- 1Division of Gastroenterology, Department of Internal Medicine, Medical School, University of Patras, Patras, Greece
- 2Division of Infectious Diseases, Department of Internal Medicine, Medical School, University of Patras, Patras, Greece
Non-alcoholic fatty liver disease (NAFLD) and Hepatitis B virus infection (HBV) constitute common chronic liver diseases with worldwide distribution. NAFLD burden is expected to grow in the coming decade, especially in western countries, considering the increased incidence of diabetes and obesity. Despite the organized HBV vaccinations and use of anti-viral therapies globally, HBV infection remains endemic and challenging public health issue. As both NAFLD and HBV have been associated with the development of progressive fibrosis, cirrhosis and hepatocellular carcinoma (HCC), the co-occurrence of both diseases has gained great research and clinical interest. The causative relationship between NAFLD and HBV infection has not been elucidated so far. Dysregulated fatty acid metabolism and lipotoxicity in NAFLD disease seems to initiate activation of signaling pathways that enhance pro-inflammatory responses and disrupt hepatocyte cell homeostasis, promoting progression of NAFLD disease to NASH, fibrosis and HCC and can affect HBV replication and immune encountering of HBV virus, which may further have impact on liver disease progression. Chronic HBV infection is suggested to have an influence on metabolic changes, which could lead to NAFLD development and the HBV-induced inflammatory responses and molecular pathways may constitute an aggravating factor in hepatic steatosis development. The observed altered immune homeostasis in both HBV infection and NAFLD could be associated with progression to HCC development. Elucidation of the possible mechanisms beyond HBV chronic infection and NAFLD diseases, which could lead to advanced liver disease or increase the risk for severe complications, in the case of HBV-NAFLD co-existence is of high clinical significance in the context of designing effective therapeutic targets.
Introduction
Non-alcoholic fatty liver disease (NAFLD) constitutes the most common chronic liver disease, affecting approximately 25% of adults globally (1). The increased incidence of diabetes and obesity in western countries, seems to contribute to the growth of NAFLD burden in the coming decade (2) considering that NAFLD has been associated with metabolic syndrome and insulin resistance (3). A spectrum of liver disease stages and complications have been attributed to NAFLD, including simple steatosis, non-alcoholic steatohepatitis (NASH), progressive fibrosis, and hepatocellular carcinoma (HCC) (4). Hepatitis B virus infection (HBV) plays also a major causative role in the development of liver pathologies, such as cirrhosis and hepatocellular carcinoma and leads to increased liver-related mortality and morbidity (5, 6). Although the worldwide uptake of HBV vaccinations may have restrained HBV transmission, HBV remains endemic and challenging public health issue, especially in low- and middle-income countries. According to World Health Organization 296 million people worldwide were estimated to live with chronic HBV infection and 820000 HBV-related deaths, mainly from cirrhosis and HCC, were reported in 2019, with 1.5 million new infections being reported each year (7). The co-occurrence of NAFLD and HBV infection has gained great research and clinical interest, regarding the chronic liver injury progression to severe complications under the effect of both diseases. Biopsy-proven NAFLD has been estimated in Chronic hepatitis B (CHB) patients to range from 14% to 30% (8, 9). Investigation of the relationship between CHB and NAFLD disease is still ongoing. Hepatic steatosis may have a favorable effect on CHB progression, by accelerating HBsAg serum clearance (10). In contrast, the co-occurrence of chronic HBV infection and NAFLD has been associated with increased risk for advanced liver disease and HCC (11). NAFLD has been remarked as causative agent of elevated ALT enzyme with a rate of 25%, in CHB patients (12). HBV has been shown to increase the risk for hepatic steatosis in vivo and specifically HBx protein has been proved to upregulate the liver fatty-acid binding proteins, promoting hepatic lipid accumulation (13). However, clinical studies have reported that only metabolic factors are independently associated with NAFLD (14). The management of patients with CHB and NAFLD post a new challenge in clinical practice, considering that little is known about the possible interaction of two liver pathologies and the pathologic outcomes of their interaction. Thus, in this review we aim to describe the possible mechanisms beyond HBV chronic infection and NAFLD diseases, which could lead to advanced liver disease or increase the risk for severe complications, in the case of HBV-NAFLD co-existence.
Possible effects of NAFLD on liver disease progression during HBV infection
The causative relationship between NAFLD and HBV infection has not been elucidated so far. Metabolic components and immune alterations which are related to NAFLD progression have been suggested to directly inhibit HBV replication or induce indirectly anti-viral immune responses. A significant increase in Th17 cell related gene expression, including cytokine IL-21, has been remarked in NASH patients (15), which may contribute to HBV clearance. IL21 levels have been found elevated and positively associated with HBV DNA and HBeAg in immune clearance phase of chronic HBV infection, compared to immune tolerance phase (16). Increased serum levels of IL-21 in HBV-related liver failure may contribute to activation of T and B cells, which will produce inflammatory cytokines and eliminate virus proliferation and subsequent liver injury. Thus, persistence of HBV infection could be probably attributed to low levels of IL-21 (17, 18).
Elevated expression of Toll-like receptors (TLRs) has been remarked in NASH stage (19, 20), which is accompanied by increased infiltration and activation of adaptive immune cells, such as CD8+ T cells and NKT cells (21). TLRs play a major role in activation and modulation of immune responses and their activity has been highlighted in the pathogenesis and progression of chronic liver diseases, including HBV and HCV infection, alcoholic liver disease, hepatic fibrosis, NAFLD/NASH, cirrhosis and hepatocellular carcinoma (22–24). TLRs are highly distributed in liver cells [hepatocytes, kupffer cells (KCs), hepatic stellate cells (HSCs), sinusoidal endothelial cells, hepatic dendritic cells (DCs)] and many other liver cell populations can respond to TLRs. TLR signaling contributes to chronic liver disease progression, by mediating inflammatory processes and liver pathologies (e.g. hepatocellular injury and regeneration, fibrosis and cirrhosis) (25). Stimulation of TLRs in HSCs, upon activation of pro-inflammatory IKK/NF-κβ signaling, c-Jun N-terminal kinase (JNK) activity and secretion of pro-inflammatory cytokines and chemokines, leads to hepatic stellate cell activation and differentiation, promoting fibrosis (26–29). TLR5 has been shown to have an impact on the progression of fibrosis, by activating NF-κB and MAPK signaling pathways (30). Activation of the NF-κB and JNK pathways have been associated with production of cytokines related to TLR-induced liver damage and HCC progression (24). Although the activation of adaptive immune cells in NASH (21) may enhance further the anti-viral immune responses in HBV-infection and prevent the HBV-related severe liver disease progression, the increased expression and activity of TLRs in NASH stage of NAFLD may aggravate liver disease progression to fibrosis and HCC.
TLRs are activated in recruited hepatic DCs in liver sinusoids during inflammation and induce production of pro-inflammatory cytokines (TNF-α, IL-6, IL-12) (31, 32). Saturated fatty acids, have been shown to induce TLR4 activation and activate immune responses through myeloid differentiation factor 88 (MyD88)-mediated pathways in obese individuals (33, 34). TLR4/MyD8 signaling results in the production of TNF-α and IL-6 cytokines which are associated with development and progression of NAFLD to NASH and HCC (35, 36). TLR4 stimulation in KCs induces the production of pro-inflammatory cytokines (TNF-α, IL-1, IL-6 and IL-8, chemokines) and profibrogenic factors (TGF-β), which will promote fibrosis by activating HSCs (26, 28). Activated Lipopolysaccharide (LPS)/TLR4 signaling in HSCs, stimulates production of chemokines, which further recruit KCs. A vicious cycle of unrestricted activation of HSCs by KCs-derived profibrogenic cytokine TGF-β is established, which leads to development of liver fibrosis (37–39). Thus, activation of TLR4 in HSCs has been suggested to be the main mediator of fibrosis and cirrhosis, by initiating collagen production (26, 40). KCs induce fibrogenesis by secreting proinflammatory and profibrogenic cytokines, whereas HSCs mainly produce extracellular matrix in the fibrotic liver (40, 41). LPS/TLR4 and TLR2 signaling has been suggested to be involved in hepatic inflammation-fibrosis-carcinoma (IFC) sequence, which is linked to viral hepatitis. LPS/TLR4 signaling induces anti-viral responses, inflammation, steatosis, fibrosis, and hepatocarcinoma, as well as hepatic fibrosis-mediated portal hypertension, which leads to bacterial overgrowth and intestinal permeability (42).
Zhang et al. investigated the role of TLR4-mediated innate immunity in pathogenesis of CHB in NAFLD subjects and the effect of TLR4 signaling on HBV replication. The TLR4/MyD88 signaling pathway was demonstrated to be activated in the HBV-transgenic mice with NAFLD and HepG2.2.15 cells with SA-induced steatosis and contributed to inhibition of HBV replication (34). It was suggested that increased LPS and free fatty acids (FFAs) in HBV transgenic mice with NAFLD, were sensed by TLR4, stimulating its signaling pathway which results in production of anti-viral cytokine IFN-β and HBV DNA reduction. IL-6 and TNF-α cytokines, which are also induced by TLR4 signaling, have been shown to inhibit HBV replication. Thus, the increased TLR activity in NAFLD, under the effect of fatty acids, seems to have a positive impact on the HBV infection course, by possibly controlling HBV replication (34). Hu et al. developed an HBV- immunocompetent model to investigate the interplay between HBV infection and fatty liver. They showed that hepatic steatosis can be associated with significantly decreased serum levels of HBeAg, hepatic HBcAg and HBsAg, HBV DNA, and pgRNA, indicating a possible positive effect of fatty liver on HBV infection course, related to inhibition of HBV replication and proliferation (43) (Table 1).
Although HBV has been shown to downregulate TLRs, chronic infection and loss of HBeAg may lead to upregulation of TLR signaling pathways which trigger hepatic inflammation and disease progression (40). The NAFLD-associated metabolic stress, could have a positive impact on CHB, as it can activate the HBV-suppressed innate and adaptive immunity [restoration of antiviral substances, such as endogenous interferons and tumor necrosis factor-α (TNF-α)] which will eliminate HBV virus and prevent severe disease progression. Metabolic alterations in NAFLD could have an effect on HBV replication. In particular, peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α), a key transcription factor in gluconeogenesis, is increased in fasting status and stimulates in vivo the HBV DNA replication (44). PGC-1α has been decreased in NAFLD and it has been negatively correlated with NAFLD severity (45). Thus, PGC-1α in NAFLD may lead to inhibition of HBV replication. Accelerated apoptosis of HBV-infected cells has also been attributed to NAFLD effects. Inhibition of autophagy and increased Fas-mediated apoptosis have been remarked in liver samples from NASH patients, indicating that NAFLD could prevent disease progression in CHB patients by eliminating HBV replication and increasing apoptosis of HBV-infected cells, resulting in HBsAg and HBV-DNA clearance (46–48). Another possible effect of NAFLD disease on HBV infection course could be associated with immune abnormalities, which have been observed in NAFLD animal models. Miyake et al. (49) used two well-characterized antigens of HBV virus (HBsAg and HBcAg) to induce adaptive immunity in NAFLD mice. They showed that the saturated fatty acid, palmitic acid, can induce impaired function of DCs, causing down-regulation of HBsAg processing and presentation of DCs. It was also suggested that impaired DC function in NAFLD mice may be attributed to the non-antigen-specific maturation of DCs in these mice, which could be linked with their inability to activate antigen-specific immunocytes (50, 51). This observation along with the fact that NAFLD mice had impaired glucose tolerance could contribute to abnormal or insufficient immune responses, increasing the possibility of a more severe disease course by impeding the HBV clearance in case of a NAFLD-HBV infection co-occurrence (52) (Figure 1A) (Table 1). It could be suggested that some NAFLD-associated metabolic and immune components may have a positive impact on HBV replication and HBV clearance and thus contribute to prevention of severe HBV-related liver disease progression. However the presence of aggravating factors such as metabolic imbalance and immune dysregulation in NAFLD disease renders the NAFLD-HBV interplay quite complicated, as these factors could enhance the progression to severe liver disease.
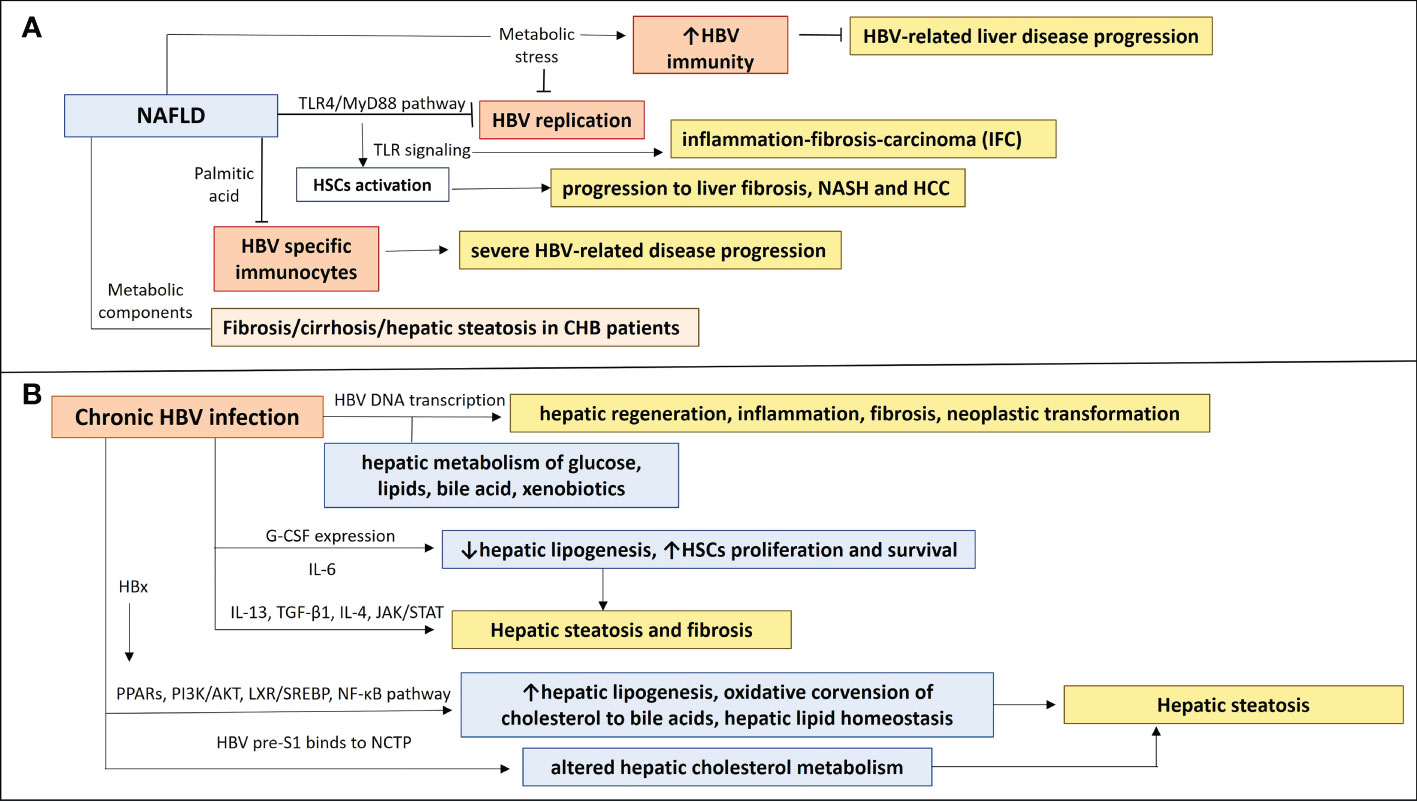
Figure 1 The NAFLD-HBV interplay in chronic liver disease progression. (A) NAFLD effects on chronic HBV infection and chronic liver disease progression. Activation of TLR4/Myd88 pathway in NAFLD inhibits HBV replication and induction of TLRs contributes to HSCs activation, leading to inflammation-fibrosis-carcinoma (IFC) and progression to liver fibrosis, NASH and HCC. Production of saturated fatty acid palmitic acid suppresses HBV specific immunocytes, resulting in insufficient immune responses, which could be associated with a more severe HBV-related disease progression. Metabolic components have been implicated in liver disease progression and NAFLD development in CHB patients, as they were correlated with fibrosis/cirrhosis and hepatic steatosis. NAFLD-associated metabolic stress restores HBV-suppressed immunity, preventing from severe HBV-related liver disease progression. (B) Chronic HBV infection effects on NAFLD and chronic liver disease progression. Transcription of HBV DNA is related to hepatic metabolism of glucose, lipids, bile acid, and xenobiotics and may play a role in the inhibition or promotion of hepatic regeneration, inflammation, fibrosis, and neoplastic transformation. A differential expression of IL-13, G-CSF, CCL11, IL-6 and IL-4 may be implicated in development of hepatic steatosis and fibrosis in HBV patients, through affecting hepatic lipogenesis and HSCs proliferation and survival. HBx protein can induce PPARs and signaling pathways (PI3K/AKT, LXR/SREBP, NF-κβ), having an impact on hepatic lipogenesis, oxidative conversion of cholesterol to bile acids, hepatic lipid homeostasis and therefore hepatic steatosis. HBV pre-S1 binds to NCTP, leading probably to altered hepatic cholesterol metabolism and hepatic steatosis.
Possible effects of HBV infection on NAFLD disease progression
The pathophysiological mechanisms beyond the association between HBV infection and NAFLD development and disease progression remains unclear. HBV-related metabolic changes, which could lead to NAFLD development have been observed in animal model studies. NAFLD pathogenesis and hepatic steatosis relies on excessive fatty acid uptake and synthesis, which cannot be balanced by oxidation (94). HBV infection could probably promote NAFLD progression to severe hepatic steatosis by affecting lipid biosynthesis. A significant upregulation of lipid biosynthesis gene expression in the liver of HBV transgenic mice, including sterol regulatory element-binding protein (SREBP) 2, ATP citrate lyase (ACLY), retinol-binding protein 1 (RBP1) and fatty acid synthase has been revealed by cDNA microarray analysis (95). Significant changes in long-chain fatty acid and polyunsaturated fat subpathways following HBV infection, along with a significant increase in glycolytic intermediates and glycogen metabolism have also been found. These alterations implied an increased pool of free fatty acid and upregulated glycolysis respectively (96). Rat primary hepatocytes transfected with the HBV genome or HBx have shown major alterations in long-chain fatty acid and polyunsaturated fat subpathways and increased glycolytic intermediates and glycogen metabolism. Thus, HBV infection could have an effect on NAFLD development by promoting significant metabolic changes, associated with NAFLD (97). However, Hu et al. have shown no specific effect of HBV infection in lipid metabolism and insulin resistance in an HBV-immunocompetent and NAFLD-induced mouse model. In particular, there were no increase in plasma and hepatic lipids or cholesterol and changes in plasma glucose and insulin levels in HBV-NAFLD co-occurrence compared with NAFLD group (43). Positive Hepatitis B core antibody (HBc) has been associated with high incidence of cirrhosis, cirrhosis complications and HCC in NAFLD patients (98).
Long noncoding RNAs (lncRNA) could constitute another factor in the interplay between HBV and NAFLD in liver disease progression. LncRNA may have a role in liver inflammation, considering its implication in the regulation of gene expression and various physiological and pathological processes (99, 100). Increased expression levels of lncRNAs have been observed in CHB patients (101). Higher expression levels of lncRNA EXOC7 have been found in liver tissues and serum of NASH patients compared to patients without steatohepatitis, and they were positively correlated with the aggravation of liver steatosis and inflammation (102). Li et al. (103) analyzed expression profiles of lncRNAs and mRNAs in treatment-naive patients with chronic HBV infection and NAFLD. Expression level of long-chain noncoding RNA-metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) was significantly higher in CHB group than NASH group, suggesting that MALAT1 plays an important role in the HBV-infection-related inflammatory response of patients with chronic HBV infection and NAFLD. An mRNA encoding thioredoxin interacting protein (TXNIP), whose expression was significantly upregulated in CHB group and was associated with MALAT1, through competing endogenous RNA, was identified, proposing a potent new regulatory pathway of MALAT1 and TXNIP, called MALAT1- micRNA-20b-5p-TXNIP (103). TXNIP is a protein complex composed of thioredoxin (TRX), reduced coenzyme II (NADPH) and thioredoxin reductase (TRX-R), which has a major impact on regulation of oxidative stress in cells. It may be associated with initiation of inflammatory responses, as it has been found to bind to the nucleotide oligomerization domain-like receptor family and pyrin domain containing 3 (NLRP3) inflammasomes, inducing its activation (104). Activated NLRP3 stimulates NF-κβ signaling pathway, resulting in upregulation of pro-inflammatory cytokines pro-IL-1β, pro-IL-18 (105). Thus, activation of NLRP3 inflammasome by MALAT1- micRNA-20b-5p-TXNIP regulatory pathway may lead to chronic HBV infection-related inflammatory responses, contributing to liver injury process. Liver immune cells, hepatic parenchymal cells, bile duct epithelial cells, and hepatic stellate cells express and activate inflammatory components under the effect of related signals. Activation of NLRP3 inflammatory components is implicated in NASH-mediated inflammatory injury and it may be related with high-mobility group box protein (HMGB), but the mechanism beyond this relationship remains unknown. As an increased ROS production has been found in HepG2 expressing full-length HBx protein, Li et al. (103) suggested that HBx protein-induced ROS in HBV-infected hepatocytes activate NLRP3, by interacting with TRX protein. Activation of NLRP3 leads to high production of IL-1β by KCs of liver tissue. IL-1β mediates the expression of immune-related genes and lymphocyte recruitment to the infection site, initiating inflammation responses which result in liver damage and increased ALT (106). The increased levels of lncRNAs in both CHB and NAFLD diseases could aggravate the tissue liver damage by enhancing inflammatory responses which lead to liver injury.
Effect of HBV viral load and specific plasma markers on NAFLD progression
An inverse correlation between HBV viral load and liver steatosis and an inverse correlation between HBsAg and fibrosis score have been remarked in some studies (46, 107, 108). We must also consider that transcription of HBV DNA in hepatocytes, is conducted under the effect of various host transcription factors (TFs) and coactivators, including CCAAT/enhancer binding protein (C/EBP), cyclic AMP response element-binding protein (CREB) (54, 55), the hepatic nuclear factor 3 (HNF3)/FoxA and HNF4 (56, 57), farnesoid X receptor (FXR), retinoid X receptor (RXR) (58, 59), peroxisome proliferator-activated receptor (PPAR) α and peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) (59, 60). Some of these TFs are implicated in hepatic metabolism of glucose, lipids, bile acid, and xenobiotics and they may play a role in the inhibition or promotion of hepatic regeneration, inflammation, fibrosis, and neoplastic transformation, by interacting with other pro-inflammatory TFs, induced by activated immune cells, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (61, 62) (Table 1).
HBV viral load has not been associated with controlled attenuation parameter (CAP) liver stiffness measurement (LSM) scores in chronic HBV patients. The implication of metabolic components in liver disease progression and NAFLD development has also been shown in CHB patients, as they had a significant positive correlation with fibrosis/cirrhosis and hepatic steatosis (53). Specific plasma markers of CHB, such as CCL11, IL-6, IL-4, IL-13 and G-CSF have been shown to have a significant influence on the CAP and LSM scores independent of metabolic components. A differential expression of IL-13, G-CSF, CCL11, IL-6 and IL-4 among patients at different stages of hepatic steatosis, highlighted a possible role of an inflammatory response in the development of hepatic steatosis and fibrosis in CHB patients. IL-13 has been shown as an independent predictor of the liver steatosis severity (53). IL-13 has been referred to play a role in liver fibrosis, as a component of a T-helper type 2 inflammatory response (63) and activates transforming growth factor 1 (TGF-β1) (64, 65). Stimulation of TGF-β1 gene expression mediates the fibrogenic effects of IL-13, which result in activation and proliferation of myofibroblasts, excessive production of extracellular matrix (ECM) and inhibition of ECM degradation (65–67). IL-13 signaling activates JAK-STAT-6 pathway (68), which results in CCL11 production in smooth muscle cells, an eosinophil chemotactic protein, which recruits eosinophils (69). Hepatic infiltration and activation of eosinophils has been associated with steatosis and fibrosis (70, 71). IL-13 has been suggested to contribute indirectly to HBV-related liver fibrogenesis by upregulating CCL11, which has a significant association with liver fibrosis (72). IL-13Rα2 receptor has been found to be overexpressed in hepatic stellate cells in sinusoidal lesions of the liver of NASH patients (65). Granulocyte colony-stimulating factor (G-CSF) has been inversely correlated with hepatic steatosis (53), as it has been related to the down-regulation of hepatic lipogenic genes and up-regulation of b-oxidative genes (73). G-CSF could ameliorate and improve hepatic steatosis by reducing the expression of SREBP-1c (74), a transcription factor, inductor of hepatic lipogenesis and mobilizing bone marrow cells, which contributes to liver regeneration (75). IL-4 and IL-6 have shown a potent protective effect on liver fibrosis (53). IL-4 has shown an anti-fibrotic effect, by activating alternatively activated macrophage, M2, which breakdown extracellular matrix (ECM), leading to resolution of liver fibrosis, by secreting matrix metalloproteinase-12 (MMP-12) (76, 77). IL-6 acts as pro-inflammatory cytokine and is implicated in liver regeneration and metabolic function (78). IL-6 receptors are expressed on all liver cell types and IL-6 signaling can affect each liver cell type separately. Hepatic KCs produce IL-6, which has been shown to promote proliferation and survival of HSCs (79). However data regarding the role of IL-6 in liver fibrosis are contradictory, depending probably on homeostasis between inhibitory and stimulatory signals during the different stages of liver fibrosis and under the effect of different etiologies of liver fibrosis (80) (Figure 1B) (Table 1).
Relationship between hepatic steatosis and HBV infection
The mechanisms beyond the increased fibrosis/cirrhosis in chronic HBV patients under the effect of severe steatosis remain to be elucidated. The interaction between viral factors and metabolic components of inflammation, underlying the NAFLD disease progression has to be investigated. There are contradictory data regarding the relationship between hepatic steatosis and HBV. In HBV infection, Hepatitis B protein X (HBx) is implicated in cellular signal transduction pathways and gene transcription related with cell growth and apoptosis. HBx has been suggested to lead to increased lipid accumulation in the liver, by affecting mitochondrial reactive oxygen species levels and oxidative stress, as it can directly interact with the mitochondrial respiratory chain complex subunit (13). Lipid accumulation is also induced in hepatocytes by HBx/fatty acid–binding protein 1/hepatocyte nuclear factor 3-b (HNF3b), CCAAT/enhancer-binding protein a (C/EBPa), and peroxisome proliferator-activated receptor a axis (PPARa), which activates FAB1 gene transcription. Over-expression of FABP1 increases the rate of fatty acid uptake (13). Elevated serum levels of FABP1 have been remarked in HBV-infected patients and HBx-transgenic mice (13). HBx protein also interacts with the liver X receptor a (LXRα) or tumor necrosis factor (TNF) receptor 1, leading to NF-κβ activation and TNF production, inhibition of apolipoprotein B secretion, and stimulation of PPARg and sterol-regulatory element-binding protein (SREBP)-1c. LXR/SREBP pathway plays a major role in hepatic steatosis, as LXRs contribute to activation of transcription of enzymes related to the synthesis of fatty acids, including the fatty acid synthase (FAS), acetyl coenzyme A acid enzymes (ACC), and SREBP-1c, and upregulation of the expression of CYP7A1, which participates in oxidative conversion of cholesterol to bile acids (81). SREBPs contribute to hepatic lipid homeostasis (82). HBx interacts with LXRa, enhancing its binding to the promoter LXREs of SREBP-1c and FAS, inducing hepatic lipogenesis (83, 84) (Figure 1B).
Induction of PPARs is another endpoint of HBx protein activity. PPARs constitute nuclear receptor proteins, playing a major role in energy metabolism and lipid oxidation, as they modulate the expression of downstream genes related to fatty acid-binding (apolipoproteins A1 and A2) and maintain lipid metabolism homeostasis, including fatty acid uptake, binding, and lipid transport (84). HBx can also upregulate PI3K/AKT signaling pathway, which is implicated in regulation of cell growth, proliferation, and differentiation (85) and can activate SREBP (86). HBx could take part in promoting hepatic steatosis via activating pro-inflammatory NF-κβ signaling pathway, as HBx interacts with tumor necrosis factor receptor (TNFR) 1 (87). The role of NF-κβ has been highlighted in promotion of hepatic steatosis and insulin resistance (88, 89). Thus, HBx protein modulates the molecular environment for initiation of inflammation and de novo lipogenesis (90).
HBV infection has also been associated with the induction of expression of cholesterol synthesis genes, which predispose to liver steatosis [e.g. 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and LDL receptor]. An inverse correlation between HBV and NAFLD has also been found. In particular, hepatitis B surface antigen (HBsAg)-positive patients have shown significantly decreased cholesterol levels, whereas increased cholesterol levels were observed in the HBsAg-negative patients (91). HBV infection can lead to an altered hepatic cholesterol metabolism. An increased expression of low-density lipoprotein receptor and 3-hydroxy-3-methylglutharyl-coenzyme A reductase (HMGCR) has been shown in HBV-transfected cells (92). Genes, related to hepatic cholesterol production and uptake, including those encoding SREBP-2, HMGCR and LDL receptor have been highly expressed in HBV-infected humanized mice. This observation could be attributed to pre-S1 domain of the HBV envelope, which by binding to Na+-taurocholate cotransporting polypeptide (NTCP) could impede NTCP-mediated bile acid (BA) uptake and lead to compensatory production and uptake of cholesterol (93). Non-alcoholic hepatic steatosis has been shown to inhibit HBV replication in a HBV-immunocompetent mouse model, as indicated by the reduction of HBV DNA and HBV-related antigens, whereas HBV replication has not been related with altered lipid metabolism in mice (43) (Table 1).
The adipose tissue: A possible linker between HBV, hepatic steatosis and liver injury
Crosstalk between adipose tissue and liver has a major effect on fatty liver disease development. Excessive fat accumulation on adipose tissue, due to obesity or alcohol consumption leads to alterations in adipose tissue endocrine functions. The function of triglyceride storage in adipocytes is disrupted, resulting in lipotoxicity and increased transfer of fatty acids in liver. This could favor the development of hepatic steatosis. Adipose tissue secretes a variety of pro- and anti-inflammatory cytokines termed adipokines, including tumor necrosis factor (TNF)-α, IL-6, resistin, leptin, and adiponectin (109). Adiponectin exerts anti-inflammatory action by inhibiting the synthesis and release of TNF-α from macrophages in adipose tissue (110). The production of adipokines by adipocytes is affected by nutritional status and plays a crucial role in biological functions and some adipokines are also produced by hepatocytes (111). Adipokines could constitute another link between HBV, hepatic steatosis and risk for liver fibrosis and HCC development. It has been speculated that the increased serum levels of TNF-α, resistin, and leptin in obese patients, as well as adiponectinemia may enhance steatosis, inflammation, fibrogenesis, or hepatocarcinogenesis in the liver (112). However the exact mechanisms beyond this association remain to be elucidated.
Adipokines can mediate the progression of liver injury. Leptin has been shown to enhance fibrotic responses to injury (113). The amelioration of adipose inflammation in NAFLD, induced by weight loss or use of thiazolidinediones (TZDs), has been shown to improve liver injury (114). TNF-α and adiponectin have been implicated in NAFLD (115, 116). The adipose tissue dysfunction, characterized by a dysregulated response of adiponectin to fat metabolism and ingestion has been shown to modulate liver injury and cardiometabolic risk in NAFLD (117). Adiponectin is decreased in NAFLD patients compared to healthy controls and physiologically suppresses fatty acid synthesis and promotes mitochondrial β -oxidation. Hepatocyte death and pro-inflammatory responses, that enhance liver injury and progression to fibrosis are also induced by TNF-α activity (114). Reduction of adiponectin levels in liver tissue of NAFLD patients has been suggested to modulate a pro-inflammatory microenvironment, resulting in increased lipotoxicity and promotion of simple steatosis to NASH and fibrosis (118). Adiponectin has been shown to limit pro-inflammatory responses in obesity by limiting IFN-γ and IL-17 producing CD4+ T cells in obesity (119). Roberts et al. have proposed a possible molecular crosstalk between liver and white adipose tissue that could lead to enhanced liver disease progression. In particular, a feed-forward loop between hepatic unconventional prefoldin RPB5 interactor (URI) and cytokine interleukin-17A (IL-17A) has been remarked to promote DNA damage and systemic inflammation leading to NASH and HCC. URI and IL-17A contribute to cross-talk between liver and white adipose tissue, where lipolysis, neutrophil infiltration and insulin resistance occur, resulting in hepatosteatosis and liver injury (120). HBV DNA has been positively correlated with serum adiponectin, which has been shown to decrease in patients with insulin resistance and hepatic steatosis (121). Serum TNF- α and IL-6 cytokines have been increased in HBV patients with significant necroinflammation (122). Wong et al. suggested that the increased production of TNF- α and/or IL-6 could mediate ongoing liver injury. TNF-α enhances survival of HSCs, immune activation and hepatocyte death, promoting liver fibrosis (123), whereas the high production of IL-6 in experimental- induced liver failure has shown to trigger immune suppression and disrupt liver repair, increasing mortality risk (122).
Viral load, HBeAg status and genotypes have not shown any association with insulin resistance and hepatic steatosis. Considering that viral factors are not associated with insulin resistance or pro-inflammatory adipokines, there is probably a separate, independent contribution of adipokines and HBV virus to liver injury (121). Serum leptin levels may be related with fibrosis progression, as they have been positively correlated with hepatic necroinflammation and are higher in cirrhosis stage in CHB patients (121, 124, 125). The potent pro-fibrogenic role of leptin could be attributed to its activity in innate and adaptive immunity, considering that leptin receptors are expressed by DCs, monocytes, neutrophils, macrophages, natural killer (NK) cells, T cells and B cells. Leptin signaling can activate a variety of signaling pathways which regulate cell activation, cell growth, cytokine production and function of immune cells (126). Adipose tissue may also have a potent major effect on HCC development in chronic HBV infection. Non-cirrhotic patients with HBV-related HCC have shown a higher visceral adipose tissue index (VATI), highlighting the VATI as an independent risk factor for HCC (127).
Possible mechanisms of HBV-NAFLD interplay leading to hepatocellular carcinoma development
Both HBV and NAFLD diseases have been associated with development of liver cancer (128, 129). Considering that hepatocellular carcinoma (HCC) accounts for 93.3% of primary liver cancers (130) and constitutes the fourth, most deadly type of cancer, investigation of early prognostic markers could be of high clinical significance, especially in cases of different liver diseases co-occurrence. A retrospective cohort study by Chan et al. showed that NAFLD is independent risk factor for HBV-associated HCC development and the presence of APOC3 gene polymorphism (related with triglycerides metabolism) increases further the risk for HCC development in CHB patients (131). The mechanisms beyond the interaction between HBV and NAFLD, which contribute to development of HCC are still not elucidated. Each liver disease has its own separate effect on progression to HCC and the possible mechanistic interplay between NAFLD and HBV could probably be illustrated by the co-occurrence of NAFLD and HBV separate activities.
HBV and NAFLD-mediated signaling pathways related to HCC
The HBV-induced chronic inflammation can lead to mutations in HBV gene and host genome, which can promote the malignant transformation of liver cells, by altering the viral biological behavior and pathogenicity, as well as the homeostasis of cell processes (132, 133). Mutated HBx has been found in HCC cases (134) and the role of HBx in progression of liver carcinogenesis is possibly attributed to its effect on abrogation of cell-cycle arrest and inhibition of apoptosis (135, 136). Hbx has been suggested to lead to increased risk of HCC, by interacting with a variety of proteins and mRNAs, related with signaling pathways and cell processes that regulate protein posttranslational modification, cell-cycle progression and apoptosis. In particular, HBx mutant protein can interact with Bcl-2, a major regulator of apoptosis and farnesoid X receptor (FXR), a major regulator of bile acid synthesis, lipid and glucose metabolism, to promote HCC development (137, 138). HBx can also lead to stabilization of transcriptional oncoproteins Myc and PAX8, by blocking their ubiquitination process (139, 140). The integration of HBV viral DNA into the host genome has been shown to have significant effect on HCC development in patients with occult HBV infection, as it has been associated with changes in tumor suppressor genes, mutations in the p53 ongogene, and genomic instability (141, 142). Thus, HBV can target a variety of ongogenes (143) and regulate the expression of different miRNAs, interfering with multiple signaling pathways, including Wnt, MAPK, STAT, P53, Akt and Notch to promote HCC development (144–146). For instance HBx can promote the proliferation and migration of HCC cells, by regulating expression of miR-1269b in an NF-κB-dependent manner (147). HBx can directly interact with MyH9 protein to activate Wnt/β-catenin/c-Jun signaling pathway, promoting metastasis, proliferation and malignant cell transformation (148, 149). HBx can also aggravate HBV-related carcinogenesis, by activating PI3K/Akt signaling pathway, regulating liver cell proliferation and malignant transformation (150, 151). HBx could enhance tumorigenesis and HCC growth, by inducing the expression of pro-ongogenic MAPK14 and Notch signaling (152, 153). Increased ROS production by HBx, HBs, and HBc HBV proteins (154) constitutes another indirect risk for HCC development. Accumulation of mutated HBs proteins in hepatocytes has been shown to induce endoplasmic reticulum (ER) stress and favor cell growth, by initiating multiple signaling pathways (155, 156). Mutated HBc protein increases production of ROS by stimulating ER stress and activates the NF-κB signaling pathway by promoting the malignant transformation of infected hepatocytes. HBc activity can mediate proliferation, glycolysis, amino acid metabolism and suppression of apoptosis and regulate the Src/PI3K/Akt pathway and blocks the TRAIL/Fas pathway or expression of p53 oncogene (157–160).
The presence of NAFLD in chronic HBV infection could be an aggravating factor in HCC development, as increased hepatic lipid storage leads to lipotoxicity, endoplasmic reticulum stress and reactive oxygen species-mediated DNA damage, which could enhance oncogenesis (161). Abnormal metabolism, dysbiosis of gut microbiota and dysregulation of immune responses have been implicated in NAFLD-mediated HCC development (162). It has been speculated that abnormal alterations in intrahepatic lipid metabolism which may establish insulin resistance and changes in signaling pathways and oncogenes, could lead to inflammation, fibrogenesis and hepatocarcinogenesis (163). Chronic lipotoxicity leads to oxidative and ER stress, which could have a causative role in NAFLD-HCC. Oxidized LDL uptake by macrophages has been shown to stimulate carcinogenetic signaling, by inducing expression of proteins, related to promotion of lipophagy and enhanced lysophosphatidic acid-enhanced Yes-associated protein (YAP) oncogenic activity (163). Similar to HBV virus, NAFLD disease components can interfere with signaling pathways, including signal transducer and activator of transcription (STAT) signaling pathways, which have been associated with HCC development (164, 165). Oxidative hepatic environment in obesity models of NAFLD has been associated with increased STAT-1 and STAT-3 signaling and inactivated STAT-1 and STAT-3 phosphatase T cell protein tyrosine phosphate (TCPTP), promoting hepatic T cell recruitment, NASH, fibrosis and HCC. STAT-1 signaling has been associated with NASH and fibrosis, whereas STAT-3 signaling has been correlated with HCC development (166). The cell cycle-related kinase (CCRK), an androgen receptor-driven oncogene can contribute to hepatocarcinogenesis via a signaling pathway dependent on β-catenin and T cell factor (TCF). CCRK has been associated with NAFLD-mediated HCC, by inducing STAT-3 and the mTORC1/4E-BP1/S6K/SREBP1 pathway (167).
The observed microbiome dysbiosis in NAFLD has been also correlated with NAFLD-mediated HCC. Liver inflammation and fibrosis in NAFLD could be attributed to altered bile acid signaling and a persistent immune activation, mediated by increased gut permeability and translocation of lipopolysaccharides (161). NAFLD-HCC patients have shown increased Bacteroides and Ruminococcaceae populations in their gut microbiome compared to patients with NALFD cirrhosis and no HCC. This microbiota profile has been associated with higher levels of cytokines and chemokines (IL- 8, IL-13, CCL3, CCL4 and CCL5) and activated monocytes in blood, indicating that microbiome changes could possibly aggravate the development of HCC, by exacerbating inflammation (168) (Table 2).
HBV and NAFLD-related immune responses and HCC
An altered immune microenvironment is present in both HBV and NAFLD. The tolerogenic status of liver turns into persistent active inflammation, which results in cellular injury and fibrosis, affecting progression to HCC (211). Various immune cells and immune-related markers have been reported in tumor microenvironment, as significant predictors of clinical outcome in cancer patients (211) and dysregulation of hepatic immune cells may have a major effect on hepatocarcinogenesis. Liver is composed of innate and adaptive immune cells, including macrophages, dendritic cells, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, CD4+ T, CD8+ T and B cells (212). The observed inhibitory effect of HBV on innate and adaptive immunity may enhance tumorigenesis. Under the effect of a suppressed immune system, the chronic HBV-induced inflammation could evolve in a persistent liver injury and promote the malignant transformation of liver cells. The tumor microenvironment in HBV-associated HCC has been characterized by a more severe immunosuppression compared to the non-HBV associated HCC (213). However, the mechanisms related to this status of HBV-HCC remains to be elucidated. HBsAg has been shown to significantly inhibit the activation and function of NK cells, by inhibiting the expression and activation of STAT3 transcription factor (169). Impaired activity of NK cells has been associated with enhanced progression of hepatitis to HCC (170, 171). HBsAg-mediated increase of monocytes induces expression of higher levels of suppressive cell surface molecules and cytokines (e.g. Tim-3, PD-1 and IL-10) in NK cells of CHB patients (172, 173). Regarding the role of macrophages in chronic HBV infection, inflammatory stimuli and viral proteins can lead to transition of macrophages into M2-like tumor macrophages, promoting HCC progression (174, 175). HBeAg has been shown to induce up-regulation of checkpoint molecular programmed death-ligand 1 (PD-L1) on macrophage, resulting in polarization to M2 protumor subtype, which impairs responses of CD8+ T cell to HBV (176). HBV-mediated macrophage release of matrix metalloproteinase 9 (MMP9) and IL-23 induces the blockade of binding of IFN-α to IFNAR1, which could contribute to tumor progression and angiogenesis (177, 178). MDSCs might affect tumor progression, by favoring immunosuppression, as they have been shown to inhibit T cell proliferation and function and induce Treg cells and tumor-associated macrophages. MDSCs expansion, induced by HBeAg, has been reported in CHB patients, and it has been associated with impaired T cell function, including T cell proliferation and IFN-γ production, as it interferes with indoleamine-2,3-dioxygenase (IDO) pathway (179). HBsAg also activates the ERK/IL-6/STAT3 signaling axis to promote differentiation of MDSCs (180). Cell cycle-related kinase (CCRK) as a regulator of androgen-receptor oncogene, has been implicated in virus-host signaling to promote tumor progression and induce polymorphonuclear MDSCs in HCC (181, 182).
T lymphocytes represent the major regulators of immune responses, which may play a crucial role in tumor development. CD4+ T cells constitute key players in anti-viral and anti-tumor immunity, as they produce cytokines and interact with other immune cells to activate CD8+ T cells and B cells. A decreased number and activity of cytotoxic T cells has been observed in advance stages of HCC and it has been linked with recurrence and poor survival in HCC patients (214). A decreased frequency and activity of specific CD4+ T follicular helper cells (CXCR5+CD4+ Tfh) in HBV-related HCC patients, along with decreased expression of their co-stimulatory molecules (ICOS) and cytokines (IL-10/IL-21), could result in impairment of naïve B cell differentiation into plasmablasts (183). An exhaustion of CD8+ T cell responses, characterized by decreased proliferation and function has been shown in HBV infection, which could further enhance disease progression to HCC, by establishing insufficient anti-tumoral immunity. CD8+ T cells have shown higher expression of inhibitory molecules (CTLA-4, PD-1 and TIM-3) in HBV and HBV-HCC and high expression of programmed cell death protein 1 (PD-1) on HBV-specific T and B cells has led to exhaustion of T cells and decreased production of antibodies (184–186). Exhausted CD8+ T cells and Tregs have been reported in HCC patients, which could further restrict antitumor immune responses (187). HBV-associated progression to HCC has been correlated with increased peripheral blood neutrophil/lymphocyte ratio (NLR) and increased number of Foxp3+ Treg cells (188, 189). In HBV infection, the increased TGF-β activity has been shown to suppress the expression of microRNA-34a, resulting in enhanced production of chemokine CCL22. Increased CCL22 recruits regulatory T (Treg) cells, promoting tumor immune escape and metastasis (190). An imbalance in Th17/Treg ratio has been proposed as indicator of liver cirrhosis process and it has been associated with increased risk for HCC in HBV patients (215). The expression of PD-1 was significantly decreased in peripheral blood CD4+ and CD8+ T cells of patients with HBV-related HCC and it has been associated with accelerated disease progression, compared to patients with HBV or cirrhosis (191). B cells play a crucial role in alleviation of immune responses and disease course in HBV infection (192). HBsAg-specific B cells have shown high expression of inhibitory molecules (PD-1, FcRL4 and FcRL5) and defective antibody production in HBV patients (186, 193). HBsAg can inhibit TLR9 expression on B cells via suppressing CREB protein, resulting in decreased proliferation of B cells and pro-inflammatory cytokine release (194). A high frequency of IL-10 producing, immunosuppressive type of B cells, has been remarked in HCC patients, which have been negatively correlated with the expression of granzyme A/B and perforin in CD4+ T cells, leading to suppressed cytotoxic activity of T cells (195).
The NAFLD progress to HCC is accompanied by recruitment and trafficking of innate and adaptive immune cells in liver during inflammation and fibrosis. Accumulated neutrophils in inflamed liver of NASH patients could induce angiogenesis, by promoting the secretion of matrix metalloproteinase-9 (196). Specific PD-L1+ monocytes, which suppress tumor-specific T cell immunity, leading to poor survival have been found in HCC patients (197). Tregs and MDSCs could favor tumor immune escape in NAFLD, as they have been shown to exert immunosuppressive effects on CD8+ T cells and NK cells in NASH (161). Dysregulation of NK cells, probably mediated by IL-15 activity, has been involved in NAFLD progression (198). The observed transformation of NK cells into less cytotoxic ILC1-like phenotype in NAFLD, has been linked with their impaired activity in killing cancer cells (199). The impaired activity of NK cells to control HSCs activity in advanced fibrosis in NAFLD, could further lead to deterioration of liver tissue in NAFLD-HCC patients (216). CCRK-AR signaling has been proved to establish a pro-tumorigenic environment in mice with obesity-associated HCC. Activated CCRK led to induction of mTORC1/4E-BP1/S6K/SREBP1 signaling pathways, resulting in recruitment of MDSCs, which enhance progression to HCC, by initiating metabolic reprogramming and modulating an immunosuppressive microenvironment (167). Lipid accumulation in liver has also been shown to promote recruitment of MDSCs and lead to increased ROS production in NASH mice model (200, 201). The interaction between liver KCs and highly activated platelets, along with platelet glycoprotein Ibα-mediated aggregation in NASH, has been shown to promote immune cell recruitment, which could enhance hepatic inflammation and HCC development (202).
In the context of adaptive immunity, the dysregulation of lipid metabolism in NAFLD has been associated with a selective loss of intrahepatic CD4+ T cells which further could lead to progression to HCC, highlighting a possible link between abnormal lipid metabolism and impaired anti-tumor immune surveillance. Progression of NAFLD to HCC has been shown to be delayed by the in vivo induction of hepatic CD4+ T cell population, mediated by ROS blockade (217). Brown et al. proposed a mechanistic role of dysregulated lipid metabolism in HCC development in NAFLD, indicating a major effect of accumulated linoleic acid on CD4+ T cells. Increased lipotoxicity and hepatocyte death induce linoleic acid release, which has been associated with increased production of ROS and CD4+ cell apoptosis (203). The presence of an exhausted, hepatic PD1+CD8+ T cell population, characterized by increased expression of C-X-C motif chemokine receptor 6 (CXCR6) and TNF-α in NASH mice, has been related with increased NASH progression to HCC, by possibly impairing immune surveillance (204). Th17 cells constitute another cell population, which have been positively associated with human fatty liver-associated HCC (205). Infiltration of Th17 in tumor microenvironment has been shown to promote tumor growth and angiogenesis, through induction of angiogenic factors (vascular endothelial growth factor/VEGF and prostaglandin E2/PGE2) and activation of oncogenic IL-6/Stat3 signaling (206, 207). An increased and highly active CD20+ B cell population has been observed in NAFLD patients (218). The number of tumor‐infiltrating B cells has been associated with tumor progression in HCC (208). Accumulated IgA+ plasma cells in NASH-related fibrosis have been shown to suppress CD8+ T cells via programmed cell death ligand 1 (PD-L1) and IL-10 expression, contributing to development of HCC in NAFLD (209). IL-10 producing, B regulatory cells have been shown to promote HCC growth, through direct interaction with tumor cells, mediated by CD40/CD154 signaling pathway (210) (Table 2).
Discussion
As both chronic HBV infection and NAFLD diseases can lead to chronic liver injury, and result in severe hepatic complications, HBV and NAFLD co-occurrence raises high concerns regarding the clinical management of patients. Dysregulated fatty acid metabolism and lipotoxicity in NAFLD disease may initiate activation of signaling pathways that enhance pro-inflammatory responses and disrupt hepatocyte cell homeostasis, which could either promote liver injury and progression of NAFLD disease to NASH, fibrosis and HCC or affect HBV replication and immune encountering of HBV virus during CHB. The metabolic dysregulation has been associated with increased cell stress and lipotoxicity in NAFLD, leading to trigger of inflammation, recruitment of immune cells in liver and hepatocyte death. Specific nuclear receptors, expressed by immune and liver parenchymal cells, are activated by inflammatory and stress stimuli and initiate signaling pathways related to fibrogenesis and hepatic steatosis (219). Fatty liver has also been linked to HBV replication, as patients with HBV-NAFLD co-occurrence have shown decreased viral replication (107). Chronic HBV infection is suggested to have an influence on metabolic changes, which could lead to NAFLD development and the HBV-induced inflammatory responses and molecular pathways may constitute an aggravating factor in hepatic steatosis development. However the role of HBV-NAFLD interplay in hepatic steatosis development might be more complicated as Xin et al. have proposed two opposite effects of HBV infection on steatosis. Specifically, CHB could be correlated with decreased risk of hyperlipidemia and lower prevalence of steatosis, probably due to an elevated serum adiponectin level and increased hepatic lipid accumulation could be induced by HBx overexpression and the observed genetic susceptibility to fatty liver in CHB patients (220). We must also consider the significance of the immune homeostasis imbalance which characterizes both HBV infection and NAFLD and its implication in liver disease progression to HCC. The disruption of immune cell function, which can be either induced by the dysregulated lipid metabolism in NAFLD, or the HBV-mediated immunosuppressed microenvironment, could impair the anti-tumor immunity and result in liver cancer progression. The presence of fatty liver has been associated with increased risk for HCC development in CHB patients (11). Further experimental studies are required to elucidate the exact mechanisms beyond the possible interaction between the inflammatory components and signaling pathways of both HBV and NAFLD and their impact on liver pathophysiology. Some studies have focused interest on clinical impact of targeting specific molecules, which are implicated in molecular signaling and immune responses on liver disease progression and response to treatment. Liu et al. have shown that serum IL-21 levels were increased at 12 week of HBV treatment, predicting early anti-viral response in patients with CHB and NAFLD (221). A phase I clinical study has investigated the therapeutic effect of OPB-111077, a novel STAT3 inhibitor, in patients with advanced hepatocellular carcinoma, which was proved to be well-tolerated (222). Restoration of miRNAs in HCC has shown to suppress tumor progression and improve chemosensitivity (223, 224). Zhong et al. suggested that blockade of T cell co-inhibitory receptor TIGIT combined with HBsAg vaccination in a mouse model of HBV-related HCC is able to recover immune homeostasis by reversing hepatic CD8+ T cell tolerance to HBsAg (225). Thus, investigation of the molecular background beyond the HBV and NAFLD co-occurrence is of high clinical significance in the context of designing effective therapeutic targets which will prevent or ameliorate the hepatic complications.
Author contributions
ET, SA and CT conceived and coordinated the study. ET and SFA did the literature search and analysis and wrote the manuscript.CT, KT, SA and MM were responsible for the revision of the manuscript for important intellectual content. ET, CT, KT, MM and SA approved the submitted version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The publication of this article has been financed by the Research Committee of the University of Patras.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lonardo A, Byrne CD, Caldwell SH, Cortez-Pinto H, Targher G. Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) 64(4):1388–9. doi: 10.1002/hep.28584
2. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, united kingdom, and united states for the period 2016-2030. J Hepatol (2018) 69(4):896–904. doi: 10.1016/j.jhep.2018.05.036
3. Cheng H-Y, Wang H-Y, Chang W-H, Lin S-C, Chu C-H, Wang T-E, et al. Nonalcoholic fatty liver disease: prevalence, influence on age and sex, and relationship with metabolic syndrome and insulin resistance. Int J Gerontology. (2013) 7(4):194–8. doi: 10.1016/j.ijge.2013.03.008
4. Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol (2019) 16(6):377–86. doi: 10.1038/s41575-019-0144-8
5. Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med (2014) 12(145):014–0145. doi: 10.1186/s12916-014-0145-y
6. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol (2013) 47(Suppl0):S2–6. doi: 10.1097/MCG.0b013e3182872f29
7. World Health Organization. Hepatitis B. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Accessed June 24, 2022].
8. Lin S, Wang M, Liu Y, Huang J, Wu Y, Zhu Y, et al. Concurrence of HBV infection and non-alcoholic fatty liver disease is associated with higher prevalence of chronic kidney disease. Clin Res Hepatol Gastroenterol (2021) 45(2):6. doi: 10.1016/j.clinre.2020.06.009
9. Huang J, Jing M, Wang C, Wang M, You S, Lin S, et al. The impact of hepatitis b virus infection status on the prevalence of nonalcoholic fatty liver disease: A population-based study. J Med Virol (2020) 92(8):1191–7. doi: 10.1002/jmv.25621
10. Chu CM, Lin DY, Liaw YF. Clinical and virological characteristics post HBsAg seroclearance in hepatitis b virus carriers with hepatic steatosis versus those without. Dig Dis Sci (2013) 58(1):275–81. doi: 10.1007/s10620-012-2343-9
11. Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis b. Clin Mol Hepatol (2019) 25(1):52–64. doi: 10.3350/cmh.2018.0040
12. Spradling PR, Bulkow L, Teshale EH, Negus S, Homan C, Simons B, et al. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis b virus infection. J Hepatol (2014) 61(4):785–91. doi: 10.1016/j.jhep.2014.05.045
13. Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN, Lin X. Hepatitis b virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J Virol (2015) 90(4):1729–40. doi: 10.1128/JVI.02604-15
14. Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J, et al. Hepatitis b virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study. Liver Int (2019) 39(1):70–80. doi: 10.1111/liv.13933
15. Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol (2011) 166(2):281–90. doi: 10.1111/j.1365-2249.2011.04471.x
16. Li HJ, Kang FB, Li BS, Yang XY, Zhang YG, Sun DX. Interleukin-21 inhibits HBV replication in vitro. Antivir Ther (2015) 20(6):583–90. doi: 10.3851/IMP2950
17. Chen HM, Liu HL, Yang YC, Cheng XL, Wang YF, Xing FF, et al. Serum IL-21 levels associated with chronic hepatitis b and hepatitis b-related liver failure. Exp Ther Med (2014) 7(4):1013–9. doi: 10.3892/etm.2014.1533
18. Pan Q, Yu Y, Tang Z, Xi M, Jiang H, Xun Y, et al. Increased levels of IL-21 responses are associated with the severity of liver injury in patients with chronic active hepatitis b. J Viral Hepat. (2014) 21(9):10. doi: 10.1111/jvh.12242
19. Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol (2018) 15(6):349–64. doi: 10.1038/s41575-018-0009-6
20. Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr (2014) 3(6):377–85. doi: 10.3978/j.issn.2304-3881.2014.12.04
21. Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell (2014) 26(4):549–64. doi: 10.1016/j.ccell.2014.09.003
22. Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. (2006) 130(6):1886–900. doi: 10.1053/j.gastro.2006.01.038
23. Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. (2008) 48(1):322–35. doi: 10.1002/hep.22306
24. Broering R, Lu M, Schlaak JF. Role of toll-like receptors in liver health and disease. Clin Sci (2011) 121(10):415–26. doi: 10.1042/CS20110065
25. Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J Hepatol (2016) 8(32):1354–69. doi: 10.4254/wjh.v8.i32.1354
26. Kesar V, Odin JA. Toll-like receptors and liver disease. Liver Int (2014) 34(2):184–96. doi: 10.1111/liv.12315
27. Chen Y, Sun R. Toll-like receptors in acute liver injury and regeneration. Int Immunopharmacol. (2011) 11(10):1433–41. doi: 10.1016/j.intimp.2011.04.023
28. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med (2007) 13(11):1324–32. doi: 10.1038/nm1663
29. Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. (2007) 46(5):1509–18. doi: 10.1002/hep.21867
30. Shu M, Huang DD, Hung ZA, Hu XR, Zhang S. Inhibition of MAPK and NF-κB signaling pathways alleviate carbon tetrachloride (CCl4)-induced liver fibrosis in toll-like receptor 5 (TLR5) deficiency mice. Biochem Biophys Res Commun (2016) 471(1):233–9. doi: 10.1016/j.bbrc.2016.01.119
31. Hsu W, Shu SA, Gershwin E, Lian ZX. The current immune function of hepatic dendritic cells. Cell Mol Immunol (2007) 4(5):321–8.
32. Shu SA, Lian ZX, Chuang YH, Yang GX, Moritoki Y, Comstock SS, et al. The role of CD11c(+) hepatic dendritic cells in the induction of innate immune responses. Clin Exp Immunol (2007) 149(2):335–43. doi: 10.1111/j.1365-2249.2007.03419.x
33. Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab (2014) 19(5):821–35. doi: 10.1016/j.cmet.2014.03.029
34. Zhang RN, Pan Q, Zhang Z, Cao HX, Shen F, Fan JG. Saturated fatty acid inhibits viral replication in chronic hepatitis b virus infection with nonalcoholic fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon. (2015) 15(5):e27909. doi: 10.5812/hepatmon.15(5)2015.27909
35. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. (2006) 116(11):3015–25. doi: 10.1172/JCI28898
36. Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol (2013) 1(0 1):38–42. doi: 10.1111/jgh.12019
37. Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int (2010) 4(4):659–72. doi: 10.1007/s12072-010-9219-x
38. Federico A, Dallio M, Godos J, Loguercio C, Salomone F. Targeting gut-liver axis for the treatment of nonalcoholic steatohepatitis: translational and clinical evidence. Transl Res (2016) 167(1):116–24. doi: 10.1016/j.trsl.2015.08.002
39. Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol (2006) 290(6):26. doi: 10.1152/ajpgi.00405.2005
40. Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. (2009) 58(5):704–20. doi: 10.1136/gut.2008.156307
41. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. (2005) 115(2):209–18. doi: 10.1172/JCI24282
42. Soares JB, Pimentel-Nunes P, Afonso L, Rolanda C, Lopes P, Roncon-Albuquerque R Jr., et al. Increased hepatic expression of TLR2 and TLR4 in the hepatic inflammation-fibrosis-carcinoma sequence. Innate Immun (2012) 18(5):700–8. doi: 10.1177/1753425912436762
43. Hu D, Wang H, Wang Y, Wan X, Yan W, Luo X, et al. Non-alcoholic hepatic steatosis attenuates hepatitis b virus replication in an HBV-immunocompetent mouse model. Hepatol Int (2018) 12(5):438–46. doi: 10.1007/s12072-018-9877-7
44. Shlomai A, Paran N, Shaul Y. PGC-1alpha controls hepatitis b virus through nutritional signals. Proc Natl Acad Sci U S A. (2006) 103(43):16003–8. doi: 10.1073/pnas.0607837103
45. Piccinin E, Villani G, Moschetta A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators. Nat Rev Gastroenterol Hepatol (2019) 16(3):160–74. doi: 10.1038/s41575-018-0089-3
46. Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, et al. Inverse relationship between hepatic steatosis and hepatitis b viremia: Results of a large case-control study. J Viral Hepat. (2018) 25(1):97–104. doi: 10.1111/jvh.12766
47. Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis b virus (HBV) surface antigen in chronic HBV infection? Int J Obes (2007) 31(5):871–5. doi: 10.1038/sj.ijo.0803479
48. Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, Rao GR, et al. Fatty liver reduces hepatitis b virus replication in a genotype b hepatitis b virus transgenic mice model. J Gastroenterol Hepatol (2012) 27(12):1858–64. doi: 10.1111/j.1440-1746.2012.07268.x
49. Miyake T, Akbar SM, Yoshida O, Chen S, Hiasa Y, Matsuura B, et al. Impaired dendritic cell functions disrupt antigen-specific adaptive immune responses in mice with nonalcoholic fatty liver disease. J Gastroenterol (2010) 45(8):859–67. doi: 10.1007/s00535-010-0218-4
50. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. (2007) 449(7161):419–26. doi: 10.1038/nature06175
51. Fazle Akbar SM, Abe M, Yoshida O, Murakami H, Onji M. Dendritic cell-based therapy as a multidisciplinary approach to cancer treatment: present limitations and future scopes. Curr Med Chem (2006) 13(26):3113–9. doi: 10.2174/092986706778742882
52. Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia (1998) 41(10):1241–8. doi: 10.1007/s001250051058
53. Wong SW, Ting YW, Yong YK, Tan HY, Barathan M, Riazalhosseini B, et al. Chronic inflammation involves CCL11 and IL-13 to facilitate the development of liver cirrhosis and fibrosis in chronic hepatitis b virus infection. Scand J Clin Lab Invest. (2021) 81(2):147–59. doi: 10.1080/00365513.2021.1876245
54. López-Cabrera M, Letovsky J, Hu KQ, Siddiqui A. Multiple liver-specific factors bind to the hepatitis b virus core/pregenomic promoter: Trans-activation and repression by CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. (1990) 87(13):5069–73. doi: 10.1073/pnas.87.13.5069
55. Kim BK, Lim SO, Park YG. Requirement of the cyclic adenosine monophosphate response element-binding protein for hepatitis b virus replication. Hepatology. (2008) 48(2):361–73. doi: 10.1002/hep.22359
56. Raney AK, Zhang P, McLachlan A. Regulation of transcription from the hepatitis b virus large surface antigen promoter by hepatocyte nuclear factor 3. J Virol (1995) 69(6):3265–72. doi: 10.1128/jvi.69.6.3265-3272.1995
57. Yu X, Mertz JE. Distinct modes of regulation of transcription of hepatitis b virus by the nuclear receptors HNF4alpha and COUP-TF1. J Virol (2003) 77(4):2489–99. doi: 10.1128/JVI.77.4.2489-2499.2003
58. Ramière C, Scholtès C, Diaz O, Icard V, Perrin-Cocon L, Trabaud MA, et al. Transactivation of the hepatitis b virus core promoter by the nuclear receptor FXRalpha. J Virol (2008) 82(21):10832–40. doi: 10.1128/JVI.00883-08
59. Reese VC, Oropeza CE, McLachlan A. Independent activation of hepatitis b virus biosynthesis by retinoids, peroxisome proliferators, and bile acids. J Virol (2013) 87(2):991–7. doi: 10.1128/JVI.01562-12
60. Tang H, McLachlan A. Transcriptional regulation of hepatitis b virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc Natl Acad Sci U S A. (2001) 98(4):1841–6. doi: 10.1073/pnas.98.4.1841
61. Ning BF, Ding J, Liu J, Yin C, Xu WP, Cong WM, et al. Hepatocyte nuclear factor 4α-nuclear factor-κB feedback circuit modulates liver cancer progression. Hepatology. (2014) 60(5):1607–19. doi: 10.1002/hep.27177
62. Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. (2008) 48(5):1632–43. doi: 10.1002/hep.22519
63. Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. (1999) 104(6):777–85. doi: 10.1172/JCI7325
64. Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med (2001) 194(6):809–21. doi: 10.1084/jem.194.6.809
65. Shimamura T, Fujisawa T, Husain SR, Kioi M, Nakajima A, Puri RK. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J Immunol (2008) 181(7):4656–65. doi: 10.4049/jimmunol.181.7.4656
66. Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta (2013) 7(60):6. doi: 10.1016/j.bbadis.2012.09.014
67. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. (2016) 12(6):325–38. doi: 10.1038/nrneph.2016.48
68. Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. (2003) 300(5625):1527–8. doi: 10.1126/science.1085458
69. Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol (2001) 108(4):594–601. doi: 10.1067/mai.2001.118600
70. Tarantino G, Cabibi D, Cammà C, Alessi N, Donatelli M, Petta S, et al. Liver eosinophilic infiltrate is a significant finding in patients with chronic hepatitis c. J Viral Hepat. (2008) 15(7):523–30. doi: 10.1111/j.1365-2893.2008.00976.x
71. Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. (2013) 57(5):2026–36. doi: 10.1002/hep.26196
72. Tacke F, Trautwein C, Yagmur E, Hellerbrand C, Wiest R, Brenner DA, et al. Up-regulated eotaxin plasma levels in chronic liver disease patients indicate hepatic inflammation, advanced fibrosis and adverse clinical course. J Gastroenterol Hepatol (2007) 22(8):1256–64. doi: 10.1111/j.1440-1746.2006.04621.x
73. Song YS, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, et al. Granulocyte-colony stimulating factor prevents the development of hepatic steatosis in rats. Ann Hepatol (2015) 14(2):243–50. doi: 10.1016/S1665-2681(19)30787-2
74. Song YS, Fang CH, So BI, Park JY, Jun DW, Kim KS. Therapeutic effects of granulocyte-colony stimulating factor on non-alcoholic hepatic steatosis in the rat. Ann Hepatol (2013) 12(1):115–22. doi: 10.1016/S1665-2681(19)31393-6
75. Jin SZ, Meng XW, Sun X, Han MZ, Liu BR, Wang XH, et al. Granulocyte colony-stimulating factor enhances bone marrow mononuclear cell homing to the liver in a mouse model of acute hepatic injury. Dig Dis Sci (2010) 55(10):2805–13. doi: 10.1007/s10620-009-1117-5
76. Weng SY, Wang X, Vijayan S, Tang Y, Kim YO, Padberg K, et al. IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. EBioMedicine. (2018) 29:92–103. doi: 10.1016/j.ebiom.2018.01.028
77. Niu H, Li Y, Li H, Chi Y, Zhuang M, Zhang T, et al. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity. Sci Rep (2016) 6:20171. doi: 10.1038/srep20171
78. Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol (2016) 64(6):1403–15. doi: 10.1016/j.jhep.2016.02.004
79. Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of rodent kupffer cells on stellate cells. Hepatology. (2006) 44(6):1487–501. doi: 10.1002/hep.21427
80. Kong X, Horiguchi N, Mori M, Gao B. Cytokines and STATs in liver fibrosis. Front Physiol (2012) 3(69). doi: 10.3389/fphys.2012.00069
81. Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov (2014) 13(6):433–44. doi: 10.1038/nrd4280
82. Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie (2004) 86(11):839–48. doi: 10.1016/j.biochi.2004.09.018
83. Kim K, Kim KH, Kim HH, Cheong J. Hepatitis b virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRalpha. Biochem J (2008) 416(2):219–30. doi: 10.1042/BJ20081336
84. Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, et al. Hepatitis b virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. (2007) 132(5):1955–67. doi: 10.1053/j.gastro.2007.03.039
85. Na TY, Shin YK, Roh KJ, Kang SA, Hong I, Oh SJ, et al. Liver X receptor mediates hepatitis b virus X protein-induced lipogenesis in hepatitis b virus-associated hepatocellular carcinoma. Hepatology. (2009) 49(4):1122–31. doi: 10.1002/hep.22740
86. Brown AJ. Viral hepatitis and fatty liver disease: how an unwelcome guest makes pâté of the host. Biochem J (2008) 416(2):e15–7. doi: 10.1042/BJ20081916
87. Kim JY, Song EH, Lee HJ, Oh YK, Choi KH, Yu DY, et al. HBx-induced hepatic steatosis and apoptosis are regulated by TNFR1- and NF-kappaB-dependent pathways. J Mol Biol (2010) 397(4):917–31. doi: 10.1016/j.jmb.2010.02.016
88. Wang XA, Zhang R, She ZG, Zhang XF, Jiang DS, Wang T, et al. Interferon regulatory factor 3 constrains IKKβ/NF-κB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology. (2014) 59(3):870–85. doi: 10.1002/hep.26751
89. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med (2005) 11(2):183–90. doi: 10.1038/nm1166
90. Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O. Nonalcoholic fatty liver disease and hepatic cirrhosis: Comparison with viral hepatitis-associated steatosis. World J Gastroenterol (2015) 21(46):12989–95. doi: 10.3748/wjg.v21.i46.12989
91. Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis b virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology. (2017) 65(3):828–35. doi: 10.1002/hep.28917
92. Li YJ, Zhu P, Liang Y, Yin WG, Xiao JH. Hepatitis b virus induces expression of cholesterol metabolism-related genes via TLR2 in HepG2 cells. World J Gastroenterol (2013) 19(14):2262–9. doi: 10.3748/wjg.v19.i14.2262
93. Oehler N, Volz T, Bhadra OD, Kah J, Allweiss L, Giersch K, et al. Binding of hepatitis b virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology. (2014) 60(5):1483–93. doi: 10.1002/hep.27159
94. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res (2009) 48(1):1–26. doi: 10.1016/j.plipres.2008.08.001
95. Hajjou M, Norel R, Carver R, Marion P, Cullen J, Rogler LE, et al. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J Med Virol (2005) 77(1):57–65. doi: 10.1002/jmv.20427
96. Shi Y-X, Huang C-J, Yang Z-G. Impact of hepatitis b virus infection on hepatic metabolic signaling pathway. World J gastroenterology. (2016) 22(36):8161. doi: 10.3748/wjg.v22.i36.8161
97. Chao HW, Chao SW, Lin H, Ku HC, Cheng CF. Homeostasis of glucose and lipid in non-alcoholic fatty liver disease. Int J Mol Sci (2019) 20(2):298. doi: 10.3390/ijms20020298
98. Chan TT, Chan WK, Wong GL, Chan AW, Nik Mustapha NR, Chan SL, et al. Positive hepatitis b core antibody is associated with cirrhosis and hepatocellular carcinoma in nonalcoholic fatty liver disease. Am J Gastroenterol (2020) 115(6):867–75. doi: 10.14309/ajg.0000000000000588
99. Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. (2013) 193(3):651–69. doi: 10.1534/genetics.112.146704
100. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet (2016) 17(1):47–62. doi: 10.1038/nrg.2015.10
101. Chen W, Lin C, Gong L, Chen J, Liang Y, Zeng P, et al. Comprehensive analysis of the mRNA-lncRNA Co-expression profile and ceRNA networks patterns in chronic hepatitis b. Curr Genomics (2019) 20(4):231–45. doi: 10.2174/1389202920666190820122126
102. Zhang L, Deng J, Zhang S. Expression and significance of the long non-coding RNA EXOC7 in nonalcoholic fatty liver disease. J Clin Hepatology. (2020) 36(2):391–4. doi: 10.3969/j.issn.1001-5256.2020.02.032
103. Li JZ, Ye LH, Wang DH, Zhang HC, Li TY, Liu ZQ, et al. The identify role and molecular mechanism of the MALAT1/hsa-mir-20b-5p/TXNIP axis in liver inflammation caused by CHB in patients with chronic HBV infection complicated with NAFLD. Virus Res (2021) 298(198405):26. doi: 10.1016/j.virusres.2021.198405
104. Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol (2012) 189(8):4175–81. doi: 10.4049/jimmunol.1201516
105. Lamkanfi M, Kanneganti TD. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol (2010) 42(6):792–5. doi: 10.1016/j.biocel.2010.01.008
106. Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicological Sci (2010) 115(2):307–21. doi: 10.1093/toxsci/kfq009
107. Ceylan B, Arslan F, Batırel A, Fincancı M, Yardımcı C, Fersan E, et al. Impact of fatty liver on hepatitis b virus replication and virologic response to tenofovir and entecavir. Turk J Gastroenterol (2016) 27(1):42–6. doi: 10.5152/tjg.2015.150348
108. Goyal SK, Jain AK, Dixit VK, Shukla SK, Kumar M, Ghosh J, et al. HBsAg level as predictor of liver fibrosis in HBeAg positive patients with chronic hepatitis b virus infection. J Clin Exp Hepatol (2015) 5(3):213–20. doi: 10.1016/j.jceh.2015.04.008
109. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol (2005) 115(5):911–9. doi: 10.1016/j.jaci.2005.02.023
110. Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-ay obese mice. Hepatology. (2004) 40(1):177–84. doi: 10.1002/hep.20282
111. Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann New York Acad Sci (2010) 1212(1):E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x
112. Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol (2008) 43(11):811–22. doi: 10.1007/s00535-008-2213-6
113. Martínez-Uña M, López-Mancheño Y, Diéguez C, Fernández-Rojo MA, Novelle MG. Unraveling the role of leptin in liver function and its relationship with liver diseases. Int J Mol Sci (2020) 21(24):9368. doi: 10.3390/ijms21249368
114. Parker R. The role of adipose tissue in fatty liver diseases. Liver Res (2018) 2(1):35–42. doi: 10.1016/j.livres.2018.02.002
115. Wong VW, Wong GL, Tsang SW, Hui AY, Chan AW, Choi PC, et al. Genetic polymorphisms of adiponectin and tumor necrosis factor-alpha and nonalcoholic fatty liver disease in Chinese people. J Gastroenterol Hepatol (2008) 23(6):914–21. doi: 10.1111/j.1440-1746.2008.05344.x
116. Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol (2006) 44(2):253–61. doi: 10.1016/j.jhep.2005.11.030
117. Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R. Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology. (2012) 56(3):933–42. doi: 10.1002/hep.25739
118. Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver - the link between adipocytes and hepatocytes. Digestion. (2011) 83(1-2):124–33. doi: 10.1159/000318741
119. Surendar J, Frohberger SJ, Karunakaran I, Schmitt V, Stamminger W, Neumann AL, et al. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol (2019) 10(2555). doi: 10.3389/fimmu.2019.02555
120. Roberts AA, Hebbard LW. Molecular cross-talk between the liver and white adipose tissue links excessive noURIshment to hepatocellular carcinoma. Trans Cancer Res (2016) 5:S1222–S6. doi: 10.21037/tcr.2016.11.16
121. Wong VW, Wong GL, Yu J, Choi PC, Chan AW, Chan HY, et al. Interaction of adipokines and hepatitis b virus on histological liver injury in the Chinese. Am J Gastroenterol (2010) 105(1):132–8. doi: 10.1038/ajg.2009.560
122. Roth K, Strickland J, Gonzalez-Pons R, Pant A, Yen T-C, Freeborn R, et al. Interleukin-6 drives key pathologic outcomes in experimental acetaminophen-induced liver failure. bioRxiv [Preprint] (2021). Available at: https://www.biorxiv.org/content/10.1101/2021.11.15.468664v1.abstract (Accessed May 20, 2022).
123. Yang YM, Seki E. TNFα in liver fibrosis. Curr Pathobiol Rep (2015) 3(4):253–61. doi: 10.1007/s40139-015-0093-z
124. Mousa N, Abdel-Razik A, Sheta T, Shabana W, Zakaria S, Awad M, et al. Serum leptin and homeostasis model assessment-IR as novel predictors of early liver fibrosis in chronic hepatitis b virus infection. Br J Biomed Science. (2018) 75(4):192–6. doi: 10.1080/09674845.2018.1505187
125. Bolukbas FF, Bolukbas C, Horoz M, Gumus M, Erdogan M, Zeyrek F, et al. Child-pugh classification dependent alterations in serum leptin levels among cirrhotic patients: a case controlled study. BMC Gastroenterol (2004) 4(23):4–23. doi: 10.1186/1471-230X-4-23
126. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev (2008) 4(2):70–9. doi: 10.2174/157339508784325046
127. Imai K, Takai K, Miwa T, Maeda T, Hanai T, Shirakami Y, et al. Higher accumulation of visceral adipose tissue is an independent risk factor for hepatocellular carcinoma among viral hepatitis patients with non-cirrhotic livers. Cancers (2021) 13(23):5980. doi: 10.3390/cancers13235980
128. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2021) 18(4):223–38. doi: 10.1038/s41575-020-00381-6
129. Liu Y, Veeraraghavan V, Pinkerton M, Fu J, Douglas MW, George J, et al. Viral biomarkers for hepatitis b virus-related hepatocellular carcinoma occurrence and recurrence. Front Microbiol (2021) 12(665201). doi: 10.3389/fmicb.2021.665201
130. Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, et al. Report of the 20th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res (2020) 50(1):15–46. doi: 10.1111/hepr.13438
131. Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis b. J Gastroenterol Hepatol (2017) 32(3):667–76. doi: 10.1111/jgh.13536
132. Wei F, Zheng Q, Li M, Wu M. The association between hepatitis b mutants and hepatocellular carcinoma: A meta-analysis. Medicine. (2017) 96(19):0000000000006835. doi: 10.1097/MD.0000000000006835
133. Liu Y, Tao S, Liao L, Li Y, Li H, Li Z, et al. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun (2020) 11(1):019–14190. doi: 10.1038/s41467-019-14190-2
134. Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, et al. Integration of tumour and viral genomic characterizations in HBV-related hepatocellular carcinomas. Gut. (2015) 64(5):820–9. doi: 10.1136/gutjnl-2013-306228
135. Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A, Zakaria MK, Fatima K, et al. Hepatitis b virus, HBx mutants and their role in hepatocellular carcinoma. World J Gastroenterol (2014) 20(30):10238–48. doi: 10.3748/wjg.v20.i30.10238
136. Kew MC. Hepatitis b virus x protein in the pathogenesis of hepatitis b virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol (2011) 1:144–52. doi: 10.1111/j.1440-1746.2010.06546.x
137. Jiang T, Liu M, Wu J, Shi Y. Structural and biochemical analysis of bcl-2 interaction with the hepatitis b virus protein HBx. Proc Natl Acad Sci U S A. (2016) 113(8):2074–9. doi: 10.1073/pnas.1525616113
138. Niu Y, Xu M, Slagle BL, Huang H, Li S, Guo GL, et al. Farnesoid X receptor ablation sensitizes mice to hepatitis b virus X protein-induced hepatocarcinogenesis. Hepatology. (2017) 65(3):893–906. doi: 10.1002/hep.28924
139. Lee S, Kim W, Ko C, Ryu WS. Hepatitis b virus X protein enhances myc stability by inhibiting SCF(Skp2) ubiquitin E3 ligase-mediated myc ubiquitination and contributes to oncogenesis. Oncogene. (2016) 35(14):1857–67. doi: 10.1038/onc.2015.251
140. Wang J, Li N, Huang ZB, Fu S, Yu SM, Fu YM, et al. HBx regulates transcription factor PAX8 stabilization to promote the progression of hepatocellular carcinoma. Oncogene. (2019) 38(40):6696–710. doi: 10.1038/s41388-019-0907-2
141. Mak LY, Wong DK, Pollicino T, Raimondo G, Hollinger FB, Yuen MF. Occult hepatitis b infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol (2020) 73(4):952–64. doi: 10.1016/j.jhep.2020.05.042
142. Zhao LH, Liu X, Yan HX, Li WY, Zeng X, Yang Y, et al. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun (2016) 7:12992. doi: 10.1038/ncomms12992
143. Sadri Nahand J, Bokharaei-Salim F, Salmaninejad A, Nesaei A, Mohajeri F, Moshtzan A, et al. microRNAs: Key players in virus-associated hepatocellular carcinoma. J Cell Physiol (2019) 234(8):12188–225. doi: 10.1002/jcp.27956
144. Tian JH, Liu WD, Zhang ZY, Tang LH, Li D, Tian ZJ, et al. Influence of miR-520e-mediated MAPK signalling pathway on HBV replication and regulation of hepatocellular carcinoma cells via targeting EphA2. J Viral Hepat (2019) 26(4):496–505. doi: 10.1111/jvh.13048
145. Lou W, Liu J, Ding B, Chen D, Xu L, Ding J, et al. Identification of potential miRNA-mRNA regulatory network contributing to pathogenesis of HBV-related HCC. J Transl Med (2019) 17(1):018–1761. doi: 10.1186/s12967-018-1761-7
146. Wang G, Dong F, Xu Z, Sharma S, Hu X, Chen D, et al. MicroRNA profile in HBV-induced infection and hepatocellular carcinoma. BMC Cancer. (2017) 17(1):017–3816. doi: 10.1186/s12885-017-3816-1
147. Kong XX, Lv YR, Shao LP, Nong XY, Zhang GL, Zhang Y, et al. HBx-induced MiR-1269b in NF-κB dependent manner upregulates cell division cycle 40 homolog (CDC40) to promote proliferation and migration in hepatoma cells. J Transl Med (2016) 14(1):016–0949. doi: 10.1186/s12967-016-0949-y
148. Lin X, Li AM, Li YH, Luo RC, Zou YJ, Liu YY, et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct Target Ther (2020) 5(1):020–0111. doi: 10.1038/s41392-020-0111-4
149. Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis b viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate wnt/β-catenin signaling. Cancer Lett (2011) 300(2):162–72. doi: 10.1016/j.canlet.2010.09.018
150. Chung TW, Lee YC, Ko JH, Kim CH. Hepatitis b virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res (2003) 63(13):3453–8.
151. Kim GW, Imam H, Khan M, Mir SA, Kim SJ, Yoon SK, et al. HBV-induced increased N6 methyladenosine modification of PTEN RNA affects innate immunity and contributes to HCC. Hepatology. (2021) 73(2):533–47. doi: 10.1002/hep.31313
152. Witt-Kehati D, Fridkin A, Alaluf MB, Zemel R, Shlomai A. Inhibition of pMAPK14 overcomes resistance to sorafenib in hepatoma cells with hepatitis b virus. Transl Oncol (2018) 11(2):511–7. doi: 10.1016/j.tranon.2018.02.015
153. Liao B, Zhou H, Liang H, Li C. Regulation of ERK and AKT pathways by hepatitis b virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int J Oncol (2017) 51(5):1449–59. doi: 10.3892/ijo.2017.4126
154. Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, et al. Oxidative stress, a trigger of hepatitis c and b virus-induced liver carcinogenesis. Oncotarget. (2017) 8(3):3895–932. doi: 10.18632/oncotarget.13904
155. Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, et al. Pre-s mutant surface antigens in chronic hepatitis b virus infection induce oxidative stress and DNA damage. Carcinogenesis (2004) 25(10):2023–32. doi: 10.1093/carcin/bgh207
156. Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis b virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol (2014) 61(2):408–17. doi: 10.1016/j.jhep.2014.04.041
157. Liu W, Guo TF, Jing ZT, Yang Z, Liu L, Yang YP, et al. Hepatitis b virus core protein promotes hepatocarcinogenesis by enhancing src expression and activating the Src/PI3K/Akt pathway. FASEB J (2018) 32(6):3033–46. doi: 10.1096/fj.201701144R
158. Liu D, Cui L, Wang Y, Yang G, He J, Hao R, et al. Hepatitis b e antigen and its precursors promote the progress of hepatocellular carcinoma by interacting with NUMB and decreasing p53 activity. Hepatology. (2016) 64(2):390–404. doi: 10.1002/hep.28594
159. Xie Q, Fan F, Wei W, Liu Y, Xu Z, Zhai L, et al. Multi-omics analyses reveal metabolic alterations regulated by hepatitis b virus core protein in hepatocellular carcinoma cells. Sci Rep (2017) 7:41089. doi: 10.1038/srep41089
160. Du J, Liang X, Liu Y, Qu Z, Gao L, Han L, et al. Hepatitis b virus core protein inhibits TRAIL-induced apoptosis of hepatocytes by blocking DR5 expression. Cell Death Differ (2009) 16(2):219–29. doi: 10.1038/cdd.2008.144
161. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol (2019) 16(7):411–28. doi: 10.1038/s41575-019-0145-7
162. Takakura K, Oikawa T, Nakano M, Saeki C, Torisu Y, Kajihara M, et al. Recent insights into the multiple pathways driving non-alcoholic steatohepatitis-derived hepatocellular carcinoma. Front Oncol (2019) 9(762). doi: 10.3389/fonc.2019.00762
163. Tian Y, Yang B, Qiu W, Hao Y, Zhang Z, Li N, et al. ER-residential nogo-b accelerates NAFLD-associated HCC mediated by metabolic reprogramming of oxLDL lipophagy. Nat Commun (2019) 10(1):019–11274. doi: 10.1038/s41467-019-11274-x
164. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of ras and Jak/Stat pathways in human HCC. Gastroenterology (2006) 130(4):1117–28. doi: 10.1053/j.gastro.2006.01.006
165. He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell (2010) 17(3):286–97. doi: 10.1016/j.ccr.2009.12.048
166. Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, et al. Obesity drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. (2018) 175(5):1289–306. doi: 10.1016/j.cell.2018.09.053
167. Sun H, Yang W, Tian Y, Zeng X, Zhou J, Mok MTS, et al. An inflammatory-CCRK circuitry drives mTORC1-dependent metabolic and immunosuppressive reprogramming in obesity-associated hepatocellular carcinoma. Nat Commun (2018) 9(1):018–07402. doi: 10.1038/s41467-018-07402-8
168. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. (2019) 69(1):107–20. doi: 10.1002/hep.30036
169. Zheng B, Yang Y, Han Q, Yin C, Pan Z, Zhang J. STAT3 directly regulates NKp46 transcription in NK cells of HBeAg-negative CHB patients. J Leukoc Biol (2019) 106(4):987–96. doi: 10.1002/JLB.2A1118-421R
170. Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol (2015) 12(3):292–302. doi: 10.1038/cmi.2014.91
171. Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol (2017) 14(8):712–20. doi: 10.1038/cmi.2015.113
172. Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T Cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis b. J Hepatol (2010) 52(3):322–9. doi: 10.1016/j.jhep.2009.12.005
173. Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. (2018) 67(11):2035–44. doi: 10.1136/gutjnl-2017-314098
174. Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. (2015) 64(10):1593–604. doi: 10.1136/gutjnl-2014-307671
175. Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, et al. Hepatitis b virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PloS Pathog (2014) 10(3):e1004032. doi: 10.1371/journal.ppat.1004032
176. Tian Y, Kuo CF, Akbari O, Ou JH. Maternal-derived hepatitis b virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity. (2016) 44(5):1204–14. doi: 10.1016/j.immuni.2016.04.008
177. Chen J, Xu W, Chen Y, Xie X, Zhang Y, Ma C, et al. Matrix metalloproteinase 9 facilitates hepatitis b virus replication through binding with type I interferon (IFN) receptor 1 to repress IFN/JAK/STAT signaling. J Virol (2017) 91(8):01824–16. doi: 10.1128/JVI.01824-16
178. Zang M, Li Y, He H, Ding H, Chen K, Du J, et al. IL-23 production of liver inflammatory macrophages to damaged hepatocytes promotes hepatocellular carcinoma development after chronic hepatitis b virus infection. Biochim Biophys Acta Mol Basis Dis (2018) 12(10):4. doi: 10.1016/j.bbadis.2018.10.004
179. Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, et al. Hepatitis b e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis b virus infection. PloS Pathog (2019) 15(4):e1007690. doi: 10.1371/journal.ppat.1007690
180. Fang Z, Li J, Yu X, Zhang D, Ren G, Shi B, et al. Polarization of monocytic myeloid-derived suppressor cells by hepatitis b surface antigen is mediated via ERK/IL-6/STAT3 signaling feedback and restrains the activation of T cells in chronic hepatitis b virus infection. J Immunol (2015) 195(10):4873–83. doi: 10.4049/jimmunol.1501362
181. Yu Z, Gao YQ, Feng H, Lee YY, Li MS, Tian Y, et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis b virus-associated hepatocarcinogenesis. Gut. (2014) 63(11):1793–804. doi: 10.1136/gutjnl-2013-305584
182. Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. (2018) 67(5):931–44. doi: 10.1136/gutjnl-2017-314032
183. Zhou ZQ, Tong DN, Guan J, Tan HW, Zhao LD, Zhu Y, et al. Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and b cell help, and is associated with advanced tumor stages. Am J Transl Res (2016) 8(7):2926–36.
184. Li Y, Tang L, Guo L, Chen C, Gu S, Zhou Y, et al. CXCL13-mediated recruitment of intrahepatic CXCR5(+)CD8(+) T cells favors viral control in chronic HBV infection. J Hepatol (2020) 72(3):420–30. doi: 10.1016/j.jhep.2019.09.031
185. Salimzadeh L, Le Bert N, Dutertre CA, Gill US, Newell EW, Frey C, et al. PD-1 blockade partially recovers dysfunctional virus-specific b cells in chronic hepatitis b infection. J Clin Invest. (2018) 128(10):4573–87. doi: 10.1172/JCI121957
186. Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral b cells are defective in hepatitis b. J Clin Invest. (2018) 128(10):4588–603. doi: 10.1172/JCI121960
187. Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. (2017) 169(7):1342–56. doi: 10.1016/j.cell.2017.05.035
188. Liu X, He L, Han J, Wang L, Li M, Jiang Y, et al. Association of neutrophil-lymphocyte ratio and T lymphocytes with the pathogenesis and progression of HBV-associated primary liver cancer. PloS One (2017) 12(2):e0170605. doi: 10.1371/journal.pone.0170605
189. Tajiri K, Baba H, Kawai K, Minemura M, Yasumura S, Takahara T, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis b virus infection. J Gastroenterol Hepatol (2016) 31(7):1291–9. doi: 10.1111/jgh.13287
190. Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, et al. TGF-β-miR-34a-CCL22 signaling-induced treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell (2012) 22(3):291–303. doi: 10.1016/j.ccr.2012.07.023
191. Liu X, Li M, Wang X, Dang Z, Jiang Y, Kong Y, et al. PD-1(+) TIGIT(+) CD8(+) T cells are associated with pathogenesis and progression of patients with hepatitis b virus-related hepatocellular carcinoma. Cancer Immunol Immunother. (2019) 68(12):2041–54. doi: 10.1007/s00262-019-02426-5
192. Zhang S, Zhao J, Zhang Z. Humoral immunity, the underestimated player in hepatitis b. Cell Mol Immunol (2018) 15(6):645–8. doi: 10.1038/cmi.2017.132
193. Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S. HBV induces inhibitory FcRL receptor on b cells and dysregulates b cell-T follicular helper cell axis. Sci Rep (2018) 8(1):018–33719. doi: 10.1038/s41598-018-33719-x
194. Tout I, Gomes M, Ainouze M, Marotel M, Pecoul T, Durantel D, et al. Hepatitis b virus blocks the CRE/CREB complex and prevents TLR9 transcription and function in human b cells. J Immunol (2018) 201(8):2331–44. doi: 10.4049/jimmunol.1701726
195. Xue H, Lin F, Tan H, Zhu ZQ, Zhang ZY, Zhao L. Overrepresentation of IL-10-Expressing b cells suppresses cytotoxic CD4+ T cell activity in HBV-induced hepatocellular carcinoma. PloS One (2016) 11(5):e0154815. doi: 10.1371/journal.pone.0154815
196. Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol (2011) 54(5):948–55. doi: 10.1016/j.jhep.2010.08.041
197. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med (2009) 206(6):1327–37. doi: 10.1084/jem.20082173
198. Cepero-Donates Y, Lacraz G, Ghobadi F, Rakotoarivelo V, Orkhis S, Mayhue M, et al. Interleukin-15-mediated inflammation promotes non-alcoholic fatty liver disease. Cytokine. (2016) 82:102–11. doi: 10.1016/j.cyto.2016.01.020
199. Cuff AO, Sillito F, Dertschnig S, Hall A, Luong TV, Chakraverty R, et al. The obese liver environment mediates conversion of NK cells to a less cytotoxic ILC1-like phenotype. Front Immunol (2019) 10(2180). doi: 10.3389/fimmu.2019.02180
200. Tsunashima H, Tsuneyama K, Moritoki Y, Hara M, Kikuchi K. Accumulated myeloid-derived suppressor cells demonstrate distinct phenotypes and functions in two non-alcoholic steatohepatitis mouse models. Hepatobiliary Surg Nutr (2015) 4(5):313–9. doi: 10.3978/j.issn.2304-3881.2015.04.08
201. Yao L, Abe M, Kawasaki K, Akbar SM, Matsuura B, Onji M, et al. Characterization of liver monocytic myeloid-derived suppressor cells and their role in a murine model of non-alcoholic fatty liver disease. PloS One (2016) 11(2):e0149948. doi: 10.1371/journal.pone.0149948
202. Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med (2019) 25(4):641–55. doi: 10.1038/s41591-019-0379-5
203. Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis (2018) 9(6):018–0687. doi: 10.1038/s41419-018-0687-6
204. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0
205. Gomes AL, Teijeiro A, Burén S, Tummala KS, Yilmaz M, Waisman A, et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell (2016) 30(1):161–75. doi: 10.1016/j.ccell.2016.05.020
206. Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol (2009) 50(5):980–9. doi: 10.1016/j.jhep.2008.12.033
207. Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol (2013) 182(1):10–20. doi: 10.1016/j.ajpath.2012.08.041
208. Faggioli F, Palagano E, Di Tommaso L, Donadon M, Marrella V, Recordati C, et al. B lymphocytes limit senescence-driven fibrosis resolution and favor hepatocarcinogenesis in mouse liver injury. Hepatology. (2018) 67(5):1970–85. doi: 10.1002/hep.29636
209. Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. (2017) 551(7680):340–5. doi: 10.1038/nature24302
210. Shao Y, Lo CM, Ling CC, Liu XB, Ng KT, Chu AC, et al. Regulatory b cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett (2014) 355(2):264–72. doi: 10.1016/j.canlet.2014.09.026
211. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
212. Campisano S, La Colla A, Echarte SM, Chisari AN. Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases. Nutr Res Rev (2019) 32(1):128–45. doi: 10.1017/S0954422418000239
213. Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis b virus-related hepatocellular carcinoma. Gut. (2019) 68(5):916–27. doi: 10.1136/gutjnl-2018-316510
214. Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. (2013) 58(1):139–49. doi: 10.1002/hep.26054
215. Li K, Liu H, Guo T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients. Clin Res Hepatol Gastroenterol (2017) 41(4):399–407. doi: 10.1016/j.clinre.2016.12.004
216. Amer J, Salhab A, Noureddin M, Doron S, Abu-Tair L, Ghantous R, et al. Insulin signaling as a potential natural killer cell checkpoint in fatty liver disease. Hepatol Commun (2018) 2(3):285–98. doi: 10.1002/hep4.1146
217. Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. (2016) 531(7593):253–7. doi: 10.1038/nature16969
218. Schwenger KJP, Chen L, Chelliah A, Da Silva HE, Teterina A, Comelli EM, et al. Markers of activated inflammatory cells are associated with disease severity and intestinal microbiota in adults with non−alcoholic fatty liver disease. Int J Mol Med (2018) 42(4):2229–37. doi: 10.3892/ijmm.2018.3800
219. Puengel T, Liu H, Guillot A, Heymann F, Tacke F, Peiseler M. Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int J Mol Sci (2022) 23(5):2668. doi: 10.3390/ijms23052668
220. Tong X, Song Y, Yin S, Wang J, Huang R, Wu C, et al. Clinical impact and mechanisms of hepatitis b virus infection concurrent with non-alcoholic fatty liver disease. Chin Med J (2022) 135(14):1653–63. doi: 10.1097/CM9.0000000000002310
221. Liu X, Shen Z, Zhang H, Liang J, Lin H. Interleukin-21 is associated with early antiviral response in patients with hepatitis b e antigen-positive chronic hepatitis b and nonalcoholic fatty liver disease. J Interferon Cytokine Res (2016) 36(6):367–73. doi: 10.1089/jir.2015.0129
222. Yoo C, Kang J, Lim HY, Kim JH, Lee MA, Lee KH, et al. Phase I dose-finding study of OPB-111077, a novel STAT3 inhibitor, in patients with advanced hepatocellular carcinoma. Cancer Res Treat (2019) 51(2):510–8. doi: 10.4143/crt.2018.226
223. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol (2015) 8(122):015–0220. doi: 10.1186/s13045-015-0220-7
224. Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. (2012) 122(8):2884–97. doi: 10.1172/JCI63455
Keywords: NAFLD, HBV, infection, hepatic steatosis, HCC, liver disease
Citation: Tourkochristou E, Assimakopoulos SF, Thomopoulos K, Marangos M and Triantos C (2022) NAFLD and HBV interplay - related mechanisms underlying liver disease progression. Front. Immunol. 13:965548. doi: 10.3389/fimmu.2022.965548
Received: 09 June 2022; Accepted: 15 November 2022;
Published: 05 December 2022.
Edited by:
Jinhang Gao, Sichuan University, ChinaReviewed by:
Guandou Yuan, the First Affiliated Hospital of Guangxi Medical University, ChinaHong Yien Tan, Xiamen University, Malaysia
Copyright © 2022 Tourkochristou, Assimakopoulos, Thomopoulos, Marangos and Triantos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stelios F. Assimakopoulos, c2Fzc2ltQHVwYXRyYXMuZ3I=
 Evanthia Tourkochristou
Evanthia Tourkochristou Stelios F. Assimakopoulos
Stelios F. Assimakopoulos Konstantinos Thomopoulos1
Konstantinos Thomopoulos1 Christos Triantos
Christos Triantos