- 1Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, NC, United States
- 2Population, Neurodevelopment and Genetics Program, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI, United States
- 3Departments of Public Health Sciences, Environmental Medicine, and Pediatrics University of Rochester School of Medicine and Dentistry, Rochester, NY, United States
- 4Social & Scientific Systems, Durham, NC, United States
- 5Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, NC, United States
Background: Between 1988 and 2012, prevalence of antinuclear antibodies (ANA) increased in the U.S., especially in adolescents and non-Hispanic Whites. Female predominance of ANA suggests a role for hormonal factors, including xenobiotic exposures that may disrupt endocrine signaling. Benzophenone-3 (BP-3) is one such chemical with increasing exposure through sunscreen use. We investigated whether urinary BP-3 levels were related to ANA in adolescents and young adults.
Methods: In a sample of 1,785 individuals ages 12-39 years in the National Health and Nutrition Examination Survey (NHANES; 2003-4, 2011-12), we examined cross-sectional associations of ANA (N=192; 3+ or 4+ at the 1:80 dilution, measured by HEp-2 immunofluorescence) with urinary BP-3, and other phenols bisphenol-A, triclosan, and parabens. Adjusted prevalence odds ratios (POR) were calculated in season-stratified models [winter (November-April) and summer (May-October)], given differences in sunscreen use and BP-3 concentrations.
Results: BP-3 concentrations (detected in >98.5% of individuals) did not differ by ANA positivity in the summer (geometric mean, GM 30.6 ng/ml ANA-positive vs. 35.3 ANA-negative; GM ratio 1.15), but in winter were higher among ANA-positives (50.2 vs. 20.1 ANA-negative; GM ratio 2.50). ANA was associated with log10BP-3 in winter (POR 1.57; 95%CI 1.07-2.30 per unit increase) but not summer (0.94; 0.61, 1.44; interaction p=0.09). Triclosan, parabens, and bisphenol-A levels were unrelated to ANA overall or by season (ORs 0.64 to 1.33).
Conclusions: The association of urinary BP-3 with ANA in the winter may reflect different exposure patterns or unmeasured confounders. Findings warrant replication in prospective studies and including past and year-round exposures.
Introduction
Recent findings from the National Health and Nutrition Examination Survey (NHANES) suggest the prevalence of autoimmunity, as measured by antinuclear antibodies (ANA; at the 1:80 dilution using HEp-2 immunofluorescence), may be increasing in the U.S (1). In clinical settings, ANA are elevated among patients with systemic autoimmune diseases and can be detected years prior to diagnosis (1, 2). In the general population, autoimmune diseases are generally rare and risk factors for ANA are not well understood. Just as systemic autoimmune diseases occur more often in women, a female predominance of ANA suggests a role for hormonal factors, which may include xenobiotic exposures with endocrine effects (3, 4).
Growing evidence suggests that autoimmune diseases and the adaptive immune response may be impacted by exposure to endocrine disrupting chemicals, which are xenobiotics that can influence the function of endogenous hormones (3, 5). One class, the environmental phenols, includes bisphenol-A (BPA), triclosan, and benzophenone-3 (BP-3, oxybenzone). BP-3, a widely used chemical sunscreen, is rapidly absorbed through the skin, excreted in urine, and was detected in nearly 97% of the U.S. population in 2003-2004 (6, 7). Measured urinary BP-3 levels in humans have increased in the U.S. population, especially in white individuals, paralleling trends in increasing sunscreen use and incorporation of sunscreens into diverse personal care products such as moistures and cosmetics (8, 9).
We hypothesized that BP-3 could be related to higher ANA prevalence. We analyzed data from two NHANES cycles (2003-2004 and 2011-2012) with available data on both BP-3 and ANA to examine their association in adolescents and young adults (ages 12-39). We also examined associations of ANA with other phenols (BPA and triclosan), as well as parabens, another class of endocrine disruptors (measured in 2003-2004) found in sunscreen and other personal care products (9–11). Given seasonal differences in sunscreen use, associations were examined separately for the summer (May-October) and winter (November-April) NHANES samples.
Methods
Design and sample
The NHANES is a nationally representative sample of the non-institutionalized U.S. population, with data collected in consecutive two-year cycles since 1999. This cross-sectional study included 1941 participants ages 12-39 with existing data on ANA and urinary phenols from two survey cycles: from 2003-2004 (N=1023) and 2011-2012 (N=918). Participants completed questionnaires and most provided a blood specimen during an in-person examination. Enrollment followed a seasonal pattern such that those in more northern states were sampled in the summertime (May-October), and at lower latitudes in the winter (November-April). The NHANES protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention (CDC). All participants provided informed consent.
ANA assessment
The study used existing data on ANA (12). Serum samples were shipped cold and stored at -80°C, until they were tested at the 1:80 dilution using the HEp-2 indirect immunofluorescence assay NOVA Lite HEp-2 ANA slide with DAPI kit (INOVA Diagnostics, San Diego, CA), and using a highly specific fluorescein isothiocyanate (FITC)-conjugated secondary antibody (goat anti-human IgG). Images were read using the NOVA View automated fluorescence microscope system (INOVA Diagnostics). Staining intensity was rated on a scale of 0 to 4; ANA positivity was defined as any signal above zero. Assays were performed in a single laboratory and evaluated independently by two experienced reviewers who agreed on > 95% of overall ratings, with differences revolved by consensus or a third reviewer. A random sample of samples were rated with over 98% concordance.
Phenols and parabens
Spot urine samples were collected and stored at -20°C until assays were performed. Exposures evaluated in the current study included three phenols (BP-3, BPA, triclosan; available on both cycles), and four parabens (butyl paraben, ethyl paraben, methyl paraben, and propyl paraben; available only in 2003-2004). Concentrations were measured by solid phase extraction, high performance liquid chromatography and tandem mass spectrometry as described: (https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/EPH_G_met.pdf. Limit of detection (LOD) was 0.4 ng/mL for BP-3 and BPA, 2.3 ng/mL for triclosan, 0.2, 1.0, 1.0, and 0.2, respectively for butyl, ethyl, methyl, and propyl paraben. Samples below the LOD were assigned by dividing the LOD by the square-root of 2. Urine concentrations were adjusted indirectly by including creatinine as a covariate in multivariable models. Modest correlations were seen for methyl paraben with BP-3 and BPA in the winter sample (r=0.30 and 0.31), and among the parabens (r range 0.31-0.84).
Covariates
Participant characteristics were collected through questionnaires and in-person visits. Covariates included age (continuous as an adjustment factor, and categorical for stratified models – ages 12-29, 20-39 years), gender (female, male), race/ethnicity (white, African American, other), education (< high school, high school grad/equivalent, some college or above), smoking, vitamin D, and body mass index (BMI). As previously described, BMI was grouped as underweight/healthy (<25 kg/m2), overweight (25>30 kg/m2)), and obese (30+ kg/m2) in adults 20 to 39 years and applying the 2000 CDC growth chart percentiles of <85, 85< 95, 95+ for those ages 12 to 19, and current smoking was based on cotinine concentrations as none (<0.05ng/ml), second-hand (0.05-15ng/ml) and active (>15 ng/ml) (12). Serum 25(OH)D levels were determined in 2003-2004 samples by radioimmunoassay (Diasorin, Stillwater, MN), and by liquid chromatography/tandem mass spectroscopy (LC-MS/MS) in 2011-2012 as described: (NHANES 2011-2012: Vitamin D Data Documentation, Codebook, and Frequencies (cdc.gov). Radioimmunoassay levels were harmonized to LC-MS/MS equivalents (13). Serum 25(OH)D levels grouped as: <50 nmol/L (20ng/ml, including deficient and insufficient), 50-75 nmol/L (20-30ng/ml, a debated threshold for insufficiency), and >75 nmol/L (>30 ng/ml, generally regarded as sufficiency) (13). Adults ages 20-39 were also asked about sunscreen use (“when you go out on a very sunny day for more than an hour, how often do you use sunscreen - never, rarely, sometimes, most of the time, always?”), and whether they had been diagnosed with psoriasis?
Analysis
Analyses were performed using SAS survey procedures (version 9.4, Cary, NC, U.S.A.), and included sampling weights and design variables to account for the complex survey design of the NHANES. We first examined participant characteristics by ANA status, including demographic factors, season, BMI, smoking, cycle, and vitamin D levels; these associations with ANA were described using logistic regression models to calculate age-adjusted prevalence odds ratios (POR) and 95% confidence intervals (CI).
All analyses of BP-3 and ANA were season-specific, given prior evidence of seasonal variability in immune and inflammatory biomarkers such as total white blood cell count and C-reactive protein in adults and children in the NHANES (1999-2012) (14), sunscreen use, as well as sunlight and other unmeasured covariates, together with differential sampling of NHANES participants (i.e., Page 9: https://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf). We first examined concentrations of BP-3 (Geometric Mean, GM, and standard deviation (GSD), by ANA status, overall and stratified by key covariates, including age, gender, race/ethnicity, vitamin D levels, and BMI. Concentrations in ANA positive versus ANA negative samples were directly compared through calculating the ratios of geometric means and 95%CI.
We estimated associations of ANA with log10BP-3 in multivariable logistic regression models, adjusting for creatinine, NHANES cycle, age, gender, race/ethnicity, education, current smoking, vitamin D, and smoking. In an overall model also including season, we also assessed potential multiplicative interaction to describe seasonal differences through addition of a product term (log10BP-3 by season); a p-value of <0.10 for the difference in -2 Log Likelihoods in the model with and without the interaction term was considered statistically significant. We also explored associations across the different demographic subgroups and vitamin D levels. Finally, we examined associations with triclosan, BPA, and parabens overall and in season-stratified models adjusting for the same model covariates except for vitamin D.
To further explore these findings, we examined the overall association by nuclear ANA pattern and staining intensity. We also conducted two sensitivity analyses for BP-3 in adults ages 20-39 years. Psoriasis is the most common autoimmune skin condition for which sunscreen might be advised; therefore, to examine potential reverse causality we excluded from the analysis individuals who reported having psoriasis who were ANA positive (4.3%) and 36 (1.7%) who were ANA-negative. We also examined associations of ANA with log10BP-3 by varying degrees of sunscreen use (always/usually, sometimes/rarely, and never).
Results
Sample characteristics
Overall, 192 (10.8%) of the study sample had detectable ANA (Table 1). The odds of being ANA positive were greater in females compared to males (age-adjusted POR 3.35;95%CI 2.14, 5.26), lower for those with a high school degree compared to those with at least some college (OR 0.45; 95%CI 0.25, 0.80), and among those who were overweight compared to normal or underweight (POR 0.53; 95%CI 0.37, 0.78). ANA did not vary significantly by race/ethnicity, season, smoking, vitamin D, or NHANES cycle.
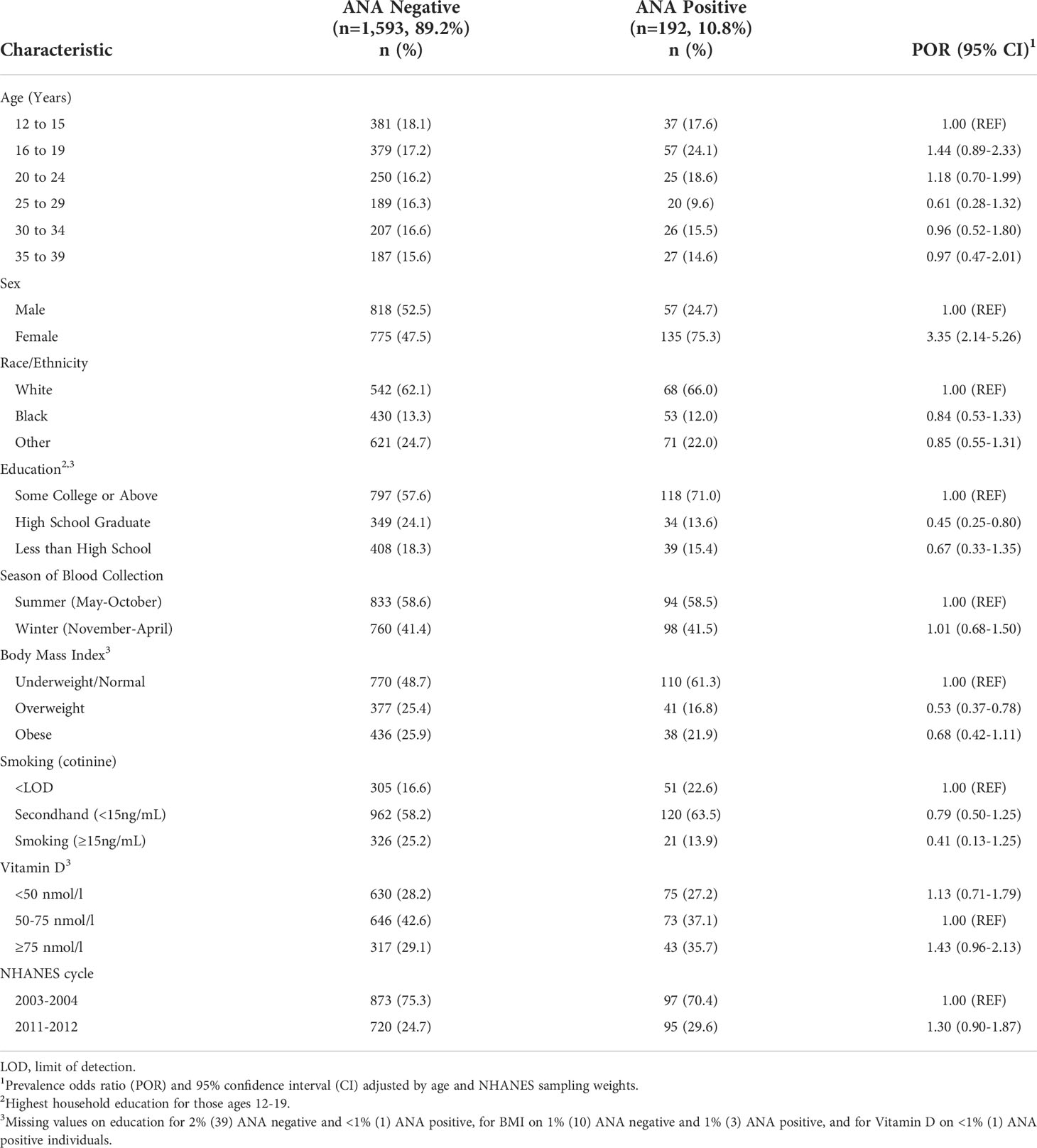
Table 1 Characteristics and weighted proportions by ANA status in the U.S. population ages 12-39, in the NHANES sample with measured urinary phenols (benzophenone, BPA, and triclosan).
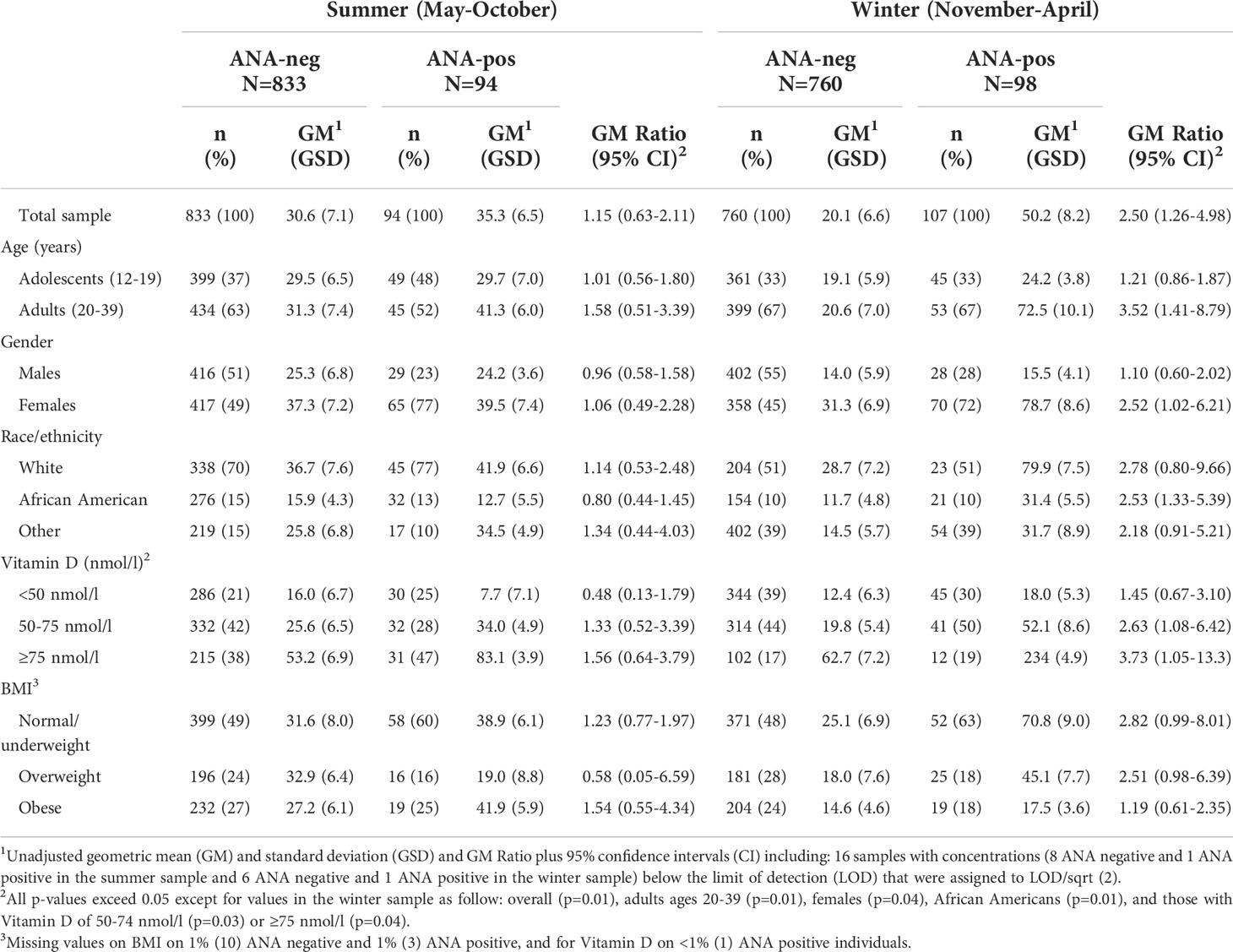
BP-3 concentrations
In the summer sample, BP-3 concentrations were somewhat higher in ANA-positive individuals (35.3 ng/ml ANA-positive, GM 30.6ng/ml ANA-negative), but the ratio of these values was not different from the null (GM Ratio 1.15; 95%CI 0.63, 2.11)(Table 2). Ratios in the summer sample were elevated (>1.5) in adults, those with higher vitamin D (≥75 nmol/l), or an obese BMI, and inverse in those with lower vitamin D (<50 nmol/l: 0.48) or an overweight BMI: (0.58). In the winter sample, BP-3 concentrations were higher in ANA-positive versus ANA-negative individuals (50.2 ng/ml vs. 20.1 ng/ml; GM Ratio 2.50; 95%CI 1.26, 4.98). Differences in the GM ratios in the winter sample compared to the summer sample were typically driven by both the lower BP-3 concentrations in ANA-negative individuals, as well as the higher levels in some ANA-positive individuals. For example, in males and ANA-negative females, BP-3 concentrations in the winter sample were lower than in the summer sample, while ANA-positive females in the winter sample had higher levels than those in the summer sample. GM ratios in the winter sample were elevated in other subgroups, except in adolescents, males, or those with lower vitamin D (<50 nmol/l) or an obese BMI.
Multivariable analyses
Figure 1 shows the multivariable adjusted odds of ANA-positivity per unit increase in urinary log10BP-3. Higher BP-3 concentrations were associated with ANA in the winter (POR 1.57; 95%CI 1.07, 2.30) but not in the summer sample (POR 0.94; 95%CI 0.61, 1.44; interaction intp=0.09). No elevated PORs were seen in the summer sample subgroups, but an inverse association (POR 0.47; 95%CI 0.21, 1.05) was seen among those with vitamin D <50 nmol/l. Associations in the winter sample were most apparent among adults (POR 1.95; 95%CI 1.17, 3.24), females (POR 1.78; 95%CI 1.10, 2.88), and those with normal/underweight BMI (POR 1.82; 95%CI 1.09, 3.03). The interaction by season was especially apparent among African Americans (intp=0.04) and those with normal/underweight BMI (intp=0.07).
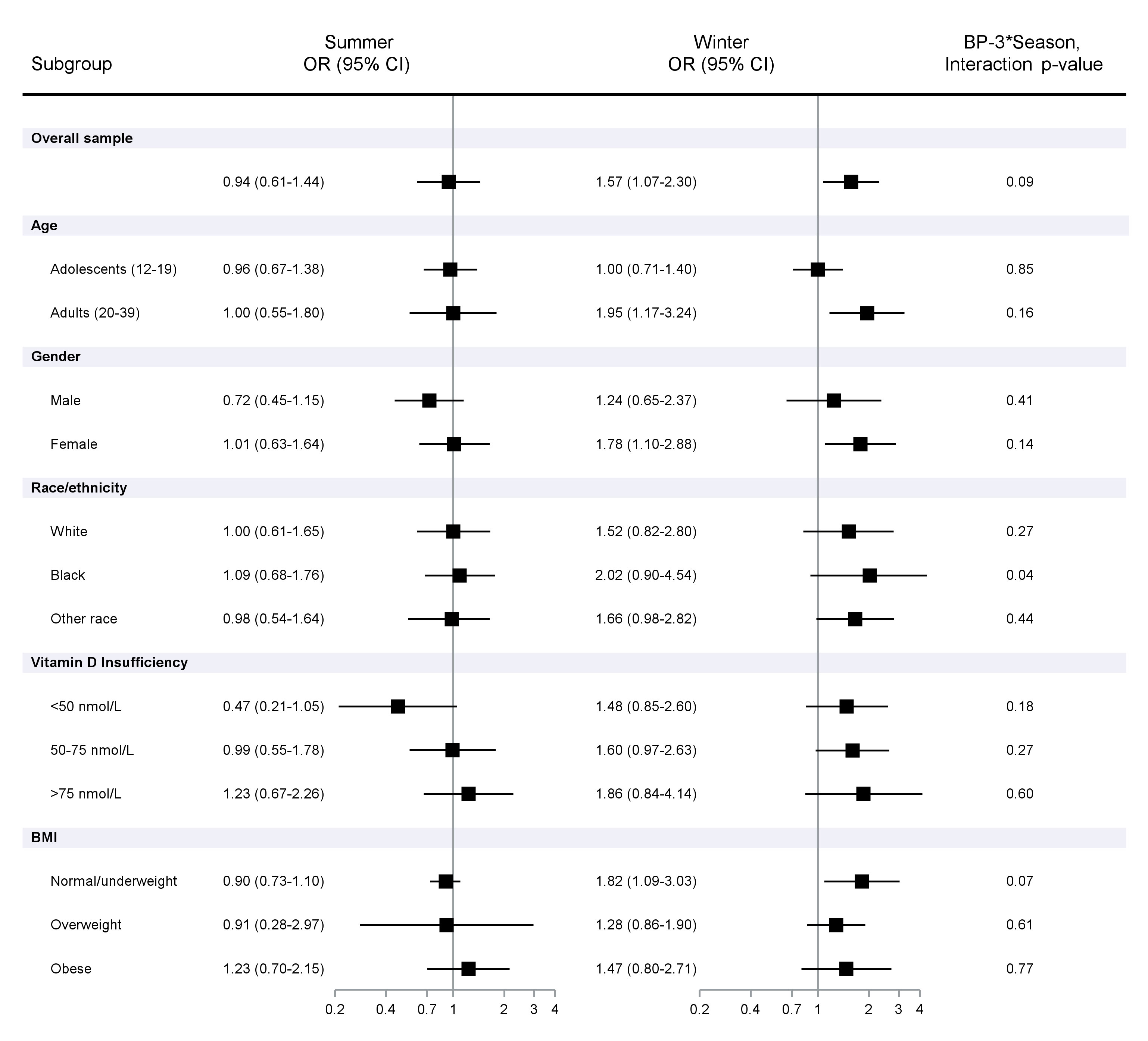
Figure 1 Association of ANA positivity per unit increase in urinary log10 BP-3 concentrations adjusted for creatine and covariates. Prevalence odds ratios and 95% Confidence Intervals calculated using logistic regression models, adjusting for urinary creatinine, NHANES cycle, age, sex, race/ethnicity, BMI, smoking (cotinine), education, and Vitamin D.
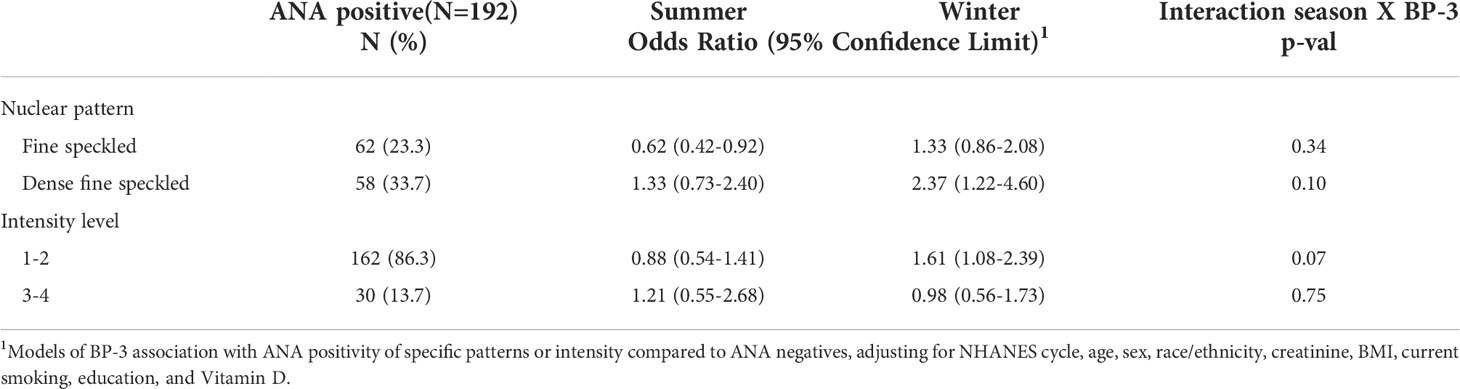
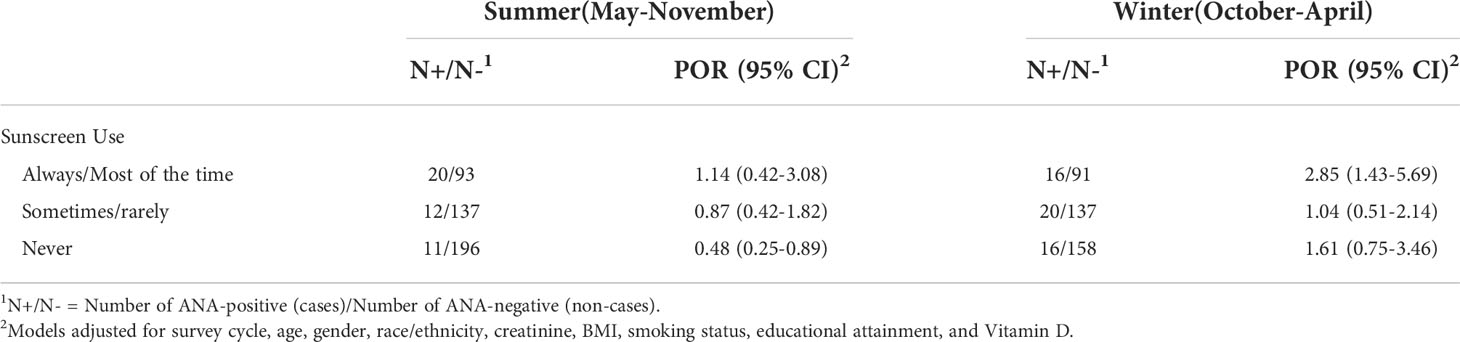
Table 3 shows that the association with BP-3 in the winter sample was most apparent for those with the dense fine speckle pattern (34% of total ANA positive individuals (POR 2.37; 95%CI 1.22, 2.08) and those with a lower staining intensity (85% of ANA positives at 1-2; POR 1.61; 95%CI 1.08, 2.39). The fine speckle pattern was inversely associated with BP-3 concentrations in the summer sample. In adults ages 20-39 with available data, a similar overall association was seen in the winter sample after excluding those with psoriasis (Table 4). Further, a stronger association was seen in the winter among those who usually or always used sunscreen (OR 2.85; 95%CI 1.43-5.69), while an inverse association was seen among those in the summer who never used sunscreen (OR 0.48; 95%CI 0.25, 0.89; Table 5).

Table 4 Association of log10BP-3 with ANA, ages 20-39: excluding participants with self-reported psoriasis.
In other analyses, ANA did not appear to be associated with the phenols, triclosan and BPA, overall or in either of the two seasonal samples, nor were associations seen in the smaller subsample for the parabens; ORs ranged from 0.64 (0.31, 1.27) for methyl paraben in the winter sample to 1.33 (0.73, 2.41) for methyl paraben in the summer (Table 6).
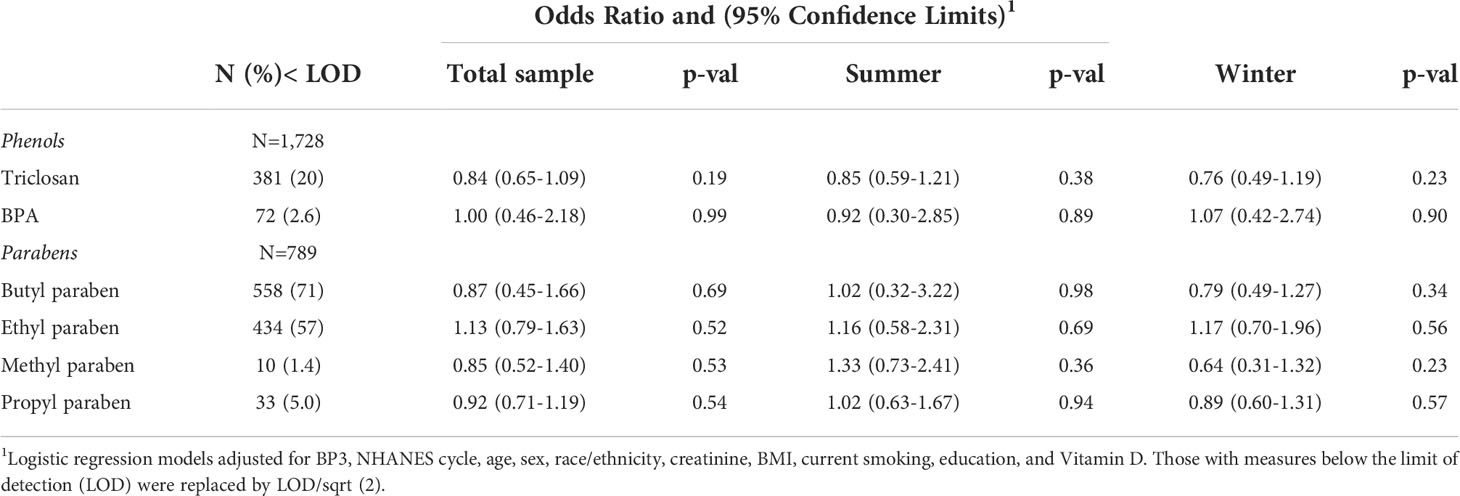
Table 6 Association of ANA with log10 triclosan, BPA, and paraben concentrations overall and by season.
Discussion
Exposure to benzophenone-3, an ingredient in chemical sunscreens for over 40 years (15), is widespread and increased in the U.S. population at the same time as rising ANA prevalence (8, 9, 12). In this cross-sectional study of NHANES participants ages 12-39, we found that elevated urinary BP-3 concentrations were related to ANA among those who were sampled in winter, but not summer. These findings were robust to confounder adjustment and covariate stratification, providing some support for the idea that BP-3 exposure may play a role in the observed increases in ANA.
Our analyses were stratified based on the a priori expectation of seasonal differences in exposure patterns and circumstances of individuals sampled in the warmer months, such as greater episodic sun exposure and sunscreen use. Indeed, BP-3 concentrations were elevated among the majority of individuals sampled in summer. While season was not associated with ANA, it significantly modified the association of BP-3 with ANA. In other NHANES adult samples from 2003-2006 and 2009-2012, BP-3 concentrations were associated with more frequent sunscreen use in both the summer and winter samples (16). Interestingly, in our sensitivity analyses the association of ANA with BP-3 in the winter sample was primarily among those using sunscreen most of the time or always. This finding was based on only 16 ANA-positive individuals, however, and caution is warranted in making comparisons, especially with those in the summer with less frequent or no sunscreen use (12 and 11 ANA-positive individuals).
In the NHANES, season determines the geographic location of sampling, which may impact opportunities for longer duration and more intense sun exposure – increasing both cumulative sunscreen use and exposure to ultraviolet (UV) radiation. Notably, UV light can suppress immunity, while at the same time periodic intense exposures (and greater damage) may stimulate existing ANA production through greater antigen exposure (17, 18). Personal sun exposure and sunburn have been associated with the development of some autoimmune diseases, including lupus erythematosus (SLE) and dermatomyositis (19, 20), while in other studies UV has appeared protective for multiple sclerosis and rheumatoid arthritis (21, 22). Further investigation is needed in a larger sample to evaluate the role of UV exposure, sunburn, and sunscreen use, in relation to ANA.
Vitamin D levels may also be influenced by UV exposure (23). Lower vitamin D levels were previously found to be associated with ANA in middle aged and older adults in the NHANES (2001-2004) (24), but in the current study sample of younger adults and adolescents, higher vitamin D appeared positively associated with ANA (age-adjusted OR 1.43 for ≥75 versus 50-75 nmol/L). We saw no evidence of confounding by vitamin D levels; though in the summer sample the association of log10BP-3 with ANA appeared to be inverse, this may be due to chance or confounding or interactions with other unmeasured factors.
Females had higher ANA prevalence compared to males, and the association of ANA with BP-3 in this winter sample was also more apparent in females. This was largely driven by the higher BP-3 concentrations in the winter sample among ANA-positive females and may reflect underlying differences in exposure sources and patterns. In a consumer survey from 2013, nearly 43% of females regularly used sunscreen on their face, compared with 18% of males (25); regular use of sunscreen-containing cosmetics may extend year-round and lead to increased cumulative or chronic exposures. Lifelong patterns of sunscreen and personal care product use may start in adolescence, at younger ages parental influences play a role – as evidenced by the strong correlation of maternal levels with their children’s ages 6-11 in a study of 145 unique pairs (26). Interestingly, BP-3 concentrations were similar among ANA-negative adolescents and adults, both in the summer and winter samples; and the elevated BP-3 levels among ANA-positive adults in the winter sample could reflect a similar scenario as suggested for females if this reflects individuals who habitually use sunscreen year round or at an even greater frequency in the summer.
We lacked data on systemic autoimmune diseases, however, these are still relatively rare and unlikely to account for the observed differences; moreover, we saw no evidence of potential reverse causality after excluding individuals reporting psoriasis. In NHANES adults, sunscreen use was previously associated with both BP-3 and triclosan levels (9). But in the current study sample, the other phenols and parabens were neither strongly correlated with BP-3, nor were they associated with ANA overall or by season. While the finding for BP-3 appeared fairly specific, we cannot rule out the role of unmeasured confounders. We did not consider mixtures or other chemicals included in personal care products, such as phthalates, or other chemical sunscreens, some of which may also impact the immune system (27, 28).
The relationship of BP-3 with ANA could be mediated by hormone-disrupting effects (e.g., estrogenic, androgenic) either directly or through its metabolites (29–31). Experimental studies of other phenols (e.g., BPA), suggest potential effects on the development of T-cells, including regulatory T-cells. Other non-endocrine mediated effects may include BP-3 phototoxicity, or through other pathways (e.g., retinoid-X receptor) (32, 33). Research in susceptible animal models may be helpful in understanding the potential effects of BP-3 on ANA development.
Limitations of the current study include the cross-sectional design and small sample size. Our findings of seasonal differences suggest that a single measured BP-3 concentrations in spot-urines may be insufficient for capturing the relevant exposure or timing relative to the development of ANA. Similarly, urinary BPA levels in spot urines are also insufficient measures for daily or longer-term exposure, thereby introducing exposure misclassification and likely obscuring potential associations (34). The use of sunscreen containing BP-3 has been shown to lead to short-term rise in plasma levels that remain above background at 7 days (6), and while concentrations in spot urines will certainly detect recent use, levels may also be compounded among individuals routinely using other BP-3 containing products, e.g., daily moisturizers or cosmetics. BP-3 has also been found in adipose tissue along with other phenols and some parabens, but the implications of accumulation and elevated body burden have not been explored (35). We saw no evidence of confounding by BMI; however, the seasonal difference was most apparent among those who were not overweight or obese. This could be due to differences in habitual uses of sunscreen (e.g., for physical activity outdoors or sunbathing), confounders, or differential processing or excretion patterns associated with elevated body fat.
The current study sample was limited to adolescents and young adults who lived the majority of their lives following approval of BP-3 containing sunscreens in the early 1980s and were likely to be exposed at younger ages. NHANES sampling is representative U.S. population, however our findings may not be generalizable to other ages and populations. The association of BP-3 with ANA was more apparent in adults ages 20-39 and females, who may be a key demographic for use of sunscreens and sunscreen-containing cosmetics (often used year-round). We lacked data on disease-specific antibodies but found that the association with BP-3 was stronger for the nuclear dense fine speckled phenotype, typically seen in healthy populations and patients with inflammatory conditions (but not autoimmune diseases) (36, 37). We noted an unexpected inverse association in the summer sample among those with the fine speckle pattern, which is seen in some systemic autoimmune diseases (38). Interestingly, we also saw an inverse association in the summer sample among those with lower vitamin D (<50 nmol/l) or who never used sunscreen; reasons for a protective association in the summer are unclear, and the numbers are too small to disentangle these findings. While the determinants and natural history of ANA in the general population are not well understood, once immune tolerance is broken the ability to produce ANA is likely to be maintained over time, though levels may wax and wane. As ANA can be present many years prior to the development of autoimmune diseases, the longer-term implications of ANA associations and potential role of BP-3 exposure at younger ages warrant further investigation.
In conclusion, our findings partially support the idea that BP-3 concentrations may be associated with ANA in the winter NHANES sample. Given the widespread use of BP-3 containing sunscreens and other products, further research is warranted in longitudinal studies following individuals with data collected across the seasons, and exposure assessment including internal measures, but also questionnaire data on past and present, along with co-exposures, other sunscreen active ingredients and diverse exposure sources (39). In particular, given the general lack of association seen in the summer sample, better understanding is needed on the role of personal sun exposures and potential pathways through which UV could mitigate the effects of BP-3 on ANA.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES), https://wwwn.cdc.gov/nchs/nhanes/Default.aspx
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Centers for Disease Control and Prevention (CDC). Written informed consent to participate in this study was provided by the participants.
Author contributions
FM, DS, HM, and CP contributed to the conception and design of the study. JW performed the statistical analyses. CP, HM, TJ, and DS contributed to the design of analyses and interpretation of results. CP wrote the first draft of the manuscript. All authors helped to revise the manuscript, read, and approved the final draft for submission. All authors contributed to the article and approved the submitted version.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES049028) and contract HHSN273201600011C to Social & Scientific Systems. HM was supported in part by the Shaw Scientist Award from the Greater Milwaukee Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med (2003) 349(16):1526–33. doi: 10.1056/NEJMoa021933
2. Bossuyt X, De Langhe E, Borghi MO, Meroni PL. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol (2020) 16(12):715–26. doi: 10.1038/s41584-020-00522-w
3. Edwards M, Dai R, Ahmed SA. Our environment shapes us: The importance of environment and sex differences in regulation of autoantibody production. Front Immunol (2018) 9:478. doi: 10.3389/fimmu.2018.00478
4. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol (2001) 2(9):777–80. doi: 10.1038/ni0901-777
5. Popescu M, Feldman TB, Chitnis T. Interplay between endocrine disruptors and immunity: Implications for diseases of autoreactive etiology. Front Pharmacol (2021) 12:626107. doi: 10.3389/fphar.2021.626107
6. Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: A randomized clinical trial. Jama (2020) 323(3):256–67. doi: 10.1001/jama.2019.20747
7. Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the united states: National health and nutrition examination survey 2003–2004. Environ Health Perspect (2008) 116(7):893–7. doi: 10.1289/ehp.11269
8. Han C, Lim YH, Hong YC. Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general U.S. population from 2003 to 2012. Environ pollut (2016) 208(Pt B):803–10. doi: 10.1016/j.envpol.2015.11.002
9. Ferguson KK, Colacino JA, Lewis RC, Meeker JD. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol (2017) 27(3):326–32. doi: 10.1038/jes.2016.27
10. Berger KP, Kogut KR, Bradman A, She J, Gavin Q, Zahedi R, et al. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J Expo Sci Environ Epidemiol (2019) 29(1):21–32. doi: 10.1038/s41370-017-0003-z
11. Bethea TN, Wesselink AK, Weuve J, McClean MD, Hauser R, Williams PL, et al. Correlates of exposure to phenols, parabens, and triclocarban in the study of environment, lifestyle and fibroids. J Expo Sci Environ Epidemiol (2020) 30(1):117–36. doi: 10.1038/s41370-019-0114-9
12. Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, et al. Increasing prevalence of antinuclear antibodies in the united states. Arthritis Rheumatol (2020) 72(6):1026–35. doi: 10.1002/art.41214
13. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, et al. The vitamin d status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin d shows recent modest increases. Am J Clin Nutr (2016) 104(2):454–61. doi: 10.3945/ajcn.115.127985
14. Liu B, Taioli E. Seasonal variations of complete blood count and inflammatory biomarkers in the US population - analysis of NHANES data. PloS One (2015) 10(11):e0142382. doi: 10.1371/journal.pone.0142382
15. Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol (2013) 69(6):867.e1–14. doi: 10.1016/j.jaad.2013.08.022
16. Zamoiski RD, Cahoon EK, Michal Freedman D, Linet MS. Self-reported sunscreen use and urinary benzophenone-3 concentrations in the united states: NHANES 2003-2006 and 2009-2012. Environ Res (2015) 142:563–7. doi: 10.1016/j.envres.2015.08.006
17. Andrade F, Casciola-Rosen LA, Rosen A. Generation of novel covalent RNA-protein complexes in cells by ultraviolet b irradiation: implications for autoimmunity. Arthritis Rheum (2005) 52(4):1160–70. doi: 10.1002/art.20992
18. Meyer T, Stockfleth E. Light and skin. Curr Probl Dermatol (2021) 55:53–61. doi: 10.1159/000517592
19. Parks CG, Wilkerson J, Rose KM, Faiq A, Noroozi Farhadi P, Long CS, et al. Association of ultraviolet radiation exposure with dermatomyositis in a national myositis patient registry. Arthritis Care Res (Hoboken) (2020) 72(11):1636–44. doi: 10.1002/acr.24059
20. Cooper GS, Wither J, Bernatsky S, Claudio JO, Clarke A, Rioux JD, et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatol (Oxford) (2010) 49(11):2172–80. doi: 10.1093/rheumatology/keq214
21. Tremlett H, Zhu F, Ascherio A, Munger KL. Sun exposure over the life course and associations with multiple sclerosis. Neurology (2018) 90(14):e1191–9. doi: 10.1212/WNL.0000000000005257
22. Arkema EV, Hart JE, Bertrand KA, Laden F, Grodstein F, Rosner BA, et al. Exposure to ultraviolet-b and risk of developing rheumatoid arthritis among women in the nurses’ health study. Ann Rheum Dis (2013) 72(4):506–11. doi: 10.1136/annrheumdis-2012-202302
23. Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, et al. Seasonal variation in 25-hydroxyvitamin d concentrations in the cardiovascular health study. Am J Epidemiol (2011) 174(12):1363–72. doi: 10.1093/aje/kwr258
24. Meier HC, Sandler DP, Simonsick EM, Parks CG. Association between vitamin d deficiency and antinuclear antibodies in middle-aged and older U.S. adults. Cancer Epidemiol Biomarkers Prev (2016) 25(12):1559–63. doi: 10.1158/1055-9965.EPI-16-0339
25. Holman DM, Berkowitz Z, Guy GP Jr., Hawkins NA, Saraiya M, Watson M. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol (2015) 73(1):83–92.e1. doi: 10.1016/j.jaad.2015.02.1112
26. Frederiksen H, Nielsen JK, Mørck TA, Hansen PW, Jensen JF, Nielsen O, et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health (2013) 216(6):772–83. doi: 10.1016/j.ijheh.2013.02.006
27. Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int (2019) 125:350–64. doi: 10.1016/j.envint.2019.01.078
28. Ferraris FK, Garcia EB, Chaves ADS, de Brito TM, Doro LH, Félix da Silva NM, et al. Exposure to the UV filter octyl methoxy cinnamate in the postnatal period induces thyroid dysregulation and perturbs the immune system of mice. Front Endocrinol (Lausanne) (2019) 10:943. doi: 10.3389/fendo.2019.00943
29. Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect (2001) 109(3):239–44. doi: 10.1289/ehp.01109239
30. Morohoshi K, Yamamoto H, Kamata R, Shiraishi F, Koda T, Morita M. Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays. Toxicol In Vitro (2005) 19(4):457–69. doi: 10.1016/j.tiv.2005.01.004
31. Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl (2012) 35(3):424–36. doi: 10.1111/j.1365-2605.2012.01280.x
32. Kim HJ, Lee E, Lee M, Ahn S, Kim J, Liu J, et al. Phosphodiesterase 4B plays a role in benzophenone-3-induced phototoxicity in normal human keratinocytes. Toxicol Appl Pharmacol (2018) 338:174–81. doi: 10.1016/j.taap.2017.11.021
33. Kim S, Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Environ Int (2014) 70:143–57. doi: 10.1016/j.envint.2014.05.015
34. LaKind JS, Naiman DQ. Temporal trends in bisphenol a exposure in the united states from 2003-2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ Res (2015) 142:84–95. doi: 10.1016/j.envres.2015.06.013
35. Artacho-Cordón F, Fernández MF, Frederiksen H, Iribarne-Durán LM, Jiménez-Díaz I, Vela-Soria F, et al. Environmental phenols and parabens in adipose tissue from hospitalized adults in southern Spain. Environ Int (2018) 119:203–11. doi: 10.1016/j.envint.2018.05.052
36. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum (2011) 63(1):191–200. doi: 10.1002/art.30084
37. Ochs RL, Mahler M, Basu A, Rios-Colon L, Sanchez TW, Andrade LE, et al. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med (2016) 16(3):273–93. doi: 10.1007/s10238-015-0367-0
38. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the international consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis (2019) 78(7):879–89. doi: 10.1136/annrheumdis-2018-214436
Keywords: cross-sectional studies (MeSH), antinuclear antibodies, oxybenzone, benzophenone-3, phenols, xenobiotics, sunscreen
Citation: Parks CG, Meier HCS, Jusko TA, Wilkerson J, Miller FW and Sandler DP (2022) Benzophenone-3 and antinuclear antibodies in U.S. adolescents and adults ages 12-39 years. Front. Immunol. 13:958527. doi: 10.3389/fimmu.2022.958527
Received: 31 May 2022; Accepted: 15 August 2022;
Published: 13 September 2022.
Edited by:
Allen Jay Rosenspire, Wayne State University, United StatesReviewed by:
Hui Wang, University of Texas Medical Branch at Galveston, United StatesBiman Saikia, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2022 Parks, Meier, Jusko, Wilkerson, Miller and Sandler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine G. Parks, cGFya3MxQG5pZWhzLm5paC5nb3Y=
 Christine G. Parks
Christine G. Parks Helen C. S. Meier
Helen C. S. Meier Todd A. Jusko
Todd A. Jusko Jesse Wilkerson4
Jesse Wilkerson4 Frederick W. Miller
Frederick W. Miller Dale P. Sandler
Dale P. Sandler