- 1Department of Hematology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 2Blood Diseases Institute, Xuzhou Medical University, Xuzhou, China
- 3Jiangsu Key Laboratory of Bone Marrow Stem Cells, Xuzhou, China
- 4Xuzhou Medical University, Xuzhou, China
Background: Glucocorticoids (GCs) are often used to treat cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). The effects of GCs on the efficacy of CAR-T cell treatment in relapsed/refractory multiple myeloma (RRMM) have not been fully established. We evaluated the impact of GCs on clinical outcomes of RRMM patients treated with CAR-T cells.
Methods: This study involved RRMM patients treated with CAR-T cells at our center between June 2017 and December 2020. Patients were stratified into GC-used group (GC-group) and non-GC-used group (NGC-group). CRS or ICANS was graded on the basis of the American Society of Transplantation and Cellular Therapy consensus grading system. Response status was evaluated by the IMWG Uniform Response Criteria. The duration of response (DOR), progression-free survival (PFS), and overall survival (OS) were calculated.
Result: A total of 71 patients were included in this study. In the NGC group (40 patients), 34 (85%) had responses to CAR-T cell therapy, including 16 (40%) stringent complete response (sCR), seven (17.5%) complete response (CR), five (12.5%) very good partial response (VGPR), and six (15%) partial response (PR). The overall response rate (ORR) and complete response rate (CRR) in the NGC group were 85% and 57.5%. In the GC group (31 patients), 29 (93.5%) had responses, including 11 (35.5%) sCR, nine (29%) CR, two (6.4%) VGPR, and seven (22.6%) PR. Differences in ORR and CRR between the two groups were insignificant. The dose, duration, and timing of GCs did not affect ORR and CRR. At a median follow-up of 28.2 months, the median PFS was 20.4 months (95% CI, 7.9 to 32.9) while the median OS was 36.6 months (95% CI, 25.9 to 47.2) for the GC group. The median PFS and OS for the NGC group were 13.7 months (95% CI, 8.8 to 18.6) and 27.5 months (95% CI, 14.1 to 41.0). There were no significant differences in either PFS or OS between the GC group and the NGC group. Differences in median DOR for the patients with CR or better in the GC group and NGC group were not significant (p = 0.17). Earlier, prolonged use and high dose of GCs were not associated with any effects on either PFS or OS. Additionally, GCs had no effects on CAR-T cell proliferation.
Conclusion: Administration of GCs, dose, timing, and duration does not influence the clinical efficacy of CAR-T cells in RRMM in this study.
Introduction
Anti–B cell maturation antigen (BCMA) CAR-T cell therapy has attained encouraging efficacy in treating relapsed/refractory multiple myeloma (RRMM) (1–6). However, CAR-T cell therapy-associated immunological toxicity remains a major clinical challenge (7). Although glucocorticoids (GCs) can mitigate severe adverse effects, the expansion and persistence of CAR-T cells may also be sacrificed, which adversely affect clinical outcomes (8–10). Clinical trial data reported are very confusing about the role of GCs for the management of CRS and/or immune effector cell-associated neurotoxicity syndrome (ICANS). Early studies showed that GCs might not impact the efficacy of CAR-T cells for B-cell acute lymphoblastic leukemia (B-ALL) (11). Recent studies reported that high cumulative dose and prolonged application of GCs negatively affected overall survival (OS) in large B-cell lymphoma (LBCL) (12). However, the effect of GCs on CAR-T cell efficacy in RRMM has not been established.
It is necessary to clarify whether the use of GCs and their dose, timing, and duration could impact clinical outcomes in clinical trials for RRMM. Herein, we carried out a retrospective analysis to investigate the impacts of GCs on outcomes of CAR-T cell therapy in RRMM.
Methods
Patient selection
This retrospective investigation was approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University. RRMM patients treated with CAR-T cells at our center between June 2017 and December 2020 were included in this study (ChiCTR1900026219, ChiCTR-OIC-17011272). All patients were infused with anti-BCMA CAR-T cells alone or combined with anti-CD19 CAR-T cells. CAR structures targeting CD19 and BCMA were as described previously (13, 14). The data cutoff for follow-up was 30 November 2021.
CRS and ICANS management
CRS or ICANS was graded according to the ASTCT consensus grading system. Fever is defined as a temperature ≥38°C after CAR-T cell infusion according to ASTCT consensus (15). Dexamethasone or methylprednisolone was administrated when antipyretics (aspirin-DI-lysine, 900 mg, each time, up to 3,600 mg per day) failed to resolve continuing fever (≥38°C) and ICANS.
Clinical outcome and follow-up
The IMWG Uniform Response Criteria for Multiple Myeloma were used to evaluate clinical response (16). Patients were monitored at 2 weeks, 1 month, 2 months, 3 months, and 6 months after CAR-T cell infusion. After 1 year, regular follow-up was conducted every 6 months. Further assessments were as previously reported (14).
Evaluation of laboratory parameters
Complete blood counts (CBC) and ferritin, IL-6, and CRP levels were assessed before lymphodepletion, on the day of CAR T-cell infusion, and at intervals following CAR-T cell infusion. CAR-T cell counts in peripheral blood were assessed by flow cytometry (FCM) (17, 18).
Statistical methods
Absolute number and percentage were used to describe categorical variables. Mann–Whitney U-test was used in the case of non-parametric data. Kaplan–Meier analysis was used to estimate the duration of response (DOR), progression-free survival (PFS), and overall survival (OS). A comparison of overall response rate (ORR) and complete response rate (CRR) between the GC-used group (GC-group) and non-GC-used group (NGC-group) was analyzed using Fisher’s exact test. All statistical analyses were performed using the IBM SPSS 25.0 software, and two-sided P values less than 0.05 were considered to be statistically significant.
Results
Patient characteristics
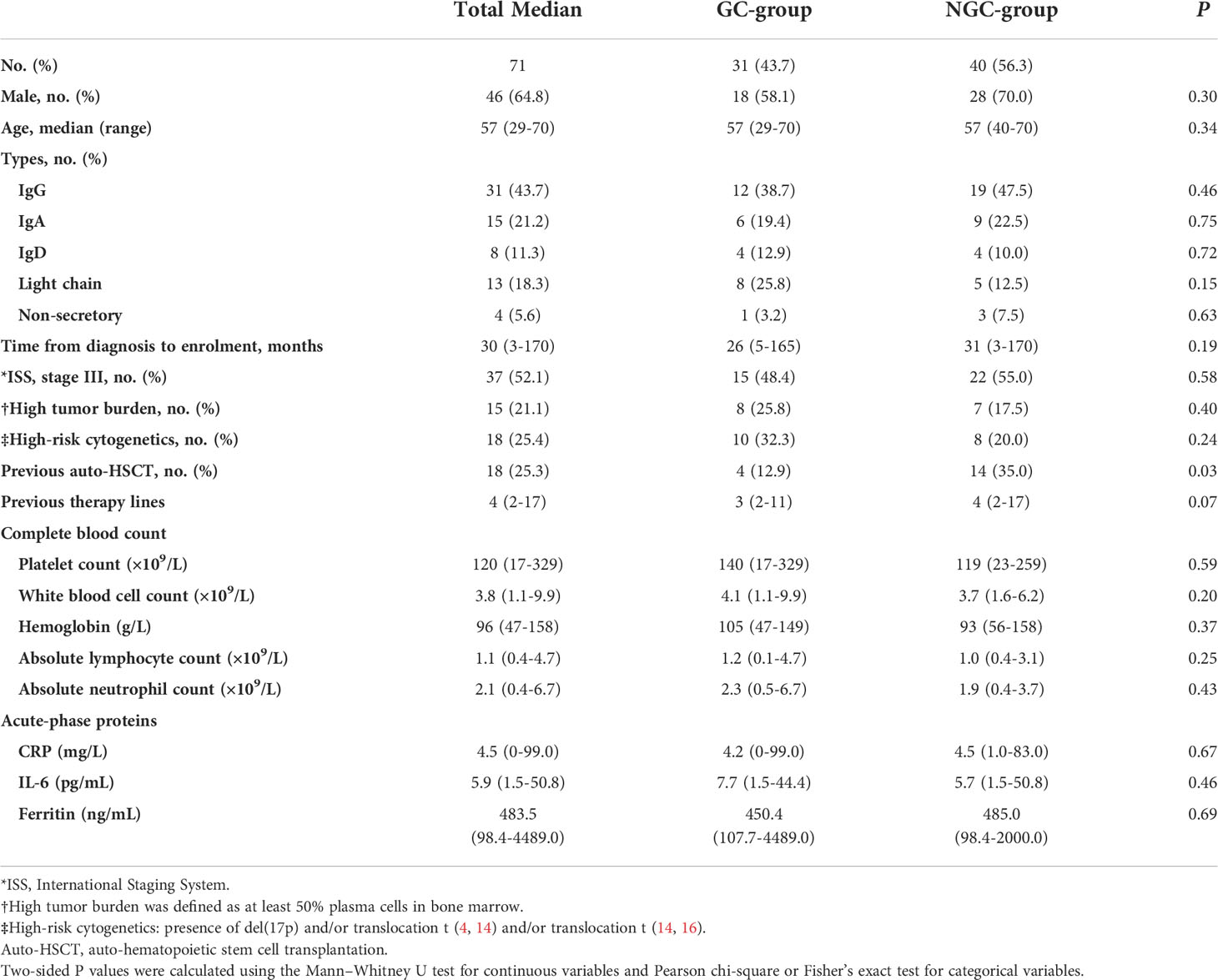
A total of 71 patients were stratified into GC group and NGC group. The median age was 57 years (range, 29 to 70), and 12.7% of patients were older than 65 years. Fourteen patients in the NGC group received auto-hematopoietic stem cell transplantation (auto-HSCT) while four patients in the GC group received auto-HSCT (p = 0.03). Other baseline characteristics were not remarkably different between the two groups (Table 1).
Management of CRS and ICANS
CRS occurred in 64 (90.1%) of 71 patients, 43 (60.6%) had grade 1, 14 (19.7%) had grade 2, and 7 (9.9%) had grade ≥3 CRS. In the NGC group (n = 40), 25 patients had grade 1, 8 had grade 2, and 7 had no CRS. In the GC group (n = 31), 18 patients had grade 1, 6 had grade 2, and 7 had grade ≥ 3 CRS (Figure 1). ICANS occurred in 5 (7.0%) of 71 patients, and 2 (2.8%) patients had grade ≥3 ICANS. In the NGC group, 8/40 patients with grade 2 CRS required the use of oxygen by low-flow nasal cannula. In the GC group, 24/31 patients received dexamethasone (5–10 mg, q6h) and 7 received methylprednisolone (1–2 mg/kg/day). The median cumulative GC dose was equivalent to 35 mg of dexamethasone (range, 5 to 450) and the median duration of GCs was 3 days (range, 1 to 34). The reason for the prolonged use of GCs was persistent symptom of CRS or ICANS. Eleven (35.5%) of 31 patients received GCs within the first 7 days post CAR-T cell infusion and 20 (64.5%) beyond day 7.
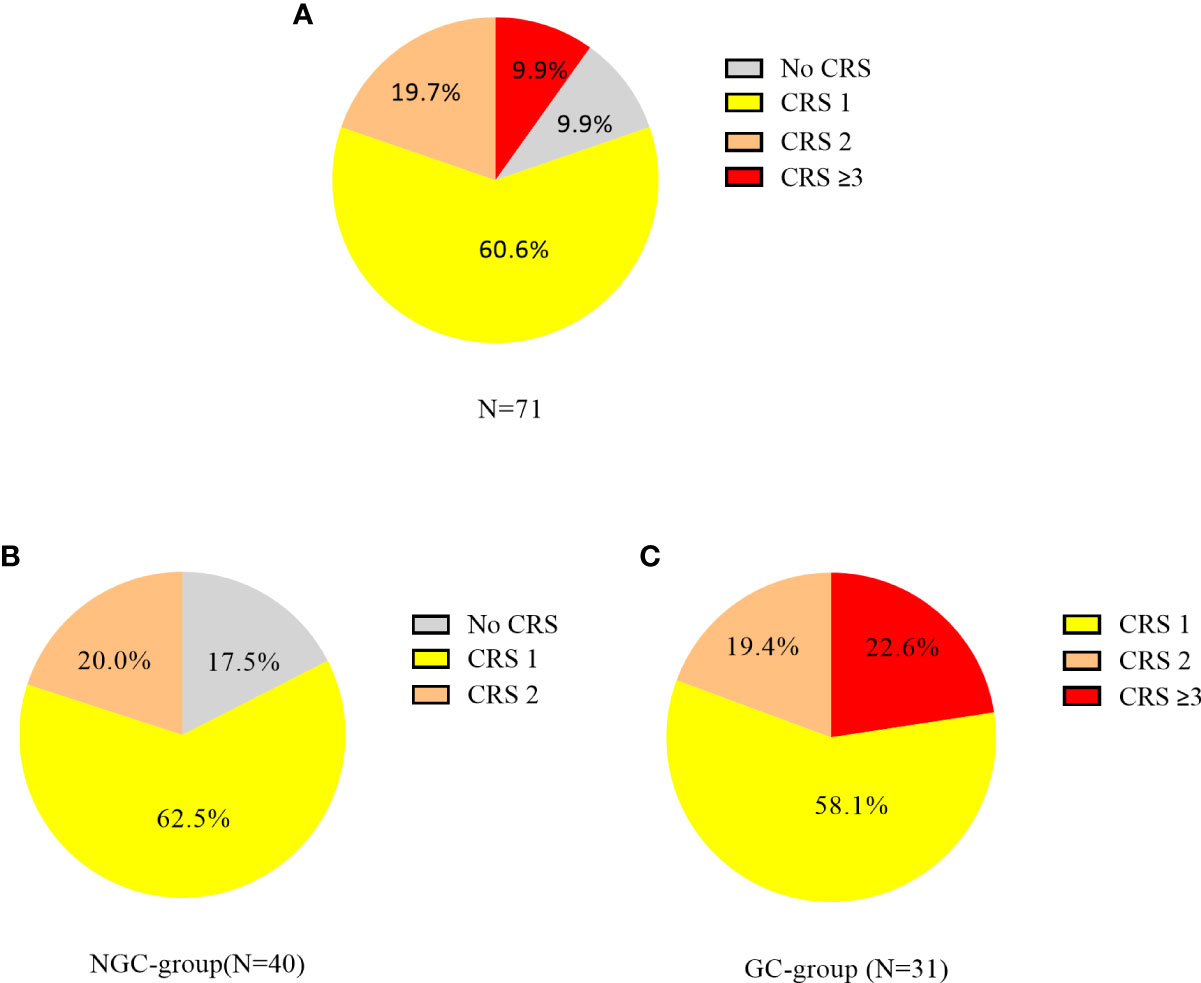
Figure 1 Cytokine release syndrome (CRS) incidence and severity in patients with CAR-T cell therapy. CRS was graded according to the ASTCT consensus grading system. (A) CRS incidence in all patients; (B) CRS incidence in the NGC group. (C) CRS incidence in the GC group.
GCs and ORR or CRR
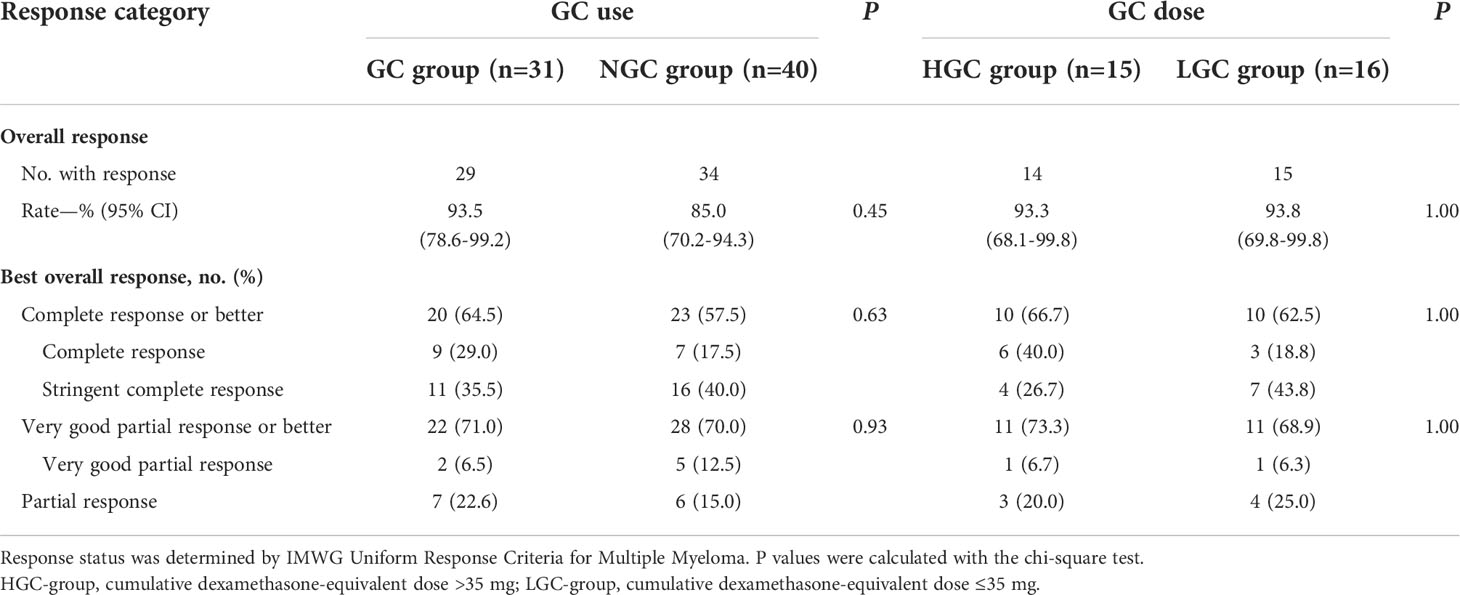
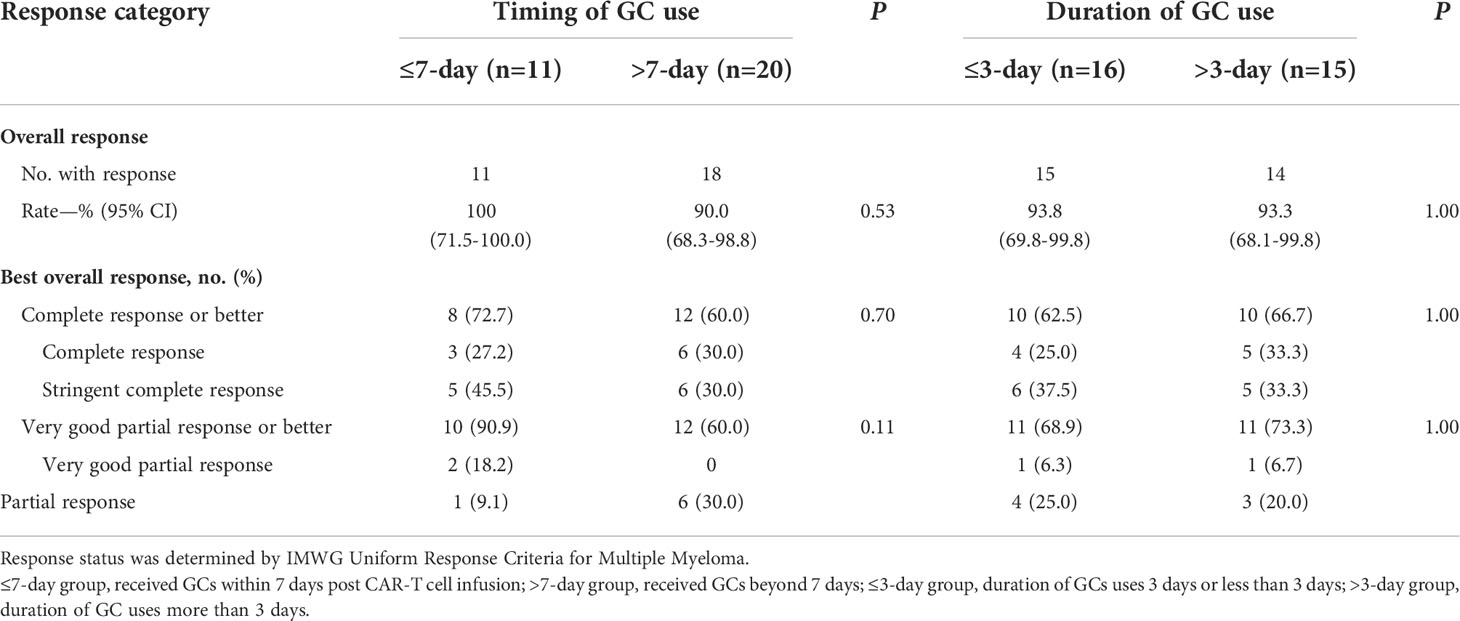
Patients achieved the best response within a median time of 70 days (range 14–207 days). In the NGC group, responses were observed in 34 patients, and ORR was 85%, including 16 (40%) stringent complete response (sCR), seven (17.5%) complete response (CR), five (12.5%) very good partial response (VGPR), and six (15%) partial response (PR). In the GC group, responses were observed in 29 patients, and ORR was 93.5%, including 11 (35.5%) sCR, nine (29%) CR, two (6.4%) VGPR, and seven (22.6%) PR. Differences in ORR and CRR between the NGC group and GC group were insignificant (Table 2). Considering the difference in the proportion of patients who received auto-HSCT between the two groups, Cochran’s and Mantel–Haenszel statistics was performed. There was no difference in ORR and CRR between the two groups. We also investigated whether GC doses were associated with ORR and CRR. According to cumulative dose, 31 patients in the GC group were divided into two subgroups: high-dose GC group (dexamethasone-equivalent dose >35 mg, HGC-group) and low-dose GC group (dexamethasone-equivalent ≤35 mg, LGC group). No significant difference in ORR and CRR was observed between the high-dose group and low-dose group (Table 2). To clarify whether the timing of GCs could affect the response, patients in the GC group were divided into two groups, 11 of 31 patients received GCs within 7 days (≤7-day group) and 20 beyond 7 days (>7-day group). There was no correlation between timing of GCs and responses (Table 3).
GCs and DOR
The median DOR for patients with CR or better was 26.8 months (95% CI, 16.7 to 36.9) (Figure 2A) . In the GC group, the median DOR for 20 patients having CR or better was not reached. In the NGC group, the median DOR for 23 patients having CR or better was 20.5 months (95% CI, 10.7 to 30.2) (Figure 2B). Differences in median DOR for the patients with CR or better in the GC group and NGC group were not significant (p = 0.17).

Figure 2 Kaplan–Meier curves of the duration of response (DOR). (A) The median DOR for patients with CR or better. (B) The median DOR for patients according to the use of GCs in patients with CR or better.
GCs and OS or PFS
At a median follow-up of 28.2 months, the median PFS in the GC group was 20.4 months (95% CI, 7.9 to 32.9) and the median OS was 36.6 months [95% CI, 25.9 to 47.2]. The median PFS and OS in the NGC group were 13.7 months (95% CI, 8.8 to 18.6) and 27.5 months (95% CI, 14.1 to 41.0). There were no significant differences in either PFS or OS between the NGC group and GC group (Figures 3A, B). The median PFS and OS in the HGC group were 11.5 months (95% CI, 0.1 to 22.9) and 28.4 months (95% CI, 0.2 to 58.4). The median PFS in the LGC group was 21.7 months (95% CI, 1.2 to 42.2), and the median OS was not reached. No significant difference was observed in PFS and OS between the HGC group and LGC group (Figures 3C, D). We did not observe a remarkable association between the time or duration of GC use and survival (Figures 3E–H).
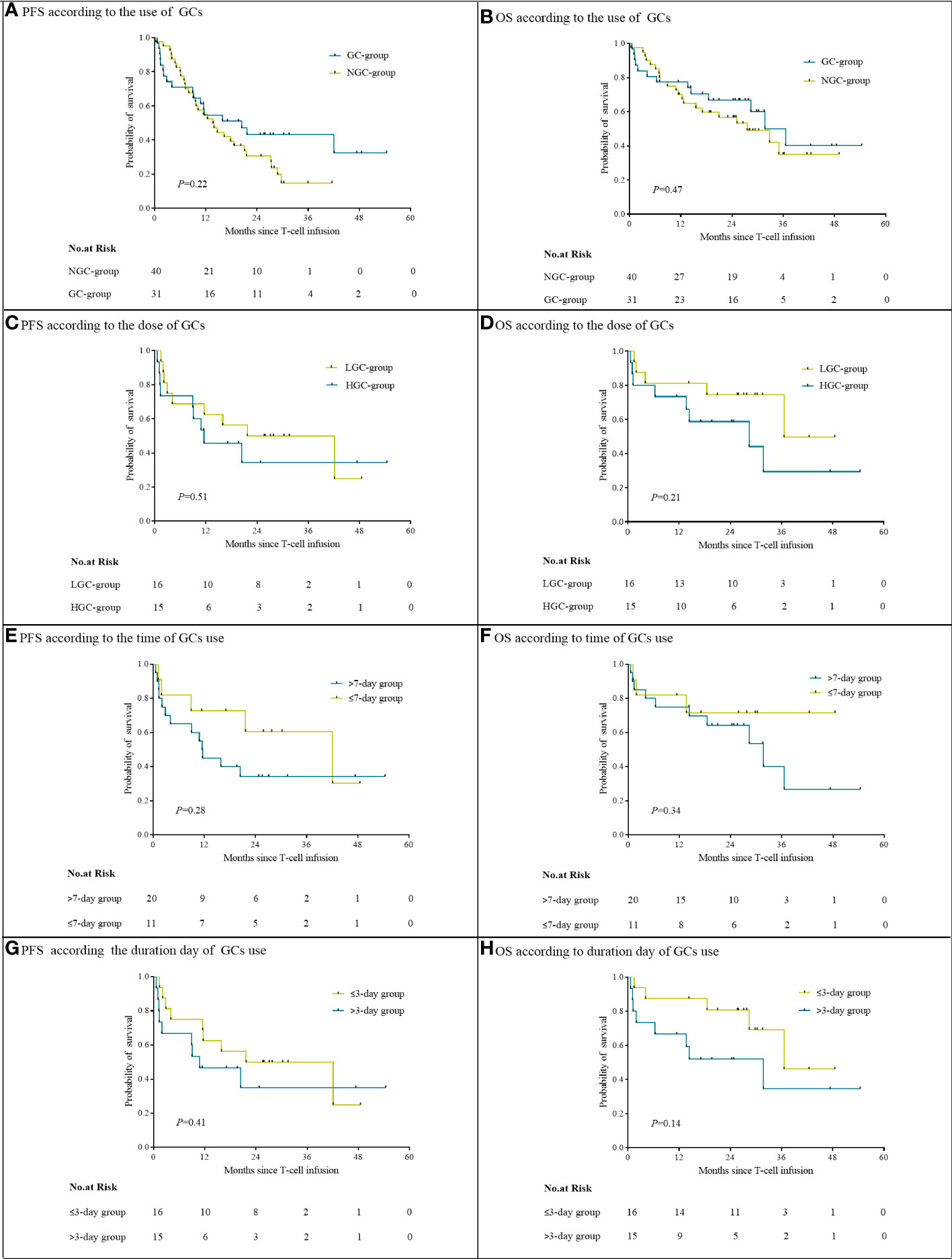
Figure 3 Progression-free survival and overall survival, according to use of GCs (A, B), cumulative GC dose (C, D), timing (E, F), and duration (G, H).
GCs and the proliferation of CAR-T cell
CAR-T cells started to proliferate within 7 days in both GC group and NGC group. The percentage and counts of CAR-T cells reached a peak at day 14 then decreased gradually. The median percentage and CAR-T cell counts in the GC group were higher than those in the NGC group at indicated time points (days 7, 14, 21, and 28 post CAR-T infusion) (Figure 4). There was a remarkable difference in median percentage and counts of CAR-T cells between the GC group and NGC group (Figure 4).
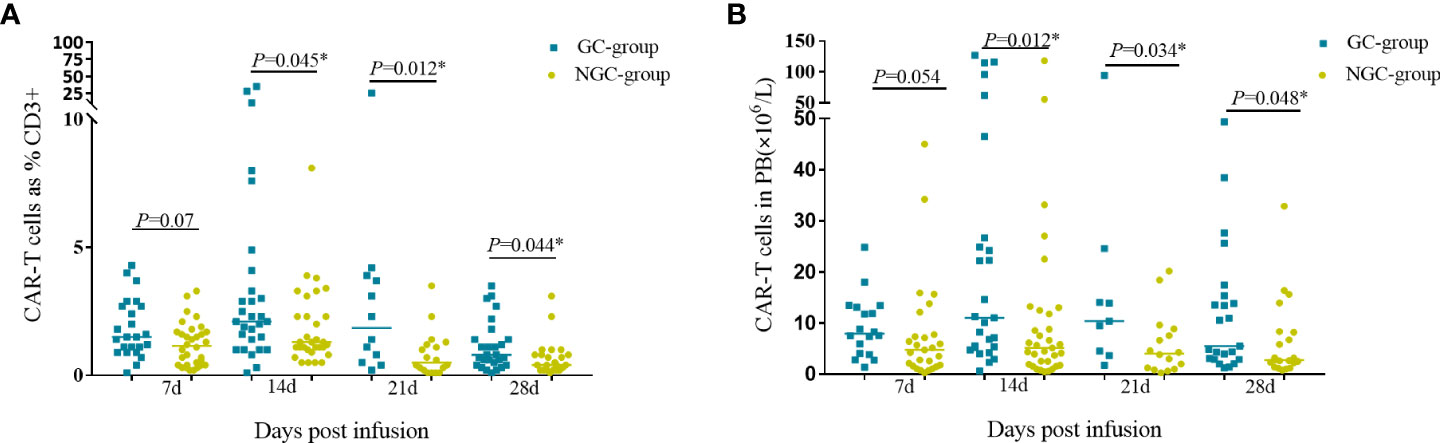
Figure 4 CAR-T cell expansion and persistence in peripheral blood. Proliferation of CAR-T cells in peripheral blood was assessed by flow cytometry on days 7, 14, 21, and 28. (A). The percentage of CAR-T cells in the peripheral blood. (B) Counts of CAR-T cells in the peripheral blood. *Represents for statistically different (P < 0.05).
Discussion
GCs are effective in reducing CAR-T-related adverse events. There are theoretical concerns about whether the use of GCs will affect the expansion of CAR T cells as well as impair their efficiency in killing tumor cells. Limited studies exploring the impact of GCs on clinical outcomes of CAR-T cell therapy are mainly from clinical trials for B-ALL and LBCL (19–21). To the best of our knowledge, this is the first retrospective study to investigate the effects of cumulative dose, timing, and duration of GCs on the efficacy of CAR-T cells in RRMM.
We found no relation between GCs and ORR or CRR, contrary to earlier reports. High dosages of GCs are effective for the treatment of CRS, generating concerns about the effectiveness of CAR-T. Our research exhibited that the use of GCs had no effects on the efficacies of CAR-T therapy, including ORR and CRR, irrespective of cumulative dosage, timing, or duration of GC use. Liu et al. (20) recently discovered that low-dose GCs for a short period of time (average 4 days) had no effect on outcomes of CAR-T cell therapy. Our findings were also consistent with other clinical trials. There are two largest investigations conducted by Strati et al. (12) and Neelapu et al. (19), respectively, none of which showed an association between GCs and ORR or CRR.
The association between GCs and long-term survival was also evaluated. We established that the dose, duration, and timing of GCs use did not impact PFS or OS. In another study, high dose, timing, and prolonged use of GCs were linked to shorter PFS and OS. We compared the differences between these two studies and found that the study by Strati et al. reported negative results from the tendency to use high doses of GCs for a long period (median cumulative dexamethasone-equivalent dose and duration were 186 mg and 9 days, respectively). However, in our study, the median cumulative GC dose was equivalent to 35 mg of dexamethasone (range, 5 to 450) while the median duration of GCs was 3 days (range, 1 to 34). Consistent with our findings, Sesques et al. and Nastoupil et al. did not find any correlation between GC use and PFS (22, 23). Additionally, Holtzman et al. (24) reported that a much longer and higher cumulative dose of GC treatment did not impact PFS or OS in patients with ICANS. In this study, patients with ICANS required GC treatment, with a median total GC dose equal to 221 mg of dexamethasone given for a median of 12.5 days. In contrast to earlier studies, neither the dosage nor the duration of GCs was correlated with a shorter PFS or OS.
Previous clinical studies have shown that for different primary diseases, the application of GCs has different effects on the clinical outcomes (12, 22). Early studies showed that GCs might impact the efficacy of CAR-T cells for B-ALL (21). Two recent investigations exhibited that early treatment with GCs for CRS or ICANS had no influence on the antitumor potency of CD19 CAR-T cells and the potential mechanisms have been postulated (25–27). Our findings illustrated that GCs, even high-dose GCs, had no effect on CAR-T cell growth. It is possible that our results differ from prior findings because we utilized high-dose GCs for a short period of time, while they were used for much longer. Limitations of our study include its retrospective nature and the small sample size, which prevented use of a propensity score matching analysis. Early GC use in CAR-T toxicity control is being explored (28), and although more prospective trials are required, our results may relieve anxiety regarding GC use in CRS treatment.
Conclusions
In summary, although limited by small sample size and likely patient selection bias, GC use was not associated with lower CRR or ORR and shorter PFS, or OS in RRMM patients treated with CAR-T cells. Moreover, GCs had no detectable influence on patient treatment outcomes, regardless of duration or cumulative dosage in RRMM patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the Affiliated Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HC and KX designed the research study. All investigators and their respective research teams recruited and followed up the patient. XW, YQ, and HL analyzed the data and wrote the first draft of the paper. All authors reviewed and critically edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the National Natural Science Foundation of China (81930005, 82070127, 81871263).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a b-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (Cartitude-1): A phase 1b/2 open-label study. Lancet (2021) 398(10297):314–24. doi: 10.1016/S0140-6736(21)00933-8
2. Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells genetically modified to express an anti-B-Cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol (2018) 36(22):2267–80. doi: 10.1200/JCO.2018.77.8084
3. Li C, Cao W, Que Y, Wang Q, Xiao Y, Gu C, et al. A phase I study of anti-bcma car T cell therapy in Relapsed/Refractory multiple myeloma and plasma cell leukemia. Clin Transl Med (2021) 11(3):e346. doi: 10.1002/ctm2.346
4. Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med (2021) 384(8):705–16. doi: 10.1056/NEJMoa2024850
5. Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, et al. Safety and clinical efficacy of bcma car-T-Cell therapy in multiple myeloma. J Hematol Oncol (2020) 13(1):164. doi: 10.1186/s13045-020-01001-1
6. Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai H, et al. A phase 1 study of a novel fully human bcma-targeting car (Ct103a) in patients with Relapsed/Refractory multiple myeloma. Blood (2021) 137(21):2890–901. doi: 10.1182/blood.2020008936
7. Abramson HN. B-Cell maturation antigen (BCMA) as a target for new drug development in relapsed and/or refractory multiple myeloma. Int J Mol Sci (2020) 21(15):5192. doi: 10.3390/ijms21155192
8. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol (2018) 15(1):47–62. doi: 10.1038/nrclinonc.2017.148
9. Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Muller-Tidow C, et al. Side-effect management of chimeric antigen receptor (Car) T-cell therapy. Ann Oncol (2021) 32(1):34–48. doi: 10.1016/j.annonc.2020.10.478
10. Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, et al. Improved cns exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood (2018) 132(6):662–6. doi: 10.1182/blood-2018-05-846428
11. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-Treat leukemia remission by Cd19 car T cells of defined formulation and dose in children and young adults. Blood (2017) 129(25):3322–31. doi: 10.1182/blood-2017-02-769208
12. Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in Large b-cell lymphoma. Blood (2021) 137(23):3272–6. doi: 10.1182/blood.2020008865
13. Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, et al. Potent anti-leukemia activities of humanized Cd19-targeted chimeric antigen receptor T (Car-T) cells in patients with Relapsed/Refractory acute lymphoblastic leukemia. Am J Hematol (2018) 93(7):851–8. doi: 10.1002/ajh.25108
14. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-Cd19 and anti-bcma car T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol (2019) 6(10):e521–e9. doi: 10.1016/S2352-3026(19)30115-2
15. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. Astct consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant (2019) 25(4):625–38. doi: 10.1016/j.bbmt.2018.12.758
16. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol (2016) 17(8):e328–46. doi: 10.1016/S1470-2045(16)30206-6
17. Nilson BH, Logdberg L, Kastern W, Bjorck L, Akerstrom B. Purification of antibodies using protein l-binding framework structures in the light chain variable domain. J Immunol Methods (1993) 164(1):33–40. doi: 10.1016/0022-1759(93)90273-a
18. Zheng Z, Chinnasamy N, Morgan RA. Protein l: A novel reagent for the detection of chimeric antigen receptor (Car) expression by flow cytometry. J Transl Med (2012) 10:29. doi: 10.1186/1479-5876-10-29
19. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel car T-cell therapy in refractory Large b-cell lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
20. Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, et al. Corticosteroids do not influence the efficacy and kinetics of car-T cells for b-cell acute lymphoblastic leukemia. Blood Cancer J (2020) 10(2):15. doi: 10.1038/s41408-020-0280-y
21. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z car T cell therapy in b cell acute lymphoblastic leukemia. Sci Transl Med (2014) 6(224):224ra25. doi: 10.1126/scitranslmed.3008226
22. Sesques P, Ferrant E, Safar V, Wallet F, Tordo J, Dhomps A, et al. Commercial anti-Cd19 car T cell therapy for patients with Relapsed/Refractory aggressive b cell lymphoma in a European center. Am J Hematol (2020) 95(11):1324–33. doi: 10.1002/ajh.25951
23. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care axicabtagene ciloleucel for relapsed or refractory Large b-cell lymphoma: Results from the us lymphoma car T consortium. J Clin Oncol (2020) 38(27):3119–28. doi: 10.1200/JCO.19.02104
24. Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: Predictive biomarkers and clinical outcomes. Neuro Oncol (2021) 23(1):112–21. doi: 10.1093/neuonc/noaa183
25. Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. Preemptive mitigation of Cd19 car T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood (2019) 134(24):2149–58. doi: 10.1182/blood.2019001463
26. Topp MS, van Meerten T, Houot R, Minnema MC, Bouabdallah K, Lugtenburg PJ, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for Large b-cell lymphoma. Br J Haematol (2021) 195(3):388–98. doi: 10.1111/bjh.17673
27. Oluwole OO, Bouabdallah K, Munoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for Large b-cell lymphoma. Br J Haematol (2021) 194(4):690–7. doi: 10.1111/bjh.17527
Keywords: chimeric antigen receptor T cell, glucocorticoids, relapsed/refractory, multiple myeloma, outcome
Citation: Wang X, Qi Y, Li H, Liu F, Cao J, Chen W, Wang Y, Qi K, Yan Z, Zhu F, Li Z, Cheng H and Xu K (2022) Impact of glucocorticoids on short-term and long-term outcomes in patients with relapsed/refractory multiple myeloma treated with CAR-T therapy. Front. Immunol. 13:943004. doi: 10.3389/fimmu.2022.943004
Received: 13 May 2022; Accepted: 01 August 2022;
Published: 23 August 2022.
Edited by:
Katy Rezvani, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Hyatt Balke-Want, Stanford University, United StatesPedro Castro Rebollo, Hospital Clinic of Barcelona, Spain
Copyright © 2022 Wang, Qi, Li, Liu, Cao, Chen, Wang, Qi, Yan, Zhu, Li, Cheng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Cheng, aGFpX2NoZW5nQHh6aG11LmVkdS5jbg==; Kailin Xu, bGlobWRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xue Wang
Xue Wang Yuekun Qi1,2,3†
Yuekun Qi1,2,3† Fengan Liu
Fengan Liu Jiang Cao
Jiang Cao Wei Chen
Wei Chen Ying Wang
Ying Wang Kunming Qi
Kunming Qi Zhiling Yan
Zhiling Yan Feng Zhu
Feng Zhu Zhenyu Li
Zhenyu Li Hai Cheng
Hai Cheng Kailin Xu
Kailin Xu