental Acute Graft-Versus-Host Disease. J Invest Dermatol (1994) 102(4):451–61. doi: 10.1111/1523-1747.ep12373016
Keywords: skin disease, allergy, autoimmunity, infection, rejection
Citation: Numata T, Harada K and Nakae S (2022) Roles of Mast Cells in Cutaneous Diseases. Front. Immunol. 13:923495. doi: 10.3389/fimmu.2022.923495
Received: 19 April 2022; Accepted: 16 June 2022;
Published: 06 July 2022.
Edited by:
Satoshi Tanaka, Kyoto Pharmaceutical University, JapanReviewed by:
Elba Mónica Vermeulen, Instituto de Biología y Medicina Experimental (CONICET), ArgentinaViktor Bugajev, Institute of Molecular Genetics (ASCR), Czechia
Copyright © 2022 Numata, Harada and Nakae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takafumi Numata, bnVtYXRhQHRva3lvLW1lZC5hYy5qcA==; Susumu Nakae, c25ha2FlQGhpcm9zaGltYS11LmFjLmpw
 Takafumi Numata
Takafumi Numata Kazutoshi Harada1
Kazutoshi Harada1 Susumu Nakae
Susumu Nakae